Book - Human Embryology and Morphology 4
| Embryology - 2 May 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Keith A. Human Embryology and Morphology. (1902) London: Edward Arnold.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Chapter IV. Development of the Organ of Hearing
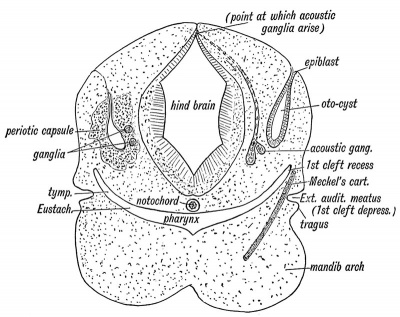
The Structures which form the Organ of Hearing.— In figure 35 is shown diagrammatically the derivation of the five elements which unite together to make up the organ of hearing. The five elements are :
- The otocyst — an area of epiblast (epithelial covering of embryo) above the first branchial cleft which becomes invaginated in a saccular form, and forms the epithelial lining of the membranous labyrinth. Some of its lining cells become differentiated into the auditory epithelium.
- A ganglion derived from the " neural crest " of the hind brain (Fig. 35). The nerve cells form the cochlear and vestibular ganglia. Each cell sends out two processes, one to become connected with the auditory epithelium of the otocyst, the other to end in groups of nerve cells in the floor of the 4th ventricle, their collective fibres forming the auditory nerve. The development of the auditory nerve thus resembles that of the posterior or sensory root of a spinal nerve.
- The otocyst (membranous labyrinth) becomes surrounded by a capsule of cartilage — the periotic capsule. This ossifies from several centres, and forms the bony labyrinth and petro-mastoid.
- The dorsal end of the first visceral cleft. — The inner recess of the 1st cleft forms the Eustachian tube, the tympanum and antrum of the mastoid ; the external cleft depression, the external auditory meatus; while out of the cleft membrane is formed the membrana tympani.
- The malleus is derived from the upper end of Meckel's cartilage, the incus from the posterior end of the palato-quadrate bar (cartilaginous skeleton of maxillary process), while the stapes is an independent formation developed round the stapedial artery. It may be derived, in part at least, from the upper end of the cartilage of the hyoid arch (see also page 10).
Fig. 35. Diagrammatic Section through the Cephalic region of an embryo, showing the origin of the Auditory System.
In lower fishes the auditory apparatus is composed of elements 1 and 2, and are in them of the simplest form. The other elements are added and specialized in the evolution of the higher vertebrates.
External Auditory Meatus
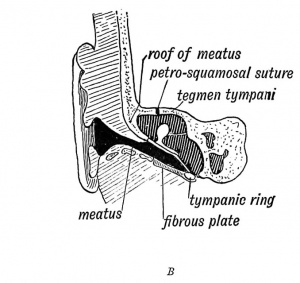
The external auditory meatus is derived from the upper part of the first cleft depression. In the adult the meatus is 1.25 in. long ; at birth it is 0.33 of an inch, and is surrounded by fibro-cartilaginous and fibrous tissue. In the adult the tympanic ring grows outwards in the fibrous tissue, as we have already seen (page IV) to form the tympanic plate and the inner two thirds of the meatal floor. The squamous part of the temporal, which is developed over in its roof, also grows outwards and forms a thick, horizontal plate in the inner two-thirds of the meatal roof (Figs. 36 A and B). Over the roof lies the third temporal convolution.

|
|
| Fig. 36 A. A Section of the External Auditory Meatus of the Adult. | Fig. 36 B. A Section of the External Auditory Meatus at Birth. (After Symington.) |
The meatus is supplied in front by the nerve of the mandibular arch (auriculo-temporal branch). Why the vagus should supply it with a branch (Arnold's nerve) is obscure. The vagus is a visceral nerve and supplies the 3rd and 4th clefts by its superior and inferior laryngeal branches. In fishes a branch of the vagus passes backwards beneath the skin on each side and supplies the sense organs of the lateral line. Many regard the auricular branch of the vagus as a vestige of such a branch.
In the newly-born child the membrana tympani is so obliquely set that its outer surface is almost in contact with the meatal floor. With the development in length of the meatus, it becomes more vertical in position. The meatus may be only partly developed or even absent, the upper part of the 1st cleft becoming completely closed like the lower part. In such a case there is commonly a corresponding absence of development of the middle and internal ear.
The External Ear
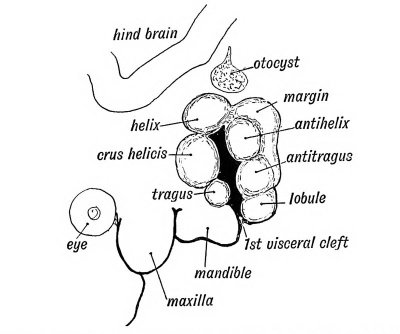
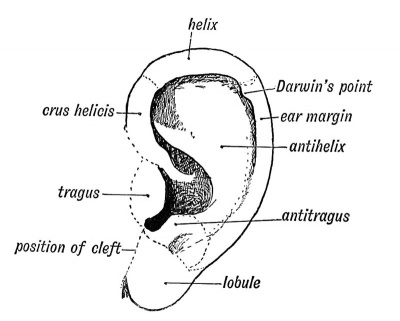
Six tubercles appear on the mandibular and hyoid arches round the 1st cleft depression and form the external ear (see Figs. 37 and 38). Two of these tubercles grow from the mandibular arch and form the tragus and crus of the helix ; three from the hyoid to form the lobule, antitragus from the tissue behind the tubercles which form the antihelix and antitragus. The auricular tubercles may not fuse completely and thus leave fistulae between them. Such fistulae are commonly seen between the tragus and root of the helix, or between the antihelix and helix. The outgrowth of the ear may be arrested at any stage. The mandibular part is supplied, as one would expect from its origin, by the third division of the 5th, while the sensory fibres of the hyoid part come from the 2nd cervical by the great auricular and small occipital nerves.
|
|
| Fig. 37. Showing the Tubercles which arise round the First Visceral Cleft to form the External Ear. | Fig. 38. Showing the part of the Adult Ear formed by each Tubercle. |
Darwin's Tubercle
The human ear appears to be derived from a form in which the margin was pointed at the posterior superior angle, such as is seen in many of the lower forms of apes and mammals generally. With the retrogression of the posterior border or descending helix and increased development of the antihelix in the human ear, the posterior margin became infolded ; hence the tip appears as a tubercle on the inturned posterior margin or welt of the human ear (Fig. 38).
Muscles of the External Ear
These are derived from the platysma sheet and are supplied by the nerve of that sheet— the Vll th or facial. The ear muscles are not so reduced in man as in some other primates, such as the orang.
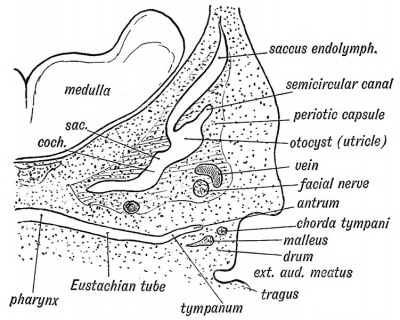
Fig. 39. Showing the condition of the Auditory Organs in a 6th week human foetus. (After Siobenmann.)
The Eustachian Tube
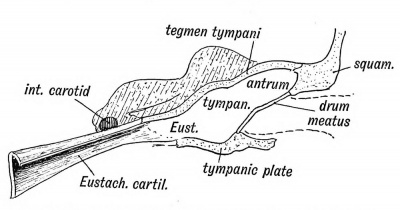
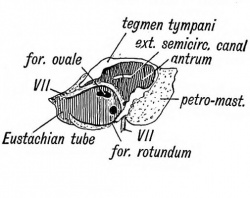
The Eustachian tube is derived from the first inner cleft recess of the pharynx (Fig. 39) and retains through life the ciliated epithelial lining of the primitive pharynx. It is 1.45 inches long. Its inner two thirds is bounded behind by a triangular plate of cartilage, which is attached at its inner or pharyngeal end to the internal pterygoid plate, a derivative, with the incus, of the pterygo-palatine bar (Fig. 10 C). The cartilage is developed in the walls of the 1st visceral cleft in the 4th month of foetal life. The tympanic plate grows inwards and forms the floor of its outer third (Fig. 40), while the periotic capsule (petro-mastoid) which is developed above and behind the 1st cleft, grows over and forms the roof of its outer third. The part of the petro-mastoid which grows over it, is the tegmen tympani ; it also forms the roof of the tympanum and antrum of the mastoid. It forms the roof of all the cavities derived from the inner recess of the first cleft (Fig. 40). The anterior edge of the tegmen tympani appears in the Glaserian fissure. The tensor tympani and tensor palati are developed on the mandibular side of the first cleft and are supplied from the nerve of the mandibular process through the otic ganglion.
Fig. 40. Showing the Cavities derived from the Inner Recess of the First Cleft.
The Tympanum
As may be seen from Fig. 39, the tympanum can scarcely be said to exist at the sixth week of foetal life. The inner cleft recess ends in the jelly-like tissue containing the cartilaginous bases of the malleus and incus. It is directed outwards and backwards between the periotic capsule to its posterior and inner side and the external cleft depression (meatus) and developing squamosal to its outer (Fig. 39). As the internal recess extends and widens outwards and backwards, the gelatinous tissue is absorbed, so that the malleus and incus and developing stapes, with the chorda tynipani, become surrounded by the hypoblasts lining of the inner cleft recess and appear to be situated within the cavity thus formed — the tympanum. The tympanic plate forms its floor, the membrana tympani and squamosal its outer wall, while the petro-mastoid forms its inner wall and roof (Fig. 40). That part of the tympanum which lies above the level of the membrana tympani is named the attic, and contains the head of the malleus and body of the incus (Fig. 36).
In carnivora and some other mammals the floor of the tympanum, formed by the tympanic plate, is inflated into a bulla, the tympanic bulla. Its meaning is unknown, but when a bulla is developed the antrum of the mastoid is small or, absent.
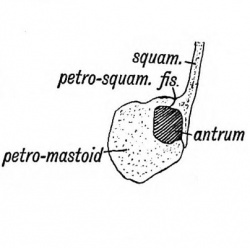
The Antrum of the Mastoid
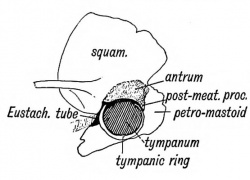
The antrum of the mastoid represents the extreme outer or posterior end of the first cleft recess (Figs. 39 and 40). It is formed at the same time and in the same manner as the tympanum. Its use is unknown, but it has frequently to be exposed by the surgeon to remove the effects of chronic middle ear disease. At birth its outer wall is formed by the thin post-auditory part of the squamosal (Figs. 41 and 42). The squamosal forming its outer wall is then only 2 mm. thick, but every year until the 20 th, or later, this plate increases nearly 1 mm. in thickness, so that by the 20th year the antrum is buried by a plate of bone about 20 mm. thick. There is a great individual variation, however, in the thickness of its outer wall. The antrum lies above and behind the level of the external auditory meatus ; the post-auditory spine and suprameatal triangle formed by the post-auditory part of the squamosal lie over it and serve as surface guides to it. The antrum opens in front into the attic of the tympanum. The tegmen tympani (Fig. 42) forms its roof and the petro-mastoid its floor and inner wall. The aqueductus Fallopii runs down the inner wall of its mouth (Fig. 43), and in its inner wall is situate the external semicircular canal. The petro-squamous suture in its roof (Fig. 42), and the masto-squamous suture on its outer wall (Fig. 14 B), become closed in the second year, and thus the escape of pus from it is rendered more difficult.
The Primitive Jugular Vein
In the petro-squamous suture a vein or sinus, frequently of considerable size, runs forwards from the lateral sinus, and commonly ends in a tributary of the middle meningeal vein. It receives as it runs along . venules from the antrum and attic and may be the means of carrying infection from the middle ear to the lateral sinus or to the meningeal veins (Cheatle). The petro-squamous sinus represents the primitive jugular vein, and may open in man, as it does in mammals generally, at the post-glenoid foramen, situated at the outer end of the Glaserian fissure, near the base of the zygoma. In the early weeks of embryonic development the primitive jugular is the chief vein from the skull, but very soon the internal jugular vein enlarges and takes its place, and thus the blood of the lateral sinus comes to pass out by the jugular foramen instead of by the petro-squamous sinus and temporal canal at the base of the zygoma.
The Membrana Tympani
As may be seen from Fig. 39, the membrana tympani is of very considerable thickness until the third month. It has an inner covering of hypoblast and an outer of epiblast. In the mesoblastic tissue between the coverings lie parts of the malleus, incus and chorda tympani. As the tympanic cavity expands the mesoblastic tissue is compressed and absorbed, and thus the handle of the malleus and chorda tympani come to appear as if they lie on the membrane, although really within it. The mucous lining of the tympanum covers them. The membrane is supported by the tympanic ring which have already been dealt with (p. arteries of the first cleft supply the drum
The Membranous Labyrinth
Origin
- (Note some text of this section is incomplete from the original scan)
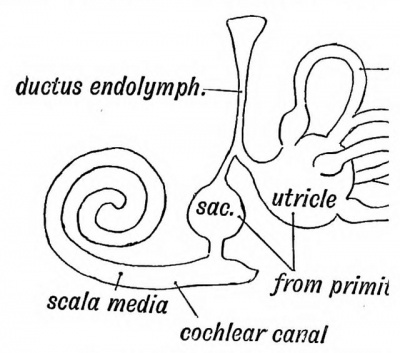
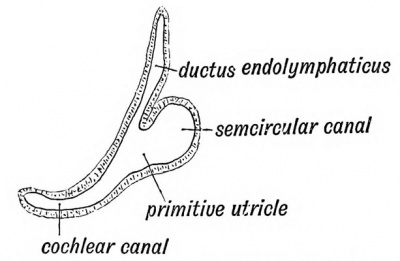
tain area of epiblast, situated above the 37), become ultimately sensitive to soun invaginated during the third week, and pyriform sac, the otocyst, which lies above in the mesoblastic tissue between the hindbrain (Figs. 37 and 39). The sac contains a flat also otoliths are found in it later. The coat of mesoblast. The epithelial cells line originally columnar, soon become flattened acousticae, where they retain the colum: hair-like processes. These become connected by the auditory nerve fibres of the cochlea. In the lower vertebrates, as in the early the higher mammals, the otocyst is of a otocyst, subdivides into the saccule and utricle (Fig. 44). The division occurs at the entrance of the endolymphatic canal, which thus comes to open into both saccule and utricle. The endolymphatic canal, which is simply the stalk of the otocyst, is enclosed in the petro-mastoid, its extremity appearing at the hiatus vestibuli, where it ends beneath the dura mater in a dilatation. It appears to be a vestige of the primitive mouth of the otocyst, which opens on the surface of the head in many fishes. Lastly, the scala media or canal of the cochlea (Fig. 44) grows out from the saccule. There is merely a rudiment of the cochlea in fishes and amphibians. In reptiles, birds, and monotremes it is a straight canal — the Lagena. Only in mammals is it arranged spirally. In it the organ of Corti is developed.
Fig. 44. Diagram of the Membranous Labyrinth
Fig. 45. The Otocyst in an Embryo of five weeks ; it shows a demarcation into the various parts of the Membranous Labyrinth.
The Petro-mastoid
Origin of the Petro-mastoid
The mesoblast surrounding the membranous labyrinth becomes cartilaginous at the end of the 3rd month of foetal life, forming the periotic capsule (Figs. 35 and 39). The tissue which immediately surrounds the membranous labyrinth does not undergo chondrification, but becomes converted into an open meshwork of cells, the intercellular spaces containing perilymph. The lymphatic space thus formed within the petro-mastoid, containing the saccule and utricle, is the vestibule. The scalae tympani and vestibuli of the cochlea are of similar origin. An oval space on the outer wall of the vestibule is not chondrified ; it contains the footplate of the stapesand forms the foramen ovale. The foramen rotundum also remains unchondrified and separates the tympanum from the: scala tympani (Fig. 43).
It will be observed that the various structures which serve to transmit the sound waves from the external auditory canal to the brain are drawn from many and very different sources. The membrana tympani is the membrane of the first cleft ; the malleus is the end of the skeleton of the first visceral arch ; the incus is part of the palato-quadrate bar; the stapes is of independent origin or drawn from the cartilage of the second arch ; the epithelial lining of the membranous labyrinth is derived from the skin ; the petro-mastoid and the perilymph spaces from the mesoblast ; the ganglia and nerves from the hindbrain.
Although the derivation of the auditory ossicles given here is the usually accepted one, there are many reasons for supposing that Gadow's views of the nature of the malleus, incus and stapes will turn out to be the right one. He regards all three bones as derived from the upper part of the hyoid arch (see page 11).
The aqueductus cochleae is the outlet of the perilymphatic space. It passes from the scala vestibuli to the anterior wall of the jugular fossa.
Ossification of the Petro-mastoid
About the end of the 4th month, four ossific centres appear in the periotic capsule; one, the pterotic, gives rise to the tegmen tympani which forms the roof of the antrum, tympanum, and Eustachian tube; the petro-squamous suture marks its outer edge ; the hiatus Fallopii marks its junction with a second centre — the opisthotic. This centre forms the posterior half of the petrous bone. The pro-otic forms the anterior half; the mastoid part, which appears on the surface of the skull, is developed from the epiotic centre.
The Mastoid
The mastoid part of the petro-mastoid is fiat at birth ; about the 2nd year the mastoid process appears as a slight knob, and it gradually grows downwards to form a cephalic lever for the sterno-mastoid, splenius and trachelo-mastoid muscles. The period of its most active growth is marked by the eruption of the permanent teeth. In most mammals the mastoid grows out as a flat wing-shaped process continuous with the occipital crest, and thus increases the basal area of the skull on which the neck muscles are inserted. The post-auditory process of the squamosal forms a considerable part of the mastoid process; it reaches to the apex and forms the anterior border (Fig. 14(7). As the mastoid process grows the diploic spaces within it enlarge into air spaces. Those round the antrum come to open into it, but the more distal remain closed. These spaces occupy the whole of the mastoid part of the temporal, but they also extend forwards in the post-auditory process of the squamosal, and may spread backwards to the occipital.
Three varieties of mastoids are recognised :
- Dense processes in which the air cells are minute or absent ;
- Containing numerous large spaces (pneumatic) ;
- An intermediate type with large cells round the antrum, and a few small ones near the surface. The third type is the commonest.
The Floccular or Subarcuate Fossa
At birth there is a fossa situate on the posterior aspect of the petro-mastoid. It is filled with a process of the dura mater, and over it, but not within it, the flocculus is situated. The posterior semicircular canal surrounds the fossa. This is the condition in most mammals throughout life, but soon after birth the fossa becomes closed in man, merely a remnant being seen above and internal to the hiatus vestibuli in the bone of the adult. Its meaning is unknown.
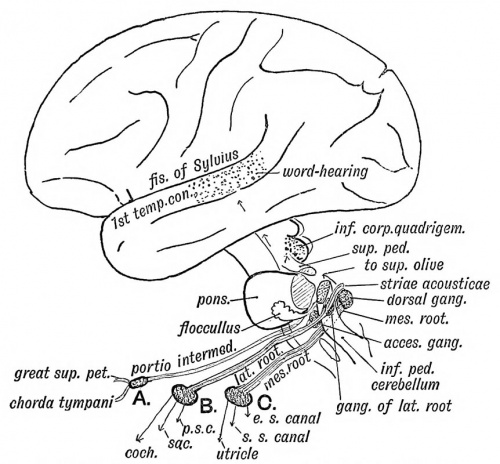
The Acoustic Ganglia
It has already been shown (Fig. 35) that the mass of nerve cells which come in contact with the otocyst arise from the neural crest of the hind brain in the same manner as the ganglion of a posterior nerve root. The mass of nerve cells is divided into three groups (A, B, C, see Fig. 46).
| One becomes the geniculate ganglion (A) ; in the formation of the petro-mastoid it is included in the aqueductus Fallopii. Its cells give rise to the great superficial petrosal nerve and chorda tympani in the same manner as the ganglion of a posterior root produces the sensory fibres of a spinal nerve (Dixon).- The pars intermedia, in part at least, represents the afferent or ingrowing root of the ganglion.
Each cell of the ventral acoustic ganglion (group B) sends efferent processes to the organ of Corti in the cochlea and the acoustic cells of the saccule and posterior semicircular canal and afferent fibres which form the dorsal or lateral root of the auditory nerve and run to the nerve cells in the opposite side of the medulla oblongata. Some of these fibres form the striae acousticae.
|
Fig. 46. Showing the Nerve Structures concerned in the Sense of Hearing. |
By uncertain tracts the central auditory terminations in the medulla are connected with : —
- The cerebellum ;
- The superior olive ;
- The posterior corpora quadrigemina ;
- The middle third of the 1st temporal convolution in which is situated the "word-hearing" centre (Fig. 46).
Internal Auditory Meatus
The internal auditory meatus is formed round the Vlllth nerve, its ganglia, and the Vllth nerve. The falciform crest separates the fibres of the lateral from the fibres of the mesial root. The meatus also contains a prolongation of the arachnoid and subarchnoid space. Fractures of the base of the skull frequently cross the petro-mastoid in the line of the internal auditory meatus, vestibule and membrana tympani. In such cases the cerebro-spinal fluid and perilymph may escape by the external auditory meatus.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Human Embryology and Morphology (1902): Development or the Face | The Nasal Cavities and Olfactory Structures | Development of the Pharynx and Neck | Development of the Organ of Hearing | Development and Morphology of the Teeth | The Skin and its Appendages | The Development of the Ovum of the Foetus from the Ovum of the Mother | The Manner in which a Connection is Established between the Foetus and Uterus | The Uro-genital System | Formation of the Pubo-femoral Region, Pelvic Floor and Fascia | The Spinal Column and Back | The Segmentation of the Body | The Cranium | Development of the Structures concerned in the Sense of Sight | The Brain and Spinal Cord | Development of the Circulatory System | The Respiratory System | The Organs of Digestion | The Body Wall, Ribs, and Sternum | The Limbs | Figures | Embryology History
Reference
Keith A. Human Embryology and Morphology. (1902) London: Edward Arnold.
Cite this page: Hill, M.A. (2024, May 2) Embryology Book - Human Embryology and Morphology 4. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Human_Embryology_and_Morphology_4
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G