User:Z3332863: Difference between revisions
(→Lab 2) |
|||
| Line 78: | Line 78: | ||
'''Copyright''' | '''Copyright''' | ||
2011 Carlo et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |||
Revision as of 15:34, 5 August 2012
Lab Attendance
Lab 1 --Z3332863 11:47, 25 July 2012 (EST)
Lab 2 --Z3332863 10:20, 1 August 2012 (EST)
Individual Assessments
Lab1
Origin of Nobel Prize & Discoverer
In 2010, Robert G. Edwards won the Nobel Prize for developing In vitro Fertilisation. IVF originated in 1950s when Edwards began fertilizing human eggs in cell culture dishes as a way of treating infertility. In 1978, Edward's IVF technology gave the world's first IVF baby. Over the next few years, Edwards and his team fine-tuned the technique of IVF.
Research paper on fertilisation:
<pubmed>22317970</pubmed>
What does this paper tell us about fertilisation?
This article looks at the rise of aneuploidies in IVF embryos from women around 40yrs of age. To do this Handyside et al, used 'microarray comparative genomic hybridisation' technology to study the chromosome copy number in the zygote, the 1st and 2nd polar bodies in older women receiving IVF treatment. Handyside et al found that:
- Most of the aneuploidies of IVF embryos arose from the 2nd meiotic division of the oocyte. This is surprising because most aneuploidies in naturally fertilized embryos arise from Meiosis I of the oocyte.
- Aneuploidies in IVF zygotes were not due to non-disjunction of chromosomes in the oocyte. Instead, these Aneuploidies were due to predivision of the chromatids in the oocyte.
- In IVF zygotes made from aged oocytes, often there were multiple aneuploidies in 1 zygote.
By looking at the origin of aneuploidies in IVF zygotes, these scientists are trying to find a way to reduce these aneuploidies.
Lab 2
Prac class work - See below for lab 2 Assessment
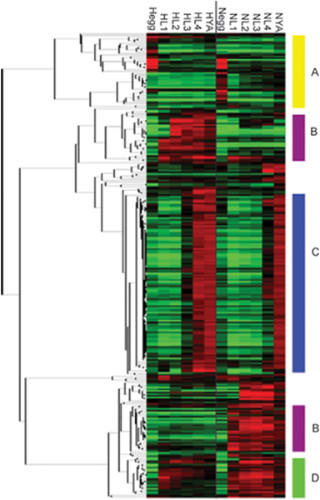
Genes that display significant strain by stage variation fall into four main categories
Genes that display significant strain by stage variation fall into four main categories. The genes that show significant variation due to strain by stage interaction were clustered hierarchically. Four distinct patterns appear in the clustered data, identified by the letters A–D. CB4856 (H) are on the left, from the egg to the young adult, while N2 (N) are on the right, from the egg to the young adult. Missing values were imputed using KNN-impute and expression values represent the average from four replicates.
Further Description
Capra et al were studying the variation in gene expression during the different stages of Development of different isolates of C. elegans. This image is a microarray result, showing genes that are expressed in different amounts in different strains of C. elegans during development. This Micrarray shows allow these differentially expressed genes to be classified into 4 groups. It’s likely the genes in the same cluster are regulated in the same way.
Reference
<pubmed>19116648</pubmed>
Copyright 2008 Capra et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
lab 2 Assessment
Q1. Paper & Image Related to Fertilization
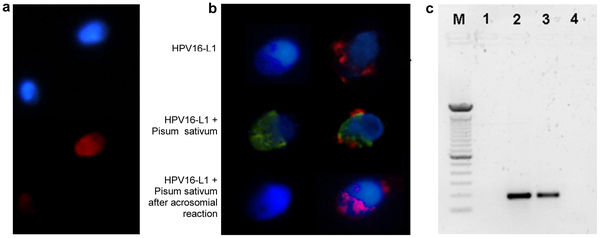
Detection and Localisation of HPV in Sperms
Detection and localization of HPV in human sperm.
a. Fluorescence in situ hybridization (fluorescence microscope) for HPV DNA on sperm from a patient with HPV16 in semen. Infected and noninfected sperm are shown. Red: HPV DNA (Texas red); blue: nuclear staining (DAPI). b. Immunofluorescence (confocal fluorescence microscope) for HPV16 capsid protein L1 on sperm from a control (left) and a patient with HPV16 in semen (right). Upper panel, L1 antibody; central panel, L1 antibody and Pisum Sativum (acrosome); lower panel, L1 antibody and Pisum Sativum after induction of the acrosome reaction. Red: HPV16 L1; green: Pisum Sativum; blue: nuclear staining (DAPI). c. PCR for HPV E7 gene from sperm DNA. Lane M: DNA marker (100 bp); 1: negative control (no template); 2: positive control (sperm transfected with recombinant plasmid pIRES2-AcGFP1-E6E7); 3: sperm from a patient with HPV16 in semen; 4: sperm from a control subject.
Reference:
<Pubmed>21408100</pubmed>
Copyright
2011 Carlo et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
- Note - This image was originally uploaded as part of an undergraduate science student project and may contain inaccuracies in either description or acknowledgements. Students have been advised in writing concerning the reuse of content and may accidentally have misunderstood the original terms of use. If image reuse on this non-commercial educational site infringes your existing copyright, please contact the site editor for immediate removal.