2016 Group Project 6: Difference between revisions
No edit summary |
No edit summary |
||
| Line 80: | Line 80: | ||
==Further Reading== | ==Further Reading== | ||
==Glossary== | ==Glossary== | ||
Revision as of 23:21, 19 October 2016
| 2016 Student Projects | ||||
|---|---|---|---|---|
| Signalling: 1 Wnt | 2 Notch | 3 FGF Receptor | 4 Hedgehog | 5 T-box | 6 TGF-Beta | ||||
| 2016 Group Project Topic - Signaling in Development
OK you are now in a group, add a topic with your student signature to the group page. | ||||
| This page is an undergraduate science embryology student project and may contain inaccuracies in either descriptions or acknowledgements. | ||||
| Group Assessment Criteria |
|---|
 Science Student Projects Science Student Projects
|
| More Information on Assessment Criteria | Science Student Projects |
TGF beta Signaling Pathway
Introduction
Transforming Growth Factor (TGF) beta is a multifunctional peptide/cytokine that controls proliferation, cellular differentiation, angiogenesis and other functions in various cell types. TGF-beta plays a dominant part in the development of the embryo and adult organism, as well as cell growth, immune function and hormone secretion.
TGF-beta superfamily
TGF-beta belongs to the Transforming Growth Factor superfamily, a large group of structurally connected cell regulatory proteins. It consists of TGF-beta 1, 2 and 3, Activins, Inhibins, Lefty, Nodal, Growth Differentiation Factors (GDFs), Bone Morphogenetic Proteins (BMPs), Glial-derived Neurotrophic Factors (GDNFs) and Mullierian Inhibiting Substance (MIS). This site will focus on the TGF-beta family. TGF betas are involved in embryogenesis. During development of the embryo, members of the TGF-beta family are essential for bone and cartilage formation, mesoderm induction and patterning and dorso-ventral patterning.
Process of TGF-beta signalling pathway
The Transforming Growth Factor (TGF) beta signalling pathway is required for regulation fof a large number of cellular processes such as cell proliferation, invasion and inflammation, it is also activated mitogen activated protein kinase signalling. There are two main routes in TGF-Beta signalling, the SMAD dependant pathway and SMAD independant pathway.
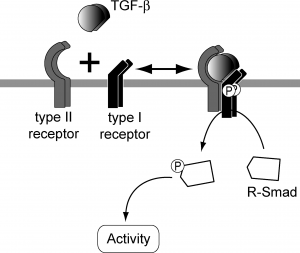
SMAD dependant TGF-beta signalling
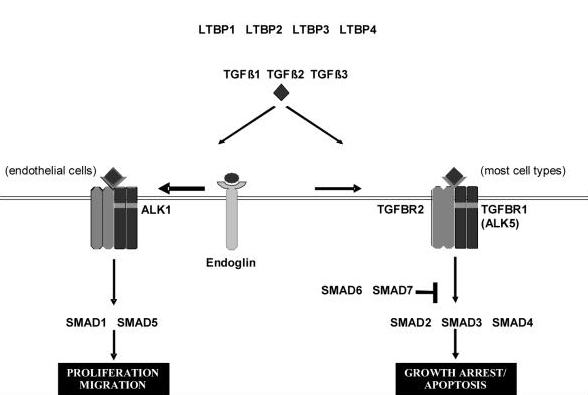
TGF beta superfamily ligands form dimers that bind to heterodimeric receptor complexes composed of two type I and two type II transmembrane receptor subunits with serine/threonine kinase domains. Following ligand binding, the Type II receptor (TGF-beta RII) phosphorylates and activates the Type I receptor (TGF-beta RI). In most cell types, this leads to recruitment and phosphorylation of SMAD2 and SMAD3. (SMAD is a family of gene regulatory proteins). SMAD1 and SMAD5 can be activated by TGF-beta signalling in some cell types depending on the Type I receptor that is expressed. Activated SMAD proteins associate with SMAD4 and translocate to the nucleus, where they accumulate (and act as transcription factors and participate in the regulation of target gene expression). They recruit additional transcriptional regulators, including DNA-binding transcription factors, co-activators, co-repressors and chromatin remodeling factors, that control the expression of numerous target genes.This initiates a SMAD-dependent signalling cascade that induces or represses transcriptional activity. SMADs are widely expressed in most adult tissue and cell types indicating that the TGF-beta signalling pathway is ubiquitous. Differential expression of these factors may be responsible for some of the cell type-specific responses to TGF-beta.
Note: On one hand, Type I cytokine receptors are also transmembrane receptors expressed on the surface of cells. They recognize and respond to cytokines with four alpha helical strands. On the other hand, Type II cytokine receptors are transmembrane proteins that are expressed on the surface of certain cells. The difference between Type I and Type II receptors is that Type II receptors do not possess the signature sequence WSXWS, which is a characteristic of Type I receptors. (Tabulate how it is involved in the various processes of embryology)
SMAD independant TGF-beta signalling
Rather than SMAD-mediated transciption TGF-β also has the potential to activate other signalling cascades for example the Erk, JNK and p38 MAPK kinase pathways. In some cases these pathways exhibit activation with slow kinetics which indicates SMAD-dependant mechanics, however there has also been rapid activation cases (5-15mins) suggesting independence from transcription mechanisms. Studies carried out with SMAD4 deficient cells and dominant-negative SMADS provide evidence that the MAPK pathway activation is independent from SMADS, as well as this it has be found that p38 MAPK signalling was activated in response to mutated TGF- β type 1 receptors, which were defective in SMAD activation[1].
The precise mechanisms and biological consequences of these SMAD-Independent pathways (Erk, JNK, p38 MAPK) are currently poorly characterized. Ras is implicated in TGF- β induced Erk signalling as there is rapid activation of Ras by TGF- β in epithelial cells. The JNK and p38 MAPK signalling are activated by various MAPK kinase kinases (MAPKKK) TGF- β kinase 1 (TAK 1) receptor is a MAPKKK family member. Further research and identification of various interactions between the small signalling molecules and receptor proteins will provide additional insight into the precise mechanism behind the activation of MAPK pathways by TGF- β ligands [1].
History
Regulation of the pathway and factors affecting it
Signalling mechanisms by TGF-β like factors are regulated in both negative and positive fashions, these are all tightly controlled through a multitude of mechanisms at extracellular, membrane, cytoplasmic and all the way to nuclear levels. Positive regulation is required to amplify signalling from TGF-β like factors, while negative regulation is important for the termination and restriction of signalling usually occurring through the mechanism of a feedback loop. There is also additional regulation of TGF-β like factors via cross-talk with other signal transduction pathways such as MAPK and JAK/STAT pathways[2].
Positive Regulation
The positive regulation of TGF-β specifically the induction of ligands and their signalling components often is triggered by the action TGF-β-like factors themselves. For example NODAL, a secretory protein of the TGF-β superfamily which plays a role in early embryogenesis and acts through activin receptors and SMAD2 is induced by nodal signalling itself[2].
Negative Regulation
Current Research
Abnormalities of the TGF-Beta Pathway
Mutations in the TGF-beta RII gene have been associated with multiple syndromes. Alterations of this signalling pathway are common in cancer. Accessory proteins such as soluble or membrane-bound regulators or co-receptors can also affect TGF-beta signalling.
Further Reading
Glossary
Apoptosis - Cytokine - a broad and loose category of small proteins that are important in cell signalling Ligands - a molecule that binds to a larger molecule CCL-64 - mink lung epithelial cell
| 1970s | It was believed that the growth of normal cells was mainly controlled by the interaction between various polypeptide hormones and hormone-like growth factors that were found in tissue fluids. Numerous new polypeptide growth factors had just been classified in cellular extracts, blood and serum.
It was also noted that malignant cells were not prone to be affected by all the same growth controls compared to normal cells and needed a lesser amount of these exogenous growth factors for optimal growth and multiplication. |
| 1980 | The “autocrine secretion” hypothesis was developed. It proposed that the putative transformed cell should produce the transforming polypeptide and have its own functional cellular receptors. This model implied that the endogenous production of growth-promoting polypeptides by a transformed cell would lessen its own requirement for an exogenous supply of similar growth factors. REWORD |
| Early 1980s | Anita Roberts found that SGF was not a particular substance, but a combination of two or more elements. One fraction was named “transforming growth factor-a”, the embryonic form of the epidermal growth factor (EGF) found in the salivary gland of an adult. The other fraction showed no competition with EGF in a receptor binding assay, but had the remarkable property of inducing the growth of many large colonies of NRK cells. It was called “transforming growth factor-b”. REWORD
It was evident that the theory that TGF’s were cancer-specific was inaccurate. In vivo studies confirmed the initial hypothesis that one of the functions of TGF-beta in normal tissues was to participate in wound healing. reword |
| 1984 | Moses and colleagues made the important discovery that TGF-beta could also inhibit cell growth if an appropriate reader cell such as CCL-64 was used
The first receptor binding assay was published |
| 1985 | TGF-beta1 was cloned by Derynck and colleagues at Genentech
It was shown that TGF-b could be multifunctional in the very same cells in which it was assayed, depending on the context of the assay |