Paper - The Comparative Behavior of Mammalian Eggs in Vivo and in Vitro
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Pincus G. and Enzmann EV. The comparative behavior of mammalian eggs in vivo and in vitro. (1935) J Exp Med. 62(5): 665-75. PMID 19870440
| Online Editor |
|---|
| This historic 1935 paper by Pincus describes the development of the rabbit oocyte.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Comparative Behavior of Mammalian Eggs In Vivo And In Vitro
I. The Activation of Ovarian Eggs
By Gregory Pincus, S.D., And E. V. Enzmann, Ph.D.
(From the Biological Laboratories, Harvard University, Cambridge)
- This investigation has been aided by a grant from the National Research Council Committee for Problems of Sex.
PLATES 29 AND 30
(Received for publication, July 17, 1935)
The eggs of most mammals are shed from the ovary with the first polar body formed. The mechanism controlling this stage of maturation has never been investigated in detail. Furthermore, under normal conditions only shed ova are fertilized. Does this indicate that the first maturation division is an essential prelude to fertilization? Or may ovarian eggs in fact be activated before the first meiotic division?
This investigation concerns itself with these problems, and falls into two parts dealing with: (1) the mechanism controlling the first meiotic division; (2) the capacity for fertilization of ovarian eggs. Superficially unrelated, these two studies are aspects of the broad problem of the fundamental nature of the activation process.
Experimental
The rabbit is especially favorable material for this study since it ovulates only after copulation. It has been established that copulation results in a stimulation of pituitary secretion, and that the amount of anterior pituitary secretion necessary to induce ovulation occurs during the 1st hour after copulation (Deansley, Fee, and Parkes, 1930). The injection of pituitary extracts or of prolan induces ovulation (Friedman, 1929); and furthermore, ovulation induced by stimulating hormones occurs at 10 hours after injection (Bellerby, 1929). Ova are normally shed with the first polar body at 10 hours after copulation. According to Heape (1905) both polar bodies are formed at 9 hours after copulation.
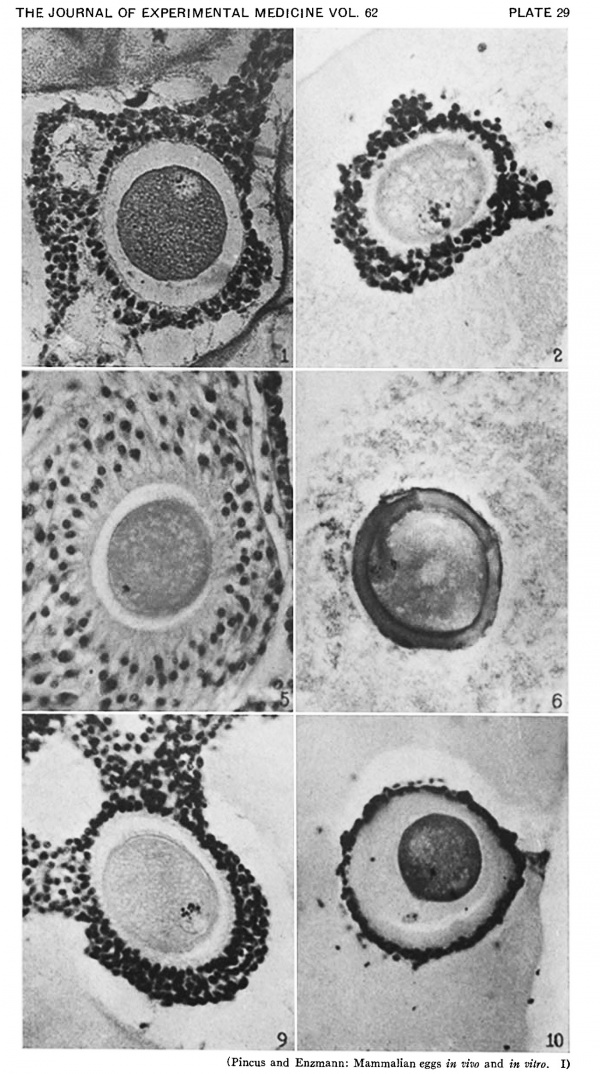
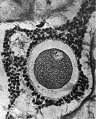
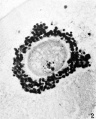
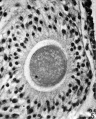
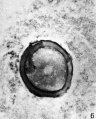
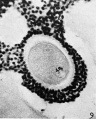
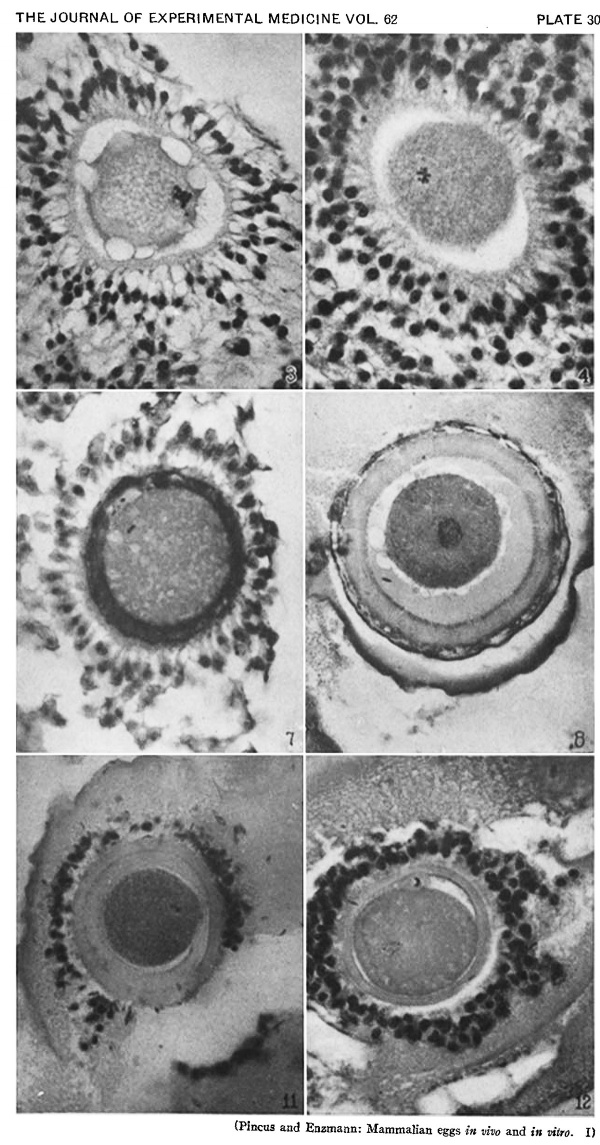
Heape’s statement is but partially correct. Only one polar body is formed. We have investigated this situation in detail, and our data are summarized in Table I. Before copulation occurs the ovum contains a single large vesicular nucleus about 30 microns in diameter (Fig. 1). At 2 hours after copulation some of the ripe ova show signs of the initiation of maturation. The diakinesis-like chromatin begins to condense into tetrads, but the nuclear membrane remains intact (Fig. 2). The separation of strands of follicle cells adjacent to the corona radiata begins to be manifest. By 4 hours after copulation the tetrads of the first polar spindle are formed and the nuclear membrane is ordinarily dissolved (Figs. 3, 4, and 5). The metaphase plate is found in all maturing ova by 6 hours after copulation, and the freeing of the egg and corona from the connecting follicular strands is almost complete. The first polar body is given off between 7 and 8 hours after copulation (Fig. 6). The second polar spindle is formed during the 9th hour post coitum and the ripe ovum (Fig. 7) is shed between 9.5 and 10.5 hours.
Table I
| Table 1 - Progressive Changes in Maturing Ovarian Eggs during the Time Interval between Capulaticm and Ovulation | |||
| Time elapsed since copulation (hrs.) | No. of cases observed | Condition of the egg | Condition of the follicles |
|---|---|---|---|
| 0 | 10 | Egg fully grown. Nucleus vesicular, in some cases vesicular tetrads present | Average diameter 970 um. The follicular epithelium forms a spider web. In some cases the egg is in a cumulus |
| 2 | 9 | The vesicular nucleus present in most cases. In all cases tetrads present | Spider web arrangement of granulosa. Liquor folliculi increasing in amount. Disintegration of follicle cells adjacent to
corona begins |
| 4 | 7 | Vesicular membranes have disappeared. Only traces present. Tetrads free in cytoplasm type | Average diameter 1045 um. Follicular epithelium changing from spider web to cumulus |
| 6 | 6 | Chromatic material decreases very much in size and forms the first spindle. No trace of nuclear membrane left | Most follicles in cumulus type. The liquor folliculi becomes increasingly viscous |
| 7 | 4 | First polar body extruded in many cases. The remaining chromatin moves sideways | As in preceding type |
| 8 | 10 | First polar body present in all cases | Average diameter 1125 um |
| 9 | 4 | First polar body present. Second spindle in place and ready to form the second polar body | Average diameter 1310 um. Egg almost free in follicle |
There is also a definite follicular enlargement during this period, our measurements of fixed material giving a maximum follicular diameter of 970 microns (average of 10 follicles) before copulation and an increase to 1125 microns (average of 10 follicles) by the 8th hour after copulation.
When pregnancy urine, antuitrin-S,‘ or saline pituitary extracts, are injected intravenously, exactly the same sequence of events ensues.
We attempted to determine whether the maturation process involved in the production of the first polar body was due to the direct action of pituitary hormones.
Ova were taken from the large follicles of unmated does and cultivated in vitro. The culture medium consisted of sterile rabbit blood plasma, to which was added in the control series, several drops of a phosphate-buffered Ringer’s solution. In the experimental series we substituted for the Ringer’s solution extracts of beef pituitary glands made in Ringer’s or a preparation of maturity hormone? The ova were rapidly dissected in a Ringer-serum solution, care being taken to remove the viscous liquor folliculi that often surrounds the cumulus oophorus. The cumulus cells were not dissected away to any extent, since it seemed desirable to reduce handling to a minimum. The ova were cultured for varying periods at 38°C. in hollow ground slides sealed with paraflin, fixed in Bouin’s solution, and prepared for microscopic examination.
The data of this experiment are summarized in Table II. It is evident that the control series difiers in no way from the experimental series. All the cultured ova had formed tetrads and in some cases the nuclear membrane had dissolved. In certain instances the nucleus resembled the fusion nucleus of normally fertilized eggs (Fig. 8), as though chromosome division had taken place without polar body formation but with subsequent refusion of the nuclear elements.
Table II
| Table 2 - Data on Ovarian Eggs Obtained by Puncture of Graafian Follicles, and Cultured in (a) Media Containing Pituitary Hormones, (b) Media of Ringer-Locke Solution | |||
| Time of culturing | No. of cultures | Medium | Results |
|---|---|---|---|
| 20 (min.) | 14 | Ringer-Locke + 1 drop beef pituitary | Vesicular tetrads in all cases |
| 2 (hrs.) | 11 | Ringer-Locke + 2 drops beef pituitary | In some cases vesicular tetrads and some free tetrads were formed. Some formed polar bodies |
| 24 | 9 | Ringer-Locke + 1 drop maturity hormone | Vesicular tetrads in all cases, except 3 which had free tetrads |
| 25 | 4 | Ringer-Locke + 2 drops maturity hormone | Vesicular tetrads formed in all cases |
| 25 | 7 | Ringer-Locke + 3 drops maturity hormone | Vesicular tetrads, free tetrads, structures resembling fusion nuclei |
| 2 | 18 | Ringer-Locke | Vesicular tetrads and free tetrads |
| 4 | 3 | Ringer-Locke | Free tetrads |
| 6 | 3 | Ringer-Locke | Rudiment of first polar spindle |
| 20 | 16 | Ringer-Locke | Vesicular tetrads, free tetrads, fusion nuclei |
These data indicate that polar body formation as a result of direct stimulation by pituitary hormones is improbable. It is possible however, that the hormone concentration in vitro was too low to effect any stimulation of the ova. In order to test this we determined the efiect of varying concentrations of the two preparations injected intravenously. The data are summarized in Table III.
Table III
| Table 3 - Effect of Injecting Varying Concentrations of Saline Beef Pituitary Extract and of Maturity Hormone upon tke Maturation o/Ovarian Eggs | ||||
| Preparation used for injection | No. of animals | Method of injection | Time between injection and autopsy | Results |
|---|---|---|---|---|
It will be seen that a dosage of 1/4 cc. of maturity hormones was sufiicient to cause polar body formation, and 2 cc. of Ringer’s extract of beef pituitary was similarly effective. Slightly less than this minimal dosage was added to the cultures receiving the maximum amount of hormone. If we consider that the minimum effective dosage in vivo is distributed throughout the organism and probably partitioned or excreted in such a manner as to make available to the ovary only a fraction of the amount injected, it seems entirely probable that a sufficient amount was available to the cultured ova.
1 We are indebted to Dr. Oliver Kamm of Parke, Davis & Co. for the antuitrin-S.
2 This preparation was supplied to us by Dr. J. B. Collip of McGill University, to whom we express our gratitude.
Since direct action of the pituitary hormone upon the ova seems to be excluded, the probability exists that a second hormone acts directly upon the eggs, and that a stimulating concentration of this second hormone occurs as the result of pituitary hormone activity. Pituitary secretions are known to be thyreotropic as well as gonadotropic, and accordingly thyroxin activity may be involved. More- over, Carter (1932) has demonstrated that thyroxin-like substances are probably concerned in the activation of sea urchin eggs. We therefore injected thyroxin intravenously, as indicated in Table IV.
Table IV
| Table 4 - Effect of Injecting Thyroxin* or Thyroproteint of Varying Doses upon the Maturation of Ovarian Eggs | ||||
| Preparation used for injection | No. of animals used | Method of injection | Time between injection and autopsy | Results |
|---|---|---|---|---|
Ovulation in no instance occurred, but a definite cytological effect was observed. This consisted of an atresia of ovarian follicles preceded or accompanied by the formation of chromosome tetrads in the ova of large follicles (Fig. 9). In a few instances polar body formation occurred but ordinarily not at 8 hours after injection of the thyroxin but some time later. The effect of thyroxin injection is therefore not as rapid as the pituitary-induced effect, nor, under the conditions of these experiments, as complete. Eggs cultured in vitro with crystalline thyroxin added to the medium (see Table V) formed polar bodies but so did the control ova cultured without added thyroxin. In one instance an ovum cultured with thyroxin formed a second polar body. With this one exception there is no detectable cytological difference between ova cultured in thyroxin-containing media and the control ova.
Table V
| Table 5 - Effect of Adding Thyroxin* to Culture Media upon the Maturation of Ovarian Eggs in Culture | |||
| Composition of the medium | No. of cultures made | Time interval of culturing | Result |
|---|---|---|---|
All of the in vitro experiments indicate that the mere process of explanting ova results in the initiation of maturation. This implies that the nutritive conditions that make for maturation in vivo are automatically duplicated in vitro. Pituitary hormones (and to a lesser extent thyroxin) must induce, in the follicle, these nutritive conditions. What these conditions are is not entirely clear, but it is obvious that in vivo the pituitary hormones initiate the events leading to their establishment.
In another experiment ovarian eggs were handled and cultured in Ringer's solution alone (Table II) to obviate the possibility that the blood plasma might contain sufficient pituitary hormone or thyroxin to stimulate maturation per se. Maturation spindles were formed in these ova whether maturity hormone was present or not. Finally, motion pictures were taken of single ovarian eggs cultured in Ringer's solution. The dissolution of the nuclear membrane and the formation of tetrads and the polar spindle occurred at the same rate as in vivo.
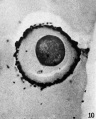
When ripe ova are removed from the follicles of unmated does and placed in sperm suspensions, sperm penetration occurs (Figs. 10 and 11), and in some instances the male pronucleus forms (Fig. 12). The first polar body is given off by these ova just as in control ova cultured without sperm, but control ova (Table II) never give off the second polar body whereas a number of these ova with sperm do (see Table VI).
Table VI
| Table 6 - Results o/Inseminating Ovarian Eggs in Culture with Sperm Suspensions | |||
| Approximate age of egg | Condition of sperm | Time of culture | Result |
|---|---|---|---|
Ovarian ova inseminated in vitro have been transplanted into the fallopian tubes of pseudopregnant rabbit does. 2 to 3 days later these ova were recovered from the tubes and examined. In a small number regular cleavage had taken place. In others cleavage was irregular or absent. The latter cases are undoubtedly due to a condition of polyspermy. It is difficult to avoid polyspermy with in vitro inseminations, since it is necessary to have sperm suspensions sufficiently concentrated to insure sperm entry and not so dilute that the sperm will die before any entry is possible. Even with fairly dilute suspensions of active sperm we have found two or more spermatozoa in the egg cytoplasm. This situation may also be due parry to the fact that a number of our insemination experiments were carried on at room temperature, when perhaps the normal fertilization reaction insuring monospermy is either inhibited or proceeds at a rate so slow that several sperm may enter before the reaction is completed.
Nonetheless, the recovery of normally cleaved ova indicates that ovarian eggs may be successfully fertilized even though they have not, at the time of the removal from the ovary, undergone the first maturation division. The fact that this occurs normally in the dog indicates that this is not as unlikely as it may seem, and implies that it does not occur normally in other mammals simply because the stimulus to maturation is attained in the follicle in these other species.
Discussion
We believe that these experiments demonstrate clearly that the ovarian egg in the ripe follicles of mammals is activatable and fertilizable at any time. in another paper (Pincus and Enzmann) we have shown that once ovarian ova have reached full size and the nucleus has entered into the dictyate stage an isolation of the egg from the follicular epithelium frequently results in the formation of polar spindles with or without subsequent polar body formation, dependent apparently upon the rate of follicular atresia or the onset of ovulation. The data of this investigation show that the isolation of ova in vitro results in a similar sequence of nuclear changes. This implies that the associated follicle cells serve either to maintain the egg in a nutritional state wherein nuclear maturation is impossible, or that they actually supply to the ovum a substance or substances which directly inhibit nuclear maturation. It is notable that the preovulatory follicle characteristically contains few or no strands connecting the ovum to the follicular epithelium (cf. Pincus and Enzmann). It is interesting to note that in the dog (Evans and Cole, 1931) rupture of the follicular epithelium does not occur until ovulation (a complex folding goes on before ovulation, and the separation of the ovum from the epithelium presumably intervenes at a short interval before ovulation)
The obvious inference from these findings is that mammalianovaries contain large numbers of fertilizable ova that never emerge from the ovary. If these ova can be obtained easily, one of the chief limitations to the direct study of mammalian eggs in vitro, namely the llmited number of eggs ovulated, will be overcome. We have, in fact, obtained large numbers of eggs by puncturing follicles of various sizes. The uses to which these eggs have been put will be described in subsequent papers.
We should, finally, consider one possible objection to the findings here reported. It may be declared that the maturation figures of our cultured ova are the results of atresia and not of activation. If that were the case then normal maturation would be also a process of atresia, since the events occurring in vivo and in vitro are indistinguishable morphologically and in the rate of their progress. True atresia may be said to occur only when an ovum is no longer fertilizable (for further discussion see Pincus, 1936).
Summary
- A definite chronological sequence of events occurs in the eggs and follicles of rabbits after mating or after the injection of ovulation-inducing substances. The follicle secretes secondary liquor folliculi, and there occurs a separation of the corona radiata from strands connecting it to the follicle ceils. The ovum goes through nuclear maturation with as climax the production of the first polar body by the 8th hour after copulation.
- Thyroxin injections cause indirectly the same effects as mating or pituitary injections but no ovulation occurs. The thyroxin effect occurs later than the pituitary effect and is due to an initiation of atresia in the follicles.
- Explantation of ova results in typical maturation phenomena which are apparently unaffected by the presence of pituitary hormones or of thyroxin in the culture medium.
- It is concluded that maturation of the ovum can be obtained simply by isolating it from the normal follicular environment.
- Normal fertilization can be secured with eggs removed from the follicles.
Bibliography
Bellerby, C. W., J. Physiol., 1929,67, 33.
Carter, G. J., Brit. Y. Exp. Biol., 1932,9, 238.
Deansley, R., Fee, A. R., and Parkes, A. S., J. Physiol., 1930, 70, 38.
Evans, H. M., and Cole,H. H., Mere. Univ. California, 1931,9, 65.
Friedman, M. H., Am. J. Physiol., 1929,90, 617.
Heape, W., Proc. Roy. Soc. London, Series B, 1905, 76, 260.
Pincus, G., The eggs of mammals, New York, The Macmillan Co., 1936, in press.
Pincus, G., and Enzmann, E., The growth, maturation and atresia of ovarian eggs of the rabbit, to be published.
Plates
Plate 1
Plate 2
FIG. 3. Ovarian egg from a doe mated 4 hours previously. Tetrads fully formed and vesicular nucleus dissolved.
FIG. 4. Ovarian egg from a doe mated 5 hours previously. The tetrads have become smaller and arranged themselves in a plate. All traces of the vesicular membrane have disappeared.
FIG. 7. Ovarian egg from a doe mated 9 hours previously. First polar body and second polar spindle.
FIG. 8. Ovarian egg cultured for 24 hours in Ringer-Locke solution containing maturity hormone. Note apparent fusion nuclei.
FIG. 11. Ovarian egg from a doe mated 6 hours previously and inseminated in vitro with normal sperm. Male and female pronuclei present side by side.
FIG. 12. Ovarian egg from a doe mated 8 hours previously and inseminated with normal sperm. The first polar body has formed and sperm penetration occurred. The entering spermatozoan has formed a male pronucleus (centre left).
Pincus G. and Enzmann EV. The comparative behavior of mammalian eggs in vivo and in vitro. (1935) J Exp Med. 62(5): 665-75. PMID 19870440
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - The Comparative Behavior of Mammalian Eggs in Vivo and in Vitro. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_Comparative_Behavior_of_Mammalian_Eggs_in_Vivo_and_in_Vitro
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G