2009 Group Project 4: Difference between revisions
No edit summary |
No edit summary |
||
| Line 186: | Line 186: | ||
Genetic maps, the road maps of genetics, are of two types: linkage and physical. The 'sign posts' on the maps are loci, any location or marker in the genome that can be detected by genetic or DNA analysis. The term 'gene' is more restrictive than loci and refers to DNA segments that encode proteins or can be linked to phenotypes. Linkage maps are recombinational maps and are constructed by carrying out linkage crosses that measure the recombination frequency between genes or loci on the same chromosome. | Genetic maps, the road maps of genetics, are of two types: linkage and physical. The 'sign posts' on the maps are loci, any location or marker in the genome that can be detected by genetic or DNA analysis. The term 'gene' is more restrictive than loci and refers to DNA segments that encode proteins or can be linked to phenotypes. Linkage maps are recombinational maps and are constructed by carrying out linkage crosses that measure the recombination frequency between genes or loci on the same chromosome. | ||
*The first genetic linkage in the mouse (and first autosomal linkage in mammals) was described in 1915 in the classic paper on the linkage of pink-eyed dilution and albino (Haldane et al., 1915) | |||
*Genetic mapping with spontaneous mutations that created visible phenotypes, such as changes in coat colour/texture or behaviour was labarious and sometimes took years because crosses between mice carrying recessive mutations yielded so few informative progeny, and genes on only one or two chromosomes could be scored in each cross. | |||
*The first real breakthrough in linkage mapping, enabling the scoring of many test markers and chromosomes in the same cross, was the discovery and use of co-dominant biochemical (isoenzyme) genes (e.g. glucose phosphate isomerase 1, Gpil; Hutton and Coleman 1969). | |||
Revision as of 14:35, 29 August 2009
MOUSE
Timeline of Development
- Development of the Mouse Embryo
Staging
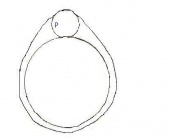
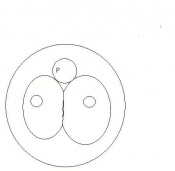
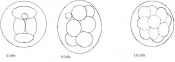
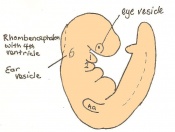
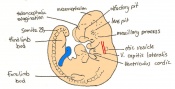
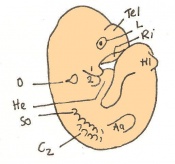
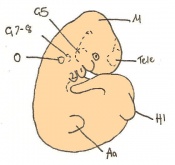
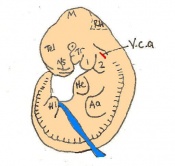
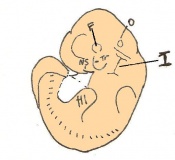
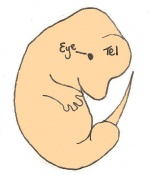
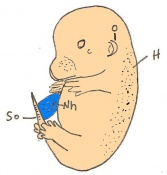
Mouse embryonic development commences once the female's egg or oocyte has become fertilized by the male's sperm. Mouse development has a gestation period of 19-21 days, and can range in different strains of mice. The development of an embryo can be categorized into different stages including cell number, somite stages and morphology. Link to movie showing mouse embryo [1] The most common method of staging is by Theiler (1989) which categorizes mouse development into prenatal and postnatal stages consisting of 26 and 2 stages respectively. Downs and Davies (1993 ) have also established a method of staging the mouse development based on morphological changes. Link to Downs and Davies [2]
| Theiler Stage | Embryonic Age in Days Post Coitum (dpc) | Stage Characteristic | Cell Characteristics | Zona Pellucida | Location | |
|---|---|---|---|---|---|---|
| 1 | 0-0.9
(range 0-0.25) |
One-celled embryo (fertilized) | One cell | Present | Ampulla | |
| 2 | 1
(range 1-2.5) |
Dividing egg | 2-4 cells
- 1st cleavage after 24hrs |
Present | Travelling down oviduct | |
| 3 | 2
(range 1-3.5) |
Morula (early to fully compacted) | 4-16 cells | Present | Oviduct. Usually near utero-tubal junction. | |
| 4 | 3
(range 2-4) |
Change from Morula to Blastocyst
-Intra-cellular matrix present -Blastocoelic cavity evident |
16-40 compacted cells
-Inner cell mass -Outer layer of trophectoderm cells |
Present | Uterine lumen | |
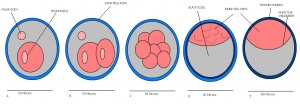
| 5 | 4
(range 3-5.5) |
Zona free Blastocyst (hatching) | Blastocyst is ready to implant as surrounding cell layer of zona pelludica is lost | Absent | Uterine lumen |
Dr Mark Hill 2009, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
Link to ultrasound of mouse embryo [3]
| Theiler Stage | Embryonic age in Days Post Coitum (dpc) | Stage Characteristic | Cell characteristics | |
|---|---|---|---|---|
| 6 | 4.5 (range 4-5.5)
Human carnegie stage: 4 |
Attachment of blastocyst
-Implantation |
Embryonic Endoderm present covering the blastocoelic cells of the inner cell mass. | |
| 7 | 5 (range 4.5-6)
Human carnegie stage: 5 |
Implantation
-Egg cylinder formation -Ectoplacental cone |
Inner cell mass increases in size
-Epiblast formation (enlarged mass) -Proximal cells are cuboidal in shape -Mural trophectoderm is lined by primary endoderm | |
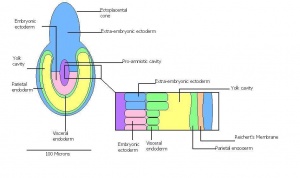
| 8 | 6 (range 5-6.5)
Human carnegie stage: 5 |
Differentiation of egg cylinder into embryonic and extra-embryonic regions
-Pro-amniotic cavity formation |
Trophoblast giant cells invade maternal tissue
-Maternal blood invades the ectoplacental cone -Reichert's membrane appears -Implantation site is 2x3mm | |
| 9 a) | Pre-streak | Advanced Endometrial and egg cylinder stage
-First evidence of embryonic axis |
Morphological difference can be seen between embryonic and extra-embryonic ectoderm
-Maternal blood further invades ectoplacental cone -Uterine crypts lose their original lumen | |
| 9 b) | Early streak | Gastrulation begins (later in stage) | First mesodermal cells produced | |
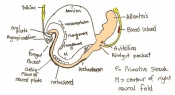
| 10 a) | 7 (range 6.5-7.5)
Mid streak to late streak Human carnegie stage: 8 |
Amnion formation | The amniotic fold starts to form from posterior tissue of primitive streak bulging.
-Allantoic bud evident -Gastrulation continues -Primitive node visible -Amnion begins to close | |
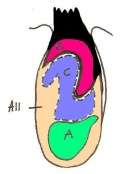
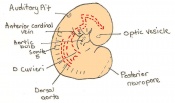
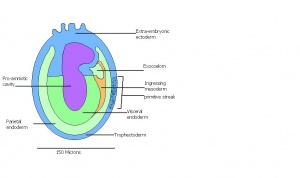
| 11 | 7.5 (range 7.25-8)
Human carnegie stage: 9 |
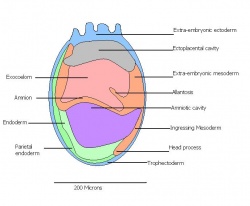
Formation of neural plate and presomites | Amniotic cavity is sealed to form 3 cavities (amniotic cavity, exocoelom and ectoplacental cleft)
-Allantoic bud elongates -Notochodal plate can be seen in the midline and subjacent to neural groove -Head form from the enlargement of the rostral end of neural plate (early head fold) -Formation of foregut pocket begins |
History of Model Use
Introduction
The mouse has contributed an important part of research and finings in the biomedical field since the middle of the sixteenth century (Hendrich et al. 2004). This research using the mouse, especially the mouse embryo, has continued into and multiplied in the 20th century.
It's popularity over time in mammalian biology, biomedicine, immunology, oncology, pathology, and genetics is a result of:
- The mouse breeding system: consistently monitoring the characterisitcs, that are precisley known, generation after generation, thereby producing highly standardised strains (Hedrich et al. 2004).
- The metabolic and internal anatomical similarities between the mouse and the human allowing for comparisons. Because of these similarities, they share similar diseases, for example, cancer, diabetes, autoimmunity, endocrine disease, and neurological dysfunctions (Hedrich et al. 2004).
1895: Dr. Johannes Sobotta
Researched the fertilisation and cleavage of the mouse's egg (Sobotta, 1895). Link to Dr. Sobotta's research [4]
What did he find?
- The corpus Luteum is formed by the enlargement of the epithelial cells of the follicle, aided by growth of connective tissue.
- There is no distinction between corpora lutea vera and corpora lutea spuria.
- The corpora lutea do not degenerate. They remain unchanged during the life of the animal, and therefore add to the size of the ovary.
1958: Professor Dame Anne McLaren
Developed the first birth of mice in-vitro. Link to Published article [5]
1962: George Todaro and Howard Green
- By studying the fibroblast cells of the mouse embryo , they explained how cells behave in a similar fashion once they have undergone transformation (Bhamrah et al. 2002).
How did they do this?
- Todaro and Green cultured the fibroblast cells from the mouse embryo.
What did they find?
- A few weeks after the culture, the growth rate of the Fibroblast cells slowed down. They thought that the cells, as with normal human fibroblast cells, would eventually stop didviding and die. However this was not the case.
- Two to three months later, the cell growth rate increased. This meant that the resulting new population of cells had undergone spontaneous transformation. The resulting permanently dividing cell line, called the '3T3 cell line', have been growing in culture for over 20 years. (Bhamrah et al. 2002)
Genetics
Genome
Sequencing of the mouse genome was completed in late 2002. Tha haploid genome is about 3 billion long (3000 Mb distributed over 20 chromosomes) and therefore equal to the size of the human genome. The current estimated gene count is 23,786 and humans are estimated to have 23,686 genes.
The genetic map of the mouse
Genetic maps, the road maps of genetics, are of two types: linkage and physical. The 'sign posts' on the maps are loci, any location or marker in the genome that can be detected by genetic or DNA analysis. The term 'gene' is more restrictive than loci and refers to DNA segments that encode proteins or can be linked to phenotypes. Linkage maps are recombinational maps and are constructed by carrying out linkage crosses that measure the recombination frequency between genes or loci on the same chromosome.
- The first genetic linkage in the mouse (and first autosomal linkage in mammals) was described in 1915 in the classic paper on the linkage of pink-eyed dilution and albino (Haldane et al., 1915)
- Genetic mapping with spontaneous mutations that created visible phenotypes, such as changes in coat colour/texture or behaviour was labarious and sometimes took years because crosses between mice carrying recessive mutations yielded so few informative progeny, and genes on only one or two chromosomes could be scored in each cross.
- The first real breakthrough in linkage mapping, enabling the scoring of many test markers and chromosomes in the same cross, was the discovery and use of co-dominant biochemical (isoenzyme) genes (e.g. glucose phosphate isomerase 1, Gpil; Hutton and Coleman 1969).
Current Embryology Research
Overview
Mice are highly used models of developmental biology, immunity, neurobiology, and human diseases in pathology. It's main contribution to developmental biology has been through transgenic and knockout technology which are both very important to the research of early development or organogenesis (Slack, 2006).
References
1. Dr Mark Hill 2009, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G [6]
2. Bard, Kaufman, Dubreuil, Brune, Burger, Baldock, Davidson (1998). An internet accessible database of mouse development anatomy based on a systemic nomenclature, Mechanisms of development, (74) 111-120.
3.Hedrich, H., Bullock, G., & Petrusz, P. (2004). The Laboratory Mouse. London: Elsevier Academic Press
ANAT2341 group projects
Project 1 - Rabbit | Project 2 - Fly | Project 3 - Zebrafish | Group Project 4 - Mouse | Project 5 - Frog | Students Page | Animal Development