Paper - The Development of the Infra-Umbilical Portion of the Abdominal Wall, with Remarks on the Aetiology of Ectopia Vesicae
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Wyburn GM. The development of the infra-umbilical portion of the abdominal wall, with remarks on the aetiology of ectopia vesicae. (1937) J Anat. 71(2): 201-31. PMID 17104636
| Online Editor |
|---|
| George McCreath Wyburn (1903-1985) was appointed regius professor of anatomy in the University of Glasgow (1948 - 1972) in succession to Prof. W. J. Hamilton, who has been appointed to the chair of anatomy at Charing Cross Hospital Medical School, London. Dr. Wyburn was a pupil of the late T. H. Bryce, and graduated at Glasgow in 1925 : after holding appointments in various hospitals, he returned to Glasgow as demonstrator in 1929. He became senior lecturer in anatomy in 1936, and was acting head of the Department during 1944-45. His researches include work on embryology, with special reference to bone formation, on the endocrinological aspects of reproduction, and on tissue-grafting (of skin, cartilage and cornea in particular). He was awarded the Struthers Gold Medal and Prize in 1939 for embryological research, and again in 1947 (with Dr. Paul Bacsich) for work done during the War on the repair of peripheral nerve injuries. (modified from Nature 162, 56-56 - 10 July 1948) |
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of the Infra-Umbilical Portion of the Abdominal Wall, with Remarks on the Aetiology of Ectopia Vesicae
By George M. Wyburn, M.B., CH.B., F.R.F.P.S.G.
Senior Demonstrator of Anatomy in the University of Glasgow
Introduction
The umbilical region of the human embryo has at all times attracted much interest as a prolific source of congenital defects and abnormalities, in spite of which, perhaps because of which, there still exists some gaps in our knowledge regarding the method of closure and completion of the belly wall.
An examination of the sections of a human embryo of 4-5 mm. in length (McIntyre II), with a favourable history, and showing the yolk sac with connecting duct, seemed to offer a fruitful field for study. The impossibility of limiting observation to the umbilicus alone was soon apparent, and it became obvious that satisfactory results could only be procured by a more comprehensive survey embracing the whole question of the development of the anterior abdominal wall.
The initial absence of a ventral wall to the embryo is found in all vertebrates with the exception of some of the Amphibia.
In the frog with its small, yolked, holoblastic egg gastrulation is complete—the blastopore forms at the equator with dorsal, lateral and ventral lips and wholly encloses the yolk cells of the vegetative pole. The yolk is thus taken into the body of the embryo and lies in the floor of the gut.
In the Elasmobranchs the large amount of yolk impedes cell division so that segmentation is meroblastic. The resulting blastoderm corresponds to the cells of the animal pole of the frog’s egg. The embryo arises from the posteromedian portion of the blastoderm, so that only the dorsal lip of the blastopore is intra-embryonic, while the lateral and ventral lips, formed of the extra-embryonic blastoderm, grow and enclose the yolk, the resultant sac being outside the body of the embryo to which it is connected by a stalk. Thus at an early stage the embryo is devoid of a ventral wall. The midgut, for a varying period, communicates with the yolk sac by the yolk stalk; this connexion is later obliterated.
In the Gymnophiona the anterior edge and the major portion of the lateral edges of the blastoderm take no part in the formation of the blastopore, and - there is not only an absence of ventral wall, but the yolk remains uncovered.
The embryonic shield of the Amniota, according to J enkinson, is the equivalent of the blastoderm of Gymnophiona. Blastopore formation affects only the shield, there being no true ventral lip. The edge of the extra-embryonic blastoderm grows steadily round the yolk entirely independent of blastopore formation, and finally encloses it at the vegetative pole. The yolk sac is extra-embryonic.
In the chick the enveloping blastoderm is continuous with the false amnion or chorion and is gradually separated from the yolk sac by the encroachment of the extra-embryonic coelom between somatopleure and splanchnopleure.
In placental mammals the egg has undergone loss of yolk and cleavage is accordingly holoblastic. The blastocyst with its surrounding trophoblast and enclosed embryonic material is possibly a modification to meet nutritional and protective requirements. The trophoblast, which from an early stage surrounds the yolk sac, is analogous with, if not the homologue of, the false amnion of the chick. This (the false amnion) is comparable to the extra-embryonic blastoderm of the Anamnia which encircles the yolk. The modified form of blastopore formation affects only the fembryonic shield. The embryo is therefore without a ventral wall and the yolk sac is extra-embryonic. The completion and closure of the anterior abdominal wall occurs at a late stage of development in placental mammals.
It is the opinion of Keith that the trophoblast in the human blastocyst can
be regarded as the precociously developed belly wall of the embryo. In View of
the comparative lack of knowledge of the earliest stages in the formation of the
human blastocyst, which acquires in a relatively short time its own salient
characteristics, and taking into account the fundamental differences in the
early ontogeny of the placental mammals which represent not a simple or
common evolutionary plan but rather a great diversity of methods of attaining
similar physiological requirements, it seems a hazardous experiment to affix a
phylogenetic label to any portion of the human blastocyst.
In the heavily yolked eggs the inert yolk substance is responsible for the
partial cleavage and limitation of gastrulation to the more active animal pole.
The placental mammals show the early differentiation of the tissues concerned
with protection and nourishment, and gastrulation is again much modified.
The absence of a true ventral wall in the early stages and the extra-embryonic position of the yolk sac may perhaps be regarded as a modification
incurred by nutritional necessity, which modification could be explained as a concomitant of incomplete and suppressed gastrulation ‘following on the more
prolonged embryological phase with its demand for “yolk” or “membranes”.
The anterior abdominal wall in its development falls naturally into three parts—supra-umbilical, umbilical andpinfra-umbilical. The present portion of the work deals with the infra-umbilical abdominal wall.
The Infra-Umbilical Abdominal Wall
This part of the ventral wall cannot be said to exist until the appearance of the genital tubercle with the subsequent development of an interval between the tubercle and lower attachment of the body stalk. In the series examined the interval first becomes apparent in an embryo of 12-5 mm. which shows a well-marked genital tubercle with a narrow dense band of mesoderm between it and the attachment of the stalk. Keibel & Mall give the first appearance of the tubercle as occurring in an embryo of 13 mm. This mesoderm, caudally merging into the genital tubercle and superiorly continuous with the tissue of the stalk, would therefore seem to be the basis of the future infra-umbilical portion of the belly wall.
An endeavour to trace the origin of this mesodermal mass through a series of younger embryos in the Glasgow Collection has involved a consideration of the primitive streak, of the cloacal membrane, of the mesoderm in general, and has led to a study of existing descriptions of early embryos in connexion with the individual points within this question.
Embryo Mcintyre I
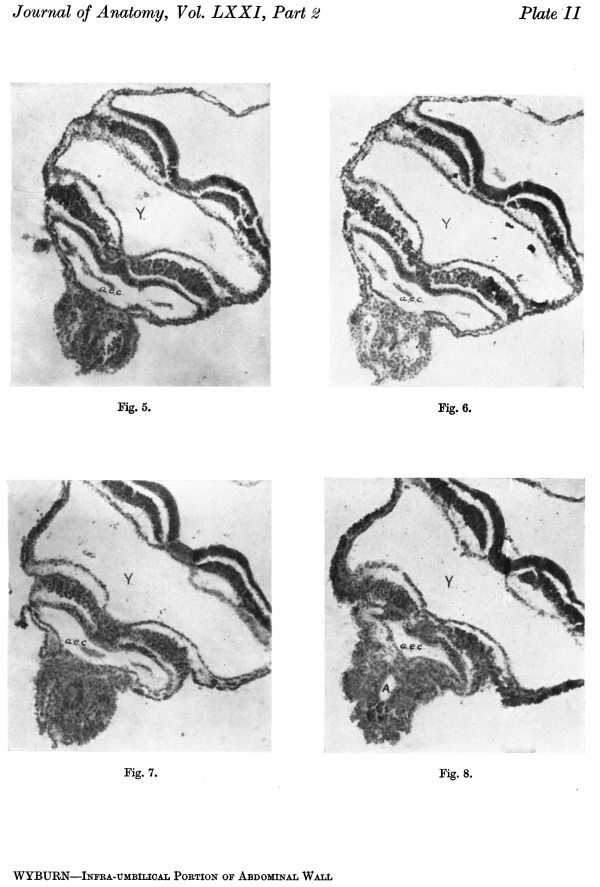
The first of the series examined was embryo McIntyre I. Observations on this embryo were published by Bryce (1924), but in his paper particular interest was centred on the more cranial sections of the embryonic shield. The embryo is of the presomite class, having a pronounced ventral curve at the hinder end, a primitiye streak measuring 0-33 mm., the major portion of which occupies the ventral aspect of the caudal flexure, and a total length of 1-4 mm., including the primitive streak.
Bryce is of the opinion that the McIntyre embryo is younger than embryo Gle (Graf v. Spee) and embryo Vull (Eternod), to which it bears a close resemblance and is probably a stage succeeding that of Ingalls in which, however, the disc has the dimensions of 2 x 0-75 mm.
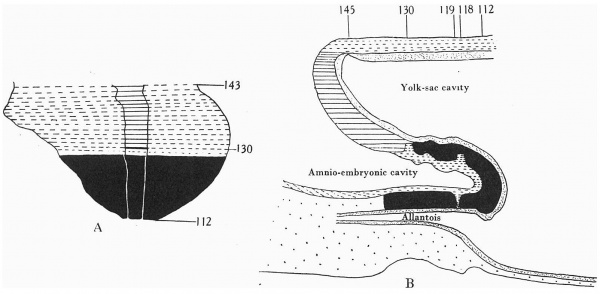
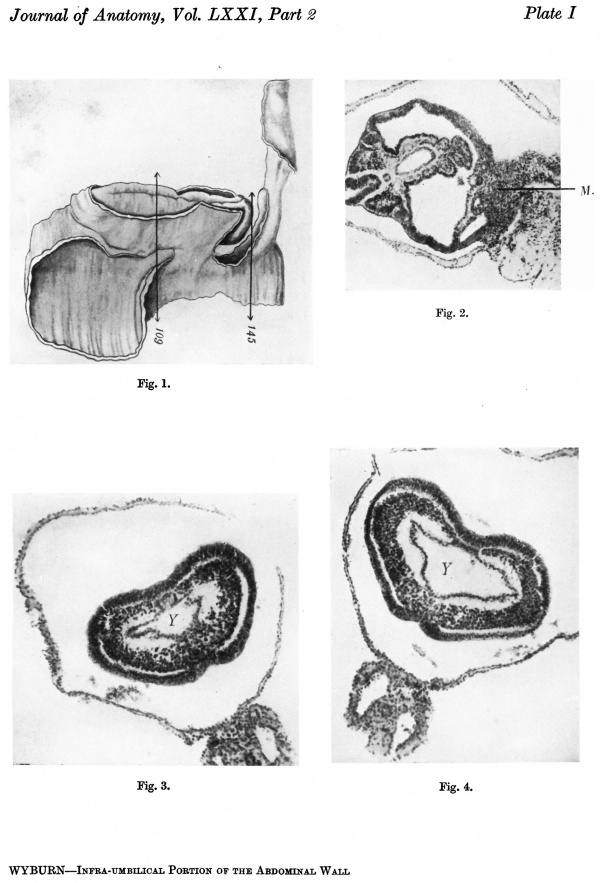
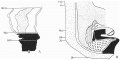
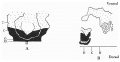
A graphic reconstruction was made of the caudal half of the embryo from sections 109-150 (Text-fig. 1A, B), -the exact position of the sections being indicated on the drawing of the wax models (Pl. I, fig. 1).
Section 146 (P1 I, fig. 3) is placed at the flexure and shows the opening of the archenteric canal. Sections 150—147 are through the region of the caudal bend, consequently there is some difficulty in interpreting the nature of the tissues and it is impossible to discriminate between ectoderm and mesoderm, but from the position it is probable that the section is cut through Hensen’s knot.
Cranial to 146 the sections show the primitive groove and primitive streak on the ventral aspect, i.e. on the caudal portion of the shield which has been bent round ventrally to become continuous with the body stalk.
From sections 145 to 142 (P1. I, fig. 4) the primitive streak appears as a
fusion between the ectoderm and mesoderm. The endoderm dorsally is in
contiguity with the mesoderm, but there is no distinct fusion of the layers. In
the sections cranial to 142 the primitive streak on the ventral aspect has the
appearance of mesoderm budding 011' from the ectoderm, more especially from
the walls of the primitive groove. The endoderm forms a distinct layer connected to the mesoderm by a few cellular strands. This appearance persists in
the next twelve sections, the only change being a gradual deepening of the
yolk—sac pocket with consequent approximation of endoderm and ectoderm,
and decrease of intervening mesoderm.
Text-fig. 1. Embryo McIntyre I. x 100. The numbers refer to the sections. A. Reconstruction of the surface of the caudal portion of the embryonic shield, i.e. the portion bent ventrally to form the tail fold. In the midline is the primitive groove. Area. in which mesoderm crosses the midline is shown in black. B. Reconstruction of a median sagittal section (see Pl. I, fig. 1). Description in text. Ectoderm—interrupted horizontal lines; endoderm—fine stippling; dense mesoderm—black; primitive streak — horizontal lines.
Section 130 (P1. II, fig. 5). The primitive groove is still present. The ectoderm forms a thickened cord of cells with the endoderm lying immediately above. There is no intervening mesoderm, but mesoderm is seen extending laterally on each side, separated by an interval from the ectoderm and endoderm. The appearance is that of a cloacal membrane, and extends only over
one section.
Sections 129-127 (Pl. II, fig. 6) again show mesoderm between the ectoderm and the endoderm with a gradual flattening out of the primitive groove.
The amount of mesoderm between the two layers gradually increases. There is no sign of budding off of the mesoderm from the ectoderm, and while the three
layers are in contiguity at the midline this may be in some measure artificial,
as both ectoderm and endoderm can be traced as distinct layers while the
mesoderm stretches across from side to side between them.
Section 126. There is no primitive groove. The ectoderm seems to be thickened in the midline, forming a plate of cells which at first glance would appear
to extend up to and establish contact with the endoderm. On further examination with an apochromatic 4 mm. lens this resemblance to a cloacal plate is found to be due to shrinkage of the mesoderm on each side leaving an apparent space between the “plate” and the mesoderm. Similar spaces can be seen in
the mesoderm more laterally as a result of formalin fixation. The ectoderm and
endoderm can again be traced as distinct layers across the middle line.
Section 125 (P1. II, fig. 7). Is similar to 126, but the resemblance to a cloacal plate is even more striking.
Sections 124.-120 (Pl. II, fig. 8). Mesoderm is again visible separating the ectoderm and endoderm.
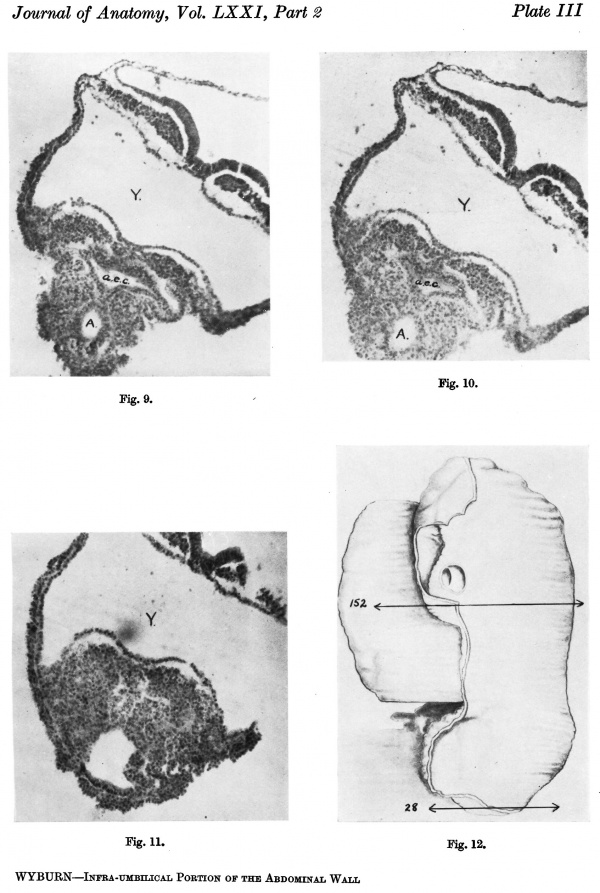
Section 119 (P1. III, fig. 9). The amniotic space between the disc and body stalk is diamond-shaped in the section. From the lower apex a distinct cord composed of a double row of cells can be made out extending down to and establishing contact with the endoderm of the allantois which at this level is oval in section and extending up the cord.
Section 118 (P1. III, fig. 10). The amniotic space is still diamond-shaped.
The cord of cells noted in the previous section is not now evident, the amnion
and allantois being separated by definite mesoderm. From the upper angle of
the space a short process extends up to but does not reach the endoderm of the
yolk-sac diverticulum, a single row of mesodermal cells intervening.
Sections 117-109 (Pl. III, fig. 11). The amniotic space gradually diminishes
and disappears, the mesoderm of the disc fusing with that of the body stalk,
the resultant mass being placed between the endoderm of the yolk sac dorsally
and that of allantois ventrally.
Bryce (1924) in the description of the embryo states that the primitive
streak extends up to section 117, from which point it is replaced by the cloacal
plate connecting the yolk-sac diverticulum with the ectoderm and found up to
the commencement of the allantoic duct proper. As before mentioned, in the
original description, interest was focused almost entirely on the cranial sections
and not on the region now examined.
No evidence of a cloacal plate is to be found in section 117, or those cranial
to that; in fact in those sections where the amniotic space is gradually closing
in, the ectoderm can be distinguished only with difliculty and ultimately dis-
appears entirely.
Definite contact between ectoderm and endoderm is established at two
points, viz. sections 130 and 119. The possibility of a cloacal plate in sections
125 and 126 has been rejected because of the alternative explanation of an
artefact during fixation. In this embryo there are therefore two points at which an undoubted cloacal membrane can be said to exist. Between these two points
mesoderm is continuous on the one hand with that of the primitive streak and
on the other with the mesodermal basis of the body stalk.
Regarding these findings there are two questions which occur—(A) the source of the mesoderm of the body stalk; (B) an explanation of the division of the cloacal membrane.
Source of Mesoderm of the Body Stalk
It is firstly necessary that there should be a clear conception of the structure of the primitive streak.
Bryce (1924) states that all three layers are fused over the length of the streak, but while the mesoderm can be observed budding off from the ectoderm, the basal part of which is indistinguishable from that layer, the endoderm, except in the most caudal section where it is not present, preserves its identity and can be traced as a distinct layer across the middle line. There is certainly no interval between the mesoderm and endoderm, but in none of the sections of the primitive streak did there appear to be any actual budding off of mesoderm from endoderm.
Hill & Florian (1931), describing the primitive streak of the “Dobbin”
embryo (0-98 mm.), state that the endoderm is clearly visible on the ventral
aspect of the streak. Ingalls (1918) in his embryo of 2 mm. describes a considerable interval between the endoderm and the primitive streak, while
Heuser (1930), in one of 16 mm., shows the streak as a fusion of ectoderm and
mesoderm, the endoderm lying quite free. Debeyre (1912), on the other hand,
states of the region of the primitive streak: “ Tout le long de la ligne primitive
ou a son niveau on remarkera l’union intime qui existe entre les feuillets.”
Florian (1934), from reconstructions of embryos W0, Op. and Fetzer, is of the
opinion that in the primitive streak primordium there is a fusion of endoderm
and mesoderm in the midline and that this persists for some time, while
Rossenbeck (1923), writing on the embryo Peh-Hochstetter, considers the
primitive streak as a fusion of ectoderm and mesoderm, the endoderm being
separated by an interval from the actual primitive streak.
This disparity in the description of the character of the streak is probably due to variations in its form according to the degree of development, not necessarily corresponding to the size of the embryonic shield; but most observers seem agreed that while there is a fusion of all three layers in the region of Henson’s knot, the actual streak itself is represented by a fusion of ectoderm and mesoderm. Grosser (1927) expresses the opinion that the primitive groove —is due to a sinking of the ectoderm on account of the heavy emigration of mesodermal cells.
The blastocyst of man and higher mammals is characterized by the precocious development of mesoderm, which being in the later stages an angioblastic tissue arises in accordance with the mode of nutrition of the embryo (Bryce).
Of three of the earliest ova showing the embryonic rudiment, in that of
Peters (1899) the amniotic and yolk-sac vesicles are separated by mesoderm
except in the middle line at the cranial end; in T.B. 1 (1908) there is a considerable interval between the two vesicles which are situated in a cavity already
filled with mesenchyme——the unusual distance between the amniotic cavity and
the yolk sac may be to some degree artificial; Streeter (1927) in a description of
section 4 of the Miller ovum—the sixth section through the rudiment—states
that the amniotic and yolk-sac cavities are in a common compartment
surrounded by mesenchyme, the ectoderm of the amnion being in contact
with the endoderm of the yolk sac with but a narrow intervening cleft.
There is, however, some doubt as to what constitutes the yolk sac in the
Miller ovum.
Fetzer (1910), describing an embryo of 0-23 mm. in length, states that there
is no primitive groove, medullary fold, or neurenteric canal. Nevertheless the
yolk-sac endoderm and ectoderm of the shield are separated by a layer of
mesenchymal cells passing towards the cranial end and fusing in the midline
with the ectoderm. According to Rossenbeck this is undoubtedly the primitive
streak.
Graf von Spee, discussing Peter’s ovum, is of the opinion that the anterior
and posterior poles of the amniotic and yolk-sac vesicles grow into the solid
mesenchyme separating them from the chorion which mesenchyme, acting as
a wedge, ultimately completely separates the ectoderm and endoderm except
for an area in the midline at the caudal end—the cloacal membrane.
It is now well known that the primary mesenchyme in the human ovum and that of the higher primates makes its appearance long before there is any indication of a primitive streak, thereby differing fundamentally from that in the lower mammals.
Streeter (1927) is of the opinion that this mesenchyme arises either from
the inner cell mass during the formation of the segmentation cavity or alternately is split off from the trophoblast. The close relation of the mesenchyme
to the trophoblast inclines him to the latter explanation.
Bryce (1924) accepts the former hypothesis and regards the differentiation
of the primitive mesenchyme as simultaneous with that of ectoderm and endoderm from the formative ceH mass.
Strahl & Beneke (1910) express the opinion that the primary mesoderm is
split off at the morula stage, the ectoderm and endoderm remaining Widely
connected in the early stages. Later this connexion is restricted to the
primitive streak region.
Hubrecht (1909) finds that in Tarsius the mesoderm of the connecting stalk is derived from a proliferation of the ectoderm at the posterior margin of the shield which Bryce (Quain) suggests is a precocious development of the primitive streak formed later as an extension of this area.
In a history of the primates Hill (1932) shows four stages in the genesis of the body stalk:
- Lemur, e.g. Loris, where the mesoderm arises relatively late (blastocyst 3 mm.) and both extra- and intra-embryonic mesoderm are derived from a primitive streak. The mesenchyme of the allantoic outgrowth which is the homologue of the connecting stalk is formed from the primitive streak, arising from its hind end and extending round the cloacal membrane.
- In Tarsius the connecting stalk mesenchyme is in the first instance a proliferation of the posterior margin of the shield ectoderm, later being reinforced by a backward extension from the primitive streak.
- In “Hapale” embryo there is an area of proliferating mesenchyme at the caudal margin of the shield which Hill suggests corresponds to the precociously developed hinder end of the primitive streak in Tarsius and which in his opinion forms the whole of the primary mesoderm, including that of the stalk. The extra-embryonic mesenchyme is differentiated some considerable time before the appearance of the primitive streak.
- The fourth stage is the human embryo.
Florian (1930) has demonstrated the existence of a proliferating area in the posteromedial margin of the shield ectoderm in the embryo Fetzer and believes this to be the source of the stalk mesenchyme and the major portion of the rest of the primary mesenchyme in the human. In a recent publication (1933) he avers to have satisfied himself as to the existence of such a region in the embryos Peters, Werner, and Von Mollendorff’s W0 and Op. This accords with the views of Rossenbeck that in the human the primitive streak forms relatively little of the mesoderm.
It is to be noted that in the embryos of Loris, Tarsius and Selenka’s Kleim S., the ectoderm and endoderm of the rudiment are in contact in the early stages; the duration of contact diminishes as the scale is ascended.
In answer to question A (p. 206) it can therefore be stated that in the human the mesenchyme of the stalk is in all probability derived from a proliferation of the caudal margin of the shield ectoderm, and one can postulate a stage, perhaps transient, when the ectoderm and endoderm are connected in the midline over a considerable extent of the embryonic shield. As seen in the sections of McIntyre I and also demonstrated by Florian (1934) in embryo Beneke (size 0-75 mm.) and by Hill and Florian (1931) in the Dobbin embryo (0.98 mm.), the mesoderm of the primitive streak is continuous round the cloacal membrane with that of the stalk.
As there is no histological diiference between the primary as compared with the secondary mesoderm it is impossible from a study of the sections to say where the one ends and the other begins, but it is reasonable to assume that the mass of mesoderm beyond the cloacal membrane and on the caudal aspect of the stalk is compounded of primary and secondary mesoderm.
Division of the Cloacal Membrane
Until the recent work of Florian, scant attention was given by embryologists to the caudal region of the human embryo. Any semblance of union between the ectoderm and endoderm in the expected region by direct apposition or indefinite cords of cells was designated “cloacal membrane”. The approximation of endoderm and ectoderm with no intervening mesoderm in section 130 of McIntyre I gives the appearance of an undoubted cloacal membrane. Similarly the connecting cord of cells from the amniotic ectoderm to the endoderm of the allantois is clearly demonstrable in section 119. On the other hand, a cursory examination of sections 125 and 126 might lead to their interpretation as cloacal plates, whereas a more careful study shows the impossi- bility of excluding mesoderm intervention.
Section 118 with the cord of cells from the amnion extending almost to the
endoderm suggests that at this point former fusion between the two layers is
being broken down by a pressing in of mesoderm from each side.
Most observers are agreed as to the difliculty in defining the limits of the cloacal membrane. Hill & Florian (1931), describing the Dobbin embryo, comment on this, more especially the differentiation between the membrane and the primitive streak. Of this region Debeyre (1912) says: “ A son extrémité postérieure comme dans l’embryon de Keibel-Frassi (1907) se trouve l’ébauche d’une membrane cloacale trés probablement au moins (bien que nous ne puissons le vérifier directement sur les coupes).” Much of this difficulty, however, arose from the failure to recognize the incompleteness of fusion in many
parts of the “membrane”.
Sternberg (1927), in an embryo of 4 somites, described a fusion of ectoderm
and hindgut endoderm, and also separate connexions between amniotic ectoderm and the endoderm of the allantois. Since then similar conditions have
been noted in other specimens—Florian (1930) in embryo Bi II (also of 4
somites); Hill and Florian (1931) in the Dobbin embryo; and West (1930),
describing an embryo of 8 somites, states that the cloacal membrane is very
indefinite, actual contact being established in only two sections.
In the Ingalls (1918) embryo there exists what the author designates as
an amnio-allantoic duct characterized by a pocketing of the amniotic cavity
towards the allantois with contiguity of ectoderm and endoderm. Rossenbeck
(1923), after noting the cloacal membrane as a cord of cells from the ectoderm
to the endoderm in the Peh-Hochstetter embryo, in a subsequent paragraph
describes an apposition of the dorsal wall of the allantoic passage with the
floor of the amniotic cavity.
From a study of McIntyre I, along with the facts available from recent work
on this subject, more especially the researches of Florian, I am of the opinion
that the primordium of the cloacal membrane is in the early stages relatively
extensive, the caudal fusion of endoderm and ectoderm being between the
amnion and the future allantois, at this stage not yet separated off from the yolk sac. At a subsequent period the allantoic portion of the connexion is
broken down by an extension of the mesoderm in towards the middle line, the
chordall part remaining as the site of the permanent cloacal membrane. This
undergoes extension later along with the growth of the cloaca and embryo as a
whole.
Table I. Table showing extent of cloacal membrane relative to the size of the embryo
| Embryo | Length of embryonic disc Presomite | Cloacal membrane |
|---|---|---|
| W.O. (V. Mollendorlf, 1925) | 0.25 mm | What von Mollendorlf describes as the cloacal membrane is, according to Florian, the primitive streak, and von Mollendorif’s allantois is the cloacal membrane of Florian |
| “Fetzer” (Fetzer & Florian, 1930) | 0.26 mm | The cloacal membrane is separated by an interval from the primitive streak |
| T. F. (Florian (1928)) | 0.4 mm | The cloacal membrane is a cord of cells which extends on to and connects the allantoic endoderm with the amniotic ectoderm |
| Bi I | ? | Primitive streak reaches the cloacal membrane |
| Bi 24 | 0.625 mm | Length 0.06 min |
| “Hugo” Stieve (1926) | 0.635 mm | Length 0.035 mm |
| “Beneke” | 0.75 mm | Cloacal membrane separated by an interval from (Strahl & Beneke, 1910) the primitive streak |
| W.A. 17 (Grosser, 1930-1) | 0.85 mm | Length 0-13 mm and extends on to the allantois |
| “Dobbin” (Hill & Florian, 1931) | 0.98 mm | Length 0.08 mm |
| Peh-Hochstetter Rossenbeck (1923) | 1.4 mm | Cloacal membrane extends well on to allantois |
| Heuser (1930) | 1.6 mm | Cloacal membrane extends well on to allantois |
| Ingalls (1918) | 2.0 mm | Length 0.12 mm, and there is an additional connexion between the allantoic endoderm and amniotic ectoderm |
| Somites | ||
| F. von Orts Llorca (1934) | 2 | Cloacal membrane in two parts—one of which is allantoic |
| H 3 (Wilson) | 2-3 | Cloacal membrane at the commencement of allantois |
| Bi II | 4 | Cloacal membrane broken up—one portion involvingallantois |
| Sternberg (1927) | 4 | Cloacal membrane is in four portions |
| Pfannenstiel-Kromer | 5-6 | Very short cloacal membrane extending some distance into the allantois |
| Dandy (1910) | 7 | Not mentioned |
| Payne (1925) | 7 | Not mentioned |
| West (1930) | 8 | Length 0.09 mm |
| Etemod (1899) | 8 | Length 0.05 mm |
| Corner (1929) | 10 | Cloacal membrane terminates before reaching the allantois |
| Embryo Bl. XI | 10 | Cloacal membrane terminates before reaching the allantois |
| Pfannenstiel III | 13-14 | Has trebled its length compared to P£annenstiel-Kramer |
| Heuser (1930) | 14 | Length 0.18 mm |
| Atwell (1930) | 17 | Length 0.18 mm Extends to the allantois |
| Davis (1924) | 20 | Length 0.16 mm |
Table I is a series of embryos arranged according to size, with, as far as can be obtained, the available data regarding the cloacal membrane.
In the earliest specimens before the appearance of the allantois the fusion of ectoderm and endoderm occurs at the caudal end of the disc cranial to the attachment of the body stalk. In later embryos of the presomite class, in every case the membrane includes the allantois, and this condition remains up to the stage of about 4 somites. At this time the connexion with the amnion is being interrupted by mesoderm, and in the immediately older embryos of about 5-8 somites the cloacal membrane will be relatively much reduced. This is exemplified in the Pfannenstiel-Kromer embryo of 5-6 somites, and may account for its apparent absence in that of Dandy (1910)—7 somites—and Payne (1925)—7 somites. After the 10-somite stage the cloacal membrane seems to undergo proportionate development, although according to Heuser there are variations in its extent which have no relation to the size of the embryo.
The inferences deduced from the table, owing to the small series and lack of
mathematical measurements, can only of course be accepted in a broad sense,
but are of interest as coinciding with the suggestions made from observations
on embryo McIntyre I. It should be noted that in the Dobbin embryo Hill
and Florian observed the presence of granules in the cells of the cloacal membrane
which in the opinion of Florian are to be regarded as indicative of degeneration of the cells. These granules occurred in the areas of incomplete fusion.
F. von Orts Llorca (1934) has demonstrated a very similar condition to that in
in McIntyre I in an embryo of 2 somites where he shows the cloacal membrane
in two parts, (1) at the junction of allantois and yolk sac, (2) between the
amnion and allantois. Regarding the membrane he states: “Da in dieser
Gegend zahlreiche absterbende Zellen vorhanden sind, scheint es, dass die
Kloakenmembran in friihen Entwicklungsstadien eine grossere Ausdehnung
besitzt und dass sie zunachst bis auf das Allantoisdivertikel iibergreift, wahrend spater der der Allantois entsprechende Teil der Kloakenmembran verschwindet und das Mesoderm zwischen das Amnion- und das Allantoisepithel vordringt.”
The terminal point of the primitive streak is still a matter of controversy.
In embryo McIntyre I, as already noted, sections 142-131 show the mesoderm
distinctly budding off from the ectoderm of the primitive groove, whereas in
the sections after the first part of the cloacal membrane, i.e. in 129-119, while
the mesoderm and ectoderm are in continuity there is no actual formation of mesoderm. One would therefore infer that the primitive streak terminates at the cloacal membrane.
1 In this paper the portion of the cloacal membrane nearest the body stalk will be designated the “allantoic end”, and the portion most distant from the body stalk the “ chordal end”.
Keibel and Elze express the opinion that the cloacal membrane should be
regarded as a modified part of the primitive streak which reaches up to the
body stalk. Their views are based on the presence of thickening of the ectoderm
which stretches down almost to the allantois. This I would regard as a partially
obliterated cloacal membrane and not primitive streak. Grosser (1927)
implies that the streak becomes divided, a part growing into the tail bud, and
the remainder being represented by the cloacal membrane. He also states that
the primitive streak does not appear until the disc is 0-5 mm. long. The cloacal
membrane has been shown to exist when the anlage of the primitive streak is
represented only by a nodal fusion of all three layers (Florian, 1934). In the
same monograph Florian has also demonstrated in E. Beneke and Fetzer an
interval between what he regards as the cloacal membrane and the primitive
streak in which all three layers are distinct and separate. In the later stages
the primitive streak grows down to the cloacal membrane.
The name primitive streak was first given to the appearance observed in
the chick embryo, the “streak” being essentially caused by the formation of
mesoderm from the ectoderm at that particular region. As there is no meso-
derm in the formation of the cloacal membrane it can be assumed that the
actual streak as observed in the chick does not include the membrane. Keibel
argues that the membrane is a secondary formation, the approximation of the
ventral rim of the cloaca and ectoderm pressing the mesoderm from the mid-
line. Recent Work has negatived this as the union of ectoderm and endoderm
at the caudal end of the shield occurs as early as, if not before, the primordium
of the primitive streak.
Grosser (1927) inclines to the view that the ectoderm and endoderm of the
embryonic disc are everywhere separated by an interrupted layer of primitive
mesoderm, but quotes von Mollendorff’s assertion of a ribbon-like connexion
between the layers in the midline caudally which Rossenbeck regards as a
connexion from the morula stage.
Strahl & Beneke (1910) express the opinion that the ectoderm and endoderm
remain widely connected in the early stages, this connexion being later restricted
to the primitive streak region.
Opinion seems unanimous that some degree of contact is maintained in the
midline, and it is not unreasonable to conclude that in man as in lower mammals
there is continuity along the length of the disc, separation being effected as
suggested.
The hypothesis advanced here is as follows. Despite the precocious de-
velopment of the primary mesenchyme in the human, it seems reasonable
from phylogeny to postulate a stage when the ectoderm and endoderm are in
contact in the midline. The appearance of the primordium of the primitive
streak representing the fused lips of the blastopore acclaims the formation of
secondary mesoderm separating the ectoderm and endoderm in this region.
The invagination of the dorsal lip with the pushing forward of the head process
intervenes between the two layers of the disc cranially. Anterior to the head
process the layers remain in contact-—the site of the buccopharyngeal membrane.
Caudal to the fused lips of the blastopore and reaching to the attachment of the stalk remains a relatively extensive area of contact, the anlage of the cloacal membrane. The yolk-sac endoderm of the caudal portion of the mem- brane is predestined to become the epithelium of the allantoic diverticulum, and, as suggested above, the ectoderm and endoderm are here later separated by incursions of mesoderm. The cloacal membrane would thus be the homologue of the ventral lip of the blastopore and adjacent area.
From the cranial end of the primitive streak wings of mesoderm extend
forwards on each side of the head process gfusing anteriorly in the region of Elie
mesodermal field of the protocardial area. Similarly from the caudal end of the
streak as seen in McIntyre I, mesodermal processes extend round the cloacal
membrane fusing with the stalk tissue in the region of the posterior mesoder-
mal field. There would therefore be a close analogy in the development of the
poles of the disc, the more rapid progress of the caudal end in the human
obscuring many of the features.
The above assumption is compatible with the facts as observed at a later stage of development.
The bearing of these findings and conclusions on the problem of ectopia vesicae will be discussed later.
Description of Embryos from 2-4 to 40 mm
2.4-mm embryo
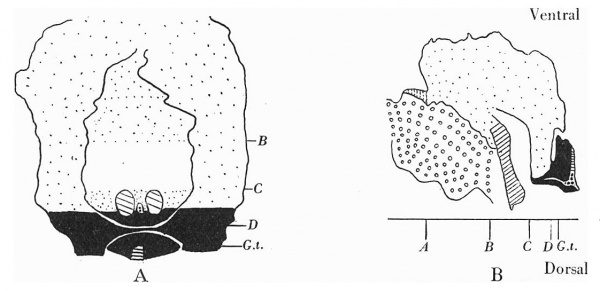
The next of the Glasgow series to be examined is an embryo of 2.4 mm. in length. This was obtained as a result of abortion, and although seeming for the most part morphologically normal the tissues show some pathological change and the region of the tail fold is abnormal. This and the plane in which the sections were cut forbid any definite statement regarding the position and size of the cloacal membrane.
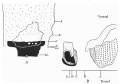
A photograph of a wax plate model of the embryo is given in P1. III, fig. 12, and it is evident that there is some degree of torsion on a longitudinal axis, the attachment of the stalk and yolk sac being twisted to the right, while the caudal extremity turns to the left.
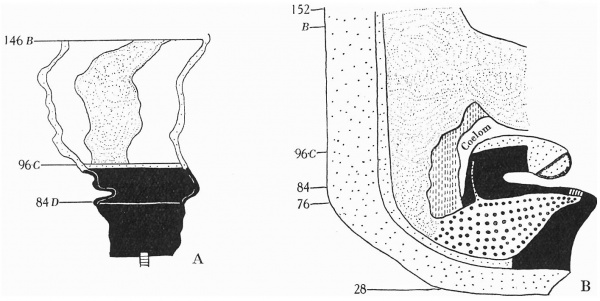
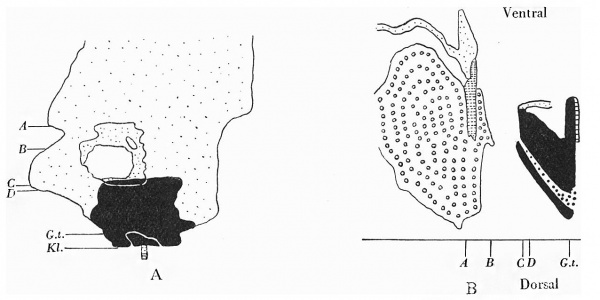
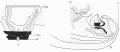
Section 84 (Pl. I, fig. 2) shows a mesodermal mass on the caudal aspect of the stalk attachment, and the graphic reconstruction of a coronal and sagittal plane give some idea of the extent of this tissue (Text-fig. 2A, B).
It envelops the embryonal end of the stalk, extending for a short distance only into the stalk substance and becoming continuous below with the mesoderm forming the ventral wall of the caudal end. As has been stated, the mesodermal mass is composed of primitive and secondary mesoderm (the secondary mesoderm extending in wing-like processes round the cloacal membrane), the two fusing and forming the tissue seen in the figure, and illustrated in the reconstruction.
It is to be noted that at this stage there is no anterior abdominal wall, the
entire ventral aspect below the septum transversum being occupied by the
wide communication with the yolk sac and the attachment of the stalk. The
allantoic end of the cloacal membrane is some distance from the stalk attachment.
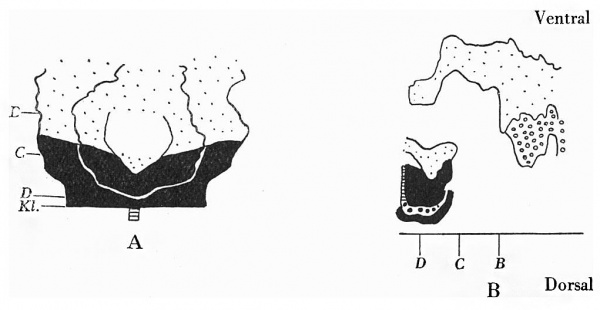
4.5-mm embryo (McIntyre II)
This embryo was obtained following a hysterectomy and sterilization in a case of mitral stenosis and incompetence with a history of cardiac trouble during previous pregnancies. When brought into the Department the Vesicle was perfectly clear and transparent with no magma present. On opening the vesicle the umbilical arteries could be seen actually pulsating. There is therefore every probability of a normal structure, and considerable weight may be attached to the findings in this case.
Text-fig. 2. Embryo 2.4 mm. x50. Numbers refer to the sections. A. Reconstruction of the ventral surface from the septum transversum to the cloacal membrane. B. Reconstruction of a median sagittal section (see P1. III, fig. 12). Description in text. Gut and yolk sac—fine stippling; cloaca and allantois—-large dots; cloacal membrane—-straight lines; dense mesoderm — black; spIanchnopleure. L—interrupted lines. Between B and 0 the ventral wall is occupied by the mouth of the yolk sac. D. = the level of the caudal attachment of the body stalk.
Pl. VI, fig. 21, is a photograph of a wax-plate reconstruction from which it is observed that the ventral wall is still occupied by the communication with the yolk sac considerably narrowed from that in the 2-4. mm. Below this is the stalk attachment.
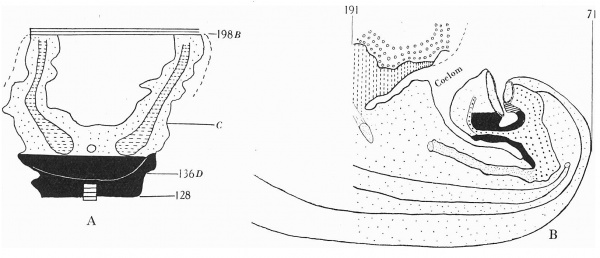
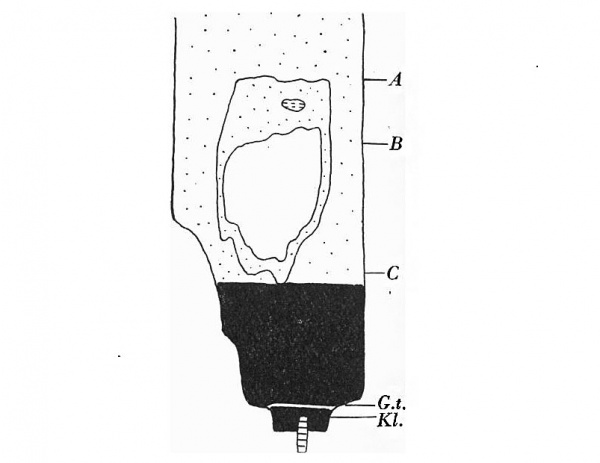
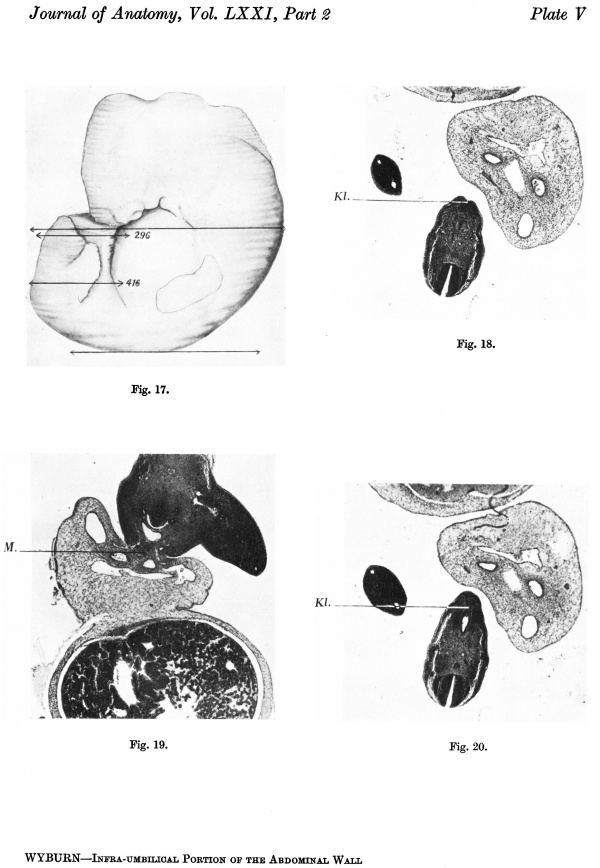
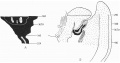
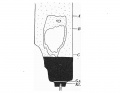
The embryo was cut transversely in a cranio-caudal direction, and Text-fig. 3A shows a graphic reconstruction of the ventral surface from the septum transversum to the region of the cloacal membrane. The caudal end is represented straightened out and the approximate position of the cloacal membrane indicated. Text-fig. 3B is a graphic reconstruction in a plane indicated in P1. VI, fig. 21. The cloacal membrane extends over twenty sections, each of 10 p., and has therefore a total length of 0-2 mm. Owing to the direction of the sections and the folding of the caudal end, the distance of the membrane from the stalk attachment cannot be accurately measured, but it is apparent from a study of the reconstruction that the allantoic end of the cloacal membrane is some distance below the lower attachment of the stalk.
Text-fig. 3. Embryo 4-5 mm. (McIntyre II). X50. The numbers refer to the sections. A. Reconstruction of the ventral surface from septum transversum to cloacal membrane. The tail is represented straightened out. B. Reconstruction of median sagittal section (see Pl. VI, fig. 21). The yolk sac is not shown. Description in text. Gut — fine stippling; cloaca and allantois — large dots; dense mesoderm — black; cloacal membrane — horizontal lines; allantoic vessels ~ interrupted horizontal lines; septum transversum — interrupted vertical lines; liver — small circles. The interval between B and 0 is the communication between extra- and intra-embryonic coelom. D=the level of the caudal attachment of the body stalk.
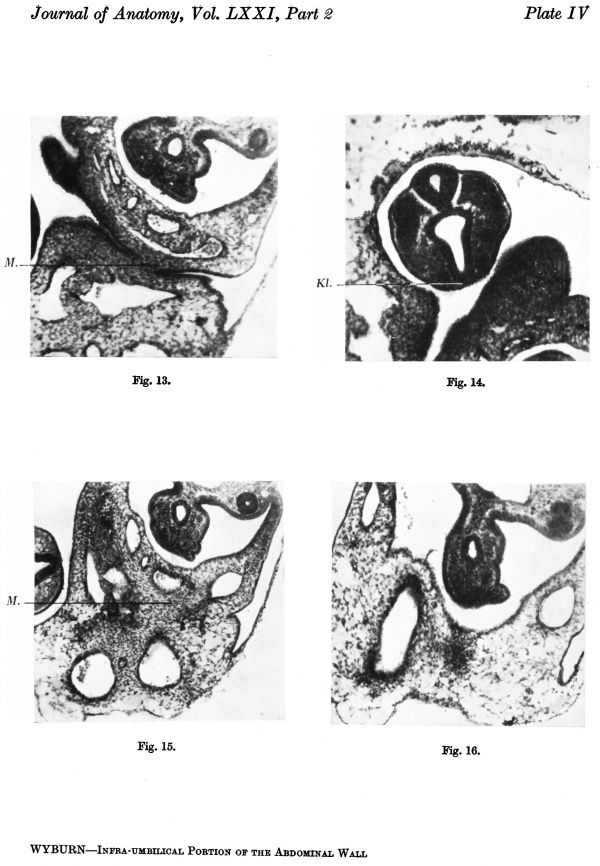
Section 136 (P1. IV, fig. 13) shows the lower attachment of the stalk and the hindgut with its dorsal mesentery. There is no indication of the cloacal membrane, indeed the cloaca itself only comes into view in the more caudal sections.
Pl. IV, fig. 14, is a photograph of section 131 and is the allantoic end of the cloacal membrane. It can be seen that the section is at the head of the tail fold and some distance from the stalk attachment.
Section 138 (P1. IV, fig. 15) is at the caudal limit of the attachment of the stalk. There is a strong mesodermal condensation at the embryonal end of the stalk. Section 144 (P1. IV, fig. 16) is the site of the cranial attachment, and the loose texture of the tissue is apparent in comparison with that in section 138.
This mesodermal condensation at the lower aspect of the stalk, as can be seen from the reconstructions, is continuous with and prolonged into the tissue of the ventral wall below the stalk attachment, extending down to the cloacal membrane. It has increased relatively from the corresponding mass in the 2-4-mm. embryo and represents the primordium of the genital tubercle and ventral parts of the infra-umbilical abdominal wall.
Keibel & Mall give a reconstruction of the embryo Pfannensteil-Kromer of 1.38 mm, showing a cloacal membrane of 0-02 mm. length extending up to the attachment of the stalk, and also a reconstruction of Pfannenstiel III of 2.6 mm — with a cloacal membrane 0-13 mm. long extending up to and a short distance along the body stalk. Most authors seem to be in agreement with this, including Bryce (Quain’s Anatomy, Vol. 1), Keith and Frazer.
In the embryo just examined there is quite a definite interval between the cloacal membrane and the stalk. In one of his later publications a similar mesodermal separation was shown by Keibel in embryo “EB ”, and has been demonstrated by Sternberg (1926) in embryo B of 18 somites and embryo F of 4 mm.
The question will be later discussed in relation to the problem of extraversion of the bladder.
7-mm embryo
This specimen was obtained following curettage and cut transversely at 10p. in a cranio-caudal direction. Pl. V, fig. 17, is a drawing of a wax model reconstruction.
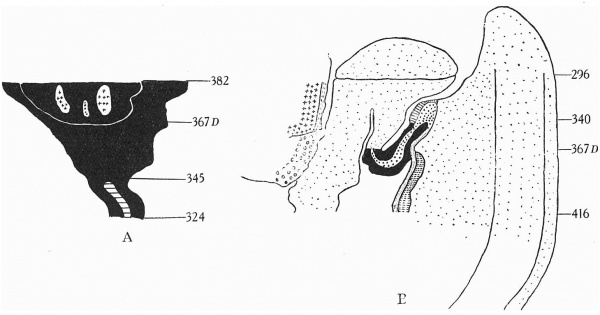
Text-fig. 4. Embryo 7 mm. Numbers refer to the sections. A. Reconstruction of the caudal portion of the ventral surface. x circa 50. The tail is straightened out. B. Reconstruction of a median sagittal section. x 25. See Pl. V, fig. I7. Gut — fine stippling; cloaca and allantois — large dots; dense mesoderm — black; cloacal membrane—ho1-izontal lines; mesentery —interrupted horizontal lines; septum transversum — interrupted vertical lines; liver — small circles; heart and allantoic vessels — crosses. D=the level of the caudal attachment of body stalk.
The cloacal membrane stretches over sixteen sections, giving a total length of 0-16 mm. Section 329 illustrates the chordal end of the membrane, and section 345 the allantoic end (P1. V, figs. 18-20), and it is to be noted that in this specimen there is no membrane as represented by the coaptation of two layers of cells, but rather as a cord or plate of cells several rows thick, the major portion of the plate seeming to be derived from the endoderm. Section 367 is through the caudal attachment of the stalk; the allantoic end of the cloacal membrane is therefore 0-24 mm. from the stalk.
Section 373 (P1. V, fig. 19) affords comparison between the caudal and cranial regions of the body stalk, the former exhibiting a fairly dense mesenchyme around and on the superficial aspect of the vessels, While the latter shows the typical loose texture of stalk tissue.
As can be observed in the graphic reconstruction of a medial sagittal
section (Text-fig. 4A, B), the mesodermal condensation in the stalk is continuous with and merges into the dense tissue intervening between the ventral
part of the cloaca and the surface. It extends on either side of the cloacal
membrane as part of the mesodermal bed of the cloaca.
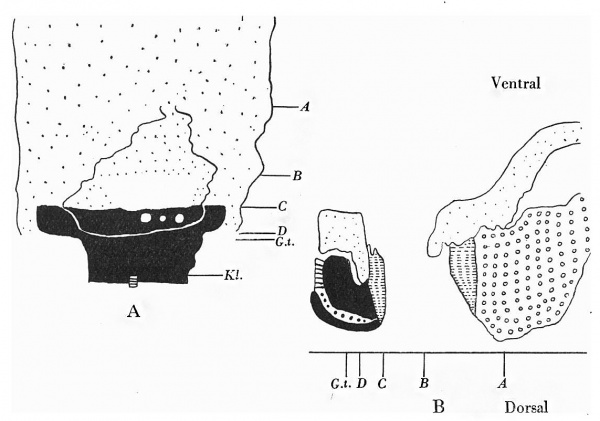
12.5-mm embryo
This embryo was obtained during operation for a ruptured ectopic preg- nancy, the history of acutehaemorrhage suggesting the recent death of the foetus.
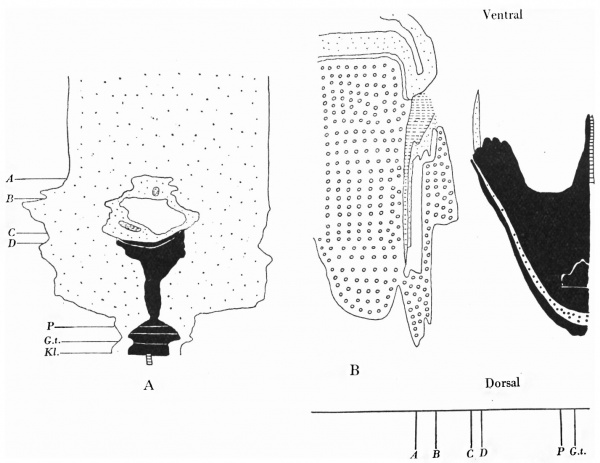
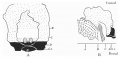
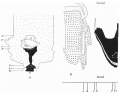
Text-fig. 5. Embryo 12-5 mm. Description in text. A. Reconstruction of ventral surface from septum transversum to cloacal membrane. x circa 12. B. Reconstruction of median sagittal section of same. x 10. Dense mesoderm — black; ventral cloaca and allantois — large dots; cloacal membrane — horizontal lines; blood vessels — oblique lines; liver — circles. A = section 675 at the level of the cranial attachment of body stalk; B = section 775; C = section 825; interval between B and C is the extension of the coelom into the stalk and occupied by mid-gut loop; D = section 875 at the level of caudal attachment of the stalk; GU. = section 888 at the level of the genital tubercle.
It is the first of the series in which the genital tubercle appears, and the small area between the tubercle and the stalk may be said to be the commencement of a true infra-umbilical portion of the abdominal wall.
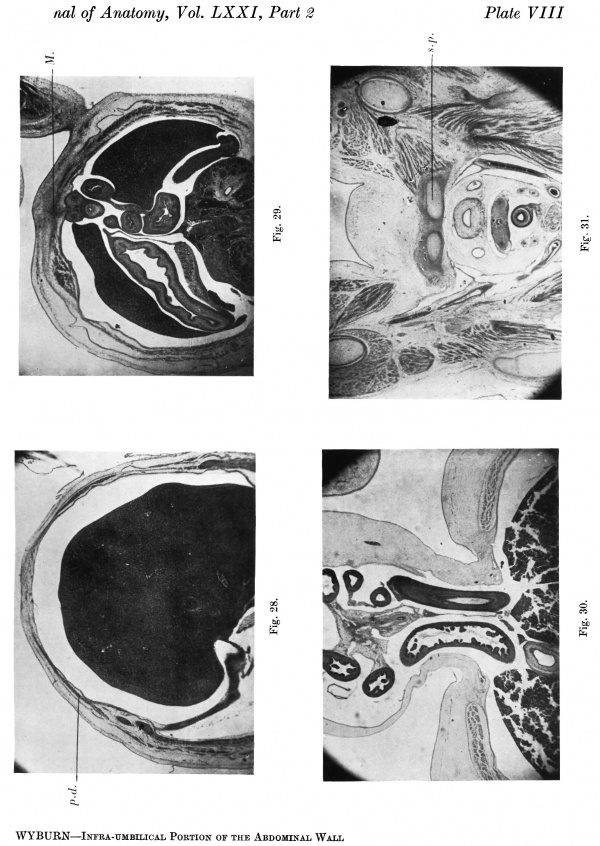
Section 864 (P1. VI, fig. 23) shows the mesodermal condensation in the lower portion of the stalk and caudal to the vessels. It is becoming more marked and extends laterally to meet the paraxial downgrowth into the abdominal wall.
The relations can be followed from the reconstructions of the ventral wall
(Text-fig. 5A) and a median sagittal section (Text-fig. 5B). The condensation
can be traced caudally as it passes round the cloacal membrane to fuse with
the general mesoderm on each side of the cloaca. It forms the basis of the
genital tubercle which carries the membrane on its lower surface. The “infra-
umbilical” area extends over eight sections measuring therefore about 0-08 mm.
13-mm embryo
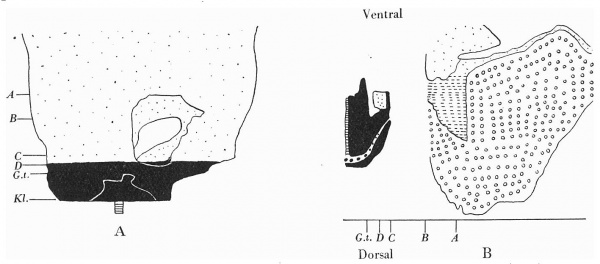
Obtained following abortion, this embryo, despite its greater size, is probably an earlier stage than the preceding. There is no genital tubercle and the tissues of the specimen show pathological changes.
The mesodermal condensation in the caudal portion of the stalk is, however, evident, and reference to the graphic reconstruction (Text-figs. 6A, B) shows again the continuation of this tissue down to and round the cloacal membrane. The allantoic end of the membrane itself is separated by a short interval from the stalk attachment.
Text-fig. 6. Embryo 13 mm. x circa 12. A. Reconstruction of lower ventral surface. B. Reconstruction of median sagittal section of ventral wall. Cloacal membrane—horizontal lines; dense mesoderm — black; ventral eloaca and allantois — large dots; liver — circles; B = section 910; C = section 954; interval between B and 0 is the extension of the coelom into the stalk and occupied by the midgut loop. D = section 1002 at the level of caudal attachment of the body stalk. Kl. = cloacal membrane.
14-mm embryo
Obtained at operation and fixed while still transparent. Before fixation this embryo measured 15-5 mm. The sections were cut transversely.
The sections cranial to the stalk and intestinal loop show the ventral downgrowths from the paraxial mesoderm. These downgrowths exhibit some tendency to divide into strata foreshadowing the muscular layers of the anterior abdominal wall (Pl: VII, fig. 24). Ventrally the wall is completed by mucoid tissue. Caudally the downgrowths from the paraxial mesoderm become less marked until they ultimately disappear entirely, but ventrally the mucoid tissue merges into a mesodermal condensation which in the lower sections is well marked, and at the level of the caudal attachment of the stalk appears as a thick band of tissue on the superficial aspect of the allantoic vessels (Pl. VII, fig. 25). More caudally the band is continuous with the genital tubercle. The extent and relations of the mesodermal mass are shown in the graphic reconstructions (Text-figs. 7A, B). The sections show clearly that the mesodermal condensation of the ventral portion of the infra—umbilical abdominal wall is not derived from the paraxial down growths, but has a separate origin. This part of the abdominal wall, as represented by the interval between the attachment of the stalk and the genital tubercle, has increased in length as compared with the 12-5-mm. embryo.
Text-fig. 7. Embryo 14 mm. x 10. A. Reconstruction of the ventral surface caudal to the septum transversum. B.Dense mesoderm — black; cloaca and allantois — large dots; cloacal membrane — horizontal lines; liver — circles; blood vessels — interrupted horizontal lines. A = section 872 at the level of cranial attachment of the stalk. B = section 972; 0 = section 1044; interval between B and 0 is the extension of the coelom occupied by the midgut loop. D = section 1052 at the level of caudal attachment of stalk; G.t. = section 1060 at the level of genital tubercle. Kl. = cloacal membrane.
16.1-mm embryo
Obtained following abortion.
The sections show the downgrowths from the paraxial mesoderm dividing into three strata.
These persist into the lower portion of the abdominal wall. In the upper sections (Pl. VII, fig. 26) the downgrowths reach about half-way round, but below the stalk they become continuous with the mesodermal thickening which commences on the caudal aspect of the stalk, and inferiorly forms the genital tubercle (Pl. VII, fig. 27). As can be seen from the graphs (Text-fig. 8A, B) this mesoderm forms the increasing infra-umbilical abdominal wall, the genital tubercle, and the tissue between the cloaca and the ventral surface. The cloacal membrane, represented by a deep cleft, is on the caudal aspect of the genital tubercle.
Text-fig. 8. Embryo 16-1 mm. x circa 12. A. Reconstruction of the ventral surface below the septum transversum. B. Reconstruction of a median sagittal section of ventral wall. Dense mesoderm — black; cloacal membrane — horizontal lines; ventral cloaca and allantois — large dots; liver — circles; blood vessel — interrupted horizontal lines. A = section 1148 at the level of the cranial attachment of the body stalk. B =section 1200. 0 = section 1256. B-C = extension of the coelom occupied by the midgut loop. D = section 1272 at the level of the caudal attachment of the stalk. G.t. = section 1292 at the level of the genital tubercle. Kl. = cloacal membrane.
23-mm embryo
No history.
The downgrowths from the dorsal mesoderm are clearly divisible into three strata uniting in front. Above the body stalk the ventral junction is some distance from the midline where the body wall is composed of tissue of loose texture (Pl. VIII, fig. 28). At the level of the body stalk the thickening forming the rectus muscle is visible; the splitting to form its sheath can be dis- cerned. Below the body stalk the rectus sheath runs into a mesodermal mass forming the midventral wall (Pl. VIII, fig. 29). The infra-umbilical abdominal wall has increased considerably from that of the 16-1 embryo.
Text-fig. 9. Embryo 23 mm. x circa 12. A. Reconstruction of the ventral surface below the septum transversum. B. Reconstruction of a median sagittal section of ventral wall. Dense mesoderm — black; cloacal membrane — horizontal lines; ventral cloaca and allantois — black dots; liver — circles; blood vessels — interrupted horizontal lines. A = section 825 at the level of the cranial attachment of body stalk. B = section 857 ; 0 =section 913. Between B and 0 is the extension of the coelom occupied by the midgut loop. D=section 921 at the level of the caudal attachment of the stalk. G.t. =section 977 at the level of the genital tubercle. Kl. = cloacal membrane.
The mesodermal mass forms not only the ventral portion of the abdominal wall but the entire thickness of tissue superficial to the allantois and bladder (Text—fig. 9A, B).
30-mm embryo
Obtained following abortion; sections cut longitudinally. The graph (Text-fig. 10) shows the extent of the mesoderm and P1. VI, fig. 22, is a section almost through the midsagittal plane, demonstrating the continuity of the stalk mesoderm with that forming the lower abdominal wall and genital tubercle.
Text-fig. 10. Embryo 30 mm. x circa 12. Reconstruction of the ventral surface below the septum transversum. Dense mesoderm — black; cloacal membrane — horizontal lines; blood vessel - interrupted horizontal lines. A = cranial attachment of body stalk; between B and C is the extension of the coelom occupied by the midgut loop; D = the caudal attachment of the stalk; G.t. = genital tubercle. Kl. = cloacal membrane.
40-mm embryo
No history.
The ventral wall again shows the three muscular sheets terminating in the rectus in front.
The increasing thickness of the abdominal wall, well marked in this embryo, appears to be due to the somatopleure mesoderm rather than the paraxial myogenic downgrowths. The umbilical cord appears to be sunk in a depression in the anterior abdominal wall (Pl. VIII, fig. 30), which may be caused by a failure of the thickening to reach the midventral line owing to fixation at the umbilicus. The interval between the recti decreases in the more caudal sections.
The mesodermal mass in the midventral portion of the infra-umbilical wall, now of considerable length, is reinforced by extensions from the rectus sheath. It is also increased in thickness, stretching from the surface to the bladder and allantois internally.
In this embryo the condensations forming the pubic portions of the pelvis are present (Pl. VIII, fig. 31), and it is apparent that these arise from the central mesodermal mass (see graphs—Text-figs. 11A, B). Caudally the mesoderm forms the genital tubercle.
Text-fig. 11. Embryo 40 mm. x circa l2. A. Reconstruction of the ventral surface below the septum transversum. B. Reconstruction of a median sagittal section of ventral wall. Dense mesoderm — black; cloacal membrane — horizontal lines; ventral cloaca and allantois —black dots; liver — circles; blood vessels — interrupted horizontal lines. A = section 1152 at the level of the cranial attachment of body stalk; B = section 1184; 0 = section 1236; between B and 0 is extension of coelom; D = section 1252 at the level of the caudal attachment of stalk; G.t. = section 1408 at the level of the genital tubercle; Kl. = cloacal membrane; P = section 1384 at the level of the pubic condensations.
Discussion
In the earliest embryo studied (the McIntyre I of 1-4 mm.) there is present a band of mesodermal tissue passing from the caudal aspect of the stalk round the cloacal membrane to the region of the termination of the primitive streak. From the graphic reconstructions of the various stages up to that of 4-0 mm. the growth and development of this band of tissue can be traced; it is apparent that from it are formed the structures in the midventral line of the infra-umbilical part of the body wall including the abdominal wall itself, the genital tubercle, cloacal fold, symphysis pubis, and the muscular coat of the bladder.
At the body stalk this mesoderm envelops the caudal aspect extending
only a short distance along the stalk and surrounding not only the proximal
portion of the allantois and allantoic vessels but furnishing a distinct band of
tissue on the deep surface of the body wall caudal to the umbilicus in which are
placedethese structures; it becomes continuous with the mesoderm round the
cloaca.
The more dense character of the tissue on this portion of the stalk as compared with the looser texture and mucoid appearance of the remainder is particularly well marked up to the 23-mm. stage. In the 30. and 40 mm., while still apparent, it does not so sharply contrast with the surrounding tissue in the stalk or upper half of the abdominal Wall, as though its strength Were becoming exhausted by a “paying-out” to its derivatives.
The question of the origin of the umbilical part of the mesoderm has already been discussed and the conclusion formed that it arises from the primitive mesoderm representing the site of the posterior mesodermal field of the embryonic shield. Extending as wing-like processes round the cloacal membrane it becomes continuous with the secondary mesoderm from the end of the primitive streak.
The observations leading to the assumption that the primitive streak
terminates at or even before the commencement of the cloacal membrane are
recorded earlier, and the reasons given for discarding the suggestion of Keibel
and others that the cloacal membrane itself is a modification of the primitive
streak which may extend as far as or even on to the body stalk.
The first indication of the existence of a body wall below the attachment of
the stalk is in the 12-5-mm. embryo, where the interval between the genital
tubercle and the stalk is occupied by the mesodermal mass under discussion.
This also shows lateral extensions passing some distance round the body wall
and on each side of the genital tubercle forming the cloacal folds.
The relative decrease in the breadth of attachment of the body stalk, with
the consequent ventral encroachment of the paraxial downgrowths into the
somatopleure in the older embryos, minimizes the actual area of abdominal
Wall formed by this mesoderm, but it can be observed in its position in the
midventral line up to the 40-mm. stage and is probably ultimately represented
in the adult body wall by the symphysis pubis, lower part of the rectus sheath
and infra-umbilical portion of the linea alba.
The extension of the ‘coelom’ into the pelvis follows a ‘taking in’ of the stalk into the body of the embryo with a prolongation of the ‘umbilical coelom ’ (i.e. that part of the body cavity passing into the stalk) between the hindgut and the ventral wall containing the allantois and the vessels. This can be seen in the reconstruction of the 4-5- and 7-mm. embryos. The mesoderm of the caudal end, in which is embedded the cloaca, remains unsplit, and thus that portion which passes from the hind end of the primitive streak to become
continuous with the downgrowth from the stalk is equivalent to somatopleure and splanchnopleure, forming not only part of the parietes, but the fibrous and muscular coats of the bladder. The condensations forming the pubes seen in the 30- and 40-mm. embryos are obviously arising in the midline from the mesoderm in this region.
Ectopia Vesicae and Epispadias
Any investigation of the embryology of this region inevitably involves the problem of ectopia vesicae and epispadias and the plethora of suggestion—much of it empirical, most of it unsatisfactory——which has been offered to account for this congenital defect.
On the question of the aetiology of extroversion of the bladder and epispadias most surgical text-books are content with the statement that the deformity can be ascribed to arrested development of the lower abdominal parietes. Others elaborate ingenious mechanical theories to account for the condition, for example, “pressure of the umbilical cord which passes between the lower limbs of the foetus to a dorsally placed placenta” (Surgery of Childhood, J. Fraser); or again, “Epispadias is really a hypospadias in which torsion of the penis has occurred at an early stage of foetal life so that the under surface becomes the upper” (System of Surgery, Choyee, vol. II, 1932).
For some time the views of Keibel were very generally accepted. According to this author the cloacal membrane should be regarded as a modified part
of the primitive streak and ectopia vesicae as a persistent portion of the
blastopore.
Keibel and Elze believe “that the primitive streak extends along the body
stalk, as in some sections the ectoderm covering the body stalk shows a local
thickening which is nearly in contact with the allantoic duct”. The description
is more akin to that of the cloacal membrane becoming obliterated by the
incursion of mesoderm than to that of a primitive streak.
Keith agrees with Berry Hart that the primitive streak forms the post-umbilical wall and suggests that extroversion of the bladder is an unclosed
condition of the streak. Should this be so, is there any reason why the non-closure of the blastopore should be confined to the allantoic portion of the
ventral wall of the cloaca? Would it not be reasonable to assume that amongst
the many recorded cases of this condition a few would show more extensive or
additional defects involving perhaps the anal region or some degree of spina
bifida which, according to Grosser (1927), is a non-fusion of the lips of the
primitive groove? Or, again, one would expect to find the condition in lower
mammals where the primitive streak forms not only the secondary but the
primary mesoderm.
Cogent reasons have earlier been advanced for the rejection of the averment that the primitive streak extends on to the body stalk. There is neither histological nor ontogenetical evidence to support the suggestion that the cloacal membrane is a modified primitive streak. The facts‘ I have recorded as observed in McIntyre I, viz., that the primitive streak terminates at or even some distance before the commencement of the cloacal membrane, is in agreement with most of the recent work already referred to.
Keibel (1896) later retracts from his original position, and, having observed
some diminution in the absolute length of the cloacal membrane in embryos of
about 6 mm., is of the opinion that ectopia vesicae may result from a failure of
shortening of the membrane at this stage and accordingly is of a later developmental period than .Was presumed in his previous observations.
Enderlen (1908) states that if such assumptions .Were correct, then the
cloacal membrane must extend up to the body stalk, and in his opinion this is
actually so up to the 9-mm. stage; the earlier the error occurs the more com-
plete the defect, ranging from complete extroversion to hypospadias.
In the embryos 4-5 and 7 mm. of this series the cloacal membrane did not
reach the body stalk, and similar observations on even earlier stages have been
recorded.
Felix (1911) found that the cloacal membrane reaches the body stalk up to 18 somites.
From the study of this region in a series of embryos ranging from 1-4 to
40 mm. the following facts have been observed and are recounted graphically
in the drawings.
Firstly, in the presomite stage the cloacal membrane involves not only the
cloaca, but exists as an area of contact between the amniotic ectoderm and
allantoic endoderm. This is seen in section 119 of the McIntyre embryo and has
been recorded by a number of observers including Florian (1930) in embryo
Bi II and Sternberg (1927) in an embryo of 4 somites. The cloacal membrane is
therefore at this stage relatively extensive reaching on to and along the proximal end of the connecting stalk. In the McIntyre embryo the “allantoic
cloacal membrane” extends only over one section, and in the intervening area
between that and the hindgut cloacal membrane, the ectoderm and endoderm
are separated by mesoderm. In the Sternberg embryo there are three separate
portions of the allantoic cloacal membrane, and two in the Bi II of Florian. In
the next embryo studied of 24 mm. the cloacal membrane is limited to the
cloaca proper, with a mesodermal interval between its allantoic extremity and
the stalk.
It is therefore reasonable to assume that the interrupted contact of allantoic endoderm and ectoderm is evidence of the disappearance of the allantoic
cloacal membrane, and not the commencement or extension of the existing
cloacal membrane, and that, primarily, the “membrane” was an area of contact of ectoderm and endoderm extending from the hindgut cranially,1 and
caudally terminating some distance along the stalk.
1 The terms “cranially” and “caudally” are here descriptive of the relations existing before the appearance of the tail fold.
The McIntyre I thus represents a stage showing the process of obliteration of the “allantoic cloacal membrane”. Florian (1930) observed signs of degeneration in this part of the membrane.
Examination of the sections of this embryo leads to the conclusion that the
separation of the ectoderm and endoderm in this region is effected by the mesoderm growing in towards and ultimately fusing in the midline. This mesoderm
seems to be derived from the wing-like processes arising from the hind end of
the primitive streak extending round on either side of the chordal portion of
the cloacal membrane, and encroaching on the midline towards the allantoic
end. One would therefore anticipate that at a later stage there would be evi-
dence of a decrease in size and extent of the membrane, and a study of Table I
discloses such a condition occurring in embryos of about 6-8 somites. It is of
interest that the actual obliteration is in progress during what is probably the
period of maximum activity of the primitive streak and when one would pre-
dict an optimum mesodermal energy.
In the 2-4 mm. and more definitely in the 4-5 mm. the cloacal membrane is placed some distance behind the caudal attachment of the stalk and is limited to the ventral aspect of the cloaca.
The processes of mesoderm encircling the membrane and fusing with the mesenchyme of the stalk form the band of denser tissue whose development has been followed up to the 40-mm. embryo. The extent covered by structures arising from this mesodermal tissue is indicated in the reconstructions by the areas ‘shown in black from which it becomes evident that failure of this tissue to develop would result in the persistence of the original extensive cloacal membrane which would form the infra-umbilical portion of the ventral abdominal wall, the absence of the genital tubercle with defective formation of the external genitals, and deficiency of the symphysis pubis. With the breaking down and absorption of the cloacal membrane which normally occurs about the second month, the condition of ectopia vesicae would then be established. Minor mesodermal failure would be responsible for the lesser defect of epispadias.
The mesodermal septum dividing the cloaca into ventral and dorsal parts reaches the surface separating the anal from the urogenital orifice so that the extroversion is limited to the ventral cloaca, and the anal region develops normally.
As the mesodermal band has been shown to consist of primary and secondary mesoderm, the fault might be apportioned to the one or the other, or again
could be accounted for by an undue adherence of amniotic ectoderm and allantoic endoderm, what Rossenbeck (1923) would term an “incomplete loosening ”
of the germinal layers preventing the mesodermal inroads.
Bearing in mind the role of the primitive streak and that it is less productive in the human than in any other form, one inclines to the View that the
aetiological factor is deficiency and non-development of the mesodermal processes which normally arise from the hind end of the primitive streak, extend on either side of the cloacal membrane, press in towards the midline, obliterate its allantoic extension, and, continuing on to the stalk, merge into the primary mesoderm.
Sternberg (1926), in a study of the cloacal membrane of young embryos,
concludes that the period of shortening of the membrane coincides with the
appearance of the first somites, and that therefore extroversion must occur
before the degeneration of the allantoic cloacal membrane. He states of the
condition: “Die teratogenetische terminationperiode der Bauchblasenspalte
und der mit ihr verwandten missbildungen ist demnach wesentlich friiher, als
man bisher angenommen hatte und zwar etwa in jenes stadium zu verlegen im
welchem sich die ersten Ursegmente ausbilden.”
Extroversion of the bladder is a condition peculiar to the human species
(Kerrnauner, 1909). This is probably due to the precocious development of the
allantois, e.g. the Peh-Hochstetter embryo (Rossenbeck, 1923), in accordance
with the mode of nutrition in the human, coupled with the diminished activity
of the primitive streak. In Tarsius (Hubrecht, 1909) the primitive streak
thickening appears as a forward extension of the proliferating caudal margin of
the embryonic shield from which is formed the mesoderm of the connecting
stalk, whereas in the human the origin of the primitive streak is much more
cranial.
The “allantoic cloacal membrane” appears to be a human characteristic
(Sternberg) and is possibly permitted by the more cranial origin of the streak
with the consequent lessened mesodermal production at this point. Again, the
extension of the cloacal membrane may be a secondary development due to the
activity of the ectodermal cells of the amnion described by Florian (1930)
which, encroaching on and destroying the mesoderm of the connecting stalk,
thereby permits of the extension of the amniotic space at the expense of the
stalk tissue.
The cloacal membrane appears at a relatively early stage in the human embryo (Fetzer-Florian embryo, 1930), and perhaps is not so much a development as an actual persistence of contact between the ectoderm and endoderm.
One concludes that ectopia vesicae is to be regarded as yet another of the
long list of congenital defects to be laid to the account of the mesoderm and in
some way occasioned by the declining activity of the primitive streak.
The determination of the causal factor effecting the mesodermal deficiency
is a problem the answer to which may be found in the complicated mechanism
of “organizers”, the initiation of “Field Gradients” with their subtle interaction and susceptibility to adverse environmental conditions.
Summary
- A study has been made of a series of embryos ranging from 1.4 to 40 mm. in length, with graphic reconstructions, tracing the development of the infra-umbilical abdominal wall.
- The infra-umbilical portion of the anterior abdominal wall, the genital tubercle, symphysis pubis, and muscular coat of the bladder, are formed from a well-defined band of mesoderm.
- This band of mesoderm has a twofold origin: (a) from the caudal margin of the embryonic shield——primary mesenchyme; (b) processes of secondary mesoderm passing round the cloacal membrane from the hind end of the primitive streak. i .
- In the embryo McIntyre I the cloacal membrane is in two parts, one of which implicates the allantoic diverticulum.
- At an early stage the cloacal membrane is a relatively large area of contact of ectoderm and endoderm extending some distance along the allantoic diverticulum.
- The allantoic cloacal membrane is later obliterated by the mesoderm pressing in towards the midventral line between the ectoderm and endoderm.
- Extroversion of the bladder is due to mesodermal deficiency, particularly of the processes of secondary mesoderm arising from the hind end of the primitive streak, following on which there is persistence of the primary extensive cloacal membrane, impaired development of the muscular coat of the bladder, of the symphysis pubis, and of the formation of external genitals and infra-umbilical portion of anterior abdominal wall.
- Epispadias is a similar mesodermal error in a minor form.
My thanks are due to my Chief, Prof. Blair, for his helpful criticism and valuable suggestion, and to Dr Norman H. W. Maclaren for his very generous assistance and advice.
The drawings of the wax plate reconstructions were made by Mr Alexander Rettie of this Department.
References
ATWELL, W. J. (1930). “A human embryo with seventeen pairs of somites.” Oameg. Instn Publ. No. 407; Contr. Embryol. No. 118.
BRYCE, T. H. (1908). Quainis Anatomy, 11th ed., vol. 1. London.
— (1924). “Observations on the early development of the human embryo.” Trans. ray. Soc. Edinb., vol. LDI, pt. iii, No. 26.
BRYCE, T. H. & TEACHER, J. H. (1908). Oontrilmtiorrw to the Study of the Early Development and Imbedding of the Human Ovum. Glasgow: Maclehose.
CHOYCE. (1932). System of Surgery, vol. II.
CORNER, G. W. (1929). “A well-preserved human embryo of ten somites.” Oarrwg. Inatn Publ. No. 394; Omar. Embryol. No. 112.
DANDY, W. E. (1910). “A human embryo with seven pairs of somites.” Amer. J. Amt. vol. 2:.
DAVIS, C. L. (1924). “Description of a human embryo having twenty paired somites.” Carmag. Instrt Publ. No. 332; Otmtr. Embryol. No. 72.
DEBEYRE, A. (1912). “Description d’un Embryon humain de 0mm. 9.” J. Anat., Paris, 1:. XLVIII.
ENDERLEN, E. (1908). “Uber Blasenektopie.” Samml. Iclirt. Vortr. Nr. 472/73.
ETERNOD, A. C. F. (1899). “Il y a un Canal notochordal dans l’Embryon humain.” Anat. Anz. Bd. XVI.
FELIX, W. (1911). “Die Entwicklung der Ham- und Geschlechtsorgane.” In Keibel-Mall, Handbuch der Enlwiclclungsgeschichte des M erwchen.
FETZER, M. (1910). “Uber ein durch Operation gewonnenes menschliches Ei.” Anat. Anz. Bd. xxxvn.
FETZER, M. & FLORIAN, J. (1930). “Der Embryo ‘Fetzer’ mit beginnender Axia.lmesodermbildung und bereits angelegter Kloakenmembran.” Z. milcr.-anat. Forsch. Bd. XXI.
FLORIAN, J. (1928). “Ein junges menschliches Ei in situ (Embryo T.F. mit Primitivstreifen, ohne Kopifortsatz).” Z. milcr.-anat. Forsch. Bd. xnr.
— (1930). “The formation of the connecting stalk and the extension of the amniotic cavity towards the tissue of the connecting stalk in young human embryos.” J. Anat., Lond., vol. LXIV, pt. iv.
— (1933). “The early development of man, with special reference to the development of the mesoderm and cloacal membrane.” J. Anat., Lond., vol. LXVII.
— (1934). “Ein Schema der Entwicklung der Axialgebilde des menschlichen Embryos bis in das Stadium Von 10 Urwirbelpaaren.” Biol. gen. Bd. X, Lief. 2.
FRAZER, J. (1926). The Surgery of Childhood. London: Arnold and Co.
FRASSI, L. (1907). “Uber ein junges menschliches Ei in situ.” Arch. mikr. anat. Bd. LXX.
FRAZER, E. (1931). Manual of Embryology. London: Bailliere, Tindall and Cox.
GROSSER, 0. (1927). Friihentwicklung, Eihautbildung und Placentatizm. Munich. : (1931). “Primitivstreifen und Kopflbrtsatz beim Menschen.” Anat. Anz. Bd. LXXI.
HEUSER, C. H. (1930). “A presomite human embryo with definite chorda canal.” Carneg. Instn Publ. No. 433; Contr. Embryol. No. 138.
— (1930). “A human embryo with fourteen pairs of somites.” Oarneg. Instn Publ. No. 441; Contr. Embryol. No. 131.
HILL, J. P. (1932). “A developmental history of the primates.” Philoa. Trans. B, vol. CCXXI.
HILL, J. P. & FLORIAN, J. (1931). “A young human embryo (Embryo Dobbin) with head process and prochordal plate.” Philos. Trans. B, vol. CCXIX.
HUBRECHT, A. A. W. (1909). “Early ontogenetic phenomena in mammals.” Quart. J. micr. Sci. vol. LIII, N.S.
INGALLS, N. W. (1918). “A human embryo before the appearance of the myotomes.” Carneg. p Instn Publ. No. 227; Oontr. Embryol. No. 23.
JENKINSON, J. W. (1913). Vertebrate Embryology. Oxford. ~
KEIBEL, F. (1896). “Zur Entwicklungsgeschichte des menschlichen Urogenitalapparats.” Arch. Artat. Phys1Iol., Anat. Abt.
KEIBEL, F. & MALL, F. (1912). Manual of Human Embryology.
KEITH, A. (1921). Manual of Embryology and Morphology, 4th ed.
KERMAUNER, F. (1909). “Die Missbildungen des Rumpfes.” In Schwalbe, Die Morphologie der M issbildurtgen des M enschen und der Tiere.
LLOBCA, F. VON Oars (1934). “ Beschreibung eines menschlichen Embryo mit 4 Urwirbelpaaren.” Z. gee. Amt. 1. Z. Anat. En/twG'e.sch. Bd. cm, Heft 6.
MOLLENDORFF, V01: (1925). “Das menschliche Ei. Wo(lfi-ing) Implantation. Verschluss der Implantationsolfnung und Keimesentwicklung beim Menschen vor Bildung des Primitivstreifens.” Z. ges. Anal. 1. Z. Anat. EntwGeach. Bd. 76.
PAYNE, F. (1925). “General description of a seven-somite human embryo.” Carneg. Instn Publ. No. 361; Contr. Ernbryol. No. 81.
PETERS, H. (1899). Uber die Einbettung des menechlichen Eies. Leipzig u. Wien: Franz Deutike.
ROSSENBECK, H. (1923). “Ein junges menschliches Ei,_ Ovum Humanum Peh-Hochstetter.” Z. gee. Amt. 1. Z. Anal. EntwG'esch. Bd. LXVIII.
STIEVE, H. (1926). “Ein 13;-Tage altes, in der Gebiirmntter erhaltenes und durch Eingriif gewonnenes menschliches Ei.” Z. mikr.-anat. Farsch. Bd. VII.
STERNBERG, H. (1926). “ Zur formalen Genese der Bauchblasenspalte.” VirchawsArch. Bd. CCLXIII.
— (1927). “Beschreibung eines menschlichen Embryos mit vier Ursegmentpaaren.” Z. ges. Anal. 1. Z. Amt. EntwGesch. Bd. Lxxxn.
STRAHL, R. & BENEKE, R. (1910). Ein junges menschliches Embryo. Wiesbaden.
STREETER, G. L. (1927). “The Miller ovum.” Carney. Irwm Publ. No. 363; Cont. Embryol. No. 92.
VEIT, O. & Escn, P. (1922). “Untersuchung eines in situ fixierten operativ gewonnenen mensch- lichen Eies der 4. Woche.” Z. gee. Amt. 1. Z. Anat. EntwG'esch. Bd. LX111.
WILSON, J. T. (1914). “ Observations upon young human embryos.” J. Anat., Lond., vol. XLVIII.
WEST, C. M. (1930). “Description of an embryo of eight somites.” Cameg. Insm Publ. No. 407; Ozmtr. Embryol. No. 119. -
Explanation of Plates
Plate I
Fig. 1. Photograph of a drawing of a wax plate reconstruction of embryo McIntyre I. x circa 40. The arrows indicate the plane of the graphic reconstruction (Text-fig. 1 B). The numbers refer to the sections limiting the area reconstructed in the graph.
Fig. 2. Section 84 of 2.4-mm embryo. Photomicrograph x circa 80. M . = mesodermal condensation at the caudal attachment of the stalk.
Fig. 3. Section 146 of embryo McIntyre I. Photomicrograph x circa 140. Description in text. Y. = cavity of the yolk sac.
Fig. 4. Section 143 of embryo McIntyre I. Photomicrograph x circa 140. Description in text. Y. = cavity of the yolk sac.
Plate II
Sections of embryo McIntyre I. Photomicrographs x circa 140. Description in text.
Fig. 5. Section 130. Y. = cavity of yolk sac. a.e.c. = amnio-embryonic cavity.
Fig. 6. Section 129. Y. = cavity of yolk sac. a.e.c. = amnio-embryonic cavity.
Fig. 7. Section 125. Y. = cavity of yolk sac. a.e.c. = amnio-embryonic cavity.
Fig. 8. Section 122. A process of ectoderm can be seen extending up to yolk sac endoderm but separated from it by a small interval in which are mesodermal cells. Y. = cavity of the yolk sac. a.e.c. = amnio-embryonic cavity. A. = allantoic diverticulum.
Plate III
Figs. 9-11 are sections of embryo McIntyre I. Photomicrographs x circa 140. Description in text.
Fig. 9. Section 119. Y. = cavity of yolk sac. a.e.c. = amnio-embryonic cavity. A. = allantoic diverticulum.
Fig. 10. Section 118. Y . = cavity of yolk sac. a.e.c.= amnio-embryonic cavity. A.= allantoic diverticulum.
Fig. 11. Section 112. Y . = cavity of yolk sac.
Fig. 12. Photograph of a drawing of a wax plate reconstruction of embryo 2-4 mm. in length. x circa 40. The arrows indicate the plane of the graphic reconstruction (Text-fig. 2B). The numbers refer to the sections limiting the area reconstructed in the graph.
Plate IV
Sections of embryo 4.5 mm (McIntyre II). Photomicrographs x circa 110. Description in text.
Fig. 13. Section 136. M .= mesodermal condensation.
Fig. 14. Section 131. Kl.= cloacal membrane.
Fig. 15. Section 138. M .= mesodermal condensation.
Fig. 16. Section 144.
Plate V
Fig. 17. Photograph of a drawing of a wax plate reconstruction of a 7-mm embryo. x circa 12. The arrows indicate the plane of the graphic reconstruction (Text-fig. 4B). The numbers refer to the sections limiting the area reconstructed in the graph.
Figs. 18-20 are sections of embryo 7 mm in length. Photomicrographs x circa 120. Description of sections in text.
Fig. 18. Section 329. Kl. = cloacal membrane.
Fig. 19. Section 373. M. = mesodermal condensation.
Fig. 20. Section 345. Kl. = cloacal membrane.
Plate VI
Fig. 21. Photograph of 9. drawing of a wax plate reconstruction of a 4-5-mm. embryo (McIntyre II). x circa 18. The arrows indicate the plane of the graphic reconstruction (Text-fig. 3B). The numbers refer to the sections limiting the area reconstructed in the graph.
Fig. 22. Longitudinal section of a 30-mm embryo. Photomicrograph x circa 7.
Fig. 23. Section 864 of 12-5-mm embryo. Photomicrograph x circa 27. Description in text. M. = mesodermal condensation.
Plate VII
Fig. 24. Section 761 of a 14-mm embryo. Photomicrograph x circa 17. Description in text. p.d. = paraxial myogenic downgrowth.
Fig. 25. Section 1081 of a 14-mm embryo. Photomicrograph x circa 17. Description in text. M. = mesodermal condensation.
Fig. 26. Section 1079 of a. 16-1-mm embryo. Photomicrograph x circa 17. Description in text.
Fig. 27 . Section 1278 of a 16-1-mm embryo. Photomicrograph x circa 17. Description in text. M. = mesodermal condensation.
Plate VIII
Fig. 28. Section 773 of a 23-mm. embryo. Photomicrograph x circa 7. Description in text. p.d. =paraxial myogenic downgrowth.
Fig. 29. Section 924 of a 23-mm. embryo. Photomicrograph x ciroa 7. Description in text. M. = mesodermal condensation.
Fig. 30. Section 1216 of a 40-mm. embryo. Photomicrograph x circa 7. Description in text.
Fig. 31. Section 1408 of a 40-mm. embryo. Photomicrograph x circa 7. Description in text. 3.12. =condensation forming the pubes.
Text-Figures
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - The Development of the Infra-Umbilical Portion of the Abdominal Wall, with Remarks on the Aetiology of Ectopia Vesicae. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_Development_of_the_Infra-Umbilical_Portion_of_the_Abdominal_Wall,_with_Remarks_on_the_Aetiology_of_Ectopia_Vesicae
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G