Book - Physiology of the Fetus 8
| Embryology - 27 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Windle WF. Physiology of the Fetus. (1940) Saunders, Philadelphia.
1940 Physiology of the Fetus: 1 Introduction | 2 Heart | 3 Circulation | 4 Blood | 5 Respiration | 6 Respiratory Movements | 7 Digestive | 8 Renal - Skin | 9 Muscles | 10 Neural Genesis | 11 Neural Activity | 12 Motor Reactions and Reflexes | 13 Senses | 14 Endocrine | 15 Nutrition and Metabolism | Figures
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Chapter VIII The Fetal Kidneys And Fluids - The Fetal Skin
Development of Kidney Function
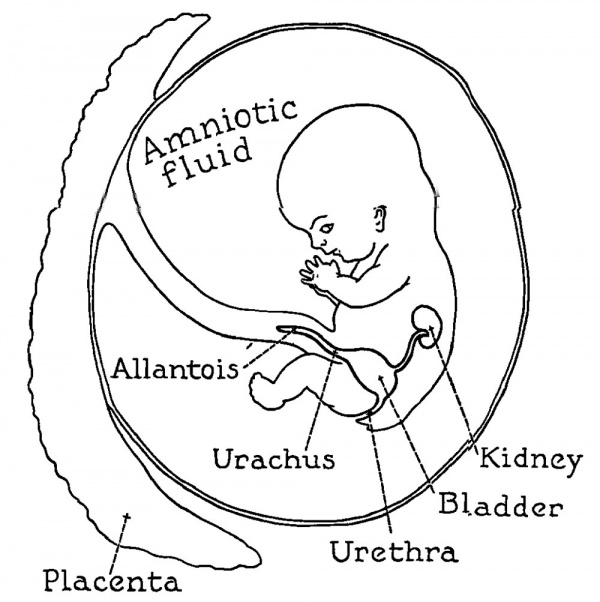
Development of renal function is related to the formation of amniotic and allantoic fluids so closely that a consideration of one invariably involves the other. The most casual observation in fetuses of laboratory animals reveals the fact that a clear fluid lills the bladder. This is known to be true in human fetuses during the kourth month. Analysis of the bladder contents later in prenatal life demonstrates that the Auid is indeed a dilute urine.1-3 An appreciable .amount of uric acid (1oo mg.) was found in the human ketal kidneys at 7 months by one investigator. 4 Urea has been detected in human amniotic fluid as early as 2.5 months gestation.5 We may infer from these observations that the fetal lcidneys do function, at 1east to a limited extent, well before they are required to perform all elimination. Excretion of nitrogenous wastes is accomplished entirely by the fetus in birds. A large allantoic sac is formed to receive the Urweconcentrate it and salvage the water which is essential to the fetus for other metabolic processes The rigid economy of water encountered in birds is not necessary in« the mammals. The placenta provides a mechanism for turning over the end-products of ketal metabolism to the maternal blood and lcidneys in a11 the true mammals. An allantois would seem to be unneeessary but one korms neverthe1ess, and kunctions to a variahle extent. The al1antois is vestigial in some mamma1s and man but is exceedingly 1arge« in others, opening into the ketal bladder through the urachus. The urethra communicates with the amniotic vesie1e. Thus both allantoic and amniotic Huids can receive the fetal urine. These relations are illustrated in Figs. 42 and 43.
Fig. 42. Relation of the bladder, amnion and allantois in the fetal calf.
Fig. 43. Relation of the bladder, amnion and vestigial allantois in the human fetus.
Although the placenta is the chiek excretory organ of mainmalian ketuses it seems probable that elimination through it varies with the intimaqy of the ketal and maternal hlood strearnz One should not expect the ketal waste products to pass through six tissue layers in the epitheliochorial placenta of? the pig as readiiy as through only three layers in the hemoschoriiil plaeenta of xnan or the single membrane which is found in the herno-endothe1ia1 p1acenta of the higher rodents. Some believe that there is no placental elimination at all in the pig, sheep and eat, anirnals in which a large allantois is present« In general it appears to be true that the species with the: simplest placentas are those with the largest allantoic vesicles, and the mammals with highly developed placentas in which the two bloods are more intimately related have very small or vxstigial allantoic sacs. Moreover it has been thought that the size of the embryonic mesonephros is related to the intimacy .of the two bloods in the placenta as well as to the development of an allantois. Bremers found that the trophoblast epithelial layer of the higher placentas becomes thin in certain places and covers the chorionic capillaries lilce Bowman’s capsule invests adult renal glomerular capillaries. He suggested that this formation of trophoblast plates takes place when the mesonephric tubules cease to function and before the true lcidney (metanephros) takes over excretion. He found no trophoblast plates in species whose mesonephric and metanephric function overlapped and concluded that in others the placenta had been forced to adapt itself to more eliicient excretory activity in order to bridge a gap in renal function. However, we shall soon learn that mesonephros and metanephros actually overlap functionally not only in lower mammals but in those with ·the most eliicient placentas as well; the precise relationship just postulated has been questioned.
Physiologic Development of the Nephric Tubules
There is no lack of evidence that the placenta is the principal excretory organ of the fetus. It can perform its duties in abnormal fetuses laclcing lcidneys and in specimens with congenital occlusion of the urinary passagesJs S Nevertheless, the fact is well established that both mesonephric and metanephric tubules are capable of elimination very early in prenatal life» of the normal fetus.
Among the older studies on fetal renal function there are a number in which chemical solutions were injected into the mother and sought in the fetal lcidneys or bladder. Preyers and NeedhamI have reviewed these. 1t may be said that placental permeability varies directly with the intimacy of contact between maternal and fetal blood streams and is highest in the hemos chorial and hemoendothelial placentas. The chem-icals used ex— perimentally were recoverable in the fetal urine after introduction int·o the maternal blood partly because their small molecular size made them capable of passing the placental membranes and the fetal nephric epithelium.
Attempts have been made to inject materials directly into the fetus in order to determine their concentration in the fetal kidney or bladder. A number of experiments in rabbits, cats, guinea pigs and swine have contributed little specific information concerning intrinsic functions of the nephron. 9-13 chiclc embryos have been used more extensively than mammals, but it is well known of course that their early nephrons do excrete, for there is no other accessory mechanism like the placenta to function in elimination. Experimental occlusion of the mesonephric (Wolffian) duct leads to hydronephrosis as early as the fourth day of incubation 14 because of pressure of the urine in the mesonephric tubules. Indigo red solutions injected into the vascular systems of chick embryos incubated five days and more appeared in the lumen of the nephric tubules. Trypan blue has been used in the chick by a number of investigatorsIS49 and it too made its appearance in the mesonephric and metanephric tubules. Chambers and his colleagues20s 21 have confirmed and extetided these earlier experiments. They found that elimination of phenol red begins in the chiclc mesonephrons at about 41zs days of incubation. They grew pieces of embryonic lcidneys in tissue cultures and used these preparations to study activities of the nephrons. The proximal convoluted tubule of both meso- and metanephrons passed an in— dicator dye, phenol red, into the lumen and the distal portions of the tubule resorbed waret. Some similar experiments have been reported in the duck.22
The most signilicant study of functional capabilities of the mammalian mesonephric and metanephric tubules is that of GershW who employed carefully controlled histochemical methods. He used a nonstoxic solution of sodium ferrocyanide as an indicator of glomerular elimination and phenol red as a test for elimination in the proximal convoluted tubule. These chemicals had been employed previously to study similar functions in adult nephrons« Administration was effected by placental transmission in the rabbit, intravenously in the chiclc embryo· and by direct injection into the fetal bodies (intraperitoneally?) of rabbits, cats, pigs and pouch—young opossums. Results indicate that the ferrocyanide was eliminated with water into the glomerular space from which it passed through the lumen of the remaining portions of the tubules of both mesonephros and metanephros just as in adult ltidneys. The phenol red was never found in the glomerular space but did appear in the cells of the proximal part of the tubule and the Iumen of the remaining portions in both meso- and metanephros. The criticism may be raised that sonie dye was passed by the glomerulus but in such a dilution that it could not be detected histologically in the Bowman’s capsule. Gersh however considered it doubtful if phenol red was eliminated at all by the glomerulus. Its presence within the cells of the proximal tubules suggested secretion. Concentration of the test substances was suAiciently marked to justify the conclusion that urine was being formed in the mesonephric as well as the metanephric tubules of the embryo.
In all the species studied the mesonephros functioned in elimi— nating ferrocyanide and phenol red for a shorter or longer time after the metanephric tubules had talcen on the same functions. Water resorption in the loop began later than did glomerular and tubular eliminationks The high uric acid content encountered in the fetal lcidney in one instance suggests that much of the water dialysate together with urea and other soluble compounds which enter the embryonic nephrons is resorbed before reaching the ca1yces.4 Fetal nephric excretion appears to be a slow cons tinuous process.
Structural difkerentiation of the nephric tubule could be correlated with onset "of elimination in Gersh’s study, but the growth of new blood vessels along the tubules bore no re1ationship to it. In the glomeruli on the other hand no immediate change in structure appeared to be related to onset of ferrocyanide elimination which, it was suggested, must be due to «some extraglomerular or extrarenal factor such as change in blood pressure, and/or osmotic pressure of the blood colloids or with a sub— microscopic change in energy capacity or permeability of the glomerular membrane." 23 The assumption of ability on the part of the lcidney to do thermodynamic worlc is accompanied by increased oxygen consumption as measured by the Warburg method. 25
That conditions in the human fetal nephrons are similar to those in the other mammals seems certain. Histologically their development is the same. 0n the basis of difkerential staining HewerW conc1uded that an histologic change which may be associated with assumption of kunctional activity talces place in the convoluted tubules at 12 weelcs and possib1y as early as 9 weelcs. Gersh23 believes that the human metanephrons begin to eliminate at the 32—mm. stage, which is about 9 weelcs fertilization age,27 and that mesonephric tubules are still functional at that time.
Cameron and Chambers 28 studied tissue cultures of bits of human fetal kidneys at 3.5 months. They observed that the cut ends of tubu1e segments healed over in the cultures, and that phenol red and orange G dye solutions which were present in the culture medium passed into and accumulated in the lumens of the proximal tubules. Furthermore, it was determined that the fluid accumulating in these tubules had a pH of approximately 7.o while that of the culture medium was from 7.4 to 7.6. In the chiclc acidification of the cultures with carbon dioxide to a pH of 5.o failed to prevent the secretion of phenol red.29
Conditions Regulating Renal Function
0ne important deduction made from the experiments with mammalian fetuses is that urine formation is remarlcably slow.23 As a matter of fact it might be considered remarlcable that urine is formed at all in fetuses of some species, in which it has been reported that a small gradient between arterial and venous blood pressures exists. In cat fetuses at term arterial pressures of at least so mm. and venous pressures somewhat less than 1o mm. of mercury may be physiologic in the large vessels. Filtration in the glomeruli must be carried out at a rather low pressure especially in view of the fact that intraureteral pressure is appreci— able.23 If these observations are correct osmotic pressure of the fetal blood colloids must be low. No information is available in the cat, but it has been found that the osmotic pressure in the dog at birth (with an arterial pressure of« 4o mm. mercury) is low.3-0
The protein nitrogen of the dog’s blood at birth amounts to 375 mg. per ioo cc. It increases, with a proportional risein blood pressure, to 8oo or goo mg. in the adult. Each gram of the newborn dog’s plasma protein has only about »three-fourths the osmotic equivalent of each gram in the adult. The osmotic pressure of the fetal blood has not been jneasured, but in the young puppy it is 16.7 cm. of water when the plasma protein nitrogen concentra— tion reaches 5 16 mg. per cent, rising to 37.7 cm. of water when the concentration attains a value of 866 mg. per cent.
In contrast to these data from carnivorous mammals the gradient between arterial and venous pressures is high in fetal sheep near term (artery 75 to 80 mm.; vein 18 mm. mercury) , and there is some reason to believe that the osmotic pressure of the fetal blood is considerably greater than in the dog. The serum of the sheep fetus has a high proportion of albumin (62 per cent) and a low proportion of globulin (38 per cent) , which would make the blood protein have, gram for gram, a higher osmotic pressure than that of the mother. But the total protein con— tent is approximately only about iiveeighths that of the mother per cc. of serumkIs Z« Consequently the osmotic pressure of the blood plasma of the sheep fetus may be no higher, if as high, as that of the mother.
In the human fetus arterial pressures as high as 110 mm. have been recorded at delivery and the pressure in the veins is no greater than 20 or 25 mm., perhaps much less under normal conditions within the uterus before labor starts (see Chapter II) . This allows enough of a pressure gradient to account for filtration in the fetal glomeruli without assuming a reduced osmotic pressure of the fetal blood colloids. In adult man we know that systemic arterial pressures greater than 75 mm. and intraureteral pressures less than zo mm. of niercury are compatible with urine formation.33
In summarizing the present state of our knowledge concerning renal function in prenatal life the following observations appear to be of greatest signiiicancm Excretion begins early in the true kidney (9 weeks in the human) and is continuous but slow. The mesonephros functions before the true kidney (metanephros) and for some time after the latter has talcen over urine production. The fetal glomerulus (capillary tuft and Bowman’s capsule) gives rise to a dialysate from the blood and the rapidity with which this forms is related to fetal capillary pressure, osmotic pressure of the fetal blood col1oids, carbon dioxide level of the fetal blood and other factors. some substances may» be contributed to the glomerular fluid» by secretory activity of the cells of the proximal convoluted tubules. shortly after elimination begins in the glomerulus and proximal tubule, resorption of water and highly soluble compounds occurs in the proximal tubules and the thin segments of Henle’s loop in response to an altered osmotic balance in the blood which has passed through the glomeruli. It seems probable that elimination in the fetus can be explained large1y on the basis of factors which bring about excretion in the adult. although secretion of tubular epithelium may play a more important role in the fetus than in the adult.
The placenta is the chief excretory organ of the human fetus, but its importance appears to vary in other species with the degree of intimacy of the fetal and maternal blood streams. An allantoic vesicle provides a receptacle for fetal urine in some mammals, notably those with the less highly developed types of placentas. The amniotic vesicle can and does receive fetal urine during part of prenatal life in all mammals.
Changes In Elimination At Birth
Provisions are made for rapidly altering many of the organism’s vital activities at the time of birth. changes are encountered in the circulatory and nervous systems and especially in the respiratory mechanism where a sudden shift from placental to lung breathing takes place. It is therefore not surprising to lind dramatic structural and functional changes in the kidneys at the time of birth.
High columnar epithelium invests the fetal renal glomerulus. This, the visceral layer of Bowman’s capsule, forms a heavy sac which coniines the glomerular tufts. Its comparatively low permeability must serve as an impediment to rapid glomerular elimination. The fetus not only does not need a mechanism for rapid tiltration in its kidneys, but such a mechanism might actually be detrimentaL for it would seriously disturb the Huid balance in utero. When placental elimination is suddenly abolished at birth the columnar epithelial investments of the renal glomeruli burst, allowing the glomerular vascular loops to expand and to come directly into contact with the capsular space. Henceforth glomerular liltration is greatly enhanced.34
The Fetal Urine
Although it has been Jdetermined that urine formation is slow nothing is known about the amount produced in any period of time. It is said that the human fetal bladder capacity varies from 4o to 7o cc. between the seventh month and birthFZ certainly the amount contained within the bladder at any moment is no measure ok the total formed because the amniotic fluid receives an indeterminable quantity.
| At term | At term | At 6 months | |
|---|---|---|---|
| Freezing point (°) | —0.141 | ... | -0.174 |
| Total N gm. % | 0.043 | 0.041 | 0.061 |
| NaCI gm. % | 0.136 | 0.263 | 0.171 |
A few chemical analyses have been made on human fetal urine. Makepeace and his colleagues2 examined samples collected at birth in two specimens and at six months gestation in one other. Jacques analyzed the bladder urine of fetal sheep 36-—47 cm. long and compared the values with adult sheep urine and other fetal Auids. Some of their data appear in Tables 161 and 172
| Adult urine | Fetal urine | Allantoic fluid | Anmiotic fluid | |
|---|---|---|---|---|
| Freezing point (°) | -1.959 | -0.255 | —0.545 | -0.470 |
| Protein gm.% | ... | 0.044 | 0.054 | ... |
| NaCl gm.% | 022 | 0.17 | 0.16 | 0.64 |
| Total ash gm.% | 0.689 | 0.37 | 0.924 | 0.84 |
| Insol. ash gm.% | 0.13 | 0.011 | 0.074 | 0.017 |
| Spl. ash gm.% | 0.56 | 0.34 | 0.85 | 0.82 |
That the contents of the bladder is urine rather than some simple transudate may be assumed from the fact that it is isotonic neither with the blood of the fetus nor with that of the motherz it is low in sodium chloride. Jacquss data show some resemblance between fetal urine and the allantoic Huid of the sheep, and it may be concluded that the latter is partly concentrated fetal urine. However, the feta1 fluids of the sheep do not contain all the excreted waste products because it is probable that some elimination takes place through the placenta. The freezing point of the sheep urine decreases after birth; at the end of 36 hours it was —1.042° in one case, in contrast to —0.255° before birth. In other words the hypotonicity of fetal urine in respect to blood (fetal blood = —0.623°; maternalsheep blood = —0.578°) disappears after birth with the assumption of full elimination by the kidneys. 1
The Allantoic Fluid
Few additional facts concerning the allantois and its contents need be mentioned. The vesicle develops as a ventral diverticulum of the embryonic bladder and maintains a patent connection with the bladder during the first half of fetal life or longer by -means of a duct, the urachus. In those animals with well developed allantois such as the sheep the amount of Huid contained in it rises sharply in early prenatal life, then dec1ines and subsequently increases again toward the end of gestation. It has a greater volume than the amniotic Huid in early prenatal life and may have more toward the end of gestation in individual cases, but the relationship does not hold throughout the middle of the period when the volume of amniotic iluid overtalces it. NeedhamT believes, on the basis of the data of a number of other investiga— tors, that there is an exchange of- Huids between amnion and allantois (the former giving up Huid to the latter) made possible by the fac·t that the contents of allantoic and amniotic sacs are separated by nothing more than a double membrane. The function of the allantois is most clearly indicated in birds where the organ receives all nitrogenous wastes, concentrates and precipitates the uric acid, and salvages the water for other uses.
The Amniotic Fluid
The most important function performed by the amniotic fluid is the provision of an aquatic environmenkfor the developing embryo.30 Were it not for this it is doubtful if uniformly even growth could take place because the very soft embryonic tissues would be molded by pressure from the surrounding structures. The Huid is said also to prevent embryonic adhesions. Protection from shoclcs and drying is provided by the Chorion, uterus and body wa11 more than by the amniotic fluid and the delicate membrane enclosing it. The «fluid-lilled amniotic sac acts as an hydraulic wedge for the descending fetal head at the time of birth and helps malce fetal postural adjustments to birth possible.
The composition of amniotic fluid has been studied in man and several other animals. 2,3,5, 37-39 but a detailed account of its chemistry would be out of place here.I It has a specific gravity of about hooögss in man and is defmitely hypotonic both to the maternal and fetal blood, containing less sodium chloride and other salts. Its urea and uric acid content gives a clue to the origin in part. The quantity of these two substances increases during prenatal life, as may be seen in Table 18.5 It follows that the amniotic iluid receives a significant contribution from the fetal kidneys throughout most of the gestation period.
| Month of gestation | Volume (cc.) | Freezing point (C) | Urea (mg. %) | Uric acid (mg. %) |
|---|---|---|---|---|
| 2.5 | 40 | -0.520 | 34 | 3.4 |
| 4.5 | 140 | -0.515 | 38 | 4.0 |
| 7.5 | 1,050 | -0.482 | 40 | 4.5 |
| 10.0 | 1,800 | -0.467 | 44 | 5.1 |
This brings us to the still unsettled controversy regarding the source and method of formation of amniotic fluid. stated in the simplest terms, two theories have developed: (a) that the fluid is a transudate or dialysate of the mother, and (b) that it is formed entirely by the fetus, some investigators holding that it is a secretory product of fetal kidneys and amniotic epithelium. A detailed consideration of all information bearing upon the subject can not be given here; and once more the reader must turn to more extensive reviews.1
In the sheep, an animal with a large allantois as well "as amnion, it has been found that the fetal urine is passed from the bladder through the urachus to the allantois (see Fig. 42) up to a little past the middle of the gestation period.3 In late fetal life however the urethra transmits urine into the amnion and the urachus ceases to supply it to the allantois. A mid-interval exists during which both vesicles receive the fetal urine.
One of the strongest arguments favoring the theory of fetal origin of amniotic fluid is based on experiments of Watson40 who found that rabbit fetuses died when the amniotic fluid was withdrawn from the pregnant does, but the maternal part of the placenta continued to grow and was vascularized normally. There was no regeneration of the amniotic fluid. 0ther evidence for at Ieast a partial fetal origin is of course the proved fact of fetal renal activity and the presence of an open pathway from the kidneys to the amniotic space. The presence of fructose and of certain proteins in fetal urine of some species of animals and in the amniotic and allantoic Huids of the same but not in others adds weight to the theory of fetal origin. 41
It has been pointed out that the chorion of the cat and the vitelline membrane of the rabbit are vascularized by fetal vessels which are interposed between the fetal Auids and Uterus. « Further— more, the cat’s endometrial epithelium is rather thoroughly re— stored during the last half of pregnancy except at the placental site. These facts make it seem unlilcely that fetal fluids can arise as a transudate from the mother’s endometrium to the amniotic sac during the last half of gestation in the cat and rabbit.
Some interesting experiments leading to production of polyhydramnios in rabbits provide rather convincing evidence that the amniotic fluid is formed by fetal structures.42- 43 After double nephrectomy of pregnant rabbits it was found that a signilicant increase in amniotic fluid volurne occurred during the latter part of the gestation period when the fluid volume is normally decreased. No edema, ascites or other transudate was encountered in the mother’s body. Average data from this study are presented in Table 19. 42
| Ave. wt. fetus (gms.) | Controls (cc.) | Nephrectomized (cc.) |
|---|---|---|
| Less than 10 | 24 | .... |
| 10-20 | 4.9 | 4.1 |
| 20-30 | 3.8 | 4.8 |
| 30-40 | 1.7 | 5.6 |
| 40-50 | 0.5 | 10.75 |
Injection of large amounts of saline so1ution into nonnephrectomized pregnants animals leads to formation of transudates in the mother’s body« cavities without increasing the amount of amniotic fluid.44
Although the strongest evidence favors the concept that amniotic Huid is formed by fetal structures it does not prove that this is its only source in all species. It is certainly not formed entirely by the fetal kidneys. Early in embryonic life before renal function becomes established we do not know its origin. The amniotic epithelium and blood vessels of the embryo have been suggested The possibility of maternal origin can not be dismissed entire1y. More than 2oo cc. may be present in the pig when the fetus is only 30 mm. longss certain chemical compounds, enzymes and antibodies present in the mother’s body appear in the placenta and amniotic fluid but not in the fetal tissues themselvess The quantity of amniotic fluid increases sharply during the early part of prenatal life in all species of animals. In some, such as the cat and« guinea pig, the rise has been found to continue to full term, but with considerable individual variations« In others, probably man and the sheep, it attains a maximum some time before the end of gestation and this volume is maintained until term. A remarlcable diminution in the quantity of amniotic fluid is encountered in the latter part of prenatal life of the rabbit. A careful comparative study of the minute volume of blood flow in the uterus correlated with the quantity of fetal fluids at different ages may help explain species differences. The great individual and species variations in volume of amniotic f1uid seen during late pregnancy may be related in part to the phenomenon of fetal swallowingkss It has been reported« that polyhydramnios in human subj ects can be reduced by stimulating swallowing by the fetus.
The Fetal Skin
Little can be said regarding the physiology of the fetal slcin and its, associated structures. The sweat g1ands are present and have developed lumens by the seventh month but it is questionable whether they actually secrete in -utero. The sebaceous glands do function before birth, adding their oily secretion to desquamated epithelium and lanugo hair to form the vernix caseosa which covers the fetus. It is usually said that this material serves to protect the living epithelial cells from becoming macerated in the amniotic Huid, a statement for which there does not appear to be the least justiftcation. 0thers have held on the basis of unsatisfactory evidence that the vernix caseosa is a deposit upon the slcin from lipids excreted by the amnion.48 The vernix is usually removed for esthetic reasons at the time of birth, but when allowed to remain it has been found that it will disappear of its own accord in about 8 hoursfs probably by absorption during drying and corniftcation of the outer epithelial layer.
Other cutaneous glands are capable of functional activity at birth. A most curious transient phenomenon is encountered in the mammary glands (see Chapter XIV). A small quantity of secretion is often observed in both sexes and to this the name «witch milk" has been applied. Lacrimal glands are well formed in prenatal life but apparently they do not function. The newborn child is said to cry without tears.
Skin pigmentation is deftcient at birth even though melanin production starts early elsewherez pigment granules start to form in the optic cup of the 7 mm. human embryo. Melanin appears to be manufactured by the fetal tissues with the aid of an oxydase. Although pigment is not found in the hair primordia of human fetuses until the ftfth month and is not present in the epidermal cells before the sixth, the enzyme is there earlier and can produce pigrnent when the dopa reagent (dihydroxyphenyla1anine) is added experimentallyåo The developmental chemistry of the skin should be an interesting and prolitable study.
The fetal skin can have no importance from the standpoint of heat regulation in utero, but it is of interest to know whether or not this function is present at birth. For more than a century it has been known that the ofkspring of some animals are incap— able of maintaining their birth temperature when removed from a warm environmentPo Young rabbits and kittens acquire ability to fully regulate their temperatures ·a"bout 15 days after birth. 0ther animals, such as the guinea pig, are born with a good coat of hair and possess a well formed heat regulatory mechanism at that time. similar species difkerences are encountered in birds, with the chick of the domestic fowl falling into the class of the guinea pigPIs 52 Many anima1s can compensate for a drop in temperature by increasing their metaboIism but are incapable ok meeting the conditions imposed by a high tempctatuke III the external environmentz the rabbit, cat and man are in this class. Others, such as the mouse, possess no form ok heilt kegulation while still others, the guinea pig being an example alt« completely homothermic at births.
References Cited
. Needhaxxk J. . Malcepeacq A. W., F. Fremontsmith M. E. Dailey Z: M. P. C3UV1I«
. saridstrorry C. J. . Gersh, I. . Gersh, I. . Flexner, L. B. E I. Gersli. i9s7. Contr. Emb., 26: ieiä
. Heu-er, E. E. i924. Quart. J. Exp. Physiol., i4: 49.
. Altschu1e, M. D. i9so. Anat. Rec., 46: 8i.»
. cameron, G. E R. chambers i9s8. Am. J. Physiol» ins: 482.
. chambers, R. E R. T. Icemptoix i9ss. J. Cel1. comp. Physiol» s: i si. . Stark, G. A. E I-I. E. I-Io1ling. i9si. J. Physiol» 7s: so5.
. Mccarthy, E. F. i9s8. Ibid., 9s: 8i.
. I-Iowe, P. E. ig25. Physiol. Reis» z: 4s9.
. smith, I-I. W. i9s7. The Physiology ok the Icidney, Oxford Univ. Press.
i9si. Chemical Embryology, Cambridge Und« Ptess.
i9si. Sarg. Gyn. E 0bst., 5s: 6s5.
Jacque, L. i9o6. Art-h. Internat. Physiol» s: 46s.
Leu-is, J. I-I. i9i6. J. Bio1. Chem., 24: 249.
Guthinanm H. E W. May. i9so. Arch. Gynä1(., i4it 450. Bremer, J. L. i9i6. Arn. Anat., i9: i79.
Engliseh, i88i. Arch. Icinderhllk L: 85.
. Preyek W. i885. speeielle Physiologie des Embryo. Gliedert- Leipzig. . Bat, P. i88i. , Recherches pour servir a Phistoire de khydkamnidss Paris«
(cited by I. Gersh, i9s7.) Fritscheltz F. i928. Ztschr. mi1c.—anat. Forsch., is: öl . Firlr.et, J. i92o. Comph Rend. soc. Biol., 8s: i2so. Wisloclci, G. B. i92i. Johns Hoplcins I-Iosp. Bul1., se: 9s. . Franlcenbergen Z. i92i.
Rozpravy Ceslce Alcademiep 302 47« (Cited by
I. Gersh, i9s7.)
. Boyden, E. A. i924. J. Exp. Zool» 4o: 4s7. . Balcounine, s. i895. Arch. Ital. Biol., es: s5o. . Zaretslcy, s. i9io. Virchows Art-h» 2oi: as. . AtwelL W. E E. B. I-Ianan. . I-Iurd, M. C. i928. Am. J. Anat., 42: i 55.
i926. Anat. Rec., se: suppL ges.
Wisloclch G. B. i92i. Anat. Rec., ge: 267.
chambers, R. E G. camerom i9s2. J. cell. comp. Physiol» 22 99chambers, R., L. V. Beclc E M. Bellcin. i9s5. Ibid.- S: 425.
i9s5. Anat. Rec., Sie: 7.
i9s7. contn Emb., 26: ss.
i9s4. Am. Physiol» io8: s55.
Gruenwald, P. E I-I. Popper. i94o. J. Urol., 4s: 45Z. Feldman, W. M. i92o. Anteqiatal and Posvnatal child Physi0l0gyLongmans, Green, N. Y. . Ginglingen A. sc. c. Ray-set.
FYEITAL KIDNBYS AND FLUIDs. FETAL sIcIN 127
. Mossman, H. W. 1937. contra Kind» as: i29. . Döderlein, A. i89o. Arclr. Gynälc., 37: i41. . Uyeno, D. 19i9. J. Biol. chern., 37: 77.
cantarom A» H. stuckert sc R. c. Das-is. i933. Sarg» Gyn. se Obst» 57- Sz . Watson, B. P. i9o6. J. Obst. Gyn. Brit. Emp., g: is. . Paton, D. H» B. P. Watson sc J. Kett.
i9o7. Trans. Roy. soc. Edin.,
46: 7i.
. Wolih B. 19o4. Arch. Gynälc., 71: 224. . Wolfh B. 19o9. Ibid., 89: i77.
. Bakounine, s. 19oo. Atti d. r. Accad. med.-chik., Napoli, 54: i. (cited by Needham, i931.")
. Wislocki, G. B. 1935. Anat. Rec., Cz: 183.
. Becken R. F» W. F. Windle, E. E. Barth sc. M. D. Schuh. i94o. Sarg.
Gyn. s: Obst» 7o: sog.
. De snoo, K. i937. Monatschtu Gebuktsh. Gynälsp los: As.
. Iceiiketz H. i926. Gynee et Obstet., 14: I.
. Bloclh B! i921. Arch. f. DermatoL syphilis, i35: 77.
. Edwakdsh F. 1824. Traite de Pinlluence des agents physiques sur la vie,
Paris. (Cited by M. s. Pembrezs 1895.)
. Pembkezs M. s. i895. J. Physiol» is: 363. . Giaja,
1925. Amt. d. Physiol. et Phys.-chem. Biolsp i: 628. by Needham, i93i.)
(cited
1929. compt Rend. soc. Biol., tot: 71 I.
Cite this page: Hill, M.A. (2026, February 27) Embryology Book - Physiology of the Fetus 8. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Physiology_of_the_Fetus_8
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G