Paper - The developmental significance of the mammalian pharyngeal tonsil - Cat
| Embryology - 1 May 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Kingsbury BF. The developmental significance of the mammalian pharyngeal tonsil - Cat. (1932) Amer. J Anat. 50(2): 201-231.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Developmental Significance of the Mammalian Pharyngeal Tonsil: Cat
Benjamin Freeman Kingsbury (1872-1946)
Laboratory of Histology and Embryology, Cornell University, Ithaca, New York
Two Text Figures And Four Plates (Twenty-Five Figures)
Introduction
The pharyngeal structures to which the term ‘tonsil’ has come to be supplied present in certain respects a unique problem both for the morphologist and for the physiologist. It is quite customary at the present day to ascribe to any specifically differentiated structure an equally specific ‘function.’ It is therefore generally felt, perhaps, that, existing as they do as an ill-defined ring in the pharynx (Waldeyer’s ring), they constitute in some Way a defense mechanism, for the protection against infection of the lower reaches of the digestive and (particularly) the respiratory passageways. The evidence supporting such an interpretation is, as the Writer views it, of the slightest. It is quite possible that in the tonsils we are dealing with structures of no adaptative significance, which is What the term ‘function’ signifies when used in this connection. It may be pointed out that the idea of function, use, purpose, can contribute nothing to the knowledge of determining factors, and that, despite the apparently favorable location which Waldeyer’s ring presents, a teleologic significance for tonsillar structures may be entirely lacking.
In any event, it is of importance to ascertain the developmental factors that underlie their appearance. The tonsils possess as their characteristic structural feature the development of lymphatic tissue beneath and intimately related to the epithelium of the pharynx. The term ‘tonsil’ is not infrequently extended so as to include similar lympho-epithelial structures in other localities (ileal, caecal, laryngeal tonsils). In all these regions the mesenchymal tissue underlying the epithelium exhibits the Well-known differentiation as ‘lymphatic tissue ’—-a cell and fiber reticulum with marked growth activity and proliferation of free cells of the lymphocyte series which is usually localized, producing the characteristic lymphatic nodule. In its general characteristics as well as in its marked proliferative activity, the lymphatic tissue suggests an essentially embryonic tissue, both in the character of the foundation tissue, the reticulum cells, and the cells formed as the result of the proliferative process——the lymphocytes.
The factors lying back of such a tissue differentiation with a retention of certain embryonic characteristics are, however, quite obscure. It is at once obvious that in ultimate analysis the conditions determining such proliferative activity must be fundamentally the same for all forms and locations of lymphatic tissue. In connection with the interpretation of tonsillar structures, Stohr (’91, p. 547) made the interesting suggestion that the presence of leucocytes (i.e., lymphocytes) in such ‘organs’ as tonsils, pronephros of lower vertebrates, the gills of Anura, thymus, processus vermiformis, so-called trachomal glands of the nictitating membrane, was due to the regressive character of these structures. This interpretation was earlier commented upon by the writer (Kingsbury, ’15; compare also Kingsley, ’24). Other instances might be cited of an intimate association of lymphocyte proliferation and regions of regressive change, and the subject is clearly Worthy of more detailed attention. It is obvious, however, that as an interpretation of the intimate relationship of lymphocyte formation and epithelium such as occurs in the tonsils, the suggestion is not thoroughly adequate in itself, since it covers in any event but a superficial aspect of the matter. The developmental factors responsible for the differentiation must lie deeper.
Of the tonsillar structures, the pharyngeal tonsil is in some respects the simplest. It occurs in the common mammals with the exception of certain of the rodents, at least the guineapig, rat, mouse. These seem to entirely lack this structure. It must, of course, be appreciated that there are many mammals which have not been examined for its presence or absence. It is reported also in reptiles and birds, although a basis for the designation and comparison with mammals is perhaps not quite clear. The location of the pharyngeal tonsil of mammals is characteristic——in the vault of the pharynx close to the base of the skull. Here the pertinent morphologic feature is the relation of the pharyngeal tonsil to the dense fibrous tissue of the fascia pharyngobasilaris. The significance of the latter structure in the complex of causative factors for tonsil development will be discussed subsequently. A second morphologic relation to be considered in an analysis of the development of the pharyngeal tonsil is the recessus pharyngis medialis and the bursa pharyngea, which will now be discussed.
A recess of the pharynx at the site of the palatine tonsil of mammals is present, usually at least, the fossa tonsillaris of human anatomic nomenclature. Since the pioneer Work of His ( ’85) and particularly as a result of the classical research of Hammar (’02), the fossa tonsillaris has been interpreted as derived from the second branchial pouch of the embryo; hence Stiihr ’s comment, already referred to in connection with the view that an element of degeneration underlies such centers of lymphocyte proliferation. The second pouch is the regressive structure in the case of the palatine tonsil. However, a recent detailed reexamination (Kingsbury and Rogers, ’27) of the development of the palatine tonsil in a particularly favorable form (calf) has shown that the tonsillar fossa is not a persistence of the second branchial pouch, but itself clearly determined by the developmental mechanics of the region.
Since it is clear that fundamentally the same morphogenetic factors must underlie the development of both the palatine and the pharyngeal tonsils, it is but natural that the pharyngeal recess within the latter tonsil’ should be considered comparable and in the last analysis of the same significance as the tonsillar fossa of the former. While it is clear that the bursa pharyngea or recessus pharyngis medialis bears no direct causative relation to the pharyngeal tonsil, it will nevertheless be of value at this point to consider in some detail the pharyngeal outpocketing in this region.
As was to be expected, due to its importance in practical medicine, knowledge of the pharyngeal tonsil has been largely gained from its structure in man.’ In the late fetus and during the first few years of childhood it is quite clear that the pharyngeal tonsil usually exhibits a quite orderly arrangement of folds about a median furrow which deepens toward its caudal end Where it terminates in a pit of variable extent, and at this point the lateral furrows tend to converge. Later on in life, with the growth of the tonsil, such a definite arrangement of tonsillar folds appears to be lacking or disguised. To the more marked depression at the caudal termination of the median furrow has been applied both the terms ‘recessus pharyngis medius’ (Ganghofner, ’79; Schwabach, ’88) and bursa pharyngea. The latter term was first applied by Mayer (’42) and described what was clearly an abnormal or exaggerated outpocketing (‘Schleimsack’) in the dorsal pharyngeal wall, of infrequent occurrence, and the term should possibly be reserved for such pathologic sacculations as Ganghofner early insisted. The matter of terminology was, however, further complicated by the application of the name ‘bursa’ to the embryonic outpocketing presently discussed.
Froriep (’82) described in the human embryo a notochordal contact with the pharyngeal epithelium, and in one instance there was present a pharyngeal outpocketing whose apex was in close relation to a group of chordal cells. To this outpocketing Froriep applied the term bursa pharyngea and suggested that the fusion of the notochord with the pharyngeal epithelium was responsible for the development of the rather tubular outpocketing—-the ‘bursa.’ To avoid duplication of terms and confusion with the bursa pharyngea of postfetal life, with which it may or may not coincide——-the evidence is not conclusive—it may perhaps be termed ‘bursa pharyngea embryonalis.’ Subsequently, investigation of a large number of human embryos at the proper stages, by Meyer (’10), Linck (’10), and particularly Huber (’12), fully confirmed the observation of Froriep that the notochord in man makes contact with the pharyngeal epithelium and that an outpocketing—-the so-called bursa—arises at this point. These authors also gave general support to the interpretation that the notochordal contact was in part at least mechanically responsible for the drawing out of the pharyngeal pocket. As supporting the intimate causal relation of notochordal contact and pharyngeal outpocketing, it may be noted that Tourneux (’12) found in a human embryo of 44 to 57 mm. length two tubular recesses or bursae, and in each case the epithelium of the inner end or apex was in contact with the notochord.
‘It is quite obvious that the development of the pharyngeal tonsil of man requires further work, both for the determination of fact and for the analysis of underlying factors. In the first connection may be cited, first of all, the relation it bears developmentally to the definitive bursa pharyngea and the correspondence of the latter to the bursa pharyngea embryonalis.
Although Killian (’88) in his classical and extensive research came to the conclusion that the bursa pharyngea is a structure sui generis and formed by the independent proliferative activity of the pharyngeal epithelium, there seems little doubt that such notochordal contacts in the human embryo determine in some way the development of the bursa pharyngea embryonalis. The question as to how such soft fusions of notochordal cellular material and pharyngeal epithelium may be efiective in a mechanical way in producing the bursa introduces another point of view. It may be pointed out that such epithelial contacts of necessity interrupt the continuity of the related mesenchyme. Hence weight attaches to the statement of Rand (’17, p. 488) that at the time when the pharyngeal epithelium becomes distinctly invaginated to form the bursa, the mesenchyme about the notochord at its point of contact is thickening to form a sheath, later incorporated with the fascia pharyngobasilaris. . . . [thus] It may be suggested that while the human bursa pharyngea regularly develops in contact with the notochord, it arises not so much through tension exerted on the pharyngeal epithelium by the notochord itself, as by the sheath of (developing) connective tissue surrounding the notochord.
This conclusion was based in part on the conditions in the pig embryo—the main object of her investigation. In this mammal no persistent contacts of notochord and pharyngeal epithelium exist; nevertheless, small outpocketings may occur in relation to the fiber strands of the pharyngobasilar fascia (compare her figs. 9 and 10). Schwabach ( ’88) had pointed out the relation of the ‘ligamentum occipito-pharyngeum’ to the apex of the ‘funnel’ and thought it quite possible that the recess was in fact due to this connective-tissue bundle. Froriep (’82) had suggested that the occipitopharyngeal fascia is a second possible factor, as did Tourneux (’12) subsequently.
Brief comment may be made on the relation of the median pharyngeal recess or bursa to the pharyngeal tonsil. Schwab— ach ( ’88) considered the recess as nothing else than the first anlage of the pharyngeal tonsil, making direct comparison in this respect with the relation of the tonsillar fossa and the palatine tonsil. In support of this interpretation it was pointed out that the infiltration with ‘leucocytes ’ (i.e., lymphocytic cells) occurred first and was densest in the immediate neighborhood of the epithelial depression—a condition also illustrated by Huber (’12, his fig. 14 a for a human embryo of 145 mm. length). Killian (’88) pointed out that such a genetic relation of recess or bursa to tonsil could not be accepted, since many mammals entirely lacked a pharyngeal outpocketing, but nevertheless, possessed well-developed pharyngeal tonsils—a position well supported by the subsequent work on the development of the pharyngeal roof in mammals other than man (Band, ’17; Atterbury, ’19; Tourneux, ’l2). As far as reported, in only one other mammal, namely, the horse (Tourneux, ’12), has a condition been encountered similar to that in the human embryo——namely, the occurrence of an outpocketing at the site of notochordal contacts With the pharyngeal epithelium.
As briefly reviewed above, and in the light of the knowledge already gained as to the development of the pharyngeal tonsil, it is obvious from the conditions in mammals other than man that the presence of an evagination of the epithelium in the vault of the pharynx cannot in itself have significance for the development of the pharyngeal tonsil, even though preceding the appearance of the latter and possessing an intimate topographic relation to it. This was the conclusion of Killian ( ’88), who considered that the bursa pharyngea, a structure sui generis and an active evagination of the epithelium not determined by mechanical factors, had nothing to do with the pharyngeal tonsil or its formation, although he recognized that in man the lymphocytic ‘infiltration’ appeared first and in greatest amount immediately ahead of the bursa. Nevertheless, it is clear that the occasional occurrence of epithelial outpocketings in these regions of the pharynx, whether in con.nection With the notochord or not, are quite clearly due to growth tensions in the development of the region. The attention is thus centered on the question of the general occurrence of such growth tensions in the roof of the pharynx and introduces for consideration the question of their interpretation as embodying possible factors operative in the structural differentiation of the region. A bursa pharyngea would thus exist only as an expression of the developmental tensions, and possess no further significance. The term would then be of descriptive value only. The comparison of any pharyngeal recess in the territory of the pharyngeal tonsil with the tonsillar fossa of the palatine tonsil may on such a basis have real significance, if — as suggested by Kingsbury and Rogers — out-pocketings in the territory of the future palatine tonsil are themselves determined through tenions in the development of the region and thus are in an indirect Way associated with the differentiation of the tonsillar tissue. In the case of the pharyngeal tonsil of man, the first appearance of the tonsillar differentiation at or near the summit of the (so-called) bursa pharyngea (Schwabach, ’88; Huber, ’12) suggests an application of the same interpretation in the analysis of the development of this tonsillar mass. It is this aspect of the problem of the pharyngeal tonsil that is presented in this paper. The question for consideration is Whether the development of the pharyngeal tonsil does or does not bear particular reference to the underlying growth tensions to which the region is subjected. As excluding entirely any possible consideration of a bursa or recess as a factor in tonsillar development and thus simplifying the problem somewhat, the cat was chosen—a form in which no outpocketing occurs in this region. The pharyngeal tonsil is, furthermore, of a quite simple type.
Observations
The general structure of the simple pharyngeal tonsil of the cat may be seen from figures 13, 19, and 20. It is a roughly triangular area in the Vault of the pharynx, the apex of the triangle marking its caudal extremity closely adherent to the base of the skull. Its pharyngeal surface is without folds or furrows or recesses and appears knobby, due to the abundant lymphatic nodules beneath the stratified or pseudostratified ciliated columnar epithelium which is, in the main, intact — that is, not infiltrated by lymphocytes from the underlying lymphatic tissue. At the borders of the tonsillar area, the change to the characteristic connective tissue of the tunica propria is usually gradual; hence there exists no sharp boundary for the tonsil. Branched glands, abundant in the pharyngeal region, are present also Within the tonsillar area (fig. 20), but are poorly developed. A dense connectivetissue membrane bounds the lymphatic tissue internally as a capsule. It is continuous with the tunica propria of the mucous membrane at the periphery of the tonsillar area, and more medially with the dense connective tissue of the cranial periosteum — as the fascia pharyngobasilaris. In figure 13 the caudal end of the tonsil is far enough from the median plane to miss the denser connective-tissue connection with the cranial periosteum, that is, the fascia pharyngobasilaris. At the cephalic end a median caudally directed pharyngeal diverticulum (H) marks the boundary of the region. This diverticulum is connected with, and possibly a differentiation of, the lower portion of the hypophyseal stalk. In the cat for a long time after birth the hypophysis maintains a connection with the ‘pharynx,’ and a craniopharyngeal canal is a striking perforation of the base of the skull in this region. At the caudal end of the tonsillar area two landmarks are the cephalic border of the pharyngeal musculature and the transverse pharyngeal vein.
In its longitudinal extent the region of the embryonic pharynx which may ‘be designated as the ‘tonsillar area’ extends from the region of the hypophysis to the point of recession of the pharynx from the closer relation to the basis cranii (figs. 9, 16, and 18). In the early embryo presumably throughout this region the notochord is embedded in the endoderm? With the increase in the mesenchyme of the region (fig. 3) the notochord soon recedes from its confluence with the entoderm, maintaining no primary contact therewith and establishing no secondary contacts through growth bendings.
At the stage of figure 3 the mesenchyme exhibits little differentiation. At a length of 12.5 mm. (fig. 4) chondrification of the basilar plate has appeared and soon (fig. 5) the condensations of the perichondrium and a tunica propria next the pharyngeal epithelium are clearly shown. The notochord, it may be noted, traverses the cranial side of the basis cranii, enters the cartilage, and at its cephalic end again attains a superficial position. Tourneux (’12 a) has pointed out that in mammals the course of the notochord in its relation to the cartilaginous basal plate is markedly different, but characteristic. Thus it courses intracranially (retrobasilar) in the rat, traverses the cartilage (intrabasilar) in the pig, and in man and horse courses to the pharyngeal aspect of the skull (antebasilar), where it may retain, or secondarily make, contacts with the pharyngeal epithelium. Only in this last group are developed a pharyngeal bursa (embryonalis) just discussed. The cat illustrates, it would appear, the second of the three conditions (figs. 3 and 5) ; hence, as has been stated, no bursae are found to occur. Immediately caudal to the site of tonsillar differentiation, there is a tendency to pocket formation which may be quite marked, as illustrated in figure 7. Such are apparently artificially produced and hardly deserve the name of bursa.
- Compare Keibel (’89), Huber (’18), and Kingsbury (’24). No eflfort has been made to determine in the cat the early morphologic relations of the notochord nor is there suggested any close connection between the extent of the first portion of the notochord and that of the pharyngeal tonsil.
By the time the embryo has attaineda length of 28 mm. (fig. 6), the tonsillar area is becoming Well delimited through three features of its development. To begin with the least significant or—more cautiously expressed—the least marked, the epithelium of the tonsillar area and more caudal reaches of the pharynx has become markedly thickened. No such distinction is apparent earlier, as may be seen on comparing figures 3, 4, and 5, but is quite evident in the 28-mm. and 35-mm. stages illustrated in figures 6, 9, 10, 22, and 23. At this stage the epithelium has not attained its characteristic differentiation, although already ciliated. It may be noted that cilia are at this stage (35 mm.) absent on the thinner epithelium to the right. With the onset of tonsillar differentiation, this difference in the pharyngeal and prepharyngeal (pretonsillar) regions is gradually lost. Inasmuch as the epithelium so thickened is entodermal, it may be that its determination is intrinsic. It is more probable, however, that it but expresses difierences in growth tensions. Compare the epithelium upon the two sides of the epiglottis and the soft palate (fig. 7).
The mesenchyme begins at this stage (figs. 6 and 10) to clearly foreshadow the lymphatic differentiation soon to follow. Even as early as the stage of figure 5 the arrangement of the cells suggests the subsequent change. The elongation of the cells marks the fibroblastic tendency and the subsequent development of the characteristically fibrous connective tissue (figs. 5, 6, 9, 10, 11, and 14) to become dense connective tissue in the perichondrium, the capsule of the tonsil, and the fascia pharyngobasilaris. Within the tonsillar region proper the mesenchyme retains its more characteristic reticulation. The cells are later to become the reticulum cells of the lymphatic tissue. Whether any of them are destined to remain ‘undifferentiated mesenchyme cells’ is perhaps an open question. figure 22 gives at higher magnification the condition in the 34—mm. stage, and even as early as this a proliferation of free cells has begun, two such being shown in this figure. For comparison there is given figure 23 from the same section, but just cephalad of the hypophyseal stalk (fig. 9), i.e., ahead of the tonsillar region.
Approximately during this period, the vascularization of the region is becoming established. In the series of stages here illustrated vascularization is just appearing at 17 mm. (fig. 5). At 28 mm. (fig. 6) the characteristic transverse pharyngeal vein is not yet established; it is present, however, in all subsequent stages (figs. 9, 10, 11, 12, etc.). The appearance of blood-vascular channels antedates slightly the advent of lymphatic vessels. Beginning with the 35-mm. stage, the larger channels are quite apparent and are shown in figures 6, 11, 14, and 22.
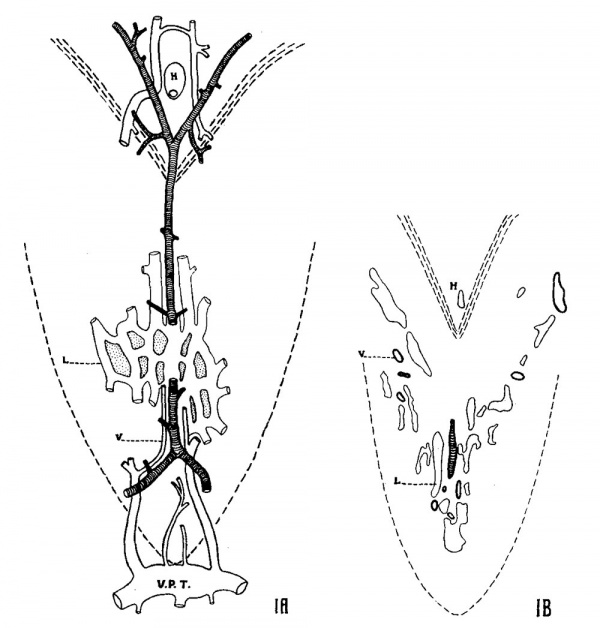
The blood supply of the dorsal pharyngeal region—i.e., that of the pharyngeal tonsil—is quite characteristic, and the arrangement is exhibited in figure 1, A. The median pharyngeal artery is located dorsally, close to the dense fibrous tissue of the capsule (fascia). Small branches pass to the tonsil. Venae comitantes accompany it caudally. Two arteries unite to form it caudally, and at its cephalic end it bifurcates, the branches passing either side of the territory of the hypophyseal stalk. The caudal arteries arise from pharyngeal arteries which leave the carotid at and within its bifurcation, therefore close to the carotid body. The arteries arising at the cephalic bifurcation anastomose with small ‘naso-palatine’ arteries, branching from the internal carotids just before they enter the cranial foramen. The blood supply of the pharyngeal tonsil is thus unique. Venous drainage is apparently both cephalad and caudad, the latter predominating, Where the transverse pharyngeal vein so apparent in the figures receives several small veins (fig. 1, A) from the tonsillar region as well as the emergent vein from the basi-occipital (figs. 17 and 18) and others. The veins into which the transverse communication drains—the occipital veins—enter the internal jugulars. The basal lymphatic channels in the tonsillar region, particularly at the time of early development of the lymphatic tissue (100 to 130 mm.), are numerous and capacious. Hence the plotting is somewhat diagrammatic. The pharyngeal artery is accompanied by a medial lymphatic channel lying ventral to it, and particularly in the more cephalic portion by lateral channels. figure 1, B, diagrams accurately a single section from the series plotted. Lymphatic drainage appears to be both caudad and cephalad.
Fig. 1 A. Flat reconstruction (semidiagrammatic) made from a. series through the pharyngeal tonsil of a cat fetus, 100-mm. length, cut in a plane parallel to the surface. Arteries cross-marked; veins (1)) with heavy outline; basal lymphatic channels (L) shown by light line (diagrammatic). V.P.1'., transverse pharyngeal vein; H, hypophyseal stalk. Caudal end is down. Boundary of the pharyngeal region is indicated by broken line. X 20. B. Reproduction of a single section from the series (100-mm. fetu), correctly plotting vascular channels. Glands and developing mesenchymal tissue not indicated. X 20.
When the fetus has attained the length of 75 mm., the proliferation which transforms the region into the pharyngeal tonsil is well under way. The source of the free cells is clearly from the local mesenchyme, and the cells form in the main cells of the ‘lymphocyte series’—cells with round nuclei and a basophile cytoplasm moderate in amount. Since, however, other cell forms appear, including myeloid cell types, and because the complexity of the blood-cell problem calls for special consideration, it seems best to consider the cytological aspect of the tonsil development in a separate communication.
The concentration of cells marking the beginnings of the lymphatic tissue appears first in the more caudal portion of the tonsillar region (figs. 12 and 14). Thence the tonsillar process ‘spreads forward (figs. 14, 16, and 18), and quite early (fig. 14) distinct centers of proliferation appear. These are at first, and until after birth, rather poorly defined. They are, in all instances in which it could be determined, close to the epithelium; or to reverse the relation, remote from the basal densed connective tissue (capsule) and the larger vascular channels. A ‘frontal’ section through a primary nodule is shown in figure 26. What in last analysis determines the localization is no more apparent here than it is in the case of nodules in other lymphatic tissue structures. No definite relation to the neighboring gland ducts could be determined, and there is much to suggest that vascular factors are intimately linked with the cell proliferation. At a stage in which no definitive lymphatic tissue has as yet appeared, it may be repeatedly observed (fig. 24) that typical small lymphocytes crowd the developing lymphatic radicles. It is apparent that the formation of this particular cell type bears a relation to the presence of lymphatic endothelium. The observations are at present insuflicient to determine clearly what underlies the intimate association: whether a growth produces at one end and the same time lymphocyte and lymphatic endothelium; whether the proliferation is determined by degeneration of a blood vessel which is also associated with the development of the lymphatic; or whether the accumulation of lymphocytes simply expresses an invasion due to a specific ‘attraction.’ As the tonsils and the associated lymphatic channels develop, the engorgement of the latter correspondingly increases (figs. 25 and 27). The illustration (fig. 27) is from a fetus of 100 mm. length. While in the 130-mm. fetus from which the plotting of figure 1 was made the larger lymphatic channels were quite free, in another specimen of the same age they were quite packed with lymphocytes, many of which, however, were clearly degenerating. As the lymphatic tissue increases in amount the engorgement of the basal lymphatic channels becomes less obvious, due possibly to the mechanical pressures of their environment. The local proliferations (fig. 26) increase in number and in size progressively with the growth of the region. It is only relatively late that germinal‘ centers (secondary nodules of Flemming) appear (figs. 19, 20, and 15), but when formed present the typical structure found in lymphatic tissue elsewhere. Their analysis will not be undertaken here, since further investigation is called for.
The glands of the tonsillar region never attain the development that characterizes them elsewhere in the pharynx. Their rudimentary condition expresses in its own way the developmental peculiarities of the region.
Comparing the location of the tonsil with the adjacent base of the skull, it is seen that it approximately underlies the basisphenoid ossification, but extends somewhat caudally. By comparing figures 12, 16, 18, and 13 it is apparent that no great relative shifting occurs during its development. This is in contrast with Killian’s statement for man, that while early in its development it underlies the sphenoid, it later occupies the level of the occipital bone.
Discussion
Certain comments may be made in conclusion. first, were it necessary, it could be easily shown that the development of the pharyngeal tonsil in the cat quite disproves the vigorously defended view of Retterer (’93) that at tonsillar sites the lymphocytes were derived from the epithelium. The epithelium takes no part in the proliferation. While lymphocytes occur in the epithelium, it is only at the height of the tonsillar growth, at sexual maturity, that this markedly occurs. Lymphocytes may appear in the epithelium at places in a relative concentration (figs. 8 and 15), and also penetrate the epithelium to the free surface. There does not occur, however, in this relatively simple tonsil, appreciably, any correlated thickening of the epithelium such as exists so constantly and characteristically in the palatine tonsil.
As is well known, Jolly (’13) created the group of ‘lymphoepithelial’ organs, including tonsillar structures, the bursa Fabricii (of birds), and thymus, in all of which there is apparent a relation and in many a correlation between epithelium and lymphatic tissue or lymphocyte formation often of complex character (mammalian thymus). Ewing (’29) has recently pointed out that the epithelial-lymphocytic correlation holds even in certain tumors from these (pharyngeal) localities.
In the pharyngeal tonsil of the cat the epithelium appears quite passive in its relation to the underlying lymphatic tissue. It would seem that the epithelium ofiers no specific attraction for the lymphocytes. It is indeed strongly suggested that in the uniform proliferation there exists no other outlet for the lymphocytes on the superficial side. The characteristic crescent in the cortex of the nodule (fig. 15) may best be understood on the same mechanical basis——namely, that whereas in the depths lymphatic channels exist to take up the lymphocytes, superficially they are lacking or inadequate. It would be expected therefore that the lymphocytes would only appear in the epithelium when the tonsil is approaching its maximum development. It might, perhaps, be expected that the uniform epithelial surface of the cat’s pharyngeal tonsil present quite different reactions from that in the depths of crypts or fold, or the completely included epithelial component of the developing thymus. The above should not be interpreted, however, as a rejection of the hypothesis that in development epithelium and underlying mesenchyme are not in some way and to some degree interrelated in their differentiations.
On the physiological or functional side, we have for consideration: 1) the theory that the pharyngeal tonsil is part of a tonsillar ring, functioning as a defense mechanism, protecting the lower reaches of the digestive and respiratory passageways; 2) the problem of a function for the lymphocyte; 3) the pharyngeal tonsil as a source of blood lymphocytes; as well as, 4) the general functional interpretations which include the tonsils along with other lymphatic tissue structures. The last need not be considered here. The nodules of the pharyngeal tonsil, when they have attained full development, possess quite the same structure as in other localities.
The development and structure of the pharyngeal tonsil in the cat furnishes no evidence of a specific function. VVhile, of course, it is entirely possible that specific chemical substances of protective significance arising in the lymphatic tissue might diffuse through the epithelium or reach the surface by Way of gland ducts, it is apparent that the more probable way in which such a function might be operative would be through the migration of lymphocytes out upon the free surface. This emigration, it has just been stated, is entirely comprehensible as an expression of the local growth proliferation under the mechanical conditions there existent.
Whether this local growth and resultant emigration of cells possesses an adaptative significance is, however, problematic, and in the light of the data from removal in man (tonsillectomy, adenotomy), quite improbable. Any distinctive function the tonsil might possess would thus be expressed through the function of lymphocyte. N 0 clear function may be ascribed to this cell form. Possessing no phagocytic property, at least in the smaller forms, it thus cannot serve as a direct destroyer of micro-organisms. The interpretation has therefore been advanced that it is through the neutralization (in some way) of toxins that it ‘serves’ the organism (Bunting and Huston, ’21). The suggestion of Bunting was in a way a corollary of his attempt to determine the fate of the lymphocyte in the body. Thus, he assumes that balancing the great influx, mainly through the lymph, there is a corresponding migration from the blood particularly out upon mucous surfaces, where the lymphocyte performs its function——the neutralization of toxins. It may be suggested that, in view of the great local production of lymphocytes within mucous membranes, the majority found Within the epithelium must be so derived. Certain it is that the concentration within the epithelium corresponds to the development of lymphatic tissue beneath. It might appear that some other fate for the lymphocyte of the blood must be sought.
As to the function of the lymphocyte, the opinion may be vouchsafed that there is no necessity for the assumption that the lymphocyte possesses any function. The evidence is fast accumulating (vide Maximow, ’27) that the lymphocyte is essentially an embryonic, i.e., undifferentiated cell type. It is a question Whether a specific function need be postulated in such case. The scanty amount of cytoplasm possessed by the prevalent (small) lymphocyte likewise suggests a functionless cell. It is in the cytoplasm that the diiferentiations which give a cell its specific character take place. In this connection reference may be made to the rather neglected observations and interpretation of Weidenreich ( ’08, ’09) that the emigrated lymphocytes undergo in the mouth (and pharynx) a difierentiation to polymorphonuclear granulecytes. Possibly therein may be found, rather indirectly it is true, a function for the tonsils. Granted the correctness of such an interpretation, it is nevertheless (in the writer’s opinion) probable that such protective value (if any) of the tonsils is more than counterbalanced by their status as ports of entry, due to the defective epithelium which the emigration of lymphocytes entails.
As a source of lymphocytes reaching the blood by Way of the lymphatics, the pharyngeal tonsils, in the cat at least, may be suspected to be of slight significance. Observations were negative, since no or few lymphocytes were observed in the lymphatics leaving the region, and this despite the fact that lymphatics within the region were frequently gorged. The anatomic relations in the vault of the pharynx seem ill adapted to facilitate the transport of lymphocytes there formed.
The writer has several times insisted that in the analysis of structure, two distinct aspects exist: the pattern, which would include the ‘functional,’ adaptative, and teleologic, and the process aspect. Whatever specific ‘function,’ if any, may ultimately be determined for the pharyngeal tonsil, will leave quite unaffected the problem of the underlying developmental processes and the accompanying factors responsible for the developmental transformation. This aspect of the problem was outlined in the introductory paragraphs, where it was pointed out that the occasional presence of bursae indicated negative tensions. With these might be linked the tonsillar differentiation which characterized the region. While it is recognized that a careful, detailed reexamination of the development of the tonsil in such a form, particularly man, was quite desirable, the cat was nevertheless chosen because of the simplicity of the morphology and the absence of a bursa. The hypothesis underlying the investigation Was that, in last analysis, tensions determined or had determined the differentiation of skeletal intercellular substance, and where these were lacking cell proliferation might be expected to predominate, as for example, in the interior of bones, where the marrow tissue is such a marked site of blood-cell proliferation. Broadly considered, there are here antithesized growth and diiferentiation as two mutually contrasted, though interrelated, aspects of development. A detailed considerar tion would involve ‘general biological theory. That the special application of the general problem has considerable complexity Will appear later. It is due to the inaccessibility of the pharyngeal region, as well as the inability to reproduce even approximately the conditions under which the difierentiation occurs, that the direct experimental approach is impracticable, although in many subsidiary phases of the problem it may be effectively applied. A detailed examination in a range of forms becomes thus important. In the cat, in the vault of the pharynx where the pharyngeal wall is in close relation to the base of the skull, a fibrous connective tissue is developed in the depth joining the region to the periosteum of the skull, particularly caudally, and continuous peripherally with the fibrous tunica propria of the mucous membrane. This leaves, however, in the included area a region of slight fibrosis, but marked proliferation of free cells. This proliferation is at first irregular and diffuse, but becomes more localized, finally producing the characteristic nodular lymphatic tissue of the tonsil.
In the analysis of the region, from the viewpoint of the hypothesis set forth, the accompanying schema (fig. 2, A) will be useful. Since the schema pictures a median plane relation, figures 9, 14, 16, and 18 may be referred to in connection therewith, and figure 17 directly compared. Since the connective-tissue fibers are interpreted as representing diiferentiations along lines of tension, double-ended arrows are used in indication. It should be appreciated that the triangle (fig. 2, B) which the schema presents is in reality a section of a low oblique cone with a roughly triangular base, the apex of the basal ‘triangle’ being at the more vertical side of the sectional triangle. Considered in connection with the anatomical relation, it is obvious that two gradients in tension would be anticipated. A center of ‘negative tension’ might be expected approximately where a perpendicular intersects the base (a; in the figures). From this point, it may be conceived, gradients of increasing tension radiate (fig. 2, B; 2, C). Secondly, tension is considered as least at or near the epithelium, increasing as the zone of fibrous diflerentiation is approached (fig. 2, B). Turning from the theoretic expectations under the negative-tension hypothesis to the actual developmental transformations in the eat, We find a marked degree of conformity. The proliferation of undifferentiated cells (i.e., those of the lymphocyte series) occurs immediately ahead of the point of recession of the pharynx from relation to the cranial floor, and spreads thence forward and finally backward, fading out gradually at the periphery. Secondly, this proliferation is at first quite close to the pharyngeal epithelium, presents there the greatest activity, and is of characteristic type. That the diiferentiation near the fibrous tissue takes on the character of myeloid tissue has been referred to and will be considered in another article.
Fig. 2 Diagram to illustrate the hypothesis of negative tension as a fundamental factor in the development of the pharyngeal tonsil. A. Schematic representation of a median-plane section in terms of fascial relations and the implied tensions. B. Simplified expression of A, in the form of a triangle. The arrows indicate gradients of increasing tension. C. An adaptation of B to A.
That growth tensions are not the only factors to be considered is obvious. The vasculogenesis of the region presents a second aspect of the problem clearly of fundamental importance not only for understanding the tonsillar structure, but the lymphatic tissue in general. Furthermore, we have here but a special application of the general problems of vasculogenesis and endothelial growth and diiferentiation, which appear far from solution at the present day. Thus, granted that growth of new endothelial channels from the preexisting does occur after differentiation is attained, a decision is nevertheless not clear between the interpretations of local mesenchymal origin in the first instance, as against such an invasional source from earlier formed vessels. Whether one inclines to the hypothesis of local vasculogenesis, or the view of proliferation from preexisting vessels and a vascularization through invasion, it is clear that the degree of vascularity and the pattern of arrangement vary greatly and characteristically in the different regions of the body. Local factors must in last analysis determine the blood supply and the lymphatics, if any, and this applies equally to the tonsillar sites.
In connection with the problem of the palatine tonsil (Kingsbury and Rogers, ’27) it was stated that “We encounter here in special instance the broader problem of the interrelation of blood cell, mesenchyme (connective tissue), and vascular channel (endothelium).” The triangular problem of interrelationship alluded to might perhaps have been better phrased as between free cell, fixed cell, and endothelial cell. Expressed in terms of process instead of cell types within the mesenchyme, the threefold problem may be expressed as proliferation, fibrosis, and vasculogenesis. Many instances of bodily reactions‘ exist in which the three aspects are presented: developmental processes (terminated by senile change); healing in defects (repair of wounds); general inflammatory reactions; reactions to transplants; reactions to epithelial tumor growths, etc. The matter need not be here reviewed. Cell proliferation and vascularization are early with fibrosis tending to be an end result. Thus in the case of the tonsil there is illustrated a localized cell proliferation of characteristic pattern and with intimate vascular correlations, which reaches a maximal intensity and fades out, the end result tending to be, in old age, a fibrosis. Again, we are led to the broader problems of the mesenchymal cell type, their interrelationship and reaction potencies.
That the covering epithelium may not be purely passive in the development of the pharyngeal tonsil is strongly suggested. Surface epithelium and the underlying connective tissue are so obviously correlated on their adaptative aspect that it would seem their differentiation must be linked. This has already been discussed (Kingsbury and Rogers, ’27) in connection with the palatine tonsil, under the term ‘epitheliomesenchymal reaction,’ and need not be further considered, since the simple relations of the pharyngeal tonsil in the cat furnish no adequate evidence. Clark et al. (’31), in their interesting study of the growth of blood vessels, find that the presence of epithelium markedly influences the development of new capillaries. What correlations there may be with the other two sides of the threefold mesenchymal reaction need not be considered here. At the tonsillar site subepithelial cell proliferation predominates; pass beyond its confines and fibrosis becomes the more striking feature.
Literature Cited
ATTERBURY, RUTH R. 1919 Bursa and tonsilla pharyngea: A note on the relations in the embryo calf. Anat. Rec., vol. 16, no. 4, pp. 251-263.
BUNTING, C. H., AND J. HUSTON 1921 Fate of the lymphocyte. J. Exp. Med., vol. 33. '
CLARK, E. R., W. J. Hrrscnwn, KIRBY-SMITH, R. O. Rmx, AND J. H. SMITH 1931 General observations on the ingrowth of new blood vessels into standardized chambers in the rabbit/s ear, and the subsequent changes in the newly grown vessels over a. period of months. Anat. Rec., vol. 50, no. 2, pp. 126-168.
EWING, JAMES 1929 Lymphoepithelioma. Am. J. Path., vol. 5, no. 2, pp. 99-107.
FRORIEP, A. 1882 Kopfteil der Chords. dorsalis bei menschlichen Embryonen. Beitréige znr Anat. u. Emb:-yo1., Festgabe Jacob Henle, Bonn. 1882, B. 26-40.
Gsncnorxm, F3. 1879 Ueber die Tonsilla und Bursa pharyngea. Sitzungsber. d. k. Akad. <1. wiss. in Wien., Bd. 78, S. 182-212.
HAMMAR, J. 1902 Studien fiber die Entwicklung des Vorderdarms und einiger angrenzenden Organe. II. Abt. Das Sehicksal der zweiten Schmidspalte. Zur vergleichenden Embryologie der Tonsille. Arch. f. mikr. Anat., Bd. 61, S. 404-458.
His, WM. 1885 Anatomie menschlicher Embryonen, III, S. 82-83. Leipzig.
HUBER, G. C. 1912 On the relation of the chorda dorsalis to the anlage of the pharyngeal bursa. or the median pharyngeal recess. Anat. Rec., vol. 6, pp. 373-404.
1918 On the anlage and morphogenesis of the chorda. dorsalis in Marnmalia. Anat. Rec., vol. 14, no. 4, pp. 217-264.
JOLLY, J. 1913 Sur les organes lymphoepitheliaux. C. R. Soc. Biol., '1‘. 74, no. 10, pp. 540-543.
KEIBEL, FRANZ 1889 Zur Entwicklungsgeschichte der Chorda. bei Siiugern (Meerschweinchen und Kaninchen). Arch. f. Anat. n. Physiol., Anat. Abt., S. 329-388.
KILLIAN, G. 1888 Ueber die Bursa und Tonsilla pharyngea. Eine entwickelungsgeschichtliche und vergleiehen-anatomische Studie. Morphol. Jahrb., Bd. 14, S. 618-711.
KING-SBURY, B. F. 1915 The development of the human pharynx. I. The pharyngeal derivatives. Am. J. Anat., vol. 13, no. 3, pp. 329-397.
1924 The developmental significance of the notochord (chorda dorsalis). Zeitsehr. f. Morphol. u. Anthropol., Bd. 24, S. 59-73.
KINGSBUEY, F. B., AND W. M. Rooms 1927 The development of the palatine tonsil: calf (Bos taurus). Am. J. Anat., vol. 39, no. 3, pp. 379-435.
KINGSLEY, D. W. 1924 Regressive structures and the lymphocyte. The plasma cell; its origin and development. A study of the mammalian nictitating membrane. Anat. Rec., vol. 29, no. 1, pp. 1-19.
LINCK 1910 Ueber die Geuese der Bursa pharyngea embryonalis. Zeitsehr. f.
Ohrenheilk. u. f. d. Krankh. d. Luftwege., Bd. 62, S. 158-181.
MAXIMOW, ALEX. A. 1927 Morphology of the mesenchymal reactions. Arch. of Path. and Lab. Med., vol. 4, pp. 557-606.
MAYER, A. F. J. C. 1842 Neue Untersuchungen aus dem Gebiete der Anatomie und Physiologic. Bonn.
MEYER, R. 1910 Ueber die Bildung des Recessus pharyngeus medius, Bursa pharyngea, in Zusammenhang mit der Chorda bei menschlichen Embryonen. Anat. Anz., Bd. 37, S. 449-453.
RAND, RUTH 1917 On the relation of the head chords. to the pharyngeal epithelium in the pig embryo, etc. Anat. Rec., vol. 13, no. 7, pp. 465-491.
RE1'1'ER.EB., E. 1893 Revue générale sur la part que prend Pepithelium a la formation de la bonrse de Fabricius, des amygdales et des plaques de Peyer. J. de l’Anat. et de la Physiol., année 29, no. 1, pp. 137-142.
SCHWABACB 1887 Ueber die Bursa pharyngea. Arch. f. mikr. Anat., Bd. 29, S. 61-74.
1888 Zur Entwiekelung der Rachentonsille. Arch. f. mikr. Anat., Bd. 32, s. 187-213.
S'r6HR., PH. 1891 Ueber die Mandeln und deren Entwickelung. Die Entwicke1ung des Adenoiden Gewebes der Zungenbalge und der Mandeln der Menschen. Anat. Anz., Bd. 6, S. 545-548.
TOURNEUX, F. AND J. P. 1912 Base cartilagineuse du crane et segment basilaire de la chorde dorsale. J. de l’Anat. et de la Physiol., année 48, pp. 57-105.
TOURNEUX, J. P. 1912 Bourse pharyngienn et récessus median de pharynx chez l’homme et chez le cheval, fossettes pharyngienn et naviculaire chez l’homme. J. de 1’Anat. et de la Physiol., année 48, pp. 516-544.
WALDEYEB. 1886 Beitriige zur normalen und vergleichenden Anatomic des Pharynx mit besonderer Beziehung auf den Schlingweg. Sitzungsber. d. k. Akad. d. Wissensch. zu Berlin, S. 233-250.
WEIDENREICH, FRANZ 1908 Ueber Speichelkiirperchen. Ein Uebei-gang von Lymphocyten in neutrophile Leukocyten. Folia Haematologica, Bd. 5, N o. 1, S. 1—7. 1909 Zur Morphologie und morphologische Stellung der ungranulier ten Leucocyten——-Lymphocyten—des Blutes. VI. Fortsetzung der “Studien fiber das Blut und die blutbildenden und zersttirenden Organe.” Arch. f. mikr. Anat., Bd. 73, S. 793-882.
Plates
Plate 1
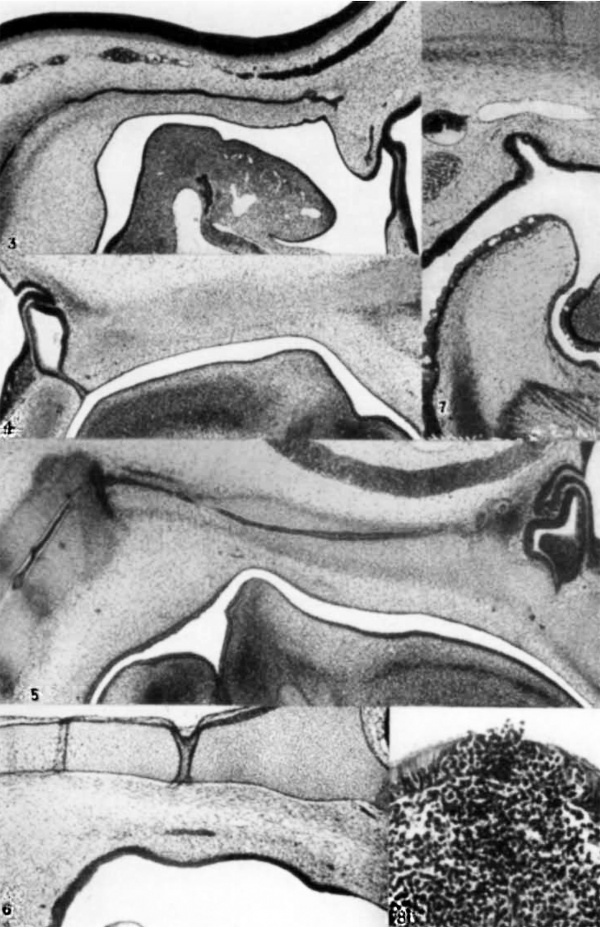
3 Cat; 7-mm. embryo. Median sagittal section, showing the anterior end and the entire pharyngeal extent of the notochord, in its relation to neural tube and pharynx. Hypophyseal region is at the right. X 375.
4 Cat; 12.5-mm. embryo. Nearly median section, showing the chondrifying basilar plate, etc. Hypophyseal region is at the left. X 37%.
5 Cat; 16~mm. embryo. Median sagittal section, showing notoehord, basilar plate, craniopharyngeal canal, etc. The anterior end of the notochord is cut twice. Hypophysis to the right. The hollow hypophyseal stalk appears two sections (20 ,u) away, roughly perpendicular to the pharyngeal epithelium which it joins. X 41.
6 Cat; 28»mm. embryo. Nearly median (parasagittal) section. Shown are perichondrium and fascia pharyngobasilaris, etc. Edge of hypophyseal fossa is at the right. Two artificial wrinkles of basilar plate are present. X 41.
7 Cat; 50-mm. embryo. Nearly median section. Caudal portion of pharyngeal region, to show an unusual bursal outpocketing. To the left is the transverse pharyngeal vein with the dorsal edge of the pharyngeal musculature immediately below. A longitudinal medial lymphatic channel is above the bursa. The basilar cartilage and perichondrium are at the top. Epiglottis occupies the lower portion. Caudal edge of the soft palate appears at the right. X 371}.
8 Cat; young adult. Vertical section of pharyngeal tonsil showing lymphatic infiltration of the ciliated epithelium over a nodule and escape of lymphocytes. X 285.
Plate 2
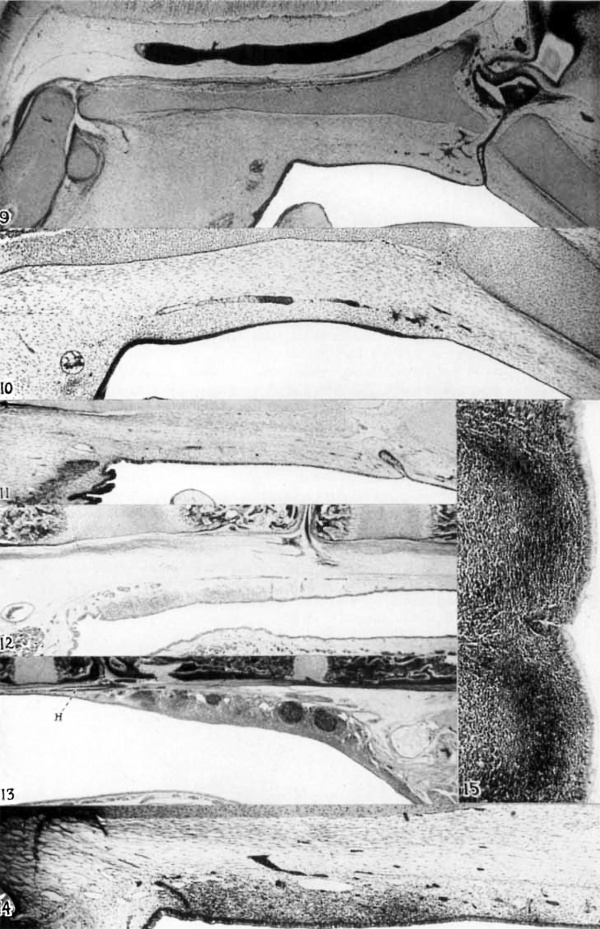
9 Cat; 35-min. embryo. Nearly median sagittal section to show the topography of the pharyngeal region shortly before tonsillar differentiation. From above downward appear: ventral edge of hindbrain, basilar artery, cartilaginous basis cranii, the pharyngeal region with transverse pharyngeal vein at the left and hypophyseal stalk and craniopharyngeal canal at the right. Dorsal edges of epiglottis and soft palate are below. X 22%.
10 The same. Parasagittal section of the pharyngeal region 60 ,u. to the side of the section above. Longitudinal veins bound the tonsillar region above. X 37:}.
11 Cat; 75-min. fetus. Nearly median section of the tonsillar region, at the beginning of tonsillar differentiation. Transverse pharyngeal vein and pharyngeal musculature at the left, hypophyseal stalk and craniopharyngeul canal at the right. ><22A_1..
12 Cat; 100—n1m. fetus. Nearly median section. Early differentiation of the tonsil. Above are os basioccipitale (left), os basisphenoidale with craniopha— ryngeal canal and included hypophyseal stalk, and 0s presphenoidale (right). Transverse pharyngeal vein and pharyngeal musculature at the left. Soft palate below. Hypophyseal stalk reaches the epithelium at a point about -3; inch from the right edge of the photograph. X 16%.
13 Kitten approaching sexual maturity (200 mm., occipital crest to root of tail). Nearly median sagittal section, cephalic end to the left. Ossification of basis cranii advanced. Transverse vein at right. H, diverticulum of hypophyseal stalk. Ventral end of canal above its caudal end. X 11.
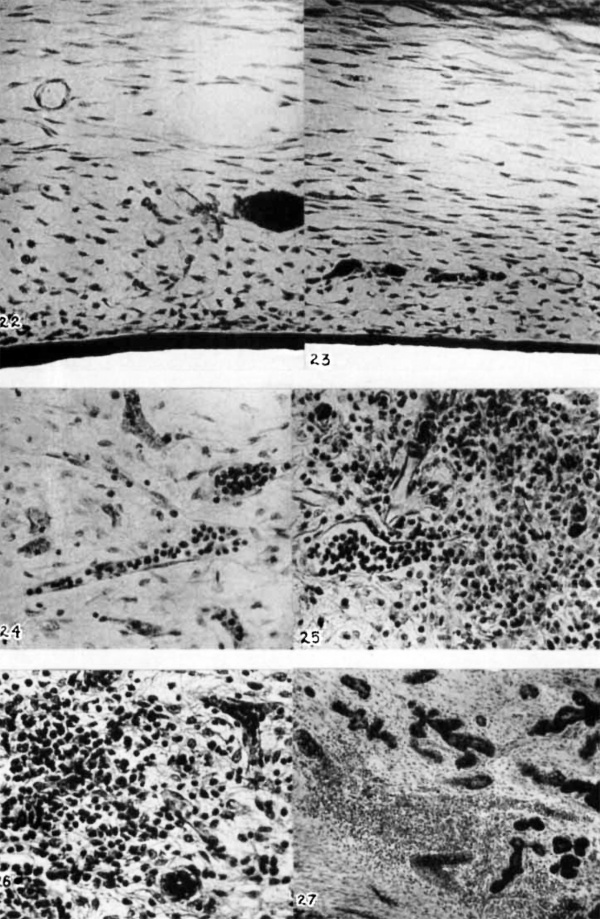
14 Cat; 85-to-90-mm. fetus. Sagittal section of developing pharyngeal tonsil. Cartilage of basis cranii above. Clear spaces are lymphatic channels. Darker areas, blood vessels and glands; a caudally c.urved gland appears toward the left. X 41.
15 Cat; young adult (310 mm., occipital crest to root of tail). Pharyngeal tonsil, surface epithelium (to the right) and superficial portions of two lymphatic nodules with crescents. Lymphatic infiltration of the epithelium in the depression near center. X 71:}.
Plate 3
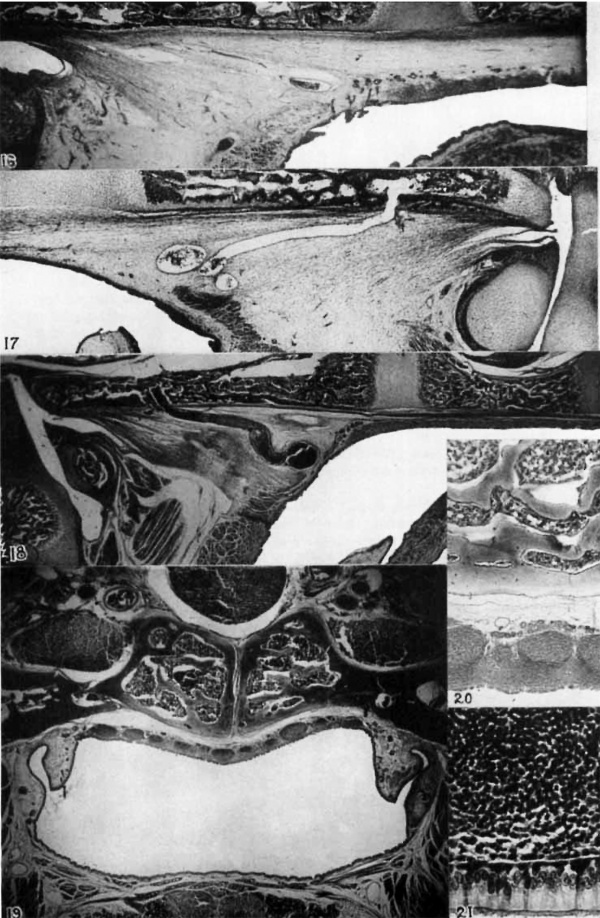
16 Cat; 100-mm. fetus. Nearly median section, caudal portion of tonsil, and fascia pharyngobasilaris. The occipital bone and basisphenoid (to the right) are above. Edge of vertebra I to the left. X 16%.
17 Cat; 83-mm. fetus. Nearly median section. Caudal portion of nasopharynx, cephalic aspect to the left. To show fascia pliaryiigobasilaris, basioecipital bone above, atlas and axis to the right. Tonsil not yet differentiated. Transverse pliaryngeal vein above dorsal edge of pliaryngeal musculature. X 22%.
18 Kitten (125 mm., occipital crest to root of tail). Nearly median section, showing fascia pliaryiigobasilaris. Relations as above, tonsil close to skull. X 11.
19 Kitten; nearly 111atu1'e (275 mm., occipital crest to root of tail). Transverse section through cephalic portion of pharyngeal tonsil. A portion of the hypopl1ysis is above; the basisphenoid with craniopliaryngeal canal underlies it. At each side of the nasopharynx the ostium tubae auditivae is shown. The soft palate is below. X 11.
20 The same, but a. more caudal level. A higher nxagnlficatioii of a portion of the tonsil underlying the base of the skull. X 22%.
21 Cat; young adult. Tonsil and pharyngeal epitlielimn (strati1‘ie.d and pseudostratified columnar ciliated). X 285.
Plate 4
22 and 23 Cat; 35-min. embryo. Three sections removed from that of figure 10. A comparison of the differentiation caudal to (fig. 22) and cephalad of (fig. 23) the hypophyseal stalk. The epithelium is below, the fibroblasts of the perichondrium are above. A large and a small lymph channel appear in figure 10; veins are filled with blood. X 240.
24 Cat; 75-mm. fetus. Frontal section of the tonsillar region. Two lymphatic radicles filled with lymphocytes. five blood channels surround the center of the picture. The photograph does not adequately distinguish erythrocytes from lymphocytes. X 285.
25 Cat; near term (130—mm. fetus). Frontal section of the tonsillar region. Lymphatic radicles filled with lymphocytes. The tonsil is now well differentiated. X 285.
26 Cat; 85—to—90~mm. fetus. Frontal section of tonsillar region. A single beginning lymphatic nodule, one of a number. All are broadly in relation to the epithelium. Blood vessels appear above and to the left of the nodule. A gland duct is beneath and to the right. X 285.
27 Cat; 100-mm. fetus. Frontal section through the tonsillar region, dorsal level. The fibrous differentiation (capsule) is at the lower left. Numerous glands are shown. The larger lymphatics are gorged with lymphocytes. Note particularly the junction of channels in the lower center. X 71.
Cite this page: Hill, M.A. (2024, May 1) Embryology Paper - The developmental significance of the mammalian pharyngeal tonsil - Cat. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_developmental_significance_of_the_mammalian_pharyngeal_tonsil_-_Cat
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G