User:Z3292017: Difference between revisions
| Line 5: | Line 5: | ||
Lab 2 - [[User:Z3292017|Z3292017]] | Lab 2 - [[User:Z3292017|Z3292017]] | ||
Lab 3 - | Lab 3 - --[[User:Z3292017|Z3292017]] 10:13, 8 August 2012 (EST) | ||
==Lab 1 - [[User:Z3292017|Z3292017]]== | ==Lab 1 - [[User:Z3292017|Z3292017]]== | ||
Revision as of 10:13, 8 August 2012
Lab Attendance
Lab 1 - Z3292017
Lab 2 - Z3292017
Lab 3 - --Z3292017 10:13, 8 August 2012 (EST)
Lab 1 - Z3292017
Lab 1 Online Assessment
Question 1: Identify the origin of In Vitro Fertilization and the 2010 nobel prize winner associated with this technique:
The origin of IVF can be dated from the late 19th Century where embryo transplantation in rabbits was discovered by Walter Heape. The development of IVF was due to a cascade of events during the 20th century. Pincus and Enzmann from Harvard University suggested the possibility that mammalian eggs are able to develop normally in vitro in 1934. In 1948 Menken and Rock exposed 138 oocytes to spermatozoa in vitro. In 1959, Change was able to provide evidence for IVF by fertilising rabbit eggs with capicated sperm and thus achieve birth. From 1965 Robert Edwards attempted to fertilise human oocytes in vitro. In 1968 Edwards successfully fertilised a human egg using a human culture media he developed. Finally after years of research and failed attempts, the first test tube baby was born in 1978. Robert Edwards was awarded the Nobel Prize in Physiology and Medicine in 2010 due to his immense development and contribution of IVF.
References
- http://www.ivf-worldwide.com/ivf-history.html
- http://en.wikipedia.org/wiki/In_vitro_fertilisation
- http://www.nobelprize.org/nobel_prizes/medicine/laureates/2010/
Question 2: Identify and add a PubMed reference link to a recent paper on fertilisation and describe its key findings:
Cleavage speed and implantation potential of early cleavage embryos in IVF or ICSI cycles by Lee, Lin and Hwu (July, 2012) attempted to determine the correlation of early embryo cleavage, its speed and the potential implantation rates for IVF. Their definition of early cleavage was embryonic mitosis occurring 25-27 hours after insemination. The embryos’ (day 3) cleavage speed was assessed and rated into 3 groups: rapid (more than 9 cells), normal (7-8 cells) and slow (less than 7 cells) along with their morphological quality being either good or poor. Normal fertilisation was determined by the presence of 2 nuclei and 2 polar bodies. 25-27 hours after IVF an early cleavage examination took place to determine which embryos had already cleaved. They embryos were then examined for their quality from 66-68 hours after IVF.
Early cleavage emrbyos developed normally in comparison to non early cleavage embryos. Notably, the early cleavage embryos produced a greater amount of “good quality” embryos and subsequently the implantation rate was sufficiently greater with early cleavage embryos. This finding is of great importance as embryo morphology is the most important tool to select the best embryo to transfer andthus increase the rates of implantation, pregnancy and live birth.
References
Lee MJ, Lee RK, Lin MH, Hwu YM Cleavage speed and implantation potential of early-cleavage embryos in IVF or ICSI cycles. J Assist Reprod Genet. 2012 Jul 25 PMID: 22825967
Lab 2 - Z3292017
Lab 2 Online Assessment
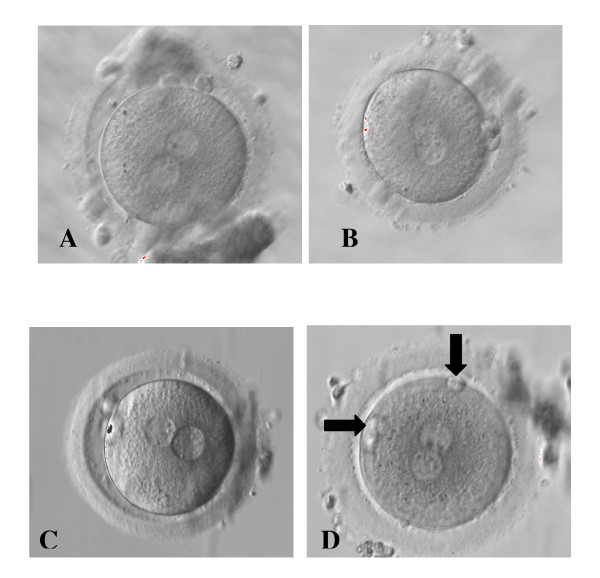
Question 1: Image from journal source
Zygotes showing different distribution of NPB in the 2PN and different PB alignment [1]
Reference
- ↑ Alessia Nicoli, Francesco Capodanno, Lucia Moscato, Ilaria Rondini, Maria T Villani, Antonella Tuzio, and Giovanni B La Sala (2010) Analysis of pronuclear zygote configurations in 459 clinical pregnancies obtained with assisted reproductive technique procedures Department of Obstetrics and Gynecology, Arcispedale Santa Maria Nuova, Reprod Biol Endocrinol. 2010; 8: 77. NCBI
Question 2: Identify a protein associated with the implantation process, including a brief description of the protein's role
Proprotein convertase 6 (PC6) is a necessary regulatory molecule for embryo implantation which generates bioactive proteins such as growth factors, peptide hormones and adhesion molecules. It is produced in the uterine stromal cells in particular at the embryo attachment site throughout early pregnancy in mice. In order for implantation to occur, the uterus ungergoes morphological and physiological changes, one being differentiation of endometrial stromal cells.
Studies have shown that PC6 mRNA is upregulated in particularly at the site of embryo attachment in the mouse uterus, being predominantly at the antimesometrial pole undergoing decidualisation. These results imply that PC6 is associated with decidualisation. From this study, the researchers were able to determine that endometrial PC6 is imperative for maternal stromal decidual response for an implanting embryo during the time of implantation. They discovered that endometrial PC6 is produced explicitly in decidual cells (the vascular and cellular changes in a uterus in preparation for pregnancy) and is not found in other cells as well as the embryo. Similarly, they noted that inhibition of the production of PC6 early on (around day 3.5) using anti-PC6 MO blocked the decidualisation and thus impeded implantation. Notably, PC6 is produced in the late secretory phase of the menstrual cycle in preparation for implantation.
Reference
Guiying Nie, Ying Li, Min Wang, Yi Xun Liu, Jock K. Findlay and Lois A. Salamonsen Inhibiting Uterine PC6 Blocks Embryo Implantation: An Obligatory Role for a Proprotein Convertase in Fertility Biology of Reproduction April 1, 2005 vol. 72 no. 4 1029-1036. Biology of Reproduction