Paper - A Young Human Embryo (Embryo Dobbin) with Head-Process and Prochordal Plate
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Hill JP. and Florian J. A young human embryo (embryo dobbin) with head-process and prochordal plate. (1931) Phil. Tran. Roy. Soc. London B, 219: 443-486.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
A Young Human Embryo (Embryo Dobbin) with Head-Process and Prochordal Plate
By J. P. HILL, D.Sc., F.R.S., and J. FLORIAN, M .D.
James Peter Hill (1873 - 1954)
(From the Department of Anatomy and Embryology, University College, London.) (Received July 27, 1931. Read November 19, 1931.)
(Plates 29-35)
Introduction
Our knowledge of the early development of Man has made marked progress during recent years, numerous fairly Well—preserved embryos having been described in greater or less detail. There are still, however, numerous gaps in our knowledge, pertaining not merely to points of detail but to fundamental questions, so that any early embryo reasonably Well preserved and reasonably Well sectioned is deserving of careful study.
Our specimen belongs to the comparatively late presomite stage represented in the literature by such embryos as K113 (GROSSER, 1913), Wa17 (GROSSER, 1931), Peh.1- Hochstetter (ROSSENBECK, 1923), and the embryo of INGALLS (1918), and characterised by the presence of an elongated primitive streak, a luminated chorda-process and a prochordal plate. We offer no apology, however, for presenting a fairly detailed, description of yet another example of this stage, since our embryo amongst other things provides a more complete picture of the cranial region of the head~process than any specimen hitherto described.
History and Treatment of the Specimen
The chorionic vesicle, the embryo of which forms the subject-matter of this paper, was presented to one of us (HILL) by Dr. ROY DOBBIN, of Cairo, through the kind offices of Professor D. E. DERBY. In appreciation of his valuable gift, We have much pleasure in associating Dr. DOBBIN’s name with the embryo.
The clinical history supplied by Dr. DOBBIN is as follows: “ Coitus, 6.10.23 ; effort probably causing abortion, 21.10.23; first bleeding, 22.10.23; abortion (painless), 23.10.23." Although an abortion, we see no reason to regard the specimen as other than perfectly normal.
The chorionic vesicle (which was preserved in spirit) was, when received, somewhat flattened and shrunken (fig. 1, Plate 29). Except over a small area on one side (approximately 3 x 2 mm in diameter), which was almost bare, the vesicle possessed a fairly uniform covering of short, close~set, branched villi (fig. 2, Plate 29), to which at one point a small fragment of blood—clot adhered. Including the villi, its dimensions in alcohol were as follows : 11.5 mm (in long diameter) x 8.5 mm (in short diameter) x 45 mm (in thickness). After clearing in oil of cedar-wood, the corresponding internal diameters were 9 mm x 5.5 mm x 2.5 mm. The vesicle, after being photographed and drawn, was dehydrated and cleared in oil of cedar-wood. A small portion of the chorion, including the bare area, was then carefully removed, and through the opening so made it was possible, fortunately enough, to locate the embryo under the binocular dissecting microscope. The embryo was then isolated along with the segment of the chorion to which it was attached, and stereo-photographs were successfully taken of it, in the cleared condition in oil of cedar—wood.
Subsequently Mr. A. K. MAXWELL, with the aid of these photographs and the camera lucida, made the beautiful drawings representing the left lateral and dorsal aspects of the embryo which are reproduced as figs. 3 and 4, Plate 29. These figures are, we believe, unique, in that they are the only illustrations extant of the dorsal and lateral views of the human embryo at this particular phase of development which have been made directly from the embryo itself, and not from models or reconstructions.
The embryo and the related piece of chorion were double—embedded in cedar-wood oil-pyroxelene and paraffin, and cut cranio—caudally by H. BARKER into a complete and really very fine series of sections at 8 (1., the sectional plane being almost exactly transverse to the long axis of the embryo. The state of preservation of the latter proved to be by no means perfect cytologically, but is sufficiently good to ustify us in giving a fairly detailed account of its structural condition. The most obvious fixation—defect is the partial disintegration of the most cranial portion of the Shield-ectoderm, the granular detritus resulting therefrom lying partly in the amniotic cavity and partly below the ectoderm, in a space enclosed between the latter and the detached basement membrane. The fact that the cranial region of the early embryo is the first part to undergo dissolution seems to be Well recognised. Inspection of fig. 3, Plate 29, will show that the embryo no longer occupies its normal position in relation to the chorion, but has been displaced in the ventral direction. Fortunately the deformation accompanying this displacement has affected only the most caudal part of the embryo and the connecting stalk, and is not of a serious character.
The following measurements were made whilst the embryo was still in oil of cedar- Wood, but must be regarded as approximate only. The lettering refers to text—fig. 2 :-
| Anterior margin, embryonal shield to region of cloacal membrane (A-Cl.) | 0.98 mm |
| Vertical diameter (D-F) of yolk-sac | 1.092 mm |
| Antero-posterior diameter of yolk-sac, near its mid-region | 0.98 mm |
| Vertical height, amnio-embryonal vesicle (D-E) | 0.468 mm |
| Length of yolk-sac process | 0.88 mm |
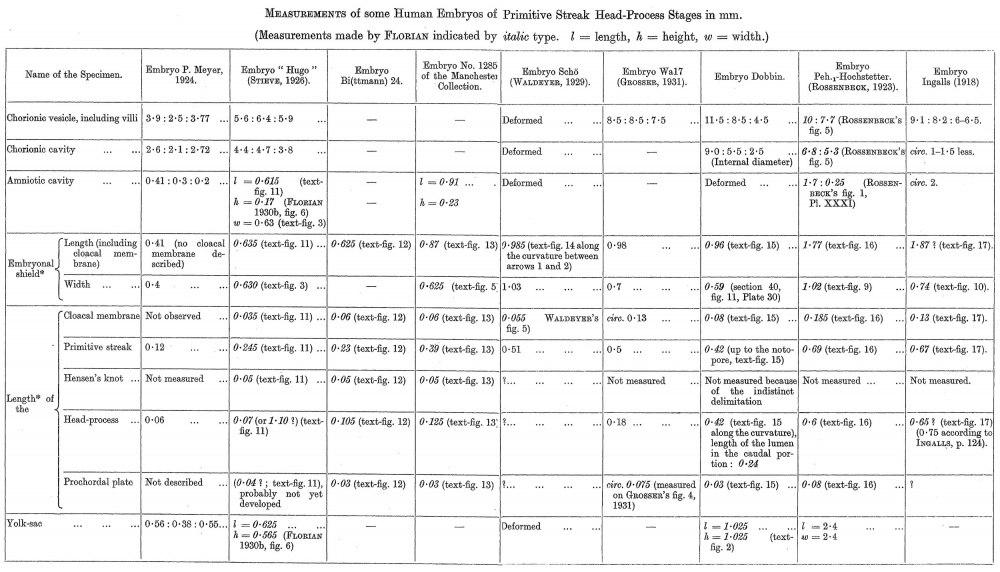
The measurements of the embryo based on the sections and graphic reconstructions are set forth, along with those of other early embryos, in the tables provided at the end of this paper (p. 480-81).
We take this opportunity of expressing our very grateful thanks to Dr. ROY DOBBIN and to Professor D. E. DERBY for the gift of this interesting embryo. We are further greatly indebted to Professor J. S. B. STOPFORD for the loan of the presomite Manchester embryo, No. 1285 ; to Professor J. C. BRASH for the opportunity of examining the sections of the Thompson-Brash embryo; to Hofrat Professor F. HOCHSTETTER for permission to study the Peh.,-Hochstetter embryo, described by ROSSENBECK, 1923 ; and to Dr. O. BITTMANN, Brno, for the gift to one of us (FLORIAN) of the Bi(ttmann) 24 embryo, to which reference is made on pp. 466—7 . We desire also to thank Mr. A. K. MAXWELL, Artist to the Department, for the care and skill he has expended on the illustrations on Plates 29-34, Which, with the exception of figs. 1 and 2, are all based on photomicrographs; and to Mr. F. J. PITTOCK for his invaluable assistance in photography.
The Embryo as a Whole
The embryonal body, represented by the two vesicles — the amnio-embryonal and yolk-sac vesicles — is attached by a very distinct connecting stalk to the chorion (fig. 3, Plate 29). It will be observed that, as has just been mentioned, the dorsal surface of the amnio~embryonal vesicle does not face the chorion, as is the rule in this stage of development, the embryonal shield forming an angle of about 125° with the inner surface of that membrane. This is evidently due to the artificial displacement of the embryonic body, including the connecting stalk, in the ventral direction. At the same time the embryo and the connecting stalk have sufiered a slight rotation round the long axis of the body towards the left side (as may be seen from fig. 4, Plate 29). As is usual at this stage, the yolk—sac vesicle is distinctly larger than the amnio-embryonal.
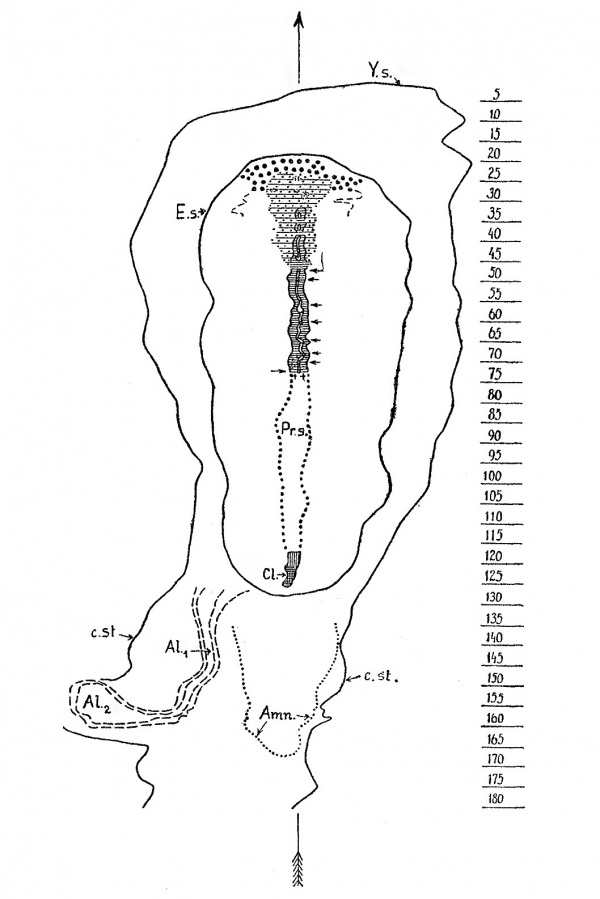
Text-fig. 1. Graphic reconstruction of the dorsal view of the embryonic formation; Dobbin embryo X 75. - Outlines of the embryonal shield (E.s.), yolk-sac (Y.s.) and connecting stalk (c.st.) indicated by a thick line-; outlines of the allantoic canal (ALI = tubular part, AL2 =2: vesicular part) indicated by interrupted lines; outline of the caudal end of the amniotic cavity (Amn.) dotted; cloacal membrane (UL) lined longitudinally where indistinct, cross—hatched where distinct ; P7‘..S‘. =2 primitive streak; chorda process and chorda canal lined transversely; lateral mesodermal bands lined transversely and dotted; ventral openings of the chorda canal marked by arrows on the right, its dorsal opening by an arrow on the left. ++ = HENSEN’s knot ; prochordal plate marked by large dots. On the scale is indicated the level of every fifth section. The median plane is indicated by an arrow.
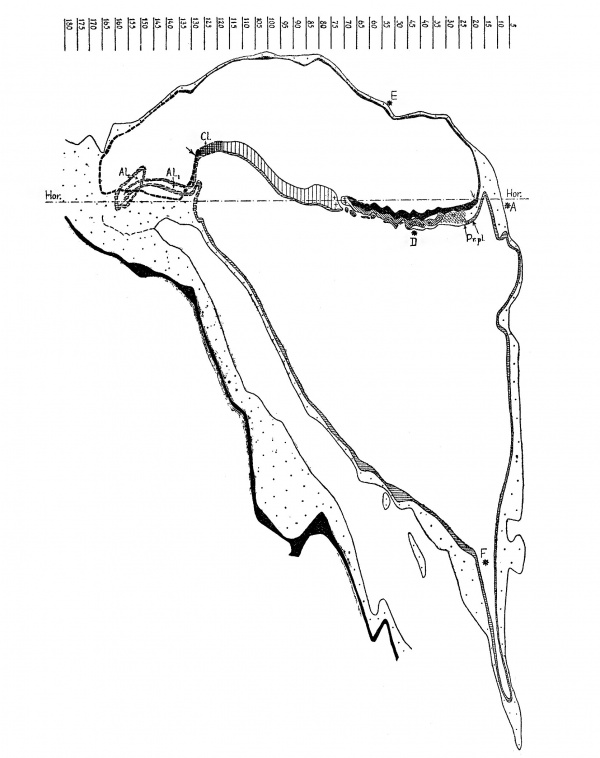
Text-fig. 2. Graphic reconstruction of the median section through the Dobbin embryo X 7 5. - Ectoderm black; endoderm lined horizontally; primitive streak lined vertically; +=HENSEN’s knot; chorda-process lined obliquely; prochordal plate (Pr.pl.) lined horizontally and dotted; mesoderm dotted; cloacal membrane (O'l.) cross—hatched. Limits of the embryonal shield marked by arrows. AL; 2: proximal (tubular) part of the allantoic canal; Al.2 == its distal (vesicular) part. Hor. —-—=. horizontal plane (the plane of the projection in text-figs. 1 and 8). The caudal part of the embryonic formation, including the cloacal membrane, the allantoic canal, the caudal part of the d amniotic cavity and the connecting stalk are projected into the median plane, and their outlines are marked by interrupted lines. , The cell-layer underneath the intermediate region and the most cranial portion of the head-process, the significance of Which is doubtful, is left White.
The embryonal shield forms in the lateral view (text-fig. 2) an open S-shaped curve. It reaches cranially up to the contour-line formed by the amniotic and yolk—sac vesicles, and ends caudally just in front of the connecting stalk.
The sections show that the embryonal shield over its cranial two-thirds is not flat as is normal at this stage, but is dorsally concave so that in the lateral view of the embryo (fig. 3, Plate 29) the ventral surface of the left half of the embryonal shield is turned towards the observer and appears much darker than the right half, which is only partly visible.
The yolk—sac is roughly conical in form; its broad base underlies the embryonal shield, whilst its apical region narrows to form a spike-like projection. This latter is prolonged into a filamentous process (about 0 - 88 mm. in length) which exhibits three enlargements, an elongated thickening close to the apex of the yolk-sac, an oval swelling near the middle of its length and a quite minute nodule close to its termination (fig. 3, Plate 29). It ended freely, without reaching the chorion. The entire process represents the yolk~sac process first described by STRAHL and BENEKE 1910, and subsequently observed in all adequately preserved early human embryos in a more or less developed condition. The process attains its maximum development in the embryos Teacher-Bryce II (BRYCE, 1924) and BENEKE, in which it reaches and is attached to the opposite wall of the chorion. The latest stage in which it has been observed in this complete condition is the embryo, G POLITZER, with seven pairs of somites (G. POLITZER, 1930). The yolk-sac at its postero-dorsal extremity is in the sections seen to be continued into the mesoderm of the connecting stalk in the form of an elongated canal (the allantoic canal). At its caudal end this enlarges to form a distinct thin-walled vesicle, which can be seen. both in the dorsal and lateral views of the embryo (figs. 3 and 4, Plate 29) as a small oval structure on the surface of the connecting stalk close to its attachment to the mesoderm of the chorion, and on the left side of the same 9 (cf. also figs. 28 and 29, Plate 32).
On the surface of the yolk-sac (fig. 3, Plate 29) a number of vessel—primordia can be clearly observed.
In the dorsal view (text-fig. 1 and fig. 4, Plate 29), the shoe—sole shaped form of the embryonal shield is apparent. Along its middle line the head-process and the primitive groove could be made out in the intact specimen. The pointed prolongation of the amniotic cavity along the dorsal surface of the connecting stalk is very distinctly seen in the dorsal view, whilst the amniotic membrane itself, forming the roof of the amnio- embryonal vesicle, is seen in the lateral View (fig. 3, Plate 29).
Embryonal Area
(i) Head-process[1] and Prochordal Plate; - In our preliminary note (HILL-FLORIAN, 1931) we distinguished three portions in the axial preblastoporic structures in our specimen. The most cranial portion we interpreted as a prochordal plate which had already produced a thick mass of prochordal mesoderm. Since the publication of our preliminary note one of us (F.), by the kind permission of Professor HOCHSTETTER, has been able to study the region in question in the embryo Peh.1-Hochstetter (RossEN~BECK, 1923). As this embryo is much better preserved in the region of the prochordal plate than the Dobbin embryo, we have been able to convince ourselves that what we have interpreted as prochordal plate in our preliminary note really represents the cranial end of the head~process. The actual prochordal plate (text-fig. 8) in the Dobbin embryo is very indistinct because of the poor preservation of this region, and it is also possible that the plate varies in difierent embryos. It is represented by an area of thickened endoderm in front of the cranial end of the head—process, presenting no distinct evidence of mesoderm formation. The thickened endoderm of this region can be traced continuously backwards underneath and laterally to the cranial part of the head-process up to section 27. Caudally it becomes indistinct. _ In the head—process itself we can distinguish three parts : (1) A caudal part forming about the caudal half of the entire formation and extending through sections 7 2-46; (2) a shorter part which extends forwards from the caudal segment and gradually widens to pass into continuity with the third part. This second portion we distinguished in our preliminary note as the intermediate region. It is comprised in sections 46 to about 32; (3) a short but broad terminal or cranial part occupying sections 31 to 23.
The caudal part (1) is formed by a typical chorda—canal, its lumen communicating by seven ventral openings with the yolk—sac cavity and by a dorsal opening on the surface of I-IENsEN’s knot with the amniotic cavity. 'I‘he_intermediate part (2) is formed by a segment of the head—process, which here has undergone differentiation into a median chorda~process and lateral mesodermal bands. The most cranial part (3) (which we distinguished as prochordal plate in our preliminary note) is formed by a distinct, dense cellular mass, Olevoid of any differentiation.
The ectoderm of the amnio-embryonal vesicle is first encountered in section 18 and its cavity in section 19 (fig. 9, Plate 30). Between the cranial end of the amnio-embryonal vesicle and the yolk—sac endoderm, there is present a sheet of mesoderm which can be traced cranially up to section 15. . Its cells are fairly compactly arranged and are connected by fine cytodesmata with the ectoderm and endoderm. The median part of this mesodermal sheet passes into continuity behind ‘with the cranial tip of the head—process, which is first visible in section 23. In front of the head-process the prochordal plate appears. The first indication of a thickened endodermal area in the median region seems to occur in section 20, but is only slight. It broadens out in section 21 (fig. 30, Plate 32), and in section 22 becomes more distinct. In section 23 (fig. 31, Plate 32) the prochordal endoderm has almost the same structure as in section 22, the nuclei being in places several deep ; in this section the cranial tip of the head-process appears in the median plane, intercalated into the mesodermal sheet. The head-process rapidly increases in size as it is traced back, and already in section 25 has assumed the appearance seen in section 27 (fig. 10, Plate 30 ; fig. 32, Plate 32). These figures show clearly the mesoderm-like character of this part of the head-process. Laterally on each side, the endoderm is still ‘distinctly thickened, but below the head- process itself it is no longer distinguishable as a separate layer. The cells of the head- process are for the most part compactly arranged and form a mass of considerable thickness, which laterally passes over without definite limit into the adjoining thin sheets of primitive streak mesoderm. The cells contain deeply staining chromatophilic granules which resemble those described by BONNET (1901, p. 287) in the “ Erganzungs- platte ” of the dog, and they are also present in the minute intercellular spaces. In places the cells tend to be arranged round small cavities (one in section 24, two in section 27, fig. 32,Plate 32, and one in section 28, fig. 33, Plate 32), but these vesicles are too indistinct and too irregularly distributed to have any important significance.
The thickened endoderm continued back from the prochordal plate can be traced on either side of the head-process up to section 27 and, as in the preceding sections, is fused with the head-process in the median line. Caudally to this section the thickened endoderm area becomes indistinct, and behind section 34 is certainly no longer present. The possible explanation. of these facts we shall discuss later in connection with the description of the same region in other human embryos (t. p. 482).
In section 30 (fig. 34, Plate 32) the head-process has become reduced, both in thickness and in width, as compared with that of the preceding sections, whilst its cells present a somewhat looser appearance, and the chromatophilic granules have almost completely disappeared. The next section (N o. 31, fig. 35, Plate 32) presents a very similar appearance, except that there are no granules. and the axial mass is more massive.
In both sections no distinct endoderm can be observed below the head-process.
Section 32 (fig. 36, Plate 32) marks the beginning of our intermediate region, since in it we encounter the tip of the chorda-process in the form of a compact circular mass, situated asymmetrically on the left side[2] and composed of cells radially arranged round a potential lumen and possessing peripheral nuclei. On the right of the chorda-process the mesoderm of the head-process forms a dense mass, but on the left it is quite diffuse. There is no clearly defined underlying layer of endoderm.
In the following section (No. 33, fig. 37, Plate 33) the chorda-process is represented by a distinct chorda—canal, with a very obvious lumen and with a mesodermal mass closely adjoining it on each side ; that on the left being more difiuse, that on the right more compact and much less massive than the mass in the corresponding position in the preceding section. Both the masses as well as the chorda-canal closely adjoin the endoderm, which is not distinct as a separate layer. We regard these lateral masses as part of the lateral mesodermal bands, characteristic of the intermediate region.
Similar conditions are observed in section 34 (fig. 38, Plate 33), except that the lumen of the chorda—-canal is much reduced, and in section 35 (fig. 39, Plate 33) only a trace of it remains. The endoderm below the axial region is not distinguishable as a separate layer. This is the case also in section 36 (fig. 40, Plate 33), where the lumen of the chorda—canal again becomes quite distinct.
In section 37 (fig. 41, Plate 33) the chorda-process is solid, and the limits of its ventral wall are difficult to determine. No endoderm is distinguishable below the head—process. The mesodermal bands are distinct, the right being more compact than the left, and are delimited from the chorda—-process dorsally by a groove. Section 38 (fig. 42, Plate 33) is very similar, except that the right mesodermal band is distinctly larger than the left. It is evident that no endoderm is distinct below the head-process. A broad shallow groove (already indicated in the two preceding sections) is now to be found underneath the latter, in contrast to the ventral convexity in the corresponding position in the most cranial region of the head-process. It can be traced back into continuity with the well-marked median groove underlying the caudal half of the head»-process.
In sections 39--43 (figs. 43--46, Plate 33) there is a continuous lumen in the chorda- canal; no definite endoderm is present below the median part of the head-process. The lateral mesodermal bands are symmetrically disposed and quite well marked.
In section 44 (fig. 47, Plate 33) the chorda-canal, broader than in fig. 46, does not show the typical characters seen in the preceding sections. Its lumen appears as a large eccentric cavity of somewhat irregular outline, whilst its cells exhibit an epithelial arrangement only over its dorsal half, those of its ventral wall being irregularly arranged and fused with the endoderm. The lateral mesodermal. bands are now much reduced in size. Essentially the same features are seen in the succeeding section (No. 45, fig. 48, Plate 33). The cells exhibit an epithelial character only on the dorsal side of the solid chorda-process. The right mesodermal band is still distinct, but small, and the left has practically disappeared. In section 46 (fig. 49, Plate 33) the sectional appearance of the axial formation has considerably altered. The chorda-process now appears more massive, owing to the compact groupingof its cells. They show practically no distinct epithelial arrangement, and are fused ventrally with the endoderm, which below the chorda-process is not distinguishable as an independent layer. There may possibly" still be a remnant of the right mesodermal band, but the left has completely disappeared. We may regard this section as marking the junction between our intermediate region and the caudal part of the axial formation, which, as we shall see, consists only‘ of a chorda—canal, devoid of lateral mesodermal bands. In section 47 (fig. 50, Plate 33) the chorda-process is massive and takes the form of a chorda-canal semicircular in outline, the lumen of which appears as a very narrow fissure with a wide opening below into the yolk—sac cavity, and whose wall is composed of cells exhibiting an epithelial arrangement. Marginally, on each side, the wall passes into continuity on the one hand with the lateral (primitive streak) mesoderm, and on the other with the endoderm. We have here in this section the most cranial ventral opening of the chorda-canal, which we shall be able to follow in the succeeding sections.
In section 48 (fig. 51, Plate 33) the chorda-canal is closed; its ventral wall is intercalated in the endoderm, and so forms a part of the dorsal wall of the yolk—sac cavity.
In section 49 a second ventral opening is present in the form of a very narrow slit. The delimitation of the canal from the adjoining mesoderm is not distinct, owing perhaps to the state of. preservation, and this is also the case in the next section (No. 50). In the latter the canal possesses a minute lumen, and this is the case also in sections 51-54 (fig. 12, Plate 30, of section 52).
In sections 55-57 (fig. 13, Plate 30, of section 57) the chorda-canal again opens into the yolk-sac cavity, but in sections 58-59 it is closed, and in section 59 (fig. 14, Plate 30) the endoderm is present below its ventral wall. In section 60 there is a very minute ventral opening, and perhaps also in section 61. In section 62, (fig. 15, Plate 30) the lumen of the canal is distinct, but closed, so also in section 63.
In sections 64 (fig. 16, Plate 31) and 65 the lumen is filled with fine granules, and opens below by a very minute opening. In section 66 the lumen, largely occupied by a nucleus, is closed, and the ventral wall of the canal appears to be fused with the endoderm.
In section 67 the canal, again with a wellemarked lumen, is delimited both from the mesoderm and the underlying endoderm. Its dorsal wall is pointed and projects into the overlying ectoderm. In the following section (No. 68) this dorsal wall is fused with the ectoderm. Its wide lumen, filled with granules of ‘varying size, opens below by a very narrow opening. In section 69 (fig. 17, Plate 31) the conditions are similar, but the lumen is closed.
In section 70 the conditions are again similar, but the lumen of the canal communicates with the yolk-sac cavity by a definite but very narrow opening, which also is indicated in section 71. We have here in these two sections the ventral opening of a very short neurenteric canal, which runs obliquely upwards and backwards to open into the amniotic cavity by a distinct aperture (blastopore or notopore) in the next section 72 (fig. 18, Plate 31).
Sections 68-7 3 include the region corresponding to HENSEN’s knot, but in this embryo there is no conspicuous thickening marking its position, and only in section 72 is the endoderm closely attached to the ventro-caudal wall of the neurenteric canal. In the other sections in this region the endoderm is separate and clearly distinguishable as an independent layer.
In section 73 (fig. 19, Plate 31) the axial ectoderm exhibits a slight groove prolonged back from the opening of the neurenteric canal, and is continuous below with a compact mass of cells, which represents the caudal wall of that canal. Laterally this mass is continuous with the lateral sheets of primitive streak mesoderm.
That completes the description of the sections through the preblastoporic axial structures in our specimen, and we may now summarise our findings.
The prochordal plate - an area of thickened endoderm - begins close (10 micron.) behind nd behind section 46 (fig. 49, Plate 33), which represents the caudal limit of our intermediate region, are no longer present.
The more caudal msegment of the chorda-canal extends from section 47 back to section 72, and thus has a length of about 208 pi. In this caudal region the chorda- canal is for the most part intercalated in the dorsal wall of the yolk-sac, endoderm being recognisable below it only in a few sections (section 59 and caudally to section 66). The lumen of the canal communicates with the yolk-sac cavity by seven openings, the most cranial of which is situated close behind the caudal limit of the intermediate region, whilst the most caudal forms the ventral opening of a very short neurenteric canal, which passes obliquely upwards and backwards through HENsEN’s knot to open dorsally on section 72, and so connects the yolk-sac cavity with the amniotic cavity.
HENSEN’s knot may be regarded as extending from section 68 - in which the chorda-canal first fuses with the ectoderm - back to section 73. In our embryo it does not form a conspicuous thickening.
The relations of the mesoderm to the chorda-canal may now be reviewed. With the disappearance of the lateral mesodermal bands at the hinder end of the intermediate region, the medial margins of the lateral sheets of primitive streak mesoderm, which are not specially thickened, come into direct relationship with the lateral margins of the chorda-canal, and in many of the sections that relationship is so intimate as to suggest actual continuity between the two. Indeed in some sections (fig. 15, Plate 30, and fig. 51, Plate 33) continuity appears to be mediated by actual extensions of the wall of the chorda-canal, so that a direct (if small) contribution from the head-process of this region to the lateral mesoderm cannot be entirely excluded. That possibility raises the question whether the “ lateral mesodermal bands ” of the intermediate region may not also be derivatives of the head-process of that region. Apart from the obvious close topographical relationship between these bands and the median chorda-process, intensive study of the sections failed to yield any positive evidence of actual genetic relationship between them, and we were consequently much exercised as to the inter- pretation we should put upon these bands.
We had examined sections of the corresponding region in other mammalian embryos without gaining much enlightenment, when, fortunately, the sections of a very well- preserved presomite embryo of Loris (Loris 49 of the collection made by Professor A. SUBBA RAU, the embryonal area of which measures 1-92 >< 1-25 mm.) became available for study, and proved invaluable for comparison with those of the Dobbin embryo. Loris 49 is in much the same developmental stage as the latter, so far as concerns the head~process, but is a little more advanced, inasmuch as the caudal portion of the chorda-canal has already become transformed into the definitive chorda-plate. Cranially, however, the chorda-canal is still intact, and a region corresponding to the intermediate region of the Dobbin embryo is clearly recognisable, whilst a well-marked prochordal plate region is also present. A detailed description of this very interesting embryo will begiven elsewhere. Here we reproduce, as figs. 55-59, Plate 34, five photomicrographs through the most important regions of the preblastoporic axial formation, for comparison with our figures of the Dobbin embryo. fig. 59, Plate 34;, shows the chorda—canal (about 0 - 19 mm.) in front of the cranial extremity of the chorda-plate It shows the typical characters. The lumen is transversely wide, and the dorsal wall is distinctly thicker and more definitely epithelial in nature than the ventral, which is closely connected with the underlying endoderm. On either side of the chorda—canal and fairly definitively delimited from it, is a thick sheet of primitive streak mesoderm.
The section here figured may be compared with the sections through the caudal region. of the chorda—canal in the Dobbin embryo, in which no ventral openings are present (figs. 12, 15, Plate 30, and fig. 16, Plate 31).
fig. 58, Plate 34, is 0-176 mm. (22 sections) in front of fig. 59, and represents a section through the caudal portion of the intermediate region. Here the axial formation is clearly distinguishable into three parts - a median chorda—canal, and two smaller luminated masses situated symmetrically one on either side of the canal and in continuity with its lateral wall. These masses, as the serial sections show, are not isolated structures, but, as in the Dobbin embryo, are simply sections of two continuous cords or bands of mesoderm which accompany the chorda—canal throughout the extent of the inter- mediate region. We regard them as the homologues of the “lateral mesodermal bands" of that embryo.
The lateral limits of the chorda—canal are somewhat indefinite, owing to its continuity with the lateral masses, but it appears to be of much the same width as in fig. 59, though its lumen is smaller. Its ventral wall also lacks a definite ventral boundary, since it is connected with the underlying endoderm 3 by somewhat loosely arranged mesenchymatous cells. The lateral masses in this section are very striking structures. Each possesses a very small central lumen, round which the enclosing cells are arranged. in a radiating fashion, after the manner of an epithelium, thus recalling the mesodermal somites of an early embryo. It is deserving of emphasis that they are in direct continuity with the chorda—canal, whilst they are very definitely delimited from the lateral sheets of primitive streak mesoderm, which are here thinner than in fig. 59. Comparison of fig. 58 with fig. 46, Plate. 33, through precisely the same region -of the Dobbin embryo, reveals at once the striking agreement between the two, the only difference being that, in the Dobbin embryo, the lateral bands are solid and less highly differentiated than in Loris 49, and are in apparent continuity with the lateral sheets of primitive streak mesoderm.
fig. 57, Plate 34, is thirteen sections in front of fig. .58. We can still recognise the median chorda—canal with a relatively small lumen and the two lateral bands, also, with slit-like lumina. Although in this section the lumen of the chorda—canal is small, in other sections it may be duplicated and may even be prolonged towards the lumina in the lateral bands. In this region the axial structures (chorda—canal and lateral mesodermal bands) are underlain by a thick zone of ‘cells, loosely arranged immediately underneath the chorda—canal, but becoming more compact below. The lowermost stratum of cells of this more compact zone directly bounds the yolk-sac cavity, so that the endoderm is not distinguishable as a separate and independent layer. Essentially similar conditions are found also in the Dobbin embryo (cf. p. 453), but in the latter the lowermost stratum of cells is not so distinctly thickened. The appearances suggest that the axial endoderm has here undergone active proliferation, and accordingly it might be regarded as a part of the prochordal plate here overlapped by the head- process. On the other hand, it may be that the cellular zone in question is to be regarded simply as the ventral part of the original head-process here indistinguishably fused with the endoderm. This latter view seems to us the more probable (of. p. 482).
Our next figure through this Loris embryo (fig. 56, Plate 34) shows a section through the cranial extremity of the head-process, where it is quite undifferentiated and much narrower than in fig. 57. The cell-layer underlying the head-process is not so thick as in the latter figure, and shows a greater tendency to an epithelial arrangement, though it still presents the appearance of proliferation. In this figure it can be seen that the marginal portion of the embryonal endoderm situated on either side of the axial region has the form of a well-defined thick layer composed of columnar cells. Traced medially this thick endoderm passes into a more or less definitely localised cell—mass, which projects upwards from the endoderm, and on the left side appears like a fold of the latter, since there is present in it an indistinct cleft—like lumen. We have to do here with two paraxial thickenings in which mesoderm is being proliferated from endoderm. These thickenings are also visible in fig. 57 and on the left side in fig. 58, and they can be traced forwards from fig. 56 into continuity with the prochordal ‘plate, so that in reality they have the form of paraxial bands, connected cranially by the pro- chordal plate.
Our last figure through this embryo (fig. 55, Plate 34) passes through the middle of the prochordal plate, which is readily distinguishable from the columnar embryonal endoderm adjoining its lateral margins by its more irregular character, and by the fact that it consists of a thinner axial portion uniting two distinct lateral thickenings. These latter are similar in character to the paraxial bands noted in fig. 56. They can be traced caudally in the series into direct continuity with them, whilst cranially the prochordal plate loses its bilateral character and appears as a single thickening.
The relations of the lateral mesodermal bands to the chorda—canal in this Loris embryo, as briefly set forth above, lead us to the conclusion that the cranial segment of the head-process in the Loris embryo, as in the human, undergoes differentiation in the caudo-cranial direction into a median chorda—canal and two lateral mesodermal bands. On the assumption that the head-process is archenteric (metenteric) in significance, these bands may be regarded as representing cephalic mesoderm of direct archenteric (metenteric) origin (so-called “ gastra ” mesoderm). It is perhaps not without significance that the Lemur embryo in this matter of the lateral mesodermal bands should provide such an exact parallel to the condition prevailing in the early human embryo.
Whilst in the Loris and Dobbin embryos, the lateral bands of “ gastral ” mesoderm are confined to the cranial region of the head-process, it is of considerable interest to recall that WILSON and HILL (1907) have shown that in early embryos of Ornitho- rhynchus, the caudal portion of the archenteric plate (head-process) extending forwards from the primitive (HENsEN’s) knot, is distinguishable into a median portion destined to form the chorda—plate and thickened lateral portions which lie in series with the lateral masses (“ protosomites ”) of the primitive knot and which are destined to become converted into “ gastral ” mesoderm. As may be seen from WILSON and HILL’s figures (of. especially figs. 63-66, Plate 12), these lateral portions of the Ornithorhynchus plate are strikingly similar to our lateral mesodermal bands in their relations. The facts suggest that the head-process was originally capable of producing “ gastral ” mesoderm along its entire length.
(ii) Mesoderm, Primitive Streak and Cloacasl M embmiie.-Laterally the most cranial part of the head-process passes on each side into continuity with a mesodermal sheet situated between the shield-ectoderm and the yolk-sac endoderm and attached marginally to the mesothelial investment of the amnio-embryonal and yolk-sac vesicles. The limits between the head-process and the said mesodermal sheets are not sharp enough to permit of the precise delimitation of the head-process, but the latter probably does not extend laterally beyond the limiting lines indicated in text-figs. 1 and 8.
The existence of the “ primary ” or extra—embryonal mesoderm in the human embryo makes the analysis of the mesoderm situated laterally to the axial structures some- what difiicult. As the cells of the “ primary ” mesoderm differ in no important respect from those of the later formed primitive streak mesoderm, the main criterion we can use as a guide in distinguishing between the two is the relative density of the layers. T We have mentioned already (p. 449) that underneath the cranial margin of the shield- ectoderm there is a mesoderm plate of such density that it can hardly be regarded as composed only of “primary ” mesoderm ; it is more probable that the primitive streak mesoderm has already reached the cranial border of the embryonal shield, and par- ticipates in the formation of the plate in question. If we follow this plate caudally we notice a curious asymmetry in the density of the plate on the two sides. On the left side (right in the embryo) the mesoderm plate is much less compact than on the right. This difference in thickness reaches approximately to section 50 in the caudal direction, i.c., a little more caudally than the caudal limit of the intermediate region (section 46). We have no other explanation of this asymmetry to offer than to suggest that it is simply an individual variation. We tried at first to use some of the breaks in the mesodermal sheets for the determination of the probable limit reached by the primitive streak mesoderm, but finally came to the conclusion that the primitive streak mesoderm on both sides extends forwards in front of the prochordal plate.
Caudally to the region of HENsEN’s knot the artificial dorsal concavity of the embryonal shield gradually diminishes, and behind section 111 (fig. 23, Plate 31) the embryonal shield assumes its normal dorsally convex form. The primitive streak is long (420 p. inlength, measured in theimedian section along the curvature) and is well developed. It extends from section 741 back‘ to the cloacal membrane, and from section 90 (over its posterior «two-thirds) a distinct primitive groove is distinguishable along it (figs. 20——23, "Plate 31). The lateral sheets of mesoderm. arising from it are thick and compact, though in some sections the cells tend to be arranged in two layers. The sheets extend beyond the margins of the embryonal shield on both sides to become continuous with the mesothelial covering of theramnio-—em.bryonal and yolk-sac. vesicles. In certain sections (figs. 20 and 21, Plate 31) a small cavity is present in the sheet at its point of attachment to the mesothelium, the significance of which is doubtful (perhaps ccelomic or vascular). The endoderm is clearly distinguishable below the whole extent of the primitive streak.
At the caudal end of the primitive streak its ectoderm passes over into that of the cloacal membrane. The transition is very gradual, so that the determination of the cranial limit of the cloacal membrane is somewhat diflicult. We may observe that the cell-proliferation from the bottom * of the primitive groove - which is very clearly marked (figs. 22 and 23, Plate 31) - gradually disappears, whilst the ectoderm thickens and grows down towards the endoderm, and finally comes into contact with the latter (section 117, fig. 52, Plate 34). It is difficult to determine whether in section 117 we have an actual cloacal membrane or not. The ectoderm is certainly in connection with the endoderm, whilst no connection with the mesoderm seems to exist, except latero-dorsally on the apparent right side, but the fusion of the ectoderm and endoderm is not so complete as it ought to be in a fully developed cloacal membrane. The conditions seen here can be explained if we assume that the cloacal membrane extends in the cranial direction partly at the cost of the primitive streak. In the following sections (118--120) the connection between the two embryonal layers is less complete than in section 117, but there are present in these sections chromatophilic granules, which BONNET (1901) first recorded as of common occurrence in this region as well as in the prochordal plate, and which have been found in human embryos of later stages by STERNBERG (1927) and FLORIAN (1930, cc). These two authors regarded these granules as products’ of cell~dissolution, but, as indicated on p. 453, they have possibly quite a different significance.
In section 121 (fig. 24, Plate 32) the mesoderm on both sides is clearly separated from the ectoderm in the median plane, and a typical cloacal membrane is certainly present ; but the fusion between the ectoderm and endoderm is not yet so complete as in section 122 (fig. 53, Plate 34). In both sections the above-mentioned granules are present in large numbers in the cloacal membrane and in the following sections (123—-125, fig. 54, Plate 34), also in the mesoderm quite close to. the cloacal membrane. In these latter sections the fusion of the ectoderm and the endoderm becomes less marked ; whether there are still traces of fusion in sections 126-127 (fig. 25, Plate 32) is difficult to determine. s In section 128 (fig. 26, Plate 32) ecto- and endoderm are widely separated.
It is worthy of note that a groove in continuity with the primitive groove is present along the dorsal surface of the cloacal membrane in our specimen. FLORIAN (1930, a) has also observed a corresponding groove in embryo Bi II.
- ↑ We employ the old term “ head—process ” for the axial formation which extends forwards from I-IENsEN’s knot, the homologue of the dorsal lip of the blastopore (notopore). This formation comprises not only the primordium of a part of the mesoderm (which does not seem to be very extensive), but also that of the chorda, and therefore such terms as “ Mesodermsackchen ” (HERTWIG, 0., 1906) or “ kranialer Mesoblastfortsatz ” (WALDEYER, 1929) are not suitable.
- ↑ Right and left in the description of the figures correspond to the reader’s right and left, but‘ actually to the left and right sides of the embryo respectively, 6.6., the apparent right side of the figure is the actual left in the embryo and vice versa. 3
Yolk-Sac
We have already described (p. 448) the form of the yolk-sac, and here we need only give a very brief account of the structure of the yolk-sac wall. We have dealt with the endoderm. in the median part of the dorsal wall in our description of the axial structures of the embryonal area. In the lateral parts of the dorsal wall the endoderm is composed of a single layer of flattened cells, which in this region is for the most part artificially detached from the mesoderm. In places where this detachment has not taken place, it is connected with the mesoderm by means of cytodesmata. In the dorsal part of the lateral wall the endoderm is also thin, but in the more ventral region it is composed of a well-marked layer of cubical or low columnar cells, the limits of which are distinct (figs. 5, 7 and 8, Plate. 29).
In the lateral and ventral portions of the wall the formation of blood-vessels is in active progress. The vessel-primordia range from solid blood-islands to vessels, each with an endothelial wall enclosing a lumen more or less completely filled by primitive blood-cells. Vessel development is more advanced in the ventral than in the more dorsal I parts of the wall, the endothelial vessels in that region often possessing large lumina, only partly filled by blood-cells. p The vessel-lumina do not as yet form a continuous network.
The preservation of our specimen is not sufficiently perfect to permit us to discuss the origin of the vascular elements.
Allantoic Canal
As is shown in our reconstruction (text-figs. 1 and 2) the yolk-sac at its caudo-dorsal extremity gives origin to a long tubular diverticulum which extends into the mesenchyme of the connecting stalk. This is the primordium of the allantoic canal. It is situated caudally to the cloacal membrane, but, as in other described embryos of about this stage, it arises not from the hind-gut as in lower Amniotes, but from that part of the yolk-sac which is destined in later stages to form the hind-gut. FLORIAN (1930, ct) has proposed to term the diverticulum of the yolk—sac - which is destined to furnish not only the allantoic canal, but also part of the hind-gut - the “ allanto—enteric diverticulum ” (cf. FLORIAN’s text—figs. 6 and 7). In our specimen the di verticulum referred to above represents the primordium of the allantoic canal alone ; it is situated behind the cloacal membrane, which is not included in it. It is distinguishable into two parts, viz., an elongated tubular cranial part and a swollen vesicular terminal part, already noted in our description of the entire embryo (p. 448), which projects on the surface of the connecting stalk (as the result probably of the artificial deformation of that structure) (figs. 28 and 29, Plate 32).
FLORIAN has called attention to the fact that, in the cranial region of the allanto- enteric diverticulum in an embryo with four paired somites (embryo Bi II), vacuoles appear in the basal parts of the endoderm-cells of the ventral wall (FLORIAN, 1930, ct, Plate I, fig. 7). In the caudal part of the diverticulum the vacuoles are present also in the cells of the dorsal wall. STERNBERG (1927), in his description of the allantoic endoderm also in an embryo with four paired somites, did not mention vacuoles, but spoke of faintly stained cytoplasm. In a later stage (embryo XI with ten paired somites) FLORIAN (1930, ct) has shown that the cells over the whole extent of the allantoic canal contain vacuoles, and in the caudal part of the canal the cells are so vacuolated that only a very thin layer of cytoplasm is left at the free surfaces of the cells. If we compare FLonIAN’s fig. 10 (Plate II) with S'rER.NBERc’s fig. 33, p. 180, it is apparent that We are dealing with a quite similar condition of the allantoic endoderm in the two embryos.
In our specimen there are vacuoles in the cells of the cranial part of the allantoic canal, but they are not so numerous and not so regularly situated as in embryo Bi II.
The tubular part of the allantoic canal passes over gradually into the terminal vesicle. In the transitional region the wall of the canal is formed by two layers of flattened cells (fig. 29, Plate 32), and the lumen can be traced continuously into the cavity of the vesicle. The wall of the latter consists in places of a single layer of cubical cells with ill-defined cell-limits, but in other places of two or even more cell layers, the inner layer being formed by flattened cells, theouter by cubical cells resembling those of the regions where the wall is single layered. It is possible, however, that the outer layer is of mesodermal origin.
Here we may call attention to certain interesting observations of STERNBERG (1927). He found in the tissue of the connecting stalk of the four-somite embryo already referred to, an epithelial vesicle, quite separated from the allantoic canal ('0. his Taf. I, Abb. 1, and Taf. II, Abb. 2) and possessing a canal—like prolongation directed towards the free end of the latter. He regarded this structure as an “ Amniongang ” (pp. 181-182). Now if we compare the terminal vesicle (fig. 28, Plate 32) of our specimen with the structure in question in STERNBERG’s embryo (STERNBERG, 1927, fig. 35, p. 181), there seems to be no essential difference between them, and if we compare figs. 34 and 33 in STERN- BERG’s paper with fig. 10, Plate II, in FLoRIAN’s paper (1930, a), the only obvious difference is in the size of the lumen. We are, therefore, convinced that the “ Amnion- gang ” in S.TERNBERG’s specimen represents the detached vesicular end of the allantoic canal. STERNBERG, it is of interest to note, describes the tubular part of the supposed “ Amniongang ” as showing signs of degeneration. Our suggestion is supported not only by the fact that STERNBERG{did not find an “ Amnionnabel ” (p. 182), but also by STREETER’s (1919) observations on the Mateer—embryo, where he describes the “ allantois ” as separated into two parts. They are so close to each other that there can be no doubt that they are parts of one and the same formation (STREETER, 1919, Plate I, fig. 6 ; text-figs. 1-2 (sections 58-7 9) and text—fig. 3), but in the Mateer-embryo the separated distal part of the allantoic canal is not enlarged. We accordingly regard the conditions in the Mateer—embryo as transitional between those seen in our embryo and in STERNBEB.G’S specimen. p These observations seem to indicate that the allantoic canal in Man, which is recognised to be a vestigial structure, may undergo separation into two parts (or perhaps more) during development, the distal part being subject to early degeneration, whilst the proximal part persists for a much longer period. In an undescribed embryo (No. 1285 of the Manchester collection) which we have studied (text-fig. 13), the allantoic canal is separated into two parts, but here the condition is certainly an artifact.
Connecting Stalk
As we have mentioned above (p. 445) the connecting stalk has been bent ventrally, and has also been slightly twisted from right to left. It is accordingly necessary to bear this deformation in mind when comparing the stalk of our embryo with that of other specimens.
As in other early embryos, two parts may be distinguished in the connecting stalk (FLORIAN-, 1930, Lt), viz., a distal part, the amnio-embryonal stalk, and a proximal, the umbilical stalk. The amnio-embryonal stalk attaches the embryonal formation as a whole to the chorion, and is continuous on the one hand with the mesoderm of the amnion, and on the other with the umbilical stalk. The umbilical stalk is the proximal part of the connecting stalk, which lies in early stages directly behind the amniotic cavity, and whose anterior or cranial surface is clothed by the continuation of the amniotic ectoderm which FLORIAN has distinguished as stalk-ectoderm. The tip of the amniotic cavity which is clearly seen in fig. 3, Plate 29, marks approximately the extent of the umbilical stalk.
In the mesenchyme of the connecting stalk numerous vascular primordia are present, but it is worthy of note that there is no direct connection between them and those of the yolk-sac. We are thus able to confirm the observations of other authors that vascular development proceeds at first quite independently in these two regions. In our specimen the primordia in the ventral wall of the yolk-sac are, if anything, slightly more advanced than those of the stalk.
The primordia take the form of solid blood—islands and irregular cell—strands either solid or possessing lumina of varying size. The larger lumina are enclosed by a layer of flattened endothelial cells, and as in some cases they can be traced through a considerable number of sections, we are justified in speaking of them as endothelial tubes. Comparison with later stages enables us to identify two of these tubes as the primordia of the umbilical arteries.
In the proximal part of the stalk, a short distance behind the caudal extremity of the yolk-sac, two fair~sized vessel-primordia make their appearance, one on either side of the allantoic canal. These we regard as the primordia of the umbilical arteries. They can be traced from section 132 caudally to section 136 (fig. 27, Plate 32, of section 135). Behind the latter, they gradually become connected by intervening cell-strands more or less luminated, and so there -is formed a plexiform arrangement, semicircular in section, between which and the amniotic cavity the allantoic canal is situated. This is the condition met with in sections 142-144. The two main vessel-primordia again separate in sections 145——15O and again become connected in sections 151-152 (fig. 28, Plate 32). In section 153 they separate once more, and from this section they can be followed, behind the caudal extremity of the allantoic canal, up to the junction of the stalk-mesenchyme with that of the chorion (fig. 29, Plate 32, of section 157).
The anastomosis between the two main vessel-primordia probably represents the future single umbilical artery (the artewia unzbildcalts commuwis) of later stages as described by POLITZER (1928, Plate XIX, figs. O, P, Q, 8).
Besides the primordia of the umbilical arteries, there is present in sections 137—142 a well-marked, compact blood-island, formed of closely aggregated spherical cells. It lies close under the stalk~ectoderm between that and the allantoic canal, and produces a slight bulging into the amniotic cavity. It occupies the position of the later primordium of the vena umbilvlcalvfis, and possibly represents it. The stalk-ectoderm above the island is distinctly flattened and, in some places, seems to lie in direct contact with it.
A similar blood-island, but of smaller size, is found close to the allantoic canal between the two umbilical arteries in sections 133-135 (fig. 27, Plate 32) and 142-144.
In the most distal part of the connecting-stalk there are present, besides the two main primordia above mentioned, many irregular cell-strands and small, irregular vesicles, which resemble vessel~primordia, but are very irregular in form and distribution and may not be vessel—primordia at all. In this connection it may be mentioned that similar structures occur in the chorionic mesoderm and the caudal part of the connecting-stalk in much earlier stages (embryo “ T.F.”, FLORIAN, 1928, a; embryo “ Fetzer,” FETZER-FLORIAN, 1930) which are certainly not vessel-primordia (FLORIAN, 1928, a, pp.566—7) finally, the relationship of the vascular primordia to the mesothelium calls for brief mention. As in other embryos, the mesothelium is only present around the proximal portion of the stalk. We can confirm the observations of certain authors (M‘INTYRE, 1926 ; STERNBERG, 1927) that the mesothelium is closely connected.-with some of the vessel—primordia. There can be little "doubt that the mesothelium is connected with a cell—island in section 125. This island is present in sections 121-128, and can be traced continuously into connection with the primordium of the left umbilical artery. An undoubted connection of the mesothelium with an island could also be observed in sections 150-151. This island extends through sections 145-158 and lies near the amniotic cavity (see figs. 28 and 29, Plate 32, on the right) ; it is solid over most of its extent, but in sections 155-156 a cavity seems to be formed in it. It becomes connected by a thin cell—strand (section 156) also with the primordium of the left umbilical artery.
Chorion
Apart from the above~mentioned primordia of the umbilical arteries which can be traced into the junctional region between the chorionic mesenchyme and that of the stalk, undoubted vessel-primordia in the mesenchyme of the chorion can only be observed in the immediate neighbourhood of the insertion of the connecting-stalk. They are situated at the inner or deep surface of the mesenchyme and take the form of cell- strands, in which cell—outlines are not distinguishable and in which the central part stains less deeply than the periphery and contains nuclei apparently in degeneration. Here and there the strands become transformed into capillaries, the endothelial wall enclosing a narrow lumen, partly occupied by more or less spherical cells, some of which are in degeneration.
In the rest of the chorionic mesenchyme, as well as in that of the villi, no structures are present which could be regarded as of the nature of vessel-primordia. The trophoblastic ectoderm covering the villi consists of the usual two layers, but there are already many small areas where LANeHAN’s layer has disappeared and the villi are covered only by syncytio-trophoblast.
Technique of the Reconstructions
(text-figs. 1-17)
Before we compare the reconstructions of our specimen with those of other early human embryos, it is perhaps advisable to explain briefly how the reconstructions of embryos not directly available to us have been made.
The reconstructions of the embryo “ Hugo ” (STIEVE, 1926) (text-figs. 3 and 11) correspond with those published by FLORIAN (1930, b), and were constructed on the basis of the figures of sections published in STIEVE’s paper. As we (FLORIAN, 1930, b) have already shown, the embryo is not cut transversely, but obliquely ; the recognition of this fact explains the asymmetry of the specimen (especially that of the primitive streak) mentioned by STIEVE. The cloacal membrane, according to FLORIAN (1928, a, p. 545; 1930, b), is to be found in sections 60-62 (figs. 31——32 in STIEVEs paper), its connection with the endoderm being close to the point designated in STIEvE’s fig. 31 as “ Sichelknoten,” the connection with the ectoderm in fig. 32 being designated as “ Blutinse1” ; the connection between these two is present in section 61, the figure of which was not published. If we observe the position of the primitive streak in STIEvE’s figures, we notice that as we pass back in the series the streak gradually approaches nearer to the right margin of the embryonal shield ; the cloacal membrane (in STIEVE’s fig. 31) being situated actually at that margin. This fact clearly indicates that the specimen is not cut transversely. We have estimated that the sectional plane forms an angle of about 64° with the median plane, and an angle of about 26° with the transverse plane, and is perpendicular to the embryonal shield. We have measured this specimen in the reconstructions made in accordance with these estimates, and naturally there are some small differences between our measurements and those of STIEVE.
The embryo Bi24 (text-figs. 4 and 12) represents a new, as yet undescribed, human embryo, handed over to FLORIAN by Dr. 0. BITTMANN, of Brno. It was cut (by H. BARKER in this Department) precisely perpendicularly to the median plane, but obliquely to the plane of the embryonal shield, the sectional plane forming an angle of 71° 5' with the latter.
The comparison of WALDEYER’s (1929) reconstructions of the Schonholz embryo with the other reconstructions was by no means easy, According to WALDEYER’s paper (p. 413), the thickness of the sections of the Schonholz embryo is 10 (L. The magnification of his text-fig. 6, p. 428, ought to be 150 x , since the reconstruction was made at magnification 200 X, and, according to the author, was reduced for repro- duction to 7‘:-. If we measure the thickness of 10 sections (including sections 25aI2 to 25aI5) on the scale on the left of the reconstruction, we do not obtain 15 mm. in correspondence with a magnification of 150 (0 -010 mm.x 10 x 150 = 15 mm.), but only 12 mm., which corresponds with a magnification of 120. According to WALDEYER’s note (p. 427 ), the length of the primitive streak in the projection (presumably in the plane of the reconstruction) is 0.51 mm, and the width 0.12 mm. If we assume that the magnification of fig. 6 is 120, the length ought to be 61 -2 mm (0 .51 x 120) and the width 14.4 mm (0.12 x 120), Which is exactly what we measure in WALDEYER’S figure. Under the same circumstances the distance between the cranial end of the embryonal shield and the cranial end of the primitive streak ought to be, according to WALDEYER (p. 427), 0.37 mm. ; in the reconstruction. this distance measures 44-4 mm., which corresponds with a magnification of 120 (0.37 x 120 = 44-4).
As WALDEYER did not mark the cloacal membrane in his reconstruction of the dorsal view of the Scho-embryo, we have indicated it only approximately ; the length of the cloacal membrane was obtained from WALDEYER’s fig. 5. The author does not indicate the prochordal plate in his reconstruction.
Text-fig. 7 (after GROSSER) shows the dorsal view of the embryonal shield of the embryo Wa17, described by him (1931). GROSSER has not marked in his figure the limit between what we call the head-process and the primitive streak mesoderm which accompanies it on either side. We cannot, accordingly, determine the limits of the cranial undifferentiated part of the head-process as defined by us and indicated in our reconstructions by dots and horizontal lines. The dotted line in text—-fig. 7 on either side laterally and caudally to the prochordal plate represents, according to GROSSER, the outline of the head-process; but see later, pp. 468-9.
As stated above (p. 445), the Dobbin embryo has suffered dislocation in the ventral direction. As the result, its caudal end is slightly distorted, so that the actual median plane in the region of the caudal end is curved to the left (text-fig. 1). In making the reconstruction of the median section (text—figs. 2 and 15) we have projected the structures which normally lie in the median plane into the plane marked by an arrow in text- fig. 1. These structures comprise the cloacal membrane, the allantoic canal and the caudal end of the amniotic cavity. Their outlines are indicated by an interrupted line in the text-figs. 2 and 15.
The reconstruction of the dorsal view of the embryonal shield of the embryo Peh.1-Hochstetter (ROSSENBECK, 1923) shown in text—fig. 9, is based partly on figs. 1-39, Plates XXXI—-XL of R0SSENBECK’s paper, taking account of the description in the text, partly on our own study of the prochordal plate head-process region in the sections. This we were permitted to do through the kind permission of Professor HOCHSTETTER. We have made a reconstruction of this region and have provided a series of photo- micrographs of the sections (figs. 63—80, Plate 35), the levels of which are indicated by horizontal lines on the right side of text-fig. 9 ; their numbers correspond with the figs. 63-80. The two indistinct ventral openings described by ROSSENBECK, but not shown in his reconstruction, are indicated ('0. 0. ?). The limit between the very high ectoderm of the embryonal shield and the flattened ectoderm of the most cranial part of the latter is indicated by a dot—and—dash line. This line probably marks the cranial limit of the medullary plate ectoderm. The thin part of the ectoderm cranially to that limit is destined to form part of the ectoderm of the head-end of the embryo.
The reconstruction of the median section of the same embryo (text-fig. 16) is also based partly on Plate XXXI, fig. I, of R0SSENBECK’S paper, partly on our own observations. According to R0SSENBECK’S statement that the “ Anlage der Kloakenmembran ’’ (p. 385) first appears in fig. 38, we have marked its cranial limit at the level of line 38 in Plate XXXI, fig. I, of R0SSENBECK’S_ paper. The reconstruction of the region of the prochordal plate and the cranial part of the head-process (between lines 5 and 16 on the scale) is based on our own studies and differs somewhat from the original recon- struction.
The reconstruction of the median section through the Ingalls embryo (text-fig. 17) is combined from text—figs. 3 and 5 of INGALLS’ paper, on the supposition that their magnification is 40 and 198 - 8 respectively. The dorsal view of the embryonal shield of the same embryo is combined from his text—figs. 2 and 4, on the assumption that their magnification is 40 and 187 respectively.
Development of the Head-Process and Prochordal Plate in Man
(A) Discussion of Specimens thus far known
The Beneke embryo is the earliest embryo in which originally the existence of a head-process was suggested (STRAHL—BENEKE, 1910), but it is now generally agreed (FLORIAN-BENEKE, 1930) that no head~process is present in this specimen.
The embryo T.F. (FLORIAN, 1928, a), the next older human embryo described,unfortunately is not well enough preserved to be utilised for the study of head-process development.
The embryo P. MEYER (1924) is the earliest human embryo in which the head-process seems to be definitely present (FLonIAN—-VoLKER, 1929 ; WALDEYER, 1929), but, unfortunately, the author provides only one figure through the head-process, and his description is quite brief.
The embryo “ Hugo ” (STIEVE, 1926) (635 (1. in length according to our measurements, which are based on a new reconstruction, text-fig. 11) certainly possesses a head-process. It is shown in our reconstruction and in section in the figures of STIEVE as a broad compact plate of cells extending forwards in front of HENsEN’s knot and apparently intercalated throughout its extent in the endoderm, 'i.e., no endodermal layer is distinguishable below it. This plate undoubtedly comprises in its median part the head—process, but whether and to what extent its lateral portions are formed by forward extensions of the primitive streak mesoderm cannot be determined from the examination of the sections figured. The head~process forms a comparatively compact mass caudally to section 28 (fig. 15 in S'rIE.VE’s paper). Already in this section its apparent right half is composed of loosened mesoderm-like cells. This character is still more distinct in section 26 (fig. 14 in STIEvE’s paper). The endoderm is obviously missing underneath this part of the head-process. In section 24 (fig. 13 in S'.1‘IEvE’s paper) the dorso—median wall of the yolk-sac is formed by an irregularly thickened epithelium, which perhaps represents the most cranial part of the head-process. Whilst the head-process in sections 32, 30 and in section 28 (on the left) is quite obvious, the structural appearances in sections 24, 26 and in section 28 (on the right) are more difficult to interpret. We were inclined at first to interpret them as indicating a prochordal plate, but the comparison of this region with that in other embryos renders this explanation improbable. We are, therefore, inclined to regard this region as a part of the head-process in accordance with STIEvE’s description. There is still another possibility. FLORIAN and BENEKE (1930) described a horse-shoe shaped area of thickened endoderm in front of the primitive streak in the Beneke embryo. The thickened endoderm) in section 24 of STIEvE’S specimen closely resembles that in the Beneke embryo, but at the moment it is impossible to solve this problem. In the recon- struction we have attempted to indicate the limits between the primitive streak mesoderm and that part of the mesoderm which seems to arise from the yolk—sac endoderm, and which STIEVE calls “ Dottersackmesoderm,” but it was impossible to determine the limits of the two with any degree of exactness.
In the embryo Bi (ttmann) 24 (625 pr. in length), which is only a little more differentiated than the “ Hugo ” embryo, HENsEN’s knot is much better marked than in the latter. In front of HENsEN’s knot, below the median region of the shield-ectoderm, there is present a plate distinguishable into a thickened median cord of cells and two thinner lateral wings of mesoderm (fig. 62, Plate 34). The underlying endoderm is rather indistinct, and in some places is represented by scattered. and mostly flattened cells with frequently pycnotic nuclei. As the plate is followed cranially, the mesodermal wings become narrow and finally disappear (fig. 61, Plate 34). In front of this level the median cell-cord can be traced into continuity with an area of thickened endoderm in which the nuclei lie close together, several deep (fig. 60, Plate 34). This area, we think, can only be interpreted as a prochordal plate in which mesoderm formation is not yet very active. (If the horse-shoe shaped area of thickened endoderm in the Beneke embryo mentioned above has nothing to do with the prochordal plate and if the axial structures in sections 24, 26 and 28 of the “ Hugo ” embryo represent parts of the head-process, then the embryo Bi24 would be the youngest human embryo in which an undoubted prochordal plate has been observed.) The median cell-cord certainly represents a head--process, the primordium of the later chorda~process. The lateral sheets of mesoderm accompanying the more caudal portion of the head-process we regard as forward extensions of the primitive streak mesoderm. The cranial part of the head-process which is not accompanied by lateral mesoderm is broader and somewhat thicker than the more caudal part. It probably represents the primordium of the intermediate region we have described in the Dobbin embryo.
The next specimen we have studied, embryo No. 1285 of the Department of Anatomy of Manchester University, was most generously placed at our disposal by Professor J. B. S. STOPFORD (text-figs. 5 and 13). It is much longer than embryo Bi24 (870 p. as compared with 625 y.), the difference in length being due mainly to the considerable increase in the length of the primitive streak, which is almost twice as long as that of the latter embryo. The cranial part of the embryonal shield, (cranially to HENSEN’S knot) measures 360 y., and is only 80 p. longer than in the embryo Bi24. Unfortunately, the fixation of the specimen is not good, so that We have had great difficulty in inter- preting the sectional appearances. Our conclusions accordingly must be accepted with all reserve and as provisional only.
So far as we can determine, the head—process is only very little longer than in Bi24, but is Wider and thicker. It is accompanied, as in the latter, by lateral sheets of primitive streak mesoderm, which are much thicker than in Bi24. Owing to the state of preservation, the limits between the lateral mesoderm and the median head-process are somewhat indistinct in the caudal region. Cranially (text—fig. 5) the mesodermal sheets become thinner, and the head—process which is still thick and compact is more distinctly delimited from them. Still more oranially, at the level of the interrupted line in text-fig. 5, the head—process broadens out and the lateral sheets of mesoderm practically disappear. We regard the just mentioned line as marking the cranial limit reached by the primitive streak mesoderm accompanying the head-process. Cranially to that line the head-process narrows forward and is surrounded anteriorly and laterally by thickened endoderm, which we interpret as prochordal plate. If this explanation is right, the prochordal plate appears to be much broader than that of Bi24, but is of about the same length in the median plane and is in a little more advanced condition.
The position in the series of the next specimen (embryo Scho, WALDEYER, 1929) is difficult to determine". According to WALDEYER’s statement, the length of the embryonal shield (without the cloacal membrane '4) is 0- 99 mm.
We tried to re-measure the length of the;embryonal shield on WALDEYER’s fig. 5 (compare our text—fig. 14). first we have controlled the magnification of this fig. 5 With WALDEYEn’s measurements of the primitive streak (0-51 mm. in the projection) and the distance between its cranial end and the cranial end of the embryonal shield (0-37 mm. in the projection) (see WALDEYER, 1929, p. 427). The total length between the cranial end of the cloacal membrane and the cranial end of the embryonal shield ought to be 88 mm. ((0-51 -1- 0-37) x 100 2: 88), but it is only 85-8 mm., which corresponds with a magnification of 97-5 ((0-51 —|~ 0-37) x 97-5 :2 85-8). Under the presumption that the magnification of WALDEYER’s fig. 5 is 97-5 and that the length of the embryonal shield without the cloacal membrane is 0-99 mm. (measured along the curvature), the cranial end of the embryonal.- shield would be as indicated by the arrow 3 in our text-fig. 14. This does not seem to be true. The median plane passes the cranial margin of the embryonal shield about the level of the section 25a11 ('0. the scale on WALDEYER’s fig. 6). The figure of this section was not published, but from the figures of the adjoining sections (section 24051114 and 25a12 ; figs. 11 and 12 in WALDEYER’s paper) we may assume that the basal outline of the ectoderm of the most cranial part of the embryonal shield corresponds with the white dotted line in our text-fig. 14, and that the cranial end of the embryonal shield is to be found in the point marked by arrow 2 in our text-figure. The total length between arrow 2 and the caudal end of the cloacal membrane (Which must be regarded as a part of the primitive streak, and therefore as a part of the embryonal shield) is 930 y. ; if measured along the curvature, 985 51.. If we now compare the length -of WALDEYER’s embryo with that of the Wa17 embryo (Gnossnn), we notice a difference of only 5 51.. The difference in the width of these two specimens, on the other hand, is very considerable, and may possibly be explained ‘as due to individual variation (see also p. 483).
According to WALDEYER, the head-process is not distinguishable into a median epithelial cord and lateral mesodermal sheets. If this is actually the case, then his embryo must be exceptional, since we have found a median epithelial cord, which we have interpreted as the chorda-process, in two smaller embryos (B124, and the Manchester embryo), and GROSSEB. found the same in embryo Wa17, which is nearly as long as embryo Scho.
The prochordal plate in the embryo Soho, according to WALDEYER, is represented by a single layer of cubical cells, and, accordingly, the production of mesoderm has not yet commenced. Unfortunately, WALDEYER did not mark the limits between the prochordal plate and the head-process in his reconstructions, which makes the comparison of this region with the corresponding region in other embryos impossible.
The embryo VVa17 (GROSSER, 1930 /1 931, 1931) (text-fig. 7) fits very well into our series of embryos, but GROSSER’s interpretation of the preblastoporic structures as set forth in his dorsal reconstructions differs somewhat from our own, inasmuch as he depicts the head-process as comprising what we regard as the lateral sheets of primitive streak mesoderm, accompanying the median head-process. In the text, however, he admits the possibility that the lateral mesodermal sheets are of primitive streak origin. He writes, p. 285 : “ Wir halten es fur gastrales Mesoderm im Sinne C. RABLS und miissten dementsprechend eigentlich annehmen, dass es aus dem Material des Zentralstranges hervorgeht. Dafiir gibt unser Objekt allerdings keine Anhaltspunkte; man konnte immerhin auch mit HILL und FLORIAN annehmen, dass diese seitlichen lV.[esoderm-massen, die ‘ Seitenfliigel des kranialen Mesoblastfortsatzes ’ im Sinne WALDEYERS, zugleich mit dem Zentralstrang aus dem vorderen Ende des Primitivstreifengebietes vorgeschoben werden.” In the figures in question (GRossER’s figs. 2 and 3) the head-process is depicted as being overlain by a layer of mesoderm. In the text, however, he suggests the possibility that this appearance may be due to the obliquity of the sectional plane through this region and with this suggestion we feel inclined to agree. The embryo I-Io (FAHRENHOLZ, 1927), which belongs somewhere among embryos with an early head-process, was so much deformed that it cannot be used for the study of the head-process. We quite agree with WALDEYER’s opinion (1929) that the formation interpreted by FAHRENHOLZ as “ Lieberkuhn’s canal ” is a mere artificial folding of the embryonal shield. On the other hand, a head-process is undoubtedly present in this specimen.
In the Mateer—embryo (STREETER, 1919) the sectional plane in the region of the head- process is so unfavourable, that the detailed study of the latter is not possible. There is no doubt that a head-process is already developed in this specimen.
Through the kindness of Professor J. C. BRASH, we have been able to examine the sections of the Thompson-Brash embryo. Although the preservation of this specimen is not satisfactory, the following facts could be recognised. The head-process is in a stage of development similar to that of the embryo Wa17 (GROSSEB). A median epithelial chorda—process can be observed in the caudal region of the head-process, accompanied by mesoderm on both sides. More cranially, the epithelial arrangement of the cells is distinguishable only in the dorsal part of the head-process. The cells of the most cranial part of the latter do not show any distinct epithelial arrangement. The prochordal plate is very distinct in this specimen as a localised area of thickened endoderm, being much more obvious than in the Dobbin embryo. It surrounds the cranial end of the head-process as in the Manchester and Dobbin embryos, and has not yet formed any mesoderm. In the original paper the authors express the opinion that there is no evidence of the formation of a “ completion plate ” in this specimen (THOMPSON-BRASH, 1923, pp. 10 and 12), but there is no doubt about the existence of a thickened endoderm area, 75.6., of a prochordal plate, 11 sections (110 p.) behind the section figured in fig. 4, p. 11, in the original paper. This area extends through four sections in front of the head-process, and the thickened endoderm is still present in about four following sections on both sides of the head-process. The section figured in fig. 7, p. 13, in THoMrsoN—-Bn;AsH’s paper is 13 sections (130 p.) caudally to the cranial end of the prochordal plate, and six sections (60 p.) in front of HENsEN’s knot.
The next stage in our series is represented by the Dobbin embryo. The main differences between this specimen and the Manchester embryo are seen in the considerable lengthening of the head-process (text-fig. 18), and in its much more advanced stage of difierentiation. The head-process is now distinguishable into three regions: (a) A caudal region (forming about a half of the whole head-process), in which it has become transformed into a chorda-canal provided with a dorsal opening into the amniotic cavity and seven ventral openings into the yolk-sac cavity; (3)) an intermediate region, in which it has undergone differentiation into an axial chorda-process and two laterally situated mesodermal bands. The chorda-process is undergoing transformation into a chorda-canal and possesses three isolated lumina. Cranially the chorda-process and the two mesodermal bands of the intermediate region pass into continuity with the most cranial region (0), where the head~process is composed of a thick mesoderm—like cell mass. No chorda-process is distinguishable in this region. The primitive streak is only very slightly longer than that of the Manchester embryo No. 1285.
The embryo Peh.1-Hochstetter (R0SSENBECK, 1923), although of greater length (1-4 mm. according to R0SSENBECK, 1 - 77 mm. according to our measurement, which includes the cloacal membrane), exhibits no great advance on the Dobbin embryo in the differentiation of the axial structures. Both the primitive streak and the head—process have increased in length almost in the same proportions. In our view, the same regions as in the Dobbin embryo are distinguishable in the axial structures situated cranially to the blastoporic opening, viz. :-
- A caudal chorda-canal region. in which only two indistinct ventral openings are described by ROSSENBECK. It is probable that the disappearance of the ventral Wall of the chorda-canal is subject to great individual gvariation; and cannot be used by itself as an infallible guide in estimating the stage of development.
- An intermediate region in which the lateral mesodermal bands are very distinct, its caudal limit lying about the level of section 76 in our text-fig. 9. Caudally to this section we find on each side an irregular, thin layer of mesoderm which seems to be fused with the endoderm, and to be distinct from the overlying sheet of primitive streak mesoderm (see the area marked by interrupted lines in our text-fig. 9 between sections 76 and 80). Whether these sheets (suggested by ROSSENBECK as possibly representing the primordia of the aortee, ROSSENBECK, 1923, p. 343) are identical in origin with the mesodermal bands of which they represent direct continuations, cannot be determined. All the sections in the region are artificially damaged, the endodermal and the mesodermal layers in question being broken off from the axial structures on either side. If we assume that the mesodermal bands are present only cranially to section 76 (indicated. by lines and dots), the proportions of the caudal and intermediate regions in both the Dobbin and Rossenbeck embryos would be practically the same. If, on the other hand, the region between sections 76 and 80 (marked by interrupted lines) belongs to the intermediate region, the latter would be proportionally twice as long in the Rossenbeck specimen as in the Dobbin embryo. The lateral mesodermal bands in the intermediate region in the Rossenbeck embryo appear to be closely attached to, if not fused, with the endoderm, whilst in the Dobbin embryo the fusion is much less distinct. The chorda-canal in RossENBEOK’s specimen is clearly distinguishable in the caudal part of the intermediate region. It possesses a continuous lumen as far forwards as section 70 (text-figs. 9, 16; figs. 70-——80, Plate 35). In some sections the lumen is indistinct (indicated by the dotted outlines of the chorda—canal in text-figs. 9 and 16), perhapsbecause of the state of preservation. In front of section 70 the median cell—cord (our chorda- process) becomes. indistinct. A lumen seems to be present only in sections 68 and 69.
- The cranial undifferentiated part of the head-process is very short in this specimen, and extends cranially only to section 65. The next sections in front (our figs. 64 and 63, Plate 35 ; and ROSSENBECK, 1923, figs. 5-7, Plate XXXI) must be regarded as possessing a prochordal plate. The latter is represented by a thickened area of endoderm in which the nuclei are arranged in two or three layers, but in its most cranial part the plate is formed by a single layer of cubical cells. The plate extends altogether through seven sections in front of what we regard as the anterior extremity of the head-process.
Before We pass to the next specimen, we may call attention to R0SSENBECK’s view that the aortae develop from the mesoderm situated immediately laterallyto the chorda~canal in sections 76 to 80 (our numbering). VVhether he had in mind also the thin mesodermal layers fused with endoderm, mentioned above, is not quite clear. In our opinion, these are probably identical with the lateral mesodermal bands with which they are in continuity.
Our lateral mesodermal bands, which we regard as a part of the head-process, ROSSENBECK interpreted as derivatives of the prochordal plate (completion plate).
The embryo Ingalls (1918) is the next specimen we need refer to. Its length, according to the author, is 1 - 8 mm., which compares with a length of 1 -4 mm. (according to R0ssENBEoK’S measurement), and 1 -77 mm. (our measurement) in R0SSENBECK'S embryo ; but if we measure the distance between the cranial limits of the cloacal membrane and the prochordal plate along the ventral outline in the median reconstructions (text-figs. 16 and 17), we find it is 1 -3 mm. in RossENBEoK’s specimen and 1 ° 33 mm. in that of INGALLs’s. The length of the primitive streak in the Ingalls embryo (670 p. measured in the reconstruction of the median section, text——fig. 17) is only 20 p. smaller than that of R0SSENBECK's embryo. We may accordingly assume that these two specimens belong to very much the same developmental stage. The Ingalls embryo seems to be slightly more differentiated, inasmuch as there are three distinct ventral openings in the caudal portion of the chorda-canal, and a continuous lumen with one ventral opening in the chorda-canal of our intermediate region. This latter region, however, Was interpreted by INGALLS as part of the “ completion plate.” We cannot determine the limit between the intermediate region and the prochordal plate in this specimen, because the description is not sufficiently detailed. As far as We can estimate, the length of the caudal portion of the chorda-canal (where it is not accom- panied by mesodermal bands) is practically the same in ROSSENBECK’S and INGALLS’ specimens.
The most developed embryo of the presomite stage in which a prochordal plate is described is the M‘Intyre embryo (BRYCE, 1924). The interpretation of the region in question in this specimen, Where a chorda-plate is already developed, is made difficult by the great obliquity of the sectional plane in this region. It is most probable that the thickened endoderm in sections 51--54 (figs. 21—24, Plate V, BRYCE, 1924) represents the prochordal plate. More difiicult is the explanation of the median structures in sections 55-61 (figs. 30——-36, Plate VII, BRYCE, 1924). It is very likely that the cavity seen in sections 55 and 56 is due to an artificial detachment of the inner germ layer from the other layers, but it should be mentioned that STERNBERG (1927) has found What he regards as a corresponding cavity in the prochordal plate of an embryo with four paired somites. Whether the two bilateral masses or “ cell-groups ” in the M‘Intyre embryo represent lateral mesodermal bands in the intermediate region or Whether they belong to the prochordal plate, We are not able to determine.
BRYCE concludes “ that the cell-groups or pouches correspond to embryonic vestiges of the head cavities of lower forms.” If that conclusion is substantiated they would unquestionably belong to the prochordal plate.
BRYCE was the first to describe and to identify correctly a prochordal (completion) plate in the human embryo. What could be regarded as a prochordal plate, or at least as prochordal mesoderm was, however, figured by WILSON (1914) in a human embryo (H3), in which the number of somites could not be exactly determined because of its poor preservation (there may be up to three pairs of somites according to WILSON), but the author described it as a constituent of a “primary pharyngeal membrane ” (WILSON, 1914, p.‘.326,fig. 11, p. 333).
Later PAYNE (1925) recognised and described a prochordal plate as a ‘,‘ thickened mass of cells continuous dorsally and posteriorly with the notochord and extending caudally along the ventral surface of the gut for at short distance ” in an embryo with seven paired somites.
In 1927 STERNBERG described a prochordal plate in an embryo with four paired somites. He found the endoderm of the foregut thickened, and in one section observed in the thickening a median cavity, which he identified With that described by BRYCE (1924) in the prochordal plate of the M‘Intyre embryo.
In 1928 LUDWIG described a fusion of the cranial extremity of What he terms “ chorda ” with the mesoderm, in an embryo with———according to LUDWIG---One paired somite, which fused area is, we think, none other than a prochordal plate.
In the same year FLORIAN (1928, 6) figured a prochordal plate in an embryo with four paired somites (embryo Bi III), but without recognising it as such. POLITZER (1930), in describing the prochordal plate in his embryo Ct with seven paired somites, has identified the structure in question in. the figures of the embryo BiIII as a prochordal (completion) plate, and this identification we accept. As a matter of fact, FLORIAN had recognised it as such before PoLrrzEn,’s paper was published. POLITZER also recognised the existence of a prochordal plate in the embryo H 197, BARTELMEZ--EVANS (1926).
The observations of the authors mentioned above indicate that the production of mesenchyme by the prochordal plate in the human embryo does not reach its maximum prior to the differentiation of the somites.
(B) Conclusions
We may now summarise the foregoing review of the A available material illustrating the development of the head-process and the prochordal plate as follows :-.
The earliest specimen in which the head-process is undoubtedly developed is the P. Meyer embryo. Here the head-process is represented by a short cell-cord with no indication of difierentiation into as median chorda-process and lateral mesodermal masses.
The head-process in S'rIEvE’s embryo “ Hugo ’ is longer and broader. A median chorda-process is also in this case not yet distinguishable. The prochordal plate, if present, is not distinguishable as a localised area of thickened endoderm. ).
In the embryo B124 the caudal part‘ of the head-process is transformed into a chorda-process. It is accompanied on either side by mesoderm (marked by dots in text-fig. 4), which we interpret as of primitive streak origin. 2 Cranially the head-process broadens (marked by horizontal lines and dots) and assumes a somewhat different character. No median chorda-process is differentiated in this part of the head-process. A very small prochordal plate, formed of thickened endoderm, is situated just in front of the head-process. This specimen is the earliest one in which we have found an undoubted prochordal plate.
In the Manchester embryo the caudal part of the head-process, transformed into a chorda-process and accompanied by primitive streak mesoderm on both sides, is slightly longer than in the preceding embryo. The cranial, undifferentiated. part of the head-process (marked by horizontal lines and dots in text—fig. 5) is surrounded cranially and laterally by prochordal plate (thickened endoderm).
In the Dobbin embryo the caudal part of the head-process is transformed into a chorda—canal, opening dorsally into the amniotic cavity and ventrally by seven openings into the yolk—sac. The intermediate region of the head-process is differentiated into a median chorda-process or chorda-canal and two lateral mesodermal bands. The cranial portion of the head-process is composed of a compact undifierentiated cell-mass, under which no endoderm is distinguishable. i Its cranio-lateral margins overlap on each side a thickened zone of endoderm, Which is continuous with the thickened endoderm of the prochordal plate situated in front of the head-process.
In the Rossenbeck embryo the same structures can be observed, only increased in size, but here the thickened endodermal area — the prochordal plate — is present only in front of the head-process. The lateral mesodermal bands and the chorda-process appear to be fused With the endoderm.
The available evidence seems to indicate that the head—process in Man very early becomes intercalated in, or fused With, the endoderm. The prochordal plate, in the earliest stage (Bi24) in which it was identified with certainty, is situated immediately in front of, and in contact with, the head-process. Whilst these facts seem to be quite certain, the developmental changes which are effected in the next following stages are not so easily interpretable. For instance, how are we to interpret the apparent semicircular form of the thickened endoderm area in the Manchester, Thompson—Brash and Dobbin embryos, whilst in the earlier stages (Bi24) and in the later stages (embryo Rossenbeck) the thickened endoderm area is situated directly in front of the head-process and is relatively smaller. Then, again, how are we to interpret the occurrence of lateral mesodermal bands in the intermediate region? finally, what is the significance of the apparent mesoderm producing, cell-layer which underlies the intermediate region of the head-process and which is so well marked in the Loris 49 embryo? The possibilities are as follows :—
- In explanation of the semicircular form of the prochordal plate in embryos Manchester, Thompson—Brash and Dobbin, it may be suggested that the cranial end of the head-process secondarily grows forward over the greater part of the plate, and becomes fused with, or intercalated in it. In this case the cell-layer beneath the chorda—canal and the lateral mesodermal bands of the intermediate region would represent prochordal endoderm which shows, especially in the Loris 49 embryo, evidence of mesoderm proliferation. Against this suggestion it may be urged (a), that it demands a prochordal plate of a very large size, overlapped in its greater part by the head-process; and (b) that in the supposed region of overlap mesoderm formation starts earlier than in the cranial region. # It may be suggested that the intercalation of the cranial part of the head-process in the endoderm in the stages in question (embryo Stieve to embryo Rossenbeck) has not yet occurred, the endoderm, though indistinguishable as a separate layer, being still present though fused with the under side of the head-process. On this view we must suppose that in the intermediate region with which we are here specially concerned, the chorda—process and its related lateral mesodermal bands differentiate from the more dorsal part of the original head-process, Whilst the ventral part of the latter remains fused with the endoderm as is suggested by the condition in the embryo Loris 49. Accordingly, the prochordal plate would not be overlapped by the head-process at all. The semicircular form of the thickened endoderm area lying cranio-laterally to the tip of the latter, might be interpreted as due to the growth and expansion of the cranial end of the l1ead—process. On the other hand, the lateral horns of the semicircular thickening might be regarded as the remnants of the paraxial endodermal thickenings, which We have described in Loris a11d which HUBRECHT (1902) originally described in Tarsius under the name “ annular zone ” or “ ringformige Mesoblastproliferation.".
In this connection, it may be recalled that in the Beneke embryo, FLORIAN and BENEKE (1930, fig. 2) have described a horse-shoe shaped zone of thickened endoderm in front of the primitive streak which is suggestive of the semicircular area in embryos Manchester, Thompson-Brash and Dobbin, the cranio-median part of which might be taken to represent the primordium of the prochordal plate. In this case we should have to regard the prochordal plate as being diflerentiated before the appearance of the head-process as HUBRECHT (1890) originally described. in the case of Sorex, and as HILL and TRIBE (1924) have suggested in the case of Felis. It must be pointed out, however, that in the embryos Hugo and Bi24, which are intermediate between the Beneke and the Manchester embryos, no such horse-shoe shaped or semicircular thickening could be observed, but if the horns of the latter are vestiges, they might very well be subject to individual variation.
As concerns the interpretation of the lateral mesodermal bands of the intermediate region, we believe we are justified in maintaining that they form parts of the head-process. The conditions in the Loris 49 embryo appear to us to substantiate the correctness of this view. We are not inclined to accept RossENBEOK’s suggestion that these bands are developed from the “ completion plate,” a suggestion which implies the derivation of the cranial and intermediate region of the head—pr0cess from this same plate.
finally, arising out of the comparison of the reconstructions of the dorsal views of the human embryos provided in this paper, we may call attention to the possibility of the existence of two different varieties of embryonal shield : one narrow (text-figs. 4, 5, 7, 8 and 10), the other broader (teXt~figs. 3, 6 and 9). If we compare S'rIEVE’s embryo (text-fig. 3) with embryo Bi24 (teXt—fig. 4), we notice that the former is slightly longer, but very much broader than the latter, at the same time Bi24 is certainly more differentiated. Again, if we compare WALDEYEB’s embryo with the Dobbin embryo, we observe the same fact : the length of both is practically the same, but the former is much broader than the latter, and the head-process is certainly much less developed. Again, if we compare the Dobbin embryo with RossENBECK’s specimen, we see only a slight difierence in the differentiation of the axial structures, but a very marked difierence in size, especially in breadth. These observations suggest that there may be two different forms of the human embryonal area : one small and comparatively narrow (what we may call a dwarf form), the other large and comparatively broad (what we may call a giant form). In this connection it is of interest to recall that two varieties of embryonal area are distinguishable in the rabbit, one in which the embryonal area is short and broad, the other in which it is long and narrow (cf. the figures of VAN BENEDEN an.d RABL).
Table 1
Text-fig. 9. Embryo Peh.1 - Hochstetter (ROSSENBECK, 1923). ‘The lines 63-80 correspond with the levels of sections shown in our figs. 63~80, Plate 35. The lines 1-39 correspond with the levels of sections shown in RossENBEoK’s paper on Plates XXXI-XL. For other explanations, see description of text-figs. 3-10, p. 470 (our own reconstruction).
Text-fig. 10. Embryo Ingalls (1918). For explanation, see description of text-figs. 3-10, p. 470.
Text-fig. 18. Schematic figures for comparison of the length of the difierent regions of the embryonal area of six human embryos. X 7 0.—-——Cloacal membrance cross-hatched ; primitive streak lined vertically; + 2 HENSEN’S knot ; c.n. = primordium of the neurenteric canal ; head—process lined obliquely; prochordal plate dotted; caudal end on the left, cranial end on the right. Between the stages of embryos “ Hugo ” and Bi 24 a rapid growth of the cloacal membrane is indicated by arrows 1 and 2 ; the cloacal membrane in the former embryo has the form of an elongated cell-band, whilst in the latter specimen it has assumed the form of a membrane. Between the stages of embryos Bi 24 and Manchester, 3. rapid growth of the primitive streak (marked by arrow 3) has occurred. Between the stages of embryos Manchester and Dobbin, the rapid elongation of the head~process is marked by arrow 4; the head-process and prochordal plate nearly reach the cranial margin of the embryonal area in the Dobbin embryo. In the region of HENsEN’S knot in the Dobbin embryo, the primordium of the neurenteric canal can be observed. Between the stages of embryos Dobbin and Peh.1— Hochstetter-—apart from the proportional increase of all parts of the embryonal area - a rapid growth of the shield-ectoderm in front of the prochordal plate is indicated by arrows 5 and 6 ; the thickened ectoderm of the medullary plate can be distinguished from the flattened ectoderm of the embryonal body in the region indicated (see p. 465). The doubtful area in the embryo “ Hugo ” (see p. 466) is designated by 3, as is also the region of the prochordal plate and the cranial limit of the embryonal area in embryo Ingalls, which cannot be determined from INGALLs’s description. To facilitate comparison of the regions, we have so placed the diagrams that the region of HENSEN’s knot (or the neurenteric canal) in the various embryos lies in the same vertical line. Naturally, we do not regard Hensen’s knot as constituting a fixed point.
List of References
BARTELMEZ, G. W., and EVANS, H. M. (1926). ‘ Carn. Contrib. to Embr.,’ vol. 17, No. 85.
BONNET, R. (1901). ‘ Anat. Hefte,’ vol. 16.
BRYCE, T. H. (1924). ‘ Trans. Roy. Soc. Edin.,’ vol. 53.
FAHRENHOLZ, C. (1927). ‘ Z. Mikr.-Amt. ForSch.,’ vol. 8.
FETZER (1910). ‘Anat. Anz.,’ vol. 37, Erg.—Heft. I
FETZER, M., and FLORIAN, J. (1930). ‘ Z. Mikr.-Anat. ForSch.,’ vol. 21.
FLORIAN, J. (1928, a). ‘ Z. Mikr.-Anat. FOrSch.,’ V01. 13.
- (1928, b). ‘ C. R. ASSOc. Ana,t., 23"” Réunion,’ Prague.
- (1930, ct). ‘ J. A.I1a.t.,’ vol. 64.
- (1930, b). ‘ Anat. Anz.,’ V01. 71. .
FLORIAN, J ., and BENEKE, R. (1930-31). ‘ Anat. Anz.,’ vol. 71, Erg.—Heft.
FLORIAN, J ., and VOLKER, O. (1929). ‘ Z. Mikr.-Anat. ForSch.,’ vol. 16.
GROSSER, O. (1913). ‘ Anat. Hefte,’ V01. 47. .
- (1930 - 31). ‘ Anat. Anz.,’ V01. 71, Ergu-Heft.
- (1931). ‘ Z. Anat. EntW.,’ V01. 94.
HERTWIG, O. (1906). Handbuch dcr Vergleichenden und experimentellen Entwickelungslehre der VVirbeltiere, Bd. 1. G. fischer, Jena.
HILL, J. P., and FLORIAN, J. (1931). ‘ J. Anat.,’ vol. 65.
HILL, J. P., and TRIBE, M. (1924). ‘ Quart. J. Micr. Sci.,’ vol. 68.
HUBREOIIT, A. A. VV. (1890). ‘ Quart. J. Micr. Sci.,’ Vol. 31.
- (1902). ‘ Verhand. K. Akad. _WctenS. Amsterdam,’ vol. 8, No. 6.
INGALLS, N. W. (1918). ‘Carn. Contrib. to Embr,’ V01. 7, N0. 23.
LUDWIG, E. (1928). ‘ Morph. J'a.hrb.,’ Vol. 59.
M‘INTYRE, D. (1926). ‘ Trans. Roy. Soc. Edin.,’ Vol. 55.
MEYER, PAUL (1924). ‘ Arch. Gryr1ék.,’ Vol. 122.
PAYNE, F. (1925). ‘Carn. Contrib. to Embr.,’ V01. 16, NO. 81. Z
POLITZER, G. (1928). ‘ Z. Anat. Entw.,’ vol. 87; :(1930). ‘ Z. Anat. EntW.,’ V01. 93.
RABL, C. (1915). ‘ Arch. Mikr. AIIa.t.,’ vol. 88.
ROSSENBEOK, H. (1923). ‘ Z. Anat. Entw.,’ Vol. 68.
STERNBERG, H. (1927). ‘Z. Anat. Entw..,’ V01. 82.
STIEVE, H. (1926). ‘ Z. Mikr. Anat. FOrSch.,’ vol. 7.
STRAHL, H., and BENEKE, R. (1910). Ein junger mensciflicher Embryo, Wiesbaden.
STREETER, G. L. (1919). ‘ Carn. Contrib. to Embr.,’ V01. 9.
THOMPSON, P., and BRASH, J. C. (1923). ‘ J. Anat.,’ vo1.58.
WALDEYER, A. (1929). ‘ Z. Anat. EI1tW.,’ V01. 90.
WILSON, J. T. (1914). ‘ J. Anat.,’ vol. 48.
WILSON, J. T., and HILL, J. P. (1907). ‘ Phil. Trans.,’ B., vol. 199.
Explanation Of The Plates
Plate 29
FiG. 1. Lateral View of chorionic vesicle. Dobbin embryo.
FiG. 2. Section of the chorion and villi. Dobbin embryo.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Published December 9, 1931. In Australia copyright has expired - creator died before 1955, provided; work was also published before 1955.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - A Young Human Embryo (Embryo Dobbin) with Head-Process and Prochordal Plate. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_A_Young_Human_Embryo_(Embryo_Dobbin)_with_Head-Process_and_Prochordal_Plate
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G