|
|
| (52 intermediate revisions by the same user not shown) |
| Line 1: |
Line 1: |
| {{Arey1924 Header}} | | {{Arey1924 Header}} |
|
| |
|
| | [[File:Arey1924 Title-page.jpg|thumb]] |
| =Developmental Anatomy - A Text-Book And Laboratory Manual Of Embryology= | | =Developmental Anatomy - A Text-Book And Laboratory Manual Of Embryology= |
|
| |
|
| Line 6: |
Line 7: |
| By | | By |
|
| |
|
| Leslie Bleainerd Arey | | Leslie Brainerd Arey (1891-1988) |
|
| |
|
|
| |
|
| Professor Of Anatomy At The Northwestern University Medical School, Chicago . | | Professor Of Anatomy At The Northwestern University Medical School, Chicago. |
|
| |
|
|
| |
|
| Line 24: |
Line 25: |
|
| |
|
| ==Preface== | | ==Preface== |
| | {| width=100%| |
| | | This book has been prepared for the use of medical students and others whose interests center primarily on man and mammals. The emphasizing of structural rather than functional aspects of Embryology is reflected in the title; such presentation is consistent both with the practical demands of modern courses and with the meagre information existant as to the physiological factors in developmcnt. |
|
| |
|
| This book has been prepared for the use of medical students and others whose interests center primarily on man and mammals. The emphasizing of structural rather than functional aspects of Embryology is reflected in the title; such presentation is consistent both with the practical demands of modern courses and with the meagre information existant as to the physiological factors in developmcnt.
| |
|
| |
|
|
| |
|
| The volume contains three sections. In the first i>art the early stages are treated comparatively and the fulCcourse of prenatal and postnatal development is outlined. The second section traces the origin and differentiation of the human organ-systems, grouped according to their germlayer derivations. The third division comprises a laboratory manual for the study of chick and pig embryos. | | The volume contains three sections. In the first part the early stages are treated comparatively and the full course of prenatal and postnatal development is outlined. The second section traces the origin and differentiation of the human organ-systems, grouped according to their germlayer derivations. The third division comprises a laboratory manual for the study of chick and pig embryos. |
| | |
|
| |
|
|
| |
|
| Many illustrations are from the earlier Prentiss-Arey text and discontinuous fragments of description have likewise been retained. Yet, in plan and content the work is essentially new. It is hoped that the developmental story has been told in an orderly and clear, but concise fashion, and that it records accurately the present state of the subject. | | Many illustrations are from the earlier Prentiss-Arey text and discontinuous fragments of description have likewise been retained. Yet, in plan and content the work is essentially new. It is hoped that the developmental story has been told in an orderly and clear, but concise fashion, and that it records accurately the present state of the subject. |
| | |
| | |
| | |
|
| |
|
|
| |
|
| Line 37: |
Line 43: |
|
| |
|
| Chicago, ill., September, 1924. | | Chicago, ill., September, 1924. |
| | | [[File:Leslie Arey.jpg|thumb|Leslie Brainerd Arey (1891-1988)]] |
| | |} |
|
| |
|
| ==Contents== | | ==Contents== |
| | | {| |
| PART I. GENERAL DEVELOPMENT | | | valign=top|PART I. GENERAL DEVELOPMENT |
| | | * Introduction |
| Introduction | | * General Features of Development |
| | | * Fundamental Conceptions |
| General Features of Development | | * The Vertebrate Groups |
| | | * Titles for Collateral Reading and Reference |
| Fundamental Conceptions | | * [[Book - Developmental Anatomy 1924-1|Chapter I. - The Germ Cells and Fertilization]] |
| | | ** The Germ Cells |
| The Vertebrate Groups | | ** Spermatogenesis, Oogenesis and Maturation |
| | | ** Ovulation and Insemination |
| Titles for Collateral Reading and Reference | | ** Fertilization |
| | | ** Heredity and Sex |
| [[Book - Developmental Anatomy 1924-1|Chapter I. - The Germ Cells and Fertilization]] | | * [[Book - Developmental Anatomy 1924-2|Chapter II. - Cleavage and the Origin of the Germ Layers]] |
| | | ** Cleavage |
| The Germ Cells | | ** The formation of Ectoderm and Entoderm (Gastrulation) |
| | | ** Origin of the Mesoderm, Notochord and Neural Tube |
| Spermatogenesis, Oogenesis and Maturation | | * [[Book - Developmental Anatomy 1924-3|Chapter III. - Implantation and Fetal Membranes]] |
| | | ** The Fetal Membranes of Reptiles and Birds |
| Ovulation and Insemination | | ** The Fetal Membranes of Mammals |
| | | ** The Fetal Membranes of Man |
| Fertilization | | ** Implantation and Early Mucosal Relations |
| | | ** The Decidual Membranes |
| Heredity and Sex | | ** The Placenta |
| | | ** Parturition |
| [[Book - Developmental Anatomy 1924-2|Chapter II. - Cleavage and the Origin of the Germ Layers]] | | * [[Book - Developmental Anatomy 1924-4|Chapter IV. - Age, Body Form and Growth Changes]] |
| | | ** Age, Size and Weight of Embryos |
| Cleavage | | ** An Outline of Prenatal Development |
| | | ** The Establishment of External Form |
| The formation of Ectoderm and Entoderm (Gastrulation) | | ** Growth Changes |
| | | | valign=top|PART II. ORGANOGENESIS |
| Origin of the Mesoderm, Notochord and Neural Tube | |
| | |
| [[Book - Developmental Anatomy 1924-3|Chapter III. - Implantation and Fetal Membranes]] | |
| | |
| The Fetal Membranes of Reptiles and Birds | |
| | |
| The Fetal Membranes of Mammals | |
| | |
| The Fetal Membranes of Man | |
| | |
| Implantation and Early Mucosal Relations | |
| | |
| The Decidual Membranes | |
| | |
| The Placenta | |
| | |
| Parturition | |
| | |
| [[Book - Developmental Anatomy 1924-4|Chapter IV. - Age, Body Form and Growth Changes]] | |
| | |
| Age, Size and Weight of Embryos | |
| | |
| An Outline of Prenatal Development | |
| | |
| The Establishment of External Form | |
| | |
| Growth Changes | |
| | |
| | |
| PART IV. ORGANOGENESIS | |
|
| |
|
| Entodermal Derivatives | | Entodermal Derivatives |
| | * [[Book - Developmental Anatomy 1924-5|Chapter V. - The Digestive System]] |
| | ** The Mouth |
| | ** The Pharynx |
| | ** The Digestive Tube |
| | ** The Liver |
| | ** The Pancreas |
| | * [[Book - Developmental Anatomy 1924-6|Chapter VI. - The Respiratory System]] |
| | ** The Larynx |
| | ** The Trachea |
| | ** The Lungs |
|
| |
|
| [[Book - Developmental Anatomy 1924-5|Chapter V. - The Digestive System]] | | Mesodermal Derivatives |
| | | * [[Book - Developmental Anatomy 1924-7|Chapter VII. - The Mesenteries and Coelom]] |
| The Mouth | | ** The Mesenteries |
| | ** The Primitive Mesentery |
| | ** Differentiation of the Dorsal Mesentery |
| | ** Differentiation of the Ventral Mesentery |
| | ** The Ccelom |
| | ** The Primitive Coelom |
| | ** The Septum TransveRsum |
| | ** The Pleuro-pcricardial and Pleuro-peritoncal Membranes |
| | ** The Pericardium and Diaphragm |
| | * [[Book - Developmental Anatomy 1924-8|Chapter VIII. - The Urogenital System]] |
| | ** The Urinary Organs |
| | ** The Pronephros |
| | ** The Mesonephros |
| | ** The Metanephros |
| | ** Differentiation of the Cloaca |
| | ** The Genital Organs |
| | ** Indifferent Stage |
| | ** Internal Sexual Transformations |
| | ** The External Genitalia |
| | ** Homologies of Internal and External Genitalia |
| | * [[Book - Developmental Anatomy 1924-9|Chapter IX. - The Vascular System]] |
| | ** Origin of the Blood Vessels and Blood Cells |
| | ** Hemopoiesis |
| | ** Development of the Heart |
| | ** The Primitive Vascular System |
| | ** Development of the Arteries |
| | ** Development of the Veins |
| | ** Fetal Circulation and the Changes at Birth |
| | ** The Lymphatic System |
| | | valign=top| |
|
| |
|
| The Pharynx | | * [[Book - Developmental Anatomy 1924-10|Chapter X. - The Skeletal System]] |
| | ** Histogenesis of the Supporting Tissues |
| | ** Connective Tissue |
| | ** Cartilage |
| | ** Bone |
| | ** Morphogenesis of the Skeleton |
| | ** The Axial Skeleton |
| | ** The Appendicular Skeleton |
| | * [[Book - Developmental Anatomy 1924-11|Chapter XI. - The Muscular System]] |
| | ** The Histogenesis of Muscle |
| | ** Morphogenesis of the Muscles |
| | ** Ectodermal Derivatives |
| | * [[Book - Developmental Anatomy 1924-12|Chapter XII. - The Integumentary System]] |
| | ** The Skin |
| | ** The Nails |
| | ** The Hair |
| | ** Sebaceous Glands |
| | ** Sweat Glands |
| | ** Mammary Glands |
| | * [[Book - Developmental Anatomy 1924-13|Chapter XIII. - The Central Nervous System]] |
| | ** Histogenesis of the Nervous Tissues |
| | ** Morphogenesis of the Central Nervous System |
| | ** The Spinal Cord |
| | ** The Brain |
| | * [[Book - Developmental Anatomy 1924-14|Chapter XIV. - The Peripheral Nervous System]] |
| | ** The Spinal Nerves |
| | ** The Cranial Nerves |
| | ** The Sympathetic Nervous System |
| | ** The Chromaffin Bodies and Suprarenal Gland |
| | * [[Book - Developmental Anatomy 1924-15|Chapter XV. - The Sense Organs]] |
| | ** General Sensory Organs |
| | ** The Gustatory Organ 292 |
| | ** The Nose |
| | ** The Eye |
| | ** The Ear |
| | | valign=top| Part III. A LABORATORY MANUAL OF EMBRYOLOGY |
| | * [[Book - Developmental Anatomy 1924-16|Chapter XVI. - The Study of Chick Embryos]] |
| | ** The Unincubated Ovum and Embryos of the First Day |
| | ** Embryo of Five Segments (Twenty-Three Hours) |
| | ** Embryo of Seven Segments (Twenty-five Hours) |
| | ** Embryo of Seventeen Segments (Thirty-eight Hours) |
| | ** Embryo of Twenty-seven Segments (Two Days) |
| | ** Embryos of Three to Four Days |
| | * [[Book - Developmental Anatomy 1924-17|Chapter XVII. - The Study of Pig Embryos]] |
| | ** The Anatomy of a Six Mm. Pig Embryo |
| | ** The Anatomy of Ten to Twelve Mm, Pig Embryos |
| | ** The Anatomy of an Eighteen Mm. Pig Embryo |
| | ** The Anatomy of a Thirty-five Mm. Pig Embryo |
| | ** Methods for the Dissection of Pig Embryos |
| | |} |
|
| |
|
| The Digestive Tube
| | ==Part I. General Development== |
|
| |
|
| The Liver
| | ===Introduction=== |
|
| |
|
| The Pancreas | | ====The Scope of Embryology==== |
| | Developmental anatomy, or embryology, traces the formative history of the individual from the origin of the germ cells to the adult condition. Although the most striking changes in human development occur while the young (called an embryo or fetus) is still inside its mother's womb, yet development by no means ceases at birth. Birth is a mere incident which occurs when the new individual is sufficiently advanced to allow its transference from a protected riterine environment to one in the external world. Some vertebrates, like fishes and amphibia, are capable of an active and independent existence at very immature stages; these free-living larvae, as they are termed, then gradually progress to adults. The human newborn, although far more complete anatomically, is still utterly dependent for food and care: many years of infancy and childhood must elapse before it becomes self-maintaining in human society. During all this period, postnatal development continues. Birth, itself, initiates anatomical changes of profound influence on the body. Throughout the entire growth period, with its uneven but steadily slowing growth rate, come the completion of some organs and a gradual remoulding of the shape of the body and its parts. Only at the age of twenty-five are these progressive changes complete. |
|
| |
|
| [[Book - Developmental Anatomy 1924-6|Chapter VI. - The Respiratory System]]
| |
|
| |
|
| The Larynx | | All vertebrate, or backboned, animals are organized upon a common anatomical plan, and even many of their structural details are comparable, though superficially disguised. Similarly, their fundamental mode of development is essentially identical. The minor variations that do occur are caused by such secondary modifying factors as the crowding yolk content of the egg or adaptations to development inside or outside the mother's body. While the comparative viewpoint is indispensable for gaining a broad understanding of embryology, it has been of especial importance in supplying missing parts of the human developmental story and in interpreting many perplexing conditions. For, the earliest human embryos known are about two weeks old and have the three primary germ layers already formed. Even invertebrate material is highly useful for demonstrating such early stages as maturation, fertilization, cleavage, and the formation of blastula and gastrula. |
|
| |
|
| The Trachea | | ====The Value of Embryology==== |
| | A general conception of how man and other animals develop from a single cell by orderly and logical processes should share in the cultural background of every educated mind. To the medical student, embryology is of primary importance because it affords a comprehensive understanding of the intricacies and variations of human anatomy, and thus is essential to sound surgical training. It also explains many anomalies and 'monstrous - conditions, and the origin of certain tumors and other pathological changes in the tissues. Obstetrics is essentially applied embryology. From the theoretical side, it is the key with which we may unlock the secrets of heredity, the determination of sex, and, in part, of organic evolution. |
|
| |
|
| The Lungs
| |
|
| |
|
| MeSodermal Derivatives
| | ====Historical==== |
| | [[File:Arey1924 fig001.jpg|thumb|Fig. 1. - Human sperm cell containing a miniature organism, according to Hartsoeker (1694).]] |
| | The science of modern embryology is comparatively new, originating with the use of the compound microscope and advancing with the improvement of microscopical technique. [[Book_-_Fathers_of_Biology#Aristotle|Aristotle]] (384-322 B. c.), however, centuries before the introduction of magnifying lenses had followed the general development of the chick, day by day. The popular belief that slime and decaying matter is capable of giving rise to living animals, as also asserted by Aristotle, was disproved by Redi (1668). |
|
| |
|
| [[Book - Developmental Anatomy 1924-7|Chapter VII. - The Mesenteries and Coelom]]
| |
|
| |
|
| | A few years after Harvey and Malpighi had published their fundamental studies on the chick embryo, Leeuwenhoek reported the discovery of the human spermatozoon by Ham in 1677 - At this period, it was believed either that fully formed animals existed in miniature in the egg, needing only the stimulus of the spermatozoon to initiate development, or that similarly preformed bodies, male and female, constituted the spermatozoa and that these merely enlarged within the ovum. According to this doctrine of preformation, all future generations were likewise encased, one inside the sex cells of the other, and serious computations were made as to the probable number of progeny (200 millon) thus present in the ovary of Mother Eve, at the exhaustion of which the human race would end! Dalenpatius (1699) and others even believed they had observed a minute human form in the spermatozoon (Fig. 1). |
|
| |
|
| The Mesenteries
| |
|
| |
|
| The Primitive Mesentery | | The preformation theory was strongly combated by [[:File:Theoria Generationis 1774.jpg|Wolff (1759)]], who saw that the organs of the early chick embryo were differentiated gradually from unspecialized living substance. This theory, known as epigenesis, was proved correct when von Baer discovered the mammalian ovum in 1827, and later demonstrated the germ-layer composition of all embryos. |
|
| |
|
| Differentiation of the Dorsal Mesentery
| |
|
| |
|
| Differentiation of the Ventral Mesentery
| | About twenty years after Schleiden and Schwann (1839) had shown the cell to be the structural unit of the organism, the ovum and spermatozoon were recognized as true cells. [[:File:Oskar Hertwig.jpg|O. Hertwig]], in was the first to observe and appreciate the events of fertilization. Henceforth, all multicellular organisms were believed to develop each from a single fertilized ovum. This conception is expressed in the famous aphorism: ''ornne vivum ex ovo'' . |
|
| |
|
| The Ccelom
| | ====Modern Embryology==== |
| | | As an organized and definite science, began with [[:File:Francis Balfour.jpg|Balfour (1874)]], who reviewed, digested, and made accessible the earlier scattered facts. Throughout this period, the experimental method of investigation has been used increasingly; without it many structural and physiological aspects of development would remain unsolved. |
| The Primitive Coelom
| |
| | |
| The Septum TransveRsum
| |
| | |
| The Pleuro-pcricardial and Pleuro-peritoncal Membranes
| |
| | |
| The Pericardium and Diaphragm
| |
| | |
| [[Book - Developmental Anatomy 1924-8|Chapter VIII. - The Urogenital System]]
| |
| | |
| The Urinary Organs
| |
|
| |
| The Pronephros
| |
| | |
| The Mesonephros
| |
| | |
| The Metanephros
| |
| | |
| Differentiation of the Cloaca
| |
| | |
| The Genital Organs
| |
| | |
| Indifferent Stage
| |
| | |
| Internal Sexual Transformations
| |
| | |
| The External Genitalia
| |
| | |
| Homologies of Internal and External Genitalia
| |
| | |
| | |
| [[Book - Developmental Anatomy 1924-9|Chapter IX. - The Vascular System]]
| |
| | |
| Origin of the Blood Vessels and Blood Cells
| |
| | |
| Hemopoiesis
| |
| | |
| Development of the Heart
| |
| | |
| The Primitive Vascular System
| |
| | |
| Development of the Arteries
| |
| | |
| Development of the Veins
| |
| | |
| Fetal Circulation and the Changes at Birth
| |
| | |
| The Lymphatic System
| |
| | |
| [[Book - Developmental Anatomy 1924-10|Chapter X. - The Skeletal System]]
| |
| | |
| Histogenesis of the Supporting Tissues
| |
| | |
| Connective Tissue
| |
| | |
| Cartilage
| |
| | |
| Bone
| |
| | |
| Morphogenesis of the Skeleton
| |
| | |
| The Axial Skeleton
| |
| | |
| The Appendicular Skeleton
| |
| | |
| [[Book - Developmental Anatomy 1924-11|Chapter XI. - The Muscular System]]
| |
| | |
| The Histogenesis of Muscle
| |
| | |
| Morphogenesis of the Muscles
| |
| | |
| Ectodermal Derivatives
| |
| | |
| [[Book - Developmental Anatomy 1924-12|Chapter XII. - The Integumentary System]]
| |
| | |
| The Skin
| |
| | |
| The Nails
| |
| | |
| The Hair
| |
| | |
| Sebaceous Glands
| |
| | |
| Sweat Glands
| |
| | |
| Mammary Glands
| |
| | |
| [[Book - Developmental Anatomy 1924-13|Chapter XIII. - The Central Nervous System]]
| |
| | |
| Histogenesis of the Nervous Tissues
| |
| | |
| Morphogenesis of the Central Nervous System
| |
| | |
| The Spinal Cord
| |
|
| |
| The Brain
| |
| | |
| [[Book - Developmental Anatomy 1924-14|Chapter XIV. - The Peripheral Nervous System]]
| |
| | |
| The Spinal Nerves
| |
| | |
| The Cranial Nerves
| |
| | |
| The Sympathetic Nervous System
| |
| | |
| The Chromaffin Bodies and Suprarenal Gland
| |
| | |
| [[Book - Developmental Anatomy 1924-15|Chapter XV. - The Sense Organs]]
| |
| | |
| General Sensory Organs
| |
| | |
| The Gustatory Organ 292
| |
| | |
| The Nose
| |
| | |
| The Eye
| |
| | |
| The Ear
| |
| | |
| | |
| Part III. A LABORATORY MANUAL OF EAIBRYOLOGY
| |
| | |
| | |
| [[Book - Developmental Anatomy 1924-16|Chapter XVI. - The Study of Chick Embryos]]
| |
|
| |
| The Unincubated Ovum and Embryos of the First Day
| |
| | |
| Embryo of Five Segments (Twenty-Three Hours)
| |
| | |
| Embryo of Seven Segments (Twenty-five Hours)
| |
|
| |
| Embryo of Seventeen Segments (Thirty-eight Hours)
| |
| | |
| Embryo of Twenty-seven Segments (Two Days)
| |
| | |
| Embryos of Three to Four Days
| |
| | |
| [[Book - Developmental Anatomy 1924-17|Chapter XVII. - The Study of Pig Embryos]]
| |
| | |
| The Anatomy of a Six Mm. Pig Embryo
| |
| | |
| The Anatomy of Ten to Twelve Mm, Pig Embryos
| |
| | |
| The Anatomy of an Eighteen Mm. Pig Embryo
| |
| | |
| The Anatomy of a Thirty-five Mm. Pig Embryo
| |
| | |
| Methods for the Dissection of Pig Embryos
| |
| | |
| | |
| PART I. GENERAL DEVELOPMENT
| |
| | |
| | |
| INTRODUCTION
| |
| | |
| | |
| The Scope of Embryology. - Developmental anatomy, or embryology, traces the formative history of the individual from the origin of the germ cells to the adult condition. Although the most striking changes in human development occur while the young (called an embryo or fetus) is still inside its mother - s womb, yet development by no means ceases at birth. Birth is a mere incident which occurs when the new individual is sufficiently advanced to allow its transference from a protected riterine environment to one in the external world. Some vertebrates, like fishes and amphibia, are capable of an active and independent existence at very immature stages; these free-living larvee, as they are termed, then gradually progress to adults. The human newborn, although far more complete anatomically, is still utterly dependent for food and care: many years of infancy and childhood must elapse before it becomes self-maintaining in human society. During all this period, postnatal development continues. Birth, itself, initiates anatomical changes of profound influence on the body. Throughout the entire growth period, with its uneven but steadily slowing growth rate, come the completion of some organs and a gradual remoulding of the shape of the body and its parts. Only at the age of twenty-five are these progressive changes complete.
| |
| | |
| | |
| All vertebrate, or backboned, animals are organized upon a common anatomical plan, and even many of their structural details are comparable, though superficially disguised. vSimilarly, their fundamental mode of development is essentially identical. The minor variations that do occur are caused by such secondary modifying factors as the crowding yolkcontent of the egg or adaptations to development inside or outside the mother - s body. While the comparative viewpoint is indispensable for gaining a broad understanding of embryology, it has been of especial importance in supplying missing parts of the human developmental story and in interpreting many perplexing conditions. For, the earliest human embryos known are about two weeks old and have the three primary germ layers already formed. Even invertebrate material is highly useful for demonstrating such early stages as maturation, fertilization, cleavage, and the formation of blastula and gastrula.
| |
| | |
| | |
| The Value of Embryology. - A general conception of how man and other animals develop from a single cell by orderly and logical processes should share in the cultural background of every educated mind. To the medical student, embryology is of primary importance because it affords a comprehensive understanding of the intricacies and variations of human anatomy, and thus is essential to sound surgical training. It also explains many anomalies and 'monstrous - conditions, and the origin of certain tumors and other pathological changes in the tissues. Obstetrics is essentially applied embryology. From the theoretical side, it is the key with which we may unlock the secrets of heredity, the determination of sex, and, in part, of organic evolution.
| |
| | |
| | |
| Histoucal. - The science of modern embryology is comparatively new, originating with the use of the compound microscope and advancing with the improvement of microscopical technique. Aristotle (384-322 B. c.), however, centuries before the introduction of magnifying lenses had followed the general development of the chick, day by day. The popular belief that slime and decaying matter is capable of giving rise to living animals, as also asserted by Aristotle, was disproved by Redi (1668).
| |
| | |
| .A few years after Harvey and Malpighi had published their fundamental studies on the chick embryo, Leeuwenhoek reported the discovery of the human Sp ermatozoon by Ham in 1677 - At this period, it was believed either that fully formed animals existed in miniature in the egg, needing only the stimulus of the spermatozoon to initiate development, or that similarly preformed bodies, male and female, constituted the spermatozoa and that these merely enlarged within the ovum. According to this doctrine of preformation, all future generations were likewise encased, one inside the sex cells of the other, and serious computations were made as to the probable number of progeny (200 millon) thus present in the ovary of Mother Eve, at the exhaustion of which the human race would end! Dalenpatius ( 1699) and others even believed they had observed a minute human form in the spermatozoon (Fig. i).
| |
| | |
| | |
| | |
| The preformation theory was strongly combated by Wolff ( 175 9), who saw that the organs of the early chick embryo were differentiated gradually from unspecialized li ving substance. This theory, known as epi genesis, was proved correct wEen von Baer discQvered the mammalian ovurn in 1 827, and later demonstrated the germ-layer composition of all embryos.
| |
| | |
| | |
| Fig. I. - Human sperm cell containing a miniature organism, according to Hartsoeker (1694).
| |
| | |
| | |
| About twenty years after Schleiden and Schwann (1839) had shown the cell to be the structural unit of the organism, the ovum and spermatozoon were recognized as true cells. O. Hertwig , in was the first to
| |
| observe and appreciate the events of fertilizat ion . Henceforth, all multicellular organisms were believed to develop each from a single fertilized ovum. This conception is expressed in the famous aphorism: - ornne_ vivum ex ovo . -
| |
| Modern embryology , as an organized and definite science, began with Balfou r (1874 ), who reviewed, digested, and made accessible the earlier scattered facts. Throughout this period, the experimental method of investigation has been used increasingly; without it many structural and physiological aspects of development would remain unsolved.
| |
| | |
| | |
| | |
| GENERAL FEATURES OF DEVELOPMENT
| |
|
| |
|
| | ===General Features Of Development=== |
|
| |
|
| A multicellular embryo results from the division of the fertilized ovum to form daughter cells. These are at first quite similar in structure, and, if separated, in some animals each may become a complete embryo (sea urchin; certain vertebrates). In general, the development of an embryo depends: (i) upon the multiplication of its cells by division; (2) upon the growth in size of the individual cells; (3) upon changes in their form and structure. | | A multicellular embryo results from the division of the fertilized ovum to form daughter cells. These are at first quite similar in structure, and, if separated, in some animals each may become a complete embryo (sea urchin; certain vertebrates). In general, the development of an embryo depends: (i) upon the multiplication of its cells by division; (2) upon the growth in size of the individual cells; (3) upon changes in their form and structure. |
|
| |
|
|
| |
|
| Cell Division. - All cells arise from pre-existing cells by division. There are two methods of cell division - amitosis and mitosis. | | Cell Division - All cells arise from pre-existing cells by division. There are two methods of cell division - amitosis and mitosis. |
| | |
| | |
| Amitosis. - Cells may divide directly by the simple fission of their nuclei and cytoplasm. This rather infrequent process is called amitosis. Amitosis is said by many to occur only in specialized or moribund cells. It is the type of cell division demonstrable in the epithelium of the bladder.
| |
| | |
| | |
| Mitosis - In the reproduction of typically active somatic cells and in all germ cells, complicated changes take place in the nucleus. These changes give rise to thread-like structures, hence the process is termed mitosis (thread) in distinction to amitosis (no threadk Mitosis is divided for convenience into four phases (Fig. 2) :
| |
| Prophase. - 1. The centrosome divides and the two minute bodies resulting from the division move apart, ultimately occup^dng positions at opposite poles of the nucleus (I-uI).
| |
| | |
| | |
| | |
| 2. Astral rays appear in the cytoplasm about each centriole. They radiate from it, and the threads of the central or achromatic spindle are formed between the two asters, thus constituting the amphiastcr (u).
| |
| | |
| | |
| 3. The nuclear membrane and nucleolus disappear, the karyoplasm and cytoplasm becoming confluent.
| |
| | |
| | |
| 4. During the above changes the chromatic network of the resting nucleus resolves itself into a skein, or spireme, which soon shortens and l;)reaks up into distinct, heavily-staining bodies, the chromosomes (u, uI). x\ definite number of chromosomes is always found in the cells of a given species, the chromosomes may be block-shaped, rod- shaped, or bent in the form of a U or V.
| |
| | |
| | |
| | |
| Fig. 2. - Diagrams of the phases of mitosis (Schafer).
| |
| | |
|
| |
|
|
| |
|
| 5. The chromosomes arrange themselves in the equatorial plane of the central spindle (IV). If U- or V-shaped, the angle of each is directed toward a common center. The amphiaster and the chromosomes together constitute a mitotic figure, and at the end of the prophase this is called a monaster.
| | Amitosis - Cells may divide directly by the simple fission of their nuclei and cytoplasm. This rather infrequent process is called amitosis. Amitosis is said by many to occur only in specialized or moribund cells. It is the type of cell division demonstrable in the epithelium of the bladder. |
|
| |
|
| | [[File:Arey1924 fig002.jpg|thumb|Fig. 2. Diagrams of the phases of mitosis (Schafer).]] |
| | ====Mitosis==== |
| | In the reproduction of typically active somatic cells and in all germ cells, complicated changes take place in the nucleus. These changes give rise to thread-like structures, hence the process is termed mitosis (thread) in distinction to amitosis (no thread). Mitosis is divided for convenience into four phases (Fig. 2) : |
|
| |
|
| Metaphase. - The longitudinal splitting of the chromosomes into exactly similar halves constitutes the metaphasc (IV). The aim of mitosis is thus accomplished, an accurate division of the chromatin between the nuclei of the daughter cells.
| | Prophase |
| | # The centrosome divides and the two minute bodies resulting from the division move apart, ultimately occupying positions at opposite poles of the nucleus (I-III). |
| | # Astral rays appear in the cytoplasm about each centriole. They radiate from it, and the threads of the central or achromatic spindle are formed between the two asters, thus constituting the amphiaster (II). |
| | # The nuclear membrane and nucleolus disappear, the karyoplasm and cytoplasm becoming confluent. |
| | # During the above changes the chromatic network of the resting nucleus resolves itself into a skein, or spireme, which soon shortens and breaks up into distinct, heavily-staining bodies, the chromosomes (II, III). The definite number of chromosomes is always found in the cells of a given species, the chromosomes may be block-shaped, rod- shaped, or bent in the form of a II or V. |
| | # The chromosomes arrange themselves in the equatorial plane of the central spindle (IV). If U- or V-shaped, the angle of each is directed toward a common center. The amphiaster and the chromosomes together constitute a mitotic figure, and at the end of the prophase this is called a monaster. |
|
| |
|
|
| |
|
| Anaphase. - The two groups of daughter chromosomes separate and move up along the central spindle fibers, each toward one of the two asters. Hence this is called the diaster stage (V, VI). Each centriole may divide in preparation for the next diviSion of the daughter cells.
| | Metaphase - The longitudinal splitting of the chromosomes into exactly similar halves constitutes the metaphasc (IV). The aim of mitosis is thus accomplished, an accurate division of the chromatin between the nuclei of the daughter cells. |
|
| |
|
| Telophase. - i. The daughter chromosomes resolve themselves into a reticulum and daughter nuclei are formed (Vu, VuI).
| |
|
| |
|
| | Anaphase - The two groups of daughter chromosomes separate and move up along the central spindle fibers, each toward one of the two asters. Hence this is called the diaster stage (V, VI). Each centriole may divide in preparation for the next diviSion of the daughter cells. |
|
| |
|
| 2. The cytoplasm divides in a plane perpendicular to the axis of the mitotic spindle (VuI). Two complete daughter cells have thus arisen from the mother cell.
| | Telophase - i. The daughter chromosomes resolve themselves into a reticulum and daughter nuclei are formed (Vu, VuI). |
|
| |
|
| The number of chromosomes is constant in the cells of a given species. The smallest assortment, two, occurs in Ascaris megalocephala univaleus, a round worm parasitic in the intestine of the horse. The largest number known is found in the brine shrimp, Artemia, where 1 68 have been counted. The chromosome enumeration for the human cell has been variously stated but the results of Winiwarter (1912), Grosser (1921 ), and Painter (1923) now agree on a relatively high number, which Painter establishes as 48 for whites and negroes of both sexes.
| |
|
| |
|
| | 2. The cytoplasm divides in a plane perpendicular to the axis of the mitotic spindle (VIII). Two complete daughter cells have thus arisen from the mother cell. |
|
| |
|
|
| |
|
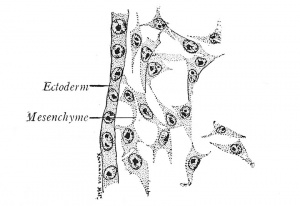
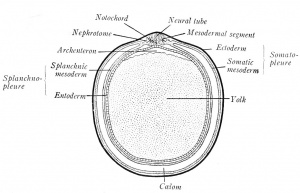
| The Germ Layers. - The first changes in the form and arrangement of the cells establish three definite plates, the primary germ layers, which are termed from their positions the ectoderm (outer skin), mesoderm (middle skin) and entoderm (inner skin) (Fig. 4). Since the ectoderm covers the body, it is primarily protective in function, but it also gives origin to the nervous system, through which sensations are received from the outer world. The entoderm, on the other hand, lines the digestive canal and is from the first nutritive. The mesoderm, lying between the other two layers, naturally performs the functions of circulation, of muscular movement, and of excretion; it also gives rise to the skeletal structures which support the body. While all three germ layers form definite sheets of cells known as epithelia, the mesoderm takes also the form of a diffuse meshwork of cells, the mesenchyme (Fig. 3). | | The number of chromosomes is constant in the cells of a given species. The smallest assortment, two, occurs in Ascaris megalocephala univaleus, a round worm parasitic in the intestine of the horse. The largest number known is found in the brine shrimp, Artemia, where 168 have been counted. The chromosome enumeration for the human cell has been variously stated but the results of Winiwarter (1912), Grosser (1921), and Painter (1923) now agree on a relatively high number, which Painter establishes as 48 for whites and negroes of both sexes. |
|
| |
|
| | ===The Germ Layers=== |
| | [[File:Arey1924 fig003.jpg|thumb|Fig. 3. Mesenchyme from a chick embryo (Prentiss). X 495.]] |
| | The first changes in the form and arrangement of the cells establish three definite plates, the primary germ layers, which are termed from their positions the ectoderm (outer skin), mesoderm (middle skin) and entoderm (inner skin) (Fig. 4). Since the ectoderm covers the body, it is primarily protective in function, but it also gives origin to the nervous system, through which sensations are received from the outer world. The entoderm, on the other hand, lines the digestive canal and is from the first nutritive. The mesoderm, lying between the other two layers, naturally performs the functions of circulation, of muscular movement, and of excretion; it also gives rise to the skeletal structures which support the body. While all three germ layers form definite sheets of cells known as epithelia, the mesoderm takes also the form of a diffuse meshwork of cells, the mesenchyme (Fig. 3). |
|
| |
|
|
| |
| Fig. 3. - Alesenchyme from a chick embryo (Prentiss). X 495.
| |
|
| |
|
|
| |
|
| Line 388: |
Line 258: |
| This series of changes - an embryonic (undifferentiated) stage; progressive functional s])ecialization ; gradual degeneration; death and removal - which tissue cells experience is designated by the term cytomorphosis. | | This series of changes - an embryonic (undifferentiated) stage; progressive functional s])ecialization ; gradual degeneration; death and removal - which tissue cells experience is designated by the term cytomorphosis. |
|
| |
|
| Derivatives of the Germ Layers. - The tissues of the adult are derived from the primary germ layers as follows: . | | ====Derivatives of the Germ Layers==== |
| | |
| | The tissues of the adult are derived from the primary germ layers as follows: |
| | |
| | {| |
| | |-bgcolor="CEDFF2" |
| | ! width=30%|Ectoderm |
| | ! width=30%|Mesoderm |
| | ! width=30%|Entoderm |
| | |- |
| | | I. Epidermis and derivatives. |
| | |
| | Hair; nails; glands. |
|
| |
|
| | Lens of eye. |
|
| |
|
| Ectoderm
| | 2. Epithelium of: |
|
| |
|
| Mesoder
| | Organs of special sense. Cornea. |
|
| |
|
| | Mouth; enamel organ. |
|
| |
|
| Entoderm Epithelium of:
| | Oral glands; hypophysis. |
| I. Pharynx and derivatives. Auditory tube.
| |
|
| |
|
| Tonsils.
| | Anus. |
|
| |
|
| Thymus.
| | Amnion; chorion. |
|
| |
|
| Thyroid.
| | 3. Nervous tissue. |
|
| |
|
| | Neuroglia. |
|
| |
|
| I. Epidermis and derivatives. Hair; nails; glands.
| | Chromaffin tissue. |
|
| |
|
| Lens of eye.
| | 4. Smooth muscle of; Iris. |
|
| |
|
| A. Mesothelium. | | Sweat glands. |
| | | A. Mesothelium. |
|
| |
|
| 1. Pericardium. | | 1. Pericardium. |
| Line 418: |
Line 303: |
| 3. Peritoneum. | | 3. Peritoneum. |
|
| |
|
| 4. LTrogenital epithelia. | | 4. Urogenital epithelia. |
|
| |
|
| 5. Striated muscle. | | 5. Striated muscle. |
|
| |
|
| | B. Mesenchyme. |
|
| |
|
| | 1 . Smooth muscle. |
|
| |
|
| 2. Epithelium of: | | 2. Notochord. |
|
| |
|
| Organs of special sense. Cornea.
| | 3. Connective tissue; cartilage; bone. |
|
| |
|
| | 4. Blood; bone marrow. |
|
| |
|
| Ectoderm
| | 5. Endothelium of blood vessels and lymphatics. |
|
| |
|
| | 6. Lymphoid organs. |
|
| |
|
| Mesoderm
| | 7. Suprarenal cortex. |
| | | Epithelium of: |
|
| |
|
| | I. Pharynx and derivatives. Auditory tube. |
|
| |
|
| Entoderm
| | Tonsils. |
|
| |
|
| | Thymus. |
|
| |
|
| Mouth; enamel organ. Oral glands; hypophysis. Anus.
| | Thyroid. |
| | |
| Amnion; chorion.
| |
| | |
| | |
| B. Mesenchyme.
| |
| | |
|
| |
|
| Parathyroid. | | Parathyroid. |
| Line 450: |
Line 336: |
| 2. Respiratory tract. | | 2. Respiratory tract. |
|
| |
|
| | | Larynx; trachea. |
| 1 . Smooth muscle.
| |
| | |
| 2. Notochord.
| |
| | |
| 3. Connective tissue; .
| |
| | |
|
| |
|
| Lungs. | | Lungs. |
| Line 462: |
Line 342: |
| 3. Digestive tract. | | 3. Digestive tract. |
|
| |
|
| Larynx; trachea.
| | Liver; pancreas. |
| | |
| 3. Nervous tissue. Neuroglia. Chromaffin tissue.
| |
| | |
| | |
| cartilage; bone.
| |
| | |
| 4. Blood; bone marrow.
| |
| | |
| 5. Endothelium of blood .
| |
| | |
|
| |
|
| Yolk sac; allantois. | | Yolk sac; allantois. |
| Line 478: |
Line 348: |
| 4. Bladder (except trigone). | | 4. Bladder (except trigone). |
|
| |
|
| 5. LTrethra (except prostatic). | | 5. Urethra (except prostatic). |
|
| |
|
| 6. Prostate. | | 6. Prostate. |
| | |} |
|
| |
|
| Liver; pancreas.
| | ===Primitive Segments - Metamerism=== |
| | | [[File:Arey1924 fig004.jpg|thumb|Fig. 4. Diagrammatic transverse section of a vertebrate embryo (Minot-Prentiss).]] |
| 4. Smooth muscle of; Iris.
| |
| | |
| Sweat glands.
| |
| | |
| vessels and lymphatics.
| |
| | |
| 6. Lymphoid organs.
| |
| | |
| 7. Suprarenal corte.
| |
| | |
| | |
| | |
| | |
| | |
| Primitive Segments - Metamerism. - A prominent feature of vertebrate embryos are the primitive segments, or metameres (Fig. 59). These segments are homologous to the serial divisions of an adult earth-worm - s body, divisions which, in the earth worm, are identical in structure, each containing a ganglion of the nerve cord, a muscle segment, or myotome, and pairs of blood vessels and nerves. In vertebrate embryos, the block like primitive segments lie next the neural tube and are known as mesodermal segments, or somites (Fig. 4). Each pair gives rise to a vertebra, to two myotomes, or muscle segments, and to paired vessels; each set of mesodermal segments is supplied by a pair of spinal nerves: consequently, the adult vertebrate body is segmented like that of the earth worm. As a worm grows by the formation of new segments at its tail-end, so the metameres of the vertebrate embryo begin to form in the head and are added tailward. There is this difference between the segments of the worm and the vertebrate embryo; the segmentation of the worm is complete, while that of the vertebrate is incomplete ventrally. | |
| | |
| | |
|
| |
|
| Fig. 4. - Diagrammatic transverse section of a vertebrate embryo (Minot-Prentiss). | | A prominent feature of vertebrate embryos are the primitive segments, or metameres (Fig. 59). These segments are homologous to the serial divisions of an adult earth-worm's body, divisions which, in the earth worm, are identical in structure, each containing a ganglion of the nerve cord, a muscle segment, or myotome, and pairs of blood vessels and nerves. In vertebrate embryos, the block like primitive segments lie next the neural tube and are known as mesodermal segments, or somites (Fig. 4). Each pair gives rise to a vertebra, to two myotomes, or muscle segments, and to paired vessels; each set of mesodermal segments is supplied by a pair of spinal nerves: consequently, the adult vertebrate body is segmented like that of the earth worm. As a worm grows by the formation of new segments at its tail-end, so the metameres of the vertebrate embryo begin to form in the head and are added tailward. There is this difference between the segments of the worm and the vertebrate embryo; the segmentation of the worm is complete, while that of the vertebrate is incomplete ventrally. |
|
| |
|
|
| |
|
| | ===Somatopleure and Splanchnopleure=== |
| | In early embryos the mesoderm splits into two layers, the somatic (dorsal) and splanchnic (ventral) mesoderm (Fig. 4). The ectoderm and somatic mesoderm constitute the body wall, which is termed the somatopleure. In the same way, the entoderm and splanchnic mesoderm combine as the splanchnopleure; it forms the mesenteries and the walls of the gut, heart, and lungs. |
|
| |
|
| Somatopleure and Splanchnopleure. In early embryos the mesoderm splits into two layers, the somatic (dorsal) and splanchnic (ventral) mesoderm (Fig. 4). The ectoderm and somatic mesoderm constitute the lu)dy wall, which is termed the somatopleure. In the same way, the entoderm and splanchnic mesoderm combine as the splanchnopleure; it forms the mesenteries and the walls of the gut, heart, and lungs.
| |
|
| |
|
| | ===Coelom=== |
| | The space between the somatopleure and splanchnopleure is the coelom, or body cavity. At the first splitting of the mesoderm, isolated clefts are produced. These unite on each side and eventually form one cavity - the coelom. With the extension of the mesoderm, the coelom surrounds the heart and gut ventrally (Fig. 4). Later, it is subdivided into the pericardial cavity about the heart, the pleural cavity of the thorax, and the peritoneal cavity of the abdominal region. The epithelia lining the several body cavities are termed mesothelia. |
|
| |
|
| Coelom. -The space between the somatopleure and splanchnopleure is the ccclom, or body cavity. At the first splitting of the mesoderm, isolated clefts are produced. These unite on each side and eventually form one cavity - the coelom. With the extension of the mesoderm, the cot'lom surrounds the heart and gut ventrally (Fig. 4). Later, it is subdivided into the pericardial caznty about the heart, the pleural cavity of the thorax, and the peritoneal cavity of the abdominal region. The ci)ithelia lining the several body cavities are termed mesothelia.
| |
|
| |
|
| | ===The Nephrotome=== |
| | The bridge of cells connecting the primitive segment with the unsegmented somatic and splanchnic layers is the nephrotome, or intermediate cell mass (Fig. 4). From these will develop the urogenital glands and ducts. |
|
| |
|
| The Nephrotome. - The bridge of cells connecting the primitive segment with the unsegmented somatic and splanchnic layers is the nephrotome, or intermediate cell mass (Fig. 4). From these will develop the urogenital glands and ducts.
| | ===Developmental Processes=== |
| | | The developing embryo exhibits a progressively complex structure, the various steps in the production of which occur in orderly sequence. There may be recognized in development a number of component mechanical processes which are used repeatedly by the embryo. The general and fundamental process conditioning ilifferentiation is cell multiplication, and the subsequent growth of the daughter cells. The more important of the specific developmental processes are the following: ( 1) cell migration; (2) localized growth, resulting in eidargements and constrictions; (3) cell aggregation, forming (a) cords, (b) sheets, [c] masses; (4) delamination, that is, the splitting of single sheets into separate layers; (5) folds, including circumscribed folds which produce (a) evaginations, or out-pocketings, (b) invaginations, or in-pocketings. |
| | |
| D evelopmental Processes. - The developing embryo exhibits a ])rogressively comjilex structure, the various steps in the production of which occur in orderly sequence. There may be recognized in development a number of component mechanical processes which are used repeatedly by the embryo. The general and fundamental process conditioning ilifferentiation is cell multiplication, and the subsequent growth of the daughter cells. The more important of the specific developmental ])rocesses are the following: ( i) cell migration; (2) localized growth, resulting in eidargements and constrictions; (3) cell aggregation, forming (a) cords, (b) sheets, [c] masses; (4) delamination, that is, the splitting of single sheets into separate layers; (5) folds, including circumscribed folds which produce ia) evaginations, or out-pocketings, (b) invaginations, or in-pocketings.
| |
|
| |
|
|
| |
|
| The production of folds, including evaginations and invaginations, due to unequal rapidity of growth, is the chief factor in moulding the organs and hence the general form of the embryo. | | The production of folds, including evaginations and invaginations, due to unequal rapidity of growth, is the chief factor in moulding the organs and hence the general form of the embryo. |
|
| |
|
| | ===Fundamental Conceptions=== |
| | ====The Anlage==== |
| | This German word, which lacks an entirely satisfactory English equivalent, is a term applied to the first discernible cell, or aggregation of cells, which is destined to form any distinct jiart or organ of the embryo. In the broad sense, the fertilized ovum is the anlage of the entire adult organism; furthermore, in the early cleavage stages of certain embryos it is possible to recognize single cells or cell groups from which definite structures will indubitably arise. The term anlage, however, is more commonly applied to the primordia that differentiate from the various germ layers. Thus the epithelial thickening over the optic vesicle is the anlage of the lens. |
|
| |
|
| | ====The Law of Genetic Restriction==== |
| | As development advances, there is a constantly increasing restriction in the kind of differentiation open to the various parts. Each emerging tissue or organ is more rigidly bound to its particular type of differentiation than was the generalized material from which it came. A line of specialization, once begun, cannot be abandoned for another type. The parent tissue, likewise, is limited by losing the capacity for duplicating anlages already formed. Thus, the primitive thyroid can never become anything but a thyroid, whereas the gut that formed it also buds off, at other levels, the lungs, liver, and pancreas. Yet if the embryonic thyroid were destroyed, the pharynx would never replace it. From mesenchyme arise connective tissue, blood cells, and smooth muscle; when once the specialization begins, there can be no retraction or transformation to another type. |
|
| |
|
| FUNDAMENTAL CONCEPTIONS
| | ====Continuity of the Germ Plasm==== |
| | According to this important conception of Weismann, the body-protoplasm, or soma, and the reproductive-protoplasm differ fundamentally. The germinal material is a legacy that has existed since the beginning of life, from which representative portions are passed on intact from one generation to the next. Around this germ plasm there develops in each successive generation a shortlived body, or soma, which serves as a vehicle for insuring its transmission and perpetuation. The reason, therefore, why offspring resembles parent is because each develops from portions of the same stuff. |
|
| |
|
| The Anlage. - This German word, which lacks an entirely satisfactory English equivalent, is a term applied to the first discernible cell, or aggregation of cells, which is destined to form any distinct jiart or organ of the embryo. In the broad sense, the fertilized ovum is the anlage of the entire adult organism; furthermore, in the early cleavage stages of certain embryos it is possible to recognize single cells or cell groups from which definite structures will indubitably arise. The term anlage, however, is more commonly applied to the primordia that differentiate from the various germ layers. Thus the epithelial thickening over the optic vesicle is the anlage of the lens.
| | ====The Law of Biogenesis==== |
| | | Of great theoretical interest is the fact, constantly observed in studying, embryos, that the individual in its development repeats hastily and incompletely the evolutionary history of its own species. This law of recapitulation was first stated clearly by Muller in 1863, and was termed by Haeckel the law of biogenesis. In accordance with it, the fertilized ovum is compared to a unicellular organism like the Ameba: the blastula is supposed to represent an adult Volvox type; the gastrula, a simple sponge; the segmented embryo, a worm-like stage ; and the embryo with gill slits may be regarded as a fishlike stage. Moreover, the blood of the human embryo in development passes through stages in which its corpuscles resemble in structure those of the fish and reptile; the heart is at first tubular, like that of the fish, and the arrangement of blood vessels is equally primitive; the kidney of the embryo is like that of the amphibian, as are also the genital ducts. Many other examples of this law may readily be observed. |
| | |
| The Law of Genetic Restriction. - As development advances, there is a constantly increasing restriction in the kind of differentiation open to the various parts. Each emerging tissue or organ is more rigidly bound to its particular type of differentiation than was the generalized material from which it came. A line of specialization, once begun, cannot be abandoned for another type. The parent tissue, likewise, is limited by losing the capacity for duplicating anlages already formed. Thus, the primitive thyroid can never become anything but a thyroid, whereas the gut that formed it also buds off, at other levels, the lungs, liver, and pancreas. Yet if the embryonic thyroid were destroyed, the pharynx would never replace it. From mesenchyme arise connective tissue, blood cells, and smooth muscle; when once the specialization begins, there can be no retraction or transformation to another type. | |
| | |
| | |
| Continuity of the Germ Plasm. - According to this important conception of Weismann, the body-protoplasm, or soma, and the reproductive-protoplasm differ fundamentally. The germinal material is a legacy that has existed since the beginning of life, from which representative portions are passed on intact from one generation to the next. Around this germ plasm there develops in each successive generation a shortlived body, or soma, which serves as a vehicle for insuring its transmission and perpetuation. The reason, therefore, why offspring resembles parent is because each develops from portions of the same stuff.
| |
| | |
| | |
| The Law of Biogenesis - Of great theoretical interest is the fact, constantly observed in studying, embryos, that the individual in its development repeats hastily and incompletely the evolutionary history of its own species. This law of recapitulation was first stated clearly by Muller in 1863, and was termed by Haeckel the law of biogenesis. In accordance with it, the fertilized ovum is compared to a unicellular organism like the Ameba: the blastula is supposed to represent an adult Volvox type; the gastrula, a simple sponge; the segmented embryo, a worm-like stage ; and the embryo with gill slits may be regarded as a fishlike stage. Moreover, the blood of the human embryo in development passes through stages in which its corpuscles resemble in structure those of the fish and reptile; the heart is at first tubular, like that of the fish, and the arrangement of blood vessels is equally primitive; the kidney of the embryo is like that of the amphibian, as are also the genital ducts. Many other examples of this law may readily be observed.
| |
|
| |
|
|
| |
|
| Some apparently useless structures appear during development, perfunctorily reminiscent of ancestral conditions; certain other parts, of use to the embryo alone, are later replaced by better-adapted, permanent organs. Representatives of either type may eventually disappear or they may persist throughout life as rudimentary organs; more than a hundred of the latter have been listed for man. Still other ancestral organs abandon their provisional embryonic function, yet are retained in the adult and utilized for new purposes. | | Some apparently useless structures appear during development, perfunctorily reminiscent of ancestral conditions; certain other parts, of use to the embryo alone, are later replaced by better-adapted, permanent organs. Representatives of either type may eventually disappear or they may persist throughout life as rudimentary organs; more than a hundred of the latter have been listed for man. Still other ancestral organs abandon their provisional embryonic function, yet are retained in the adult and utilized for new purposes. |
|
| |
|
| | | ===The Vertebrate Groups=== |
| THE VERTEBRATE GROUPS
| |
|
| |
|
| There are five vertebrate classes, the higher characterized by the possession of an enveloping embryonic membrane, called the amnion, and another embryonic appendage, known as the allantois: | | There are five vertebrate classes, the higher characterized by the possession of an enveloping embryonic membrane, called the amnion, and another embryonic appendage, known as the allantois: |
|
| |
|
| (R) Anamniota (amnion absent). | | (A) '''Anamniota''' (amnion absent). |
| | | : 1. ''Fishes'' - lamprey; sturgeon; shark; bony fishes; lung fish. |
| 1. Fishes - lamprey; sturgeon; shark; bony fishes; lung fish. | | : 2. ''Amphibia'' - salamander; frog; toad; etc. |
| | |
| 2. Amphibia - ^salamander; frog; toad; etc. | |
| | |
| {B) Amniota (amnion present).
| |
| | |
| 3. Reptiles - lizard; crocodile; snake; turtle.
| |
| | |
| 4. Birds.
| |
| | |
| 5. hlammals. Characterized by hair and mammary glands, (a) Monotremes - duck-bill; primitive mammals that have a
| |
| cloaca and lay eggs with shells.
| |
| | |
| | |
| (C) Marsupials - oppossum; kangaroo; etc. The young are born immature and are sheltered in an integumentary pouch, (r)Placentalia. All other mammals whose young are nourished in the uterus by a placenta.
| |
| | |
| Ungulate series. Hoofed mammals (cattle; sheep; pig; deer; horse; etc.).
| |
|
| |
|
| Unguiculate series. Clawed mammals (mole; bat; rat; rabbit; cat; dog; etc.). The highest order is the Primates (lemur; monkey; ape, man). | | (B) '''Amniota''' (amnion present). |
| | : 3. ''Reptiles'' - lizard; crocodile; snake; turtle. |
| | : 4. ''Birds.'' |
| | : 5. ''Mammals''. Characterized by hair and mammary glands. |
| | :: (a) Monotremes - duck-bill; primitive mammals that have a cloaca and lay eggs with shells. |
| | :: (b) Marsupials - oppossum; kangaroo; etc. The young are born immature and are sheltered in an integumentary pouch. |
| | :: (c) Placentalia. All other mammals whose young are nourished in the uterus by a placenta. |
| | ::: Ungulate series. Hoofed mammals (cattle; sheep; pig; deer; horse; etc.). |
| | ::: Unguiculate series. Clawed mammals (mole; bat; rat; rabbit; cat; dog; etc.). The highest order is the Primates (lemur; monkey; ape, man). |
|
| |
|
| The Vertebrate Body Plan. - All vertebrate animals are constructed in accordance with a common body plan. The distinctive characteristics of the vertebrate type include: . | | ====The Vertebrate Body Plan==== |
| | All vertebrate animals are constructed in accordance with a common body plan. The distinctive characteristics of the vertebrate type include: . |
|
| |
|
| 1. A tubular central nervous system, dorsally placed (Fig. 4).
| | # A tubular central nervous system, dorsally placed (Fig. 4). |
| | # A notochord, between the neural tube and gut (Fig. 4). This cellular |3rimitive-axis is replaced, wholly or in part, by the vertebral column. |
| | # A pharynx, which develops paired pouches and clefts that determine the positions of important nerves, muscles and blood vessels (Fig. 91). |
| | # The position of the mouth. Unlike the condition in many invertebrates, it is not surrounded by a circumoral ring of nervous tissue which connects a dorsal - brain - with a ventral chain of ganglia. |
| | # The limbs, Two pairs, with an internal skeleton (Fig. 227). |
| | # A coelom, which is divided into a dorsal, segmental part (cavities of the somites), and a ventral, unsegmented part, partitioned by the septum transversum (diaphragm) into thoracic and abdominal portions (Fig. 4). |
|
| |
|
| 2. A notochord, between the neural tube and gut (Fig. 4). This cellular |3rimitive-axis is replaced, wholly or in part, by the vertebral column.
| | ==Titles for Collateral Reading and Reference== |
| | |
| 3. A pharynx, which develops paired pouches and clefts that determine the positions of important nerves, muscles and blood vessels (Fig. 91).
| |
| | |
| 4. The position of the mouth. Unlike the condition in many invertebrates, it is not surrounded by a circumoral ring of nervous tissue which connects a dorsal - brain - with a ventral chain of ganglia.
| |
| | |
| 5. The limbs. Two pairs, with an internal skeleton (Fig. 227).
| |
| | |
| 6. A coelom, which is divided into a dorsal, segmental part (cavities of the somites), and a ventral, unsegmented part, partitioned by the septum transversum (diaphragm) into thoracic and abdominal portions
| |
| (Fig. 4 • .
| |
| | |
| | |
| TITLES FOR COLLATERAL READING AND REFERENCE
| |
|
| |
|
| Broman. Normale und abnorme Entwicklung des Menschen. | | Broman. Normale und abnorme Entwicklung des Menschen. |
| Line 589: |
Line 428: |
| Duval. Atlas D - Embryologie. | | Duval. Atlas D - Embryologie. |
|
| |
|
| Hertwig. Handbuch der Entwicklungslehre der Wirbeltiere. | | Hertwig. [[Book - Text-Book of the Embryology of Man and Mammals|'''Handbuch der Entwicklungslehre der Wirbeltiere''']]. |
|
| |
|
| Keibel and Mall. Human Embryology. | | Keibel and Mall. [[Book - Manual of Human Embryology|'''Human Embryology''']]. |
|
| |
|
| Kellicott. A Textbook of General Embryology. | | Kellicott. [[Book - Outlines of Chordate Development|'''A Textbook of General Embryology''']]. |
|
| |
|
| Kollmann. Handatlas der Entwicklungsgeschichte des Menschen. | | Kollmann. [[Atlas_of_the_Development_of_Man_1|'''Handatlas der Entwicklungsgeschichte des Menschen''']]. |
|
| |
|
| Lillie. The Development of the Chick. | | Lillie. The Development of the Chick. |
|
| |
|
| Minot. A Laboratory Text-book of Embryology. | | Minot. [[Book - A Laboratory Text-Book of Embryology (1903)|'''A Laboratory Text-book of Embryology''']]. |
|
| |
|
| McMurrich. The Development of the Human Body. | | McMurrich. The Development of the Human Body. |
|
| |
|
| Patten. The Early Embryology of the Chick. | | Patten. [[Book - The Early Embryology of the Chick|'''The Early Embryology of the Chick''']]. |
|
| |
|
| Wilson. The Cell in Development and Inheritance. | | Wilson. The Cell in Development and Inheritance. |
|
| |
|
| |
|
| |
|
| |
|
| |
| CHAPTER I . THE GERM CELLS AND FERTILIZATION THE GERM CELLS .
| |
|
| |
|
| |
| All multicellulai' animals, except a few invertebrates, result from the union of two ripe sex cells. These are re])resentative portions of the germ plasm stored in the male and female sex glands, and are termed spermatozoon and ovinn respectively. In form and function they are quite unlike, for each is adapted to a specific purpose. It will be simplest first to describe these elements fully-formed, and then to show how they develop, mature, meet, and unite.
| |
|
| |
|
| |
|
| |
| The Ovum. - The female germ cell, or ovum, is a typical animal cell produced in the ovary. Although always large, its exact size is correlated with the amount of stored food substance. The smallest eggs are those of the mouse and deer (about 0.07 mm.). The largest have a diameter measurable in inches (birds; a shark). Most ova are nearly spherical in form and posSess a nucleus with nucleolus, chromatin network, and nuclear membrane (Figs. 5 and 7). The nucleus is essential to the life, growth, and reproduction of the cell. The function of the nucleolus is unknown; the chromatin bears the hereditary qualities. The cytoplasm is distinctly granular and contains more or less numerous yolk granules, mitochondria, and rarely a minute centrosome.
| |
|
| |
|
| |
|
| |
| Fig. 5. - Ovum of monkey (Prentiss). X 430.
| |
|
| |
|
| |
|
| |
| The yolk, or deutoplasm, containing a fatty substance termed lecithin, furnishes nutriment for the developing embryo. It is doubtful if any ovum is totally devoid of yolk, yet it is useful as a basis for classifying eggs. Those ova which contain relatively little yolk, uniformly distributed, are termed isolecithal. Examples are found among various invertebrates and in all placental mammals, for such embryos either attain an independent existence quickly or are sheltered and nourished within the uterine wall of the mother. If the yolk collects at one end (called the vcgdal pole in contrast to the more jmrely jirotoplasmic animal pole) the ova are said to he telolecuhal. Many invertebrates and all vertebrates lower than the Placentalia illustrate this type. The so-called yolk of the hen - s egg (Fig. 6) is the ovum proper and its yellow color is due to the large amount of lecithin it contains. Finally, among the arthropods the yolk is centrally located and surrounded by a peripheral shell of clear cytoplasm; such eggs are centrolccithal.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 6. - Diagrammatic longitudinal section of a hen - s egg (Thomson in Heisler).
| |
|
| |
|
| |
| Fig. 7. - . 4 , Human ovum, approaching maturity, examined fresh in the liquor folliculi (Waldeyer). X 415. The zona pellucida appears as a clear girdle surrounded by the cells of the corona radiata. Yolk granules in the cytoplasm enclose the nucleus and nucleolus. 5 , A human spermatozoon correspondingly enlarged.
| |
|
| |
|
| |
|
| |
| Most ova become enclosed within protective membranes, or envelopes. The vitelline membrane, secreted by the egg itself, is a primary membrane (Fig. 5). The follicle cells about the ovum usually furnish other secondary membranes, such as the zona pellucida. In lower vertebrates tertiary membranes may be added as the egg passes through the oviduct and uterus ; the albumen and shell of the hen - s egg (Fig. 6) or the jelly of the frog - s egg are of this sort.
| |
|
| |
|
| |
| The Human Ovum. - This is relatively of small size, measuring about 0.2 mm. in diameter (Fig. 7). It conforms closely to the isolecithal mammalian type, but has fine yolk granules somewhat condensed centrally. There is apparently a very delicate vitelline membrane, and outside it a thick, radially-striate membrane, the zona pellucida. The striate appearance is said to be due to fine canals through which nutriment is transferred from smaller follicle cells during the growth of the ovum within the ovary.
| |
|
| |
|
| |
| The Spermatozoon. - -In a few instances only, does the mature male element, or spermatozoon, resemble a typical cell. Most are slender, elongate structures which develop a flagellum to accomplish the active swimming that characterizes the cell. Unlike the ovum, which is the largest cell of an organism, the spermatozoon is usually the smallest. The extremes of size range from 0.018 mm. in Amphioxus to 2.0 mm. in an amphibian. The commonest shape is that of an elongate tadpole, with an enlarged head, short neck (and connecting piece), and thread-like tail (Fig. 8).
| |
|
| |
|
| |
| Fig. 8. - .1, Diagram of a human spermatozoon, surface view (Meves). B, Human spermatozoa, from life, in edge and surface view. X 700.
| |
|
| |
|
| |
|
| |
| The Human Spermatozoon. - The sperm of man is of average size (0.055 mm.) and shape (Fig. 8). Compared to the ovum its volume is as i : 200,000 (Fig. 7). The /zcah is about 0.005 mm. in length. It appears oval in surface view, pear-shaped in profile. When stained, the anterior two-thirds of the^head may be seen to constitute a cap, and the sharp border of this cap is the so-called perforatorium. The head contains the nuclear elements of the sperm cell. The disc-shaped neck includes the anterior centrosonial body. The tail begins with the posterior centrosonial body and is divided into a short connecting piece, a chief piece, or flagellum, which forms about four-fifths of the length of the sperm cell, and a short end piece, or terminal filament. The connecting piece is marked off from the chief piece by the annulus. The connecting piece is traversed by the axial filament {fdvLm principale), and is surrounded: (i) by the sheath common to it and to the flagellum; (2) by a sheath containing a spiral filament; and (3) by a mitochondrial sheath. The chief piece is composed of the axial filament, surrounded by a cytoplasmic sheath, while the end piece comprises the naked continuation of the axial filament.
| |
|
| |
|
| |
| Atypical spermatozoa occur in some individuals. These include giant and dwarf forms, and elements with multiple heads or tails.
| |
|
| |
|
| |
| Comparison of the Ovum and Spermatozoon. - The dissimilar male and female sexual cells are admirably adapted to their respective functions, and illustrate nicely the modifications that accompany a physiological division of labor. Each has the same amount of chromatin, although in the sperm it is more compactly stored. The cells thus participate equally in heredity. The egg contains an abundance of cytoplasm (but nO' centrosome), and often a still greater supply of stored food. As a result, it is large and passive, yet closely approximates the typical cell. On the contrary, the sperm is small, and at casual inspection bears slight resemblance to an ordinary cell. Its cytoplasm is reduced to a bare minimum and contains no deutoplasm. Structurally, all is subordinated to a motile existence. Correlated with small size is an extraordinary increase in numbers, for the greater the total liberated the more surely will the ovum be found. Hence, apart from its role in heredity, the chief function of the spermatozoon is to seek the ovum and activate it to divide.
| |
|
| |
| SPERMATOGENESIS, OOGENESIS AND MATURATION
| |
|
| |
|
| |
| In becoming specialized germ cells, the ovum and spermatozoon pass through parallel stages. The general process of sperm formation is designated spermatogenesis; that of egg formation, oogenesis. An essential feature of lioth is a component process, termed maturation, which is important for the following reason. Since reproduction in vertebrates depends upon the union of male and female germ cells, it is manifest that without special provision this union would necessarily double the number of chromosomes at each generation. Such progressive increase is prevented by the events of maturation. This may be defined as a form of cell division during which the number of chromosomes in the germ cells is reduced to one-half the number characteristic for the species. Its significance in the mechanism of inheritance is discussed on p. 28.
| |
|
| |
|
| |
| Spermatogenesis. - The spermatozoa originate in the epithelial lining of the testis tubules. Two types of cells are recognizable : the sustentacular cells (of Sertoli), and the male germ cells (Fig. 9). All the latter are descendants of primordial germ cells, which, by division, first form spermatogonia. These in turn |3roliferate and produce numerous generations of like cells. Ultimately the spermatogonia enter a growth period, at the end of which they are termed primary spermatocytes. Each contains the full number of chromosomes typical for the male of the species. Next ensues the process of maturation. This comprises two cell divisions, each primary spermatocyte producing two secondary spermatocytes, and these in turn four cells known as spermatids. During these cell divisions the number of chromosomes is reduced to half the original number in the spermatogonia.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 9. - Stages in the spermatogenesis of man arranged in a composite to represent a portion of a seminiferous tubule sectioned transversely. X 900.
| |
|
| |
|
| |
|
| |
| The spermatids now attach to Sertoli cells, from which they appear to receive nutriment, and become transformed into mature spermatozoa (Fig. 10). The nucleus forms almost all the head; the centrosome divides, the resulting particles passing to the extremities of the neck. The posterior centrosome differentiates the annulus and is prolonged to become the axial filament. The cytoplasm forms the sheaths of the neck and tail, whereas the spiral filament of the connecting piece is derived from cytoplasmic mitochondria. When the transformation is complete, the spermatozoa detach from the sustentacular cells and are set free in the lumen of the seminiferous tubule.
| |
|
| |
|
| |
|
| |
| Maturation in Ascaris. - The way the number of chromosomes is reduced may be seen in the spermatogenesis of Ascaris (Fig. 1 1). Four chromosomes are typical for Ascaris megalocephala hivalens, and each spermatogonical cell contains this number. In the early prophase of the primary spermatocyte there appears a spireme thread consisting of four parallel rows of granules (B). This thread breaks in two and forms two quadruple structures, known as tetrads (D-F) ; each is equivalent to two original chromosomes, paired side by side and split lengthwdse to make a bundle of four. At the metaphase {G}, a tetrad divides into its two original chromosomes which already show evidence of longitudinal fission and are termed dyads. One pair of dyads goes to each of the daughter cells, or secondary spermatocytes (G-I). Without the formation of a nuclear membrane, the second maturation spindle appears at once, the two dyads split into four monads, and each daughter spermatid receives two single chromosomes (monads), or one-half the number characteristic for the species. The tetrad, therefore, represents a precocious division of the chromosomes in preparation for two rapidly succeeding cell divisions which occur without the intervention of the customary resting periods. The easily understood tetrads are not formed in most animals, although the outcome of maturation is identical in either case. A diagram of maturation is shown in Fig. 12. The first maturation division in Ascaris is probably reductional, each daughter.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 10. Diagrams of the development of spermatozoa (Meves in Lewis and Stohr). a.c, Anterior centrosome; a.f., axial filament; c.p., connecting piece; ch.p., chief piece; g.c., cap; «., nucleus; nk., neck; p., cytoplasm; p.c., posterior centrosome.
| |
|
| |
|
| |
|
| |
| Fig. 11. - Reduction of chromosomes in the spermatogenesis of Ascaris megalocephala bivalens (Brauer in Wilsonj. X about i loo. A-G, Successive stages in the division of the primary spermatocyte. The original reticulum undergoes a very early division of the chromatin granules which then form a quadruply split spireme (B, in profile). This becomes shorter (C, in profile), and then breaks in two to form two tetrads (D, in profile), (E, on end). F, G, H, first division to form two secondary spermatocytes, each receiving two dyads. I, Secondary spermatocyte. J, K, The same dividing. L, Two resulting spermatids, each containing two monads or chromosomes.
| |
|
| |
|
| |
|
| |
| nucleus receiving two complete chromosomes of the original four, whereas in the second maturation division, as in ordinary mitosis, each daughter nucleus receives a half of each of the two chromosomes, these being split lengthwise. The latter division is equauonal and the daughter nuceli receive chromosomes bearing similar hereditary qualities.
| |
|
| |
| Some animals reverse the sequence of events, reduction occurring at the second maturation division.
| |
|
| |
|
| |
|
| |
| Maturation in Man - All spermatogonia, like the somatic cells, contain 48 chromosomes. The primary spermatocytes form tetrads and their division separates the mated chromosomal pairs into 24 single chromosomes of the secondary spermatocyte. Hence, this mitosis is reductional. The secondary spermatocytes then divide equationally into spermatids, each of which also contains 24 single chromosomes. Transformation into spermatozoa ensues (Figs. 9 and 10). Those details of maturation which pertain to sex determination are explained on p. 29.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 12. - Diagrams of maturation in spermatogenesis and oogenesis (Boveri).
| |
|
| |
|
| |
|
| |
| Oogeneiss. - The ova, like the male elements, arise from the multiplication of primordial germ cells in the ovary (cf. p. 156). At birth, or shortly after, human ova cease forming. The number at this time in both ovaries has been placed between 100,000 and 800,000. Cellular degeneration reduces this supply until, at 18 years, the total is from 35,000 to 70,000 and several years after the menopause no more are to be found.
| |
|
| |
|
| |
| Late in fetal life, indifferent cells, by surrounding the young ova ioogonia) of the cortex, produce primordial follicles (Fig. 13 A). Some begin growth at once, others are quiescent until childhood or adult life is attained. During the slow growth period, the small, nutritive follicle cells increase in number and the oogonium gains greatly in size. When the follicle cells are several layers deep, a cavity appears between them. This enlarges, and there reSults a sac, the vesicular, or Graafian follicle, filled with fluid, the liquor folliculli (Fig. 13 5 ). As growth continues, the oogonium becomes located more and more eccentrically until it lies at one side of the follicle, buried in a mound of follicular cells termed the cumulus odphorus (egg-bearing hillock) (Fig. 14). Around the stratified follicle cells, now designated the stratum granulosum, there is differentiated from the stroma of the ovary the theca folliculi. This is composed of an inner, vascular tunica interna, and an outer, fibrous and muscular tunica externa.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 13. A, Two primordial human follicles and one early in growth (De Lee). X200. B, Section of a human ovarian cortex with ten primordial follicles and one young Graafian follicle (Piersol). X 90.
| |
|
| |
|
| |
| .At the end of the growth period, the follicle has enlarged from a structure 0.04 to 0.06 mm. in diameter to one 5 to 12 mm. (Fig. 16 A); similarly, the primordial ovum measured 0.04 to 0.05 mm. whereas it now has a diameter of about 0.2 mm. In harmony with the terminology for the male cell, the grown oogonium is designated a primary oocyte. The final stages of oogenesis are maturative. As in spermatogenesis, two cell divisions take place, but with this difference: the cytoplasm is divided unequally, and instead of four cells of equal size resulting, there are formed one large ripe ovum, or ootid, and three rudimentary or abortive ova, known as polar bodies, or polocytes (Fig. 15). The number of chromosomes is reduced in the same manner as in the male, so that the ripe ovum and each polar cell contain one-half the number of chromosomes found in the oogonium or primary oocyte.
| |
|
| |
|
| |
|
| |
| Fig. 14. - An advanced Graafian follicle and ovum from a girl of fifteen (Prentiss). X 30.
| |
|
| |
|
| |
| Fig. 15. - . 4 , Formation of the first polar cell in the mouse ovum (Sobotta). X 1500. B, Separation of the second polar cell in the bat ovum (after Van der Stricht).
| |
|
| |
|
| |
| During maturation the ovum and first polocyte are termed secondary oocytes (comparable to secondary spermatocytes) ; the mature ovum (ootid) and second polocyte, with the daughter cells of the first polocyte. are comparable to the spermatids (Fig. 12). Each spermatid, however may form a mature spermatozoon, but only one of the four daughter cells of the primary oocyte becomes functional. The ovum develops at the ex])ense of the three ])olocytes which are abortive and degenerate eventually, though it has been shown that in some insects the polar cell may be fertilized and segment several times like a normal ovum. In most animals, the actual division of the first polocyte into two daughter cells is suppressed (cf. Fig. 1 5 B). The nucleus of the ovum after maturation is known as the jcniale pronndcus.
| |
|
| |
|
| |
| Maturation in the Mouse. - Typical maturation occurs in the mouse. 1 'he first jiolocyte is formed while the ovum is still in the Graafian follicle. Neither astral rays nor typical centrosomes have been observed; the chromosomes are V-shaped. The finst polar cell is constricted from the ovum and lies beneath the zona pellucida as a spherical mass about 25 micra in diameter (Fig. 15 A). Both ovum and polar cell (secondary oocytes) contain 20 chromosomes, or half the number normal for the mouse. The first maturation division is the reductional one and the chromosomes take the form of tetrads.
| |
|
| |
|
| |
| After ovulation has taken place, the ovum lies in the ampulla of the uterine tube. If fertilization occurs, a second polocyte is cut off, the nucleus of the ovum not having regained its membrane between the production of the first and second polar bodies (Figs. 15 A and 17 A, D). The second maturation spindle and second polar cell are smaller than the first. Immediately after the appearance of the second polar cell, the chromosomes resolve themselves into a reticulum and the female pronucleus is complete [Mig. 17 D).
| |
|
| |
|
| |
| Maturation in Man. - -The only observations are those of Thompson (1919), who believes to have identified stages in the formation of all three polar cells prior to ovulation or fertilization. The evidence presented, however, can hardly be accepted as conclusive. Yet, in Tarsius, a low primate, both polar cells have been observed.
| |
|
| |
|
| |
| OVULATION AND INSEMINATION
| |
|
| |
| The ripe germinal products are next released from their respective sex glands and then brought together.
| |
|
| |
| Ovulation. - The discharge of the ovum from its follicle comprises ovulation. A few animals breed continuously, but commonly there is a seasonal or annual spawning period. The several mammalian groups show various gradations between an almost continuous breeding period (oestrus) and an annual one. In man ovulation is periodic, at intervals of four weeks, beginning at puberty and ending with the menopause. However, fully formed Graafian follicles appear in the ovary during the second year of infancy, and, in some individuals, even before birth.
| |
|
| |
|
| |
| Ovulation may occur at this time, but usually these precociously formed follicles degenerate with their contained ova. Generally, only one follicle and ovum mature each month, the ovaries roughly alternating. Yet, ordinary multiple births depend on the rupture of two or more follicles. Rarely in man, but frequently in the monkey, follicles contain more than one egg. Thus, from the thousands of potential ova, only about 200 ripen in each ovary during the 30 years of sexual activity.
| |
|
| |
|
| |
| The completed follicle is from 5 to 12 mm. in diameter. It makes a bud-like protuberance from the surface of the ovary, and at this point the ovarian wall is very thin (Fig. 16 A). Internally, the follicle contains fluid, probably under vascular and muscular tension. The precise factors which cause rupture are not positively known, but they doubtless include mechanical pressure, perhaps combined with a weakening of the follicular wall by the digestive influence of the contained fluid (Schochet, 1920).
| |
|
| |
|
| |
| Fig. 16. A , Human uterine tube and ovary with mature Graafian follicle] (RibemontDessaignes). B, Sectioned human ovary with a corpus luteum verum and two corpora albicantia. X 1.5.
| |
|
| |
|
| |
|
| |
| When the follicle bursts, the fluid gushes out, carrying with it the ovum torn loose from its cumulus oophorus. The adhering follicular cells, immediately investing the ovum, constitute the corona radiata (Fig. 6). The ovum is swept into the uterine tube by inwardly stroking cilia of the tubal Ambriae. Although the ovum is now ready to be fertilized, it is not yet technically - mature, - for the last polar division awaits the stimulus of fertilization.
| |
|
| |
|
| |
| The Corpus Luteum - After ovulation, a blood clot, the corpus liemorrhagicum, forms within the empty follicle. The follicle cells of the stratum granulosum proliferate, enlarge, and produce a yellow pigment. The w'hole structure, composed of lutein cells and connective-tissue strands, is termed the corpus luteum, or yellow body (Fig. 16 B). If pregnancy does not supervene, the corpus luteum spurium reaches its greatest development within two weeks and then gradually is replaced by fibrous tissue ; the resultant white scar is known as the corpus albicans. In pregnancy the corpus /zhtvuKuer/oH continues its growth until, at the thirteenth week, it reaches a maximal diameter of 1 5 to 30 mm. ; at term it is still a prominent structure in the ovary. The corpus luteum is believed to produce an important internal secretion, for if removed the ovum fails to attach to the wall of the uterus, or if the ovum is already embedded, development ceases (Fraenkel). An influence in retarding ovulation and stimulating the mammary gland function has also been shown experimentally (L. Loeb; O - Donoghue).
| |
|
| |
|
| |
|
| |
| Relation of Ovulation and Menstruation. - Since human ovulation and menstruation both begin with puberty, recur at about twenty-eight day intervals, and discontinue during pregnancy and at the menopause, a close relation has long been inferred. The cessation of the menses after ovarian removal further indicates dependence. For many years the two processes were supposed to be synehronous. This belief was based upon clinical oliservations by Leopold, Ravano and others who tried to correlate the ages of corpora lutea with known menstrual histories. Since then, Meyer, Ruge, Schroder, Fraenkel, and Halban, utilizing better standardized corpora lutea, have presented convincing evidence that ovulation occurs most often between the fourth and fourteenth day after the menstrual onset. While correct as a generalization, this correlation is not rigid and often ova are liberated at other times. Moreover, in young girls ovulation may precede the inception of menstruation and it may occur in women during pregnancy and lactation or after the menopause.
| |
|
| |
|
| |
|
| |
| Coitus and Insemination. - In most aquatic animals the eggs and sperm are discharged externally at about the same time and place. Their meeting depends largely upon chance, enhanced by the production of immense numbers of spermatozoa. Some animals increase the certainty of such cell union by a pscudocopulation ; thus, the male frog clasps the female and jiours his milt over the eggs as they are extruded. Many invertebrates and all amniote vertebrates have their sex cells unite inside the female - s body. This is effected by the sexual embrace termed copulation, or coitus. In general, those animals whose offspring reach maturity with reasonable surety (as the result of internal fertilization and postnatal care) produce fewer germ cells, especially ova, than those that leave fertilization to chance and development to hazard. The codfish produces 10,000,000 eggs in a breeding period, a sea urchin 20,000,000; in certain birds and mammals only a single egg is matured, yet the stock of each remains constant.
| |
|
| |
|
| |
| The purpose of coitus is to introduce spermatozoa into the vagina. The completed human sperm detach from the Sertoli cells, and clusters are moved along the efferent ductules into the epididymis. Here they become separate and motile, due to a secretion of the duct epithelium. The seminal fluid accumulates about the ampulla of the ductus deferens; its storage in the seminal vesicles is much questioned. At the climax of coitus ejaculation occurs and the spermatozoa, suspended in seminal fluid, are forcibly ejected. The seminal fluid, or semen, is a mixture chiefly of the secretions of the seminal vesicles, prostate, and bulbourethal glands, in which occur the spermatozoa. The volume of the ejaculate is about 3 c.c. and in it swim over 200,000,000 spermatozoa.
| |
|
| |
|
| |
| The outstanding functional feature of spermatozoa is their flagellate swimming. Because of this they were once regarded as parasites living in the seminal fluid. Forward progress is at the rate of about 2.5 mm. a minute, which, length for length, compares with the ordinary gait of man. An acid environment, such as the vagina, is deleterious or fatal; an alkaline medium, as furnished by the uterus, is favorable. Spermatozoa tend always to swim against feeble currents. This is important, as the outwardl^^ stroking cilia of the uterine tubes and uterus direct the spermatozoa by the shortest route to the ovum. They probably reach the ampulla of the uterine tube two hours or more after coitus.
| |
|
| |
|
| |
| Spermatozoa have been found motile in the uterine tube nine days after the admission of a patient to the clinic, and, according to her statement, three and one-half w^eeks after coitus. They have been kept alive eight days outside the body. It is not known for how long spermatozoa are capable of fertilizing ova. Keibel holds that this would certainly be more than a week. However, Lillie (1915) has shown with sea urchins that the ability to fertilize is lost long before vitality or motility is impaired, and Mall (1918) concludes that the duration of the fertilizing power of human spermatozoa is safely less than the corresponding period in the ovum, which is probably for fully 24 hours after ovulation. In the hen, spermatozoa remain functional three weeks; in bats six months; in bees five years.
| |
|
| |
|
| |
|
| |
| FERTILIZATION
| |
|
| |
| The formation, maturation, and meeting of the male and female germ cells are all preliminary to their actual union which definitely marks the beginning of a new individual. This penetration of ovum by spermatozoon and the fusion of their - pronuclei - constitute the process oi jertilization. In practically all animals, fertilization also starts the ovum dividing and thus initiates development in the ordinary sense. A few invertebrates, however, can develop without the aid of fertilization; this method is styled parthenogenesis, and in such eggs there is usually but one polar cell and hence no chromosome reduction.
| |
|
| |
| Random movements of the sperm bring them in contact with ova. It is very doubtful whether there is any chemical attraction. In some forms, as for example fishes, tactile response keeps -the spermatozoa in contact with anything touched. In mammals, amphibia, and many invertebrates, the ovum is either naked or surrounded by a delicate vitelline membrane. Spermatozoa can enter such eggs at any point. Ova that are invested with heavy membranes usually have a definite funnel-shaped aperture, the micro pyle, through which the male cell must enter. Only motile spermatozoa are able to attach to the surface of an egg; it is probable that forces allied to phagocytosis, rather than vibrational energy, accomplish the actual - penetration. -
| |
| In general, only one spermatozoon normally enters an egg; how others, endeavoring to penetrate, are thereafter excluded is not entirely clear. If accident or im]iaired vitality admits more than one sperm, development is abnormal and soon ends. On the contrary, some sharks, amphibia, reptiles, and birds normally exhibit such polyspermy. In all these cases, however, only one spermatozoon unites with the female pronucleus.
| |
|
| |
|
| |
| The fertilized ovum derives its nuclear substance equally from both parents, the cytoplasm (and yolk) almost entirely from the mother, the centrosome probably from the father.
| |
|
| |
|
| |
| The fundamental results of fertilization are: (i) the union of male and female pronuclei to form the cleavage nucleus (thus restoring the original number of chromosome pairs); (2) the initiation of cell division, â– or cleavage, in which all male and female chromosomes take part.
| |
|
| |
|
| |
| These two factors are separate and independent phenomena. It has been shown by Boveri and others that fragments of sea urchin - s ova containing no part of the nucleus may be fertilized by spermatozoa, segment, and develop into larvae. The female chromosomes are thus not essential to the process of cleavage. Loeb, on the other hand, proved that the ova of invertebrates may be made to develop by chemical and mechanical means without the cooperation of the spermatozoon {artificial parthenogenesis). Even adult frogs have been reared from mechanically stimulated eggs. These facts show that the actual union of the male and female pronuclei is not the means of initiating the development of the ova. In all vertebrates it is, nevertheless, the end and aim of fertilization.
| |
|
| |
|
| |
| Lillie maintains that the cortex of a sea urchin - s ovum produces a substance, fertilizin. This he regards as an amboceptor essential to fertilization, with one side chain which agglutinates and attracts the spermatozoa, and another side chain which activates the cytoplasm and initiates the cleavage of the ovum. According to Loeb, agglutination is proved in but few forms and Lillie - s interpretation fails to meet all the facts. Loeb holds that the spermatozoon actually activates the ovum to develop by increasing its oxidations and by rendering it immune to the toxic effects of oxidation.
| |
|
| |
|
| |
| Fertilization in the Mouse. - Normally, a single spermatozoon enters the ovum six to ten hours after coitus. While the second polar cell is forming, the spermatozoon penetrates the ovum and loses its tail (Fig. 17 A-C). Its head enlarges and is converted into the male projiuclcits (D). The pronuclei, male and female, approach (E) and resolve first into a spireme stage (F), then into two groups of 20 chromosomes (G). A centrosome, possibly that of the male cell (cf. Fig. 15 B), appears between them, divides into two, and soon the first cleavage spindle is formed (F-H). The 20 male and 20 female chromosomes arrange themselves in the equatorial plane of the spindle, thus making the original number of 40 (H). Fertilization is now complete and the ovum divides in the ordinay way ( 7 , /), the daughter cells each receiving equal numbers of maternal and paternal chromosomes.
| |
|
| |
|
| |
| Fertilization in Man. - The union of the human germ cells is believed usually to take place in the ampulla of the uterine tube, although it never has been observed in any primate except Tarsius. This conclusion is supported by direct observations on other mammals and by the frequency of tubal pregnancies at this site. Rarely ova become fertilized before entering the tube, but the possibility of fertilization after they have reached the uterus is usually denied.
| |
|
| |
|
| |
|
| |
| Fig. 17. - Fertilization of the ovum of the mouse (Sobotta). X 500. A-D, Entrance of the spermatozoon and formation of the polar cells; D-E, development of the pronuclei ; F - J, union of chromosomes and the first cleavage spindle.
| |
|
| |
|
| |
|
| |
| To be fruitful, the time of coitus and ovulation must roughly agree (p. 22), and, on the average, about one day is supposed to elapse between insemination and fertilization. Most conceptions occur during the week or ten days following menstruation; this is in harmony with the known data on ovulation time (p. 24).
| |
|
| |
|
| |
| While there are no direct observations on fertilization in man, the ]irocess has been studied throughly in several mammals. In all essentials it undoubtedly follows the common course as described for the mouse.
| |
|
| |
|
| |
| Superfetation.- If an ovum is liberated by a pregnant woman and fertilized at a later coitus, it may develop into a second, younger fetus. This rare condition, called sit perjctation, is often denied, yet in the early weeks of ])regnancy it is theoretically possible. Superfetation should not be confused with strikingly unequal twin development, due to nutritional or other inequalities.
| |
|
| |
|
| |
|
| |
| HEREDITY AND SEX
| |
|
| |
|
| |
| The Significance of Mitosis and Maturation. - The complicated processes of mitosis serve the purpose of dividing accurately the chromatic substance of the nucleus in such a way that the self-pcrjietuating chromosomes of each daughter cell may be the same, both quantitatively and ciualitatively. This is important since it is believed by most students of heredity that chromatin particles, or genes, in the chromosomes bear the hereditary characters, and that these are arranged in definite linear order in particular chromosomes. At maturation there is a side by side union of like chromosomes, one member of each pair having come from the father, the other from the mother of the preceding generation; each member, however, carries the same general set of hereditary charaeters as its mate. At this stage of chromosomal conjugation there may be an interchange, or - crossing over, - of corresponding genes, resulting in new hereditary combinations. The reducing division of maturation separates whole chromosomes of each pair, but chance alone governs the actual assortment of paternal and maternal members to the daughter cells; this mitosis obviously halves the chromosome number characteristic for the species. The significance of the ecjuational maturation mitosis, beyond accomplishing mere cellular multiplication, is obscure.
| |
|
| |
|
| |
|
| |
| Mendel’s Law of Heredity. - Experiments show that hereditary characters fall into two opposing groups, the contrasted pairs of which are termed allelomorphs. As an example, we may take the hereditary tendencies for dark and blue eyes. It is believed that there are paired chromatic particles, or genes, which are responsible for these hereditary tendencies, and that paired spermatogonial chromosomes bear one each of these genes. Each chromosome pair in separate germ cells may possess similar genes, both bearing dark-eyed tendencies or both blue-eyed tendencies, or opposing genes, bearing the one dark-, the other blue-eyed tendencies. It is assumed that at maturation these paired genes are separated along with the chromosomes, and that one only of each pair is retained in each germ cell.
| |
|
| |
|
| |
| In our example, either a blue-eyed or a dark-eyed tendency-bearing particle would be retained. At fertilization, the segregated genes of one sex may enter into new combinations with those from the other sex. Three combinations are possible. If the color of the eyes be taken as the hereditary character: (i) two - dark - germ cells may unite; (2) two - blue - germ cells may unite; (3) a - dark - germ cell may unite with a - blue - germ cell. The offspring in (i) will all have dark eyes, and, if interbred, their progeny will likewise inherit dark eyes exclusively. Similarly, the offspring in (2), and if these are interbred their progeny as well, will include nothing but blue-eyed individuals. The first generation from the cross in (3) will have dark eyes solely, for black in the present example is dominant, as it is termed. Such dark-eyed individuals, nevertheless, possess both dark- and blueeyed bearing genes in their germ cells; in the progeny resulting from the interbreeding of this class, the original condition is repeated - pure darks, impure darks which hold blue recessive, and pure blues will be formed in the ratio of 1:2:1 respectively. It is thus seen that blue-eyed children may be born of dark-eyed parents, whereas blue-eyed parents can never have dark-eyed offspring. Many such allelomorphic pairs of hereditary characters are known.
| |
|
| |
|
| |
| Cytoplasmic Inheritance. - Certain eggs show distinct cytoplasmic zones which cleavage later segregates into groups of cells destined to form definite organs or parts. In a sense this represents a refined sort of preformation, but prelocalization is a more exact term. From these facts Conklin and Loeb argue that the cytoplasm is really the embryo in the rough, the nucleus, through Mendelian heredity, adding only the finer details. Morgan, among others, refuses to admit the validity of this interpretation.
| |
|
| |
|
| |
| The Determination of Sex. - The sex-determining power lies in a chromosome that can be identified in many animals. This chromosome is termed the accessory, X, or sex chromosome. According to Painter (1923), humair obgonia contain 46 ordinary chromosomes and two X -chromosomes. At maturation the number is halved, and all oocytes and polocytes contain 23 -f X. The spermatogonia, on the contrary, contain 46 ordinary chromosomes, one X-chromosome and its diminutive mate, called the Y-chromosome. After maturation, therefore, half the spermatids have 23 -j- X, the remaining half have 23 -p y. When a spermatozoon with 23 + X fertilizes an ovum, the number is restored to 46 -p 2X and a female results. When a spermatozoon with 23 -p Y fertilizes, the outcome is 46 -p X -p Y and a male results.
| |
|
| |
|
| |
| Many animals lack the Y, and the male cells contain an odd number of chromosomes. Reduction then forms two classes of spermatozoa, those with the extra chromosome being female producing. In certain birds and moths the system is the exact reverse, inasmuch as the spermatozoa are all alike in chromosomal constitution while the eggs are of two sorts.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| ++++++++++++++++++++++++++++++++.
| |
|
| |
| Chapter II Cleavage And The Origin Of The Germ Layers
| |
|
| |
|
| |
| Cleavage
| |
|
| |
| The fertilized ovum promptly begins to form the new, multicellular individual by a process termed cleavage, or segmentation . This comprises orderly and rapid successions of mitoses which result in an aggregate of smaller cells, called blastomercs. Every blastomere receives the full assortment of chromosomes, half from each parent (Fig. 17 F-J).
| |
|
| |
| The abundance and distribution of yolk in the egg so influences mitosis as to allow the following classification of cleavage:
| |
| (A) Total. Entire ovum divides; holoblastic ova.
| |
|
| |
| 1. Equal. In isolecithal ova; blastomeres are of equal size; e.g., amphioxus and mammals.
| |
|
| |
|
| |
| 2. Unequal. In moderately telolecithal ova; yolk accumulated at vegetal pole retards mitosis, and fewer but larger blastomeres form there; e.g., lower fishes and amphibia.
| |
|
| |
|
| |
| {B) Partial. Protoplasmic regions alone cleave; meroblastic ova.
| |
|
| |
| 1. Discoidal. In highly telolecithal ova; mitosis restricted to anim^d ]Jole; e.g., higher fishes, reptiles, and birds.
| |
|
| |
| 2. Superficial. In centrolecithal ova; mitosis restricted to the peri])heral cytoplasmic investment ; arthropods.
| |
|
| |
|
| |
|
| |
|
| |
| Cleavage in Amphioxus. - The early processes of development are easily understood in a primitive, fish-like form, Amphioxus. About one hour after fertilization, its essentially isolecithal ovum divides vertically into two nearly equal blastomeres (Fig. 18, 2). Within the next hour the daughter cells again cleave in the vertical plane, at right angles to the first division, thus forming four cells (3). Fifteen minutes later a third division takes place in a horizontal plane (4). As the yolk is somewhat more abundant at the vegetal poles of the four cells, the mitotic spindles lie nearer the animal pole. Consequently, in the eight-celled stage the upper tier of four cells is slightly smaller than the lower four. By successive cleavages, first in the vertical, then in the horizontal ]3lane, a 16- and 3 2 -celled embryo is formed (5, 6). The upper two tiers are now smaller, and a cavity, the blastocode, is enclosed by the cells. The embryo at this stage is sometimes called a morula because of its resemblance to a mulberry. In subsequent cleavages, as development proceeds, the size of the cells is diminished, while the cavity enlarges (7, 8). The embryo is now a blastnla, nearly spherical in form and about four hours old. The cleavage of the holoblastic Amphioxus ovum is thus total and nearly equal.
| |
|
| |
|
| |
| Fig. 18. - Cleavage in Amphioxus, viewed laterally (Hatschek). X 200. i. Mature egg, with one polar body (P. 5 .) ; the other missing. 2. Ovum partly divided into two blastomeres. 3. Four blastomeres. 4. Eight blastomeres. 5. Sixteen blastomeres. 6. Thirty-two blastomeres, hemisected to show the blastocoele, B. 7, 8. Total and hemisected blastul®.
| |
|
| |
|
| |
|
| |
|
| |
| Cleavage in Lower Fishes and Amphibia. - These ova contain enough yolk so that the nucleus and most of the cytoplasm lie nearer the upper, or animal pole. The first cleavage spindle appears eccentrically in this cytoplasm. The first two cleavage planes are vertical and at right angles, and the four resulting cells are equal. The spindles for the third cleavage are located near the animal pole, and the division takes place in a horizontal plane. As a result, the upper four cells are much smaller than the lower four (Fig. ig A). The large, yolk-laden cells divide more slowly than the upper, small cells (B-D). At the blastula stage, the cavity is small, and the cells of the vegetal pole are many times larger than those of the animal pole {E, F). The cleavage is thus total but unequal vertical but the inert yolk does not cleave. The segmentation is thus partial and discoidal. In the bird - s ovum, the cytoplasm is divided by successive vertical furrows into a mosaic of cells, which, as it increases in size, forms a cap-like structure upon the surface of the yolk (Fig. 20 A). These cells are separated from the yolk beneath by horizontal cleavage.
| |
|
| |
|
| |
|
| |
| Fig. 20. - Cleavage of the pigeon - s ovum (redrawn from Blount). A, Blastoderm in surface view; B, in vertical section.
| |
|
| |
|
| |
|
| |
| Fig. 19. - Cleavage and gastrulation in the frog. X 12. A-D, Cleavage stages; E, blastula; F, hemisection of E; G, early gastrula; u, hemisection of G. an., Animal cells; arch., archenteron; b'c., blastocoele; b - p., blastopore; ect., ectoderm; ent., entoderm; v - g., vegetal cells.
| |
|
| |
|
| |
|
| |
| Cleavage in Higher Fishes, Reptiles and Birds. - The ova of these vertebrates contain a large amount of yolk. There is very little pure cytoplasm except at the animal pole, and here the nucleus is located (Fig. 6). When segmentation begins, the first plane of separation is furrows, and successive horizontal cleavages give rise to several layers of cells (Fig. 20 B). The space between cells and yolk mass may be compared to the blastula cavity of Amphioxus and the frog (Fig. 22). The cellular cap is termed the germinal disc, or blastoderm. The yolk mass, which forms the floor of the blastula cavity and the greater part of the ovum, may be compared to the large, yolk-laden cells at the vegetal pole of the frog - s blastula. The main yolk mass never divides but is gradually used up in supplying nutriment to the embryo which is developed from the cells of the germinal disc. At the periphery of the blastoderm, new cells form progressively until they enclose the yolk (Fig. 22 C).
| |
|
| |
|
| |
|
| |
| Fig. 21. - Diagrams of cleavage ami the blastodermic vesicle in the raut^it (Thomson, after van Beneden). X 200.
| |
|
| |
|
| |
|
| |
| Cleavage in Mammals. - The ovum of all the higher mammals, including man, is isolecithal and nearly microscopic in size. Its cleavage has been studied in several forms, but the rabbit - s ovum will serve as an example. The cleavage is complete and nearly equal (Fig. 21), a cluster of approximately uniform cells being formed within the zona pellucida. This corresponds to the morula stage of Amphioxus. Next, an inner mass of cells is formed that is equivalent to the germinal disc, or blastoderm of the chick embryo. The inner cell mass is overgrown by an outer layer which is termed the trophectoderm, because it later supplies nutriment to the embryo from the uterine wall. Fluid then appears between the outer layer and the inner cell mass, thereby separating the two except at the animal pole. As the fluid increases in amount, a hollow blastodermic vesicle results, its walls composed of the single-layered trophectoderm, except where this is in contact with the inner cell mass. It is usually spherical or ovoid in form, as in the rabbit, and probably such is the form of the human ovum at this stage. In the rabbit, the vesicle is 4.5 mm. long before it becomes embedded in the wall of the uterus; among ungulates, or hoofed animals, the vesicle is greatly elongated and attains a length of several centimeters, as in the pig.
| |
|
| |
|
| |
|
| |
| Fig. 22 . - Diagrams of blastula homologies (Prentiss). . 4 , Amphioxus; B, frog; C, chick; D, mammal.
| |
|
| |
|
| |
|
| |
|
| |
| Comparing the mammalian blastodermic vesicle with the blastula stages of Amphioxus, the frog, and the bird, it will be seen that it is to be homologized with the bird - s blastula, not with that of Amphioxus (Fig. 22). In each case there is an inner cell mass of the germinal disc. The trophectoderm of the mammal represents a ])recocious development of cells, which, in the bird, later enveloji the yolk. The cavity of the vesicle is to be compared, not with the blastula cavity of Amphioxus and the frog, but with the yolk mass pins the cleft-like blastocoele of the bird's ovum. The higher mammalian ovum, although almost devoid of yolk, thus develops a - blastula - resembling that attained by the yolk-laden ova of reptiles and birds. That this similarity has an evolutionary significance is attested by discoidal cleavage in the highly telolecithal eggs of present-day monotreme mammals.
| |
|
| |
|
| |
| In the low primate Tarsius, cleavage and the blastodermic vesicle are well known. A four-celled Macacus ovum, with blastomeres nearly equal and oval in form, is the only cleavage stage yet observed among higher jirimates. In all placental mammals, segmentation of the ovum occurs during its passage down the uterine tube.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| THE FORMATION OF ECTODERM AND ENTODERM (GASTRULATION)
| |
|
| |
|
| |
| The blastula and early blastodermic vesicle show no differentiation into layers. Such differentiation next takes place, giving rise first to the ectoderm and entoderm, and finally to the mesoderm. From these three primary germ layers all tissues and organs of the body are derived.
| |
|
| |
| The processes of gastridation, by which ectoderm and entoderm arise, and of mesoderm formation will be treated separately.
| |
|
| |
| Amphioxus and Amphibia. - The larger cells at the vegetal pole of the Amphioxus blastula fold inward (Fig. 23 A, B). Eventually, these invaginating cells obliterate the blastula cavity and come in contact with the outer layer (Fig. 23 C). The new cavity, thus formed, is the primitive gut, or archenteron, and its narrowed mouth is the blastopore. The outer layer of cells is the ectoderm, the inner, newly formed layer is the entoderm. The entodermal cells are henceforth concerned in the nutrition of the body. The embryo is now termed a gastrula (little stomach).
| |
|
| |
|
| |
| In amphibia, invagination begins at the junction of animal and vegetal cells (Fig. ig G). Externally, the blastopore appears as a crescentic groove. Since the vegetal cells are large and the blastocoele is relatively small, simple invagination fails. Hence, archenteron formation is aided by a lip-like overgrowth of rapidly dividing cells from the animal pole (Fig. ig H).
| |
|
| |
|
| |
| Fig. 23. - Gastrulation in Amphioxus. X 200.
| |
|
| |
|
| |
|
| |
| Reptiles and Birds. - ^The germinal disc, or blastoderm, in these animals lies like a cap on the surface of inert yolk (Fig. 6). Since the enormous amount of yolk makes gastrulation as in Amphioxus and amphibians impossible, the process exhibits marked modifications.
| |
|
| |
|
| |
| There appears caudally on the blastoderm of reptiles a pit-like depression. From this invagination, a proliferation of cells forms a layer which spreads beneath the ectoderm. The inner layer, originating in this manner, is the entoderm, and the region of the pit, where ectoderm and entoderm are continuous, is the blastopore. In Fig. 27 A these changes are complete.
| |
|
| |
|
| |
|
| |
| Fig. 24. - Gastrulation in the pigeon, as shown by a longitudinal section of the blastoderm (redrawn after Patterson). X 50.
| |
|
| |
|
| |
| In birds, the caudal portion of the blastoderm is rolled or tucked under, the inner layer formed in this way constituting the entoderm (Fig. 24). The marginal region, where ectoderm and entoderm meet, bounds the blastopore, while the space between entoderm and yolk is the archenteron.
| |
|
| |
|
| |
|
| |
| Mammals. - Cells on the under surface of the inner cell mass become arranged in a definite sheet, the entoderm (Fig. 2^ A). It is usually said to arise by s])litting, or delamination, although there are attempts to prove ingrowth from a - blastopore. - In most mammals, the entoderm spreads rapidly and lines the blastodermic vesicle (Fig. 38) but in Tarsius, the entoderm forms a much smaller sac (Fig. 25 B, C). The youngest human embryos known (Fig. 40) indicate a previous origin of entoderm much as in Tarsius.
| |
|
| |
|
| |
|
| |
|
| |
| Fig 23. - Gastrulation in the low primate, Tarsius, as demonstrated by sections of the blastodermic vesicle (redrawn after Hubrecht). X 260.
| |
|
| |
|
| |
|
| |
| ORIGIN OF THE MESODERM, NOTOCHORD AND NEURAL TUBE .
| |
|
| |
|
| |
| Amphioxus and Amphibia. - The dorsal portion of the inner sheet, which forms the roof of the archenteron in Amphioxus, gives rise to paired, lateral diverticula, the ccdonuc pouches (Fig. 26). These separate both from a mid-dorsal plate of cells (the future notochord), and from the entoderm of the gut, and become the primary mesoderm. The mesodermal pouches grow ventrad and their cavities form the coelom, or body cavity. Their outer layers, with the ectoderm, constitute the body wall, or somatopleure; their inner layers, with the gut entoderm, form the intestinal wall, or splanchnopleure. In the meantime, a dorsal plate, cut off from the ectoderm, folds into the neural tube (anlage of the nervous system), and the notochordal plate becomes a cord, or cylinder, of cells (axial skeleton) extending the length of the embryo. In this simple fashion the ground plan of the chordate body is attained.
| |
|
| |
|
| |
| Fig. 26. - Origin of the mesoderm in Amphioxus (Hatschek). X 425. a/, Lumen of gut; r//., notochord : ai\, ccelom; crrl.p., coelomic pouch; ect., ectoderm; ent., entoderm; n.c., neural canal; n.g., neural groove.
| |
|
| |
|
| |
|
| |
| Fig. 27. - Longitudinal sections of the snake - s blastoderm, at various stages, to show the origin of the notochordal plate (adapted after Hertwig).
| |
|
| |
|
| |
|
| |
| In amphibia, solid mesodermal plates arise in a similar location and extend laterally between the ectoderm and entoderm. Later, these plates split into two layers and the cavity so formed is the coelom (cf .3S)' The notochord also originates as in Amphioxus.
| |
|
| |
|
| |
| Reptiles, - The same pocket-like depression in the caudal portion of the blastoderm, that gave rise to the cells of the entodermal layer, now invaginates more extensively and forms a pouch which pushes forward between ectoderm and entoderm (Fig. 27 A and B). The size of the invagination cavity varies in different species; in some it is elongate and narrow, lieing confined to the middle line of the blastoderm. The floor of this pouch soon fuses with the underlying entoderm, and the two thin, rupture, and disappear, thus putting the cavity of the pouch temporarily in communication with the space (archenteron) beneath the entoderm (Fig. 27 C). The cells of the roof persist as the notochordal plate, which later liccomes the notochord. The neural folds arise before the mouth of the pouch (blastopore) closes, and, fusing to form the neural tube, incorporate the blastopore into its floor. This temporary communication between the neural tube and the primitive enteric cavity is the neurenteric canal (cf. Fig. 27 C) \ it is found in all the vertebrate groups (cf. Fig. 58). A transverse section through the invaginated pouch, at the time of rupture of its floor, and through the underlying entoderm will make clear the lateral extent of these changes (Fig. 28).
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 28. - Transverse section of a snake - s blastoderm, at a level corresponding to the middle of
| |
|
| |
|
| |
| Fig. 27 C (adapted after Hertwig).
| |
|
| |
|
| |
|
| |
|
| |
| From about the blastopore, and from the walls of the pouch, mesodermal plates arise and extend like wings between the ectoderm and entoderm (Fig. 28). As in amphibia, they later separate into outer (somatic) and inner (splanchnic) layers enclosing the Primitive groove coelom. The relation between notochordal plate, mesoderm, and entoderm, shown in Fig. 28, resembles strikingly the conditions in Amphioxus (Fig. 26 A).
| |
|
| |
|
| |
| Birds - Due to the modified gastrulation in reptiles, birds, and mammals through the influence of yolk, a structure known as the primitive streak becomes important. An account of its formation and significance, based on conditions found in the bird, may be introduced conveniently at this place.
| |
|
| |
|
| |
| Shortly after the formation of entoderm, an opaque band appears in the median line at the more caudal portion of the blastoderm (Fig. 29).
| |
|
| |
|
| |
|
| |
| Fig. 29. - BlaStoderm of a chick cmliryo at the stage of the primitive streak. X 20.
| |
|
| |
|
| |
| Along this primitive streak, which is at first merely a linear ectodermal thickening, there forms a shallow primitive groove, and at its forward end the streak ends in a knob, the primitive knot, or node (of Hensen). The primitive streak becomes highly significant when interpreted in the light of the theory of concrescence, a theory of general application in vertebrate development. It will be remembered that the entoderm of birds arises by a rolling under of the outer layer along the caudal margin of the blastoderm. As the blastoderm expands, it is believed that a middle point on this margin remains fixed (Fig. 30 A) while the edges of the margin on eaeh side are carried caudad and brought together (B, C). Thus, a crescentic margin is transformed into a longitudinal slit. Since this marginal lip originally bounded the blastopore (p. 35), the longitudinal slit must also be an elongated blastopore whose direction has merely been changed. The lips of the slit fuse, forming the primitive streak (D). The teachings of comparative embryology support these conclusions, for the neurenteric canal arises at the cranial end of the primitive streak, the anus at its caudal end, while the primary germ layers fuse in its substance. All these relations exist at the blastopore of the lower animals.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 30. - Diagrams to illustrate the formation of the primitive streak according to the theorjof concrescence. The expanding blastoderm is indicated by dotted circles.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 31. - Median longitudinal section of a chick embryo at the stage of the primitive streak and head process. X 100.
| |
|
| |
|
| |
| From the thickened ectoderm of the primitive streak a proliferation of cells takes place, and there grows out laterally and caudally between the ectoderm and entoderm a solid plate of mesoderm which soon splits into somatic and splanchnic layers (Fig. 316). An axial growth, the head process, or notochordal plate, likewise extends forward from the primitive knot and fuses at once with the entoderm (Figs. 31 and 317). Since the primitive streak represents a modified blastopore, it is evident that this cranial extension, the head process, corresponds to the pouch-like invagination concerned in the formation of notochord and mesoderm in reptiles. In birds, the fusion of the head process with the entoderm, the relation of mesodermal sheets to it laterally, the formation of the notochord from its tissue and the occasional traces in it of a cavity continuous with the primitive pit (that is, a notochordal canal), all recall the conditions described for the less modified invagination in reptiles. The primitive groove is the visible result of mesoderm proliferation from the tissue of the streak.
| |
|
| |
|
| |
| Fig. 32. - . 1 , Embryonic disc of the Mateer human embryo, at the stage of the primitive streak (after Streeter). X 50. B, Embryonic disc of the Ingalls human embryo, with primitive streak and head process. X 26.
| |
|
| |
|
| |
|
| |
| Mammals - A typical primitive streak appears on the blastoderm of mammals (Fig. 32 A). The under side of its ectodermal thickening proliferates mesodermal cells which grow laterally and caudally (Fig. 33). All three germ layers fuse in the primitive knot and from it a head process soon extends forward (Fig. 32 B).
| |
|
| |
|
| |
|
| |
| Fig. 33. - Transverse section through the primitive streak of the Mateer human embryo (Fig. 32 ,1 ) to show the origin of mesoderm (redrawn after Streeter). X 185.
| |
|
| |
|
| |
| The head process of many mammalian embryos contains a cavity {notochordal canal), which in some cases is of considerable size, opening at the primitive pit (Fig. 34). As in reptiles, the floor of this cavity fuses with the entoderm, and the two rupture and disappear. Portions of the floor, still persistent, are shown in Fig. 34. Thus a canal, later enclosed by the neural folds, and then known as the neurenteric canal, puts the dorsal surface of the blastoderm in communication with the enteric cavity beneath the entoderm (Figs. 57 and 58). The roof of the head process, or notochordal plate, is for a time associated closely with the lateral mesoderm (compare these relations in reptiles. Fig. 28), but eventually it becomes the notochord.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 34. - Median sagittal section through the primitive knot and head process of the Ingalls human embryo (Fig. 32 5 ). X 200.
| |
|
| |
|
| |
| The mesoderm grows rapidly around the wall of the blastodermic vesicle, until Anally the two wings fuse ventrally. The single sheet then splits into two layers, the cavity between being the coelom, or body cavity (Fig. 35). The outer mesodermal layer (somatic), with the ectoderm, forms the somatopleure, or body wall; the inner splanchnic layer, with the entoderm, forms the intestinal wall, or splanchnoplcure. The neural tube having in the meantime arisen from the neural folds of the ectoderm, there is present the ground plan of the vertebrate body, the same in man as in Amphioxus (Fig. 35 A).
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 35. - Diagrammatic transverse sections of mammalian embryos. . A , The origin and spread of mesoderm (Brjme); B, the further differentiation of mesoderm, and the body plan (Prentiss).
| |
|
| |
|
| |
|
| |
|
| |
| Mesoderm, but not a coelom, is already present in the youngest human embryo yet examined (Fig. 40 A). In Tarsius, a low primate, the mesoderm has two sources: (i) From the splitting of ectoderm at the caudal edge of the blastoderm; this constitutes the extra-embryonic mesoderm and takes no part in forming the body of the embryo. (2) The intra-embryonic mesodertu, which gives rise to body tissues, takes its origin from the primitive streak as in the chick and lower mammals. The origin in the human embryo is probably much the same as in Tarsius.
| |
|
| |
|
| |
| Homologies of Mesoderm and Notochord. - In Amphioxus and amphibia, transverse sections (Fig. 26) apparently show that the mesoderm and notochord are folded directly from dorsal gut-entoderm. Yet such is illusory, for the roof of the archenteron grows from the dorsal lip of the blastopore. Longitudinal sections prove that as the embryo elongates, this caudal, formative region progressively adds to the roof of the primitive gut. Hence, both notochord and mesoderm originate from the indifferent tissue at the blastopore where ectoderm and entoderm meet. In reptiles, birds, and mammals the mesoderm arises as lateral proliferations from the primitive streak, whereas the notochordal plate (head process) is a - growth - from its anterior end (cf. p. 40). But, as the primitive streak is a modified, fused blastopore (p. 39), their origin is fundamentally like that in Amphioxus and amphibia. From its external position and developmental relations the parent blastoporic tissue is often styled ectoderm; especially in embryos with a primitive streak this is convenient and unobjectionable. It will be evident, therefore, that although the ultimate source of both mesoderm and notochord is from an indifferent - ectoderm, - the notochord, once formed, is true mesoderm.
| |
|
| |
|
| |
| The Notochord or Chorda Dorsalis. - As the primitive streak recedes caudad during development, the head process is progressively lengthened at its expense. Ultimately, the primitive streak becomes restricted to the tail region and serves as a growth zone there, whereas the entire remainder of the body is built around the head process as an axis. The original position of the primitive knot corresponds to the junction of head and neck in the future body. In later stages, the rod-like notochord extends from head to tail in the midplane (Fig. 91). It becomes enclosed in the centra of the vertebrae and in the base of the cranium, and eventually degenerates. In Amphioxus, the notochord forms the only axial skeleton, and it is persistent in the vertebrae of fishes and amphibians. In adult man, traces are found as - pulpy nuclei - in the intervertebral discs.
| |
|
| |
|
| |
| Twinning. - LTsually but one human ovum is produced and fertilized at coitus. The simultaneous development of two or more embryos is due commonly to the ripening, expulsion, and subsequent fertilization of an equal number of ova. In such cases ordinary, or fraternal livins, triplets, and so on, of the same or opposite sex result; properly speaking, they are not twins at all. Identical, or duplicate twins, that is, those true twins always of the same sex and strikingly similar in form and feature, arise from two growing points on the embryonic cell mass, each of which develops as a separate embryo within the common chorion. The identical quadruplets of certain armadillos are known to result from the division of a single blastoderm into four parts. Separate development of the cleavage cells can also be produced experimentally in many of the lower animals.
| |
|
| |
| Occasionally twins are conjoined. All degrees of union, from almost complete separation to fusion throughout the entire body-length, are known. If there is considerable disparity in size, the smaller is termed the parasite; in such cases the extent of attachment and dependency grades down to included twin (fetus in fetu) and tumor-like fetal inclusions. In some - monsters - the duplication is partial, as doubling of the head or legs. All of these terata, like identical twins, are the products of a single ovum, but variably fused in accordance with their original degree of separation on the blastodermic mass.
| |
|
| |
| Stockard reduces the primary cause of all non-hereditary abnormal developments, including twins, to a single factor - developmental inhibition or arrest; the exact type of deformity that results depends solely on the precise moment when the interruption occurs. A slowing of the developmental rate at the critical moment (gastrulation) when one of several potential embryonic axes is about to assert its dominance, causes it to lose its original advantage and one or more neighboring points may then appear as additional axes. The direct cause of the arrest is referred to retarded oxidations.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| CHAPTER III. IMPLANTATION AND FETAL MEMBRANES
| |
|
| |
|
| |
| The conditions under which vertebrate eggs develop vary markedly. In all vcrtel)rates below mammals the eggs are laid and develop in the surrounding medium, aided sometimes (especially in reptiles and birds) by parental protection and incubation. As a group, the mammals alone develop their young within the genital tract of the mother.
| |
|
| |
|
| |
| The embryos of fishes and amphibia grow rapidly to immature forms capable of independent existence. All other vertebrates are much farther advanced at Ihrth and accordingly form various organs, of use during develoi)ment only. Especially in higher mammals has the absence of yolk, and the resulting physiological dependence upon the mother, led to the greatest elaboration of these ajopendages.
| |
|
| |
|
| |
| Such fetal organs include the yolk sac and stalk, the allantois, amnion, and chorion. They have to do with the nutrition and respiration of the embryo, and the elimination of katabolic wastes. In higher mammals, the chorion is associated intimately with the uterine mucosa and forms with it an important organ called the placenta.
| |
|
| |
|
| |
| THE FETAL MEMBRANES OF REPTILES AND BIRDS
| |
|
| |
| Development is similar in both classes. The chick illustrates typically the manner of membrane formation.
| |
|
| |
|
| |
| Amnion and Chorion. - The embryo develops in the center of the blastoderm, which first lies like a disc upon the massive yolk (Fig. 6). Later, the periphery of the blastoderm, not concerned in embryo formation, expands and encloses the yolk mass. This envelope consists of somatopleure and splanchnopleure, separated by the coelom (Fig. 4). The amnion and chorion arise from the somatopleure. This double layer (ectoderm and somatic mesoderm) is thrown up into crescentic folds, just in front of and behind the embryo (Fig. 36 A). Gradually, the hood-like folds close in from all sides until they meet and fuse over the embryo ( Fig. 36 B-C ) . The inner somatopleuric layer, thus formed, is the amnion; it constitutes a protective sac, lined with ectoderm and soon filled with fluid, within which the embryo is suspended. The outer of the two somatopleuric sheets is the chorion. It lies next the shell and is separated by the extra-embryonic coelom from the enclosed embryo and its other membranes (Fig. 37).
| |
|
| |
|
| |
| Yolk Sac. - As the embryo enlarges, its original connection with the extra-embryonic blastoderm becomes a slender stalk, uniting embryo and yolk (Fig. 37). It is designated the yolk stalk, whereas the yolk, enveloped by extra-embryonic blastoderm, is the yolk sac. Vitelline blood vessels ramify on the surface of the yolk sac and through them all the food material of the liquefied yolk is conveyed to the chick during the incubation period.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 36. - Diagrams in a sagittal plane illustrating the development of the fetal membranes of most amniotes (after Gegenbaur in McMurrich). Ectoderm, mesoderm, and entoderm are represented by heavy, light, and dotted lines respectively. .1/., Allantois: Am., amniotic cavity; I 5 .yolk sac.
| |
|
| |
|
| |
|
| |
| Fig. 37. - Diagram of a five-day chick embryo and its membranes (Marshall). X 1.5.
| |
|
| |
|
| |
| Allantois. - There is an early outpouching of the ventral floor of the gut, near its hind end. This entodermal diverticulum pushes outward into the extra-embryonic coelom, carrying before it an investment of splanchnic mesoderm (Fig. 36). It forms a vesicle, known as the allantois, which develops rapidly into a large sac, connected to the hind-gut by the narrower allantoic stalk (Fig. 37). Finally, the allantois flattens and fuses with the chorion, just underlying the porous shell (Fig. 36 D). The blood vessels that ramify in the combined mesodermal wall are situated favorably for gaseous interchange, and the allantois becomes the embryonic respiratory organ. The allantoic cavity also serves in its primitive capacity as a reservoir for the excreta of the embryonic kidneys, and the wall assists in the absorption_of albumen.
| |
|
| |
|
| |
| THE FETAL MEMBRANES OF MAMMALS
| |
|
| |
| Amnion and Chorion. - In most mammals these membranes arise by folding, as in reptiles and birds. Some (guinea pig; hedgehog; bat; primates) form an amnion precociously in an entirely different manner. In the bat, fluid-filled clefts appear in the interior of the embryonic cell mass; these coalesce and constitute the amnion cavity (Fig. 38). Later, a layer of somatic mesoderm envelops its ectodermal roof and the structural outcome is identical with the type derived by folding. The deer and sheep show a method transitional between these extremes; the embryonic mass hollows and its roof ruptures; then the definitive amnion develops by folding. The same group that derives an amnion by dehiscence, forms a chorion from the outer trophectoderm layer of the blastodermic vesicle, to which somatic mesoderm is added (Fig. 40).
| |
|
| |
|
| |
| Yolk Sac. - The yolk sac of monotremes resembles that of birds, but in higher forms an actual yolk mass is lacking. There are numerous early developmental variations. In the majority, the yolk-sac entoderm spreads beneath the trophectoderm shell and for a time lines it (Fig. 38) ; when the extra-embryonic mesoderm and coelom appear, the entoderm becomes clothed with the splanchnic layer and the sac is reduced in relative size. On the contrary, the yolk sac of primates is small from the first and remains as a diminutive central vesicle (Figs. 25 and 40). In rodents, carnivores, and split-hoofed mammals it early attains a large size, but ceases growth as the allantois comes to prominence. The splanchnic mesoderm of all groups bears the vitelline blood vessels. Many animals with a highly developed yolk sac effect an intimate association (through union with the chorion) with the uterine mucosa. There is thus formed a transitory yolk-sac placenta. In some marsupials and insectivores this relation persists.
| |
|
| |
|
| |
|
| |
| Allantois. - Alany mammals, like reptiles and birds, form an allantois by the sacculation of gut-splanchnopleure into the extra-embryonic coelom. In some it remains small, and, especially in certain marsupials, does not come in contact with the chorion. On the contrary, in carnivores and ungulates it becomes very large and lines the chorionic sac (Fig. 39). A goat embryo of two inches has an allantois two feet long.
| |
|
| |
|
| |
|
| |
| Fig. 38. - Stages of amnion formation in the bat (Van Beneden). X about 160.
| |
|
| |
|
| |
|
| |
| Primates have a tiny, tubular allantois; it grows into and lies within the body stalk, which is a bridge of mesoderm connecting the embryo to he chorion (Fig. 40 D). Allantoic, or umbilical blood vessels accompany he allantois.
| |
|
| |
|
| |
| The Placenta. - The egg-laying monotremes develop under the same nutritive and respiratory conditions as do reptiles and birds. The marsupials, after a brief gestation period, give birth to immature young; their chorion, therefore, remains as a smooth membrane but in close apposition with the vascular uterine mucosa. The yolk sac is large and in some forms it unites with the chorion, apparently to serve as a nutritive path from uterus to embryo.
| |
|
| |
|
| |
| In all higher mammals, the chorion is beset with vascular villi and there is a more or less intimate relation, which persists throughout gestation, between the uterine mucosa and the chorionic vesicle. This arrangement results in the formation of an organ, the placenta, specialized for the nutrition of the embryo and for its respiration and excretion.
| |
|
| |
|
| |
|
| |
| Fig. 39. - Diagram of the fetal membranes and allantoic placenta of a pig embryo, in median sagittal section (adapted by Prentiss).
| |
|
| |
|
| |
| The form and extent of the placenta vary in accordance with the final distribution of the chorionic villi. The pig and horse have villi diffusely scattered over the entire chorion. In ruminants, they occur in broadly scattered tufts, interspaced with smooth stretches of chorion. The villi of carnivores constitute a girdle-like band about the chorionic sac. In rodents, insectivores, bats, and primates, the villi are limited to a patchlike disc (Fig. 48).
| |
|
| |
|
| |
| There is likewise a structural series, based on the degree of fetal maternal intimacy. At the bottom of the scale stands the mere apposition of the uterine mucosa and the avillous chorion of marsupials.
| |
|
| |
|
| |
| Simplest of the forms with chorionic villi is the condition illustrated by the pig or horse (Fig. 39). The allantois, developing as in the chick, comes in contact and fuses with the chorion. Allantoic vessels lie in the combined mesoderm. Meanwhile, the external ectoderm of the amnion has closely applied itself to the uterine epithelium, and, when the chorionic villi appear, they fit into corresponding pits in the mucosa. Nutritive substances and oxygen from the maternal blood must pass through both layers of epithelium before entering the allantoic vessels. In the same manner, waste products from the embryo pass in the reverse direction. The allantois has therefore become important, not only as an organ of respiration and excretion, as in reptiles and birds, but also as an organ of nutrition. Through its vessels it has taken on the function belonging to the yolk sac of lower vertebrates, and the rudimentary, yolkless sac of higher mammals is now explained.
| |
|
| |
|
| |
| This general scheme of the ungulates is modified by an advance among its ruminant subgroup. Here the villi penetrate deeper and come in closer relation with the connective tissue about the maternal vessels by a partial destruction of the uterine epithelium. At the end of gestation the chorionic villi of the pig, horse, and ruminant are merely withdrawn, and the maternal mucosa is not lost.
| |
|
| |
|
| |
| In carnivores there is marked destruction of the mucosa, so that the chorionic epithelium about the maternal vessels is separated from the circulating blood by endothelium alone.
| |
|
| |
|
| |
| The highest type, as in rodents and primates, is characterized by a superficial erosion and destruction of the uterine mucosa, so that the chorionic villi, dangling in cavernous spaces, are bathed by the maternal blood which issues from eroded vessels (Fig. 34). In this and the preceding type, the changes are so profound and the fusions so intimate that the mucosa is largely sloughed at birth as a decidua. The chorion was important in the ungulate chiefly as it brought the allantois into close relation with the uterine wall, but in man and most unguiculates it assumes the several placental functions, and the allantois, now superseded like the yolk sac, in turn becomes rudimentary.
| |
|
| |
|
| |
| THE FETAL MEMBRANES OF MAN
| |
|
| |
|
| |
| Amnion. - -In the youngest known human embryo (Miller) the embryonic mass is solid (Fig. 40 A), but its ectoderm indicates a stage preparatory to the formation of amnion clefts. As an amniotic cavity is present in the slightly older embryo described by Bryce-Teacher (Fig. 40 B), the method of origin must be by direct splitting as in the bat (p. 46). These specimens likewise lack a coelom in the precociously formed extra-embryonic mesoderm, whereas all older embryos possess somatic and splanchnic layers bounding a more or less extensive coelomic cleft (Fig. 40 C). Somatic mesoderm then covers the primitive ectodermal roof of the amniotic cavity (Fig. 40 D) ; this order of layering is identical in all amniotes.
| |
|
| |
|
| |
|
| |
| At first there is a broad union between the amnion and the external shell of t rophectoderm (Fig. 40 C), but this becomes reduced by the continued extension of the coelomic cavity until presently it is limited to the caudal end of the embryo alone (Fig. 40 D). This narrow, mesodermal bridge, into which the allantois and its vessels grow, is the body stalk (Fig. 43).
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 40. - Diagrams of early human embryos (adapted by Prentiss). A, Miller (modified); B, Bryce-Teacher (modified); C, Peters; D, Spee.
| |
|
| |
|
| |
|
| |
| Hence, from the first, the human amniotic cavity is closed. The base of the amnion is attached to the periphery of the embryonic disc, which also constitutes the floor of the cavity (Fig. 32 A). The amnion becomes a thin, pellucid, non-vascular membrane, lined with a simple epithelium (Figs. 43, 61 and 65). The amniotic cavity enlarges rapidly at the expense of the extra-embryonic coelom, and, at the end of the second month, fills the chorionic sac (Fig. 51). It then attaches loosely to the chorionic wall, thereby obliterating the extra-embryonic body cavity (Fig. 50).
| |
|
| |
|
| |
| Amniotic fluid fills the sac. Its immediate origin (fetal or maternal) is disputed. During the early months of pregnancy the embryo is suspended by the umbilical cord in this fluid (Fig. 51). Throughout gestation the amniotic fluid serves as a protective water cushion, equalizing pressures and preventing adherence of the amnion. At parturition, it acts as a fluid wedge to dilate the uterine cervix. The embryo is protected from maceration by a fatty skin-secretion, the vernix caseosa.
| |
|
| |
|
| |
| During the early stages of childbirth the membranes usually rupture, and about a liter of amniotic fluid escapes as the - waters. - If the tough amnion fails to burst, the head is delivered enveloped in it, and it is then popularly known as the - caul. -
| |
| Anomalies. - When the amniotic fluid is excessive in volume, the condition is designated ' hydramuios. - If less than the optimal amount is present, the amnion may adhere to the embryo and cause malformations. Fibrous bands sometimes extend across the amnion cavity. As pressure increases during growth, they may cause scars and the splitting or even amputation of parts.
| |
|
| |
|
| |
|
| |
| Fig. 41. - Section of Peters - 0.19 mm. human embryo (about fifteen da}^s). The portion of extra-embryonic ccelom shown is limited below by a strand of the magma reticulare.
| |
|
| |
|
| |
|
| |
| Yolk Sac. - The entodermal portion of the Miller embryo is solid (Fig. 40 A), but in all other early specimens it forms a small vesicle, lined with a single layer of entoderm and covered with splanchnic mesoderm (Figs. 40 B-D, 41 and 43). In embryos of 1.5 to 2.0 mm., the entodermal roof of this vesicle begins to form the fore- and hind-gut which are then connected by a slightly narrowed region to the yolk sac proper (Figs. 43 . and 44). With the growth of the head- and tail regions of the embryo there is an apparent progressive constriction of the yolk sac (Figs. 60, 61 and 64). This, however, is a deception. Both embryo and yolk sac enlarge, whereas the region of union lags in transverse development but elongates into the slender yolk stalk (Fig. 42).
| |
|
| |
|
| |
| The yolk stalk becomes incorporated in the umbilical cord (Figs. 45, 64 and 65). It loses its attachment with the gut in embryos of 7 mm. and soon degenerates. Even earlier, the yolk sac has attained its final diameter of about i cm. ; it persists and may be found at birth adherent to the amnion in the placental region (Fig. 55). The yolk sac of man is a vestige containing a coagulum but no yolk (Fig. 41). Blood vessels arise very early in its mesoderm (Figs. 43 and 44) and institute a vitelline circulation with the embryo.
| |
|
| |
|
| |
|
| |
| Fig. 42. - Yolk sac and Stalk of a 20 mm. human embryo (Prentiss). X u.
| |
|
| |
|
| |
|
| |
| Anomalies. If that portion of the yolk stalk between the intestine and umbilicus remains pervious it constitutes a fecal fistula through which intestinal contents may escape.
| |
|
| |
| In 2 per cent of all adults there is a persistence of the proximal end of the yolk stalk, to form a pouch, Meckel - s diverticulum of the ileum. This varies between 3 and 9 or more cm. in length and lies about 80 cm. above the colic valve. The divei'ticulum is important surgically as it sometimes telescopes into the intestinal lumen and occludes it.
| |
|
| |
|
| |
|
| |
| Allantois. - Although the allantois is absent in the youngest embryos known, it nevertheless appears very early - even before the gut. In the Spee specimen, the allantois is a slender tube extending into the mesoderm of the body stalk (Fig. 43). It never becomes saccular, as in most lower amniotes. Since the human allantois arises so precociously, it does not develop as an evagination of the hind-gut into the extra-embryonic coelom; yet the body stalk, which contains the allantois, represents mesoderm into which the coelom has failed to penetrate.
| |
|
| |
|
| |
| Elongation extends the allantoic tube as far as the chorion (Figs. 44, 71 and 184), and, when the developing umbilical cord includes the allantois as a component, it at first is as long as the cord (Figs. 45 and 51). Soon, however, growth ceases and at birth the only remnant is a tenuous, and generally discontinuous, solid strand.
| |
|
| |
|
| |
|
| |
| Fig. 44. - Mali - s 2.0 mm. human embryo in median sagittal section (adapted by Prentiss).X 23.
| |
|
| |
|
| |
| Umbilical blood vessels accompany the allantois; these also reach the chorion and vascularize it (Figs. 51 and 184). When the chorion becomes a part of the placenta it performs all the functions of nutrition, respiration, and excretion. Like the yolk sac, the allantois is a superseded rudiment.
| |
|
| |
|
| |
|
| |
| Chorion. - The human chorion is derived directly from the trophectoderm layer of the blastodermic vesicle to which is added extra-embryonic mesoderm (Fig. 40). The trophectoderm of the youngest known embryos has already given rise to an outer syncytial layer, the irophodcrm, but the mesoderm is solid. In slightly older specimens, the mesoderm is cleft by the extra-embryonic cmlom and its outer, or somatic, layer lines the chorion (Figs. 40 B-I) and 46). The chorion forms villous processess (Fig. 48). At first these are solid ectoderm, the primary villi (Fig. 40 C), but soon the chorionic mesoderm invades them as central cores (Fig. 43) and allantoic, or umbilical blood vessels ramify in their branches. Such villi are secondary, or true villi (Figs. 51 and 65). The further history of the chorion is inseparable from placental development (p. 62).
| |
|
| |
|
| |
|
| |
| Umbilical Cord. - As the embryo enlarges, its ventral, unclosed area, bounded by the edge of the amnion, becomes relatively smaller (Fig. 45 A, 5 ). For a time the amnion attaches close to the embryo, but, during the sixth week, growth of the adjoining body wall, accompanied by an elongation of the body stalk, causes the amnion to recede from the umhilicus. The tubular structure thus formed is the itmhilical cord (Fig. 45 C). It encloses both yolk stalk and allantois, and includes a portion of the coelom. Henceforth, the umbilical cord connects the embryo to that part of the chorion which constitutes the fetal half of the placenta (Figs. 51 and 55). The umbilical cord is actually an embryonic growth, and the amnion merely attaches to its distal end ( Fig. 65).
| |
|
| |
|
| |
| The cord is covered with ectodermal epithelium and contains, embedded in mucous tissue (jelly of Wharton): (i) the yolk stalk (and in early stages its vitelline vessels); (2) the allantois; (3) the allantoic or umbilical vessels (two arteries and a single, large vein ) . The mucous tissue, peculiar to the umbilical cord, comes from mesenchyme; it bears neither capillaries nor nerves. Between the sixth and tenth weeks, the gut extends into the coelom of the cord and forms a temporary umbilical hernia there (Fig. 96). After it is withdrawn, the cavity of the cord disappears.
| |
|
| |
|
| |
| The mature cord is about 1 .5 cm. in diameter and attains an average length of 50 cm. Its insertion is usually near the center of the placenta (Fig. 56), but may be marginal or even on the adjoining membranes. A spiral twist appears (Fig. 53), just how is not known, and the blood vessels sometimes curl in masses which cause external bulgings, designated - false knots. - True knots are known also. The cord may wind about the neck or extremities of a fetus and induce atrophy or even amputation.
| |
|
| |
|
| |
|
| |
| Fig. 45. - Diagrams of the development of the human umbilical cord (DeLee). a.c., Amniotic cavity; exc., extra-embryonic coelom.
| |
|
| |
|
| |
|
| |
| Implantation and Early Mucosal Relations During the events of cleavage and the formation of a morula and blastodermic vesicle, the ciliated lining of the uterine tube steadily transports the ovum downward. Early in this period of migration and development, the ovum loses its corona radiata cells and pellucid membrane. In about eight days it probably reaches the uterus, having attained a stage something like Fig. 40 A, although the vesicle is only about 0.2 mm. in diameter. It is evident, therefore, that the foregoing sections of this chapter describe changes which occur largely after implantation, rather than before it.
| |
|
| |
|
| |
| Fig. 46. - Section through a human embryo of 0.19 mm., embedded in the uterine mucosa (semidiagrammatic after Peters), am., Amniotic cavity; hS., body stalk; eel., ectoderm of embryo; ent., entoderm; ?nes., mesoderm; yS., yolk sac.
| |
|
| |
|
| |
| Implantation comprises the process by which the embryonic vesicle becomes embedded in the uterine mucosa. Actual observations on the human ovum are lacking, but from careful studies on the earliest specimens, and from more complete observations on other mammals, the course of events is reasonably certain.
| |
|
| |
|
| |
| The ovum penetrates the mucosa as would a parasite, the trophoderm supposedly producing an enzyme which digests away the maternal tissues until the embryo is entirely embedded. The Peters specimen, shown in Fig. 46, is well established and the chorionic vesicle has an internal diameter of more than a millimeter. Its point of entrance is marked by the customary fibrin clot which soon disappears, and the defect is repaired.
| |
|
| |
|
| |
|
| |
| Continued rapid growth of the embryo necessitates a correspondingly progressive erosion of the maternal tissues. This causes extravasations of blood which collect in large vacuoles in the invading trophoderm and form blood lacunae (Fig. 46). The lacunce break up the trophoderm into solid cords, composed of both the inner cellular and outer syncytial layers. These constitute the primary villi. It is the syncytial layer that is active in the destruction of the uterine tissues, and probably also in the absorption of blood and tissue products (emhryotroph) for the early nutrition of the embryo.
| |
|
| |
|
| |
|
| |
| Next, there are changes leading to the definitive [hemotrophic) type of nutrition. Chorionic mesoderm extends into the primary villi, and branching secondary or true villi result (Figs. 43 and 47). During the development of villi the blood lacunas in the original trophoderm shell expand, run together, and produce intervillous spaces which surround the villi and bathe their epithelium (Fig. 47). The formerly Spongy trophoderm is now reduced to a continuous layer covering the outer surfaces of the villi and chorion. Branches of the umbilical vessels develop in the mesoderm of the chorion and villi (Fig. 51). The mesodermal core of each villus and its branches is then covered by a two-layered epithelium; an inner, ectodermal layer (of Langhans) with distinctly outlined cuboidal cells, and an outer, syncytial trophoderm layer (Figs. 47 and 53 A). The epithelium also forms solid columns of cells which anchor the ends of certain villi to the uterine wall (Fig. 47),
| |
|
| |
|
| |
|
| |
| Fig. 47. - Diagram of the early development of chorionic villi and placenta (after Peters).
| |
|
| |
|
| |
| In the vessels of the chorionic villi, the chorionic circulation of the embryo is established. The blood vessels of the uterus open into the intervillous blood s]iaces, and here the maternal blood circulates and bathes the syncytial trophoderm of the villi (Figs. 47 and 54). The transfer of nutritive substances and oxygen to the fetal blood takes place through the walls of the chorionic villi, whereas fetal wastes pass in the reverse direction. The tro])hoderm, like endothelium, prevents the coagulation of maternal blood. According to Mall, it also forms a wall which dams or ulugs the maternal blood vessels as soon as eroded, and, with the decidua (p. 62), limits the flow of blood into the intervillous spaces.
| |
|
| |
|
| |
| Villi at first cover the entire surface of the chorion (Fig. 40 D). As the embryo enlarges, the villi next the uterine cavity become both compresSed and remote from the Idood supply (Fig. 51). During the fourth week these villi atrophy and disappear (Fig. 48). This leaves a smooth surface, called the chorion Laeve. The villi adjacent to the uterine wall persist as the chorion frondosum and become the fetal part of the placenta (Fig. 49),
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 48. - Human chorionic vesicles of five and seven weeks (De Lee). The chorion laeve and chorion frondosum are apparent. Natural size.
| |
|
| |
|
| |
| The Decidual Membranes
| |
|
| |
|
| |
| Two sets of important changes take place normally in the uterine mucosa. One of these is periodic, between puberty and the menopause, and is the cause of menstruation. It is comparable to the oestrus cycle in lower animals, and may also be regarded as preparatory to the second set of changes which appear only in pregnancy and give rise to the decidual membranes and placenta.
| |
|
| |
|
| |
| Menstruation. - The periodic changes that accompany the phenomenon of menstruation form a cycle which occupies twenty-eight days. This period is divisible into four phases ; .
| |
|
| |
|
| |
| 1. Tumefaction (six days). The uterine mucosa thickens both because of vascular congestion and cellular multiplication. Blood escapes from the enlarged capillaries and forms subepithelial masses. The uterine glands elongate and their deeper portions especially are convoluted and dilated with secretion. The mucosa thus shows a superficial, compact layer and a deep, spongy layer.
| |
|
| |
|
| |
| 2. Menstruation proper (four days). The superficial blood vessels rupture and add to the blood and glandular discharge which is escaping into the uterine cavity. The surface epithelium and a portion of the underlying tissue may or may not be desquamated.
| |
|
| |
| 3. Restoration (five days). The vascular engorgement disappears. Extravasated blood corpuscles are resorbed or cast off. The epithelium, glands, and capillaries are repaired.
| |
|
| |
|
| |
| 4. Intermenstruum (thirteen days). An interval of rest. Since ovulation occurs most often postmenstruum, Grosser believes that the embryo reaches the uterus during the premenstrual stage. The congestion and loosening of the uterine tissue at this time would seemingly favor the implantation of the embryo, and the glandular secretion might afford nutriment for its growth until implantation occurred. The first phase of menstruation, according to this view, prepares the uterine mucosa for the reception of the embryo. If pregnancy supervenes, it soon inhibits any further premenstrual changes so that menstruation does not occur. Menstruation proper would then represent an over-ripe condition of the mucosa and the abortion of an unfertilized ovum.
| |
|
| |
| The Deciduae. - The intimate fusions between fetal and maternal tissues necessitate an extensive sloughing of the uterine lining at birth.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 50. - Vertical section through the decidua vera of about seven months, with the attached membranes in situ (Schaper in Lewis and Stohr). X 30.
| |
|
| |
|
| |
| The mucosa of the pregnant uterus is, therefore, designated the decidua. Its preparation and continuance during gestation, and the long deferred loss and repair at parturition, only exaggerate the events of an ordinary menstrual cycle. The two processes show undoubted fundamental similarities.
| |
|
| |
|
| |
| The chorionic vesicle lies embedded in part of the uterine wall only (Fig. 49). This allows three regions to be recognized : (i) a portion not in direct contact with the ovum, the decidua vera; (2) a portion which constitutes a superficial covering or arching dome, the decidua capsularis; (3) a portion underlying the embryo and between it and the muscularis, the decidua basalis.
| |
|
| |
|
| |
| Decidua Vera. - The premenstrual, superficial compact layer and deep spongy layer are still further emphasized in pregnancy (Fig. 50). The compact layer contains the straight but dilated segments of the uterine glands. Its surface epithelium disappears by the end of the third month. The spongy layer is characterized by the greatly enlarged and tortuous portions of the glands of pregnancy.
| |
|
| |
|
| |
| A prominent constituent are the decidual cells that occur chiefly in the stratum compactum (Fig. 50). They are modified stroma cells, frequently multinucleate, which become about 50^ in diameter. Athough diagnostic of pregnancy their function is in doubt. Many degenerate during the later months.
| |
|
| |
|
| |
| Fig. 51. - Diagrammatic section through a pregnant uterus of two months (Thomson). c, Uterine tube; c', mucous plug in cervi.x; dv, decidua vera; dr, decidua capsularis; ds, decidua basalis; ch, chorion (its longer villi constitute the chorion frondosum) ; am, amnion; u, umbilical cord; al, allantois; y,y', yolk sac and stalk.
| |
|
| |
|
| |
|
| |
| During the first two months of gestation the long axes of the glands are vertical. Later, as the decidua is stretched and compressed, owing to the growth of the fetus, the glands are broadened and shortened, and their cavities become elongated clefts parallel to each other and to the surface of the decidua (Fig. 50). Similarly, the gland cells stretch, and flatten until they resemble endothelium. The decidua vera attains a maximum thickness of about i cm., but in the latter half of pregnancy pressure causes it to thin and lose much of its early vascularity. The cervix uteri does not form a decidua; its glands secrete a mucous plug which closes the uterus until the beginning of labor (Fig. 51).
| |
|
| |
|
| |
| Decidua Capsularis . - In the earlier stages of development, glands and lilood vessels occur in its substance and the surface epithelium is continuous with that of the decidua vera (Fig. 49). As the chorion expands, the capsularis grows thin and atrophic. During the fourth month it comes into contact with the decidua vera, with which it fuses, thereliy obliterating the uterine cavity (Figs. 51 and 55). Soon after, the capsularis degenerates and disappears. This allows the chorion Ireve to become adherent to the decidua vera (Fig. 50).
| |
|
| |
|
| |
| Decidua Basalis - During the first four months of pregnancy this portion of the mucosa resembles the decidua vera in structure (Fig. 49). Both compact and spongy layers are represented, although there are superficial erosions and blood extravasations caused by the activity of the chorionic trophoderm. The decidua basalis does not share in the degeneration common to the other deciduae but persists until birth as a component of the nutritional organ termed the placenta (Figs. 52 and 55). The decidua is said to help in preventing excessive hemorrhage during the earlier part of pregnancy by acting as a dam between the chorionic villi and the eroded uterus (p. 58).
| |
|
| |
|
| |
| The Placenta
| |
|
| |
| The placenta has a double origin. The chorion frondosum is the fetal portion and the decidua basalis is the maternal contribution (Fig. 49). The area of persistent frondosum villi is somewhat circular in form, so that the placenta becomes disc-shaped (Fig. 56). Near the middle of its fetal surface is attached the umbilical cord ; the surface itself is covered by glistening amnion that has fused with the subjacent chorion (Fig. 52).
| |
|
| |
|
| |
| The Placenta Fetalis. - The villi of this portion of the chorion form profusely branched, tree-like structures which lie in the intervillous spaces (Figs. 52 and 54). The ends of some of the villi are attached to the wall of the decidua basalis and are known as anchoring villi, in contrast to the floating free villi. In the connective-tissue core of each villus are commonly two arteries and two veins (branches of the umbilical vessels), cells like lymphocytes, and special ceils of Hofbauer apparently phagocytic in function. Lymphatics are also present. The epithelium of the villi is at first composed of a layer of trophectoderm, with the outlines of its cuboidal cells sharply defined (Fig. 53 A). This layer (of Langhans ) forms and is covered by a syncytium, the trophoderm. In the later months of pregnancy, as the villi grow, the trophectoderm is used up in forming the syncytium, so that at term the trophoderm is the only continuous epithelial layer of the villi (Fig. 53 B). About the margin of the placenta the trophectoderm persists as the closing ring, which is continuous with the epithelium of the chorion Ireve.
| |
|
| |
|
| |
|
| |
| The Plaecnta Materna. - This, like the decidua vera, is differentiated into a basal plate, which is the remains of the compact layer and forms the floor of the intervillous spaces, and into a deep spongy layer (Figs. 52 and 54 )
| |
| 1 he basal plate is composed of a connective-tissue stroma, containing decidual cells, canalized fibrin, and persisting portions of the epithelium of the villi. The - canalized fibrin - (Fig. 47) forms chiefly by a fibrinoid necrosis of the mucosa, but the fibrin of the maternal blood and the chorionic trophoderm also participate (Mall, 1915). Septa extend from the basal plate into the intervillous spaces but do not unite with the chorion frondosum (Grosser). Near term, these constitute the septa placentae (Fig. 54) which incompletely divide the placenta into lobules, or cotyledons (Fig. 56 B).
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 53. - Transverse sections of chorionic villi (Schaper in Lewis and Stohr). A, At the fourth week; B, C, at the end of pregnancy.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| The maternal arteries and veins pass through the basal plate, taking a sinuous course and opening into the intervillous spaces (Fig. 54). Near their entrance they proceed obliquety and lose all but their endothelial layers. The original openings of the vessels into the intervillous spaces were formed during the implantation of the ovum when their walls were eroded by the invading trophoderm of the villi (Fig. 47). As the placenta increases in size, the vessels grow larger. The ends of the villi frequently are sucked into the veins and interfere with the placental circulation.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 54. - Scheme of placental circulation (Kollmann). Arrows indicate the blood flow in the intervillous spaces.
| |
|
| |
|
| |
|
| |
| At the periphery of the placenta is an enlarged intervillous space that varies in extent but never circumscribes the placenta completely. This space is the marginal sinus through which blood is carried away from the placenta by the maternal veins (Fig. 55). The blood of the mother and fetus does not mix, although the epithelial cells of the villi are instrumental in transferring nutritive substances to the blood of the fetus and in eliminating wastes from the fetal circulation into the maternal blood stream of the intervillous spaces.
| |
|
| |
|
| |
|
| |
| Mall (1915) states that there is little evidence of an actual intervillous circulation; the decidua and trophoderm are active in preventing this (pp. 58 and 62). Some authorities hold that the intervillous circulation is peculiar to the second half of pregnancy. In summary, Mall regards the entire question as still open.
| |
|
| |
|
| |
| Parturition
| |
|
| |
| Before birth, the placenta is concave on its amniotic surface, its curvature corresponding to that of the uterus (Fig. 55). At term, the duration of which is taken as ten lunar months, the muscular contractions of the uterus, termed - pains, - bring about a dilation of the cervix uteri, the rupture of the amnion and chorion lasve, and cause the extrusion of the child. With the rupture of the membranes the amniotic liquor is expelled, but the fetal membranes remain behind, attached to the deciduae. The pains of labor begin the detachment of the decidual membranes, the plane of their separation lying in the spongy layer of the decidua basalis and decidua vera, where there are only thin-walled partitions between the enlarged glands (Figs. 50 and 52). Following the birth of the child, the tension of the umbilical cord and the - after pains - which diminish the size of the uterus normally complete the separation of the decidual membranes from the wall of the uterus. The uterine contractions serve also to diminish the size of the ruptured placental vessels and prevent extensive hemorrhage. From the persisting portions of the spongy layer and from the epithelium of the glands are regenerated' the tunica propria, glands, and epithelium of the uterine mucosa.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 56. - Mature placenta (Heisler). A, Entire fetal surface with membranes attached to its periphery; B, detail of maternal surface showing cotyledons.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 55. - Section of the uterus, illustrating the relation of an advanced fetus to the placenta and membranes (Ahlfeld).
| |
|
| |
|
| |
| PARTURITION .
| |
|
| |
|
| |
| The decidual membranes, and the structures attached to them when expelled, constitute the - after birth. - The placenta is disc-shaped, about 17 cm. in diameter, 2 cm. thick, and weighs 500 gm. It is usually everted so that its amniotic surface is convex, its maternal surface concave (Fig. 56). The placenta is composed of the amnion, chorion frondosum (chorionic villi with intervillous spaces divided incompletely by the septa into cotyledons), and includes on the maternal side the basal plate and a portion of the spongy layer of the decidua basalis (Fig. 52). Near the center is attached the umbilical cord, and at its margins the placenta is continuous with the decidua vera and the remains of the chorion Iseve and decidua capsularis. The amnion lines all the deciduae (Fig. 55).
| |
|
| |
|
| |
|
| |
| Gross Changes in the Uterus. - During pregnancy the uterus enlarges enormously, due chiefly to the hypertrophy of its muscle fibers, and the fundus reaches the level of the xiphoid process. After birth, it undergoes rapid involution; at the end of one week it has lost one-half its weight, and in the eighth week the return is complete. The mucosa is regenerated in two or three weeks from the remains of the spongy layer (Fig. 52).
| |
|
| |
|
| |
|
| |
| Position of the Placenta and Its Variations. - The position of the placenta is determined by the point at which the embryo is implanted. In most cases it is situated on either the dorsal or ventral wall of the uterus. Occasionally it is lateral in position, and, very rarely, it is located near the cervix and covers the internal os uteri, constituting a placenta prcBvia. A partially or wholly duplicated placenta, or accessory {succenturiate) placentas may be formed from persistent patches of villi on the chorion lseve.
| |
|
| |
|
| |
|
| |
| Ectopic Pregnancy. - If the ovum becomes implanted and develops elsewhere than in the uterus, the condition is known as an extra-uterine, or ectopic pregnancy. The commonest site is the uterine tube, tubal pregnancy. Attachment to the peritoneum, abdominal pregnancy, and the development of an unexpelled ovum within the ruptured follicle, ovarian pregnancy, are known also.
| |
|
| |
|
| |
|
| |
| Plural Pregnancy. - Twins occur once in 85 births; triplets, once in 7000; quadruplets, once in 750,000. Each member of ordinary double-ovum - twins - (p. 42) has its own amnion, chorion, and umbilical cord. The placenta and decidua capsularis are also individual, except in those cases where the original proximity of implantation leads to secondary fusions. Single-ovum, identical twins comprise only 15 per cent of the entire twin group; the chorion, placenta, and decidua capsularis are necessarily common, but the cord and usually the amnion are double.
| |
|
| |
|
| |
| CHAPTER IV . AGE, BODY FORM AND GROWTH CHANGES AGE. SIZE AND WEIGHT OF EMBRYOS
| |
|
| |
|
| |
|
| |
| The age of a human embryo can not be determined with certainty, because too little is known of the time relations existing between ovulation and menstruation, and between ovulation, coitus, and fertilization (p. 27). This lack of a reliable basis makes any computation approximate, although the errors thus introduced are significant only in young specimens.
| |
|
| |
|
| |
| From numerous clinical observations it is certain that ovulation does not immediately precede menstruation, as was long held, but on the contrary follows it (p. 24). Experience proves that most pregnancies date from a coitus within a week or ten days after the menses cease. Hence, it is a])proximately correct to compute the age of an embryo from the tenth Jay after the onset of the last menstruation.
| |
|
| |
|
| |
| Careful studies on embryos which were accompanied by adequate data as to menstruation, coitus, and clinical history have led to the establishment of certain age-norms. By comparing a given specimen with such standards its age can be determined with reasonable accuracy. It is simplest to make these comparisons on the basis of size, although young embryos vary sufficiently so that structure must be taken into account as well. Embryos are measured in two ways. Commonest is the crown-rump length (designated CR), or sitting height; this is the measure from vertex to breech. The second is the crown-heel length (CH). or standing height.
| |
|
| |
| The following table, based on data by Mall and Scammon, lists the size and weight of human embryos corresponding to definite ages :
| |
| Ratio of increase .
| |
|
| |
| .
| |
|
| |
|
| |
| Crown-rump length .
| |
|
| |
| Crown-heel length .
| |
|
| |
| .
| |
|
| |
| to weight at .
| |
|
| |
| .
| |
|
| |
| (.CR), or sitting .
| |
|
| |
| (CH). or standing .
| |
|
| |
| Weight in .
| |
|
| |
| beginning of .
| |
|
| |
| Age .
| |
|
| |
| height (mm.).
| |
|
| |
| .
| |
| height (mm.) .
| |
|
| |
| grams .
| |
|
| |
| month .
| |
|
| |
| Three weeks .
| |
|
| |
| 0.5 .
| |
|
| |
| 0.5 .
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| Four weeks .
| |
|
| |
| 2.5 .
| |
|
| |
| 2-5 .
| |
|
| |
| .
| |
|
| |
| 8000.00 .
| |
|
| |
| Five weeks .
| |
|
| |
| 5-5 .
| |
|
| |
| 5-5 .
| |
|
| |
| . 004 .
| |
|
| |
| .
| |
|
| |
| Six weeks .
| |
|
| |
| I I . 0 .
| |
|
| |
| 11.0 .
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| Seven weeks .
| |
|
| |
| 17.0 .
| |
|
| |
| 19 . 0 .
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| Second lunar month .
| |
|
| |
| 25.0 .
| |
|
| |
| 30. 0 .
| |
|
| |
| 2 .
| |
|
| |
| 499 . 00 .
| |
|
| |
| Third lunar month .
| |
|
| |
| 68.0 .
| |
|
| |
| 98 . 0 .
| |
|
| |
| 24 .
| |
|
| |
| I I . 00 .
| |
|
| |
| Fourth lunar month .
| |
|
| |
| I 2 I . 0 .
| |
|
| |
| 180.0 .
| |
|
| |
| 120 .
| |
|
| |
| 4.00 .
| |
|
| |
| Fifth lunar month .
| |
|
| |
| 167 . 0 .
| |
|
| |
| 250.0 .
| |
|
| |
| 330 .
| |
|
| |
| 1-75 .
| |
|
| |
| Sixth lunar month .
| |
|
| |
| 210.0 .
| |
|
| |
| 3130 .
| |
|
| |
| 600 .
| |
|
| |
| 0.82 .
| |
|
| |
| Seventh lunar month ,.
| |
|
| |
| .
| |
| 245 . 0 .
| |
|
| |
| 370.0 .
| |
|
| |
| 1000 .
| |
|
| |
| 0.67 .
| |
|
| |
| Eighth lunar month .
| |
|
| |
| 284.0 .
| |
|
| |
| 425 0 .
| |
|
| |
| 1600 .
| |
|
| |
| 0.60 .
| |
|
| |
| Ninth lunar month .
| |
|
| |
| 316.0 .
| |
|
| |
| 470.0 .
| |
|
| |
| 2400 .
| |
|
| |
| 0.50 .
| |
|
| |
| Tenth lunar month .
| |
|
| |
| 345 â– 0 .
| |
|
| |
| 500. 0 .
| |
|
| |
| 3200 .
| |
|
| |
| 0.33 .
| |
|
| |
|
| |
| 68 .
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| For estimating the age of an embryo when its size is known, or the reverse, the following rules are useful ;
| |
|
| |
| Standing height (in cm.) X 0.2 = Age {in months)
| |
|
| |
| Sitting height (in cm.) X 0.3 = ,-lg^ {in months)
| |
| (For embryos less than 10 cm. long, add one month to the result)
| |
|
| |
| Age (in months) ^ 0.2 = Standing height (in cm.)
| |
|
| |
| Age {in months) -u 0.3 = Sitting height {in cm.)
| |
| (For embryos of the first 3 months, subtract 4 cm. from the result)
| |
|
| |
| Of practical interest is the determination of the date of delivery of a pregnant woman. Most labors occur ten lunar months, or 280 days, from the first day of the last menstrual period. The month and day of this date are easily found by counting back three months from the first day of the last period, and then adding one week. As some women menstruate once or more after becoming pregnant this computation is not infallible.
| |
|
| |
|
| |
| For comparison and reference, the gestation periods of a few representative mammals are appended ;
| |
|
| |
| Opossum 13 days Pig
| |
| Mouse 20 days Sheep
| |
| Rat 21 days Cow
| |
| Rabbit 30 days Horse
| |
| Cat 8 W - eeks Rhinoceros
| |
| Dog, guinea pig 9 weeks Elephant . .
| |
|
| |
|
| |
|
| |
|
| |
| The early history of the human ovum, including implantation and the development of membranes for its protection and nutrition, has been described on previous pages. The present section will deal with the appearance of the embryo and fetus at successive stages of uterine existence.
| |
|
| |
|
| |
| Period of the Embryo
| |
|
| |
| Embryos of the Second Week. - The youngest known embryo is the Miller specimen. It is somewhat like the diagram represented in Fig. 40 A. The central embryonic anlage is solid, without amnion cavity or yolk sac; it measures oI mm. in length. The extra-embryonic mesoderm is unsplit by a coelom. The chorion has both syncytial and Langhans layers, but true mesodermal villi are absent; its internal cavity' measures 0.44 mm.
| |
|
| |
| The Bryce-Teacher ovum (Fig. 40 B) differs from the foregoing specimen chiefly by possessing an amniotic cavity^ and y'olk sac.
| |
|
| |
|
| |
| A well-defined extra-embryonic coelom divides the mesoderm of Peter - s specimen into somatic and splanchnic layers, and there is also the beginning of true villi (Figs. 40 C and 46). The ectodermal embryonic disc measures 0.19 mm.; it is thickened and separated from the entoderm by a layer of mesoderm (Fig. 41). Strands of mesoderm, known as the magma reticulare, bridge the extra-embryonic body cavity, which is 0.9 X 1.6 mm. in diameter (Fig. 41).
| |
|
| |
|
| |
| 17 weeks 21 weeks 41 weeks 48 weeks
| |
| 18 months 20 months .
| |
|
| |
|
| |
| These ova all belong to the latter part of the second week. The yolk sac is smaller than the amnion and the villi are mostly unbranched. The embryo is merely a plate combined from the three germ layers. Neither primitive streak nor allantois has appeared. Even in the oldest, a broad zone of mesoderm connects embryo to chorion.
| |
|
| |
|
| |
| Embryos of the Third Week. - The Mateer ovum is shown as Fig. 32 A. It possesses a distinct primitive groove and allantois. The embryonic disc is 0.9 mm. in length.
| |
|
| |
|
| |
|
| |
| Fig. 57 - Dorsal view of a human embryo of 1.54 mm. (Spee). X 23.
| |
|
| |
|
| |
|
| |
| A head process with its contained notochordal canal features the advance illustrated by the Ingalls embryo (Fig. 32 B). There is also the beginning of a neural groove. The chorionic vesicle has an internal diameter of 7 mm.
| |
|
| |
|
| |
| Spec’s specimen has progressed still further (Fig. 57). The embryonic disc measures 1.54 mm. and is slightly constricted from the yolk sac. The primitive streak is confined to the caudal end of the embryonic disc, the neural folds are well-marked, and a neurenteric canal opens as a pore into the primitive intestinal cavity. In longitudinal section it is evident that the floor of the head process has disappeared, leaving its roof as the notochordal plate (Figs. 40 D and 43). The fore-gut is forming and there are indications of a future heart anlage.
| |
|
| |
|
| |
| In this group as a whole, the continued extension of the extra-embryonic coelom has separated the embryo from the chorion except in the region of the body stalk, which constitutes a bridge that contains the allantois. The yolk sac is now larger than the amnion. The chorionic villi branch freely and there is evidence of blood-vessel formation in the wall of the yolk sac (Fig. 43), and, usually, in the body stalk and chorion.
| |
|
| |
|
| |
| Embryos of the Fourth Week. - Embryos of this period are early characterized by the presence of high neural folds (Fig. 58) whose edges soon unite along part of their extent to form a tube which is the anlage of the brain and spinal cord (Figs. 59 and 245). The expansive brain portion is already recognizable. The mesoderm of each side of the midplane becomes arranged in blocks, the primitive (mesodermal) segments, visible externally.
| |
|
| |
| In the embryo shown in Fig. 245 there are 14 pairs. The primitive streak is now insignificant (Figs. 44 and s8).
| |
|
| |
|
| |
|
| |
| Fig. 58. - Kromer human embryo of 1.8 mm., in dorsal view (after Keibel and Elze). X 20.
| |
|
| |
|
| |
| Fig. 59. - Human cmbrjm of 2.1 1 mm. in dorsal view (Eternod). X 35.
| |
|
| |
|
| |
| Growth at the head and tail regions appears to constrict the embryo from the yolk (Figs. 58 and 245). In a longitudinal section of an embryo at the middle of this period (Fig. 44), both fore- and hind-gut are evident and the heart is conspicuous. A system of blood vessels is established connecting with the heart (Figs. 180 and 181). The embryo is now cylindrical, its body wall encloses two more or less complete tubes (neural and enteric) with the axial notochord between. During this period there is an increase in length from 0.5 to 2.5 mm.
| |
|
| |
|
| |
| Embryos of the Fifth Week. - Specimens corresponding to Figs. 60 and 61 stand at the turn between the fourth and fifth weeks, whereas one like Fig. 62 is more representative of this period. The progressive separation of embryo from yolk sac is evident. The primitive segments have increased until the 2.6 mm. specimen (Fig. 61) has 35 of the definitive 38 pairs. The convex curvature of the back is characteristic. External swellings indicate the three primary brain vesicles and the head becomes hexed at a right angle in the mid-brain region. On each side of the future neck appear branchial arches, separated by grooves. The hrst pair of arches bifurcates into maxillary and mandibular processes that will form the upper and lower jaws; between them is a depression, the oral fossa or stomodeum, where the mouth will be. The heart is large and flexed. The body ends in a blunt tail, and, toward the end of the period, bud-like outgrowths indicate the anlages of the upper and lower limbs. An idea of the extent of internal organization may be gained by examining Figs. 91, 183 and 184.
| |
|
| |
|
| |
| Embryos of Six to Eight Weeks. - These embryos range between 5.5 and 25 mm. and show marked changes. Their external form comes to resemble more the adult condition, and, after the second month, the developing young is designated a fetus. This external metamorphosis may be followed by referring to the illustrations of embryos of 7 mm. (Fig. 63), 9 mm. (Fig. 227), 12 mm. (Fig. 64), 18 mm. (Fig. 65), and 23 mm. (Fig. 66; two months). It is due principally to the following factors: (i) Changes in the flexures of the body; the dorsal convexity is lost, the head becomes erect, and the body straight. (2) The face develops (also illustrated in Fig. 68). (3) The external structures of the eye, ear, and
| |
| nose appear. (4) The prominent tail of the sixth week regresses and becomes inconspicuous, largely through concealment by the growing buttocks. (5) The umbilical cord encloses both yolk stalk and body stalk and constitutes the sole attachment, limited to the region of the umbilicus. (6) The heart, which formed the chief ventral prominence in earlier embryos, now shares this distinction with the rapidly growing liver, and the two determine the ventral body shape until the eighth week when the gut dominates the belly cavity and the contour of the abdomen is more evenly rotund. (7) The appearance of a neck region, due chiefly to the settling of the heart caudad and the loss of the branchial arches. (8) The external genitalia appear in their - sexless - condition.
| |
|
| |
|
| |
|
| |
| Fig. 62. - Human embryo of 4.2 mm. (His). X 15.
| |
|
| |
|
| |
| Fig. 63. - Human embryo of 7 mm. (Mall in Kollman). X 14. 1, u, HI, Branchial arches; u, lit., heart; L, liver; 0, otic vesicle; R, olfactory placode.
| |
|
| |
|
| |
| Fig. 64 . - Human embryo of 12 mm. (Prentiss). X 4 .
| |
|
| |
|
| |
|
| |
|
| |
| Period of the Fetus
| |
|
| |
|
| |
| During the third month the fetus definitely resembles a human being, but the head is still disproportionately large (Fig. 66); the umbilical herniation is reduced by the return of the intestine into the abdomen; the eyelids fuse, nail anlages form, and sex can now be distinguished readily. In the fourth month, the muscles become active and cause fetal movements; lanugo hair makes its appearance (Fig. 66). At five months, hair is present on the head. During the sixth month the eye brows and lashes grow and vernix caseosa forms; the body is lean but in better proportion. At seven months, the fetus looks like a dried-up, old person with red, wrinkled skin; the eyelids reopen. In the eighth month, the testes usually are in the scrotum ; infants of this age born prematurely may generally be reared. In the ninth month, the dull redness of the skin fades, wrinkles smooth out, the panniculus adiposus develops, the limbs become rounded, and nails extend to the finger tips. At ten months, the child is - at full term, - ready to cope with an extrauterine existence (Fig. 55).
| |
|
| |
|
| |
| Fig. 65. - Human embryo of 18 mm. with its membranes. X 2. The chorion is opened and reflected; the upper half of the amnion has been cut away.
| |
|
| |
|
| |
|
| |
| Fig. 66. - Human embryos of three weeks to two months (His), and fetuses of three and four months (De Lee). Natural size.
| |
|
| |
|
| |
| +++++++++++++++++++++++++++++++++.
| |
|
| |
| THE ESTABLISHMENT OF EXTERNAL FORM
| |
|
| |
|
| |
| Although the preceding section deals largely with the aquisition of fetal form, this topic requires supplementary treatment.
| |
|
| |
| The Head and Neck
| |
| Since development in the cephalic region maintains its early advantage, the head and neck of an embryo are for a long time disproportionate^ large. In Fig. 63 the last cervical segment is midway on the body. The gradual adjustment of size relations may be traced in Fig. 6g .
| |
|
| |
|
| |
| Anomalies. - Many grossly abnormal embryos are found at operation or spontaneous abortion. Various pathological conditions in the embryo commonly accompany those disturVjances which induce its stunting or death. Degenerative changes are common also in the fetal membranes, although the chorionic sac sometimes continues to grow quite normally after the embryo has died or disappeared. Dead, retained fetuses are usually resorbed, but they may mummify and persist indefinitely,
| |
|
| |
|
| |
| The head is composed of two portions almost from the start. One is neural in nature and includes the brain, eyes, and internal ears, and their supporting structures. The other is the facial, or visceral, part that contains the cephalic ends of the alimentary and respiratory tracts. The neural portion is much the larger in young embryos and this superiority is never lost completely, although the subsequent differentiation and growth of the nose, jaws, and pharynx reduces the early disparity.
| |
|
| |
| Branchial Arches. - -The formation of the face and neck involves the history of the branchial arches. These are bar-like prominences, separated by grooves, which occur on the lateral surfaces of the neck (Figs. 6 1 to 63). They correspond to the gill-bearing arches of fishes that are separated by clefts through which respiratory water flows. In amniotes they never assume a respiratory function, but occur as transitory vestiges that are applied to various purposes, then disappear. The human embryo develops five such arches, separated by four ectodermal grooves; subjacent to these grooves the entoderm of the pharynx bulges correspondingly (Fig. 87). The thin plates thus formed by the union of ectoderm and entoderm sometimes rupture to make temporary openings, reminiscent of the gill-slit condition.
| |
|
| |
|
| |
| The last arch lies caudal to the fourth cleft and is poorly defined along its posterior margin. Toward the end of the sixth week, the first and second arches overlap the other three and obScure them. Fig. 63 shows the beginning of this process. Fig. 227 an advanced stage, and in Fig. 64 it is complete. The caudal arches sink into a triangular depression called the cervical sinus. When the posterior edge of the second arch fuses with the thoracic wall, the sinus and its contained arches are closed off. This cavity eventually degenerates.
| |
|
| |
|
| |
| Various muscles and bones form from the arches, and from the entodermal pouches certain glandular organs arise. The completion of this metamorphosis marks the appearance of a neck (Fig. 65) which is characteristic of amniotes alone.
| |
|
| |
|
| |
| Anomalies. - Imperfect closure of the branchial clefts (usually the second) leads to the formation of cysts, diverticula, or even fistulae. Such structures may be derived either from an ectodermal groove or the complementary entodermal pouch.
| |
|
| |
|
| |
| The Face. - Pig embryos show clearly how the face forms. In Fig. 369 the expansive jronto-nasal process represents much of the front of the head. The olfactory pits are present, and the first branchial arches have not only bifurcated into maxillary and mandibular processes but the mandibular segments have already united as the lower jaw. Laterally, the olfactory pits subdivide the fronto-nasal process into paired lateral and median nasal processes (Figs. 394 and 67 A). Soon, the median nasal processes fuse with each other and with the maxillary processes; this constitutes the upper jaw (Fig. 67 B). The lateral nasal processes likewise join the maxillary process, thereby obliterating the lacrimal groove, and forming the wings and margins of the nose and the adjacent cheek region. Meanwhile, the mesial portion of the original fronto-nasal process becomes the forehead and the septum and bridge of the nose.
| |
|
| |
|
| |
| The early development of the human face is essentially the same. These changes may be followed in Fig. 68. At first the nose is broad and fiat, with the nostrils set far apart and directed forward (Fig. 68 C). In the later fetal months the bridge is elevated and prolonged into the apex, and the nostrils look downward (Fig. 68 D). The line of fusion of the median nasal processes is evident in the adult as the philtrum . The chin is a median projection from the fused mandibular processes. During the formation of the jaws the originally broad mouth opening is reduced in its lateral extent. Epithelial ingrowths begin to separate the lips from the alveolar portions of the jaws at the fifth week (Fig. 79); at birth the inner edges of the lips bear numerous villosities. Progressive modelling of the face continues until the individual becomes fully grown.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 67. - Development of the pig - s face (Prentiss). X 7. A, 12 mm.; B, 14 mm.
| |
|
| |
|
| |
|
| |
|
| |
| Anomalies. - A common facial defect is hare lip. This is usually unilateral and on the left side. It may involve both lip and maxilla. Hare lip is attributed to the failure to fuse of the median nasal and maxillary processes (Kdlliker), or the lateral and median nasal processes (Albrecht).
| |
|
| |
|
| |
| Fig. 68. - Stages in the development of the human face (adapted). A, Five weeks; B, six weeks; C, eight weeks; D, si.xteen weeks. The fronto-nasal process is indicated by parallel lines, the median nasal processes by circles, and the lateral nasal processes by dots.
| |
|
| |
|
| |
| The Sense Organs. - The eye, ear, and nose will be considered in detail in Chapter XV. The external nose has just been described. The eye makes its appearance in the early weeks, and, by the second month, lids are present. For a time the e^-es are placed laterally and far apart, but gradually this distance is reduced (Fig. 68). The external ear is developed around the first branchial groove by the appearance of small tubercles which form the auricle (Figs. 64, 65 and 31 1). The groove itself becomes the external auditory meatus.
| |
|
| |
|
| |
| The Trunk
| |
|
| |
| In young embryos the trunk is like a cylinder, flattened by lateral compression (Fig. 63). Its external contour is determined by the modelling of the viscera within. During the fetal period, this visceral mass becomes more rounded and the muscles and skeleton of the trunk appear, d'he trunk then assumes an ovoid form, circular in section, and largest at the umbilicus (Fig. 66). From the third fetal month through early infancy there is relative!}^ little change in the trunk ])roportions. When erect i)osture is assumed, the dominance of the thorax and abdomen is reduced and the lumbar region gains in prominence and relative length. The thorax of the newborn is rather conical, with its base below, due to the ril:)s l)cing more horizontal. In the adult the thorax is barrel-shaped, that is, broadest in its middle. The characteristic curves of the spinal column are absent at birth. They appear partly through the drag of body weight, ])artly through the pull of the muscles, and are not pronounced until the posture becomes erect.
| |
|
| |
|
| |
|
| |
| Anomalies. - The embryonic tail sometimes persists and develops beyond its ordinary size. Specimens as long as 8 cm. have been recorded in the newborn. Most are soft and ileshy, but a few have contained skeletal elements. Some tumors of the coccygeal region are attributed to the activity of residual primitive-streak tissue.
| |
|
| |
|
| |
| The Appendages
| |
|
| |
| The limbs appear during the fifth w^eek as lateral buds. In a 4 mm. embryo (Fig. 62) limb buds may be recognized, but due to the early expanse of the head-neck region they seem to be located far down the body. The distal ends flatten (Fig. 63) and a constriction divides this paddle-like portion from the proximal, rounded segment (Fig. 196). Later, a second constriction separates the cylindrical part into two further segments (Figs. 64 and 65), and the three divisions of arm, forearm, and hand, or thigh, leg, and foot are respectively formed. Radial ridges, separated by grooves, first foretell the formation of digits (Figs. 64 and 65). These elongate as the definitive fingers and toes, and rapidly project beyond the original plates; the latter by a slower rate of growth become confined as webs about the basal ends of the digits (Fig. 66). The thumb and great toe early separate widely from the index finger and second toe.
| |
|
| |
|
| |
| The limbs as a whole undergo several changes of position. At the very start they point caudad (Figs. ig6 and 64), but soon project outward at right angles to the bod\^ wall. Next, they are bent ventrad so that the thumb (radial) side of the arm and the great toe ( tibia!) side of the leg are directed forward; the palmar and plantar surfaces face the body; the elbow turns outward and somewhat caudad, the knee outward and slightly cephalad (Fig. 65). Finally, both sets of limbs undergo a torsion of 90° about their long axes, but in opposite directions. As a result, the radial side of the arm is outward (when radius and ulna are parallel) and the palm faces ventrad; on the contrary, the tibal side of the leg is the inner side, while the sole faces dorsad. By following through these changes it will be seen that the radial and tibial sides of arm and leg are homologous, as are palm and sole, elbow and knee.
| |
|
| |
|
| |
| The upper limb buds arise first and they maintain a slight advance in differentiation. Not until the second year of childhood are the two equal in length.
| |
|
| |
|
| |
| Anomalies. - The extremities may either fail to develop, or become mere stubs; the hands and feet may join the body like flippers. Rarely, the hands or feet are partially duplicated or reduced. The presence of extra digits is polydactyly; a fusion of digits constitutes syndactyly. More or less complete union of the legs occurs as sympodia.
| |
|
| |
|
| |
|
| |
| The developmental period of man is divided by the incident of birth into prenatal and postnatal periods. At birth the infant is sufficiently advanced to be cared for outside its mother - s body, yet its development is far from complete. In its new environment differentiation and growth, especially marked by changes in form and proportion, continue until the beginning of the third decade ; only then is full size and mature structure attained.
| |
|
| |
|
| |
| The several divisions of the developmental period are listed as follows by Scammon, from whose account much of the material of the succeeding paragraphs is taken : .
| |
|
| |
|
| |
| Divisions of the Develop.mental Period in Man
| |
| ^Period of the ovum (Fertilization to end of second week)
| |
| Prenatal life - Period of the embryo (Second to eighth week)
| |
| \Period of the fetus (Second to tenth month)
| |
| Birth
| |
| Period of the newborn (Neonatal period; birth to end of second week)
| |
| 1 Infancy (Second week until assumption of erect posture at 13 to 14 months) j 1 Early childhood (Milk-tooth period; first to sixth year)
| |
| j - idliood childhood (Sixth to ninth or tenth year)
| |
| †\ Later childhood (Prepubertal period; from 9 or 10 years to 12-15
| |
| Postnatal life ! I years in females and 13-16 years in males)
| |
| I Puberty
| |
| I (Fourteenth year in females: sixteenth year in males)
| |
| I Adolescence (From puberty to the last years of the second decade in females I and to the first years of the third decade in males) .
| |
|
| |
|
| |
| Changes in Form. If an adult maintained the chubby newborn shape his weight would be twice the actual amount. Fig. 69 shows the proportions of the body at various developmental periods, all drawn as of the same height. Note: the great decrease in the size of the head; the constancy of the trunk length; the early completion of the arms and the tardier growth of the legs; the upward shift of the umbilicus and symphysis pubis, and the downward trend of the midpoint of the body.
| |
|
| |
|
| |
| Fig. 6g. - DiagramS to illustrate the changing proportions of the body during prenatal and postnatal growth (Scammon after Stratz).
| |
|
| |
|
| |
|
| |
| Certain of these facts may be tabulated in terms of per cent of the total body volume:
| |
|
| |
| Growth in Relative Volume of the Parts of the Body .
| |
|
| |
|
| |
| In per cent of the total body volume .
| |
|
| |
|
| |
| Age .
| |
|
| |
| Head and neck .
| |
|
| |
| Trunk .
| |
|
| |
| Arms .
| |
|
| |
| Legs .
| |
|
| |
| Second fetal month .
| |
|
| |
| 45 .
| |
|
| |
| 50 .
| |
|
| |
| 3 .
| |
|
| |
| 3 .
| |
|
| |
| Sixth fetal month .
| |
|
| |
| 37 .
| |
|
| |
| 40 .
| |
|
| |
| 8 .
| |
|
| |
| 15 .
| |
|
| |
| Birth .
| |
|
| |
| 27 .
| |
|
| |
| 49 .
| |
|
| |
| 9 .
| |
|
| |
| 15 .
| |
|
| |
| Two years .
| |
|
| |
| 22 1 .
| |
|
| |
| 50.5 .
| |
|
| |
| 9 .
| |
|
| |
| 17-5 .
| |
|
| |
| Six years .
| |
|
| |
| 15 .
| |
|
| |
| 51 .
| |
|
| |
| 9 .
| |
|
| |
| 25 .
| |
|
| |
| ^Maturity .
| |
|
| |
| 7 .
| |
|
| |
| 53 .
| |
|
| |
| 10 .
| |
|
| |
| 30 .
| |
|
| |
|
| |
| Increase in Surface Area. - The relation of surface area to body mass or volume has a profound influence on metabolism. This relation changes greatly during the postnatal period. At birth, the surface area is about 2500 sq. cm. This is doubled in the first year, tripled by the middle of childhood, and increases rapidly before puberty. At maturity, the total gain is seven-fold. Since, however, the weight of the body has increased some twenty-fold in the same time it is obvious that there has been a relative loss. Thus, in the newborn there are over 800 sq. cm. per kilogram of body weight, whereas in the adult there are less than 300 sq. cm.
| |
|
| |
|
| |
| Growth in Weight. - During prenatal life the weight of the body increases several billion times, whereas from birth to maturity the increment is only twenty-fold. In absolute mass, however, 95 per cent of the final weight is acquired after birth. The ratio of increase during each fetal month to the weight at the beginning of that month is shown in the table on p. 68.
| |
|
| |
|
| |
| Growth in Length. - Growth in length and in weight have certain features in common, although the relative increase in length is obviously smaller since weight is a three dimensional phenomenon. The increase in the second fetal month is ten-fold but thereafter the relative rate of growth gradually declines. The data of prenatal growth are given in the table on p. 68. The total postnatal increment is 3.3 times. During the first six months after birth, length increases 30 per cent; in the first year, 50 per cent. Throughout the most of childhood the linear increase is very slow, but at the prepubertal period there is an acceleration; as with weight, this is begun and ended earlier in girls than in boys. Growth is complete at about 18 years in females and soon after 20 in males. The body is heaviest in proportion to its length during late fetal life and early infancy. From the middle of the first year until after puberty there is a decline in relative weight. Thereafter there is an increase in relative mass which may continue throughout life. During infancy and childhood girls are relative^ lighter than boys, but after puberty the reverse is true.
| |
|
| |
|
| |
| Growth of Organ Systems. - The skeleton grows rather slowly until the ninth and tenth fetal months, when it shows an acceleration. At birth, it constitutes from 13 to 20 per cent of the body weight. Postnatal growth apparently parallels that of the body as a whole and shows neither relative loss nor gain. The musculature likewise grows slowly at first, but forms about 2 5 per cent of the weight of the newborn and 40 to 45 per cent of the adult. The central nervous system, on the contrary, is relatively huge in the young embryo. It decreases from about 25 per cent in the second month to about 15 per cent at birth and 2 to 2.5 per cent in the adult. Incomplete data on the peripheral nervous system and skin indicate a considerable reduction in relative weight during the postnatal years. As a whole, the visceral group decreases slowly and steadily in relative weight after the first two embryonic months. In the second month they comprise about 15 per cent of the body weight, about 9 per cent at birth, and from 5 to 7 per cent in the adult.
| |
|
| |
|
| |
| Growth of the Organs. - Although the general course of relative growth in the individual organs follows that of the visceral group, each has its characteristic curve. Each usually increases more or less rapidly to a maximum relative size and then decreases in relative size through the subsequent prenatal and postnatal periods.
| |
|
| |
| During fetal life the curves of absolute growth are quite similar. The various organs have an initial period of slow increase, followed after the fifth month by a terminal phase of rapid growth. However, this uniformity disappears at birth, and most of the organs can be arranged in four main divisions. The splanchnic group includes the digestive, respiratory, and urinary organs, and the heart, thyroid, and spleen. The nervous group comprises the brain, cord, and eyeballs. The genital group excludes the ovary and uterus which have special curves. The lymphoid group includes all but the spleen. Fig. 70 shows these relations graphically from embryo to adult.
| |
|
| |
|
| |
|
| |
| Fig. 70. - Chart showing the course of growth in the various organ groups (after Scammon).
| |
|
| |
| Growth is calculated in per cent of adult weight.
| |
|
| |
|
| |
|
| |
|
| |
| Anomalies. - Giants and dwarfs may be of monstrous size when born at full term, or the acceleration or slowing may be secondary at some later period. This abnormal size is sometimes unilateral or even confined to specific parts of the body.
| |
|
| |
|
| |
| ++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
| |
|
| |
| PART II. ORGANOGENESIS ENTODERMAL DERIVATIVES
| |
|
| |
| CHAPTER V THE DIGESTIVE SYSTEM
| |
|
| |
|
| |
|
| |
|
| |
| The entoderm of the embryonic disc is at first directly continuous with the entodermal lining of the yolk sac, and merely forms a roof to that organ (Fig. 40 C). As the embryo grows and expands, while its connection with the yolk sac lags in development, the entoderm necessarily takes the form of a blind tube within the cylindrical body. This extends first into the head region as the fore-gut (Figs. 40 C and 43), then tailward as the hind-gut (Fig. 44). The intermediate region, open ventrally through the narrower yolk stalk into the yolk sac, is sometimes termed the mid-gut (Fig. 71), but its existence is brief for the yolk stalk loses its connection with the gut during the sixth week.
| |
|
| |
|
| |
| At each end, the gut comes into direct contact ventrally with the ectoderm. The plates thus formed are the pharyngeal and cloacal membranes (Fig. 71). The pharyngeal membrane forms the floor of a depression known as the oral fossa, or stomodeum ; this fossa is bounded by the fronto-nasal, maxillary, and mandibular processes (Figs. 61 and 62). At the beginning of the fifth week (2.5 to 3 mm. embryos), the pharyngeal membrane ruptures and the oral fossa and fore-gut become continuous. The oral fossa develops into the front part of the mouth cavity, which is therefore ectodermal. The remainder of the mouth cavity, the respiratory tract, and the alimentary canal to a point well along the small intestine are all derived from the entodermal fore-gut.
| |
|
| |
|
| |
| The caudal end of the entodermal tube comprises the cloaca, which soon receives the allantoic, urinary, and genital ducts (Figs. 87, 91 and 94). The cloaca promptly begins to subdivide into a dorsal rectum and a ventral urogenital sinus (Figs. 139 to 142). At the same time, the cloacal membrane is separatedinto anal and urogenitalmembranes (Figs. 71, 95 and 96). The anal membrane ruptures at about the ninth week, and an external depression, the proctodeum, therefore becomes continuous with the hindgut (Figs. 96 and 142). It constitutes the anal canal, which, like the front part of the mouth cavity, is lined with ectoderm. The hind-gut itself forms some of the small intestine, the colon, and the rest of the rectum (Figs. 93 to 96). It will be noticed that the primitive entodermal tube extends a little beyond the cloacal membrane (Figs. 71, 91 and 139); this tail-gut, or postanal gut, soon dwindles and disappears.
| |
|
| |
| The entoderm forms only the epithelial lining of these organs. AU other coats develop from the investing splanchnic mesoderm. The original low epithelium of the gut differentiates into the several types of simple epithelium formed in the digestive and respiratory systems, as well as into the pseudostratified and stratified forms. The various glands are primarily epithelial outgrowths.
| |
|
| |
|
| |
|
| |
| Fig. 71. - Diagrams showing the human alimentary canal in median sagittal section. X 35. A, 2 mm. (modified after His): B, 2.5 mm. (after Thompson).
| |
|
| |
|
| |
|
| |
| It is impossible to determine the exact junction of ectoderm and entoderm in the mouth, but in general the roof and peripheral portions are ectodermal. The salivary glands are considered to be from ectoderm, as are the enamel of the teeth, a portion of the tongue epithelium, and much of the lining of the nose and palate. Although these structures do not strictly belong with entodermal derivatives, it is simplest to consider them with the systems of which they are integral parts.
| |
|
| |
|
| |
|
| |
| THE MOUTH
| |
|
| |
|
| |
| Lips and Cheeks. - During the fifth week, these separate from the jaws proper by the ingrowth of epithelial plates which promptly begin to thin and form the vestibule (Figs. 74, 76 and 79).
| |
|
| |
|
| |
| The Palate. - -The roof of the original mouth cavity is the base of the skull. When the membranes which separate the olfactory pits from the mouth rupture, their orifices, the primitive choance, also open into the common oral cavity. The nasal passages next become separate by partitioning off a portion of the mouth cavity and adding it to their original extent. They then communicate with the pharynx by the secondary, definitive choance. The horizontal septum which thus divides mouth from nasal ^passage is the palate. The details of its formation follow ; At first the jaws are closed and the tongue extends up between shelf like folds of the maxilla, the lateral palatine processes (Figs. 73 A and 74), which project downward (Fig. 72 A). Soon the mandible drops, owing to growth changes, and the tongue is withdrawn. This allows the palatine folds to bend upward to the horizontal plane (Fig. 75), approach, and fuse (Fig. 72 B). The shift of the lateral palatine processes is an active bending, due to cellular proliferation on their under sides (Fig. 75). The union of the halves of the palate begins about the end of the second month and progresses backward toward the pharynx (Fig. 73 B). Coincidently, bone appears in the front part and forms the hard palate; more caudad, ossification fails, and this region constitutes the soft palate and its free apex the uvida. The unfused, backward prolongations of the palatine folds give rise to the pharyngo-palatine arches, which delimit oral cavity from pharynx. The palate shows a median seam, and, for a time, the uvula is notched; both are indicative of the mode of origin.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 72. - Sections through the jaws of pig embryos, to show the development of the palate (Prentiss). X 8. A, 22 mm.; B, 34 mm.
| |
|
| |
|
| |
|
| |
| Fig. 73 - Dissections to show the development of the palate in pig embryos (Prentiss). X 5. A , The upper jaw and palatine processes of a 22 mm. embryo in ventral view; B, fusion of the palatine processes in a 35 mm. embryo.
| |
|
| |
|
| |
|
| |
| 74. - The roof of the mouth of a two-months - human embryo, groove, primitive choanse and developing palate (after His). showing the labial ,X 9 .
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 75. - Section through the jaws of a 25 mm. pig embryo, to show the change in position of one palatine process due to unequal growth (Prentiss).
| |
|
| |
|
| |
|
| |
|
| |
| The median nasal lobes of the original fronto-nasal process also develop median palatine processes, so-called, which do not contribute to the palate but form the premaxillary portion of the upper jaw (Figs. 73 and 74). Fusion with the palate is incomplete and in the midplane there is a gap, the incisive foramen, flanked by the incisive canals (of Stenson). These become covered with mucous membrane, although they sometimes are patent at birth.
| |
|
| |
|
| |
|
| |
| Fig. 76. - Early stages in the development of the teeth (Rose). . 4 , Seven weeks (X 90); B,
| |
| nine weeks (X 45).
| |
|
| |
|
| |
| Anomalies. - The lateral palatine processes occasionally fail to unite in the middle line, producing a defect known as deft palate. The extent of the defect varies considerably, in some cases involving only the soft palate, while in other cases both soft and hard palate are cleft. It may be associated also with hare lip.
| |
|
| |
|
| |
| The Teeth. - The teeth have a double origin. The enamel is from ectoderm ; the dentine, pulp, and cement are mesodermal.
| |
|
| |
|
| |
| The Enamel Organ. - When the labial groove is forming in embryos of six weeks, a horizontal shelf develops from it and extends backward into the substance of the jaw (Fig. 76). This curved dental ridge, or lamina, is parallel with the adjacent labial groove and lies mesial to it (Fig. 82). At intervals a series of thickenings develop, the anlages of the enamel organs; these will form enamel and serve as the moulds of the future teeth (Figs. 76 B and 77). Early in the third month the ventral side of each enamel organ becomes concave, like an inverted cup, and the concavity is occupied by dense mesenchymal tissue, the dental papilla, which will differentiate into dentine and pulp (Figs. 77 and 78). An enamel organ and its associated dental papilla is the basis of each tooth (Fig. 79). Ten such anlages of the decidual, or milk teeth, are present in each jaw (Fig. 82). Their connection with the dental ridge is eventually lost.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 77. - Models of the early development of three teeth, one in section (Lewis and StohrL .
| |
|
| |
|
| |
|
| |
| Fig. 78. - Section through an upper incisor from a fetus of three months (Prentiss). X 70.
| |
|
| |
|
| |
|
| |
| The compact internal cells of the enamel organ transform into a reticulum resembling mesenchyme, termed the enamel pulp (Fig. 78). The outer enamel cells, at first cuboidal, flatten out as a fibrous layer. Neither of these components contributes to tooth formation. The inner enamel cells line the cup-shaped concavity of the enamel organ. Over the crown of the tooth these cells are designated amelohlasis, for they become columnar and produce the enamel layer along their basal ends (Fig. 80). The enamel is laid down first as an uncalcified, fibrillar layer which then calcifies in the form of enamel prisms, one for each ameloblast. The enamel is deposited first at the apex of the crown and then downward .
| |
|
| |
|
| |
|
| |
| Fig. 79. - Parasagittal section through the mandible and tongue of a three-months - fetus, showing the relations of the first incisor anlage (Prentiss). X 14.
| |
|
| |
|
| |
|
| |
| Fig. 80. - Section through a portion of the crown of a developing tooth, showing the various
| |
| layers (Tourneux in Heisler).
| |
|
| |
|
| |
|
| |
| Toward the root. The enamel cells about the future root of the tooth remain cuboidal or low columnar in form, come into contact with the outer enamel cells, and the two layers constitute the epithelial sheath of the root (Fig. 81); it does not produce enamel prisms.
| |
|
| |
|
| |
| The Dental Papilla - At the end of the fourth month, the outermost cells of the dental i)apilla arrange themselves as a definite layer of columnar epithelium. Since they produce the dentine, or dental bone, these cells are known as odontoblasts (Fig. 8i). When the dentine layer is deposited, the odontoblast cells remain internal to it, but branched processes from them (the dentinal fibers of Tomes) extend into the dentine and occupy the dental canalicnli (Fig. 80). Internal to the odontoblasts, the remaining mesenchymal cells differentiate into the dental pulp, popularly known as the - nerve - of the tooth. This is composed of a framework of reticular tissue in which are found blood vessels, lym.phatics, and nerve fibers. The odontoblast layer persists throughout life and intermittently lays down dentine, so that eventually the root canal may be obliterated,
| |
|
| |
|
| |
|
| |
| Fig. 81. - Longitudinal section of a decidual tooth of a newborn dog. X 42. Above the enamel, on either side, are artificial shrinkage spaces (Lewis and Stohr).
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| The Dental Sac. - The mesenchymal tissue surrounding the anlage of the tooth gives rise to a dense outer layer and a more open inner layer of fibrous connective tissue. These form the dental sac (Fig. 8i). Over the root of the tooth a layer of osteoblasts, or bone forming cells, develops, and when the epithelial sheath of the enamel organ disintegrates, they deposit about the dentine an investment of specialized bone, known as the cement. Cementum contains typical bone cells but no Haversian systems. As the tooth grows and fills its alveolar socket, the dental sac.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 82. - Dental lamina and anlages of the upper milk teeth in a three-months - fetus (Rose).
| |
|
| |
|
| |
| Becomes a thin, vascular layer, the peridental membrane. This has fibrous attachments to both the alveolar bone and the cement and holds the tooth in place.
| |
|
| |
|
| |
|
| |
| Eruption. - ^When the crown of the tooth is fully developed the enamel organ disintegrates, and, as the root continues to grow, the crown approaches the surface and breaks through the gum. The periods of eruption of the various milk, or decidual teeth vary with race, climate, and nutritive conditions Usually they are cut in the following sequence: .
| |
|
| |
|
| |
| Median Incisors sixth to eighth month.
| |
|
| |
| Lateral Incisors eighth to twelfth month.
| |
|
| |
| First Molars twelfth to sixteenth month.
| |
|
| |
| Canines seventeenth to twentieth month.
| |
|
| |
| Second Molars twentieth to thirty-sixth month.
| |
|
| |
|
| |
| The permanent teeth develop precisely like the temporary set. The anlages of those permanent teeth which correspond to the milk dentition arise in another series along the free edge of the dental lamina (Fig. 77 H) and come to lie mesad of the decidual teeth (Fig. 83). In addition, three permanent molars are developed on each side, both above and below, from a backward or alioral extension of the dental lamina, entirely free from the oral epithelium (Fig. 82). The anlages of the first permanent molars appear at the end of the fourth month, those of the second molars at six weeks after birth, while the anlages of the third permanent molars, or wisdom teeth, are not found until the fifth year. The permanent dentition of thirty-two teeth is then complete.
| |
|
| |
|
| |
| Before the permanent teeth begin to erupt, the roots of the milk teeth undergo partial resorption, their dental pulp dies, and they are eventually shed. Toward the sixth year, before the loss of the decidual teeth begins, each jaw may contain twenty-six teeth (Fig. 83). The permanent teeth are cut as follows : .
| |
|
| |
| First Molars
| |
|
| |
| Median Incisors
| |
|
| |
| Lateral Incisors
| |
|
| |
| First Premolars
| |
|
| |
| Second Premolars
| |
|
| |
| Canines I
| |
|
| |
| Second Molars )
| |
|
| |
| Third Molars (Wisdom Teeth)
| |
|
| |
|
| |
|
| |
|
| |
| seventh year, eighth year, ninth year, tenth year, eleventh year.
| |
|
| |
| Thirteenth to fourteenth year, seventeenth to fortieth year.
| |
|
| |
|
| |
| Fig 83. - Skull of a live-year-old child, showing the positions of the decidual and permanent teeth (Sobotta-McMurrich).
| |
|
| |
|
| |
|
| |
| The teeth of vertebrates are homologues of the placoid scales of elasmobranch fishes (sharks and skates). The teeth of the shark resemble enlarged scales, and many generations are produced in the adult fish. In some mammalian embryos, three, or even four, dentitions are present. The primitive teeth of mammals were of the canine type, and from this conical tooth the incisors and molars have arisen. Just how the cusped tooth differentiated - whether by the fusion of originally separate units, or by the development of cusps on a single primitive tooth - is debated.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 84. - Dissections showing the development of the tongue in pig embryos (Prentiss). X 12. A, 7 mm.; B, 9 mm.; C, 13 mm.
| |
|
| |
|
| |
| Anomalies. - Dental anomalies are frequent. They may consist in the congenital absence of some or all of the teeth, or in the production of more than the normal number. Defective teeth are frequently associated with hare lip. Cases have been noted in which, owing to a defect of the enamel organ, the enamel was wanting. Third dentitions have been recorded, and occasionally fourth molars are developed behind the wisdom teeth.
| |
|
| |
|
| |
|
| |
| The Tongue. - The tongue develo])s as two distinct portions, the body and the root, separated from each other by a V-sha]ied groove, the sulcus tcnninalis (Fig. 85 B). In both human and pig embryos, the body of the tongue is represented by three anlages that appear in front of the second branchial arches. These are the median, somewhat triangular iuhcrculitm uupar, and the paired lateral swellings of the first, or mandibular arches - all of which are present in human embryos of 5 mm. (Figs. 84 A and 85 . 4 ). At this stage, a median ventral elevation, formed by the union of the second branchial arches, constitutes the copula. This, with the ])ortions of the second arches lateral to it, forms later the root of the tongue. Between it and the tuberculum impar is the point of evagination of the thyroid gland, represented in the adult by the foramen cecum (Fig. 85). I'he copula also connects the tuberculum impar with a rounded prominence that is developed in the midventral line from the bases of the third and fourth branchial arches. This is the anlage of the epiglottis (Figs. 84 and 85).
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 85. - Stages in the development of the human tongue (adapted). A, 6 mm.; B, 15 mm. Contributions from the first three branchial arches are indicated respectively by parallel lines, dots and crosses; the tuberculum impar is marked by circles.
| |
|
| |
|
| |
|
| |
| In later stages (Figs. 84 B, C and 85 B), the lateral mandibular anlages, bounded laterally by the alveolo-lingual grooves, increase rapidly in size and fuse with the tuberculum impar, which lags behind in development, and, according to recent investigators, atrophies completely. The epiglottis enlarges and becomes concave on its ventral surface. Caudad, and in early stages continuous with it, are two thick, rounded folds, the arytenoid ridges. Between these is the slit-like glottis, leading into the laryn.
| |
|
| |
|
| |
| In fetuses of 11 weeks, the fungiform and filiform papilla; may be distinguished as elevations. Taste buds appear in the fungiform papillae at 14 weeks and are much more numerous in the fetus than in the adult.
| |
|
| |
|
| |
|
| |
|
| |
| The vallate papilla: develop on a V-shaped epithelial ridge whose apex corresponds to the site of the thyroid evagination (Fig. 85 B). After the thirteenth week, circular epithelial down-growths occur at intervals along the ridges and take the form of inverted and hollow truncated cones (Fig. 86 A). During the fourth month circular clefts appears in the epithelial down-growths, thus separating the walls of the vallate papillae from the surrounding epithelium and forming the trench from which this type of papilla derives its name (Fig. 86 B). At the same time, lateral outgrowths arise from the bases of the epithelial cones, hollow out and form the ducts and glands of Ebner (Fig. 86 C). The taste buds of the vallate papillae also are formed early, appearing in embryos of three months. Foliate papilla: probably develop at about six months.
| |
|
| |
|
| |
| The foregoing account applies to the early origin of the mucous membrane alone. The musculature of the tongue is supplied chiefly by the hypoglossal nerve, and both nerve and muscles belong historically to the post-branchial region. If not in the development of each present-day embryo, at least in the past the musculature has migrated cephalad and invaded the branchial region beneath the mucous membrane (cf. p. 229). At the same time, the tongue may be said to extend caudad until its root is covered by the epithelium of the third and fourth branchial arches. This is shown by the fact that the sensory portions of the nn. trigeminus a.nA facialis, the nerves of the first and second arches, supply the body of the tongue, while the nn. glossopharyngeus and vagus, the nerves of the third and fourth arches, supply chiefly the root.
| |
|
| |
|
| |
|
| |
| Fig. 86. - Diagrams showing the development of the vallate papillae of the tongue (Graberg in McMurrich). a, Valley; b, von Ebner's gland.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Anomalies. - Faulty development or incomplete fusion of the several anlages causes variable degrees of absence or bifurcation of the tongue.
| |
|
| |
|
| |
| The Salivary Glands. - The glands of the mouth are all regarded as derivatives of the ectodermal epithelium. They complete their differentiation only after birth.
| |
|
| |
|
| |
| The parotid is the first to develop. Its anlage has been observed in 8 mm. embryos, near the angle of the mouth, as a keel-like flange in the floor of the groove which divides cheek from jaw. The flange elongates, and, in embryos of seven weeks, separates from the parent epithelium, forming a tubular structure that opens into the mouth cavit\" near the front end of the original furrow. The tube grows back into the region of the external ear, branches, and forms the main body of the gland in this region, while the stem portion of the tube becomes the parotid duct. Acinus cells are present at five months.
| |
|
| |
|
| |
| The submaxillary gland arises at n mm. as an epithelial ridge in the groove between the jaw and the tongue, its cephalic end located near the frenulum. The caudal end of the ridge soon begins to separate from the epithelium and extend backward and ventrad into the submaxillary region, where it enlarges and branches to form the gland proper ; its cephalic, unbranched portion, persisting as the duct, soon hollows out (Fig. 79).
| |
|
| |
|
| |
|
| |
| Fig. 87. - Diagrammatic ventral view of pharynx, digestive tube and mesonephroi of a 4 to 5 mm. embryo (adapted by Prentiss). X about 30. The liver and yolk sac are cut away. The tubules of the right mesonephros are shown diagrammatically.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| The sublingual gland appears by the eighth week as several solid evaginations of epithelium from the jaw-tongue groove (Fig. 79). This group, usually regarded as a sublingual gland, really consists of the sublingual proper, with its ductus major, and of about ten equivalent alveololingual glands. Mucin cells have appeared by the sixteenth week.
| |
|
| |
|
| |
|
| |
| Pharyngeal Pouches. - There are developed early from the lateral wall of the entodermal pharynx paired outpocketings which are formed in succession cephalo-caudad. In 4 to 5 mm. embryms, five pairs of such pharyngeal {branchial) pouches are present, the fifth pair being rudimentary (Figs. 87 and 91). Meanwhile, the pharynx has flattened and broadened, so that it is triangular in ventral view (Figs. 87 and 88).
| |
|
| |
|
| |
|
| |
| From each pharyngeal pouch develop small dorsal and large ventral diverticula. All five pouches come into contact with the ectoderm of corresponding branchial grooves, fuse with it, and form the closing plates. Although the closing plates become perforate in human embryos only occasionally, these pouches and grooves, nevertheless, are homologous to the functional branchial clefts of fishes and tailed amphibia. The first and second pharytigeal pouches soon connect with the pharyngeal cavity through wide common openings. The third and fourth pouches grow laterad and their diverticula communicate with the pharynx through narrow ducts in lo to 12 mm. embryos (Fig. 88). When the cervical sinus (p. 77) is formed, the ectoderm of the second, third, and fourth branchial clefts is drawn out to produce the transient branchial and cervical ducts and the cervical vesicle. These are fused at the closing plates with the entoderm of the corresponding pharyngeal pouches, the fate of the entodermal pouches is varied and spectacular. Although they do not continue as parts of the digestive apparatus, their embryonic relations justify their inclusion in the present section. The first differentiates into the tympanic cavity of the middle ear and into the auditory (Eustachian) tube. The second becomes the palatine tonsil in part. The third, fourth, and fifth pouches give rise to a series of ductless glands: the thymus, parathyroids, and the ultimobranchial bodies.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 88. - Reconstruction of the pharynx and fore-gut of a 12 mm. human embryo, seen in dorsal view (Hammar-Prentiss). The ectodermal structures are stippled.
| |
|
| |
|
| |
|
| |
|
| |
| The Tonsils. - By the growth and lateral expansion of the pharynx, the second pouch is absorbed into the pharyngeal wall, its dorsal angle alone persisting, to be transformed into the tonsillar and su proton sillar fossae. Crypts arise at the end of the third month by the hollowing of solid epithelial ingrowths, whereas a mound of mesodermal lymphoid tissue hrst presses against the epithelium at the middle of the fourth month. This association constitutes the palatine tonsil.
| |
|
| |
|
| |
| A Subepithelial infiltration of lymphocytes during the sixth month gives rise to the median pharyngeal tonsil, which like the lingual tonsil is not of pharyngeal pouch origin. Immediately caudad is a recess, the pharyngeal bursa, formed by a protracted connection of the epithelium with the notochord (Huber). It bears no relation to the original blind termination of the fore-gut known as Seesel - s pouch. According to Hammar, the lateral pharyngeal recess (of Rosenmfiller) is not a persistent portion of the second pouch, as His asserted.
| |
|
| |
|
| |
| The Thymus. - The thymus anlages appear in 10 mm. embryos as ventral and medial prolongations of the third pair of pouches (Figs. 88 and 89). The ducts connecting the diverticula with the pharynx soon disappear so that the anlages are set free. At first, they are hollow tubes which soon lose their cavities and migrate caudally into the thorax, usually passing ventral to the left innominate vein. Their upper ends become attentuate and atrophy, but may persist as accessory thymus lobes. The enlarged lower ends of the anlages form the body of the gland, which is thus a paired structure (Fig. 90). At 1 1 weeks the thymus still contains solid cords and small closed vesicles of entodermal cells. From this stage on, the gland becomes more and more lymphoid in character. Its final position is in the thorax, dorsal to the upper end of the sternum. It grows under normal conditions until puberty, after which involution begins. This process proceeds slowly in healthy individuals, rapidly in case of disease. True atrophy of the parenchyma enters at about the fiftieth year.
| |
|
| |
|
| |
| The ventral diverticulum of the fourth pouch is a rudimentary thymic anlage which usually atrophies.
| |
|
| |
| It is now generally believed that the entodermal epithelium of the thymus is converted into reticular tissue and thymic corpuscles. The latter are the atrophic and hyalinized remains of embryonic tubules and cords (Marine, 1915). The lymphoid cells were regarded by Stohr as entodermal in origin, but most observers derive them from the mesoderm.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 89. - Diagram of the pharynx and its derivatives (adapted by Prentiss). I-V, first to fifth pharjmgeal pouches.
| |
|
| |
|
| |
|
| |
| The Parathyroid Glands. - Each dorsal diverticulum of the third and fourth pharyngeal pouches gives rise to a small mass of epithelial cells termed a parathyroid gland (Fig. 89). Two pairs of these bodies are thus formed, and, with the atrophy of the ducts of the pharyngeal pouches, they are set free and migrate caudalw^ard. The}* eventually lodge in the dorsal surface of the thyroid gland; the pair from the third pouches lies, one on each side, at its caudal border, the ]iair from the fourth pouches at the cranial border (Fig. go). Their solid bodies are broken up into masses and cords of jiolyhedral entodermal cells intermingled with blood vessels. In postfetal life, lumina may appear in the cell masses and fill with a colloid-like secretion.
| |
|
| |
|
| |
|
| |
| Fig. 90. - Reconstruction of the thymus, thyroid and parathj-roid glands in a human embryo of two months (Tourneaux and Verdun). X 15.
| |
|
| |
|
| |
|
| |
| The Ultimobranchial Bodies. - These bodies, also called postbranchial, are usually rated as derivatives of the fifth pharyngeal pouches (Fig. 89).
| |
|
| |
|
| |
| Fig. 91. - Reconstruction of a 4.2 human embryo (His- Prentiss). X 25.
| |
|
| |
|
| |
|
| |
| By the atrophy of the ducts of the fourth pouches they are set free and migrate caudad with the parathyroids. Each forms a hollow vesicle which has been erroneously termed the lateral thyroid. It takes no part in forming thyroid tissue, but atrophies. Kingsbury (1915) denies the origin of the ultimobranchial body from any specific pouch, and asserts it is “merely formed by a continued growth activity in the branchial entoderm .
| |
|
| |
|
| |
|
| |
| The Thyroid Gland. - In embryos with five to six primitive segments (1.4 mm.) there appears in the midventral wall of the pharynx, between the first and second branchial arches, a small outpocketing, the thyroid milage. In 2.5 mm. embryos it has become a stalked vesicle (Figs. 71 j 5 and 87). Its stalk, the thyroglossal duct, opens at the aboral border of the tuberculum impar of the tongue (Figs. 85 B); this spot is represented permanently by the foramen cecum (Fig. g6). The duct soon atrophies and the bilobled gland anlage (Fig. 89) loses its lumen and breaks up into irregular, solid, anastomosing plates of tissue as it migrates caudad. The thyroid assumes a transverse position with a lobe on each side of the trachea and larynx (Fig. 90). In embryos of eight weeks, discontinuous lumina begin to appear in swollen portions of the plates; these represent the primitive thyroid follicles. Colloid soon forms.
| |
|
| |
|
| |
|
| |
| Anomalies. - Persistent portion of the thyroglossal duct may form cysts or even fistulce.
| |
|
| |
|
| |
|
| |
| THE DIGESTIVE TUBE.
| |
|
| |
|
| |
| The several accessory coats of the digestive tube are all derived from splanchnic mesoderm wTich invests the entoderm of the primitive gut. In each division of the tube the circular muscle layer develops before the longitudinal layer.
| |
|
| |
|
| |
|
| |
| The Esophagus. - The esophagus in 4 to 5 mm. embryos is a very short tube, extending from pharynx to stomach (Fig. 91). As the heart and diaphragm recede into the thorax, it grows rapidly in length (Figs. 95 and 96). In embryos of 8 mm. the esophageal epithelium is composed of two layers of columnar cells, but at birth they number nine or ten. The esophagus remains so broadly attached to the dorsal body wall that there is never a distinct mesentery (Fig. 124).
| |
|
| |
|
| |
| During the eighth week vacuoles appear in the epithelium and increase the size of the lumen, which, however, is at no time occluded. Glands begin to develop at four months. The circular muscle layer is indicated at six weeks but the longitudinal fibers do not form a definite layer until u weeks.
| |
|
| |
|
| |
|
| |
| Anomalies. - There may be atresia. This usually involves a fistulous relation with the trachea; the esophagus is divided transversely, the trachea opening into the lower segment, while the upper portion ends as a blind sac.
| |
|
| |
|
| |
| The Stomach. - The stomach appears in embryos of 4 to 5 mm. as a laterally flattened, fusiform enlargement of the fore-gut, caudal to the lung anlages (Figs. 93 and 94). Its wall is composed of three layers: the entodermal epithelium, a thick mesenchymal layer, and the peritoneal mesothelium (Fig. 114). The stomach is attached dorsally to the body wall by its mesentery, the greater omentum, and ventrally to the liver by the lesser omentum (Fig. 112 B). The dorsal border of the stomach soon bulges locally to form the fundus, and also grows more rapidly than the ventral wall throughout its extent, thus producing the convex greater curvature (Fig. 92). The whole stomach becomes curved, and its cranial end is displaced to the left by the enlarging liver (Fig. 88). This forms a ventral concavity, the lesser curvature, and produces the first flexure of the duodenum.
| |
|
| |
|
| |
|
| |
| Fig. 93. - Median sagittal section of a 5 mm. human embryo, to show the digestive canal
| |
| (Ingalls-Prentiss). X 14.
| |
|
| |
|
| |
|
| |
| The rapid growth of the gastric wall along the greater curvature also causes the stomach to rotate about a long axis until its greater curvature, or primitive dorsal wall, lies to the left, its lesser curvature, or ventral wall, to the right (Fig. 114). The original right side is now dorsal, the left side ventral in position, and the caudal, or pyloric end of the stomach is ventral and to the right of its cardiac, or cephalic end. The whole organ extends obliquely across the peritoneal cavity from left to right.
| |
|
| |
|
| |
|
| |
| These changes in position progress rapidly and are already completed early in the second month.
| |
|
| |
| The rotation of the stomach explains the asymmetrical position of the vagus nerves of the adult organ, the left nerve supplying the ventral wall of the stomach, originally the left wall, while the right vagus supplies the dorsal wall, originally the right. At the end of the seventh week the stomach has reached its permanent position, the cardia having descended through about ten segments, the pylorus through six or seven.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 94. - Reconstruction of a 5 mm. human embryo, showing the entodermal canal and its derivatives (His in Kollmann). X 25.
| |
|
| |
|
| |
| Gastric pits are indicated in embryos of seven weeks, and at 14 weeks the glands begin to differentiate. The gastric pits number 270,000 at birth but increase by fission to nearly seven million in the adult. At seven w'eeks, the circular muscle layer is indicated by condensed mesenchyme; a heavier ring forms the pyloric sphincter. During the fourth month the cardiac region shows a few longitudinal muscles fibers, which become distinct in the pyloric region at seven months.
| |
|
| |
|
| |
|
| |
| The Intestine. - ^In 5 mm. embryos (Fig. 93), the intestine, beginning at the stomach, consists of the duodenum (from which are given off the hepatic diverticulum and dorsal pancreas), and the cephalic and caudal limbs of the intestinal loop, which bends ventrad and connects with the yolk stalk. Caudally, the intestinal tube expands into the cloaca. It is sup]:)orted from the dorsal body wall by the mesentery (Fig. 94).
| |
|
| |
|
| |
| From 5 to 9 mm., the ventral flexing of the intestinal loop becomes more marked and the attachment of the yolk stalk to it normally disappears (Fig. 95). At this stage there is formed in the caudal limb of the intestinal loop an enlargement, due to a ventral bulging of the gut wall, that marks the anlage of the cecum and the boundary line between the large and small intestine. Succeeding changes in the intestine consist; (i) in its torsion and coiling, due to rapid elongation, and (2) in the differentiation of its several regions. As the gut elongates in 9 to 10 mm. embryos, the intestinal loop rotates. As a result, the originally caudal limb lies at the left and cranial to its cephalic limb (Fig. 95) of the small intestine soon lengthens so rapidly that the coelom can no longer accommodate it, and, at seven weeks, it protrudes into the umbilical cord and forms loops there (Fig. 96). This constitutes a normal umbilical hernia. Six primary loops occur and these may be recognized in the arrangement of the adult intestine. In embryos of ten weeks, spatial readjustments have allowed the intestine to return from the umbilical cord into the abdominal cavity; the coelom of the cord is obliterated soon after.
| |
|
| |
|
| |
| Fig. 95. - Median sagittal section of a 9 mm. human embryo, showing the digestive canal (Mall-Prentiss). X 9.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Vacuoles appear in the duodenal wall of embryos six to nine weeks old and epithelial septa completely block its lumen. The remainder of the small intestine becomes vacuolated but not occluded. Villi develop as rounded elevations of the epithelium at eight weeks. They begin to form at the cephalic end of the jejunum, and, at four months are found throughout the small intestine. Intestinal glands appear as ingrowths of the epithelium about the bases of the villi. They develop first in the duodenum at 14 weeks. The duodenal glands (of Brunner) are said to appear during the fourth month. In embryos of six weeks the circular muscle layer of the intestine first forms, but the longitudinal layer is not distinct until the end of the third month.
| |
|
| |
|
| |
|
| |
| Fig. 96. - Median sagittal section of a 17 mm. human embryo, showing the digestive canal
| |
| (Mall-Prentiss). X 5.
| |
|
| |
|
| |
| The large intestine, as seen in 9 mm. embryos (Fig 95), forms a tube extending from the cecum to the cloaca. It does not lengthen so rapidly as the small intestine, and, when the intestine is withdrawn from the umbilical cord, its cranial, or cecal end lies on the right side and dorsal to the small intestine (Fig. 97). It extends across to the left side as the transverse colon, then, bending abruptly caudad as the descending colon, returns by its sigmoid segment to the median plane and continues into the rectum. In fetuses from three to six months old, the lengthening of the colon causes the cecum and cephalic end of the colon to descend toward the pelvis (Fig. 97). The ascending colon is thus established in the position which it occupies in the adult. The distal end of the cecal anlage continues to elongate, but early lags in transverse development; as a result, the vermiform process is distinct from the cecum at the end of the third month. These structures make a sharp U-shaped bend with the colon at ten weeks, and this flexure gives rise to the colic valve.
| |
|
| |
|
| |
|
| |
| Fig. 97. - Later changes in the intestine and dorsal mesentery of the human fetus (Tourneux in Heisler). i. Stomach; 2, duodenum; 3, small intestine; 4, colon, 5, yolk stalk; 6, cecum; 7, greater omentum; 8, mesoduodenum; 9, mesentery, 10, mesocolon. The arrow points to the orifice of the omental bursa. The ventral mesentery is not shown.
| |
|
| |
|
| |
| Fig. 98. - Development of the cecum and vermiform process (adapted from Kollman and Paterson). A, Two months; B, three months; C, early infancy; D, five years.
| |
|
| |
|
| |
| The Rectum. - The terminal portion of the intestine is derived by the horizontal division of the cloaca; Figs. 95 and 96, and 140 to 142 illustrate the process which is described in full on p. 145. When the anal membrane ruptures at the ninth week, the ectodermal proctodeum is added to the entodermal rectum.
| |
|
| |
|
| |
|
| |
| The circular muscle layer of the large intestine appears first at two months, the longitudinal layer at three months. Between the third and seventh months villi are present.
| |
|
| |
|
| |
| Glandular secretions and desquamated entodermal cells, together with swallowed amniotic fluid, containing lanugo hairs and vernix caseosa, collect in the fetal intestine. This mass, yellow to brown in color, is known as meconium. At birth the intestine and its contents are perfectly sterile, but a bacterial flora is promptly acquired.
| |
|
| |
|
| |
|
| |
| Anomalies. - The intestine may show atresia. This occurs most often in the duodenum as a retention of the embryonic occlusion. When the anal membrane fails to rupture, an imperforate anus results. If the rectum does not separate completely from the cloaca, a common urogenital and rectal cavity remains. Rarely there is nonrotation of the intestine and the colon lies on the left side. Two per cent of all adults show a persistence of the proximal end of the yolk stalk to form a pouch, Meckel - s diverticulum of the ileum (p. 52). Congenital umbilical hernia is due either to the continuance of the normally transitory embryonic condition or to a secondary protrusion of the viscera. Other hernias are explained on pp. 134 and 163.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 99. - Model of the liver anlage of a 4 mm. human embryo (Bremer). X 160. In., Intestine; Pa., pancreas; V., veins in contact with liver trabeculse.
| |
|
| |
|
| |
|
| |
| In embryos of 2.5 mm., the liver anlage is present as a median ventral outgrowth from the entoderm of the fore-gut, just cranial to the yolk stalk (Fig. 71). Its thick walls enclose a cavity which is continuous with that of the gut. This hepatic diverticitluni becomes embedded at once in a mass of splanchnic mesoderm, the septum transversum (Fig. 91). Cranially, the septum will contribute later to the formation of the diaphragm; caudally, in the region of the liver anlage, it becomes Glisson - s capsule and the ventral mesentery (Figs, no and in). Thus, from the first, the liver is in close relation to the septum transversum, and later when the septum becomes the diaphragm, the liver remains attached to it (Fig. 113).
| |
|
| |
|
| |
| In embryos 4 to 5 mm. long, solid cords of cells proliferate from the ventral and cranial portion of the hepatic diverticulum (Fig. 91). These cords anastomose and form a crescentic mass with wings extending u]iward on either side of the gut (Fig. 93). This mass, a network of solid trabeula?, is the glandular portion of the liver, whereas the primitive, hollow diverticulum differentiates later into the gall bladder and the large biliary ducts. Referring to Fig. 183, it will be seen that the early liver anlage lies between the vitelline veins and is in close proximity to them laterally. The veins send anastomosing branches into the ventral mesentery. The trabeculce of the expanding liver grow between and about these venous plexuses, and the plexuses in turn make their way .
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 100. - The trabeculae and sinusoids of the liver in section (after Minot). X 300. Tr., Trabeculae of liver cells; Si., sinusoids.
| |
|
| |
|
| |
| Between and around the liver cords (Fig. 99). The vitelline veins, on their way to the heart, are thus surrounded by the liver and largely subdivide into a network of vessels, termed sinusoids. The endothelium of the sinusoids is closely applied to the cords of liver cells, which, in the early stages, contain no bile capillaries (Fig. 100).
| |
|
| |
| The glandular portion of the liver grows rapidly, and, in embryos of 7 to 8 mm., is connected with the primitive hepatic diverticulum by a single cord of cells only, the hepatic duct (Fig. loi A). That portion of the hepatic diverticulum distal to the hepatic duct is now differentiated into the terminal, solid gall bladder and its cystic duct; the proximal portion forms the ductus choledochus. In embryos of 10 mm. (Fig. loi B), the gall bladder and ducts have become longer and more slender and the hepatic duct receives a right and left branch from the corresponding lobes of the liver. The gall bladder is without a lumen up to the 15 mm stage, but later its cavity appears, surrounded by a wall of high, columnar epithelium.
| |
|
| |
|
| |
| The glandular portion of the liver develops fast and is largest relative to the size of the body at nine weeks. In certain regions the liver tissue undergoes degeneration, and especially is this true in the peripheral portion of the left lobe. In general, the external lobes of the liver are moulded under the influence of the fetal vitelline and umbilical trunks. The development of the ligaments of the liver is described on p. 126.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 101. - Reconstructions of the hepatic diverticulum and pancreatic anlages in human embryos. A, 7.5 mm. (Thyng). X 50; B, 10 mm. (Prentiss). X 33.
| |
|
| |
|
| |
| Fig. 102. - Diagrams illustrating the formation of liver lobules (Mall), a, Hepatic side; d, portal side; b and c, successive stages of the hepatic vein; e and f, successive stages of the portal vein.
| |
|
| |
|
| |
|
| |
| During the development of the liver the endothelial cells of the sinusoids become stellate in outline, and thus form an incomplete layer. From the second month of fetal life to some time after birth, blood cells are actively differentiating between the hepatic cells and the endothelium of the sinusoids. At eight weeks hollow interlobular ducts appear spreading inward from the hepatic duct along the larger branches of the portal vein. In fetuses of ten weeks bile capillaries with cuticular borders are present, most numerous near the interlobular ducts with which some of them connect. At birth, or shortly after, the number of liver cells surrounding a bile capillary is reduced to two, three, or four. Secretion of the bile commences at about the end of the third fetal month.
| |
|
| |
|
| |
| The lobules, or vascular units of the liver, are formed, according to Mall, by the peculiar and regular manner in which the veins of the liver branch. The primary branches of the portal vein extend along the periphery of each primitive lobule, parallel to similar branches of the hepatic veins that drain the blood from the center of the lobule (Fig. 102). As development proceeds, each primary branch becomes a stem, giving off on either side secondary branches which bear the same relation to each other and to new lobules as did the primary branches to the first lobule. This process is repeated during fetal and early postnatal life until thousands of liver lobules are developed until the 20 mm. stage, the portal vein alone supplies the liver. The hepatic artery, from the cadiac axis, comes into relation first with the hepatic duct and gall bladder. Later, it grows into the connective tissue about the larger bile ducts and the branches of the portal vein, and also supplies the capsule of the liver.
| |
|
| |
|
| |
|
| |
| Anomalies. - A common anomaly of the liver consists in its subdivision into multiple lobes. Absence or duplication of the gall bladder and of the ducts may occur. In some animals (horse; elephant) the gall bladder is absent normally.
| |
|
| |
|
| |
|
| |
| THE PANCREAS
| |
|
| |
|
| |
| Two pancreatic anlages are developed almost simultaneously in embryos of 3 to 4 mm. The dorsal pancreas arises as a hollow outpocketing of the dorsal duodenal wall, just cranial to the hepatic diverticulum (Figs. 93 and 94). At 7.5 mm., it is separated from the duodenum by a slight constriction and extends into the dorsal mesentery (Fig. 10 1 A). The ventral pancreas develops in the inferior angle between the hepatic diverticulum and the gut, and its wall is at first continuous with both. With the elongation of the ductus choledochus, its origin is transferred to this portion of the diverticulum.
| |
|
| |
|
| |
|
| |
| Fig. 103. - Stages in the development of the human pancreas (Kollman). 4 , 8 mm. ; B, 20 mm.
| |
|
| |
|
| |
|
| |
|
| |
| Of the two pancreatic anlages, the dorsal grows more rapidly, and, in 10 mm. embryos, forms an elongated structure with a central duct and irregular nodules upon its surface (Fig. loi B). The ventral pancreas is smaller and develops a short, slender duct that opens into the ductus choledochus. When the stomach and duodenum rotate, the pancreatic ducts shift their positions as well. At the same time, growth and bending of the bile duct to the right bring the ventral pancreas into close proximity with the dorsal pancreas (Figs. 101 and 103).
| |
|
| |
|
| |
| In embryos of 20 mm., the tubules of the dorsal and ventral pancreatic anlages interlock (Fig. 103 B). Eventually, anastomosis takes place between the two ducts, and the duct of the ventral pancreas, plus the distal segment of the dorsal duct, persists as the functional pancreatic duct (of Wirsung) of the adult. The proximal portion of the dorsal pancreatic duct forms the accessory duct (of Santorini), which remains pervious, but becomes a tributary of the chief pancreatic duct. The ventral pancreas forms part of the head and uncinate process of the adult gland. The dorsal pancreas participates in forming the head and uncinate process, and comprises the whole of the body and tail.
| |
|
| |
|
| |
| In 10 mm. embryos the portal vein separates the two pancreatic anlages, and later they partially surround the vein. The alveoli of the gland are derived from the ducts as darkly staining cellular buds in fetuses of ten weeks. The islands, characteristic of the pancreas, also bud from the ducts (and alevoli, Mironescu, 1910) and appear first in the tail a week later. Owing to the shift in the position of the stomach and duodenum during development, the pancreas takes up a transverse position.
| |
|
| |
|
| |
|
| |
| Anomalies. - The ventral pancreas may arise directly from the intestinal wall, and paired ventral anlages also occur. Accessory pancreases are not uncommon. Both the dorsal and ventral ducts persist in the horse and dog; in the sheep and man, the ventral duct normally becomes of chief importance; in the pig and ox, the dorsal duct.
| |
|
| |
|
| |
| ++++++++++++++++++++++++++++++++++++++++++++.
| |
|
| |
| CHAPTER VI THE RESPIRATORY SYSTEM .
| |
|
| |
|
| |
| The development of the nose and pharynx are described elsewhere. Accordingly, the present account will deal exclusively with the origin of the other respiratory organs. In embryos of 23 segments, the anlage of this apparatus appears as a laryngo-tracheal groove in the floor of the entodermal tube, just caudal to the pharyngeal pouches. This groove produces an external ridge on the ventral wall of the tube which promptly becomes larger and rounded at its caudal end (Fig. 104). The groove and .
| |
|
| |
|
| |
|
| |
| Fig. 104. - Stages in the early development of the trachea and lungs of human embryos (adapted by Prentiss). X about 50. A, 2.5 mm.; B, 4 mm.; C, stage B in side view; D, 5 mm. ; E, 7 mm.
| |
|
| |
| The ridge are the anlages of the larynx and trachea. The rounded end of the ridge is the unpaired anlage of the lungs; in embryos of 4 to 5 mm. it becomes bilobed.
| |
|
| |
|
| |
|
| |
| Externally, two lateral longitudinal grooves mark off the dorsal esophagus from the ventral respiratory anlages. A fusion of the lateral furrows, progressing cephalad, constricts first the lung anlages and then the trachea from the esophagus. At the same time the laryngeal portion of the groove and ridge advances cranially until it lies between the fourth branchial arches (Fig. 87). At 5 mm., the respiratory apparatus consists of the laryngeal groove and ridge, the tubular trachea, and the two lung buds (Fig. 104 D).
| |
|
| |
|
| |
| The Larynx. - In embryos of 5 to 6 mm., the oral end of the laryngeal groove is bounded on either side by two rounded prominences, the arytenoid swellings, which are continuous orally with a transverse ridge to form the furcula of His (Fig. 85). The transverse ridge becomes the epiglottis, derived from the third and fourth branchial arches (p. g6). In embryos of 15 mm., the arytenoid swellings are bent near the middle; their caudal portions lie parallel, while their cephalic segments diverge nearly at right angles (Fig. 105). The glottis, opening into the larynx, thus becomes T-shaped and ends blindly, as the laryngeal epithelium has fused. In fetuses of ten weeks this fusion is dissolved, the arytenoid swellings are withdrawn from contact with the epiglottis, and the entrance to the larynx becomes oval in form (Fig. 106). At eight weeks the ventndes of the larynx appear, and, during the tenth week, their margins indicate the position of the vocal cords. The elastic and muscle fibers of the cords are developed by the fifth month.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 105. - The larynx of a 16 mm. human embryo (Kallius). X 15.
| |
|
| |
|
| |
|
| |
| At the end of the sixth week, the cartilaginous skeleton of the larynx is indicated by surrounding condensations of mesenchyme, derived from the fourth and fifth pairs of branchial arches (p. 221). The cartilage of the epiglottis appears relatively late. The thyroid cartilage is formed from the fusion of two lateral plates, each of which has two centers of chondrification. The anlages of the cricoid and arytenoid cartilages are originally continuous; later, separate cartilage centers develop for the arytenoids. The cricoid is at first incomplete dorsad, but eventually forms a complete ring; it therefore may be regarded as a modified tracheal ring. The corniculatc cartilages represent separated portions of the arytenoids. The cuneiform cartilages are derived from the cartilage of the epiglottis. The laryngeal muscles originate in the fourth and fifth branchial arches and are consequently innervated by the vagus nerve which supplies those arches.
| |
|
| |
|
| |
| The Trachea. - This tube gradually elongates during development, and its columnar epithelium becomes ciliated. Muscle fibers and the anlages of the cartilaginous rings appear in the condensed mesenchyme at the end of the seventh week. The glands develop as ingrowths of the epithelium during the last five months of fetal life.
| |
|
| |
|
| |
| The Lungs. - Soon after the lung anlages, or stem buds, are formed (in 5 mm. embryos), the right bronchial bud becomes larger and is directed .
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 107. - Ventral and dorsal views of the lungs from a human embryo of about 9 mm. (after Merkel). Ap., Apical bronchus; Di, D2, etc., dorsal, V i. V2, ventral bronchi; Jc., infracardiac bronchus.
| |
|
| |
|
| |
| Straighter caudad (Fig. 104). At 7 mm. the stem bronchi give rise to two bronchial buds on the right side, to one on the left. The smaller bronchial bud on the right side is the apical {eparterial) hud. The right and left chief buds, known as ventral bronchi, soon bifurcate. There are thus formed three bronchial rami on the right side and two on the left; these correspond to the primitive lobes of the lungs (Figs. 88 and 107). On the left side, an apical bud is interpreted as being derived from the first ventral bronchus (Fig. 107). It remains small so that there is no separate lobe corresponding to the upper lobe of the right lung. The absence of this upper left lobe may be an adaptation to permit the normal caudal regression of the aortic arch (p. 190).
| |
|
| |
|
| |
| The bronchial anlages continue to branch in srich a way that the stem bud is retained as the main bronchial stem (Fig. 107). That is, the branching is monopodial, not dichotomous, lateral buds being given off from the stem bud proximal to its growing tip. Only in the later stages of development has dichotomous branching of the bronchi and the formation of two equal buds been described. Such buds, formed dichotomously, do not remain of equal size (Flint). The inclination of the heart to the left suppresses one of the larger ventral bronchial rami on that side, but at the same time it affords opportunity for an excessive development of the corresponding right ramus which then projects into the space between the heart and diaphragm as the infrasardiac bronchus (Fig. 107, Jc).
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 108. - Transverse section through the lungs and pleural cavities of a 10 mm human embryo (Prentiss). X 23.
| |
|
| |
|
| |
|
| |
| The entodermal anlages of the lungs and trachea are developed in a median mass of mesenchyme, dorsal and cranial to the peritoneal cavity. This tissue forms a broad mesentery, termed the mediastinum (Fig. 108). The right and left stem buds of the lungs grow out laterad, carrying with them folds of the mesoderm. The branching of the bronchial buds takes place within this tissue which is covered by the mesothelial lining of the future pleural cavity. The terminal branches of the bronchi are lined with entodermal cells; these flatten out and form the respiratory epithelium of the adult lungs. The surrounding mesenchyme differentiates into the muscle, connective tissue, and cartilage plates of the lung, tracheal, and bronchial walls. Into it grow blood vessels and nerve fibers. When the pleural cavities are separated from the pericardial and ])eritoneal cavities, the mesothelium covering the lungs, with the connective tissue underl3ung it, becomes the visceral pleura. The corresponding layers lining the thoracic wall form the parietal pleura. These layers are derived respectively from the visceral (splanchnic) and parietal (somatic) mesoderm of the embryo.
| |
|
| |
|
| |
| In 11 mm. embryos, the two pulmonary arteries, from the sixth pair of aortic arches, course first lateral then dorsal to the stem bronchi (Fig. 109). d he right pulmonary artery passes ventral to the apical (epar
| |
| terial) bronchus of the right lung. The single pulmonary vein receives two branches from each lung: a larger vein from each lower lobe, a smaller vein from each upper lobe, including the middle lobe of the right side. These four pulmonary branches course ventrad and drain into the pulmonary trunk. When this common stem is taken up into the wall of the left atrium, the four pulmonary veins open directly in to the latter.
| |
|
| |
|
| |
| According to Kolliker, the air cells, or alveoli, of the lungs begin to form in the sixth month and their developnent is completed during pregnancy. Elastic tissue appears during the fourth month in the largest bronchi. The abundant connective tissue found between the bronchial branches in early fetal life becomes reduced in its relative amount as the alveoli of the lungs are developed.
| |
|
| |
|
| |
| Until birth the lungs are relatively small, compact, and possess sharp margins. They lie in the dorsal portion of the pleural cavities. After birth the lungs normally fill with air, expanding and completely filling the pleural cavities. Their margins are then rounded and the compact, fetal lung tissue, which resembles that of a gland in strrrcture, becomes light and spongy, owing to the enormous increase in the size of the alveoli and blood vessels. Because of the greater amount of blood admitted to the lungs after birth, their weight is suddenly increased.
| |
|
| |
|
| |
| Anomalies. - Variations occur in the size and number of lobes of the lungs; rarely there is a third lobe on the left side. The most common anomaly involving both esophagus and trachea is described on p. 104.
| |
|
| |
|
| |
| A striking malformation of the viscera in general is situs visccrum inversus, in which the various organs are transposed in position, right for left and left for right, as in a mirror image. This reversal may effect all the internal organs, or an independent transposition of the thoracic or abdominal viscera alone may occur. The early influence of the larger left great venous trunks is thought to be chiefly responsible for the usual positions and asymmetrical relations of the viscera.
| |
|
| |
|
| |
|
| |
| Fig. 109. - Ventral view of the lungs of a 10.5 mm. embryo, showing the pulmonary arteries and veins (His in McMurrich). X 27. Ep., Apical bronchus; I, u, primary bronchi.
| |
|
| |
|
| |
|
| |
| +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++.
| |
|
| |
|
| |
| CHAPTER Vu THE MESENTERIES AND COELOM
| |
|
| |
|
| |
| I. THE MESENTERIES
| |
|
| |
|
| |
| The Primitive Mesentery. - -The gut arises when the entoderm is folded into a tube (Fig. 165). At the same time, the lateral expanse of superposed splanchnic mesoderm swings inward from each side toward the midplane and forms a double-layered sheet, extending from the roof of the coelom to the midventral body wall and containing the gut between its layers (Figs, no and in). This membrane is the primitive mesentery. The covering layers of the gut (and other viscera), mesenteries, and body wall are continuous with each other and consist of a mesothelium, overlying connective tissue (Fig. ui). The parietal lining is derived from the somatic layer of mesoderm and the visceral covering from the splanchnic layer (Fig. 165).
| |
|
| |
|
| |
|
| |
| Fig. 110. - Diagram showing the primitive mesenteries of an early human embryo in median sagittal section (Prentiss). The broken lines (A, B, and C) indicate the level of sections A , B, and C, in Fig. 111.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 111. - Diagrammatic transverse sections of an early human embryo (Prentiss). A, Through the heart and pericardial cavities; B, through the fore-gut and liver; C, through the intestine and peritoneal cavity. (Compare guide lines A, B, C, Fig. uo.) .
| |
|
| |
|
| |
|
| |
| Differentiation of the Dorsal Mesentery. - At first, the gut is broadly attached dorsad and its roof lies directly beneath the notochord and descending aortee (Fig. 165). Presently this region of attachment becomes relatively narrow, and the gut is then suspended throughout most its length by a definite dorsal mesentery which extends like a curtain in the midplane. The esophagus lies in the mediastinum and has no typical mesentery in the adult (Fig. 124). On the contrary, that portion of the digestive canal which passes through the peritoneal cavity is contained in an originally continuous dorsal mesentery. Later, distinctive names are given to its several regions (Fig. no): thus, there is the dorsal mesogastrium (or greater omentum), of the stomach, the mesoduodenum, the mesentery proper of the small intestine, the mesocolon, and the mesorecinm.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 112. - Diagrammatic model cf an 8 mm. human embryo, showing the primitive omental bursa (Prentiss). The model is sectioned transversely, caudal to the liver, so the observer looks cephalad at the caudal surfaces of the liver and sectioned body.
| |
|
| |
|
| |
|
| |
| The Omental Bursa. - The history of the mesogastrium is chiefly concerned with the development of a huge sacculation known as the omental bursa, or lesser peritoneal sac. According to Broman, its first indication in a 3 mm. embryo is a peritoneal pocket which extends cranially into the dorsal mesentery, to the right of the esophagus. A similar pocket, present on the left side, has disappeared in 4 mm. embryos. Lateral to the opening of the primitive bursa, a lip-like fold of the mesentery is continued caudally along the dorsal body wall into the mesonephric fold as the caval mesentery, in which the inferior vena cava develops later (Fig. 1 12). Furthermore, it will be remembered that the liver grows out into the ventral mesentery from the fore-gut, and, expanding laterally and ventrally, takes the form of a cresent. Its right lobe comes into relation with the caval mesentery, and, growing rapidly caudad, forms with this fold a partition between the lesser sac and the peritoneal cavity. Thus, the cavity of the omental bursa is extended caudally from a point opposite the bifurcation of the lungs to the level of the pyloric end of the stomach. In 5 to 10 mm. embryos, it is crescent-shaped in cross section (cf. Fig. Ill) and is bounded mesially by the greater omentum (dorsal mesentery) and the right wall of the stomach, laterally by the liver and caval mesentery, and ventrally by the lesser omentum (ventral mesentery) (Fig. 1 14). It communicates to the right with the peritoneal cavity through an opening between the liver ventrally and the caval mesentery dorsally (Figs. 114 and 116). This aperture is the epiploic foramen (of Winslow). When the dorsal wall of the stomach rotates to the left, the greater omentum is carried with it to the left of its dorsal attachment. The omental tissue grows actively to this side and caudally, and gives the omentum an appearance of being folded on itself between the stomach and the dorsal body wall (Fig. 113). The cavity of the omental bursa is carried out between the folds of the greater omentum as the inferior recess (Figs. 97 and 116).
| |
|
| |
|
| |
| From the cranial end of the sac there is constricted off a small closed cavity which is frequently persistent in the adult. This is the infra-cardiac bursa and may be regarded as a third pleural cavity. It lies at the right of the esophagus in the mediastinum when the stomach changes its position and form so that its midventral line becomes the lesser curvature and lies to the right, the position of the lesser omentum is also shifted. From its primitive location in a median sagittal plane, with its free edge directed caudally, the lesser omentum is rotated through 90° until it lies in a coronal plane with its free margin facing to the right. The epiploic foramen then forms a slitlike opening leading from the peritoneal cavity into the vestibule of the omental bursa (Fig. 114). The foramen is bounded ventrally by the edge of the lesser omentum, dorsally by the inferior vena cava, cranially by the caudate process of the liver, and caudally by the wall of the duodenum.
| |
|
| |
|
| |
|
| |
| Fig. 113. - Diagrammatic model of a 14 mm. human embryo (adapted by Prentiss). The figure shows the caudal surface of a section through the stomach and spleen, a ventral view of the stomach, the liver having been cut away to leave the sectioned edges of the omental bursa and caval mesentery, and the caudal surface of the septum transversum and pleuro-peritoneal membrane. Upon the surface of the septum is indicated the attachment of the liver
| |
|
| |
|
| |
|
| |
| During fetal life the greater omentum grows rapidly to the left and caudad, in the form of a sac, flattened dorso-ventrally. It overlies the intestines ventrally and contains the inferior recess of the omental bursa (Fig. 1 1 5). In the fourth month, the dorsal wall of the sac usually fuses with the transverse colon and mesocolon where it overlies them (Fig 115 B). The transverse mesocolon of the adult is conseciuently a double structure and the omental connection between stomach and colon becomes the gastro-coUc ligament. Caudal to this attachment, the walls of the omental bursa commonly unite and obliterate its cavity. The inferior recess of the omental bursa thus may be limited in the adult chiefly to a space between the stomach and the dorsal fold of the great omentum, which latter is largely fused to the peritoneum of the dorsal body wall. The s[)leen develops in the cranial portion of the great omentum; that stretch of the omentum extending between the stomach and spleen is known as the gastro-splenic ligament (Fig. 113), while its continuation beyond the spleen is the spleno-renal ligament.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 114. - Transverse section through a 10 mm. human embryo at the level of the stomach and epiploic foramen (Prentiss). X 33.
| |
|
| |
|
| |
|
| |
| Fig. 115. - Diagrams showing the developmental relations of the greater omentum (Hertwig). A, Illustrates the beginning of the greater omentum and its independence of the transverse mesocolon: in B the two come into contact; in C they have fused. A, Stomach; B, transverse colon; C, small intestine; D, duodenum; E, pancreas; F, greater omentum; G, greater sac; H, omental bursa.
| |
|
| |
|
| |
|
| |
| Other Changes in the Dorsal Mesentery. - As long as the gut remains a straight tube, the dorsal mesentery is a simple sheet whose two attached edges are equal in length. But when the intestine starts to elongate faster than the body wall, and forms first a loop and then coils, the intestinal attachment of the mesentery grows correspondingly and is carried out into the umbilical cord between the intestinal limbs. Even before this herniation of the intestine occurs, its limbs are so shifted that the cecal end of the large intestine comes to lie cranially and to the left, and the small intestine caudally and to the right; in this position the future duodenum and colon cross in close proximity to each other (Fig. 95). On the return of the intestinal loop into the abdomen, the cecal end of the colon is carried over to the right, and the transverse colon crosses the duodenum ventrally and cranially (Fig. 116 A). The primary loops of the small intestine lie caudal and to the left of the ascending colon. There has thus been a torsion of the mesentery about the origin of the superior mesenteric artery as an axis, which is accentuated as the limb of the ascending colon elongates toward the pelvis (Fig. 116 B). From this focal point, the mesentery of the small intestine and colon spreads out like a fan or funnel.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 116. - Diagrams showing the later history of the dorsal mesentery in ventral view (Tourneux-Prentiss). *, Cut edge of greater omentum; a, h, area of ascending and descending mesocolon fused to dorsal body wall. The arrow emerges from the omental bursa.
| |
|
| |
|
| |
|
| |
|
| |
| Previous to the middle of the fourth month, the gut is freely movable within the scope of its restraining mesentery, but soon secondary fusions occur which attach certain segments. The ascending and descending colon are applied against the body wall on the right and left side respectively. The flat surfaces of their mesenteries fuse with the adjacent dorsal peritoneum, and these two limbs of the colon become permanently anchored (Fig. 1 16). vSince the transverse colon passes ventral to the duodenum (Fig. 1 16), its mesentery remains distinct; but in the region of crossing, the base of the mesocolon fuses with the surface of the duodenum and pancreas. In accordance with its final position, this mesentery is now known as the transverse mesocolon. The line of attachment of the mesocolon presses the duodenum firmly against the dorsal body wall and obliterates its mesentery, thereby fixing this portion of the small intestine. The pancreas, which primarily is an outgrowth of the duodenum into the mesoduodenum, necessarily assumes also a retroperitoneal position behind the root of the transverse mesocolon. The mutual union of the lamellae of the greater omentum and its fusion to the transverse colon and the dorsal body wall have been mentioned. The mesentery proper of the small intestine is thrown into numerous folds, corresponding to the loops of the intestine, but normally does not exhibit secondary attachments; the sigmoid mesocolon likewise remains free.
| |
|
| |
|
| |
| Differentiation of the Ventral Mesentery. - The same splanchnic mesodermal layers that invest the entoderm and form the dorsal mesentery, also combine beneath the gut as the ventral mesentery (Figs, no and uI). The ventral mesentery is associated intimately with the development of two important organs. One is the heart, which becomes a single tube by the union of paired anlages lying one in each lateral fold of splanchnic mesoderm (Fig. 165). Hence the heart is supported by the ventral mesentery both above and below (Figs, no and in A). The other organ, the liver, grows downward into the ventral mesogastrium, splitting apart its component lamellae and then having similar mesenterial relations as the primitive heart (Figs, no and in B). Caudal to the yolk sac the ventral mesentery does not persist, even in young embryos (Figs, no and in C).
| |
|
| |
|
| |
| Most cephalad, the heart is suspended in the ventral mesentery which is there designated the dorsal and ventral mesocardium (Figs, -no and uI * 4 ). The latter is transitory and the dorsal mesocardium also disapjiears somewhat later, leaving the heart unsupported in the pericardial cavity (Fig. i66).
| |
|
| |
|
| |
|
| |
| Ligaments of the Liver. - From the first, the liver is enclosed by the lamelte of the ventral mesogastrium, which, as the liver increases in size, give rise to its capsule and ligaments (Figs, no and in B). Wherever the liver is unattached, the enveloping mesodermal layers form the capsule (of Glisson), a fibrous layer covered by mesothelium, continuous with that of the jjeritoneum (Fig. in B). Along its mid-dorsal and midventral line, the liver remains connected to the ventral mesentery. That portion of the mesentery between the liver, stomach, and duodenum is the lesser omentum (Fig. 113). This in the adult is differentiated into the hepato-gastric and he pato-duodcnal ligaments. The mesogastrial attachment of the liver to the ventral body wall extends caudally from diaphragm to umbilicus and constitutes the falciform ligament.
| |
|
| |
|
| |
| In its early development, the liver abuts upon the primitive diaphragm, and, in 4 to 5 mm. embryos, is attached to it along its cephalic and ventral surfaces. Soon, dorsal prolongations of the lateral liver lobes, the coronary appendages, come into relation with the diaphragm dorsally and laterally (Fig. 124). The attachment of the liver to the septum transversum now has the form of a crescent, the dorsal horns of which are the coronary appendages (Fig. 113). This union becomes the coronary ligament of the adult liver. The dorso-ventral extent of the coronary ligament is reduced during development, and, at five months, its lateral extensions upon the diaphragm give rise to the triangular ligaments of each side.
| |
|
| |
|
| |
| The right lobe of the liver, comes into relation along its dorsal surface with the caval mesentery of 9 mm. embryos (Figs. 112 and 113). This attachment extends the coronary ligament caudally on the right side and makes possible the connection between the veins of the liver and mesonephros which contributes to the formation of the inferior vena cava. The portion of the liver included between the caval mesentery and the lesser omentum is the caudate lobe. The umbilical vein (later the ligamentum teres) courses in a deep groove along the ventral surface of the liver, and, with the portal vein and gall bladder, bounds the quadrate lobe.
| |
|
| |
| In general, the several displacements and secondary fusions of the primitive mesentery cause its line of peritoneal attachment to depart throughout most of its extent from the original midsagittal position.
| |
|
| |
|
| |
|
| |
| The special mesenterial supports of the urogenital organs will be described in the next chapter.
| |
|
| |
|
| |
|
| |
| Anomalies. - The mesenteries may show malformations, due to the persistence of the simpler embryonic conditions, usually correlated with the defective development of the intestinal canal. In about 30 per cent of cases the ascending and descending mesocolon are more or less free, having failed to fuse with the dorsal peritoneum. The primary sheets of the greater omentum may also fail to unite, so that the inferior recess extends to the caudal end of the greater omentum, as is normal in many mammals.
| |
|
| |
|
| |
|
| |
| II. THE COELOM .
| |
|
| |
|
| |
| Pericardial cavity Surface of fore-gut .
| |
|
| |
|
| |
| The Primitive Coelom. - The first occurrence of a coelom is in the extra-embryonic mesoderm (Fig. 40 C). vShortly after, numerous clefts appear in the embryonic mesoderm of each side and split it into somatic and splanchnic layers (cf. Fig. 325).
| |
|
| |
| These clefts coalesce in the cardiac region and form two elongated pericardial cavities, lateral to the paired heart tubes (Fig 165 A, B). Similarly, right and left pleuro-peritoneal cavities are formed between the mesoderm layers caudal to the heart. The paired pericardial cavities extend toward the midplane cranial to the heart and presently communicate with each other (Figs. 117 and 165 C).
| |
|
| |
|
| |
| Laterally, they are not continuous with the extra-embryonic coelom, for in this region the head of the embryo has already separated from the underlying blastoderm .
| |
|
| |
| The pericardial cavities, nevertheless, are prolonged caudally until they open into the pleuro-peritoneal cavities where these in turn communicate laterally with the extra-embryonic coelom. In an embryo of 2 mm., the coelom thus consists of a U-shaped pericardial cavity, the right and left limbs of which are continued caudally into the paired pleuro-peritoneal cavities; these extend out into the extraembryonic coelom (Fig. 117).
| |
|
| |
|
| |
| The primitive coelom lies in the horizontal plane (Fig. 117). Coincident with the caudal regression of the primitive diaphragm, the pericardial cavity is bent ventrad and enlarged (Fig. 118). The ventral mesocardium, attaching the heart to the ventral body wall, disappears, and the right and left limbs of the U-shaped cavity become confluent, ventral to the heart. The result is a single, large pericardial chamber, the long axis of which now lies in a dorso-ventral plane, nearly at right angles to the plane of the pleuro-peritoneal cavities, and connected with them dorsally by the right and left pleuro-pericardial canals. On account of the more rapid growth of the embryo, there is an apparent constriction at the yolk stalk, and, with the development of the umbilical cord, the peritoneal cavity is separated definitely from the extra-embryonic coelom (Fig. 45). Dorsally, the pleural and peritoneal cavities are permanently partitioned lengthwise by the dorsal mesentery.
| |
|
| |
|
| |
| Pleuro-peritoneal canal Entoderm of gut Peritoneal cavity Extra-embryonic coelom Wall of yolk sac
| |
|
| |
| Fig. 117. - Diagrammatic model of the fore-gut and coelom in an early human embryo, viewed from above and behind (Robinson-Prentiss)
| |
|
| |
| the cavities of the mesodermal segments are regarded as portions of the coelom, but in man they disappear early. The development of the vaginal sacs, which grow out from the inguinal region of the peritoneal cavity into the scrotum, will be described in Chapter VuI the division of the primitive coelom into separate cavities is accomplished by the development of three types of membrane that join on each side in a Y-shaped fashion (Figs. 122 and 123); (i) the unpaired septum transversum, which separates partially the pericardial and pleural cavities from the peritoneal cavity; (2) the paired pleuro-pericardial membranes, which complete the division between pericardial and pleural cavities; (3) the paired pleuro- peritoneal membranes, which complete the partition between each pleural cavity and the peritoneal cavity.
| |
|
| |
|
| |
|
| |
| Fig. 118. - Reconstruction, cut at the left of the median sagittal plane of a 3 mm. human emhryo, showing the body cavities and septum transversum (Kollmann).
| |
|
| |
|
| |
|
| |
| The Septum Transversum. - The vitelline veins, on their way to the heart, course in the splanchnic mesoderm lateral to the fore-gut (Fig. 183). In embryos of 2 to 3 mm., these large vessels bulge into the coelom until they meet and fuse with the somatic mesoderm (Fig. 381). Thus, there is formed caudal to the heart a transverse partition, filling the space between the sinus venosus of the heart, the gut, and the ventral body wall, and separating the pericardial and peritoneal cavities from each other ventral to the gut (Fig. i66). This mesodermal partition was termed by His the septum transversum. In Fig. u8 it comprises both a cranial portion (designated - septum transversum - that is the anlage of a large part of the diaphragm, and a caudal portion, the ventral mesentery, into which the liver is growing.
| |
|
| |
|
| |
|
| |
| At first the septum transversum does not extend dorsal to the gut, but leaves on either side a pleura- peritoneal canal through which the pericardial and pleuro-peritoneal cavities communicate (Fig. 1 1 8). In embryos of 4 to 5 mm., the lungs develop in the median walls of these canals and bulge laterally into them (Fig. 120). Thus the canals become the pleural cavities, and will be so termed hereafter.
| |
|
| |
|
| |
| The septum transversum of 2 mm. embryos occupies a transverse position in the middle cervical region (Fig. 119,
| |
| 2). It then migrates caudally, the ventral portion at first moving more rapidly so that its position becomes oblique. In 5 mm. embryos (Fig. 119, 5) the septum is opposite the fifth cervical segment, at which level it receives the phrenic nerve. During a second period of migration the dorsal attachment travels faster than the ventral portion, and as a result the septum rotates to a position nearly at right angles to its plane at 7 mm. The final location, opposite the first lumbar segments, is attained in an embryo of two months.
| |
|
| |
|
| |
| The Pleuro-pericardial and Pleuro-peritoneal Membranes. - The common cardinal veins (ducts of Cuvier), on their way to the heart, curve around the pleural cavities laterally in the somatic body wall (Fig. 118). In embryos of 7 mm., each vein, with the overlying mesoderm, forms a ridge that projects from the body wall mesially into the adjacent pleural canal. This ridge, the pulmonary ridge (of Mall), is the anlage of both the pleuro-pericardial and pleuro-peritoneal membrane (Figs. 118 and 120). Later, it broadens and thickens cranio-caudally (Fig. 121), forming a triangular structure whose apex is continuous with the septum transversum (Fig. 122). Its cranial side constitutes the pleuro-pericardial membrane, and, in g to 10 mm. embryos, reduces the opening between the pleural and pericardial cavities to a mere slit. Its caudal side becomes the pleuro-peritoneal membrane, which later completes the partition dorsally between the pleural and peritoneal cavities (Fig. 123).
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 119. - Diagram showing the migration of the septum transversum (MallPrentiss). Numerals indicate the length of the embryo at each position of the septum. The letters and numbers at the right represent the occipital, cervical, thoracic and lumbar segments.
| |
|
| |
|
| |
|
| |
| Fig. 120. - Reconstruction of a 7.5 mm. human embryo, cut across and viewed caudally to show the body cavities and pulmonary ridge (Kollman).
| |
|
| |
|
| |
|
| |
| Fig. 121. - Reconstruction of a 7 mm. human embryo, showing from the left side the pleuro-pericardial membrane, the pleuro-peritoneal membrane and the septum transversum (Mall in Prentiss). X 20. The phrenic nerve courses in the pleuro-pericardial membrane. An arrow jiasses from pericardial to peritoneal cavity through the pleuro-pericardial canal.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 122. - Reconstruction of an 1 1 mm. human embryo, to show the structures of Fig. 121 at a later stage (Mall in Prentiss). X 14.
| |
|
| |
| The two sets of membranes at first lie nearly in the sagittal plane, and a portion of each lung is caudal to the corresponding pleuro-peritoneal membrane (Fig. 121). Between the stages of 7 and u mm. the dorsal attachment of the septum transversum shifts caudad more rapidly than its ventral portion, and carries the pleuro-peritoneal membrane with it until the latter lies caudad to the lung (Figs, ug, 121 and 122). Each lung then occupies a spherical triangle between pleuro-pericardial and pleuro-peritoneal membranes (Fig 122). During this rotation the dorsal end of the pleuro-pericardial membrane lags behind, anchored by the phrenic nerve which courses through it, and so takes up a position in a coronal plane nearly at right angles to the septum transversum (Figs. 122 and 123). In 1 1 mm. embryos, the pleuro-pericar dial membranes have fused completely on each side with the median walls of the pleural cavities (Fig. 123).
| |
|
| |
|
| |
| The pleuro-peritoneal membranes are continuous dorsally and caudally with the mesonephric folds; ventrally and caudally, they fuse later with the dorsal pillars of the diaphragm, or coronary appendages of the liver (Figs. 113 and 124). Between the free margins of the membranes and the mesentery a temporary opening is left on each side, through which the pleural and peritoneal cavities communicate (Figs. 108, 113 and 122). Owing to the caudal migration of the septum transversum and the growth of the lungs and liver, the pleuro-peritoneal membrane, at first lying in a nearly sagittal plane (Figs. 120 and 12 1), is shifted to a horizontal position (Fig. 122), and gradually its free margin unites with the dorsal pillars of the diaphragm and with the dorsal mesentery. The opening between the pleural and peritoneal cavities is thus narrowed and finally closed in embryos of 19 to 20 mm.
| |
|
| |
|
| |
|
| |
| Fig. 123. - Transverse section through a 10 mm. human embryo, showing the pleuro-pericardial and pleuro-peritoneal membranes (Prentiss). X 33.
| |
|
| |
|
| |
|
| |
| The Pericardium and Diaphragm. - The lungs grow and expand, not only cranially and caudally but also laterally and ventrally (Fig. 125). Room is made for them by the obliteration of the very loose, spongy mesenchyme of the adjacent body wall (Fig. 124). As the lungs burrow laterally and ventrally into the body wall around the pericardial cavity, the pleuro-pericardial membranes enlarge at the expense of this tissue and more and more the heart comes to lie in a mesial position .
| |
|
| |
|
| |
|
| |
| Fig. 124. - Transverse section through a 10 mm. human embryo, showing the fusion of the pleuro-peritoneal membranes with the coronary appendages (Prentiss). X 16.
| |
|
| |
| Between the lungs, but separated from them by the pericardium (Fig. 125 B). The pleural cavities thus increase rapidly in size at the same time the liver grows enormously, and on either side a portion of the body wall is taken up into the septum transversum and pleuro-peritoneal membranes. The diaphragm, according to Broman, is thus derived from four sources (Fig. 126): (i) its ventral pericardial portion from the septum transversum; its lateral portions from (2) the pleuro-peritoneal membranes, plus (3) derivatives from the body wall; (4) lastly, a median dorsal portion is formed from the dorsal mesentery. In addition to these, the striated muscle of the diaphragm takes its origin from a pair of premuscle masses, which, in 9 mm. embryos, lie one on each side opposite the fifth cervical segment (Bardeen). This is the level at which the phrenic nerve enters the septum transversum (Fig. 124). The exact origin of these muscle anlages is in doubt, but they probably represent portions of the cervical myotomes of this region. The muscle masses migrate caudally with the septum transversum and develop chiefly in the dorsal portion of the diaphragm.
| |
|
| |
|
| |
|
| |
| Fig, 125. - Diagrams showing the development of the lungs and the formation of the pericardium (Robinson-Prentiss). A, Coronal section; B, transverse section.
| |
|
| |
|
| |
| Fig. 126. - Diagram showing the contributions to the diaphragm (Broman). i. Septum transversum; 2, 3, dorsal mesentery; 4, 4, pleuro-peritoneal membranes; 5, 5, bodj' wall; A, aorta; Oe, esophagus; VC, inferior vena cava.
| |
|
| |
|
| |
| Anomalies. - The persistence of a dorsal opening in the diaphragm, more commonly on the left side, finds its explanation in the imperfect development of the pleuro-peritoneal membrane. Such a defect may lead to diaphragmatic hernia, the abdominal viscera projecting to a greater or less extent into the pleural cavity. Similarly, faulty development of the left pleuro-pericardial membrane sometimes causes the heart and left lung to occupy a common cavity. An intact diaphragm, locally deficient in muscle, may herniate,
| |
|
| |
|
| |
| ++++++++++++++++++++++++++++++++++++++++++++++++.
| |
|
| |
|
| |
| CHAPTER VIII THE UROGENITAL SYSTEM
| |
|
| |
| The urinary and reproductive systems are associated intimately in development. Both arise from the mesoderm of the intermediate cell mass fnephrotome), which unites the primitive segments with the lateral layers of somatic and splanchnic mesoderm (Figs. 35 S and 128). In the course of development these anlages bulge into the coelom as paired longitudinal ridges, termed the urogenital folds (Figs. 131, 143 and 144).
| |
|
| |
|
| |
| Vertebrates possess excretory organs of three district types. The pronephros is the functional kidney of amphioxus and certain lampreys, but appears in immature fishes and amphibians only to be replaced by the mesonephros. The embryos of amniotes (reptiles, birds, and mammals) develop first a pronephros, and then a mesonephros, whereas the permanent kidney is a new organ, the metanephros. Whether these glands represent modifications of an originally continuous organ, or whether they are three distinct structures, is undecided, but however this may be the promeso-, and metanephroi of amniotes develop successively, one caudad of the other, in the order named.
| |
|
| |
|
| |
|
| |
| I. THE URINARY ORGANS
| |
|
| |
| The Pronephros. - When functional, the pronephros consists of paired, segmentally arranged pronephric tubules; one end of each tubule opens into the coelom, the other into a longitudinal collecting duct which drains into the cloaca (Fig. 128 A). Near the nephrostome (the funnel-like opening into the coelom), knots of arteries project into the coelom, forming glomeruli. These filter wastes from the blood into the coelomic fluid which is then taken up by the tubules and carried by ciliary movement into the excretory ducts.
| |
|
| |
|
| |
| The human pronephros is vestigial. It consists of about seven pairs of rudimentary pronephric tubules, formed as dorsal sprouts from the nephrotomes in each segment, from the seventh to the fourteenth, and perhaps from more cranial segments as well (Fig. 35 B). Y"et the earliest tubules begin to degenerate before the last appear. The nodules hollow out and open into the coelom (Fig. 128 B). Dorsally and laterally, the tubules of each side bend backward and unite to form a longitudinal pronephric collecting duct (Fig. 12S B, A). Caudal to the fourteenth segment no pronephric tubules are developed, but the free end of the collecting duct, by a process of terminal growth, extends caudad beneath the ectoderm (and lateral to the nephrogenic cord) until it reaches the lateral wall of the cloaca and perforates it (Fig. 87). Thus are formed the paired primary excretory {prone phric) ducts. The pronephric tubules begin to appear in embryos of 1.7 mm., with nine or ten primitive segments; at 2.5 mm. (23 segments), all the tubules have developed and the primary excretory duct is nearly complete. In 4.3 mm. embryos, the duct has reached the wall of the cloaca and soon after fuses with it (Fig. 87). The pronephric tubules promptly degenerate, but the primary excretory ducts persist and become the ducts of the mesonephroi.
| |
|
| |
|
| |
|
| |
| Fig. 127. - Transverse section of a 2.4 mm. human embryo, showing the intermediate cell mass or nephrotome (Kollmann).
| |
|
| |
|
| |
|
| |
| Fig. 128. - Diagrams illustrating the development of the proncphric duct and proncphric tubules (Felix-Prentiss). A, represents a later stage than B.
| |
|
| |
|
| |
| The Mesonephros. - The mesonephros, like the pronephros, consists essentially of a series of tubules, each of which at one end is related to a knot of blood vessels and at the other end opens into the primary excretory duct (Fig. 87). But the mesonephric tubule differs in two important respects: ( i) the glomerulus indents one end of the tubule, and excreta from the blood pass directly into its lumen; (2) the nephrostomes are transitory and never open into the mesonephric chamber. The mesonephric tubules arise just caudal to the pronephros and from the same general source, that is, the nephrotomes. Only a few of the more cranial tubules, however, are formed from distinct intermediate cell masses, for, caudal to the tenth pair of segments, this mesoderm fuses into unsegmented, paired nephrogenic cords which may extend as far as the twenty-eighth segment (Fig. 132). The primary excretory ducts lie lateral to the nephrogenic cords.
| |
|
| |
|
| |
| When the developing mesonephric tubules begin to expand, there is not room for them in the dorsal body wall, which as a result bulges ventrally into the eoelom. Thus is produced on each side of the dorsal mesentery a longitudinal urogenital fold, which may extend from the sixth cervical to the third lumbar segment (Fig. 143). Later, this ridge is divided into a lateral mesonephric fold and into a median genital fold, the anlage of the genital gland (Figs. 13 1 and 144).
| |
|
| |
|
| |
| Differentiation of the Tidmlcs. - The nephrogenic cord in 2.5 mm. embryos first divides into spherical masses of cells, the anlages of the mesonephric tubules. As many as four of these are formed in a single segment. Appearing first in the thirteenth to fifteenth segments, the anlages of the tubules differentiate both cranially and caudally. In 5.3 mm. embryos the cephalic limit is reached in the sixth cervical segment, and thereafter degeneration begins at this end (Fig. 130). Hence, the more cranial tubules overlap those of the pronephros. In 7 mm. embryos, the caudal limit is reached in the third lumbar segment.
| |
|
| |
|
| |
| Differentiation of the tubule anlages progresses in a cephalo-caudad direction (Fig. 130). First, veSicles with lumina are formed (4.3 mm.) from the spherical masses (Fig. i2g AB). Next, the vesicles elongate laterally, unite with the primary excretory ducts, and become S-shaped (Fig. 129 B, C). The free, vesicular end of the tubule enlarges, becomes thin walled, and into this wall grows a knot of arteries to form the glomerulus (5 to 7 mm. ; Fig. 129 D). The wall of the vesicle about the glomerulus is Bowman 's capsule and the two constitute a renal corpuscle of the mesonephros (Fig. 131). The tubule, which was at first solid, is now lined with a low columnar epithelium. In the human embryo the tubules do not branch or coil as in the pig, consequently the mesonephros is relatively smaller. At 10 mm., about 35 tubules are present in each mesonephros and the glomeruli are conspicuous (Fig. 130). Each tubule shows a distal secretory portion and a proximal collecting part which connects with the duct (Fig. 13 1). The glomeruli form a single median column in the gland; the tubules are dorsal and the duct is lateral in position. Ventro-lateral branches from the aorta supply the glomeruli (Fig. 212), while the posterior cardinal veins (Fig. 145), dorsal in position, break up into a network of sinusoids about the tubules.
| |
|
| |
|
| |
|
| |
| Fig. 129. - Diagrams showing the differ- Fig. 130. - The anlages of the urinary or entiation of a mesonephric tubule (Felix- gans in a 10 mm. human embryo, as seen Prentiss). L. lateral; M. median. from the left side (adapted by Prentiss).
| |
|
| |
|
| |
|
| |
|
| |
| The pronephric duct, now termed the mesonephric, or Wolffian duct, is solid in 4.3 mm. embryos. A lumen is formed at 7 mm., wider opposite the openings of the tubules. The duct is important, as the ureteric anlage of the permanent kidney grows out from its caudal end, while the tube itself is transformed into the chief genital duct of the male.
| |
|
| |
| That the human mesonephros is a functional excretory organ is plausible (Bremer, 1916), but not proved. Degeneration proceeds rapidly in embryos between 10 and 20 mm. long, beginning cranially (Fig. 148). New tubules are formed at the same time caudally (Fig. 130). In all 83 pairs of tubules arise, of which only 26 pairs persist at 21 mm., and these are usually broken at the angle between the collecting and secretory regions. How the genital system utilizes them for new purposes will be traced in a later section (p. 156).
| |
|
| |
|
| |
|
| |
| The Metanephros. - The essential parts of the permanent kidney are the renal corpuscles (glomeruli with Bow^man - s capsules), secretory tubules, and collecting tubules. Like the mesonephros, the metanephros is of double origin. The ureter, pelvis, calyces, and collecting tubules are outgrowths of the mesonephric duct. The secretory tubules and the capsules of the renal corpuscles are differentiated from the isolated, caudal end of the nephrogenic cord and thus have an origin similar to that of the mesonephric tubules.
| |
|
| |
|
| |
| In embryos of about 5 mm. the mesonephric duct makes a sharp bend just before it joins the cloaca, and it is at this angle that the ureteric evagination appears, dorsal and somewhat median in position (Fig. 139 B, C). The bud grows at first dorsally, then cephalad. Its distal end expands and forms the primitive pelvis; its proximal elongated portion is the ureter. The pelvic anlage grows into the lower end of the nephrogenic cord (Fig. 132), which, during the third month, becomes separated from the cranial end. The nephrogenic tissue forms a cap about the primitive pelvis, and, as the pelvis grows cranially, is carried along with it.
| |
|
| |
|
| |
|
| |
| Fig. 132. - Reconstruction of the metancphric anlages in a human embryo of about 9 mm (after Schreiner).
| |
|
| |
|
| |
| In embryos of g to 13 mm. the pelvis, having advanced cephalad through three segments, attains a position in the retroperitoneal tissue dorsal to the mesonephros and opposite the second lumbar segment. Thereafter, the kidney enlarges both cranially and caudally without shifting its mean position (Fig. 154).
| |
|
| |
|
| |
|
| |
| Fig. 133. - Diagrams showing the development of the primitive pelvis, calyces and collecting tubules of the metanephros (adapted by Prentiss).
| |
|
| |
| .
| |
|
| |
|
| |
|
| |
| .
| |
|
| |
| Differentiation of the Ureteric Anlage. - Primary collecting tubules grow out from the primitive pelvis in 10 mm. embryos. Of the first two, one is cranial, the other caudal in position, and, between these, two others usually appear (Fig. 133 B, C). From an ampullary enlargement, at the end of each primary tubule sprout off two, three, or four secondary tubules. These in turn give rise to tertiary tubules (Fig. 133 D) and repeated until the fifth month of fetal life, when it is estimated that twelve generations of tubules have been developed.
| |
|
| |
| The pelvis and the primary and secondary tubules enlarge greatly during development. The two primary expansions become the major calyces, and the secondary tubules opening into them form the minor calyces (Fig. 134). The tubules of the third and fourth orders are taken up into the walls of the enlarged secondary tubules so that the tubules of the fifth order, 20 to 30 in number, open into the minor calyces as papillary ducts. The remaining orders of tubules constitute the collecting tubules which form the greater part of the medulla of the adult kidney.
| |
|
| |
|
| |
| When the four to six primary tubules develop, the nephrogenic cap about the primitive pelvis is subdivided and its four to six parts cover the end of each primary tubule.
| |
|
| |
|
| |
| As new orders of tubules arise, each mass of nephrogenic tissue increases in amount and is further subdivided until finally it forms a peripheral layer about the tips of the branches tributary to a primary tubule. The converging branches of such a tubular - tree - constitute a primary renal unit, or pyramid, with its base at the periphery of the kidney and its apex projecting pnto the pelvis. The apices of the pyramids are termed renal papilla:, and through them the papillary ducts open. The nephrogenic tissue forms the cortex of the kidney, and each subdivision of it, covering the tubules of a pyramid peripherally, is marked off on the surface of the organ by grooves or depressions. The human fetal kidney is thus distinctly lobed, the lobations persisting for several years after birth; this condition is permanent in reptiles, birds, and some mammals (whale; bear; ox). The primary p^ - ramids are subdivided into several secondary and tertiary pyramids. Between the pyramids, the cortex of nephrogenic tissue dips down to the pelvis, forming the renal columns (of Bertin). The collecting tubules, on the other hand, extend out into the cortex as the cortical rays, or pars radiata of the cortex. In these rays, and in the medulla of the kidney, the collecting tubules run parallel and converge to the papillae.
| |
|
| |
|
| |
|
| |
| Fig. 134 - Reconstruction of the ureter, pelvis, calyces and their branches from a 16 mm. human embryo (Huber). X 50.
| |
|
| |
|
| |
|
| |
| Differentiation of the Nephrogenic Tissue. - In stages from 13 to 19 mm., the nephrogenic tissue about the ends of the collecting tubules condenses into spherical masses that lie in the angles between the buds of new collecting tubules and their parent stems (Fig. 135). One such metanephric sphere is formed for each new tubule. The spheres are converted into vesicles with eccentrically jilaced lumina. The vesicle elongates, its thicker outer wall forming an S-shaped tubule which unites with a collecting tubule, its thin inner wall becoming the capsule (Bowman - s) of a renal corpuscle.
| |
|
| |
| The uriniferous tubules of the adult kidney have a definite and peculiar structure and arrangement (Fig. 136 4 l). Beginning with a renal corpuscle, each tubule forms a proximal convoluted portion, a \J-shaped loop (of Henle) with descending and ascending limbs, a connecting piece, which lies close to the renal corpuscle, and a distal convoluted portion continuous with the collecting tubule. These parts are derived from the S-shaped anlage, which is composed of a lower, middle, and upper limb. The middle limb, somewhat U-shaped, bulges into the concavity of Bowman - s capsule (Fig. 136 B). By differentiation the lower portion of the lower limb is converted into Bowman - s capsule, and ingrowing arteries form the glomerulus (Fig. 1^6 B, C). The upper part of the same limb by enlargement, elongation, and coiling becomes the proximal convoluted tubule. The neighboring portion of the middle limb forms the primitive loop (of Stoerck) ; the base of the middle limb gives rise to the connecting piece, and the rest of it, rvith the upper limb of the S, comprises the distal convoluted tubule. The primitive loop of Stoerck includes both the descending and ascending limbs of Henle - s loop and a portion of the proximal convoluted tubule as well. Henle - s loop is differentiated during the fourth fetal month and extends from the pars radiata of the cortex into the medulla (Fig. 137). The concavity of Bowman - s capsule, into which grow the arterial loops of the glomerulus, is at first shallow. Eventually, the walls of the capsule grow about and enclose the vascular knot, except at the point where the arterioles enter and emerge (Fig. 135,4 and 5). Renal corpuscles are first fully formed at the end of the second month. The newer corpuscles differentiate peripherally from persisting nephrogenic tissue, and this may continue for some time after birth; hence, in the adult, the oldest corpuscles are those next the medulla. Reconstructions of the various stages in the development of the uriniferous tubules are shown in Fig. 138.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 135 - (Huber). The left half of each figure represents an earlier stage than the right half.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 136. - Diagrams showing the differentiation of the various parts of a human uriniferous tubule (adapted by Prentiss). A, From an adult; B, C, from embryos.
| |
|
| |
|
| |
| Fig. 137. - Diagram showing the relation of Bowman - s capsule and the uriniferous tubule to the collecting tubules of the metanephros (Huber), c, Collecting tubule; e, end branches of collecting tubules; r, renal corpuscles; n, neck; pc, pro.ximal convoluted tubule; dl,al, descending and ascending limbs of Henle - s loop, /; dc, distal convoluted tubule; j, junctional tubule.
| |
|
| |
|
| |
|
| |
| Fig. 138. - Reconstructed stages in the development of the human metanephric tubule at
| |
| the seventh month (Huber). X 16.
| |
|
| |
|
| |
|
| |
| Anomalies. - The kidneys may fail to ascend from their embryonic position in the pelvis. Absence of one kidney is not infrequent. The kidneys sometimes fuse, either completely into a disc-shaped mass, or partially by cortical union (V/cm - -i/zoe ;
| |
| in such cases the ducts usually are bilateral. Double or cleft ureters and pelves occur.
| |
|
| |
|
| |
|
| |
| Renal Cysts (cystic kidney) result from the primary non-union of uriniferous and collecting tubules, or by the cystic degeneration of secondarily detached tubules (Kampmeier, 1923).
| |
|
| |
|
| |
| Differentiation of the Cloaca. - In embryos of i . 4 mm., the cloaca, a caudal expansion of the primitive entodermal canal, is in contact ventrally with the ectoderm, and the area of union constitutes the cloacal membrane (Fig. 139 A). This membrane at first extends from the tail bud to the body stalk (Figs. 71 and 95), and occupies a region corresponding to the hind end of the primitive streak (Figs. 44 and 58). Later, .
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 139. - Reconstructions of the early human cloaca (Pohlman-Prentiss). X about 50. .4 3.5 mm.; B, 4 mm.; C, 5 mm.; D, 7 mm.
| |
|
| |
|
| |
| Its expanse is diminished in both directions (Figs. 96, 141 and 142). Ventro-cephalad, the cloaca gives off the allantoic stalk, receives the mesonephric ducts laterally, and is prolonged caudally as the tail-gut (Fig. 139 B).
| |
|
| |
| The saddle-like partition between the intestine and allantois grows caudally, dividing the cloaca into a dorsal rectum and ventral, primitive urogenital sinus (Figs. 139 to 142). The division is complete in embryos of u to 15 mm., and at the same time the partition, fusing with the cloacal membrane, divides it into the anal membrane of the gut and the urogenital membrane (Fig. 142). The intermediate tissue represents the body of the primitive perineum. At u mm., according to Felix, the primitive urogenital sinus by elongation and constriction is differentiated into two regions: (1) a dorsal vesico-urethral anlage which receives the allantois and mesonephric duct, and is connected by the constriction with (2) the phallic portion (Figs. 140 and 141). The latter extends into the phallus of both sexes and forms a greater part of the male urethra (Fig. 142), as described on p. 164.
| |
|
| |
|
| |
|
| |
| Fig. 140. - Reconstruction from a 12 mm. human embryo, showing the partial division of the cloaca into rectum and urogenital sinus (Pohlman-Prentiss). X 65.
| |
|
| |
|
| |
|
| |
| Fig. 141. - Reconstruction of the caudal portion of an 11.5 mm. human embryo, showing the differentiating rectum, bladder and urethra (Keibel-Prentiss). X 25.
| |
|
| |
|
| |
| The vesico-urethral anlage enlarges and transforms into the bladder and into either the entire female urethra or the prostatic and membranous male urethra. In 7 mm. embryos the proximal ends of the mesonephric ducts are funnel shaped, and, at 10 mm., eoincident with the enlargement of the bladder, these ends are taken up into its wall until the ureters and mesonephric ducts acquire separate openings (Figs. 141 and 142). The ureters, having previously shifted their openings into the mesonephric ducts from a dorsal to lateral position, now open into the vesico-urethral anlage lateral to the mesonephric ducts. The lateral walls of the bladder anlage grow more rapidly than its dorso-median urethral wall; hence the ureters are carried cranially and laterally upon the wall of the bladder while the mesonephric ducts open close together on a hilloek, Muller's tubercle, into the dorsal wall of the urethra (Fig. 142). Thus an area, roughly bounded by the openings of the ureters and the mesonephric (ejaculatory) ducts, is mesodermal. Besides the trigone of the bladder the area includes a proximal segment of the urethra (Fig. 160 C). In the male, this stretch corresponds to the upper portion of the prostatic urethra; in the female, it includes much of the shorter definitive urethra.
| |
|
| |
|
| |
|
| |
| Fig. 142. - Reconstructions of the caudal end of a two-months - human embryo, showing the complete separation of the rectum and urogenital sinus and the relations of the urogenital ducts (Keibel-Prentiss). X 15.
| |
|
| |
|
| |
| The narrowed apex of the bladder, continuous with the allantoic stalk at the umbilicus, is known as the urachus (Fig. 157). It persists as the solid, fibrous middle umbilical ligament (Fig. 199). Contrary to earlier views, the allantois contributes nothing to the bladder or urachus (Felix, 1912).
| |
|
| |
|
| |
| The transitional epithelium of the bladder appears at 10 weeks. The circular and outer longitudinal layers of muscle develop at the end of the second month. The inner longitudinal muscle layer is found at 10 weeks and the sphincter vesicse in fetuses of three months.
| |
|
| |
|
| |
| Anomalies. - A conspicuous malformation is that of a persistent cloaca, due to the failure of the rectum and urogenital sinus to separate. The bladder sometimes opens widely onto the ventral body wall and is everted through the fissure; a urogenital aperture corresponding to the upper extent of the primitive cloacal membrane would cause this condition (Fig. 139 C, D). At times, the urachus remains a patent tube, opening at the umbilicus as a urinary fistula. Portions of its epithelium which fail to degenerate may form cysts.
| |
|
| |
|
| |
| Fig. 143. - Reconstruction of the male urethra and associated parts, from a fetus of four months (after Broman). X 13.
| |
|
| |
|
| |
| Accessory Genital Glands. - The prostate gland develops in both sexes as outgrowths of the urethra, both above and below the entrance of the male ducts (Fig. 143). Hence, the upper portion, at least, must be mesodermal in origin. The tubules arise at ten weeks in five distinct groups and total an average number of 63. The surrounding mesenchyme differentiates both connective tissue and smooth muscle fibers, into which the anlages of the prostate grow. In the female, the homologue is rudimentary; these isolated para-urethral ducts (of Skene) number at most three.
| |
|
| |
|
| |
|
| |
| The bulbo-urethral glands (of Cowper) arise in male embryos of nine weeks as solid, paired epithelial buds from the entoderm of the urethra (Fig. 143). The buds penetrate through the mesenchyme of the corpus cavernosum urethree, about which they enlarge. The glands branch, and, at four months, the epithelium becomes glandular. The vestibular glands (of Bartholin) are the homologues in the female of the bulbo-urethral glands. They appear at the same age as the male glands, grow until after puberty, and degenerate after the climacterium.
| |
|
| |
|
| |
| u. THE GENITAL ORGANS .
| |
|
| |
| A. Indifferent Stage .
| |
|
| |
|
| |
| The Gonads. - In origin and early development, the ovary and testis are identical. The urogenital fold (p. 135) is the anlage of both the mesonephros and the genital gland (Figs. 392 and 144). At first two-layered, its epithelium in embryos of 5 mm. thickens over the ventro-median surface of the fold, becomes rciany-layered, and bulges into the coelom ventrally to produce the longitudinal genital fold (Fig. 13 1). The genital fold thus lies mesial and parallel to the mesonephric fold. Large primordial germ cells are found in the entoderm of the future intestinal tract; at 3.5 mm., these migrate into the dorsal mesenteric epithelium and thence into the epithelium of the genital fold. It is undecided whether or not the definitive germ cells of the genital glands are descendants of such elements. At 10 to 12 mm., the genital anlage shows no distinctive sexual differentiation (Fig. 145); there is a superficial eph/je/ia/ layer and an inner epithelial mass of somewhat open structure owing to the great development of the suprarenal glands and metanephroi, the cranial portions of the urogenital folds, at first parallel and close together, are displaeed laterally. This produces a double bend in each fold, which, in 20 mm. embryos, shows a cranial longitudinal portion, a transverse middle portion between the bends, and a longitudinal caudal portion (Fig. 160 A). In the last-named segment, the mesonephric ducts course to the urogenital sinus, and here the right and left folds fuse, producing the genital cord (h^ig. 154). As the genital glands increase in size, they become constricted from the mesonephric fold by lateral and mesial grooves until the originally broad base of the genital fold is converted into a stalk (Figs. 149 to 151). This mesenterial attachment extends lengthwise and forms in the male the mesorchium , in the female the mesovarium.
| |
|
| |
|
| |
|
| |
| Fig. 144. - Ventral view of the urogenital folds in a human embryo of 9 mm. (Kollmann).
| |
|
| |
|
| |
|
| |
| Fig. 145. - Transverse section through the mesonephros, genital gland and suprarenal gland of the right side; from a 12 mm. human embryo (Prentissf. X 165.
| |
|
| |
|
| |
| The Primitive Genital Ducts. - The mesonephric ducts, with the degeneration of the mesonephroi, become the male genital ducts; their origin and early history have been deseribed (pp. 137 and 139).
| |
|
| |
|
| |
| Both sexes also develop a pair of female ducts. In embryos of 10 mm., these Mullerian ducts arise as thickened ventro-lateral grooves in the urogenital epithelium, near the eranial ends of the mesonephroi (Fig. 146 A).
| |
|
| |
|
| |
|
| |
| Fig. 146. - Transverse sections through the anlage of the right Mullerian duct from a 10 mm. human embryo (Prentiss). X 250. A, Cranial end of groove; B, three sections caudad.
| |
|
| |
|
| |
| Fig. 147. - X - entral dissection of an 18 mm. pig embryo, to show the growing Mullerian ducts (Prentiss). X 7.
| |
|
| |
|
| |
| Fig. 148. - Ventral dissection of a 24 mm. pig embryo, showing a later stage in growth of the Mullerian ducts (Prentiss). X 6.
| |
|
| |
| .
| |
|
| |
| Fig. 149. - Section through the left testis and mesonephros of a 20 mm. human embryo (Prentiss). X 250.
| |
|
| |
| .
| |
|
| |
| Caudally, the dorsal and ventral lips of the groove close and form a tube which separates from the epithelium and lies beneath it (Fig. 146 B). Cranially, the tube remains open as the funnel-shaped ostium abdominale of the Mullerian duct. The solid end of the tube grows caudalward, beneath the epithelium and lateral to the mesonephric, or male ducts (Figs. 147 to 149). Eventually, by way of the genital cord, the Mullerian ducts reach the median dorsal wall of the urogenital sinus and open into it (Figs. 142 and 160 A). In the lov/est vertebrates, the Mullerian duct arises by a longitudinal splitting of the mesonephric duct.
| |
|
| |
|
| |
| Embryos not longer than 12 mm. are thus characterized by the possession of indifferent genital glands and both male and female genital ducts. There is as yet no sexual differentiation.
| |
|
| |
|
| |
| B. Internal Sexual Transformations .
| |
|
| |
|
| |
| Differentiation of the Testis. - In male embryos of 13 mm., the genital glands show two characters which mark them as testes: (1) the occurrence of branched, anastomosing cords of cells, the testis cords; (2) the occurrence between epithelium and testis cords of a layer of tissue, the anlage of the tunica albuginea (Fig. 149). According to Felix (1912), the testis cords of man are developed suddenly from the loose, inner epithelial mass by a condensation of its cells; on the contrary, xMlen (1904) holds that in the pig and rabbit they grow in from the surface epithelium The cords converge towards the mesorchium, where they form the dense, epithelial anlage of the slenderer rete testis. Two or three layers of loosely arranged cells between the testis cords and the epithelium constitute the future tunica albuginea.
| |
|
| |
| .
| |
|
| |
| Fig. 150. - Section through the left testis of a fetus of fourteen weeks (Prentiss). X 44.
| |
|
| |
| .
| |
|
| |
| The testis cords soon become rounded and are marked off by connective-tissue sheaths from the intermediate cords, which are columns of undifferentiated tissue lying between them (Fig. 150). Toward the rete testis, the sheaths of the testis cords unite to form the anlage of the mediastinum testis. The testis cords are composed chiefly of indifferent cells, with a few larger germ cells. The cells gradually arrange themselves radially about the inside of the conneetive-tissue sheath as a many-layered epithelium; during the seventh month, a lumen ajDpears and extends toward the rete testis to meet lumina which have formed there. Thus the solid cords of both are converted into tubules. The distal portions of the testis tubules anastomose and form the tuhnli contorti. Their proximal portions remain straight, as the tuhuli recti. The rete testis beeomes a network of small tubules that finally unite with the efferent duetules.
| |
|
| |
|
| |
| The primordial germ cells of the testis eords form the spermatogonia of the seminiferous tubules, and from these, at puberty, are probably developed the later generations of spermatogonia, although some claim that the early germ cells all disappear, to be replaced later from the indifferent elements. The indifterent cells of the tubules become the sustcntacnlar cells (of Sertoli) of the adult testis. Certain cells of the intermediate cords, epithelial in origin, are transformed into large, pale cells, which, after puberty, are numerous in the interstitial connective tissue and hence are designated interstitial cells. The intermediate cords, as such, disappear, I:)ut the connective-tissue sheaths of the tubules unite to form septula which extend from the mediastinum testis to the fibrous tunica albuginea.
| |
|
| |
| .
| |
|
| |
| Differentiation of the Ovary. - The primitive ovary, like the testis, consists of an inner epithelial mass, bounded by the parent peritoneal epithelium. The ovarian characters ajjpear much more slowly than those of the testis. In fetuses of ten to eleven weeks, the inner ei^ithelial mass, composed of indifferent cells and primordial germ cells, becomes less dense centrally and bulges into the mesovarium (Fig. 151). There may be distinguished a dense, outer cortex beneath the epithelium, a clearer medullary zone containing large germ cells, and a dense, cellular anlage in the mesovarium, the primitive rete ovaru, which is the homologue of the rete testis. Neither epithelial cords nor tunica albuginea are developed at this stage, as in the testis.
| |
|
| |
| .Later, three important changes take place: (i) There is an ingrowth of connective tissue and blood vessels from the hilus, resulting in the formation of mediastinum and septula. (2) Most of the cells derived from the inner epithelial mass are transformed into young ova, the process extending from the rete ovaru peripherally (Fig. 151). (3) In fetuses of three to
| |
| five months, the ovary grows rapidly, owing to the formation of a new peripheral zone of cells, derived perhaps in part from the peritoneal epithelium. At the end of this period the septula line the epithelium with a fibrous sheath, the anlage of the tunica albuginea. Hereafter, such folds of the epithelium as form do not penetrate beyond the tunica albuginea and all cells derived from this source subsequently degenerate. This new peripheral zone, according to Felix, is always a single cellular mass in man, cords, or - Pfluger - s tubes, - never growing in from the epithelium. Generally, it has been believed that the primary follicles are derived from the subdivision of such cords.
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| Fig. 152. - Section through the ovarian cortex of a five-months' fetus (De Lee).
| |
|
| |
| .
| |
|
| |
| Fig. 151. -Section through the left ovary of a three-months - fetus (Prentiss). X 44.
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
|
| |
| Coincident with the origin of a new zone of cells at the periphery of the ovary, goes the degeneration of young ova in the medulla. Invading connective tissue separates these germ cells into clusters, or cords, which degenerate and leave only a stroma of fibrous tissue in the medulla. Late in fetal life, indifferent cells, by surrounding the young ova of the cortex, produce primordial follicles (Fig. 13 A) whose differentiation into vesicular follicles is described in an earlier chapter (p. 20). In opposition to this classic concept, Allen ( 1923) and others contend that the definitive ova do not represent grown primordial ones but that they are new cells proliferated periodically in the adult from the germinal (peritoneal) epithelium.
| |
|
| |
| .
| |
|
| |
| Anomalies. - Congenital absence or duplication of the testes and ovaries is very rare. Fused testes and lobed ovaries are also known,
| |
|
| |
|
| |
| Teratomata . - These peculiar tumor-like growths occur rather frequently in the ovarv, less often in the testis and other regions. The simpler types, called dermoid cysts, contain such ectodermal derivatives as skin, hair, nails, teeth, and sebaceous glands. They grade into comple.Kes consisting of organ-like masses, from all three germ layers, intermingled without order. Misshapen representatives of all tissues and organs may be present. Among other explanations of the cause, the isolation and subsequent faulty development of blastomeres has been advanced.
| |
|
| |
|
| |
| Transformation of the Mesonephric Tubules and Ducts. - In both male and female embryos of 21 mm., the mesonephros has degenerated until only twenty-six tubules at most persist, and these are separated into a cranial and a caudal group. In the cranial group of 5 to 12 tubules, the collecting jiortions have broken apart from the secretory portions. The free ends of these collecting tubules project against that part of the inner epithelial mass which gives rise to the rete tubules of either testis or ovary (Figs. 149 and 151). The cords of the rete develop in contact with the collecting tubules of the mesonephros and unite with them in fetuses of 10 weeks.
| |
|
| |
|
| |
| In the male, the lumina of rete and collecting tubules become continuous and the cranial collecting group is transformed into the ductuli efcrentcs of the epididymis. During the fifth month of pregnancy the efferent ductules coil at their proximal ends, and, when surrounded by connective tissue, they are known as lobuli epididymidis. The lower group of collecting tubules persist as the vesitigial paradidymis and duciuli abherautes (Fig. 160 G). The efferent ductules convey spermatozoa from the testis tubules into the mesonephric duct, which thus becomes the male genital duct. The cranial portion of the mesonephric duct coils and forms the ductus epididymidis; its blind cranial end persists as the appendix epididymidis. The caudal portion of the male duct remains straight, and, as the ductus deferens and ejaculatory duct, extends from the epididymis to the urethra. Near its opening into the latter it dilates to form the ampulla, from the wall of which is evaginated the sacculated seminal vesicle in fetuses of three months (Fig. 143).
| |
|
| |
|
| |
| In the female, the rete ovaru is always vestigial, yet some time before birth it becomes tubular and unites with the cranial persisting group of mesonephric collecting tubules which forms a rudimentary structure, the epodphoron (Fig. 160 B). The caudal group of mesonephric tubules constitutes the paroophoron. Usually the greater part of the mesonephric ducts atrophy in the female, the process beginning early in the third month, but portions persist as Gartner - s ducts of the epodphoron.
| |
|
| |
|
| |
| Gartner - s ducts may extend as vestigial structures from the epodphoron to the lateral walls of the vagina, passing through the broad ligament and the wall of the uterus. They open into the vagina close to the free border of the hymen. The ducts are rarely present throughout their entire length and are absent in two-thirds to three-quarters of the cases examined.
| |
|
| |
|
| |
| Transformation of the Mullerian Ducts. - The Mullerian, or female ducts, follow the course of the mesonephric ducts (Fig. 148). At first lateral in position, the Mullerian ducts cross the mesonephric ducts and enter the genital cord median to them (Fig. 160 A). In embryos of two months their caudal ends are dorsal to the urogenital sinus and extend as far as the Mullerian tubercle, a projection into the median dorsal wall of the primitive urethra formed by the earlier entrance of the mesonephric ducts (Fig. 142). This tubercle marks also the position of the future hymen. In fetuses of u weeks the Mullerian ducts break through the wall of the urethra and open into its cavity. Before this takes place, their caudal ends, which are pressed close together between the mesonephric ducts in the genital cord, fuse, and in both male and female embryos of two months give rise to the single anlage of the uterus and vagina (Figs. 142 and 153 A). The paired cranial portions of the Mullerian ducts become the uterine tubes. During development, the ostial ends of the uterine tubes undergo a true descensus from the third thoracic to the fourth lumbar vertebra.
| |
|
| |
|
| |
| In the male, these parts are rudimentary. Those portions of the Mullerian ducts corresponding to the uterine tubes and uterus begin to degenerate at the beginning of the third month. The vaginal segment remains as a pouch on the dorsal wall of the urethra, the vagina masculina, or prostatic utricle (Fig. 143). The extreme cranial end of each Mullerian duct constitutes a so-called appendix testis (Fig. 160 C).
| |
|
| |
|
| |
| The Uterus and Vagina. - Since the Mullerian ducts develop in the urogenital folds, they make two bends in their course (Fig. 153 A) corresponding to those of the folds (p. 150). Each duct consists of a cranial longitudinal ])ortion, a middle transverse portion, and a caudal longitudinal jiortion which is fused with its fellow to form the utero-vaginal anlagc. At the angle between the cranial and middle segments is attached the inguinal Jold, the future round ligament of the uterus (Figs. 154 and 155). The mesenchyme condenses about the utero-vaginal anlage and the middle transverse portion of the Mullerian ducts, forming a thick, sharply defined layer, from which is differentiated later the muscle and connective tissue of these organs (Fig. 153 A). As development proceeds, the cranial wall between the transverse limbs of the Mullerian ducts bulges outward, so that its original cranial concavity becomes convex (Fig. 153 B). The middle, transverse portions of the ducts are thus taken up into the wall of the uterus to form its JunJus, while the narrow cervix of the uterus and the vagina arise from the original utero-vaginal anlage. A distinction between uterus and vagina is not evident until the middle of the fourth month. The entrance to the vagina is originally some distance above the outlet of the urogenital sinus (Fig. 160 A). This intervening stretch of sinus hereafter elongates relatively little and so becomes the shallow vestibule into which both urethra and vagina open (Fig. 160 B),
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 153- - Diagrams illustrating the development of the uterus and vagina (Felix-Prentiss).
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
|
| |
| The lower limit of the vagina lies at the level of Muller - s tubercle, where the utero-vaginal anlage breaks through the wall of the urogenital sinus. The tubercle is compressed into a disc, lined internally by the vaginal epithelium and externally by the epithelium of the urogenital sinus, or future vestibule. These layers, with the mesenchyme between them, constitute the hymen, which thus guards the opening into the vagina (Fig. 160 A, B). A cireular aperture in the hymen is for a time closed by a knob of epithelial cells, but later, when the hymen becomes funnel-shaped, the opening is compressed laterally to form a sagittal slit.
| |
|
| |
| .
| |
|
| |
|
| |
| Muller - s tubercle persists in the male as the colliculus seminalis, from the summit of which leads off the prostatic utricle.
| |
|
| |
|
| |
| At 10 weeks, the serosa, muscularis, and mucosa are indicated. The first circular muscle fibers appear during the fifth month; the other muscle layers develop later. The epithelium of the uterine tubes and corpus remains simple; that of the cervix and vagina becomes stratified at nine weeks. The tubular glands of the corpus appear about the seventh month. The uterus shortens greatly soon after birth and does not fully recoup this loss until the eleventh year. The virginal size is attained by a short period of rapid grovdh, chiefly before puberty. The vagina is for a time without a lumen, and solid epithelium fills its fornices. The vaginal lum.en reappears in fetuses of about five months through degeneration of the central epithelial cells.
| |
|
| |
| .
| |
|
| |
|
| |
| Anomalies. - Many cases of abnormal uterus and vagina occur. The more common anomalies are: (i) Complete duplication of the uterus and vagina, due to the failure of the Mullerian ducts to fuse. (2) Uterus bicornis, due to the incomplete fusion of the ducts. Combined with these defects, the lumen of the uterus and vagina may fail, partly or completely, to develop and the vaginal canal may not open to the exterior (imperforate hymen) . (3) The body of the uterus may remain flat (uterus planifundus; Fig. 153 . 1 ) or fail to grow to normal size (uterus fetalis and infantalis). (4) Congenital absence of one or both uterine tubes, or of the uterus or vagina, rarely occurs, but may be associated with hermaphroditism of the external genitalia. The hymen is of variable shape.
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 154. - Ventral dissection of the urogenital organs in a human embryo of two months (Prentiss). The right suprarenal gland has been removed to show the metanephros.
| |
|
| |
| .
| |
|
| |
|
| |
| Ligaments of the Internal Genitalia. - Female. - The ovary is primarily suspended by a short mesentery, termed the mesovarium (Fig. 151). A further support is furnished by the terminal portion of the primitive genital fold, which unites the caudal end of the ovary first to the genital cord and then to the uterus that develops in it. This connection becomes fibrous and is known as the proper ligament of the ovary (Fig. 15 s). With the degeneration of the mesonephric system, the uterine tube lies in a fold, the mesosalpinx (Fig. 151).
| |
|
| |
|
| |
| The mutual fusion of the caudal portions of the urogenital folds, as the genital cord, forms a mesenchymal shelf bridging in the coronal plane between the two lateral body walls and containing the uterus in its center (Fig. 154). It persists as the sheet-like broad ligaments of the uterus.
| |
|
| |
| In embryos of 14 mm., a band, called the inguinal fold, joins the urogenital fold to the inguinal crest, which is merely a prominence on the adjoining al)dominal wall (Fig. 154). Within the inguinal crest is differentiated the chorda gubcrnaculi, which later becomes fibrous. The abdominal muscles develop around it and form a tube, the inguinal canal. At the outer end of the canal the external obliciue muscle leaves a foramen, through which the chorda connects with a second cord that extends to the genital swelling and is hence designated the ligamentum labiale. The chorda gubernaculi and the ligamentum labiale thus form a continuous cable from the labium majus to the uterus, which in the meantime has been developing in the fused urogenital folds; the two together constitute the round ligament of the uterus (F'ig. 155).
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 155. - Ventral dissection of the urogenital organs in a female fetus of nine weeks (Prentiss).
| |
|
| |
| .
| |
|
| |
|
| |
| .Male. - The primitive mesentery of the testis is the mesorchium (Figs. 149 and 150). It is represented in the adult as the fold between the epididymis and testis. The degenerating cephalic end of the mesonephros for a time constitutes the so-called diaphragmatic ligament of the mesonephros (Figs. 154 and 155).
| |
|
| |
| .
| |
|
| |
| The ligamentum testis, like the ligamentum ovaru, develops in the lower end of the genital fold and extends from the caudal pole of the testis to the mesonephric fold at a point adjacent to the attachment of the bridgelike inguinal fold (cf. Fig. 155). As in the female, the inguinal fold connects with the chorda guhernaculi within the inguinal crest, and this in turn is continued by way of the liganieutum scroti to the integument of the scrotum. A cord differentiates in the mesonephric fold and unites the ligamentum testis to the chorda gubernaculum. Thus there is formed a continuous ligament, the gubernaculum testis, extending from the caudal end of the testis through the inguinal canal to the scrotal integument. The gubernaculum is composed of the ligamentum testis, a mesonephric cord, the chorda gubernaculi, and the ligamentum scroti. It is the homologue of the ovarian ligament plus the round ligament of the uterus, between which the uterus intervenes (Fig. 155).
| |
|
| |
| Descent of the Testis and Ovary. - The original positions of the testis and ovary change during development. At first they are elongate structures, extending in the abdominal cavity from the diaphragm toward the pelvis (Fig. 144). Since their caudal ends continue to grow and enlarge while their cranial portions atrophy, there is a progressive, wave-like shifting of the glands caudad. Yet an actual internal descent by mass movement does not occur. When the process of growth and degeneration is complete, the caudal ends of the testes lie at the boundary line between the abdomen and pelvis, whereas the ovaries are located in the pelvis itself, a position which they retain. Owing to the rotation of the o\mry about its middle point as an axis, it takes up a transverse position. The ovary also rotates nearly 180° about the Mullerian duct as an axis, and thus comes to lie caudal to the uterine tube.
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 156. - Diagrams illustrating the descent of the testis, p.v., Processus vaginalis: .v.,
| |
| obliterated vaginal sac.
| |
|
| |
|
| |
| In addition to its early apparent migration, the testes normally leave the abdominal cavity and descend bodily into the scrotum. At the beginning of the third month, while the testes are still fairly high in the abdomen, sac-like pockets appear in each side of the ventral abdominal wall. These are the anlages of the vaginal sacs, and during the fourth, fifth, and sixth fetal months the testes lie near them without change of position. Each processus {saccns) vaginalis evaginates over the pubis, through the inguinal canal, and into the scrotum (Fig. 156). During the seventh to ninth months the testes also descend rapidly along the same path (Fig. 157). Although the factors involved are not sufficiently understood, it is clear that the gubernaculum testis plays an important part. From the caudal pole of each testis the corresponding gubernaculum extends through the inguinal canal to the scrotal wall. During the seventh month the gubernaculum not only ceases growth but actually shortens one-half. The resultant relative and actual shortening serves to draw the testes into the scrotum (Fig. 157), where they usually are found by the ninth month, or at least before birth. It must be understood that the testis and gubernaculum are covered by the peritoneum before the descent begins; consequently the testis follows the gubernaculum along the inguinal canal dorsal to the peritoneum, and, when it reaches the scrotum, is invaginated into the processus vaginalis, but does not lie within the cavity of the coelomic extension (Fig. 156). The gubernaculum of a newborn is but one-fourth the length when descensus begins; after birth it atrophies almost completely within a few months after birth the narrow canal connecting the processus vaginalis with the abdominal cavity becomes solid and its epithelium is resorbed. The vaginal sac, now isolated, represents the tunica vaginalis of the testis. Its visceral layer is closely applied to the testis and its parietal layer forms the lining of the scrotal sac. The ductus deferens, and the spermatic vessels and nerves, are carried down into the scrotum with the testis and epididymis. They are surrounded by connective tissue and constitute the spermatic cord. Owing to the path taken by the testis, the ductus deferens loops over the ureter in the abdomen (Fig. 160 C).
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 157. - Ventral dissection of a full-term fetus to demonstrate the relation of the descended testis to the processus vaginalis (partly after Kollmann and Corning). On the left the peritoneum is intact; on the right the peritoneum and its sacculation are opened and the testis is rotated 90°.
| |
|
| |
| .
| |
|
| |
|
| |
| In the female, shallow peritoneal pockets, frequently persistent as the diverticula of Nuck, correspond to the vaginal sacs of the male. Rarely, a miOre or less complete descent of the ovary into the labium majus occurs. The interposition of the uterus between the ovarian and round ligaments is responsible for the normal retention of the ovaries in the abdomen (Fig. 155).
| |
|
| |
| .
| |
|
| |
|
| |
| Anomalies. - At times, the testes remain in the abdomen, undescended, a condition known as cryptorchism and associated with sterility in man. In some mammals (whale; elephant) it is the normal condition. When the inguinal canals of man remain open, conditions are favorable for one type of inguinal hernia of the intestine. Open inguinal canals, with a periodic descent during the breeding season, occur normally in some animals (rodents; bats).
| |
|
| |
| .
| |
|
| |
|
| |
| C. The External Genitalia
| |
| Recent investigation (Spaulding, 1921) proves that the external genitalia exhibit recognizable sex differences almost from their first appearance. In embryos of 8 mm., a rounded genital tubercle develops in the midline of the ventral body wall, between the umbilical cord and tail (Fig. 144). Its caudal slope bears the shallow urethral groove which is separated from the anal pit by a transverse ridge (Figs. 158 A and 159 A ) ; this ridge comprises the primitive perineum. The margins of the groove are slightly elevated as the urethral folds. Embryos of about 15 mm. show rupture of the urethral membrane in the floor of the groove, and the genital tubercle becomes more conical. Sex can now be recognized by the length of the urethral groove which in males extends from the base of the tubercle nearly to its apex (Fig. 158 A, B), whereas in females it is shorter and terminates some distance below the apex (Fig. 159 A, B)\ this diagnostic feature prevails until the definitive modelling begins.
| |
|
| |
|
| |
| At about 16 mm. (seven weeks) the genital tubercle has elongated into a somewhat cylindrical phallus, bearing at its tip the rounded glans which is set off by a constricted neck from the shaft-like body (Figs. 158 A and 159 A). On either side of the base of the phallus, and separated from it by a groove, are lateral, rounded ridges; these are the labioscrotal swellings, possibly represented much earlier by certain indefinite elevations.
| |
|
| |
| .
| |
|
| |
| Male. - Embryos of ten weeks are at the beginning of the definitive stage. In the male, the edges of the urethral groove progressively fold together and thus transform the open urogenital sinus into the tubular urethra (Figs. 158 B, C and 142). The fused edges constitute the raphe (Fig. 158 D). The scrotal swellings shift caudad to their final position where each becomes a half of the scrotum, separated from its mate by the raphe and underlying septum scroti. In the meantime, the shaft of the penis elongates, and, by the fourteenth week, the urethra has closed as far as the glans. The urethra is then continued along an epithelial plate which represents a solid part of the original urethral anlage incompletely partitioning the glans; by splitting, the plate is first converted into a trough which promptly recloses into a tube that continues the urethra to the definitive opening at the tip of the glans. A cylindrical collar of the surface epithelium, incomplete on the anal side, grows deep into the end of the primitive glans. By the disappearance of the central cells of the epithelial downgrowth, an outer cylindrical mantle, the prepncumi, or foreskin, is formed about the spheroidal glans penis (cf. Fig. 86). Where the epithelial downgrowth is incomplete the glans and fore-skin remain connected by the frenulum prepucu. The coropora cavernosa penis arise as paired mesenchymal columns. The corpus cavernosuni urethra: results from the linking of similar, unpaired anlages, one in the glans the other in the shaft.
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| Fig. 158. - Stages in the development of the male external genitalia (redrawn after S]3aulding). . 1 , Nearly seven weeks (X 15); B, nearly eight weeks (X 12): C, ten weeks (X 8); D, twelve weeks ( X 8).
| |
|
| |
| .
| |
|
| |
| Fig. 159. - Stages in the development of the female external genitalia (redrawn after Spaulding). , 4 , Nearly seven weeks (X i8); B, nearly eight weeks (X 15); C, ten weeks (X u): twelve weeks (X 8).
| |
|
| |
| .
| |
|
| |
|
| |
| Female. - Changes in the female are less profound, yet slower (Fig. 159). The phallus lags in development and becomes the clitoris, with its homologous glans clitoridis and prepucium. The shorter urethral groove never extends onto the glans, as in the male. It remains open as the vestibule. The urethral folds which flank the original groove constitute the labia nuiwra. The primitive labio-scrbtal swellings grow caudad and fuse in front of the anus as the posterior commissure (embryos of u weeks), while the original lateral portions enlarge into the labia majora; these parts now form a horse-shoe shaped rim, open toward the umbilicus. The mans pubis, which arises later, appears to develop independently.
| |
|
| |
| Besides the sexual difference in the length of the urethral groove already mentioned, male embryos of more than 20 mm. are characterized by a phallus which stands at right angles to the body, whereas in the female it curves downward.
| |
|
| |
| ,
| |
|
| |
| HOMOLOGIES OF INTERNAL AND EXTERNAL GENITALIA .
| |
|
| |
|
| |
| Male .
| |
|
| |
| Indifferent stage .
| |
|
| |
| Female .
| |
|
| |
| TestiS
| |
| Rete testis.
| |
|
| |
| .
| |
| Gonad.
| |
|
| |
| .
| |
| Ovary
| |
| [Rete ovaru].
| |
|
| |
| .
| |
| Ligamentum testis. Gubernaculum testis.
| |
|
| |
| .
| |
| Primitive ligaments.
| |
|
| |
| .
| |
| Ligamentum ovaru proprium. Lig. ovaru -|- Lig. teres.
| |
|
| |
| .
| |
| Ductuli efferentes. Paradidymis.
| |
|
| |
| .
| |
| Mesonephric collecting tubules. Cranial group.
| |
|
| |
| .Caudal group.
| |
|
| |
| .
| |
| Epobphoron.
| |
|
| |
| .Paroophoron.
| |
|
| |
| .
| |
| Appendix epididymidis Ductus epididymidis. Ductus deferens. Seminal vesicle. Ejaculatory duct.
| |
|
| |
| .
| |
| Mesonephric duct.
| |
|
| |
| .
| |
| Gartner - s duct.
| |
|
| |
| .
| |
| (1) Appendix testis.
| |
|
| |
| .(2)
| |
| (3) Utriculus prostaticus (Vagina masculina).
| |
|
| |
| .
| |
| Mullerian duct.
| |
|
| |
| .
| |
| (0 Uterine tube.
| |
|
| |
| .( 2 ) Uterus.
| |
|
| |
| .(3) Vagina.
| |
|
| |
| .
| |
| Colliculus seminalis.
| |
|
| |
| .
| |
| Muller - s tubercle.
| |
|
| |
| .
| |
| Hymen.
| |
|
| |
| .
| |
| (1) Prostatic and membranous urethra.
| |
|
| |
| .(2) Cavernous urethra.
| |
|
| |
| .(3) Prostate gland.
| |
|
| |
| .(4) Bulbo-urethral glands.
| |
|
| |
| .
| |
| Urogenital sinus.
| |
|
| |
| .
| |
| (1) Urethra and vestibule.
| |
|
| |
| .(2)
| |
| (3) Para-urethral ducts.
| |
|
| |
| .(4) Vestibular glands.
| |
|
| |
| .
| |
| Penis.
| |
|
| |
| .Gians penis.
| |
|
| |
| .Anal surface of penis.
| |
|
| |
| .
| |
| Phallus.
| |
|
| |
| .Gians.
| |
|
| |
| .Lips of urethral groove.
| |
|
| |
| .
| |
| Clitoris.
| |
|
| |
| .Gians clitoridis. Labia minora.
| |
|
| |
| .
| |
| Scrotum.
| |
|
| |
| .
| |
| Labio-scrotal swellings.
| |
|
| |
| .
| |
| Labia majora. Posterior commissure.
| |
|
| |
|
| |
| Epididymis .
| |
|
| |
| .
| |
|
| |
| Fig. 160. - Diagrams to show the development of male and female genital organs from a common type (after Thompson).
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| Anomalies.- If the lips of the slit-like urogenital opening on the under surface of the penis fail to fuse, hypospadias results. Rarely, there is a similar defect on the upper surface - epispadias; it is usually associated with vesico-abdominal fissure.
| |
|
| |
|
| |
| True hermaphroditism consists in the presence of both testis and ovary in the same invividual. It is of rare occurrence in birds and mammals, is not uncommon in the lower dertelirates, and is the normal condition in many invertebrates (worms; molluscs). In man there are five authentic cases with combined ovotestis and four cases with separate ovary and testis. The internal genitalia are faultily bisexual. The external genitalia show mixed male and female characteristics. The secondary sexual characters (beard, mamm^, voice, etc.) are usually intermediate, tending now one way, now the other.
| |
|
| |
| .
| |
|
| |
| False hermaphroditism is characterized by the presence of the genital glands of one sex in an individual whose secondary sexual characters and external or internal genitalia resemble those of the opposite sex. In masculine hermaphroditism, an individual possesses testes, often undescended, but the external genitals (by retarded development) and secondary characters are like thoSe of the female. In feminine hermaphroditism, ovaries are present, and sometimes descended, but the other sexual characters, such as enlarged clitoris or fused laldae, simulate the male. The cause of hermaphroditism is unknown.
| |
|
| |
| .
| |
|
| |
|
| |
| +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++.
| |
|
| |
|
| |
| CHAPTER IX THE VASCULAR SYSTEM ORIGIN OF THE BLOOD VESSELS AND BLOOD CELLS .
| |
|
| |
|
| |
| Both the primitive blood cells and blood vessels arise from a tissue termed the angioblast. Its germ-layer origin has long been disputed, but the majority of recent investigations agree on the mesoderm. In certain regions, such as the body stalk of human embryos, any other interpretation is precluded. The angioblast consists initially of isolated, solid cords and masses of cells which appear first in the splanchnic mesoderm of the body stalk and yolk sac (Fig. 43). These strands soon hollow out, the peripheral cells forming the flattened endothelium of the primitive vessels, the inner cells, bathed by a clear fluid, persisting as the primitive blood cells (Fig. 326). At intervals, clusters of the latter elements adhere to the sides of the vessels and constitute the temporary blood islands (Figs. 44 and 323).
| |
|
| |
|
| |
| By the growth and union of the isolated spaces, the original anlages are converted into a vascular plexus which is present on the yolk sac, body stalk, and chorion of human embryos of i mm. In the wall of the yolk sac this network comprises the area vasculosa which later envelops the entire sac.
| |
|
| |
| The first vessels within the embryo itself appear at about 1.5 mm. Many have held that they develop as continuations of the extra-embryonic angioblast which progressively invade the embryo, but it is now agreed that the fundamental origin of intra-embryonic vessels is from discrete local anlages like those on the yolk sac. Growth by sprouting, rapidly extends the primitive vascular channels.
| |
|
| |
| .
| |
|
| |
|
| |
| HEMOPOIESIS .
| |
|
| |
| Two sharply contrasted views are held as to the mode of origin (hemopoiesis) of the various blood elements. According to the monophyletic theory, a common mother cell gives rise to all types of blood elements, both red and white. The polyphylctic theory, on the contrary, asserts that the erythroplastids are derived from one mother cell while the several kinds of white cells trace their ancestry to one or more distinct stem cells. The total evidence favors the monophyletic view.
| |
|
| |
| The earliest blood cells that originate from the angioblast are viewed by some as the parent elements from wTich all later blood cells are derived. Although it is recognized that various organs of the embryo successively serve as blood-forming centers, they are interpreted as mere depots where the primitive angioblastic cells are first deposited from the circulating blood and subsequently proliferate. On the contrary, it is urged by many that there is evidence of the continued new formation of blood cells from the mesenchyme and endothelium of the embryo and from the connective tissue of the adult. This is the more popular interpretation.
| |
|
| |
| The primitive blood cells multiply rapidly by mitosis, and differentiate successively in the following locations; (i) yolk sac; (2) mesenchyme and blood vessels of the embryo; (3) liver and spleen (assisted by lymphoid organs in lymphocyte production) ; (4) bone marrow. There is a certain degree of overlap in the activities of these foci, which, one by one, give up blood formation until the red marrow alone remains as the permanent source of all types of blood cells. Yet every lymphoid organ continues the production of lymphocytes throughout life, and in certain diseases the spleen assumes again its full hemopoietic function.
| |
|
| |
| The primitive blood cell has been given various names, such as mcsameboid, primary lymphocyte, and hemoblast. It shows a large, vesicular nucleus surrounded by a small amount of finely granular cytoplasm (Fig. 1 61, a). There is no distinct cell membrane and the cell is assumed to be ameboid. From such parent cells, according to the monophyletic view, all blood elements arise. Specialization proceeds in divergent directions; one line leads to the red corpuscles, the other to the leucocyte series.
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 161. - Blood cells from luiman embryos (Prentiss). X 1160. a. Primitive hemoblasts; h, mcgaloblasts; c, d, e, normoblasts: /, erythrocytes, (a-c, 12 mm.; d-f, 20 mm.) .
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 162. - Human blood cells (Todd). X 1000. i, Erytliroplastid; 2, normoblasts; 3, megaloblast and normoblast; 4, blood platelets, one lying on a I'ed corpuscle; 5, lymphocytes, large and small; 6, 7, large mononuclear leucocytes, polar and profile views; 8, neutrophilic leucocytes; 9, eosinophilic leucocytes; 10, basophilic leucocyte; u, neutrophilic myelocyte; 12, eosinophilic megalocyte; 13, basophilic myelocyte.
| |
|
| |
| .
| |
|
| |
| Origin of the Erythrocyte. - The red blood corpuscles, arising from the hemoblast type of cell, are first formed in the mesenchyme and blood vessels of the e/mbryo and then in the liver, spleen, and bone marrow. Soon after birth, the red marrow is the only normal source of new corpuscles. In each of these sites the manner of transformation from the parent hemoblast is identical. There are recognized three principle stages : .
| |
|
| |
|
| |
| 1. Megaloblasts. These are sometimes called erythroblasts and they have also been termed ichthyoid blood cells, because of their resemblance to the typical red blood cell of fishes. They are characterized by the presence of hemoglobin in the homogeneous cytoplasm, which is thus colored red. The nuclei are veSicular, with granular chromatin (Figs. 16 1, b and 162, 3). There is a definite cell membrane. For the first six weeks of development (12 mm.) the megaloblast is the only red blood cell found, and, like its progenitor, multiplies in the circulating blood. After the third month it practically disappears from the blood stream.
| |
|
| |
|
| |
| 2. Normoblasts, also termed sauroid blood cells because they resemble the red blood cells of adult reptiles and birds, are first transformed in the liver from the megaloblasts, and are predominant in embryos of two months. They are distinguished by their small, round nuclei with dense chromatin which stains so heavily that little or no structure can be seen (Figs. 16 1, c, d, e and 162, 2, 3). The cytoplasm is large in amount and contains more hemoglobin than before, but the normoblast may still undergo corpuscles in the cat mitosis. The final state is often listed as a (Howell), a, Successive stages in separate stage, the erythrohlast. Until the the development of a normoblast; seventh month many normoblasts occur in ^^trusion of the nucleus.
| |
|
| |
|
| |
| The circulating blood.
| |
|
| |
|
| |
| 3. Erythrocytes (red blood corpuscles; erythroplastids) are developed in mammals from normoblasts which lose their nuclei. The way in wTich the nucleus disappears is disputed. It is usually said to be extruded as a whole or in fragments (Fig. 163), but some claim it is absorbed and others state that the cytoplasm buds away from the nucleated remnant.
| |
|
| |
| The first red blood corpuscles are spherical and are formed during the second month, chiefly in the liver. During the third month, the enucleated erythrocytes predominate (Fig. 161, /). Although usually cup-like in preserved material, their normal adult shape is that of a biconcave disc about 7.5 m in diameter. Mature erythroplastids are believed to exist not more than a month.
| |
|
| |
| Origin of the Leucocytes. - The white blood cells are divided into non-granular and granular groups (Fig. 162). According to the monophyletic view, it is held that both types are derived from the hemoblastic mother cells.
| |
|
| |
| .
| |
|
| |
| I. Non-granular Leucocytes: .
| |
|
| |
| 1. Lymphocytes are ordinarily about the size of a red corpuscle but some are twice as large ( Fig. 162, 5). The small lymphocytes are supposed to be the daughter cells of large lymphocytes; the large are the small ones grown up. Their spherical nucleus, containing numerous small masses of chromatin, stains darkly and is surrounded by a narrow zone of clear, faintly baso])hilic cytoplasm. Lymphocytes constitute from 22 to 25 per cent of the leucocytes in adult Idood and are developed both in the marrow and in the lymphoid organs.
| |
|
| |
|
| |
| 2. Large monomiclcar leucocytes are two or three times the size of a red corpuscle (Fig. 162, 5, 6). They possess a clear nucleus, usually indented, and considerable faintly basophilic cytoplasm. The large mononuclears are notably phagocytic. They comprise i to 3 per cent of all leucocytes and are developed from the reticular cells of lymph glands, and, perhaps, from endothelium as well.
| |
|
| |
| ,
| |
|
| |
|
| |
| Fig. 164. - (lianl cell from the bone marrow of a kitten, showing pseudopodia extending into a blood vessel (F), and giving rise to blood platelets {hp) (Wright).
| |
|
| |
|
| |
| u. Granular or Polymorphonuclear Leucocytes: .
| |
|
| |
| The generalized blood-forming cells lodged in the red bone marrow also give rise to myelocytes, cells with round or crescentic nuclei and granular cytoplasm (Fig. 162, 11-13). By undergoing changes in the form and structure of their nuclei, and in the size and staining qualities of their cytoplasmic granules, the myelocytes transform into three types of granular leucocytes; .
| |
|
| |
| 1. Neutrophils (70 to 72 per cent of all leucocytes; Fig. 162, 8). These have a hnely granular cytoplasm which is neutral in its staining reactions, coloring by the interaction of both acid and basic stains. In development, their nuclei take up an eccentric position and become crescentic, horse-shoe shaped, and, in the older stages, lobate. As it changes in form, the nucleus undergoes pyknosis and stains intensely.
| |
|
| |
| .
| |
|
| |
| 2. Eosinophils (2 to 4 per cent of all leucocytes; Fig. 162, 9). These are characterized by coarse cytoplasmic granules that stain intensely with acid dyes. The granules apparently differentiate intracellularly although they have been interpreted as ingested fragments of red corpuscles or muscle. In development the nucleus becomes bilobed.
| |
|
| |
|
| |
| 3. Basophils or Mast Leucocytes (0.5 per cent of all leucocytes; Fig. 162, 10). Their nuclei are very irregular in form and may be broken down into several pieces which stain intensely. The cytoplasmic granules are variable in number, size, and form, and often stain so heavily with basic dyes as to obscure the nucleus. Basophiles are often regarded as degenerating granular leucocytes, but this view is not entirely convincing. They are distinct from the - mast cells - of the tissues.
| |
|
| |
|
| |
| Origin of the Blood Platelets. - In the bone marrow are giant cells known as megakaryocytes, the cytoplasm of which shows a darkly-staining, granular endoplasm and a clear, hyaline ectoplasm (Fig. 164). They originate like leucocytes but follow a distinct course of specialization. It has been demonstrated that the blood platelets represent the tips of cytoplasmic processes which have been detached from the giant cells. The central granular mass of the platelets represents a portion of the endoplasm. Genuine giant cells and blood platelets occur only in mammals,
| |
|
| |
| .
| |
|
| |
|
| |
| DEVELOPMENT OF THE HEART .
| |
|
| |
| The heart of the lower fishes and of amphibians develops directly within the ventral mesentery of the fore-gut. A tubular cavity first appears, about which the cells differentiate into endo-, myo-, and epicardium.
| |
|
| |
| .While the embryo of bony fishes, reptiles, birds, and mammals is still flattened on the surface of the yolk, paired heart anages arise which secondarily grow mesad and fuse. These anlages are first composed of aggregates of mesodermal cells which appear between the entoderm and splanchnic mesoderm; such paired cellular masses are present in the Spee 1.54 human embryo (Fig. 43). They soon form thin-walled endothelial tubes and are flanked by folds of splanchnic mesoderm that bulge laterally into the coelomic cavity (Figs. 165 A and 327). As the embryo grows away from the yolk and the fore-gut is formed, the entoderm withdraws from between the endothelial tubes, allowing first these and then the mesodermal folds to fuse (Figs. 165 5 , C; 328 and 329).
| |
|
| |
|
| |
| The heart is now a single endothelial tube, lying in the folds of the splanchnic mesoderm (Fig. ui A). When the ventral mesenterial attachment presently disappears, the heart is left suspended by a temporary dorsal mesocarduim in a common pericardial chamber (Fig. 165 C). The endothelial tube forms the endocardium; the splanchnic mesoderm later gives rise to the epicardium and myocardium. This type of heart occursin human embryos of 2 mm. ( 5 or 6 somites. Fig. 1 66) and shows three regions; (i) the atrium, which receives the blood from the primitive veins; (2) the ventricle; (3) the Imlh, from which is given off the ventral aorta.
| |
|
| |
|
| |
| As the cardiac tube soon grows faster than the pericardial cavity in which it lies, it bends to the right, thereby throwing the bulbus and ventricle into a U-shaped loop (Fig. 167). Four regions may then be distinguished ; ( i ) the sinus Eel. venosus; (2) the atrium, also thinwalled and lying cranial to the Ent. sinus; (3) the ihick-waXled ventricular limb, ventrad and caudad in position; (4) the bulbar limb, cranial to the ventricular limb and separated from it by the bulbo-ventricular cleft. Next, the bulbo-ventricular loop further shifts its position until its base is directed caudad and the loop as a whole lies ventrad (Fig. 167 B). At the same time, the sinus venosus is brought dorsal to the atrium and the two assume a position cephalad of the bulbo-ventricular loop (Fig. 168 A). These changes thus result in an essential reversal of the primitive positional relations.
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| Fig. 165. - Diagrams to illustrate the origin of Fig. 166. - The heart of a a mm.
| |
|
| |
| The mammalian heart. Eel., Ectoderm; End., endo- human embryo in ventral view (Mall), thelial tubes; Ent., entoderm; Eg., fore-gut; MscD., X 65. The open tube is the fore-gut. dorsal mesocardium; MsSpl., splanchnic mesoderm (epi- and myocardium).
| |
|
| |
| .
| |
|
| |
| The right fDortion of the sinus venosus now begins to grow more rapidly than the left, this being due to a shift in the flow of blood from the left umbilical vein through the liver to its right side. As a result, the enlarged right horn of the sinus opens into the right dorsal wall of the atrium through a longitudinally oval foramen, guarded on each side by valve-like folds (Fig. 170). The atrium is constricted dorsally by the gut, ventrad by the bulbus. It therefore can enlarge only laterally, and in so doing forms sacculations which become the future right and left atria (Fig. 168 A, B) the deep, external groove between the atria and the bulbo-ventricular part of the heart is the coronary sulcus. As the bulboventricular region increases in size, the duplication of the wall between the two limbs lags in development and finally disappears (Fig. 169), leaving the proximal portion of the bulb and the ventricular limb to form a single chamber, the primitive ventricle. In an embryo of 5 mm., the heart is thus composed of three undivided chambers: (i) the sinus venosus, opening dorsad into the right dilatation of the atrium; (2) the, bilaterally dilated atrium, communicating by the single transverse atrial canal with (3) the .
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 167. - Ventral views of the early human heart (His). A, 2.15 mm.; B, 3 mm.
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 168. - Ventral views of the early .
| |
|
| |
| .
| |
|
| |
|
| |
| THE VASCULAR SYSTEM .
| |
|
| |
|
| |
| primitive undivided ventricle. The three-chambered heart is persistent in adult fishes, but in birds and mammals a four-chambered heart is developed, in which venous blood circulates on the right side and arterial l:)lood on the left. In amphibians and reptiles, transitional types occur.
| |
|
| |
|
| |
| The important changes next to be considered, leading to the formation of the four-chambered heart, are: (i) the complete partitioning of the atrium and ventricle, each into right and left side chambers; (2) the incorporation of the sinus venosus into the wall of the right atrium; (3) the longitudinal division of the bulb and its distal continuation, the truncus arteriosus, into the aorta and pulmonary artery; (4) the development of the semilunar and atrio-ventricular valves. The heart of an embryo â– of two months has attained its general structural characteristics.
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 169. - The incorporation of the bulbus into the right ventricle through the slower development of the bulbo-vcntricular fold.
| |
|
| |
| .
| |
|
| |
| Origin of the 'Right and Left Atria. - In human embryos of 6 mm. there develops a thin, sickle-shaped membrane from the mid-dorsal wall of the atrium (Figs. 170 and 171). This is called the septum primnm (I), for it grows toward the ventricle as a partition. Simultaneously, endothelial thickenings appear in the dorsal and ventral walls of the canal which connects atrium with ventricle (Figs. 171 A, B). These endocardial cushions later fuse, and divide the. single atrial canal into right and left atrio-ventricular canals (Fig. 176). The atrium is now partly divided into right and left atria, which, however, still communicate ventrad through the interatrial Joranien. Next, the septum I thins out in one region, and a secondary opening, the foramen ovale, appears there (Figs. 170 and 171 B). The atria are then connected by two openings, the oval and interatrial foramina. Soon, the ventral and caudal edge of septum / fuses with the endocardial cushions, which have in turn united with each other (Figs. 170 and 1 7 1 C). The temporary interatrial foramen is thus obliterated, but the foramen ovale persists until after birth. In â– embryos of 9 mm., the septum secundum ( 11 ) is developed from the dorsal and cephalic wall of the atrium, just to the right of the septum primum (Fig. 170 (T). It is important, as it later fuses with the left valve of the sinus venosus, whence the two join with septum I to complete the atrial septum of the late fetal and adult heart.
| |
|
| |
| .
| |
|
| |
| Fate of the Sinus Venosus and its Valves . - The opening of the sinus venosus into the dorsal wall of the right atrium is guarded by a right and left valvular fold (Fig. 170). Along the dorsal and cephalic wall of the atrium these unite to form the so-called septum spuruim; caudally, the valves flatten out on the floor of the atrium. In embryos of six to eight weeks, the atria increase rapidly in size and the lagging right horn of the sinus venosus is taken up into the wall of the right atrium. By this absorption the superior vena cava of necessity drains directly into the cephalic wall of the atrium, the inferior vena cava into its caudal wall (Fig. 1 71 C). The transverse portion of the sinus venosus, persisting as the coronary sinus in part, likewise opens into the posterior wall of the atrium (Figs. 173 and 174).
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| Fig. 170. - Horizontal sections through the chambers of the human heart (adapted by Prentiss). X about 50. A, 6 mm.; B, 9 mm.; C, 12 mm.
| |
|
| |
| .
| |
|
| |
| Fig. 171. - Lateral dissections of the human heart, viewed from the left side (Prentiss). X about 38. A, 6 mm.; B, 9 mm.; C, 12 mm. Cor. sin., Coronary sinus; D. end. c., dorsal endocardial cushion; For. ov., foramen ovale; Int. for., interatrial foramen; I. v. c., inferior vena cava; L. air., left atrium; L. va. s. v., left valve of sinus venosus; L. vent., left ventricle; Pid. a., pulmonary artery; Pul. v., pulmonary vein; Sept. I, Sept. u, septum primum, septum secundum; Sup. V. c., superior vena cava; V. end. c., ventral endocardial cushion.
| |
|
| |
| .
| |
|
| |
| Fig. 172. - Lateral dissection of the heart of a three-months - fetus, viewed from the right
| |
| side (Prentiss). X 12 .
| |
|
| |
| .
| |
|
| |
| The right valve of the sinus venosus is very high until the end of the third month and nearly divides the atrium into two chambers (Fig. 172), but later it diminishes greatly in relative size. Its cephalic portion becomes the rudimentary crista terminalis (Fig. 173); the remainder is divided by a ridge into two parts, of which the larger cephalic division persists as the valve of the inferior vena cava (Eustachian valve), located at the right of the opening of the vein, and the smaller caudal portion becomes the valve of the coronary sinus (Thebesian valve).
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| Fig. 173. - Lateral dissection of the heart of a four-months - fetus, viewed from the right side
| |
| (Prentiss). X 7.
| |
|
| |
| .
| |
|
| |
|
| |
| The left valve of the sinus venosus unites with the septum u, and, after the second month, the two bound an oval opening whose rim is the limbus ovalis (Figs. 173 to 175).
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 174 - Lateral dissection of the heart of a three-months - fetus, viewed from the left side
| |
| (Prentiss). X 8.
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 175. - Lateral dissections of the human heart, viewed from the left side (Prentiss). A , two months; B, four months. Bic. va., Bicuspid valve; Cor. sin., coronary sinus; For. ov., foramen ovale; I.v.c., inferior vena cava; L. atr. vent, c., left atrio-ventricular canal; L. vent., left ventricle; Pul. a., jiulmonary artery; Sept. I, Sept. u, septum primum and septum secundum.
| |
|
| |
| .
| |
|
| |
|
| |
| Closure of the Foramen Ovale. - -The growth of the primitive atrial septa proceeds in such a manner that the free edge of septum u overlaps the foramen ovale in septum I (Figs. 171 C, 174 and 175). During fetal life the left atrium receives little blood from the lungs, so that the pressure is much greater in the right atrium. As a result, the septum I is pushed to the left and the blood flows from the right into the left atrium through the foramen ovale. After birth, the left atrium receives from the expanding lungs as much blood as the right atrium, hence the septum I is pressed against the limbus of the previously fused septum u and left sinus valve, and unites with it. The depression formed by the thinner walled septum I is the fossa avails.
| |
|
| |
|
| |
| The Pulmonary Veins. - In embryos of about 6 mm., a single vein drains into the caudal wall of the left atrium at the left of the septum I { Fig. I 7 1 C) . This vessel bifurcates into right and left pulmonary veins which in turn divide so that two branches extend to each lung. fVs the atrium grows, these pulmonary vessels are progressively taken up into the atrial wall. As a result, at first two, then four pulmonary veins open into the left atrium.
| |
|
| |
|
| |
| Origin of the Aorta and Pulmonary Artery. - In embryos of 5 mm. there arise in the aortic bulb (including its distal truncus arteriosus) longitudinal thickenings, four in the distal half, two in the proximal half. Of the four distal thickenings (Fig. 176), two, which may be designated a and c, are larger than the other thickenings, b and d. Thickenings a and c, which distally occupy left and right positions in the bulb, meet, fuse, and divide the bulb into a dorsally placed aorta and ventrally placed pulmonary trunk (Fig. 177). Traced proximally, they pursue a clockwise, spiral course, a shifting from left to ventral, and c from right to dorsal, both becoming continuous with the proximal swellings. Thickenings b and d are also prominent at one point proximally; Avhen the bulb in this region is divided by ingrowing connective tissue into the aorta and pulmonary artery, the aorta contains the whole of the thickenings b and half of a and c, while the pulmonary trunk contains the whole of d and half of a and c (Fig. 176). Distally, the three thickenings now present in each vessel disappear, but proximally they enlarge, hollow out on their distal surfaces and eventually form the thin-walled 5c?n?7«»ar ra/t'C5 (Figs. 172 and 176). The anlages of these valves are prominent in embryos of six to seven weeks as clump swellings projecting into the lumina of the aorta and pulmonary artery.
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 176. - Scheme showing the division of the bulbus and its thickenings into aorta and pulmonary artery with their valves.
| |
|
| |
| .
| |
|
| |
|
| |
| The two proximal bulbar swellings, continuous with a and c, fuse and extend the spiral division of the bulb toward the interventricular septum in such a way that the base of the pulmonary trunk, now ventrad and to the right, opens into the right ventricle, while the base of the aorta, now lying to the left and dorsad, opens into the left ventricle (Fig. 1 77 i?).
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 177. - Ventral views of the human heart, showing the division of the bulbus and ventricle
| |
| (Kollman). A, 5 mm.; B, 7.5 mm.
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| Origin of the Right and Left Ventricles. - Coincident with the division of the aortic bulb there appears at the base of the primitive ventricular cavity a sagittally placed elevation, the interventricular septum (Fig. 170 5 ). It grows toward the endocardial cushions, and temporarily forms an incomplete partition between the right and left ventricles which still communicate through the persisting interventricular foramen (Fig. 177 B). Corresponding to the internal attachment of the septum, there is formed externally the interventricular sulcus (Fig. 177 A); this marks the external line of separation between the large left ventricle and the smaller right ventricle. The interventricular foramen in embryos of seven weeks is bounded: (i) by the interventricular septum; (2) by the proximal bulbar septum; and (3) by the dorsal portion of the fused endocardial cushions (Fig. 177). Soon these structures are approximated and fuse, thereby forming the septum memhranaceum, which closes the interventricular foramen and completes the partition.
| |
|
| |
| .
| |
|
| |
| Loosely-arranged muscle bundles compose the uniformly spongy wall of the early ventricle (Fig. 178 D). Soon there is a condensation, especially at the periphery. As a result, the tissue next the surface becomes compact, whereas the muscular cords near the lumen retain an open arrangement for a longer period (Fig. 178 B). Some cords are attached to the anlages of the atrio-ventricular valves. These latter arise as thickenings of the endocardium and endocardial cushions, about the atrio-ventricular foramina (Figs. 170 and 171). Three such flaps are formed on the right, two on the left. The size of the primitive valvular cusps is presently increased by an undermining process whereby the muscular cords beneath become less numerous and wider spaced (Fig. 1 7 8 S) . Degeneration ensues both in the muscle tissue of the valve anlages and in that of the subjacent muscle cords. As a result, the valve cusps become fibrous and connect with similarly transformed chordce tendinece, which in turn continue into the unaffected papillary muscles. Thus there are developed the three cusps of the tricuspid valve between the right chambers of the heart (Fig. 173) and the two flaps of the bicuspid {mitral) valve between the left chambers (Fig. 174). The irregular muscle bundles that ])ersist next the ventricular cavities constitute the trabecula: carnce.
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| Fig. 178. - Diagrams of the development of the ventricular wall and atrio-ventricular valves.
| |
|
| |
| .
| |
|
| |
| Differentiation of the Heart Wall. - The primitive folds of splanchnic mesoderm form both the thick myocardium, with its specialized type of muscle, and the serous epicardial coat. The myocardial layers, at first continuous over the surface of the heart, become divided by connective tissue at the atrioventricular canal, leaving a small bridge alone. 'I'his connecting strand, located behind the posterior endocardial cushion, is the atriovaitriadar hiuidlc. The endothelial lining becomes the chief constituent of the endocardium. Originally a simple sac, it later dips between the trabeculae and wraps about the papillary muscles.
| |
|
| |
| .
| |
|
| |
| Fig. 179. - The caudal end of a chick embryo of 32 somites (Evans). The sciatic artery will differentiate from the primary capillary plexus of each limb Irud; aortae have already formed from the mesial margins.
| |
|
| |
|
| |
| Descent of the Heart. - At first the heart lies far cephalad in the cervical region, but it gradually recedes during development until it assumes a permanent position in the thorax. This migration is attested in the adult by the courses of the recurrent and cardiac nerves. After the diaphragm reaches its final location (Fig. 1 19), the heart rotates so that the ventricles, which previously were ventral to the atria, now become caudal.
| |
|
| |
| .
| |
|
| |
| Anomalies. - Dextrocardia is associated with a general transposition of the viscera (p. 1 1 8). The aorta and pulmonary artery may also be transposed in the absence of dextrocardia. Rarely, the paired anlages form a double heart. Of the complete or partial defects of the septa, most common is a patent foramen ovale. If the foramen fails to close after birth, the mixed blood produces a purplish hue in the child which is known popularly as a - blue baby. - This condition may be persistent in adult life. Incomplete closure occurs in about one in four cases, but actual mingling of the blood is rare, due to an approximation of the overlapping septal folds during atrial contraction. Valvular anomalies occur ; those of the semilunar valves result from an atypical division of the bulbus,
| |
|
| |
| .
| |
|
| |
| THE PRIMITIVE VASCULAR SYSTEM .
| |
|
| |
|
| |
| The vascular system of all higher mammals develops precociously. This is due to the absence of nutritive yolk, and the consequent need of vessels that will extract nourishment and oxygen from the maternal circulation and distribute them to the tissues of the embryo.
| |
|
| |
|
| |
| Delicate injections show that capillary plexuses precede the formation of definite arterial and venous trunks (Fig. 179). Only by the selection, enlargement, and differentiation of appropriate paths do the definitive vessels arise, whereas those capillaries from which the flow has been diverted, atrophy. Both inheritance and the hydrodynamic factors incident to the blood flow participate in the selection of channels from the capillary bed,
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| Fig. 180. - Diagram, in lateral view, of the blood vessels in human embryos of 1.5 to 2 mm.
| |
|
| |
| .
| |
|
| |
| The first paired vessels of human embryos are formed as longitudinal anastomoses of capillary networks that originate first in the angioblast of the yolk sac and chorion (p. 169). In the Eternod embryo of 1.3 mm., in which the somites are still undeveloped, such paired vessels are already formed (cf. Fig. iSo). The umbilical veins emerge from the chorion, fuse in the body stalk, then, separating again, course in the somatopleure to the paired, tubular heart anlages. From the heart tubes, paired vessels, the ventral aorta:, extend cephalad, then bend upward around the first aortic arches and continue caudad as the descending aorta: . The latter give off the umbilical arteries which bend sharply ventrad into the body stalk and branch in the wall of the chorion. The chorionic circulation is thus the first to be established in embryos 2 to 2.5 mm. long (5 to 8 somites), the heart has become a single tube (Fig. 181). From the yolk sac, numerous veins converge cephalad and form a pair of vitelline veins. These join the umbilical veins, whereupon the combined vitcllo-umhilical trunks traverse the septum transversum and open into the sinus venosus. The cranial portions of the descending aortas give off several pairs of dorsal intersegmental arteries, the caudal portions a ventral series of vitelline arteries to the yolk sac. The umbilical arteries now take their origin from a plexus of ventral vessels, in series with the vitelline arteries. At this stage, the vitelline circulation of the yolk sac is established,
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 181. - Diagram, in lateral view, of the blood vessels in human embryos of 2 to 2.5 mm.
| |
|
| |
| .
| |
|
| |
| Fig. 182. - Diagram, in lateral view, of the blood vessels in a human embryo of 2,6 mm. (Felix-Prentiss).
| |
|
| |
| .
| |
|
| |
| Fig. 183. Ventral reconstruction of the blood vessels in a 3,2 mm. human embryo (His).
| |
|
| |
| .
| |
|
| |
| Fig. 184. - Lateral reconstruction of the blood vessels in a 4.2 mm. human embryo (His). nix., Maxillary process; jv., precardinal vein; cv., postcardinal vein; ot., otocyst.
| |
|
| |
| .
| |
|
| |
|
| |
| In embryos of 15 to 23 somites (Fig. 182), the veins of the embryo proper develop as longitudinal anastomoses of branches from the segmental arteries. The paired precardinal (or anterior cardinal) veins of the head are developed first (Fig. 18 1); coursing back on either side of the brain, they join the vitello-umbilical trunk. In embryos of 23 somites, the postcardinals are present (Fig. 182). They lie dorsal to the nephrotomes, and, running cephalad, join the anterior cardinal veins to form the common cardinal veins. Owing to the later enlargement of the sinus venosus, the proximal portions of the common venous trunks are taken up into its wall, and thus three veins open into each horn of the sinus venosus: (1) the umbilical veins from the chorion; (2) the vitelline veins from the yolk sac; (3) the common cardinal veins from the body of the embryo. The descending aorta: fuse below the level of the seventh intersegmental arteries and form a single dorsal aorta as far caudad as the origin of the umbilical arteries. Of the numerous vitelline arteries, one pair is prominent; its halves unite into a single vessel which courses in the mesentery and later becomes the superior mesenteric artery. By the enlargement of capillaries connecting the ventral and dorsal aorta}, a second pair of aortic arches is formed at this stage,
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 185. - Lateral reconstruction of the arteries and cardinal veins in a 4.9 mm. human
| |
| embryo (Ingalls-Prentiss). X 20.
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| In embryos 4 to 5 mm. in length, five pairs of aortic arches are successively developed: the first, second, third, fourth, and sixth (Figs. 184 to 185). An additional pair of transitory vessels, which extend from the ventral aorta to the sixth arch, appear later in embryos of 7 mm., but soon degenerate (Fig. 186 B). They are interpreted as being the fifth pair in the series. From each dorsal, or descending aorta there develop cranially the internal carotid arteries (Fig. 184). These extend toward the optic stalks where they bend first dorsad and then caudad, and connect finally with the first intersegmental arteries of each side (Fig. 185). The descending aortae are now fused to their extreme caudal ends and the umbilical arteries thereby originate from the single vessel. Twenty-seven pairs of dorsal intersegmental arteries are jjresent ; from the seventh cervical pair, the subclavian arteries of the upper limbs arise. Of the ventral vitelline vessels, three are now prominent : the coeliac artery in the stomach pancreas region, the superior mesenteric in the small-intestine region, and the inferior mesenteric of the large-intestine region,
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 186. - Reconstructions of the human aortic arches and pharyngeal pouches (Tandler; .A, 5 mm.; B, 7 mm.
| |
|
| |
|
| |
| The embryonic plan of primitive vessels is altered profoundly in later stages. The sections that follow will describe these changes in detail,
| |
|
| |
| .
| |
|
| |
|
| |
| DEVELOPMENT OF THE ARTERIES .
| |
|
| |
| .
| |
|
| |
| Transformation of the Aortic Arches. - Both the ventral and descending aortae, and the ancestral aortic arches which interconnect them (Fig. 186 A), are early transformed into more appropriate vessels. In embryos of 7 mm., the first and second pairs of aortic arches drop out (Figs. 186 B and 187), but the subjacent ventral aortae persist as the external carotid arteries; similarly, the descending aortse at this level, together with the third aortic arches, become the internal carotids. The continuations of the ventral aorte between the third and fourth arches remain as the common carotid stems, whereas the corresponding segments of the descending aortae obliterate. The fourth pair of aortic arches are important ; the left is converted into the permanent aortic arch: on the right side, the fourth arch persists with the descending aorta as far as the seventh intersegmental artery and forms the first part of the right subclavian artery, which is thus a more complex vessel than its mate. The segment of the fourth arch proximal to the right common carotid becomes the innominate artery. The fifth arches of amniotes are rudimentary (p. 188). On the right side, the distal portion of the sixth arch is lost; on the left, it persists as the ductus arteriosus and its lumen is obliterated only after birth. The proximal portion of the right sixth arch forms the stem of the right pulmonary artery, but the proximal portion of the left arch is incorporated in the pulmonary trunk. Most of the pulmonary artery arises from a postbranchial plexus whose union with the sixth arch is acquired secondarily (Huntington, 1919). In 15 mm. embryos, the primitive bulbus cordis has been divided into distinct aortic and pulmonary trunks which open respectively into the left and right ventricles,
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 187. - Diagram, in ventral view, of the transformation of the human aortic arches.
| |
|
| |
| .
| |
|
| |
| The aortic arches of the embryo are of especial importance comparatively. Five arches are formed in connection with the functional gills of fishes. In adult tailed amphibia, three or four arches, and in some reptiles, two arches, are represented on either side. In birds the right, in mammals the left fourth arch persists as the arch of the aorta.
| |
|
| |
|
| |
| The different courses of the recurrent laryngeal nerves are easily explained. The vagus early gives off paired branches which reach the larynx by passing caudal to the primitive fourth aortic arches. When the latter, through growth changes, descend into the chest, loops of both nerves are carried with them. Hence, after the transformation of the fourth arches, the left recurrent nerve remains looped around the arch of the aorta, the right around the right subclavian artery (cf. Fig. 187).
| |
|
| |
| .
| |
|
| |
| Branches of the Dorsal Aorta. - ^From each primitive aorta arise dorsal, lateral, and ventral branches in three paired longitudinal series (Fig. 188); .
| |
|
| |
| .
| |
|
| |
| Fig. 188. - Transverse section of the trunk, illustrating the arrangement of the segmental aortic branches.
| |
|
| |
| .
| |
|
| |
| I. The dorsal branches are intersegmental in arrangement and develop small dorsal and large ventral rami.
| |
|
| |
|
| |
| From the dorsal rami are given off neurdl branches which bifurcate and form directly the dorsal and ventral spinal arteries. The vertebral arteries arise by longitudinal, postcostal anastomoses (Fig. 188) of the first seven pairs of dorsal rami (Fig. 189). The original stems of the first six pairs are lost, so that the vertebrals then take their origin from the seventh intersegmental arteries (Fig. 190). In embryos of 9 mm., the vertebral arteries fuse at the level of the cerebellum to form a single midventral vessel, the basilar artery; since the internal carotids are recurved cranially at 5 mm. (Fig. 185), and terminate in union with the first intersegmental arteries, the basilar is now connected cranially with the internal carotids and caudad with the definitive vertebral arteries.
| |
|
| |
| The internal carotids (Fig. 185), after branching off the ophthalmic arteries, give rise cranially to the anterior cerebral artery, from which develop later the middle cerebral and anterior chorioidal arteries; all of these supply the brain. Caudalward there are many small branches to the brain wall which ultimately form a true posterior cerebral artery,
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 189. - Origin of the vertebral and subclavian arteries and the costo-cervical trunk in a young rabbit embryo (modified after Hochstetter). Ill AB.-IV AB., Aortic arches; A.v.cB., cephalic portion of vertebral artery; CD. and C.v., internal and external carotid arteries.
| |
|
| |
|
| |
| The ventral rami of the dorsal intersegmental arteries become prominent in the thoracic and lumbar regions and persist as the intercostal and lumbar arteries, segmentally arranged in the adult. Longitudinal, precostal anastomoses (Fig. 188) constitute the costo-cervical and thyrocervical trunks (Fig. 189). The subclavian and a portion of the internal mammary artery are derived from the ventral ramus of the seventh cervical segmental artery (Fig. 189). The remainder of the internal mammary, and the superior and injerior epigastric arteries, are formed by longitudinal ventral anastomoses (Fig. 1 88) between the extremities of the ventral rami from the thoracic and lumbar intersegmental arteries, beginning with the second or third thoracic (Fig. 191).
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 190. - Arterial system of a human embryo of lo mm. (His). X i8. Ic, Internal carotid artery; P, pulmonary artery; IV, vertebral artery; 111 - VI, persistent aortic arches.
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
|
| |
| 2. The lateral {visceral) branches of the descending aortae are not segmentally arranged. They supply structures arising from the nephrotome region (mesonephros, sex glands, metanephros, and suprarenal glands). From them arise the renal, suprarenal, inferior phrenic, and internal spermatic or ovarian arteries.
| |
|
| |
| Bremer (1915) derives the renal arteries not from transformed mesonephric vessels, as did Broman (1906), but from a plexus of multiple aortic origin. There cire frequent variations in the selection of ])ermanent channels.
| |
|
| |
| .
| |
|
| |
| 3. The ventral {splanchnic) branches are imperfectly segmental. Primitively, they form the paired vitelline arteries to the yolk sac (Figs. 180 to 182). Coincident with the degeneration of the yolk sac, the prolongations of the ventral vessels to its walls disappear, and the paired arteries
| |
| that persist and pass in the mesentery to the gut fuse to form unpaired vessels. From these, three large arteries are derived; the coeliac artery, the superior mesenteric, and the inferior mesenteric (Figs. 185 and 192),
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 191. - The development of the internal mammary and epigastric arteries in a human embryo of 13 mm. (Mall in McYIurrich).
| |
|
| |
| .
| |
|
| |
|
| |
| The primitive coeliac axis arises opposite the seventh intersegmental artery. It then migrates caudad until eventually its origin is opposite the twelfth thoracic segment (Fig. 192). This transference, according to Evans, is due to the unequal growdh of the dorsal and ventral walls of the aorta. Similarly, the superior mesenteric artery is displaced caudad ten segments, the inferior mesenteric artery three segments,
| |
|
| |
|
| |
| The umbilical arteries arise in embryos of 2 to 2.5 mm. from the primitive aortae opposite the fourth cervical segment. They take origin in a plexus of ventral vessels of the vitelline series (Fig. 18 1), and are gradually shifted caudad until they arise from the dorsal aorta opposite the twenty-third segment (fourth lumbar). In 5 mm. embryos, the umbilical arteries develop secondary, lateral connections with the aorta (Fig 192 A). The new vessels pass lateral to the mesonephric ducts, and, at 7 mm., the primitive ventral stem-artery has disappeared (Fig. 192 B). The segment of this new trunk, proximal to the origin of the external iliac artery which soon arises from it, becomes the common iliac,
| |
|
| |
| .
| |
|
| |
| Fig. 192. - Reconstructions of the human aorta and its branches (Tandler-Prentiss). - 1 , 5 mm. ; B, g mm.
| |
|
| |
|
| |
| The remainder of the umbilical trunk constitutes the hypogastric artery. When the placental circulation ceases at birth, the distal portions of the hypogastric arteries, from bladder to umbilicus, atrophy, forming the lateral umbilical ligaments of adult anatomy (Fig. 199).
| |
|
| |
|
| |
| The middle sacral artery is the direct caudal continuation of the aorta. Its dorsal position in the adult is the result of secondary growth changes,
| |
|
| |
| .
| |
|
| |
| Arteries of the Extremities.- It is assumed that in man, as in observed birds and mammals, the first vessels of the limb buds form a capillary jrlexus (Fig. i yg).
| |
|
| |
| .
| |
|
| |
| Upper Extremity. - The capillary plexus takes origin by several lateral branches from the aorta. In human embryos of 5 mm. but one connecting vessel remains, and this arises secondarily as the ventral ramus of the seventh dorsal intersegment al artery (Fig. iSSb The portion of this vessel in the future free arm is plexiform at first, but later becomes a single axis which forms successively the subclavian, axillary, brachial, and inicrosscous arteries. Subseciuently, the median, radial, and ulnar arteries develop.
| |
|
| |
| .
| |
|
| |
| Lower Extremity. - In embryos of 7 mm. a branch, known as the sciatic artery, is given off from the future common iliac. It is the cluef arterial stem of the lower extremity and includes the definitive popliteal and peroneal arteries. At 15.5 mm. it is largely superseded by the external iliac -and femoral arteric';, of which the latter annexes the branches of the sciatic distal to the middle of the thigh. The sciatic artery persists proximally as the inferior gluteal artery,
| |
|
| |
| .
| |
|
| |
| DEVELOPMENT OF THE VEINS .
| |
|
| |
| Three systems of paired veins are present in embryos of 23 somites ( Fig. 182) : the umbilical veins from the chorion; the vitelline veins from the yolk sac; and the prccarditial and postcardinal veins, which unite in the common cardinal veins, from the body of the embryo. Thus, three veins open into the right horn of the sinus venosus, and three into the left.
| |
|
| |
|
| |
| Transformation of the Vitelline and Umbilical Veins. - Accompanying the increase in size of the primitive liver is a mutual intergrowth l)etween the hepatic cords and the endothelium of the vitelline A^eins. As a result, these vessels form in the liver a network of sinusoids (Figs. 183 and 193), and each vein is thereby divided into a distal portion which ])asses from the yolk sac to the liver, and into a proximal portion which carries blood from the liver sinusoids to the Sinus venosus. Soon, the proximal part of the left vitelline vein is largely absorbed into the hepatic sinusoids and shifts its blood flow to the right horn of the sinus venosus. Moreover, in 5 mm. embryos, the parallel vitelline v - eins communicate by three cross anastomoses (Figs. 194 and 195): (i) a cranial transverse connection in the liver, ventral to the duodenum; (2) a middle one, dorsal to the duodenum; and (3) a caudal one, ventral to it. There are thus formed about the gut a cranial and a caudal venous ring. A new vessel, the superior mesenteric, now develops in the mesentery of the intestinal loop and joins the left vitelline vein just caudal to its middle anastomosis (Fig. 195). Coincident with the atrophy of the yolk sac, the vitelline veins degenerate caudal to the junction of the superior mesenteric vein. The persisting trunk between the latter vessel and the liver sinusoids is the portal vein, and thus represents: (i) a portion of the left vitelline vein in the left limb of the caudal ring; (2) the middle transverse anastomosis between the vitelline veins; (3) that segment of the right vitelline vein which forms the right limb of the cranial ring,
| |
|
| |
| .
| |
|
| |
| Fig. 194. - Ventral reconstruction of the veins of the liver in a 4.9 mm. human embrj'o (Ingalls).
| |
|
| |
| .
| |
|
| |
| According to Mall, the intrahepatic portion of the right vitelline vein persists proxinially as the right ramus of the hepatic vein, and distally as the ramus arcuatus of the portal vein. The intrahepatic jjortion of the left vitelline vein drains secondarily into the right horn of the sinus venosus, and proximally forms later the left hepatic ramus. Distally, where it is connected with the left umbilical vein, it becomes the ramus angidaris of the portal vein. In this way two primitive portal, or supplying trunks, and two hepatic, or draining trunks, originate. Later, there are differentiated first four, then six, such apposed trunks within the liver, and the six primary lobes supplied and drained by these vessels may be recognized in the adult.
| |
|
| |
|
| |
| While these changes have been progressing, the liver tissue grows laterad, comes in contact with the umbilical veins, and taps them so that their blood is diverted more directly to the heart through the sinusoids of the liver (Fig. 194). As the channel of the right proximal vitelline is larger, the blood from the left umbilical vein flows diagonal!}^ to the right horn of the sinus venosus. When all the umbilical blood enters the liver, as in embryos of 5 to 6 mm., the proximal segments of the umbilical veins atrophy (Fig. 195). At 7 mm. the left umbilical is large, while the corresponding right vein has degenerated and soon disappears. The left persists during fetal life, shifts to the midplane, and courses in the free edge of the falciform ligament. After birth its lumen is obliterated, and from the umbilicus to the liver it constitutes the ligamentum teres,
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 195. - The origin of the portal vein and ductus venosus as illustrated by a human embryo of 7 mm. (modified after Flis).
| |
|
| |
|
| |
| In the liver, the portal vein, through its cranial anastomosis between the primitive vitelline veins, is connected with the left umbilical vein (Fig. 195). As the right lobe of the liver grows, the course of the umbilical and portal blood through the intrahepatic portion of the right vitelline vein becomes circuitous, and hence a new, direct channel to the sinus venosus is formed through the hepatic sinusoids. This is the ductus venosus (Fig. 195), which is obliterated after birth and forms the ligamentum venosum of the postnatal liver.
| |
|
| |
| Transformation of the Precardinal Veins. - Each precardinal (anterior cardinal) vein consists of two parts (Fig. 185): (i) the primary head vein, which extends into the unsegmented head proper and courses ventrolateral to the brain wall; (2) the true precardinal, located laterad in the segmented portion of the head and neck and draining into the common cardinal vein.
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 196. - Veins of the head in a g mm. human embryo (after Mall). X 9,
| |
|
| |
|
| |
| The primary head veins have three pairs of tributary plexuses (Fig. 196) which presently extend dorsad over the brain. From this primitive arrangement the various veins and sinuses of the brain are developed.
| |
|
| |
|
| |
| The true precardinals communicate during the eighth week by a transverse venous channel which carries the blood from the left side of the head into the right vein (Fig. 197 D). As a result, the left precardinal soon loses its connection with the common cardinal on the same side and degenerates (E). The stump of the left common cardinal comprises the inconstant oblique vein of the left atrium: it also joins with the transverse sinus venosus in forming the coronary sinus. The right common cardinal and the right precardinal, as far as its cross anatomosis, become the superior vena cava. The anatomosis itself forms the left innominate vein, while that portion of the right precardinal between the anastomosis and the right subclavian vein is known as the right innominate. The distal segments of the precardinals become the internal jugular veins of the adult, whereas the external jugular and subclavian veins are vessels which develop somewhat later (C-E).
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| Fig. I97. - Diagrams to illustrate the transformation of the pre-, post-, sub-, and supracardinal veins (adapted after Huntington and McClure). A, 6 mm.; B, 10 mm.; , 15 mm., D, 18 mm,
| |
|
| |
| .
| |
|
| |
|
| |
| Transformation of the Post-, Sub-, and Supracardinal Veins. - The primitive postcardinal veins course cephalad along the dorsal sides of the mesonephroi and open into the common cardinals (Fig. 197 *d). Each receives tributaries from the posterior extremities, mesonephroi, and body wall. Median and ventral to the mesonephros are developed the suhcardinal veins, which connect at intervals with the postcardinals through the mesonephric sinusoids, and with each other by anastomoses ventral to the aorta. Thus, all the blood from the lower body is in early stages drained by the postcardinal veins alone. Soon, the postcardinals are divided midwayinto cranial and caudal segments (S). Cranial to their interruption, these vessels atrophy (C). The caudal portions are associated with the mesonephroi and persist longer, but finally disappear with those organs {D, E). The sole permanent remnants of the postcardinal system are small contributions to the azygos and sex veins,
| |
|
| |
| Of the subcardinals, only the middle regions, at about the final level of the kidneys, are retained. Here the two vessels communicate by a broad anastomosis, and here each is similarty connected with the postcardinal of the same side [B). Below this level the subcardinals presently disappear, except for portions which supply the sex glands {C-E) . Above, the left drops out, its lower stump alone transforming into the left suprarenal vein; the corresponding part of the right subcardinal remains as the right suprarenal and also as an important component of the inferior vena cava.
| |
|
| |
| In the meantime, a new pair of anastomosing veins, the su pracardinals, make their appearance (C). They lie dorso-mesial to the postcardinals, and,' in a sense, replace them. The supracardinal veins originally extend from near the common cardinals to the union of the primitive iliac vessels, but they soon break at the level of the kidneys (D). The cranial halves midway develop a prominent anastomosis and become the azygos and hemiazygos of the adult (£). Opposite the kidneys, the caudal segments form a permanent, broad union with the right subcardinal and a temporary one with the left (C, D] in yellow). The right main supracardinal channel, with the annexed right subcardinal, constitutes the lower half of the inferior vena cava {D, E) (Huntington and McClure, 1920).
| |
|
| |
| The development of the unpaired inferior vena cava begins when communication is established between the right hepatic and right subcardinal veins. The liver on the right side becomes attached to the dorsal body wall, and from its point of union a ridge, the caval mesentery (Fig. 112), extends caudad. Capillaries from the subcardinal vein invade the mesentery, and, growing cranially, meet and fuse with capillaries extending caudad from the liver sinusoids. Thus is formed the vein of the caval mesentery (. 4 , B), which is already present in human embryos of 10 mm. 44 ie blood from the lower trunk and leg region soon becomes drained by the complex inferior vena cava, which is composed of the following veins (E) : (1 ) the common hepatic and right hepatic veins (primitive right vitelline) ; (2) the connecting vein of the caval mesentery; (3) an inter-renal portion of the right subcardinal vein (and its adjoining anastomoses with the right post- and su])racardinal and the left subcardinal) ; (4) the right supracardinal vein, below the level of the kidneys.
| |
|
| |
|
| |
| The permanent kidneys take up their positions opposite the great anastomosis between the subcardinals, and, at this point, the renal veins are developed [D, E)\ the longer left renal vein differs from the right in that proximally it represents a left portion of the anastomosis itself. A cephalic segment of the left subcardinal vein persists as the left suprarenal vein, which thus opens into the left renal instead of joining the inferior vena cava as does the right suprarenal vein of similar subcardinal origin (E). The spermatic or ovarian veins contain both postcardinal and subcardinal components. The left early drains into the left caudal border of the great subcardinal anastomosis, which, as already described, contributes to the left renal vein; the right opens into that portion of the right subcardinal which is incorporated into the inferior vena cava. The posterior intercostal and lumbar veins are at first tributaries of the postcardinals. As the latter vessels degenerate, these tributaries connect secondarily with the replacing supracardinal veins; later, they of necessity drain respectively into the azygos veins and inferior vena cava. The history of the common iliacs is similar, the stem of the longer left representing a caudal anastomosis between the primitive paired supracardinals (C-E).
| |
|
| |
|
| |
| Veins of the Extremities. - The primitive capillary plexus of the flattened limb buds gives rise to a peripheral border vein (Fig. 227). In the upper extremity, its ulnar portion persists, forming at different points the subclavian, axillary, brachial, and basilic veins. At 10 mm. the border vein opens into the dorsal wall of the postcardinal, but, as the heart shifts caudad, it Anally drains by a ventral connection into the xjrecardinal, or internal jugular vein. The cephalic vein develops secondarily in connection with the ulnar border vein; later, in embryos of 23 mm., it anastomoses with the external jugular and finally drains into the axillary vein, as in the adult. AVith the development of the digits, the vv. ccphalica and basilica become distinct (35 mm.), but later are again connected by a plexus on the dorsum of the hand.
| |
|
| |
| In the lower extremity, the fibular portion of the primitive border vein persists. Later, the V. saphena niagna arises separately from the postcardinal, gives off the vv. femoralis and tibialis posterior, and annexes the fibular border vein at the level of the knee. Distal to this junction, the border vein persists as the v. tibialis anterior, and, probably, the V. saphena parva; proximally, it becomes greatly reduced, forming the v. glutea inferior,
| |
|
| |
| .
| |
|
| |
| Anomalies. - Anomalous blood vessels are of common occurrence. They may be due: (i) to the choice of unusual paths in the primitive vascular plexuses; (2) to the persistence of vessels normally obliterated; (3) to the disappearance of vessels normally retained; (4) to incomplete development; (5) to fusions and absorptions of parts usually distinct.
| |
|
| |
|
| |
| FETAL CIRCULATION AND THE CHANGES AT BIRTH .
| |
|
| |
| During fetal life oxygenated placental blood enters the embryo by way of the large umbilical vein and is conveyed to the liver where it mingles with that brought in by the portal vein (Fig. 198). Thence it flows to the inferior vena cava either directly, through the ductus venosus, or indirectly through the liver sinusoids and hepatic vein. The impure blood of the inferior vena cava and portal vein contaminates but slightly the greater volume of pure placental blood. According to common belief, the blood from the inferior vena cava is directed by the valve of that vein across the right atrium and through the foramen ovale into the left atrium (following the path of the sounds in Figs. 172 to 174), which, before birth, receives little venous blood from the lungs. This purer blood of the left atrium then enters the left ventricle and is driven out through the aorta, to be distributed chiefly to the head and upper extremities,
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 198. - Diagram of the circulation before birth (Heisler). Arrows point out the course of the blood current; colors show the older conception of the character of the blood carried by various vessels, whereas experimentation indicates a thorough mixing within the heart,
| |
|
| |
|
| |
| The venous blood of the superior vena cava is supposed to flow from the right atrium into the right ventricle, whence it passes out by the pulmonary artery. A small amount is conveyed to the lungs, but, as the fetal lungs do not function, most of it enters the dorsal aorta by way of the ductus arteriosus. Since the ductus is caudal to the origin of the subclavian and carotid arteries, its less pure blood is distributed to the trunk, viscera, and lower extremities. The placental circuit is completed through the hypogastric, or umbilical arteries.
| |
|
| |
|
| |
| In spite of the apparent anatomical arrangement in the heart to prevent the mixing of pure and impure blood, actual experiments indicate .
| |
|
| |
| .
| |
|
| |
| Fig. 199. - Diagram of the circulation after birth (Heisler). Obliterated fetal passages are indicated by roman type,
| |
|
| |
| .
| |
|
| |
| That, contrary to the prevalent view, there is thorough mingling of the blood which enters the right atrium through the two caval veins. Hence, there can be no difference in the quality of the blood distributed to the various parts of the body. Circulatory efficiency must then depend on the relatively large quantity of swiftly moving blood.
| |
|
| |
| Changes at Birth. - When the lungs become functional, the placental circulation ceases quickly. This transfer of the seat of oxygenation not only changes the character of the blood in many vessels but throws important fetal vessels and parts into disuse (Fig. 199). In general, physiological occlusion follows immediately but anatomical obliteration is slower,
| |
|
| |
| .
| |
|
| |
| The large amount of blood returned to the heart from the functional lungs equalizes the pressure in the two atria (p. 181). As a result, the septum primum, or valve of the forcuucn ovale, is pressed against the septum secundum, thereby closing the foramen. Eventually, the two septa fuse - in one-third of all cases within three months, in three-fourths by maturity (p. 185).
| |
|
| |
| The ductus arteriosus also ceases to function, as all the blood from the pulmonary arterial trunk is conveyed to the expanded lungs. In four out of five cases the ductus becomes impervious within three months and persists as a solid, fibrous cord, the Ugamentum arteriosum.
| |
|
| |
| The umbilical vessels contract and their lumina are obliterated by fibrous invasion. The process advances proximad during the first two or three months of postfetal life. The cord-like vein is persistent as the Ugamentum teres of the liver; the arteries become the lateral umbilical ligaments.
| |
|
| |
| The ductus venosus likewise atrophies, and, within two months, transforms into the fibrous Ugamentum venosum, embedded in the wall of the liver,
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
|
| |
| THE LYMPHATIC SYSTEM .
| |
|
| |
| .
| |
|
| |
| The lymphatics originate independently of blood Y - essels from discrete mesenchymal spaces which become lined with an endothelium of transformed border cells. Temporary venous connections are now generally believed to be acquired secondarily. By the progressive fusion and budding of such local anlages, the lymphatic system grows to its final form.
| |
|
| |
| The first plexus of lymphatic capillaries is distributed along the primitive, main venous trunks. The dilatation and coalescence of this network at definite regions gives rise to five lymph sacs (Fig. 200): (i, 2) Paired jugular sacs appear in 10 mm. embryos, lateral to the internal jugular veins. (3) At 23 mm., the unpaired retroperitoneal sac develops at the root of the mesentery, adjacent to the suprarenal glands, and the cisterna chyli also differentiates (4, 5). Paired posterior sacs arise in relation to the sciatic veins of embryos 24 mm. long. All these sacs at first contain blood which they soon discharge into neighboring veins, thereupon losing their venous connections. With relation to the lymph sacs as centers, the thoracic duct (at 30 mm.) and the peripheral lymphatics develop. Thus, lymphatic vessels grow to the head, neck, and arm from the jugular sacs; to the hip, back, and leg from the posterior sacs, and to the mesentery from the retroperitoneal sac. The jugular sacs alone acquire permanent connections with the internal jugular veins that are later utilized by the thoracic and right lymphatic ducts. The various sacs themselves are eventually replaced by chains of lymph glands.
| |
|
| |
| ,
| |
|
| |
| 204 .
| |
|
| |
|
| |
| THE VASCULAR SYSTEM .
| |
|
| |
|
| |
| Lymph Glands. - -Paired lymph glands appear during the third month, first in the axillary, iliac, and maxillary regions (Fig. 200). Those replacing the lymph sacs develop later. Primitive sinuses, with simple connective-tissue septa, mark the primary stage of development. Ordinarily it has been believed that the sinuses represent lymphatic plexuses, but recent investigators (Downey, 1922) claim they are channels in the reticulum, originating as clefts in the mesenchyme and acquiring secondary .
| |
|
| |
| .
| |
|
| |
| Fir,. 200. - Profile reconstruction of the primitive lymphatic system in a human embryo of two months (redrawn after Sabin). X 3.
| |
|
| |
| .lymphatic connections. Lymphocytes collect in the stroma, forming cortical nodules which become associated with blood capillaries and after birth acquire germinal centers (Fig. 201 A). The peripheral sinus organizes and connects with afferent and efferent lymphatics; the central sinuses cut the lymphoid tissue into medullary cords (Fig. 201 B). The connective tissue differentiates into a fibrous capsule from which trabeculce dip into the gland.
| |
|
| |
| .Hemal (Hemolymph) Glands.- -The orgin of hemal glands is traced by Meyer (1917) to condensations of mesenchyme which develop in relation to blood vessels, not lymphatics. The peripheral sinus arises independently; its vascular connections are secondary.
| |
|
| |
| ,
| |
|
| |
| A .
| |
|
| |
|
| |
| Afferent lymphatic vessels .
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| Living nf sinus .
| |
|
| |
|
| |
| Peripheral lymph sinus .
| |
|
| |
| .
| |
|
| |
| Lymphatic vessel Blood vessels .
| |
|
| |
|
| |
| Lymphatic vessel .
| |
|
| |
|
| |
| B
| |
| Afferent lymphatic vessels .
| |
|
| |
| .
| |
|
| |
| Efferent lymphatic vessels
| |
| Fig. 201 . - Diagrams representing four stages in the development of lymph glands. The earlier stages are shown on the left side of each figure (Lewis and Stohr).
| |
|
| |
| ,
| |
|
| |
| THE LYMPHATIC SYSTEM .
| |
|
| |
|
| |
| 205 .
| |
|
| |
|
| |
| The Spleen. - Embryos of 9 mm. exhibit a swelling on the left side of the dorsal mesogastrium, near the dorsal pancreas (Fig. 202 ^d). The thickening is due to a temporary proliferation and invasion of mesothelial cells into the underlying mesenchyme, which, meanwhile, has also undergone local enlargement and vascularization. These cells from the peritoneal epithelium give rise to a large part, at least, of the future spleen. The union of the splenic anlage with the mesogastrium (Fig. 202 B) is ultimately reduced to a narrow band.
| |
|
| |
| .At first the blood vessels constitute a closed system. The peculiar adult circulation is acquired relatwely late. Lymphoid tissue first appears as ellipsoids about the smallest arteries in fetuses of four months. At .
| |
|
| |
| .
| |
|
| |
| Fig. 202. - Developmental stages of the human spleen (redrawn from Kollman and Tonkoff).
| |
|
| |
| .A, 10.5 mm.; B, 20 mm.
| |
|
| |
| Seven months, the ovoid splenic corpuscles form nodules about the larger arteries. The capsule, trabeculce, and reticulum differentiate from the cells of the common anlage. During the last half of fetal life, red blood corpuscles are developed actively in the splenic capillaries.
| |
|
| |
| The Glomus Coccygeum. - The coccygeal body develops from the wall of the middle sacral artery. It appears at the apex of the coccyx in the third month, and, during the fourth month, is an encapsulated cluster of polyhedral cells. Later, it becomes lobulated by the ingrowth of connective tissue trabeculae and receives a rich vascular supply. Affinities are obscure, but at no time does it resemble chromaffin tissue, as is often stated.
| |
|
| |
| Tonsils and Thymus. - ^For their development see p. 100 .
| |
|
| |
|
| |
| CHAPTER X .
| |
|
| |
|
| |
| THE SKELETAL SYSTEM I. Histogenesis of the Supporting Tissues
| |
| Connective tissue, cartilage, and bone all differentiate from that type of diffuse mesoderm known mesenchyme (Fig. 3). Mesenchyme arises directly from the primitive streak, and secondarily from mesodermal segments and the lateral somatic and splanchnic layers (Fig. 211). It is a spongy mesh work composed of branching and anastomosing cells; between these occur open spaces filled with a ground substance of coagulable fluid. In early embryos the mesenchyme constitutes an unspecialized packing material between the external and internal epithelia (Fig. 212), but it soon differentiates into various tissues and organs (p. 7). (jf these, the inert supporting tissues are peculiar in that a fibrous, hyaline, or calcified matrix forms, which becomes bulkier than the persisting cellular elements. In each type the origin of such matrix, whether inter- or intracellular, is disputed.
| |
|
| |
| .Connective Tissue
| |
| Discordant views exist as to the precise manner in which connectivetissue fibers differentiate. Some maintain that the fibrils develop within the cytoplasm of mesenchyme cells and are subsequently extruded into the adjoining matrix. A modification of this theory, proposed by Mall, traces the origin of the fibrils to a hyaline, ectoplasmic layer of the syncytial mesenchyme; this layer then transforms into matrix, while the nuclei and granular endoplasm remain unaffected as definitive connective-tissue cells.
| |
|
| |
| .A rival theory, strongly su]>ported of late, interprets the primitive matrix as a lifeless, gelatinous ground substance, secreted by the mesenchyme. In it fibers are formed by a gradual process of organization, which, according to Baitsell (iq2i), is structurally identical with the transformation of a plasma clot into fibrin.
| |
|
| |
| Reticular Tissue. - Except for the jelly-like mucous tissue of the umbilical cord, reticular tissue departs least from the embryonal type. The fine reticular fibrils remain embedded within the cytoplasm of the cells (Downey, 1922).
| |
|
| |
| .White Fibrous Tissue. -^The differentiation of this tissue may be divided into two phases: (i j a prefibrous stage, marked by the appearance
| |
| 206 .
| |
|
| |
|
| |
| CONXECTIVE TISSUE .
| |
|
| |
|
| |
| 207 .
| |
|
| |
|
| |
| of fibrils resembling those of reticular tissue (Fig. 203, at top); (2) the fibrils take the form of parallel bundles and are converted, through a chemical change, into typical white fibers (Fig. 203, at middle). The early, spindle-shaped cells transform into the several types characteristic .
| |
|
| |
| .
| |
|
| |
| Fig. 203. - The differentiation of white fibers in the Fig. 204. - The differentia
| |
| skin of a 5 cm. pig embryo (after Mall). X 270. tion of elastic fibers in the um
| |
| bilical cord of a 7 cm. pig embryo (after Mall). X 270.
| |
|
| |
| ,
| |
|
| |
| of the adult. In areolar tissue, the bundles of white fibers are interwoven to form a meshwork; in tendon, ligaments, and fascias they are arranged in compact, parallel fascicles.
| |
|
| |
| D .
| |
|
| |
|
| |
| Elastic Tissue. - Yellow elastic fibers develop in association with the white variety (Fig. 204). They originate singly after the general manner of white fibers, but may coalesce, as in the fenestrated membranes of arteries.
| |
|
| |
| .Adipose Tissue. - Certain of the mesenchymal cells give rise, not to fibroblasts, but to fat cells. They secrete within their cytoplasm droplets I of fat which increase in size and become confluent (Fig. 205). Finally,
| |
| i a single globule fills the cell, and the nucleus and C3 - 'toplasm are pressed .
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 206. - Two interpretations of the development of cartilage (Lewis and Stohr). . 1 , Studnicka; B, Mall.
| |
|
| |
| ,
| |
|
| |
| Mes. Precartilaqe Carulage.
| |
|
| |
| ,
| |
|
| |
| THE SKELETAL SYSTEM .
| |
|
| |
|
| |
| 2oS
| |
| to the periphery. Fat cells are most numerous along the course of blood ' vessels in areolar tissue and a])pear first during the fourth month.
| |
|
| |
| In several locations there are groups of distinctive, granular lipo
| |
| blasts, termed adipose glands, but at infancy they become indistinguish- I
| |
| able from the ordinary fat cells. 15
| |
| 'i
| |
| CARTILAGE \
| |
| A preliminary stage in the development of cartilage begins as early ^
| |
| as the fifth week with the enlargement of mesenchymal cells to form a -
| |
| compact, cellular tiSsue, designated prccartilage (Fig. 206). The origin ^ of mat fix is interpreted in two ways: (i) Some claim that it appears between the cells from thickened and transformed ectoplasmic walls (Fig. 206 A). (2) According to Mall, mesenchymal cells give rise first
| |
| to an ectoplasm in which fibrillce develop. Next, the cells increase in size and are gradually extruded until they lie in the intercellular spaces (Fig. 206 B). Simultaneously, the ectoplasm undergoes both a chemical and structural change and is converted into the hyaline matrix peculiar to cartilage.
| |
|
| |
| The matrix of hyaline cartilage remains homogeneous. In fibro- i cartilage, white fibers also are dejjosited within the matrix, in elastic f cartilage, yellow elastic fibers. Cartilage grows both internally and • externally. Interstitial growth results from the proliferation of cartilage cells and the production of new matrix by them. Ap positional growth takes place through the mitotic activity of the connective-tissue sheath, y the perichondrium; its inner cells are transformed into young cartilage cells.
| |
|
| |
| BONE
| |
| Bone begins to appear after the sixth week. There are two types: the membrane bones of the face and cranium which develop directly within fibrous sheets, and the cartilage bones which replace the earlier cartilaginous skeleton. The mode of histogenesis, however, is identical in each. Bone matrix forms through the activity of specialized, connective-tissue cells, named osteoblasts (bone-formers). First, fibrilla2 and then interfibrillar lime salts differentiate (Fig. 207). Whether these constituents '. are transformed ectoplasm or intercellular deposits is debated.
| |
|
| |
| Development of Membrane Bone. - The flat bones of the face and cranial vault are preceded by connective-tissue membrane. At one â– I or more central points intramembranous ossification begins. Such centers of ossification are characterized by the appearance of osteoblasts which ;
| |
| promptly deposit bone matrix in the form of spicules (Fig. 207 A). These |
| |
| unite into a mesh work of trabeculjc that spreads radialty in all directions. :i Since the osteoblasts are arranged in an epithelioid layer upon the surface J of a spicule, the latter grows both in thickness and at its tip (Fig. 207). ; !| .
| |
|
| |
|
| |
| BOXE .
| |
|
| |
|
| |
| 2og .
| |
|
| |
|
| |
| As the matrix is progressively laid down, some osteoblasts become trapped and remain imprisoned as bone cells; these are lodged in spaces termed lacnncc.
| |
|
| |
| Somewhat later, the mesenchyme next; the flat surfaces of the spongy plate thus formed condenses into a fibrous membrane, the periosteum (Fig. 207 B). Osteoblasts arise on its inner surface and deposit parallel lamellte of compact bone. This process is known as periosteal ossification.
| |
|
| |
| ,
| |
|
| |
| .
| |
|
| |
| Bone matrix .
| |
|
| |
|
| |
| A
| |
| Bone matrix .
| |
|
| |
|
| |
| Bone cell .
| |
|
| |
|
| |
| C' .
| |
|
| |
|
| |
| â– mil .
| |
|
| |
|
| |
| Osteoclast in bone matrix .
| |
|
| |
|
| |
| Fig. 207. - Two stages in the development of bone. . 4 , Section through the frontal bone of a 20 mm. pig embryo (after Mall). X 270. B, Section through the periosteum and bone lamellse of the mandible of a three-months - fetus (Prentiss). X 325.
| |
|
| |
| ,
| |
|
| |
| Periosteum .
| |
|
| |
|
| |
| Osteoblast .
| |
|
| |
|
| |
| In such a manner are developed the dense inner and outer tables, joined by the spongy diploe already described.
| |
|
| |
| .Much bone that is first formed is provisional, and so is resorbed and replaced in varying degrees as the bone grows and assumes its final modelling. At this time, large, multinucleate cells appear upon the surface of the bone matrix (Fig. 207 B). These giant cells are named osteoclasts, that is, bone destroyers. There is, however, no positive evidence that the osteoclasts are responsible for bone dissolution; more likel}^ they are degenerating, fused osteoblasts and freed bone cells (Arey, 1920). The open spaces of spongy bone are filled with derivatives of the mesen
| |
| 14 .
| |
|
| |
|
| |
| 2 10 .
| |
|
| |
|
| |
| THE SKELETAL SYSTEJI .
| |
|
| |
|
| |
| chyme. Such reticular tissue, fat cells, blood vessels, and developing blood cells constitute the red hone marroiv.
| |
|
| |
| Development of Cartilage Bone. - The shape of a cartilage bone is determined by the transitory cartilage model which precedes it (Fig. 209). The chief peculiarity of this method of bone formation is the preliminary destruction of the cartilage. For this reason, these skeletal elements are often designated replacement bones. Thereafter, the course of events is essentially as in the development of a membrane bone. Ossification occurs both within the eroded cartilage and peripherally beneath its perichondrium (Fig. 208). In the first case, the process is intracartilaginous or endochondral, in the second instance, perichondral, or better, periosteal.
| |
|
| |
| .Endochondral Bone Formation. - In the center of the cartilage the cells enlarge, become arranged in characteristic radial rows, and lime is deposited in their matrix (Fig. 208). The cartilage cells and part of the calcified matrix then disintegrate and disappear, thereby forming primordial marrow cavities. This destruction apparently is caused by the vascular primary marrow tissue which simultaneously invades the cartilage. It arises from the inner, cellular layer of the perichondrium and burrows into the cartilage in bud-like cords. Such eruptive tissue gives rise to osteoblasts and bone marrow which occupy the primordial marrow cavities. The osteoblasts first de])osit matrix directly upon persisting spicules of cartilage, hence endochondral bone is spongy. Similarly, the hitherto intact regions of the cartilage undergo progressive invasion, destruction, and replacement until eventually the entire cartilage is superseded by cancellous bone.
| |
|
| |
| .Periosteal Bone Formation. - While the foregoing changes are occurring within the cartilage, compact bone develops about it (Fig. 208). This process is identical with the formation of the tables of the flat bones, and likewise is due to the activity of the inner osteogenetic layer of the perichondrium which now converts directly into the periosteum. Those bone lamelkv deposited about blood vessels that course in hollowed grooves are concentrically arranged into Flaversian systems.
| |
|
| |
| .Growth of Bones. - Flat membrane bones increase in lateral extent by continued marginal ossification from osteoblast-rich connective tissue at the site of the later sutures. Both cartilage and membrane bones grow in thickness by the further deposition of periosteal matrix. In a long bone, this superficial accretion is accompanied by a central resorption which destroys not only the endochondral osseous tissue but also much of the earlier periosteal layers. As a result, cancellous bone, and its associated red bone marrow, persist only at the ends, whereas in the middle region an extensive open cavity develops. The latter is filled with yellow bone marrow, composed chiefly of fat cells.
| |
|
| |
| ,
| |
|
| |
|
| |
| Zone of cartilage erosion and endochondral ossification .
| |
|
| |
|
| |
| ^ Zone of calcified cartilage I (Cells swollen and in rows) .
| |
|
| |
|
| |
| â– j! .
| |
|
| |
|
| |
| / Zone if unmodified cartilage .
| |
|
| |
|
| |
| - Level of diarthroidal joint anlage .
| |
|
| |
|
| |
| Periosteal bone .
| |
|
| |
|
| |
| Marrow cavity .
| |
|
| |
|
| |
| Endochondral hone deposited OH remains of cartilage .
| |
|
| |
|
| |
| Position of later epiphysis .
| |
|
| |
|
| |
| Fig. 208. Cartilage bone development as illustrated in the finger of a five-months - fetus (Sobotta). X 15. Longitudinal section.
| |
|
| |
| ,
| |
|
| |
|
| |
| J .
| |
|
| |
|
| |
| BONE .
| |
|
| |
|
| |
| 2u .
| |
|
| |
|
| |
| Most bones, especially those preformed in cartilage, have more than one center of ossification (Fig. 209). In all, there are over 8co such centers, half of which first appear after birth. On the average, therefore, there are four centers to each mature bone. Many cartilage bones, such as occur in the extremities, vertebree and ribs, lack periosteum on their articular surfaces. The consequent inability to increase in length by ordinary means has led to an interesting adaptation. The cartilage at either end grows rapidly, and progressively ossifies, but sometime between birth and puberty, or even later, osteogenetic tissue invades these cartilages and secondary ossification centers, the epiphyses, are established (Fig. 209 C, D). Both surfaces of the intervening cartilaginous plate continue to develop new cartilage as long as the bone lengthens, and this in turn is steadily replaced by bone matrix. Finally, when the adult length is attained, the cartilage ceases proliferation, ossifies, and, the epiphyses are .
| |
|
| |
| .
| |
|
| |
| Fig. 209. - Diagrams to illustrate the method of growth in a long bone (Prentiss).
| |
|
| |
| ,
| |
|
| |
| firmly united to the central mass. The so-called epi physeal lines mark this union.
| |
|
| |
| .Joints. - The joints occur at regions where the original mesenchyme fails to differentiate into skeletal elements. Such articulations include two general groups; (i) synarthroses, in which little movement is allowed; (2) diarthroses, or freely movable joints.
| |
|
| |
| In joints of the synarthroidal type, the mesenchyme differentiates into a uniting layer of connective-tissue {suture, syndesmosis) or cartilage {synchondrosis) .
| |
|
| |
| Diarthroidal joints are characterized by a prominent joint cavity, between the movable skeletal parts, and a ligamentous capside at the periphery (Fig. 210 B). The joint cavity arises from a cleft in the open mesenchyme; the capsule from the denser external tissue continuous with the periosteum (Figs. 208 and 210). The cells on the inner surface of the capsule flatten into the epithelioid synovial membrane. Ligaments or tendons which apparently course through the adult joint cavities represent .
| |
|
| |
|
| |
| 212 .
| |
|
| |
|
| |
| THE SKELETAL SYSTEM .
| |
|
| |
|
| |
| secondary invasions, covered with reflexed synovial membrane, and hence are really external to the cavity. Sesamoid bones develop in relation to tendons, and, usually, joints; they commonly arise in the substance of the primitive joint capsule and may exhibit a cartilaginous stage.
| |
|
| |
| ,
| |
|
| |
|
| |
| Cartilage Joint cleft Perichondrium Mesenchyme .
| |
|
| |
|
| |
| Marrmv cav
| |
| Synovial membrane
| |
| Joint cavity
| |
| Artictdar cartilage Joint ca psnle
| |
| Spongy bone Periosteum Compact bone .
| |
|
| |
|
| |
| Fi(', 2 10. - Stages in the development of a diarthroidal joint.
| |
|
| |
| ,
| |
|
| |
| u. MORPHOGENESIS OF THE SKELETON
| |
| The skeleton comprises: (i) the axial skeleton (skull, vertebrae, ribs, and sternum), and (2) the appendicular skeleton (pectoral and pelvic girdles and the limb bones). Except for the flat bones of the face and cranial vault, the l)ones of the mammalian skeleton exhibit first a blastemal, or membranous stage, next a cartilaginous phase, and Anally a permanent, osseous condition. A comparalole ascending series occurs among adult chordates of the present day. It seems that the bones of the higher vertebrates that are descended from the cartilaginous skeleton of Ashes pass through a reminiscent cartilaginous stage, whereas those additional bones made necessary by the increased size of the brain develop directly in membrane.
| |
|
| |
| THE AXIAL SKELETON
| |
| The primitive axial support of all vertebrates is the notochord, or chorda dorsalis, the origin of which has been traced on ])p. 40 and 42. The notochord constitutes the only skeleton of Amphioxus, whereas in Ashes and amphibians it is replaced in part, and in higher animals almost entirely, by the permanent axial skeleton. Among mammals, this supporting rod is transient, except at the intervertebral discs where it persists as the nuclei pitlposi.
| |
|
| |
| The axial skeleton differentiates from mesenchyme, most of which comes from the adjacent pairs of mesodermal segments. Toward the .
| |
|
| |
|
| |
| THE AXIAL SKELETON .
| |
|
| |
|
| |
| 213 .
| |
|
| |
| .
| |
|
| |
| Somatic 7nesoderm .
| |
|
| |
|
| |
| Splanchnic
| |
| 7)t€Soderm .
| |
|
| |
|
| |
| Fig. 21 1. - Transverse section of a 4.5 mm. human embryo, showing the development of the sclerotomes (Kollmann). X about 300.
| |
|
| |
| ,
| |
|
| |
| Spinal ganglion .
| |
|
| |
|
| |
| Dermatome (?) .
| |
|
| |
|
| |
| M votome .
| |
|
| |
|
| |
| Spinal nerve .
| |
|
| |
|
| |
| Arm bud .
| |
|
| |
|
| |
| Proliferating cells of myotome .
| |
|
| |
|
| |
| Mesonephric duct
| |
| Mesonephric tubule and glomerulus
| |
| Ccelom Somatic mesoderm .
| |
|
| |
| .
| |
|
| |
| - S ® a ®9 4
| |
| s'a Q 6 Q 0a
| |
| Of 99 I
| |
| ® Q .
| |
|
| |
|
| |
| Fig. 212. - Transverse section of a 10.3 mm. monkey embryo, showing the sclerotome, myotome and dermatome (Kollmann). A, aorta; *, sclerotome.
| |
|
| |
| ,
| |
|
| |
| 214 .
| |
|
| |
|
| |
| THE SKELETAL SYSTEM .
| |
|
| |
|
| |
| end of the third week, their temporary cavities fill with diffuse, spindleshaped cells, derived from the surrounding walls (Fig. 21 1). The median side of the segment then opens and its mesenchymal content, designated a sclerotome, migrates mesad (Fig. 212). The sclerotomes are the anlages of verte!:>ra2 and ribs.
| |
|
| |
| The Vertebrae and Ribs.- -The sclerotomic mesenchyme comes to lie in paired segmental masses on either side of the notochord, separated from similar masses before and behind by the intersegmenial arteries (Fig. 212). In embryos of about 4 mm., each sclerotome differentiates into a caudal, compact portion and a cranial, less dense half (Fig. 213 A). From the caudal portions, horizontal tissue masses now grow toward the median plane and enclose the notochord, thus establishing the body of each vertebra (Figs. 123 and 214). Similarly, dorsal extensions pass dorsad around the neural tube to form the vertebral arch, and ventro-lateral outgrowths constitute the costal processes. The looser tissue of the cranial halves .
| |
|
| |
|
| |
| Fig. 213. - Frontal sections through the left mesodermal segments of human embryos. A, at about 4 mm., showing the differentiation of the sclerotomes into less dense and denser regions; B , at about 5 mm., illustrating the union of the halves of successive sclerotomes to form the anlages of the vertebne.
| |
|
| |
| .also grows mesad and fills in the intervals between successive denser regions.
| |
|
| |
| The denser, caudal half of each sclerotomic mass presently unites with the less dense, cranial half of the sclerotome next caudad to form the anlages of the definitive vertebra: (Fig. 213 B). Mesenchymal tissue, filling the new intervertebral fissure thus formed, gives rise to the intervertebral discs. Since a vertebra is formed from parts of two adjacent sclerotomes, it is evident that the intersegmental artery must now pass over the body of a vertebra, and the myotomes and vertebra alternate in position.
| |
|
| |
| .Following this blastcmal stage, centers of chondrification appear, two centers in the vertebral body, one in each half of the vertebral arch, and one in each costal process (Fig. 214). These centers enlarge and fuse into a solid cartilaginous vertebra. The original union of the costal processes, which will give rise to ribs, with the vertebral body is temporary, for an .
| |
|
| |
| .
| |
|
| |
| â– T i â– â– â– â– Notochord
| |
| Hi'
| |
| I ,> A nlage of vertebra
| |
| it
| |
| 'â– 'â– i:. $,. Intervertebral fissure C-i i
| |
| ^ - Intersegmental artery .
| |
|
| |
|
| |
| B .
| |
|
| |
|
| |
| THE AXIAL SKELETON .
| |
|
| |
|
| |
| 215 .
| |
|
| |
|
| |
| articulation next the head develops subsequently. T ransverse and articular processes grow out from the vertebral arch, and the rib cartilages, having in the meantime formed tubercles, articulate with the transverse processes somewhat later. The various ligaments of the vertebral column arise from mesenchyme surrounding the vertebree.
| |
|
| |
| .Finally, at the end of the eighth week, the stage of ossification sets in (Fig. 222). A single center appears in the body, one in each half of the arch, and one near the angle of each rib. The replacement of cartilage by bone is not completed until several years after birth. At about the seventeenth year, secondary centers arise in the cartilage still covering the cranial and caudal ends of the vertebral body and form the disc-like, bony epiphyses. These unite with the vertebra proper to constitute a single mass at about the twentieth year.
| |
|
| |
| ,
| |
|
| |
| Neural tube .
| |
|
| |
| .
| |
|
| |
| Fig. 214. - Transverse section through the blastemal stages of vertebra and ribs from a 13 mm. human embryo (partly after Bardeen). X 18. The light areas are centers of chondrification.
| |
|
| |
| ,
| |
|
| |
| While the foregoing account holds for vertebrae in general, a few' deviations occur. When the atlas is formed, a body differentiates as w'ell, but it is appropriated by the body of the epistropheus (axis), thereafter serving as the tooth-like dens of the latter. The sacral and coccygeal vertebrae represent types wdth reduced vertebral arches. At about the tw'entyfifth year the sacral vertebrae unite to form a single bony mass, and a similar fusion occurs between the rudimentary coccygeal vertebra.
| |
|
| |
| The ribs originate in the costal processes wTich are ventro-lateral outgrow - ths from the vertebral bodies (Fig. 214). Each has an early center of chondrification and ossification (Fig. 222). About puberty, two epiphyseal centers appear in the tubercle and one in the head. The highest development of ribs is realized in the thoracic region. In the cervical region they are short; their tubercles fuse wdth the transverse processes and their heads wfith the vertebral bodies, thus leaving intervals, the transverse foramina, through wTich the vertebral vessels course. In the lumbar region, the ribs are again diminutive and are fused to the trans.
| |
|
| |
|
| |
| THE SKELETAL SYSTEM .
| |
|
| |
|
| |
| 216
| |
| verse ])rocesses. The rudimentary ribs of the sacral vertebras are represented by flat plates which unite on each side to form a pars lateralis of the sacrum, (Inly in the first of the coccygeal vertebrae are there traces of ribs.
| |
|
| |
| The Sternum. -Modern studies prove that the sternal anlages arise as paired mesenchymal bands, with which the first eight or nine thoracic ribs fuse secondarily. After the heart descends into the thorax, these cartilaginous sternal bars, as they now may be termed, unite in a craniocaudal direction to form the sternum, at the same time incorporating a smaller, mesial sternal anlage (Fig. 215). Ultimately, one or two pairs of the most caudal ribs lose their sternal connections, the corresponding .
| |
|
| |
| .
| |
|
| |
| Fig. 215. - The sternum of a human fetus Fig. 216. - Sternum of a child, showing centduring the third month. ers of ossification.
| |
|
| |
| .portion of the sternum constituting the xiphoid process in part. At the cranial end of the sternum there are two imperfectly separated episternal cartilages with which the clavicles articulate. These usually unite with the longitudinal bars and contribute to the formation of the manubrium. Variations in the ossification centers are not uncommon, although a primitive, bilateral, segmental arrangement is evident (Fig. 216). In the two cranial segments, however, unpaired centers occur.
| |
|
| |
| The Skull. -The head skeleton includes three primary components: (i) the brain case; (2) capsular investments of the sense organs; (3) a branchial-arch skeleton, derived from the peculiar arches that enclose the first part of the alimentary tract in all embryos and in adult fishes and tailed amphibia (cf. p. 77). Apart from exceptions in the third group, these elements unite intimately into a composite mammalian skull.
| |
|
| |
| The notochord originally extends into the head as far as the pharyngeal membrane. Not only is the skull built around it, but the accommoda.
| |
|
| |
|
| |
| THE AXIAL SKELETON .
| |
|
| |
|
| |
| 217 .
| |
|
| |
|
| |
| tion of the cerebral hemispheres has made necessary a prechordal development which includes those bones in front of the sella turcica.
| |
|
| |
| The earliest anlage of the skull is a mass of dense mesenchyme, which, at the end of the first month, envelops the cranial end of the notochord and extends cephalad into the nasal region. Laterally, it forms wings which enclose the neural tube. Mesodermal segments do not form in front of the otocysts, so, except in the occipital region, where there are indications of the incorporation of three or four vertebrae, the skull is from the first devoid of segmentation.
| |
|
| |
| .Early in the second month chondrification begins mesially in the future occipital and sphenoidal regions, and extends cephalad and to a slight .
| |
|
| |
| .
| |
|
| |
|
| |
| - Interparietal
| |
| Supraoccipital --Exoccipital Condyle
| |
| - Basioccipital
| |
| Fig. 2 18. - Occipital bone of a human fetus of four months. The portions still cartilaginous are shown as a homogeneous background.
| |
|
| |
| ,
| |
|
| |
| Fig. 217. - The chondrocranium of a 14 mm. human embryo (Levi in McMurrich). as, Alisphenoid; bo, basioccipital; bs, basisphenoid; eo, exoccipital; ni, Meckel - s cartilage; os, orbitosphenoid; p, periotic; ps, presphenoid: so, sella turcica; s, supraoccipital.
| |
|
| |
| ,
| |
|
| |
| extent dorsad. At the same time, the internal ears become invested with cartilaginous periotic capsules which eventually unite with the occipital and sphenoidal cartilages (Fig. 217). The chondrocranium, as it is termed, is thus confined chiefly to the base of the skull, whereas the bones of the sides, roof, and the face are of membranous origin. Chondrification also occurs more or less extensively in the branchial arches.
| |
|
| |
| In the period of ossification, which now ensues, it beeomes evident that some bones which are separate in adult lower animals fuse to form compound bones in the human skull. The sphenoid and temporal bones, for example, represent five primitive pairs each. As such components may arise either in membrane or cartilage, the mixed nature of various adult bones is explained.
| |
|
| |
| .A striking feature of the fetal skull is the great relative size of the neural portion. The ratio of cranial to facial volume decreases from 8; i at birth to 2.5:1 in the adult.
| |
|
| |
| ,
| |
|
| |
| 2i8 .
| |
|
| |
|
| |
| THE SKELETAL SYSTEM .
| |
|
| |
|
| |
| Ossification of the Chondrocranimu . - The Occipital Bone. - Ossification begins in the occipital region during the third month (Fig. 222). Four centers a])pear at right angles about the foramen magnum (Fig. 218). From the ventral center arises the basilar (hasioccipital) part of the future bone; from the lateral centers the lateral {cxocci pital) portions which bear the condyles; and from the dorsal, originally paired center, the squamous {supraocci pital) part below the superior nuchal line. The squamous .
| |
|
| |
|
| |
| Ala magna Ala parva
| |
| i.-Uisphcnoid) Presphenoid [Orbilosphcnoid) .
| |
|
| |
| .
| |
|
| |
| Basisphenoid process .
| |
|
| |
|
| |
| Fig. 219. - Sphenoid bone of a human fetus of nearly four months. Parts still cartilaginous are represented in stipple.
| |
|
| |
| ,
| |
|
| |
|
| |
| Fig. 220 . - Ethmoid bone of a human fetus of four months.
| |
|
| |
| ,
| |
|
| |
| Squamosum .
| |
|
| |
|
| |
| {interparietal) area above that line is an addition of intramembranous origin. These several components do not fuse completely until about the seventh year.
| |
|
| |
| The Sphenoid Bone. - Ten principal centers arise in the cartilage that corresponds to this bone (Fig. 219): (i and 2) in each ala magna {ali
| |
| sphenoid) - , (3 and 4) in each ala parva {orbitosphenoid ) ; (5 and 6) in the corpus between the alee magnac (basisphenoid) (7 and 8) in each lingula - , (9 and 10) in the corpus between the alee parvae (presphenoid). Intramembranous bone also enters into its composition, forming the orbital and temporal portion of each ala magna and the mesial laminae of each pterygoid Fig. 221. - The left temporal pi'occss (except the hamulus). Fusion of the bone at birth. The portion of various regions is Completed during the first intracartilaginous origin is repre- y0g^j
| |
| suited in stipple. The Ethmoid Bonc. - The ethmoid cartilage
| |
| consists of a mesial mass, which extends from the sphenoid to the tip of the nasal process, and of paired masses lateral to the olfactory fossai. The lower part of the mesial mass persists as the cartilaginous nasal septum, but ossification of the upper portion produces the lamina per pendicularis and the crista galli (Fig. 220). The lateral masses ossify at first into the spongy bone of the ethmoidal labyrinths. From this, the definitive honeycomb structure (ethmoidal cells) and .
| |
|
| |
| .
| |
|
| |
| THE AXIAL SKELETON .
| |
|
| |
|
| |
| 219 .
| |
|
| |
|
| |
| the concha; are formed through evaginations of the nasal mucous membrane and the coincident resorption of bone. (Similar invasions of the mucous membrane and dissolution of bone produce the frontal, sphenoidal, and maxillary sinuses: p. 297.) Fibers of the
| |
| olfactory nerve at first course between the unjoined mesial and lateral masses. Later, cartilaginous, and finally, bony trabeculse surround these bundles of nerve fibers; as the cribriform plates, they . interconnect the three masses.
| |
|
| |
| The Temporal Bone. - Several centers of ossification in the periotic capsule unite to form a single center from which the whole cartilage is .
| |
|
| |
|
| |
| 0
| |
| c
| |
| c
| |
| 1 P i I a I .
| |
|
| |
|
| |
| Fig.
| |
|
| |
| ,
| |
|
| |
| Parietal .
| |
|
| |
| .
| |
|
| |
| ^Interparietal
| |
| Basi- and exoccipital Tympanic .
| |
|
| |
|
| |
| Vertebra .
| |
|
| |
|
| |
| Hill m .
| |
|
| |
|
| |
| Frontal .
| |
|
| |
|
| |
| Temporal Nasal Maxilla Mandible u u merits .
| |
|
| |
|
| |
| -Metacarpal
| |
| -Phalanges .
| |
|
| |
|
| |
| Radius
| |
| Ulna .
| |
|
| |
|
| |
| - Fibula Tibia .
| |
|
| |
|
| |
| \ .
| |
|
| |
|
| |
| - Metatarsal â– Phalanx .
| |
|
| |
|
| |
| 222. - The extent of ossification in a fetus of u weeks (after Broman).
| |
|
| |
| ,
| |
|
| |
| X 1.5.
| |
|
| |
| ,
| |
|
| |
| transformed into the petrous and mastoid portions of the temporal bone (Figs. 221 and 222). The mastoid process is formed after birth by a bulging of the petrous bone; its internal cavities, the mastoid cells. are formed and lined by the evaginated epithelial lining of the middle ear. The squamosal and tympanic portions of the temporal bone are of intramembranous origin, while the styloid process originates from the proximal end of the second, or hyoid branchial arch.
| |
|
| |
| .Membrane Bones of the Skull. - From the preceding account it is evident that, although the bones forming the base of the skull arise chiefly .
| |
|
| |
|
| |
| 220 .
| |
|
| |
|
| |
| THE SKELETAL SYSTEM .
| |
|
| |
|
| |
| in cartilage, they receive substantial contributions from membrane bones. The remainder of the sides and roof of the skull is wholly of intramembranous origin, each of the parietals forming from a single center, the frontal from paired centers (Fig. 222). At the incomplete angles between the ])arietals and their adjacent bones, union is delayed for some time after birth. These membrane-covered spaces constitute the fontanellcs, or - soft spots - .
| |
|
| |
| The vonier forms from two centers in the connective tissue flanking the lower border of the lamina perpendicularis of the ethmoid. The cartilage of the ethmoid thus invested undergoes resorption. Single centers of ossification in the mesenchyme of the facial region give rise to .
| |
|
| |
|
| |
| Fig.
| |
|
| |
| ,
| |
|
| |
|
| |
| Cricoid cartilage ( Meckel's cartilage (1)
| |
| Hyoid cartilage [lesser horn) (u)
| |
| Hyoid cartilage (greater horn) (uI) Thyroid cartilage (IV -|- V)
| |
| -Lateral dissection of the head of a human fetus, showing the derivatives of the branchial arches (after Kollmann).
| |
|
| |
| ,
| |
|
| |
| Malleus (I)
| |
| Incus (I) .
| |
|
| |
|
| |
| Temporal squama .
| |
|
| |
|
| |
| Stapes (u) .
| |
|
| |
|
| |
| Styloid process (u
| |
| Tympanic ring Stylo-hyoid lig. (u) .
| |
|
| |
|
| |
| Mandible .
| |
|
| |
|
| |
| the nasal, lacrimal, and zygomatic, all pure membrane bones. The maxillary and palate bones are described in the next paragraph.
| |
|
| |
| Branchial-Arch Derivatives. - The first branchial arch on each side forks into an upper maxillary and a lower mandibular process (Fig. 64). Cartilage fails to appear in the maxillary processes, due to accelerated development, hence the palate bones and the maxillcr arise directly in membrane (Fig. 222). Each palate bone develops from a single center of ossification. According to recent investigations, two centers contribute to the formation of each maxilla ; one gives rise to the portion bearing the incisor teeth, the other to the remainder of the maxilla.
| |
|
| |
| The entire core of the mandibular process becomes a cartilaginous bar, Meckel - s cartilage, which extends proximally into the tympanic cavity of the ear (Figs. 222 and 223). Alembrane bone, developing distally in the body of the future lower jaw, encloses Meckel - s cartilage and the inferior .
| |
|
| |
|
| |
| THE APPENDICULAR SKELETON .
| |
|
| |
|
| |
| 221 .
| |
|
| |
|
| |
| alveolar nerve, whereas proximally in the ramus the membrane bone merely lies lateral to these structures - hence the position of the adult mandibular foramen. The portion of Meckel - s cartilage invested by bone disappears, while the cartilage proximal to the mandibular foramen becomes in order, the s pheno-mandibular ligament, the malleus, and the incus (p. 310 and Fig. 310).
| |
|
| |
| .Each second branchial arch enters into relation proximally with the periotic capsule. This upper segment of the cartilage becomes the stapes and the styloid process of the temporal bone (Figs. 223 and 310). The succeeding distal portion is transformed into the stylo-hyoid ligament; it connects the stjdoid process with the distal end of the arch, which also undergoes intracartilaginous ossification to form the lesser horn of the hyoid bone.
| |
|
| |
| The cartilage of the third branchial arches ossifies and gives origin to the greater horns of the hyoid bone, while a plate connecting the two arches becomes its body.
| |
|
| |
| The fourth branchial arches differentiate into the cuneiform cartilages and most of the thyroid cartilage.
| |
|
| |
| The fifth branchial arches appear to contribute to the thyroid cartilage and to form the corniculate, arytenoid, and cricoid cartilages.
| |
|
| |
| THE APPENDICULAR SKELETON
| |
| The appendicular skeleton apparently is derived from the unsegmented somatic mesenchyme, and not from the sclerotomes. In embryos of 9 mm., mesenchymal condensations have formed definite blastemal cores in the primitive limb buds (Figs. 212 and 227). Following this condition, the various bones pass through cartilaginous and osseous stages.
| |
|
| |
| The Upper Extremity. - The clavicle is the first bone of the skeleton to ossify, centers appearing at each end (Fig. 222). Prior to ossification, it is composed of a peculiar tissue which makes it difficult to decide whether the bone is intramembranous or intracartilaginous in origin.
| |
|
| |
| The scapula arises as a single plate with two chief centers of ossification (Fig. 222). An early center forms the body and spine. The other, after birth, gives rise to the rudimentary coraeoid process, which in lower vertebrates extends from the scapula to the sternum. Union between the coracoid process and the body is delayed until about the fifteenth year.
| |
|
| |
| The humerus, radius, and ulna ossify from single primary centers and two or more epiphyseal centers (Figs. 209 and 222).
| |
|
| |
| In the cartilaginous carpus there is a proximal row of three, and a distal row of four elements. Other inconstant cartilages may appear, and subsequently disappear or become incorporated into the carpal bones.
| |
|
| |
| ,
| |
|
| |
| 222 .
| |
|
| |
|
| |
| THE SKELETAL SYSTEM .
| |
|
| |
|
| |
| The mctacar pals and phalanges develop from single primary and epiphyseal centers.
| |
|
| |
| The Lower Extremity.- - The cartilaginous plate of the coxal, or hip bone is at first so placed that its long axis is perpendicular to the vertebral column (Fig. 227). Later, it rotates to a position parallel with the vertebral column, and shifts slightly caudad to come into relation with the first three sacral vertebrae (Fig. 222). A retention of the membranous condition in the lower half of each primitive cartilaginous plate accounts for the obturator membrane which closes the foramen of the same name. Three centers of ossification ajjpear, forming the ilium, ischium, and pubis. The three bones do not fuse completely until about puberty.
| |
|
| |
| The general development of the femur, tibia, fibula, tarsus, metatarsus, and phalanges is c|uite similar to that of the corresponding bones of the upper extremity. The patella, like the pisiform of the carpus, is regarded as a sesamoid bone; both develop within tendons.
| |
|
| |
| .Anomalies. - Variations in the size, shape, and number of skeletal parts are common. Developmental arrest and over- development are the prime causative factors. Variations in the number of vertebra? (except cervical) are not infrequent. The last cervical and first lumbar vertebra? occasionally bear ribs, due to the continued development of the primitive costal processes. Cleft sternum or cleft xiphoid process represents an incomplete fusion of the sternal bars. Additional fingers or toes (polydactyly) may occur; the cause is obscure. More rarely, there is fusion between two or more digits {syndactyly). Hare lip and cleft palate are described in an earlier chapter (pp. yq; 8q).
| |
|
| |
| ,
| |
|
| |
| CHAPTER XI .
| |
|
| |
|
| |
| THE MUSCULAR SYSTEM I. THE HISTOGENESIS OF MUSCLE
| |
| The muscular system is composed of specialized cells, called muscle fibers; these form a tissue in which contractility has become the predominant function. The fibers are of three types: (i) smooth, found principally in the walls of the viscera and blood vessels; (2) cardiac, forming the myocardium of the heart; (3) skeletal, chiefly attached to the elements of the skeleton. Of these, cardiac and skeletal muscle are banded with cross stripes ; only skeletal fibers are under voluntary control. All three differentiate from myoblasts of the mesoderm; the only exceptions are the smooth muscles of the iris and sweat glands, which are ectodermal.
| |
|
| |
| Smooth Muscle. - Certain stellate cells of the mesenchyme enlarge and elongate. The resulting, spindle-shaped cells remain attached to each other by cytoplasmic bridges. In the superficial layer of their cytoplasm coalesced granules form coarse, non-contractile myoglia fibrils, similar to the primitive fibrillge of connective tissue (Fig. 224 A). Fine myofibrils then differentiate uniformly throughout the cytoplasm of the myoblasts (Fig. 224 B). They increase in number as development proceeds, while the coarse type diminishes. The cytoplasmic bridges later give origin to white connective-tissue fibers which envelop the muscle cells and bind them together. In older fetuses new muscle elements also arise by mitotic division of existing fibers and by the transformation of apparent interstitial cells.
| |
|
| |
|
| |
| Fig. 224. - Stages in the histogenesis of smooth muscle (adapted from (McGill). . 4 , 13 mm. pig embryo ( X 550) : coalescing granules give rise to coarse myoglia fibrils. B. 27 mm. pig embryo (X 850): both myoglia fibrils and fine myofibrils are present.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Cardiac Muscle. - The cardiac type of involuntary muscle develops from the splanchnic mesoderm that invests the primitive heart tubes
| |
|
| |
|
| |
|
| |
|
| |
| THE MUSCULAR SYSTEjM
| |
|
| |
|
| |
| (Fig. 155). The cells of this myocardial anlage at first form a syncytium in which myojibrils differentiate from the linear union of cytoplasmic granules (Fig. 225, A, B). The myofibrillae arise at the periphery of the syncytial strands of cytoplasm and soon extend long distances through the syncytium (D). They multiply ra])idly (C) and form alternate light and dark bands, as in skeletal muscle. The syncytial character of cardiac muscle })ersists in the adult and the nuclei remain central in position. The intercalated discs, typical of adult cardiac muscle, probably appear in the early months of fetal life.
| |
|
| |
|
| |
| Skeletal Muscle. - All striated voluntary muscle is derived from the mesoderm - ^either from portion of the mesodermal segments (muscles of the trunk, and, possibly, limbs), or from the mesenchyme (muscles of the head). According to Bardeen, the remainder of the primitive segment not involved in forming skeletal tissue constitutes the myotome, and its cells become myoblasts (Fig. 211). On the contrary, Williams finds that in the chick only the cells of the dorsal and mesial walls of a mesodermal segment comprise the myotome (Fig. 212).
| |
|
| |
|
| |
|
| |
| As to the composition of the individual muscle fibers, there is also a difference of opinion. It is generally believed that the myoblasts elongate, and, by the repeated mitotic division of their nuclei, become multi nucleate. Godlewski, however, holds that several myoblasts unite to form a single muscle fiber. At the beginning of differentiation the nuclei lie centrally, surrounded by granular sarcoplasm (Fig. 226 A). These granules become consolidated in rows as the myofibrillce, which increase in number by longitudinal splitting (Fig. 226 B, C). The myofibrillae soon acquire the characteristic transverse bands, and the individual fibrils become so grouped that, in the third month, their dark and light stripes coincide (Fig. 226 C). During development, the muscle fibers increase enormously in size, the nuclei migrate to the surface, and the myofil)rilla} are arranged in bundles, or muscle columns. The fibrils of each column are said to result from the longitudinal splitting of single, primitive myofibrils. For a time new muscle fibers arise also by the division of those already formed.
| |
|
| |
|
| |
|
| |
| u. MORPHOGENESIS OF THE MUSCLES
| |
|
| |
|
| |
|
| |
| The muscles of the body are distributed in two systems ; the visceral musculature, and the skeletal musculature.
| |
|
| |
| The Visceral Musculature. - This group is associated chiefly with the hollow viscera and is under the involuntary control of the sympathetic nervous sytem. Except for the striated cardiac muscle in the wall of the heart, the visceral muscles are smooth. Their commonest arrangement is in orderly sheets or interlacing bundles.
| |
|
| |
|
| |
|
| |
| The Skeletal Musculature. - As the name indicates, these striated muscles come in intimate relation to the skeleton. With the exception of those muscles attached to the branchial arches, they originate from that portion of mesodermal segments designated a myotome, or muscle plate (p. 7; Figs. 21 1 and 212). Mesodermal segments first appear in the occipital region of embryos about 1.5 mm. long (Fig. 58), and the full number of nearly forty is acquired at 6 mm.( Fig. 63). At the latter stage of about five weeks, the myotomes first formed begin the differentiation of muscles. It will be convenient to consider their morphogenesis under three divisions: the muscles of the trunk, limbs, and head.
| |
|
| |
|
| |
|
| |
| Fig. 225. - The histogenesis of cardiac muscle in a 9 mm. rabbit embryo (adapted after Godlewski). A, Linear arrangement of granules; B, coalescence of granules into a fibril; C, fibril splitting; £>, long fibrils extending through syncytium.
| |
|
| |
|
| |
|
| |
| Fig. 226. - Stages in the histogenesis of skeletal muscle (after Godlewski). . 4 , Myoblast of a 13 mm. sheep embryo; B, myofibrils in a myoblast of a lo mm. guinea pig embryo; C, myoblast with longitudinally-splitting, striated myofibrils from an 8.5 mm. rabbit embryo.
| |
|
| |
| .Fundamental Processes . - Although the primitive segmental arrangement of the myotomes is, for the most part, soon lost, their original innervation by the segmental spinal nerves is retained throughout life. For this reason, the history of adult muscles formed by fusion, splitting, or other modifications may be traced with considerable certainty.
| |
|
| |
| ,
| |
|
| |
| 226 .
| |
|
| |
|
| |
| THE MUSCULAR SYSTEM .
| |
|
| |
|
| |
| The changes occurring in the myotonies during the formation of adult muscles are referable to the operation of the following factors;
| |
| 1. A change in direction of muscle fibers from the original craniocaudal orientation in the myotome. The fibers of but few muscles retain their initial orientation parallel to the long axis of the body.
| |
|
| |
| .2. A 'migration of myotonies, wholly or in part, to more or less remote regions. Thus, the latissinius dorsi originates from cervical myotonies, but finally attaches to the lower thoracic and lumbar vertebrae and to the crest of the ilium. Other examples are the serratus anterior and the trajiezius.
| |
|
| |
| .3. A fusion of portions of successive myotonies. The rectus abdominis and sacro-spinalis illustrate this process.
| |
|
| |
| .4. A longitudinal splitting of myotonies into several portions. Examples are found in the sterno- and omo-hyoid and in the trapezius and sterno-niastoid.
| |
|
| |
| .5. A tangential splitting into two or more layers. The oblique and the transverse muscles of the abdomen are formed in this common way.
| |
|
| |
| .6. A degeneration of myotomes, wholly or in part. By this process fascias, ligaments, and a poneuroscs may be produced.
| |
|
| |
| .M uscles of the I'runk.- -Ventral extensions grow out from the cervical and thoracic myotonies (Fig. 212), and a fusion that is well advanced superficially occurs between all the myotonies in embryos of 10 mm. A dorsal, longitudinal column of fused myotonies, however, can still be distinguished from the sheet formed from the combined ventral prolongations (Fig. 227).
| |
|
| |
| .From the su])erficial portions of the dorsal column there arise by longitudinal and tangential splitting the various long muscles of the back and neck, innervated by the dorsal rami of the spinal nerves (Fig. 2 28). The deep portions of the myotomes do not fuse, but give rise to the several intervertebral muscles, which thus retain their primitive segmental arrangement.
| |
|
| |
| Ddie muscles of the neck, other than those innervated by the dorsal rami and those arising from the branchial arches, differentiate from ventral extensions of the cervical myotomes. The muscles of the diaphragm, which in early stages lies at this level, appear to have a like origin. In the same manner, the thoraco-abdominal muscles arise from the more pronounced ventral prolongations of the thoracic myotomes that grow into the body wall along with the ribs (Fig. 228).
| |
|
| |
| The ventral extensions of the lumbar myotomes (except the first) and of the first two sacral myotomes do not participate in the formation of the body wall. If they persist at all, it is possible that they contribute to the formation of the lower limb. The ventral portions of the third .
| |
|
| |
|
| |
| MORPHOGENESIS OF THE MUSCLES .
| |
|
| |
|
| |
| 227 .
| |
|
| |
| .
| |
|
| |
| and fourth sacral myotomes give rise to the muscles of the perineal region.
| |
|
| |
| .Muscles of the Limbs . - It is commonly stated that the muscles of the extremities develop from buds of the myotomes which grow into .
| |
|
| |
|
| |
| Fig. 227. - Reconstruction of a 9 mm. human embryo, to show the partially fused myotomes and the premuscle masses of the limbs (Bardeen and Lewis). X 13. Distally, in the upper extremity, the radius, ulna and hand plate are disclosed; in the lower extremity the hip bone and the border vein show.
| |
|
| |
| The limb anlages. In sharks this is clearly the case, but in birds and mammals distinct buds of this sort do not occur. The segmental nerve supply of the limb muscles of higher animals is merely suggestive, not proof, of a myotomic origin. Nevertheless, a diffuse migration of cells from the ventral portion of human myotomes has been recorded by various observers, recently by Ingalls. These cells soon lose their epithelial character and blend with the undifferentiated mesench}mie of the limb .
| |
|
| |
|
| |
| 228 .
| |
|
| |
|
| |
| THE MUSCULAR SYSTEM .
| |
|
| |
|
| |
| buds (Figs. 212 and 227). From this diffuse tissue, which at about 9 mm. forms condensed, premuscle masses, the limb muscles are differentiated, the proximal ones being the first to appear. The progressive differentiation into distinct muscles reaches the level of the hand and foot in embryos of seven weeks (Fig. 228). The upper limbs naturally maintain an advance over the lower throughout development.
| |
|
| |
| I\uisclcs of the Head . - Distinct mesodermal segments do not occur in the head region. It is possible, however, that a premuscle mass, from .
| |
|
| |
| .
| |
|
| |
| Fig. 228. - Reconstruction of the superficial muscles of a 20 mm. human embryo (Bardeen and
| |
| Lewis in Bailey and Miller). X 4.5.
| |
|
| |
| .which the eye muscles of man are developed, is comparable to three myotomic ^segments having a similar fate in the shark. The muscles of the eyes are activated by the third, fourth, and sixth pairs of cerebral nerves.
| |
|
| |
| The remaining muscles of the head differ from all other skeletal muscles in that they arise from the splanchnic mesoderm of the branchial arches and are innervated by nerves (visceral) of a different category than those (somatic) which supply myotomic muscles (p. 275). The muscles derived from the several arches retain their primitive branchial-arch innervation (Fig. 367). Hence it follows that the mesoderm of the first branchial arch gives rise to the muscles of mastication and to all other muscles associated with the (fifth) trigeminal nerve. Similarly, the muscles of .
| |
|
| |
|
| |
| MORPHOGENESIS OF THE MUSCLES .
| |
|
| |
|
| |
| 229 .
| |
|
| |
|
| |
| expression, and other muscles supplied by the (seventh) facial nerve, originate from the second, or hyoid arch. The third arch appears to be the source of muscles, like the pharyngeal constrictors, which receive branches of the (ninth) glossopharyngeal nerve. The fourth and fifth arches shave the (tenth) vagus nerve; it innervates their derivatives, such as the laryngeal muscles and part of the pharyngeal and palate group.
| |
|
| |
| The muscles of the tongue are supplied by the hypoglossal nerve, originally a member of the spinal series. For this reason, it is assumed that, at least historically, they are derived from myotonies of the occipital region. Yet, according to Lewis, “there is no direct evidence whatever for this statement, and we are inclined to believe from our studies that the tongue musculature is derived from the mesoderm of the floor of the mouth.â€
| |
| Segmentation of the Vertebrate Head. - The vertebrate head undoubtedly consists of fused segments. This was suggested to the earlier workers by the arrangement of the branchial arches {hranckiomerism), by the presence of supposedly significant - neuromeres' in the brain wall (p. 257), and by the discovery, in the embryos of lower vertebrates, of so-called head cavities, homologous with mesodermal segments.
| |
|
| |
| Only the first three head cavities persist; they form the eye muscles. All the remaining muscles of the head are derived from branchiomeres. Even assuming that the branchiomeres represent portions of the primary head somites - and there are recent observations which tend to disprove this - their segmentation still is not comparable to that of the trunk, for the branchial arches are formed by the serial division of splanchnic mesoderm, tissue which in the trunk never segments. The branchial arches, therefore, represent a different sort of metamerism. From what has been said, it is evident that one cannot compare the relation of the cranial nerves to the branchiomeric muscles with the relation of a spinal nerve to its myotomic muscles. For this reason, the cerebral nerves furnish unreliable evidence as to the primitive number of cephalic segments. A'arious investigators have set this number between eight and nineteen.
| |
|
| |
| .Anomalies. - Variations in the form, position, and attachments of the muscles are common. Most of these anomalies are referable to the variable action of the several developmental factors listed on p. 226.
| |
|
| |
| ,
| |
|
| |
| ECTODERMAL DERIVATIVES .
| |
|
| |
|
| |
| CHAPTER Xu
| |
| THE INTEGUMENTARY SYSTEM
| |
| The contribution of ectoderm to the front part of the oral and nasal cavities, and specifically to the development of teeth, tongue, and salivary glands, is described in earlier chapters. Here will be presented the histogenesis of the skin and the development of its accessory epidermal structures. The integument is a double-layered organ; only its epithelium is derived from eetoderm, whereas the fibrous corium is mesodermal.
| |
|
| |
| THE SKIN
| |
| The Epidermis. - The embryonic ectoderm is originally a single sheet of cuboidal cells (Fig. 212), but, at the end of the first month, it consists of .
| |
|
| |
| .
| |
|
| |
|
| |
| Fig. 229 . - Sections of the integument from a three-months - fetus (Prentiss). X 440- -E From the neck, showing at the right a two-layered epidermis and at tlie left the beginning of an intermediate layer; B, from the chin, w'ith three well -developed cjudermal layers.
| |
|
| |
| Two layers. The outer, somewhat flattened cells compose the transient periderm; the hasal cells, of cuboidal or low columnar shape, are the reproducing elements which gradually give rise to new strata above (Fig. 229 A).
| |
|
| |
| During the third and fourth months, the epidermis is typically threelayered, an intermediate stratum being interposed between the basal and periderm cells (Fig. 220 /?). After the fourth month, the epidermis becomes .
| |
|
| |
|
| |
| THE NAILS .
| |
|
| |
|
| |
| highly stratified. The inner layers of actively dividing cells then constitute the definitive stratum germinativum. The outer layers cornify progressively toward the surface. Thus, above the germinative cells is the thin stratum granulosum, containing keratohyalin granules. Next higher, lies the clear stratum lucidum whose fluid, eleidin content is supposed to represent softened keratohyalin. Still nearer the surface, the thickened ectoplasm becomes cornifled, and in the cytoplasm a fatty substance collects; these gradually flattened cells comprise the stratum corneum.
| |
|
| |
| .When the hairs emerge, at about the sixth month, they do not penetrate the outer periderm of the epidermis, but lift it off. Hence, in mammals, this layer is known also as the epitrichuim (layer upon the hair). Desquamated epitrichial and epidermal cells mingle with cast-off lanugo hairs and sebaceous secretions to form the pasty vernix caseosa that smears the fetal skin. Pigment granules appear soon after birth in the cells of the stratum germinativum; these granules are probably elaborated by the cytoplasm. Negro infants are quite light in color at birth, but within six weeks their integument reaches the final degree of pigmentation.
| |
|
| |
| The Derma or Corium. - -The origin of the fibrous layers of the chick - s integument may be traced to that lateral portion of a mesodermal segment termed the cutis plate or dermatome (Fig. 212). It is now claimed that mammals lack a dermatome and that the region usually so designated really is a part of the myotome. In this event, the connective-tissue corium differentiates directly from the mesenchyme subjacent to the epidermis. At about the end of the third month, a distinction between the compact corium proper and the looser subcutaneous tissue becomes recognizable. From the corium, papillae project into the germinative stratum.
| |
|
| |
| .Anomalies. - The deposition of pigment in the epidermis and elsewhere may fail {albinism), or be over-abundant {melanism). The defects of pigmentation sometimes affect local areas only. Navi are either pigmented spots Cmoles - ), or purple discolorations {'birthmarks - ) caused by cavernous vascular plexuses in the corium. Ichthyosis represents an excessive thickening of the stratum corneuni. In severe cases, horny plates, separated by deep cracks, are formed. Dermoid cysts (p. 156), resulting from epidermal inclusions, are not infrequent along the lines of fusion of embryonic structures (e.g., branchial grooves, mid-dorsal and mid-ventral body wall).
| |
|
| |
| THE NAILS
| |
| Nails are modifications of the epidermis that correspond to the claws and hoofs of lower mammals. The nail anlage is recognizable in fetuses of 10 weeks as an epidermal, pouch-like fold that soon extends from the proximal border of the future exposed plate almost to the articulation of the terminal phalanx (Fig. 230 C) \ this proximal nail fold also continues laterally on either side as the lateral nail folds (Fig. 230 A, B).
| |
|
| |
| ,
| |
|
| |
| 2^2 .
| |
|
| |
|
| |
| TJIE INTEGUMENTARY SYSTEM .
| |
|
| |
|
| |
| The material of the nail is developed in the lower lamina of the ]:>roximal nail fold (Tig. 230 C). Certain of the epidermal cells, which, according to Bowen, represent a modified stratum lucidum, develop keratin fil)rils during the fifth fetal month. These appear without the preliminary keratohyalin stage, as is the case in ordinary epidermis. The cells flatten and form the compact mass of which the nail plate is composed. Thus, the nail substance differentiates in the proximal nail fold as far distad as the outer edge of the Imuila (the whitish crescent at the base of the adult nail). Beyond the lunule, the underlying epidermis takes .
| |
|
| |
|
| |
| A B .
| |
|
| |
| .
| |
|
| |
| Fk;. 230. - The develo])ment of the human nail (Kollman). . 1 , 10 weeks (X 20); B, 14 weeks (X 1,3); C, longitudinal section at 14 weeks (X 24).
| |
|
| |
| .no active part in development. The stratum corneum and periderm of the epidermis for a time cover completely the free nail and are termed the eponychium (Fig. 230 C). In late fetuses this is lost, but portions^ of the horny layer persist as the curved rim of epidermis which adheres to the base of the adult nail. During life the nail constantly grows at its base (proximally), is shifted distally over the nail bed, and projects at the tip of the digit. The coriuni throws its surface of contact with the nail into parallel longitudinal folds that produce the characteristic ridging.
| |
|
| |
| THE HAIR
| |
| Hairs are specialized epidermal gowths. The earliest begin to develop at the end of the second month on the eyebrows, upper lip, and chin; those of the general integument appear at the beginning of the fourth month.
| |
|
| |
| The first evidence of a hair anlage is the crowding and elongation of a cluster of germinative cells (Fig. 231, A). Their bases sink bud-like into .
| |
|
| |
|
| |
| THE H.AIR .
| |
|
| |
|
| |
| 233 .
| |
|
| |
|
| |
| the corium, and active proliferation soon produces a cylindrical epithelial plug (Fig. 23 ] , 5 , C). This hair anlage consists of an outer wall of colum.
| |
|
| |
| .
| |
|
| |
| Ccnlral cells
| |
| Epidermal anlage of hair B
| |
| Anlage of hair papilla .
| |
|
| |
|
| |
| Epitrichium .
| |
|
| |
|
| |
| Epidermal anlage hair A .
| |
|
| |
|
| |
| Anlage of hair papilla .
| |
|
| |
|
| |
| Epidermal anlage of hair C .
| |
|
| |
|
| |
| Mesefichymal Hair Hair papilla' .
| |
|
| |
|
| |
| Fig. 231. - Section through the facial integument of a three-months - fetus, showing three stages in the early development of hair (Prentiss). X 330.
| |
|
| |
| ,
| |
|
| |
|
| |
| Fig. 232. - Longitudinal section through a developing hair from a five and one-half months -
| |
| fetus (Stdhr in Prentiss). X 220.
| |
|
| |
| .nar cells, continuous with the basal layer of the epidermis, and an internal mass of polyhedral cells. About the whole is a mesenchymal sheath, and at its base the mesenchyme condenses into a mound-like papilla.
| |
|
| |
| ,
| |
|
| |
| 2,34 .
| |
|
| |
|
| |
| THE INTEGUMENTARY SYSTEM .
| |
|
| |
|
| |
| As development proceeds and the hair anlage pushes deeper into the corium, its base enlarges into the bulb which becomes moulded over the papilla (Fig. 232). The actual hair substance is a proliferation from the basal epidermal cells next the papilla. These cells give rise to an axial core, destined to become the inner sheath and shaft, which grows toward the surface (Fig. 232). Entirely distinct are the peripheral cells of the original anlage which constitute the outer sheath.
| |
|
| |
| The hair grows at its base and is pushed up through the central cells of the primordial downgrowth. Above the level of the bulb, the cells of the hair shaft cornify and differentiate into an outer cuticle, middle cortex, and central, inconstant medulla. Two swellings of the outer hair sheath appear on the lower side of the obliquely directed follicle. (Fig. 232). The upper of these becomes the associated sebaceous gland; the deeper swelling is the epithelial bed, a region of rapid mitosis that contributes to the growth of the hair follicle. Mesenchymal tissue near the epithelial bed transforms into the smooth fibers of the arrcctor pili muscle. Pigment granules develop in the basal cells of the hair and cause its characteristic coloration.
| |
|
| |
| The first generation of dense, silky - lanugo - hairs are short-lived, all except those covering the face being cast off soon after birth. The coarser, replacing hairs develop, at least in part, from new follicles. Thereafter, hair is shed and regenerated periodically throughout life.
| |
|
| |
|
| |
|
| |
|
| |
| Anomalies. -- Hypertrichosis refers to excessive hairiness which may be general or local, as in exhibited - hairy monsters. - In the rare hypotrichosis, the congenital absence of hair is usually associated with defective teeth and nails.
| |
|
| |
|
| |
|
| |
| SEBACEOUS GLANDS
| |
|
| |
| Nine-tenths of all sebaceous glands accompany hairs but independent ones occur also, such as those around the nostrils, anus, and eyelids. They appear first in the fifth month as solid buds of the epidermis, especially that of the hair follicles (Fig. 232). The anlage becomes a lobulated sac. A lumen forms by the fatty degeneration of the central cells, and the resultant oih" secretion is an important constitutent of vernix caseosa (p. 231).
| |
|
| |
|
| |
|
| |
|
| |
| SWEAT GLANDS
| |
|
| |
| Sudoriferous glands begin to develop in the fourth month from the epidermis of the finger tips, the palms of the hands, and the soles of the feet. They are formed as solid downgrowths from the epidermis, but differ from hair anlages in being more compact and in lacking the mesenchymal papillie at their bases (Fig. 233, A, B). During the sixth month the simple cords coil, and, in the seventh month, their lumina appear (Fig. 233, C, D). The inner layer of eells forms the gland cells, while the outer cells become transformed into smooth muscle fibers, which, accordingly, are ectodermal. In the axilla, eyelids, and external acoustic meatus the sweat glands are large and branched.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 233. - Vertical sections of the integument, illustrating four stages in the development of a sweat gland (adapted from Kollman). A, B, Four months; C, D, seven months.
| |
|
| |
|
| |
| MAMMARY GLANDS
| |
|
| |
|
| |
| Mammary glands are peculiar to mammals. In embryos of about 9 mm. paired ectodermal thickenings extend lengthwise between the bases of the limb buds (Fig. 234). This linear ridge is the milk line. In man it often is inconspicuous except in the pectoral region, but in lower mammals, like the pig, that have serially repeated glands, a prominent thickening reaches from axilla to groin (Fig. 389).
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 234. - Human embryo of 13.5 mm. with a prominent milk line (after Kollman). X 5.
| |
|
| |
|
| |
|
| |
| THE INTEGUMENTARY SYSTEM .
| |
|
| |
|
| |
| Each human mammary gland begins as a thickening and downgrowth from the epidermal milk line in the region of the future breast (Fig. 235 ^)During the fifth month, from 15 to 20 solid cords bud off {B). These anlages of the milk ducts branch in the mesenchymal tissue of the corium, hollow out, and eventually produce the alveolar oid pieces (C). Where the milk ducts open on the surface the epidermis is elevated to form the nipple, but this may be delayed until after birth. The glands yield a little secretion ( - witch milk - ) at birth; they enlarge rapidly at puberty and are further augmented during pregnancy, while two or three days after parturition they become functionally active.
| |
|
| |
|
| |
| The mammary glands are regarded by most authorities as modified sweat glands. This homology is made because their development is similar, and because in the lower mammals their structure is the same. Rudimentary mammary glands (areolar glands of Montgomery), which also resemble sweat glands, occur in the areola about the nipple. In many mammals, numerous pairs of mammary glands are developed along the milk line (pig; dog) ; in some a single pair occurs in the pectoral region (primates; elephant); in others, they are confined to the inguinal region (sheep; cow; horse).
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 235. - Sections rei>rcsenting three stages in the development of the human mammary gland (Tourneux). A, Two months; B, four months; C, seven months.
| |
|
| |
|
| |
|
| |
|
| |
| Anomalies.- -Supernumerary mammary glands (hypermaslia) or nipples (liy perihelia) between the axilla and groin are common. These represent independent differentiations along the primitive milk line, such as occur normally in some mammals.
| |
|
| |
|
| |
|
| |
| ++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
| |
|
| |
| CHAPTER XuI .
| |
|
| |
|
| |
| THE CENTRAL NERVOUS SYSTEM I. HISTOGENESIS OF THE NERVOUS TISSUES .
| |
|
| |
|
| |
| The nervous tissues and sensory epithelia are derived from portions of the primitive integument. The anlage of the entire nervous system is a thickened band of ectoderm along the mid-dorsal line of the embryo. This is the neural plate (Figs. 57 and 58), which, in embryos of 2 mm., develops a deep neural groove, bounded laterally by paired neural folds (Fig. 236 A-C). The folds presently meet and fuse, thereby forming the neural tube (D) which lies below the surface of the general ectoderm and becomes separated from it (Fig. 241). The cells of this tube, and its associtaed ganglion crests, give rise to all the nervous tissues, with the single exception of the nerve cells and fibers of the olfactory epithelium.
| |
|
| |
|
| |
| Fig. 236. - Sections of the developing neural tube in human embryos (adapted by Prentiss). A, An early stage; B, 2 mm.; C, 2 mm.; D, 2.7 mm.
| |
|
| |
|
| |
|
| |
| The cells of the neural tube differentiate into two products. These are nerve cells, in which irritability and conductivity have become the predominant functions, and neuroglia cells, which constitute the distinctive supporting tissue of the nervous system. The wall of the neural tube, consisting at first of a single layer of columnar cells (Fig. 237 A), becomes many-layered; the com]jonent cells lose their sharp outlines and form a comijact syncytium which is bounded, on its outer and inner surfaces, by an external and lutcrual limiting membrane (B, C ). In lo mm. embrvos, the cellular strands of the syncytium are arranged radially and nearly parallel (D). The nuclei are now so grouped that there may be distinguished three layers: (i) an inner ependymal zone, with cells abutting on the internal limiting membrane and their processes extending peripherally; (2) a middle, nucleated mantle zone; and (3) an outer, non-cellular marginal zone, into which nerve fibers grow. The ependymal zone contributes cells for the development of the mantle layer (A~D). The cellular mantle layer forms the gray substance of the central nervous system, while the fibrous marginal layer constitutes the white substance.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 237. - The differentiation of the neural tube (Hardesty). X 690. . 4 , From a rabbit
| |
| embryo before the closure of the neural tube; B, from a 5 mm. pig embryo after closure; C, from a 7 mm. pig embryo; /t, from a 10 mm. pig embryo. *, Boundary between mantle and marginal layers.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| The primitive germinal cells of the neural tube divide by mitosis and give rise to the ependymal cells of the ependymal zone and to indifferent cells of the mantle layer (Fig. 238). From these latter differentiate spongioblasts and neuroblasts. The spongioblasts transform into neuroglia cells Sind fibers, which become the supporting tissue of the central nervous system ; the neuroblasts are primitive nerve cells, which develop cell processes and are converted into neurons. A neuron is the structural and functional unit of nervous tissue.
| |
|
| |
|
| |
| The Differentiation of Neuroblasts. - ^The nerve fibers develop as outgrowths from the neuroblasts, and a nerve cell with all its processes constitutes a neuron. The origin of the nerve fibers as processes of the neuroblasts is seen best in the development of the root fibers of the spinal nerves.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 238. - Diagrams showing the differentiation of the cells of the neural tube (after Schaper).
| |
|
| |
|
| |
|
| |
| The Development of Efferent Neurons. - ^At the end of the first month, clusters of neuroblasts separate from the general syncytium in the mantle layer of the neural tube. The neuroblasts become pear-shaped, and from the small end of the cell a slender, primary process grows out (Fig. 239 A, - F'\ B). This process is the axon, or axis cylinder. Such primary processes may course in the marginal layer of the neural tube (Fig. 239 A, a), or, converging, may penetrate the marginal layer ventro-laterally and form the ventral roots of the spinal nerves (Fig. 240). Similarly, the efferent fibers of the cerebral nerves grow out from neuroblasts of the brain wall. Within the cytoplasm of young nerve cells and their primary processes, strands of fine fibrils occur (Fig. 239 A). These, the ncurofibrillcc, are usually assumed to be the conducting elements of the neurons. The cell bodies of the efferent neurons soon become multipolar by the development of branched secondary processes, the dendrons or dendrites.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 239. - The differentiation of neuroblasts in chick embryos of the third day (Cajal). A, Transverse section through the spinal cord, showing axons (F) growing from neuroblasts into the ventral root, and from bipolar ganglion cells (i/) into the dorsal root. B Single neuroblasts, showing neurofibrils and growing tip(*).
| |
|
| |
|
| |
|
| |
| Fig. 240. - Transverse section of the spinal cord from a human embryo of five weeks, showing the origin of ventral root fibers from neuroblasts (His). X 130.
| |
|
| |
| ,
| |
|
| |
| HISTOGENESIS OF THE NERVOUS TISSUES .
| |
|
| |
|
| |
| 241 .
| |
|
| |
|
| |
| Development of the Spinal Ganglia and Afferent Neurons. - After the formation of the neural plate and groove, a longitudinal ridge of cells appears on each side where the ectoderm and neural plate join (Fig. 241 A). This ridge of ectodermal cells is the neural, or ganglion crest. When the neural tube closes and the ectoderm separates from it, the cells of the ganglion crest overlie the neural tube dorso-laterally (Fig. 241 B, C). As development continues they separate into right and left linear crests, distinct from the neural tube, and migrate ventro-laterally to a position .
| |
|
| |
|
| |
| Neural crest .
| |
|
| |
| .
| |
|
| |
| Fig. 241. - The development of the ganglion crest in a 2.5 mm. human embryo (after
| |
| Lenhossek).
| |
|
| |
| Between the neural tube and myotomes (Fig. 212). In this position, the ganglion crest forms a band of cells extending the whole length of the spinal cord and as far cephalad as the otic vesicles. At regular intervals in its course along the spinal cord, the proliferating cells of the crest give _-,i. - /,â– ,
| |
| ri^se to enlargements, the spinal ganglia (Figs. 278 and 279). The spinal a -/V ganglia are arranged segmentally and are connected at first by cellular bridges that later disappear. In the hind-brain region, certain ganglia •
| |
| of the cranial nerves develop also from the crest but are not segmentally arranged.
| |
|
| |
| The cells of the spinal ganglia differentiate into ganglion cells and supporting cells, groups which are comparable to the neuroblasts and spongioblasts of the neural tube. The neuroblasts of the ganglia become fusiform and develop a primary process at each pole; thus, these neurons
| |
| 16 .
| |
|
| |
|
| |
| />• -j-f .
| |
|
| |
| .C uW a. No fo ^ I .
| |
|
| |
|
| |
| C , 7 - ^£^2 ^ y .
| |
|
| |
| ,
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| .
| |
|
| |
| 2_12 .
| |
|
| |
|
| |
| THE CENTRAL NERVOUS SYSTEM .
| |
|
| |
|
| |
| are of the bipolar type (Fig. 23Q A, d). The centrally directed processes of the ganglion cells converge, and, by elongation, form the dorsal roots. They penetrate the dorso-lateral wall of the neural tube, bifurcate, and course cranially and caudally in the marginal layer of the spinal cord. By means of branched processes they come in contact with the neurons of the mantle layer. The peripheral processes of the ganglion cells, as the dorsal spinal roots, join the ventral roots, and with them constitute the trunks of the spinal nerves (Fig. 246).
| |
|
| |
| .At first bijjolar (Fig. 242, A), the majority of the ganglion cells become unipolar, either by the fusion of the two primary processes or by .
| |
|
| |
| .
| |
|
| |
| Fig. 242. - Stages in the formation of unipolar ganglion cells (Cajal). From a human fetus of
| |
| ten weeks.
| |
|
| |
| ,
| |
|
| |
| the bifurcation of a single process (C). The process of the unipolar ganglion is then T-sha])ed (B)- Many of the bipolar ganglion cells persist in the adult, while others develop several secondary processes and thus become multipolar in form. In addition to forming the spinal and cranial ganglion cells, neuroblasts of the ganglion crest are believed to migrate ventrally and form the sympathetic ganglia (Fig. 246).
| |
|
| |
| Differentiation of the Supporting Elements. In the Neural Tube . - â– The si)ongioblasts of the neural tube differentiate into the supporting tissue of the central nervous system. This includes the ependymal cells, which line the neural cavity and constitute one of the primary layers of the neural tube, and neuroglia cells and their fibers.
| |
|
| |
| ,
| |
|
| |
| HISTOGENESIS OF THE NERVOUS TISSUES .
| |
|
| |
|
| |
| 243 .
| |
|
| |
|
| |
| A preceding paragraph describes how the strands of the syncytium, formed by the spongioblasts, become arranged radially in the neural tube of early embryos (Fig. 237 D). As the wall thickens, the strands elongate equally and form a radiating, branched framework (Fig. 243). The group of spongioblasts which lines the neural cavity constitutes the ependymal layer. Processes from these cells extend through the neural tube, even to its periphery. The cell bodies are columnar and persist as the lining of the central cavities of the spinal cord and brain (Fig. 244).
| |
|
| |
| .Near the midplane of the adult spinal cord, both dorsally and ventrally, the supporting tissue retains its primitive ependymal structure .
| |
|
| |
|
| |
| B .
| |
|
| |
| .
| |
|
| |
| Fig. 243. - Ependymal cells from the neural tube of a chick (Cajal). . 4 , Embryo of first day;
| |
| B, of third day.
| |
|
| |
| ,
| |
|
| |
| (Fig. 244). Elsewhere, the supporting framework is differentiated into neuroglia cells and fibers. The neuroglia cells form part of the spongioblastic syncytium and are scattered through the mantle and marginal layers of the neural tube. By proliferation they increase in number, and their form depends upon the pressure of the nerve cells and fibers which develop around them. Neuroglia fibers are differentiated in a manner comparable to that of connective-tissue fibers (Fig. 203). As the cytoplasmic processes of the neuroglia cells primarily form a syncytium, the fibers may extend from cell to cell. The neuroglia fibers develop late in fetal life and undergo a chemical transformation into neurokeratin, the same substance that is found in the sheaths of myelinated fibers.
| |
|
| |
| ,
| |
|
| |
| 244 .
| |
|
| |
|
| |
| THE CENTRAL NERVOUS SYSTEM .
| |
|
| |
|
| |
| Supporting Elements of the Ganglia. - The supporting cells of thespinal ganglia at first form a syncytium, in the meshes of which are found the neuroblasts. They differentiate into flattened capsule eells, which encapsulate the ganglion cells, and into sheath cells, which envelop the axon processes of both dorsal and ventral root fibers and are continuous with the capsules of the ganglion. It is certain that many of the sheath cells migrate peripherally along with the developing nerve fibers. They are .
| |
|
| |
| .
| |
|
| |
| Fig. 244. - Ependymal cells of the spinal cord, from a fetus of ten weeks (Cajal). /I, Floor plate; B, central canal; C, line of future fusion of neural walls; E, ependymal cells; *, neuroglia cells and fibers.
| |
|
| |
| ,
| |
|
| |
| at first spindle-shaped, and, as primary sheaths, enclose bundles of nerve fibers. Later, by the proliferation of the sheath cells, the bundles are separated into single fibers, each with its sheath of Schwann, or neurilemma. Every sheath cell forms a segment of the neurilemma, the limits of contiguous cells being indicated by constrictions, the nodes of Ranvier.
| |
|
| |
| The Myelin Sheath. - -During the fourth month an inner myelin, or medullary sheath appears about many nerve fibers. This consists of a spongy framework of neurokeratin in the interstices of which a fatty substance, myelin, is deposited. The origin of the myelin sheath is in doubt.
| |
|
| |
| ,
| |
|
| |
| MORPHOGENESIS OF THE CENTRAL NERVOUS SYSTEM .
| |
|
| |
|
| |
| 245 .
| |
|
| |
|
| |
| By some ( Ranvier) it is believed to be a differentiation of the neurilemima, the myelin being deposited in the substance of the nucleated sheath cell. Others regard the myelin as a direct product of the axis cylinder (Kolliker), or as an intercellular substance precipitated through its influence (Bardeen). The integrity of myelin is dependent at least upon the nerve cell and axis cylinder, for, when a nerve is cut, it very soon shows degenerative changes. In the central nervous system there is no distinct neurilemma sheath investing the fibers. However, sheath cells are said to be present and most numerous during the period when myelin is developed. Hardesty traces their origin to the spongioblastic supporting cells of the neural tube, and believes that the myelin of the fibers arises in the interspaces.
| |
|
| |
| The myelinated fibers, those with a myelin sheath, have a glistening white appearance and give the characteristic color to the white substance of the central nervous system and to the peripheral nerves. The fibers which are first functional receive their myelin sheaths first. This process is only completed during the third year of infancy. Many of the peripheral fibers, especially those of the sympathetic system, remain unmyelinated but are supplied with a neurilemma sheath. Large numbers of unmyelinated fibers occur also in the peripheral nerves and spinal cord.
| |
|
| |
| The Neuron Doctrine. - The neuron concept of the development of nerve fibers is the one generally adopted at the present time. It assumes that all axons and dendrites are formed as outgrowths from nerve cells, an hypothesis first promulgated by His. The embryological evidence is supported by experiment. It has long been known from the work of Waller that if nerves are severed, the fibers distal to the point of section, and thus isolated from their nerve cells, will degenerate, but that regeneration will take place from the central stumps of cut nerves, the fibers of which are still connected with their cells. IMore recently, Harrison, experimenting on amphibian larvse, has shown that peripheral nerves do not develop if the neural tube and crest are removed, and that isolated ganglion cells growing in clotted lymph will give rise to long axon processes in the course of four or five hours.
| |
|
| |
| .A second theory, supported by Schwann, Balfour, Dohm, and Bethe, but not widely credited, assumes that the nerve fibers are in part differentiated from a chain of cells, so that the neuron would represent a multicellular, not a unicellular structure. Apathy and 0. Schulze modified this cell-chain theory by assuming that the nerve fibers differentiate in a syncytium which intervenes between the neural tube and the peripheral end organs. Held further modified the theory by claiming that the proximal portion of a nerve fiber is derived from the neuroblast or ganglion cell and that this grows into a syncj-ffium which gives rise to the peripheral portion .
| |
|
| |
| u. Morphogenesis of the Central Nervous System
| |
| The primitive neural tube is formed by the folding of the neural plate into an epithelial tube, as described in the previous section. The groove begins to close in embryos of 2 mm. along the mid-dorsal line, near the middle of the body, and the closure advances in both directions (Fig. 245).
| |
|
| |
| ,
| |
|
| |
| 246 .
| |
|
| |
|
| |
| THE CENTRAL NERVOUS SYSTEM .
| |
|
| |
|
| |
| Until after the fourth week, however, there still persists a neuroporic opening at each end of the neural tube, somewhat dorsad (Fig. 251). But before the closure of the neuropores, even in embryos of 2.5 mm. or less, the cranial end of the neural tube has enlarged and constricted at two points to form the three primary brain vesicles. The caudal two-thirds .
| |
|
| |
| .
| |
|
| |
| Fig. 245. - Human embryo of 2.4 mm., with a ])artially closed neural tube (Kollmanni. X 30.
| |
|
| |
| Of the neural tube, which remains smaller in diameter, is the anlage of the spinal cord.
| |
|
| |
| THE SPINAL CORD
| |
| The spinal portion of the neural tube is at first nearly straight, but as the embryo flexes it also is bent into a curve, convex dorsally (Fig. 282). Its wall gradually thickens during the first month and the diameter of the central canal is diminished from side to side. By the end of the first month, three layers have been developed in the manner already described (Fig. 246). These layers are the inner ependymal layer, which forms a narrow zone about the neural cavity, the middle, cellular mantle layer, and the outer, fibrous marginal layer.
| |
|
| |
| The Ependymal Layer differentiates into a dorsal roof plate and a ventral floor plate (Fig. 247). Laterally, its proliferating cells contribute .
| |
|
| |
|
| |
| THE SPINAL CORD .
| |
|
| |
|
| |
| 247 .
| |
|
| |
| .
| |
|
| |
| Mantle layer .
| |
|
| |
|
| |
| Dorsal ramus .
| |
|
| |
|
| |
| Ventral
| |
| root .
| |
|
| |
|
| |
| Marginal layer .
| |
|
| |
|
| |
| Dorsal Ependymal layer Spinal .
| |
|
| |
|
| |
| Neural cavity .
| |
|
| |
|
| |
| Nerve trunk Sympathetic ganglion
| |
| Fig. 246. - Transverse section through a 10 mm. human embryo at the level of the arm budsshowing the spinal cord and a spinal nerve (Prentiss). X 44.
| |
|
| |
| ,
| |
|
| |
|
| |
| X 44 .
| |
|
| |
| 248 .
| |
|
| |
|
| |
| THE CENTRAL NERVOUS SYSTEM .
| |
|
| |
|
| |
| neuroblasts and neuroglia cells to the mantle layer. This proliferation ceases first in the ventral floor, which is thus narrower than the dorsal portion in 10 to 20 mm. embryos (Figs. 246 and 247). The neural cavity is for a time somewhat rhomboidal in transverse section, wider dorsally than ventrally. Its lateral angle forms the sulcus limitans (Fig. 255), which marks the subdivision of the lateral walls of the neural tube into the donsal alar plate (sensory) and ventral basal plate (motor). When the ependymal layer ceases to contribute new cells to the mantle layer, its walls are a])proximated dorsally (Fig. 247). At about nine weeks, .
| |
|
| |
| .
| |
|
| |
| Fi<;. 248. - Transverse section of the s])inal cord and ganglion from a fetus of nine weeks
| |
| (Prentiss). X 44.
| |
|
| |
| These walls fuse and the dorsal portion of the neural cavity is obliterated (Fig. 248); in a fetus of three months, the persisting cavity is becoming rounded into the definite central canal (Fig. 249). The cells lining the central canal are ependymal cells proper. Those in the floor of the canal form the persistent floor plate. Their fibers extend ventrad, reaching the surface of the cord in the depression of the ventral median fissure.
| |
|
| |
| .When the right and left walls of the ependymal layer fuse, the ependymal cells of the roof plate no longer radiate, but form a median septum (Fig. 248). Later, as the marginal layers of either side thicken and are approximated, the median septum is extended dorsally. Thus, the roof plate is converted into part of the dorsal median septum of the adult spinal cord (Fig. 249).
| |
|
| |
| The Mantle Layer, as already described, receives contributions from the proliferating cells of the ependymal layer. A ventro-lateral thickening first becomes prominent in embryos of 10 to 15 mm. (Fig. 246). This is the ventral {anterior) gray colunm, which in later stages is subdivided.
| |
|
| |
| ,
| |
|
| |
| THE SPINAL CORD .
| |
|
| |
|
| |
| 249 .
| |
|
| |
|
| |
| forming also a lateral gray colmnu (Fig. 249). It is a derivative of the basal plate. In embryos of 20 mm., a dorso-lateral thickening of the mantle layer is seen, the cells of which constitute the dorsal (posterior) gray column (Figs. 248 and 249); about these cells the collaterals of the dorsal root fibers end. The cells of the dorsal gray column, derivatives of the alar plate of the cord, thus form terminal nuclei for the afferent spinal nerve fibers. Dorsal and ventral to the central canal, the mantle layer forms the dorsal and ventral gray commissures. In the ventral floor plate, nerve fibers cross from both sides of the cord as the ventral white commisstire.
| |
|
| |
| ,
| |
|
| |
| Dorsal median septum Fasciculus gracilis .
| |
|
| |
| .
| |
|
| |
| Fig. 249. - Transverse section of the spinal cord from a fetus of three months (Prentiss).
| |
|
| |
| .X 44 .
| |
|
| |
| The Marginal Layer is composed primarily of a framework of neurogliaand ependymal-cell processes. Into this framework grow the axons of nerve cells, so that the thickening of the marginal layer is due to the increasing number of nerve fibers contributed to it by extrinsic ganglion cells and neuroblasts. When their myelin develops, these fibers form the white substance of the spinal cord.
| |
|
| |
| The fibers have three sources (Fig. 281) ; (i) they may arise from the spinal ganglion cells, entering as dorsal root fibers and counsing cranially and caudally in the marginal layer; (2) they may arise from neuroblasts in the mantle layer of the spinal cord, (a) as fibers which connect adjacent nuclei of the cord (fasiculi propru or ground bundles), or (b) as fibers which extend upward to the brain; (3) they may arise from neuroblasts of the brain, (a) as descending tracts from the brain stem, or (6) as long, descending corticospinal tracts from the cortex of the cerebrum. Of these fiber tracts, (i) and (2 a) appear during the first month; (2 b) and (3 a) dui'ing the third month; (3 b) at the end of the fifth month.
| |
|
| |
| ,
| |
|
| |
| THE CENTRAL NERVOUS SYSTEM .
| |
|
| |
|
| |
| 250 .
| |
|
| |
|
| |
| The dorsal root fibers from the spinal ganglion cells, entering the cord dorso-laterally, subdivide the white substance of the marginal layer into dorsal and lateral funiculi (Fig. 248). The lateral funiculus is marked off by the ventral root fibers from the ventral funiculus (Fig. 246). The ventral root fibers, as we have seen, take their origin from the neuroblasts of the ventral gray column in the mantle layer. They are thus derivatives of the basal plate.
| |
|
| |
| The dorsal funiculus is formed chiefly by the dorsal root fibers of the ganglion cells, and is subdivided into two distinct bundles, the fasicul us f^racilis, median in position, and the fascicul us nnicalus, lateral (Fig. 240). The dorsal funiculi are separated only by the dorsal median septum.
| |
|
| |
| The lateral and ventral funiculi are composed: (i) of fasciculi propru, or ground bundles, originating in the spinal cord; (2) of ascending tracts from the cord to the brain; (3) of the descending fiber tracts from the brain. The fibers of these fasciculi intermingle and the fasciculi are thus without sharp boumlaries. The floor plate of ependymal cells lags behind in its development, and, as it is interposed between the thickening right and left walls of the ventral funiculi, these do not meet and the ventral median fissure is produced (cf. Figs. 246 and 240).
| |
|
| |
| The development of myelin in the nerve fibers of the cord begins late in the fourth month of fetal life and is completed between the fifteenth and twentieth years. Myelin appears first in the root fibers of the spinal nerves and in those of the ventral commisSure,
| |
| next in the ground fjundles and dorsal funiculi. The corticospinal (pyramidal) fasciculi are the tardiest; they become myelinated during the first and second years. As myelin appears in the various fiber tracts at different periods, this condition has been utilized in tracing the extent and origin of the various fasciculi in the central nervous system.
| |
|
| |
| The Cervical and Lumbar Enlargements. -
| |
| The spinal cord enlarges at the levels of the two nerve plexuses supplying the upper and lower extremities. As the fibers to the muscles of the extremities arise from nerve cells in the ventral gray column, the number of these cells and the mass of the gray substance is increased ; since larger numbers of fibers from the integument of the limbs also enter the cord at this level, there are likewise present more cells about which sensory Ihe brain and cord of a three- ^^ers terminate. Consequently, there is formed months - fetus (Kouiker). at the level of the origin of the nerves of the
| |
| brachial plexus the cervical enlargement, and opposite the origins of the nerves of the lumbo-sacral plexus the lumbar enlargement (Fig. 250).
| |
|
| |
|
| |
| After the third month, the vertebral column grows faster than the spinal cord. Since the cord is anchored to the brain, the vertebrae and the associated roots and ganglia of the spinal nerves shift caudally along the cord. For this reason, the origin of the coccygeal nerves in the adult is opposite the first lumbar vertebra and the nerves course obliquely downward, nearly parallel to the spinal cord. The tip of the neural tubje is attached to the coccyx during this period of unequal growth, so its caudal portion becomes stretched into the slender, solid cord known as the filum terminale. The obliquely coursing spinal nerves, with the filum terminate, constitute the cauda equina. Traces of the original saccular termination of the neural tube in the integument are recognizable at birth.
| |
|
| |
|
| |
|
| |
|
| |
| THE BRAIN
| |
|
| |
|
| |
|
| |
| Primary Divisions. - The neural tube in embryos of 2 to 2.5 mm. is nearly straight, but its cranial end is enlarged to form the anlage of the brain (Fig. 245). Three regions of expansion, separated by two retarded zones of apparent constriction, subdivide the brain into three primary brain vesicles - the fore-brain (prosencephalon) , mid-brain (mesencephalon) , and hind-brain (rhombencephalon).
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 251. - Reconstructions of the brain of a 3.2 mm. human embryo (His-Prentiss). X about 35. . 4 , Lateral surface; B, median sagittal section.
| |
|
| |
|
| |
|
| |
|
| |
| Both the fore- and hind-brain vesicles promptly give rise to two secondary vesicles, whereas the mid-brain remains undivided. In embryos of about 3 mm. (four weeks), the fore-brain shows indication dorsally of a fold which subdivides it into the telencephalon, with its primitive cerebral hemispheres, and the dicncephalon, which bears the optic vesicles (Fig. 251). The mid-brain retains its original designation, the mesencephalon . At 7 mm. (five weeks), the neuropores have closed and the hind-brain constricts into the mctencephalon , or future region of the cerebellum and pans, and into the myclencephalon, or medulla oblongata. The further sej)aration of these vesicles may be followed easily in stages of lo mm. (six weeks) (Figs. 262 and 265), and 14 mm. (nearly seven weeks) (Fig. 253).
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 252. - Reconstructions of the brain of a 7 mm. human embryo (His-Prentiss). A, Lateral surface; B, median sagittal section.
| |
|
| |
|
| |
|
| |
| Fig. 253. - Brain of a 14 mm. human embryo in median sagittal section (His in Sobotta). I, Optic recess: 2, ridge formed by 3, the optic chiasma; 4, infundibular recess.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Cavities. - The lumen of the simple neural tube undergoes less change than the walls (Figs. 251 to 253). The cavity of the telencephalon extends into the paired hemispheres as the lateral ventricles; that of the diencephalon (and the median portion of the telencephalon) is designated the third ventricle; the narrow canal of the mesencephalon becomes the cerebral aqueduct; the lumen of the metencephalon and myelencephalon is the fourth ventricle. The latter is continuous with the central canal of the spinal cord.
| |
|
| |
|
| |
|
| |
|
| |
| Flexures. - ^ While the several divisions of the brain are differentiating, certain flexures appear in its roof and floor, due largely to unequal growth processes. In part, these correspond to those external bendings seen in the head and neck regions of young embryos. The first, or cephalic flexure appears as a sharp bend in the mid-brain region of embryos about 3 mm. long (Figs. 61 and 251). Soon, the angle is so acute that the long axes of the fore- and hind-brains are nearly parallel (Figs. 252 and 282). Next, two other flexures become evident at about the same time. These are the cervical flexure at the junction of brain and spinal cord, and the pontine flexure in the region of the future pons (Figs. 251 and 254). The cervical, like the cephalic flexure, corresponds to a similar bend in the gross embryo (Fig. 64). It is produced by the entire head flexing ventrad. On the contrary, the ])ontine flexure is peculiar to the brain; it bends in the opposite direction to the others and involves the floor only. Eventually, the pontine flexure straightens and disappears; the cervical flexure is nearly lost, but the cephalic flexure, somewhat reduced, persists.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 254. - Brains of human embryos (after His). . 4 , 4.2 mm. (X 20); B, 7 mm. (X 16): C, 19 mm. (X 4).
| |
|
| |
|
| |
|
| |
|
| |
| The history of the flexures of the brain and the relative growth of its different regions may be followed by comparing the brains of embryos of four, five, and seven weeks (Fig. 254), 11 weeks (Fig. 269), and 14 weeks (Fig. 274). In the adjoining table are listed the primary subdivisions of the neural tube and the parts derived from them.
| |
|
| |
|
| |
| DERIVATIVES OF THE NEURAL TUBE .
| |
|
| |
|
| |
| Primary vesicles .
| |
|
| |
| Subdivisions .
| |
|
| |
| Derivatives .
| |
|
| |
| Cavities .
| |
|
| |
| .
| |
|
| |
| Telencephalon .
| |
|
| |
| Rhinencephalon Cerebral cortex Corpora striata Pars optica hypothalami .
| |
|
| |
| Lateral ventricles Cranial portion of third ventricle .
| |
|
| |
| Prosencephalon .
| |
|
| |
| Dicncephalon .
| |
|
| |
| Epithalamus Thalamus Metathalamus Hypothalamus Hypophysis Tuber cinereum Mammillary bodies .
| |
|
| |
| Remainder of third ventricle .
| |
|
| |
| Mesencephalon .
| |
|
| |
| Mesencephalon .
| |
|
| |
| Corpora quadrigemina
| |
| Tegmentum
| |
| Crura cerebri .
| |
|
| |
| Acjuaeductus cerebri .
| |
|
| |
| Rhombencephalon .
| |
|
| |
| Metencephalon .
| |
|
| |
| Cerebellum
| |
| Pons .
| |
|
| |
| Fourth ventricle .
| |
|
| |
| Myelencephalon .
| |
|
| |
| Medulla oblongata
| |
|
| |
| Spinal cord
| |
|
| |
| Spinal cord .
| |
|
| |
| Central canal.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| The Myelencephalon. - The wall of the myelencephalon, like that of the spinal cord, differentiates dorsally and ventrally into roof- and floor plates, laterally into the alar- and basal plates (Fig. 255). The boundary line between the alar and basal plates is the sulcus Umitans. The myelencephalon differs from the spinal cord, however, in that its roof plate is a broad, thin, and flattened ependymal layer (Figs. 255 5 and 256). In the alar and basal plates, the marginal, mantle, and ependymal zones are differentiated as in the spinal cord (Fig. 256). Owing to the formation of the pontine flexure at the beginning of the second month, the roof plate is broadened, especially in the cranial portion of the myelencephalon, and the alar plates bulge laterally (Figs. 257 and 258 . 4 ). The cavity of the myelencephalon is thus widened from side to side, and flattened dorso-ventrally. This is most marked cranially, where, between the alar plates of the myelencephalon and metencephalon, are formed the lateral recesses of the fourth ventricle (Figs. 258 A and 275). Blood vessels grow into the ependymal roof of the myelencephalon, and, invading the lateral recesses, form there the chorioid plexus of the fourth ventricle. This plexus consists of small, finger-like folds of the ependymal layer and its vascular, mesenchymal cover. The line of attachment of the ependymal layer to the alar plate is known as the rhombic lip (Fig. 258 D); it becomes later the tcenia and obex of the fourth ventricle (B).
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 255. - Transverse sections from a 10 mm. human embryo (Prentiss). X 44. . 4 , Through the upper spinal cord; B, through the lower myelencephalon.
| |
|
| |
|
| |
|
| |
| Fig. 256. - Transverse sections through the mj^elencephalon of a 10 mm. human embryo (His). X 37. - 1 , Through the nuclei of Nn. XI and A'//; B, through the nuclei of Nn. A - and Xu.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 258. - Dorsal views of the developing cerebellum (. 4 , His; B-D, Prentiss). A, Six weeks; B, two months; C, four months; D, five months.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| In early stages, the floor of the myelencephalon is furrowed transversely by the so-called rhombic grooves, six in number; the intervals between successive grooves are neuromeres (cf. Figs. 368 and 392). Some view these as evidential of a former segmentation of the head, similar to that of the trunk (p. 229). It is more probable, however, that they merely stand in relation to certain cranial nerves and hence the segmental arrangement is secondary.
| |
|
| |
| The further growth of the myelencephalon is due : to the rapid formation of neuroblasts, derived from the ependymal and mantle layers; to the development of nerve fibers from these neuroblasts; and to the invasion of fibers from neuroblasts in other parts of the brain and spinal cord.
| |
|
| |
| The neuroblasts of the basal plates give rise to the efferent fibers of the cranial nerves (Fig. 256). In embryos of the sixth week, they thus constitute niotor nuclei of origin for the trigeminal, abducens, facial, glossopharyngeal, vagus complex, and hypoglossal nerves - nuclei corresponding to the ventral and lateral gray colmnns of the spinal cord. The basal plate likewise produces the reticular formation, which is derived in part also from the neuroblasts of the alar plate (Fig. 257). Some axons cross as external and internal arcuate fibers and constitute a portion of the median longitudinal bundle, a fasciculus corresponding to the ventral ground bundles of the spinal cord. Other axons grow into the marginal zone of the same side and form intersegmental fiber tracts. The reticular formation is thus differentiated into a gray portion, situated in the mantle zone, and into a white portion located in the marginal zone (Fig. 257). The marginal zone is added to further by the ascending fiber tracts from the spinal cord and the descending pyramidal tracts from the brain. As in the cord, the marginal layers of each side remain distinct, separated by the cells of the floor plate.
| |
|
| |
| The alar plates differentiate a httle later than the basal plates. The afferent fibers of the cranial nerves first enter the mantle layer, and, coursing upward and downward, form definite tracts (tractus solitarius; spinal tract of fifth nerve) (Fig. 257). To these are added tracts from the spinal cord, so that an inner gray- and outer white substance is formed. Soon, however, the cells of the mantle layer proliferate, migrate into the marginal zone, and surround the tracts. These neuroblasts of the alar plate form groups of cells along the terminal tracts of the afferent cranial nerves (which correspond to the dorsal root fibers of the spinal nerves) and constitute the receptive, or terminal nuclei of the fifth, seventh, eighth, ninth, and tenth cranial nerves. Caudally, the nucleus gracilis and nucleus cuneatus are developed from the alar plates as the terminal nuclei for the afferent fibers which ascend from the dorsal funculi of the spinal cord. The axons of the neuroblasts in these receptive nuclei decussate through the reticular formation, chiefly as internal arcuate fibers, and ascend to the thalamus as the median lemniscus. Still other nuclei differentiate, the axons of which connect the brain stem, cerebellum, and fore-brain. Of these, the most conspicuous is the inferior olivary nucleus (Fig. 271).
| |
|
| |
|
| |
|
| |
| The characteristic form of the adult myeleneephalon is determined by the further growth of the above-mentioned structures. The nuclei of origin of th cranial nerves, derived from the basal plate, produce swellings in the floor of the fourth ventricle that are bounded laterally by the sulcus limitans. The terminal nuclei of the mixed and sensory cranial nerves lie lateral to this sulcus. The enlarged cuneate and gracile nuclei bound the ventricle caudally and laterally as the cuneits and clava (Fig. 275). The inferior olivary nuclei produee lateral, rounded prominences, the olives, and ventral to these are the large cortico-spinal traets, or pyramids (Fig. 271).
| |
|
| |
| The Metencephalon. - The alar plates feature prominently in the differentiation of the metencephalon. Cranial to the lateral recesses of the fourth ventricle, their cells proliferate ventrally and form the numerous and relatively large nuclei of the pons (cf. Fig. 274). The axons from the cells of these nuclei mostly cross to the opposite side and become the hrachinm pontis of the cerebellum. Many cerebral fibers from the cerebral peduncles end about the cells of the pontine nuclei; others pass through the pons as fascicles of the cortico-spinal tracts.
| |
|
| |
|
| |
|
| |
| Fig. 259. - Median sagittal sections of the metencephalon (Prentiss). A, Two months; B, at middle of fifth month.
| |
|
| |
|
| |
|
| |
| The Cerebellum. - When the alar plates of the cranial end of the myeleneephalon are bent out laterally by the pontine flexure, their direct continuations into the metencephalic region assume a transverse position also (Fig. 258 A). During the second month, the alar plates thicken and bulge into the ventricle (Fig. 258 A). Near the midline, paired swellings indicate the anlages of the vermis, while the remaining lateral portions represent the future cerebellar hemispheres (Figs. 258 and 275).
| |
|
| |
|
| |
| The cerebellar anlages grow rapidly in length, so that their surfaces are folded transversely. During the third month their walls bulge outward and form on either side a convex hemisphere connected with the pons by the hrachuini pontis (Fig. 258 C). In the meantime, the anlages of the vermis have fused in the midline, producing a single structure marked by transverse fissures. The rhombic lip gives rise to the flocculus and nodulus. Between the third and fifth months the cerebellar cortex grows faster than the deeper layers, and the principal lobes and fissures are formed (Fig. 258 C, D). The hemispheres are the last to be differentiated; their fissures do not appear until the fifth month.
| |
|
| |
|
| |
| The wall of the neural tube remains thin both in front and behind the cerebellum; it constitutes respectively the anterior- and posterior medullary velum of the adult (Fig. 259 B). The points of attachment of the vela remain approximately fixed, while the cerebellar cortex grows enormously. As a result, the vela are folded in under the expanding cerebellum.
| |
|
| |
|
| |
|
| |
| Fig. 260. - Transverse sections through the mesencephalon of a 10 mm. human embryo (His). A, Through the nucleus of N. IV; B, through the nucleus of .V. uI.
| |
|
| |
|
| |
| The anlages of the cerebellum show at first differentiation into the same three layers which are typical for the neural tube. During the second and third months, cells from the ependymal, and perhaps from the mantle layer of the rhombic lip migrate to the surface of the cerebellar cortex and give rise to the molecular and granular layers which are characteristic of the adult cerebellar cortex. The later dift'erentiation of the cortex is not completed until after birth. The cells of the granular layer become unipolar by a process of unilateral growth. The axons of Purkinje cells and those of entering afferent fibers form the deep medullary layer of the cerebellum.
| |
|
| |
|
| |
|
| |
|
| |
| Many cells of the mantle layer take no part in the development of the cerebellar cortex, but give rise to neuroglia tissue and to the internal nuclei. Of these, the dentate nucleus is seen at the end of the third month; later, its cellular layer becomes so folded as to produce characteristic convolutions. The fibers arising from its cells form the greater part of the brachium conjunctivum.
| |
|
| |
|
| |
|
| |
| The Mesencephalon. - ^Distinct basal and alar plates can be recognized in this subdivision of the brain, and each differentiates into the three primitive layers (Fig. 260). At the end of the first month, the neuroblasts of the basal plate give rise to the axons of motor nerves - the oculomotor .
| |
|
| |
|
| |
|
| |
| cranial in position, the trochlear caudal (Fig. 260). In addition to these nuclei of origin, the red nucleus develops; its early history is not well understood. The mantle layer is enclosed ventrally and laterally by fiber tracts which develop in the marginal zone. These include the median and lateral leninisci, and the descending tracts from the cerebral cortex which together constitute the cerebral peduncles.
| |
|
| |
| The alar plates form the paired superior and inferior colliculi, jointly known as the corpora quadrigemina (Figs. 258 5 and 269). The plates thicken and neuroblasts migrate to their surfaces, forming stratified ganglionic layers comparable to the cortical layers of the cerebellum and the cerebellar nuclei. With the development of the superior and inferior colliculi the cavity of the mesencephalic region decreases in size and becomes the cerebral aqueduct.
| |
|
| |
|
| |
|
| |
| Fig. 261. - Transverse section through the diencephalon of a 14 mm. human embryo (His). .X 29.
| |
|
| |
|
| |
|
| |
|
| |
| The Diencephalon. - The wall of the diencephalon differentiates a dorsal roof plate, and paired alar plates which include both the lateral and ventral regions (Fig. 261). It is doubtful if the basal- and floor plates of lower levels extend into the diencephalon (Kingsbury, 1922). The roof plate becomes a thin ependymal lining to the folded tela chorioidea. Blood vessels grow into the tela and form the chorioid plexus of the third ventricle (Fig. 261). At the junction of the caudal portion of the roof plate with the alar |date is an area termed the epithalamus (Fig. 253). From it the epiphysis, or pineal body, evaginates during the seventh week (Fig. 266) and later incorporates a certain amount of mesenchymal tissue (Fig. 263). The solid, conical epiphysis corresponds but partially to the pineal eye of reptiles.
| |
|
| |
|
| |
|
| |
| Each thickened alar plate is divided by the sulcus limitans (Fig. 262) into the dorsal thalamus and metathalamus and ventral hypothalamus (Figs. 253 and 263). The metathalamus, really a part of the definitive thalamus, gives rise to the geniculate bodies. Several structures develop from the hypothalamic floor. Passing caudad, these are the infundibulum, tuber cinereum, and mammillary recess (Fig. 262). The lateral walls of the latter enlarge into paired mammillary bodies (Fig. 267).
| |
|
| |
|
| |
|
| |
| Fig. 262. - Median sagittal section through the fore- and mid-brain of a 10 mm. human embryo (His).
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 263. - Median sagittal section of the brain from a fetus of the third month (His in Sobotta).
| |
|
| |
|
| |
| The third ventricle lies largely in the diencephalon and is at first relatively broad. Owing to the thickening of its lateral walls, it is compressed to a narrow, vertical cleft (Fig. 270). The thalami are approximated, and often fuse; the niassa intermedia, thus formed, is encircled by the cavity of the ventricle (Fig. 273 B).
| |
|
| |
|
| |
|
| |
| Fig. 21)4. - (Jblique section through the di- and telencephalon of a 10 mm. human embryo
| |
| (Prentiss). X 61.
| |
|
| |
|
| |
| Fig. 265. - Lateral view of the fore- and mid-brain of a 10 mm. human emliryo (His).
| |
|
| |
|
| |
|
| |
| The Hypophysis . - The hypophysis, or pituitary body, has a double origin. Its glandular portions develop from the ectodermal Rathke - s pouch, which appears at about 3 mm. just in front of the pharyngeal membrane (Fig. 91). This ])Ouch early comes in contact with a saclike extension of the infimdibuhun, the anlage of the neural hypophyseal lobe (Figs. 262 to 264, and 392). Rathke - s pouch, at first flat, grows laterally and caudally about the neural lobe, and loses its stalked connection with the oral epithelium at the end of the second month (Fig. 415). The original cavity of the pouch becomes the residual lumen of the adult gland. In embryos of about seven weeks, its walls differentiate into the glandular cords of the anterior lobe. That portion of the wall between the lumen and the neural lobe remains thin and constitutes the pars intermedia. Recently, a further glandular portion, the pars tnberalis, has been recognized, lying along the tuber cinereum; it develops from the fusion of paired lateral lobes, at the base and in front of Rathke - s pouch. The anlage of the neural lobe is transformed into a solid mass of neuroglia tissue which remains connected to the diencephalon by a permanent infundibular stalk (Fig. 418). The anterior lobe and the pars intermedia elaborate important internal secretions.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 266. - Dorsal surface of the fore- and mid-brain of a 14 nim. human embryo (His). The pallium of the telencephalon is cut away, exposing the lateral ventricle.
| |
|
| |
|
| |
|
| |
|
| |
| The Telencephalon. - Like the diencephalon, this specialized division of the neural tube represents, for the most part, greatly expanded alar plates. It is convenient to regard the telencephalon as consisting of a median portion, continuous with the diencephalon and containing the cranial part of the third ventricle, and of lateral hemispheric outgrowths (Fig. 266). Toward the end of the first month, each cerebral hemisphere differentiates into corpus striatum (a ventral portion continuous with the thalamus), pallium (primitive cerebral cortex), and rhinencephalon (olfac.
| |
|
| |
|
| |
|
| |
| Fig. 268. - Transverse section through the fore-brain of a 15 mm. human embryo (His).
| |
|
| |
|
| |
|
| |
| The Corpus Striatum . - The floor of each hemisphere produces a thickening (Fig. 252 B), which, at six weeks, bulges prominently into the lateral ventricle (Figs. 266 and 268). The corpus striatum, so formed, is in line caudally with the thalamus of the diencephalon and is closely connected with it, both developmentally and functionally. The corpus striatum elongates in company with the cerebral hemisphere, its caudal portion curving around to the tip of the inferior horn of the lateral ventricle and forming the slender tail of the caudate nucleus (Figs. 269 and 272). The thickening of the corpus striatum is due to an active proliferation of cells in the ependymal layer which give rise to a prominent mass of mantle layer cells. Nerve fibers passing in both directions between the thalamus and the cerebral cortex course through the corpus striatum as laminae which are arranged in the form of a wide V, open laterally. This V-shaped tract of white fibers is the internal capsule. Its cranial limb partly divides the corpus striatum into the caudate and lenticular nuclei; the caudal limb of the capsule extends between the lenticular nucleus and the thalamus (Fig. 270). The thalamus and corpus striatum are separated by a deep groove until the end of the third month (Fig. 268). As the structures enlarge, the groove between them disappears and they form one continuous mass (Fig. 270). According to some investigators there is direct fusion between the two.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 269. - The fetal brain at nearly three months (His). IMost of the right pallium is removed.
| |
|
| |
|
| |
|
| |
| The Pallium. - The i)allial walls expand rapidly until they overlap and conceal much of the other brain structures (Figs. 267, 274 and 277). During this growth the median lamina between the two hemispheres lags in development, and thus there is formed the longitudinal fissure (Fig. 266). The lamina extends from the ventrally situated optic chiasma upward and backward to the roof plate of the diencephalon; it becomes the lamina terminalis, the cranial boundary of the third ventricle (Fig. 263). The lateral ventricles, or cavities of the hemispheres, at first communicate broadly with the third ventricle through the interventricular foramina (of Monro) (Fig. 264). Later, each foramen is narrowed to a slit, not by constriction, but because its boundaries grow more slowly than the rest of the telencephalon (Fig. 268).
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 270. - Horizontal section through the fore-brain of a five-months - fetus (His).
| |
|
| |
|
| |
| The Rlunencephalon . - During the sixth week a swelling appears on the ventral surface of each cerebral hemisphere (Fig. 265). These enlarge into distinct olfactory lobes, which, however, remain small in man (Figs. 267 and 271). Each lobe includes an anterior and posterior division. The pars anterior is the anlage of the olfactory bulb and tract; the latter receives the backward-growing olfactory fibers, and the original lumen is lost. The pars posterior is a thickening of the brain wall which later constitutes the anterior perforated substance and the parolfactory area (Figs. 271 and 277).
| |
|
| |
|
| |
| The olfactory apparatus includes also a pallial portion. It is termed the ar chi pallium, because it forms the entire primitive wall of the cerebrum, a condition permanent in fishes and amphibia. In mammals, the neopallium, or adult cortex, becomes dominant and the archipallium is represented by the hippocampus (Figs. 266 and 269), a portion of the hippocampal gyrus (Fig. 271), and the dentate gyrus (Fig. 272). It resembles the rest of the cerebral cortex in the arrangement of its cells.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 271. - Ventral view of the brain of a three-months - fetus, to show the rhinencephalon (Kollmann).
| |
|
| |
|
| |
| The Chorioid Plexus of the Lateral Ventricles. - ^Just as the chorioid plexus of the third ventricle develops in the folds of the roof plate of the dieneephalon, so the thin, median wall of the pallium, at its junction with the wall of the diencephalon, is folded into each lateral ventricle. A vascular plexus, continuous with that of the third ventricle, grows into this fold, and projects into the corresponding lateral ventricle (Figs. 266 and 268). The fold of the pallial wall forms the chorioid fissure (Fig. 269), and the vascular plexus is the chorioid plexus of the lateral ventricle (Fig. 272). This is a paired structure, which, with the plexus of the third ventricle, makes a T-shaped figure, the stem of the T overlying the third ventricle and its curved arms projecting into the lateral ventricles just caudal to the interventricular foramen. Later, as the pallium expands, the chorioid plexus of the lateral ventricles and the chorioidal fissures are elongated extensively into the temporal lobes and inferior horns of the lateral ventricles (Figs. 270 and 272).
| |
|
| |
|
| |
|
| |
| Commissures of the Telencephalon. - The important commissures are the fornix, anterior commissure, and corpus callosum. The first two are older commissures of the archipallium, while the larger corpus callosum is the great transverse bridge of the neopallium, or cerebral cortex. The commissures develop in relation to the lamina terminalis, crossing partly in its wall and partly in the fused adjacent portions of the median pallial walls. Owing to the union of the pallial walls dorsal and cranial to it, the lamina thickens rapidly during the fourth and fifth months. It is at this time that the significant development of the commissures occurs.
| |
|
| |
|
| |
| Fig. 272. - Transverse section through the telencephalon of a three-months - fetus (His). Th, Thalamus; Cs, corpus striatum.
| |
|
| |
|
| |
| The fornix takes its origin early, chiefly from cells in the hippocampus. The fibers course along the chorioidal side of the hippocampus cranially (cf. Fig. 269), passing dorsal to the foramen of Monro (Fig. 273 A). In the cranial portion of the lamina terminalis, fibers are both given off to the basal portion of the rhinencephalon and received from it. In this region, fibers crossing the midplane form the hippocampal commissure (Fig. 273 A)\ with the later growth of the corpus callosum it shifts further caudad (Fig. 272 B). Other fibers, as the diverging columns of the fornix, curve ventrad and end in the mammillary body of the hypothalamus (Fig. 273 B).
| |
|
| |
|
| |
| The fibers of the anterior commissure cross in the lamina terminalis, ventral to the primitive hippocampal commissure (Fig. 273 ^ 4 ). They arise in paired cranial and caudal divisions. The fibers of the former interconnect the olfactory bulbs in a horse-shoe bow. The fibers of the caudal division pass ventrally between the corpora striata and the cortex, and may be derived from one or both of these regions.
| |
|
| |
|
| |
| The corpus callosum appears, cranial and dorsal to the primitive hippocampal commissure, in the roof of the thickened lamina terminalis (Fig. 273 A). Through its fibers, which arise from neuroblasts in the wall of the neopallium, nearly all regions of one hemisphere are associated eventually with corresponding regions of the other. The fibers, found first in the corpus callosum, arise in the median wall of the hemispheres. AsHhe pallium expands, interstitial fibers develop which extend the corpus callosum both cranially and caudally (Fig. 273 B). In fetuses of five months, this great commissure is a conspicuous structure and shows the form which is characteristic of the adult (Figs. 272 5 and 277).
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 273. - The cerebral commissures in median section (adapted by Prentiss), .1, Three months; B, four months.
| |
|
| |
|
| |
|
| |
| The triangular interval between the fornix and corpus callosum contains a thin partition which separates the two lateral ventricles (Fig. 273 B). This scplitm pelluciditm is a membranous portion of the lamina terminalis and really is thinned, median pallial wall. As a result of stretching, caused by the growth of the corpus callosum, a cavity sometimes forms between the laminae of the septum; it is designated the space of the septum pcUncidum, or often, falsely, the^///z ventricle (Fig. 277).
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 374. ^Lateral view of the l>rain in situ, at the middle of the fourth month (His.)
| |
|
| |
|
| |
|
| |
| External Configuration of the Hemispheres. - The telencephalon so - expands cranially, caudally, and ventrally that four lobes may be distinguished (Fig. 274): (i) a cranial frontal lobe; (2) a dorsal parietal lobe; (3) a caudal occipital lobe; and (4) a ventro-lateral temporal lobe. The ventricle extends into each of these regions and forms respectively the anterior horn, the body, the posterior horn, and the inferior horn of the lateral ventricle.
| |
|
| |
| The surface extent of the cerebral wall, the thin gray cortex, increases more rapidly than the underlying, white medullary layer. As a result the cortex is folded, producing convolutions, between which are prominent furrows, termed fissures. The chorioid fissure is formed, as already explained (p. 267), by the ingrowth of the chorioid plexus (Fig. 267). During the third month, the rhinal (Fig. 277) and hippocampal fissures develop in association with the rhinencephalon. The latter fissure represents a curved infolding along the median wall of the temporal lobe (Fig. 272); the corresponding elevation on the inner surface of the pallium is the hippocampus (Figs. 266 and 269). At the same time, the lateral fissure (of Sylvius) makes its appearance in the following way (Fig. 274): The
| |
| cortex overlying the corpus striatum develops more slowly than the surrounding areas and is thus gradually overgrown by opercular folds of the frontal, parietal, and temporal lobes. The area thus covered is the insula (island of Reil), and the depression so formed is the lateral fissure (Fig. 276). These opercula are not approximated over the insula until after birth.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 275. - Dorsal view of the brain from a three months - fetus (Kollmann).
| |
|
| |
|
| |
|
| |
| Fig. 276. - Lateral view of the right cerebral hemisphere from a seven-months - fetus (Kollmann).
| |
|
| |
|
| |
|
| |
| Fig. 277. - Median surface of the right cerebral hemisphere from a seven-months - fetus (Kollmann).
| |
|
| |
|
| |
| In fetuses of six to seven months, four other neopallial depressions ; appear which later form important landmarks in the cerebral topography. ( They are: (i) the central sulcus, or fissure of Rolando, which forms the ^ dorso-lateral boundary line between the frontal and parietal lobes (Fig. '* 276); (2) the parieto-occipital fissure, which, on the median wall of the cerebrum, is the line of separation between the occipital and parietal lobes (Fig. 277); (3) the calcarine fissure, which marks the position of the • visual area of the cerebrum (Fig. 277) and internally causes the convexity termed the calcar avis; (4) the collateral fissure on the ventral surface of the temporal lobe, which produces the inward bulging on the floor of the posterior horn of the ventricle known as the collateral eminence.
| |
|
| |
|
| |
| Simultaneously with the development of the latter group of fissures/ appear other shallower depressions known as sulci. These have a definite arrangement, and, with the fissures, mark off from each other the various functional areas of the cerebrum. The surface convolutions between the depressions constitute the gyri of the adult cerebrum.
| |
|
| |
|
| |
|
| |
| Histogenesis of the Cerebral Cortex . - In the wall of the pallium are differentiated the three primitive zones typical of the neural tube; the ependymal, mantle, and marginal layers. During the first two months the cortex remains thin and differentiation is slow. At eight weeks, neuroblasts migrate from the ependymal and mantle zones into the superficial marginal zone and give rise to layers of pyramidal and other cells typical of the cerebrum. The differentiation of these layers is most active during the third and fourth months, but probably continues until after birth. From the fourth month on, the cerebral wall thickens rapidly, owing to the development of fibers from the thalamus and corpus striatum, and of endogenous fibers from the neuroblasts of the cortex. The fibers form a white, inner medullary layer, surrounded by the gray cortex. Myelination begins shortly before birth, but some fibers may not acquire their sheaths until after the twentieth year. As the cerebral wall increases in thickness, the size of the lateral ventricle relatively diminishes; especially is this true of its lateral diameter.
| |
|
| |
|
| |
|
| |
| Anomalies. - There are numerous types of defective neural tube development - most the result of arrest. These usually involve the bony investments as well, and produce conspicuous malformations.
| |
|
| |
|
| |
| The more or less extensive failure of the neural groove to close produces cranioschisis or rachischisis, depending on whether the region of the head or vertebral column is affected. In such instances the roof of the skull is lacking [acrania; liemicrania), or there are clefts in the vertebral canal. If the defect contains a sac-like protrusion of the membranes, the condition is known as meningocoelc; if the neural wall alone protrudes, it is cncephaloccele (brain) or myeloccele (spinal cord) ; if, as is most common, both are involved, it is meningoencepJialoccele, or meningo-myeloccele. Such a hernial condition of the spine is often called spina bifida and is most frequent in the lumbo-sacral region, where the sac may become the size of a child’s head.
| |
|
| |
|
| |
| An excessive fluid content in the brain cavities causes both brain and skull to enlarge, producing hydrocephaly The virtual absence of a brain is anencephaly; of the spinal cord, a my el us.
| |
|
| |
|
| |
|
| |
| ++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
| |
|
| |
|
| |
| CHAPTER XIV THE PERIPHERAL NERVOUS SYSTEM
| |
|
| |
|
| |
|
| |
|
| |
| The peripheral nervous system consists of bundles of myelinated and unmyelinated nerve fibers, and aggregations of nerve , cells, the ganglia. The fibers are of two types: afferent fibers, which carry sensory impulses to the central nervous system, and efferent fibers, which carry motor impulses away from the nervous centers. The peripheral afferent fibers originate from nerve cells located in the ganglion crest (p. 241) outside the neural tube. The peripheral efferent fibers grow from neuroblasts of the basal plate and emerge ventro-laterally out of the neural tube. Fibers of one or both sorts converge into distinct segmental cords called nerves. These belong to two main systems: the cerebro-spinal series and the sympathetic division.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 278. - Diagrammatic section through the embryonic myelencephalon, showing the arrangement of the functional cell columns and the origin, course and termination of the functional components of the cranial nerves (Ranson).
| |
|
| |
|
| |
|
| |
| Functional Classification of Fibers. - -The early observation that sensory impulses travel in the dorsal root fibers and motor impulses in ventral root fibers (Fig. 281) has been supplemented by a more complete analysis (Fig. 278). All neurons fall within four chief functional groups, which are in turn subdivided as indicated in the accompanying table. No single nerve contains representatives of every fiber type; those components designated - special - are peculiar to the cranial nerves alone.
| |
|
| |
| .1. Somatic afferent.
| |
|
| |
| .(а) General (Fibers ending chiefly in the integument).
| |
|
| |
| .(б) Special (Neurons of the eye and ear).
| |
|
| |
| .2. Visceral afferent.
| |
|
| |
| .(а) General (Sympathetic fibers conducting sensory impulses from the viscera).
| |
|
| |
| .(б) Special (Fibers to the olfactory membrane and taste buds).
| |
|
| |
| .3. Somatic efferent. (Fibers ending on skeletal muscle).
| |
|
| |
| .4. Visceral efferent.
| |
|
| |
| .(а) General (Fibers ending about sympathetic ganglion cells, which in turn control smooth and cardiac muscle and glandular tissue).
| |
|
| |
| .(б) Special (Cranial nerve fibers ending directly on striated, branchial-arch musculature) .
| |
|
| |
| THE SPINAL NERVES
| |
|
| |
|
| |
| The spinal nerves are arranged segmentally, and each is attached to the spinal cord by a dorsal (posterior) root, with which is associated a
| |
|
| |
|
| |
| N. IX N. X-XI ganglion crest .
| |
|
| |
|
| |
| Fig. 279. - The cerebro-spinal nerves of a 4 mm. human embryo (Streeter). X 17. Ci-6,
| |
|
| |
|
| |
| Ventral roots of cervical spinal nerves.
| |
|
| |
| Spinal ganglion, and a ventral (anterior) root (Fig. 246). In embryos of 4 mm., the ventral roots are already developing as outgrowths of neuroblasts in the mantle layer of the spinal cord (Fig. 279). The spinal ganglia are represented as enlargements along the continuous ganglion crest. At the stage of 7 mm., or five weeks, the cells of the spinal ganglia begin to develop centrally directed processes which enter the marginal zone of the cord as dorsal root fibers (Fig. 280). Peripheral processes of the ganglion cells soon join the ventral root fibers in the trunk of the nerve (Fig. 246).
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 2S0. - The cerebro-spinal nerves of a 7 ihiti. human embryo (Streeter). X i7
| |
|
| |
|
| |
| Pig. 281. - Transverse section of a 10 mm. human embryo, showing a spinal nerve and functional components (Prentiss).
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| At 10 mm. (Fig. 282), the cellular bridges of the ganglion crest, which hitherto interconnect spinal ganglia, have begun to disappear, and the several parts of a typical spinal nerve are evident (Figs. 246 and 281). The trunk of the nerve, just ventral to the union of the dorsal and ventral roots, gives off laterally the dorsal ramus, the fibers of which supply the dorsal muscles and integument. The ventral ramus, continuing, gives off mesially the ramus communicans to the sympathetic ganglion, and divides into the lateral and ventral terminal rami. The efferent fibers of these rami supply the muscles of the lateral and ventral body wall, and the afferent fibers end in the integument of the same regions.
| |
|
| |
|
| |
| Fig. 282. - The nervous system of a 10 mm. human embryo (Streeter). X 12.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Nerve Plexuses . - ^At the points where the anterior and lateral terminal rami arise, connecting loops may extend from one spinal nerve to another. Thus, in the neck region, superficial and deep cervical plexuses are formed. The deep cervical jilexus gives rise to the ansa hypoglossi and the phrenic nerve (Fig. 282).
| |
|
| |
|
| |
| The nerves supplying the arm and leg also form plexuses that first appear at 7 mm. (Fig. 280) and are clearly indicated in embryos of 10 mm. (Fig. 282). The trunks of the last four cervical nerves and of the first thoracic unite into a flattened ])late, the anlage of the brachial plexus. From this ]>late nervous cords extend into the intermuscular spaces and end in the premuscle masses. The developing skeleton of the shoulder splits the Brachial plexus into dorsal and ventral laminae. From the dorsal lamina arise the musculo-cutaneous, median, and ulna nerves; from the ventral lamina, the axillary and radial nerves.
| |
|
| |
|
| |
| The lumbar and sacral nerves to the leg unite in a plate-like structure, the anlage of the lumbosacral plexus (Fig. 282). The plate is divided by the skeletal elements of the pelvis and femur into two lateral and two median trunks. Of the cranial pair, the lateral becomes the femoral nerve; the median, the obturator nerve. The caudal pair constitutes the primitive sciatic nerve; the lateral trunk will be the peroneal nerve, the median trunk the tibial.
| |
|
| |
|
| |
|
| |
| Twelve pairs of cranial nerves appear at about the end of the first month. They are not arranged segmentally and attempts to interpret them as serial homologues of spinal nerves fail. In addition to the general sensory and motor components of spinal nerves, the cranial group contains special fibers to the major sense organs and to muscles derived from branchial arches. The several sensory and motor nuclei are arranged in definite longitudinal columns within their respective alar and basal plates (Fig. 278). Unlike the spinal series, the cranial nerves vary widely in functional composition. Those of the first two groups in the subjoined table have but a single kind of fiber; on the contrary, the members of the third group are all mixed, as witness the ninth and tenth which contain five different types each. The cranial nerves fall roughly into three functional groups; .
| |
|
| |
|
| |
| THE CRANIAL NERVES .
| |
|
| |
|
| |
| Special
| |
| Sensouv .
| |
|
| |
|
| |
| Somatic
| |
| Motor .
| |
|
| |
|
| |
| Visceral Sensory .
| |
|
| |
|
| |
| AND Motor .
| |
|
| |
|
| |
| I. Olfactory. u. Optic.
| |
|
| |
| ,
| |
|
| |
| uI. Oculomotor.
| |
|
| |
| ,
| |
|
| |
| V. Trigeminal.
| |
|
| |
| .Vu. Facial.
| |
|
| |
| IX. Glossopharyngeal.
| |
|
| |
| .X. Vagus comple.x (including XI. Spinal Accessory).
| |
|
| |
| ,
| |
|
| |
| VuI. Acoustic.
| |
|
| |
| ,
| |
|
| |
| IV. Trochlear. VI. Abducens.
| |
|
| |
| ,
| |
|
| |
| Xu. Hypoglossal.
| |
|
| |
|
| |
| (A) The Special Sensory Nerves
| |
|
| |
|
| |
| 1. The Olfactory Nerve, though purely sensory, has no ganglion. Its nerve cells lie at first in the epithelium of the nose and are of the bipolar type. From them, peripheral processes develop which end directly at the surface of the olfactory epithelium (Fig. 283). Central processes grow backward during the fifth week and form the strands of the olfactory nerve, around which the cribriform plate later develops. They end in the glomeruli of the olfactory bulb in contact with dendrites of the mitral cells, or olfactory neurons of the second order. Some olfactory cells migrate from the epithelium, with which, however, they retain peripheral connections. Such bipolar elements, found along the entire course of the nerve, resemble ordinary dorsal ganglion cells. The olfactory nerve fibers are peculiar in that they remain unmyelinated. Nerve fibers from the epithelium of the vestigial vomero-nasal organ (of Jacobson) also end in the olfactory bulb.
| |
|
| |
|
| |
| The ganglionated terminal nerve courses in close association with the olfactory nerve. Its unmyelinated fibers end in the epithelium of the vomero-nasal organ and of the septum.
| |
|
| |
|
| |
| Although evidently a distinct nerve, its relations and significance are obscure.
| |
|
| |
|
| |
| 2. The Optic Nerve is formed by fibers which grow from neuroblasts in the nervous layer of the retina. Since the retina differentiates from the evaginated wall of the fore-brain (Fig. 264), the optic nerve is not a true peripheral nerve, but belongs to the central system of tracts. The neuroblasts from which the optic nerve fibers develop constitute the ganglion cell layer of the retina (Fig. 301). During the sixth and seventh weeks these cells give rise to central processes which form a nerve fiber layer on the inner side of the retina. The optic fibers converge to the optic stalk and grow through its wall back to the brain (Fig. 284 A). The cells of the optic stalk are converted into a neuroglia framework and its cavity is obliterated {B). In the floor of the fore-brain, at the boundar}" between telencephalon and diencephalon, the fibers from the median half of each retina at about the end of the second month cross to the opposite side, and this decussation constitutes the optic cluasma. The crossed and uncrossed fibers constitute the optic tract (Fig. 271).
| |
|
| |
|
| |
| Efferent fibers, terminating in the inner reticular layer of the retina, are present also. In certain fishes, where their function has been studied, these fibers resemble visceral efferent components (Arey, 1916).
| |
|
| |
|
| |
|
| |
| Fig. 283. - Diagram of the relations of the fibers in the olfactory nerve.
| |
|
| |
|
| |
| 8. The Acoustic Nerve is composed of fibers which grow from the acoustic ganglion. Its cells arise directly from the brain wall of 2 mm. embryos (Bartelmez, 1922) and soon lie just cranial to the otic vesicle (Fig. 305). The cells become bipolar, central processes uniting the ganglion to the tuberculum acusticum of the myelencephalon and peripheral fibers connecting it with the wall of the otocyst.
| |
|
| |
| The acoustic ganglion is differentiated into vestibula?- and spiral ganglia (Fig. 285). The original ganglion elongates and is subdivided into superior and inferior portions in 7 mm. embryos. The superior part supplies fibers to the utriculus and to the ampullae of the anterior and lateral semicircular ducts. Part of the inferior portion innervates the sacculus and the ampulla of the posterior semicircular duct, and this portion, together with the entire pars superior, constitutes the vestibular ganglion. Most of the pars inferior, however, differentiates into the spiral ganglion, the peripheral fibers of which innervate the hair cells of the spiral organ (of Corti) in the cochlea. The spiral ganglion appears in 9 mm. embryos and conforms to the spiral turns of the cochlea, hence its name. Its central nerve fibers form the cochlear division of the acoustic nerve. This is distinctly separated from the central fibers of the vestibular ganglion which constitute the vestibular division of the acoustic nerve, the fibers of which are equilibratory in function. The pars inferior of the vestibular ganglion becomes closely connected with the n. cochlearis, and thus in the adult it appears as though the sacculus and posterior ampulla were supplied by the cochlear nerve.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 284. - Transverse sections through the human optic stalk during its transformation into the optic nerve (redrawn after Bach and Seefelder). /I, 14.5 mm.; B, 19 mm.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 285. - The development of the acoustic ganglion and nerve (Streeter). The vestibular ganglion is finely stippled, the spiral ganglion coarsely stippled.
| |
|
| |
|
| |
|
| |
|
| |
| (B) The Somatic Motor Nerves
| |
|
| |
|
| |
| This group, consisting of the three nerves to the eye muscles and the n. hypoglossus, is purely motor, the fibers originating from neuroblasts of the basal plate of the brain stem, near the midplane. They are regarded as homologues of the ventral motor roots of the spinal cord, but they have lost their segmental arrangement and are otherwise modified. The nuclei of origin of these nerves are colored red in Fig. 287.
| |
|
| |
|
| |
| 3. The Oculomotor Nerve develops from neuroblasts in the basal plate of the mesencephalon (Fig. 260 B). The fibers emerge as small fascicles on the ventral surface of the mid-brain, in the concavity due to the cephalic flexure (Figs. 282 and 287). The fascicles converge, form the trunk of the nerve, and end in the premuscle masses of the eye. The nerve eventually supplies all of the extrinsic muscles of the eye, save the superior oblique and external rectus.
| |
|
| |
|
| |
| 4. The Trochlear Nerve fibers arise from neuroblasts of the basal plate, located just caudal to the nucleus of origin of the oculomotor nerve (Fig. 287). They are directed dorsad, curve around the cerebral aqueduct, and, crossing in its roof, emerge at the isthmus (Fig. 260 A). From this superficial origin, each passes ventrad as a slender nerve which connects with the anlage of the superior oblique muscle of the eye (Fig. 282).
| |
|
| |
|
| |
| 6. The Abducens Nerve takes origin from a nucleus of cells in the basal plate of the myelencephalon, located directly beneath the fourth neuromere (Figs. 282 and 287). The converging fibers emerge ventrally at a point caudal to the future pons, and, as a single trunk, course cranially, mesial to the semilunar ganglion, finally ending in the anlage of the external rectus muscle of the eye. Vestigial rootlets of the abducens and hypoglossal nerve tend to fill in the gap between these two nerves.
| |
|
| |
|
| |
| 12. The Hypoglossal Nerve results from the fusion of the ventral root fibers of three to five precervical nerves. Its fibers originate from neuroblasts of the basal plate and emerge from the ventral wall of the myelencephalon in several groups (Figs. 279 and 287). In embryos of 7 mm., the fibers have converged ventrally to form the trunk of the nerve (Fig. 289). Later, they grow cranially, lateral to the ganglion nodosum, and eventually end in the muscle fibers of the tongue (Fig. 282).^ The nerve in its development unites with the first three cervical nerves to form the ansa hypoglossi.
| |
|
| |
| That the hypoglossus is a composite nerve, homologous with the ventral roots of the spinal nerves, is shown: (i) by the segmental origin of its fibers; (2) from the fact that its nucleus of origin is a cranial continuation of the ventral gray column, or nucleus of origin for the ventral spinal roots; (3) from the fact that in mammalian embryos (pig; sheep; cat; etc.) rudimentary dorsal ganglia are developed, one of which at least (Froriep - s ganglion) sends a dorsal root to the hypoglossus. In human embryos, Froriep - s ganglion may be present as a rudimentary structure (Figs. 282 and 286), or it may be absent and the ganglion of the first cervical nerve may also degenerate and disappear. In pig embryos there are one to four accessory ganglia (including Froriep - s) from which dorsal roots extend to the root fascicles of the hypoglossal nerve (Fig. 391).
| |
|
| |
|
| |
|
| |
|
| |
| (C) The Visceral Mixed Nerves
| |
|
| |
|
| |
| The motor roots of this group arise in a lateral series, distinct from the dorsal and ventral roots already described (Figs. 256 and 278). The trigeminal nerve contains not only visceral fibers but numerous somatic sensory neurons which supply the integument of the head and face. The facial, glossopharyngeal, and vagus nerves are essentially visceral in function. Their sensory fibers innervate the sense organs of the branchial arches and viscera. A few somatic sensory fibers, having the same origin and course in the myelencephalon, pass to the adjacent integument.
| |
|
| |
|
| |
|
| |
|
| |
| 5. The Trigeminal Nerve is chiefly sensory. Its large semilunar ganglion, a derivative of the ganglion crest, arises at the very beginning of the hind brain (Fig. 280). Centrally directed processes form the large sensory root that enters the wall of the hind-brain at the level of the pontine flexure (Fig. 282). These fibers fork, and then course cranially and caudally in the alar plate of the myelencephalon; the caudal fibers constitute the descending spinal tract of the trigeminal nerve (Fig. 287). The peripheral processes separate into three large divisions, the ophthalmic, maxillary, and mandibular nerves, and supply the integument of the head and face and the epithelium of the mouth and tongue.
| |
|
| |
|
| |
| The motor fibers of the trigeminal nerve arise largely from a dorsal motor nucleus that lies opjiosite the point at which the sensory fibers enter the brain wall (Fig. 287). In the embryo, these fibers emerge as a separate motor root, course along the mesial side of the semilunar ganglion, and, as a distinct trunk, supply the premuscle masses which later form the muscles of mastication. From the chief motor nucleus, a line of cells, extending cranially into the mesencephalon, constitutes a second source of origin for motor fibers. In the adult, the motor fibers form a part of the mandibular division of the nerve.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 287 - Reconstruction of the nuclei of origin and termination of the cranial nerves in a 10 mm. human embryo (Streeter). X 30. The somatic motor nuclei are colored red.
| |
|
| |
|
| |
|
| |
| 7. The Facial Nerve is composed for the most part of efferent fibers that supply the facial muscles of expression. In 10 mm. embryos these â– fibers arise from a cluster of neuroblasts in the basal plate of the myelen- - 7 cephalon, located beneath the third neuromere (Fig. 287). The fibers-pass laterally, and emerge just mesial to the acoustic ganglion. The motor trunk then continues caudally and is lost in the tissue of the hyoid branchial arch, tissue which later gives rise to the muscles of expression (Fig. 282). The sensory fibers of the facial nerve arise from the eells of the geniculate ganglion, which Bartelmez (1922) asserts is a derivative of the brain wall rather than of the ganglion crest. This ganglion is present in 7 mm. embryos (Fig. 280), located cranial to the acoustic ganglion. The centrally directed processes of the geniculate ganglion enter the alar plate and form part of the solitary tract. Some peripheral fibers course with motor fibers in the chorda tympani, join the mandibular branch of the trigeminal nerve, and end in the sense organs of the tongue. Other sensory fibers form later the great superficial petrosal nerve, which extends to the spheno-palatine ganglion.
| |
|
| |
|
| |
| The motor fibers of the facialis at first grow straight laterad, passing cranial to the nucleus of the abducens. The nuclei of the two nerves later shift their positions, that of the facial nerve moving caudad and laterad, while the nucleus of the abducens shifts cephalad. x\s a result, the motor root of the facial nerve bends around the nucleus of the abducens, producing the genu, or knee, of the former. Together, they produce the rounded eminence in the floor of the fourth ventricle known as the facial colliculus.
| |
|
| |
|
| |
| 9. The Glossopharyngeal Nerve takes its superficial origin just caudal to the otic vesicle (Figs. 280, 286 and 288). Its few motor fibers arise from neuroblasts in the basal plate beneath the fifth neuromeric groove. These neuroblasts form part of the nucleus amhiguus, a nucleus of origin which the glossopharyngeal shares with the vagus (Fig. 287). The motor fibers course laterally beneath the spinal tract of the trigeminal nerve and emerge to form the trunk of the nerve. These fibers later supply the muscles of the pharynx derived from the third branchial arch.
| |
|
| |
|
| |
| The sensory fibers of the glossopharyngeal nerve arise from two ganglia, the su perior, or root ganglion, and the petrosal, or trunk ganglion (Figs. 282 and 288). These fibers constitute the greater part of the nerve, and divide peripherally to form the tympanic and lingual rami to the second and third branchial arches. Centrally, the sensory fibers enter the alar plate of the myelencephalon and join similar fibers of the facial nerve coursing caudally in the solitary tract.
| |
|
| |
|
| |
| 10. u. The Vagus and Spinal Accessory. - The vagus, like the hypoglossus, is composite. It represents the union of several nerves which supply the branchial arches of aquatic vertebrates (Figs. 282 and 288). The more caudal fascicles of motor fibers take their origin in the lateral gray column of the cervical cord, as far back as the fourth cervical segment. These fibers emerge laterally, and, as the spinal accessory trunk (in anatomy a distinct nerve), course cephalad along the line of the neural crest (Figs. 280, 282 and 288). Other motor fibers originate from the neuroblasts of the nucleus ambiguus of the myelencephalon (Fig. 287). Still others arise from a dorsal motor nucleus that lies median in position. The fibers from these two sources emerge laterally as separate fascicles and join the fibers of the spinal accessory in the trunk of the vagus nerve. The accessory fibers soon leave the trunk of the vagus and are distributed laterally and caudally to the vdsceral premuscle masses which later form the sterno-mastoid and trapezius muscles of the shoulder (Fig. 282). Other motor fibers of the vagus supply muscle fibers of the pharynx and laryn.
| |
|
| |
|
| |
| XAs the vagus is a composite nerve, it has several root ganglia which arise as enlargements along the course of the ganglion crest (Figs. 282 and 288). The more cranial of these is the jugular ganglion. The others, termed accessory ganglia, are vestigial structures and not segmentally arranged. In addition to the root ganglia of the vagus, there is the nodose ganglion of the trunk (Fig. 288). The trunk ganglia of both the vagus and glossopharyngeal nerves are believed to be derivatives of the ganglion crest, their cells migrating ventrad in early stages. The central processes from the neuroblasts of the vagus ganglia enter the wall of the myelencephalon, turn caudad, and, with the sensory fibers of the facial and glossopharyngeal nerves, complete the solitary tract. The peripheral processes of the ganglion cells form the greater part of the vagus trunks after the separation from it of the spinal accessory fibers.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 288. - The peripheral nerves in the occipital region of an 18 mm. human embryo (Streeter).X 17
| |
|
| |
|
| |
|
| |
| Placodes. - In aquatic vertebrates, special somatic sensory fibers from the lateral line organs join the facial, glossopharyngeal, and vagus nerves, and their ganglion cells form parts of the geniculate, petrosal, and nodose ganglia. In human embryos, the organs of the lateral line are represented by ectodermal thickenings, or placodes, which occur temporarily over these ganglia. The placode of the hyoid arch is said to contribute cells to the substance of the geniculate ganglion, and possibly the ganglia of the ninth and tenth nerves receive similar additions (Bartelmez, 1922).
| |
|
| |
|
| |
| Neurobiotaxis. - The positions of the motor nuclei vary widely in the several vertebrate groups. This is because the cell bodies of motor neurons migrate during development toward the centers from which their principal afferent impulses proceed. Such a reSponse to some unknown attractive force is called neurobiotaxis.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| THE SYMPATHETIC NERVOUS SYSTEM
| |
|
| |
|
| |
| The sympathetic nervous system is composed of a series of ganglia and peripheral nerves, the fibers of which supply gland cells and the cardiac- and smooth muscle fibers of the viscera and blood vessels. The nerve cells are of the multipolar ganglion type and their axons remain unmyelinated.
| |
|
| |
|
| |
| Sympathetic ganglia arise from cells of the ganglion crest (and the neural tube), which, at 10 mm., migrate distally along the nerve roots and accumulate in masses dorso-lateral to the aorta (Figs. 281 and 406). In the region of the trunk, these paired, segmental clusters unite from segment to segment to form longitudinal cords, which, at 10 mm., are converted into nerve fibers that thereafter link the ganglia in a commissural manner (Fig. 289). The resultant ganglionated cords are the sympathetic trunks.
| |
|
| |
| Root fibers from the cerebro-spinal nerves pass into the adjacent ganglia of the sympathetic trunks (Figs. 289 and 409). Some are efferent and terminate about the ganglion cells, whence their impulses are relayed by unmyelinated sympathetic neurons to their destination (Fig. 281). Others are afferent, bringing visceral sensory impulses directly from the viscera to the spinal ganglia and central nervous system. Both fiber types acquire myelin sheaths and so constitute the white communicating rami. Unmyelinated sympathetic fibers also grow back into the spinal nerves by separate gray communicating rami. These are efferent in function and are distributed with the spinal nerves.
| |
|
| |
| In addition to the primary ganglia of the paired sympathetic trunks, there are other more peripheral ones, known as collateral ganglia, belonging to the great prevertebral plexuses, such as the cardiac, coeliac, and hypogastric (Fig. 289). Still further distad, are the terminal ganglia, located near or even within the structures they innervate; this group includes the ciliary and cardiac ganglia and the small ganglion masses of the myenteric and submucous plexuses. Each cell in these several types of ganglion is in direct relation to the axon of a cerebro-spinal cell, so that every sympathe.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 289. - The sympathetic system of a 16 mm. human embryo (Streeter). X 7. The ganglionated trunk is heavily shaded, cil., Ciliary ganglion; coe., cceliac artery and plexus; lit., heart and cardiac plexus; ot., otic ganglion; pet., petrosal ganglion; s-vi., submaxillary ganglion; sph-p., spheno-jmlatine ganglion.
| |
|
| |
|
| |
|
| |
| The neuron forms a terminal link in a chain whose first link is a neuron belonging to the central nervous system.
| |
|
| |
| The ganglion cells of the prevertebral plexuses originate, in embryos of 7 mm., like those of the sympathetic trunks, and differ only in migrating greater distances. The terminal ganglia related to the cardiac, pulmonary, and upper enteric plexuses arise at about the same time from cells of cerebro-spinal origin which advance peripherally along the vagus nerves.
| |
|
| |
|
| |
| The adult cervical sympathetic ganglia represent fusions between the primitive ganglionic masses of this region (Figs. 288 and 289). The sympathetic ganglia related to the brain are from the first unsegmental. They are derived chiefly from the primitive semilunar ganglia, although the brain wall and the geniculate and petrosal ganglia also contribute (Fig. 289).
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| THE CHROMAFFIN BODIES AND SUPRARENAL GLAND
| |
|
| |
|
| |
| Certain cells of the sympathetic ganglia are transformed into peculiar glands, rather than into neurones. The internal secretion formed by these elements causes them to stain brown when treated with chrome salts - hence the designation, chromaffin cells. Cells of this type, derived from the ganglionated cord of the sympathetic system, give rise to structures known as chromaffin bodies. Chromaffin derivatives of the coeliac plexus, together with mesenchymal tissue, also form the suprarenal gland.
| |
|
| |
|
| |
|
| |
| Fig. 290. - Section through a chromaffin body in a human fetus of ten weeks (after Kohn). .X 450.
| |
|
| |
|
| |
|
| |
| The chromaffin bodies of the ganglionated cords are rounded, cellular masses partly embedded in the dorsal surfaces of the ganglia, and so termed paraganglia (Fig. 290). They appear during the third month, and, at birth, may attain a diameter of i to 1.5 mm. In number they vary from one to several for each ganglion. Similar chromaffin bodies may occur in all the larger sympathetic plexuses. The largest, found in the abdominal sympathetic plexuses, are the aortic chromaffin bodies{ of Zuckerkandl). These occur in embryos of seven weeks, on either side of the inferior mesenteric artery, ventral to the aorta and mesial to the metanejihros. At birth, they attain a length of about i cm. and are composed of cords of chromaffin cells intermingled with strands of connective tissue, the whole being surrounded by a connective-tissue capsule. After birth the chromaffin bodies degenerate, but do not disappear entirely. Associated with the intercarotid sympathetic plexus is a highly vascular chromaffin organ known as the carotid body. Its analge has been first observed in emliryos of seven weeks.
| |
|
| |
|
| |
| The Suprarenal Gland has a double origin. The cortex is derived from mesoderm, the medulla from chromaffin tissue. In an embryo of 6 mm., the anlage of the cortex begins to form from ingrowing buds of the peritoneal mesothelium; this proliferation occurs on each side of the mesentery, near its root. At about 9 mm. the paired glands are definite organs and their vascular structure is evident (Fig. 114). The anlages of the suprarenals early project from the dorsal wall of the coelom, between the mesonephros and mesentery; here they become relatively huge organs (Figs. 145, 154 and 155). The differentiation of the cortex into its three characteristic layers is not completed until between the second and third years. The inner reticular zone is formed first, the fasciculate zone next, and finally the glomerular zone appears during the third month.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 291. - Section through the right suprarenal gland of a 16 mm. human embryo (Bryce). *, Invading groups of chromaffin cells.
| |
|
| |
|
| |
|
| |
|
| |
| The chromaffin cells of the medulla are derived from the coeliac plexus of the sympathetic system. In embryos of seven weeks, whien the cortex is already prominent, masses of these cells begin to migrate from the median side of the suprarenal anlage to a central position (Fig. 291). Such penetration probably continues until after birth. The primitive chromaffin cells are small and stain intensely.
| |
|
| |
|
| |
|
| |
| Anomalies. - Portions of the suprarenal anlage separate frequently from the parent gland and form accessory suprarcnals. As a rule, such accessory glands are composed only of cortical substance; they may migrate some distance from their original position, accompanying the genital glands. In fishes, the cortex and medulla occur normally as separate organs.
| |
|
| |
|
| |
|
| |
|
| |
| +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
| |
|
| |
|
| |
|
| |
|
| |
| CHAPTER XV THE SENSE ORGANS
| |
|
| |
|
| |
| The sense cells of primitive animals, such as worms, are ectodermal in origin and position. Only those of the vertebrate olfactory organ have retained this primitive positional relation, although the germ-layer origin is unchanged. During ]jhylogenesis the cell-bodies of all other such primary sensory neurones, except smell, migrated inward to form the dorsal ganglion (Parker), hence their peripheral processes either end freely in the epithelium, are associated with various sensory corpuscles, or appropriate new cells to serve as sensory receiptors (taste; hearing).
| |
|
| |
|
| |
|
| |
| Among the sense organs are receptive elements of general sensibility which Ipelong to the integument, muscles, tendons, and viscera; these mediate such sensations as touch, pressure, muscle and tendon sensibility, temperature, and pain. Other organs, of a special sensory nature, are responsible for the sensations of taste, smell, vision, and hearing. Each is tuned to a specific and exclusive kind of stimulus. The organs of smell, vision, and hearing are distance receptors, in contrast to all others which collect information from the organism itself and especially from its integument. The apparatus for smell and taste consists of little more than the ' special sensory cells alone, whereas the eye and ear possess elaborate accessory mechanisms for receiving the external stimulus and converting it into a form suitable to affect the sensory cells proper.
| |
|
| |
|
| |
|
| |
|
| |
| GENERAL SENSORY ORGANS
| |
|
| |
| Free nerve terminations form the great majority of all the general sensory organs. When no sensory corpuscle is developed, the neurofibrils of the sensory nerve fibers separate and end among the cells of the epithelia.
| |
|
| |
|
| |
| Laniellated corpuscles first arise during the fifth month as masses of mesodermal cells clustered around a nerve termination. The cells multiply, flatten, and give rise to concentric lamellce. In the cat, these corpuscles increase in number by budding.
| |
|
| |
| Tactile corpuscles are said to develop from mesenchymal cells and | branching nerve fibrils during the first six months after birth.
| |
|
| |
|
| |
| THE GUSTATORY ORGAN
| |
|
| |
| In fetuses of three months, thickenings of the lingual epithelium represent the future taste buds. The parent tissue is quite clearly entoderm, yet an ectodermal origin is sometimes asserted. The cells of an anlage lengthen and extend to the surface of the epithelium. The ovoid mass then differentiates into sensory taste cells, with modified cuticular tips, and into supporting cells. Taste buds are supplied by nerve fibers of the seventh, ninth, and tenth cranial nerves; the fibers branch and end in contact with the periphery of the taste cells.
| |
|
| |
| Between the fifth and seventh fetal months, taste buds are more widely distributed than in the adult. They are found in the walls of the vallate, fungiform, and foliate papillae of the tongue, on the under surface of the tongue, on both surfaces of the epiglottis, on the palatine tonsils and arches, and on the soft palate. After birth, many taste buds degenerate, only those on the lateral walls of the vallate and foliate papillae, on a few fungiform papillae, and on the laryngeal surface of the epiglottis persisting. The development of the several papillae has been described in an earlier chapter (p. 96).
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 292. - Sections through the olfactory anlages of human embryos (adapted by Prentiss). X about 15. A, 4.9 mm.; B, 6.5 mm.; C, 8.8 mm.; D and E, 10 mm.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| THE NOSE
| |
|
| |
|
| |
| The olfactory epithelium arises in embryos of about 4 mm. as paired ectodermal thickenings, on the ventro-lateral sides of the head (Fig. 292. 294 .y 4 ). Specimens 8 mm. long show these placodes depressed into olfactory pits, or fosscc, about which the nose develops (Figs. 184 and 292 B, C).
| |
|
| |
|
| |
|
| |
| The detailed history of the olfactory organ is associated with that of the face. It will be remembered (p. 77) that each first branchial arch forks into a maxillary and mandibular process. Dorsal to the mouth is the fronto-nasal process of the head, lateral to it the maxillary processes, and ventral to it are the mandibular processes (Fig. 3 6g). With the appearance of the nasal pits, the lower part of the fronto-nasal process necessarily is subdivided into paired lateral and median nasal processes (Fig. 293 A) The nasal depressions are at first grooves, each bounded mesially by the median nasal process and laterally by the lateral nasal and maxillary processes. The prompt fusion of the maxillary processes with the median nasal processes converts the nasal grooves into blind pits, opening by external nares (Fig. 293 A), and separated from the mouth cavity by ectodermal plates (Fig. 293 D, E). The mutual union of the median and lateral nasal processes reduces still further the size of the external nares (Fig. 293 B). The epithelial plates which separate the nasal fossse from the primitive mouth cavity become thin, membranous structures caudally, and, rupturing, produce two internal nasal openings, the primitive choana: (Fig. 74). The front part of the plate is invaded by mesoderm, thereby forming the primitive palate (Fig. 292 D) ; the latter becomes the lip and the premaxillary palate. The nasal fossae now open externally through the external nares, and internally into the roof of the mouth cavity through the primitive choanae.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 295, - Stages in the development of the human face. A, 10.5 mm. (Peter); B, 11.3 mm. (Rabl).
| |
|
| |
|
| |
|
| |
|
| |
| Coincident with these changes, the median frontal process has become relatively narrower, and that portion of it between the nasal fossae serves as the nasal septum (Fig. 293). By the development and fusion of the palate anlages (p. 87), the dorsal portion of the mouth cavity is presently partitioned off as the nasal passages (Figs. 294 and 295). The passages of the two sides for a time communicate through the space between the hard palate and the nasal septum (Fig. 294), but later, the ventral border of the septum fuses with the hard palate and separates them completely (Fig. 295). The definitive nasal passages thus consist of the primitive nasal fossae plus a portion of the primitive mouth cavity which has been appropriated secondarily by the development of the hard palate. Their internal opening into the pharynx is by Secondar}", permanent choance. From the second to the sixth month the external nares are closed by epithelial plugs.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 294. - Section through the nose and mouth of a human embryo of seven weeks (Prentiss).X 30.
| |
|
| |
|
| |
|
| |
| The lining of the upper part of the primitive fossae is transformed into olfactory epithelium (Figs. 294 and 295). Many of its ciliated cells become elongate sensory elements with olfactory nerve fibers growing from their basal ends (Fig. 283). The rest of the nasal epithelium, originally a part of the mouth cavity and now respiratory in function, covers the conchas and lines the vomero-nasal organ, ethmoidal cells, and paranasal sinuses (Fig. 296).
| |
|
| |
|
| |
|
| |
| Fig. 295. - Section through the nasal passages of a three-months - fetus (Prentiss). X 14 .
| |
|
| |
|
| |
|
| |
|
| |
| The Vomero-nasal Organs (oj Jacobson) are rudimentary epithelial structures which first appear in 9 mm. embryos as paired grooves on the median walls of the nasal fossse (Fig. 292 C, E). The grooves deepen and close caudally to form tubular sacs, opening toward the front of the nasal septum (Fig. 294). Nerve fibers, arising from the epithelial cells of the organ, join the olfactory nerve, and other fibers from the terminal nerve are received into it. During the sixth month the vomero-nasal organ attains a length of 4 mm. and special cartilages are developed early for its support (Fig. 294). In late fetal stages it often degenerates, but may persist in the adult. This organ is not functional in man, but in many animals it evidently constitutes a special olfactory apparatus.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 296. - Right nasal passage of a fetus at term (Killian-Prentiss). I, Maxillo-turbinal; 11-VI, ethmo-turbinals. The slight elevation at the left of I and u is the naso-turbinal.
| |
|
| |
|
| |
|
| |
| The human Concha; are poorly developed. They include several elevated folds on the lateral and median walls of the nasal foss?e. The maxillo-turhinal is developed first, followed by five ethmo-turhinals arranged in order of decreasing size (Figs. 294 to 296). According to Peter, the ethmo-turbinals arise on the median walls of the fossae, and, by a process of unequal growth, are transferred to the lateral walls. The naso-turbinal is very rudimentary and appears as a slight elevation dorsal and cranial to the maxillo-turbinal (Fig. 296). In adult anatomy, the inferior concha forms from the maxillo-turbinal (I), the middle concha from the first ethmoturbinal {u), and the superior concha from the second and third ethmoturbinals {uI, ID). The naso-turbinal becomes the agger nasi.
| |
|
| |
| In communication with the nasal cavity are several irregular chambers, known collectively as the paranasal sinuses. The ethmoidal cells develop in the grooves between the ethmo-turbinals. During the third month the maxillary sinus begins to evaginate from the groove between I and u (Fig. 296), and, after birth, the superior portion of the same furrow gives rise to the frontal sinus. The caudal end of each nasal fossa is set aside during the third month as a sphenoidal sinus which secondarily invades the sphenoid bone to accommodate its increasing size. These cells and sinuses represent excavations of bone which become lined wdth simultaneously advancing epithelium.
| |
|
| |
|
| |
|
| |
|
| |
| THE EYE
| |
|
| |
|
| |
|
| |
| The eye is a derivative of the fore-brain. In embryos of 2.5 mm., eY^en before this region of the neural groove closes, the evaginated anlages are recognizable (Fig. 305). Soon, at 4 mm., distinct optic vesicles are attached to the brain wall by hollow optic stalks (Figs. 251 and 297), and this condition is followed promptly by the stage of the optic cup in which there is an invagination of the distal wall of the vesicle to form a double-layered crater (Figs. 252 A, 297 B-D, and 299). The optic cup is destined to become the retina, or the essential sensory epithelium of the eye, and the optic nerve. Meanwhile, the surface ectoderm, overlying the optic vesicle, thickens into a placode (Fig. 297 B) that presently pockets inward to produce the leus vesicle, or anlage of the lens (Fig. 297 C, D). The accessory vascular and fibrous coats differentiate from the adjacent mesoderm (Fig. 299). With this introductory explanation for a background, the details of development may now' be set forth.
| |
|
| |
|
| |
| The experiments of Stockard suggest that the earliest optic anlage may be a median area of the fore-brain wall which separates into two placodes that migrate laterad to the positions where they are usually first recognized.
| |
|
| |
| The invagination of the optic vesicle is a self-governed process. On the contrary, contact of the optic vesicle with the overlying ectoderm stimulates the latter to lens formation, even in regions that normally never differentiate a lens (Lewis, 1907). It is possible, however, for a lens to arise independently of this contact stimulus (Stockard, 1910).
| |
|
| |
|
| |
|
| |
| Fig. 297. - Stages in the early development of the human eye (Keibel and Elze-Prentiss). X about 23. /I, B, 4 mm.; C, 5 mm.; D, 6.3 mm.
| |
|
| |
|
| |
| Fig. 298. - The optic stalk, cup and lens of a human embryo of 12.5 mm. (after Hochstetter)X 90. The chorioid fissure has closed along the optic stalk.
| |
|
| |
|
| |
| Differentiation of the Optic Cup. - From the first, the optic cup is imperfect, inasmuch as its region of invagination extends also ventrally along the optic stalk (Fig. 252 A). This produces a defect in the rim of the cup, continuous with a furrow-like groove of the stalk known as the chorioid fissure (Fig. 298). As a necessary result, both the inner and outer layer of the optic cup are continued into the stalk (Fig. 299). During the sixth or seventh week the lips of the chorioid fissure close, so that the distal rim of the optic cup then forms a complete circle.
| |
|
| |
| The development of the optic cup obliterates the cavity of the primitive spherical vesicle (Figs. 297 and 299). Its two component layers lie in apposition and transform into the epithelial retina. The outer, thinner layer becomes the pigment layer. Pigment granules appear in its cells in embryos of 7 mm. and the pigmentation is soon dense (Fig. 303).
| |
|
| |
|
| |
|
| |
| Mesenchyme Lens vesicle Vitreous body Optic stalk Optic recess of brain .
| |
|
| |
|
| |
| Epithelium of cornea Pigment layer of retina Nervous layer of retina
| |
|
| |
|
| |
| Fig. 299. - Section through the optic cup, stalk and lens of a 10 mm. human embryo (Prentiss).X 100.
| |
|
| |
|
| |
| The inner, thicker layer of the optic cup is the retinal layer proper. In it may be recognized the pars cceca, a non-nervous zone bordering the rim, and the pars optica, or the true nervous portion. The line of demarcation between these two regions is a scalloped circle, the ora serrata. By the development of the mesodermal ciliary bodies, the pars caeca is subdivided into a pars ciliaris and pars iridica. The former, with a corresponding zone of the pigment layer, covers the ciliary bodies. The pars iridica blends intimately with the pigment layer and beeomes similarly pigmented (Fig. 304). It forms the inner eovering of the iris.
| |
|
| |
| The pars optica, or nervous portion of the retina, begins to differentiate near the optic stalk and the differentation extends peripherally. An outer, cellular layer (next the pigment coat) and an inner, fibrous layer may be distinguished in 12 mm. embryos (Fig. 299). These correspond to the cellular layer (ependymal and mantle zones) and marginal layer of the neural tube. At three montlis, the retina shows three strata, large ganglion cells having migrated in from the outer cellular layer of rods and cones (Fig. 300). In a fetus of the seventh month, all the layers of the adult retina may be recognized (Fig. 301). As in the wall of the neural tube, both supporting and nervous tissue appear. The supporting elements, of fibers of Midler, resemble ependymal cells and are arranged radially (Figs. 300 and 301). Their terminations form internal and external limiting membranes. The outermost neuroblasts of the retina differentiate into rod and cone cells, the receptive visual cells of the retina, which are at first unipolar (Fig. 301). Next in position comes an intermediate layer of bipolar cells. The inner stratum of multipolar cells constitutes the ganglion cell layer; axons from its cells form the nerve fiber layer. These converge to the optic stalk, and, in embryos of 15 mm., grow back in its wall to the brain (Fig. 284 N). The cells of the optic stalk are converted into a scaffolding of neuroglia' supporting tissue, and the cavity in the stalk is gradually obliterated (Fig. 284 5 ). The optic stalk is thus transformed into the optic nerve, containing a central artery and vein which originally coursed along its open groove (Fig. 303; cf. p. 299).
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 300. - Section of the nervous layer of the retina from a fetus of three months (Prentiss). X 440. At the left are the component elements according to Cajal.
| |
|
| |
|
| |
|
| |
| Fig. 301. - Section through the retina of a seven-months - fetus (Prentiss). X 440 .
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 302. - Section through the lens and corneal ectoderm of a 16 mni. pig embryo (Prentiss). .X 140.
| |
|
| |
|
| |
|
| |
| The Lens. - For a short time the lens vesicle nearly fills the cavity of the optic cup and is attached to the parent ectoderm. In embryos of 8 mm., it lies free of both surface ectoderm and optic cup as a sac whose proximal wall is thicker than the distal one (Fig. 299). The cells of the distal wall remain of a low columnar type, and constitute the lens epithelium (Fig. 302). The cells of the inner wall increase rapidly in height (Fig. 303), and, at about seven weeks, obliterate the original cavity (Fig. 302). These cells transform into lens fibers and their nuclei degenerate. Toward the end of the third month, the primary lens fibers attain a length of 018 mm., whereupon they cease forming new fibers by cell division. All additional fibers arise from the cells of the epithelial layer at its equatorial junction with the lens-fiber mass. Lens sutures are formed on the proximal and distal faces of the lens when the longer, newly formed, peripheral fibers overlap the ends of the shorter, central fibers (Fig. 304). By an intricate but orderly arrangement of fibers these sutures are later transformed into lens- stars of three, and finally of six or nine rays. The structureless capsule of the lens is apparently derived from the lens cells. The fetal lens is spherical and relatively large (Fig. 304).
| |
|
| |
|
| |
|
| |
| Fig. 303. - Section through the optic cup and chorioid fissure of a 12.5 mm. human embryo (Prentiss). X 105.
| |
|
| |
|
| |
| The Vitreous Body and Intraocular Vessels. - The space between the > retina and lens becomes filled with a peculiar hyaline tissue, designated the > vitreous body (Figs. 303 and 304). Modern investigations agree that this substance is primarily a product of the retina, formed in the following way: Processes, probably derived from the early supporting cells of i
| |
| Muller, project from the surface of the retina and constitute a fine, 'â– fibrillar reticulum. This is the primitive vitreous (Fig. 299). Those fibers formed by the pars ciliaris retinae seemingly become the zonula y ciliaris, or suspensory ligament of the lens. Whether the lens itself . participates in vitreous development is disputed.
| |
|
| |
|
| |
| Only when the primitive vitreous body is partly formed does mesenchyme first appear within the optic cup. It enters with the central artery, which, in embryos of 6 mm., courses along the gutter-like groove in the optic stalk, and extends as the hyaloid artery through the chorioid fissure of the optic cup toward the lens (Fig. 303). The fate of this invading mesenchyme - whether it contributes to the structure of the vitreous, or whether it degenerates - is not yet decided beyond question.
| |
|
| |
|
| |
| The hyaloid artery and its accompanying mesenchyme vascularize the back surface of the lens (Fig. 302). Other vessels from the peripheral chorioid supply the front of the lens in the corresponding pupillary membrane (Fig. 304). The investment as a whole constitutes the vascular tunic of the lens. Its highest development is attained in the seventh month, whereas at birth the tunic has usually disappeared. The hyaloid i artery also degenerates completely, the only trace being the space of the hyaloid canal.
| |
|
| |
|
| |
|
| |
| Fig. 304. - Section through the distal half of the eyeball and eyelids of a three-months - fetus (Prentiss). X 35.
| |
|
| |
|
| |
|
| |
|
| |
| The Fibrous and Vascular Coats. - AVhen the lens detaches from the overlying ectoderm, migrant mesenchymal cells fill the intervening space (Figs. 299 and 303), and both lens and optic cup become invested with a i double layer of condensed mesenchyme. The outer, more compact sheath is the anlage of the fibrous coat which differentiates into the sclera and cornea (Fig. 304). The inner, looser sheath will form the vascular coat which includes the iris, ciliary body, and chorioid.
| |
|
| |
| The tough, fibrous sclera covers the base and sides of the eyeball. It corresponds to the dura mater of the brain. During the eighth week, fluid-filled clefts appear in the mesenchyme between the lens and the surface ectoderm; these coalesce into a larger cavity, the anterior chamber of the eye (Fig. 304). The mesodermal layer then located in front of the chamber, and continuous with the sclera, is the cornea (Fig. 304). Externally, it is covered with ectoderm, and the whole area becomes transparent at the end of the fourth month. The mesodermal tissue between the lens and the anterior chamber is the temporary pupillary membrane. The continued lateral extension of the anterior chamber presently separates the iris from the cornea (Fig. 304).
| |
|
| |
| The inner mesenchymal investment, between the anlage of the sclerotic and the pigment layer of the retina, acquires a high vascularity during the sixth week. Its cells become stellate and pigmented, so that the tissue is loose and reticulate. This vascular tissue constitutes the chorioid, in which course the chief vessels of the eye; it corresponds to the pia mater of the brain. Distal to the level of the ora serrata, the vascular coat differentiates into; ( i) the vascular folds of the ciliary bodies; (2) the smooth fibers of the ciliary muscle; (3) the stroma of the iris. The pigmented layers of the iris are derived both from the pars iridica retinae and from a corresponding zone of the pigment layer. Of these, the pigmentlayer cells give rise to the pupillary muscles of the iris. These smooth muscle fibers are thus of ectodermal origin.
| |
|
| |
|
| |
|
| |
| Accessory Apparatus. - The Eyelids develop as folds of the integument bordering the eyeball. The folds appear at the end of the seventh week, and two weeks later their edges have met and fused (Fig. 304). This epidermal union persists until the seventh or eighth month. A third, rudimentary eyelid, corresponding to the functional nictitating membrane of lower vertebrates, constitutes the adult plica semilunaris. The epidermis of the lid is reflected as a mucous membrane over the inner surface, where it is known as the conjunctiva ; this in turn is continuous with the conjunctival epithelium of the cornea. The cilia, or eyelashes, develop like ordinary hairs at the edges of the lids (Fig. 304); they are provided with both sebaceous glands (of Zeiss) and modified sweat glands (of Moll). About 30 tarsal glands also arise along the edge of each lid; these Meibomian glands are sebaceous in nature. The cilia and small glands just mentioned all develop while the eyelids are still fused.
| |
|
| |
|
| |
| The Lacrimal Glands appear during the ninth week as approximately six knobbed ingrowths of the conjunctiva. They lie dorsad near the external angle of the eye. At first solid epithelial cords, they soon branch and acquire lumina.
| |
|
| |
|
| |
| The Naso-lacrimal Duct arises in 12 mm. embryos as a ridge-like thickening of the epithelial lining of the naso-lacrimal groove (Fig. 227), which, it will be remembered, extends from the inner angle of the eye to the primitive olfactory fossa. This thickening becomes cut off, and, as a solid cord, sinks into the underlying mesoderm (Figs. 294 and 295). Secondary sprouts, growing out to each eyelid, comprise the lacrimal ducts.
| |
|
| |
|
| |
| Anomalies. - Lack of pigment in the retina and iris is usually associated with general albinism. A retention of the pupillary membrane causes congenital atresia of the pupil. If the chorioid fissure fails to close properly, there results a gaping, and hence unpigmented defect, or coloboma, in the iris, ciliary body, or chorioid. In cyclopia, a single median eye replaces the usual paired condition. All intergrades exist from closely approximated, separate eyes to perfect unity. The mode of genesis, whether from the fusion of separate •eyes or from the inhibited separation of a common anlage into its bilateral derivatives, is in dispute. In cases of cyclopia the nose is usually a cylindrical proboscis, situated above the median eye.
| |
|
| |
|
| |
|
| |
|
| |
| THE EAR
| |
|
| |
|
| |
| The human ear consists of a sound-conducting apparatus and a receptive organ. The transmission of sound is the function of the external and middle ears. The end organ proper is the internal ear, with auditory reception residing in the cochlear duct. Besides an acoustic function the labyrinthine portion of the internal ear serves as an organ of equilibration.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 305. - Horizontal sections through the early auditory anlages (Keibel and Elze-Prentiss) X 30. A, 2 mm.; B, 4 mm.
| |
|
| |
|
| |
| The Internal Ear. - -The epithelium of the internal ear is derived from the ectoderm. Its anlage appears in embryos of 2 mm. as a thickened, ectodermal plate, the auditory placode, located midway along the side of the hind-brain (Fig. 303 . 4 ). The paired placodes are invaginated to form hollow vesicles which close at about the stage of 3 mm., but remain in temporary union with the ectoderm (Fig. 305 B).
| |
|
| |
|
| |
| The otocyst, or auditory vesicle, when closed and detached, is nearly spherical. Approximately at the point where it joined the ectoderm, a recess, the endolymph duct, is formed and then shifted to a mesial position (Figs. 306 and 307 a). The endolymph duct corresponds to that of selachian fishes, which remains permanently open to the exterior. In man, its extremity is closed and dilated into the endolymph sac (Fig. 307 /).
| |
|
| |
|
| |
| In an embryo of 7 mm., the vesicle has elongated, its narrower ventral process constituting the anlage of the cochlear duct (Figs. 306 and 307 a). The wider, dorsal portion of the otocyst is the vestibular anlage , which, shows indications dorsally of the developing semicircular ducts (Fig. 307 a). These are formed in 11 mm. embryos as two pouches - the anterior and posterior ducts from a single] pouch at the dorsal border of the otocyst, the lateral duct later from a horizontal out-pocketing (Fig. 307 c). Centrally, the walls of these pouches flatten and fuse into epithelial plates, but canals are left peripherally, communicating with the cavity of the vestibule. Soon, the solid, central portions of the epithelial plates are resorbed, leaving the semicircular ducts as in Fig. 307 d, c. Dorsally, a notch separates the anterior and posterior ducts. Of these, the anterior is completed before the posterior; the lateral duct is the last to develop.
| |
|
| |
|
| |
| In a 20 mm. embryo ^Fig. 307 e), the three semicircular ducts are present and the cochlear duct has begun to coil like a snail shell. It will be seen that the anterior and posterior ducts have a common opening dorsally into the vestibule, while their opposite ends, and the cranial end of the lateral duct, are dilated to form ampulla:. In each ampulla is located an end organ, the crista ampullaris, which will be referred to later. By a constriction of its wall the vestibule is differentiated into a dorsal portion, the utriculus, to which are attached the semicircular ducts, and a ventral portion, the sacculus, connected with the cochlear duct (Fig. 307 e). At 30 mm., the adult condition is nearly attained (Fig. 307 /). The sacculus and utriculus are more completely separated, the semicircular ducts are relatively longer, their ampullae more prominent, and the cochlear duct is coiled about two and a half turns. In the adult, the utriculus and sacculus become completely separated from each other, but each remains attached to the endolymph duct by a slender canal that represents the prolongation of their respective walls. Similarly, the cochlear duct is constricted from the sacculus ; the basal end of the former becomes a blind process, and a canal, the ductus reuniens, alone connects the two.
| |
|
| |
|
| |
|
| |
| Fig. 306. - Section through the otic vesicle of a 7 mm. human embryo (His).
| |
|
| |
|
| |
| Fig. 307. - Stages in the development of the internal ear (Streeter). X 25. The figures show lateral views of models of the left membranous labyrinth - a at 6.6 mm. ; 6 at 9 mm.; c at 1 1 mm.; d at 13 mm.; e at 20 mm.; and / at 30 mm. The colors, yellow and red, are used to indicate respectively the cochlear and vestibular divisions of the acoustic nerve and its ganglia, absorp. focus, Area of wall where absorption is complete; crus, crus commune; cSc.lat., ductus semicircularis lateralis; c. sc. post., ductus semicircularis posterior; c. sc. sup., ductus semicularis superior or anterior; cochlea, ductus cochlearis; coch. pouch, cochlear anlage; endolymph., endolymph duct; sacc., sacculus; sac. endol., endolymph sac; utric., utriculus.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| The epithelium of the membranous labyrinth is composed at first of a single layer of low columnar cells. At an early stage, fibers from the acoustic nerve grow between the epithelial cells in certain regions, and these become modified into special sense organs. Such end organs are the crista: ampuUares in the ampullce of the semicircular ducts, the macula acustica in the utriculus and sacculus, and the spiral organ (of Corti) in the cochlear duct.
| |
|
| |
|
| |
|
| |
| The criste and maculae are static organs, or sense organs for maintaining equilibrium.
| |
|
| |
| In each ampulla, transverse to the long axis of the duct, the epithelium and underlying tissue form a curved ridge, the crista (Fig. 309). The cells of the epithelium are differentiated into sense cells, with bristle-like hairs at their ends, and supporting cells. Arborizing about the bases of the sensory cells are fibers from the vestibular division of the acoustic nerve (Fig. 307/). The maculae resemble the cristae in their development, save that larger areas of the epithelium are differentiated into cushion-like end organs. Over the maculae, concretions of lime salts may form otoconia which remain attached to the sensory bristles.
| |
|
| |
|
| |
| The true organ of hearing, the spiral organ, is developed in the basal epithelium of the cochlear duct, basal having reference here to the base of the cochlea. The development of the spiral organ has been studied carefully only in the lower mammals. According to
| |
| I Prentiss (1913), in pig embryos of 5 cm. the basal epithelium is thickened, the cells . becoming highly columnar and the nuclei forming several layers. In later stages, 7 to 9 cm.,
| |
| u inner and outer epithelial thickenings are differentiated, the boundary line between them 1 being the future spiral tunnel (Fig. 308 . 4 ). At the free ends of the cells of the epithelial I swellings there is formed a cuticular structure, the tectorial membrane, which appears first
| |
| in embryos of 4 to 5 cm. The cells of the inner (axial) thickening give rise to the epithelium of the spiral limbus, to the cells lining the internal spiral sulcus, and to the supporting cells and inner hair cells of the spiral organ (Fig. 308 B, C). The outer epithelial I thickening forms the pillars of Corti, the outer hair cells, and supporting cells of the spiral
| |
| I organ. Differentiation begins in the basal turn of the cochlea and proceeds toward the |i apex. The internal spiral sulcus is formed by the degeneration and metamorphosis of the |i cells of the inner epithelial thickening which lie between the labium vestibulare and 1| the spiral organ (Fig. 308 B, C). These cells become cuboidal or flat, and fine the spiral
| |
| I I sulcus, while the tectorial membrane loses its attachment with them.
| |
|
| |
|
| |
| I From what is known of the development of the spiral organ in human embryos, it > I follows the same lines of development as described for the pig. It must differentiate i relatively late, however, for, in the cochlear duct of a newborn child figured by Krause, the spiral sulcus and the spiral tunnel are not yet present. The development of the acoustic nerve and the distribution of its vestibular and cochlear divisions are described on p. 2S0 and illustrated in Figs. 285 and 307.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. S08. - Stages in the differentiation of the spiral organ of the pig (Prentiss). X about 130. A, 8.5 cm.; B, 20 cm.; C, 30 cm. (near term), epSSp., Epithelium of spiral sulcus; h.c., hair cells; i.ep.c., inner epithelial thickening; i.li.c., inner hair cells; i.pil., inner pillar of Corti; lab. vest., labium vestibulare; limb, sp., limbus spiralis; mBas., basilar membrane; m. tect., membrana tectoria; 7 u.vest., vestibular membrane; n.coch., cochlear division of acoustic nerve; o.ep.c., outer epithelial thickening; o.h.c., outer hair cells; sSp., sulcus spiralis; scTymp., scala tympani; stu, stripe of Hensen; tSp. spiral tunnel.
| |
|
| |
|
| |
| The mesenchyme surrounding the membranous labyrinth is differentiated into a ffbrous basement membrane, which lies next the Epithelium, and into cartilage which envelops the whole labyrinth. At about the tenth week, the cartilage bordering the labyrinth then begins a secondary reversal of development whereby it returns first to precartilage and next to a syncytial reticulum which becomes the open tissue of the perilymph spaces (Streeter, 1918) (Fig. 309). The membranous labyrinth is thus suspended in the fluid of the perilymph space. The cochlear duct appears triangular in section, for its lateral wall remains attached to the peripheral bony labyrinth, wTile its inner angle is adherent to the modiolus. Large perilymph spaces are formed above and below the cochlear duct ; the upper is the scala vestibidi, the lower the scala tympani. The thin wall separating the cavity of the cochlear duct from that of the scala vestibuli is the vestibular membrane (of Reissner) (Fig. 308). Beneath the basal epithelium of the cochlear duct, a ffbrous structure, the basilar membrane, is differentiated by the mesenchyme. The bony labyrinth is produced by the conversion of the cartilage capsule into bone. The modiolus is exceptional, however, in that it develops directly from mesenchyme as a membrane bone.
| |
|
| |
|
| |
|
| |
| Fig. 309. - Development of the perilymph space about a semicircular duct of a four-months - human fetus (after Streeter). X 85.
| |
|
| |
|
| |
|
| |
| The Middle Ear. - The middle ear cavity is differentiated from the first pair of pharyngeal pouches, which appear in embryos of 3 mm. (Fig. 87). The entodermal pouches enlarge rapidly, ffatten horizontally, and are in temporary contact with the ectoderm (Fig. SS). During the latter part of the second month, the proximal wall of each pouch constricts to form the auditory tube. This canal lengthens and its lumen becomes slit-like during the fourth month. The blind end of the pouch enlarges into the tytn panic cavity; it is surrounded by loose connective tissue, in which the auditory ossicles are developed and for a time lie embedded. Even in the adult, the ossicles, muscles, and chorda tympani nerve retain a covering of mucous epithelium continuous with that lining the tympanic cavity. The pneumatic cells of the mastoid wall are evaginations formed at the close of fetal life.
| |
|
| |
|
| |
| The auditory ossicles develop from the condensed mesenchyme of the first and second branchial arches. Of these, the malleus and incus are differentiated serially from the dorsal end of the first arch (Figs. 233 and 310). The cartilaginous anlage of the malleus becomes disconnected from Aleckel - s cartilage of the mandible when ossification begins. A portion of the incus, which in early stages joins the stapes, becomes the crus longum. Articulations develop where the three ossicles touch.
| |
|
| |
|
| |
| The stapes is derived from the second branchial arch (Fig. 310). Its mesenchymal and cartilaginous anlages are perforated by the stapedial artery, and consequently become ring-shaped. This form persists until the middle of the third month, when the adult structure is assumed and the stapedial artery disappears.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 310. - Diagram showing the branchial arch origin of the auditory ossicles.
| |
|
| |
|
| |
| The muscle of the malleus, the tensor tympani, is derived from the first branchial arch; the stapedial muscle from the second arch. The further fact that these muscles are innervated by the trigeminal and facial nerves, which are the nerves of the first and second arches respectively, points toward a similar origin for the ear ossicles. These relations strengthen the belief in a branchial arch origin, as maintained by most modern investigators. Fuchs (1905) is among those who deny that the ossicles are derived from the arches.
| |
|
| |
|
| |
|
| |
| The External Ear. - -The external ear is developed from the first ectodermal branchial groove and its adjoining arches (Fig. 64). The external acoustic meatus represents the groove itself, which, for a time, is in contact with the entoderm of the first pharyngeal pouch. Later, however, this contact is lost, and, toward the end of the second month, the groove deepens centralh* to form a funnel-shaped canal which corresponds to the outer portion of the definitive meatus (Fig. 65). From the inner, ectodermal surface a cellular plate grows back and reaches the tympanic cavity. During the seventh month the plate splits, and the space thus added constitutes the inner portion of the external meatus.
| |
|
| |
|
| |
| The tympanic membrane forms by a thinning out of the mesodermal tissue in the region where the wall of the external auditory meatus abuts upon the wall of the tympanic cavity. Hence, it is covered externally b}^ ectodermal epithelium and internally by entoderm.
| |
|
| |
|
| |
| The auricle arises from six elevations, which appear, three on the mandibular arch, and three on the hyoid arch (Fig. 31 1). Modern accounts of the transformation of these hillocks into the adult auricle agree in the main; caudal to the hyoid anlages, a fold of the hyoid integument is formed, the auricular fold, or hyoid helix. A similar fold, dorsal to the first branchial groove, appears later, and unites with the auricular fold to form with it the free margin of the auricle. The point of fusion of these two folds marks the position of the satyr tubercle, according to Schwalbe. Darwin's tubercle occurs at about the middle of the margin of 1 the free auricular fold, and corresponds to the apex of the auricle in lower 1 mammals. The tragus is derived from mandibular hillock i ; the helix from S mandibular hillocks 2 and 3 ; the antihelix from hyoid hillocks 4 and 5 ; t the antitragus from hyoid hillock 6. The lobule represents the lower end y. of the auricular fold.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 311. - Stages in the development of the auricle (adapted in part after His.) . 4 , u mm.; B, 13.6 mm.; C, 15 mm.; D, adult, i, 2, 3, Elevations on the mandibular arch; 4, 5, 6, elevations on the hyoid arch; af, auricular fold; ov, otic vesicle; i, tragus; 2, 3, helix; 4, 5, antihelix; 6, antitragus.
| |
|
| |
|
| |
|
| |
|
| |
| +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
| |
|
| |
|
| |
|
| |
| PART uI. A LABORATORY MANUAL OF EMBRYOLOGY
| |
|
| |
|
| |
| CHAPTER XVI
| |
|
| |
|
| |
| THE STUDY OF CHICK EMBRYOS (A) THE UNINCUBATED OVUM AND EMBRYOS OF THE FIRST DAY
| |
|
| |
| The Unincubated Egg. - The - yolk - of the hen - s egg is a single ovum, enormously expanded with stored food material. AVhen this egg cell is expelled from the ovary, at the time of ovulation, it is enveloped by the vitelline membrane, secreted by the cytoplasm, and by the delicated zona pellucida, a product of the follicle cells (Fig. 312). By the time the liberated ovum passes into the oviduct, the process of maturation has progressed to the point where one polar cell is given off (cf. Fig. 15 A). Fertilization by mature, waiting spermatozoa now ensues, and, coincidently, the second polar cell is extruded to complete maturation (cf. Figs. 15 B and 17). As the egg passes down the oviduct, the albumen, shell membrane, and shell are added as accessory investments. The ovum is ready to be laid one day after its discharge from the ovary; at this time, the appearance is as indicated in Fig. 312. The cytoplasmic area, already started toward the formation of an embryo, is the familiar whitish disc, technically designated the blastoderm.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| ^ A majority of the illustrations for this section and the skeleton of many descriptions have been adapted from the manual published by Professor C. W. Prentiss.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 312. - Diagrammatic longitudinal section of a hen - s egg before incubation (Thomson in Heisler).
| |
|
| |
|
| |
| Fig. 314. - Gastrulation in the pigeon, as shown by a longitudinal section of the blastoderm (redrawn after Patterson). X 50.
| |
|
| |
|
| |
|
| |
|
| |
| Cleavage, Blastula and Gastrula. - Fertilization promptly initiates a series of cell divisions which divide the blastoderm into a cellular disc, separated from the yolk by a cleftlike space (Fig. 313). Such mitoses comprise the process of cleavage, and the resulting, asymmetrical, hollow sphere is the blastula. During the period of gastrulation which follows, the blastoderm becomes two-layered. This is accomplished by the rolling under of cells at the future caudal margin of the blastoderm (Fig. 314). Such proliferation and undertucking gives rise to an inner layer, the entoderm; the original surface layer is the ectoderm.
| |
|
| |
|
| |
|
| |
| Primitive Streak and Mesoderm.
| |
|
| |
| The first conspicuous structure on the blastoderm is an opaque band which is named the primitive streak (Fig. 315). It appears after 16 hours incubation, lying somewhat caudad in the future midline. The primitive streak is interpreted as the margin of the germinal disc where entoderm formation just occurred; the changed appearance and direction are due to the swinging together and fusion of its two lateral halves (Fig. 30). Directly following the earliest appearance of the streak, a primitive groove courses lengthwise along its surface. Cephalad, this ends in the deeper primitive pit, while at the extreme cranial end is an area not indented by the groove, known as the primitive knot (of Hensen).
| |
|
| |
|
| |
|
| |
| Fig. 315. - Chick blastoderm at the stage of the primitive streak (16 hours). X 20.
| |
|
| |
|
| |
|
| |
|
| |
| Transverse sections across the primitive streak prove that it is a thickening of the ectoderm and the site of origin for the middle germ layer - -as it was for the entoderm at an earlier stage (Fig. 316). When the mesoderm cells first arise, they are sparse, migratory elements which soon associate into distinct plates extending laterad and caudad. Later, the mesoderm invades the region in front of the streak. At the primitive knot all three germ layers fuse intimately (Fig. 316 A)\ in the caudal half of the streak the entoderm is free (Fig. 316 B). But at both levels the mesoderm represents lateral proliferations from the primitive streak. It appears that the primitive groove is the mechanical result of this rapid growth, or invagination, of mesoderm. From the three germ layers, thus formed, all the tissues and organs will develop, as listed on p. 6.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 316. - Transverse sections of a chick embryo at the stage of the primitive streak. X 165., 4 , Through the primitive knot; B, through the primitive groove.
| |
|
| |
|
| |
|
| |
|
| |
| Head Process and Head Fold. - Embryos of about 19 hours - incubation show an axial strand of cells extending forward from the primitive knot (Fig. 317). This is the so-called head process; it is also termed the notochordal plate because it becomes the cylindrical notochord which serves as the primitive axis about which the embryo differentiates. Although the head process is often described as an outgrowth from the primitive knot, it more probably represents a later stage of the cephalic end of the primitive streak, and grows progressively at the expense of the primitive streak, as the latter, still maintaining its regional characteristics, recedes caudad. A longitudinal section shows the relation of head process to primitive knot (P^ig. 318); a transverse section demonstrates it as a median, thicker mass, continuous laterally with mesoderm which has grown into this region (Fig. 319). Both sections illustrate the independence of the head process from the ectoderm above, and the temporary fusion with the entoderm below.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 317. - Chick blastoderm and embryo at the stage of the head process and head fold ( 19 hours). X 19.
| |
|
| |
|
| |
|
| |
|
| |
| After the head process is established, a curved fold appears cephalad to it (Fig. 317). This is the head fold which at first involves ectoderm and entoderm alone (Fig. 318). The future development of this important structure will establish the gut internally and definitely delimit the upper body externally.
| |
|
| |
|
| |
|
| |
| Fig. 318. - Median sagittal section of a chick embryo at the stage of the head process and head fold (19 hours). X 100.
| |
|
| |
|
| |
|
| |
|
| |
| Neural Groove and Mesodermal Segments. - Even embryos of the previous stage exhibit a broad zone of thickening in the ectoderm overlying the head process. This region constitutes the neural plate (Fig. 319). In an embryo of 21 hours, the plate folds lengthwise to form a gutter-like trough, called the neural groove, which shortly will become rolled into the tubular brain and spinal cord (Fig. 320). The notochord now shows through the ectoderm at the bottom of the groove; laterally, the groove is flanked by elevated ridges, the neural folds.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 319. - Transverse section through the head process of a 19 hour chick embryo. X 165.
| |
|
| |
|
| |
| The wings of mesoderm which grew from the sides of the primitive streak have spread cephalad to the extent indicated by the darker shading in Fig. 32c. Next the notochord, the mesoderm is thick, and in it have appeared two pairs of vertical clefts;- these separate the mesoderm into successive masses (the first incomplete cranially), which will be seen better in older stages. The}^ are mesodermal segments.
| |
|
| |
|
| |
| Fig. 320. - Chick embryo at the stage of the neural groove and first two pairs of segments (21 hours) (after Duval). X 13. The head process is visible through the bottom of the neural groove.
| |
|
| |
|
| |
| (B) EMBRYO OF FIVE SEGMENTS (TWENTY -THREE HOURS)
| |
|
| |
|
| |
|
| |
| It is evident that an embryonic and an extra-embryonic region of the blastoderm are becoming more sharply defined (Fig. 321). Of the extraembryonic territory, that nearest the embryo comprises the clearer area pellucida; peripherad lies the area opaca, darker because of its adherence to the yolk beneath. In the more proximal zone of the opaque area are mottled masses, the blood isla>ids, already observed in younger stages but now fusing into an incomplete network. This mesh is best developed caudally; it is the area vascidosa.
| |
|
| |
|
| |
|
| |
| At this period the head is growing rapidly. It rises above the blastoderm and projects ccphalad as a somewhat cylindrical part of the embryo, which, at its cephalic end, is entirely free (Fig. 321). In accomplishing this result, the shallow head fold of earlier stages appears to have grown j caudad and liberated the head by undercutting (Fig. 322) ; the real factor, however, is a true forward growth on the part of the head itself. Simul- ;i taneously with the extension of the head, the entodermal component of the original head fold is elongated into an internal tubular pocket of | roughly corresponding shape; this is the primitive j ore-gut. Cranially, j it is a blind sac; caudally, it opens out onto the yolk through an arched 1 aperture termed the intestinal portal. In Fig. 321 the lateral limits of the darker fore-gut and its relation to the crescentic intestinal portal are shown plainly; Fig. 322 illustrates how the entoderm is reflected into the fore-gut at the level of the portal.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 321. - Dorsal view of a chick embryo with hve segments (23 hours). X 14- 1
| |
|
| |
|
| |
|
| |
|
| |
| The neural groove is broad and deep (Fig. 321). Midway, its lateral folds are approximated and ready to fuse. Caudally, the folds diverge and become increasingly indistinct.
| |
|
| |
|
| |
| The mesodermal segments are clearly defined and block-like. The notochord shows through the transparent ectoderm, and the x^rimitive streak is shorter, both relatively and actually. Later, when the body form is further indicated by the formation of the tail fold, the primitive streak will disappear. It is a notable fact that the head not only arises soonest but retains its early advantage over lower levels of the body. The progressive advance of differentiation first reaches the end of the trunk at a considerably later period.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 322. - Median longitudinal section of a five-segment chick embryo (redrawn from Patten). X 25.
| |
|
| |
|
| |
|
| |
|
| |
| (C) EMBRYO OF SEVEN SEGMENTS (TWENTY-FIVE HOURS)
| |
|
| |
|
| |
|
| |
| A surface view of a chick embryo at this stage resembles the one last described, but shows certain distinct advances (Fig. 323) ; yet the descriptions that follow will apply in all essentials to embryos having from five to ten primitive segments. The vascular area of the blastoderm is better organized than before and extends far cephalad. In front of the head is a light area, not yet invaded by mesoderm and known by the unsuitable name proamnion. The primitive streak is still prominent caudally and measures about one-fourth the length of the embryo. The notochord may be followed cephalad from the primitive knot until it is lost beneath the neural tube.
| |
|
| |
|
| |
| Neural Tube. - The lips of the neural folds have met throughout the cranial two-thirds of the embryo, but have not fused to any extent. The neural tube, formed thus by the closing of the ectodermal folds, is open at each end; the delayed closure of the cranial extremity leaves a temporary opening to the outside, designated the anterior neuropore. In succeeding stages, the more caudal regions of the present neural groove will be rolled progressively into a tube and added to that already completed. At the head end, the neural tube has begun to expand into the brain vesicles. Of these, only ihe forc-hrain is prominent, and from it the optic vesicles are budding out laterally.
| |
|
| |
|
| |
| Fore -gut. except for an increase in size, the fore-gut is little changed. Near its blind end, the floor of the gut is applied to the ectoderm and the two comprise the temporary pharyngeal membrane (cf. Fig. 335). The fore-gut will ultimately constitute the alimentary canal as far as the middle of the small intestine, the way in which the entoderm is folded up from the blastoderm and forward into the head is shown well in Figs. 322 and-335 Mesoderm and Coelom. - The tissue of the middle germ layer assumes two different forms. Throughout most of the head region it comprises a diffuse meshwork of cells which fills in the spaces between the various epithelial layers. This tissue is mesenchyme (Fig. 331). In the caudal part of the head, and in the remainder of the body, the mesoderm at this stage is organizing more definitely. Nearest the midplane, it is divided by transverse furrows into seven block-like primitive segments, four of which belong to the future head (Figs. 323 and 324). Caudad, between the segments and the primitive streak, there is the undifferentiated mesoderm of the segmental zone, but new pairs of segments will develop progressively throughout this region. Lateral to each segment is a plate of unsegmented mesoderm, termed the intermediate cell mass; it is also called the neplirotome because it will play an important role in the development of the excretory system (Fig. 324). The nephrotome plate serves as a bridge between the segments and the unsegmented lateral mesoderm. The lateral mesoderm, when first formed, aggregates into two solid plates (Fig. 316) each of which splits secondarily into two lamellce, separated by a space (Figs. 324 and 325). The dorsal layer comprises the somatic mesoderm, the ventral layer the splanchnic mesoderm.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 323. - Dorsal view of a chick embryo with seven segments (25 hours). X 20. numbered lines indicate the levels of the sections. Figs. 325-332.
| |
|
| |
|
| |
|
| |
| Fig. 324. - Diagrammatic transverse section through a hen - s egg at an early stage of development (Minot-Prentiss).
| |
|
| |
|
| |
|
| |
| The space between the two layers first occurs as isolated clefts, which soon unite to form the body cavity, or coelom (Fig. 324). The originally bilateral coelomic chambers will later become confluent v^entrally, as in the adult (Fig. 324). In the region of the heart, the ccelom is already enlarged locally, anticipating its destiny as the pericardial cavity. Other portions will become the pleural cavities of the thorax, and the peritoneal cavity of the abdomen.
| |
|
| |
|
| |
|
| |
| Heart and Blood Vessels. - The heart is a simple, straight tube, l3ung in the midplane, ventral to the gut. In a dorsal view of the total embr^m it is inconspicuous because largely concealed by overlying structures.
| |
|
| |
|
| |
|
| |
| Caudally, it is continuous with the converging vitelline veins which enter the body by following along the margins of the intestinal portal: the two veins unite as they join the heart (Fig. 323). From the cephalic end of the heart is given off the ventral aorta; dorsal to the gut course paired descending aortae.
| |
|
| |
|
| |
| Transverse Sections
| |
|
| |
| The first embryo to be studied in serial section is easiest understood if the student begins caudad, where differentiation is least, and works toward the head. Important facts pertaining to the germ layers and the jirinciples underlying the development of the neural tube, gut, heart, and head are then made simple. The following illustrations and descriptions may be used to interpret sections of chick embryos between the stages of five and ten segments. The level of each section may be determined from the numbered lines on Fig. 323.
| |
|
| |
| Sections through the Primitive Streak and Knot. - Conditions are essentially the same , as in the younger embryos already examined (Fig. 316). Section through the Fifth Primitive Segment (Fig. 325). - This general level is characterized l.)y the differentiation of the mesoderm, the approximation of the neural folds, and the presence of two vessels, the descending aortir, one on each side between the mesodermal segments and the entoderm. The neural folds are thick, as is the adjoining ectoderm to a less degree. The jwtochord is a sharply defined oval mass of cells. The mesodermal segments are somewhat triangular in outline and connected by the intermediate cell mass, or j ucplirotomc, with, the lateral mesoderm. The lateral mesoderm is partially divided by irregu- j lar, flattened spaces into two sheets, the dorsal of which is the somatic layer, the ventral the splanchnic layer. Later, the spaces unite to form the coelom, or primitive body cavity, J and the mesodermal lining then becomes mcsothelium.
| |
|
| |
|
| |
|
| |
| Fig. 325. - Transverse section through the fifth pair of mesodermal segments of a seven segment chick embryo. X 90.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Through the higher segments in the series the differentiation of mesoderm and ccelom is more advanced (cf. Fig. 343). Caudal to the seventh segment, in the region of the segmental zone, the mesoderm forms solid plates (cf. Fig. 344).
| |
|
| |
| Sections through the Area Vasculosa (Fig. 326). - The illustrations show a little of the extra-embryonic territory, peripheral to the area pellucida. In this region of the area 0 paca , the entoderm is associated intimately with the coarsely granular yolk. The splanchnic mesoderm contains aggregations of cells known as Mood islands, many of which are fusing into the network that characterizes the area vasculosa (Figs. 323 and 326 I). The cellular thickenings of the blood islands undergo differentiation into two cell types : fluidfilled vacuoles appear and expand so as to set free the innermost cells which later separate and float about as primitive Mood corpuscles; the same process flattens the peripheral cells into an endothelium {B, C). The endothelial spaces both coalesce and form new vascular sprouts, and in this way the system of extra-embryonic vessels is extended. All blood vessels at first consist of an endothelial layer only.
| |
|
| |
|
| |
|
| |
| Fig. 326. - Transverse sections through the area vasculosa of a seven-segment chick embryo.X 300.
| |
|
| |
|
| |
|
| |
|
| |
| Section Caudal to the Intestinal Portal (Fig. 327). - The section is characterized: (i) by the meeting of the neural folds preparatory to closing the neural tube; (2) by the arching of the entoderm, which, a few sections nearer the head end, forms the fore-gut; (3) by the presence of the vitelline veins laterally between the entoderm and splanchnic mesoderm; .
| |
|
| |
|
| |
| Fig. 327. - Transverse section caudal to the intestinal portal of a seven-segment chick embryo.X 90.
| |
|
| |
|
| |
|
| |
| (4) by the wide separation of the somatic and splanchnic mesoderm and the consequent increase in the size of the coelom. In this location the coelom later surrounds the heart and is converted into the pericardial cavity.
| |
|
| |
| The neural tube at this level is transforming into the third brain vesicle, or hind-brain. The neural folds have not yet fused, and at their dorsal angles are the neural crests, the anlages of the spinal ganglia. Mesodermal segments never develop as far cephalad as this region; instead, diffuse masses of mesenchyme occupy comparable positions adjacent to the neural tube. On the left of the section, an asterisk marks the junction of somatic and splanchnic mesoderm.
| |
|
| |
|
| |
| Section through the Intestinal Portal (Fig. 328). - This section passes through a vertical fold of entoderm at the exact point where the latter is reflected into the head as the forc-gut (cf. Figs. 322 and 335). The entoderm forms a continuous bridge of tissue between the vitelline veins, thereby closing the fore-gut ventrally. The splanchnic mesoderm is differentiated into a thick-walled pouch on each side, lateral to the endothelial layer of the veins.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 328. - Transverse section through the intestinal portal of a seven-segment chick embryo.X 90.
| |
|
| |
|
| |
|
| |
|
| |
| A few sections cephalad, the gut separates from the general entoderm; this will allow first the endothelial heart tubes to meet, and then the flanking folds of splanchnic mesoderm.
| |
|
| |
|
| |
| Fig. 329. - Transverse section through the heart of a seven-segment chick embryo. X 90.
| |
|
| |
| Section through the Heart (Fig. 320). - Passing cephalad in the series, the vitelline veins open into the heart just cranial to the intestinal portal. The entoderm in the head fold now lines the crescentic pharynx of the fore-gut, and is separated by the heart, ccelom, and splanchnic mesoderm from the entoderm of the germinal disc. The descending aortae are larger, making conspicuous spaces between the neural tube {hind-brain) and the pharynx. The heart, as will be seen, results from the union of two endothelial tubes, continuous with those constituting the vitelline veins in the preceding sections. The median walls of these tubes disappear at a slightly later stage and thereby establish a single tube, the endocardium. Thickened layers of splanchnic mesoderm, which, in the preceding section, invested the vitelline veins laterally, now form the mesothelial wall of the heart; this tissue will become the later myocardium and epicardium. In the median ventral plane, the layers of splanchnic mesoderm of each side have fused and separated from the splanchnic mesoderm of the germinal disc; thus, the two pericardial cavities are put in communication. Dorsally, the splanchnic mesoderm, as the dorsal mesocarduim, suspends the heart, while still more dorsally it is continuous with the somatic mesoderm at the point where the mesenchyme of the head extends to the coelom.
| |
|
| |
|
| |
|
| |
| Fig. 330. - Transverse section through the head fold of a seven-segment chick embryo. X 90.
| |
|
| |
|
| |
|
| |
| Origin of the Heart and Embryonic Vessels. - From the two sections last described, it is seen that the heart arises as a pair of endothelial tubes lying in folds of the splanchnic mesoderm. Later, the endothelial tubes fuse and the mesodermal folds are also brought together. The heart then consists of a single endothelial tube within a thick-walled investment of mesoderm. The endothelial cells of the heart often appear to arise from the entoderm but this is perhaps a deception, for elsewhere endothelium is mesodermal in origin. The vascular system is primitively a paired system, the heart arising as a double tube with two veins entering and two arteries leaving it (cf. Figs. 180 and 181). The blood vessels of the body are delicate endothelial channels which originate as clefts in the mesenchyme. Coalescence and budding produce a plexus from which definite vessels are selected (Fig. 179).
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 331. - Transverse section through the pharyngeal membrane of a seven-segment chick embryo. X 90.
| |
|
| |
|
| |
| Section through the Head Fold (Fig. 330). - It will be remembered that a crescentic ectodermal fold lies both beneath the head and lateral to it, and that the portion of the body cephalad to this head (old is free from the blastoderm (Figs. 322 and 323). The present section, from a level just cephalad of the heart, is located at the critical region where these folds meet. The inspection of a few sections in each direction will demonstrate how the embryonic and extra-embryonic territories are related and how they become separate. The coelom does not extend into the head. Midway of the blastoderm is a space which lacks mesoderm; it is the proamnion. Ventral to the pharynx, the ventral aorta are becoming separate vessels as they leave the heart.
| |
|
| |
|
| |
| Section through the Pharyngeal Membrane (Fig. 331). - This section shows the head free from the underlying blastoderm (cf. Fig. 322). The ectoderm surrounds the head, and near the midventral line it is bent dorsad, is somewhat thickened, and comes in contact with the thick entoderm of the pharynx. The area of contact between ectoderm and pharyngeal entoderm constitutes the pharyngeal membrane. Later, this plate breaks .
| |
|
| |
|
| |
|
| |
| Fig. 332. - Transverse section through the fore-brain and optic vesicles of a seven-segment chick embryo. X 90.
| |
|
| |
|
| |
| Through and establishes the oral cavity, which, accordingly, is partly ectodermal. The expanded neural tube is closed and forms the middle brain vesicle, or mid-brain; the |i superficial ectoderm is entirely separate from it. The descending aortse appear as small vessels dorsal to the lateral folds of the pharynx. The blastoderm in the region beneath the head is composed of ectoderm and entoderm only; this is the proamnion. Laterad may be seen the layers of the mesoderm.
| |
|
| |
|
| |
|
| |
| Section through the Fore -brain and Optic Vesicle (Fig. 332). - The neural tube is open here and constitutes the first brain vesicle, or fore-brain. The opening is the temporary anterior neuroporc. The ectoderm is composed of two or three layers of nuclei and is continuous at the neuropore with the much thicker wall of the fore-brain. The two ectodermal layers are in contact with each other except in the midventral region, where the mesenchyme is beginning to penetrate and separate them. The lateral expansions of the : fore-brain are the optic vesicles, which eventually give rise to the retina of the eye.
| |
|
| |
|
| |
|
| |
| (D) EMBRYO OF SEVENTEEN SEGMENTS (THIRTY-EIGHT HOURS)
| |
|
| |
|
| |
| The stage selected as a type for illustrating the significant advances j since the seven-segment embryo is a chick of about 38 hours incubation which possesses 17 primitive segments. At this time, the segments are j| developing rapidly and the descriptions that follow will apply satisfactorily i to embryos between 33 hours (12 segments) and 40 hours (18 segments).
| |
|
| |
| The long axis of the embryo is still nearly straight, but specimens of full 17 segments should show a flexing of the head ventrad (Fig. 335) and a slight turning of the tip of the head on its left side. Fig. 333 does not illustrate this feature. The area pellucida is dumb-bell shaped and is developing a vascular network. The extra-embryonic vessels of the area opaca are well differentiated, and the vascular area is bordered by a terminal sinus. Opposite the caudal end of the heart, the vascular networks converge and become continuous with the stems of the vitelline veins. Connections have been established also between the descending aortae and the vascular area at the level of the lowest segments, but as yet the vitelline arteries have not appeared as distinct trunks (Fig. 334). The tubular heart is bent to the embryo - s right; the head is more prominent and the three primary vesicles of the brain are evident; the proamniotic area is reduced to a small region in front of the head ; the primitive streak is inconspicuous.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 333. - Dorsal view of a chick embr\^o with seventeen segments (38 hours). X 20.
| |
|
| |
|
| |
|
| |
| Central Nervous System and Sense Organs. - ^The tardy closure of the anterior neuropore has occurred, and the neural tube is complete save at its caudal end where the divergent neural folds form the so-called rhomboidal sinus (Fig. 333)- In the head, the neural tube is differentiated into three brain vesicles, marked off from each other by constrictions. The fore-brain (prosencephalon) is characterized by the outgrowing optic vesicles. The mid-brain (mesencephalon) is a simple dilatation. The elongate hind-brain (rhombencephalon) gradually merges with the spinal cord; it shows a number of secondary constrictions, the neuromeres ectoderm of the ventral surface of the head and the entoderm caudal to the intestinal portal have been removed. Numbered lines indicate the levels of Figs. 337 - 344.
| |
|
| |
|
| |
| The ectoderm is thickened laterally over the optic vesicles to form the lens placode of the eye (Fig. 337). The optic vesicle flattens at this point and will soon invaginate to produce the optic cup. Dorso-laterally, in the hind-brain region, the ectoderm is thickened and indented as the auditory placodes (Fig. 334). Each placode will become an otocyst, or otic vesicle, from which differentiates the sensory epithelium of the internal ear (membranous labyrinth).
| |
|
| |
|
| |
|
| |
| Fore -gut. - The entoderm is still flattened over the surface of the yolk, caudal to the intestinal portal. In Fig 334, the greater part of the entoderm is cut away. The broad fore-gut, folded inward at the portal, shows indications of three lateral diverticula, the pharyngeal pouches. Cephalad, the pharynx is closed ventrally by the pharyngeal membrane, and the ectodermal depression external to it is the stomodeum (Fig. 335).
| |
|
| |
|
| |
|
| |
| Fig. 335. - Median sagittal section of the head end of a seventeen-segment chick embryo..X about 50.
| |
|
| |
|
| |
| Heart and Blood Vessels. - After receiving the vitelline veins just cephalad of the intestinal portal, the venous end of the heart tube dilates into the ventricle which bends ventrad and to the embryo - s right (Fig. 334). It then is flexed dorsad and to the median plane, and narrows to form the bulbns, and its continuation, the ventral aorta. The ventral aorta lies beneath the pharynx and divides into two divisions. These diverge and course dorsad around the pharynx as the first pair of aortic arches. Before reaching the optic vesicles they bend sharply caudad, and, as the paired descending aorta:, may be traced to a point opposite the last primitive segments. In the region of the intestinal portal they lie close together and have fused to form a single vessel, the dorsal aorta. Below this level they separate again, and, opposite the last primitive segments, connect by numerous capillaries with the vascular network. In this region, the trunks of the paired vitelline arteries presently will be differentiated. The heart beats spasmodically at this stage; the blood flows from the vascular area by way of the vitelline veins to the heart, thence by the aortse and vitelline arteries back again. This constitutes the vitelline circulation, and through it the embryo receives nutriment from the yolk for its future development.
| |
|
| |
|
| |
|
| |
| Heretofore, the body of the embryo has been without definite veins, but now two pairs of vessels are developing for the purpose of returning lilood to the heart. The anterior cardinal veins drain blood from the head region; the posterior cardinals, just appearing at this stage, will perform a similar function in the lower body (cf. Fig. 348). The two vessels unite on each side into a common cardinal (duct of Cuvier) which enters the venous end of the heart.
| |
|
| |
|
| |
| Differentiation of Mesoderm. -The production of early mesodermal segments, and the addition of new ones by a progressive furrowing of the segmental zone, has been observed in previous stages. The segments thus formed are block-like with rounded corners when viewed dorsally, the ectoderm is removed from the dorsal surface.
| |
|
| |
|
| |
|
| |
| Fig. 336. - Reconstruction through the lower mesodermal segments of a two-day chick embryo.
| |
|
| |
|
| |
|
| |
|
| |
| Triangular in transverse section (Fig. 336). In higher vertebrates, the segments contain at most only indications of a cavity; in the chick there is a minute central space, representing a portion of the coelom, which is filled with a cellular core, while the other cells of the segment form a thick, radially-arranged layer about it (Fig. 343). The ventral wall and a portion of the median wall of each primitive segment break down into a mass of mesenchyme termed the sclerotome (Fig. 2 1 1 ) ; these later surround the notochord and neural tube, and transform into the axial skeleton. The remaining portions of the segment constitute the dernio-myotome (Figs. 212 and 340). The cells of the dorso-mesial wall of the plate, the myotome, eventually give rise to the skeletal musculature of the body. The lateral plate is the dermatome which is destined to furnish the deeper layers of the integument.
| |
|
| |
|
| |
|
| |
| The bridge of cells connecting a primitive segment with the lateral mesodermal layer constitutes the intermediate cell mass, or nephrotome (Fig. 336). In the chick, the nephrotomes of the fifth to sixteenth segments give rise to segmental pairs of bud-like sprouts which extend dorsad (Fig. 343). These are the prone phric kidney tubules. Although rudimentary, their ends unite to form a tube, known as the pronephric duct, which grows to the cloaca (Fig. 128). More caudal nephrotomes will soon form the embryonic kidney, or mesonephros, whose tubules open into the pronephric duct, then called the mesonephric duct (Fig. 336). Later still, the permanent kidney develops partly from the pronephric duct and partly from nephrotome tissue. Accordingly, the intermediate cell masses may be regarded as the anlages of the urogenital glands and ducts - all mesodermal in origin.
| |
|
| |
|
| |
| In the embryo of seven primitive segments, the lateral mesoderm was observed to split into two layers, the dorsal somatic and the ventral splanchnic mesoderm (Fig. 336). These layers persist, the somatic mesoderm giving rise to the pericardium, parietal pleura, and peritoneum, while the splanchnic layer forms the epi-myocardium, the visceral pleura, and the mesenteries and mesodermal layers of the gut. The somatic mesoderm and ectoderm are closely associated in development and together are designated the somatopleure (Fig. 324) ; it forms the body wall. Similarly, the splanchnic mesoderm and entoderm are jointly termed the splanchnoplcure. Both the mesodermal segments and the unsegmented mesodermal layers contribute the mesenchymal cells which play such an important part in development.
| |
|
| |
|
| |
| Transverse Sections
| |
|
| |
| In studying serial sections of an embryo it is not sufficient merely to identify the structures seen. The student should determine also the exact level of each significant section with respect to the illustrations of the total embryo, as indicated for this series along the margins of Fig. 334, and trace the organs from section to section in the series. He is then ready to reconstruct mentally the complete picture of a part and to interpret its origin and relations.
| |
|
| |
|
| |
| The following sections are drawn, viewed from the cephalic surface; hence, the right side of the embryo is at the reader - s left. These illustrations and descriptions may be used for the stud}" of chick embryos between 33 hours (12 segments) and 40 hours (18 segments).
| |
|
| |
|
| |
| Section through the Fore-brain and Optic Vesicles (Fig. 337). - The optic stalks connect the optic vesicles laterally with the ventral portion of the fore-brain. Dorsally. the section passes through the mid-brain, due to the somewhat ventrally flexed head (cf. fig- 335)- The lens placodes are thickenings of the surface ectoderm over the optic vesicles. Note that there is now a considerable amount of mesenchyme between the ectoderm and the neural tube; the small spaces are terminal branches of the anterior cardinal veins. Layers of mesoderm are present in the underlying blastoderm.
| |
|
| |
|
| |
| Section through the Pharyngeal Membrane and Mid-brain (Fig. 338). - In the midventral line, the thickened ectoderm bends up into contact with the entoderm of the rounded pharynx of the fore-gut. The resulting ectodermal pit is the stomodeum, and the two apposed layers represent the pharyngeal membrane. At this point the oral opening will break through. ( )n either side of the pharynx a pair of large vessels is seen; the ventral pair are the ventral aorta. Two sections cephalad, their cavities open into those of the dorsal pair, the descending aorta. The section is thus just caudad of the first aortic arches. The caudal end of the mesencephalon is the portion of the neural tube showing; it is thick walled, with an oval cavity. Note the large amount of undifferentiated mesenchyme throughout the section. The structure of the blastoderm is complicated by the presence of collapsed blood vessels.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 337. - Transverse section through the fore-brain of a seventeen-segment chick embryo.X 75 .
| |
|
| |
|
| |
| Fig. 338. - Transverse section through the pharyngeal membrane of a seventeen-segment chick embryo. X 7,3.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Section through the Hind-brain and Auditory Placodes (Fig. 339). - This section is characterized by: (i) the auditory placodes, which represent the anlages of the internal ear; .
| |
|
| |
|
| |
|
| |
| Fig. 339. - Transverse section through the hind-brain and auditory placodes of a seventeen
| |
| segment chick embryo. X 75.
| |
|
| |
|
| |
|
| |
|
| |
| (2) the large hind-brain, somewhat thin and flattened dorsad; (3) the broad pharynx, cut through the second pair of pharyngeal pouches, above which on each side lie the descending aortae; (4) the presence of the bulbar and ventricular portions of the heart. The bulbus is suspended dorsally by the mesoderm, which here forms the dorsal mesocardium. The ventricle lies on the right side of the embryo; a few sections caudad in the series it is continuous with the bulbus (cf. Fig. 334). Between the somatic and splanchnic mesoderm is the large pericardial cavity, surrounding the heart. Yentro-lateral to the brain are the anterior cardinal veins, which return blood from the head region.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 340. - Transverse section through the caudal end of the heart of a seventeen-segment chick embryo. X 73.
| |
|
| |
|
| |
|
| |
| Section through the Caudal End of the Heart (Fig. 340). - The section still includes the hind-brain. The descending aortce are separated only by a thin septum which is ruptured at this level. The anterior cardinal veins are cut where they bend ventrad to enter the heart. The mesothelial wall of the heart is continuous through the dorsal mesocarduim with the splanchnic mesoderm. On the right side of the section there is fusion between the epi-myocardium of the heart and the somatic mesoderm. iMesodermal segments were not observed at higher levels, but now they appear lateral to the hind-brain.
| |
|
| |
|
| |
|
| |
| Fig, 342. - TranSverse section caudal to the intestinal portal of a seventeen-segment chick embryo. X 90.
| |
|
| |
|
| |
|
| |
| The ventro-mesial part of the segment is breaking down into the sclerotome; the dorsomesial wall represents the myotome, and the lateral plate the dermatome.
| |
|
| |
|
| |
| Section through the Intestinal Portal (Fig. 341). - The descending aortre now form a single vessel, the dorsal aorta, the medium septum having disappeared. The section passes through the entoderm at the point where it is folded dorsad and cephalad into the head as the fore-gut (cf. Fig. 335). Two sections caudad is found the opening {intestinal portal) where the fore-gut communicates with the flattened open gut between the entoderm and the yolk. On each side of the fore-gut are the large vitelline veins, sectioned obliquely. The splanchnic mesoderm overlying these veins is pressed by them against the somatic mesoderm, and the cavity of the ccelom is thus interrupted on each side. The section is close to the level where the common cardinal veins open into the venous end of the heart.
| |
|
| |
|
| |
|
| |
| Section Caudal to the Intestinal Portal (Fig. 342). - In general, this section resembles the preceding save that the primitive gut is without a ventral wall, and, therefore, may be called mid-gut. The right vitelline vein is still large. Lateral to the enclosed coelom, on each side, are spaces which represent the posterior cardinal veins, just differentiating.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 343. - Transverse section through the fourteenth pair of mesodermal segments of a seventeen-segment chick embryo. X 90.
| |
|
| |
|
| |
| Section through the Fourteenth Pair of Primitive Segments (Fig. 343). - The body of the embryo is now flattened on the surface of the yolk. Here the descending aorta: are again separate and occupy arched spaces under the primitive segments. The section is characterized by the notochord and the differentiated mesoderm which forms typical primitive segments, nephrotomes, and somatic and splanchnic mesoderm. Arising from the nephrotomes are sprout -like pronephric tubules. The tips of these hollow out and unite to I form the primary excretory, or pronephric duct.
| |
|
| |
|
| |
|
| |
| Fig. 344. - Transverse section through the segmental zone of a seventeen-segment chick embryo. X 90.
| |
|
| |
|
| |
|
| |
| I Section through the Segmental Zone (Fig. 344). - The section is at the level of the
| |
| segmental zone, where mesodermal segments have not formed as yet. The mesodermal ' plates are splitting laterally into layers, but the ccelomic cavities are mere slits. Between I the splanchnic mesoderm and the entoderm, blood vessels may be seen. The open neural 1 groove is called the rhomboidal sinus. The ectoderm is characterized by the columnar form of its cells. At the point where the ectoderm joins the neural fold, a ridge of cells projects ventrally on either side. These projecting cells constitute the neural crests, and from them the spinal ganglia are formed in older embryos.
| |
|
| |
|
| |
| Section through the Primitive (Hensen - s) Knot (Fig. 345). - The three germ layers fuse inseparably at the 'knot - into a mass of undifferentiated tissue. The lateral mesoderm is split into somatic and splanchnic layers; the latter contains numerous small blood vessels of the vascular network.
| |
|
| |
|
| |
|
| |
| Fig. 345- Transverse section through the primitive knot of a seventeen-segment chick embryo. X 90.
| |
|
| |
|
| |
| Section through[the Primitive Streak (Fig. 346). - In the mid-dorsal line is the primitive groove. The germ layers may be seen taking their origin from the undifferentiated tissue of the primitive streak, beneath. Laterad, between the splanchnic mesoderm and entoderm, lilood vesSels are present as in the preceding sections.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 346. - Transverse section through the primitive streak of a seventeen-segment chick embryo. X 90. h'
| |
|
| |
|
| |
|
| |
| (E) EMBRYO OF TWENTY-SEVEN SEGMENTS (TWO DAYS)
| |
|
| |
|
| |
|
| |
| Although a chick embryo with 27 segments is chosen as the norm, the descriptions which follow are applicable to stages between 45 hours (23 segments) and 60 hours (32 segments).
| |
|
| |
| During the latter half of the second day a remarkable change occurs in the appearance of the embryo and in its positional relation to the blastoderm (Fig. 56). The bending of the head, already begun in the stage last studied, has continued until the fore- and hind-brains are nearly parallel. This marked cephalic flexure occurs at the region of the mid-brain. As long as the embryo retained its original prone position with respect to the yolk, it is manifest that the head could not bend greatly ventrad, so, in order that the flexion might proceed to completion, the upper body has twisted about its long axis until the left side lies squarely next the yolk. In a dorsal view, therefore, one sees the right side of the head but the dorsal side of the lower body. The actual zone of torsion, now half way down the trunk, will advance progressively until the whole embryo is turned, after which additional curvatures will make it assume the shape of the letter C (Fig. 363). One of these flexures is already appearing opposite the lower end of the heart, at the junction of head and trunk; for this reason it bears the name cervical flexure.
| |
|
| |
|
| |
| Most of the body is rather sharply delimited from the blastoderm; the head is free; much of the midbody is bounded by deep lateral folds; caudally, the tail bud represents the future hind end of the body and is bordered by a tail fold. The further combined activities of head-, lateral-, and tail folds will constrict the embryo from the extra-embryonic blastoderm.
| |
|
| |
| The head is now covered by a double fold of the somatopleure, the head fold of the amnion; it envelops the upper half of the body like a veil. The heart bends in the form of a letter S, and the extra-embryonic vascular ple.xus is profuse. Three ectodermal furrows form branchial grooves on the sides of the neck. Eye and ear anlages are prominent. Primitive segments extend far down the former segmental zone.
| |
|
| |
|
| |
|
| |
| Central Nervous System and Sense Organs. - The brain region of the neural tube is separated by constrictions into five vesicles. The first subdivision of the primitive fore-brain is the telencephalon; the rest constitutes the diencephalon. The mesencephalon remains undivided but is bent at its middle by the cephalic flexure. The hind-brain shows two indistinct regions of differentiation; a short section with a thick roof adjoining the mid-brain is the metencephalon, the thin-roofed remainder is the myelencephalon. The spinal cord is now closed to its extreme end and consequently the rhomboidal sinus no longer exists.
| |
|
| |
|
| |
| The lens anlage has assumed the form of a lens vesicle; coincident with its invagination the outer wall of the optic vesicle also folds inward, thereby making a double-walled structure, the optic cup. The latter is not a complete cup, for on one side a segment of the wall is missing; this chorioid fissure gives the cup a horse-shoe outline in surface view (Fig. 347). The auditory placode of earlier stages has become a sac, the otocyst or otic vesicle, which, however, retains a connection with the body ectoderm.
| |
|
| |
| Digestive System. - The entodermal canal shows three regional divisions. Of these, the fore-gut is best differentiated and will be referred to again. In Fig. 348 most of the entoderm has been removed, so that the open mid-gut scarcely shows; it extends from the intestinal portal to the tail bud, and, without a ventral wall, overlies the yolk. Caudad, a small portal leads into the hind-gut which is just beginning to invaginate into the tail fold; in development and relations it duplicates the fore- gut. The pharyngeal membrane now lies at the bottom of a deep pit, the stomodeum, formed by depressed ectoderm. A median ectodermal sac, just in front of the pharyngeal membrane and lying next the brain wall, is Rathke's pouch; it is the anlage of the epithelial portions of the hypophysis. The entodermal pharynx bears three pairs of lateral out-pocketings known as the pharyngeal pouches. They occur opposite the three external branchial grooves, and here ectoderm and entoderm are in contact, forming closing plates. At about this stage the first pair of plates ruptures, thereby making a free opening, or branchial cleft, into the pharynx. These transitory apertures correspond to the gill clefts in lower aquatic vertebrates. Between the successive pouches lie solid, bar-like portions of the body wall, the branchial arches; in animals with aquatic respiration the arches bear gills, and even in higher embryos, like the chick, an aortic arch courses through each (cf. Fig. 184). At the level of the second pair of pouches, a broadly open pocket grows from the median floor of the pharynx; it is the anlage of the thyroid gland (Fig. 353). Toward the intestinal portal the fore-gut is flattened from side to side, and before it opens out into the mid-gut there is budded off a bilobed liver diverticulum (Figs. 348 and 355). It lies between the vitelline veins which later break up into the sinusoidal spaces of the liver.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 347. - Dorsal view of a chick embryo with twenty-seven segments (two days). X 14.
| |
|
| |
|
| |
| Fig. 348. - Ventral reconstruction of a twenty-seven segment chick enbryo. X 18. The ectoderm of the upper body and the entoderm of the lower body have been mostly removed. Numbered lines indicate the levels of Figs. 350-361.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Vascular System. - The disappearance of the dorsal mesocardium leaves the huge, tubular heart attached only at its two ends. Since the heart tube is growing faster than the surrounding body, it of necessity bends like the letter S, when seen from the ventral side (Fig. 348). Four regions may be distinguished: (i) the sinus venosus, into which the veins ; open; (2) a dilated dorsal chamber, the atrium; (3) a tubular ventral portion, bent in the form of a U, of which the left limb is the ventricle, the right limb (4) the bulbus cordis. From the bulbus is given off the ventral ;! aorta. There are now three pairs of aortic arches which open into the paired descending aorta - . The first aortic arch extends through the first branchial arch, cranial to the first pharyngeal pouch, and is the primitive connecting vessel seen in the thirty-eight-hour embryo (cf. Fig. 184). The second and third aortic arches course in the second and third branchial arches on either side of the second pharyngeal pouch. They are developed by the enlargement of channels in primitive capillary networks between ventral and descending aortre. Opposite the sinus venosus, the paired aortic trunks fuse to form the single dorsal aorta which extends as far back as the fifteenth pair of primitive segments. At this point the aortae again separate, and, o])posite the twentieth segments, each connects with the trunk of a vitelline artery which conveys blood to the vascular area (Fig. 348). Caudal to the vitelline arteries, the aortas decrease rapidly in size and soon end.
| |
|
| |
|
| |
| As in the previous stage, the blood is returned from the vascular area to the heart by the vitelline veins, now two large trunks (Fig. 348).
| |
|
| |
| In the body of the embryo, the anterior cardinal veins course ventrolateral to the brain and already are of large size. The smaller posterior cardinal veins are developing caudal to the atrium. They lie in the mesenchyme of the somatopleure, laterad in position ( Fig. 355). Opposite the sinus venosus, the anterior and posterior cardinal veins of each side unite and form the common cardinal veins (ducts of Cuvier) which open into the dorsal wall of the sinus venosus (Fig. 348). The primitive veins are thus paired like the arteries, and like them develop by the enlargement of channels in a network of capillaries.
| |
|
| |
| Differentiation of Mesoderm. - The formation of new mesodermal segments and the progressive differentiation of older ones into sclerotome, myotome, and dermatome continue as described for the preceding embryo (p. 330).
| |
|
| |
| The ne])hrotome region shows the beginning of additional features. The prone phric duct has continued beyond its original site of formation, and as a blind, growing cord extends tailward (Fig. 336). A set of new mesonephric tubules is now starting to differentiate, caudal to the pronephric group, between the thirteenth and thirtieth segments. They arise from the intermediate cell masses as vesicles that will become tubules and join the pronephric (hereafter called mesonephric) duct. They constitute the embryonic, but not the definitive kidney. Additional information concerning the pro- and mesonephroi may be found on pp. 135-139
| |
|
| |
|
| |
|
| |
| The splanchnopleure is chiefly involved in gut formation. The somatopleure is deeply folded into the lateral body folds whose union will progressively close the ventral body wall (Fig. 356). The establishment of a complete body wall, at any level, of necessity separates embryonic from extra-embryonic coelom.
| |
|
| |
|
| |
|
| |
|
| |
| Amnion and Chorion. - At the end of the second day, two extra-embryonic, protective membranes have become prominent. They are theawnion, which will form a membranous, fluid-filled sac about the embryo itself, and the chorion which eventually encloses both embryo and all extra-embryonic structures (Fig. 37). The two membranes arise simultaneously from the extra-embryonic somatopleure by a single process of folding (Fig. 349). In front of the embryo a fold of the somatopleure is thrown up, followed later by others lateral and caudal to the embryo (A). These hood-like, arching folds close in from all sides until they meet and fuse over the embryo (B-D). The inner layer of somatopleure is the amnion; the remainder constitutes the chorion, of little importance to the chick. It should be noted that the folding brings the mesodermal components of these membranes facing each other, but separated by the extra-embryonic coelom.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 349. - Diagrams illustrating the development of the amnion, chorion and allantois in longitudinal section (after Gegenbauer in McMurrich). Ectoderm, mesoderm and entoderm are represented by heavy, light and dotted lines respectively. .4/., Allantois; Am., amniotic cavity; lA., yolk sac.
| |
|
| |
|
| |
|
| |
| The head fold of the amnion had begun in the chick of the previous stage (Fig- 335) : at the end of the second day it is continuous along a crescentic margin with the lateral folds, and envelops the upper half of the body (Figs. 347 and 356). As yet the tail fold has scarcely started.
| |
|
| |
| Transverse Sections
| |
|
| |
|
| |
| The following series of transverse sections from a two-day chick shows the fundamentally important structures; they are equally applicable to the study of embryos between 45 hours (23 segments) and 60 hours (32 Segments). The sections are drawn from the caudal surface; hence, the left side of the embryo is at the reader - s left. The precise level of each significant section should be ascertained with respect to Figs. 347 and 348, as has been indicated for this series along the margins of Fig. 348. Since the head is bending rajudly during the last hours of the second day, minor variations in the appearance of different series of sections are unavoidable; this, however, is chiefly a question of what particular structures happen to appear together in the fore-brain and hind-brain portions of a section.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 350. - Transverse section through the eyes and first aortic arches of a twenty-sevensegment chick embryo. X 50.
| |
|
| |
|
| |
|
| |
| Sections through the Cephalic Flexure. - Due to the flexed brain, the first sections encountered pass through the mesencephalon, but soon the hind-hrain and then the diencephalon are included. The blood vessels seen are the anterior cardinals. Presently the brain becomes cut twice in each section; the myelencephalon may be recognized always by its thin roof and by its close association with the notochord. Note that in these sections through the bent head, progress is caudad down the hind-brain half of the section, but cephalad toward the tip of the fore-brain.
| |
|
| |
|
| |
| Section through the Eyes and First Aortic Arches (Fig. 350). - The section passes cephalad of the optic stalks, consequently the optic vesicles appear unconnected with the fore-brain. The adjacent, thickened ectoderm is invaguiated to form the anlages of the lens vesicles. The thicker wall of the optic vesicle, next the lens anlage, will give rise to the nervous layer of the retina; the thinner outer wall becomes the pigment layer of the retina. Ventrad in the section are the wall and cavity of the fore-brain, dorsad the myelencephalon of the hind-brain with its thin, dorsal ependymal layer. Between the brain vesicles, on either side, are longitudinal sections of the first aortic arches, and lateral to the hind-brain are the smaller, paired anterior cardinal veins, which convey the blood from the head to the heart. The splanchnopleure (that is, the yolk side of the blastoderm) is characterized in this and subsequent sections by the presence of blood vessels in its mesodermal layer. The entire head is enveloped by the amnion; the chorion passes along the right side of the head, and, continuing, surrounds both embryo and yolk. In these membranes the mesodermal components face each other across the extra-embryonic ccelom.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 351. - Transverse section through the optic stalks and hypophysis of a twenty-seven segment chick embryo. X 50.
| |
|
| |
|
| |
| Section through the Optic Stalks and Hypophysis (Fig. 351). - The section passes just caudal to the lens. The optic vesicles are connected with the wall of the fore-brain by the optic stalks, which later form the path through which the fibers of the optic nerve grow from the retina to the brain; sections cut in this plane do not show the chorioid fissure. Both the ventral and the descending aortae are seen about the cephalic end of the pharynx. Between the ventral wall of the fore-brain and the pharynx is an invagination of the ectoderm. Rathke's pouch, which will become the epithelial hypophysis; a few sections farther along it o])ens externally into the stomodcum, close to the pharyngeal membrane.
| |
|
| |
|
| |
|
| |
| Passing caudad in the series a short distance, the fore-brain region of the bent head liecomes isolated from the body and soon the tip of the head is reached.
| |
|
| |
|
| |
| Section through the Otocysts and Second Aortic Arch (Fig. 352). - The otic vesicles arc sectioned caudal to their apertures, and so appear as cloSed sacs, lateral to the wall of the hind-biain. The cavity of the pharynx is somewhat triangular and its dorsal wall is thin. The anterior cardinal veins pass between the otocysts and the wall of the hind-brain.
| |
|
| |
|
| |
| Fig, 352. - Transverse section through the otocysts and second aortic arches of a twenty-seven segment chick embryo. X50.
| |
|
| |
|
| |
| Ventral to the pharynx, the bulbus cordis is sectioned obliquely where it leaves the heart, and at this level gives off the second pair of aortic arches which connect dorsad with the descending aorta. Surrounding the bulbus cordis is the large pericardial cavity, not yet enclosed by the body wall. The student should note that in the sections so far studied, the mesenchyme of the head is undifferentiated, the tissues peculiar to the adult not yet having formed. The amnion attached to the right side of the embryo is folded. This is because the primitive amnion folds fuse over the original dorsal line, regardless of the turning of the embryo; consequently, on the right there is - slack. - .
| |
|
| |
|
| |
|
| |
| Section through the Second Pharyngeal Pouches and Thyroid Anlage (Fig. 353). - As this section is taken at a level between the second and third aortic arches, the descending aortae and heart are unconnected. Tangential shavings have been cut from the walls of the otocysts. Extending laterally from the pharynx are the second pair of pharyngeal pouches which have already come in contact with the ectoderm to form closi)ig plates. A pocket-like depression in the midventral floor of the pharynx represents the thyroid anlage; later, it becomes saccular and loses its connection with the pharyngeal entoderm. The splanchnic mesodermal wall of the heart is destined to give rise later to the epi- and myocardium. A short distance caudad in the series, the large, looped ventricle is met; it is not attached by the former dorsal mesocardium.
| |
|
| |
|
| |
| Section through the Sinus Venosus and Common Cardinal Veins (Fig. 354). - At this level, the common cardinal trunk, formed by the union of anterior and posterior cardinal veins, opens into the thin-walled sinus venosus. The sinus receives all of the blood passing to the heart and is separated from the larger atrium by a slight constriction only.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 353. - Transverse section through the second pharyngeal pouches and thyroid anlage of twenty-seven-segment chick embryo. X50.
| |
|
| |
|
| |
| The descending aortae have united to form the single dorsal aorta. On either side of the pharynx are subdivisions of the coelom which will form the pleural cavities when the lung buds appear. These cavities are separated from the pericardial cavity by the septum transversum (anlage of diaphragm) in which the common cardinal veins cross to the sinus venosus. Since the last section, the myelencephalon has merged into the spinal cord, and the dermo-myotomes of the first mesodermal segments are seen. The mesodermal components of the amnion folds are not fused at this or subsequent levels.
| |
|
| |
|
| |
| Section through the Liver Anlage (Fig. 355). - In this section, the fore-gut is flattened from side to side and its cavity is narrow. Ventrad, there are evaginated from the entoderm two diverticula which constitute the earliest anlage of the liver. On either side are sections of the vitelline veins (the left swinging in from the blastoderm), on their way to the sinus venosus at a higher level in the series. This primitive liver anlage does not always appear bilobed; at a slightly later stage it is found ventral to the united vitelline veins and a second anlage, more cephalad in origin, lies dorsal to the vein. Note the intimate relation between the entodermal epithelium of the liver and the endothelium of the vitelline veins. In later stages, as the liver anlages branch, there is a mutual intergrowth between the entodermal cells constituting the liver and of the vascular endothelium of the vitelline veins. Thus are formed the hepatic sinusoids of the portal system, which surround the cords of hepatic cells.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 354. - Transverse section through the sinus venosus and common cardinal veins of a twenty-seven-segment chick embryo. X 50.
| |
|
| |
|
| |
|
| |
| Fig. 355. - Transverse section through the liver anlage of a twenty-seven-segment chick embryo. X 50.
| |
|
| |
|
| |
|
| |
| The septum transversum is still present at this level, and lateral to the fore-gut are small body cavities. Lateral to the body cavities appear branches of the posterior cardinal veins.
| |
|
| |
|
| |
| Section through the Open Gut and Amnion Folds (Fig. 356). - The intestine is now open ventrad, its splanchnopleure passing directly over to that of the vascular area. The dorsal aorta is again divided by a septum into its primitive components, the right and left descending aortae. Lateral to the aortae are the small posterior cardinal veins. The coelom is in communication with the extra-embryonic body cavity. Deep lateral body folds of somatopleure indicate how, by their ventral union, the body becomes established free from the blastoderm. The folds of the amnion have not joined, thus leaving the amniotic cavity open; (some variation may be found in the exact level where this condition occurs). In such a section, the somatopleuric components of the amnion and chorion are easily traced, and, a few sections cephalad, the manner of union of the two folds is illustrated.
| |
|
| |
|
| |
|
| |
| Fig. 356. - Transverse section through the open gut and amnion folds of a twenty-seven segment chick embryo. X 50.
| |
|
| |
|
| |
| Fig. 357. - Transverse section through the seventeenth pair of mesodermal segments of a twenty-seven-segment chick embryo. X 50.
| |
|
| |
|
| |
|
| |
| Section through the Seventeenth Pair of Mesodermal Segments (Fig. 357). - The body of the embryo is no longer rotated. On the left side of the figure, the mesodermal segment shows a der mo -myotome plate. The median and ventral portion of the segment is being converted into sclerotomic mesenchyme. On the right side, near the upper angle of the coelom, appears a section of the proncpliric (mesonephric) duct. The open space above it is the posterior cardinal vein; some sections show the median nephrotome tissue organizing into mesonephric tubule anlages. The embryonic somatoplcure is arched and will form the future ventro-lateral body wall of the embryo. The lateral infoldings of the somatouleure give indication of the later approximation of the ventral body walls, by which the embryo it separated from the underlying layers of the blastoderm.
| |
|
| |
|
| |
| Section through the Origin of the Vitelline Arteries (Fig. 358). - At this level, the embryo is more flattened and simple in Structure, as at higher levels in earlier embryos.
| |
|
| |
|
| |
|
| |
| Fig. 358. - Transverse section through the origin of the vitelline arteries of a twenty-seven segment chick embryo. X 50.
| |
|
| |
|
| |
|
| |
|
| |
| Mesodermal segments, nephrotomes, and lateral layers of somatic and splanchnic mesoderm are little differentiated. The amniotic folds have not appeared. On the left side of thehgure, the vitelline artery leaves the aorta; on the right side, the connection of the vitelline artery with the aorta does not show, as the section is cut somewhat obliquely. The posterior cardinal vein is present just laterad of the right mesonephric duct. The small clusters of cells dorso-lateral to the spinal cord are the neural crests which will differentiate into spinal ganglia.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 359. - Transverse section through the segmental zone of a twenty-seven-segment chick embryo. X 50.
| |
|
| |
|
| |
| Section through the Segmental Zone (Fig. 359). - The mesodermal segments are replaced by the segmental zone, a somewhat triangular mass of undifferentiated mesoderm from which later are formed the segments and nephrotomes. The notochord is larger the aorta smaller, and a few sections caudad they disappear. Laterally, the somatoplcure and splanchnopleure are straight and separated by the slit-like ccelom.
| |
|
| |
|
| |
| Section through the Tail Bud, Cranial to the Hind-gut (Fig. 360). - With the exception of the ectoderm, the structures near the median plane are merged into an undifferentiated mass of dense tissue, the notochordal plate. The cavity of the closed neural tube and its dorsal outline may, however, still be seen. Laterally, the segmental zone and the various layers are differentiated.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 360. - Transverse section through the tail-bud of a twenty-seven-segment chick embryo.X 50 .
| |
|
| |
|
| |
|
| |
| Section through the Hind-gut and Primitive Streak (Fig. 361). - In this embryo, the caudal evagination to form the hind-gut has just begun. The section shows the small cavity of the hind-gut in the midplane. Its wall is composed of columnar entodermal cells and it is an outgrowth of the entodermal layer. A few sections cephalad in the series, the hind-gut opens by its own posterior intestinal portal. Dorsal to the hind-gut may be seen undifferentiated cells of the primitive streak, continuous dorsad with the ectoderm, ventrad with the entoderm of the hind-gut, and laterally with the mesoderm.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 361. - Transverse section through the hind-gut of a twenty-seven-segment chick embryo.X 50
| |
|
| |
|
| |
|
| |
|
| |
| (F) EMBRYOS OF THREE TO FOUR DAYS
| |
|
| |
|
| |
|
| |
| During the third and fourth days of incubation the chick attains a stage of development corresponding to the younger of the pig embryos customarily studied. It is advisable, therefore, to describe only such essential features of developmental advance in these older chick embryos as are necessary for introducing the detailed pig studies which follow.
| |
|
| |
| .External Form. - ^The whole body shows the effect of torsion, and the embryo now lies on its left side (Fig. 362). The former flexures, especially the cervical, are pronounced, and new dorsal and caudal flexures have appeared; as a result, the embryo becomes so curved that its head and tail approach. The final number of 42 primitive segments is present and the body ends in a distinct tail. Up]ier and lower limb buds extend from the body wall, and the saccular allantois projects through the unclosed lower abdomen. Four branchial clefts show, separated by prominent branchial arches. The continued undercutting of the body folds, especially the more recent tail fold, has reduced the area of attachment with the yolk sac to a relatively narrow yolk stalk (Fig. 363).
| |
|
| |
|
| |
|
| |
| Central Nervous System and Sense Organs. - The five secondary divisions of the brain are easily identified; the telencephalon bears lateral hemispheres and the distinction between metencephalon and myelencephalon is now plain. Most of the cranial nerves and ganglia have begun j to appear (Fig. 362). From the roof of the diencephalon is the evagination of the epiphysis, in its floor the anlage of the neural lobe of the hypophysis (Fig. 363). The eye is a prominent organ, with its lens freed from the ectoderm if but with the narrowed chorioid fissure still showing (Fig. 362). The otic vesicle is a detached, closed sac from which the tubular endolymph duct is growing. Olfactory anlages, not seen hitherto, have appeared as ectodermal placodes on the ventro-lateral sides of the head; they are now depressed as olfactory pits.
| |
|
| |
| Digestive and Respiratory Systems. - The fore-gut and hind-gut are complete tubes, and the open mid-gut is a relatively short segment connected by the yolk stalk with the yolk sac (Fig. 363). As the pharyngeal membrane has ruptured, the stomodeum becomes an integral part of the mouth cavity. Y over pharyngeal pouches are prominent; in all but the fourth, the closing plates perforate and form temporary branchial clefts. At this time, the median, thyroid diverticulum loses connection with the floor of the pharynx. The trachea has arisen from a midventral groove which separates from the caudal end of the pharynx and bifurcates into two lung buds. The esophagus is a short, narrowed segment and the stomach a slightly spindle-shaped dilatation. Both liver anlages have fused into a branching mass and at the same level the pancreas is appearing.
| |
|
| |
| .Except for the attachment of the yolk sac. there are no additional features of interest above the caudal end of the hind-gut. Here the gut is separated from an ectodermal pit, the proctodeum, by a thin cloacal membranc which later perforates (Fig. 363). The mesonephric ducts join the hind-gut, and a stalked vesicle, the allantois, grows from its ventral floor. This common chamber, which receives the contents of the intestine and the secretions of the urinary and reproductive glands, is the cloaca.
| |
|
| |
|
| |
|
| |
|
| |
| Urinary System.- -The pronephric tubules disappear on the fourth day. Mesonephric tubules are still developing and consist of elongate, | coiled tubules associated with a glomerulus at one end and with the mesonephric duct at the other. The metanephros, or permanent kidney anlage, is just appearing; its collecting tubules and ureter arise as a bud from the mesonephric duct near the cloaca; the secretory tubules will develop from caudal nephrotome tissue.
| |
|
| |
|
| |
|
| |
| Vascular System. - The ventricular loop has moved caudad and the atrial region cephalad, thus reversing the original positional relations of these jiarts (Fig. 362). Both atrium and ventricle show external indications of a beginning division into right and left chambers, and the myocardial wall is assuming the characteristics of muscle cells. As a whole, the heart has sunk caudad considerably from its early cephalic position.
| |
|
| |
|
| |
| Below the heart, the primitive aortae are fused throughout their lengths. Since the second day, a fourth, a rudimentary fifth, and a . sixth pair of arches have developed; of the full set, only the third (carotid), fourth (aortic), and sixth (pulmonary) arches remain. The cardinal veins i' are well developed, and the paired vitelline arteries and veins have both , fused inside the body into single vessels. New umbilical arteries pass to the allantois, and umbilical veins return the blood by way of the lateral i! body wall to the heart. These will become still more important in the mammal.
| |
|
| |
|
| |
|
| |
|
| |
| Extra-embryonic Membranes. - During the third day the tail-fold of the amnion develops, and soon the embryo becomes enclosed by the fluidfilled amnion sac which is protective in function (Figs. 37 and 349); the chorion, formed by the same process, but of little significance, ultimately surrounds the embryo and all extra-embryonic structures. Much of the yolk sac is covered by advancing splanchnopleure which is continuous over a relatively narrow yolk stalk with that of the gut (Fig. 363). As the embryo elongates, the yolk stalk appears relatively narrower. Through the vitelline vessels the yolk supplies all the food material for embryonic growth. The allantois arises late in the third day as a diverticulum of the splanchnopleuric floor of the hind-gut (Figs. 349 and 363). It later becomes a large, stalked sac occupying the space beneath the shell. Umbilical vessels ramify in its walls and the allantois serves as the principal organ of respiration and excretion.
| |
|
| |
|
| |
|
| |
|
| |
| ++++++++++++++++++++++++++++++++++++++++++++++++++++
| |
|
| |
|
| |
|
| |
| CHAPTER XVII. THE STUDY OF PIG EMBRYOS .
| |
|
| |
|
| |
| A very young pig embryos of the primitive streak and neural fold stages are shown in Fig. 364. The closure of the neural tube and the progressive appearance of mesodermal segments are likewise illustrated in Fig. 365. The fundamental similarity of these embryos to the early chicks already studied is apparent. For a short time, succeeding stages are complicated by flexion and spiral twisting which make sections difficult for the beginner to interpret. In embryos about 6 mm. long, the twist of .
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 364. - Early pig embryos (Keibel). X 20. A, Blastoderm with primitive streak and knot; B, blastoderm with primitive streak and neural groove.
| |
|
| |
|
| |
|
| |
| The body has disappeared sufficiently so that its structure may be studied to better advantage. At this time the state of development is generally comparable to that of a four day chick (Fig. 366).
| |
|
| |
|
| |
|
| |
| The fetal membranes of the pig stand somewhat intermediate between the chick and man. The amnion, chorion, and allantois develop very much as in the chick (Fig. 349). The yolk sac is small and rudimentary, so its functions are transferred to the allantois which fuses with the chorion ; the two constitute a placenta which is the organ of fetal respiration, nutrition, and excretion (Fig. 39). The development and relations of these extra-embryonic structures are described on pp. 46-49.
| |
|
| |
|
| |
| In this manual, series of transverse sections are figured and described only. Lateral and sagittal dissections show the longitudinal relations more clearly than serial sections cut in these planes; if, however, sagittal or frontal sections are used, they may be interpreted readily from the corresponding dissections and reconstructions.
| |
|
| |
|
| |
|
| |
| Fig. 365. - Dorsal views of pig embryos, with the amnion cut away (Keibel). X 20. A, Embryo of seven segments; B, embryo of eleven segments.
| |
|
| |
|
| |
|
| |
| (A) THE ANATOMY OF A SIX MM. PIG EMBRYO
| |
|
| |
|
| |
| The descriptions given here are applicable to the study of embryos between 5 and 8 mm. Due to a shorter term of development, a 6 mm. pig embryo is slightly farther advanced in most respects than a human embryo of the same size (Fig. 61).
| |
|
| |
|
| |
| External Form. - Both head and body are bent in an even curve, convex along the dorsal line, and the tail is recurved sharply (Fig. 366). The cephalic flexure forms an acute angle at the mesencephalon, and there is also a marked cervical flexure. As a result, the head is somewhat triangular in shape. Lateral to the dorsal line may be seen the segments, which become larger and more differentiated toward the head. At the tip of the head, a shallow depression marks the olfactory pit. The lens vesicle of the eye is open to the exterior. Caudal to the eyes, at the sides of the head, are four branchial arches separated by three branchial grooves. The fourth arch is partly concealed in a triangular depression, the cervical sinus, formed by the more rapid growth of the first and second arches (cf. Fig. 369). The first, or mandibular arch, forks ventrally into two parts, a smaller maxillary- and a larger mandibular process; the latter, with^its. fellow, forms the lower jaw. The position of the mouth is indicated by the space between these processes. The furrow from the eye to the mouth is the lacrimal groove. The second, or hyoid arch is separated from the mandibular arch by an ectodermal groove which persists as the external acoustic meatus.
| |
|
| |
|
| |
| The heart is large, and through the transparent body wall may be seen the dorsal atrium and ventral ventricle. Caudal to the heart, a convexity indicates the position of the liver, dorsal to which is the bud of the upper limb. Extending caudad from the limb bud, a curved convexity indicates the position of the left mesonephros, large and precociously developed in the pig. At its end is the anlage of the lower limb. The amnion has been dissected away along the line of its attachment, ventral to the mesonephros. There is as yet no distinct umbilical cord, and a portion of the body stalk is attached to the embryo.
| |
|
| |
|
| |
|
| |
| Nervous System and Sense Organs. - The brain is differentiated into its five regions; telencephalon, diencephalon, mesencephalon, metencephalon, and myelencephalon (Fig. 367). The spinal cord is cylindrical and tapers off gradually to the tail. The anlage of the cranial and spinal ganglia and the main nerve trunks are shown. The olfactory, optic, and trochlear nerves have not yet differentiated, and the oculomotor nerve is just beginning to appear from the ventral wall of the mesencephalon. Ventrodateral to the metencephalon and myelencephalon occur in order: the semilunar ganglion and three branches of the trigeminal nerve; the geniculate ganglion and nerve trunk of the n. facialis; the ganglionic anlage of the 11. acusticus. Caudal to the otocyst, a continuous chain of cells extends lateral to the neural tube into the tail region. Cellular enlargements along this neural crest represent developing cranial and spinal ganglia. They are, in order: the superior, or root ganglion of the glossopharyngeal nerve with its distal petrosal ganglion; the ganglion jugulare and distal ganglion nodosum of the vagus nerve; the ganglionic crest and the proximal portion of the spinal accessory nerve; and the anlage of Froriep - s ganglion, an enlargement on the neural crest just cranial to the first cervical ganglion. Between the vagus and Froriep - s ganglion may be seen the numerous root fascicles of the hypoglossal nerve, which take their origin along the ventro-lateral wall of the myelencephalon and unite to form a single trunk. The posterior roots of the spinal ganglia are very short ; their anterior, or ventral roots are concealed. It should be observed that the fifth, seventh, ninth, and tenth cranial nerves pass to the four branchial arches in the order named. This primitive relation is maintained in the adult when the nerves innervate the derivatives of these arches.
| |
|
| |
|
| |
| The olfactory pits are merely slight depressions in the thickened ectoderm of the ventral head. There are stalked but the resides
| |
| still open to the exterior. The otocysts are oval, ectodermal vesicles with endolymph ducts just appearing as dorso-mesial outgrowths.
| |
|
| |
|
| |
| Digestive and Respiratory Systems. - The month lies between the mandible, the median fronto-nasal process of the head, and the maxillary processes at the sides (Fig. 369). The diverticulum of the epithelial hypophysis {Rathke's pouch) extends along the ventral wall of the forebrain (Fig. 368) ; near its distal end, the wall of the brain is thickened, and later the posterior lobe of the hypophysis will develop at this point.
| |
|
| |
|
| |
| The pharynx is flattened dorso-ventrally and is widest near the mouth. It narrows caudad, and, opposite the third branchial arch, makes an abrupt bend, which corresponds to the cervical flexure of the embryo - s body (Fig. 368). In the roof of the phar^mx, just caudal to Rathke - s pouch, is the somewhat cone-shaped pocket known as Sessell’s pouch, which may be interpreted as the blind, cephalic end of the fore-gut (Fig. 376). The lateral and ventral walls of the pharynx and oral cavity are shown in Fig. S4 A. Of the four arches, the mandibular is the largest, and a groove partly separates the tongue anlages of the two sides. Posterior to this groove and extending in the median plane to the hyoid arch is a triangular elevation, the tuberculmn impar; it later vanishes, apparently contributing nothing to the tongue. At an earlier stage, the median thyroid anlage grew out from the midventral wall of the pharynx just caudal to the tuberculum impar. The ventral ends of the second arches fuse in the midventral plane and form a prominence, the copula (Fig. 84 B). This connects the tuberculum impar with a rounded tubercle derived from the third and fourth pairs of arches, the anlage of the epiglottis. Its cephalic portion forms the root of the tongue. Caudal to the epiglottis are the arytenoid ridges, and a slit between them, the glottis, leads into the trachea.
| |
|
| |
|
| |
| The branchial arches converge caudad, and the pharynx narrows rapidly before it is differentiated into the trachea and esophagus. Laterally and ventrally, between the arches, are the four paired outpocketings of the pharyngeal pouches (Fig. 375). The pouches have each a dorsal and ventral diverticulum. The dorsal diverticula are large and wing-like; they meet the ectoderm of the branchial grooves and fuse with it to form the closing plates. Between the ventral diverticula of the third pair of pouches lies the median thyroid anlage (Fig. 376). The fourth pouch is smaller than the others; its dorsal diverticulum just meets the ectoderm; its ventral portion is small, tubular in form, and is directed parallel to the esophagus.
| |
|
| |
| The groove on the floor of the pharynx, caudal to the epiglottis, is continuous with the tracheal groove. More caudally, opposite the atrium of the heart, the trachea has separated from the esophagus (Fig. 368). The trachea at once bifurcates to form the primary bronchi and the anlages of the lungs (Fig. 369). The latter consist merely of the dilated ends of the bronchi, surrounded by a layer of splanchnic mesoderm. They bud out laterally on each side of the esophagus near the cardiac end of the stomach, and project into the pleural coelom. The esophagus is short, and widens dorso-ventrally to form the stomach. The long axis of the stomach is nearly straight, but its entodermal walls are compressed and it has revolved on its long axis so that the original dorsal border lies to the left, the ventral border to the right (Fig. 382).
| |
|
| |
|
| |
|
| |
| Caudal to the pyloric end of the stomach, and to its right, is given off from the duodenum the hepatic diverticulum (Fig. 368). This is a sac of elongated oval form from which the liver and part of the pancreas take origin, and which later gives rise to the gall bladder, cystic duct, and common bile duct. It is connected by several cords of cells with the trabeculae of the liver; the latter is divided incompletely into four lobes, a small dorsal and a large ventral lobe on each side (Fig. 367).
| |
|
| |
|
| |
| The pancreas is represented by two outgrowths. The ventral pancreas originates from the hepatic diverticulum near its attachment to the duodenum (Fig. 368). It grows to the right of the duodenum and ventral to the portal vein. The dorsal pancreas takes origin from the dorsal side of the duodenum, caudal to the hepatic diverticulum, and grows dorsally into the substance of the gastric mesentery (Fig. 376). It is larger than the ventral pancreas, and its posterior lobules grow to the right and dorsal to the portal vein, and in later stages anastomose with the lobules of the ventral pancreas.
| |
|
| |
|
| |
| The intestine of both fore-gut and hind-gut has elongated and curves ventrally into the region of the future umbilical cord wdiere the yolk stalk has nari'ow^ed at its point of attachment to the gut (Fig. 368). The cloaca, a dorso-ventrally expanded portion of the hind-gut, gives off cephalad and ventrad the allantoic stalk. This is at first a narrow tube, but soon expands into a vesicle of large size, a portion of which is seen in Fig. 368. Dorso-laterad, the cloaca receives the mesonephric ducts. The hind-gut is continued into the tail as the transitory tail- gut, or postanal gut, which dilates at its extremity. The midventral wall of the cloaca is fused to the adjacent ectoderm to form the cloacal membrane. In this region the anus will appear.
| |
|
| |
|
| |
| Coelom and Mesenteries. - The coelom communicates throughout, but already consists of a single, large pericardial cavity, paired pleural canals, and a common peritoneal chamber. The septum transversum, which will form most of the diaphragm, is prominent and serves to separate partially the heart cavity from the remainder of the coelom (Fig. 368). The primitive dorsal mesentery is a thick, double layer of splanchnic mesoderm investing the gut and attaching it to the median roof of the peritoneal cavity. As the intestine bends out toward the yolk sac, the dorsal mesentery grows at an equal rate and suspends it (Fig. 367). The liver lies in the ventral mesentery, between the stomach-duodenum and the midventral body wall. Between this level and the yolk stalk, the mesentery is beginning to disappear; the caudal limb of the intestine is already free ventrad (Fig. 376).
| |
|
| |
|
| |
|
| |
| Urogenital System. - The form of the mesonephroi is seen in Figs. 367 to 369. Each consists of large, vascular glomeruli, associated with coiled tubules which are lined with cuboidal epithelium and open into the mesonephric duct (Fig. 13 1). The mesonephric {Wolffian) duct, beginning at the anterior end of the mesonephros, curves at first along its ventral, then along its lateral surface. At the caudal end, each duct bends ventrad and to the midplane where it opens into a lateral expansion of the cloaca (Fig. 368). Before this junction takes place, an evagination into the mesenchyme from the dorsal wall of each mesonephric duct gives rise to the anlage of the mctanephros, or permanent kidney. The allantois is a prominent, stalked sac communicating with the ventral part of the cloaca. A slight thickening of the mesothelium along the median and ventral surface of each mesonephros forms a light-colored area, the genital fold (Fig. 368). This ridge is pointed at either end and confined to the middle third of the kidney. It is the anlage of the genital gland.
| |
|
| |
|
| |
| Vascular System. - The heart lies in the pericardial cavity, as seen in Fig. 368. The atrial region (Fig. 370), now includes two lateral sacs, the right and left atria. Similarly, the bulbo-ventricular loop has become differentiated into right and left ventricles, much thicker walled than the atria. The right ventricle is the smaller, and from it the bulbus passes between the atria and is continued as the ventral aorta. Viewed from the caudal and dorsal aspect (Fig. 371), the sinus venosus is seen dorsal to the atria. It opens into the right atrium and receives from the right and left sides the paired common cardinal veins. These veins drain the blood from the body of the embryo. Caudally, the sinus venosus receives the two vitelline veins. Of these, the left is small in the liver and later disappears. The right vitelline vein, now the common hepatic, carries most of the blood to the heart from the umbilical veins, and from the liver sinusoids, gut, and yolk sac.
| |
|
| |
|
| |
|
| |
| Fig. 370. - Ventral and cranial surface of the heart from a 6 mm. pig embryo. X 14.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 371. - Dorsal and caudal view of the heart from a 6 mm. pig embryo. X ^i.
| |
|
| |
|
| |
| Transverse sections of the embryo through the four chambers of the heart show the atria in communication with the ventricles through the atrio-ventricular foramina, and the sinus venosus opening into the right atrium (Fig. 380). This opening is guarded by the right and left valves of the sinus venosus. Septa partition the two atria and the two ventricles incompletely. In Fig. 380 the atrial septum {septum primum) appears complete, due to the plane of the section, but in Fig. 372, from a slightly smaller embryo, it is seen that the septum primum grows from the dorsal atrial wall of the heart and does not yet meet the endocardial cushions between the atrio-ventricular canals. This opening between the atria is known as the interatrial foramen. Before it closes, another aperture appears in the septum, dorsal in position; this is the foramen ovale which persists during fetal life. In Fig. 372 both openings may be seen, as may also the dorsal and ventral endocardial cushions bounding the atrio-ventricular foramina. The mesothelial layer of the ventricles has become much thicker than that of the atria. It forms the epicardimn and the myocardium; the sponge-like meshes of the latter are now being developed.
| |
|
| |
|
| |
|
| |
| The Arteries . - Beginning with the ventral aorta, which takes origin from the bulbus cordis, pairs of aortic arches are given off. These run dorsad in the five branchial arches (Figs. 375 and 376) and join the paired descending aorta. The first and second pairs of aortic arches are very small and originate from the small common trunks formed by the bifurcation of the ventral aorta just caudal to the thyroid gland. The fourth aortic arch is the largest. From the apparent fifth arch small pulmonary arteries are developing. There is evidence that this pulmonary arch is really the sixth in the series, the fifth having been suppressed in development (cf. Fig. 186 B). Cranial to the first pair of aortic arches, the descending aortal are continued forward into the maxillary processes as the internal carotids. Caudal to the aortic arches, the descending aortae converge, unite opposite the cardiac end of the stomach, and form the median dorsal aorta (Fig. 376). Both the dorsal and descending aortae give off paired, dorsal intersegmental arteries. From the seventh pair of these arteries (the first set to arise from the medial dorsal aorta) there are developed a pair of lateral branches to the upper limb buds. These vessels are the subclavian arteries. The dorsal aorta also sprouts ventrolateral arteries to the glomeruli of the mesonephros, and median ventral arteries to the gut (Fig. 385). Of the latter, the coeliac artery arises opposite the origin of the hepatic diverticulum. The vitelline artery takes origin by two or three trunks caudal to the dorsal pancreas; of these, the posterior is the larger and persists as the superior mesenteric artery.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 372. - Dissection of the heart from a 5.5 mm. pig embryo, viewed from the left side. X 14.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Opposite the lower limb buds, the dorsal aorta is divided for a short distance. From each division there arise, laterad, three short trunks which unite to form the single umbilical artery on each side. The middle vessel is the largest and apparent^ becomes the common iliac artery. A pair of short caudal arteries, much smaller in size, continue the descending aortae into the tail region.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 373. - Ventral reconstruction of a 6 mm. pig embryo, showing the vitelline and opened umbilical veins (Vehe). X 22. In the small orientation figure (cf. Fig. 376) the various planes are indicated by broken lines - * *.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| The Veins. - - The vitelline veins, originally paired throughout, are now represented distally by a single vessel, which, ramifying in the wall of the yolk sac, enters the embryo and courses cephalad of the intestinal loop (Fig. 373). Crossing to the left side of the intestine and ventral to it, it is joined by the superior mesenteric vein which has developed in the mesentery of the intestinal loop. The trunk formed by the union of these two vessels becomes the portal vein. It passes along the left side of the gut in the mesentery, and, opposite the stem of the dorsal pancreas, gives off a small branch, a rudimentary continuation of the left vitelline vein, which continues cephalad and in earlier stages connects with the sinusoids of the liver. The portal vein then bends sharply to the right, dorsal to the duodenum, and, in the course of the right vitelline vein (passing between the dorsal and ventral pancreas to the right of the duodenum) it soon enters the liver and connects with the liver sinusoids. The portal trunk is thus formed by persisting portions of both vitelline veins, and receives a new vessel, the superior mesenteric vein. The middle portions of the primitive vitelline veins subdivide into the network of liver sinusoids. Their proximal vitelline trunks drain the blood from the liver and open into the sinus venosus of the heart. The right member of this pair is much the larger (Fig. 371) and persists as the proximal portion of the future inferior vena cava.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 374. - Ventral reconstruction of the cardinal and subcardinal veins in a 6 mm. pig embryo, showing the early development of the inferior vena cava ( Vehe). X 22. In the small orientation figure (cf. Fig. 376) the various jdanes are indicated by broken lines.
| |
|
| |
|
| |
|
| |
| The umbilical veins, originating in the walls of the chorion and allantoic vesicle, fuse and lie caudal and lateral to the allantoic stalk (cf. Fig. 184). Before the stalk enters the body, they separate again and run lateral to the umbilical arteries. The left vein is much the larger. Both, after receiving branches from the posterior limb buds and from the body wall, pass cephalad' in the somatopleure of each side (Figs. 373 and 375). Their course is first cephalad, then dorsad, until they enter the liver. The left vein enters a wide channel, the ductus venosus, which carries its blood through the liver and thence to the heart by way of the right vitelline trunk. The right vein joins a large sinusoidal continuation of the portal vein in the liver. This common trunk drains into the ductus venosus.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 375. - Reconstruction of a 7.8 mm. pig embryo, showing the veins and aortic arches from the left side (Thyng). X 15. Ph.P. i, 2, 3, 4, Pharyngeal pouches.
| |
|
| |
|
| |
|
| |
| The anterior cardinal veins (Figs. 374 and 375) are formed to drain the plexus of veins on each side of the head. These vessels extend caudad and lie ventro-lateral to the myelencephalon. Each receives branches from the sides of the myelencephalon, then curves ventrad, is joined by the linguo-facial vein from the branchial arches and at once unites with the posterior cardinal of the same side to form the common cardinal vein. This, as already explained, opens into the sinus venosus.
| |
|
| |
| A posterior cardinal vein develops dorso-lateral to each mesonephros (Figs. 374 and 375). Running cephalad, they join the anterior cardinal veins. When the mesonephroi become prominent, as at this stage, the middle third of each posterior cardinal is broken up into sinusoids. Sinusoids extend from the posterior cardinal vein ventrally around both the lateral and medial surfaces of the mesonephros. The median sinusoids anastomose longitudinally and form the subcardinal veins, right and left.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 376. - Median sagittal reconstruction of a 6 mm. pig embryo (Vehe). X 16.5. The numbered heavy lines indicate the levels of the transverse sections shown in Figs. 377-388. The broken lines mark the outline of the left mesonephros and the course of the left umbilical artery and vein ; the latter may be traced from the umbilical cord to the liver where it is sectioned longitudinally.
| |
|
| |
|
| |
|
| |
|
| |
| The subcardinals lie along the median surfaces of the mesonephroi, more ventrad than the posterior cardinals with which they are connected at either end. There is a transverse capillary anastomosis between the two subcardinals, cranial and caudal to the permanent trunk of the vitelline artery (Fig. 374). The right vessel is connected with the liver sinusoids through a small vein (which develops in the mesenchyme of the caval mesentery) located to the right of the mesogastrium (Fig. 383). This vein now carries blood direct to the heart from the right posterior cardinal and right subcardinal, by way of the liver sinusoids and the right vitelline trunk (common hepatic vein). Eventually, the unpaired inferior vena cava forms in the course of these four vessels.
| |
|
| |
|
| |
| Transverse Sections of a Six Mm. Pig Embryo
| |
|
| |
|
| |
| Having acquainted himself with the anatomy of the embryo from the study of dissections and reconstructions, the student is prepared to examine serial sections. To interpret the structures encountered, there must be constant references to the figures already described which show the positions of the organs. Determine the exact plane of a section with reference to these illustrations, and especially Fig. 376. Representative levels are described on the following pages; the position of each is indicated by the heavy, numbered lines on Fig. 376. These sections are drawn from the cephalic surface, so that the right side of the embryo is at the readers left.
| |
|
| |
|
| |
| Sections through the Cephalic Flexure. - The earliest sections cut the mesencephalon, metencephalon, and thin-roofed myelencephalon. At levels which include two separate portions of the brain, the smaller is the diencephalon, the larger a longitudinal section of the myelencephalon. In the intermediate mass of mesenchyme lie the internal carotid arteries (cf. Fig. 377). Lateral to them are numerous branches of the anterior cardinal veins. Midway along the sides of the hind-brain are the apices of the otocysts.
| |
|
| |
|
| |
| Section through the Myelencephalon and Otocysts (Fig. 377). - As the head is bent nearly at right angles to the body, the brain is cut twice ; this section passes lengthwise through the myelencephalon and transversely through the diencephalon. The cellular walls of the myelencephalon show a series of six paired constrictions, the neuromeres. Lateral to the fourth pair of neuromeres are the otocysts, which show a median outpocketing at the point of entrance of the endolymph duct. The ganglia of the nn. trigeminus, facialis, acusticus, and the superior ganglion of the glossopharyngeal nerve occur in order on each side. Sections of the anterior cardinal vein appear in several places, and ventral to the diencephalon are the internal carotid arteries.
| |
|
| |
|
| |
| Passing along down the series into the pharynx region, observe the first, second, and third pharyngeal pouches. Their dorsal diverticula come into contact with the ectoderm , of the branchial grooves and form the closing plates.
| |
|
| |
|
| |
| Section through the Branchial Arches and the Eyes (Fig. 378). - The section passes lengthwise through the four branchial arches, the fourth sunken in the cervical sinus. The mandibular processes of the first arch have united to form the mandible, or lower jaw; i the maxillary processes of the future upper jaw lie across the stomodeal space in the separate section of the bent head. Dorsad is the spinal cord, with the first pair of cervical ganglia, and laterad the first cervical myotonies. The pharynx is cut across between the third and fourth branchial pouches. In its floor is a prominence, the anlage of the epiglottis. Ventral to the pharynx, the ventral aorta gives off two pairs of vessels. The larger pair are the fourth aortic arches w'hich curve dorsad around the pharynx to enter the descending aortce. The smaller third aortic arches enter the third branchial arches on each side. A few sections higher up in the series, the ventral aorta bifurcates, and the right and left trunks thus formed give off the first and second pair of aortic arches. Cranially, in the angle between their common trunks, lies the median thyroid anlage. The anterior cardinal veins are located dorso-lateral to the descending aortae. The end of the head is cut through the diencephalon and the optic vesicles. On the left side of the figure, the lens vesicle may be seen still connected with the ectoderm. The corresponding optic cup appears asymmetrical because it is cut through the chorioid fissure. The cup is differentiated into a thick inner, and a thin outer layer ; these form the nervous and pigment layers of the retina respectively.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 378. - Transverse section through the branchial arches and eyes of a 6 mm. pig embryo. X 26.5. .Y, aortic arch 4.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Section through the Tracheal Groove, Bulbus Cordis and Olfactory Pits (Fig. 379). - The ventral portion of the figure shows a section through the tip of the head. The telencephalon is not prominent. The ectoderm is thickened and sUghtly invaginated ventrolaterad to form the anlages of the olfactory pits. These deepen in later stages and become the nasal cavities. In the dorsal portion of the section may be seen the cervical spinal cord, the notochord just ventral to it, the descending aortce, and ventro-lateral to them the anterior cardinal veins. The naso-pharynx now is small with a vertical groove in its floor. This is the tracheal groove and more caudad it will become the cavity of the trachea. The bulbus cordis lies in the large pericardial cavity. On either side the section cuts through the cephalic portions of the atria. These will become larger farther caudad in the series.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 379. - Transverse section through the bulbus cordis and olfactory pits of a 6 mm. pig
| |
| embryo. X 26.5.
| |
|
| |
|
| |
|
| |
|
| |
| Section through the Heart (Fig. 380). - The heart lies in the pericardial cavity. Both the atrium and ventricle are divided incompletely into two chambers. A partial interventricular septum leaves the ventricles in communication dorsad. The septum primum is complete in this section, but cephalad in the series there is an interatrial foramen (cf. Fig. 372). 'T\\q foramen ovale has not formed in this particular embryo. The myocardium of
| |
| the ventricles is a spongy layer, much thicker than that of the atrial wall. Lateral to the descending aortae are the common cardinal veins. The right common cardinal opens into the sinus venosus which in turn empties into the right atrium, its opening being guarded by the two valves of the sinus venosus. The entrance of the left common cardinal into the sinus venosus is somewhat more caudad in the series. The trachea has now separated from the esophagus and lies ventral to it. Both trachea and esophagus are surrounded by a condensation of mesenchyme which will transform into the muscular and fibrous coats.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 380. - Transverse section through the four chambers of the heart of a 6 mm. pig embryo.X 26.5.
| |
|
| |
|
| |
|
| |
|
| |
| Section through the Lung Buds and Septum Transversum (Fig. 381). - The section passes through the bases of the upper limh buds. A pair of spinal nerves, with ganglia and roots, extends from the spinal cord into these anlages. The tips of the ventricles, lying in the pericardial cavity, still show in this section. Dorsally, the pericardial cavity has given place to the pleuro-pcritoueal cavity. Projecting ventrad into this cavity are the cranial ends of the mesonephric folds in which the posterior cardinal veins partly lie. Into the floor of the pleuro-peritoneal cavities bulge the dorsal lobes of the liver, embedded in splanchnic mesenchyme. This mesenchyme is continuous with that of the somatopleure, and forms a transverse partition between the liver and heart, complete ventrally. This is the septum transversum which takes part in forming the ligaments of the liver and is the chief anlage of the diaphragm. The two proximal trunks of the vitelline veins pass through the septum. Projecting laterally into the pleuro-peritoneal cavities are ridges of mesenchymecovered by splanchnic mesoderm in which the lungs develop as lateral buds from the caudal end of the trachea. The right lung bud is shown in the figure. Between the esophagus and the lung is a crescent-shaped cavity, the cranial end of the omental bursa.
| |
|
| |
|
| |
|
| |
| Fig. 381. - Transverse section through the right lung bud and septum transversum of a 6 mm.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 382. - Transverse section through the stomach of a 6 mm. pig embryo. X 26.5.
| |
|
| |
|
| |
|
| |
|
| |
| Section through the Stomach (Fig. 382). - The section passes through the upper limb buds and just caudal to the point at which the descending aortas unite to form the median dorsal aorta. As the liver develops in early stages, it comes into relation with the caval mesentery, along the dorsal body wall, at the right side of the dorsal mesogastrium. The space between the liver and plica to the right, and the stomach and its omenta to the left, is a caudal continuation of the omental bursa. The dorsal wall of the stomach is rotated to the embryo - s left, its ventral wall to the right. The liver shows a pair of dorsal lobes, and contains large blood spaces and networks of sinusoids lined with endothelium. Ventral to the liver, the tips of the ventricles still appear.
| |
|
| |
| Section through the Hepatic Diverticulum (Fig. 383). - The upper limb buds are prominent in this section. The mesonephric folds show the tubules and glomeruli of the mesonephroi, and the posterior cardinal veins are connected with the mesonephric sinusoids. From the dorsal attachment of the liver a ridge continues down into this section it lies on the dorsal body wall just to the right (left in figure) of the mesentery. In this ridge is a small vein which connects cranially with the liver sinusoids, caudally with the right subcardinal vein. As it later forms a portion of the inferior vena cava, the ridge in which it lies is termed the caval mesentery. The right dorsal lobe of the liver contains a large blood space into which the portal vein opens. The duodenum is ventral to the position occupied by the stomach in the previous section. There is given off from it, ventrad and to the right, the hepatic diverticulum. In sections cephalad, small ducts from the liver trabeculae may be traced into connection with it. In the left ventral lobe of the liver, a large blood space indicates the position of the left umbilical vein on its way to the ductus venosus. The mesothelial investment of the liver is the tissue of the expanded ventral mesentery into which it grew. Between the stomach and liver, the ventral mesentery is called the lesser omentum; between the liver and ventral body wall, the falciform ligament.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 383. - Transverse section through the hepatic diverticulum of a 6 mm. pig embryo.X 26.5.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Section through the Pancreatic Anlages (Fig. 3S4). - At this level, the upper limb buds still show. The mesonephroi are prominent and marked by their large Bowman - s capsules and glomeruli, located mesad. The right posterior cardinal vein is broken up into mesonephric sinusoids. The vein in the caval mesentary will connect with the right subcardinal vein a few sections lower. The anlage of the dorsal pancreas is seen extending from the duodenum dorsad into the mesenchyme of the mesentery. It soon bifurcates into a dorsal and right lobe, of which the latter is slightly lobulated. Ventro-lateral to the duodenum, the anlage of the ventral pancreas is cut across; it may be traced cephalad in the series to its origin from the hepatic diverticulum. To the right of the ventral pancreas lies the portal vein (at this level the portion contributed by the right vitelline). To the left of the dorsal pancreas is seen the remains of the left vitelline vein. The ventral lobes of the liver are just disappearing at this level. In the mesenchyme that connects the liver with the ventral body wall lie on each side the umbilical veins, the left being the larger. Between the veins is the extremity of the hepatic diverticulum which becomes the gall bladder. The body wall is continued ventrad to form a short umbilical cord.
| |
|
| |
|
| |
|
| |
| Fig. 384. - Transverse section through the pancreatic anlages of a 6 mm. pig embryo. X 26.5.
| |
|
| |
|
| |
| Section through the Intestinal Loop and Lower Limb Buds (Fig. 385). - As the posterior half of the embryo is curved in the form of a half circle, sections caudal to the liver, like this one, pass also through the lower end of the body. Two sections of the embryo are thus seen in one, their ventral aspects facing each other and connected by the lateral body wall. In the dorsal part of the section the mesonephroi are prominent, with large posterior cardinal veins lying dorsal to them and mesonephric arteries branching laterally from the aorta and passing to the glomeruli. The trunk of the vitelline artery is the delicate tube taking origin ventrally from the aorta. It may be traced into the mesentery, and through it onto the wall of the yolk sac. On either side of the vitelline artery are the subcardinal veins, the right being the larger. In the mesentery may be seen two sections of the intestinal loop (the small intestine being cut lengthwise, the large intestine transversely), and also sections of the vitelline artery and veins. In the lateral body walls, ventral to the mesonephros, occur the umbilical veins. The left vein is large and cut lengthwise' the right vein is cut obliquely twice.
| |
|
| |
|
| |
| In the ventral portion of the section, the lower limb buds are prominent laterally. A pair of large arteries, the common iliacs, branch from the aorta and may be traced into connection with the umbilical arteries, d'he colon, supported by a short mesocolon, lies in the colom near the mid plane. On each side are the caudal ends of the mesonephric folds, here small and each showing a section of the mesonephric duct and a single vesicular anlage of the mesonephric tubules. The mesonephric ducts are cut as they curve around from their position in the dorsal portion of the section. The tip of the recurved tail shows.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 385. - Transverse section through the intestinal loop and lower limb buds of a 6 mm. pig embryo. X 26.5.
| |
|
| |
|
| |
| Section through the Primitive Segments and Spinal Cord (Fig. 386). - In the interval since the level last described are encountered sections which show symmetrical longitudinal views of the body wall, mesonephroi, mesentery, aorta, and notochord. This section is near the end of the series, and, as the body is here curved, it is really a frontal section. At the left side of the spinal cord, the oval cellular masses are the spinal ganglia cut across.
| |
|
| |
|
| |
|
| |
| The ectoderm, arching over the segments, indicates their position. Each segment shows an outer dense layer, the dermatome, lying just beneath the ectoderm. This plate curves lateral to the spindle-shaped myotome, which gives rise to the voluntary muscle. Next comes a diffuse mass of mesenchyme, the sclerotome, which, with its fellow of the opposite side, eventually, surrounds the spinal cord and forms the anlage of a vertebra. A pair of spinal nerves and spinal ganglia are developed opposite each somite, and pairs of small vessels are seen between the segments. These are dorsal intersegmental arteries.
| |
|
| |
| Section through the Cloaca and Metanephric Anlages (Fig 387). - Having now studied sections at various levels to near the end of the series, the next step is to examine sections through the caudal region and study the anlages of the urogenital organs. Owing to the curvature of the embryo, it is necessary to go cephalad in the series. The metanephroi appear as dorsal evaginations from the mesonephric ducts, just before their entrance into the cloaca.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 386. - Transverse section through the primitive segments and spinal cord of a 6 mm. pig embryo. X 45.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 387. - Transverse section metanephroi of a 6 mm. pig embryo. X 45 .
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 388. - Transverse section through the umbilical vessels, allantois and cloaca of a 6 mm pig embryo. X 45 .
| |
|
| |
|
| |
|
| |
|
| |
| Each consists of an epithelial layer surrounded by a condensation of mesenchyme. Traced a few sections cephalad, the mesonephric ducts open into the lateral diverticula of the cloaca, which, irregular in outline because it is sectioned obliquely, lies ventral to them and receives dorsad the rectum. Caudal to the cloaca, in this embryo, the tail bends abruptly cephalad and to the right. The blind prolongation of the hindgut may be traced out into this portion of the tail until it ends in a sac-like dilatation.
| |
|
| |
|
| |
| Section through the Umbilical Vessels, Allantois and Cloaca (Fig. 388). - The present section passes through the bases of the limb buds at the level where the allantoic stalk, curving inward from the umbilical cord, opens into the cloaca. At either side of the allantoic stalk may be seen oblique sections of the umbilical arteries, and lateral to these the large left and small right â– umbilical vein. The mesonephric ducts occupy the mesonephric ridges which project into small caudal prolongations of the coelom. Midway between the ducts lies the rectum, dorsal to the cloaca. The tip of the tail is seen in section at the left of the figure.
| |
|
| |
|
| |
|
| |
|
| |
| (B) THE ANATOMY OF TEN TO TWELVE MM. PIG EMBRYOS
| |
|
| |
|
| |
| This is the most instructive single stage of development. The anlages and relations of nearly all the important organs are represented, and yet the embryo is not so complex as to confuse a beginner. Embryos between 8 and 14 mm. long may be used equally well in conjunction with the appended descriptions. A human embryo of 12 mm. is shown in Fig. 64; its internal anatomy is fundamentally the same.
| |
|
| |
|
| |
|
| |
| External Form. - The head is relatively large on account of the increased size of the brain (Fig. 389). The third branchial arch is still visible in the embryo, but the fourth arch has sunken in the cervical sinus; usually, both disappear at a slightly later stage. The olfactory pits form elongated grooves on the under surface of the head, and the lens of the eye lies beneath the ectoderm, surrounded by the optic cup. The maxillary and mandibular processes of the first branchial arch are large; the former show signs of fusing with the median nasal processes to form the upper jaw, while the mandibular processes have united already. Small tubercles, the anlages of the external ear, bound the first branchial groove, which itself becomes the external acoustic meatus.
| |
|
| |
|
| |
|
| |
| At the cervical bend, the head is flexed at right angles with the body, bringing the ventral surface of the head close to the trunk, and it is probably owing to this flexure that the third and fourth branchial arches buckle inward to form the cervical sinus. Dorsad, the trunk forms a long curve, more marked opposite the posterior extremities. The reduction in the trunk flexures results from the increased size of the heart, liver, and mesonephroi. These organs are indicated through the translucent body wall, and the position of the septum transversum may be noted between the heart and the liver (cf. Fig. 390). The limb buds are larger and the umbilical cord is prominent ventrad. Dorsally, the mesodermal segments occur, and, extending in a curve between the bases of the limb buds, is the milk line, a thickened ridge of ectoderm which forms the anlages of the mammary glands. The tail is long and tapering. Between its base and umbilical cord is the genital tubercle (Fig. 390).
| |
|
| |
|
| |
| Fig. 389. - Pig embryo of 10 mm. X 7.
| |
|
| |
|
| |
| Fig. 390. - Lateral dissection of a 10 mm. pig embryo. X 10.5.
| |
|
| |
|
| |
|
| |
|
| |
| .Nervous System and Sense Organs. - The Brain. - Five distinct regions may be distinguished (Figs. 390 to 392): (i) The telencephalon, with its rounded lateral outgrowths, the cerebral hemispheres. Their cavities, the atcral ventricles, communicate by interventricular foramina with the third ventricle. (2) The dience phaion shows a laterally flattened cavity, the third ventricle. Ventro-laterally from the diencephalon pass off the optic stalks, and. an evagination of the midventral wall is the anlage of the posterior hypophyseal lobe. (3) The mesencephalon is undivided, but its cavity becomes the cerebral aqueduct leading caudally into the fourth ventricle. (4) The metencephalon is separated from the mesencephalon by a constriction, the isthmus. Dorso-laterally it becomes the cerebellum, ventrally the pons. (5) The elongated myelencephalon is roofed over by a thin, non-nervous ependymal layer. Its ventro-lateral wall is thickened and still gives internal indication of the neuromeres. The cavity of the metencephalon and myelencephalon is the fourth ventricle.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 391. - Dissection of the postotic cranial nerves and ganglia of a 15 mm. pig embryo, viewed from the right side, X 25.
| |
|
| |
|
| |
|
| |
| The Cranial Nerves. - Of the twelve cranial nerves, all but the first (olfactory) and sixth (abducens) are represented in Fig. 390; (2) The optic nerve is represented by the optic stalk, cut through in this illustration. (3) The oculomotor, a motor nerve to four of the eye muscles, takes origin from the ventro-lateral wall of the mesencephalon. (4) The trochlear nerve fibers, motor, to the superior oblique muscle of the eye, arise from the ventral wall of the mesencephalon, turn dorsad and cross at the isthmus, thus emerging on the opposite side. From the myelencephalon appear in order: (5) the n. trigeminus, mixed, with its semilunar ganglion and three branches, the ophthalmic, maxillary, and mandibtilar; (6) the n. abducens, motor, from the ventral wmll to the external rectus muscle of the eye; (7) the n. facialis, mixed, with its geniculate ganglion and its chorda tympani, facial, and superficial petrosal branches in the order named; (8) the n. acusticus, sensory, arising cranial to the otocyst, with its acoustic ganglion and sensory fibers to the internal ear; (9) caudal to the otocyst the n. glossopharyngeus, mixed, with its superior and petrosal ganglia; (10) the n. vagus, sensory, with its jugular and nodose ganglia; ( 1 1) the n. accessorius, whose motor fibers take origin from the lateral wall of the spinal cord and myelencephalon between the jugular and sixth cervical ganglia; the internal branch of the n. accessorius accompanies the vagus; the external branch leaves it between the jugular and nodose ganglia and supplies the sterno-mastoid and trapezius muscles; (12) the n. hypoglossus, motor, arising by five or six fascicles from the ventral wall of the myelencephalon ; its trunk passes lateral to the nodose ganglion and supplies the muscles of the tongue.
| |
|
| |
|
| |
| The orderly innervation of the four branchial arches by the fifth, seventh, ninth, and tenth nerves is not so diagrammatic as in the 6 mm. embryo but these relations continue nevertheless.
| |
|
| |
|
| |
|
| |
| A nodular chain of ganglion cells extends caudad from the jugular ganglion of the vagus (Fig. 391). These have been interpreted as accessory vagus ganglia. They may, however, be continuous with Froriep - s ganglion which sends sensory fibers to the n. hypoglossus. In pig embryos of 1 5 mm. this chain is frequently divided into four or five ganglionic masses, of which occasionally two or three (including Froriep - s ganglion) send fibers to the root fascicles of the hypoglossal nerve (Fig. 391).
| |
|
| |
|
| |
|
| |
| The Spinal Nerves. - Each of these has its own spinal ganglion, from which the dorsal root fibers are developed (Figs. 390 and 406). The motor fibers take origin from the ventral cells of the neural tube and form the ventral roots which join the dorsal roots in the nerve trunk.
| |
|
| |
|
| |
|
| |
| The Sense Organs. - -The olfactory pits are deep foss®, flanked by the nasal processes. The stalked optic cup is prominent and the lens vesicle detached. The otocyst is a compressed oval vesicle with a tubular endolymph duct growing dorsad from its median side.
| |
|
| |
| Digestive and Respiratory Systems. - Pharynx. - Dorsally, the anterior lobe of the hypophysis is long and forks at its end (Figs. 392 and 393). In the floor of the pharynx are the anlages of the tongue and epiglottis (Fig. 84 B). From the mandibular arches arise elongated thickenings that will become the body of the tongue. Between, and fused to these thickenings, is the temporary tuberculum impar. The opening of the thyroglossai duct, between the tuberculum impar and the second arch, is obliterated early. A median ridge, or copula, between the second arches represents the root of the tongue and connects the tuberculum impar with the epiglottis, which develops from the bases of the third and fourth branchial arches. On either side of the slit-like glottis are the arytenoid folds of the larynx. The pharyngeal pouches are now larger than in the 6 mm. pig (Fig. 393). The first pouch persists as the auditory tube and middle ear cavity, the - closing plate - beetwen it and the first branchial groove forming the tympanic membrane . The second pouch later largely disappears; about it, develops the palatine tonsil. The third pouch is tubular, directed at right angles to the pharynx, and meets the ectoderm to form a closing plate. The ventral diverticulum of the third pouch is the anlage of the thymus gland; its dorsal diverticulum forms a parathyroid gland. The fourth pouch is smaller and its dorsal diverticulum gives rise to a second parathyroid body ; the ventral diverticulum is a rudimentary thymus anlage. A tubular outgrowth, caudal to the fourth pouch, is regarded as a fifth pharyngeal pouch in human embryos; it^forms the ultimohranchial body on each side. The thyroid gland, composed of branched cellular cords, is located in the midplane between the second and third branchial arches (Fig. 393).
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 392. - Median sagittal dissection of a 10 mm. pig embryo. X 10.5.
| |
|
| |
|
| |
| Fig. 393. - Reconstruction of a 10 mm. pig embryo. X 10. The veins are not included; broken lines indicate the outline of the left mesonephros and the positions of the limb buds.
| |
|
| |
|
| |
|
| |
| Trachea and Lungs. - Caudal to the fourth pharyngeal pouches, the esophagus and trachea separate and form entodermal tubes (Figs. 392 and 393). Cephalad of the point where the trachea bifurcates to form rhe primary bronchi, there appears on its tight side the tracheal bud of the upper lobe of the right lung (Fig. 394). This bronchial bud is developed only on the right side and appears in embryos of 8 to 9 mm. Two secondary bronchial buds arise from the primary bronchus of each lung, and form the anlages of the symmetrical lobes of each lung.
| |
|
| |
|
| |
|
| |
|
| |
| Esophagus and Stomach. - -The esophagus extends as a narrow tube past the lungs, where it dilates into the stomach. The stomach is wide from its greater to its lesser curvature and shows a cardiac diverticulum. As a whole, it has rotated so the original dorsal border, now the greater curvature, lies to the left, the ventral border (lesser curvature) to the right (Fig. 408).
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 394. - Ventral dissection of a 9 mm. pig embryo. X 9. The head is bent dorsad.
| |
|
| |
|
| |
| Intestine. - -The pyloric end of the stomach opens into the duodenum, from which the liver and pancreas develop. The liver, with its four lobes, fills in the space between the heart, stomach, and duodenum (Figs. 390 and 392). Extending from the right side of the duodenum along the dorsal and caudal surface of the liver is the hepatic diverticulum. It lies to the right of the midplane and its extremity is the saccular gall bladder. Several ducts connect the diverticulum with the liver cords. One of these persists as the hepatic duct which joins the cystic duct of the gall bladder. The portion of the diverticulum proximal to this union becomes the common bile duct, or ductus choledochus. The ventral pancreas arises from the common bile duct, near its point of origin (Fig. 393). It is directed dorsad and caudad, to the right of the duodenum. The dorsal pancreas arises more caudally from the dorsal wall of the duodenum, and its larger, lobulated body grows dorsally and cranially (Figs. 393, 397 and 410). Between the pancreatic anlages courses the portal vein. In the pig, the duct of the dorsal pancreas persists as the functional duct.
| |
|
| |
|
| |
|
| |
| Caudal to the duodenum, the intestinal loop extends well into the umbilical cord (Figs. 392 and 393). At the bend of the intestinal loop is the slender yolk stalk. The cephalic limb of the intestine lies to the right, owing to the rotation of the loop. The small intestine extends as far as a slight enlargement of the caudal limb of the loop, the anlage of the cecum (Fig. 392). This anlage marks the beginning of the large intestine (colon and rectum). The cloaca is now nearly separated into the rectum and urogenital sinus; the cavity of the former is almost occluded by epithelial cells.
| |
|
| |
|
| |
| Coelom and Mesenteries. - The coelom is still a communicating system which includes the single pericardial cavity, the paired pleural canals, and the common peritoneal chamber. Between the heart and liver is a prominent partition, the septum transversum, which will form most of the diaphragm (Fig. 392). The attachment of the liver to it is retained as the coronary and triangular ligaments. The double sheet of splanchnic mesoderm that serves as the dorsal mesentery follows the intestinal loop into the umbilical cord (Fig. 393). The primitive ventral mesentery has mostly disappeared except at the level of the liver where it will persist as the lesser omentum a.ml jalciform ligament.
| |
|
| |
|
| |
| Urogenital System. - The mesonephros is much larger and more highly differentiated than in the 6 mm. embryo (Figs. 390 and 394). Along the middle of its ventro-median surface, the genital j old is now more prominent (Fig. 392). In a ventral dissection (Fig. 394) the course of the mesonephric ducts may be traced. They open into the urogenital sinus, which also receives the allantoic stalk (Fig. 392).
| |
|
| |
| The meianephros, or permanent kidney anlage, lies just mesial to the umbilical arteries where they leave the aorta (Fig. 393). Its epithelial portion, derived from the mesonephric duct, is differentiated into a proximal, slender duct, the ureter, and into a distal, dilated pelvis. From this grow out later the calyces and collecting tubules of the kidney. Surrounding the pelvis is a layer of condensed mesenchyme, or nephrogenic tissue, which is the anlage of the secretory tubules of the kidney.
| |
|
| |
|
| |
|
| |
| Vascular System. - The Heart. - In Fig. 395 the cardiac chambers of the right side are opened. The septum primuni, between the atria, is perforated dorsad and cephalad by the foramen ovale. The inferior vena cava is seen opening into the sinus venosus, which in turn communicates with the right atrium through a sagittal slit guarded by the right and left valves of the sinus venosus. The right valve is the higher and its dorsal half is cut away. The valves were united cephalad as the septum spurium. Between the left valve and the septum primum, the sickle-like fold of the septum secundum is forming; the fusion of these three components gives rise later to the adult atrial septum. The aortic bulb is divided distally into the aorta and the pulmonary artery, the latter connecting with the sixth (apparent fifth) pair of aortic arches. Proximally, the bulb is undivided. The interventricular septum is complete except for the interventricular foramen which leads from the left ventricle into the aortic side of the bulb. Of the bulbar swellings which divide the bulb into aorta and pulmonary trunk, the left joins the interventricular septum, while the right extends to the endocardial cushion. These folds eventually fuse, and the partitioning of the ventricular portion of the heart is completed.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 395. - Heart of a 12 mm. pig embryo, dissected from the right side.
| |
|
| |
|
| |
| The endocardium, at the atrio-ventricular foramina, is already undermined to form the anlages of the tricuspid and bicuspid valves. From the caudal wall of the left atrium there is given off a single pulmonary vein.
| |
|
| |
| The Arteries. - As seen in Fig. 393, the first two aortic arches have disappeared. Cranial to the third arch, the ventral aortae become the external carotids. The third aortic arches and the cephalic portions of the descending aortae constitute the internal carotid arteries. The ventral aortae, between the third and fourth aortic arches, persist as the common carotid arteries. The descending aortae in the same region are slender and eventually atrophy. The fourth aortic arch is largest, and, on the left side, will form the aortic arch of the adult; from the right fourth arch caudad, the right descending aorta is smaller than the left. Opposite the eighth segment, the two aortae unite and continue caudally as the median dorsal aorta. The sixth aortic arches (cf. p. 362) are connected with the pulmonary trunk, and from them arise small pulmonary arteries to the lungs. Intersegmental arteries extend dorsad, six pairs from the descending aortae, others from the dorsal aorta. From the seventh pair, which originate just where the descending aortae fuse, the subclavian arteries pass off to the upper limb buds and the vertebral arteries to the head. The latter are formed by a longitudinal anastomosis between the first seven pairs of intersegmental arteries on each side, after which the stems of the first six pairs atrophy.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 396. - Reconstruction of a 12 mm. pig embryo, to show the veins and heart from the left side (after Lewis). X 9.
| |
|
| |
|
| |
|
| |
|
| |
| Ventro-lateral arteries from the dorsal aorta supply the mesonephros and genital ridge (Fig. 393). Ventral arteries form the cceliac artery to the stomach region, the vitelline, or superior mesenteric artery, to the small intestine, and the inferior mesenteric artery to the large intestine.
| |
|
| |
| The umbilical arteries now arise laterally from secondary trunks which persist as the common iliac arteries.
| |
|
| |
|
| |
| The Veins. - The veins of the head drain into the anterior cardinal veins, which become the internal jugular veins of the adult (Fig. 396). After receiving the newer external jugular veins and the subclavian veins from the ujiper limb buds, the anterior cardinals open into the common cardinal veins (ducts of Cuvier) wduch in turn empty into the right atrium.
| |
|
| |
| The posterior cardinal veins arise in the caudal region, counse dorsal to the mesonephroi, and drain the mesonephric sinusoids. The cardinal veins anastomose just caudal to the origin of the superior mesenteric artery, and the posterior cardinals are interrupted at this level. The proximal portions of the posterior cardinals open into the common cardinal veins, as in the 6 mm. embryo. Of the two subcardinal veins, the right has become very large through its connection with the right posterior cardinal vein and the common hepatic vein, and now forms the middle portion of the inferior vena cava.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 397. - Ventral reconstruction of a 10 mm. pig embryo, to show the umbilical and vitelline veins. X 15. In the small orientation figure (cf. Fig. 393) the various planes are indicated by broken lines - * *.
| |
|
| |
|
| |
| The umbilical veins (Figs. 396 and 397) anastomose in the umbilical cord, separate on entering the embryo, and course cephalad in the ventrolateral body wall of each side to the ventral lobes of the liver. The left vein is much the larger, and, after entering the liver, its course is to the right and dorsad. After connecting with the portal vein, it continues as the ductus venosus and joins the proximal end of the inferior vena cava. The smaller, right umbilical vein enters the liver and breaks up into sinusoids. It soon atrophies, while the left vein persists until after birth.
| |
|
| |
|
| |
| The Vitelline Veins. - Of these, a distal portion of the left and a proximal portion of the right are persistent. The tw'o fused vessels course from the yolk sac cephalad of the intestinal loop. Near a dorsal anastomosis between the right and left vitelline veins, just caudal to the duct of the dorsal pancreas, the left receives the superior mesenteric vein, a new vessel arising in the mesentery of the intestinal loop. Cranial to this junction, the left vitelline (with the dorsal anatomosis and the proximal portion of the right vitelline vein) forms the portal vein, which gives off branches to the hepatic sinusoids and connects with the left umbilical vein.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 398. - Reconstruction of a 10 mm. pig embryo (cf. Fig. 393). X 8. The numbered lines indicate the levels of transverse sections shown as Figs. 399-413.
| |
|
| |
|
| |
|
| |
| Transverse Sections of a Ten Mm. Pig Embryo The more important levels, as indicated by guide lines on Fig. 398, are illustrated and described. These are useful for the identification of organs, but the student must interpret his sections with reference to the dissections and reconstructions, and especially Fig. 393. All the sections figured are drawn from the ccuhalic surface; accordingly, the right side of the embryo is at the reader - s left.
| |
|
| |
|
| |
| Sections through the Cephalic Flexure. - Due to the flexed head, the sections first encountered pass through the mesencephalon and metencephalon. Soon, the former becomes continuous with the thin-roofed myelencephalon, and then the mid-brain gives way to the dieucephalon as the brain becomes cut twice. At the latter level, several interesting structures may be identified in the mesenchyme between the two portions of the brain. In the midplane, but nearer the myelencephalon, is the single basilar artery; ventro-lateral to the diencephalon are the paired internal carotids (cf. Fig. 399). A little cephalad in the series, the three vessels unite at the location of the future arterial circle (of Willis). About halfway between the midplane and the lateral wall appear branches of the anterior cardinal veins, and the oculomotor and trochlear nerves. Of the two nerves, the trochlear is smaller and slightly more laterad, but in some series it is inconspicious. The origin and relations of these nerves show plainly in Fig. 390.
| |
|
| |
|
| |
|
| |
| Fig. 399. - Transverse section through the eyes and otocysts of a 10 mm. pig embryo. X 22.5.
| |
|
| |
|
| |
|
| |
| Section through the Trigeminal Nerve and Apex of the Otocyst. - The general appearance is like Fig. 377 of the 6 mm. stage. The dicucephalon is somewhat oblong in outline; the niyelencepbalon is sectioned lengthwise and its wall still shows the neuromeric notches. At the beginning of the hind-brain is the large scmilimar ganglion of the trigeminal nerve; from its median side nerve fibers join the brain wall. This ganglion, always situated at the angle of the myelencephalon, constitutes one of the most important landmarks of the embryonic head. Midway along each side of the myelencephalon will be seen the apex of an otocyst, and mesial to it the endolyniph duct; the duct opens into the otocyst at a slightly lower level. Sections cut in the plane of this series do not include other structures of importance except those already described as occurring in the mesenchyme between the two portions of the brain.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 400. - Transverse section through the first and second pharyngeal pouches of a 10 mm. pig embryo. X 22.5.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Section through the Eyes and Otocysts (Fig. 390). - The brain is sectioned twice, lengthwise through the myelencephalon, transversely through the fore-hrain. The brain wall shows differentiation into three layers: (i) an inner ependymal layer, densely cellular; (2) a middle mantle layer of nerve cells and fibers; (3) an outer marginal layer, chiefly fibrous. These same three layers are developed in the spinal cord. A thin, vascular layer, differentiated from the mesenchyme, surrounds the brain wall and is the anlage of the pia mater. The myelencephalon exhibits three neuromeres in this section. The telencephalon is repreSented by the paired cerebral hemispheres, their cavities, the lateral ventricles, connecting through the interventricular joramina with the third ventricle of the diencephalon. Close to the ventral wall of the diencephalon is a section of the epithelial lobe of the hypophysis (Rath he - s pouch), near which are the internal carotid and basilar arteries. Lateral to the diencephalon are the optic cup and lens vesicle of the eye, which are sectioned caudal to the optic stalk. The outer layer of the optic cup forms the thin pigment layer; the inner, thicker layer is the nervous layer of the retina. The lens is now a closed vesicle, distinct from the overlying corneal ectoderm.
| |
|
| |
|
| |
| The large vascular spaces are the cavernous sinuses, which drain into the internal jugular veins (anterior cardinals). Transverse sections of the maxillary and mandibular branches of the n. trigeminus may be seen; the n. abducens is sectioned longitudinally.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 401. - Transverse section through the third pharyngeal pouches and olfactory pits of a 10 mm. pig embryo. X 22.5.
| |
|
| |
|
| |
| In front of the otocyst occur the geniculate and acoustic ganglia of the nn. facialis and acustieus. The wall of the otocyst forms a sharply defined epithelial layer which makes a convenient landmark in identifying ganglia and nerves. Caudal to the otocyst, the n. glossopharyngeus and The jugular ganglion of the vagus are cut transversely while the trunk of the n. accessorius is sectioned lengthwise.
| |
|
| |
|
| |
| Section through the First and Second Pharyngeal Pouches (Fig. 400). - The end of the head, with parts of the telencephalon and olfactory pits, is now distinct from the rest of the section. Higher in the series, Rathke's pouch opens into the stomodeum between the jaws; this level is at the actual oral opening. The pharynx shows portions of the first and second pharyngeal pouches. Opposite*the first pouch, externally, is the first branchial groove. A shaving from the tubcrcidum impar of the tongue lies the midplane in the pharyngeal cavity. The neural tube is sectioned dorsally at the level of Froriep 's ganglion. Between the neural tube and the pharynx may be seen on each side the several root fascicles of the n. hypoglossus, the fibers of the nn. vagus and accessorius, and the petrosal ganglion of the n. glossopharyngeus. Mesial to the ganglia are the descending aortce, andlateral to the vagus is the internal jugular vein.
| |
|
| |
| Section through the Third Pharyngeal Pouches (Fig. 401). - The tip of the head is now small and includes on either side the deep olfactory pits lined with thickened olfactorv epithelium. Each pit is bordered by a lateral and median nasal process. The first, second, and third branchial arches show; the first pair are fused as the mandible, the third pair are slightly sunken in the cervical sinus. The dorsal diverticula of the third pharyngeal pouches extend toward the ectoderm of the third branchial groove. The ventral diverticula, or thymic anlages, may be traced caudad in the series. The floor of the pharynx is sectioned through the epiglottis. Ventral to the pharyn.x are portions of the third aortic arches and the solid cords of the thyroid gland. Dorsally, the section passes through the spinal cord and first pair of cervical ganglia. Betw'een the cord and pharynx, named in order, are the internal jugular veins, the hypoglossal nerve, and the nodose ganglion of the vagus. Lateral to the ganglion is the external branch of the n. accessorius, and mesial to the ganglia are the small descending aortce.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 402. - Transverse section through the fourth pharyngeal pouches of a 10 mm. pig embryo.X 22.5.
| |
|
| |
|
| |
|
| |
| Section through the Fourth Pharyngeal Pouches (Fig. 402). - This region is marked by the disappearance of the head, and the appearance of the heart in the pericardial cavity. The tips of the atria are sectioned as they project on either side of the bulbus cordis. The bulbus is divided into the aorta and pulmonary artery, the latter connected with the right ventricle, which has spongy muscular walls. The crescentic pharynx is continued laterally as the small fourth pharyngeal pouches, and into its midventral wall opens the vertical slit of the trachea. A section of the vagus complex is located between the descending aorta and the internal jugular vein. At this level, the jugular vein receives the linguo-facial vein. The left descending aorta is larger than the right in anticipation of its conversion into the permanent arch of the aorta. The ventral aorta may be traced cranially in the series to the fourth aortic arches. The pulmonary artery, if followed caudad, connects with the sixth aortic arches as in Fig. 403.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 403. - Transverse section through the sixth pair of aortic arches and bulbus cordis of a 10 mm. pig embryo. X 22.5.
| |
|
| |
|
| |
|
| |
| Section through the Sixth Aortic Arches (Fig. 403). - The sixth aortic arch (see p. 362) is complete on the left side of the embryo. From these pulmonary arches small pulmonary arteries may be traced caudad in the series to the lung anlages. The esophagus, now separate from the trachea, forms a curved horizontal slit. All four chambers of the heart are represented, but the aorta and pulmonary artery are divided incompletely by the right and left bulbar swellings, or folds. The vagus nerves are prominent. Ventro-lateral to the spinal cord are diffuse myotome masses.
| |
|
| |
| Section through the Sinus Venosus and the Heart (Fig. 404). - The section is marked by the symmetrically placed atria and ventricles of the heart and by the presence of the upper limb buds. Dorsal to the atria are the common cardinal veins, the right vein forming part of the sinus venosus, the left connecting at a lower level. The sinus venosus drains into the right atrium through a sht-like opening in the dorsal and caudal atrial wall. The opening is guarded by the right and left valves of the sinus venosus, which project into the atrium. The septum primum completely divides the right and left atria at this level, which is caudal to the foramen ovale and the atrio-ventricular openings. The septum joins the fused endocardial cushions. Note that the esophagus and trachea are now tubular and that the left descending aorta is much larger than the right. Around the epithelium of both trachea and esophagus are condensations of mesenchyme, from which their outer layers are differentiated; laterad in this mass lie the vagi.
| |
|
| |
| Section through the Foramen Ovale of the Heart (Fig. 405).- - The level of this section is cranial to that of the previous figure and shows the septum primum interrupted dorsally to form the foramen ovale. Each atrium communicates with the ventricle of the same side through the atrio-ventricular foramen. Between these openings is the endocardial cushion, which in part forms the anlages of the tricuspid and bicuspid valves. The atria are marked off externally from the ventricles by the coronary sulcus. Between the two ventricles is the interventricular septum. The ventricular walls are thick and spongy, forming a network of muscular cords, or trabeculce, surrounded by blood spaces, or sinusoids. The trabeculce are composed of muscle cells, which later become striated and constitute the myocardium. They are surrounded by an endothelial layer, the endocardium. The mammalian heart receives all its nourishment from the blood circulating in the sinusoids, until later, when the coronary vessels of the heart wall are developed. The heart is surrounded by a layer of mesothelium, the epicardium, which is continuous with the pericardial mesothelium lining the body wall form the primary bronchi of the lungs. The limb buds are composed of dense, undifferentiated mesenchyme, surrounded by ectoderm which is thickened at their tips. The seventh pair of cervical ganglia and nerves are cut lengthwise, showing the spindle-shaped ganglia with the dorsal root fibers taking origin from their cells. The ventral root fibers arise from the ventral cells of the mantle layer and join the dorsal root to form the nerve trunk. On the right side, a short dorsal ramus supplies the anlage of the dorsal muscle mass. The much larger ventral ramus unites with those of other nerves at this level to form the brachial plexus.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 404. - Transverse section through the sinus venosus and heart of a 10 mm. pig embryo.X 22.5.
| |
|
| |
|
| |
| Fig. 405. - Transverse section through the foramen ovale and heart of a 10 mm. pig embryo.X 22.5.
| |
|
| |
|
| |
|
| |
| Fig. 406. - Transverse section through the liver and upper limb buds of a 10 mm. pig embryo, at the level of the tracheal bifurcation. X 22.5.
| |
|
| |
|
| |
|
| |
|
| |
| The descending aorta have now fused, and the seventh pair of dorsal intersegmental arteries arise from the dorsal aorta. From these intersegmental arteries the subclavian arteries are given off two sections caudad in the series. Lateral to the aorta are the posterior cardinal veins. The esophagus, ventral to the aorta, shows a very small lumen, while that of the trachea is continued into the bronchi on either side. Adjacent to the esophagus are the cut vagus nerves. The lung anlages project laterally into the crescentic pleural cavities, of which the left is sepaxated from the peritoneal cavity by the septum transversum. The liver, with its fine network of trabecula and sinusoids, is large and nearly fills the peritoneal cavity. The liver cords are composed of liver cells surrounded by the endothelium of the sinusoids. Red blood cells are developing in the liver at this stage. The large vein, from the liver to the heart, penetrating the septum transversum, is the proximal portion of the inferior vena cava, originally the right vitelline vein. Ventral to the bronchi may be seen sections of the pulmonary veins.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 407. - Dorsal half of a transverse section through the lung buds of a 10 mm. pig embryo.X 22.5.
| |
|
| |
|
| |
| Section through the Lung Buds (Fig. 407). - The lungs are sectioned through their caudal ends, and the esophagus is just beginning to dilate into the stomach. On either side of the circular dorsal aorta are the mesonephroi, while dorso-laterally are sympathetic ganglia. The pleural cavities now communicate freely on both sides with the peritoneal cavity. A section of the omental bursa appears as a crescent -shaped slit at the right of the stomach. In the right dorsal lobe of the liver is located the inferior vena cava. Near the median plane, ventral to the omental bursa, is the large ductus venosus.
| |
|
| |
| Section through the Stomach and Mesonephros (Fig. 408). - Prominent in the body cavity are the mesonephroi and liver lobes. The mesonephroi show sections of coiled tubules lined wdth cuboidal epithelium. Glomeruli of the renal corpuscles, median in position, have developed as knots of small arteries which grow into the ends of the tubules. The thickened epithelium along the median and ventral surface of the mesonephros is the anlage of the genital gland. The body wall is thin and lined with mesothelium continuous with that which covers the mesenteries and organs. The mesothelial layer becomes the epithelium of the adult peritoneum, mesenteries, and serous layer of the viscera. The stomach lies on its left side and is attached dorsally by the greater omentum, ventrally to the liver by the lesser omentum. The right dorsal lobe of the liver is attached dorsally to the right of the greater omentum. In the liver, ventral to this fusion, courses the inferior vena cava, and the connection forms the caval mesentery. Between the attachments of the stomach and liver, and to the right of the stomach, is the omental bursa. In the liver, to the left of the midplane, is the ductus vawsus, sectioned just at the point where it receives the left umbilical vein and a branch from the portal vein. The ventral attachment of the liver later becomes the falciform ligament.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 408. - Transverse section through the stomach and mesonephros of a 10 mm. pig embryo. X 22.5.
| |
|
| |
|
| |
| Section through the Hepatic Diverticulum (Fig. 409). - The section passes through the pyloric end of the stomach and duodenum, near the attachment of the hepatic diverticulum. The greater omentum of the stomach is larger than in the previous section, and to its right, in the caval mesentery, lies the inferior vena cava. Ventral to the inferior vena cava is a section of the portal vein. The ventral and dorsal lobes of the liver are now separate, and, in the right ventral lobe, is embedded the saccular end of the hepatic diverticulum which forms the gall bladder. To the right of the stomach, the common duct segment of the diverticulum is sectioned just as it enters the duodenum. Ventrally, the left tunbilical vein is entering the left ventral lobe of the liver. It is much larger than the right vein, which still courses in the body wall. On the left side of the embryo the spinal nerve shows, in addition to its dorsal and ventral rami, a sympathetic ramus, the fibers of which passtoa cluster of ganglion cells located dorso-lateral to the aorta. These cells form one of a pair of sympathetic ganglia. On each dorso-lateral surface of the trunk is a thickened ectodermal ridge, representing the milk line from which mammary glands differentiate.
| |
|
| |
|
| |
| Section through the Pancreatic and Genital Glands (Fig. 410). - The omental bursa, just above the level of this section, has opened into the peritoneal cavity through the epiploic foramen (of Winslow). The mesonephric ducts are now prominent ventrally in the mesonephroi, and along the mesial surfaces are the thickened anlages of the genital glands. The duct of the dorsal pancreas is sectioned tangentially at the point where it takes origin from the d uoden u m. F rom the duct , the lobulated gland may be traced dorsad in the mesentery. To the right of the dorsal pancreatic duct is a section of the ventral pancreas, which may be traced cephalad in the series to its origin from the hepatic diverticulum. Dorsal to the ventral pancreas is a section of the portal vein. The inferior vena cava appears as a vertical slit in the caval mesentery.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 409. - Transverse section through the hepatic diverticulum of a 10 mm. pig embryo.
| |
|
| |
|
| |
|
| |
|
| |
| Sections through the Lower Body. - Due to the curvature of the lower half of the body, sections transverse to the main axis include two portions of the embryo, facing each other and connected by the lateral body wall. The general appearance is much like Fig. 385 of the 6 mm. pig. In these sections, the intestinal loop may be found in the free mesentery. Besides the gut and allantois, the vitelline artery (superior mesenteric), umbilical arteries, superior mesenteric vein, and umbilical veins may be traced to the umbilical cord. Vcntro-lateral arteries are given off from the aorta to the mesonephros. Tracing the inferior vena cava caudad from the level illustrated in Fig. 410, it becomes continuous with the right subcardinal, now a component of the vena cava (cf. Fig. 385). The left subcardinal is smaller and the two connect by anastomoses.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 410. - Transverse section through the pancreatic and genital glands of a 10 mm. pig embryo. X 22.5.
| |
|
| |
|
| |
|
| |
| Section through the Cloaca. - At a level intermediate between Figs. 410 and 41 1 (cf. Fig. 3 q 8.), the cloaca and cloacal membrane will be encountered in the tail portions of sections. These relations should be compared with Fig. 387 of the 6 mm. pig series.
| |
|
| |
|
| |
|
| |
| Fig. 411. - Transverse section through the urogenital sinus and rectum of a 10 mm. pig embryo. .X 22.5.
| |
|
| |
|
| |
| Section through the Urogenital Sinus and Rectum (Fig. 41 1). - The figure shows only the caudal end of a section, in the dorsal portion of which the mesonephroi were sectioned at the level of the subcardinal anastomosis. A portion of the mesentery is shown with a section of the colon. In the body wall are veins that drain into the umbilical veins, and on each side are the umbilical arteries, just entering the body from the umbilical cord. Between them, in sections cranial to this, the allantoic stalk is located. Here it has opened into the crescentic urogenital sinus. Dorsal to the urogenital sinus (dorsal now being at the bottom of the figure, owing to the curvature of the caudal region), is a section of the rectum, separated from the sinus by a curved prolongation of the coelom. From the ends of the urogenital sinus, as we trace cephalad in the embryo {downward in the series), are given off the mesonephric ducts.
| |
|
| |
|
| |
|
| |
| Fig. 412. - Transverse section through the lower limb buds and ureters of a 10 mm. pig embryo. X 22.5.
| |
|
| |
|
| |
|
| |
| Section through the Lower Limb Buds and Origin of the Ureter (Fig. 412). - The section cuts through the middle of both lower limb buds. Mesial to their bases are the umbilical arteries, which lie lateral to the mesonephric ducts. From the dorsal wall of the left mesonephric duct is given off the ureter, or duct of the metanephros. Tracing the sections down in the series, both ureters appear as minute tubes in transverse section. They soon dilate to form the pelvis of the kidney, at the level of Fig. 413. Note the undifferentiated mesenchyme of the lower limb buds and their thickened ectodermal tips.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 413. - Transverse section through the metanephric anlages of a 10 mm. pig embryo. X 22.5.
| |
|
| |
|
| |
| Section through the Metanephroi and Umbilical Arteries (Fig. 413). - The section passes caudal to the mesonephric ducts, which curve along the ventral surfaces of the mesonephroi (Fig. 393). The umbilical arteries course lateral to the metanephroi. The latter consist merely of the thickened epithelium of the renal pelvis surrounded by a layer of condensed mesenchyme, the nephrogenic tissue, which will differentiate into secretory tubules.
| |
|
| |
|
| |
| Section through the Vertebral Anlages. - Near the caudal end of the series, the sections pass tangentially through the curved back of the embryo. The appearance is much like Fig. 386. Slightly cephalad of this particular level, the aorta is sectioned lengthwise, and a little higher still the longitudinal notochord appears surrounded by segmental masses of condensed mesenchyme. These are the anlages of vertebvcB differentiating from the union of paired sclerotomes.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| (C) THE ANATOMY OF AN EIGHTEEN MM. PIG EMBRYO
| |
|
| |
|
| |
|
| |
|
| |
| The anlages of the important organs are formed in 12 mm. embryos. Older stages are chiefly instructive, therefore, to demonstrate the growth their relative growth, and changes of position. Since the illustrations indicate better than descriptions the several structures and their states of development, only certain features will be mentioned.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 414. - Lateral dissection of an 18 mm. pig embryo. X 8.
| |
|
| |
|
| |
|
| |
|
| |
| External Form (cf. Fig. 414). - The neck and back are much straighter than before, but the ventral body is exceedingly convex. The head is relatively larger, the umbilical cord smaller. The sense organs are prominent, and the face, with snout and jaws, plain. The branchial grooves and cervical sinus have disappeared from the neck. The limbs show indications of proximal and distal divisions, and the hand and foot are paddlelike. Several mammary gland anlages occur along the milk lines, now located more ventrad. The genital tubercle has become a distinct phallus.
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 415. - Median sagittal dissection of an 18 mm. pig embryo. X 8.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Lateral Dissection (Fig. 414). - The cerebral hemispheres are larger and the cerebellum is appearing. Beneath the cerebellum is the pontine flexure of the lirain, ])ointing ventrad. Nerves and ganglia show clearly; the brachial and lumbo-sacral plexuses, opposite the limbs, are noteworthy. The liver and lungs are relatively larger, the heart and mesonephros smaller.
| |
|
| |
|
| |
|
| |
| Median Sagittal Dissection (Fig. 415). - The corpus striatum has developed in the floor of the cerebral hemisphere, a chorioid plexus invades the fourth ventricle, and the neural (posterior) lobe of the hypophysis is growing into association with the detached Rathke - s pouch. Sclerotomic anlages of vertebrae condense about the notochord. The viscera show only quantitative changes from the 12 mm. stage, but the urogenital sinus and rectum are now separate. The relation of the intestine and its temporary herniation into the umbilical cord are apparent.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 416. - Ventral dissection of a 15 mm. jug embryo. X 6. The heart and liver have been removed and the lungs are viewed through the transparent pericardium.
| |
|
| |
|
| |
|
| |
| Ventral Dissection (Figs. 416, 147 and 148). - In the first two illustrations the lungs appear to lie in the pericardial cavity but in reality they are viewed through its transparent dorsal wall. The three figures show successive stages in the growth of the Mullerian ducts toward the urogenital sinus, and also in the lobation of the lungs.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| (D) THE ANATOMY OF A THIRTY-FIVE MM. PIG EMBRYO External Form (cf. Fig. 417).
| |
|
| |
|
| |
|
| |
| The embryo is straighter and its ventral surface less protuberant. The head, with its prominent snout, is shaping like that of a lower mammal, and the neck becomes distinct. Digits have appeared on the elongate extremities.
| |
|
| |
| .Lateral Dissection (Fig. 417). - The spinal cord and brain are relatively smaller, but the latter is becoming highly specialized and folded. The cerebral hemispheres are large and olfactory lobes extend forward from the rhinencephalon. The body of the embryo elongates faster than the spinal cord, so that the spinal nerves, at first directed at right angles, course obliquely in the lumbo-sacral region. Note especially how the viscera have receded caudad (cf. Figs. 367 and 390) and how the liver dominates the abdomen. The kidney is exceptional in that it shifts cephalad.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 417. - Lateral dissection of a 35 mm. pig embryo. X 4.
| |
|
| |
|
| |
|
| |
| Median Sagittal Section (Fig. 418). - New features of the brain are the olfactory lobes, the chorioid plexus of the third and lateral ventricles, the thalami, the epiphysis, and consolidated hypophysis. The primitive mouth cavity is now divided by the palatine folds into upper nasal passages .
| |
|
| |
|
| |
| Fig. 418. - Median sagittal dissection of a 35 mm. embryo. X 4 .
| |
|
| |
| and lower oral cavity. Of the viscera, the distinct genital and suprarenal glands and the enlarged metanephros command attention, as does the coiling of the intestine. The ureter has acquired a separate opening at the base of the bladder, and the urethra may be traced into the phallus.
| |
|
| |
|
| |
|
| |
|
| |
| (E) METHODS FOR THE DISSECTION OF PIG EMBRYOS
| |
| A prominent feature of the laboratory manual prepared by Professor C. W. Prentiss was the emphasizing of dissections as an aid to the study of mammalian embryos. For reference, the methods which he developed so successfully will be appended without material change.
| |
|
| |
|
| |
|
| |
| Preparation of Material. - Pig embryos, 10 mm. or more in length, may be dissected easily, mounted as opaque objects, and used for several years. Success in dissecting such small embryos depends; (i) on the fixation and hardening of the material employed; (2) on starting the dissection with a clean cut in the right plane; (3) on a knowledge of the anatomy of the parts to be dissected.
| |
|
| |
|
| |
|
| |
| Embryos fixed in Zenker - s fluid have given the best results. They should then be so hardened in 95 per cent alcohol that the more diffuse mesenchyme will separate readily from the surfaces of the various organs, yet the organs must not be so brittle that they will crumble and break. Embryos well hardened and then kept for two weeks in 80 per cent alcohol dissect well. Old material is usually too brittle ; that just fixed and hardened may prove too soft. As a test, determine whether the mesenchyme separates readily from the cervical ganglia and their roots.
| |
|
| |
|
| |
| Dissecting instruments include a binocular dissecting microscope, a sharp safety razor blade, large, curved, blunt-pointed dissecting needles, pairs of small, sharp-pointed forceps, and straight dissecting needles, small and large.
| |
|
| |
|
| |
| In general, it is best to begin the dissection with a clean, smooth cut made by a single stroke with the safety -razor blade, which should be flooded with 80 per cent alcohol. The section is made free hand, holding the embryo, protected by a fold of absorbent cotton, between the thumb and index finger. Having made preliminary cuts in this way, the embryo may be affixed with thin celloidin to a cover glass and immersed in a watch glass containing alcohol. We prefer not to affix the embryo, as the celloidin used for this purpose may interfere with the dissection. Instead, a cut is made parallel to the plane of the dissection so that the embryo, resting in the watch glass upon this flat surface, will be in a fairly stable position. It may thus be held in any convenient position by resting the convex surface of a curved, blunt dissecting needle upon some part not easily injured. The dissection is then carried on under the binocular microscope, using the fine pointed forceps, dissecting needles, and a small pipette to wash away fragments of tissue.
| |
|
| |
|
| |
|
| |
| Whole Embryos. - For the study of the exterior, whole embryos may be affixed with celloidin to the bottoms of watch glasses which then stack in wide-mouthed jars of 80 per cent alcohol. Such specimens last several years, at a saving of both time and material. Preliminary treatment consists in immersion in 9 5 per cent alcohol one hour, in ether and absolute alcohol at least thirty minutes, in thin celloidin one hour or more. Pour enough thin celloidin into a Syracuse watch glass to cover its bottom, and immerse in this a circle of black mat paper, first wet with ether and absolute alcohol. Pour off any surplus celloidin, mount embryo in desired position, and immerse watch glass in 80 per cent alcohol, in which the specimen may be kept indefinitely. Embryos may also be mounted in gelatin-formalin solution in small, sealed glass jars.
| |
|
| |
|
| |
|
| |
| Lateral Dissections of the Viscera. - Skill is required to demonstrate most of the cranial nerves, but the central nervous system, cranial and spinal ganglia, and viscera may easily be exposed. Starting dorsally, make a sagittal section of the embryo, sHghtly to one side of the median line and avoiding the umbilical cord ventrally. With the embryo resting on the flat, sectioned surface, begin at the cervical flexure, and with fine forceps grasp the ectoderm and dural anlage at its cut edge, separate it from the neural tube and pia mater, and strip it off ventralwards, exposing the myelencephalon and cervical portion of the cord. As the mesenchyme is pulled away, the ganglia and roots of the cranial nerves will be exposed. The mesenchyme between the ganglia and along the nerves may be removed with the end of a small blunt needle. Care must be exercised in working over the mesencephalon and telencephalon of the brain not to injure the brain wall, which may be Vjrittle. By starting with a clean dissection dorsally, and gradually working ventrad, the more important organs may l>e laid bare without injury. The beginner should compare his specimen with the dissections figured, and also previously study the reconstructions.
| |
|
| |
|
| |
|
| |
|
| |
| Median Sagittal Dissections. - Preliminary to the dissection, a cut is made dorsally, as near as possible to the median sagittal plane. Beginning caudally, at the mid-dorsal line, an incision is started which extends in depth through the neural tube and the anlages of the vertebriE. This incision is carried to the cervical flexure, cranial to which point the head and brain are halved as accurately as possible. The blade is then carried ventrally and caudally, cutting through the heart and liver, to the right of the mid plane and umbilical cord, until the starting point is reached. A parasagittal section is next made, well to the left of the median sagittal plane, and the sectioned portion is removed, leaving on the left side of the emlmyo a plane surface. With the embryo resting upon this flat surface, the dissection is begun by removing with forceps the right half of the head. In pulling this away caudalwards, half of the dorsal body wall, the whole of the lateral body wall, and the parts of the heart and liver lying to the right of the midplane will be removed, leaving the other structures intact. If the plane of section were accurate, the brain and spinal cord will be halved in the median sagittal plane. Wash out the cavities of the brain with a pipette, and its internal structure may be seen. Dissect away the mesenchyme between the esophagus and trachea and expose the lung. Remove the right mesonephros, leaving the proximal part of its duct attached to the urogenital sinus. The right dorsal lobe of the liver will overlie the stomach and pancreas. Pick it away with forceps and expose these organs. Dissect away the caudal portion of the liver until the hepatic diverticulum is laid bare. It is whitish in color and may thus be distinguished from the brownish liver. Beginning at the base of the umbilical cord, carefully pull away its right wall with forceps, thus exposing the intestinal loop and its attachment to the yolk stalk. If the umbilical artery is removed in the caudal portion of the umbilical cord, the allantoic stalk may be dissected out. To see the anlage of the genital gland, break through and remove a part of the mesentery, exposing the me,sial surface of the left mesonephros and the genital fold. The dissection of the metanephros and ureter is difficult in small embryos. In lo to 12 mm. embryos, the umbilical artery, just after it leaves the aorta, passes lateral to the metanephros and thus locates it. By working carefully with fine needles, the surface of the metanephros may be laid bare and the delicate ureter may be traced to the base of the mesonephric duct. The extent of the dorsal aorta may also be seen by removing the surrounding mesenchyme. With a few trials, such dissections are made in a short time.
| |
|
| |
|
| |
|
| |
| Ventral Dissections. - Ventral dissections of the viscera are very easily made. With the safety razor blade, start a cut in a coronal plane through the caudal end of the embryo and the lower limb buds. Extend this cut laterad and cephalad through the body wall and the upper limb bud. The head may be cut away in the same plane of section, and the cut continued through the body wall and upper limb bud of the opposite side back caudally to the starting point. Section the embryo in a coronal plane, parallel with the first section and near the back, so that the embryo will rest upon the flattened surface. With forceps, now remove the ventral body wall. By tearing open the wall of the umbilical cord along one side, it may be removed, leaving the intestinal loop intact. Pull away the heart, noting its external structure. The liver may also be removed, leaving the stomach and intestine uninjured. A portion of the septum transversum covering the lungs may be carefully stripped away and the lungs thus laid bare.
| |
|
| |
|
| |
| Dissections made in this way show the trachea and lungs, the esophagus, stomach and dorsal attachment of the septum transversum, the course of the intestinal canal, and also the mesonephroi and their ducts. Favorable sections through the caudal end of the body may show the urogenital sinus, rectum, and sections of the umbilical arteries and allantois. In late stages, by removing the digestive organs, the urogenital ducts and glands are beautifully demonstrated (Figs. 147 and 148).
| |
|
| |
|
| |
|
| |
| Development of the Face. - The heads of pig embryos have long been used for the study of the development of the face. The head should be removed by passing the razor blade between the heart and the adjacent surface of the head, thus severing the neck. Next, cut away the dorsal part of the head by a section parallel to the ventral surface, the razor blade passing dorsal to the branchial clefts and eyes. Mount, ventral side up, three stages from embryos. 6, 12, and 14 mm. long, as shown in Figs. 369 and 67.
| |
|
| |
|
| |
| Development of the Palate. - This may be studied advantageously in pig embryos of two stages; (a) 20 to 25 mm. long; {h) 28 to 35 mm. long. Dissections are made by carrying a shallow incision from the anlage of the mouth back to the external ear on each side (Fig. 73). The incisions are then continued through the neck in a plane parallel to the hard palate. Before mounting the preparation, remove the top of the head by a section cutting through the eyes and nostrils, parallel to the first plane of section. Transverse sections through the snout may also be prepared to show the positions of tongue and palatine folds before and after the fusion of the latter (Fig. 72).
| |
|
| |
|
| |
| Development of the Tongue. - The development of the tongue may be studied from dissections of pig embryos 6, 9, and 13 mm. long. As the pharynx is bent nearly at right angles, it is necessary to cut away its roof by two pairs of sections passing in different planes. The first plane of section cuts through the eye and first two branchial arches just above the cervical sinus (Fig. 419, 1 ). From the surface, the razor blade should be directed obliquely dorsad in cutting toward the median line. Cuts in this plane should be made from either side. In the same way, make sections on each side in a plane forming an obtuse angle with the first section and passing dorsal to the cervical sinus {u). Now sever the remaining portion of the head from the body by a transverse section in a plane parallel to the first {uI). Place the ventral portion of the head in a watch glass of alcohol, and, under the dissecting microscope, remove that part of the preparation cranial to the mandibular arches. Looking down upon the floor of the pharynx, remove any portions of the lateral pharyngeal wall which may still interfere with a clear view of the pharyngeal arches, as seen in Fig. 84. Permanent mounts of the three stages mentioned above may be made and used for study by the student.
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Fig. 419. - Diagram to illustrate the planes of section made in dissecting the tongue.
| |
|
| |
|
| |
|
|
| |
|
| {{Arey1924 Footer}} | | {{Arey1924 Footer}} |
| | [[Category:1920's]] |