Book - Comparative Embryology of the Vertebrates 1-2
| Embryology - 23 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Nelsen OE. Comparative embryology of the vertebrates (1953) Mcgraw-Hill Book Company, New York.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Part I The Period of Preparation
Part I - The Period of Preparation: 1. The Testis and Its Relation to Reproduction | 2. The Vertebrate Ovary and Its Relation to Reproduction | 3. The Development of the Gametes or Sex Cells
The Vertetrate Ovary and Its Relationship to Reproduction
A. The Ovary and Its Importance
One of the editions of the treatise on development, “Exercitationes de Generatione Animalium,†by William Harvey (1578-1657) contains a picture of Jupiter on a throne opening an egg from which various animals, including man, are emerging (fig. 25). Upon the egg (ovum) are engraved the words ovo omnia. At the heading of chapter 62 of this work Harvey placed a caption which explains the phrase ex ovo omnia more explicitly. This heading reads: “Ovum esse primordium commune omnibus animalibus†— the egg is the primordium common to all animals. Published in 1651, this statement still maintains its descriptive force.
Many individual animals arise by asexual reproduction, that is, through a process of division or separation from a parent organism. In the phylum Chordata asexual reproduction is found among the Urochordata, where new individuals may arise by budding from a stolon -like base of the parent (fig. 27). This process often is called gemmation, the formation of a new individual by a protrusion of a mass of cells from the parental body followed by its partial or complete separation. It is a prominent method of reproduction among the lower Metazoa, particularly the coelenterates and sponges. Nevertheless, all animal species among the Metazoa ultimately utilize an egg as the primordium from which the new individual arises. Sexual reproduction, generally associated with the fertilization of an egg by a sperm element, appears to be a needful biological process.
Fig. 25. Copy of the engraved title appearing in one edition of Harvey’s dissertation
on generation as shown on p. 139 of Early Theories of Sexual Generation by E. J. Cole.
Observe the words “ex ovo omnia†upon the egg which Jupiter is opening. Various animals
are emerging from the egg.
Fig. 26. Copy of Hartsoeker’s figure of human spermatozoan, containing the homonculus or “little man,†published in 1694. This figure represents a marked preformationist conception of development. However, it is to be noted that Hartsoeker later abandoned the preformationist concept as a result of his studies on regeneration.
True as the general statement made by Harvey may be, it is not clear what
is meant by the word ovum or egg. We know certain of its characteristics,
but, for the most part, it must be accepted as an accomplished fact enshrouded
in mystery. To Harvey the egg was an indefinite, unorganized association of
substance plus a “primordial generative principle†(see Cole, F. J., ’30, p.
140), Other minds have conceived of other meanings. Nevertheless, descriptive
and experimental embryology has forced the conclusion that the egg, during
its development within the ovary, experiences a profound process of differentiation, resulting in the formation of an invisible organization. Although this organization is invisible, it is imbued with an invincibility which, when
set in motion at the time of fertilization, drives the developmental processes
onward until final fulfillment is achieved in the fully formed body of the
adult organism.
Fig, 27. Forms of asexual reproduction in the subphylum Urochordata 9 #
Chordata. (From MacBride: Textbook of Embryology, Vol. 1, Londo/rt', ^
(A) Budding from "stolon of Perophora listeri, from MacBride after (jR) , (C)
Two stages of budding in an ascidian, from MacBride after Pizon
Beyond the fundamental changes effected in the developing egg while in the ovary, the latter structure has still other roles to maintain. Through the mediation of the hormones produced within the confines of the ovarian substance, the female parent is prepared to assume the responsibilities of reproduction. In addition, in many vertebrates the further responsibility of taking care of the young during the embryonic period stems from the hormones produced in the ovary. In some vertebrates, the instinct of parental care of the young after hatching or after birth indirectly is linked to ovarian-pituitary relationships. Because of these profound and far-reaching influences which the ovary possesses in producing the new individual, it must be regarded as the dynamic center of reproduction for most animal species.
B. Preformationism, Past and Present
The above statement relative to the importance of ovarian influences and of the female parent is a position far removed from that held by some in the past. An ancient belief elevated the male parent and his “seed†or semen. As Cole, F. J., ’30, p. 38, so aptly places the thinking of certain learned sources during the 16th century: “The uterus is regarded as the ‘till’d ground for to sow the seeds on’ — a popular idea, based obviously on the analogy with plants, which prevailed long before and after this period. The seed of the male is therefore the chief agent in generation, but cannot produce an embryo without the cooperation of the female, and whether the result is male or female depends on which side of the uterus the seed falls, the time of the year, temperature, and the incidence of menstruation.†Or, in reference to the Leeuwenhoek’s belief in an intangible preformationism, Cole, F. J., ’30, p. 57, states: “He asserts that every spermatic animalcule of the ram contains a lamb, but it does not assume the external appearance of a lamb until it has been nourished and grown in the uterus of the female.†This statement of A. van Leeuwenhoek (1632-1723) was made as a criticism of N. Hartsoeker (1656-1725) whose extreme adherence to a seminal preformationism led him to picture the preformed body of the human individual, the homonculus, encased within the head of the spermatozoon (fig. 26). Hartsoeker, however, later abandoned this idea.
In fairness it should be observed that the egg during these years did not lack champions who extolled its importance. While the Animalculists considered the sperm cell as the vital element in reproduction, the Ovists, such as Swammerdam (1637-80), Haller (1708-77), Bonnet (1720-93) and Spallanzani (1729-99) believed that the pre-existing parts of the new individual were contained or preformed within the egg.
An extreme form of preformationism was advocated by certain thinkers
during this period. For example, Bonnet championed the idea of encasement
or “emboitement.†To quote from Bonnet:
The term “emboitement†suggests an idea which is not altogether correct. The germs are not enclosed like boxes or cases one within the other, but a germ forms part of another germ as a seed is a part of the plant on which it develops. This seed encloses a small plant which also has its seeds, in each of which is found a plantule of corresponding smallness. This plantule itself has its seeds and the latter bears plantules incomparably smaller, and so on, and the whole of this ever diminishing series of organized beings formed a part of the first plant, and thus arose its first growths. (Cole, ’30, p. 99.)
On the other hand, there were those who maintained that for some animals, neither the sperm nor the egg were important as “many animals are bred without seed and arise from filth and corruption, such as mice, rats, snails, shell fish, caterpillars, moths, weevils, frogs, and eels†(Cole, ’30, p. 38). This concept was a part of the theory of spontaneous generation of living organisms -a theory ably disproved by the experimental contributions of three men: Redi (1626-97); Spallanzani; and Louis Pasteur (1822-95).
Modern embryology embraces a kind of preformationism, a preformationism which does not see the formed parts of the new individual within the egg or sperm but wi.ich does see within the egg a vital, profound, and highly complex physiochemical organization capable of producing a new individual by a gradual process of development. This organization, this selfdetermining mechanism, is resident in the nucleus with its genes and the organized cytoplasm of the fully developed oocyte or egg. However, as shown later, this organization is dependent upon a series of activating agencies or substances for its ultimate realization. Some of these activating substances come from without, but many of them are produced within the developing organism itself.
C. General Structure of the Reproductive System of the Vertebrate Female
1. General Structure of the Ovary
Morphologically, the ovary presents a series of contrasts in the different vertebrate classes. In teleost fishes the size of the ovary is enormous compared to the body of the female (fig. 28), while in the human (fig. 29), cow, sow, etc., it is a small structure in comparison to the adult body. Again, it may contain millions of mature eggs in the ling, cod and conger, during each breeding season, whereas only a single egg commonly is matured at a time in the cow, elephant, or human. During the reproductive season the ovary may assume a condition of striking colored effects as in the bird, reptile, shark, and frog, only to recede into an appearance drab, shrunken, and disheveled in the non-breeding season.
Fig. 28. Dissection of female specimen of the common flounder, Limanda ferruginea. It particularly shows the ovary with its laterally placed ovarian sinus. Observe that the ovary, during the breeding season, is an elongated structure which extends backward into the tail. There are two ovaries, one on either side of the hemal processes of the caudal vertebrae.
Its shape, also, is most variable in different species. In mammals it is a flattened ovoid structure in the resting condition, but during the reproductive phase it may assume a rounded appearance, containing mound-like protrusions. In birds and reptiles it has the general form of a bunch of grapes. In the amphibia it may be composed of a series of lobes, each of which is a mass of eggs during the breeding season, and in teleost and ganoid fishes it is an elongated structure extending over a considerable area of the body.
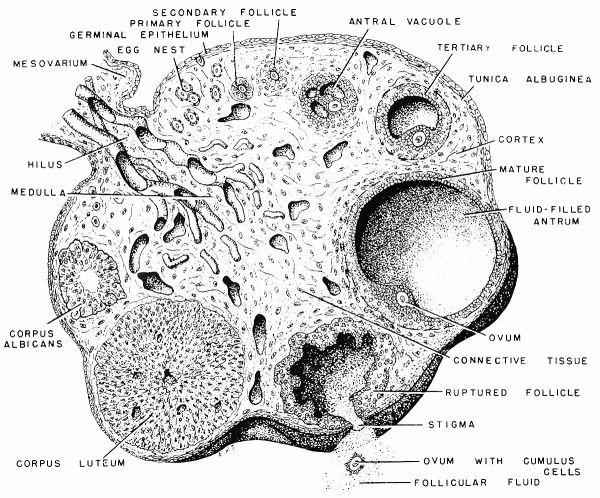
Regardless of their many shapes and sizes, the ovaries of vertebrates may be divided morphologically into two main types, namely, compact and saccular forms. The compact type of ovary is found in teleost, elasmobranch, cyclostome, ganoid, and dipnoan fishes, as well as in reptiles, birds and mammals. It has the following regions (figs. 30, 31):
- the medulla, an inner zone containing relatively large blood and lymph vessels;
- the cortex, an area outside of and surrounding the medulla (except at the hilus), containing many ova in various stages of development;
- a tunica albuginea or connective-tissue layer surrounding the cortex;
and
- the germinal epithelium or the covering epithelium of the ovary.
The germinal epithelium is continuous with the mesovarium, the peritoneal support of the ovary, and the particular area where the mesovarium attaches to the ovary is known as the hilus. Within the mesovarium and passing through the hilus are to be found the blood and lymph vessels which supply the ovary (fig. 30).
The ovary of the teleost fish is a specialized, compact type of ovary adapted to the ovulation of many thousands, and in pelagic species, millions of eggs at one time. It has an elongate hilar aspect which permits blood vessels to enter the ovarian tissue along one surface of the ovary, whereas the opposite side is the ovulating area. In many teleosts the ovulating surface possesses a special sinus-like space or lumen (fig. 28) which continues posteriad to join the very short oviduct. At the time of ovulation the eggs are discharged into this space and move caudally as the ovarian tissue contracts. In other teleosts this ovulatory space is not a permanent structure but is formed only at the time of ovulation. In Tilapia macrocephala, for example, the ovulatory lumen is formed on the side of the ovary opposite the area where the blood vessels enter. The formation of this space at the time of ovulation is described by Aronson and Holz-Tucker (’49) as a rupture of the elastic follicles during ovulation whereupon the follicle walls shrink toward the ovarian midline.
Fig. 29. Diagrammatic representation of a midsagittal section of the reproductive organs of the human female. (Slightly modified from Morris: Human Anatomy, Philadelphia, Blakiston.)
Fig. 30. Schematic three-dimensional representation of the cyclic changes which occur in the mammalian ovary.
carrying the interstitial tissue and immature ova. This shrinking away of the
tissues of the ovary leaves a space between these tissues and the outside
ovarian wall. A lumen thus is formed along the lateral aspect of the ovary
which is continuous with the oviduct. Many teleosts have two ovaries (e.g.,
flounder); in others there is but one (e.g., perch).
The amphibia possess a true saccular ovary (fig. 32). It has a cortex and germinal epithelium somewhat similar to the compact ovarian variety, but the area which forms the medulla in the compact ovary is here represented by a large lymph space. During early development, the amphibian ovary is a compact structure, but later there is a hollowing out and disappearance of the compact medullary portion, and the cortical area remains as a relatively thin, peripheral region (Burns, ’31; Humphrey, ’29).
Histologically the vertebrate ovary is composed of two general cellular groups, namely:
(1) germ cells, and
(2) general tissue cells of various kinds, such as epithelium, connective tissue, smooth muscle fibers, and the complex of elements comprising the vascular system of the ovary (figs. 30, 32). Some of the general cells form the so-called interstitial tissue of the ovary.
The germ cells differ from the general cells in that each of them has a latent potency for developing a new individual. This latent condition is converted into active potentiality during the differentiation of the primitive germ cell into the mature egg or ovum.
2. General Structure of the Accessory Reproductive Organs
The accessory reproductive structures of the female vertebrate may be separated into three general types, viz.:
( 1 ) the total absence of or the presence of a pair of short funnel-like structures which convey the eggs from the peritoneal cavity through
Fig. 31 . Three-dimensional representation of the bird ovary together with the funnel portion (infundibulum) of the oviduct. Recently ovulated egg is shown in the process of engulfment by the infundibulum. Various stages of developing eggs are shown.
Fig. 32. Anterior half of the saccular ovary of Necturus maculosus.
an opening into the urogenital sinus and thence to the outside as in cyclostome fishes,
(2) a short sinus-like tube attached to each ovary and to the urogenital sinus or to a separate body opening as in many teleost fishes (fig. 28), and
(3) two elongated oviducal tubes variously modified (figs. 29, 33, 34, 35, 36, 37).
Except in the teleost fishes the cephalic end of each oviduct generally is open and is placed near the ovary but not united directly with it (figs. 29, 33) although in some species, such as the rat, it is united with an ovarian capsule (fig. 37). In some vertebrates the anterior orifice of the oviduct may be located a considerable distance from the ovary, as in frogs, toads, and salamanders. In many vertebrates, as in birds and snakes, there is but one oviduct in the adult.
In some vertebrates the oviduct is an elongated glandular tube, as in certain urodele amphibia (fig. 33) and in ganoid fishes; in others, such as frogs, birds or mammals, it is composed of two main parts: ( 1 ) an anterior glandular structure and (2) a more caudally placed uterine portion. The latter may unite directly with the cloaca, as in the frog (fig. 38) or by means of a third portion, the vaginal canal or vagina located between the uterus and the cloaca, as in elasmobranch fishes, reptiles, and birds, or between the uterus and the external urogenital sinus, as in mammals (figs. 35, 36, 37). The vaginal canal may be single, as in eutherian mammals, or double, as in metatherian mammals (figs. 35, 36). In metatherian (marsupial) mammals it appears that a third connection with the oviducts is made by the addition of a birth passageway. This birth canal represents a secondary modification of a portion of the vaginal canals and associated structures (figs. 34, 35, 114). (See Nelsen and Maxwell, ’42.) One of the main functions of the vagina or vaginal canal is to receive the intromittent organ of the male during copulation.
The anterior opening of the oviduct is the ostium tubae abdominale, a
funnel-shaped aperture generally referred to as the infundibulum. In the
transport of the egg from the ovary to the oviduct the infundibulum, in
many species, actually engulfs and swallows the egg.
The portion of the oviduct anterior to the uterus often is called the convoluted glandular part; it is highly twisted and convoluted in many species. In amphibians, reptiles, birds, and in some mammals the glandular portion
Fig. 33. Diagrammatic representation of the reproductive structures of female urodele,
Necturus maculosus.
Fig. 34. Diagrammatic lateral view of female reproductive system of the opossum,
showing pseudo-vaginal birth canal.
Fig. 35. Reproductive structures of female opossum shown from the ventral view. Observe that the ovary and infundibular portion of the Fallopian tube lie dorsal to the horn of the uterus.
functions to secrete an albuminous coating which is applied to the egg during its passage through this region. In amphibians, reptiles, and birds it forms the major portion of the oviduct, but in mammals it is much reduced in size and extent. In the latter group it is referred to as the uterine or Fallopian tube.
The uterus is a muscular, posterior segment of the oviduct. Like the anterior glandular portion of the oviduct, it also has glandular functions, but these are subservient to its more particular property of expanding into an enlarged compartment where the egg or developing embryo may be retained. The protection and care of the egg or of the embryo during a part or all of its development, is the main function of the uterus in most vertebrates. In the frogs and toads, however, this structure seems to be concerned with a “ripening†process of the egg. Large numbers of eggs are stored in the uterine sac of the frog for a period of time before spawning.
Various degrees of union between the uterine segments of the two oviducts are found in mammals. In the primates they fuse to form a single uterine compartment with two anterior uterine tubes (fig. 29). In carnivores, there is a caudal body of the uterus with two horns extending forward to unite with the uterine tubes (fig. 36). In the rat and mouse, the uterine segments may be entirely separate, coming together and joining the single vaginal chamber (fig. 37). In the opossum the uterine segments are entirely separated, joining a dual vaginal canal system posteriorly (figs. 34, 35, 114).
D. Dependency of the Female Reproductive System on General Body
Conditions
1. Inanition
In the immature female mammal continued underfeeding results in general retardation of sexual development. The younger follicles may develop, but the later stages of follicular development are repressed. In the adult female, inanition produces marked follicular degeneration and atresia as shown by many records of retarded sexual development, reduced fertility, even cessation of the cyclic activities of menstruation and estrus occurring in man and domestic animals during war-produced or natural famine (Mason in Allen, Danforth, and Doisy, ’39, p. 1153). The ovary thus seems to be especially susceptible to starvation conditions, even more so than the testis. As the condition and well-being of the secondary reproductive structures are dependent upon proper ovarian function, this part of the reproductive system suffers marked changes as a result of ovarian dysfunction during prolonged starvation.
Fig. 36. Schematic representation of reproductive organs of the female cat. On the left side of the illustration, the body of the uterus and uterine horn have been cut open, and the Fallopian tube and ovary are highly schematized. Observe the partial ovarian capsule around the ovary shown on the right and the relatively fixed condition of the infundibular opening of the oviduct lateral to the ovary.
Fig. 37. Diagrammatic representation of the reproductive organs of the female rat,
showing the bursa ovarica around each ovary. Observe that uteri open directly into the
vagina. (Modified from Turner, ’48.)
Fig. 38. Diagrammatic representation of reproductive structures of the female frog. Observe that the ostium of the oviduct is not an open, mouth-like structure. It remains constricted until the egg starts to pass through.
2. Vitamins
a. Vitamin A
The ovary is not immediately sensitive to a lack in vitamin A in the diet but general epithelial changes in the reproductive tract occur which may aid in producing sterility (Mason, ’39).
b. Vitamin B
Ovarian and uterine atrophy occur as a result of deficiency of this vitamin in monkey, rabbit, mouse and rat (Mason, ’39). This effect may be mediated, at least partly, through the effect of B-deficiency upon the pituitary gland.
c. Vitamin C
During the earlier stages reproductive activity is maintained, but advanced stages of C-deficiency produce regressive effects (Mason, ’39).
d. Vitamin E
E-deficiency in the female rat does not upset the ovarian and general reproductive behavior. However, established pregnancies are disturbed and are terminated by resorption of the embryo (Mason, ’39). In the domestic fowl, unless sufficient amount of vitamin E is present in the egg, embryonic death occurs during early incubation periods of the egg.
3. The Hypophysis (Pituitary Gland)
The ovaries experience pronounced atrophy as a result of hypophysectomy in mammals and non-mammalian species. The earlier stages of follicle formation in the higher mammalian ovary up to the stage of beginning antrum formation are not so much affected, but later follicular development and interstitial tissue growth are inhibited (Smith, P. E., ’39). (See fig. 40.)
E. Activities of the Ovary in Producing the Reproductive State
1. The Ovary as a "Storehouse"of Oogonia
The cortex of the ovary contains many young ova in various stages of development. In the human ovary shortly after birth, the number of oogonia in the cortex of each ovary has been estimated to reach a number as high as 300,000. This figure should not be taken too literally, as the amount of variability in the ovary from time to time is great and degeneration of ova is a common episode. Haggstrdm (’21 ) estimated that each ovary of a 22-yearold woman contained 200,000 young ova. In the ovaries of young rats, Arai (’20, a and b) estimated that there were on the average around 5,000 ova under 20 /x in diameter.
Without entering into the controversy (Chap. 3) relative to the rhythmic origin of germ cells in the ovary, one must accept the conclusion that the normal ovary has within it at all times during its reproductive life large numbers of oogonia in various stages of development. Thus the ovary, aside from its other activities, functions as a storehouse and nursery for young oogonia. Relatively few of these oogonia develop into mature eggs in the mammals. For example, the reproductive life of the human female occurs from about the age of 10 or 14 years to about 48 years. If one egg per monthly cycle is discharged from the ovary which is functional during that cycle, only about 400 eggs would be matured in this way. The number would be less if pregnancies intervened. If one accepts the figures given by Haggstrom, an enormous number of eggs of the human ovary never reach their potential goal. Similarly, according to Corner (’43): “The most prolific egg producer among mammals, the sow, might possibly shed a total of 3,000 to 3,500 eggs, allowing ten years of ovarian activity not interrupted by pregnancy, and assuming the very high average of 20 eggs at each three weekly cycle, but she has vastly more than this in the ovaries at birth.â€
2 . Position Occupied by the Primitive Female Germ Cells in the Ovarian Cortex
Within the cortex the definitive germ cells or oogonia are found in or near the germinal epithelium (figs. 39, 64). Some authors regard the oogonium as originating from the cells of the germinal epithelium. (See Chap. 3, section on “germ cell origin.â€) The definitive germ cell soon becomes associated with small epithelial cells (fig. 41). This complex of a germ cell with its associated epithelial cells is found somewhat deeper in the cortex, within or below the tunica albuginea. As the oogonium begins to experience the changes propelling it toward a state of maturity, it is regarded as an oocyte (Chap. 3).
Characteristics of the primitive oocyte are:
- an enlargement of the nucleus,
- changes within the chromatin material of the nucleus pertaining to meiosis (Chap. 3), and
- a growth and increase in the cytoplasmic substances (fig. 41).
Fig. 40. Effects produced by hypophysectomy on the rat ovary and of replacement therapy utilizing injections of pituitary gonadotrophins. (After Evans, Simpson, and Penchaez: Symposia of Quantitative Biology, Vol. 5, 1937. The Biological Laboratory, Cold Spring Harbor, L. 1., N. Y.) (A) Ovary of hypophysectomized animal. Observe
that Graafian follicles are small. They do not proceed further in their development than the beginning of antral vacuole formation unless replacement therapy is applied. (B) Ovarian condition of hypophysectomized animal receiving replacement therapy in the form of injections of the LH (ICSH) gonadotrophic factor of the anterior lobe of the hypophysis. Interstitial tissue is well developed. (C) Ovarian condition of hypophysectomized animal receiving the FSH gonadotrophic factor. Note follicular growth and antral vacuole formation; interstitial tissue between the follicles remains somewhat deficient. (D) Ovarian condition of hypophysectomized animal receiving injections of FSH plus LH. Corpora lutea are evident (as well as enlarged follicles not shown in the figure). Interstitial tissue remains deficient.
Fig. 41. Development of primary condition of the Graafian follicle in the opossum ovary. (A) Young oocyte with associated epithelial (granulosa) cells which in (B) have encapsulated the oocyte. (C) Encapsulating granulosa cells have increased in number and are assuming a cuboidal shape. (D) Fully developed condition of the primary Graafian follicle. Cf. secondary condition shown in fig. 42.
Fig. 42. Secondary conditions of the Graafian follicle in the opossum ovary. Cf. that of the rat ovary in fig. 40.
As these changes are initiated, the associated epithelial cells increase in
number and eventually encapsulate the oocyte (fig. 41B). This complex of
the oocyte with its surrounding layer of follicle cells is known as an egg follicle.
3. Primary, Secondary, and Tertiary Follicles of de Graaf
In the mammalian ovary the developing egg with its associated cells is called the Graafian follicle, so named after the Dutch scientist, Reinier de Graaf (fig. 1), who first described this structure in mammals in 1672-1673. De Graaf was in error, partly, for he believed that the whole follicular complex was the egg. The mammalian egg as such was first described in 1827 by Karl Ernst von Baer (1792-1876). The following statement is taken from de Graaf relative to egg follicles.
We may assert confidently that eggs are found in all kinds of animals, since they may be observed not only in birds, in fishes, both oviparous and viviparous, but very clearly also in quadrupeds and even in man himself. Since it is known to everyone that eggs are found in birds and fishes, this needs no investigation; but also in rabbits, hares, dogs, swine, sheep, cows, and other animals which we have dissected, those structures similar to vesicles exhibit themselves to the eyes of the dissectors like the germs of eggs in birds. Occurring in the superficial parts of the testicles, they push up the common tunic, and sometimes shine through it, as if their exit from the testis is impending. (See fig. 48; also Corner, ’43, page 128.)
The mammalian egg with a single layer of epithelial cells surrounding it is known as a primary Graafian follicle (fig. 41B-D). As the egg and follicle grow, the number of epithelial cells increase and eventually there are several
Fig. 43. Tertiary conditions of the Graafian follicle in the opossum ovary. Similar conditions are found in other mammalian ovaries. (A) Follicle in which the antral vacuoles are beginning to form. (B) This is a follicle in which the antral vacuoles are more numerous and are beginning to coalesce. (C) Condition of the Graafian follicle in the opossum ovary approaching maturity. Observe that the antral space is large and is filled with fluid, the liquor folliculi, while the egg and its surrounding cumulus cells are located at one end of the follicle. The thecal tissue around the follicle is well developed.
Fig. 44. Cellular wall of the mature Graafian follicle in the opossum.
layers of epithelial or granulosa cells surrounding the egg. It may now be regarded as a secondary Graafian follicle (fig. 42 A, B). When a stage is reached where the granulosa cells form a layer five to seven or more cells in thickness extending outward from the egg to the forming thecal layers, small antral vacuoles begin to appear among the granulosa cells. The latter follicle, which is capable of forming antral vacuoles, may be regarded as a tertiary Graafian follicle (fig. 43A).
4. Hormonal Factors Concerned with the Development of Egg Follicles
The ovary with its contained egg follicles is greatly affected by the gonadotrophic hormones produced in the pituitary body. The removal of the pituitary body (hypophysectomy) causes profound regression of the ovary and accessory reproductive structures. Accordingly, the response of the ovarian tissues to these hormonal substances produced by the hypophysis is responsible for development of the Graafian follicle beyond the early tertiary stage. (See fig. 40 A.) The relationships between the pituitary hormones and the ovary have been studied most intimately in the mammals; the pituitary and eggfollicle relationship in lower vertebrates is more obscure, and probably varies with the particular group.
a. Effects Produced by the Gonadotrophic Hormones on the Development of the Mammalian Egg Follicle
The follicle-stimulating hormone, FSH, appears to increase the number of oogonia and to aid the growth and differentiation of the older follicles. It is possible that some of the effects of FSH upon follicular growth are mediated through its ability, together with small amounts of the luteinizing hormone, LH (ICSH), to cause the formation of estrogen or the female sex hormone, although some investigators believe that estrogen production depends mainly upon the action of LH (ICSH). (See Evans and Simpson in Pincus and Thimann, ’50, p. 355.) In harmony with the idea that estrogen is involved in follicular growth there is some evidence which suggests that introduction of estrogens into the peritoneal cavities of fishes and mammals results in a stimulation of mitotic activity in the germinal epithelium of the ovary. It also has been shown that estrogenic substances retard ovarian atrophy in hypophysectomized immature rats.
When the Graafian follicles of the mammalian ovary reach the proper morphological and physiological conditions (i.e., when they reach the tertiary follicular stage) an increased sensitivity of the follicle cells to FSH occurs. As a result, antral vacuoles filled with fluid appear among the granulosa cells; these eventually coalesce and form the large antral cavity typical of the mature Graafian follicle of the mctatherian and eutherian mammal (fig. 43). The presence of LH (ICSH) is necessary to augment the action of FSH during the latter part of follicle development. The beneficial action of FSH and LH together in later follicular development is shown by the fact that the injection of pure FSH alone is incapable of stimulating growth of the follicle to its full size or to initiate an increased secretion of estrogen. LH aids the maturing process of the follicle only when present in very minimal amounts during the early stages of follicle development and in larger amounts during the later stages of follicular growth. Large amounts of LH in the earlier phases of the follicle’s development bring about a premature luteinization of the follicle with ultimate atresia. A proper quantitative balance of these hormones, therefore, is necessary, with FSH being in the ascendency during the earlier phases of follicle development, followed by increased amounts of LH with decreasing amounts of FSH as the follicle reaches maturity (figs. 22, 53, 59). (For references, consult Evans and Simpson, ’50; Turner, ’48.)
h. Stimulating Effects of the Pituitary Gonadotrophins on the Ovaries of Other Vertebrates
The hormonal control of the developing follicle of other vertebrate ovaries follows similar principles to those outlined above for the mammalian ovary, although data obtained from studies upon other vertebrates in no way compares with the large quantity of information obtained in mammalian studies. In the hen, FSH and LH injected together cause a rapid development of the follicles and premature discharge of the egg from the ovary (Fraps, Olsen, and Neher, ’42). However, in the pigeon. Riddle (’38) reports that another pituitary hormone, prolactin, appears to decrease the production of these hormones and stops egg production with a subsequent atrophy of the ovary. This may be a special means which reduces the number of eggs laid at each nesting period. In regard to accessory reproductive structures, an estrogenic hormone is produced in the ovary of the hen which has profound stimulating effects upon the growth of the oviduct (Romanoff and Romanoff, ’49, pp. 242-244). In the frog, Rana pipiens, mammalian pituitary gonadotrophins are able to effect ovulation (Wright and Hisaw, ’46). Pituitary gonadotrophins have been shown also to have profound stimulative effects on the ovaries of fishes, salamanders, and reptiles.
5. Structure of the Vertebrate, Mature Egg Follicle
As a result of the differentiation and growth induced by the gonadotrophic hormones of the anterior lobe of the hypophysis described in the preceding paragraphs, the egg follicle reaches a state of maturity (fig. 43C). This state is achieved when the follicle is about to rupture with the resultant discharge of the egg. The size of the mature egg follicle varies greatly in different metatherian and eutherian mammals, although the size of the follicle is not related to the size of the egg. On the other hand the size of the mature egg follicle in prototherian mammals and in other vertebrate species shows great divergences, being dependent in this group upon the size of the egg at the time of ovulation (fig. 46).
a. Structure of the Mature Follicle in Metatherian and Eutherian Mammals"'^
The structural pattern of the mature Graafian follicle in the human is strikingly similar to the follicles in other members of this group. It is a vesicular structure with a diameter approximating five millimeters. Externally, the follicle is composed of two connective-tissue layers, an inner cellular layer containing blood capillaries, the theca interna, and an external, fibrous layer, the theca externa (figs. 43C, 44). These two layers are not clearly separable. Passing inward from the theca interna is the basement membrane. Resting upon this membrane are several layers of epithelial cells comprising the membrana granulosa. The latter membrane borders the cavity or antrum of the follicle, which is filled with the liquor folliculi. This liquid is under considerable pressure in the follicle at the time of egg discharge or ovulation.
Projecting inward into the antrum on one side is a small, mound-like mass of granulosa cells, the cumulus oophorus (fig. 43C). Within this hillock of epithelium, is the egg, which measures in the human about 130 /x to 140 fx in diameter. In the opossum, the fully developed Graafian follicle is about 1.25 by 2 mm. in diameter, while the slightly oval egg approximates 120 by 135 ii. The egg of the rat and mouse is small, having a diameter of 75 ju, while that of the dog is about 140 /x; sow, 120 to 140 /x; rabbit, 120 to 130 /x; monkey, 110 to 120 /x; deer, 115 /x; cat, 120 to 130 (x\ mare, 135 /x; armadillo, 80 /X (Hartman, ’29).
- According to Strauss, ’39, the mature Graafian follicle of Erkulus is not a vesicular
structure, as in other higher mammals, but is filled with a loose meshwork of granulosa cells.
While one Graafian follicle in only one ovary is generally developed in the human, monkey, cow, ewe, elephant, etc., at each reproductive period, a multiple condition is found in many other mammals. Each ovary in the opossum may ripen seven or more follicles, in the bitch (female dog) from 2 to 7 follicles, and in the sow from 4 to 10 follicles at each reproductive period.
b. Structure of the Prototherian Egg Follicle
The follicle of the prototherian mammals contains a relatively large egg, while the surrounding fluid and follicular tissue in comparison is small in quantity (fig. 46). In these mammals the egg fills most of the follicular cavity, with the exception of a small fluid-filled space intervening between it and the zona pellucida which lies contiguous to the granulosa cells. Internal and external thecal tissues surround the granulosa cells as in the Graafian follicle of the higher mammals.
c. Egg Follicles of Other Vertebrates
The fully-developed egg follicle in most vertebrates is similar to that found in the prototherian mammals in that the egg tends to fill the entire follicle. The general structural relationships also are similar (figs. 45, 47).
6. Ovulatory Process; Possible Factors Controlling Ovulation
The following description of the ovulatory process in the mammal and in other vertebrates should not be construed as a description of the mechanism, as the exact mechanism is unknown. However, a certain amount of general information has been obtained concerning ovulation and the factors involved. Much of this information has been obtained from studies of the ovulatory
Fig. 45. (A) Young egg follicle of Cryptobranchus alleganiensis, a urodele. (From Noble: “Biology of the Amphibia,†New York, McGraw-Hill, after Smith.) (B) Diagrammatic representation of ovarian events in the frog resulting in egg discharge. (From Turner: “General Endocrinology,†Philadelphia, W. B. Saunders, slightly modified.)
Fig. 46. Diagrammatic representation of the egg of the prototherian mammal. Echidna.
Fig. 47. Diagrammatic drawings of the pendent egg follicle in the ovary of the hen. (A) Low magnification of the entire egg follicle. (B) More detailed view of the blastodisc portion of the egg, nearing maturity, in relation to the pedicle. The latter supports the follicle and permits the blood vessels to pass into and out of the follicle. Compiled from sections of the developing ovary of the hen.
process in higher mammals, especially the rabbit. Among other vertebrates
ovulation in the hen and frog have been the objects of considerable study.
a. Process of Ovulation in Higher Mammals
1) Changing Tissue Conditions Cuhninating in Egg Discharge from the Ovary. As the Graafian follicle enlarges and matures under the influence of the follicle-stimulating and luteinizing hormones, it moves closer to the ovarian surface (fig. 30). The surface of the ovary over the ripening follicle bulges outward, forming a mound-like protuberance (fig. 30). In the rabbit as shown by Walton and Hammond (’28) and Hill, Allen, and Cramer (’35) the central part of the original protuberance pushes out still further and forms a papilla-like swelling (fig. 48A-D). As the papilla develops, it becomes avascuiar, and the underlying tissues become thin and greatly distended. The tunica albuginea of the ovary and the two thecal layers of the follicle also are involved in this thinning-out process. As the distended papillary area continues to grow thinner, a small amount of blood followed by some of the follicular fluid containing the egg emerges from the follicle and passes into the surrounding area in close proximity to the infundibulum of the Fallopian tube (fig. 48 E, F). The entire process is a gradual one and may be described as gently but not violently explosive (Hill, Allen, and Cramer, ’35). It is of interest and significance to observe that Burr, Hill, and Allen (’35) were able to detect a change in electromotive force preceding and during the known period of ovulation.
Fig. 48. Process of ovulation in the rabbit. (A-C) Early external changes of the
surface of the ovary overlying the bulging Graafian follicle. (D) Formation of a secondary papilla. (E) Rupture of the secondary papilla with discharge of egg and follicular fluid, the latter oozing down over ovarian surface of the follicle. (F) Area of
rupture with oozing follicular fluid and egg greatly magnified. (G) Follicle after egg
discharge. (A-E and G, slightly modified from Walton and Hammond, Brit. J. Exp.
Biol., 6; F, modifier from Hill, Allen, and Kramer, Anat. Rec., 63.)
The process of papillary rupture in the rabbit occupies about five seconds;
egg discharge with the surrounding liquor folliculi occurs in approximately
30 to 60 seconds. After the egg has emerged, the follicle as a whole may
collapse. The slit-like opening through which the egg and follicular fluid
passed during ovulation soon is filled with a clot composed of coagulated
blood and follicular fluid (fig. 48G).
While the foregoing processes, visible on the ovarian surface, are consummated, certain internal changes occur which form a part of the ovulatory procedure. These changes arc as follows: At about the time the egg is to be extruded, the follicular fluid reaches its maximum in quantity. This increase produces considerable follicular turgidity which may be associated with an endosmotic effect due to an increase in the salt content of the contained fluid. Shortly before the surface of the follicle ruptures, the cumulus begins to disintegrate, and the egg lies free in the antral fluid. At about this time the first maturation division of the oocyte occurs in the majority of mammals, and the first polar body is extruded.
Concerning the internal changes accompanying rupture of the mammalian follicle, passing mention should be made of the theory that bursting blood vessels discharge their contents into the follicular fluid and thus cause sufficient pressure to rupture the follicle (Heape, ’05). Considerable blood discharge into the follicle seems to be present in some forms, e.g., the mare, quite absent in others such as the human, and present slightly in the opossum.
2) Hormonal Control of the Ovulatory Process. The hormonal mechanism involved in ovulation in the spontaneously-ovulating mammals probably is as follows: The follicle-stimulating hormone causes the growth and development of the follicle or follicles. Estrogen is released by the growing follicles and possibly by other ovarian tissues due to the presence of small amounts of LH, and, in consequence, the estrogenic hormone reaches a higher level in the blood stream (figs. 53; 59).
In the meantime, it is probable that the corpus luteum hormone, progesterone, is produced in small amounts. The exact source of this hormone is not clear. It may be produced by old corpora lutea or by the interstitial tissue of the ovary under the influence of luteotrophin, LTH. The presence of progesterone, in small quantities together with increasing amounts of estrogen, stimulates the anterior lobe to discharge increased amounts of the luteinizing hormone, LH (ICSH). (See figs. 22, 53, 59.) The elevated level of estrogen, according to this theory also causes a decreased output of FSH until it reaches a minimal level at the period shortly before egg discharge (figs. 53, 59). As a result, the increased quantity of LH together with FSH has an added effect upon the follicle which brings about the chain of events leading to egg discharge. Evans and Simpson in Pincus and Thimann (’50) give the proportion of 10 parts of FSH to 1 of LH (ICSH) as the proper hormonal balance in effecting ovulation in the hypophysectomized rat.
In those mammalian species where ovulation is dependent upon the act of copulation, a nervous stimulus is involved which increases the output from the pituitary gland of the gonadotrophic factors, particularly LH.
b. Ovulation in Vertebrate Groups Other Than the Higher Mammals
The physical mechanism involved in the ovulatory procedure in the lower vertebrate classes is different from that found in higher mammals. Two forms, the hen and the frog, have been studied in detail. These two animals represent somewhat different types of ovulatory behavior.
1) Hen. As the hen’s egg develops in the ovary, it gradually pushes the ovarian surface outward; it ultimately becomes suspended from the general surface of the ovary by means of a narrowing stalk, the pedicle (figs. 31, 47). When the ovulatory changes are initiated, the musculature of the ovarian wall overlying the outer surface of the egg appears to contract, and an elongated narrow area along this outer surface becomes avascular. This avascular area represents the place where the ovarian surface eventually ruptures to permit the egg to leave the ovary; it is called variously, the rupture area, stigma, or cicatrix. Gradually, the cicatrix widens and finally a slit-like opening is formed by a tearing apart of tissues in the central region of the cicatrix. Contractions of the smooth muscle fibers appear to be responsible for this tearing procedure (Phillips and Warren, ’37). The egg eventually is expelled through the opening and in many instances it rolls into the infundibular funnel of the oviduct which at this time is actively engaged in an endeavor to engulf or “swallow†the egg (fig. 31).
2) Frog. The egg of the frog projects into the ovarian cavity within the ovary and is attached to the ovarian wall by means of a broad area or stalk (fig. 45B). As the egg enlarges, it tends to push the ovarian surface outward, and the egg and its follicle thus forms a mound-like protuberance from the ovarian surface (figs. 45A, B; 72F). The egg and the surrounding ovarian tissue thus lies exposed on one aspect to the outer surface of the ovary. The outer surface of exposure is the stigma or area of rupture, and in the older follicles this area does not contain blood vessels (fig. 72F). As ovulation
approaches, an opening suddenly appears in the area of rupture. The musculature within the theca interna around the follicle then contracts, and the
egg rolls out through the opening in the rupture area like a big ameba (fig. 45B). As the egg passes through the aperture, it may assume an hourglass shape (Smith, B. G., T6). After the egg is discharged, the follicle contracts to a much smaller size (fig. 45B). It has been suggested that the rupture of the external surface of the follicle might be produced by a digestive enzyme (Rugh, ’35, a and b).
3) Hormonal Control of Ovulation in Lower Vertebrates. The hormonal mechanism regulating ovarian rupture and egg discharge in the lower vertebrate groups has not been as thoroughly explored in all of the vertebrate groups as it has in the mammals. However, sufficient work has been done to demonstrate that pituitary hormones are responsible in all of the major vertebrate groups, including the fishes. Amphibian pituitary implants under the skin or macerated anterior-lobe pituitary tissue injected into the peritoneal cavity of various amphibia have been effective in producing ovulatory phenomena (Rugh, ’35a). More recently, purified mammalian follicle-stimulating hormone, FSH, and luteinizing hormone, LH, have been used to stimulate egg discharge in frog ovarian fragments, as well as in normal and hypophysectomized females. However, the follicle-stimulating hormone alone will not elicit ovulation (Wright, ’45; Wright and Hisaw, ’46). Accordingly, both factors are necessary in the frog, as in mammals. In the hen, these two pituitary hormones have been shown to bring about ovulation when injected intravenously (Fraps, Olsen, and Ncher, ’42; Romanoff and Romanoff, ’49, pp. 208-215). Also, Neher and Fraps (’50) present evidence which suggests that progesterone plays a part in the physiological chain which elicits ovulation in the hen. A close relationship between the physiological procedures effecting ovulation in the hen and the mammal thus appears to exist.
c. Comparison of the Immediate Factors Effecting Egg Discharge in the
Vertebrate Group
In the vertebrates thus far studied contraction of muscle tissue of the follicle following the rupture of surface tissues presumably is the main factor which brings about egg expulsion. In higher mammals, associated with muscle contracture, there also may be an increase in follicular turgidity due to endosmotic phenomena associated with the contained follicular fluid (Walton and Hammond, ’28). In the frog, hen, and mammal the changes involved in the surface tissues leading to their rupture are associated with the following sequence of events:
( 1 ) avascularity of the surface tissues,
(2) a thinning of the surface tissues, and finally
(3) a rupture of these tissues.
7. Internal Conditions of the Ovary as an Ovulatory Factor
Internal conditions of the ovary undoubtedly are important in controlling follicular growth and ovulation. For example, in the Northern fur seal, Callorhinus ur sinus, the female begins to breed at the age of two years. These seals travel north once a year to the Pribilof Islands in the Bering Sea where they go on land to give birth to the single young and also to breed. Most of the cows arrive between the middle of June and the middle of July. Heavy with young, the females give birth to their offspring within a few hours or days after their arrival. Breeding again takes place about six days after parturition. However, lactation continues, and the young are taken care of during the summer months.
Accordingly, these seals mate each year and it appears that for any particular year the mating behavior and ovulation of the egg are controlled by the ovary, which does not have a corpus luteum. As the corpus luteum, which forms after ovulation in the site of the Graafian follicle, from which the egg is discharged, remains intact for a considerable portion of the year, the ovary which does not have the corpus luteum develops the Graafian follicle for the next summer period. The following year the other ovary will function, and so on, alternating each year (Enders, et al., ’46). Thus, the corpus luteum appears to function as a suppressor of follicular growth within the ovary in which it lies. In the human female, one ovary functions to produce an egg one month, while the following month the other ovary ovulates its single egg. It is possible that here also the large corpus luteum suppresses follicular growth within the particular ovary concerned.
During gestation, the presence of the corpus luteum and its hormone, progesterone, suppresses follicle growth and ovulation in most of the mammalian group. (The placenta may be the source of progesterone during the later phases of pregnancy in forms such as the human.) On the other hand, in the mare, according to Cole, Howell, and Hart (’31 ), ovulation may occur during pregnancy. Species differences, therefore, exist relative to the control of ovulation by the corpus luteum and its hormone, progesterone.
8. Number of Eggs Produced by Different Vertebrate Ovaries
The number of eggs produced during the lifetime of the female varies with the species and is correlated generally with the amount of care given to the young. In many fishes which experience little or no parental care, enormous numbers of eggs may be produced, as for example, in the cod where several millions of eggs are spawned in one season. However, in many of the elasmobranch fishes (i.e., the shark group) the eggs develop within the oviduct, and the young are born alive. Therefore, only six to a dozen eggs produced each reproductive period is sufficient to keep the shark species plentiful. In the hen, where careful breeding and selection have been carried out with a view to egg production, a good layer will lay from 250 to 300 eggs a year. The deer, moose, fur seal, etc., ovulate one egg per year over a life span of a few years. As stated previously, the human female might ovulate as many as 400 eggs in a lifetime. In some species the reproductive life is brief. For example, in the Pacific salmon (Oncorhynchus) females and males die after their single spawning season, and a similar demise occurs in the eel (Anguilla).
9. Spontaneous and Dependent Ovulation in the Mammals and in Other Vertebrates
Spontaneous ovulation without apparent stimulation from external sources occurs commonly throughout the vertebrate series. However, dependent ovulation conditioned by psychic or other nervous stimuli also is found extensively. In certain mammals ovulation has been shown to be dependent upon the stimulus induced by copulation, as, for example, the ferret, mink, rabbit, cat, shrew, etc. The stimulus, carried through the nervous system, affects in some way the anterior lobe of the pituitary gland which then produces increased amounts of LH in addition to FSH. These females experience estrus spontaneously, but later follicle growth and egg discharge are dependent upon the added stimulation afforded by copulation.
The element of nervous stimulation has a fundamental relationship to the ovulatory phenomena in the vertebrates. Dependent ovulation occurs in certain birds, such as the pigeon, where mating provides a psychic or nervous stimulation which effects ovulation. The presence of two eggs in the nest tends to suppress ovulation. The removal of these eggs will arouse the ovulatory procedures. However, the pigeon may sometimes lay eggs without the presence of a male. In wild birds in general, the mating reaction is linked to the stimulus for egg laying. The hen, on the other hand, is not dependent upon copulation, but in many of the domestic varieties the presence of a number of eggs in the nest appears to suppress egg laying. In the lower vertebrates nervous stimuli also appear to have an influence upon ovulation. The mating antics of many fish and amphibia may be connected with ovulatory phenomena.
10. Egg Viability after Discharge from the Ovary
The length of time that the egg may survive and retain its capacity for fertilization after leaving the ovary depends upon the nature of the egg and its membrane and the surrounding environment. In the urochordate, Styela, the egg may remain for 3 to 4 hours after it is discharged into the sea water and still be capable of fertilization. In the elasmobranch fishes, reptiles, and birds the conditions of the oviduct are such that fertilization must take place in the upper part of the oviduct within a few seconds or minutes after the egg reaches the infundibular portion. In Fundulus hetewclitus and possibly many other teleost fishes, the egg must be fertilized within 15 to 20 minutes after spawning. In the frog, the egg passes to the uterus at the lower end of the oviduct shortly after it leaves the ovary. Under ordinary reproductive temperatures which obtain in the spring, the egg may remain there for 3 to 5 days without producing abnormalities. If kept at very cool temperatures, the period may be extended. Among the mammals the viability after ovulation varies considerably. In the mare, fertilization must occur within about 2 to 4 hours; rabbit, 2 to 4 hours (Hammond and Marshall, ’25); rat, about 10 hours; mouse, 12 to 24 hours (Long, ’12; Charlton, ’17); opossum, probably within the first hour or so because of the deposition of the albuminous coating in the oviduct; fox, probably only a few hours; sow, about 24 hours or less; man, probably 24 hours or less. In the guinea pig, functional degeneration may begin within 4 to 8 hours after ovulation (Blandau and Young, ’39) .
11. History of the Egg Follicle after Ovulation
a. Follicles Which Do Not Develop a Post-ovulatory Body
The changes which occur within the egg follicle after the egg has departed are most variable in different vertebrate species. In most of the fish group the ovary as a whole shrinks to a fraction of its previous size, and many very small, immature eggs, interstitial tissue, and collapsed, contracted, empty follicles make up its composition. Similarly, in frogs, toads, and salamanders the collapsed follicle which follows ovulation does not develop an organized structure. The thecal tissue contracts into a small rounded form within which are a few follicle cells (fig. 45B). These bodies soon disappear.
In many snakes and in turtles, the follicle collapses after ovulation, and it is questionable whether organized bodies develop in the site of the ovulated follicle. A similar condition appears to be the case in birds. However, Pearl and Boring (’18) described an abbreviated form of a corpus luteum in the hen in both discharged and atretic follicles. Also, Rothschild and Traps (’44) found that the removal of the recently ruptured follicle or of this follicle together with the oldest maturing follicle, at a time when the egg which originated from the ruptured follicle is in the oviduct, retarded the laying of the egg from 1 to 7 days. Removal of other portions of the ovary in control hens “practically never†resulted in egg-laying retardation. The ruptured follicle, therefore, is believed, by these investigators, to have some influence on the time of lay of the egg. Whether the hormone progesterone or something similar to it may be produced by the ruptured follicle of the hen is questionable, although present evidence appears to suggest that it does (Neher and Traps, ’50).
b. Follicles Which Develop a Post-ovulatory Body; Formation of the
Corpus Luteum
Post-ovulatory bodies or corpora lutea (yellow bodies) develop in the ovaries of elasmobranch fishes which give birth to their young alive. Also in viviparous snakes of the genera Natrix, Storeria, and Thamnophis, it has been shown that the removal of the ovaries with their corpora lutea invariably results in resorption of the young during the first part of gestation and abortion of the young during the midgestational period, while their removal during the close of gestation permits normal birth to occur (Clausen, ’40). The differentiation of the corpus luteum in the snake involves the granulosa cells of the follicle and possibly the theca interna. The differentiated organ appears similar to that of the mammal (Rahn, ’39).
The function of the corpus luteum which develops in the site of the ruptured follicle in all mammals, including the Prototheria (fig. 49), has been the subject of a long series of studies. (See Brambell, ’30, Chap. 9; Corner, ’43, Chap. V.) Its function during the reproductive period of the female mammal is described below under the section of the ovarian hormones. The events leading to the formation of the corpus luteum in the mammalian ovary may be described as follows: After the discharge of the egg, the follicle collapses. The opening of the follicle at the ovarian surface through which the egg emerged begins to heal. A slight amount of blood may be deposited within the antrum of the follicle during the ovulation process in some mammals. If so, the follicle in this condition is known as the corpus hemorrhagicum.
Fig. 49. (A) Luteal cells of the corpus luteum of the opossum. The cellular conditions
in other higher mammals are similar. The centsal core has not yet been invaded and resorbed by the phagocytes accompanying the ingrowing luteal cells and blood vessels. This
central core is composed of coagulated blood, blood cells, and connective tissue fibrils.
(B) Corpus luteum of the platypus (Ornithorhynchiis).
Then, under the influence of the luteinizing hormone, LH, the granulosa cells
of the follicle and also cells from the theca interna, together with blood capillaries, proliferate and grow inward into the antral space (figs. 22, 30, 49).
Phagocytes remove the blood clot within the antral space if present, during
the inward growth of these structures. As the ingression of cells and capillaries into the follicle continues, the granulosa cells begin to form large, polyhedral lutein cells, while the epithelioid cells of the theca interna form a
mass of smaller cells which resemble the true lutein cells; the latter are formed
in the peripheral area of the corpus luteum and are called paralutein cells.
The small spindle-shaped cells of the theca interna, together with blood capillaries, become dispersed between the lutein cells, forming a framework for
the latter.
If the egg is fertilized, the corpus luteum persists and is known as the corpus luteum of pregnancy; if fertilization does not take place, it is called the corpus luteum of ovulation. The latter body soon degenerates. Histologically, both types of corpora are identical when first formed. Eventually the corpus luteum undergoes involution, and its site becomes infiltrated with connective tissue. The latter structure is sometimes referred to as the corpus albicans.
12. Hormones of the Ovary and Their Activities in Effecting the Reproductive Condition
The ovary produces two important hormones which have a profound effect upon the reproductive process. These two hormones are the female sex hormone, estrogen, and the gestational hormone, progesterone.
a. Estrogenic Hormone
1) Definition and Source of Production. The induction of estrus (see p. 93 ) or conditions simulating this state is a property of a relatively large number of organic compounds. Because of this estrus-inducing power, they are spoken of as estrogenic substances or estrogens. Estrogens are widely distributed in nature. Two of the most potent natural estrogens are estradiol and estrone (theelin). Both have been extracted from the mammalian ovary and are regarded as primary estrogenic hormones. The most powerful estrogen is estradiol, and it is regarded at present as the compound secreted by the ovary. During pregnancy it also is found in the placenta. These structures are not the only sources of estrogens, however, for it is possible to extract them from urine after ovariectomy, and they occur in the urine of males as well as that of females. The urine of the stallion is one of the richest sources of estrogens, and the testis contains a high estrogenic content (Pincus and Thimann, ’48, p. 381 ). Estrogens are found also in various plants, such as the potato, pussy willow, etc.
The structural formulae of estradiol and of estrone are as follows:
OH ()
Estradiol Estrone
2) The Ovary as the Normal Source of Estrogen in the Non-pregnant
Female. Aside from the fact that estradiol and estrone are readily extracted
from the ovary, certain experiments tend to focus attention on the ovary as
an important site of estrogen production. For example, the removal of the
ovaries of a normal, adult female mammal causes the accessory reproductive
organs to undergo profound atrophy. The administration of appropriate
amounts of estrogen will restore the accessories of such a female to the condition normal for the resting state. (Consult Pincus, ’50, in Pincus and
Thimann, Chap. I.) The injection of follicle-stimulating hormone with small
amounts of the luteinizing hormone into the diestrous (i.e., sexually-resting)
female with intact ovaries results in follicular development within the ovaries,
accompanied by hypertrophy of the accessory reproductive organs to the full
estrous condition (Nelsen and White, ’41 ; Pincus, ’50, in Pincus and Thimann) .
These and similar experiments point to the ovary as the main site of estrogen
formation in the body of the non-pregnant female.
The exact structures of the ovary responsible for estrogen elaboration are not easily determined. Estrogen is found in all parts of the ovary, but certain observations and experimental results suggest that it is formed in relation to the follicular tissues and also by the so-called interstitial tissue of the ovary. For example, when tumors occur within the thecal tissue of the egg follicle in women who have experienced the menopause, there is often an accompanying hypertrophy of the accessory organs. This relationship suggests that thecal gland tissue of the follicle may have the ability to elaborate estrogen (Geist and Spielman, ’43). On the other hand, the normal hypertrophy of the granulosa cells of the egg follicle during the normal reproductive cycle, with the presence of follicular fluid containing estrogen in the antral space of the follicle, points to the granulosa cells as a possible source of estrogen. Also, it has been observed that tumorous growths of the granulosa cells of the follicle produce an excess of estrogenic substance (Geist and Spielman, ’43). Thus, these observations point to the granulosa cells of the egg follicle of the ovary as being capable of estrogen formation. Another possible source of estrogen secretion in the ovary is the interstitial cells, derived in part from theca interna tissue and atretic follicles. These cells are large polyhedral epithelioid cells scattered between the follicles. Their growth appears to be directly stimulated by the injection of pure luteinizing hormone (LH; ICSH) in hypophysectomized rats (fig. 40). A rapid production of estrogen results from such injections and this may mean that these cells are involved in estrogen production within the ovary (Evans and Simpson in Pincus and Thimann, ’50).
In the pregnant female mammal the placenta appears to be a source of estrogen production (Pincus and Thimann, ’48, p. 380; Turner, ’48, p. 422). This is suggested by the successful extraction of estrogen from the placenta of the human and the mare and also by the fact that in these females removal of the ovaries during the middle or latter phase of gestation does not result in estrogen diminution in urinary excretion.
3) Pituitary Control of Estrogen Formation. The removal of the anterior lobe of the pituitary gland of the female results in marked atrophy of ovarian structures (figs. 40, 50) and of the accessory reproductive organs. Replacement therapy (i.e., the injections of the pituitary gonadotrophins, FSH and LH) produces a normal reconstitution of the ovarian and reproductive duct tissues, effecting a normal appearance and functioning of these structures
Fig. 50. Follicular atresia in guinea pig ovary. (Redrawn from Asdell, ’46.) This atresia
is a sporadic but not uncommon event in the normal ovary of the mammal. However,
after removal of the pituitary gland, marked atresia and degeneration of the more mature
follicles occur. (A) Fragmentation of granulosa cells is shown. (B) Beginning invasion of the antral space by theca interna tissue is depicted. (Cf. fig. 40A.) (C) Late stage of atresia with invasion of the antral space by internal thecal cells.
Fig. 51. Effects of estradiol (estrogen) upon the female genital tract of the opossum. (After Risman, J. Morphol., 81.) (A) Reproductive tract of an ovariectomized female.
(B) Hypertrophied condition of a female experiencing the normal estrous changes. (C) Reproductive tract of an ovariectomized female injected with estradiol (0.9 mm.) 36 days after the ovaries were removed.
(fig. 40). This evidence suggests that the pituitary gonadotrophins, FSH and
LH, control the development of the ovary and, through their influence upon
the ovarian tissues, promote the secretion of estrogen with the subsequent
hypertrophy of the female accessory reproductive structures. It is to be observed that it is not at all clear that FSH in pure form is able to elicit estrogen
production without the presence of LH (ICSH). (See Evans and Simpson
in Pincus and Thimann, ’50, p. 355.)
4) Effect of Estrogen upon the Female Mammal. The changes in the mammalian accessory reproductive organs produced by estrogen are marked. An increase in vascularity and great hypertrophy of the accessory structures result from its injection into ovariectomized females. (See figs. 51, 52, 53.) Increased irritability and activity of the accessory structures also occur. This increased activity appears to be an important factor in the transportation of sperm upward within the female accessory organs to the region where the egg awaits the sperm’s arrival.
The alterations in behavior of the female as a result of estrogen stimulation may be considerable. Females actually seek the presence of a male during the period of strong estrogenic influence. The long journey of the female fur seal to the mating grounds in the Bering Sea, the bellowing and tireless search of the cow moose, the almost uncontrollable demeanor of seeking the male on the part of the female dog or of the cow in “heat†— these are a few illustrations of the regnant power of this stimulant upon the female mammal.
The culmination of these changes in behavior, resulting in a receptive attitude
toward the male, is reached at about the time when the egg is discharged
from the ovary in many mammalian species. In certain other mammals the
period of heat may precede the ovulatory phenomena.
5) Effects of Estrogen in Other Vertebrates. In the hen, estrogenic hormone causes enlargement and functional activity of the oviduct. Estrogenic substance, when injected into female chicks from the eighteenth to the fortieth day, causes an enlargement of the oviduct to about 48 times the natural size. Estrogen also has a profound effect upon the activities of the full-grown hen and aids in egg production (Romanoff and Romanoff, ’49; Herrick, ’44). Estrogen has a pronounced effect upon the oviducts of other vertebrate forms.
b. Progesterone - The Hormone of the Corpus Luteum
1) Production of Progesterone. The luteinizing hormone, LH, of the anterior lobe of the pituitary gland is concerned not only with the development of the egg follicle, but also, after ovulation or the discharge of the egg from the egg follicle, the remaining granulosa cells, and also, some of the theca interna cells of the follicle are induced by the LH factor to form the corpus luteum (figs. 30, 49). Corpora lutea also may be induced by estrogens. This, however, appears to be an indirect stimulus aroused through estrogenic stimulation of the pituitary gland to secrete added amounts of the LH factor (Evans and Simpson in Pincus and Thimann, ’50, p. 359).
Fig. 52. Characteristic histological changes in the female reproductive tract under the influence of estrogen and progesterone. (A-C) Vaginal cyclic changes in the rat. In (A) is shown the condition of the vaginal wall in the diestrus (resting) condition; (B) shows changes in vaginal wall structure during estrus. Observe cornification of outer layer of cells; (C) shows vaginal wall tissue immediately following estrus, i.e., during metestrus. The presence of progesterone tends to suppress the action of estrogen. (After Turner: General Endocrinology, Philadelphia, Saunders.) (D, E) Cyclic changes of the Fallopian tube of the human female during the reproductive cycle. In (D) is shown the midinterval of the cycle, i.e,, at a time paralleling estrus in mammals in general; (E) shows the cellular condition of the lining tissue of the Fallopian tube just before menstruation. In (D) the tissue has responded to the presence of estrogen; (E) effect of progesterone is shown. (After Maximow and Bloom: A Textbook of Histology, Philadelphia, Saunders.) (F, G) Cyclic changes in the uterine-wall tissue during the reproductive cycle in the human female. In (F) is shown general character of the uterine wall during the follicular phase, i.e., responses to estrogen; (G) shows the general condition of the uterine wall following ovulation. The uterus is now responding to the presence of progesterone added to the follicular or estrogenic stimulation. (After Maximow and Bloom: A Textbook of Histology, Philadelphia, Saunders.)
A further pituitary principle, however, seems to be involved in the functional behavior of the corpus luteum. This principle, referred to as luteotrophin (LTH), is associated with the lactogenic-hormone complex produced by the anterior lobe of the pituitary body; it induces the morphologically developed corpus luteum to secrete progesterone. (Consult Evans and Simpson in Pincus and Thimann, ’50, pp. 359, 360; Turner, ’48, p. 379, for references.)
The structural formula of progesterone is as follows:
2) Effects of Progesterone. Progesterone reduces the irritability of the accessory structures and stimulates the mucosa of the uterus to undergo further development. This increased developmental and functional condition of the
Fig. 53. Relationship of the pituitary gonadotrophins and ovarian hormones to the developing Graafian follicle and reproductive-duct change in a polyestrous female mammal.
The Graafian follicle responds to the pituitary gonadotrophins, FSH and LH, with the subsequent growth and ultimate rupture of the follicle and ovulation. Ovulation terminates the follicular phase of the cycle. Under the influence of the LH factor the corpus luteum is established. The latter becomes functional as a result of stimulation by the luteotrophic (lactogenic) hormone. The progestational hormone (progesterone) then is elaborated by the luteal cells. The activity of the latter together with estrogen controls the luteal phase of the cycle.
The rising level of estrogen in the blood suppresses FSH secretion, and together possibly with small amounts of progesterone stimulates LH secretion. Estrogen and small amounts of progesterone also probably stimulate the secretion of large quantities of LTH, and the latter stimulates the secretion of progesterone from the recently formed corpus luteum. When the estrogen level falls, FSH again is secreted.
When the estrogen level rises, the endometrium of the uterus and vaginal mucosa are stimulated. The presence of progesterone suppresses vaginal development, but the uterine mucosa is stimulated to greater activity. Observe that the involution of the endometrial lining in most mammals is gradual but in primates it is precipitous and violent, resulting in menstruation (Cf. fig. 59). (The diestrous period on this chart is shown as a relatively brief period compared to the other aspects of the reproductive cycle. However, it may be very long in females which do not experience a polyestrous condition and in some species it may last a good portion of a year.) (Compiled from various sources in the literature. The portion of the chart showing pituitary and gonadal hormonal relationships is based on data obtained from The Schering Corporation, Bloomfield, N. J.)
accessory reproductive structures added normally to the estrogenic effects
during the reproductive cycle constitutes the luteal phase of the cycle. In this
phase of the cycle the uterine glands elongate and begin secretion, and the
uterus as a whole is prepared for gestation as a result of the action of the
progestational hormone, progesterone, associated with estrogen. (See figs.
53, 59.)
F. Reproductive State and Its Relation to the Reproductive Cycle in Female Vertebrates
The changes in the female reproductive organs resulting in structural growth and development referred to above (70-74, 85-88) are consummated in the ability of the female to fulfill the reproductive functions. The phase of the reproductive events characterized by the ability to reproduce is known as the reproductive climax. This period of culmination remains for a brief period, to be followed by recession and involution once again to a resting condition. This developmental progression to a state of reproductive climax followed by regression to a resting condition constitutes a cycle of changing events. When conditions again are right, the cycle is repeated. Each of these cyclic periods is known as a reproductive or sexual cycle (figs. 53-59). The reproductive life of all female vertebrates is characterized by this series of cyclic changes.
In most vertebrate species, the female experiences one sexual cycle per year, which corresponds to the seasonal cycle in the male. However, in various mammals and in certain birds, such as the domestic hen, several or many reproductive cycles may occur during the year. The male, under these conditions, is a continuous breeder; that is, he produces sperm continuously throughout the year.
1. Sexual Cycle in the Female Mammal a. Characteristics and Phases of the Reproductive Cycle
The estrous cycle in mammals is a complex affair composed of a number of integrated subcycles. The changes occurring in the ovary are called the ovarian cycle; the cellular changes in the uterine (Fallopian tube) form a cycle; the responses in the mammary glands constitute the mammary cycle; the cyclic events in the uterus make up the uterine cycle, while those in the vagina form the vaginal cycle (figs. 53, 54, 57).
The entire estrous cycle may be divided by ovarian changes into two main phases: the follicular phase and the luteal phase (fig. 53). The former is under the immediate influence of the enlarging Graafian follicle, which in turn is stimulated by the follicle-stimulating and luteinizing hormones of the pituitary gland, with the subsequent production of estrogen. It is probable that the luteinizing hormone, LH, is mainly responsible for estrogen secretion. (See Evans and Simpson in Pincus and Thimann, ’50, p. 355.) The luteal phase on the other hand is controlled by the activities of the corpus luteum, which has replaced the Graafian follicle under the influence of the luteinizing hormone. The production of progesterone by the corpus luteum is effected as stated previously by the pituitary hormone, luteotrophin (LTH). Ovulation is the pivotal point interposed between these two phases. The follicular phase may occur without ovulation, but the true luteal phase of a normal or fertile reproductive cycle is dependent upon the ovulatory phenomena. Certain luteal conditions may be elaborated in an anovulatory cycle, but we are here concerned with the normal events of the fertile reproductive cycle.
The follicular phase includes that portion of the reproductive cycle known as proestrus and a considerable part of estrus. Proestrus is the period of rapid follicular growth and elaboration of the estrogenic substance which precedes the period of estrus. Estrogen stimulates developmental changes in the cellular structure of the accessory reproductive organs, particularly the vagina and the uterus (figs. 52, 53). Estrus represents the climax of the follicular phase. As such, it is a period of sexual receptivity of the male, and, in spontaneously ovulating forms, of ovulation. During other periods of the cycle the female is indifferent or even antagonistic to the male. The period of estrus is often called period of heat, or period of rut. Estrus is followed by pregnancy if mating is allowed and is successful, or, in many species, by a period of pscudopregnancy if mating is not permitted or if the mating is sterile (figs. 53-57). In some animals, such as the dog, pseudopregnancy is a prolonged normal event even if mating does not occur, continuing over a period almost as long as that of normal pregnancy (fig. 54). In other animals, such as the opossum, pseudopregnancy forms but a brief episode.
Pseudopregnancy is, generally speaking, intermediate in duration between that of a normal luteal phase of the cycle and that of gestation. In those female mammals where it does not occur normally, it is aroused by such procedures as sucking of the nipples, stimulation of the vagina and cervix by the natural mating process, or by artificially stimulating these structures. In some forms, such as the rabbit, pseudopregnancy is aroused by mere handling or even by sight of a male. (For discussion, see Selye, ’48, p. 813.)
The general changes of growth and development of the accessory organs which occur during pregnancy and pseudopregnancy are controlled largely by the secretions of the corpus luteum. The conditions thus imposed by the corpus luteum comprise the luteal or progestational phai^e of the cycle (fig. 57).
In most mammals, if pregnancy does not occur, the ovary and accessory organs again gradually return to the sexually-resting condition known as diestrus (fig. 53). In man and other primates the changes within the uterus are not gradual but are precipitous, and most of the endometrial lining, together with considerable amounts of blood, is discharged to the outside (figs. 53, 59). This phenomenon is called menstruation. The causes of menstruation are largely problematical; it is related to the fall of the level of either or both of the ovarian hormones, progesterone and estrogen. Why certain mammals should experience violent endometrial changes evident in menstruation and others a gradual involution and resorption is a question for the future. The general period of change following estrus in a non-fertile cycle is known as metestrus (fig. 53). In the rat and mouse, metestrus is short, about one or two days; in the human and opossum it occupies approximately ten days to two weeks of the cycle; in the dog, about 40 to 50 days, depending upon the pseudopregnant conditions experienced in different females. The word anestrus is applied to a prolonged diestrus or sexual quiescence between two sexual cycles. However, the involution experienced by the sexual organs in anestrus is somewhat more profound than that prevailing during a brief diestrus. The term lactational diestrus is used to refer to the prolonged diestrous condition in forms such as the rat, wherein estrus is suppressed in the mother while suckling the young.
The length of the sexual cycle varies with the species. When females of the rat or mouse are kept away from a male, the estrous or sexual cycle will repeat itself every 4 to 5 days. In the sow it occurs every 17 to 20 days. In the opossum there is a prolonged anestrous period during the summer and autumn months followed by a polyestrous period during the winter and spring when the estrous cycle reoccurs about every 28 days. In the human female, the sexual cycle occupies about 28 days, and there arc probably about ten normal ovulatory cycles in a year. Some human females may have more, while others experience a slightly smaller number of true ovulatory cycles per year.
Many mammals have one estrous cycle per year. This condition, known as monestrus, is true of most wild mammals, such as the deer, wolf, fox, moose, and coyote. In the shrew, mink, and ferret the moncstrous period may be prolonged if the female is kept away from the male.
Various types of polyestrous conditions exist. In the female dog, for example, there are two or three estrous periods per year about 4 to 6 months apart. In the cat there are several cycles about two weeks apart during the autumn, winter, and spring. In the domestic sheep there is a polyestrous period from September to February in which the cycles occur about every 17 days, followed by an anestrous period from early March to September. In the mare in North America, estrous cycles of about 19 to 23 days occur from March to August. In South America the breeding season is reversed, corresponding to the reversed seasonal conditions south of the equator. In England many mares breed in autumn and winter (Asdell, ’46).
In some mammals estrus may follow immediately after parturition or birth of the young. This may occur occasionally in the rat. Under normal conditions in the fur seal, the female lactates and gestates simultaneously. It is not a common procedure.
It should be observed that there are two aspects of the female reproductive cycle of the mammal relative to fertilization or the bringing together of the male and female reproductive cell. One aspect is the sexual receptivity of the female; the other is the time of ovulation of the egg. In most female mammals sexual receptivity and ovulation are intimately associated and occur spontaneously in the cycle; in others the two events may be separated. In the former group, the development of “heat†and the maturing of the egg follicle are closely associated, while in the latter the conditions favoring sexual receptivity or heat are developed considerably in advance of the maturation of the follicle, as noted in the table below.
b. Relation of Estrus and Ovulation in Some Common Mammals
1) Spontaneously Ovulating Forms (Sexual Receptivity of Male Occurs at
or near Time of Ovulation):
Length of Estrus or Period of Heat
Time of Ovulation
Dog
True period of heat about 5-10 days in the middle of a 21 -day estrous period
Variable: 1st day; 2nd day; 5th day; etc., of true period of heat
Guinea pig
6-1 1 hrs.
Views vary: 1-2 hrs. after heat or estrus begins; 10 hrs. after; at end of estrus
Man
Receptivity not always related to cyclic events
12-17 days after onset of preceding menstruation; average around 14th day
Mare
2-11 days; average length 5-6 days
About 1-2 days before end of estrus; best breeding about 3 days after heat begins
Sheep
About 36 hrs.
Late in estrus or just after estrus
ends; presumably about 20-36
hrs. after estrus begins
Sow
Silver fox
Rat
15 days
1-5 days; occurs once a year in February
One determination estimates estrus to be 9-20 hrs.; most receptive to male about first
3 hrs. of heat. Another determination estimates estrus to be 12-18 hrs.
About 1-3 days after onset of estrus 1st or 2nd day of estrus
8 -11 hrs. after beginning of heat
2) Dependent Ovulatory Forms (Sexual Receptivity (Heatl Occurs Previous to Time of Ovulation);
Length of Estrus or Period
of Heat
Time of Ovulation
Cat
2-3 days
Time of ovulation uncertain but is
dependent upon copulation
Length of Estrus or Period of Heat
Time of Ovulation
Rabbit (tame)
Estrus prolonged indefinitely during the breeding season from spring to summer; a series of different sets of egg follicles matured; each series lasts about a week, then becomes atretic
Ovulation 10-14 hrs. after mating
Shrew
Estrus prolonged
About 55 -70 hrs. after mating
Ferret
Estrus prolonged
About 30 hrs. after mating
If ovulation and subsequent pregnancy are not permitted by mating, ovarian
involution occurs, and an anestrous interlude is established. Anestrus in the
common rabbit, Oryctolagus cuniculus, occurs from October to March, but
is not absolute.
c. Non-ovulatory (Anovulatory) Sexual Cycles
Not all of the cyclic changes referred to above in those species which normally experience spontaneous ovulation are related to definite egg discharge. Some cycles occur, more or less abortively, without ovulation of the egg. This may happen in the human or in other mammals, such as the dog and monkey. Cycles without ovulations are called non-ovulatory cycles. Menstruation may follow non-ovulatory cycles in the human female.
d. Control of the Estrous Cycle in the Female Mammal
In the control of a reproductive cycle in the vertebrate animal, three main categories of factors appear to influence its appearance and course. These are:
(1) external environmental factors, such as light and temperature,
(2) external factors governing food supply, and
(3) internal factors resulting from an interplay of the activities of the pituitary gland, the ovary, general body health, and of the particular hereditary constitution of the animal.
These factors should be considered not alone in terms of the immediate production of fertile conditions in the parent, but rather, in view of the total end to be achieved, namely, the production of a new individual of the species. For example, the reproductive cycle in the deer reaches its climax or estrus in the autumn after a long period of lush feeding for the mother. The young are born the next spring amid favorable temperatures, followed by another period of bountiful food supply for the mother during lactation and for the fawn as it is weaned. A receding light factor in the late summer and early fall thus may be correlated with the period of heat, which in turn proves to be an optimum time of the year for conception with the resulting birth the following spring. Similarly, light ascendency is a factor in producing fertility in many birds. Here the incubation period for the young is short and a plentiful supply of food awaits the parents and young when it is needed. In other words, the factors which induce the onset of the reproductive state are correlated with the conditions which enhance the end to be achieved, namely, the production of a new individual.
Let us consider next the internal factors which induce the breeding state in the female mammal. The commonly held theory regarding the pituitaryovarian relationship governing the control of the reproductive periods in the mammal which ovulates spontaneously is as follows (figs. 53 and 59) :
( 1 ) FSH of the pituitary gland stimulates later follicular growth. This factor probably is aided by small amounts of the luteinizing factor, LH, to effect an increased production by the ovarian tissues of the estrogenic hormone. Early follicle growth probably occurs without FSH.
(2) Estrogen output by the ovary rises steadily during the period previous to ovulation.
(3) Old corpora lutea or other ovarian tissue possibly secrete minimal amounts of progesterone under the influence of lutcotrophin, LTH.
(4) As the quantity of estrogen rises in the blood stream, it inhibits the production of FSH and together with small quantities of progesterone, increases the output of LH from the pituitary gland. This combination also may cause an increased outflow of the luteotrophic factor.
(5) An increased amount of LH aids in effecting ovulation and the subsequent luteinization of the follicle. As the follicle becomes converted into the corpus luteum, the presence of the luteotrophic factor brings about the formation of increased quantities of progesterone and maintains for a time the corpus luteum and the functional luteal phase of the cycle.
(6) In those mammals possessing a scries of repeating sexual cycles, it is assumed that the fall of estrogen in the blood stream after ovulation suppresses the LH outflow and permits a fresh liberation of FSH from the anterior lobe of the pituitary gland, thus starting a new cycle. The lowering of the estrogen level may be particularly and immediately effective in forms such as the rat and mouse, which have a short metestrus or luteal phase in the estrous cycle.
e. Reproductive Cycle in Lower Vertebrate Females
While the words estrus, heat, or rut are generally applied to the mammalian groups, the recurrent periods of sexual excitement in lower vertebrates are fundamentally the same sort of reaction, although the changes in the reproductive tract associated with ovarian events are not always the same as in mammals. However, similar cyclic changes in the ovary and reproductive tract are present in the lower vertebrates, and their correlation with the activities of the pituitary gland is an established fact. Consequently, the words estrus, rut, sex excitement, and heat basically designate the same thing throughout the vertebrate series — namely, a period during which the physiology and metabolism of the parental body is prepared to undertake the reproductive functions. In this sense, the words estrus, anestrus, heat, etc. also may be applied to the male as well as to the female when the male experiences periodic expressions of the sexual state.
Although the reproductive cycle in all vertebrates represents basically a periodic development of the reproductive functions, there is a marked difference between the estrous cycle in the female mammal and the reproductive cycle in most of the other female vertebrates with the exception of viviparous forms among the snakes, lizards, and certain fishes. This difference is due to the absence of a true luteal phase in the cycle. The follicular phase and elaboration of estrogen appears to be much the same in birds, amphibia, and fishes as in the mammals, but the phase of the cycle governed by progesterone secretion, associated with a gestational condition in the accessory reproductive organs, is found only among those vertebrates which give birth to their young alive.
The reproductive cycles in certain vertebrates may be changed by selective breeding and domestication. For example, the domestic hen is derived from the wild jungle fowl. The jungle fowl conform to the general stimuli of nature as do most wild birds, and the reproductive cycle is associated with a particular season of the year. However, domestication and selection by man of certain laying strains have altered the original hereditary pattern of seasonal laying. Consequently, good layers will lay eggs over an extended period of the year, although there is a strong tendency to follow the ancestral plan by laying most of the eggs during the spring and summer months; during the fall and winter months, a smaller number of eggs are laid. Some of the varieties of the domestic hen conform more closely to the ancestral condition than do other strains. Similar changes may be produced in the buffalo, which in nature breeds in middle to late summer but in captivity has estrous periods three weeks apart throughout the year (Asdell, ’46).
G. Role of the Ovary in Gestation (Pregnancy)
1. Control of Implantation and the Maintenance of Pregnancy in Mammals
The ruling power of the ovary over the processes involved in pregnancy is absolute, particularly during its earlier phases. In the first place, the corpusluteum hormone, progesterone, is necessary to change the uterus already conditioned by the estrogenic hormone into a functionally active state. The latter condition is necessary for the nutrition and care of the embryo. A second change which the gestational hormone imposes upon the genital tract of the female is to quiet the active, irritable condition aroused by the estrogenic factor. Progesterone thus serves to neutralize or antagonize the effects of the estrogenic hormone. A placid condition of the uterus must be maintained during the period immediately following copulation if the fertilized egg is to be cared for within the uterine structure. Large doses of estrogens injected into mammals shortly after copulation prevent implantation of the embryo in all species thus far studied. (See Selye, ’48, p. 822.)
A third effect of the presence of progesterone is the inhibition of the copulatory responses. Immediately following estrus and ovulation, the female dog will fight off the aggressiveness of the male — an aggressiveness which she invited a day or two previously. This change in behavior is introduced by the development of the corpora lutea and the initiation of the luteal phase of the reproductive cycle. Similar anaphrodisiac changes are sometimes mentioned in the behavior of the human female during the luteal phase of the cycle. Progesterone injections also inhibit the copulatory responses in the ferret (Marshall and Hammond, ’44). All of the above-mentioned activities of progesterone thus inhibit or antagonize the condition aroused by estrogenic stimulation.
However, aside from these immediate metestrous and post-ovulatory changes in behavior induced by progesterone, one of its most essential aetivities is concerned with the maintenance of gestation or pregnancy. Ovariectomy or the removal of the ovaries at any time during the gestational period in the rat, mouse, and goat results in death and abortion of the embryo. During the first part of pregnancy in the rabbit, the ovaries must be left intact but may be removed in the closing phase without endangering the gestational process. In the human female, and also in the mare, cat, dog, guinea pig, and monkey, the ovaries may be removed during the latter half of pregnancy without danger to the offspring. However, ovariectomy performed in the early stages of pregnancy in these animals, as well as in all other mammals thus far studied, produces abortion (Pincus, ’36; Selye, ’48, p. 820). The corpus luteum hormone, therefore, is essential in the early phases of gestation in all mammals, and it appears to be necessary during most of the pregnant period in many other mammals.
It is highly probable that the placenta takes over the elaboration of progesterone in those mammals where ovariectomy is possible after the first part of pregnancy has elapsed. In the human female the corpus luteum normally involutes at about the third month of pregnancy, but progesterone may be extracted from the placenta after this period.
Although certain effects of the estrogenic hormone appear to be neutralized (or antagonized) by progesterone during the early phases of reproduction, other effects of estrogen in relation to progesterone are important for the maintenance of the pregnant condition. In this connection the estrogenic hormone appears to suppress some of the growth-promoting effects of progesterone. The two hormones thus work together to promote a gradual development of the uterine tissue and maintain a regulated, balanced condition throughout pregnancy. The placenta, through its ability to elaborate progesterone and estrogen during the latter phases of pregnancy, is an important feature regulating pregnancy in some mammals.
It should be emphasized in connection with the above statements that the presence of the fertilized egg and its subsequent development in some manner affects the maintenance of the corpus luteum. The mechanism by which this influence is conveyed to the ovary is unknown.
2. Gestation Periods, in Days, of Some Common Mammals*
- Adapted from Asdell, ’46; Cahalane, ’47; Kenneth, ’43.
Armadillo (Dasypus novemcinctus)
150
Bear, black (Ursiis americanus)
210
Bear, polar (Thalarctos maritimus)
240
Beaver, Canadian (Castor canadensis)
94-100
Bison (Bison bison)
276
Cat, domestic (Felis catus)
60
Cattle (Bos taurus)
282
Chimpanzee (Pan satyrus)
250
Deer, Virginian (Odocoileus virginianus)
160-200
Dog, domestic (Canis familiaris)
58-65
Donkey, domestic (Eqiius asinus)
365-380
Elephant (Elephas africanus)
641
Elephant (Elephas indicus)
607-641
Elk (A Ices alces)
250
Ferret (Putorius faro)
42
Fox, arctic (Alopex lagopus)
60
Fox, red (Vulpes vulpes and V. fulva)
52-63
Giraffe (Giraffa Camelopardalis)
450
Goat, domestic (Capra hircus)
140-160
Guinea pig (Cavia porcellus)
68-71
Horse (Equus cabaltus)
330-380
Man (Homo sapiens)
270-295
Lion (Felis leo)
106
Lynx (Lynx canadensis)
63
Marten, American (Martes americana)
267-280
Mink (Mustela vison)
42-76
Mole (Talpa europaea)
30
Monkey, macaque (Macaca mulato)
160-179
Mouse, house (Mas rnusculus)
20-21
Opossum (Didelphis virginiana)
13
Pig (Sus scrofa)
115-120
Rabbit (Lepus; Sylvilagus; Oryctolagus)
30-43
Rats (Various species)
21-25
Seal, fur (Callorhinus sp.)
340-350
Sheep, domestic (Ovis aries)
144-160
Skunk, common (Mephitis mephitis)
63
Squirrel, red (Tamiasciurus sp.) 30-40
Tiger (Felis tigris) 106
Whale (Various species) 334-365
Wolf (Canis lupus) 63
Woodchuck (Marmota monax) 35-42
Zebra, mountain (Equus zebra) 300-345
3. Maintenance of Pregnancy in Reptiles and Other
Vertebrates
In certain viviparous species of the genera Storeria, Matrix and Thamnophis, Clausen (’40) reports that ovariectomy during gestation results in resorption of the embryo when performed during the earlier phases of gestation and abortion during the middle of gestation, but during the terminal portion of pregnancy the process is unaffected and the young are born normally. These results are similar to those obtained from the rabbit as noted previously.
While experimental evidence is lacking in other vertebrate groups which give birth to the young alive, the evidence obtained from reptilian and mammalian studies suggests that hormones are responsible for the maintenance of pregnancy. In harmony with this statement, it may be pointed out that in the viviparous elasmobranch fishes (e.g., sharks) corpora lutea are developed in the ovaries.
H. Role of the Ovary in Parturition or Birth of the Young
The real factors bringing about parturition are not known, and any explanation of the matter largely is theoretical. However, certain aspects of the subject have been explored. For example, it was observed above that progesterone appears to antagonize the action of estrogen with the result that the uterus stimulated to irritability and contractility under the influence of estrogen is made placid by the action of progesterone. In harmony with this action studies have shown that estrogen tends to increase during the final stages of normal gestation, while progesterone appears to decrease, accompanied by an involution of the corpora lutea. Consequently, the foregoing facts have suggested the “estrogen theory,†which postulates that activities of the uterine musculature are increased by the added amounts of estrogen in the presence of decreasing amounts of progesterone during the latter phases of pregnancy. In confirmation of this theory, it has been shown that progesterone injected into a pregnant rabbit near the end of the gestation period will tend to prolong gestation. A second theory of parturitional behavior assumes that the posterior lobe of the pituitary gland elaborates oxytocin which induces increased uterine activity, resulting in birth contractions (Waring and Landgrebe in Pincus and Thimann, ’50). Again, a third concept emphasizes Ihe possibility that the placenta may produce substances which bring about contractions necessary for the expulsion of the young (Turner, ’48, p. 428). Oxytocic substances have been extracted from the placenta, which suggests the validity of this theory.
Fig. 54. Changes occurring in the reproductive organs and mammary glands of the
bitch during the reproductive cycle. The student is referred to Asdell (’46), pp. 150-156
and Dukes (’43), pp. 678-682, for detailed description and references pertaining to the
data supporting this chart. The gestation period is based upon data supplied by Kenneth
(’43) and the author’s personal experience with dogs.
Fig. 55. Reproductive and pregnancy cycles in the sow. (Modified from data supplied
by Corner, Carnegie Inst., Washington, pub. 276, Contrib. to Embryol., 13; the parturition
data derived from Kenneth, ’43.)
The specific functions of the ovary in parturition probably are more pronounced in those forms where it is essential throughout most of the gestational period, such as the viviparous snakes, and among the mammals, such
forms as the opossum, rat, mouse, and rabbit. The waning of corpus-luteum
activity in these species may serve to lower the level of progesterone in the
body and thus permit some of the other factors, such as estrogen or the
pituitary principle, to activate the uterus.
Another factor associated with the ovary and parturition is the hormone relaxin. This substance was first reported by Hisaw and further studied by this investigator and his associates (Hisaw, ’25, ’29; and Hisaw, et al., ’44).
Fig. 56. Reproductive and pregnancy cycles in the mare. (Parturition period based upon data supplied by Kenneth (’43); other data supplied by Asdell (’46) and Dukes (’43).) It is to be noted that the first corpus luteum of pregnancy degenerates after about 35 days; the second “crop of corpora lutea†(Asdell) degenerate by 150 days. The ovaries may be removed after 200 days of pregnancy without causing abortion of young.
Relaxin aids in the production of a relaxed condition of the pelvic girdle, a necessity for the formation of a normal birth passageway for the young. Relaxin somehow is associated in its formation with the presence of progesterone in the blood stream and also with the intact reproductive system. Relaxin together with estrogen and progesterone establishes a relaxed condition of the tissues in the pubic area of the pelvic girdle.
I. Importance of the Ovary in Mammary-Gland Development and Lactation
Estrogen and progesterone together with the lactogenic hormone, luteotrophin, of the pituitary gland are necessary in mammary-gland development. The entire story of the relationship of these and of other factors in all mammals or in any particular mammal is not known. However, according to one theory of mammary-gland development and function, the suggestive roles played by these hormones presumably are as follows (fig. 58): Estradiol and other estrogens bring about the development of the mammary-gland ducts; as a result a tree-like branching of the ducts is effected from a simple im
Fig. 57. Reproductive and pregnancy cycles in the cow. (Parturition period based upon
data supplied by Kenneth (’43), also by Asdell (’46), Other data for chart derived from
Asdell (’46).
Three main characteristics of heat or estrous period are evident: (1) A duration of heat of only about 10 to 18 hours; (2) abundant secretion during heat of a “stringy mucus,’’ derived from mucoid epithelium of vagina and from sealing plug of cervix when cow not in estrus (Asdell); and (3) ovulation occurs from 13Vi to 15Vi hours after termination of estrus (Asdell), Variation in time of ovulation may be considerable, from 2 hours before end of estrus to 26 hours after (Asdell).
mature pattern established during earlier development (fig. 5 8 A, A', B). The male mammary gland may remain similar to the condition shown in fig. 58A. The maturing of the egg follicles within the ovary and the concomitant formation of estrogen which accompanies sexual maturity is linked with the more complex state of the mammary-gland system shown in fig. 58B.
The next step of mammary-gland development is carried out under the influence of progesterone. Progesterone is necessary for the development of the terminal glandular tissue or alveoli associated with these ducts (fig. 58C, D). Finally, the pituitary lactogenic hormone (luteotrophin [LTH]; prolactin) stimulates the actual secretion of milk (fig. 58E). Recent research also has shown that the lactogenic hormone collaborates in some way with estrogen and progesterone in the development of the mammary-gland tissue.
Fig. 58. Mammary gland changes in relation to reproduction. (Figures are a modification of a figure by Corner: Hormones in Human Reproduction, Princeton, Princeton
University Press. The figure in the latter work was based on a figure by C. D. Turner:
Chap. XI of Sex and Internal Secretions, by Allen, et al., Baltimore, Williams & Wilkins,
1939.) Factors involved in mammary gland development and secretion are somewhat as
follows: (A, A') Condition of the young, infantile gland. (B) Development from a
simple, branched, tubular gland of the immature animal (A') into a compound tubular
gland presumably under the direct stimulation of estrogen, according to one theory, or
by the action of estrogen upon the pituitary gland which then releases mammogen I,
producing these changes, according to Turner, et al.: Chap. XI, Sex and Internal Secretions, by Allen, et al., Baltimore, Williams & Wilkins. (C) Transformation of the compound tubular gland into a compound tubulo-alveolar gland under the influence of progesterone, during the first part of pregnancy, or, according to Turner, et al., by the influence
of estrogen plus progesterone which causes the pituitary to release a second mammogen
which produces the alveolar transformation. (D) Effect of the latter part of pregnancy
is to bring about a development of the cells of the acini of the acinous or alveolar system.
The unit shown in (D) represents a simple, branched, acinous gland, in which there are
six alveoli or acini associated with the duct. (E) Affect of parturition is to release the
lactogenic hormone (prolactin; luteotrophin) from the pituitary gland which brings about
milk secretion. During pregnancy the high levels of estrogen presumably inhibit milk
secretion. However, following pregnancy the level of estrogen is lowered permitting
lactogenic-hormone action upon the alveoli of the gland.
The removal of the placenta and embryo at any time during gestation permits milk flow, provided the mammary glands are sufficiently developed. In the human, any remains of the placenta after birth inhibit milk secretion, probably because the estrogenic hormone is elaborated by the placental remnants. (See Selye, ’48, p. 829.)
In the rabbit, estrogen and progesterone are necessary for the elaboration of the duct and secretory acini; in the guinea pig and goat, and to some extent in the primates, including the human female, estrogen alone is capable of producing the development of the entire duct and acinous system. (See Turner, ’48, p. 430.)
During pregnancy, the actual secretion of milk is inhibited by the estrogenic
hormone produced by the ovary and the placenta. The role of estrogen as
an inhibitor of lactation is suggested by the fact that, after lactation has started
following normal parturition, it is possible in the cow and human to suppress
milk flow by the administration of estrogens. After parturition, however,
estrogen is no longer present in^sufflcient amounts to suppress the secretion
of milk, and the mammary gland begins to function. (In the fur seal a postpartum estrus with ovulation follows a short time after parturition. However,
the amount of estrogen produced by this reproductive cycle is not sufficient
to curb lactation.) The neurohumoral reflex, or “suckling reflex,†produced
by the sucking young appears to maintain the flow of milk over a period of
time. Probably this reflex causes a continuous discharge of the lactogenic hormone from the anterior lobe of the hypophysis.
Another theory of mammary-gland development maintains that estrogen stimulates the anterior pituitary gland to release mammogen, which causes development of the duct system, and estrogen plus progesterone induce a second mammogen which stimulates lobule-alveolar development. The lactogenic hormone produces the actual secretion of milk. The ovary thus assumes considerable importance in controlling the (morphological) development of the mammary glands in mammals, particularly in those forms in which the functional condition of the ovary is maintained throughout most of the gestational period, e.g., rat, rabbit, dog, etc. In other species, such as the human, mare, etc., the placenta through its ability to duplicate the production of the ovarian hormones, assumes a role during the latter phase of pregnancy. (For further details, consult Folley and Malpress in Pincus and Thimann, ’48; Selye, ’48, pp. 828-832; and Turner, ’48, pp. 428-448.)
Fig. 59. Stages in the reproductive cycle of the human female and its pituitary-ovarianendometrial relationships (Cf. fig. 53). (Compiled from various sources in the literature.) (a) As shown at the extreme right of the figure, a fall in the level of estrogen and progesterone in the blood stream, either or both, is associated with endometrial necrosis, bleeding, and discharge (menstruation), (b) The lowering of the estrogen level is associated with a new outflow of the follicle-stimulating hormone (FSH), as shown at the right of the figure, (c) In the left side of the figure, the influence of FSH induces egg follicles, probably several, to grow. Antral spaces appear and enlarge. The presence of a small amount of the luteinizing hormone (LH) together with FSH stimulates the secretion of estrogen by the ovarian tissues, possibly by the follicles and interstitial tissue between the follicles, (d) In consequence, the estrogen level rises in the blood stream, and menstruation subsides by the fourth day. (e) The continued influence of estrogen produces endometrial growth, and probably increases the outflow of LH from the pituitary (fig. 53). It is probable, also, that the increased estrogen level stimulates a release of the luteotrophic hormone from the pituitary, which in turn stimulates the formation of a small quantity of progesterone by either the interstitial tissue of the ovary or in old corpora lutea. (f) Some of the developing egg follicles degenerate, while one continues to develop, (g) The elevation of estrogen suppresses the outflow of FSH as indicated by the heavy broken line to the left, (h) The elevated level of estrogen together possibly with small amounts of progesterone evokes an increased outflow of LH and LTH as indicated by the heavy broken line to the right, (i) LH and FSH bring about ovulation at about the fourteenth day. (j) LH causes development of corpus luteum. (k) LTH elicits secretion of progesterone by corpus luteum. Possibly some estrogen is secreted also by corpus luteum. (1) Progesterone and estrogen stimulate added development of endometrium, (m) In the absence of fertilization of the egg, the corpus luteum regresses, with a subsequent fall of progesterone and estrogen levels in the blood stream, terminating the cycle and permitting a new menstrual procedure.
In the dog or opossum during each reproductive cycle, the mammary glands
are stimulated to grow and may even secrete milk (dog). These changes
closely parallel the ovarian activities, particularly the luteal phase of the cycle.
In the human, functional growth changes occur in pregnancy, but, pending
the events of the ordinary cycle, alterations in the duct system are slight although the breasts may be turgid due to increased blood flow and connectivetissue development.
J. Other Possible Developmental Functions Produced by the Ovary
As the eggs of the opossum and rabbit travel through the uterine (Fallopian) tube toward the uterus, they are coated with an albuminous, jelly-like coating. Similar jelly coatings are added to the eggs of the bird, reptile, frog, toad, and salamander. These coatings or membranes added to the egg as it travels through the oviduct are known as tertiary egg membranes.
In the toad, the secretion of the protective jelly by the oviduct can be elicited by the lactogenic hormone present in beef pituitary glands. The secretion of the albuminous jelly coatings around the eggs of frogs, salamanders, reptiles, and birds may be related to this hormone. The formation of the crop milk of pigeons has been shown by Riddle and Bates (’39) to be dependent upon the presence of the lactogenic hormone.
The function of the ovary in influencing the outflow of the lactogenic hormone from the pituitary, if present in the above cases of glandular secretion, must be an indirect one. Evans and Simpson in Pincus and Thimann (’50) ascribe the outflow of the “lactogenic hormone (luteotrophic hormone)†of the mammalian pituitary to estrin produced by the ovary. It is possible that in the salamanders, frogs, toads, and the birds an indirect ovarian influence may similarly induce secretion of the lactogenic hormone which in turn governs the elaboration of the albuminous jelly deposited around the egg in transit through the oviduct.
K. Determinative Tests for Pregnancy
Various tests have been used to determine the probability of pregnancy in the human female. These tests are discussed in Chapter 22.
Bibliography
Arai, H. 1920a. On the postnatal development of the ovary (albino rat) with especial reference to the number of ova. Am. J. Anat. 27:405.
. 1920b. On the cause of hypertrophy of the surviving ovary after semispraying (albino rat) and the number of ova in it. Am. J. Anat. 28:59.
Aronson, L. R. and Holz-Tucker, M. 1949. Ovulation in the mouthbreeding cichlid fish, Tilapid macrocephala (Bleeker). Anat. Rec. 105:568.
Asdell, S. A. 1946. Patterns of Mammalian Reproduction. Comstock Publishing Co., Inc., Ithaca, New York.
Blandau, R. J. and Young, W. C. 1939. The effects of delayed fertilization on the development of the guinea pig ovum. Am. J. Anat. 64:303.
Brambell, F. W. R. 1930. The Development of Sex in Vertebrates. The Macmillan Co., New York.
Burns, R. K., Jr. 1931. The process of sex transformation in parabiotic Amblystoma. II. Transformation from male to female. J. Exper. Zool. 60:339.
Burr, H. S., Hill, R. T., and Allen, E. 1935. Detection of ovulation in the intact rabbit. Proc. Soc. Exper. Biol. & Med. 33:109.
Cahalane, V. H. 1947. Mammals of North America. The Macmillan Co., New York.
Charlton, H. H. 1917. The fate of the unfertilized egg in the white mouse. Biol. Bull. 33:321.
Clausen, H. J. 1940, Studies on the effect of ovariotomy and hypophysectomy on gestation in snakes. Endocrinology. 27:700.
Cole, F. J. 1930. Early Theories of Sexual Generation. Oxford University Press, The Clarendon Press, New York.
Cole, H. H., Howell, C. E., and Hart, G. H. 1931. The changes occurring in the ovary of the mare during pregnancy. Anat. Rec. 49:199.
Corner, G. W. 1943. The Hormones in Human Reproduction. Princeton University Press, Princeton, New Jersey.
Dukes, H. H. 1943. The Physiology of
Domestic Animals. Comstock Publishing
Co., Inc., Ithaca, New York.
Enders, R. K., Pearson, O. P., and Pearson,
A. K. 1946. Certain aspects of reproduction in the fur seal. Anat. Rec. 94:213,
Evans, H. M. and Simpson, M. E. 1950. Chap. VI. Physiology of the gonadotrophins in The Hormones, Vol. II., by Pincus and Thimann. Academic Press, Inc., New York.
Folley, S. J. and Malpress, F. H. 1948. Chaps. 15, 16. Hormonal control of mammary growth and lactation in The Hormones, Vol. 1., by Pincus and Thimann. Academic Press, Inc., New York. Fraps, R. M., Olsen, M. W., and Neher,
B. H. 1942. Forced ovulation of normal ovarian follicles in the domestic fowl. Proc. Soc. Exper. Biol. & Med. 50:308.
Geist, S. H. and Spielman, F. 1943. Endocrine tumors of the ovary. J. Clin. Endocrinol. 3:281.
Haggstrom, P. 1921. Zahlenmassige Analyse der Ovarien eines 22-jahrigen gesunden Weibes. Upsala Lakaref. Forh. 26:1.
Hammond, J. and Marshall, F. H. A. 1925. Reproduction in the Rabbit. Oliver & Boyd, Ltd., Edinburgh.
Hartman, C. G. 1929. How large is the mammalian egg? Quart. Rev. Biol. 4:373.
Heape, W. 1905. Ovulation and degeneration of ova in the rabbit, Proc. Roy. Soc. London, s.B. 76:260,
Herrick, E. H. 1944. Some influences of stilbestrol, estrone and testosterone propionate on the genital tract of young female fowls. Poul. Sc. 23:65.
Hill, R. T., Allen, E., and Kramer, T. C. 1935. Cinemicrographic studies of rabbit ovulation. Anat. Rec. 63:239.
Hisaw, F. L. 1925. The influence of the ovary on the resorption of the pubic bones of the pocket gopher, G corny s bursarius (Shaw). J. Exper. Zool. 42:411.
. 1929. The corpus luteum hormone. I. Experimental relaxation of the pelvic ligaments of the guinea pig. Physiol. Zool. 2:59.
, Zarrow, M. X., Money, W. L.,
Talmadge, R. V. N., and Abramowitz, A. A. 1944. Importance of the female reproductive tract in the formation of relaxin. Endocrinology. 34:122. Humphrey, R. R. 1929. Studies on sex reversal in Amblystoma. I. Bisexuality and sex reversal in larval males uninfluenced by ovarian hormones. Anat. Rec. 42:119.
Kenneth, J. H. 1943. Gestation Periods.
Oliver & Boyd, Ltd., Edinburgh.
Long, J. A. 1912. The living eggs of rats and mice with a description of apparatus for obtaining and observing them. Lfniv. California Publ., Zool. 9(3): 105. Marshall, F. H. A. and Hammond, J., Jr. 1944. Experimental control by hormone action of the oestrous cycle in the ferret. J. Endocrinol. 4:159.
Mason, K. E. 1939. Chapter 22 in Allen, et al.. Sex and Internal Secretions. 2d ed., The Williams & Wilkins Co., Baltimore.
Meher, B. H. and Fraps, R. M. 1950. The addition of eggs to the hen’s clutch by repeated injections of ovulation-inducing hormones. Endocrinology. 46:482.
- 4elsen, O. E. and Maxwell, N. 1942. The
structure and function of the urogenital region in the female opossum compared with the same region in other marsupials. J. Morphol. 71:463.
and White, E. L. 1941. A method
for inducing ovulation in the anoestrous opossum (Didelphys virginiana). Anat. Rec. 81:529.
earl, R. and Boring, A. M. 1918. The corpus luteum in the ovary of the domestic fowl. Am. J. Anat. 23:1. hillips, R. E. and Warren, D. C. 1937. Observations concerning the mechanics of ovulation in the fowl. J. Exper. Zool. 76:117.
incus, G. 1936. The Eggs of Mammals. The Macmillan Co., New York.
. 1950. The Physiology of Ovarian
Hormones, Chap. I. The Hormones, Vol. II, in Pincus and Thimann, Academic Press, Inc., New York.
and Thimann, K. V. 1948. The
Hormones. Vol. I. Academic Press, Inc., New York.
Rahn, H. 1939. Structure and function of placenta and corpus luteum in viviparous snakes. Proc. Soc. Exper. Biol. & Med. 40:381.
Riddle, O. 1938. Prolactin, a product of the anterior pituitary, and the part it plays in vital processes. Scient. Monthly. 47:97.
and Bates, R. W. 193^. Chap. 20.
The preparation, assay and actions of the lactogenic hormone in Allen, et al., Sex and Internal Secretions. 2d ed., The Williams & Wilkins Co., Baltimore.
Romanoff, A. L. and Romanoff, A. J. 1949. The Avian Egg. John Wiley & Sons, Inc., New York.
Rothchild, 1. and Fraps, R. M. 1944. On the function of the ruptured ovarian follicle of the domestic fowl. Proc. Soc. Exper, Biol. & Med. 56:79.
Rugh, R. 1935a. Ovulation in the frog. I. Pituitary relations in induced ovulation. J. Exper. Zool. 71:149.
. 1935b. Ovulation in the frog.
II. Follicular rupture to fertilization. J. Exper. Zool. 71:163.
Ryder, J. A. 1885. On the development of viviparous osseous fishes. Proc. U. S. Nat. Mus. 8: No. 9, 128.
Selye, H. 1948. Textbook of Endocrinology. Acta Endocrinologica. Universite de Montreal, Montreal.
Smith, B, G. 1916. The process of ovulation in Amphibia. Michigan Acad. Sc., 18th Ann. Rep. p. 102.
Smith, P. E. 1939. Chap. XVI. The effect on the gonads of ablation and implantation of the hypophysis and the potency of the hypophysis under various conditions. Allen, et al.. Sex and Internal Secretions. 2d ed.. The Williams & Wilkins Co., Baltimore.
Strauss, F. 1939. Die Befruchtung und der Vorgang der Ovulation bei Ericulus aus der Familie der Centetiden. Biomorphosis. 1:281.
Turner, C. D. 1948. General Endocrinology. W. B. Saunders Co., Philadelphia.
Walton, A. and Hammond, J. 1928. Observations on ovulation in the rabbit. British J. Exper. Biol. 6:190.
Waring, H. and Landgrebe, F. W. 1950. Chap. VIII. Hormones of the posterior pituitary in The Hormones, Vol. II, by Pincus and Thimann. Academic Press, Inc., New York.
Wright, P. A. 1945. Factors affecting in
vitro ovulation in the frog. J. Exper.
Zool. 100:565.
and Hisaw, F. L. 1946. Effect of mammalian pituitary gonadotrophins on ovulation in the frog, Rana pipiens. Endocrinology. 39:247.
Cite this page: Hill, M.A. (2024, April 23) Embryology Book - Comparative Embryology of the Vertebrates 1-2. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Comparative_Embryology_of_the_Vertebrates_1-2
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G