2016 Group Project 6: Difference between revisions
No edit summary |
No edit summary |
||
| Line 11: | Line 11: | ||
TGF-beta belongs to the Transforming Growth Factor superfamily, a large group of structurally connected cell regulatory proteins. It consists of TGF-beta 1, 2 and 3, Activins, Inhibins, Lefty, Nodal, Growth Differentiation Factors (GDFs), Bone Morphogenetic Proteins (BMPs), Glial-derived Neurotrophic Factors (GDNFs) and Mullierian Inhibiting Substance (MIS). | TGF-beta belongs to the Transforming Growth Factor superfamily, a large group of structurally connected cell regulatory proteins. It consists of TGF-beta 1, 2 and 3, Activins, Inhibins, Lefty, Nodal, Growth Differentiation Factors (GDFs), Bone Morphogenetic Proteins (BMPs), Glial-derived Neurotrophic Factors (GDNFs) and Mullierian Inhibiting Substance (MIS). | ||
This site will focus on the TGF-beta family. TGF betas are involved in embryogenesis. During development of the embryo, members of the TGF-beta family are essential for bone and cartilage formation, mesoderm induction and patterning and dorso-ventral patterning. | This site will focus on the TGF-beta family. TGF betas are involved in embryogenesis. During development of the embryo, members of the TGF-beta family are essential for bone and cartilage formation, mesoderm induction and patterning and dorso-ventral patterning. | ||
<br><br> | |||
<html5media width="560" height="315">https://www.youtube.com/watch?v=GuKjUearIUI</html5media> | |||
<br><br> | |||
==Process of TGF-beta signalling pathway== | ==Process of TGF-beta signalling pathway== | ||
Revision as of 00:42, 20 October 2016
| 2016 Student Projects | ||||
|---|---|---|---|---|
| Signalling: 1 Wnt | 2 Notch | 3 FGF Receptor | 4 Hedgehog | 5 T-box | 6 TGF-Beta | ||||
| 2016 Group Project Topic - Signaling in Development
OK you are now in a group, add a topic with your student signature to the group page. | ||||
| This page is an undergraduate science embryology student project and may contain inaccuracies in either descriptions or acknowledgements. | ||||
| Group Assessment Criteria |
|---|
 Science Student Projects Science Student Projects
|
| More Information on Assessment Criteria | Science Student Projects |
Transforming Growth Factor (TGF) Beta Signalling Pathway
Introduction
Transforming Growth Factor (TGF) beta is a multifunctional peptide/cytokine that controls proliferation, cellular differentiation, angiogenesis and other functions in various cell types. TGF-beta plays a dominant part in the development of the embryo and adult organism, as well as cell growth, immune function and hormone secretion.
TGF-beta belongs to the Transforming Growth Factor superfamily, a large group of structurally connected cell regulatory proteins. It consists of TGF-beta 1, 2 and 3, Activins, Inhibins, Lefty, Nodal, Growth Differentiation Factors (GDFs), Bone Morphogenetic Proteins (BMPs), Glial-derived Neurotrophic Factors (GDNFs) and Mullierian Inhibiting Substance (MIS). This site will focus on the TGF-beta family. TGF betas are involved in embryogenesis. During development of the embryo, members of the TGF-beta family are essential for bone and cartilage formation, mesoderm induction and patterning and dorso-ventral patterning.
<html5media width="560" height="315">https://www.youtube.com/watch?v=GuKjUearIUI</html5media>
Process of TGF-beta signalling pathway
The Transforming Growth Factor (TGF) beta signalling pathway is required for regulation of a large number of cellular processes such as cell proliferation, invasion and inflammation. It is also activated mitogen activated protein kinase signalling. There are two main routes in TGF-Beta signalling; the SMAD dependant pathway and SMAD independent pathway.
SMAD dependant TGF-beta signalling
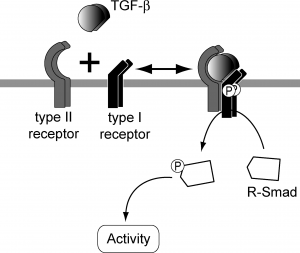
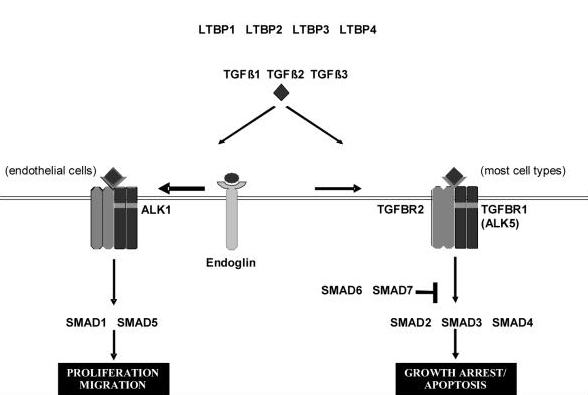
TGF beta superfamily ligands form dimers that bind to heterodimeric receptor complexes composed of two type I and two type II transmembrane receptor subunits with serine/threonine kinase domains. Following ligand binding, the Type II receptor (TGF-beta RII) phosphorylates and activates the Type I receptor (TGF-beta RI). In most cell types, this leads to recruitment and phosphorylation of SMAD2 and SMAD3. (SMAD is a family of gene regulatory proteins). SMAD1 and SMAD5 can be activated by TGF-beta signalling in some cell types depending on the Type I receptor that is expressed. Activated SMAD proteins associate with SMAD4 and translocate to the nucleus, where they accumulate (and act as transcription factors and participate in the regulation of target gene expression). They recruit additional transcriptional regulators, including DNA-binding transcription factors, co-activators, co-repressors and chromatin remodeling factors, that control the expression of numerous target genes.This initiates a SMAD-dependent signalling cascade that induces or represses transcriptional activity. SMADs are widely expressed in most adult tissue and cell types indicating that the TGF-beta signalling pathway is ubiquitous. Differential expression of these factors may be responsible for some of the cell type-specific responses to TGF-beta.
Note: Type I cytokine receptors are also transmembrane receptors expressed on the surface of cells. They recognize and respond to cytokines with four alpha helical strands. Type II cytokine receptors are transmembrane proteins that are expressed on the surface of certain cells. The difference between Type I and Type II receptors is that Type II receptors do not possess the signature sequence WSXWS, which is a characteristic of Type I receptors.
(Tabulate how it is involved in the various processes of embryology)
SMAD independant TGF-beta signalling
Rather than SMAD-mediated transciption TGF-β also has the potential to activate other signalling cascades for example the Erk, JNK and p38 MAPK kinase pathways. In some cases these pathways exhibit activation with slow kinetics which indicates SMAD-dependant mechanics, however there has also been rapid activation cases (5-15mins) suggesting independence from transcription mechanisms. Studies carried out with SMAD4 deficient cells and dominant-negative SMADS provide evidence that the MAPK pathway activation is independent from SMADS, as well as this it has be found that p38 MAPK signalling was activated in response to mutated TGF- β type 1 receptors, which were defective in SMAD activation[1].
The precise mechanisms and biological consequences of these SMAD-Independent pathways (Erk, JNK, p38 MAPK) are currently poorly characterized. Ras is implicated in TGF- β induced Erk signalling as there is rapid activation of Ras by TGF- β in epithelial cells. The JNK and p38 MAPK signalling are activated by various MAPK kinase kinases (MAPKKK) TGF- β kinase 1 (TAK 1) receptor is a MAPKKK family member. Further research and identification of various interactions between the small signalling molecules and receptor proteins will provide additional insight into the precise mechanism behind the activation of MAPK pathways by TGF- β ligands [1].
Regulation of the pathway and factors affecting it
Signalling mechanisms by TGF-β like factors are regulated in both negative and positive fashions, these are all tightly controlled through a multitude of mechanisms at extracellular, membrane, cytoplasmic and all the way to nuclear levels. Positive regulation is required to amplify signalling from TGF-β like factors, while negative regulation is important for the termination and restriction of signalling usually occurring through the mechanism of a feedback loop. There is also additional regulation of TGF-β like factors via cross-talk with other signal transduction pathways such as MAPK and JAK/STAT pathways[2].
Positive Regulation
The positive regulation of TGF-β specifically the induction of ligands and their signalling components often is triggered by the action TGF-β-like factors themselves. For example NODAL, a secretory protein of the TGF-β superfamily which plays a role in early embryogenesis and acts through activin receptors and SMAD2 is induced by nodal signalling itself[2].
Negative Regulation
Current Research
Abnormalities of the TGF-Beta Pathway
Mutations in the TGF-beta RII gene have been associated with multiple syndromes. Alterations of this signalling pathway are common in cancer. Accessory proteins such as soluble or membrane-bound regulators or co-receptors can also affect TGF-beta signalling.
Further Reading
Glossary
Apoptosis - cell death which occurs as a normal and controlled part of an organism's growth or development
Cytokine - a broad and loose category of small proteins that are important in cell signalling
Ligands - a molecule that binds to a larger molecule
CCL-64 - mink lung epithelial cell
History
From the early stages of TGF beta to the present day, research and studies on the signaling pathway have radically increased. SMAD signaling and the three receptors for TGF-beta are two of the many fields of interest regarding the topic. In medicine and specific areas such as cancer, cardiovascular disease and inflammatory bowel disease, there are numerous alternatives for drugs that can either heighten or suppress the activity of TGF-beta.
| 1970s | It was believed that the growth of normal cells was mainly controlled by the interaction between various polypeptide hormones and hormone-like growth factors that were found in tissue fluids. Numerous new polypeptide growth factors had just been classified in cellular extracts, blood and serum.
It was also noted that malignant cells were not prone to be affected by all the same growth controls compared to normal cells and needed a lesser amount of these exogenous growth factors for optimal growth and multiplication. |
| Early 1980s | Anita Roberts found that SGF was not a particular substance, but a combination of two or more elements. One fraction was named “transforming growth factor-a”, the embryonic form of the epidermal growth factor (EGF) found in the salivary gland of an adult. The other fraction showed no rivalry with EGF in a receptor binding assay, but had the striking quality of generating the growth of various large colonies of NRK cells and was called “transforming growth factor-b”.
The theory that TGF’s were cancer-specific was proved inaccurate. In vivo studies established the initial hypothesis that one of the functions of TGF-beta in normal tissues was to be involved in the process of wound healing. |
| 1980 | The “autocrine secretion” hypothesis was developed. It proposed that the supposed transformed cell should produce the transforming polypeptide and have its own functional cellular receptors. This model implied that the endogenous production of growth-promoting polypeptides by a transformed cell would minimize its own need for an exogenous supply of alike growth factors. |
| 1984 | Moses and colleagues made a significant finding that TGF-beta could hinder cell growth if an suitable reader cell such as CCL-64 was used
The first receptor binding assay was published |
| 1985 | TGF-beta1 was cloned by Derynck and colleagues at Genentech
It was shown that TGF-beta could be multifunctional in the exact cells in which it was assayed, based on the context of the assay |