2012 Group Project 6
Hearing Development
Introduction
CAN YOU HEAR ME! – Hearing is one of the most important sense and an inherent part of human life. It widens our scope of life and allows us to work, relax, communicate, learn and form memories. The sense of hearing has evolved through the years in vertebrates is inherent for both hunting and surviving. The sound energy produced has be converted into an electrical signal for us to make sense of it. For successful transmission the correct development of the ear is of utmost important. Not only formation of the structures but also their correct assembly is imperative to normal ear functioning. In this project we will discuss the development of human ear from implantation to birth by shining light on the differentiation of cells and signalling mechanisms that lead to normal development. We will also talk about some abnormal processes and mutations which lead to various structural and functional diseases.
History
| Date | Description |
| 1704 | The book De aure humana tractatus with information regarding the anatomy, physiology and pathology of the ear is published.[1] Valsalva. |
| 1707 | It is proposed that the labyrinth contains fluid instead of air. [1] Valsalva. |
| 1714 | It is confirmed that the labyrinth contains fluid. Vieussens. |
| 1789 | The membranous labyrinth is shown to consist of semicircular canals and the vestibular sacs. These form one system and are different to the periosteum. Scarpa. |
| time | text |
| 17th Century | The Metal was one of the first hearing aids created. It was placed over the ear to channel sound into the ear. |
| 1831 | It was discovered that the membranous labyrinth develops from a pit in the skin.It was also discovered that the spiral lamina is hollow. Huschke. |
| 1898 | The first electric hearing aid, the Akouphone, was developed. A carbon transmitter allowed the hearing aid to be portable. Miller Reese Hutchison. |
| time | text |
| 1978 | For the first time, a cochlear implant allowed a person to hear again. Professor Graeme Clark. |
| time | text |
| time | text |
Adult Anatomy and Histology
Outer ear: Pinna, Auricle and Tympanic membrane
Middle ear: Ossicles (Malleus, Incus and Stapes) and Muscles (Tensor Tympani and Stapedius)
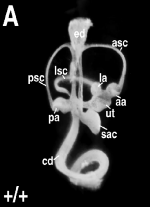
Inner ear: Bony and Membranous Labyrinth - Cochlea containing the Organ of corti, Vestibule containing Utricle and Saccule and Semi-circular canals containing semi-circular ducts
Development
The development of outer ear is attributed to the first pharyngeal arch. All the germ layer namely endoderm, mesoderm and ectoderm contribute to its formation. [2]
The pharyngeal arches arise as a series of bulges arising laterally from the embryo head around 3-4 weeks of human development. The arches have a consistent organisation of the endoderm, ectoderm and mesoderm. The ectoderm forms the outer surface of the arch with the core made up of mesoderm. Next to the mesoderm core on the opposite the ectoderm is the endoderm. All three layers contribute to the formation of outer, middle and inner ear structures hence contributing to hearing. In between the arches the ectoderm and endoderm come in contact with each other forming a continuous sheath on either side of the mesoderm, forming groves externally and arches internally. Inside the mesoderm core of each arch lies a specific cell population that goes onto develop into a nerve, cartilage and artery.
Outer Ear
Pinna
Post development the anatomy of the external ear simply consists of the pinna or the auricle and external acoustic/auditory meatus. The auricle develops around the first and second pharyngeal arches as a series of auricular enlargements or hillocks around the 5th week of development. Gradually by the 6th week the hillocks grow in size and increase to six in number, three on the first arch and 3 on the second arch, the first of which starts at the bottom anterior side going in a clockwise direction. In the 7th week the hillocks enlarge further and each of the arches contributes to a specific part of the pinna. The first arch gives rise to the tragus, helix and cymba concha whereas arch 2 gives rise to concha, antihelix and antitragus. The differentiation starts around the neck region but as the mandible develops the pinna structures move more cranially. It is not until the 12th week that the fusion of these various parts occur and is completed by week 20th.
Various genetic markers and coordinated signalling mechanisms are required for normal development of the pinna. One of which is the EYA1 gene, which is imperative for formation of the pinna. Mice with a homologous Eya1 null gene either has malformed or absent ears and since Eya1 gene plays a role in the formation of cartilage, its absence doesn’t allow the ear mesenchyme to convert into ear cartilage. Another gene important for pinna cartilage formation is the Bmp5 gene that is expressed later in development, mutation in which causes malformation of the perichondrium and thus cartilage formation. Another gene important for early patterning of the pinna is the Hox2 gene, mutations in which leads to formation of a shapeless protuberance rather than a normal shaped pinna. Hox2 gene is expressed in the second pharyngeal arch and the malformations in the ear are contributed to the parts derived from the second pharyngeal arch like the antitragus, antihelix and concha. [3] [4]
External Auditory Meatus
The External Auditory Meatus (EAM) is derived from ectoderm that forms the first pharyngeal groove situated between the 1st and the 2nd pharyngeal arches. At 8 weeks of gestation a c-shaped skeletal structure develops from ectoderm of the first pharyngeal cleft migrating and meeting the mesoderm of the first grove called the tympanic ring that controls and coordinates the invagination of the ectoderm. The tympanic ring is a transient embryological structure, which eventually gets integrated into the temporal bone and provides anchorage to the eardrum. Medial to the ring lays the endoderm of the first pharyngeal arch that contributes to the formation of the tympanic or middle ear cavity. At 12 weeks of gestation the tympanic ring begins to ossify in a sequential fashion via endomembranous ossification forming the bony part of the EAM. The ring starts to condense at the proximal end of the 1st pharyngeal arch, goes around the circumference of the cleft and invades the 2nd arch. Starting at the tip of the 1st pharyngeal cleft the ectodermal cells begin to proliferate filling the lumen of the meatus forming a meatal plug which medially extends in a disc like fashion during week 10. The proliferation follows the path of the tympanic ring with the mesoderm tissue between the ring and the meatus forming a fibrous layer. In week 13 of development the innermost part of the meatal plug makes a contact with malleus. This innermost part of the disc in week 15 splits leaving a thin endoderm layer behind which contributes to formation of the tympanic membrane. By the middle of 16th week the meatus although full in length is still narrow and curved and it is not until week 18 that it gets fully expanded and complete.
Since the correct development of EAM is heavily dependent on the tympanic ring, hence any mutation in the tympanic ring causes an abnormal growth of the EAM. Many genes are required to coordinate and control tympanic ring formation most prominent being the Gsc and Prx1 genes. Chimeric studies have revealed that tympanic ring in Gsc null mice fails to ossify whereas the ring whereas mice with null Prx1 gene have an over ossified tympanic ring. In both cases the meatus fails to form indicating a delicate balance between the expressions of both genes is required for proper ring development hence correct formation of EAM. Likewise for pinna formation Hox2 gene is also essential for differentiation of pharyngeal arch into the tympanic ring and the formation of EAM. [5] [6] [7]
Middle Ear
Inner Ear
The entire inner ear, as well as the neurons which innervate the sensory organ, are derived from the otic placode.
The otic placode is a thickened portion of ectoderm located on the each side of the developing head of the embryo, next to the hindbrain. It is generally visible after gastrulation, once the first 5 to 10 pairs of somites have formed. Invagination occurs next, which creates the otocyst - a vesicle which will develop into the different components of the inner ear: the cochlea, the semicircular canals with cristae, the utricle, the saccule and the vestibulo-acoustic ganglion. [8]
The Otic Placode
Induction of the otic placode
Experiments with molecular markers have revealed that several steps are needed for induction of the otic placode.
We will briefly consider the three major steps:
1 Pre-placodal domain
The pre-placodal domain is a narrow strip of the ectoderm adjacent to the anterior neural plate after gastrulation. Different placodes arise from the pre-placodal domain. All the craniofacial sensory organs, including the ear, develop from these different placodes located at the periphery of the neural plate.
Various evidence indicates the existence of this pre-placodal region:
- Morphology indicates a thickened band of ectoderm around the anterior neural plate in some species, including mice and humans. As time progresses, this thickening will only be present at the locations where the different craniofacial placodes differentiate - including the otic placode.[9]
- Experiments have also shown that the placodes will only develop in the correct location, if rotation of the ectoderm along the anteroposterior axis takes place at the open neural plate stage. If rotation takes place at a later time, the placodes will form at incorrect places.[10]
- Gene expression has also indicated that particular genes are present in the pre-placodal domain. These genes belong to the Dlx, Six, Eya, Iro, BMP, Foxi and Msx families. Glavic et al. (2004) has shown that 'loss and gain of function of some of these genes resulted in the widening or reduction of the pre-placodal field'. Linked to this was also the domain of expression of some placode-specific genes; which either enlarged or diminished.[11]
2 Pre-otic field
Once the general placodal state has been established, the identity of each placode is induced by local signals. The optic placode is induced by various signals, including Pax8, Pax2, Fibroblast Growth Factors (FGFs), and many transciption factors.
In particular the FGFs are significant otic inducers. Signalling occurs from various rhombomeres from the hindbrain and the cranial paraxial mesoderm located beneath the area of the otic placode. For example, in mice FGF3 is expressed in rhombomeres 5 and 6, whereas FGF10 is expressed in the underlying mesoderm.[12] [13] Mutations of FGF3 and FGF10 have been investigated. Results showed that mice with a mutation of either FGF3 or FGF10 developed an abnormal otic vesicle, and a combination of the two mutants resulted in failure to form an otic vesicle.[13]
--> picture
3 Otic placode/epidermis fate decision
In the presence of FGF signalling, Wnt signalling can significantly influence the next step, which is the otic placode/epidermis fate decision. According to the review article by Ohyama et al. (2007) ‘Cells receiving high levels of Wnt signalling differentiate as otic placode, while cells receiving little or no Wnt signalling differentiate as epidermis.’[8]
The first evidence regarding the contribution of Wnt signalling came from experiments with the otic ectoderm of chicks.
- Data showed that specific marker genes, such as Pax2, were induced to a greater extend with FGF19 and Wnt8c present as compared to FGF19 alone [14]. Ladher et al.(2000) hypothesised that FGF19 induced Wnt8c, and together they induced the otic gene markers.
Another possibility is the independent action of FGFs and Wnt signalling.
- Studies have shown that Wnt signalling onto Pax2+ cells results in differentiation of those cells into otic placode tissue. Pax2+ cells that were not exposed to Wnt signalling differentiate as epidermis [15].
- Wnt signalling also suppresses Foxi2, resulting in a Foxi2-negative area of particular size, which then allows for FGFs to induce otic genes [15]. In this case, Wnt signalling determined the size of the otic placode, yet acted independently from FGF signalling.
- Phillips et al. (2004) studied the role of FGF and Wnt signalling; specifically looking at FGF3, FGF8 and Wnt8. Their data showed that Wnt8 is not absolutely necessary for otic induction, however it is required for timely initiation of the otic field. [16]
The neural domain
During the early stages of embryonic development, a neural competent domain is established. This domain will eventually give rise to neurons and hair cells. Various signalling pathways are required to initially create this domain and to maintain it later on. [17]
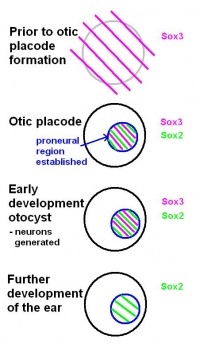
- FGF and Sox
FGF signalling and the Sox genes are essential for the establishment of the neural competent domain. An important aspect of Sox genes is that they have a state of self-renewal, as well as a state of neural commitment. [18] As investigated by Rex et al. (1997) and Pevny and Placzek (2005), "SoxB1 genes (Sox1, Sox2, Sox3) have been linked directly to ectodermal cells that are competent to acquire neural fate, and the commitment of cells to a neural fate".[19] [20]
Before the otic placode becomes distinct, Sox3 is already expressed in a broad area around the location of the future otic placode. Later in it will only be found in the proneural region of the otic placode. Based on this, the review by Alsina et al. (2009) suggests that a neural fate acquisition occurs prior to otic placode formation.[17] During the early stages of development of the otocyst, Sox2 and Sox3 are found in proliferating cells within the proneural region. Both are expressed when neurons are generated, however, Sox3 switches off and only Sox2 remains during further development of the ear. This suggests that the ongoing expression of Sox2 plays a role in sensory cell development, as explained in the review by Alsina et al (2009).[17]
-We will look into this in further detail when describing the development of the elementary sensory unit of the ear-
- Notch signalling
Notch signalling is required for several developmental processes, including the maintenance of the neural competent domain.
In the notch signalling pathway, notch is the receptor, with most of its ligands being transmembrane proteins. Signalling is therefore restricted to neighbouring cells. Research by Daudet and Lewis (2005) reveiled how notch signalling plays a role in inner ear development, including:
- Notch signalling mediates lateral inhibition and thereby controls the differentiation of hair cells and supporting cells - in particular in the vestibular regions [21]
- An early phase of Notch activity promotes formation of prosensory patches [21]
- Other signalling pathways are likely to cooperate with Notch to specify prosensory regions of the otocyst [21]
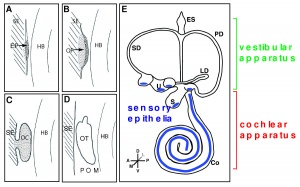
The Otocyst
Patterning of the otocyst
The otocyst, also known as the otic vesicle, is present once invagination of the otic placode has occurred. Regionalisation of the otocyst results in the topological organisation of the ear. The neural tube affects the patterning of the otocyst, and FGF, Wnt and Hh signalling pathways are also known to play a role. FGF and Wnt rely on signals from the hindbrain. [22]
Prosensory patches emerge within the otocyst. These develop as a differentiate from the neural competent domain.[23]
- haircells (sensory patch)
- neurons (neural competent domain)
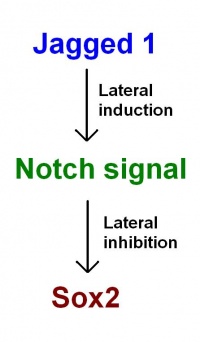
As proposed in recent models by Neves et al. (2011): Jagged 1 functions through lateral induction to activate Notch signalling. Notch signalling then functions through lateral inhibition and regulates Sox2 expression. Sox2 specifies sensory fate within the prosensory domains. "This confines sensory competence to the prosensory patches, ensuring the development of sensory organs of the correct size and location." [23]
Establishing polarity and formation of inner ear structures
The different axis of the inner ear are fixed at different points in time. This means different signals are involved to establish the polarity and allow for development.[24] The dorsal-ventral polarity is very significant in the development of inner ear structures. The ventral inner ear consists of the cochlea and saccule, and the dorsal inner ear is made up of semicircular canals, endolymphatic duct, cristae and utricle.
The neural tube plays a role in patterning of the placode and it has been shown similar signals from the neural tube are also needed to establish the dorso-ventricular axis of the inner ear.[25]
- Shh signalling
The notochord produces Shh, which helps in patterning the dorso-ventricular axis of the neural tube. [26] This signal diffuses further and also affects the developing otocyst, where a gradient of Shh receptors is located from the dorsal to ventral aspect. [27] Dorso-ventricular patterning and development of inner ear structures – in particular ventral structures – is achieved by the graded response to Shh.
As reviewed by Grooves and Fekete (2012) “data suggests that Shh acts on the ventral otocyst directly to regulate cochlear development, and that dorsal development can be regulated by signals from tissues adjacent to the otocyst that require Shh signaling for their normal development.”[28]
- Wnt signalling
Shh signalling for the ventral aspect is complemented by signals for the dorsal aspect of the inner ear. Wnt signalling takes places in the otic placode, as described above. This is initially as a gradient from medial to lateral [29], which later in the otocyst will be a gradient from doral to ventral [30]
As further suggested in the review by Grooves and Fekete (2012) “Wnt and Shh signals regulate different inner ear genes in different ways, with opposing gradients of Shh and Wnt signaling regulating the spatial localization of transcription factors, ultimately leading to the differentiation of a correctly patterned inner ear.” [31]
- Hedgehog signalling
It is also crucial for normal development of inner ear structures that Hedgehog signalling is repressed. [32]
Abnormal Hearing
Genetic
Non syndromic
1 Mutation of GJB2 gene
Approximately 60% of congenital deafness in developed nations is caused by genetic factors. More than 50 loci have been found to be responsible for the genetic causes of non syndromic deafness (isolated hearing loss with no affects to other parts of the body) called NSD and accounts for more than 80% of genetic related congenital deafness. GJB2 gene which instructs the protein Connexin 26 to be formed, is the most common cause of NSD. Connexin 26 is found all over the body, with a great number on the skin and in the inner ear. Over 90 of the GJB2 gene mutations have been associated with NSD.
Non syndromic autosomal recessive deafness (DFNB).
2 Autosomal dominant hearing loss
Autosomal dominant hearing loss is transferred directly though the previous generations and can usually be detected when reviewing at a family tree. There is a 50% probability that the child will also have hearing loss, and subsequently increases if both parents have the dominant gene. Research has shown that the phenotype due to GJB2 varies due to the great range of the degree of deafness in the patients. This alludes to the fact that other environmental causes have also contributed to the patients hearing impairment. M34T missense mutation has been found in families where there are both affected and non affected people thus indicating its contribution to autosomal deafness both dominant and recessive deafness. Similarly, studies have shown additional missense mutations R75W, D66H and G59A are also causes of DNFA however they have been connected to other clinical manifestations.
3 Autosomal recessive hearing loss
Autosomal recessive deafness is an inheritance of both the mother and father’s recessive genes of connexin 26 mutation. There is a 25% chance that the child will have hearing loss. However normally as both parents have normal hearing, it is difficult to foresee if the child will inherit hearing loss. The most prevalent connexin 26 gene mutation in the majority of ethnic groups for DNFB is the 35delG. Mutations in the connexin 26 genes equate to half of the DNFB cases. (Hearing loss associated with 35delG mutation in Connexin-26 (GJB2) gene: audiogram analysis.)
4 X linked hearing loss
X linked hearing loss is carried by the mother and passed down to both the male and female children. Generally only the male children are affected if they receive the affected X chromosome while the daughters will be the carrier of the gene. The hearing loss can be a combination of both conductive (damage to the outer or middle ear) and sensorineural hearing loss (malfunction of the cochlea and or the hearing nerve). (X-linked recessive inheritance of sensorineural hearing loss expressed during adolescence.)
5 Mitochondrial hearing loss
Mitochondrial hearing loss is passed down solely through the mother. It is caused by mutations in either MT-RNR1 or MT-TS1 leading to moderate to profound hearing loss. MT RNR1 is generally correlated to aminoglycoside ototoxicity and sensorineural hearing loss independent of aminoglycoside ototoxicity occurring usually in the 20’s. Aminoglycoside ototoxicity refers to the increased chance of hearing loss after consuming antibiotics and is irreversible. MT-TS1 is sensorineural hearing loss occurring during childhood and is generally considered nonsyndromic.
(Nonsyndromic Hearing Loss and Deafness, Mitochondrial, Arti Pandya, MD, MBA http://ghr.nlm.nih.gov/gene/GJB2)
NOTE: DEFINITIONS OF SYNDROMIC AND NON SYNDROMIC HEARING LOSS
Syndromic
Environmental
DISCUSS TORCH ORGANISMS
Infections
1 Toxoplasmosis
Toxoplasma gondii is an intracellular protozoan and can infect humans through undercooked meat containing bradyzoites or by legumes infected by oocysts (greatly infectious). A study published in 1988 collected data from 23,000 pregnancies which found mothers with an IgG antibody to toxoplasmosis during pregnancy doubled the incidence of deafness for the child. This was identified along with other symptoms such as microcephaly and low IQ scoring. It was also identified that 15 of the 23,000 pregnancies vertical transmission of the child contracting congenital toxoplasmosis. Congenital toxoplasmosis also results in mental retardation and deafness along with blindness and usually multiple organs affected such as Chorioretinitis. Notably, the infection risk differs during the gestation time: “1% at less than 6 weeks, 4–6% at 6–16 weeks, 20–40% at 16–25 weeks and 60–80% at 36 weeks of gestation” (http://www.marmaramedicaljournal.org/pdf/pdf_MMJ_446.pdf) Detection of toxoplasmosis is achived through amniocentesis and subsequently using PCR to determine a positive result from DNA amplification. (Toxoplasmosis: Maternal and Pediatric Findings in 23,000 Pregnancies, John Sever).
Although presently there are no known vaccines to prevent congenital toxoplasmosis, the treatment of pyrimethamine-sulfadiazine or spiramycin had been recommended to reduce the chance of the presumed infected mother passing on the disease to the child. (Peyron F. Congenital toxoplasmosis: systematic review of evidence of efficacy of treatment in pregnancy.)
There have been inconclusive and inconsistent treatment investigations suggested to be caused by limited randomised controlled trials. Although presently there are no known vaccines to prevent congenital toxoplasmosis, the treatment of pyrimethamine-sulfadiazine or spiramycin had been recommended to reduce the chance of the presumed infected mother passing on the disease to the child. Notably, although this was an uncontrolled trial, this prenatal treatment was proven to be of some benefit. (Peyron F. Congenital toxoplasmosis: systematic review of evidence of efficacy of treatment in pregnancy.) (Gilbert R, Gras L. Effect of timing and type of treatment on the risk of mother to child transmission of Toxoplasma gondii. Bjog 2003; 110: 112-120. ([Fetal toxoplasmosis. In utero treatment with pyrimethamine sulfamides].)
Foulon W, Villena I, Stray-Pedersen B, et al. Treatment of toxoplasmosis during pregnancy: a multicenter study of impact on fetal transmission and children's sequelae at age 1 year. Gras L, Gilbert RE, Ades AE, Dunn DT. Effect of prenatal treatment on the risk of intracranial and ocular lesions in children with congenital toxoplasmosis. Gilbert R, Dunn D, Wallon M, Hayde M, Prusa A, Lebech M, et al. Ecological comparison of the risks of mother-to-child transmission and clinical manifestations of congenital toxoplasmosis according to prenatal treatment protocol
2 Rubella
In 1979 an article was published in the Lancet providing clear evidence of the direct link between rubella and sensorineural deafness finding 24% of the hearing impaired children tested had the rubella antibody. Studies have subsequently shown that the fetal infection risk will differ throughout the duration of the pregnancy. During week 1 to 10, there is a 100% fetal transmission rate, leading to an 81% rate during the first trimester, due to the immaturity of the fetal defense mechanisms and solely relying on maternal immunoglobulin G. This decreases throughout the second and third trimester, however the infection rate goes back to 100% in the last month of pregnancy. The sudden decrease is thought to be due by the maternal antibodies along with fetal cell mediated immune responses along with the humoral responses.
Rubella infects the placenta via maternal viraemia which leads to necrotic areas in the chorionic villae epithelium. (G Tondury, DW Smith Fetal rubella pathology). As the cells appear desquamated within the vessel lumens, it is thought that the rubella virus is passed through the fetal circulation via endothelial cell emboli, which in turn leads to an increase in infection and damage to the developing fetal organs. Fetal organ development may be compromised by the virus prompting apoptosis. Studies indicate that the caspid protein dependent mechanism of the Rubella virus leads to apoptosis. (Rubella Virus Capsid Protein Induces Apoptosis in Transfected RK13 Cells, Robert Duncan). Rubella infected cells which do not undergo apoptosis result in a reduced growth rate and a shorten life span due to a decreased mitotic activity. Hearing loss is considered the most frequent defect of congenital Rubella followed by mental retardation. As Rubella has long been a known congential disease, through the Rubella vaccine, this has almost completely eliminated the disease in western countries. Thus as this is an entirely preventable disease, future programs should of Rubella prevention should lead to Rubella being a disease of the past.
(Rubella, the lancet, DWG Brown AND KV Pugachev, TK Frey Rubella virus induces apoptosis in culture cells)
(Onset and severity of hearing loss due to congenital rubella infection)
3 Cytomegalovirus Infection
Cytomegalovirus (CMV) is an enveloped herpes virus which is passed to the fetus via vertical transmission. As 95% of pregnant women are asymptomatic, it is incredibly hard to diagnose. The virus can be classified into acute and non acute infections. Acute maternal infection can be verified by low IgG avidity levels. There are 2 methods to determine if the fetus has contracted CMV. A cordocentesis can be conducted to obtain fetal blood for sampling however issues have been raised regarding its safety and notably accessibility. (Weiner C.P.: Cordocentesis. Obstet Gynecol Clin North Am 15. 283-301.1988). Amniocentesis can also diagnose the CMV via PCR and is considered the better diagnostic tool due to the quick and reliable results it gives. High DNA copy numbers for the CMV infection relates to a greater severity of the disease and hence could indicate the hearing impairment outcome.
A recent study has noted the role of CMV causing sensorineural hearing loss and subsequently being a high risk factor for hearing impairment. The results showed that more than half of the children participating had CMV IgG antibodies, with 20% being found as CMV DNA positive which indicates that the child has an active infection. (Congenital cytomegalovirus infection – a common cause of hearing loss of unknown aetiology, Eva Karltorp).
There is no current treatment protocol for CMV, however a non random sampling trial has suggested the treatment of hyperimmune globulin giving promising results in the study. (Nigro G., Adler S.P., La Torre R., et al: Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 353. 1350-1362.2005; ). Another study showed that treatment of the symptomatic cases of CMV infection with intravenous ganciclovir (or Valganciclovir orally) for 6 weeks has indicated improvement in the child’s hearing. (Oliver SE, Cloud GA, Sanchez PJ, Demmler GJ, Dankner W, Shelton M, et al. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J Clin Virol 2009;46S:S22-S6.)
2 Herpes
Drugs
1 Alcohol consumption during pregnancy
2 Chemotherapy
3 Accutane
Structural malformation of the ear
1 Stenosis
2 Enlarged vestibular aqueduct
3 Auricular Appendages
4 Absence of the Auricle
5 Microtia
6 Preauricular Sinuses and fistulas
7 Atresia of External Acoustic Meatus
8Absence of external acoustic meatus
9 Congenital cholesteatoma
Technologies to detect
Technologies to overcome the problems
Current Research
Glossary
- Cristae: the sensory organ of rotation located in the semicircular canal of the inner ear
- Ectoderm: the outermost layer of the three primary germ cell layers in the very early embryo
- Epidermis: surface epithelium of the skin, superficial to the dermis
- Gastrulation: The inward migration of cells
- Hindbrain: The lower part of the brainstem, comprising the cerebellum, pons, and medulla oblongata
- Lateral inhibition: process whereby one cell takes on a state and the adjacent cells takes on the opposite state
- Mesoderm: the middle layer of the three primary germ cell layers in the very early embryo
- Neural plate: a thickened plate of ectoderm along the dorsal midline of the early vertebrate embryo that gives rise to the neural tube and crests
- Neural tube: A hollow structure formed after gastrulation, from which the brain and spinal cord form
- Open neural plate stage: stage of the neural plate before closure into the neural tube
- Otic placode: a thickening of the ectoderm on the outer surface of a developing embryo from which the ear develops
- Otocyst – otic vesicle: The structure formed by invagination of the embryonic ectodermal tissue that develops into the inner ear
- Pre-placodal domain: An ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia
- Proneural region – neural competent domain: a region of the otic placode involved in neurogenesis
- Prosensory region: region containing a population of cells that can develop into hair cells or or supporting cells
- Rhombomere: a segment of the developing rhombencephalon (hindbrain segment)
- Saccule: one of two otolith organs. The smaller of the two fluid-filled cavities forming part of the labyrinth of the inner ear
- Somite: A segmental mass of mesoderm in the vertebrate embryo, occurring in pairs along the notochord
- Utricle: one of two otolith organs. The larger of the two fluid-filled cavities forming part of the labyrinth of the inner ear
- Vestibular region: the region of the inner ear close to the cochlea. Here the semicircular canals converge
- Vestibulo-acoustic ganglion: The cranial ganglion of cranial nerve 8
References
- ↑ 1.0 1.1 <pubmed>PMC1142106</pubmed>
- ↑ <pubmed>8287791</pubmed>
- ↑ <pubmed>14674478</pubmed>
- ↑ <pubmed>17104502</pubmed>
- ↑ <pubmed>10976045</pubmed>
- ↑ <pubmed>14674478</pubmed>
- ↑ <pubmed>1441991</pubmed>
- ↑ 8.0 8.1 <pubmed>17891709</pubmed>
- ↑ <pubmed>15531360</pubmed>
- ↑ <pubmed>14100031</pubmed>
- ↑ <pubmed>15242793</pubmed>
- ↑ <pubmed>7789270</pubmed>
- ↑ 13.0 13.1 <pubmed>12810586</pubmed>
- ↑ <pubmed>11110663</pubmed>
- ↑ 15.0 15.1 <pubmed>16452098</pubmed>
- ↑ <pubmed>14757644</pubmed>
- ↑ 17.0 17.1 17.2 <pubmed>19247974</pubmed>
- ↑ <pubmed>15863505</pubmed>
- ↑ <pubmed>9215646</pubmed>
- ↑ <pubmed>15721738</pubmed>
- ↑ 21.0 21.1 21.2 <pubmed>15634704</pubmed>
- ↑ <pubmed>17891710</pubmed>
- ↑ 23.0 23.1 <pubmed>21266409</pubmed>
- ↑ <pubmed>9389659</pubmed>
- ↑ <pubmed>16325169 </pubmed>
- ↑ <pubmed>18621990</pubmed>
- ↑ <pubmed>12231626</pubmed>
- ↑ <pubmed>22186725</pubmed>
- ↑ <pubmed>20171206 </pubmed>
- ↑ <pubmed>16452098</pubmed>
- ↑ <pubmed>22186725</pubmed>
- ↑ <pubmed>20223756</pubmed>
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
--Mark Hill 12:22, 15 August 2012 (EST) Please leave the content listed below the line at the bottom of your project page.
2012 Projects: Vision | Somatosensory | Taste | Olfaction | Abnormal Vision | Hearing