Paper - The development of the hypophysis cerebri in man (1926)
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Atwell WJ. The development of the hypophysis cerebri in man, with special reference to the pars tuberalis. (1926) Amer. J Anat. 37: 139-193.
| Online Editor |
|---|
| Wayne J. Attwell (1889 - 1941) student of GC. Huber.
Note the historic term "entoderm" refers to endoderm, and we now know that this does not contribute to the hypophysis (pituitary). Pars tuberalis (pars tuberalis of the hypophysis) anatomically is the region of anterior pituitary (adenohypophysis) extending along the anterior and lateral surfaces of the hypophyseal stalk. Tuberalis principal cells are low columnar, with cytoplasm containing lipid droplets, glycogen granules, and some colloid droplets. Region also contains part of the hypophyseal portal system. In some species (sheep) melatonin acts on these cells through melatonin (MT1) receptors to regulate prolactin secretion. See also Atwell WJ. The development of the hypophysis cerebri of the rabbit (Lepus Cuniculus L.). (1918) Amer. J Anat. 24(2): 271-337
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of the Hypophysis Cerebri In Man, with Special Reference to the Pars Tuberalis
Wayne J. Atwell
Department of Anatomy, University of Buffalo
Twenty-Six Figures
Aided by grant no. 210 from the Bache Fund of the National Academy of Sciences.
Our knowledge of the development of the hypophysis cerebri has been based largely upon studies on the lower mammals and other vertebrate classes. There have been comparatively few attempts to study its morphogenesis in man. Rudel (’18) remarks on the brevity of the treatment allotted this important gland in the Keibel and Mall “Embryology.”
Recently a distinct third lobe of epithelial origin has been recognized and described by a number of writers. This lobe—the pars tuberalis of Tilney—is distinguished by a number of features (Atwell, ’18b) among which may be mentioned its paired origin, its characteristic position in relation to the diaphragma sellae, and its distinct histological structure.
Although the pars tuberalis has been clearly differentiated and its development traced in a number of lower forms, it has not been generally recognized as a distinct component of the human hypophysis. This has been due in part to the small size of the lobe in the fully developed gland, but perhaps more largely to the almost entire lack of careful and comprehensive morphogenetic studies for the hypophysis of man. The former confusion of the pars tuberalis with the pars intermedia (compare Lothringer’s (/86) term ‘Fortsatz dos lflpitlielsaums’ and Herring’s (’08 a) ‘tongue—like process of the pars intermedia’ to designate what we now know as the pars tuberalis) has not been entirely cleared away. This is evidenced by the following quotation from a recent work (Cowdry, ’22, p. 709): “Tl1e posterior, or intermediate portion begins to extend upward along the infundibular stalk and probably gives rise to the tissue which has been recently called the pars tuberalis.” Elsewhere, however (p. 707), this writer seems to relate the pars tuberalis to the anterior lobe: “The pars tuberalis, which is so distinct in lower forms, is only represented in man by an inconspicuous prolongation of the tissue of the anterior lobe along the infundibulum.”
This study was undertaken with the object of tracing rather completely the morphogenesis of all the lobes of the hypophysis in man, but with attention directed particularly to the little known pars tuberalis. A beginning was made while the writer was at the University of hlichigan, but the study has been largely carried out and brought to completion at the University of Buffalo. It has been delayed by the great amount of time required for the collection and preparation of the human embryos contained in the embryological collection of the latter institution.
My sincere thanks are due to Prof. G. Carl Huber for the loan of six embryos from his collection and for his continued interest in this study.
Historical - Pars Tuberalis
No attempt will be made here to abstract the voluminous literature on the general features of the development of the hypophysis. The more directly pertinent contributions have been referred to in a previous paper (Atwell, ’18). Rather complete bibliographies are to be found in Stendell’s monograph (’14) and in “Endocrinology and Metabolism” (’22, vol. 5).
It may be appropriate, however, to bring together the more important references to that lobe of the hypophysis which we now designate the pars tuberalis. That the lobe was seen by the earlier embryologists now seems certain, but its recognition as a distinct part of the gland embryologically and histologically has come but recently.
W. Miiller (’71), Mihalkovics (’75), and Kraushaar (’85) noted a ‘tongue-like’ extension of the hypophysis and called it merely the ‘anterior process’ of the hypophysis.
Lothinger (’86) observed a small process spread out under the tuber cinereum which he named the ‘Fortsatz des Epithelsaums auf den Trichter.’ The terms ‘vorderer Lappen’ and ‘vorderer Fortsatz’ have been employed by Haller at various times (’97, ’09, ’10) to designate a thin part of the hypopl1ysis which extends forward and is closely applied to the brain wall.
Salzer (’98) figured and described a solid anterior process which consists of glandular substance and which extends toward the optic chiasm.
Joris (’07) saw in the meninges of the brain a mass of glandular cells attached to the anterior end of the hypophysis, and extending from the base of the infundibulum to the optic chiasm. The cell mass divides into two branches. These diverge and make an angle, open posteriorly, to embrace the neck of the infundibulum. To this cell mass Joris gave the name of ‘lobule de la tige.’
Staderini (’08) speaks of a ‘lobus chiasmaticus’ which extends forward and a ‘lobus praemammillaris,’ the cells of which are within the brain coverings and which surround the infundibular neck.
Herring ( ’08 a) describes a portion of the hypophysis which extends forward and is closely applied to the brain wall. This part he terms the ‘tongue—like process of the pars intermedia,’ although he recognizes that it is distinctly more Vascular than the pars intermedia proper. Blair Bell (’19) proposes a slightly different terminology to designate this lobe. He illustrates (p. 68) ‘the reticulated portion of the pars intermedia’ of the cat in contrast to the more compact portion of the pars intermedia which ‘abuts on the cleft.’
Bolk (’10, ’17) and VVoerdeman (’14, ’18) speak of the ‘lobulus bifurcatus’ found in certain stages of primate embryos. This ‘lobulus bifurcatus’ or ‘gabellappen’ has its two prongs directed dorsocaudally to surround the neck of the infundibulum. One is struck by the similarity to J oris’ description of his ‘lobule de la tige.’ .
Tilney (’13) applied the term ‘pars tuberalis’ to the thin epithelial layer which surrounds the infundibulum and spreads out under the tuber cinereum extending from the mammillary bodies to the optic chiasm. Tilney further gives an account of the development of the pars tuberalis in the cat and the chick. He first clearly showed that the pars tuberalis is derived from a pair of lateral lobes or ‘tuberal processes,’ although a similar origin had been suggested by Gisi (’07) for certain reptiles.
That Tilney’s pars tuberalis is identical with the ‘lobule de la tige’ of Joris, Staderini’s ‘lobus praemammillaris’ and ‘lobus chiasmaticus,’ collectively, and with Bolk’s ‘lobulus bifurcatus’ has been suggested by Woerdeman (’14). Baumgartner (’16) and the Writer have concurred in this view. The writer (Atwell, ’18) has called attention also to the likelihood that Tilney’s ‘tuberal processes’ are identical with the ‘Lateralknospen’ seen by Gaupp (’93) in lizard embryos, the ‘lobi laterali’ noted by Chiarugi (’94) in Cavia cobaya, and by Bruni (’14) in the Sauropsida and the mammals, the ‘bourrelets lateraux’ of Weber (’98), the ‘Seitensprossen’ of Economo (’99) and the ‘lobuli laterali’ of Rossi (’96), Bruni (’14, ’16), and others.
Since Tilney described the development of the pars tuberalis in the cat and the chick, several authors have given more complete accounts of its ontogeny in several other forms. Among these may be mentioned Baumgartner (’16, reptiles), Parker (’17, marsupials), and the writer (’18 a, rabbit; ’18 b, Anura, and ’21, tailed amphibia).
To examine carefully the development of the hypophysis in man with a View to determining the ontogenetic history of the pars tuberalis has been one of the principal objects of the present study, as has been stated in the introductory paragraph.
Materials, Methods, and Terminology
This study is based upon twenty-three series of sections of human embryos and fetuses, ranging in size from 4.68 mm. to 285 mm. (C. R. or sitting height). Six of these series are from the collection of Professor Huber (table 1, ‘Huber Col.’). The remainder are contained in the University of Buffalo Embryological Collection (table 1, ‘U.B.E.C.’). In addition sagittal sections of the adult hypophysis were utilized.
Wax-plate reconstructions have been made from nineteen of the above series at magnifications of 200 diameters for the younger stages (up to 25 mm.) and 100 or 50 diameters for the older stages.
The crown-rump length, collection number, and plane of section of the embryos studied are given in table 1.
The terminology here used for the several lobes of the hypophysis is that previously employed by the writer (’18 a, et seq.). The parts of neural origin are the infundibulum, the neural stalk, and the neural lobe or processus infundibuli. The lobes arising from the epithelial or buccal portion are the pars anterior proprior, the pars intermedia, and the pars tuberalis.
Observations
Early development of the buccal lobe In the smallest human embryo available for this study (4.68 mm., U.B.E.C., no. 3) the buccal lobe of the hypophysis, Rathke’s pocket, is present as a shallow diverticulum. In transverse section (fig. 1) it shows rather distinct lateral angles. A waX—plate reconstruction made from this embryo fails to show any remains of the pharyngeal membrane. There is no means of determining where entoderm ceases and ectoderm of the mouth begins. Seessel’s pouch is not prominent. Both nasally and caudally, Rathke’s pocket flattens very gradually into the roof of the mouth invagination.
In a 9.5-mm. embryo (U.B.E.C., no. 15) Rathke’s pocket is well-formed, although its communication with the mouth cavity has not begun to be constricted (fig. 2). Closely similar conditions exist in a 10-mm. embryo (Huber Col., 110. II) except that a reconstruction shows some constriction near the mouth epithelium, particularly at the sides. Considerably more constriction is noticeable in a 10.5-mm. embryo (U.B.E.C., no. 26, figs. 3 and 12).
These three embryos, though apparently very closely staged, show important differences. In the 9.5-mm. and the 10—mm. embryos careful search has failed to reveal any definite evidences of the lateral lobes, which, as will be shown, are taken to be the anlagen of the pars tuberalis. In the 10.5—mm. embryos, however, protuberances, or ridges, on the sides of the hypophysial pouch near its attachment to the mouth epithelium are the first unmistakable evidences of the lateral lobes. This stage corresponds quite closely to that of the 12-day rabbit embryo described in a previous study (Atwell, ’18).
Diverticula are often found on the dorsal wall of Rathke’s pocket in these stages. One of these is shown in figure 2. Careful count shows that there are three such diverticula or growths in the 9.5-mm. embryo, while in the 10-mm. embryo there is only one on the dorsal wall. This one is not situated in the midline, but somewhat to the left. Another prominent epithelial bud projects directly lateralward, to the left, in this specimen. It has no mate on the opposite side. The 10.5—mm. embryo exhibits no fewer than four dorsal diVerticula. Some of these may have been in relation with the cephalic end of the notochord in earlier stages, but it does not seem probable that all are to be so interpreted. The great irregularity of these diverticula does not favor their interpretation as important components of the developing hypophysis. In the three embryos just described these diverticula are not found arranged in a definite anteroposterior series like those described by Bruni (’16), but are distributed from side to side on the dorsal wall or are irregularly placed.
Formatlion and fate of the epithelial stalk Further reduction in the size of the communication between the cavity of Rathke’s pocket and the mouth is seen in a 12—mm. embryo, and a 14-mm. embryo shows the stalk reduced In a 17—mm. embryo the stalk is still length, but in an 18.5—mm. specimen it to a cylindrical tube. patent throughout it is solid for most of its extent. The youngest of my specimens to show the epithelial stalk detached is the 19—mm. embryo (no. XXXIII, Huber Col. ).
Table 1
| C.R. Length in mm. | Collection and Number | Plane of Section |
|---|---|---|
| 4.68 | No. 3, U. B. E. C. | Transverse |
| 9.5 | No. 15, U. B. E. C. | Sagittal |
| 10 | No. 11, Huber Collection | Sagittal |
| 10.5 | No. 26, U. B. E. C. | Sagittal |
| 12 | No. 32, U. B. E. C. | Transverse |
| 14 | No. 46, U. B. E. C. | Sagittal |
| 16 | No. V, Huber Collection | Sagittal |
| 17 | No. 42, U. B. E. C. | Sagittal |
| 18.5 | No. 47, U. B. E. C. (Bentz, B.) | Transverse |
| 19 | No. XXXIII, Huber Collection | Sagittal |
| 22.5 | No. 28, U. B. E. C. | Transverse |
| 23 | No. 31, U. B. E. C. | Sagittal |
| 25 | No. 33, U. B. E. C. | Sagittal |
| 26 | No. 1, U. B. E. C. | Sagittal |
| 30 | No. XLVII, Huber Collection | Sagittal |
| 36.5 | No. 9, U. B. E. C. | Sagittal |
| 39 | No. XVII, Huber Collection | Sagittal |
| 45 | No. XVIII, Huber Collection | Transverse |
| 55 | No. 25,U.B.E.C. | Sagittal |
| 102 | No. 17, U. B. E. C. | Sagittal |
| 110 | No. 16, U. B. E. C. | Sagittal |
| 177 | No. 36, U. B. E. C. | Sagittal |
| 285 | No. 45, U. B. E. C. | Sagittal |
In a 22.5-mm. embryo (no. 28, U.B.E.C.), the stalk remains continuous with mouth epithelium, but does not connect with the gland through the sphenoid cartilage. In a 23—mm. embryo (no. 31, U.B.E.C.) a thin solid epithelial stalk is seen which is interrupted about halfway through the sphenoid. At 25 mm. (no. 33, U.B.E.C.) the interrupted stalk merely indents the sphenoid cartilage above and below. An epithelial mass of considerable size is present. at the point of attachment of the inferior remnant to the epithelium. This may be termed the ‘pharyngeal hypophysis.’ In a 26-mm. fetus (no. 1, U.B.E.C.) there is a short tapering epithelial stalk attached at the nasal margin of the gland.
There is an isolated remnant of the stalk immediately below the sphenoid and also a rather large remnant attached to the mouth epithelium. A 36.5—mm. fetus (no. 9, U.B.E.C.) shows remains of the stalk, attached both to the gland above the sphenoid and to the epithelium inferiorly. There is a large pharyngeal hypophysis in this specimen and also iii a 55—mm. fetus (no. 25, U.B.E.C.).
A 102—mm. fetus (no. 17, U.B.E.C) shows a pharyngeal hypophysis with what appears to be a development of glandular trabeculae. It lies in contact with the epithelium of the nasopharynx inferior to the sphenoid cartilage. VVithin the sella, however, the point of attachment of the stalk to the gland is identified later by the attachments of the pars tuberalis. This will be traced later and will be shown to proceed from the ventral to the nasal side of the gland and finally to lie dorsal to the gland only a short distance in front of the neck of the neural lobe. My sections from older fetuses do not contain the region of the epithelial stalk.
Differentiation of the Epithelial Lobes
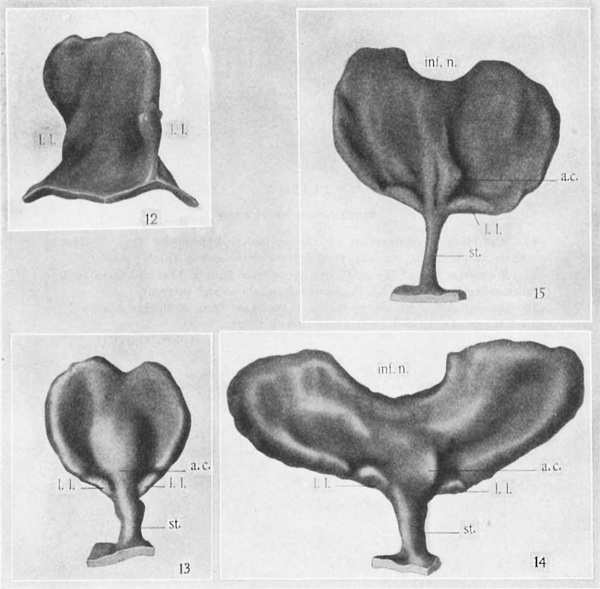
Wax-plate reconstructions of the epithelial hypophysis of 14-mm., 16—mm., and 17—mm. embryos are shown in figures 13 to 15, viewed from in front. It is to be noted that this portion of the gland is flattened and slightly concave on the surface which it presents toward the brain. The infundibular notch for the neural lobe becomes progressively deeper. There is a prominent protuberance forward in the midline not far from the attachment of the epithelial stalk. This I have called the ‘anterior chamber,’ in recognition of its similarity to the structure described by Gaupp, Bruni, and others. It must be emphatically stated, however, that this ‘chamber’ contains a cavity only in early stages of the human embryo (compare fig. 7).
The presence and position of the lateral lobes is of special interest, since they are to be traced to the formation of the pars tuberalis. At these stages the lateral lobes are to be found constantly at the ventral margin of the gland close to the attachment of the stalk (l.l., figs. 13 to 15). They exist as epithelial shelves thinner than any other part of the margin of the epithelial hypophysis. In position they correspond to the lateral lobes described by Chiarugi (’94) for the guineapig, by the writer (Atwell, ’18) for the rabbit, and to the tuberal process of the cat (Tilney, ’13). In sagittal sections a shelf-like lateral lobe is readily mistaken for a slightly oblique section of the epithelial stalk at its attachment to the gland (compare fig. 6). Reconstructions, however, show clearly the nature of the lateral lobes at this stage (fig. 15).
The growth of the neural lobe (figs. 4 and 5) is accompanied by an apparent flattening of the apex of Rathke’s pocket. The neural lobe then lies in the infundibular notch. The contact of the neural lobe with Rathke’s pocket is intimate from very early stages, and the portion of the hypophysial sack thus in contact with the neural lobe is the earliest beginning of the pars intermedia (fig. 5).
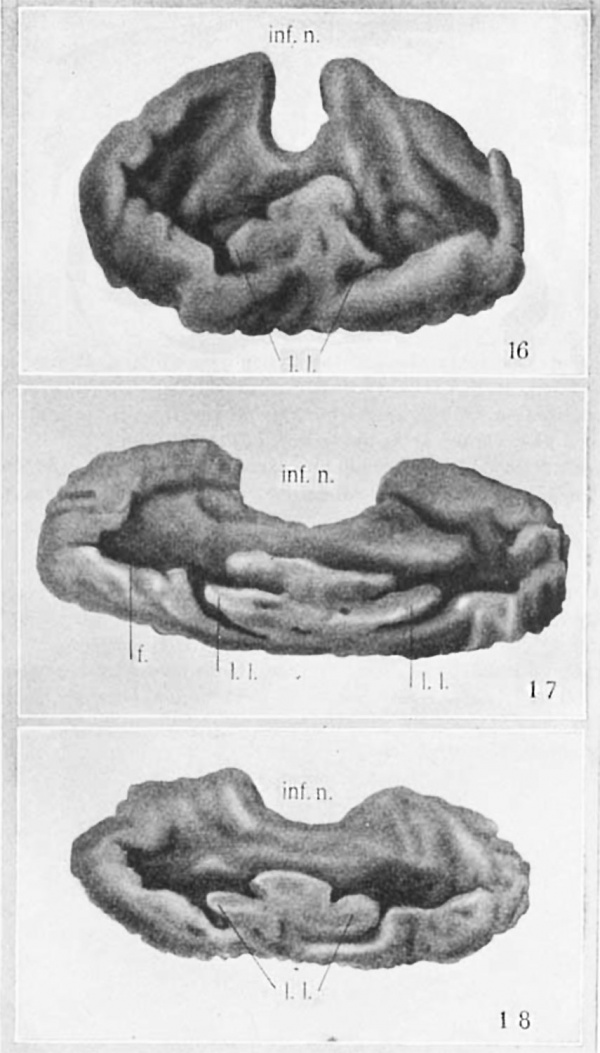
The slight concavity on the cranial side of the epithelial hypophysis now becomes much more marked. This ‘cupping’ of the gland with its cavity toward the brain floor is correlated with the separation of the epithelial stalk. Whether one factor is causal to the other can only be conjectured. The ‘cupping’ of the gland brings the lateral lobes nearer to the brain. They are seen at the nasal rim of the ‘cup’ (figs. 16, 17, 18), beyond which, at this stage, they do not project.
The presence of a ridge in the midline bearing at its nasal end the elevation which has been called the ‘anterior chamber’ divides the cavity of the hypophysial cup into two lateral compartments. These I have termed the ‘fossae’ (f., fig. 17). These fossae contain connective tissue (c.t., fig. 9), which is invaded by the trabeculae of the growing hypophysis. The two centers of connective tissue which result from thereduc— tion of the early fossae are readily discernible in frontal and transverse sections of the adult gland.
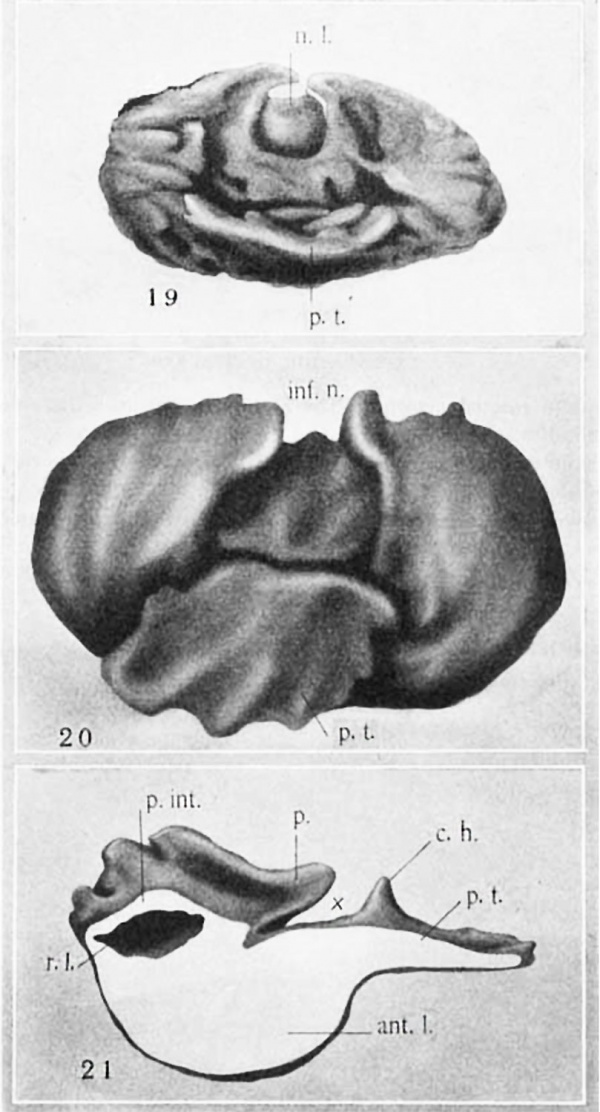
In a 4.5-mm. fetus (no. XVIII, Huber Col.) the hypophysis shows (fig. 19) the lateral lobes continuous with each other across the midlineiand forming a comparatively thin epithelial layer which projects slightly nasalward. The structure so formed may now be termed the pars tuberalis, since it is in proximity with the tuber cinereum and has begun to spread out under it.
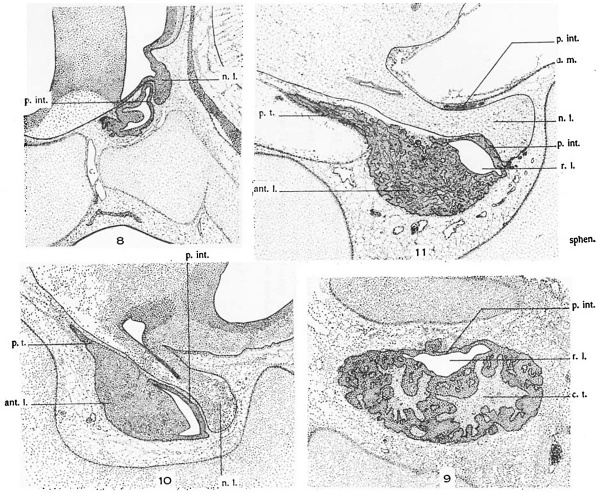
The pars tuberalisgrows forward rapidly (figs. 8, 10, 11, and 20). The entrance to the fossa on each side becomes constricted to a narrow slit or incisure (X, figs. 21 to 23), which persists for a. considerable time. This incisure is of the greatest importance as marking the boundary between the nasal limit of the pars intermedia and the caudal limit of the pars tuberalis.
Study of a reconstruction made from the 102-mm. fetus (no. 17, U.B.E.C.) proves highly instructive in tracing the further history of the pars tuberalis (figs. 21 to 23). This part is sharply delimited from the pars intermedia by the incisure (X, figs. 21 to 23). The pars tuberalis presents short horns which project caudalward and make the beginning of an attempt to surround the neck of the neural lobe. At this stage the pars tuberalis begins to merit the name ‘lobus bifurcatus’ in the sense in which that term was applied by Bolk and by Woerdeman.
These two caudally directed horns of the pars tuberalis must be distinguished carefully from the sturdier processes of the pars intermedia which grow dorsally around the neck of the neural lobe (p., figs. 21 to 23; see also figs. 24 and 11). The latter have been present from much earlier stages and are formed by upgrowth of the margins of the infundibular notch. VVhile these are called processes of the pars intermedia, it is to be realized that they contain extensions of the residual lumen of Ratl1ke’s pocket and that onlythe wall in contact with the neural lobe develops true pars intermedia structure. The outer wall differentiates into typical anterior-lobe tissue.
The pars intermedia develops from the dorsocaudal tip of Rathke’s pocket. Its limit is first indicated when the neural lobe comes into contact with Rathke’s pocket, soon causing it to be indented. The extent of the pars intermedia is gradually increased for a time by the growth of the neural lobe, which thus apparently comes to be in contact with more of the wall of Rathke’s pocket. Also, there is the above—noted growth of the pars intermedia dorsalward by sending a process around each side of the neural lobe, especially at its neck (p., figs. 21 to 23). The pars intermedia has its free surface facing the residual lumen of Rathke’s pocket. A portion of the residual lumen nearest the epithelial stalk disappears early, but the remainder serves to separate the pars i.nter— media from the anterior lobe proper, at least until later fetal stages in man. The lumen may almost entirely disappear in later adult life, but its presence during all stages of development is important in distinguishing between the pars intermedia and the pars tuberalis. Developmentally, the pars intermedia may be defined as that portion of the wall of Rathke’s pouch which early comes into contact with the neural lobe, remains relatively thin and epithelium like, faces the residual lumen, and does not become strongly vascularized. The pars tuberalis, as has been indicated, has quite a different derivation. It arises at quite the opposite end of Rathke’s pocket, that is, near the attachment of the pouch to the mouth epithelium, from a pair of lateral lobes. Due to the ‘cupping’ of the hypophysis with its concavity toward the brain after the severance of the epithelial stalk, the lateral lobes come to lie close to the brain floor not far from the infundibulum. They meet across the midline and grow forward and backward surrounding the infundibulum and spreading out on the tuber cinereum as a thin epithelial layer.
The lateral lobes may early contain cavities continuous with the cavity of Rathke’s pocket. These are lost very early, however, and thereafter the lateral lobes and the pars tuberalis which they form are never adjacent to the residual lumen. The remainder of the epithelial hypophysis becomes the anterior lobe proper. It constitutes by far the major portion of the gland. In embryonic and early fetal stages its greatest diameter is from side to side.
While this study has not been concerned primarily with the histological differentiation of the several parts of the hypophysis, it is of interest to note that certain distinctions are discernible in fetal stages. Vascularization of Rathke’s pocket and coincident trabecula formation, which is noticeable from the 18.5-mm. stages, does not involve the pars intermedia, which remains a thin epithelial layer, of practically uniform thickness, with few vessels. In the 102-mm. fetus the pars tuberalis begins to stain differently from the anterior lobe proper, which it previously most resembled. From early stages the pars tuberalis is distinctly more vascular than the pars intermedia—a feature which is one of the characteristics of its adult histology.
Discussion
Entodermal origin of a part of the hypophysis
- Online Editor - Note the historic term "entoderm" refers to endoderm, and we now know that this does not contribute to the hypophysis (pituitary).
The question as to the part played by the entoderm in the formation of the hypophysis has been renewed by Bruni (’16), who states that the material destined to form the glandular portion of the human hypophysis is represented by four diverticula, which in order anteroposteriorly in the roof of the oropharynx are: 1) a diverticulum marking the cavita anteriore (Vorraum) of Woerdeman; 2) the pouch of Rathke; 3) the diverticolo medio, and, 4) the pouch of Seessel. The first two mentioned form in front of the pharyngeal membrane and belong to the ectoderm, while the other two, forming behind the pharyngeal membrane, belong to the entoderm.
It must be noted that the smallest embryo utilized by Bruni in his study of the development of the human hypophysis was 7 mm. long. This stage would seem much too late to deter mine accurately the line of separation between ectoderm and entoderm.
The material for the present study, lacking as it does stages before, during, and just after the rupture of the oral membrane, is frankly inadequate to furnish an answer to this question. A careful examination of the smaller embryos, beginning with 4.68 mm., fails to yield any convincing confirmation of Bruni’s views. Attention has been directed in a previous paragraph to the great variability in the form and location of diverticula from the dorsal and even lateral walls of Rathke’s pouch. I have been unable to satisfy myself that Seessel’s pouch becomes incorporated with or even makes a considerable contribution to the developing human hypophysis.
The origin of the pars tuberalis
The smallest human embryo in which I have been able to identify the lateral lobes is 10.5 mm. (U.B.E.C., no. 26), and here they are not strongly indicated. In slightly younger stages (9.5— and 10-mm. embryos) the lateral lobes are not evident, at least in sagittal sections and reconstructions from these sections. It is possible that transverse or frontal sections of these and even younger embryos might yield indications of the lateral lobes, or ridges, as they are in the 10.5-mm. stage. In the 4.68-mm. embryo, which is sectioned transversely, the sharp lateral angles of the buccal hypophysis are the only structures which might be identified as the beginnings of the lateral lobes. I have not seen, in these younger embryos, the definite ‘tri—partite’ hypophysis fundament spoken of by VVoerdeman (’14).
Tilney (’13), from his studies on the development of the pars tuberalis iii the chick and the cat, concluded that the tuberal processes (lateral lobes) arise comparatively late in development. Atwell and Sitler (’18) later showed, however, that the lateral lobes may be identified very early in the chick and traced continuously to the formation of the definitive pars tuberalis.
Woerdeman (’14) holds that phylogenetically the lateral lobes of the mammals are derived from the ventral sacs of the elasmobranch hypophysis, and are homologous with the ‘lateral—knospen’ of the reptile hypophysis (Graupp, ’93). In tracing the comparative development of the hypophysis through the vertebrate classes Woerdeman called attention to tl1e lack of morphogenetic studies on the hypophysis of the telcost fishes and of the amphibia. The data for the amphibia have since been supplied (Atwell, ’18 b, ’21), but have not thrown much light on the question of the early origin of the lateral lobes, due largely to the fact that in these forms the epithelial hypophysis is solid at all stages of development.
Woerdeman has held that the lateral lobes arise separately from Rathke’s pouch and are later added to it. Rathke’s pouch proper, then, would be only that part of the hypophysial evagination dorsal to the attachment of the lateral lobes. The early stages of the lateral lobes in man, however, are so much reduced and the evidence that these lobes arise separately from the central hypophysial pouch is so insufficient that l have retained the term Rathke’s pouch for the entire eVagination.
As regards the formation of the definitive pars tuberalis from the several parts of the more primitive vertebrate hypophysis, 1 cannot agree with Woerdeman (’14) that the anterior part of the pars tuberalis is derived from the ‘Vorraum.’ In the rabbit (Atwell, ’18) distinct paired nasal horns are seen which later fuse to form the nasal end of the pars tuberalis. A similar process, although somewhat less clear—cut, takes place in man. Tt appears, then, that the pars tuberalis is derived almost entirely from the lateral lobes. The ‘Vorraum,’ or anterior chamber, very much reduced, is probably located at the place where the pars tuberalis is attached to the anterior lobe proper.
The meningeal relations of the hypophysis
The relation of the hypophysis to the fold of dura mater known as the diaphragma sellae is well known. The relations of the pia mater seem to have been less frequently discussed. Stendell (’14) and lVoerdeman (’18) both represent the pia as investing only the tissue of neural origin. Their figures show it as surrounding the infundibulum and the neural lobe, and separating the pars intermedia from the latter. This statement seems to be based largely upon theoretical considerations.
Koller (’22) advances another somewhat theoretical View to explain the varying relation of the diaphragma sellae in man and several of the domestic animals. He does not discuss the relations of the leptomeninges. Positing that the connective tissue which is to form the dura mater early lies between neural and epithelial lobes, Koller states that this is later perforated to permit the intimate union of neural lobe and pars intermedia which occurs in the so many animals.
Attention has been directed by Woerdeman (’18) and the writer (Atwell, ’18 b) to the fact that While the anterior lobe proper, the pars intermedia, and the neural lobe are contained in the sella turcica under cover of the diaphragma, the pars tuberalis (lobulus bifurcatus, Bolk) lies superior to the diaphragma, embedded in the pia mater of the brain floor. Arachnoid spaces thus intervene between the pars tuberalis and the superior surface of the diaphragma.
Cowdry (’22) gives diagrammatic figures (p. 706), based upon a survey of the literature, which show the dura lining the sella, and the pia surrounding the neural lobe only. He does not trace the arachnoid into the sella.
Lewis and Knavel (’13) state that occasionally “the arachnoid passes below the diaphragm of the sella to spreadvover the surface of the hypophysis.” In such cases there is greater danger of meningitis through opening the subarachnoid space during operation.
Recently Hughson (’22, ’24) has shown by two methods of injection that spaces which communicate with the general arachnoid spaces of the brain surround the entire hypophysis. The obvious conclusion would seem to be that the sella turcica is lined by all three cranial membranes instead of by merely the parietal layer of the dura, as has been the general teaching.
The normal circulation of cerebrospinal fluid through these spaces must be very slow. In order to infiltrate them with his injection fluids, it was necessary for Hughson either to increase the normal pressure by 50 to 80 mm. of the fluid or to reduce the normal cerebrospinal fluid pressure to below zero (by intravenous injection of a strong solution of sodium chloride), and thus cause the injection fluid to be drawn in. Simple replacement of the cerebrospinal fluid by injection fluids seemed inadequate to cause entrance into the spaces of the sella.
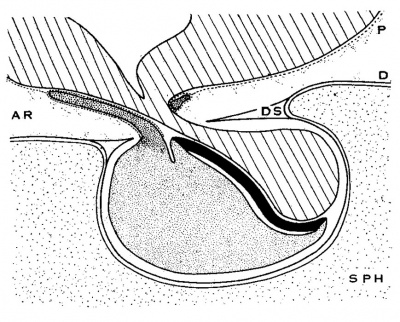
Text fig. A Diagrammatic sagittal section of hypophysis, showing relation of pars tuberalis to the meninges. Lined, brain floor and pars neuralis; fine stipple, pars anterior proprior; coarse stipple, pars tnberalis; solid black, pars interniedia. SPH, sphenoid bone; P, pia lnator; D, (lura; DS, diaphragma sellae; AR, arachnoid spaces.
Even if Hughson’s view be accepted, it in no way invalidates the usefulness, for general descriptive purposes, of speaking of the pars tuberalis as “embedded in the pia mater of the infundibular and brain floor.” This, it would seem, serves to locate the pars tuberalis rather definitely on the cranial side of the diaphragma sellae and thus to differentiate it in its position from the pars intermedia and the pars anterior proprior.
Text figure A represents the relations of the hypophysis to the dura mater and of the pars tuberalis to the pia mater. No attempt has been made to represent the pia inside the sella, either according to the theoretical View advocated by Steudell and others or according to the recent view of Hughson.
The pathology of the pars tuberalis
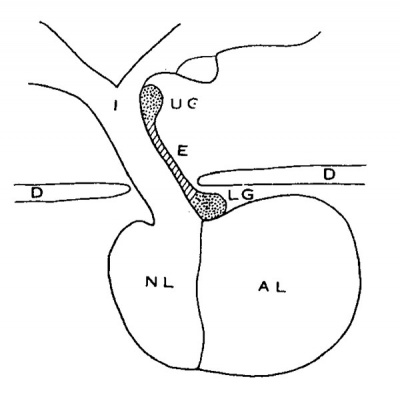
Erdheim (’04) has recognized a Very definite type of tumor in the region of the hypophysis which is said to develop from ‘rests’ of the embryonic craniopharyngeal duct. Simonds (’22) gives a diagrammatic figure to show the place of origin of two Varieties of this type of tumor. His figure (redrawn) has been reproduced as text figure B. As described by Simonds: along the anterior surface of the infundibulum there is a thin layer, or tongue—like process of cells, extending upward from the anterior lobe. This process ends high up on the infundibuluni in an ‘endswelling’, which in about eighty per cent of adults contains small groups of squamous cells on the front surface of the anterior lobe at the place of attachment of the infundibular process. Such squamous cells are not found elsewhere in the anterior lobe. The tumors described and differentiated by Erdheim originate from one or the other of these two groups of squamous cells. Their location, and their gross and microscopic appearance are characteristic and pathognomonic.
Text fig. B Diagrammatic mid-sagittal section of the hypophysis, o show the origin of Erdhelm’s craniopharyngeal duet tumors (after Simonds), nasal end at the right. AL, anterior lobe; NL, neural lobe; I, infundibulum; E, epithelial layer (pars tuberalis) extending upward on anterior surface of the infundibulnm; UG, upper group of cells; LG, lower group of cells.
It is recognized that these tumors vary somewhat, depending upon Whether they are benign or malignant and whether they originate from the upper or the lower group of cells.
From an embryological viewpoint, it is apparent at once that the epithelial layer described above is the pars tuberalis of the hypophysis. It is necessary to hold clearly in mind the fact that only the lower groups of cells marks the site of the earlier attachment of the epithelial stalk or ‘eranio— pharyngeal duct.’ It seems quite likely, in the opinion of the writer, that two different kinds of tumors are grouped under the single classification given above, and that growtlis arising from the upper group of cells (and perhaps a certain number of those from the lower group) are to be regarded as tumors of the pars tuberal.is.
The region of the lower cell group is of further interest when considered embryologically. In addition to marking the site of attachment of the pars tuberalis to the anterior lobe and the point of attachment of the epithelial stalk, it locates the earlier position of the ‘anterior chamber’ or ‘Vorraum.’ It seems quite possible that this lobe, which is important in the fishes, but decadent in the highest vertebrates, may occasionally contribute to tumor formation.
A careful analysis of tumors arising in the neighborhood of the infundibulum, in the light of the embryology and the histology of the pars tuberalis, should be productive of a clearer understanding of their differences.
Summary
- The development of the hypophysis has been studied by serial sections and reconstructions from twenty-three human embryos and fetuses ranging from 4.68 mm. to 285 mm. crown-rump length.
- The earliest appearance of the paired lateral lobes which form the pars tuberalis has been observed in a 10.5-mm. embryo. At this stage the lateral lobes exist as ridges at the anterolateral angles of the hypophysial evagination near its attachment to the mouth epithelium.
- In stages in which the epithelial stalk is a small tube or is solid (14-, 16-, 17-, and 18.5-mm. embryos) the lateral lobes are constantly identified as a pair of thin epithelial shelves located one at each side of the attachment of the epithelial stalk to the body of the gland. In sagittal sections a shelf is readily mistaken for a slightly oblique section of the epithelial stalk.
- Following the separation of the epithelial stalk and the ‘cupping’ of the epithelial hypophysis with its concavity toward the diencephalic floor, the lateral lobes lie at the nasal brim of the hypophysial cup.
- The lateral lobes have fused across the midline forming the pars tuberalis, and this has begun to grow forward by the 45—mm. stage. The pars tuberalis grows forward and backward, surrounding the infundibulum and spreading out under the tuber cinereum.
- The pars intermedia develops from the apex of Rathke’s pouch and lies in relation to the residual lumen, at least until late fetal stages. The pars tuberalis, on the other hand, is never in relation to the cavity of Rathke’s pouch after very early stages.
- A deep incisure serves to separate the pars tuberalis from the pars intermedia until late fetal stages.
- It seems not unlikely that certain of the tumors described by Erdheim as arising from ‘rests’ of the craniopharyngeal duct are in fact tumors of the pars tuberalis.
Bibliography
Atwell WJ. The development of the hypophysis cerebri of the rabbit (Lepus Cuniculus L.). (1918) Amer. J Anat. 24(2): 271-337
- 1918b The development of the hypophysis of the Anura. Anat. Rec., vol. 15, pp. 73-92.
- 1921 The morphogenesis of the hypophysis in the tailed amphibia. Anat. Rec., vol. 22, pp. 373-390.
ATWELL, WAYNE J., AND SITLER, IDA 1918 The early appearance of the anlagen of the pars tuberalis in the hypophysis of the chick. Anat. Rec., vol. 15, pp. 181-187.
BAUMGARTNER, E. A. 1916 The development of the hypophysis in reptiles. Jour. M0rph., vol. 28.
BELL, W. BLAIR 1919 The pituitary. VVm. VVood, New York.
BOLK, L. 1910 Over de ontwikkeling der Hypophyse van den Primaten in ’t bijzonder bij Tarsins en den Mensch. Verslzig Kon. Akad. V. VVetensch., Amsterdam.
- 1917 Same title. Nede1'l.'indsch. Tijdschr. G'O1’1(‘(‘Sk., vol. 2, pp. 667-675.
BRUNI, ANGELO CESARE 1914 Sullo svilnppo (lel lobo ghiandolare dell’ ipofisi negli Amnioti. Internut. Monatsehr. f. Anat. u. Physiol., Bd. 31.
- 1916 Sullo sviluppo della porzione ghiandolare dell’ ipofisi nc-ll’ uomo. Arch. ital. di anat. e di e1nbrio]., vol. 15, pp. 139-189.
CHAKUGI, G. 1894 Sull’ esistenza 'di una gemma bilaterale nell’ abbozzo della ipofisi dei inammiferi. Monitore zool. ital., vol. 5, pp. 184-188.
COWDRY, E. V. 1922 Endocrinology and Metabolism, vol. 1, p. 705, et seq.
ECoNoMo, CONSTANTIN J. 1889 Zur Entwioklung der Vogelhypopliyse. Sitz— ungsber. (1. k. Akad. d. W'issenscl1., Bd. 108, Teil 3, S. 281.
ERDHEIM, J. 1904 Ueber Hypophysenganggesehwiilste und Hirneholesteatome. Sitzungsber. d. k. Aknd. d. Wissenscli. in Wien, Bd. 113, S. 537.
GAUPP, E. 1893 Ueber die Anlage der H_\'pophyso bei Saurien. Arch. f. mikr, An:1t.., Bd. 42, S. 569.
GISI, JULIA 1907 Due Gehirn V011 Hatteria punctata. Diss., Basel.
HALLER. B. 1897 Untersuchungon iiber die Hypophysc und die lnfundibula1'— organe. Morph. Jahrb., Bd. ‘.35.
- 1909 llber die Hypophyso niederer Plaeentalier und den Saeviis vasculosus der urodelen Amphibien. Arch. f. Inikr. An:1t., Bd. 7-}, S. 812.
- 1910 Uher (‘lie ()ntogenese des Succus vasculosus und der l'l_\'p0pl1ysc der S‘.'Lngetie1'e. Aunt. Anz., Bd. 37, S. 242.
Herring PT. The histological appearances of the mammalian pituitary body. (1908) Quar. Jour. Ex. Physiol. 1: 121-159.
- 1908b The development of the mammalian pituitary and its morphological significance. Ibid., p. 161.
HUGHSON, WALTER 1922 Meningeal relations of hypophysis eo:-rebri. (Ab~ stmct.) Anat. Rec., vol. 23, p. 21.
- 1924 Meningeal relations of the liypophysis eereln-i. Bull. Johns Hopkins H0sp., vol. 35, pp. 232-234.
Joms, H. 1907 Contribution :1 l’étu<le de l’hypophyse. Mém, cou1-_ autr, pub], Aead. Roy. Med. Belgique, T. 19, fasc. 6.
KOLLER, RAPHAEL 1922 Zur vergleiclieildeil Anatomic der Hypophysennn1ge~ bung. Ztschr. f. d. ges. Anat,., 1 Al)t., Ztsclx. f. Anat. u. Entwicklngmechn., Bd. 65, S. 183-203.
KRAUSHAAR, R. 1885 Entwieklung der Hypophysis bei Nagethieron. Zeitschr, f. wiss. Zoiil., Bd. 41, S. 79.
LEWIS, DEAN, AND KNAVEL, A. B. 1913 Surgery of the Hypophyse (pituitary gland). ln Keen ’s Surgery, vol. 6.
LOTHRINGER, SALOMON 1886 Untersuchungen an der Hypophyse einiger Stingethiere und des Menschen. Arch. f. mikr. Anat., Bd. 28, S. 257.
DE MEURON, MARCEL 1915 Observations snr 1’Hyp0pl1yse, a l’état embryonnaire chez l’Homme et chez quelques Mammiféres. These, Lausanne.
v. MIHALKOVICS, VICTOR 1875 Wirbelsaite und Hirnanhang. Arch. f. mikr. Anat., Bd. 11, S. 389.
MULLER, ‘W. 1871 Uber Entwieklung und Bau der Hypophysis und des Proeessus infundibuli. Jenaische Zeitschr. 1:‘. Med. 11. Naturwiss., Bd. 6, S. 354.
PARKER, KATHERINE M. 1917 The development of the hypophysis cerebri, pi-e« oral gut and related structures in the Marsupialia. J. Anat., "01. 51.
RUDEL, E. 1918 Formentwickelung der menschlichcn Hypophysis cerebri. Anat. Hefte, Bd. 55, S. 187. Rossl, U. 1896 Sui lobi laterali della ipopfisi. Monitore zool. ital., vol. 7, pp. 240-243.
SALZER, HANS 1898 Zur Entwicklung der Hypophyse bei Sfiugern. Arch. f. mikr. Anat., Bd. 51, S. 55.
SIMONDS, J. P. 1922 Endocrinology and metabolism, vol. 1.
STADERINI, RUTILIO 1908 Di un prolungamento ghiandolare dell’ ipofisi accolto in uno speciale recesso pre-mammillare nel cervello del gatto adulto. Anat. Anz., Bd. 33, S. 271.
STENDELL, WALTER 1913 Zur vergleichenden Anatomie und Histologie der Hypophysis cerebri. Arch. 1’. mikr. Anat., Bd. 82. 1914 Die Hypophysis eerebri. Bd. 8 in Oppel’s Lehrb. d. vergleieh. mikr. Anat. d. Wirb. Fischer, Jena.
TILNEY, FREDERICK 1913 An analysis of the juxta-neural epithelial portion of the hypophysis cerebri with an embryological and histological account of an hitherto undescribed part of the organ. Internat. Monatschr. f. Anat. u. Physiol., Bd. 30.
TOURNEUX ET SOULIE 1898 Sur les premier développements de la pituitaire chez l’homme. Compt. rend. Soc. de bio1., ser. 10, T. 5, p. 896.
WEBER, A. 1898 Observations sur les premieres phases du développement de Phypophyse ehez les Chéiroptéres. Bibliogr. Anat., fase. 3.
WOERDEMAN, MARTIN W. 1914 Vergleichende Ontogenie der Hypophysis. Arch.’ f. mikr. Anat., Bd. 86. 1917 Overontwikkelingsverschijnselen in het hypophysegebied. Nederlandseh. Tijsehr. v. Geneesk., vol. 1, pp. 955-963.
- 1918 Over een weinig bekend Gedeelte der Hypophyse. Ibid., vol. 1, pp. 215-221.
Plates
Plate 1
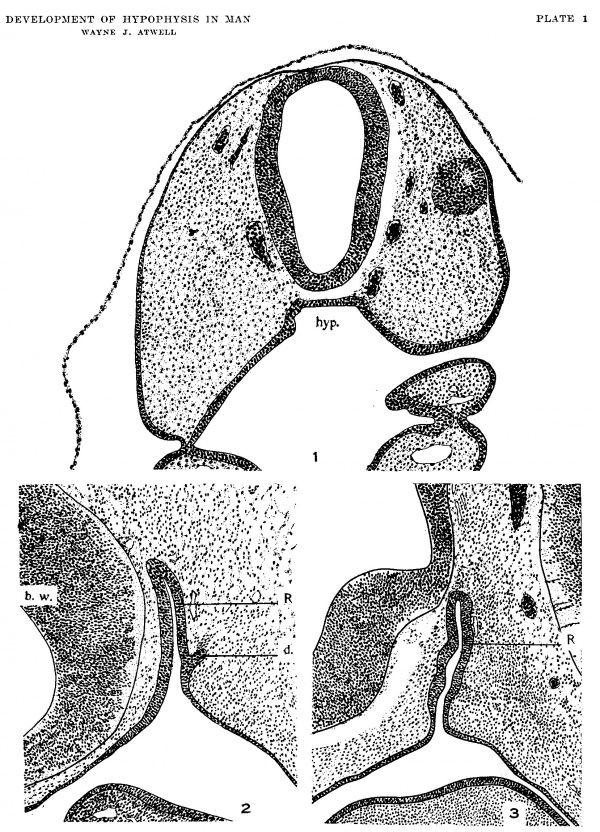
1 Transverse section, showing the oral hypophysis in a 4.G8—n1m. human (‘l]lbl'_V() (U.B.E.C., no. 3). X 100.
2 Sagittal section through hypophysis region of a 9.5~m1n. human embryo (U.B.E.C., no. 15). Nasal end at left. X 100.
3 Sagittal section through hypophysis region of 10.5-mm. human elnbryo (U.B.E.C., no. 26). Nasal end at left. X 100.
Abbreviations b.w., brain wall h_1/1)., hypophysis relation 1x’., Rathke’s pouch with ru1te1'ior extremity of notor-hard (1., divorticulum, perhaps in
Plate 2
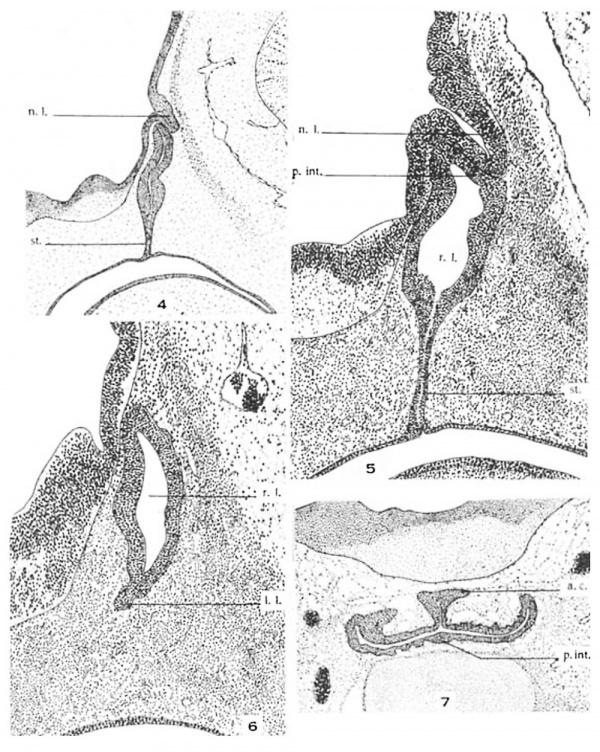
4 Sagittal section through hypophysis region of a 14-mm. human embryo (U.B.E.C., no. 46). Nasal end at left. X 50.
5 Midsagittal section through hypophysis region of a 17-mm. human embryo (U.B.E.C., no. 42). Nasal end at left. X 100.
6 Paramedian sagittal section through hypophysis of 3. 17-mm. human embryo (U.B.E.C., no. 42). Nasal end at left. X 100.
7 Transverse section through hypophysis region of a 22.5—mm. human embryo (U.B.E.C., no. 28). X 50.
Abbreviations a.c., anterior chamber p.1'nt., pars intermcdia l.l., lateral lobe r.l., residual lumen of Rat11ke’s pouch n.I., neural lobe st, epithelial stalk
Plate 3
8 Sagittal sevtion through hypophysis region of a 26-mm. human (U.B.E. 1, no. 1). Nasal end at left. X 30.
9 Transverse section through hypophysis of a 45-min. human L'I11l)1‘y0 Collection, no. XVITI). X 30.
10 Sagittal section through hypophysis region of a 55-mni. human (U.B.E. C., no. 25). Nasal end at left. X 30.
11 Sagittal section through hypopllysis region of a 102-mm. human (U.B.E.C., no. 17). Nzisul end at left. X 30.
Abbreviations m1t.l., anterior lobe c.t., (‘0I1l1C‘(‘tiV(‘, tissue p.int., pars intermedia p.t., pars tuberalis enlbryo (Huber embryo embryo rl.m., dura mater of diaphragina sellac r.l., residual lumen of Ratl1ke’s pouch 1z,.l., neural lobe sphm., sphenoid cartilage
Plate 4
12 Wax-plate reconstruction of epithelial hypophysis from a human embryo of 10.5 mm. (U.B.E.C., no.26), viewed from in front. X 65.
13 Reeonstruetiori of the epithelial hypophysis from a 14—mm. human embryo (U.B.E.C., no. 46), viewed from in front. X 65.
14 Roconst1'uetio11 of the epithelial hypophysis from a 16-mm. human embryo (Huber collection, 110. IV), viewed from in front. X 50.
15 Reoonstruetion of the epitllelial hypophysis from a 17—mm. human embryo (U.B.E.C., no. 42), viewed from in front. X 65.
Abbreviations a.c., ‘anterior chamber’ l.l., lateral lobe «L, diverticulum st, epithelial stalk 1'nf.rI .. infundibular notch
Plate 5
16 Wax~platc reconstruction of the epithelial hypophysis from a. 25-mm. human fetus (U.B.E.C., no. 33), viewed from above and in front. X 65.
17 Reconstruction of the epithelial hypophysis from a 19—mm. human embryo (Huber collection, no. XXXIH), viewed from above and in front. X 50.
18 Reconstruction of the epithelial hypophysis from a 26-mm. human fetus (U.B.E.C., 110. 1), viewed from above and in front. X 50.
Abbreviations f., fossa; inf.n., infundibular notch; l.l., lateral lobe 188
Plate 6
19 Wax-plalte reconstruction of the hypophysis from :1. 45-mm. human fetus (Huber collection, no. XVIII), viewed from above and in front. X 35.
20 Reconstruction of the epithelial hypophysis from a 55»mm. human fetus (U.B.E.C., no. 25), viewed from above and in front. X 35.
21 Reconstruction of epitllclial hypophysis from a 102-mm. human fetus (U.B.E.C., no. 17), viewed in rnidsagittal section; nasal, and at the right. X 35.
Abbreviations (ml.l., an‘ro1'ior lobe p.int., pars intermedizi p.t., pars tuberalis rJ., residual lumen xx, incisure separating pars interme-di:1 and pars tuberalis 0.11.. ca,u<la,lly directed horn of pairs tuboralis i71f.n., infuiidibular 110t(‘.ll 11.1., neural lobe 1)., process of pars interinedizi g1'0win;_r up around neck of neural lobe 1:90
Plate 7
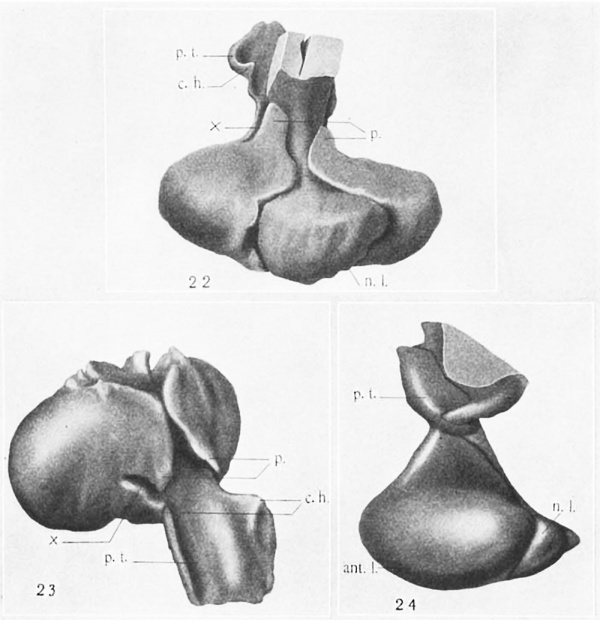
22 Wax-plate reconstruction of the hypophysis from a 102-mm. human fetus (U.B.E.C., no. 17), viewed dorsally and from above. X 35.
23 The same model as shown in figure 22 with the neural lobe l‘(’Ill0\’('(l, viewed from above and in front. X 35.
24 R4-vonstrtwtion of tho lxypophyeis from 9. human fetus of 28.5 cm. (U.B.E.C., , no. 45), viewed from the left side. X 15.
Abbreviations rm.I.t., anterior lobe ' 1)., process surrounding neck of neural c.h. «caudally directed horn of para t.u- Iobo lneralis p.t., pars tuberalis n..l., neural lobe 3'-., incisure separating pars inte-rnu.-diu and pars tuberalis
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - The development of the hypophysis cerebri in man (1926). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_development_of_the_hypophysis_cerebri_in_man_(1926)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G