Paper - The Later Development of the Notochord in Mammals
| Embryology - 1 May 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Williams LW. The later development of the notochord in mammals. (1908) J. Anat. 8:251-284.
| Online Editor |
|---|
| This historic 1808 paper by Williams describes the fate of the notochord in mammals.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Later Development of the Notochord in Mammals
By
Leonard W. Williams.
From the Laboratory of Comparative Anatomy, Harvard Medical School.
The fate of the notochord in mammals has received, in recent years, very scant attention. This is well exemplified both by the briefness of the discussions of the subject in recent text—books and also in reference works, and by the contradictory statements found in different, or even in a single book.
The following shades of opinion are found in volumes which have appeared very recently:
“The notochord here” (in mammals) “persists longer intervertebrally than intravertebrally, but it disappears entirely by the time the adult condition is reached.” (_Wiedersheim’s Comparative Anatomy, 6th lrerman edition, 1906, p. 62, and 3d English edition, 1907, p. 60.)
“They (the intervertebral discs) are developed, like the bodies around the notochord, persisting parts of this structure forming a central core to each. disc.” (Pierso1’s Anatomy, 1907’, p. 132.)
“The notochordal remains lying between each pair of vertebrae with the perichordal tissue grow and remain throughout life as the nuclei pulposi of the intervertebral discs.” (Bcnnet, Entwicklungsgeschichto, 1907, p._ 381.)
“The notochord is essentially an embryonic structure in mammals, although it does not completely disappear, for traces of it are to be found throughout life in the middle of the intervertebral discs. When fully developed it is a cylindrical rod composed of clear epithelium-like cells, enclosed within a special sheath of homogeneous substance. These cells, although they may become considerably enlarged and vacuolated, undergo no marked histogenetic change and take no part in the formation of any tissue of the adult. . . . Within the (intervertebral) disc the notochord is enlarged and afterwards converted in each, along with the surrounding tissue, into the nucleus pulposus.” (Bryce, in Quain’s Anatomy, Vol. I, Embryology, 1908, p. 49 and 252.)
“The notochord not only remains intervertebrally, but grows cont inuously at that point, showing therewith the tendency (Neigung) after the loss of its sheath, to fuse with the surrounding connective tissue. (Leboucq, 1880.) . . . The nucleus pulposus or gelatinosus of the intervertebral ligament (intervertebral disc) consists in every case in the adult mammal, of such common growth of the notochord and of the tissue lying next to it. G. Jéiger is indeed right when he compares, as mentioned ahove, the intervertebral longitudinal ligament (Langsband) of birds with the pulpy nucleus of the disc of mammals.” (Schauinsland, in Hertwig’s Handbuch d. vergl. u. exper. Entwickelungslehre der Wirbeltiere, Bd. 3, Teil 2, 1906, p.. 517.) The statement referred to is as follows: “Only inconspicuous remnants of it remain finally in the interior of the intervertebral ligament; they lie there enclosed in a longitudinal band which, as the ‘ligamentum suspensorium,’ binds together the successive vertebrae Jager, 1858).”
“finally the notochord disappears from the vertebral regions, although a canal, representing its former position, traverses each body for a considerable time, and in the intervertebral regions it persists as relatively large flat discs, forming the pulpy nuclei of the fibro—cartilages.” (McMurrich, The Development of the Human Body, 1907, p. 170.)
“La corde dorsale, par exemple, constitue a elle seule tout le squelette axial chex les Chordés primitifs, tandis qu’elle disparait entiercment dans les formes superieures.” (L. Vialleton, Un Probleme de l’Evo1ution, 1908, p. 87.)
The cause of this unflattering state of our knowledge is that the theory concerning the fate of the notochord of mammals, which was widely accepted years ago, was not well founded and does not explain the known facts. In the three decades between 1850 and 1880 the subject aroused considerable interest. Two theories were in the field: one proposed in 1852 by Luschka, and advocated by Kolliker, I-In Muller and Ldweg the other originated by Virchow, and defended by Luschka after 1856, Robin, Dursy, and Leboucq.
The nucleus pulposus in man was carefully described, in 1852, in the first edition of “Die Halbgelenke,” by Luschka, who found that it arises from the intervertebral expansion of the notochord.
Virchow later contended that the nucleus pulposus of the new-born child is formed by the liquefaction of the central portion of the connective tissue of the intervertebral disc. He also described a tumor found upon the inner surface of the base of the skull, and, believing it to be a growth of cartilage, named it Ecchondrosis physalifom.
Luschka, in 1856, adopting in part Virchow’s theory of the origin of the nucleus pulposus, found that its characteristic tissue, which consists of whitish clusters of vacuolated cells in a transparent gelatinous matrix, arises primarily by the liquefaction of the intervertebral notochord expansion, but that it is augmented by the liquefaction of the surrounding fibro—cartilage. He also accepted Virchow’s theory of the nature of Ecchondrosis physalifom.
Two years later, 1858, Luschka published the second edition of his “Halbgelenke,” in which he described the nucleus pulposus at various ages. He found further evidence of the close relationship of notochordal tissue and cartilage. The sharp boundary line between the notochord and the intervertebral disc which occurs in the new-born child, owing to the formation of papillae of fibro-cartilage which project into the notochordal tissue, disappears during childhood. Liquefaction finally leads to the obliteration of the boundary between the two tissues. In old age the nucleus pulposus becomes of dirty green or gray color. Tt loses its gelatinous and elastic character while cheesy masses, the products of fatty degeneration, as well as calcareous masses, appear in it. A large number of cartilage cells with thick, strongly laminated walls are found in it. Some of these are immense mother cells with innumerable daughter cells. Fat vacuoles are visible in many cells.
In the same year Heinrich Muller noted that portions of the notochord persist for a long time after birth in the base of the skull, in the odontoid process of the axis and in the coccyx. He tried to demonstrate that Ecchondrosis physalifom is produced by excessive growth of notochordal tissue.
Kolliker says in the fifth edition of his “Histology:”——“In 1858 I pointed out that the intervertebral ligament of a child of one year contains ordinarily a pear-shaped cavity which is filled with the continuously growing mass of the notochord; and that this mass, which consists of a soft matrix and many cells with characteristic vacuoles arranged in clusters or in a network of strands, forms in the adult a large part of the nucleus pulposus in which, in certain cases, the‘characteristic foetal notochordal cells can be recognized. . . . This soft mass” (of fibro-cartilage, which Kolliker regards as the peripheral 254 Leonard W. Williams part of the nucleus pulposus) “which often bears the irregular processes, first described by Luschka, surrounds the notochordal remnants (Reste) so that the two structures interlock with one another and a sharply marked cavity, such as occurs in the child, does not exist.” The original article is in the Verh. phys. med. Gesellsclr, Wiirzburg, 1859, IX, pp. 193-242.
This clear and precise statement by so acknowledged a leader as liiillilzer did not suflice to settle the question, for in the following year Robin, describing carefully a large series of stages in the development of the notochord of mammals, maintained that the notochord undergoes mucoid degeneration and that its intervertebral expansion is gradually invaded by papillae of fibro-cartilage which, meeting at its center and expanding gradually, finally replace the degenerated notochordal tissue.
K6lliker’s theory of the origin of the nucleus pulposus was again attacked in 1869. Dursy found that the nucleus pulposus is formed primarily by the liquefaction of the connective tissue of the intervertebral disc and that the notochord takes no part in the formation of the definitive nucleus pulposus. This view is like Luschka’s second theory, except that he found that the notochord is liquefied first and later fuses with the liquefied connective tissue.
Ten years later Lowe entered the field in defense of K6llil:er’s theory. He agreed essentially with Kolliker, but found that the entire nucleus pulposus of the adult rat is formed by the notochord. This difference is merely one of definition, for, as was noted above, Kolliker considered that the looser fibro—cartilage of the disc should be regarded as the peripheral portion of the nucleus pulposus, which, by this definition, is composed of the notochordal tissue and the loose fibro—cartilage of the disc.
Heiberg, 1878, says: “dann muss man zu dem Schluss kommen dass die Chorda dorsalis beim Menschen keinen Antheil an der Bildung der Pulpa des Intervertebralligamentes nimm .”
In 1880 Leboucq published the last considerable article upon the question of the fate of the mammalian notochord. He found that in the human and in other mammalian embryos, the notochordal tissue is practically destroyed long before birth. In the vertebra the notochord degenerates and is absorbed, but the intervertebral notochordal expansion is first invaded by connective tissue and is ultimately replaced by it.
This piece of Work, coming after a long controversy, Was widely accepted in spite of the fact that it contradicted practically all previous writers, for it indicated that the notochord disappears much earlier than
had been believed by others.
Fric, however, in his “Handbuch der Gelenke” (1904), accepts Kelliker’s work and, figuring the nucleus pulposus, describes its peculiar tissue as the remnant (Rest) of the notochord.
Turning now to Miiller’s contention that Virchow’s tumor, Ecch0ndrosis physaZv,'fo7'a, actually arises from the notochord, not from cartilage, we find Virchow’s view universally accepted until 1894, when H. Steiner, working under Ribbert, published a careful study of a case of the tumor and found that Muller was right in believing it to be an abnormal growth of the notochord.
Ribbert, a year later, proved that the tumor can be produced experimentally in the rabbit, by puncturing the intervertebral ligament so a-s to allow a portion of the nucleus pulposus to escape. This tissue, lying in the connective tissue and muscle near the ligament, grows for some time and forms a characteristic Ecclzondrosis physalifom. This knowledge led Ribbert in his “Geschwiilstlehre,” 1904, to propose and use the name chordoma, instead of Virchow’s name for the tumor. fischer and Steiner, working in Ribbert’s laboratory, described a case of malign chordoma in 1906.
The generally accepted interpretation of the formation of the vertebrae in the Amniota is the theory of Remak of the resegmentation of the vertebrae. Among recent exponents of Remak’s attractive generalization are Schultze, Schauinsland, Weiss, and Bardeen. According to this theory the vertebrae are formed by the division of the sclerotomes and by the subsequent fusion of the sclerotome halves adjacent to each intersegmental plane to form an intersegmcntal vertebra. The fissure of von Ebncr, or the intervertebral fissure, a mid-segmental transverse diverticulum of the myoccele which in mammals, however, arises independently of the myocoele and after its disappearance, divides the sclerotome into half segments. The anterior half of each sclerotome is formed of looser tissue than the posterior half. The latter is apparently a mesenchymal condensation and has been called “the primitive vertebra” by Remak, the “sclcromere” by Bardeen. Its central portion is the “hypochordal rod” of Froriep, the “horizontal plate” of Weiss, and the “primitive disc” of Bardeen. Its lateral portions are the “vertebral arches” of Froriep, and the “costal and neural processes” of Bardeen. Weiss divides the lateral portion of the primitive vertebra into a “vertical plate” which extends backward from the outer end of the “horizontal plate” to the intersegmental artery, and the “vertebral arch” which extends outward from that artery between the myotomes. Weiss finds that the transverse portion of the primitive vertebra forms the annulus fibrosus of the intervertebral disc; Schultze and Bardeen, that it forms part of that structure and also the anterior portion of the vertebra. Schultze asserts that the rib belongs to the posterior half of the segment, and Bardeen that from this point of origin it moves into an intersegmental position. Weiss maintains that the vertical plate and the arch fuse with the vertebra, Bardeen that only the arch does so.
In this paper I have undertaken to trace the development of the notoehord, in the pig, from the time of the appearance of its segmental Waves. It was also found necessary to review the formation of the vertebrae in order to determine the exact relation between the notochordal waves and other metameric structures.
The development of the notoehord in man, the rabbit, cat, dog and opossum has also been studied, but in less detail. The shape of the intervetebral notochordal expansions has been found to be sufficiently characteristic in each species to justify a brief description of the form of the notochordal enlargements.
The study has been made possible by the extensive series of mammalian embryos in the Harvard Embryological Collection, and the series referred to by number belong to this collection.
I am greatly indebted to Prof. Charles S. Minot for many helpful suggestions for the prosecution of the work.
The Formation of the Notochordal Sheaths and of the Pre-cartilaginous Vertebra of the Pig
The notochord of a very young pig embryo (5.5 mm, H.E.C. Nos. 915, 916, 917) is a dorso—ventrally flattened rod with major and minor axes approximately 25 and 50 micra long. Each cross section contains about eight wedge-shaped cells whose exposed walls form a thin notochordal sheath. As was shown by Dr. Minot in 1907, the notoehord and the floor of the spinal cord of young mammalian embryos are thrown into a series of segmental undulations. In the pig the crests or dorsal curves of the notoehord are nearly intersegmental, for they occur very slightly in front of the transverse plane of the intersegmental vessels, the troughs being nearly mid-segmental.
In the head the notochord is nearly midway between the medulla and the pharynx, but posteriorly it gradually approaches the cord until, in about the 25th segment, the two are in contact. In front of this point, the mesenchyma of the selerotomes extends from the myotomes to the notochord as a dense mass which is interrupted at the level of the notochord by scarcely perceptible light transverse zones which connect each pair of intersegmental vessels, and by a narrow longitudinal median zone of similarly light tissue.
In order to determine accurately the density of the nuclei I have counted the nuclei in an area 24 microns square in sections 10 microns thick, or in 5,760 cubic microns. In one or two cases the series are cut at a different thickness, and it was necessary to calculate the number of nuclei in this volume from the data for another volume. The percentage of error in this calculation is less than that of the counting. In every case the number given is the mean between at least two counts in different places. At the level of the notochord there are 36 nuclei in 5,7 60 cubic microns in the transverse light zones, 48 in the longitudinal zone and 62 in the denser regions. Below the notochord the mesenchyma is of nearly uniform density and has 54 nuclei in 5,760 cubic microns.
Higher up, beside the spinal cord, the structures of the anterior half of the segment, the spinal ganglion and the roots of the spinal nerves, are surrounded by loose mesenchyma; and the dense tissue, like that below the spinal cord, is confined to the posterior half of the segment. The cavities of the myotomes have closed. The fissure of Von Ebner, which in the Sauropsida divides the sclerotome into anterior and posterior halves, has not appeared and consequently the sclerotome is not divided into anterior and posterior portions.
Behind the point where the spinal cord and notochord first come in contact, there is a region in which the notochord is not in contact with the sclerotomes, but lies in a small cavity. Farther back the notochord is in contact with the spinal cord, the post-anal gut, and the somites.
Slight condensations of mesenchyma become visible in embryos of 6 mm. They occur in the middle of the segments and lie just under and perhaps a trifle behind the troughs of the notochordal Waves. They are produced by the extension of the intersegmental zones of loose tissue, forward and backward toward the centers of the sclerotomes, and are the intervertebral discs of Froriep or the primitive discs of Bardeen. Their position is mid-segmental in the pig as in the cow (Froriep), not in the posterior half of the sclerotome as in man (Bardeen).
These structures are more clearly defined in still later embryos, 7.8 mm. (Fig. 1), and it is evident that the differentiation of the axial structures is brought about as much by the spreading apart of the mesenchymal cells as by their aggregation or condensation. There are in 5,760 cubic microns about 100 nuclei in the disc and 29 in the light transverse zones. The enumeration of nuclei in the dense regions is too difficult to yield reliable results, and the approximate number only can be given. Below the level of the notochord the tissue of the disc is much less dense than at the level of the notoehord. The dense tissue of the discs does not extend much above the notoclxord, but the discs are now united by a median cord of dense tissue, the perichordal septum, which surrounds the notochord and forms a dense band below it. The rounded intersegmental zones of looser tissue are deeply con-stricted between the notochord above and perichordal septum below, but the two lateral portions of each seem always to be connected at least by a slender cord of loose tissue which passes under the crest of the corresponding notochordal undulation.
The notochordal sheath appears first in embryos of 7 mm. and is apparently fully formed in embryos of 7.8 mm. It is an anhistic membrane about 1 to 1.5 microns thick and it is faintly striated concentrically. The formation of this sheath, or of the inner sheath which appears later, does not affect the proper Walls of the notochordal cells which can still be seen inside the sheath.
The loosening up of the axial mesenchyma reaches its maximum in embryos of 9 mm., for there are 20 nuclei in 5,760 cubic microns in the light zones and 63 in the discs (Fig. 2). In embryos of 10 mm. the mesenchyma is denser, and the loose ti.ssue of the vertebrae has 50 nuclei in the same volume. The mesenchymal tissue above the notochord has increased largely, for in the 5.5 mm. embryo the notochord was separated from the spinal cord by a small plate of mesenchyma scarcelythieker than the notochord; it is now separated by more than thrice its diameter. The perichordal septum surrounds the notochord and the vertebral anlages. The septum, nevertheless, is incomplete, for, as stated above, a small isthmus under the notochordal crest connects the lateral masses of the vertebral anlage.
The multiplication of the cells of the discs and of the perichordal septum has been sufficiently rapid, up to this age, to maintain approximately the same density of tissue in them despite the rapid increase in volume of this part of the embryo, but the cells of the vertebrae do not keep pace with the general growth and are consequently drawn apart.
The notochord is larger and about 15 cells occur at the periphery and 3 or 4 at the center of each transverse plane.
The fissure of von Ebner is present in the trunk. It scarcely reaches downward to the level of the notochord and it does not reach inward as far as the intersegmental arteries. Its position is such that, if it were extended downward and inward, it would divide the intervertebral disc. Bardeen finds that in man this fissure is mid-segmental and that the “primitive disc” lies in the posterior half of the sclerotome in early stages, but that the intervertebral disc is later formed upon the site of the fissure of von Ebner. In the pig, however, the fissure of von Ebner does not divide the sclerotome into anterior and posterior portions; on the contrary, the sclerotomes fuse with one another in the median line and longitudinally, as we saw in the 5.5 mm. embryo, and, in the axial rod thus formed, appear the loose transverse zones which will form later the bodies of the vertebrae.
The dense mesenchyma of the intervertebral disc extends outward to the spinal nerves and then divides into an anterior and a posterior process. The former, the interdiscal membrane of Bardeen, extends’ forward on the inner side of the spinal nerve to the preceding disc. The latter, representing the “costal and neural processes” of Bardeen, extends outward and backward, and downward and upward. The upper, or “neural” process, extends upward behind the spinal ganglion and upon the inner side of the posterior half of the myotome. The lower, or “costal” process, extends downward and outward between the divergent lower ends of the myotomes.
As Bardeen found in man, all the axial mesenchyma is as yet blastemal, and I believe that, although this tissue has greatly increased in volume, almost all visible differentiation has been effected by the separation of its cells from one another. The apparent condensations give rise “to cartilage, perichondrium and ligaments” (Bardeen) and consequently the blastemal “scleromere” of Bardeen, which is com.posed of the intervertebral discs with the costal and neural processes, cannot justly be regarded as a morphological skeletal unit. In short, definite skeletal differentiation, the formation of cartilage or precartilage, has not as yet begun in the “primitive vertebrae,” but is foreshadowed in the definitive vertebrae by the loosening up of the blastemal tissue which, at least in the spine of the pig, always precedes the condensation that forms precartilage.
An extraordinary multiplication of the cells of the vertebra, disc, and neural and costal processes has begun in pig embryos of 11 mm. A further differentiation of the discs and the vertebrae accompanies this new phase of growth. The tissue of the vertebrae has become precartilage, for the nuclei stain less intensely and, although the protoplasmic network remains, it becomes attenuated and stains less readily than elsewhere. The cells and cytoplasm of the discs, on the other hand, continue to take stains as before.
The edges of the discs are continuous with a similar but less dense tissue which completely surrounds the vertebra and, in the medial line, fuses with the upper and lower edges of the perichordal septum. The neural processes are possibly separated from the vertebral bodies for a time by this sheet of tissue, but long before chondrilication begins the neural processes or arches are united to the vertebrae.
The notochordal cells have lost all definite arrangement and are more or less vacuolated. They are flattened antero-posteriorly and are closely packed together.
The number of cell divisions in the vertebrae apparently reaches a maximum in embryos of 12 mm. (Fig. 3), and there are 54 nuclei in 5,760 cubic microns. In addition to the exceptionally large number of mitoses, one sees many elongated and dumb—bell-shaped nuclei, as well as a few pairs of top-shaped nuclei united by their points at a. large acute or an obtuse angle. The three nuclei in Fig. 13, as well as a number of similar nuclei, were found in a single section of one vertebra (Section 894, I-I. E. C. No. 5). This embryo is well preserved, and similar nuclei occur in two other 12 mm. embryos (Nos. 6 and 518), but seem to be absent from a fourth embryo (No. '7). I am inclined to believe that the rapid cell division which accompanies the transformation of blastemal tissue into precartilage is partly mitotic and partly amitotic. Rod- and dumb-bell-shaped nuclei occur in embryos of 10 and 14 mm., but they are rare and do not furnish acceptable evidence of amitosis.
The precartilage of embryos of 12 mm. has reached its maximum density. The nuclei are surroundedby small quantities of cytoplasm which forms a delicate network.
A considerable quantity of loose mesenchyma separates the vertebrae and intervertebral discs from the spinal cord. Anteriorly the base of the spinal cord has lost its segmental undulations, but posteriorly they persist even in embryos of 14 mm. Only a small part of the neural and costal processes has been transformed into precartilage. At the level of the base of the spinal cord, the neural process of the scleromere is approximately triangular in frontal section. Two acute and equal a.ngles are directed inward and outward and its obtuse angle is directed forward. The last lies close behind the spinal nerve and is continuous with the “interdorsal” and the “interdiscal ligaments” (of Bardeen) which lie respectively upon the outer and inner sides of the nerve. The outer angle projects between the myotomic muscles and is separated from the blastemal tissue of the anterior end of the interdorsal. and interdiscal ligaments of the next segment by the ramus dorsalis of the spinal nerve. Above the ramus dorsalis there is a rounded blastemal mass which cannot be assigned to one or the other segment. The outer angle of the neural process remains blasternal for some time and seems finally to form the myoseptum. The inneriangle is continuous below and anteriorly with the costal process and with the periphery of the intervertebral disc. The precartilage of the neural arch appears upon the posterior side of the inner angle of the blastemal neural process. It reaches only to the upper edge of the myotomes. The costal process is largely blastemal, but it contains the small elongated precartilage of the rib.
In embryos of 14 mm (Fig. 4) the vertebrae are larger and are more definitely outlined. A sheet of elongated, closely—placed nuclei, formed by the extension of the interdiscal ligament and by its fusion with the perichordal septum, surrounds the vertebra and binds together the successive intervertebral discs. It represents the fibrous tissue of the perichondrium and of the dorsal and ventral common ligaments. A mass of dense blastemal tissue, which is perforated by the ramus dorsalis, extends from the neural arch to the rib.
The vacuolization of the notochord has continued and an inner sheath. which is much thicker than the outer sheath, has been formed. The inner sheath and the vacuoles of the notochord are composed of mucin or a mucin—like substance, for they are stained by mucicarmine. For convenience this substance is referred to hereafter in this paper as mucin, but I do not intend to convey the impression that its composition is even approximately known. The notochord is surrounded within its inner sheath by an apparently continuous Wall which is formed by the exposed walls of its superficial cells.
The intervertebral disc begins a little later, in a pig of 14.2 mm., to differentiate into a looser central portion, with nuclei irregular both in shape and arrangement, and an outer and larger region with nuclei which are elongated longitudinally and are united by strands of protoplasm into layers concentric with the center of the disc. The inner portion later (in 24 mm. embryos) forms the cartilage which serves, as has been shown by Schultze, Minot, and Weiss, to bind together the successive vertebrae in a continuous rod of cartilage, the chondrostyle. It should be noted in passing that the development of the cartilage of the intervertebral disc at this late period, as compared with the vertebral cartilage, is another indication that the apparent condensation, the scleromere, is not the first but the last portion of the vertebral column to be differentiated. It is undifferentiated rather than precociously differentiated tissue.
The vertebral precartilage is still further differentiated in embryos of 17 mm. Each nucleus is now surrounded by a small cell body in which are enclosed one, or more commonly two, vacuoles, each of which is nearly or quite as large as the nucleus. The cytoplasmic network has disappeared and the cells lie in a homogeneous matrix.
The perichordal septum has ceased to be recognizable as such, but its upper portion now remains as the fundament of the dorsal common vertebral ligament. It lies in a deep groove in the precartilage of the body of the vertebra. The ribs and vertebrae are sharply marked off by the perichondrium from surrounding tissues.
Chondrification of the vertebrae begins before embryos are 20 mm. long. (Fig. 5.) At this time the vertebral cells have much the same character as before, but the vacuoles are less conspicuous and the cytoplasm is more granular and stains more heavily. Each cell, however, is now separated from the matrix by a heavily stained capsule. A considerable space often separates the cell from its capsule; this, however, may be due to shrinkage.
The cartilage is now surrounded on all sides by a layer of small, closely-packed, rounded nuclei. This layer, together with the fibrous tissue surrounding the vertebra, forms the embryonic perichondrium. The central portion of the intervertebral disc, which includes from one-third to one—quarter of its diameter, is now preeartilaginous. The outer portion of the disc is gradually becoming more fibrous. The interdorsal ligament is now well difierentiated from the blastemal portion of the ligament and from the neural arch. Its upper edge and outer portion still. remain blastemal. The same is true of the upper end and outer portion of the neural process. The upper blastemal tissue will later differentiate into the upper portion of the interdorsal ligaments and of the neural arch which now extends but to the middle of the side of the spinal cord. The outer blastemal tissue of the neural process, as noted above, seems to form the myoseptum. A column of blastemal tissue from which will be formed the transverse process of the vertebra, the tubercle of the rib, the costo-transverse ligaments, etc., extends from the rib to the neural arch.
The notochord has also undergone fundamental alteration. The cell walls, which up to this time have remained intact, are now breaking down (or are being absorbed) and the mucin from the cell vacuoles escapes. The cells are united by strands of cytoplasm and the notochordal tissue now resembles mesenchyma. A part of the mucin remains in the cytoplasmic mesh, some of it, escaping, helps to thicken the inner sheath of the notochord, and a large quantity collects within the intervertebral portion of the notochord whose sheaths are compressed slightly by the intervertebral disc. The vertebral portion of the notochord, owing to the escape of the mucin from its vacuoles into the inner sheath, or into the intervertebral portion of the notochord, is much reduced in size and is much denser than before, but the corresponding portion of its sheaths is dilated. The notochord is thus dilated intervertebrally and contracted vertebrally, but the reverse is true of its sheaths, the outer sheath being of greater diameter, and the inner sheath both of greater diameter and also of greater thickness in the vertebra. The notochordal undulations are obliterated by these changes in the notochord and vertebrae.
A brief discussion and summary of the relation of the notochordal undulations and the ribs and vertebrae to the segments is desirable at this point.
Dr. Minot has shown that the segmental waves of the notochord of the pig are somewhat difierent from those of other mammals. The crests of the undulations are a very short distance in front of the intersegmental arteries, and are in the posterior fourth of the segment; the troughs are in the second fourth; the ascending or anterior slope in the third; and the posterior slope in the first fourth of the segment. The intervertebral disc, which is formed from the transverse portion of the primitive vertebra, is mid-segmental, and lies just behind the trough of each notochordal undulation. The edge of the blastemal intervertebral disc abuts upon the ‘posterior edge of the spinal nerve and from this point the dense tissue of the‘ primitive arch extends backward and outward into the neural and costal processes which belong to the posterior part of the segment. These relations persist until the formation of precartilage begins, when the blastemal primitive vertebra breaks up into the intervertebral disc, the neural arches, ribs, myosepta and such diverse structures as cartilage, fibrocartilage, fibrous connective tissue, and perichondrium.
Finally, I am convinced that the delimitation of the “primitive vertebra” is not due to its becoming differentiated before the surrounding structures, but to the more rapid differentiation of the definitive vertebrm which leaves the more slowly developing blastemal tissue between the successive vertebrae as the “primitive vertebra.” These considerations suggest that the scleromere is not a morphological unit or anlage ; it is rather a residual mass of undifferentiated sclerotomic tissue which later forms such diverse morphological units as the annulus fibrosus and the fibro—cartilage of the intervertebral discs, the rib, the neural arch and the myoseptuin. In short, the “primitive vertebra” is a lager rather than an anlage, a store of rudiments, not a rudiment. If this is true, the conception of the resegmentation of the primitive vertebrae is without foundation, for the “primitive vertebra” is not a vertebra at all. Moreover, the evidence presented by Bardeen in support of his belief that the intervertebral disc is formed by the union of the tissue from the anterior surface of the primitive disc and from the posterior surface of the anterior half of the sclerotome, is not conclusive. He says (p. 165) : “During the period of differentiation of the scleromeres the myotomes undergo a rapid development. The median surface of each myotome gradually protrudes opposite the fissure of von Ebner. The dorsal and ventral processes of each scleromere are then slowly forced into the interval between the belly of the myotome to which it belongs and the one next posterior, and thus finally they come to occupy an intersegmental position.” Again (_ p. 166), “During the development of the interdiscal membranes, the primitive discs become hollowed out on the posterior surface. A comparison of Fig. 2 with Fig. 3 demonstrates this.” On page 167, “Each primitive disc has become further hollowed out at its posterior surface, owing in all probability to the conversion of its tissue into that of the area between the discs. The tissue of each segment immediately anterior to the primitive disc has become greatly thickened and the line between it and the disc indistinct.” These facts are summarized on page 167, “The primitive discs become hollowed out posteriorly by a loosening up of their tissue and strengthened anteriorly by a condensation of the tissue immediately bounding the fissure of von Ebner.” The evidence for the most important point, whether or not the primitive disc is partitioned between the vertebra and the intervertebral disc, is very slight; and if the process described in the first and second quotations were to continue for some time, it would perfectly account for the fact stated in the third and fourth quotations. However, Weiss found in the white rat, and I find in the pig, that the primitive disc becomes the intervertebral disc. The fundamental mistake, I believe, in all work upon the primitive vertebrae, is the assumption that the primary sclerotomic condensations either are precartilage or are skeletal anlages. The fact is that they are neither the one nor the other. Precartilage, of the mammalian vertebrae at least, arises from such primary condensations only after a preliminary loosening up and subsequent condensation.
The Segmentation of the Notochord
The notochord shows a most marked change in pig embryos of 24 mm. (Fig. 6). The advancing chondrification of the vertebrae is the apparent cause of a considerable expansion which, on the one hand, presses the notochord, together with the greater part of its semifluid inner sheath, from the vertebra toward the intervertebral discs; and on the other hand draws the disc away from the notochord so that a cavity is formed within the disc for the reception of the notochord. The outer sheath seems not to be broken and the inner sheath adheres to it so that, at no point, does the notochordal tissue come in contact with the cartilage of the intervertebral disc or even with the outer sheath. The intervertebral cavity is fusiform and the notochordal enlargement is irregularly diamond-shaped. The dense tissue from the vertebral portion of the notochord usually forms two slender cones whose broadened bases, opposing the bases of similar cones from the adjacent vertebrae, compress the intervertebral part of the notochordal tissue and flatten antero-posteriorly the masses of mucin within it. The two notochordal sheaths are very much compressed at the center of the vertebra, but less and less so the farther from its center. The greater part of the mucin which forms the inner sheath is forced into the intervertebral cavity; but a small part of it, and occasionally a few notochordal cells, are retained within the vertebrae. The notochordal tissue retains its syncytial character, and there begins at this time a more rapid increase of its mass, affecting alike nuclei, cytoplasm and mucin.
The chondrostyle is now complete anteriorly, for the tissue of the inner portion of the intervertebral disc is passing in this stage into true cartilage. The outer part of the disc is more and more fibrillar and is clearly destined to form the cumulus fibrosus. The edge of the disc is still attached to the head of the rib, but the formation of the articulation is indicated by a small cavity which has now appeared between the two structures.
The cartilage of the vertebrae is more advanced. A large quantity of matrix intervenes between the cell-capsules, and many cells, owing to division, have two nuclei. The notoehord forms approximately .2, the cartilage .3, and the fibrous tissue .5 of the diameter of the intervertebral disc.
Comparatively slight changes are seen in the notoehord of an embryo of 39 mm. The syncytial network has been enlarged both by growth and by the formation of a relatively greater number of vacuoles of mucin, and, consequently, the nuclei are farther apart. The cytoplasm of the syncytium forms in places a regular continuous boundary, but at other points the vacuoles seem about to escape to the exterior. The small fragments of the notoehord which have been enclosed within the vertebrae are degenerating. The first step in this process is apparently indicated by the separation of the cells from one another and by a change in the cytoplasm and nucleus which causes the former to take Orange G. more intensely and the latter to stain more deeply with haematoxylin. Somewhat later the nucleus becomes deeply and irregularly constricted and it is finally broken up into small pieces. The cytoplasm later is fragmented in the same manner.
Calcification of the centers of the vertebrae and of the notochordal sheaths within them begins in embryos of 32 mm. (Series 136) and has affected the greater part of the vertebral bodies in an embryo of 39 mm. Within the intervertebral disc, the outer notochordal sheath is no longer recognizable in the larger embryos, having been, in all probability, stretched to excessive thinness. The inner sheath has the same character and the same relative size as before.
Mitotic figures are very frequent in the cartilage just outside the region of calcification, and many characteristic cell-nests have been formed.
The cartilaginous portion of the intcrvertebral disc has increased in Volume with advancing chondrification much more rapidly than its fibrous portion, and now forms about .41 of the disc, the notoehord forming about .25 and the fibrous tissue .33. The cartilage of the disc now has the structure which was earlier (17 mm. embryos) characteristic of the cartilage of the vertebrae; that is, each cell contains one or two vacuoles as large as or larger than the nucleus. A few cell capsules contain two nuclei. The fibrous tissue has begun to assume its characteristic arrangement in alternating layers whose fibers almost form right angles with one another and are inclined at an angle of 15° or 20° to the longitudinal axis of the spine.
A great change in the shape of the notochordal enlargement has occurred in an embryo of '75 mm. (Fig. 7). It is flattened antero-posteriorly and in vertical or horizontal section it is elliptical except for slight projections forward and backward into the open ends of its sheaths. The notochord now forms .41 of the diameter of the disc, the fibrous tissue .36, and the cartilage .23. The notochordal syncytium is larger but retains the same general character. lt is bounded by a clearly defined cytoplasmic layer which has fewer vacuoles and more nuclei than the central portion of the notochordal tissue. The cells of the fibrocartilage of the disc are flattened as though by the radial pressure produced by the growth of the notochord, and the cells of the numerous cell—clusters are arranged in rows parallel to the adjacent portion of the notochordal sheath. Periosteal buds have filled the centers of the vertebrae and bone formation has begun. In a few places the calcified notochordal sheaths have been destroyed by the periosteal buds. This process finally destroys completely the vertebral portion of the notochord and hereafter the notochord is confined to the intervertebral discs.
The Formation of the Nucleus Pulpous of the Adult Pig
The flattening of the notochordal enlargement continues until, in the cervical region of an embryo of 150 mm, it is thrice as broad as thick and forms one-half the diameter of the disc, the cartilage having shrunken to .15 and the fibrous tissue having remained of the same relative size (.36). The notochord in an embryo of 250 mm forms .58, the cartilage only .08 and the fibrous tissue .34 of the disc’s diameter. In short, the notochord is expanding at the expense of the fibro—cartilage, which, being attached to the cartilaginous faces of the vertebra near their common axis, is stretched over their faces by the expanding notochordal tissue. Consequently the cartilage finally forms a thin capsule which surrounds the notochordal disc. The diameter of the mass of notochordal tissue is about six times as great as its thickness. The peripheral portion of the notochordal syncytium is more continuous and regular, and is also denser. The notochordal tissue (Fig. 15) contains a few very large vacuoles at its center, but elsewhere is filled with a multitude of small vacuoles.
The vast increase in nuclei which accompanies the growth of the notochord is apparently due entirely to mitotic division, for in well preserved material mitotic figures are abundant and there is no suggestion of amitotic division. In poorly preserved tissue mitosis cannot be recognized, and the irregularity of certain nuclei suggests amitosis, but this condition is probably due entirely to improper fixation.
In a larger embryo (250 mm) the large central vacuoles of the notochord are apparently moving toward the periphery, and in a few places they have broken through the dense peripheral layer into the inner sheath. As they reach the surface these vacuoles often tear off portions of the dense peripheral layer which form rounded isolated masses. I find upon the lower edge of a single disc in this embryo, an interdigitation of processes of the cartilage and notochord such as Kiilliker and Luschka describe in the adult man. The cartilage has constricted off a few small nodules of notochordal tissue which are assuming the structure characteristic of adult notochordal tissue. Large vacuoles which do not consist of mucin are forming in the cells which have become visible in the syncytium.
In the half-grown pig the notochord has encroached yet farther upon the remainder of the disc and forms about .74, and the fibrous tissue and fibro—cartilage .26 of the diameter of the disc. In shape the notochordal expansion is a very thin, lenticular disc. It is still surrounded by its inner sheath of mucin, which has become more dense, and after fixation in Zenker’s solution it appears very finely and somewhat irregularly fibrillar. It now stains with haernatoxylin more strongly than the fibro-cartilage. The formerly continuous peripheral sheet of dense syncytial tissue is now broken in many places by large masses of mucin, and in other regions it contains large vacuoles which seem about to escape into the inner sheath. The formation of mucin has continued until the center of the notochordal mass consists of a large quantity of mucin in which the slender syncytial network seems suspended. Between the very loose central mass of the notochord and the much broken dense peripheral layer, there is a zone which contains moderately small vacuoles in a syncytial mass. The mucin is gradually replacing a large portion of the syncytial tissue and, beginning at the center, is gradually coming to surround the notochordal tissue instead of being surrounded by it. The mucin vacuoles at first are imbedded in the syncytium; the vacuoles, enlarging, touch and finally unite with one another, leaving a coarse network of syncytium; the strands gradually become attenuated and finally break, so that masses of notochordal tissue, which are very small near the center and large at the periphery of the nucleus pulposus, are isolated.
The cartilaginous portion of the intervertebral disc is represented by a very thin sheet of fibro—cartilage which lines the cavity in which the notochord lies. The portion of this sheet which lies upon the calcified cartilage of the epiphysis is more fibrillar than the portion which stretches around the notochord from vertebra to vertebra. The fibrous portion of the disc is very dense, and its inner portion, like the cartilaginous portion of the disc at an earlier stage, is being pressed radially outward by the notochord and forms a capsule around the notochord.
In the adult (Fig. 8) the notochordal enlargement is relatively thicker than in the half-grown pig. It is also relatively smaller, for it now forms but .4 of the diameter of the disc, the fibrous tissue forming the remaining .6. The expansion of the fibrous tissue is produced by the thickening of its various layers, not by the addition of new layers. The fibro-cartilage forms, as before, a thin lining of the notochordal cavity.
The mucin of the notochord retains the same character, but the tissue has undergone a most astonishing modification. The mucin now divides the notochordal tissue into a great number of relatively large masses which in turn are divided by smaller quantities of mucin into subsidiary masses. Each of the latter consists of a number of very peculiar cells or cell-like structures (Fig. 16) which are bound together by small quantities of syncytial cytoplasm. These cells each contain two, or more rarely one or three, large vacuoles which are surrounded by thin cytoplasmic walls and are separated by a small amount of cytoplasm in which lie, in the great majority of, if not in all cells, two small nuclei. All efforts to determine the nature of these vacuoles have failed. No stains affect them. They do not shrink in absolute alcohol but they do swell in water. Each cell of material which has been fixed in formalin and immersed in water for some time, owing to the swelling of the vacuoles, becomes elongated and constricted in the middle. Cells with but one vacuole resemble fat cells, but the vacuoles are not fat, for they are not stained by osmic acid, Sharlach R, or Sudan III-. The cytoplasm of these cells contains granules of glycogen.
It is a cause of regret that I have not been able to obtain notochordal tissue from immature pigs of various ages and from very old animals in order to follow carefully the process of formation of these cells and the ultimate modifications of the nucleus pulposus. It should be noted that although there are several points of similarity between notochordal tissue and cartilage in the pig and, as we shall find later, greater similarity in other animals, nevertheless, they are distinct tissues. The nucleus pulposus is formed entirely by the notochord.
Kolliker describes and figures clusters of cells from a child of one year which are essentially like the cell clusters of the adult pig ; and Fric describes and pictures the same tissue from the nucleus pulposus of the human adult. Both men, however, consider that this notochordal tissue forms only the central part of the nucleus pulposus and that the weak fibro-cartilage of the disc is the peripheral portion of the nucleus pulposus. No transitions occur between the two tissues, and the inclusion of the fibro-cartilage in the nucleus pulposus is merely a matter of definition, not a question of fact; but unfortunately the description of the nucleus pulposus as formed of notochord and cartilage has led many to believe that it isproduced by a fusion of the two tissues. The two tissues remain as distinct in the adult, despite their interlocking papilla, as in the new-born child, in which they are separated by a sharp boundary. Both Kolliker and Fric call the notochordal tissue of the nucleus pulposus a “remnant” (Rest) of the notochord. This terminology is not allowable because it makes the remnant of a part greater than the Whole; for the notochordal tissue of a single disc is much greater than the entire notochordal rod of the embryo.
The Notochord in Other Mammals
It has not been possible to follow out the development of the notochord in other mammals as carefully as in the pig, nor is it necessary, for unless there appears in the literature of the subject or in the tissues of the adult some confusion or doubtful evidence, it is permissible to assume that similar structures in mammals have a similar developmental history.
The notochord of an opossum embryo of 7.5 mm. is a slender rod without segmental undulations. Anteriorly it is surrounded by an anhistic sheath, and its tissue is syncytial and vacuolated. At the base of the tail, the notochord is cellular, vacuolated, and the somewhat thickened exposed cell—wal1s form its only sheath. In the tail, vacuoles are absent, and I am unable to find either cell-walls or sheath. A mesenchymal sheath surrounds the notochord in the tail and in the posterior part of the trunk, but there is no indication of a perichordal septum.
The dense sclerotomic tissue is interrupted, at the level of the notochord, by broad intersegmental zones of looser tissue.» The myocoele has closed, and I do not find the fissure of von Ebner. The lighter zones are broad anteriorly, and in the neck and thorax they have become precartilage. The intervertebral discs lie in the third and fourth fifths of the segments, and are consequently a trifle farther back than in the pig. In an embryo of 8 mm, the centers of the discs of the anterior part of the spine have become precartilage.
The chondrostyle is well formed in an embryo of 11 mm. (Series . 925), and is a cylindrical rod which encloses the notochord and bears the ribs and neural arches. Near the tip of the tail, the vertebrae are represented by broad zones of loose blastemal tissue which are separated by the denser tissue of the intervertebral discs. In the middle of the tail the notochordal undulations have appeared, and the crest of each undulation lies in an intervertebral disc, the trough in the precartilage of the vertebra. The center of each intervertebral disc in the sacral region is now precartilage, and the peripheral portion of each disc forms a slight thickening of the continuous perichondrium of the chondrostyle. The enlargement of the vertebrae, which is caused by their chondrification, is forcing the notochord in the lumbar region from the vertebrae into the intervertebral discs and is also both compressing the cells of the disc and carrying the lower and lateral parts of the disc away from the notochord. This process makes the vertebrae, which before chondrification are smaller than the intervertebral discs, larger than the intervening discs. In the trunk an.d neck, however, the chondrification of the cartilage of the discs has caused them to become of nearly as great diameter as the vertebrw; In this region, therefore, the discs are recognizable only by the notochordal enlargement, by a slight compression of the cartilage cells, and by the slight thickening of the perichondrium which represents the fibrous portion of the disc. The result of all these processesis that the spine of this embryo is represented anteriorly by the nearly cylindrical chondrostyle; in the posterior part of the trunk by the cartilaginous vertebrae and the constricted precartilaginous or blastemal intervertebral discs; and posteriorly by the constricted blastemal vertebrae and the dense blastemal discs.
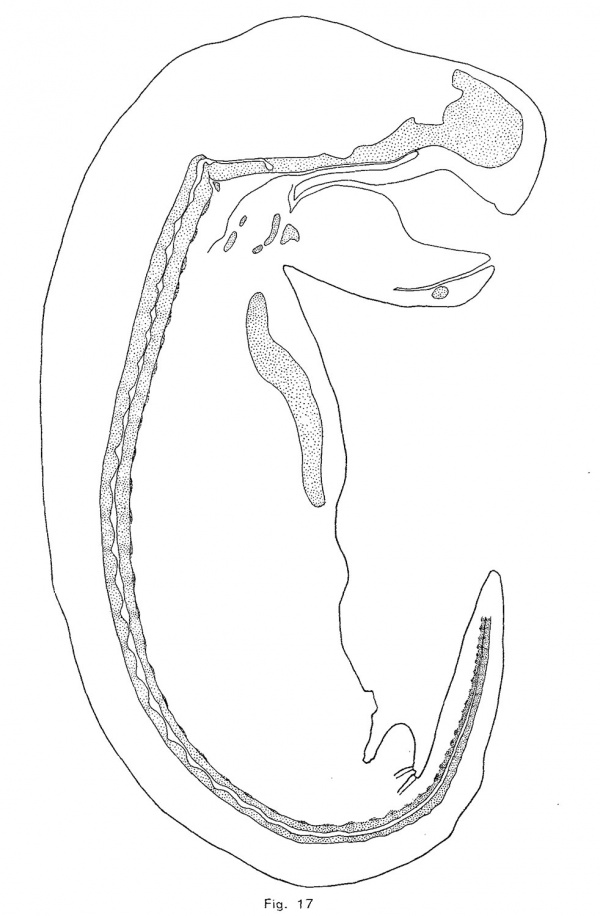
The notochordal crests correspond, at first, quite closely with the intervertebral discs; but later (assuming that the posterior portion of the notochord represents an earlier, and the anterior part a later phase of identical processes) the crests appear to be a little in front of the centers of the discs. As the notochord is driven from the vertebrae, it forces its sheath downward and outward so that its lower limit in the intervertebral disc is brought down as far as the troughs of the notochordal undulations (Fig. 10). The enlargement consequently becomes irregularly fusiforrn, its lower surface being flat. As more tissue is forced into the disc, the enlargement bulges downward sharply at a point near the middle of the disc and somewhat behind the crest of the notochordal wave. This process continues until, in an embryo of 12 mm., the notochordal enlargements are roughly diamond—shaped (Fig. 1'7). The lower angle is always less acute and prominent than the upper or primary angle, which represents the crest of the notochordal wave. The notochordal sheath is not broken by the expansion of the notochord. The inner notochordal sheath appears late and is relatively thin. The accumulation of mucin within the intervertebral enlargement of the notochord is quite large. The anterior end of the notochord terminates, at a point midway between the hypophysial fossa and the foramen magnum, in a rounded knob. The cranial portion of the notochord, with the exception of the knob just mentioned, lies upon the upper surface of the cartilage, under the perichondrium, and forms a distinct ridge. The head of each rib is continuous with the intervertebral disc, and the tubercle is continuous with the neural process of the vertebra behind the disc. The transverse process (or cervical rib) of each cervical vertebra is continuous with the neural arch above and with the body of the vertebra at the base of the arch. The head of the rib seems to be displaced forward in the trunk of the opossum.
The process of difierentiation of the vertebral column of the opossum seems to be identical with that in the pig, but the scantiness of the material at hand does not permit a precise determination of the conditions in the opossum. I am quite sure, however, that in both animals there should be recognized four distinct processes of vertebral differentiation: the blastemal stage, in which the mesenchymal tissue is loosening up; the precartilaginous, in which a rapid multiplication of cells occurs; the cartilaginous and the osseous stage.
In the adult opossum the nucleus pulposus forms a large part of the intervertebral disc. In the tail, it forms 63 per cent of the disc; the remainder being formed, as would be inferred from the great size of its cartilaginous portion in the embryo, almost entirely of fibro-cartilage. The notochordal tissue has much the same character as in the pig, but the cells have ordinarily but one nucleus and the vacuoles are smaller and of very variable ‘number. The cell or tissue clusters are much larger than in the pig.
The notochord of an adult mouse forms about 62 per cent of the cervical intervertebral discs. Of the remainder, the portion below the nucleus pulposus is of about twice the diameter of the part above it. fibro-cartilage forms about two-thirds of the former, and but onethird of the latter. The notochordal tissue forms practically a single mass which contains very little mucin, but is surrounded by a thick layer of it, the inner sheath. The cells have usually one nucleus and relatively small vacuoles.
The structure of the nucleus pulposus of the guinea—pig is quite different from that of the mouse. Its relatively small and nearly spherical cells form small strands or clusters that are suspended in a large mass of mucin. They commonly contain small vacuoles. The cartilaginous portion of the disc, as in the pig, is very small. In a few places the notochord interdigitates with the fibro-cartilage.
In the adult dog (of advanced but unknown age) the annulus fibrosus encloses a mass of soft friable tissue of yellowish color which appears, at first sight, to be homogeneous. A small rounded but irregular mass, however, forms the center of the disc. This is quite distinct, and can be lifted out whole, leaving a cavity of sharp and smooth contour. Sections of the soft center of the disc at first seem to be composed of a single tissue, but it is seen that the cell clusters at the center of the disc are of much more variable size than those of the peripheral part and also that they are of different composition. The peripheral portion of the center of the disc is clearly cartilage containing immense cell nests. Not having been able to make out fibrils in the matrix, I am inclined to believe that this is hyaline cartilage with a very soft matrix. Luschka figures and describes, in the human adult, papillae of fibro—cartilage which project into the notochordal tissue and which contain similar but much smaller nests of cells. The central mass consists of a firm and apparently fibrillar matrix in which are embedded, Without any regularity, clusters of cells of various size and appearance. Certain clusters contain small non-vacuolated cells which are from 10 to 14 microns in diameter and stain intensely with Orange G. The nuclei have a diameter about one-third as large as that of the cells and each contains small masses of chromatin and a nucleolus. Many cells contain nuclei, and the cells are often arranged in pairs or fours like cartilage cells. These cells are probably non-vacuolated notochordal cells. Other cell clusters are larger and are enclosed in a definite rounded cavity. Many of the cells in these clusters are of the type just described, the others contain vacuoles of various sizes. The cells with large vacuoles are similar to the notochordal cells described by Luschka, Kolliker and Fric in man, and are somewhat like those of the pig’s notochord. There can be little doubt that these are also notochordal cells. The boundary of the central mass or notochord can be recognized under the microscope by a slight difierence in staining property and texture between the matrix of the cartilage and of the notochord. Not having studied the formation of the intervertebral disc of the dog, I am unable to assert more than the probability that its soft center is formed of notochordal and cartilaginous tissue, and that as age advances the two tissues become more and more alike.
The morphological and physiological meaning of the segmentation of the notochord is quite clear. The notochord and its membranous, cartilaginous or bony sheath have been assumed to be developed in inverse ratio to one another: the former being the predominant structure in less, and the latter in more specialized. forms. This is, in a general way, true, but the notochord does not degenerate in mammals. On the contrary, while it loses its primary continuity and surrenders to the vertebrae a part of its primary function, nevertheless the notochord continues to perform a part of its primary function, but in a somewhat different way and in connection with the segmented spine. The notochord is primarily surrounded by a continuous sheath of connective tissue, in which later appear isolated metameric cartilaginous elements. In mammals the cartilaginous elements unite, as we have seen, at such an early stage in development that they may scarcely be said to exist as separate units. The chondrostyle is deeply constricted near the center of each segment by the fibrous tissue of the intervertebral disc. Without these constrictions the chondrostyle would be too rigid to admit ready flexion, and with them the chondrostyle would not offer a suflicient resistance to axial stresses; hence the need of the nucleus pulposus which, being incompressible and also being closely invested by the fibro—cartilage and fibrous tissue of the disc, serves as a pad upon which the vertebrae turn. When the spine is unbent, the nucleus pulposus forms a rounded mass which is bound in on all sides by the fibrous tissue and fibro—cartilage of the disc, which, being attached to the heads of the vertebrae, forms a capsule whose layers are concentric with the nucleus pulposus. When the spine is bent forward, for example, the posterior portion of the annulus fibrosus is stretched straight, forcing the nucleus pulposus forward as a wedge-shaped mass between the inclined faces of the vertebrae, while the anterior part of the disc is pushed forward, its surfaces being drawn together, in a sharp curve or in one or more folds. Corresponding changes occur as the spine is flexed in other directions or is circumducted. The nucleus pulposus contributes largely to the strength of the spine and to its flexibility. The Chondrostyle is partially replaced by bone, but its intervertebral portions persist.
The tissue of the notochord is at first cellular and epithelial. Later it becomes syncytial and resembles closely mucoid connective tissue. It finally becomes cellular a second time and then is very similar to cartilage. Notochordal tissue is perfectly distinct from all other tissues of mammals, and passes through a very characteristic cytomorphosis.
The Shape of the Notochordal Enlargements in Mammals
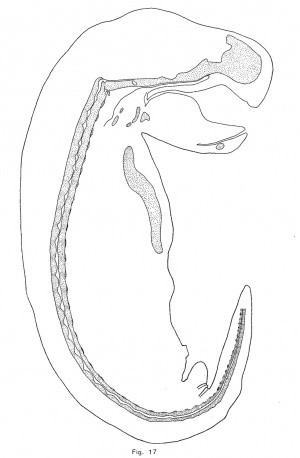
A description of the shape of the notochordal enlargements is very unsatisfactory alike because of the difficulty of accurate description; . because the shape of any particular dilation changes with growth; and because, although the shape of the enlargement in each species is remarkably characteristic, the great amount of variation which occurs renders it difficult to determine the normal type of expansion. In the opossum, as has been noted above, the crest of the notochordal undulation makes the upper contour of the expansion. From this boundary the enlargement grows downward to the level of the troughs (figs. 10 and 17). Later a small ventral process appears somewhat behind the crest of the enlargement and, as this process enlarges, the expansion becomes somewhat diamond-shaped, but the upper angle always remains in advance of the lower. Thus, although the shape of the notochordal enlargements is constantly changing, it is characteristic of the opossum at each stage.
In the pig, the trough of the notochordal wave lies slightly in front of the center of the intervertebral discs, and, as in the opossum, the convex wall of the notochord gives the cue to subsequent changes of form. The upper or concave wall of the notochord moves upward and, at the same time, the chondrification of the vertebra forces, or at least seems to force, the notochordal crest or summit forward until it comes to lie at the posterior edge of the disc. The notochord now makes a sharp descent from the posterior to the anterior edge of the intervertebral disc, and as it gradually expands the upper point moves forward to the center of the disc and the lower moves backward until a symmetrical diamond—shaped expansion is formed.
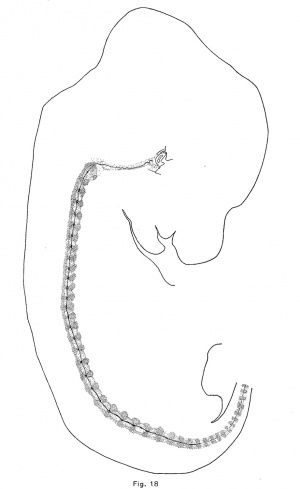
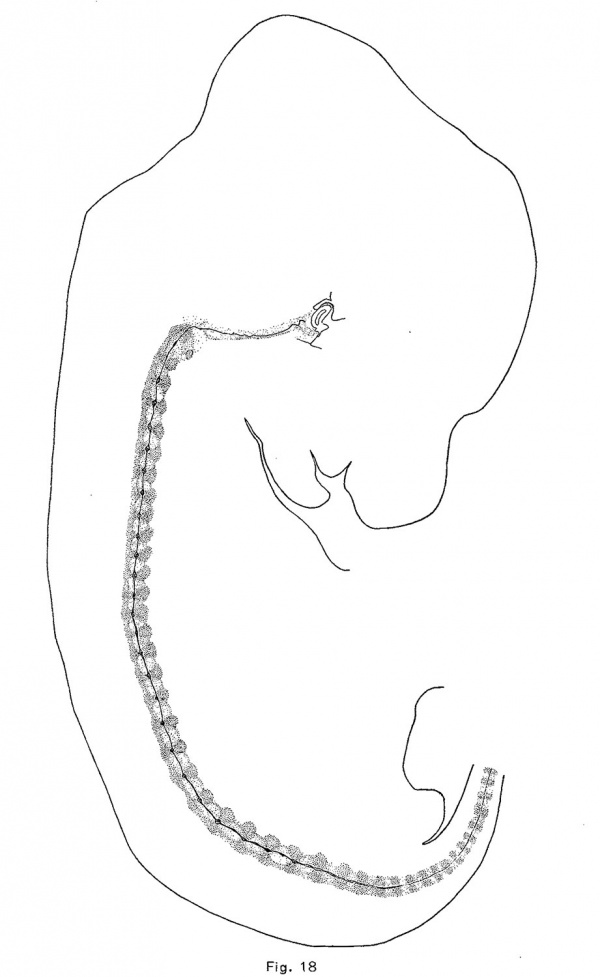
The notochord of the rabbit is first enlarged vertebrally, as in the pig and opossum, but to a greater extent. These dilations appear in the anterior vertebrae of an embryo of 10.5 mm which are just passing over into precartilage. The convex side (the lower) of the notochord is usually more expanded than the upper, and there is usually formed a. sharp ventral expansion of its sheath. The vertebral enlargements are larger and more symmetrical in an embryo of 12 mm., in which the vertebrae are precartilaginous. They occur throughout the trunk and they have apparently obliterated the notochordal undulations. Chondrification forces the notochord from the vertebrae into the intervertebral expansions, and in an embryo of 14.5 mm. (Fig. 18) the vertebral expansions have disappeared. The centers of the intervertebral discs are now loosening up in preparation for the formation of precartilage. The notochord first expands upward in the disc and later downward also. In an embryo of 18.8 mm., the enlargements are quite rounded and somewhat later they become of greater diameter than length. The upper moiety is usually somewhat larger than the lower, and somewhat in front of it (Fig. 9). The notochord is vastly more vacuolated in the embryo of the rabbit, and the vacuoles are more evenly distributed than in the other mammals studied.
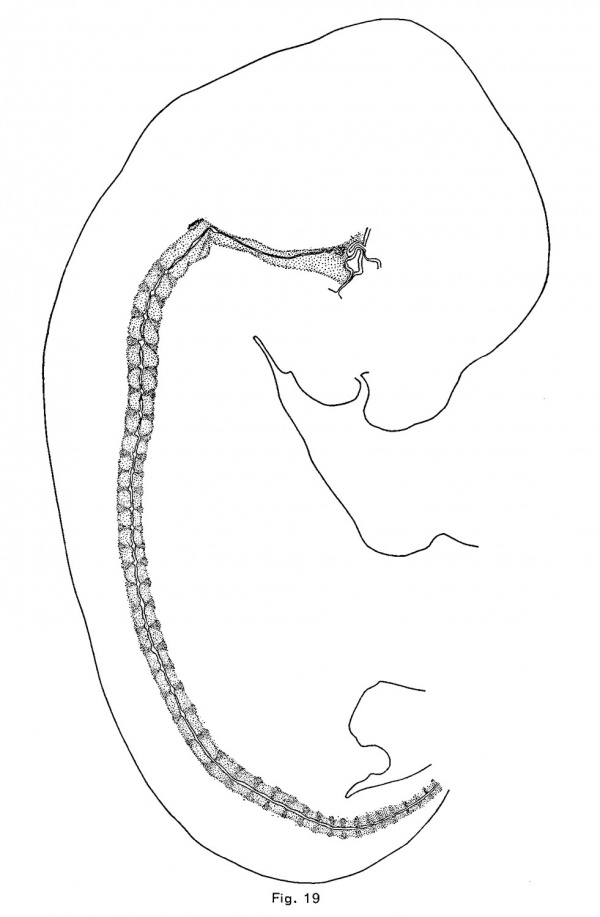
In the cat, as in the pig, opossum and rabbit, the first notochordal expansions are vertebral. These appear in an embryo of 10.7 mm. in the anterior vertebrae which consist of very loose mesenchymal tissue; and in an embryo of 12 mm they are larger and more numerous, the vertebrae being precartilaginous. The vertebrae are cartilaginous in an embryo of 15 mm, and the discs are precartilaginous. The intervertebral expansions (Fig. 19) are forming, but, unlike those of other mammals, the vertebral expansions persist at least until the cartilaginous vertebrae are calcified (in embryos of 39 mm) (Fig. 11). The first indication of the intervertebral expansion is a slight angular point which appears upon the convex or upper side of the crest of the notochord at the middle or near the anterior edge of the disc. As this increases in size, a similar but smaller and usually more rounded ventral point appears somewhat behind the first. The two angles usually become nearly equal, but they retain for a long time their asymmetrical position, and the enlargements are usually more flattened antero—posteriorly than ' in other mammals. There is also greater variation of the shape of the enlargement than in other mammals.
It should be noted in passing that a large part of the upper edge of the annulus fibrosus of the eighth to the seventeenth discs of the cat, and of the eighth to the sixteenth in the rabbit is converted into a transverse intercostal ligament which binds together the heads of each pair of ribs. In older rabbit embryos these ligaments are less distinct than in younger embryos.
The notochord of a sheep embryo of 14.6 mm. is of uniform diameter, and the chondrification of the vertebrae has just begun. In an embryo of 16.1 mm. enlargements have appeared in the first few vertebrae, but at 1’? mm. the enlargements are being compressed. The chondrification of the vertebrae is far advanced in the next older embryo in the collection, 26.1 mm. (Fig. 14). The centers of the intervertebral discs are precartilaginous and a somewhat top-shaped enlargement of the notochord has formed in each at the summit of the notochordal wave. The vertebral enlargement has been divided and the parts have been driven forward and backward toward, but not into the adjacent discs. The notochordal enlargements are thus intervertebral, but each is a deeply constricted cord., consisting of-the top-shaped central or intervertebral lobe and a pair of somewhat irregular and larger. lobes which lie at a considerably lower level in the ends of the adjacent vertebrae.
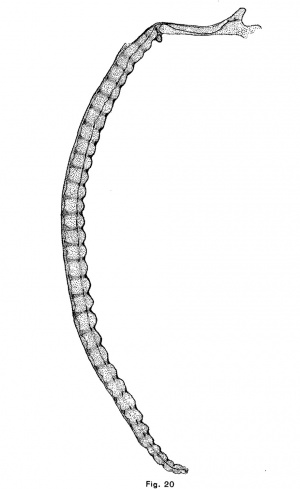
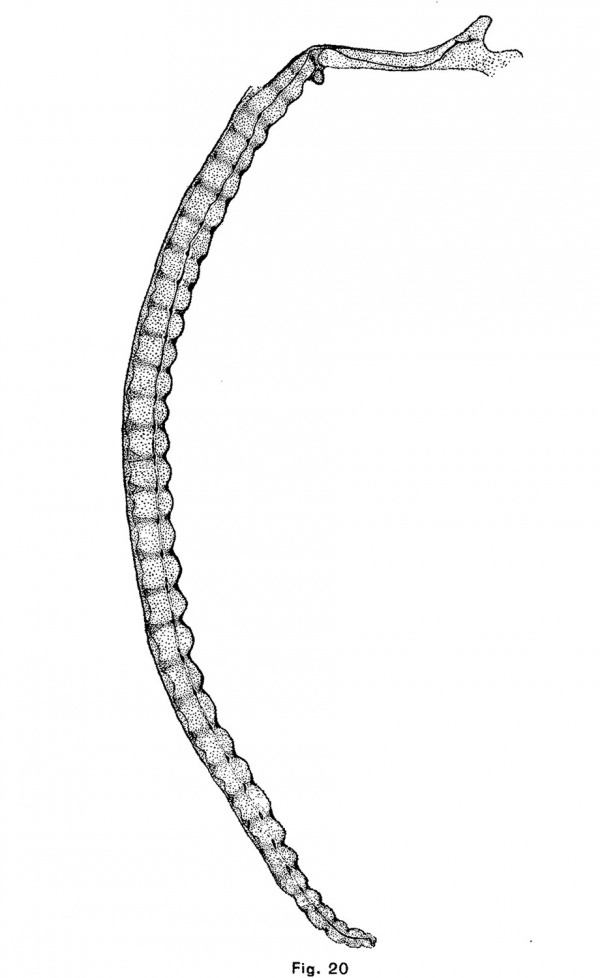
The human notochordal expansions are of yet another type. The notochord is situated considerably below the center of the vertebral column and vertebral expansions do not occur. In an embryo of 22 mm. chondrification of the vertebrae has advanced considerably and the notochord is sharply compressed in the center of each vertebra. It is correspondingly and symmetrically dilated intervertebrally. The centers of the discs are composed of precartilage. In an embryo of 32 mm (figs. 12 and 20) the chondrostyle is practically complete. The otherwise fusiform intervertebral expansion of the notochord is compressed above as by the sha.rp edges of the vertebrae, and consequently bears dorsally a small angular process which projects into the broad but thin cartilage of the disc.
The shape of the notochordal enlargements, in the mammals which have been studied, is perfectly characteristic at each stage of their development until they are transformed into the nuclei pulposi of the intervertebral discs.
The Relation of the Notochord to Chordoma
The course of the notochord in the skull of the human embryo, taken in connection with the results that have been reached in the preceding part of this paper, offers some suggestions as to the origin and nature of chordomla. It will be seen in Fig. 20 that the notochord makes a single large sigmoid curve in the base of the skull and that it lies near the surface of the cartilage at four points. It is near the upper surface, in the hypophysial fossa, a short distance behind the fossa, and near the foramen magnum and near the lower surface at a point midway between the hypophysial fossa and the foramen magnum. This is the normal course of the notochord in the skull of human embryos. Its curve is due to the fact that after the formation of the notochord, the mesenchyina, growing inward between the base of the brain and the pharynx, surrounds the anterior and posterior ends of the cranial portion of the notochord ; but, since the notochord is attached to the epithelium of the vault of the pharynx longer than elsewhere (until embryos a.re 9 or 10 mm. long), it collects above the central portion of the notochord. As the parachordal cartilages unite, they surround the notochord and hold it in this position. These facts were discovered by Froriep (1882), who described the later history of the notochord in the skull, and Gaupp cites_.,them in his article upon the skull in Hertwig’s “Handbuch der Entwickelungslehre.” The middle section bears a large but Variable number of kinks, short branches, thickenings, and other irregularities. These I find often involve the pharyngeal epithelium, which is here thickened and often invaginated. This section of the notochord has been found by Froriep to degenerate first, but in one of the embryos in the H. E; C., No. 851, 22 mm., it forms small masses of tissue imbedded in the retropharyngeal connective tissue which are very similar both to the adult notochordal tissue pictured by K6lliker and Fric and to the tissue of chordoma. The posterior part of the notochord is forced upward and backward and forms a large mass upon the upper surface of the skull-base‘a short distance in front of the tip of the tooth of the axis. The anterior part of the notochord is forced forward and, forming a large mass between the cartilage and the perichondrium of the hypophysial fossa, persists longer than elsewhere in the skull. We have seen that notochordal tissue, which is enclosed in a large mass of cartilage, is either forced from the cartilage or is enclosed in it and degenerates. If the tissue escapes from the cartilage it undergoes a typical cytomorphosis and forms adult notochordal tissue which is in all essentials like chordomal tissue. If this same process takes place in the skull we should expect to find notochordal tissue forced by the first chondrification of this region, that of the dorsum sellae, either forward into the hypophysial fossa, or backward and upward upon the dorsum sellce. The chondrification of the posterior end of the parachordal plate would, under the same conditions, force the notochord backward toward the apex of the odontoid process of the axis, or forward. In the latter case the notochordal tissue would be forced either out under the skull or forward to the junction of the sphenoidal and occipital cartilages or bones. Chordoma occurs at all these points, except between the pharyngeal epithelium and the skull, and only at these points. It occurs most frequently upon the dorsum sellw, less frequently in the hypophysial fossa and at the spheno—occipital junction, and in the malignant case reported by Fischer and Steiner it was found upon the upper surface of the basi-occipital bone. It should be noted also that the tumors which occur at the spheno-occipital junction lie in the marrow spaces, as though the tissue of the tumor had been forced into the bone under great pressure, as would be the case if notochordal tissue were compressed by the growth of both bones.
It seems to me probable that at least the majority of chordomas are comparable to cranial nuclei pulposi, and that chordoma should not be regarded as an abnormal growth of notochordal tissue, but merely a normal growth in an abnormal position. I am confident that chordorna also occurs beneath as well as above the spheno—occipital junction, but no such cases have been reported.
Summary
- The primitive vertebra of Remak or the scleromere of Bardeen is not a morphological unit and, in the pig, it is not resegmented to form the posterior part of the intervertebral disc and the anterior part of the following vertebra. Its central part forms the annulus fibrosus and the intervertebral portion of the chondrostyle from which arises the fibro-cartilage of the intervertebral disc. Its lateral portions form a large variety of structures, among which may be mentioned the ribs, the neural arches (or parts of them), the costo-transverse articulations, ligaments, myosepta, and perichondrium. In short, the primitive vertebra is a mass of undifferentiated mesenchyma which is never segmented longitudinally.
- The cartilage of the vertebra does not arise from a primary condensation of mesenchyma but from a secondary condensation which follows a loosening up of the relatively dense tissue of the scleromere. In the pig, mesenchymal tissue of nearly uniform density collects around the notochord of the trunk before the embryo is 7 mm. long. From this time until the embryo is 9 mm. long, the intersegmental or vertebral portions of this tissue become constantly looser while the midsegmental or intervertebral portions probably become slightly denser. A secondary condensation of the vertebral tissue takes place as the embryo grows from 9 to 12 mm., and at the same time there occurs a loosening up of the central part of the intervertebral disc preparatory to its secondary condensation. The secondary condensation of the tissue of the vertebrae and of the intervertebral discs produces precartilage. Chondrification begins when embryos are 14 to 17 mm. long.
- The notochord expands slightly in each vertebra at the time of the formation of precartilage in all mammals studied except possibly man. This vertebral expansion is usually obliterated as the vertebra chondrifies, and the vertebral portion of the notochordal sheaths and small pieces of notochordal tissue occasionally or regularly retained in the vertebra are destroyed before the ossification of the vertebra. Most of the notochordal tissue is forced into the intervertebral disc and, growing, forms the nucleus pulposus.
- Notochordal tissue undergoes a characteristic cytomorphosis. It is primarily cellular and epithelial; later it becomes a syncytial network with a mucin-like substance in its vacuoles; and finally it becomes cellular and closely resembles cartilage.
- The form of the notochordal enlargements in each species studied, is characteristic of that species at each stage of its development.
Bibliography
Bardeen CR. Studies of the development of the human skeleton. (1905) Amer. J Anat. 4:265-302.
BARDEEN, C. R., 1905. The development of the thoracic vertebra in man. Amer. Jour. Anat., IV, 163-174.
CARIUS, F., 1888. Ueber die Entwickelung der Chorda und der primitiven Rachenhaut bei Meerschweinchen und Kaninchen. Diss. Marburgn 33 pp.
DURSY, E., 1869. Zur Entwickelungsgeschichte des Kopfes, pp. 232, Atlas. Tiibingen.
FISCHER, B., und STEINER, 1906. Ueber ein malingnes Chordom der Sch'aide1Riickgratshiihle. Beitr. z. Path. Anat., Jena, XL, 109-119.
FRIC, R., 1904. Handbuch der Gelenke, Jena. 512 pp., 161 figs.
FRORIEP, A., 1882. Kopfteil der Chorda dorsalis bei menschlichen Embryonen. Beitr. z. Anat. u. Embryo}, als Festschrift 1. J. Henle.
1883. Zur Entwickelungsgeschichte der Wirbelséxule, insbesondere des Atlas und Epistropheus und dcr Occipitalregion. I. Beobachtung an Hiihnerembryonen. Arch. f. Anat. U. Phys, Anat_ Abt.
- 1886. II. Beobachtung an Séiugetierembryonen. Ibid.
GAUPP, E., 1905. Die Entwickelung des Kopfskelettes, Hertwig’s Handbuch der vergl. u. experimentalen Entwickelungslehre der Wirbeltiere. Jena. Bd. 3, Teil 2, 573-874.
GRAHL, OTTO, 1903. Eine Ecchondrosis physalifora spheno-occipitalis ungewohnlichen Umfangs mit interessanten klinisch-en Folgen. I. D. Gottingen.
HEIBERG, J., 1878. Ueber die Zwischenwirbelgelenke und Knochenkerne der Wirbelsiiule. Mitth. a. d. Embryo}. Inst. (1. K. K. Univ. Wien. I,
- 1880. 119-129.
JKGER, G., 1858. Das Wirbelktirpergelenk der Viigel. Sltz. Wiener Akad. XXXIII.
KEIBEL, F12, 1889. Zur Entwickelungsgeschichte der Chorda bei Siiugei-n. Arch. f. Anat. u. Phys., Anat. Abt., 329-388.
KLEBS, 1864. Ein Fall von Ecchondrosis spheno-occipitalis amylacea. Virchow’s Arch., XXXI, p. 396-399.
KOLLIKER, A., 1859. Ueber die Beziehungen der Chorda dorsalis zur Bildung der Wirbel, etc. Verh. phys.-med. Ges. Wiirzburg, IX, 193-242, T. I-III.
- 1867. Handbuch d. Gewebelehre des Menschen. Leipzig, 5th ed. 7-19 pp., 524 figs.
- 1883. Chordahiihle und Bildung der Chorda beim Kaninchen. Sitz. phys.-med. Ges. Wiirzburg, pp. 2-9.
LEBOUCQ, H., 1880. Recherches sur la mode de disparition de la corde dorsale chez les vertébrés supérieurs. Arch. de Bio1., I, 718-736, pl. 29, figs. 1-19.
LIEBERKUHN, 1882. Ueber die Chorda bei Siiugetl1ie1'en. Arch. f. Anat. u. Phys, Anat. Abth., 396-436. 1884. Ibid. 435-452.
Lijwzz, L., 1879. Zur Kenntniss der Siiugethierchorda. Arch. f. mikr. Anat., XVI, 597-612, pl. 29.
LUSCHKA, H., 1852. Die Halbgelenke.
- 1856. Die Altersverfiind. der Zwischenwirbelknorpel. Virchow’s Arch., IX., 309-327.
- 1857. Ueber gallertartige Auswiichse am Clivus Blumenbachii. Vircho\v’s Al'Ch., XI., 8-12, 4 figs.
- 1858. Die Halbgelenke, pp. 144, T. VI. Berlin.
MINOT, C. S., 1907. Segmental flexures of the Notochord. Anat. Rec, No. 3, 1907, 42-56, 6 figs.
MULLER, H., 1858. Ueber das Vorkommen von Resten der Chorda dorsalis beim Menschen. Zeitschr. f. rat. Med., II., 202, Taf. III.
RIBBERT, H., 1895. Ueber die experimentelle E-rzeugung einer Eechondrosis physalifora. Verh. XIII, Congr. f. innere Med., 455-464, pl. V.
1904. Geschwiilstlehre. Bonn. 662 pp., 596 figs.
ROBIN, C., 1868. Memoire sur Pévolution de la notocorde. Paris, 212 pp., 12 pls., 63 figs.
ROSENBERG, E., 1875. Ueber die Entwickelung der Wirbelsiiule und des centrale carpi des Menschen. Morphol. Jahrb., L, 83-197, Pl. III-V.
SCHAUINSLAND, H., 1905. Die Entwickelung der Wirbelsiiule. Hertwig’s Handbuch der vergl. und experimentalen Entwickelungslehre der Wirbelthiere. Jena. Bd. 3, Teil 2, 339-672.
SCHULTZE, 0., 1896. Ueber embryonale und bleibende Segmentierung. Vern. anat. Gesellsch. Berlin. 87-93. 1 fig.
- 1897. Grundriss der Entwickelungsgescliichte des Mienschen und der Séiugethiere. Leipzig.
STEINER, H., 1894. Ueber die Ecchondrosis physalifora spheno-occipitalis. Centralbl. f. path. Anat., V., 457-461.
Vmcnow, R., 1857. Ueber die Entwicklung des Schiidelgrundes. Berlin.
WEISS, A., 1901. Entwick. der Wirbelsiiule der weissen Ratte. Zeit. f. wiss. Zool., 69, p. 492-532, pl. 38-39.
Explanation of Figures
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Figs. 1 to 3 are photographs of the same magnification of similar frontal sections of pig embryos of 7.8, 9, and 12 mm. The notochord, the spinal nerves, the intersegmental vessels, the loose tissue of the vertebrae and the dense tissue of the “scleromere” should be noted.
Fig. 1. 7.8 mm pig embryo, section 381, H.E.C., N0. 430, X 306.
Fig. 2. 9 mm pig embryo, section 595, H.E.C., No. 54, X 306.
Fig. 3. 12 mm pig embryo, section 562, H.E.C., N0. 6, X 308.
Figs. 4 to 7 are photographs of median sagittal sections of vertebra of pig embryos of 14, 20, 24, and 75 mm.
Fig. 4. Fourth dorsal vertebra, embryo of 14 mm H.E.C., No. 66, section 134, X 133. '
Fig. 5. first dorsal vertebra, embryo of 20 mm H.E.C., No. 60, section 249, X 110.
Fig. 6. fifth vertebra, embryo of 24 mm. H.E.C.. No. 63, section 28, X 76.
Fig. 7. Eleventh dorsal vertebra, embryo of 75 mm. X 30.
Fig. 8. The nucleus pulposus and portions of the cumulus fibrosus and of the epiphyses of an adult pig. X 10.
Figs. 9 to 12 are photographs of median sagittal sections of vertebrae of embryos of the rabbit, opossum, cat and man.
Fig. 9. Twelfth dorsal vertebra of a rabbit embryo of 29 mm H.E.C., No. 171, section 182, X 60.
Fig. 10. Fourteenth dorsal vertebra of an opossum embryo of 12 mm H.E.C., No. 616, section 196, X 112.
Fig. 11. first dorsal vertebra of a cat embryo of 39 mm H.E.C., No. 394, section 195, X 56.
Fig. 12. Eighth dorsal vertebra, human embryo of 32 mm H.E.C., No. 292, section 176, X 56.
Fig. 13. Nuclei, wliich are possibly undergoing amitotic division, from a vertebra of a pig embryo of 12 mm H.E.C., No. 5, section 894, X 1000.
Fig. 14. Third dorsal vertebra of a sheep embryo of 26.1 mm H.E.C., No. 1112, section 235, X 36. The trilobate character of the intervertebrul notochordal enlargement is shown.
Fig. 15. Notochordal syncytium with mncin-filled spaces from a pig embryo of 150 mm. X 800.
Fig. 16. Three vacuolated cells from the nucleus pulposus of an adult pig. X 452.
Figs. 17 to 20
Reconstructions of a median section of the notochord and chondrostyle of embryos of the opossum, rabbit, cat and man.
Fig. 17. From an opossum embryo of 12 mm H.E.C., No. 616, X 16.
Fig. 18. From a rabbit embryo of 14.5 mm H.E.C., No. 162, X 13.
Fig. 19. From a cat embryo of 15 mm H.E.C., No. 437, X 12.
Fig. 20. From a human embryo of 32 mm H.E.C., No. 292, X 7.
Cite this page: Hill, M.A. (2024, May 1) Embryology Paper - The Later Development of the Notochord in Mammals. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_Later_Development_of_the_Notochord_in_Mammals
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G