Human Embryology and Morphology 2
Keith, A. Human Embryology And Morphology (1921) Longmans, Green & Co.:New York.
Human Embryology and Morphology: 1 Early Ovum and Embryo | 2 Connection between Foetus and Uterus | 3 Primitive Streak Notochord and Somites | 4 Age Changes | 5 Spinal Column and Back | 6 Body Segmentation | 7 Spinal Cord | 8 Mid- and Hind-Brains | 9 Fore-Brain | 10 Fore-Brain Cerebral Vesicles | 11 Cranium | 12 Face | 13 Teeth and Mastication | 14 Nasal and Olfactory | 15 Sense OF Sight | 16 Hearing | 17 Pharynx and Neck | 18 Tongue, Thyroid and Pharynx | 19 Organs of Digestion | 20 Circulatory System | 21 Circulatory System (continued) | 22 Respiratory System | 23 Urogenital System | 24 Urogenital System (Continued) | 25 Body Wall and Pelvic Floor | 26 Limb Buds | 27 Limbs | 28 Skin and Appendages | Figures
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Chapter II. The Manner in Which a Connection is Established Between the Foetus and Uterus
The Decidua
Every menstrual period, the mucous membrane which lines the cavity of the uterus becomes hypertrophied and its vessels congested.[1] If the ovum be not fertilized, then the surface layer of the mucous membrane dies and is cast off, but if fertilization occur then the process of hypertrophy proceeds and the mucous membrane now receives the name of decidua. The formation of the decidua is characterized by (1) the production of decidual cells — cells with a more or less rounded outline, large cell-body and relatively small nucleus— from the- connective tissue cells which lie beneath the epithelial lining of the mucous membrane and between the tubular glands embedded in the mucous membrane (Fig. 17, p. 15) ; (2) the epithelial lining proliferates, the surface of the mucous membrane becoming rugose with pits and depressions ; (3) the uterine glands become elongated and branched ; ^ their mouths are closed by the growth of the decidual cells ; their fundi, abutting against the muscular coat, undergo no change ; the elongated bodies of the tubes, between their mouths and fundi form cavernous spaces ; (4) the vessels of the uterus increase in size and the capillaries of its mucous membrane are dilated. In this manner the uterus is prepared to receive the fertilized ovum. It is highly probable that these changes are influenced by an ovarian secretion, for, when the ovaries are removed, these changes soon cease to occur. The internal secretion of the corpus luteum exercises a stimulant action on the uterine tissues.
Implantation of the Ovum[2]
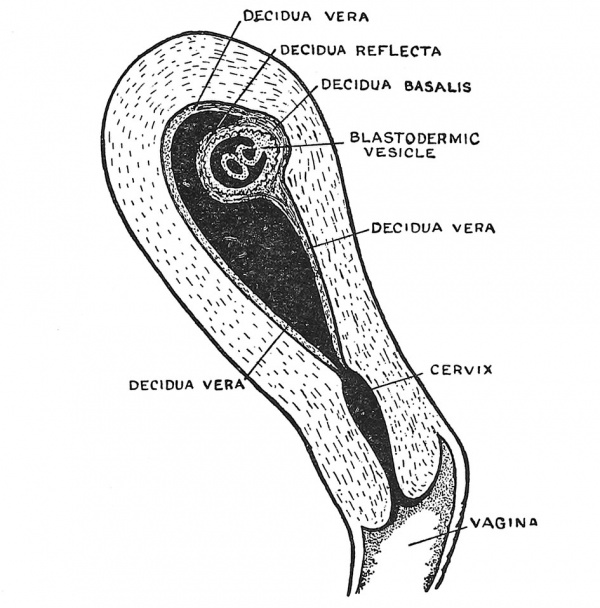
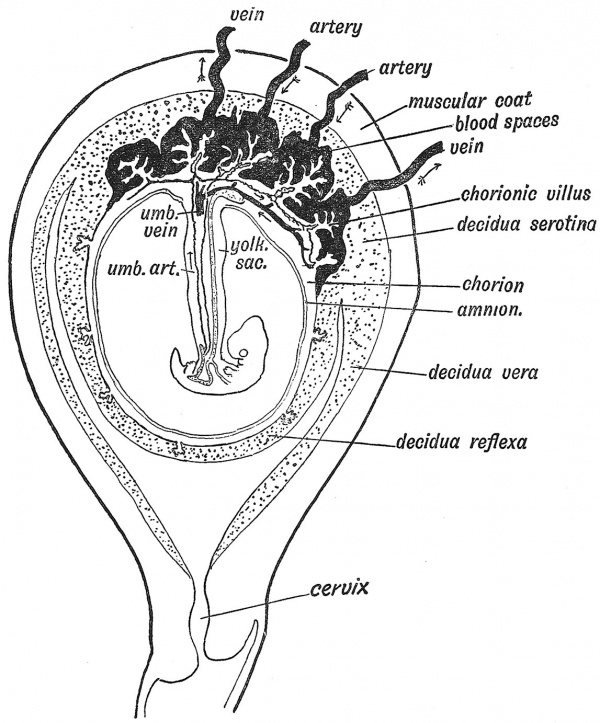
When the fertilized ovum reaches the cavity of the uterus it has already attained the blastocyst form (Fig. 14, p. 12). The inner cell mass, from which the embryo will arise, projects within the cavity and is protected by the enveloping layer or trophoblast of the blastocyst, the whole ovum measuring about '5 mm. in diameter. Implantation occurs in one of the pits of the mucous membrane usually on the posterior wall of the cavity near the fundus of the uterus, but it may occur anywhere, that form being especially dangerous in which implantation occurs in the neighbourhood of the internal mouth of the uterus. The area of the trophoblast in contact with the uterine pit grows rapidly and throws off proliferating masses of syncytium (Fig. 17, p. 15) which burrow into the decidua, thus embedding and anchoring the blastocyst and by the absorption of the decidual tissue, providing nourishment for it. The blastocyst is peculiar in man and the anthropoids in that it becomes completely buried in the decidua. The parts of the decidua are thus distinguished : (1) the decidua serotina or basalis, the part to which the ovum became attached and into which the processes of syncytium grow (Figs. 17 and 24) ; (2) the decidua capsularis or refiexa, the part which covers the ovum and is stretched as the ovum grows ; (3) the decidua vera, which lines the rest of the uterus. The decidua vera ends at the internal os, the canal of the cervix producing no true decidual layer.
^ See references, p. 13.
Fig. 24. Section of the Uterus showing in a diagrammatic manner the Embedded Ovum and the differentiation of the Decidua into Three Parts.
With the growth of the embryo the decidua refiexa is brought in contact with the decidua vera. By the fifth month they have fused together, become flattened and partially atrophied. The decidua serotina, on the other hand, forms the basis in which the placenta is developed.
Nourishment of the Early Ovum[3]
The ova of birds and reptiles are laden with yolk and on this the developing embryo lives — the yolk being absorbed by the entodermal cells lining the archenteron. Primitive forms of mammals, such as the Duckbill and Echidna, have also large supplies of yolk in their ova, but in all other mammals the ova contain only a small supply of yolk ; hence the developing ovum has to draw its nourishment from the uterus. The secretion of the uterine glands contains a proteid (Emrys Roberts) which probably affords nourishment to the ovum. The decidual cells contain vacuoles of fat and glycogen (J. W. Jenkinson) and these cells and their contents are absorbed by the trophoblast and passed on to the growing tissue.
Evolution of the Foetal Membranes
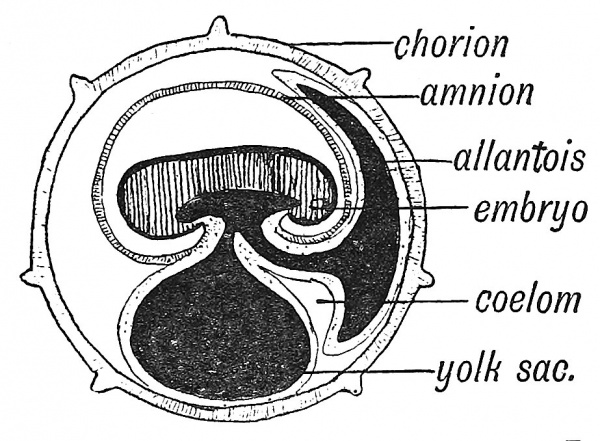
In the first chapter we have seen that, almost from the beginning of development the embryogenic mass of cells is enclosed within a vesicle formed of trophoblastic cells. With the addition of a mesodermal tissue the trophoblastic vesicle becomes the chorion, the most important of the membranes which envelop the foetus ; it comes soon to serve the foetus as lungs, stomach and kidneys. We have also seen the origin of the amniotic cavity and its surrounding membrane, the amnion ; we saw, too, how the yolk sac arose from the primitive gut cavity or archenteron, and also how a diverticulum, known as the Allantois, arose from the hinder end of the archenteron. They all appear so simply and in so regular a sequence that we are apt to forget, from an evolutionary point of view, that they are relatively of recent origin. We can only understand their true nature by an appeal to comparative anatomy. The structures just named are not found in the lower vertebrates — amphibians and fishes, only in the higher — reptiles, birds and mammals ; yet we are certain that the higher were evolved from the lower and that therefore these structures were evolved during the early history of the higher vertebrates. We can see that such a highly evolved structure as the human body is at birth could not have come into existence unless provision had been made for maintaining the individual during the months of embryonic and foetal life. Nature evidently accomplished this miracle without calling into being any new kind of structure ; the chorion, amnion, allantois and yolk sac of higher vertebrates were produced from structures already in existence in their lower vertebrate ancestors. The yolk sac, we shall see, is part of the bowel which has undergone an exaggerated and precocious development and is cut off from the rest of the bowel and cast away by a species of natural surgery, when it has served its purpose in the upbuilding of the embryo. The allantois has been evolved by a precocious development and overgrowth of the apical portion of the primitive bladder ; when this apical part has served its foetal purpose it too is sacrificed and the site of its separation closed. More marvellous still is the origin of amnion and chorion ; they represent parts of the ventral body wall which have been so hurried forwards in point of time of development that they are actually produced in the human blastocyst before the main part of the embryonic body has commenced to form. The membranes which envelop the foetus — the amnion and chorion — are precocious overgrowths of a part of the body wall which is removed at birth, the umbilicus representing the scar which marks the site of amputation.
The Yolk Sac
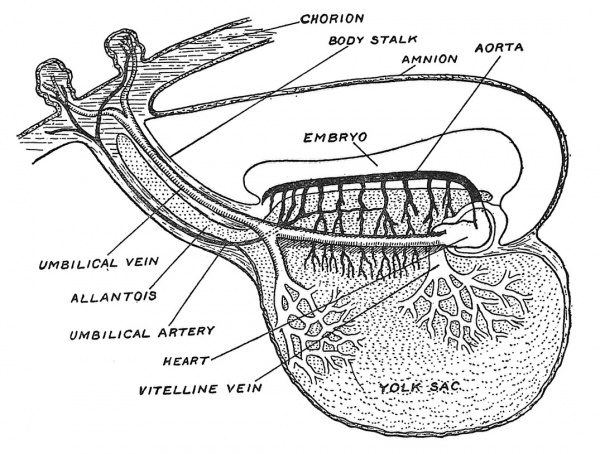
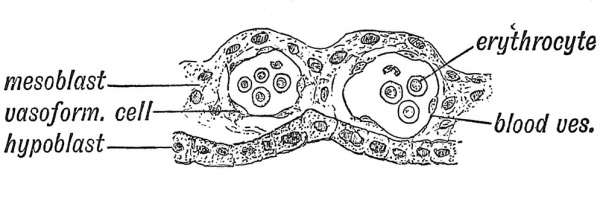
The most ancient method of providing for the growth of the embryo is by loading the ovum with yolk or vitellus. Everyone is familiar with the embryonic provision stored within a fowl's egg ; in birds and reptiles the vitelline system reaches its highest development. The meal or yolk is already in the egg before there is an embryonic stomach to digest, absorb and serve it up as nourishment to the growing embryonic structures. Hence one of the earliest efforts in the developing chick is to throw a containing wall — the archenteron — round the yolk. Even in the human embryo the yolk sac plays a very important part in the upbuilding of the body. In Fig. 25 is shown, in a somewhat diagrammatic form, the yolk sac of a human embryo at the end of the third week of development when the yolk sac measures 2 mm. in its longest diameter and is at its point of maximum importance. A circulation has not yet been established, but blood vessels and blood islands are being rapidly formed in the mesodermal or mesenchymal tissues covering its entodermal lining (Fig. 26). The aortae — right and left — are being laid down and numerous communications are being opened up between the aortae and the vascular-plexus system of the yolk-sac and also between the yolk-sac system and the venous end of the cardiac tube where the vitelline veins are forming. We see all the parts being prepared for the establishment of a vitelline circulatory system. By the end of the fifth week of development the yolk-sac lies outside the body of the embryo (Fig. 22, p. 20) and is now joined to the bowel by a narrow canal — the vitello-intestinal duct. Very soon after this, the duct closes and atrophies, but the sac itself continues to grow until it reaches a diameter of 4 or 5 mm. Its further history we shall examine later (p. 88), but here we may state that when the umbilical cord is fashioned the remains of the vitello-intestinal duct are enclosed within it, while the sac itself will be found at or near the placental end of the cord. In Fig. 26 is shown a section across a small part of the wall of the yolk sac to illustrate the manner in which embryonic blood corpuscles (erythrocytes) and blood vessels are formed in the mesodermal or mesoblastic stratum of its wall. The lining entoderm or hypoblast also gives rise to glandular structures.
Fig. 25. The Yolk Sac and early vessels of the human embryo about the end of the 3rd week of development. (Modified from Eternod.)
Fig. 26. Section across the wall of the Yolk Sac, showing blood vessels and nucleated red blood corpuscles forming in its mesoblastic layer. (After Selenka.)
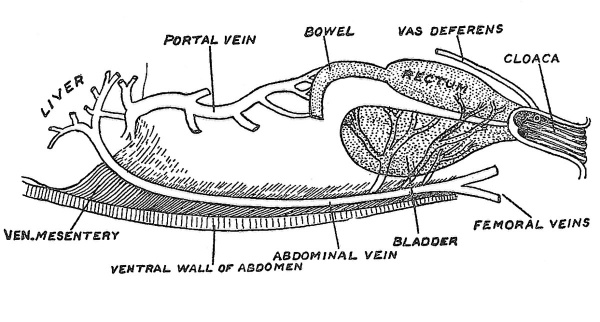
The Allantois. — The allantois appears during the third week of development of the human embryo as an outgrowth from the hinder end of the archenteron or primitive gut cavity. To understand its true nature we must examine the structures in the lower vertebrates from which the allantois has been evolved. These parts are represented in Fig. 27 — depicting a condition found in amphibia. The rectum and also the ducts of the testes — ^the two Wolffian ducts — end in a terminal passage — the cloaca. An expansion or diverticulum of the cloaca has been established as a receptaculum for urine — the bladder. The blood supply is peculiar.
Fig. 27. The cloaca, bladder and abdominal vein of a frog.
A large vein passes along the inner aspect of the ventral wall of the belly, draining the blood from the bladder and from the ventral wall of the belly, as well as from the hind-limbs and ending with the vein from the bowels and stomach in the portal system of the liver. Originally this ventral abdominal vein is double, there being a right and left vein which convey the blood of the bladder and of the ventral wall — but not that of the limbs — the connection of the femoral veins is secondary — direct to the heart. The arteries which supply the bladder and ventral wall spring from the common iliac arteries — these latter vessels representing direct continuation of the right and left primitive aortae. If, then, the allantois represents a precocious outgrowth from the apical region of the bladder and the chorion and amnion enormous and premature expansions from the ventral belly wall, we expect that their arteries would arise from the hinder ends of the embryonic aortae and their veins pass forwards on the body wall to terminate at first in the heart and afterwards in the liver. That is exactly what we do find, as may be seen from a reference to Fig-. 25.
To see the allantois in its complete form one must examine the developing chick embryo (Fig. 28). The young of animals which are developed within a shell, need a recej^taculum for the secretion from their kidneys ; for this reason alone one can understand the expansion of the embryonic bladder.
But even in the chick its use as a store place for urinary excretions has become of minor importance ; the mesodermal tissue which clothes the bladder has become the most important element ; it has grown exceedingly rich in vascular tissue. As the allantois expands in the developing chick its vascular surface becomes applied to the inner aspect of the chorion through which it can absorb oxygen and discharge carbon dioxide. The apical part of the bladder has thus become converted into a " foetal lung," but its vessels are those we have just noted in the ventral area of the frog ; its arteries — the umbilical arteries appear to be direct continuations of the two aortae, and its veins — the umbilical veins, pass to the heart and afterwards to the liver, just as in the frog.
In the human embryo, as is the case in all developing primates, the cavity of the allantois is never represented by more than a tubular outgrowth into the body stalk (see Figs. 18 and 25) and even this degenerates very soon. The human embryo has no need for a bladder, as it can discharge its urinary excretion into the maternal circulation as soon as the chorionic circulation is established. It is otherwise with the mesodermal covering of the allantois ; we shall see that this element takes the chief part in the vascularization of the chorion. In Fig. 25 it will be seen that the umbilical vein is connected with the vascular system of the yolk sac at the root of the allantoic diverticulum. We may regard the allantoic circulation as an enormous expansion from the more primitive circulation of the archenteron.
Fig. 28. The primitive form of the Allantois. (After Turner.)
The Evolution of the Amnion and Chorion
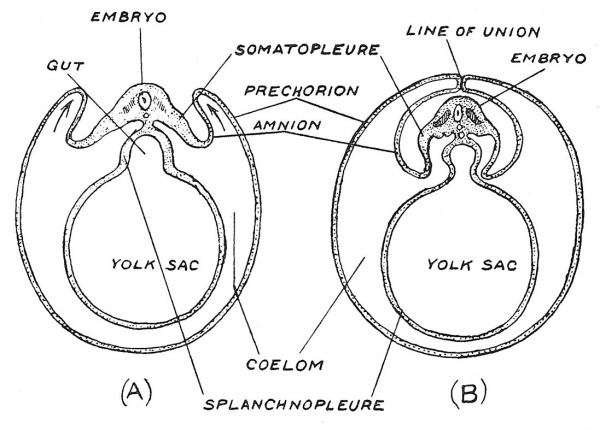
If our knowledge were confined to the highly specialized processes which give rise to the amnion and chorion, the enveloping membranes of the human embryo, it would be almost impossible for us to guess that these structures represent, in reality, folds of the embryo's own belly wall. They come into existence before even the embryo itself is apparent. Their very humble but marvellous origin is illuminated when we examine the manner in which they arise in reptiles, birds and the lowest mammals. In Fig. 29, which represents diagrammatic sections across chick embryos, the origin of the enveloping membranes is set out in a pictorial form. The somatopleure or body wall is seen to arise as a fold at each side of the embryo and pointing upwards ultimately meet and fuse along the median dorsal line. The inner fold separates from the outer and forms the amnion ; the outer remains as a membrane enveloping the embryo, amnion, yolk sac and allantois and is the basis of the chorion — the prechorion it is named in the chick. From the diagrams one would infer that the greater part of the chick's body wall was folded oif to form the enveloping membranes but when we remember that the yolk sac represents a premature but enormous development of a localized part of the bowel, we may justly conclude that the enveloping part of the somatopleure represents a limited area of the ventral part of the abdominal wall — the part drained by the ventral abdominal vein, which has become greatly expanded. The assignation of part of the somatopleure to form the enveloping membranes involves no sacrifice of muscle or nerve in the belly wall of the embryo ; we shall see that these elements invade the somatopleure long after the membranes have separated. Only two elements of the belly wall have been utilized in the formation of the amnion and chorion : (].) the epithelial or ectodermal covering of the skin which takes on a phagocytic action ; (2) the mesodermal element which gives rise to connective tissue, blood vessels and blood cells. Prof. J. P. Hill[4] has demonstrated that in the developing marsupial ovum, when only three cell-divisions have occurred and only 16 or fewer cells are formed, those which are to give rise to the epithelial covering of the chorion and amnion can be distinguished from the smaller number which is to form the embryo. In the human ovum it is also so ; we have seen that the epithelial covering of the chorion — the trophoblast — is the first structure to be differentiated in the development of the blastocyst. In early days primitive man required no scaffolding or machinery to build his rude hut ; in great modern building extensive scaffolding and elaborate macliiues have to be erected before ever building has begun. The chorion and amnion are the scaffolding thrown up for the development of the higher vertebrates and they were evolved out of simple parts of the belly wall.
Fig. 29. Illustrating the manner in which the chorion and amnion arise in the chick embryo from folds of the somatopleure — the body wall of the embryo. In A, the folds are seen in the act of growing upwards to cover the embryo ; on B, they have met over the embryo.
The amnion which contains a fluid in which the embryo floats and has its very delicate growing tissues equally supported on all sides, is not required in the development of fishes or amphibians ; their eggs are hatched in water and the larvae live in water and have therefore no need of an amnion. This structure became necessary when the ancestry of the higher vertebrates took to a life on land. To allow their young to develop in the ancestral medium the amnion was evolved from a duplicature of the embryo's body wall. Having thus given a clue to the evolutionary history of these marvels of adaptation — the amnion and chorion — we return to note stages by which the placenta is produced from the chorion and a foetal circulation established.
Chorionic Villi[5]
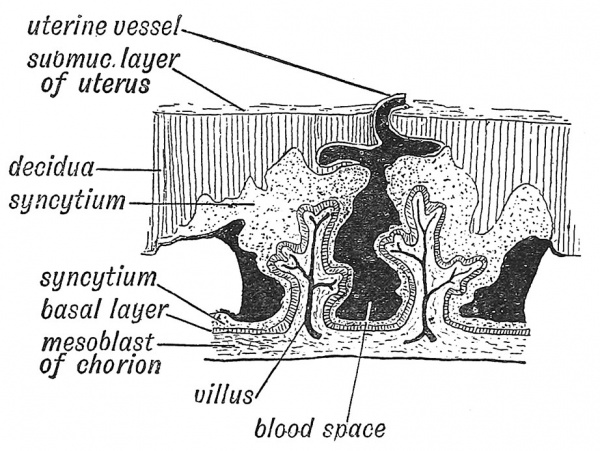
The origin of the chorion from a combination of two elements — the trophoblast (enveloping layer of ectoderm) and an extension from the somatic mesoderm — has been already traced (p. 14). The division of the trophoblast into a basal layer and syncytium was also mentioned. As soon as the ovum is embedded in the decidua, processes of syncytium invade not only the serotinal but also the reflected or capsular part (Fig. 17, p. 15). Villi, containing a core of mesoblast and a covering of the basal layer of chorionic epithelium, grow out into the syncytial masses (Fig. 30). The villi continue to divide and redivide thus becoming arborescent. During the third week the mesodermal tissue of the chorion, particularly of its villi, becomes the site of active formation of blood vessels, blood cells being developed within the vascular lumina. Similar formations are taking place in the body stalk, in the wall of the yolk sac and also in the embryo itself, so that by the end of the third week a tubular heart, dorsal aortae, vitelline and umbilical veins communicating with a great capillary network have been laid down (Fig. 25, p. 25). About the end of the third week or beginning of the fourth a circulation of blood has been established in the chorion. Direct prolongations of the two dorsal aortae now extend through the body-stalk to the chorion — these extensions forming the umbilical arteries (Young and Robinson). The umbilical veins carry the blood from the chorion through the body stalk to the embryonic heart. The chorionic circulation replaces functionally that of the yolk sac. Through the chorionic circulation the embryo is nourished.
Fig. 30. Diagrammatic Section of the Decidua Serotina (formed frcm the mucous membrane of uterus) and Chorion, to show the manner in which the placental blood spaces are formed.
Formation of Placental Blood Spaces
The decidual nutriment only affords a temporary supply. In the last few years the researches of a number of German investigators, but especially of Peters and Selenka, have shown that the maternal circulation is placed at the disposal of the chorionic villi in a simple manner. The syncytium, as it burrows into and replaces the serotinal part of the decidua (Figs. 24, 30), invades the maternal blood vessels, and replaces their walls by its own tissue. The masses of syncytium between the main villi break down and thus form large spaces into which the decidual vessels, which were enclosed by the syncytium, freely open (Fig. 30). Through these spaces the maternal blood circulates, supplied by the uterine arteries and carried away by the uterine veins. The trophoblast contains a ferment which prevents coagulation of the blood in the intervillous spaces thus formed (Young). The extension of the syncytium, the formation of villi and of blood spaces, go on until the 5th month. By that time the basal and syncytial layers of epithelium on the villi are replaced by a single flattened layer of cells. The vascular villi of the chorion hang within the decidual blood spaces, and draw from the maternal blood oxygen and nutriment for the supply of the embryo. Processes and partitions derived from the syncytium remain to bind the chorion to the uterine wall.
Formation of the Umbilical Cord
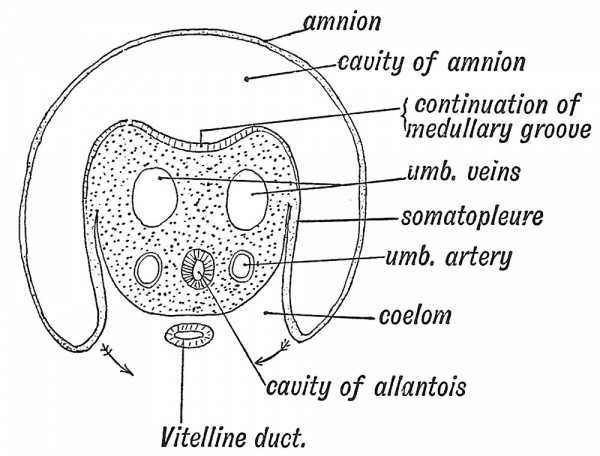
At the end of the third week of development (see Figs. 18, p. 16, 25, p. 25), when the embryo forms a cap on the yolk sac and a plate in the floor of the amniotic cavity, neither umbilicus nor umbilical cord are differentiated. The body-stalk unites the caudal end of the embryo to the inner wall of the chorion, and appears to represent a posterior extension of the embryonic body, but in reality it is formed out of a reflection of part of its ventral wall. It serves the purposes of an umbilical cord to the early embryo. A section across the body-stalk (Fig. 31) shows that two umbilical arteries, two umbilical veins, and the canal of the allantois lie in its mesoblastic basis, and while its upper epiblastic surface projects, like the rest of the embryo, within the cavity of the amnion, its lower surface lies in the wall of the extra-embryonic coelom, in contact with the yolk sac. The structures in the body-stalk are those which we find in the ventral belly-wall of the frog (Fig. 27).
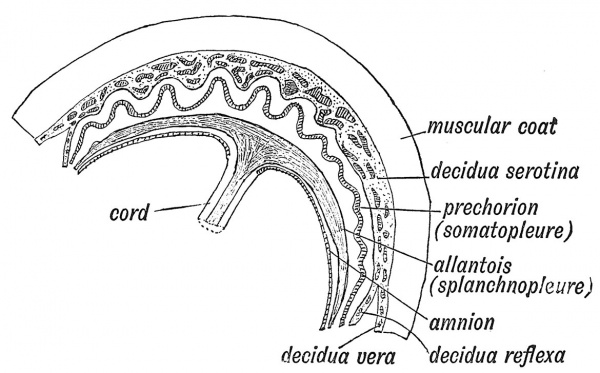
To understand the origin of tlic umbilical cord one must observe closely the attachment of the amnion at this early stage. It is attached to the circumference of the embryo and body-stalk (Figs. 20 and 21) ; to the zone of somatopleure which unites the embryo and the amnion, the name of junctional ring may be given, with the clear understanding that the bodystalk enters into the formation of the posterior part of the ring. From the junctional ring the umbilical cord is developed. While the embryo grows rapidly and expands within the amnion the junctional ring retains its embryonic size (see Fig. 2). The parts of the yolk sac and coelom which are surrounded by the ring now appear to be constricted (Fig. 21). In the second month the junctional ring begins to elongate and form a cordlike structure, in which an umbilical and a placental extremity can be recognized (Fig. 32). The amnion is attached at its j)lacental extremity.
Fig. 31. Section across the Body-Stalk. Wilhelm His (1831-1904)
The mesoderm of the junctional ring forms the jelly-like tissue (Wharton's jelly) of the umbilical cord in which are embedded the umbilical arteries and one umbilical vein, formed by the fusion of the right and left vein. By the third month the cord measures 12 cm. ; and 40 cm. by the ninth month. The elongation of the junctional ring to form the cord necessarily aiiects all those structures which lie within the ring — the neck of the yolk sac (vitello-intestinal duct), the coelomic space or primitive peritoneal space, the cavity of the allantois. All of these are included within the cord, and are obliterated during its elongation. The coelomic or peritoneal space at the umbilical end of the cord closes in the third month, but it may remain open to birth and form the seat of a congenital umbilical hernia. As an exceptional occurrence, the intra-embryonic parts of the allantois or of the vitello-intestinal canal may remain patent as far as the umbilicus, and with the removal of the cord at birth give rise to a urinary or a faecal fistula.
The elements entering into the formation of the placenta are diagrammatically shown in Fig. 33. They are : 1st. The decidua serotina, formed from the mucous membrane of the uterus. It is almost completely replaced by the syncytium and chorionic villi. Only the basal layer remains to furnish a new lining to the uterus when the membranes and placenta are shed after the birth of the child.
Fig. 32. Showing the arrangement of the Amnion, Chorion, and Decidua in the 3rd month and the Formation of the Placenta,
Formation of the Placenta
The condition of the membranes in the third month (Fig. 32) differs from that of the first month (Fig. 18) by the formation of the placenta. In the first month the chorion is uniformly covered by shaggy villi, this being the permanent condition in low primates (Lemurs). In man the chorionic villi which project within the decidua serotina hypertrophy, while those within the decidua reflexa atrophy, and in this way the discoidal placenta of man is formed (Fig. 32). In lower primates (Monkeys) there are two discs (bi-discoidal), and this form occasionally occurs in man.
2nd. The chorion, or, strictly, prechorion.
3rd. An allantoic element which is fused with the mesoderm of the chorion in the hiiman ovum. In the human placenta it is impossible to distinguish the 2nd from the 3rd element ; both are fused in the mesoderm of the chorion from the beginning.
4th. The amnion, which becomes applied to the inner surface of the chorion, thereby obliterating the extra-embryonic coelom (Figs. 32, 33). Thus it will be seen that almost the entire placenta is produced from the ovum and is truly a part of the foetal structures. The decidua, the only maternal element, merely affords a nidus or suitable bed for the development of the foetal structures.
From the inner surface of the fully-formed placenta, the amnion, a thin transparent membrane, is easily stripped off. The outer or uterine surface of the placenta is rough and shaggy, being mainly composed of the greatly hypertrophied villi developed from the serotinal or attached area of the chorion. The villi are grouped in clumps or cotyledons, between which are fibrous strands and partitions, which pass through the whole thickness of the placenta and thus maintain its fixation to the uterus. The manner in which the trophoblast covering the villi becomes changed until it forms
Fig. 33. Diagrammatic Section to show the Elements which enter into the formation of the Placenta. The trophoblast on the outer side of the prechorion has been omitted for the sake of simplicity.
merely a thin epithelial covering has been already described (p. 29). Into the villi pass branches of the umbilical arteries, ultimately forming a fine capillary network, from which the arterialized blood is returned to the foetus by the umbilical veins. Everywhere the blood of the foetus is separated from that of the mother by a thin capillary wall and a layer of flat epithelial cells ; through this wall exchanges between the foetal and maternal circulation take place. The villi project within great blood spaces formed in the decidua serotina (Fig. 32). The ovarian and uterine arteries end in these blood sinuses, and the ovarian and uterine veins begin in them.
At full term all the membranes of embryonic origin come away in the after-birth ; also the decidua, except a thin, deep layer next the uterine muscle, Avhich contains the deepest parts of the uterine glands. From this layer the mucous membrane of the uterus is regenerated.
The establishment of the developing ovum within the uterus of the mother constitutes one of the most marvellous chapters of Embryology.
It is apparent that in the evolution of the higher mammals the young have become modified to pass the first stage of life as uterine parasites. In this chapter we have seen that the ovum has already reached a considerable degree of development when it enters the uterus from the Fallopian tube. All the earlier steps in development are directed towards the formation of the structure necessary for the protection of the embryo — the chorion, amnion, yolk sac, allantois and placenta.
- ↑ See Baumgartner, Amer. Journ. Anat. 1920, vol. 27, p. 203.
- ↑ or literature on very early human embryos see T. H. Bryce and J. H. Teacher, Contributions to the Study of the Early Development and Embedding of the Human Ovum, Glasgow,1908 the more recent literature and data are given by Dr. Geo. L. Streeter, ;Contributions to Embryology, 1920, vol. 9, p. 389.
- ↑ E. Emrys Roberts, Journ. Anat. and Physiol. 1910, vol. 44, p. 192 (Embedding of Ovum and Nutrition — Guinea-pig) ; Emrys Roberts, Proc. Roy. Soc. May 21, 1908.
- ↑ Quart. Journ. Mic. Sc. 1918, vol, 63, p.91.
- ↑ For details and literature relating to the formation of placental structures see A. C. F. Eternod, L'oiuf humain, Geneva, 1909 ; A. Etemod, Compt. Reyid. Congres internal. d'Anat. 1905, p. 197 ; Compt. Rend. Assoc, des Anatomistes, 1909, p. 1 ; A. W. Hubrecht, Anat. Anz. 1905, vol. 31, No. 13 (Nature of trophoblast) ; J. W. Jenkinson, Vertebrate Embryology, comprising the early history of the embryo and its foetal membranes, 1913.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Human Embryology and Morphology: 1 Early Ovum and Embryo | 2 Connection between Foetus and Uterus | 3 Primitive Streak Notochord and Somites | 4 Age Changes | 5 Spinal Column and Back | 6 Body Segmentation | 7 Spinal Cord | 8 Mid- and Hind-Brains | 9 Fore-Brain | 10 Fore-Brain Cerebral Vesicles | 11 Cranium | 12 Face | 13 Teeth and Mastication | 14 Nasal and Olfactory | 15 Sense OF Sight | 16 Hearing | 17 Pharynx and Neck | 18 Tongue, Thyroid and Pharynx | 19 Organs of Digestion | 20 Circulatory System | 21 Circulatory System (continued) | 22 Respiratory System | 23 Urogenital System | 24 Urogenital System (Continued) | 25 Body Wall and Pelvic Floor | 26 Limb Buds | 27 Limbs | 28 Skin and Appendages | Figures