Human Embryology and Morphology 3
Keith, A. Human Embryology And Morphology (1921) Longmans, Green & Co.:New York.
Human Embryology and Morphology: 1 Early Ovum and Embryo | 2 Connection between Foetus and Uterus | 3 Primitive Streak Notochord and Somites | 4 Age Changes | 5 Spinal Column and Back | 6 Body Segmentation | 7 Spinal Cord | 8 Mid- and Hind-Brains | 9 Fore-Brain | 10 Fore-Brain Cerebral Vesicles | 11 Cranium | 12 Face | 13 Teeth and Mastication | 14 Nasal and Olfactory | 15 Sense OF Sight | 16 Hearing | 17 Pharynx and Neck | 18 Tongue, Thyroid and Pharynx | 19 Organs of Digestion | 20 Circulatory System | 21 Circulatory System (continued) | 22 Respiratory System | 23 Urogenital System | 24 Urogenital System (Continued) | 25 Body Wall and Pelvic Floor | 26 Limb Buds | 27 Limbs | 28 Skin and Appendages | Figures
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Chapter III. The Primitive Streak, Notochord and Somites
Law of Recapitulation
The pioneers of Embryology began in the hope of discovering the stages in the evolution of the human body by an accurate study of its development. It was expected that the ovum, as it became transformed into the embryo, and the embryo as it changed into the foetus, would recapitulate man's evolutionary history. From what has been related in the two jDrevious chapters it is plain that we see no resemblance between the successive stages of the human embryo and the succession of types which compose the scale of the Animal Kingdom. Those who expected the law of recapitulation to hold true in all its details forgot that the human embryo is radically modified in order that the first nine months of development may be spent parasitically in the womb of the mother. The storage of yolk in the ovum, the precocious development of trophoblast, chorion, amnion and allantois, have transformed the orderly manifestation of evolutionary stages. Yet to a certain degree the law remains true ; the human body begins as a single ceU, similar in constitution to the simplest form of animal life — a protozoon ; it becomes a globular cluster of cells in its morula stage, similar to the simple forms of multicellular organisms. Further, there are numerous features seen during the development of the embryo which can only be explained by supposing that the human body, in the course of its evolution, has passed through those stages which we see represented in simpler Invertebrate forms — such as the Hydra and the worm. The first of these obscure embryonic manifestations is the primitive streak and groove.
The Primitive Streak
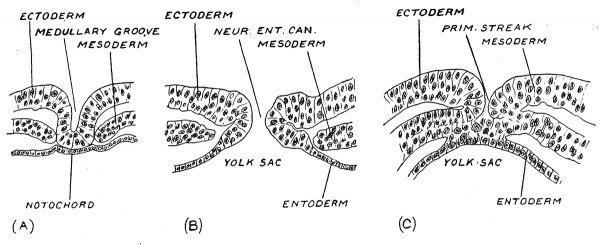
In the third week when the embryonic plate lies on the upper surface of the yolk sac and measures only about 1 mm. (1/25 in.) in length, there appears along the median line of its hinder half a linear demarcation known as the primitive streak. This line becomes the site of developmental processes of the highest significance. No sooner has the primitive streak appeared than there is formed a perforation or canal at its crania] or anterior end — the neurenteric canal (Fig. 35, ^). If a section is made across the embryonic plate at the site of the canal, the entoderm lining the archenteron is seen to be continuous with the ectoderm on the dorsal surface of the plate, the cavity of the primitive gut thus opening or having a mouth, on the dorsal surface of the embryo (Fig. 31, B). If a section be made further back, across the region of the primitive streak (Fig. 34, C) it is seen that the entoderm fuses with the ectoderm and that, at the line of fusion the mesoderniic plates are continuous with both entoderm and ectoderm. Along the line of fusion there is a vigorous production of mesoderm. A section in front of the neurenteric canal (Fig. 34, A) shows still other appearances ; the ectoderm, now being differentiated into the neural plate, is moulded to form the rudiment of the medullary furrow ; beneath the furrow there is a plate of cells — the notochordal plate (Fig. 34, A) which although apparently continuous with the entoderm is yet of different origin. The notochordal plate will form the notochord, the supporting or skeletal rod of the medullary plate. At the neurenteric canal and in front of it the mesoderm is no longer continuous with the ectoderm or entoderm ; it has grown forwards from the site of 23roduction at the primitive streak.
Fig. 34. Section across a human embryonic plate measuring 1.5 mm in length. (Graf Spee.)
A. In front of the neurenteric canal.
B. At the neurenteric canal.
C. Across the primitive streak, behind the neurenteric canal.
Fig. 35. Sections along the median line of two embryonic plates, figured by Graf Spee, to show the shifting backwards of the neurenteric canal and primitive streak as growth takes place.
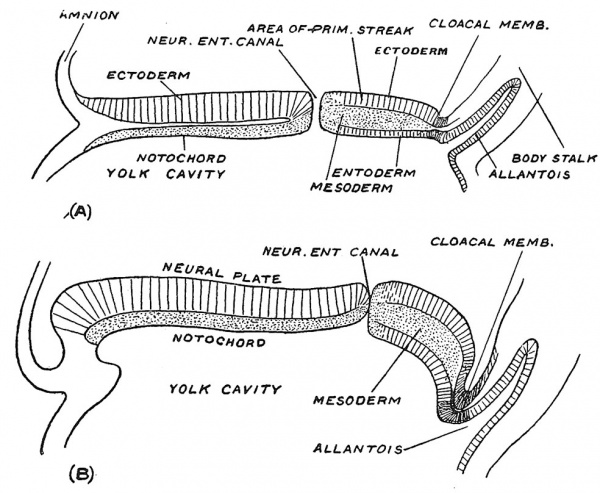
If sections are made along the embryonic plate (Fig. 35, A and B) further light is thrown on the relationship of the neurenteric canal and primitive streak to the growth of the embryo. In Fig. 35, A the neurenteric canal is seen to be placed near the middle point of the plate — which has a total length of a little over 1 mm. while in the older embryo which measures r7 mm. in length, it is pushed backwards by the rapid growth and extension of the j^recanalicular part of the embryonic plate. The region of the primitive streak — the postcanalicular part of the embryonic plate — although the site of mesodermal production, has undergone a lesser degree of growth and is being pushed to the hinder end of the embryonic plate. The exact manner in which the precanalicular part expands we are not certain of, but it will be noted that at the anterior lip of the neurenteric canal the neural plate becomes continuous with the notochordal plate and we suspect that this lip represents a growing edge. The first formed part of the precanalicular plate represents the hinder cranial region ; as the plate grows the neurenteric canal moves backwards through the cervical and dorsal regions until it reaches the lumbar region early in the fourth week, the embryo then being less than 3 mm. in length. If, as sometimes occurs, the neurenteric canal remains unclosed, a fistula from the bowel opens on the lumbar region of the back. In Fig. 34 it will be seen that while the neurenteric canal lies at the anterior end of the primitive streak a very important structure — the cloacal membrane — marks its posterior end. The cloacal membrane, lying at the foot of the body stalk, marks the site of the anus and vulval cleft. Thus the whole of the hinder end of the human body is developed on each side of the primitive streak, a relationship which must be understood if certain malformations of the human body are to be adequately explained.
|
|
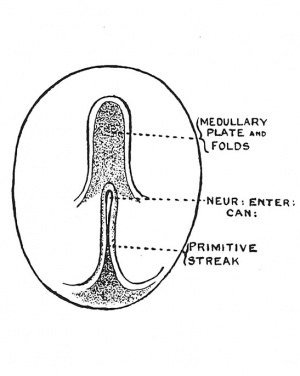
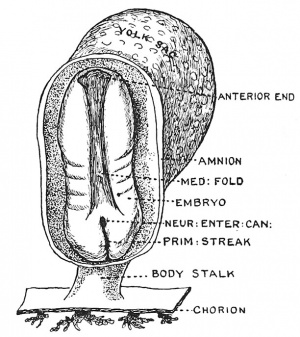
| Fig. 36. Diagram of the Embryogenic area of an Embryonic plate viewed from above. | Fig. 37. The Medullary Plate and Primitive Streak on an Embryo towards the end of the 3rd week. (After Graf Spee.) |
In the third week of development, when the primitive streak is being pushed backward on the embryonic plate, the medullary folds appear on its anterior part, the hinder ends of the folds, as they spread backwards, coming to enclose the neurenteric canal and anterior end of the primitive streak (Fig. 37). The early relationship of the medullary folds to the primitive streak is shown diagrammatically in Fig. 36. It will be seen that as the medullary folds invade the postcanalicular part of the plate the neurenteric canal and anterior end of the streak will be included within them and eventually lie in the hinder part of the spinal cord. The hinder end of the streak is carried away from the cloacal membrane by the formation of the tail.
The Blastopore
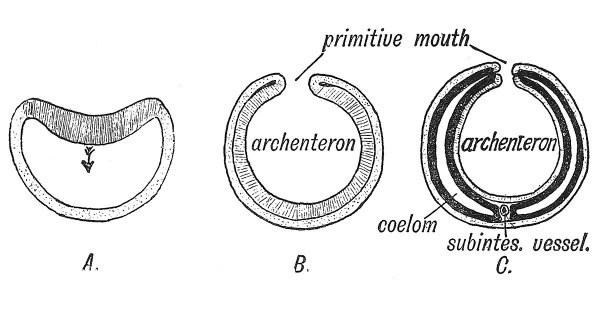
The primitive streak with the neurenteric canal at its front end and the cloacal membrane at its hind, can be best explained by supposing that they represent the primitive mouth or blastopore of lower invertebrate animal types. Its formation in the vertebrate body is best studied in amphioxus (Fig. 38). At an early stage of its segmentation the ovum of this animal forms a hollow sphere (Fig. 38, A) ; one part of the sphere becomes invaginated to form the entoderm, the uninvaginated or outer layer becoming the ectoderm. The brim of the bilaminar flask (gastrula or cup) thus formed served as a mouth or blastopore to the cavity of the entoderm (archenteron) (Fig. 38, B). The primitive streak and groove seen in the embryos of all vertebrates are believed to arise from a linear fusion of the lips of the blastopore. The neurenteric canal is a part of the blastopore which retains its patency for a few days only in the human embryo. The process of invagination or gastrulation, which is seen to occur in the development of amphioxus — by far the most primitive of vertebrate forms — has become masked and obscured in the embryos of higher vertebrates. The process has been profoundly modified by the accumulation of yolk in the ovum and the precocious development of the embryonic membranes. The embryonic plate situated on the archenteron (Fig. 37) may be regarded as the modified gastrula stage of amphioxus and the primitive streak as a modified blastopore. We shall see that some of the primary processes of development are initiated at the margins of the primitive streak.[1]
Fig. 38. Diagram showing three stages in the early development of Amphioxus.
A. Invagination of the entoderm (shaded) within the ectoderm (stippled).
B. Formation of archenteron and primitive mouth (blastopore).
C. Origin of mesoderm (black) and coelom from margin of primitive mouth, with formation of a ventral mesentery round the subintestinal vein. (After Robinson.)
Origin of the Mesoderm and Coelom
In the developing ova of higher vertebrates the mesoderm is known to originate at each side of the primitive streak, but it is difficult to follow the exact manner of its development (Fig 34, G). In amphioxus it arises as a bilateral series of diverticula from the margin of the gastrular mouth or blastopore, along the line at which the ectoderm and entoderm are continuous (Fig. 38, C). The diverticula expand and their cavities fuse together between the two primary layers to form the coelom ; the right and left series of diverticula meet below the archenteron and form a ventral median mesentery (Fig. 38, C). In higher vertebrate ova, the ectoderm and entoderm are fused together along the primitive groove just as round the primitive mouth of a Hydra. The mesoderm arises, as we have seen, from the line of union, and spreads outwards between the two primary layers. The coelom is formed, not as a diverticular cavity, but by a cleavage of the mesoderm, into outer and inner layers. In the human embryo the mesoderm appears at an extremely early stage ; long before the primitive streak has been formed mesoderm appears in the human blastocyst (see Fig. 15, p. 13). The coelom appears first as a cleavage of the blastocystic mesoderm (Fig. 16, p. 14), so altered have developmental processes become owing to the early formation of the ectodermal wall in the human ovum yet the primitive streak remains the chief site of mesodermal production.
Differentiation of Mesoderm
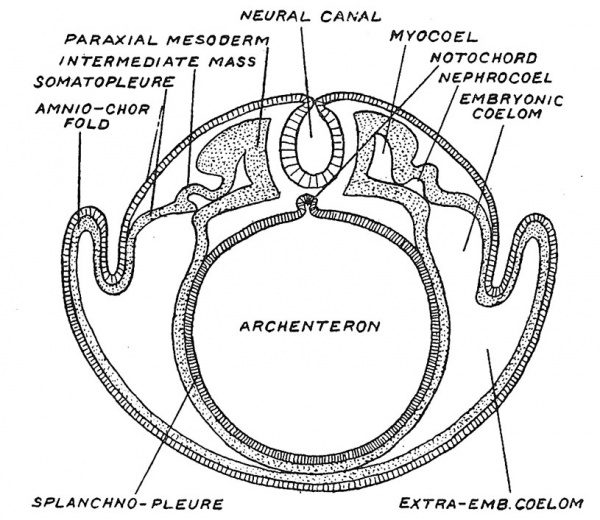
In Fig. 39, a diagrammatic representation is given of the parts into which the mesoderm, and the cavity of the mesoderm — the coelom — become differentiated. Along each side of the medullary tube lies the paraxial mesoderm ; that part we shall see becomes divided into somites and gives rise to muscles and vertebrae ; lateral to the paraxial mass, comes the intermediate cell mass ; then, lateral to the intermediate mass in which the urino-genital glands are formed, the mesoderm is cleft into an outer and inner layer^one joining the ectoderm to form the somatopleure, the other, the entoderm, to form the splanchnopleure. The cleft between these laminae is the coelomic space ; part becomes enclosed within the embryo to form the pericardial, pleural and peritoneal cavities ; the extraembryonic part (Fig. 39) is carried away and obliterated between the foetal membranes. Within the intermediate cell mass and within the paraxial mass there are extensions of the original coelom — known as the nephrocoel and myocoel. From the mesoderm arise the great mass of tissues which constitute the human body — the tissues of locomotion — muscles, bones, ligaments and connective cells. And also the circulatory and mobile systems — the heart, blood vessels, blood cells of all kinds and all forms of moving tissue cells. To this latter element of the mesoderm — the cells which form vessels, blood, connective tissue and mobile cells is given the name of Mesenchyme.
Fig. 39. Diagrammatic section across a vertebrate embryo to show the parts of the mesoderm, of the coelom, and also the origin of the neural canal and notochord.
Fig. 40. A. Diagrammatic longitudinal section of a larval Polypterus — a ganoid fish — to show the relations of the notochord. (After Graham Kerr.)
B. The larval form of Lepidosteus, another ganoid fish, to show the segmented vertebral musculature covering the notochord. (After Graham Kerr.)
Notochord
In its origin the notochord, the forerunner of the spinal column, is closely related to the primitive streak (see Fig. 40, A). Amongst the structures produced at its anterior end where the ectoderm turns in to join the entoderm is a plate of cells which comes to lie along the median line on the roof of the archenteron or primitive gut-cavity (Fig. 35). Presently the plate becomes folded ofi from the roof of the archenteron (Fig. 39) to form a rod of peculiar cells — the notochord. The posterior part of the notochord never forms part of the gut-cavity, but is developed from the lateral margins of the primitive streak. It will thus be seen that the first representation of a skeleton is produced at an extremely early date, and that it appears as a support for the medullary plate when that plate is folded in to form a tube. Its continuity with the primitive gut seems accidental, for it is hard to believe that a mesodermal skeletal structure such as the notochord could have been evolved from the alimentary system.
Segmentation
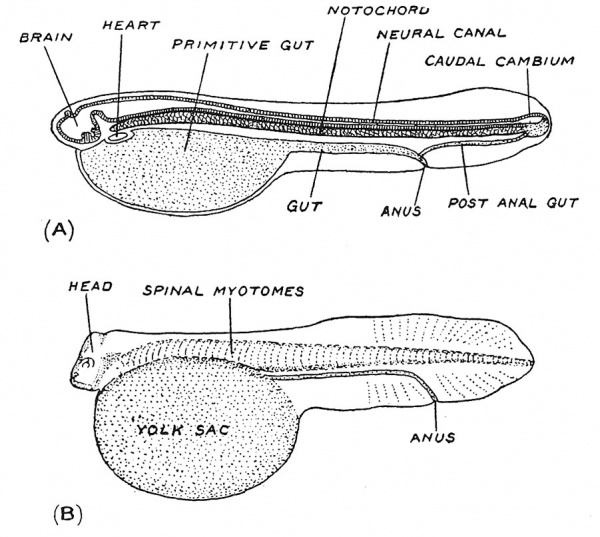
We have seen that the medullary folds rise up towards the end of the third week of development when the embryonic plate is only about 1.5 mm. in length. No sooner do they commence to fuse and thus enclose the neural plate than that part of the mesoderm which has been laid down by the side of the neural tube — the paraxial mesoderm (Fig. 39) begins to be divided, from before backwards, into segmental blocks or somites. Segmentation which begins at what will become the occipital region of the head, is confined to the paraxial mesoderm. In the embryo shown in Fig. 19, p. 17, five somites have been formed ; by the end of the fourth week, when the embryo has grown to a length of about 3 mm. (Fig. 20) the process has reached the first caudal or coccygeal segment, there being at this time 3 occipital and 30 body somites. Thereafter segmentation proceeds slowly in the caudal region, there being 8 or 10 caudal somites at the end of the sixth week, when the tail has reached its maximum development and the embryo is about 11 mm. long.
To understand the meaning of segmentation we must again appeal to comparative anatomy. Segmentation marks the onset of vertebral characterization in the human embryo. In Fig. 40, A a diagrammatic longitudinal section of a fish larva is reproduced to show the relations of the notochord ; it and the neural tube we have seen are formed first in the head region and then grow backwards. In Fig. 40, B another fish larva is depicted, with the notochord clothed with muscle segments or myotomes. A mere glance at such diagrams shows that the notochord or primitive vertebral column and the segmented spinal musculature represent a great sculling apparatus — the locomotory machine of the lowest and oldest vertebrates. Gill arches also appear in the human embryo very soon after segmentation has commenced (see Fig. 20), but even without their guidance one would infer, on the evidence of segmentation alone, that the human embryo in the fourth week is passing through a fish stage and that our vertebral column and spinal musculature represent a former locomotory system. The gill-segmentation is different and apparently older than the body-segmentation ; and as we shall see, the gills are not fashioned out of the paraxial mesoderm.
Experimental Embryology
In recent years those who study the development of the body have resorted to experiment in order to obtain a more direct knowledge of the laws and conditions of development. Loeb has shown that the ova of some invertebrate animals may be stimulated to development by chemical substances which thus simulate the action of spermatozoa. Dareste, fifty years ago, discovered that eggs hatched at abnormal temjDeratures often gave rise to malformed embryos. In more recent years it has been discovered that the addition of certain salts produces one form of malformation, while another group of salt solutions added to the water, in which the embryos of invertebrate animals are being hatched, will produce another set of abnormalities. Stockard[2] discovered that the addition of magnesium chloride to the sea water in which fishembryos are being hatched will lead to half of the larvae becoming cyclops. It has been found that embryonic structures can be transplanted or grown on artificial media. The original experiments of Dr. Ross Harrison,[3] in which he transplanted and studied parts of the living embryonic spinal cord, did much to open up this method of enquiry. In embryonic structures thus transplanted the development of nerve and other cells has been successfully studied. It has also been found that by dividing the ovum at an early stage after fertilization, or by separating the cells, it is possible to produce, in lower animal forms, an embryo from each part or cell separated, but the embryos so produced are small in size, and do not reach adult life. In other cases the cells thus separated only produce part of an embryo. Those who wish to obtain information on this important branch of embryology will find some of the more recent papers by Ross Harrison, W. H. Lewis and others in the American Journal of Anatomy and Anatomical Record.
Twins, Perfect and Imperfect
The study of early stages in the development of the ovum throws some light on the manner in which, twins arise, and especially on the production of human monsters by the incomplete separation of twins.[4] Three theories are held concerning the production of Twins : (1) There may be two or more ova shed and fertilized. (2) That each of the cells produced by the first division of the ovum gives rise to an embryo. Assheton found two inner cell masses in the blastocyst stage (Stage II.) of a sheep, each of which would have formed an embryo. A blastocyst is necessarily the product of one ovum. (3) Beard regards the cells formed by the early divisions of the ovum as indeterminate in nature — a thaUus from which a brood of germinal cells are produced. One of these germinal cells becomes the embryo, in the genital ridges of which the remaining germ cells find a nidus and form ova or spermatozoa. If two of these germinal cells become embryos, twins are produced, if three, triplets. Twins are produced once in every 89 births, but it is probable that twin pregnancies are more frequent than is suggested by birth statistics. Dr. Streeter found a vestigial twin in a human pregnancy of the third week.[5] Dr. Crawford Watt[6] has described a normal twin pregnancy of the fourth week.
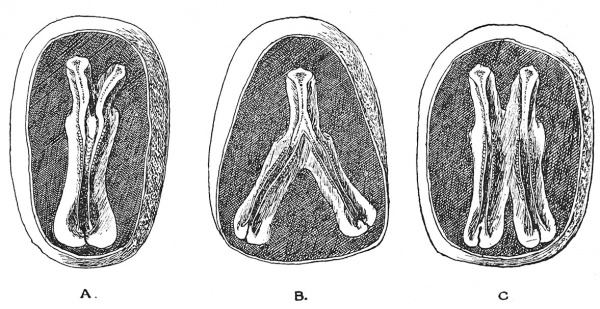
" Identical " twins[7] are produced by the division of a single ovum. They are contained within the same enveloping membranes, are of the same sex and so alike in features that, to the casual observer, they are hard to distinguish. In the production of identical twins, the embryonic plates (see Figs. A, B, C) may remain unseparated, and in this manner most of the numerous forms of human monsters are produced. The embryos may remain attached to a common yolk sac, thus forming a " Siamese " twin — the two individuals remaining attached in the region of the umbilicus. The union may affect only the lower body and limbs, or only the upper part and arms. All kinds and degrees of union occur — head to head, buttocks to buttocks, but the most common is a -ventral union effected through a common yolk sac. In some cases one twin becomes a “parasite," and dependent on the other — the " host " twin — for its circulation and nourishment. Only part of the parasitic twin may develop, and then remains attached as an appendage to the body of the host twin. At an early stage of development the parasitic twin, arrested and delayed in development, may become included within the body of the host twin. There are two examples of this condition in the Museum of the Royal College of Surgeons, England.
Fig. 41. Division of the Embryonic Plate, forming imperfect twins. A, Anterior dichotomy ; B, posterior dichotomy ; C, intermediate union.
Duplication and Atrophy of Parts
Parts of the body, such as a digit or the penis may be duplicated. In such cases we suppose that the group of cells which give rise to the part undergo a division or dichotomy, just as the growing point of a plant stem may undergo branching. Those parts of the body which arise as outgrowths, such as the nasal processes, the extremities, or segments of the extremities, may be partially or completely arrested at a very early stage of development. The embryo itself may be retarded in development or completely blasted while the membranes go on developing, giving rise to the developmental product known as a " mole." It is known that if eggs are incubated in abnormal conditions as regards temperature or atmosphere, such malformations occur more frequently than usual. Practically nothing is known of the circumstances or influences which give rise to abnormalities in the Human Embryo. We know that such abnormalities tend to occur in certain families ; they are hereditary, but we do not know the circumstances which give rise to them.
Knowledge relating to deformed and monstrous foetuses is known as Teratology. Mention will be made of various congenital deformities as we proceed.
- ↑ For details relating to the nature of the blastopore see Text-Booh of Embryology . Vol. I. Invertebrata, by Prof. E. W. MacBride. Vol. II. Vertebrata, by J. Graham Kerr, 1919 ; Profs. J. T. Wilson and J. P. Hill, PJiil. Trans. 1908, Ser. B, vol. 199, p. 31 ; The late Dr. E. Assheton, Quart. Journ. Mic Sc. 1910, vol. 54, pp. 221, 631,
- ↑ Journ. Exper. Zool 1909, vol. 6, p. 285.
- ↑ Journ. Exper. Zool 1907, vol. 4, p. 239.
- ↑ For literature on malformations of the body see : J. W. Ballantyne, Antenatal Pathology, London, 1904 ; Die Morphologie der Missbildungen des Menschen und der Tiere, edited by Ernst Schwalbe, Jena, 1906-1912 ; Prof. F. P. Mall, Journ. of Morphology, 1908, vol. 19, p. 3 (Description of a large collection of malformed human embryos with references to the more recent literature on the causation of the various kinds of maldevelopment). Also, later, Amer. Journ. Anat. 1917, vol. 22, p. 49.
- ↑ Contributions to Embryology, 1920, vol. 9, p. 389.
- ↑ Contributions to Embryology, 1915, vol. 2, p. 5.
- ↑ D. Berry Hart, Proc. Roy. Soc. Edin. July 1909 ; J. F. Gemmill, Teratology of Fishes, Glasgow, 1912 ; G. W. Tannreuther, Anat. Rec. 1919, vol. 6, p. 355.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Human Embryology and Morphology: 1 Early Ovum and Embryo | 2 Connection between Foetus and Uterus | 3 Primitive Streak Notochord and Somites | 4 Age Changes | 5 Spinal Column and Back | 6 Body Segmentation | 7 Spinal Cord | 8 Mid- and Hind-Brains | 9 Fore-Brain | 10 Fore-Brain Cerebral Vesicles | 11 Cranium | 12 Face | 13 Teeth and Mastication | 14 Nasal and Olfactory | 15 Sense OF Sight | 16 Hearing | 17 Pharynx and Neck | 18 Tongue, Thyroid and Pharynx | 19 Organs of Digestion | 20 Circulatory System | 21 Circulatory System (continued) | 22 Respiratory System | 23 Urogenital System | 24 Urogenital System (Continued) | 25 Body Wall and Pelvic Floor | 26 Limb Buds | 27 Limbs | 28 Skin and Appendages | Figures