Paper - A presomite human embryo showing a yolk-sac duct
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Lewis BV. and Harrison RG. A presomite human embryo showing a yolk-sac duct. (1966) J Anat. 100(2): 389-96. PMID: 5954785
| Online Editor | ||
|---|---|---|
|
Embryo Liverpool I was previously described in - Lewis BV. and Harrison RG. A presomite human embryo showing an early stage of the primitive streak. (1953) J Anat. 87(2):124-9. PMID: 13044724
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
A Presomite Human Embryo showing a Yolk-Sac Duct
By B. V. Lewis And R. G. Harrison
Department of Anatomy, University of Liverpool
With 8 figures
B. V. Lewis Present address: Department of Obstetrics and Gynaecology, Hammersmith Hospital, Postgraduate Medical School of London.
Introduction
Whilst several human embryos demonstrating an early stage of the primitive streak are known and recognized, since many of them show specific characteristics, it is important to record such embryos in the literature in the hope that they may shed some light on embryogenesis at this stage. One such embryo, which shows an interesting appearance of the yolk-sac, was discovered by one of us (B.V. L.) and considered worthy of documentation. It is proposed to designate the embryo ‘Liverpool II’.
Clinical History
The embryo was recovered from a woman aged thirty—five who had had eight previous pregnancies, of which four were full-term normal deliveries, two terminated in neonatal death (one from prematurity at 28 weeks, the other from congenital heart disease), and two abortions.
The mother’s menarche commenced at the age of twelve, and she had regular periods up to the time of her last confinement in 1958. Thirteen months later, and also in 1959, she was treated with methyl testosterone for epimenorrhagia, her cycle being 5/14~21.
In 1963 she had further heavy menses. A period commenced on 7 September 1963 and on the fifth day she started taking Norlestrin (Parke, Davis :_ 2-5 mg norethisterone acetate with 0-05 mg ethinyl oestradiol) one tablet daily. Seven days after starting the tablets she had a heavy blood loss per vaginam which lasted for 6 days, i.e. from 19 to 25 September 1963. On 21 October 1963 a total abdominal hysterectomy with conservation of both ovaries was performed. The operation was therefore carried out on the 32nd day of the cycle. Coitus occurred on several occasions but unfortunately the patient was unable to remember the exact dates.
At operation the uterus appeared normal. A corpus luteum of pregnancy was'not observed since pregnancy was not suspected until the uterus was opened at the end of the operation. On transecting the uterus, the endometrium was noted to be very thick and very suggestive of a decidual reaction. On the posterior wall, about 1 cm from the left lateral angle, was a small dark nodule about 2 mm in diameter, embedded in the endometrium. The possibility of an early pregnancy was considered, and therefore the specimen was placed in freshly prepared Orth’s solution and submitted to histological examination. The pathological report on the uterus described no macroscopic abnormality. A representative section from the body of the uterus shows a late secretory endometrium and a normal myometrium, whilst histologically the cervix shows dilated N abothian glands but no other abnormality.
The Blastocyst
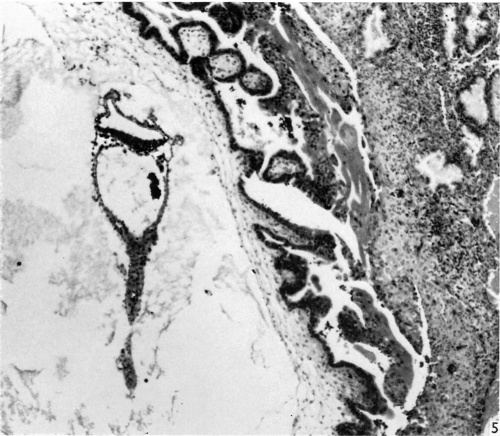
The block of tissue containing the blastocyst was sectioned serially at a thickness of 8 ,u, almost exactly in the transverse plane of the embryo. The external dimensions of the blastocyst (including chorionic villi) are 2-72 x 2-35 x 1-54 mm. The blastocyst is elevated from the surface of the endometrium and is covered by a thin layer of endometrium, without uterine epithelium (Fig. 1); the remains of a closing plug may be demarcated within this endometrium. Chorionie villi, localized to the embryonic pole of the blastocyst and completely absent from the abembryonic pole, are well developed, consisting of cytotrophoblast and extraembryonic mesoderm, with very little syncytiotrophoblast. In sections of the blastoeyst treated with the P.A.S. technique, P.A.S. positive granules are localized to the cytotrophoblast and are absent from the syncytiotrophoblast. Isolated vascular primordia formed by coalescence of angioblasts are visible within the chorionic mesoderm of the villi. The villi are branched; lacunae and intervillous spaces have formed (Fig. 1). Cyt0trophoblastic proliferations similar to those described in other embryos of this age (see Hamilton & Boyd, 1960) are clearly visible. The stroma of the endometrium at the site of implantation is oedematous, and shows a pre-decidual reaction, no definite ‘deeidual’ stromal cells having yet appeared. The endometrial arteries are spiral, and the glands tortuous. The epithelial cells of the uterine glands bordering on the site of implantation show the degenerative changes of karyorrhexis and cytoplasmic vacuolation (Fig. 5). Some syncytiotrophoblast streaming is occurring in an attempt to form a trophoblastie shell.
Embryo
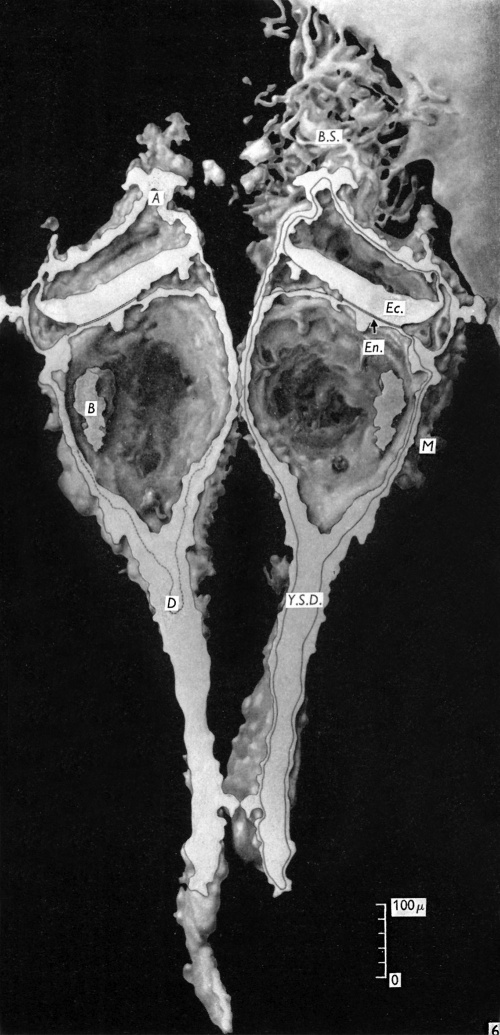
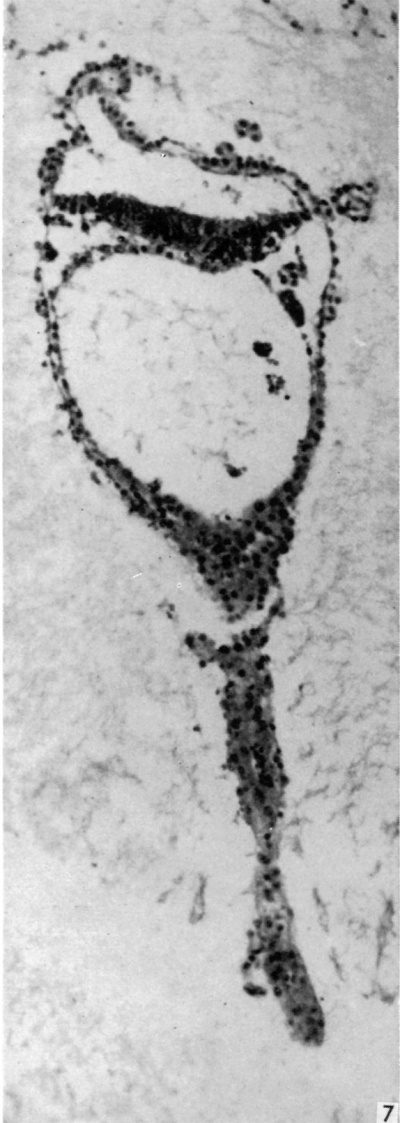
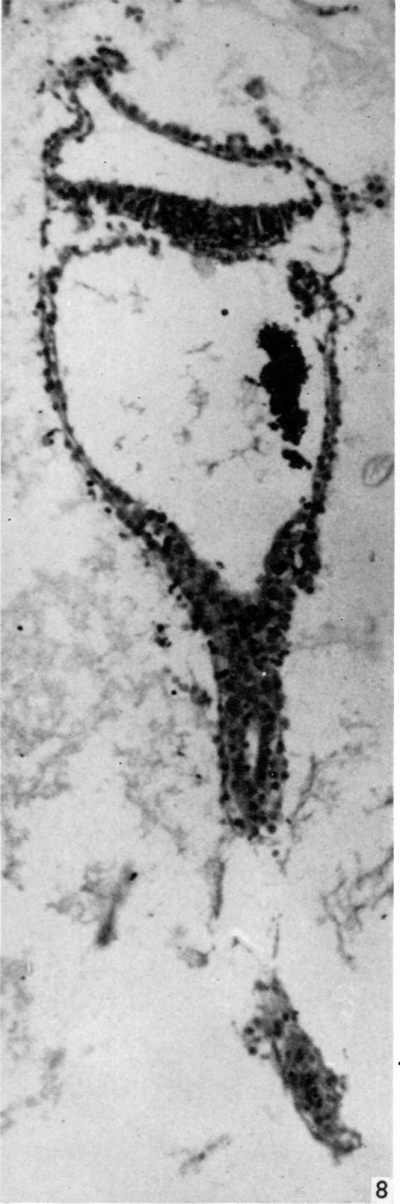
The embryonic plate is 0-264 mm in length and its transverse diameter 0-22 mm at the widest part. A primitive streak is present, recognizable in three sections at the caudal end of the embryonic plate as a slight median downgrowth two or three cells thick on the ventral aspect of the ectoderm (Fig. 2), which demonstrates a shallow primitive groove on its dorsal surface. This is immediately followed by an elevation into the amniotic cavity which extends over six sections (Fig. 3); a small allantoic diverticulum is present and an amniotic duct clearly visible (Figs. 2, 4). The allantoic diverticulum is quite small, extending over two sections only, being 25 /4 in diameter and embedded in the connecting stalk (Fig. 4). The amniotic duct, however, is much larger (approximately 55 ,u in length) and can be seen in fifteen consecutive sections. The most interesting feature of the embryo, however, is the continuation of the yolk-sac into an almost solid tubular duet, approximately ()-45 mm in length (Fig. 5). This is very obvious in a reconstruction model of the embryo, prepared from marine (resin bonded) plywood 4 mm in thickness at a magnification of 500 x (Fig. 6). This duct is lined by cuboidal cells which are much larger than the endodermal cells of the embryonic plate, and shows evidence of being constricted off from the embryo. There is a marked constriction at the origin of the duct (Fig. 7) and a lateral process of its distal end is being nipped off from the remainder (Fig. 8). The yolk-sac contains a collection of erythrocytes in its lumen; since these are anucleate it is presumed that they are of maternal origin. The embryonic disc is bilaminar, but a few intraembryonic mesoderm cells are visible interposed between ectoderm and endoderm at the lateral margins of the disc. Mitotic activity is present in certain of the ectodermal cells. The extraembryonic mesoderm forms a single layer of cells around the embryo, including the yolk-sac duct, and forms a connecting stalk at the caudal end of the embryo. In this region, in the splanchnopleuric extraembryonic mesoderm and that of the stalk, angiogenesis is proceeding actively.
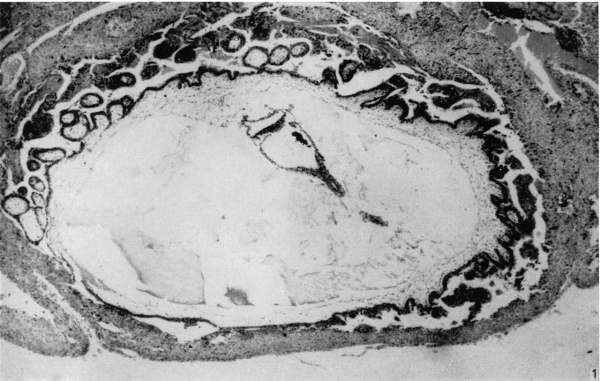
Fig. 1. The appearance of the blastocyst, showing the endometrium covering its surface, and the branched villi at the embryonic pole, absent from the abembryonic pole. The general characteristics of the embryo are visible. Part of the yolk-sac duct has constricted off from the remainder. x 44.
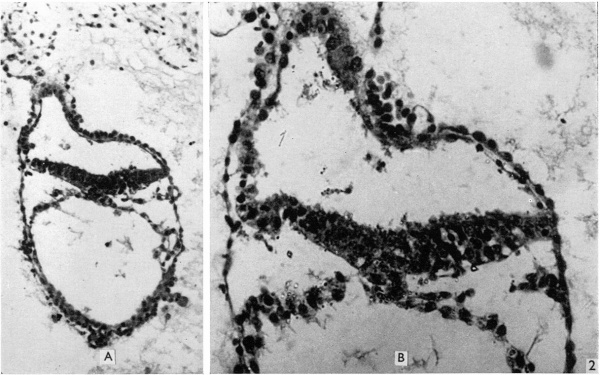
Fig. 2. The first section of the primitive streak, shown at low magnification (A, X 139), and the third section (B) at a magnification of 320. The streak forms a median downgrowth from the ectoderm of the embryonic plate towards the endoderm and fusing with it. Dorsal to the streak is a shallow primitive groove. In the upper part of A the body stalk can be seen attached to the amniotic duet, which is also clearly visible in B.
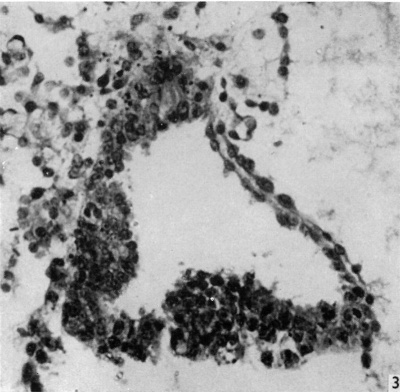
Fig. 3. Section of amniotic cavity at caudal end of embryo, caudal to the primitive streak, showing the eminence bulging into the floor of the cavity. x 320.
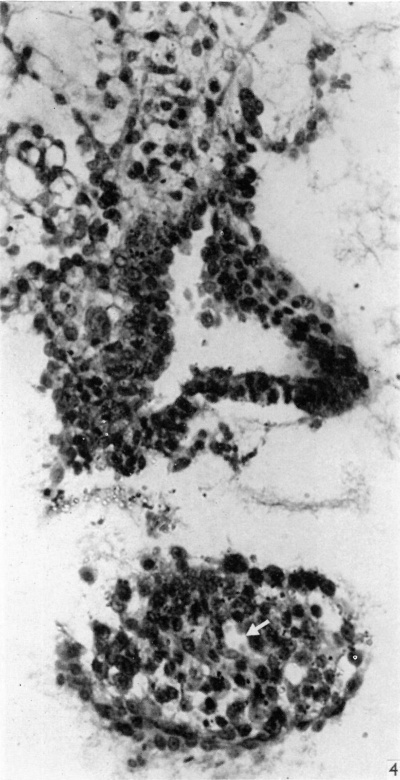
Fig. 4. Section through the embryo caudal to that in Fig. 3. The amniotic cavity is separated from the portion of the connecting stalk attached to the yolk-sac, seen in the lower part of the figure, which contains the allantoic diverticulum, indicated by an arrow. x320.
Fig. 5. Appearance of the embryo with its yolk-sac duct, and the implantation site. The yolk-sac contains a collection of erythrocytes in its lumen. The distal portion of the yolksac duct is being constricted off from the remainder. Chorionic villi in the upper part of the figure can be seen to be branching. Streamers of syncytiotrophoblast are in process of forming a trophoblastic shell. Some degeneration of the uterine glands is visible. x 71.
Discussion
The irregular menstrual history, complicated by hormone therapy, makes exact assessment of embryonic age very difficult. The dimensions, degree of differentiation and decidual appearances, however, place the embryo in Streeter’s (1942) group VII. The presence of the yolk-sac duct, in addition, considerably narrows the field of similarity to already reported embryos. The Teacher—Bryce II embryo (Bryce, 1924) possesses a yolk-sac duct which is more elongated and attached to the abembryonic pole of the blastocyst. Its lumen is prolonged through nearly the whole of the duct. Such an appearance is reminiscent of a yolk-sac placenta, for the splanchnopleuric extra-embryonic mesoderm covering the duct is in direct continuity with the somatopleure. The Biggart ovum (Morton, 1949) shows a yolk-sac duct lined by large cuboidal cells, but differs from the present specimen by possessing a large distal expansion of the duct attached to the somatopleuric extra-embryonic mesoderm at the abembryonic pole of the blastocyst. The Liverpool II embryo does not have such an expansion, but there is a large chorionic vesicle and cytoplasmic strand connecting it to the duct. A similar appearance was described by Heuser, Rock & Hertig (1945) in embryo no. 7801, in which a cytoplasmic strand connects a chorionic vesicle to the yolk-sac which, however, does not possess a duct. The general characteristics, and these latter features in particular, therefore point to an age of about 13; days. If it is accepted that the yolk-sac duct indicates a stage of differentiation of the yolk-sac from the exocoelomic membrane (primary yolk-sac), the present embryo is clearly contemporaneous with the Teacher—Bryce II embryo, Biggart ovum and embryo no. 7801. Since the age of the latter two embryos is estimated as 134} days, the process of transformation of the human exocoelomic membrane into yolk-sac must occur rapidly, and not over a period of several days as estimated by Heuser & Streeter (1941) for the macaque monkey. This would account for the comparative rarity of embryos displaying such features.
Fig. 6. Diagram of a reconstruction model of the embryo. A, Amniotic duct; B, collection of blood cells in yolk-sac lumen; B.S., connecting stalk; D, diverticulum of yolk-sac passing into yolk-sac duct (Y.S.D.); Ec., Ectoderm; En., endoderm; M, extra-embryonic mesoderm.
The above conclusions do not, of course, take into account the possible influence of coincident hormone therapy on developmental processes. This is probably the first presomite human embryo recovered under such conditions. There is, however, no histological evidence to suggest an adverse effect on the embryo of such therapy, and the degree of its preservation is even better than that in the Liverpool I embryo (Harrison & Jeffcoate, 1953).
Summary
An early presomite human embryo showing an elongated yolk-sac duct, in process of being constricted off from the embryo, is described and illustrated. The embryo, which is about 131} days old, was recovered by hysterectomy from a woman subjected to Norlestrin therapy, and shows no abnormality except the presence of erythrocytes in the yolk-sac lumen.
We are grateful to Mr D. J. Kidd for his invaluable assistance in reconstructing the embryo, to Mr L. G. Cooper in sectioning the blastocyst, and to Mr D. L. Reeve for photomicrographic assistance.
References
Bryce TH. Observations on the early development of the human embryo. (1924) Trans. Roy. Soc. Edinburgh, 53: 533-567.
Hamilton WJ. and Boyd JD. Development of the human placenta in the first three months of gestation. (1960) J. Anat., 94: 297-328 PMID 14399291
Lewis BV. and Harrison RG. A presomite human embryo showing an early stage of the primitive streak. (1953) J Anat. 87(2):124-9. PMID: 13044724
Heuser CH. Rock J. and Hertig AT. Two human embryos showing early stages of the definitive yolk sac. (1945) Contrib. Embryol., Carnegie Inst. Wash. Publ. 557, 31: 85-99.
Heuser CH. and Streeter GL. Development of the macaque embryo. (1941) Contrib. Embryol., Carnegie Inst. Wash. Publ. 525, 29: 15-55.
Morton WRM. Two early human embryos. (1949) J. Anat., 83: 308-314.
Streeter GL. Developmental horizons in human embryos. Description of age group XI, 13 to 20 somites, and age group XII, 21 to 29 somites. (1942) Contrib. Embryol., Carnegie Inst. Wash. Publ. 541, 30: 211-245.
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - A presomite human embryo showing a yolk-sac duct. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_A_presomite_human_embryo_showing_a_yolk-sac_duct
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G