User:Z3465141
Attendance
Lab 1 --Z3465141 (talk) 12:45, 6 August 2014 (EST)
Lab 2 --Z3465141 (talk) 11:56, 13 August 2014 (EST)
Lab 3 --Z3465141 (talk) 11:59, 20 August 2014 (EST)
Lab 4 --Z3465141 (talk) 11:19, 27 August 2014 (EST)
Lab 5 --Z3465141 (talk) 11:41, 3 September 2014 (EST)
Lab 6 --Z3465141 (talk) 11:46, 10 September 2014 (EST)
Lab 7 --Z3465141 (talk) 11:21, 17 September 2014 (EST)
[[1]]
Assessment 1
<pubmed>25077107</pubmed>
The article above aim was to determine whether vitamin D levels effects women’s clinical pregnancy rates following in vitro fertilization (IVF) treatment. A total of 173 infertile women participated in the study that met the following criteria: being in the age category of 18-41 years, follicle-stimulating hormone level 12 IU/L or lower, as well as consent.
Participants of this study were divided into two categories based on their Vitamin D via the serum 25-hydroxy-vitamin D (25[OH]D) levels. Sufficient levels were classified for women to have ≥ 75 nmol/L of vitamin D whereas insufficient levels were classed as being < 75 nmol/L vitamin D levels. Successful patients IVF cycles resulted in a clinical pregnancy, which is defined as a visible intrauterine sac upon ultrasound.
The study concluded that the womens clinical pregnancy rates were subsequently higher per IVF cycle if the patient had a sufficient level of Vitamin D. Thus forming a relationship between serum 25-hydroxy-vitamin D (25[OH]D) levels and clinical pregnancy rates.
Assessment 2
<pubmed>24618008</pubmed>
Assessment 3
<pubmed>18631884</pubmed> <pubmed>20807610</pubmed> <pubmed>20388228</pubmed> <pubmed>21079243</pubmed>
Assessment 4
<pubmed>23998127</pubmed>
Pre-mature ovarian failure (POF) is currently classified into two categories, these include: there are little to no remaining follicles or there is a copious quantity of follicles present in the ovaries. POF in women has been commonly treated by hormone replacement therapy, even though the treatment increases the risks of other complications including the formation of blood clots such as DVT’s and cancers such as ovarian and breast cancer. The study undertaken by Wang et. al. attempted to investigate whether Mesenchymal stem cells utilized from the human umbilical cord “umbilical cord matrix stem cells” or (UCMSCs) originating in Wharton’s Jelly has any therapeutic use for the treatment of premature ovarian failure in mice.
Wang et al. collected and isolated UCMSCs from full term umbilical cords following the drainage of the cord blood. The umbilical cords were then dissected into sections of 5-6 grams of tissue manually and treated chemically in preparation to be cultured and then harvested after 10 days. The mice were then divided into 3 categories, each consisting of 15 mice each, which included the POF and UCMSC groups. Mice in the UCMSC were intravenously injected with 1 x 10^6 hUCMSCs in 100 𝜇L PBS, whereas the mice in the POF group were exclusively injected with 100𝜇L PBS. These groups then received daily injections of intraperitoneal CTX (50mg/kg) for a total of 15 days, instigating the development of POF models of chemotherapy-induced ovarian damage.
The study concluded that following the transplantation of UCMSCs in mice in the chemotherapy treated group, the mice had a decrease in apoptosis of cumulus cells as well as restoring the normal function of the ovary. Mice treated with UCMSCs also reportedly had a significant increase in their sex hormone levels, leading to an increase in follicles present in the treated mice in comparison to the control group. In essence, the study conveyed UCMSCs could successfully restore the function of damaged ovaries as well as significantly decreasing apoptosis of granulosa cells in the developing follicles.
Vascular shunts
• Ductus venosus - connects the pulmonary artery to the proximal segment of the arotic arch allowing oxygenated blood to travel from the left umbilical vein to the inferior vena cava, thus allowing bypass of the liver. This shunt is then closed postnatally and becomes ligamentum venous.
• Foramen ovale - an opening located between the right and left atrium that directs highly oxygenated blood flow entering from the right atrium to the left atrium. This is then closed at birth and become the fossa ovalis. The remnant of a foramen ovale that had not closed after birth is known as a patent foramen ovale.
• Ductus arteriosus - connects the pulmonary artery to the proximal descending aorta. This blood vessel prevents the output of the right ventricle from entering the non-functioning and fluid filled lungs of the fetus. Ductus arteriosus then becomes the ligamentum arteriosum postnatally.
Assessment 5
Gastrochisis
Gastrochisis is a development abnormality of the anterior abdominal wall, where the bowel protrudes without a covering sac between the developing rectus muscles, occurring slightly lateral and towards the right of the fetal umbilicus. Gastrochisis commonly occurs as an isolated malformation, occurring in approximately 2.5 in 10’000 births.
During the fourth week of normal fetal development, the lateral body of the fetus folds, moving ventrally and fusing in the midline to form the anterior body wall. In Gastrochisis it has been theorized that the incomplete fusion of the midline results in this abnormality, resulting in the abdominal viscera to protrude through the abdominal wall, herniating through the rectus muscle. This is one of many theories linked to Gastrochisis as the cause is still unclear. Other theories include, the failure of mesoderm to form in the body wall, rupture of the amnion around the umbilical ring with subsequent herniation of the bowel, abnormal involution of the right umbilical vein resulting in a weakening of the body wall and thus resulting in herniation of the bowel, and disruption of the right vitelline (yolk sac) artery with consequent body wall damage and gut herniation.
<pubmed>25059025</pubmed>
<pubmed>17230493</pubmed>
<pubmed>19419415</pubmed>
Assessment 6
Thyroid Development
<pubmed>19389367</pubmed> The current study by Lania et. al. demonstrates that the developmental mechanisms of the endocrine gland, the thyroid, is regulated by genetic networks. Tbx1 is a prominent gene involved in the embryonic development of the thyroid gland, as well as many of the pharyngeal apparatus derivatives. In this study, the role of Tbx1 is emphasized as a key factor for regulating the size of the thyroid in early development of mice. Knockout mechanisms were preformed in mice embryos to remove the expression of Fgf8, in the mesoderm, which is regulated by Tbx1. The lack of Fgf8 thus subsequently leads to cause thyroid hypoplasia in the subjects. Thyroid sizes of the mouse embryos were measured following the removal of Fgf8, in 2 different stages of embryonic stages of development, with both stages showing a significant decrease in thyroid size. These results suggest that a Tbx1-NFgf8 pathway is a key factor in determining the size of he thyroid glands in mammalians.
In addition, mutant phenotypes were observed due to the lack of function of Tbx1. The mutant embryos presented with a hypoplastic thyroid, though the positioning of the organ was predominantly normal. Lania et. al. went on to observe a slightly larger than normal lumen present in the thyroid follicles of the mutants. These results were then supported further by Immunohistochemistry analysis of these embryos, which demonstrated that Nkx2-1 is typically expressed and that thyroglobulin is typically produced by mutant follicles.
Tooth Development
• Odontoblasts - derived from neural crest mesenchymal cells, and are differentiated under the influence of the enamel epithelium. Odontoblasts secrete predentin throughout life, which calcifies to form dentin, located under enamel.
• Ameloblasts - derived from oral epithelium of ectodermal origin. They produce enamel after the first production of dentin layer by odontoblasts and form the outermost layer of the tooth.
• Periodontal ligament - a specialised connective tissue layer that acts as an anchor for tooth in its bony socket and surrounds the tooth root coating of cementum.
Assessment 7
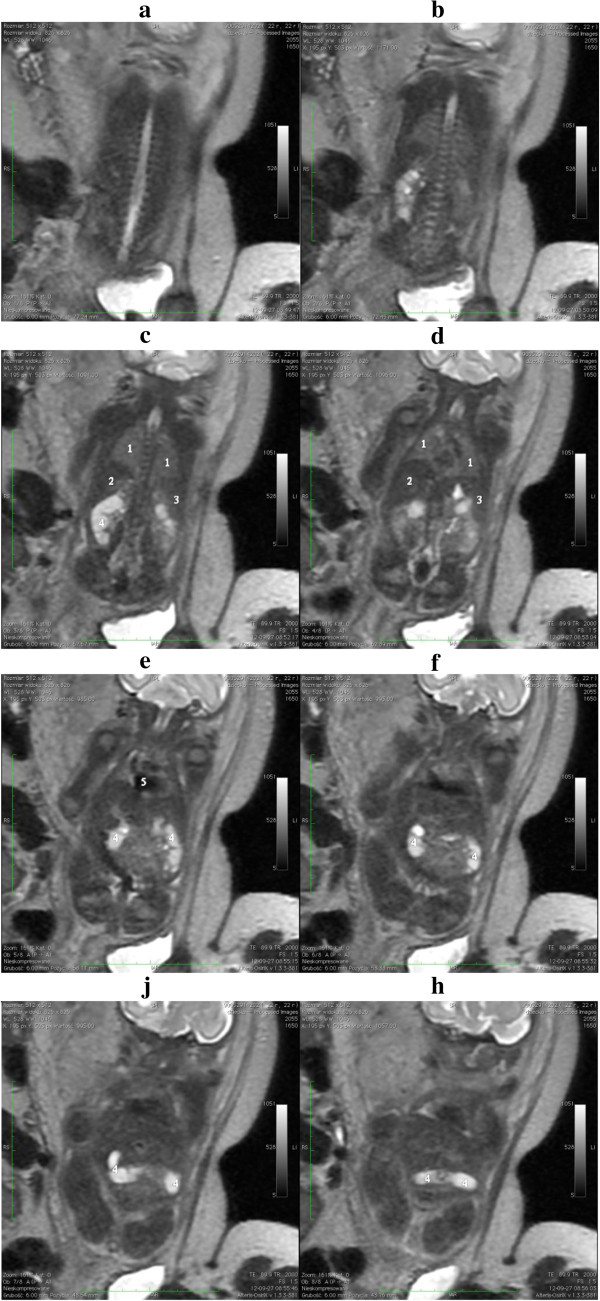
Ovary Development
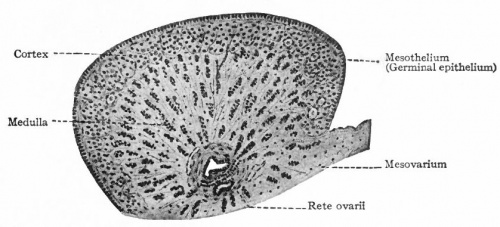
Initially, the origins of gonadal development are morphologically similar for both the testes and ovaries. It is only when the indifferent stage of sexual development occurs when the differential stages of ovary development begins. Both gonads have derivatives from the structures; Mesothelium, which lines the posterior abdominal wall, as well as underlying mesenchyme and primordial germ cells that form the earliest, undifferentiated sex cells.
During week 5 the development of ovaries begins, when a thickened area of mesothelium develops on the medial side of the mesonephros, a primitive kidney. This is then continued by the development of gonadal ridges, which results from proliferation of the mesothelium and the underlying mesenchymal tissue, as it produces a bulge on the medial mesonephros. Finger like epithelial cords then grow into the underlying mesenchyme forming the gonadal cords. In females (XX), the cortex of the indifferent gonad differentiates into an ovary, and the medulla regresses.
Primordial germ cells dwell amongst dorsal endodermal cells of the umbilical vesicle, where they first populate. They then begin to migrate to the gonadal ridge along the hindgut’s dorsal mesentery where they then migrate to the gonadal ridges during the folding process of the embryo. In week 6, the primordial germ cells enter the underlying mesenchyme and are incorporated in the gonadal cords.
Ovary development is a slow process in female embryos, and It is not until week 10, when the ovaries become histologically recognizable.
<pubmed>15664455</pubmed> Moore: The Developing Human Chapter 12