Book - Developmental Anatomy 1924-9
| Embryology - 27 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Arey LB. Developmental Anatomy. (1924) W.B. Saunders Company, Philadelphia.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Chapter IX The Vascular System
Origin Of The Blood Vessels And Blood Cells
Both the primitive blood cells and blood vessels arise from a tissue termed the angioblast. Its germ-layer origin has long been disputed, but the majority of recent investigations agree on the mesoderm. In certain regions, such as the body stalk of human embryos, any other interpretation is precluded. The angioblast consists initially of isolated, solid cords and masses of cells which appear first in the splanchnic mesoderm of the body stalk and yolk sac (Fig. 43). These strands soon hollow out, the peripheral cells forming the flattened endothelium of the primitive vessels, the inner cells, bathed by a clear fluid, persisting as the primitive blood cells (Fig. 326). At intervals, clusters of the latter elements adhere to the sides of the vessels and constitute the temporary blood islands (Figs. 44 and 323).
By the growth and union of the isolated spaces, the original anlages are converted into a vascular plexus which is present on the yolk sac, body stalk, and chorion of human embryos of i mm. In the wall of the yolk sac this network comprises the area vasculosa which later envelops the entire sac.
The first vessels within the embryo itself appear at about 1.5 mm. Many have held that they develop as continuations of the extra-embryonic angioblast which progressively invade the embryo, but it is now agreed that the fundamental origin of intra-embryonic vessels is from discrete local anlages like those on the yolk sac. Growth by sprouting, rapidly extends the primitive vascular channels.
.
Hemopoiesis
Two sharply contrasted views are held as to the mode of origin (hemopoiesis) of the various blood elements. According to the monophyletic theory, a common mother cell gives rise to all types of blood elements, both red and white. The polyphylctic theory, on the contrary, asserts that the erythroplastids are derived from one mother cell while the several kinds of white cells trace their ancestry to one or more distinct stem cells. The total evidence favors the monophyletic view.
The earliest blood cells that originate from the angioblast are viewed by some as the parent elements from wTich all later blood cells are derived. Although it is recognized that various organs of the embryo successively serve as blood-forming centers, they are interpreted as mere depots where the primitive angioblastic cells are first deposited from the circulating blood and subsequently proliferate. On the contrary, it is urged by many that there is evidence of the continued new formation of blood cells from the mesenchyme and endothelium of the embryo and from the connective tissue of the adult. This is the more popular interpretation.
The primitive blood cells multiply rapidly by mitosis, and differentiate successively in the following locations; (i) yolk sac; (2) mesenchyme and blood vessels of the embryo; (3) liver and spleen (assisted by lymphoid organs in lymphocyte production) ; (4) bone marrow. There is a certain degree of overlap in the activities of these foci, which, one by one, give up blood formation until the red marrow alone remains as the permanent source of all types of blood cells. Yet every lymphoid organ continues the production of lymphocytes throughout life, and in certain diseases the spleen assumes again its full hemopoietic function.
The primitive blood cell has been given various names, such as mcsameboid, primary lymphocyte, and hemoblast. It shows a large, vesicular nucleus surrounded by a small amount of finely granular cytoplasm (Fig. 1 61, a). There is no distinct cell membrane and the cell is assumed to be ameboid. From such parent cells, according to the monophyletic view, all blood elements arise. Specialization proceeds in divergent directions; one line leads to the red corpuscles, the other to the leucocyte series.
Fig. 161. Blood cells from luiman embryos (Prentiss). X 1160. a. Primitive hemoblasts; h, mcgaloblasts; c, d, e, normoblasts: /, erythrocytes, (a-c, 12 mm.; d-f, 20 mm.) .
Fig. 162. Human blood cells (Todd). X 1000. i, Erytliroplastid; 2, normoblasts; 3, megaloblast and normoblast; 4, blood platelets, one lying on a red corpuscle; 5, lymphocytes, large and small; 6, 7, large mononuclear leucocytes, polar and profile views; 8, neutrophilic leucocytes; 9, eosinophilic leucocytes; 10, basophilic leucocyte; u, neutrophilic myelocyte; 12, eosinophilic megalocyte; 13, basophilic myelocyte.
Origin of the Erythrocyte
The red blood corpuscles, arising from the hemoblast type of cell, are first formed in the mesenchyme and blood vessels of the e/mbryo and then in the liver, spleen, and bone marrow. Soon after birth, the red marrow is the only normal source of new corpuscles. In each of these sites the manner of transformation from the parent hemoblast is identical. There are recognized three principle stages : .
1. Megaloblasts. These are sometimes called erythroblasts and they have also been termed ichthyoid blood cells, because of their resemblance to the typical red blood cell of fishes. They are characterized by the presence of hemoglobin in the homogeneous cytoplasm, which is thus colored red. The nuclei are veSicular, with granular chromatin (Figs. 16 1, b and 162, 3). There is a definite cell membrane. For the first six weeks of development (12 mm.) the megaloblast is the only red blood cell found, and, like its progenitor, multiplies in the circulating blood. After the third month it practically disappears from the blood stream.
2. Normoblasts, also termed sauroid blood cells because they resemble the red blood cells of adult reptiles and birds, are first transformed in the liver from the megaloblasts, and are predominant in embryos of two months. They are distinguished by their small, round nuclei with dense chromatin which stains so heavily that little or no structure can be seen (Figs. 16 1, c, d, e and 162, 2, 3). The cytoplasm is large in amount and contains more hemoglobin than before, but the normoblast may still undergo corpuscles in the cat mitosis. The final state is often listed as a (Howell), a, Successive stages in separate stage, the erythrohlast. Until the the development of a normoblast; seventh month many normoblasts occur in ^^trusion of the nucleus.
The circulating blood.
3. Erythrocytes (red blood corpuscles; erythroplastids) are developed in mammals from normoblasts which lose their nuclei. The way in wTich the nucleus disappears is disputed. It is usually said to be extruded as a whole or in fragments (Fig. 163), but some claim it is absorbed and others state that the cytoplasm buds away from the nucleated remnant.
The first red blood corpuscles are spherical and are formed during the second month, chiefly in the liver. During the third month, the enucleated erythrocytes predominate (Fig. 161, /). Although usually cup-like in preserved material, their normal adult shape is that of a biconcave disc about 7.5 m in diameter. Mature erythroplastids are believed to exist not more than a month.
Origin of the Leucocytes. - The white blood cells are divided into non-granular and granular groups (Fig. 162). According to the monophyletic view, it is held that both types are derived from the hemoblastic mother cells.
I. Non-granular Leucocytes:
1. Lymphocytes are ordinarily about the size of a red corpuscle but some are twice as large ( Fig. 162, 5). The small lymphocytes are supposed to be the daughter cells of large lymphocytes; the large are the small ones grown up. Their spherical nucleus, containing numerous small masses of chromatin, stains darkly and is surrounded by a narrow zone of clear, faintly baso])hilic cytoplasm. Lymphocytes constitute from 22 to 25 per cent of the leucocytes in adult Idood and are developed both in the marrow and in the lymphoid organs.
2. Large monomiclcar leucocytes are two or three times the size of a red corpuscle (Fig. 162, 5, 6). They possess a clear nucleus, usually indented, and considerable faintly basophilic cytoplasm. The large mononuclears are notably phagocytic. They comprise i to 3 per cent of all leucocytes and are developed from the reticular cells of lymph glands, and, perhaps, from endothelium as well.
Fig. 164. (lianl cell from the bone marrow of a kitten, showing pseudopodia extending into a blood vessel (F), and giving rise to blood platelets {hp) (Wright).
II. Granular or Polymorphonuclear Leucocytes:
The generalized blood-forming cells lodged in the red bone marrow also give rise to myelocytes, cells with round or crescentic nuclei and granular cytoplasm (Fig. 162, 11-13). By undergoing changes in the form and structure of their nuclei, and in the size and staining qualities of their cytoplasmic granules, the myelocytes transform into three types of granular leucocytes; .
1. Neutrophils (70 to 72 per cent of all leucocytes; Fig. 162, 8). These have a hnely granular cytoplasm which is neutral in its staining reactions, coloring by the interaction of both acid and basic stains. In development, their nuclei take up an eccentric position and become crescentic, horse-shoe shaped, and, in the older stages, lobate. As it changes in form, the nucleus undergoes pyknosis and stains intensely.
2. Eosinophils (2 to 4 per cent of all leucocytes; Fig. 162, 9). These are characterized by coarse cytoplasmic granules that stain intensely with acid dyes. The granules apparently differentiate intracellularly although they have been interpreted as ingested fragments of red corpuscles or muscle. In development the nucleus becomes bilobed.
3. Basophils or Mast Leucocytes (0.5 per cent of all leucocytes; Fig. 162, 10). Their nuclei are very irregular in form and may be broken down into several pieces which stain intensely. The cytoplasmic granules are variable in number, size, and form, and often stain so heavily with basic dyes as to obscure the nucleus. Basophiles are often regarded as degenerating granular leucocytes, but this view is not entirely convincing. They are distinct from the - mast cells - of the tissues.
Origin of the Blood Platelets. - In the bone marrow are giant cells known as megakaryocytes, the cytoplasm of which shows a darkly-staining, granular endoplasm and a clear, hyaline ectoplasm (Fig. 164). They originate like leucocytes but follow a distinct course of specialization. It has been demonstrated that the blood platelets represent the tips of cytoplasmic processes which have been detached from the giant cells. The central granular mass of the platelets represents a portion of the endoplasm. Genuine giant cells and blood platelets occur only in mammals.
Development Of The Heart
The heart of the lower fishes and of amphibians develops directly within the ventral mesentery of the fore-gut. A tubular cavity first appears, about which the cells differentiate into endo-, myo-, and epicardium.
.While the embryo of bony fishes, reptiles, birds, and mammals is still flattened on the surface of the yolk, paired heart anages arise which secondarily grow mesad and fuse. These anlages are first composed of aggregates of mesodermal cells which appear between the entoderm and splanchnic mesoderm; such paired cellular masses are present in the Spee 1.54 human embryo (Fig. 43). They soon form thin-walled endothelial tubes and are flanked by folds of splanchnic mesoderm that bulge laterally into the coelomic cavity (Figs. 165 A and 327). As the embryo grows away from the yolk and the fore-gut is formed, the entoderm withdraws from between the endothelial tubes, allowing first these and then the mesodermal folds to fuse (Figs. 165 5 , C; 328 and 329).
The heart is now a single endothelial tube, lying in the folds of the splanchnic mesoderm (Fig. ui A). When the ventral mesenterial attachment presently disappears, the heart is left suspended by a temporary dorsal mesocarduim in a common pericardial chamber (Fig. 165 C). The endothelial tube forms the endocardium; the splanchnic mesoderm later gives rise to the epicardium and myocardium. This type of heart occursin human embryos of 2 mm. ( 5 or 6 somites. Fig. 1 66) and shows three regions; (i) the atrium, which receives the blood from the primitive veins; (2) the ventricle; (3) the Imlh, from which is given off the ventral aorta.
As the cardiac tube soon grows faster than the pericardial cavity in which it lies, it bends to the right, thereby throwing the bulbus and ventricle into a U-shaped loop (Fig. 167). Four regions may then be distinguished ; ( i ) the sinus Eel. venosus; (2) the atrium, also thinwalled and lying cranial to the Ent. sinus; (3) the ihick-waXled ventricular limb, ventrad and caudad in position; (4) the bulbar limb, cranial to the ventricular limb and separated from it by the bulbo-ventricular cleft. Next, the bulbo-ventricular loop further shifts its position until its base is directed caudad and the loop as a whole lies ventrad (Fig. 167 B). At the same time, the sinus venosus is brought dorsal to the atrium and the two assume a position cephalad of the bulbo-ventricular loop (Fig. 168 A). These changes thus result in an essential reversal of the primitive positional relations.
Fig. 165. Diagrams to illustrate the origin of Fig. 166. - The heart of a a mm. The mammalian heart. Eel., Ectoderm; End., endo- human embryo in ventral view (Mall), thelial tubes; Ent., entoderm; Eg., fore-gut; MscD., X 65. The open tube is the fore-gut. dorsal mesocardium; MsSpl., splanchnic mesoderm (epi- and myocardium).
The right fDortion of the sinus venosus now begins to grow more rapidly than the left, this being due to a shift in the flow of blood from the left umbilical vein through the liver to its right side. As a result, the enlarged right horn of the sinus opens into the right dorsal wall of the atrium through a longitudinally oval foramen, guarded on each side by valve-like folds (Fig. 170). The atrium is constricted dorsally by the gut, ventrad by the bulbus. It therefore can enlarge only laterally, and in so doing forms sacculations which become the future right and left atria (Fig. 168 A, B) the deep, external groove between the atria and the bulbo-ventricular part of the heart is the coronary sulcus. As the bulboventricular region increases in size, the duplication of the wall between the two limbs lags in development and finally disappears (Fig. 169), leaving the proximal portion of the bulb and the ventricular limb to form a single chamber, the primitive ventricle. In an embryo of 5 mm., the heart is thus composed of three undivided chambers: (i) the sinus venosus, opening dorsad into the right dilatation of the atrium; (2) the, bilaterally dilated atrium, communicating by the single transverse atrial canal with (3) the primitive undivided ventricle. The three-chambered heart is persistent in adult fishes, but in birds and mammals a four-chambered heart is developed, in which venous blood circulates on the right side and arterial l:)lood on the left. In amphibians and reptiles, transitional types occur.
Fig. 167. Ventral views of the early human heart (His). A, 2.15 mm.; B, 3 mm.
Fig. 168. Ventral views of the early .
The important changes next to be considered, leading to the formation of the four-chambered heart, are: (i) the complete partitioning of the atrium and ventricle, each into right and left side chambers; (2) the incorporation of the sinus venosus into the wall of the right atrium; (3) the longitudinal division of the bulb and its distal continuation, the truncus arteriosus, into the aorta and pulmonary artery; (4) the development of the semilunar and atrio-ventricular valves. The heart of an embryo of two months has attained its general structural characteristics.
Fig. 169. - The incorporation of the bulbus into the right ventricle through the slower development of the bulbo-vcntricular fold.
Origin of the 'Right and Left Atria. - In human embryos of 6 mm. there develops a thin, sickle-shaped membrane from the mid-dorsal wall of the atrium (Figs. 170 and 171). This is called the septum primnm (I), for it grows toward the ventricle as a partition. Simultaneously, endothelial thickenings appear in the dorsal and ventral walls of the canal which connects atrium with ventricle (Figs. 171 A, B). These endocardial cushions later fuse, and divide the. single atrial canal into right and left atrio-ventricular canals (Fig. 176). The atrium is now partly divided into right and left atria, which, however, still communicate ventrad through the interatrial Joranien. Next, the septum I thins out in one region, and a secondary opening, the foramen ovale, appears there (Figs. 170 and 171 B). The atria are then connected by two openings, the oval and interatrial foramina. Soon, the ventral and caudal edge of septum / fuses with the endocardial cushions, which have in turn united with each other (Figs. 170 and 1 7 1 C). The temporary interatrial foramen is thus obliterated, but the foramen ovale persists until after birth. In â– embryos of 9 mm., the septum secundum ( 11 ) is developed from the dorsal and cephalic wall of the atrium, just to the right of the septum primum (Fig. 170 (T). It is important, as it later fuses with the left valve of the sinus venosus, whence the two join with septum I to complete the atrial septum of the late fetal and adult heart.
Fate of the Sinus Venosus and its Valves . - The opening of the sinus venosus into the dorsal wall of the right atrium is guarded by a right and left valvular fold (Fig. 170). Along the dorsal and cephalic wall of the atrium these unite to form the so-called septum spuruim; caudally, the valves flatten out on the floor of the atrium. In embryos of six to eight weeks, the atria increase rapidly in size and the lagging right horn of the sinus venosus is taken up into the wall of the right atrium. By this absorption the superior vena cava of necessity drains directly into the cephalic wall of the atrium, the inferior vena cava into its caudal wall (Fig. 1 71 C). The transverse portion of the sinus venosus, persisting as the coronary sinus in part, likewise opens into the posterior wall of the atrium (Figs. 173 and 174).
Fig. 170. Horizontal sections through the chambers of the human heart (adapted by Prentiss). X about 50. A, 6 mm.; B, 9 mm.; C, 12 mm.
Fig. 171. Lateral dissections of the human heart, viewed from the left side (Prentiss). X about 38. A, 6 mm.; B, 9 mm.; C, 12 mm. Cor. sin., Coronary sinus; D. end. c., dorsal endocardial cushion; For. ov., foramen ovale; Int. for., interatrial foramen; I. v. c., inferior vena cava; L. air., left atrium; L. va. s. v., left valve of sinus venosus; L. vent., left ventricle; Pid. a., pulmonary artery; Pul. v., pulmonary vein; Sept. I, Sept. u, septum primum, septum secundum; Sup. V. c., superior vena cava; V. end. c., ventral endocardial cushion.
Fig. 172. - Lateral dissection of the heart of a three-months - fetus, viewed from the right side (Prentiss). X 12 .
The right valve of the sinus venosus is very high until the end of the third month and nearly divides the atrium into two chambers (Fig. 172), but later it diminishes greatly in relative size. Its cephalic portion becomes the rudimentary crista terminalis (Fig. 173); the remainder is divided by a ridge into two parts, of which the larger cephalic division persists as the valve of the inferior vena cava (Eustachian valve), located at the right of the opening of the vein, and the smaller caudal portion becomes the valve of the coronary sinus (Thebesian valve).
Fig. 173. - Lateral dissection of the heart of a four-months - fetus, viewed from the right side (Prentiss). X 7.
The left valve of the sinus venosus unites with the septum u, and, after the second month, the two bound an oval opening whose rim is the limbus ovalis (Figs. 173 to 175).
Fig. 174 - Lateral dissection of the heart of a three-months - fetus, viewed from the left side (Prentiss). X 8.
Fig. 175. - Lateral dissections of the human heart, viewed from the left side (Prentiss). A , two months; B, four months. Bic. va., Bicuspid valve; Cor. sin., coronary sinus; For. ov., foramen ovale; I.v.c., inferior vena cava; L. atr. vent, c., left atrio-ventricular canal; L. vent., left ventricle; Pul. a., jiulmonary artery; Sept. I, Sept. u, septum primum and septum secundum.
Closure of the Foramen Ovale. - The growth of the primitive atrial septa proceeds in such a manner that the free edge of septum u overlaps the foramen ovale in septum I (Figs. 171 C, 174 and 175). During fetal life the left atrium receives little blood from the lungs, so that the pressure is much greater in the right atrium. As a result, the septum I is pushed to the left and the blood flows from the right into the left atrium through the foramen ovale. After birth, the left atrium receives from the expanding lungs as much blood as the right atrium, hence the septum I is pressed against the limbus of the previously fused septum u and left sinus valve, and unites with it. The depression formed by the thinner walled septum I is the fossa avails.
The Pulmonary Veins. - In embryos of about 6 mm., a single vein drains into the caudal wall of the left atrium at the left of the septum I { Fig. I 7 1 C) . This vessel bifurcates into right and left pulmonary veins which in turn divide so that two branches extend to each lung. fVs the atrium grows, these pulmonary vessels are progressively taken up into the atrial wall. As a result, at first two, then four pulmonary veins open into the left atrium.
Origin of the Aorta and Pulmonary Artery. - In embryos of 5 mm. there arise in the aortic bulb (including its distal truncus arteriosus) longitudinal thickenings, four in the distal half, two in the proximal half. Of the four distal thickenings (Fig. 176), two, which may be designated a and c, are larger than the other thickenings, b and d. Thickenings a and c, which distally occupy left and right positions in the bulb, meet, fuse, and divide the bulb into a dorsally placed aorta and ventrally placed pulmonary trunk (Fig. 177). Traced proximally, they pursue a clockwise, spiral course, a shifting from left to ventral, and c from right to dorsal, both becoming continuous with the proximal swellings. Thickenings b and d are also prominent at one point proximally; Avhen the bulb in this region is divided by ingrowing connective tissue into the aorta and pulmonary artery, the aorta contains the whole of the thickenings b and half of a and c, while the pulmonary trunk contains the whole of d and half of a and c (Fig. 176). Distally, the three thickenings now present in each vessel disappear, but proximally they enlarge, hollow out on their distal surfaces and eventually form the thin-walled 5c?n?7«»ar ra/t'C5 (Figs. 172 and 176). The anlages of these valves are prominent in embryos of six to seven weeks as clump swellings projecting into the lumina of the aorta and pulmonary artery.
Fig. 176. - Scheme showing the division of the bulbus and its thickenings into aorta and pulmonary artery with their valves.
The two proximal bulbar swellings, continuous with a and c, fuse and extend the spiral division of the bulb toward the interventricular septum in such a way that the base of the pulmonary trunk, now ventrad and to the right, opens into the right ventricle, while the base of the aorta, now lying to the left and dorsad, opens into the left ventricle (Fig. 1 77 i?).
Fig. 177. - Ventral views of the human heart, showing the division of the bulbus and ventricle (Kollman). A, 5 mm.; B, 7.5 mm.
Origin of the Right and Left Ventricles. - Coincident with the division of the aortic bulb there appears at the base of the primitive ventricular cavity a sagittally placed elevation, the interventricular septum (Fig. 170 5 ). It grows toward the endocardial cushions, and temporarily forms an incomplete partition between the right and left ventricles which still communicate through the persisting interventricular foramen (Fig. 177 B). Corresponding to the internal attachment of the septum, there is formed externally the interventricular sulcus (Fig. 177 A); this marks the external line of separation between the large left ventricle and the smaller right ventricle. The interventricular foramen in embryos of seven weeks is bounded: (i) by the interventricular septum; (2) by the proximal bulbar septum; and (3) by the dorsal portion of the fused endocardial cushions (Fig. 177). Soon these structures are approximated and fuse, thereby forming the septum memhranaceum, which closes the interventricular foramen and completes the partition.
Loosely-arranged muscle bundles compose the uniformly spongy wall of the early ventricle (Fig. 178 D). Soon there is a condensation, especially at the periphery. As a result, the tissue next the surface becomes compact, whereas the muscular cords near the lumen retain an open arrangement for a longer period (Fig. 178 B). Some cords are attached to the anlages of the atrio-ventricular valves. These latter arise as thickenings of the endocardium and endocardial cushions, about the atrio-ventricular foramina (Figs. 170 and 171). Three such flaps are formed on the right, two on the left. The size of the primitive valvular cusps is presently increased by an undermining process whereby the muscular cords beneath become less numerous and wider spaced (Fig. 1 7 8 S) . Degeneration ensues both in the muscle tissue of the valve anlages and in that of the subjacent muscle cords. As a result, the valve cusps become fibrous and connect with similarly transformed chordce tendinece, which in turn continue into the unaffected papillary muscles. Thus there are developed the three cusps of the tricuspid valve between the right chambers of the heart (Fig. 173) and the two flaps of the bicuspid {mitral) valve between the left chambers (Fig. 174). The irregular muscle bundles that ])ersist next the ventricular cavities constitute the trabecula: carnce.
Fig. 178. - Diagrams of the development of the ventricular wall and atrio-ventricular valves.
Differentiation of the Heart Wall. - The primitive folds of splanchnic mesoderm form both the thick myocardium, with its specialized type of muscle, and the serous epicardial coat. The myocardial layers, at first continuous over the surface of the heart, become divided by connective tissue at the atrioventricular canal, leaving a small bridge alone. 'I'his connecting strand, located behind the posterior endocardial cushion, is the atriovaitriadar hiuidlc. The endothelial lining becomes the chief constituent of the endocardium. Originally a simple sac, it later dips between the trabeculae and wraps about the papillary muscles.
Fig. 179. - The caudal end of a chick embryo of 32 somites (Evans). The sciatic artery will differentiate from the primary capillary plexus of each limb Irud; aortae have already formed from the mesial margins.
Descent of the Heart. - At first the heart lies far cephalad in the cervical region, but it gradually recedes during development until it assumes a permanent position in the thorax. This migration is attested in the adult by the courses of the recurrent and cardiac nerves. After the diaphragm reaches its final location (Fig. 1 19), the heart rotates so that the ventricles, which previously were ventral to the atria, now become caudal.
Anomalies. - Dextrocardia is associated with a general transposition of the viscera (p. 1 1 8). The aorta and pulmonary artery may also be transposed in the absence of dextrocardia. Rarely, the paired anlages form a double heart. Of the complete or partial defects of the septa, most common is a patent foramen ovale. If the foramen fails to close after birth, the mixed blood produces a purplish hue in the child which is known popularly as a - blue baby. - This condition may be persistent in adult life. Incomplete closure occurs in about one in four cases, but actual mingling of the blood is rare, due to an approximation of the overlapping septal folds during atrial contraction. Valvular anomalies occur ; those of the semilunar valves result from an atypical division of the bulbus,
The Primitive Vascular System
The vascular system of all higher mammals develops precociously. This is due to the absence of nutritive yolk, and the consequent need of vessels that will extract nourishment and oxygen from the maternal circulation and distribute them to the tissues of the embryo.
Delicate injections show that capillary plexuses precede the formation of definite arterial and venous trunks (Fig. 179). Only by the selection, enlargement, and differentiation of appropriate paths do the definitive vessels arise, whereas those capillaries from which the flow has been diverted, atrophy. Both inheritance and the hydrodynamic factors incident to the blood flow participate in the selection of channels from the capillary bed,
Fig. 180. Diagram, in lateral view, of the blood vessels in human embryos of 1.5 to 2 mm.
The first paired vessels of human embryos are formed as longitudinal anastomoses of capillary networks that originate first in the angioblast of the yolk sac and chorion (p. 169). In the Eternod embryo of 1.3 mm., in which the somites are still undeveloped, such paired vessels are already formed (cf. Fig. iSo). The umbilical veins emerge from the chorion, fuse in the body stalk, then, separating again, course in the somatopleure to the paired, tubular heart anlages. From the heart tubes, paired vessels, the ventral aorta:, extend cephalad, then bend upward around the first aortic arches and continue caudad as the descending aorta: . The latter give off the umbilical arteries which bend sharply ventrad into the body stalk and branch in the wall of the chorion. The chorionic circulation is thus the first to be established in embryos 2 to 2.5 mm. long (5 to 8 somites), the heart has become a single tube (Fig. 181). From the yolk sac, numerous veins converge cephalad and form a pair of vitelline veins. These join the umbilical veins, whereupon the combined vitcllo-umhilical trunks traverse the septum transversum and open into the sinus venosus. The cranial portions of the descending aortas give off several pairs of dorsal intersegmental arteries, the caudal portions a ventral series of vitelline arteries to the yolk sac. The umbilical arteries now take their origin from a plexus of ventral vessels, in series with the vitelline arteries. At this stage, the vitelline circulation of the yolk sac is established,
Fig. 181. Diagram, in lateral view, of the blood vessels in human embryos of 2 to 2.5 mm.
Fig. 182. Diagram, in lateral view, of the blood vessels in a human embryo of 2,6 mm. (Felix-Prentiss).
Fig. 183. Ventral reconstruction of the blood vessels in a 3,2 mm. human embryo (His).
Fig. 184. Lateral reconstruction of the blood vessels in a 4.2 mm. human embryo (His). nix., Maxillary process; jv., precardinal vein; cv., postcardinal vein; ot., otocyst.
In embryos of 15 to 23 somites (Fig. 182), the veins of the embryo proper develop as longitudinal anastomoses of branches from the segmental arteries. The paired precardinal (or anterior cardinal) veins of the head are developed first (Fig. 18 1); coursing back on either side of the brain, they join the vitello-umbilical trunk. In embryos of 23 somites, the postcardinals are present (Fig. 182). They lie dorsal to the nephrotomes, and, running cephalad, join the anterior cardinal veins to form the common cardinal veins. Owing to the later enlargement of the sinus venosus, the proximal portions of the common venous trunks are taken up into its wall, and thus three veins open into each horn of the sinus venosus: (1) the umbilical veins from the chorion; (2) the vitelline veins from the yolk sac; (3) the common cardinal veins from the body of the embryo. The descending aorta: fuse below the level of the seventh intersegmental arteries and form a single dorsal aorta as far caudad as the origin of the umbilical arteries. Of the numerous vitelline arteries, one pair is prominent; its halves unite into a single vessel which courses in the mesentery and later becomes the superior mesenteric artery. By the enlargement of capillaries connecting the ventral and dorsal aorta}, a second pair of aortic arches is formed at this stage,
Fig. 185. Lateral reconstruction of the arteries and cardinal veins in a 4.9 mm. human embryo (Ingalls-Prentiss). X 20.
In embryos 4 to 5 mm. in length, five pairs of aortic arches are successively developed: the first, second, third, fourth, and sixth (Figs. 184 to 185). An additional pair of transitory vessels, which extend from the ventral aorta to the sixth arch, appear later in embryos of 7 mm., but soon degenerate (Fig. 186 B). They are interpreted as being the fifth pair in the series. From each dorsal, or descending aorta there develop cranially the internal carotid arteries (Fig. 184). These extend toward the optic stalks where they bend first dorsad and then caudad, and connect finally with the first intersegmental arteries of each side (Fig. 185). The descending aortae are now fused to their extreme caudal ends and the umbilical arteries thereby originate from the single vessel. Twenty-seven pairs of dorsal intersegmental arteries are jjresent ; from the seventh cervical pair, the subclavian arteries of the upper limbs arise. Of the ventral vitelline vessels, three are now prominent : the coeliac artery in the stomach pancreas region, the superior mesenteric in the small-intestine region, and the inferior mesenteric of the large-intestine region.
Fig. 186. Reconstructions of the human aortic arches and pharyngeal pouches (Tandler; .A, 5 mm.; B, 7 mm.
The embryonic plan of primitive vessels is altered profoundly in later stages. The sections that follow will describe these changes in detail.
Development Of The Arteries
Transformation of the Aortic Arches
Both the ventral and descending aortae, and the ancestral aortic arches which interconnect them (Fig. 186 A), are early transformed into more appropriate vessels. In embryos of 7 mm., the first and second pairs of aortic arches drop out (Figs. 186 B and 187), but the subjacent ventral aortae persist as the external carotid arteries; similarly, the descending aortse at this level, together with the third aortic arches, become the internal carotids. The continuations of the ventral aorte between the third and fourth arches remain as the common carotid stems, whereas the corresponding segments of the descending aortae obliterate. The fourth pair of aortic arches are important ; the left is converted into the permanent aortic arch: on the right side, the fourth arch persists with the descending aorta as far as the seventh intersegmental artery and forms the first part of the right subclavian artery, which is thus a more complex vessel than its mate. The segment of the fourth arch proximal to the right common carotid becomes the innominate artery. The fifth arches of amniotes are rudimentary (p. 188). On the right side, the distal portion of the sixth arch is lost; on the left, it persists as the ductus arteriosus and its lumen is obliterated only after birth. The proximal portion of the right sixth arch forms the stem of the right pulmonary artery, but the proximal portion of the left arch is incorporated in the pulmonary trunk. Most of the pulmonary artery arises from a postbranchial plexus whose union with the sixth arch is acquired secondarily (Huntington, 1919). In 15 mm. embryos, the primitive bulbus cordis has been divided into distinct aortic and pulmonary trunks which open respectively into the left and right ventricles,
Fig. 187. Diagram, in ventral view, of the transformation of the human aortic arches.
The aortic arches of the embryo are of especial importance comparatively. Five arches are formed in connection with the functional gills of fishes. In adult tailed amphibia, three or four arches, and in some reptiles, two arches, are represented on either side. In birds the right, in mammals the left fourth arch persists as the arch of the aorta.
The different courses of the recurrent laryngeal nerves are easily explained. The vagus early gives off paired branches which reach the larynx by passing caudal to the primitive fourth aortic arches. When the latter, through growth changes, descend into the chest, loops of both nerves are carried with them. Hence, after the transformation of the fourth arches, the left recurrent nerve remains looped around the arch of the aorta, the right around the right subclavian artery (cf. Fig. 187).
Branches of the Dorsal Aorta. - From each primitive aorta arise dorsal, lateral, and ventral branches in three paired longitudinal series (Fig. 188); .
Fig. 188. Transverse section of the trunk, illustrating the arrangement of the segmental aortic branches.
I. The dorsal branches are intersegmental in arrangement and develop small dorsal and large ventral rami.
From the dorsal rami are given off neurdl branches which bifurcate and form directly the dorsal and ventral spinal arteries. The vertebral arteries arise by longitudinal, postcostal anastomoses (Fig. 188) of the first seven pairs of dorsal rami (Fig. 189). The original stems of the first six pairs are lost, so that the vertebrals then take their origin from the seventh intersegmental arteries (Fig. 190). In embryos of 9 mm., the vertebral arteries fuse at the level of the cerebellum to form a single midventral vessel, the basilar artery; since the internal carotids are recurved cranially at 5 mm. (Fig. 185), and terminate in union with the first intersegmental arteries, the basilar is now connected cranially with the internal carotids and caudad with the definitive vertebral arteries.
The internal carotids (Fig. 185), after branching off the ophthalmic arteries, give rise cranially to the anterior cerebral artery, from which develop later the middle cerebral and anterior chorioidal arteries; all of these supply the brain. Caudalward there are many small branches to the brain wall which ultimately form a true posterior cerebral artery,
Fig. 189. Origin of the vertebral and subclavian arteries and the costo-cervical trunk in a young rabbit embryo (modified after Hochstetter). Ill AB.-IV AB., Aortic arches; A.v.cB., cephalic portion of vertebral artery; CD. and C.v., internal and external carotid arteries.
The ventral rami of the dorsal intersegmental arteries become prominent in the thoracic and lumbar regions and persist as the intercostal and lumbar arteries, segmentally arranged in the adult. Longitudinal, precostal anastomoses (Fig. 188) constitute the costo-cervical and thyrocervical trunks (Fig. 189). The subclavian and a portion of the internal mammary artery are derived from the ventral ramus of the seventh cervical segmental artery (Fig. 189). The remainder of the internal mammary, and the superior and injerior epigastric arteries, are formed by longitudinal ventral anastomoses (Fig. 188) between the extremities of the ventral rami from the thoracic and lumbar intersegmental arteries, beginning with the second or third thoracic (Fig. 191).
Fig. 190. Arterial system of a human embryo of 10 mm. (His). X i8. Ic, Internal carotid artery; P, pulmonary artery; IV, vertebral artery; III - VI, persistent aortic arches.
2. The lateral {visceral) branches of the descending aortae are not segmentally arranged. They supply structures arising from the nephrotome region (mesonephros, sex glands, metanephros, and suprarenal glands). From them arise the renal, suprarenal, inferior phrenic, and internal spermatic or ovarian arteries.
Bremer (1915) derives the renal arteries not from transformed mesonephric vessels, as did Broman (1906), but from a plexus of multiple aortic origin. There cire frequent variations in the selection of ])ermanent channels.
3. The ventral {splanchnic) branches are imperfectly segmental. Primitively, they form the paired vitelline arteries to the yolk sac (Figs. 180 to 182). Coincident with the degeneration of the yolk sac, the prolongations of the ventral vessels to its walls disappear, and the paired arteries
that persist and pass in the mesentery to the gut fuse to form unpaired vessels. From these, three large arteries are derived; the coeliac artery, the superior mesenteric, and the inferior mesenteric (Figs. 185 and 192),
Fig. 191. The development of the internal mammary and epigastric arteries in a human embryo of 13 mm. (Mall in McYIurrich).
The primitive coeliac axis arises opposite the seventh intersegmental artery. It then migrates caudad until eventually its origin is opposite the twelfth thoracic segment (Fig. 192). This transference, according to Evans, is due to the unequal growdh of the dorsal and ventral walls of the aorta. Similarly, the superior mesenteric artery is displaced caudad ten segments, the inferior mesenteric artery three segments.
The umbilical arteries arise in embryos of 2 to 2.5 mm. from the primitive aortae opposite the fourth cervical segment. They take origin in a plexus of ventral vessels of the vitelline series (Fig. 18 1), and are gradually shifted caudad until they arise from the dorsal aorta opposite the twenty-third segment (fourth lumbar). In 5 mm. embryos, the umbilical arteries develop secondary, lateral connections with the aorta (Fig 192 A). The new vessels pass lateral to the mesonephric ducts, and, at 7 mm., the primitive ventral stem-artery has disappeared (Fig. 192 B). The segment of this new trunk, proximal to the origin of the external iliac artery which soon arises from it, becomes the common iliac,
Fig. 192. Reconstructions of the human aorta and its branches (Tandler-Prentiss). - 1 , 5 mm. ; B, 9 mm.
The remainder of the umbilical trunk constitutes the hypogastric artery. When the placental circulation ceases at birth, the distal portions of the hypogastric arteries, from bladder to umbilicus, atrophy, forming the lateral umbilical ligaments of adult anatomy (Fig. 199).
The middle sacral artery is the direct caudal continuation of the aorta. Its dorsal position in the adult is the result of secondary growth changes.
Arteries of the Extremities
It is assumed that in man, as in observed birds and mammals, the first vessels of the limb buds form a capillary jrlexus (Fig. i yg).
Upper Extremity. - The capillary plexus takes origin by several lateral branches from the aorta. In human embryos of 5 mm. but one connecting vessel remains, and this arises secondarily as the ventral ramus of the seventh dorsal intersegment al artery (Fig. iSSb The portion of this vessel in the future free arm is plexiform at first, but later becomes a single axis which forms successively the subclavian, axillary, brachial, and inicrosscous arteries. Subseciuently, the median, radial, and ulnar arteries develop.
Lower Extremity. - In embryos of 7 mm. a branch, known as the sciatic artery, is given off from the future common iliac. It is the cluef arterial stem of the lower extremity and includes the definitive popliteal and peroneal arteries. At 15.5 mm. it is largely superseded by the external iliac -and femoral arteric, of which the latter annexes the branches of the sciatic distal to the middle of the thigh. The sciatic artery persists proximally as the inferior gluteal artery,
Development of the Veins
Three systems of paired veins are present in embryos of 23 somites ( Fig. 182) : the umbilical veins from the chorion; the vitelline veins from the yolk sac; and the prccarditial and postcardinal veins, which unite in the common cardinal veins, from the body of the embryo. Thus, three veins open into the right horn of the sinus venosus, and three into the left.
Transformation of the Vitelline and Umbilical Veins
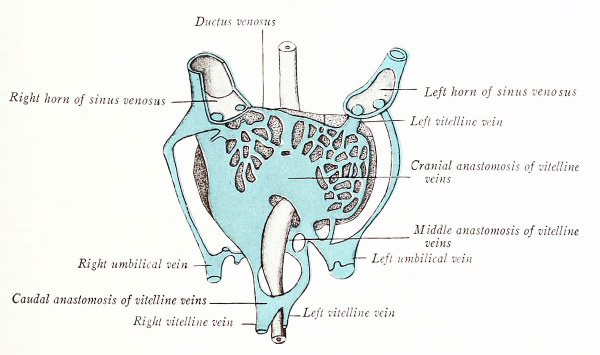
Accompanying the increase in size of the primitive liver is a mutual intergrowth between the hepatic cords and the endothelium of the vitelline veins. As a result, these vessels form in the liver a network of sinusoids (Figs. 183 and 193), and each vein is thereby divided into a distal portion which passes from the yolk sac to the liver, and into a proximal portion which carries blood from the liver sinusoids to the Sinus venosus. Soon, the proximal part of the left vitelline vein is largely absorbed into the hepatic sinusoids and shifts its blood flow to the right horn of the sinus venosus. Moreover, in 5 mm. embryos, the parallel vitelline veins communicate by three cross anastomoses (Figs. 194 and 195): (1) a cranial transverse connection in the liver, ventral to the duodenum; (2) a middle one, dorsal to the duodenum; and (3) a caudal one, ventral to it. There are thus formed about the gut a cranial and a caudal venous ring. A new vessel, the superior mesenteric, now develops in the mesentery of the intestinal loop and joins the left vitelline vein just caudal to its middle anastomosis (Fig. 195).
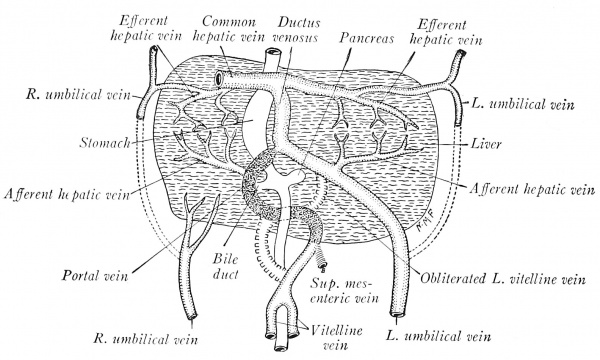
Coincident with the atrophy of the yolk sac, the vitelline veins degenerate caudal to the junction of the superior mesenteric vein. The persisting trunk between the latter vessel and the liver sinusoids is the portal vein, and thus represents: (1) a portion of the left vitelline vein in the left limb of the caudal ring; (2) the middle transverse anastomosis between the vitelline veins; (3) that segment of the right vitelline vein which forms the right limb of the cranial ring. According to Mall, the intrahepatic portion of the right vitelline vein persists proxinially as the right ramus of the hepatic vein, and distally as the ramus arcuatus of the portal vein. The intrahepatic portion of the left vitelline vein drains secondarily into the right horn of the sinus venosus, and proximally forms later the left hepatic ramus. Distally, where it is connected with the left umbilical vein, it becomes the ramus angidaris of the portal vein. In this way two primitive portal, or supplying trunks, and two hepatic, or draining trunks, originate. Later, there are differentiated first four, then six, such apposed trunks within the liver, and the six primary lobes supplied and drained by these vessels may be recognized in the adult.
Fig. 193. Ventral reconstruction of the blood vessels in a 4.2 mm human embryo Wilhelm His (1831-1904)
Fig. 194. Ventral reconstruction of the veins of the liver in a 4.9 mm. human embryo (Ingalls).
While these changes have been progressing, the liver tissue grows laterad, comes in contact with the umbilical veins, and taps them so that their blood is diverted more directly to the heart through the sinusoids of the liver (Fig. 194). As the channel of the right proximal vitelline is larger, the blood from the left umbilical vein flows diagonally to the right horn of the sinus venosus. When all the umbilical blood enters the liver, as in embryos of 5 to 6 mm., the proximal segments of the umbilical veins atrophy (Fig. 195). At 7 mm. the left umbilical is large, while the corresponding right vein has degenerated and soon disappears. The left persists during fetal life, shifts to the midplane, and courses in the free edge of the falciform ligament. After birth its lumen is obliterated, and from the umbilicus to the liver it constitutes the ligamentum teres,
Fig. 195. The origin of the portal vein and ductus venosus as illustrated by a human embryo of 7 mm. (modified after Wilhelm His (1831-1904)).
In the liver, the portal vein, through its cranial anastomosis between the primitive vitelline veins, is connected with the left umbilical vein (Fig. 195). As the right lobe of the liver grows, the course of the umbilical and portal blood through the intrahepatic portion of the right vitelline vein becomes circuitous, and hence a new, direct channel to the sinus venosus is formed through the hepatic sinusoids. This is the ductus venosus (Fig. 195), which is obliterated after birth and forms the ligamentum venosum of the postnatal liver.
Transformation of the Precardinal Veins
Each precardinal (anterior cardinal) vein consists of two parts (Fig. 185): (i) the primary head vein, which extends into the unsegmented head proper and courses ventrolateral to the brain wall; (2) the true precardinal, located laterad in the segmented portion of the head and neck and draining into the common cardinal vein.
Fig. 196. Veins of the head in a 9 mm human embryo (after Mall). X 9,
The primary head veins have three pairs of tributary plexuses (Fig. 196) which presently extend dorsad over the brain. From this primitive arrangement the various veins and sinuses of the brain are developed.
The true precardinals communicate during the eighth week by a transverse venous channel which carries the blood from the left side of the head into the right vein (Fig. 197 D). As a result, the left precardinal soon loses its connection with the common cardinal on the same side and degenerates (E). The stump of the left common cardinal comprises the inconstant oblique vein of the left atrium: it also joins with the transverse sinus venosus in forming the coronary sinus. The right common cardinal and the right precardinal, as far as its cross anatomosis, become the superior vena cava. The anatomosis itself forms the left innominate vein, while that portion of the right precardinal between the anastomosis and the right subclavian vein is known as the right innominate. The distal segments of the precardinals become the internal jugular veins of the adult, whereas the external jugular and subclavian veins are vessels which develop somewhat later (C-E).
Fig. 197. Diagrams to illustrate the transformation of the pre-, post-, sub-, and supracardinal veins (adapted after Huntington and McClure). A, 6 mm; B, 10 mm; , 15 mm, D, 18 mm.
Transformation of the Post-, Sub-, and Supracardinal Veins
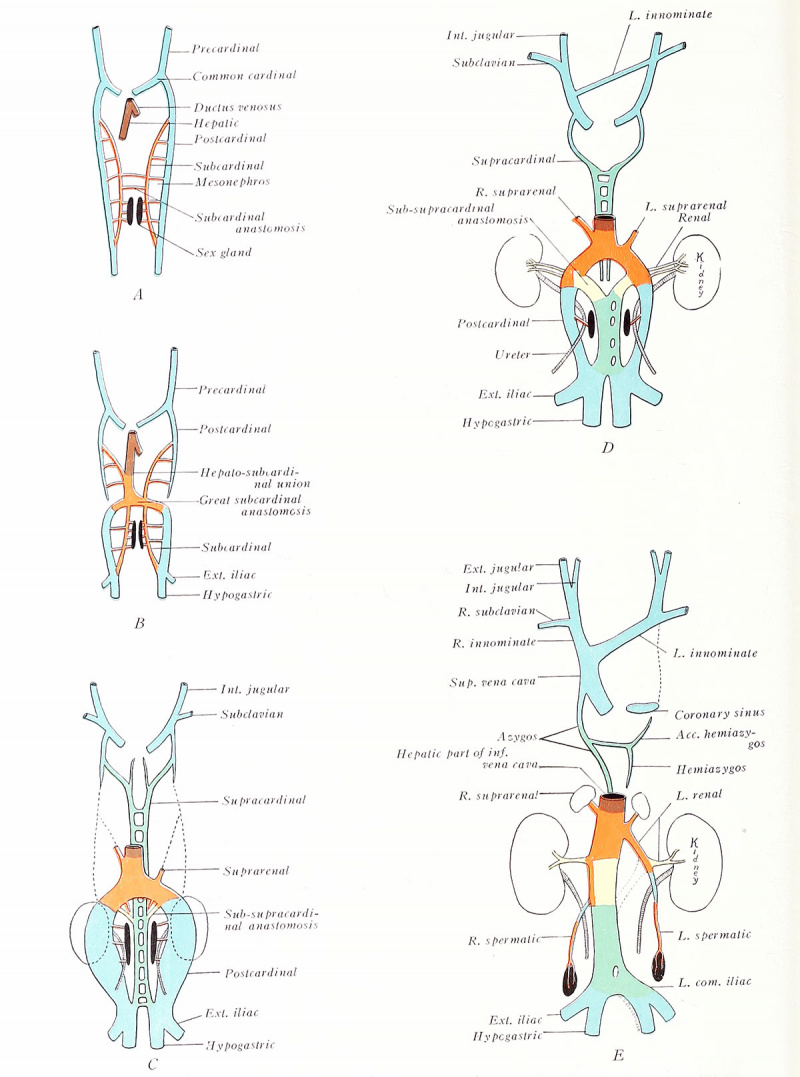
The primitive postcardinal veins course cephalad along the dorsal sides of the mesonephroi and open into the common cardinals (Fig. 197 A). Each receives tributaries from the posterior extremities, mesonephroi, and body wall. Median and ventral to the mesonephros are developed the suhcardinal veins, which connect at intervals with the postcardinals through the mesonephric sinusoids, and with each other by anastomoses ventral to the aorta. Thus, all the blood from the lower body is in early stages drained by the postcardinal veins alone. Soon, the postcardinals are divided midwayinto cranial and caudal segments (S). Cranial to their interruption, these vessels atrophy (C). The caudal portions are associated with the mesonephroi and persist longer, but finally disappear with those organs {D, E). The sole permanent remnants of the postcardinal system are small contributions to the azygos and sex veins,
Of the subcardinals, only the middle regions, at about the final level of the kidneys, are retained. Here the two vessels communicate by a broad anastomosis, and here each is similarty connected with the postcardinal of the same side [B). Below this level the subcardinals presently disappear, except for portions which supply the sex glands {C-E) . Above, the left drops out, its lower stump alone transforming into the left suprarenal vein; the corresponding part of the right subcardinal remains as the right suprarenal and also as an important component of the inferior vena cava.
In the meantime, a new pair of anastomosing veins, the su pracardinals, make their appearance (C). They lie dorso-mesial to the postcardinals, and, in a sense, replace them. The supracardinal veins originally extend from near the common cardinals to the union of the primitive iliac vessels, but they soon break at the level of the kidneys (D). The cranial halves midway develop a prominent anastomosis and become the azygos and hemiazygos of the adult (A, B). Opposite the kidneys, the caudal segments form a permanent, broad union with the right subcardinal and a temporary one with the left (C, D] in yellow). The right main supracardinal channel, with the annexed right subcardinal, constitutes the lower half of the inferior vena cava {D, E) (Huntington and McClure, 1920).
The development of the unpaired inferior vena cava begins when communication is established between the right hepatic and right subcardinal veins. The liver on the right side becomes attached to the dorsal body wall, and from its point of union a ridge, the caval mesentery (Fig. 112), extends caudad. Capillaries from the subcardinal vein invade the mesentery, and, growing cranially, meet and fuse with capillaries extending caudad from the liver sinusoids. Thus is formed the vein of the caval mesentery (. 4 , B), which is already present in human embryos of 10 mm. 44 ie blood from the lower trunk and leg region soon becomes drained by the complex inferior vena cava, which is composed of the following veins (E) : (1 ) the common hepatic and right hepatic veins (primitive right vitelline) ; (2) the connecting vein of the caval mesentery; (3) an inter-renal portion of the right subcardinal vein (and its adjoining anastomoses with the right post- and supracardinal and the left subcardinal) ; (4) the right supracardinal vein, below the level of the kidneys.
The permanent kidneys take up their positions opposite the great anastomosis between the subcardinals, and, at this point, the renal veins are developed [D, E)\ the longer left renal vein differs from the right in that proximally it represents a left portion of the anastomosis itself. A cephalic segment of the left subcardinal vein persists as the left suprarenal vein, which thus opens into the left renal instead of joining the inferior vena cava as does the right suprarenal vein of similar subcardinal origin (E). The spermatic or ovarian veins contain both postcardinal and subcardinal components. The left early drains into the left caudal border of the great subcardinal anastomosis, which, as already described, contributes to the left renal vein; the right opens into that portion of the right subcardinal which is incorporated into the inferior vena cava. The posterior intercostal and lumbar veins are at first tributaries of the postcardinals. As the latter vessels degenerate, these tributaries connect secondarily with the replacing supracardinal veins; later, they of necessity drain respectively into the azygos veins and inferior vena cava. The history of the common iliacs is similar, the stem of the longer left representing a caudal anastomosis between the primitive paired supracardinals (C-E).
Veins of the Extremities
The primitive capillary plexus of the flattened limb buds gives rise to a peripheral border vein (Fig. 227). In the upper extremity, its ulnar portion persists, forming at different points the subclavian, axillary, brachial, and basilic veins. At 10 mm. the border vein opens into the dorsal wall of the postcardinal, but, as the heart shifts caudad, it Anally drains by a ventral connection into the xjrecardinal, or internal jugular vein. The cephalic vein develops secondarily in connection with the ulnar border vein; later, in embryos of 23 mm., it anastomoses with the external jugular and finally drains into the axillary vein, as in the adult. AVith the development of the digits, the vv. ccphalica and basilica become distinct (35 mm.), but later are again connected by a plexus on the dorsum of the hand.
In the lower extremity, the fibular portion of the primitive border vein persists. Later, the V. saphena niagna arises separately from the postcardinal, gives off the vv. femoralis and tibialis posterior, and annexes the fibular border vein at the level of the knee. Distal to this junction, the border vein persists as the v. tibialis anterior, and, probably, the V. saphena parva; proximally, it becomes greatly reduced, forming the v. glutea inferior,
Anomalies
Anomalous blood vessels are of common occurrence. They may be due: (i) to the choice of unusual paths in the primitive vascular plexuses; (2) to the persistence of vessels normally obliterated; (3) to the disappearance of vessels normally retained; (4) to incomplete development; (5) to fusions and absorptions of parts usually distinct.
Fetal Circulation and the Changes at Birth
During fetal life oxygenated placental blood enters the embryo by way of the large umbilical vein and is conveyed to the liver where it mingles with that brought in by the portal vein (Fig. 198). Thence it flows to the inferior vena cava either directly, through the ductus venosus, or indirectly through the liver sinusoids and hepatic vein. The impure blood of the inferior vena cava and portal vein contaminates but slightly the greater volume of pure placental blood. According to common belief, the blood from the inferior vena cava is directed by the valve of that vein across the right atrium and through the foramen ovale into the left atrium (following the path of the sounds in Figs. 172 to 174), which, before birth, receives little venous blood from the lungs. This purer blood of the left atrium then enters the left ventricle and is driven out through the aorta, to be distributed chiefly to the head and upper extremities,
Fig. 198. Diagram of the circulation before birth (Heisler). Arrows point out the course of the blood current; colors show the older conception of the character of the blood carried by various vessels, whereas experimentation indicates a thorough mixing within the heart.
The venous blood of the superior vena cava is supposed to flow from the right atrium into the right ventricle, whence it passes out by the pulmonary artery. A small amount is conveyed to the lungs, but, as the fetal lungs do not function, most of it enters the dorsal aorta by way of the ductus arteriosus. Since the ductus is caudal to the origin of the subclavian and carotid arteries, its less pure blood is distributed to the trunk, viscera, and lower extremities. The placental circuit is completed through the hypogastric, or umbilical arteries.
In spite of the apparent anatomical arrangement in the heart to prevent the mixing of pure and impure blood, actual experiments indicate.
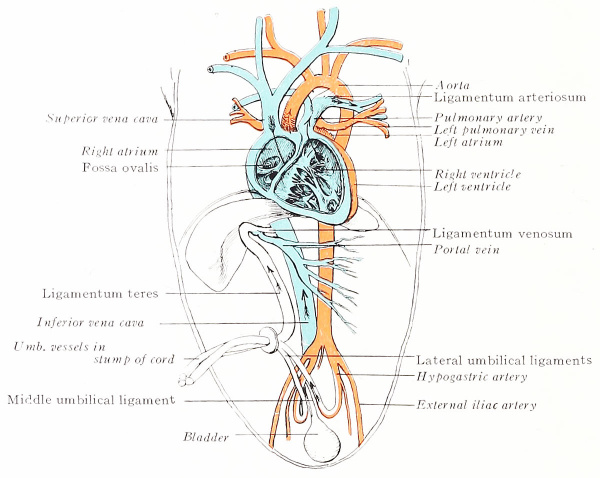
Fig. 199. Diagram of the circulation after birth (Heisler). Obliterated fetal passages are indicated by roman type.
That, contrary to the prevalent view, there is thorough mingling of the blood which enters the right atrium through the two caval veins. Hence, there can be no difference in the quality of the blood distributed to the various parts of the body. Circulatory efficiency must then depend on the relatively large quantity of swiftly moving blood.
Changes at Birth
When the lungs become functional, the placental circulation ceases quickly. This transfer of the seat of oxygenation not only changes the character of the blood in many vessels but throws important fetal vessels and parts into disuse (Fig. 199). In general, physiological occlusion follows immediately but anatomical obliteration is slower,
The large amount of blood returned to the heart from the functional lungs equalizes the pressure in the two atria (p. 181). As a result, the septum primum, or valve of the forcuucn ovale, is pressed against the septum secundum, thereby closing the foramen. Eventually, the two septa fuse - in one-third of all cases within three months, in three-fourths by maturity (p. 185).
The ductus arteriosus also ceases to function, as all the blood from the pulmonary arterial trunk is conveyed to the expanded lungs. In four out of five cases the ductus becomes impervious within three months and persists as a solid, fibrous cord, the Ugamentum arteriosum.
The umbilical vessels contract and their lumina are obliterated by fibrous invasion. The process advances proximad during the first two or three months of postfetal life. The cord-like vein is persistent as the Ugamentum teres of the liver; the arteries become the lateral umbilical ligaments.
The ductus venosus likewise atrophies, and, within two months, transforms into the fibrous ligamentum venosum, embedded in the wall of the liver,
The lymphatics originate independently of blood vessels from discrete mesenchymal spaces which become lined with an endothelium of transformed border cells. Temporary venous connections are now generally believed to be acquired secondarily. By the progressive fusion and budding of such local anlages, the lymphatic system grows to its final form.
The first plexus of lymphatic capillaries is distributed along the primitive, main venous trunks. The dilatation and coalescence of this network at definite regions gives rise to five lymph sacs (Fig. 200): (i, 2) Paired jugular sacs appear in 10 mm. embryos, lateral to the internal jugular veins. (3) At 23 mm., the unpaired retroperitoneal sac develops at the root of the mesentery, adjacent to the suprarenal glands, and the cisterna chyli also differentiates (4, 5). Paired posterior sacs arise in relation to the sciatic veins of embryos 24 mm. long. All these sacs at first contain blood which they soon discharge into neighboring veins, thereupon losing their venous connections. With relation to the lymph sacs as centers, the thoracic duct (at 30 mm.) and the peripheral lymphatics develop. Thus, lymphatic vessels grow to the head, neck, and arm from the jugular sacs; to the hip, back, and leg from the posterior sacs, and to the mesentery from the retroperitoneal sac. The jugular sacs alone acquire permanent connections with the internal jugular veins that are later utilized by the thoracic and right lymphatic ducts. The various sacs themselves are eventually replaced by chains of lymph glands.
Lymph Glands
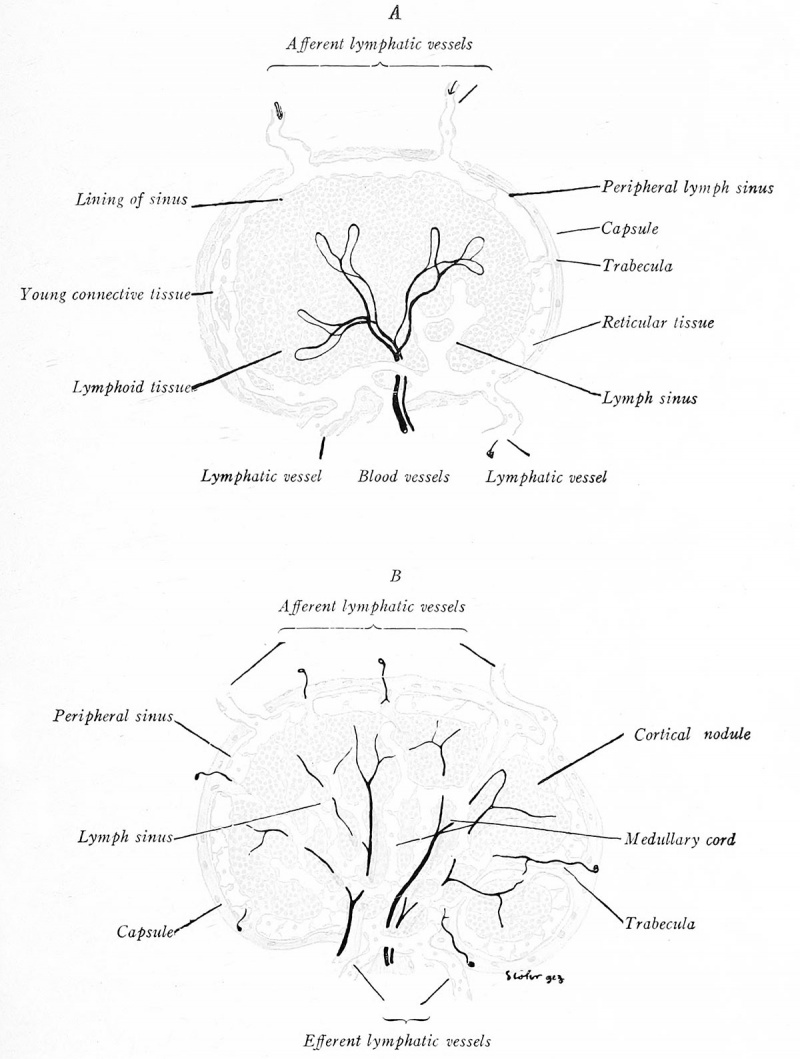
Paired lymph glands appear during the third month, first in the axillary, iliac, and maxillary regions (Fig. 200). Those replacing the lymph sacs develop later. Primitive sinuses, with simple connective-tissue septa, mark the primary stage of development. Ordinarily it has been believed that the sinuses represent lymphatic plexuses, but recent investigators (Downey, 1922) claim they are channels in the reticulum, originating as clefts in the mesenchyme and acquiring secondary lymphatic connections. Lymphocytes collect in the stroma, forming cortical nodules which become associated with blood capillaries and after birth acquire germinal centers (Fig. 201 A). The peripheral sinus organizes and connects with afferent and efferent lymphatics; the central sinuses cut the lymphoid tissue into medullary cords (Fig. 201 B). The connective tissue differentiates into a fibrous capsule from which trabeculce dip into the gland.
Fig. 200. Profile reconstruction of the primitive lymphatic system in a human embryo of two months (redrawn after Sabin). X 3.
Hemal (Hemolymph) Glands
The orgin of hemal glands is traced by Meyer (1917) to condensations of mesenchyme which develop in relation to blood vessels, not lymphatics. The peripheral sinus arises independently; its vascular connections are secondary.
Fig. 201. Diagrams representing four stages in the development of lymph glands. The earlier stages are shown on the left side of each figure (Lewis and Stohr).
The Spleen
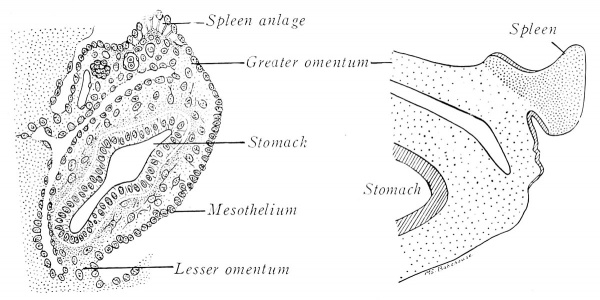
Embryos of 9 mm. exhibit a swelling on the left side of the dorsal mesogastrium, near the dorsal pancreas (Fig. 202 A). The thickening is due to a temporary proliferation and invasion of mesothelial cells into the underlying mesenchyme, which, meanwhile, has also undergone local enlargement and vascularization. These cells from the peritoneal epithelium give rise to a large part, at least, of the future spleen. The union of the splenic anlage with the mesogastrium (Fig. 202 B) is ultimately reduced to a narrow band.
At first the blood vessels constitute a closed system. The peculiar adult circulation is acquired relatively late. Lymphoid tissue first appears as ellipsoids about the smallest arteries in fetuses of four months. At seven months, the ovoid splenic corpuscles form nodules about the larger arteries. The capsule, trabeculce, and reticulum differentiate from the cells of the common anlage. During the last half of fetal life, red blood corpuscles are developed actively in the splenic capillaries.
Fig. 202. Developmental stages of the human spleen (redrawn from Kollman and Tonkoff). A, 10.5 mm.; B, 20 mm.
The Glomus Coccygeum
The coccygeal body develops from the wall of the middle sacral artery. It appears at the apex of the coccyx in the third month, and, during the fourth month, is an encapsulated cluster of polyhedral cells. Later, it becomes lobulated by the ingrowth of connective tissue trabeculae and receives a rich vascular supply. Affinities are obscure, but at no time does it resemble chromaffin tissue, as is often stated.
Tonsils and Thymus. - For their development see p. 100 .
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Reference
Arey LB. Developmental Anatomy. (1924) W.B. Saunders Company, Philadelphia.
Cite this page: Hill, M.A. (2026, February 27) Embryology Book - Developmental Anatomy 1924-9. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Developmental_Anatomy_1924-9
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G