Human Embryology and Morphology 10
Keith, A. Human Embryology And Morphology (1921) Longmans, Green & Co.:New York.
Human Embryology and Morphology: 1 Early Ovum and Embryo | 2 Connection between Foetus and Uterus | 3 Primitive Streak Notochord and Somites | 4 Age Changes | 5 Spinal Column and Back | 6 Body Segmentation | 7 Spinal Cord | 8 Mid- and Hind-Brains | 9 Fore-Brain | 10 Fore-Brain Cerebral Vesicles | 11 Cranium | 12 Face | 13 Teeth and Mastication | 14 Nasal and Olfactory | 15 Sense OF Sight | 16 Hearing | 17 Pharynx and Neck | 18 Tongue, Thyroid and Pharynx | 19 Organs of Digestion | 20 Circulatory System | 21 Circulatory System (continued) | 22 Respiratory System | 23 Urogenital System | 24 Urogenital System (Continued) | 25 Body Wall and Pelvic Floor | 26 Limb Buds | 27 Limbs | 28 Skin and Appendages | Figures
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Chapter X. The Fore-Brain or Prosencephalon (continued)
Cerebral Vesicles
We are now to follow the development of the organ which has given men the domination of the world — the cerebrum proper, comprising the right and left cerebral hemispheres. Nothing could be simpler than the cerebral vesicles at the end of the 1st month of development ; they are merely button-like bulges on the right and left walls of the fore-brain (Fig. 96). Each button-like vesicle may be demarcated into three areas — a relatively small olfactory area in front, which will be evaginated to form the olfactory vesicle, afterwards converted into the olfactory bulb and tract ; a striate area in which that great basal mass of nerve nuclei, known as the corpus striatum, will be developed ; and a pallial or mantle area in which the cortical centres, which make up the great mass of the cerebral hemispheres, are produced. In each vesicle there is also a fourth or secretory area, which, however, does not become defined until the middle of the 2nd month, when it is folded within the cavity of the vesicle to form the glandular covering of the choroid plexus of the lateral ventricle.
It is important to note the manner in which the cerebral vesicles are connected to the walls of the 3rd ventricle and to each other. At wha;t may be called its posterior border each vesicle is continuous with the optic thalamus (Figs. 96, 97) ; at its lower border, with the hypothalamic region. At both of these borders it is the striate area which joins with the thalamic regions ; nerve tracts which arise in the nuclei of these regions and pass to the mantle areas must traverse the striate zone ; there is no other route. Hence the corpus striatum becomes the bond which links each cerebral vesicle to the thalamencephalon ; it becomes the highway for the internal capsule, the name given to the great afferent and efferent nerve-tracts which link the lower nerve centres to the cortex and the cortex to the lower centres.
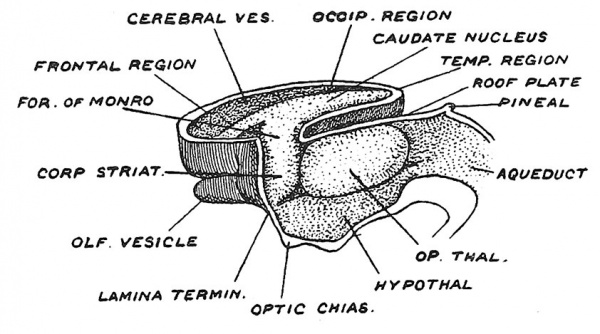
Having thus examined the connections of the cerebral vesicles along their posterior and inferior borders, we now turn to the remaining two — the anterior and superior borders (Figs. 96, 97). Along the anterior border one cerebral vesicle is united to the other by the lamina terminalis, which, we shall see, becomes enormously distorted by the development within it of, (1) the anterior commissure, (2) the hippocampal commissure and (3) the corpus callosum (see Figs. 116, 117, 118). Along the superior border the vesicles are united by a roof plate ; posteriorly the vesicular roof plate is continuous with the roof plate of the 3rd ventricle, which at the 6th week becomes transformed into choroidal ependyma (Fig. 108). A similar change affects the vesicular roof plate and a neighbouring area of the vesicular wall to form the velum interpositum. The vesicular roof plate lies over the widely gaping orifice of the cavity of the cerebral vesicle (Fig. 96). By the end of the 6th week (Fig. 97) the expansion of the cerebral vesicles has commenced ; we can then name the cavity of each vesicle — lateral ventricle, and its constricted communication with the 3rd ventricle, the interventricular opening or foramen of Monro.
Expansion of the Cerebral Vesicles
By the middle of the second month the rapid expansion of the cerebral vesicles has commenced, as may be seen in Fig. 106. Already the posterior or temporal region is passing backwards and downwards on the side and roof of the thalamencephalon (Fig. 107) ; the frontal region is bulging forwards over the olfactory bulb, while the roof of the vesicle, which is cut away in Fig. 106 to expose the corpus striatum in the floor, is rising up so that between the right and left vesicles there now exists a fissure — the commencement of the longitudinal fissure, which will become deeper and longer as the vesicles expand. We have already seen that the striate area of each vesicle is continuous with the thalamic areas of the fore-brain, and thus as the corpus striatum becomes differentiated it is continuous with the optic thalamus (Fig. 106), and hence this striate-thalamic junction may be looked on as the stalk or hilum from which the cerebral expansion takes place. The corpus striatum occupies the floor of the vesicle, so that in the fully formed brain we find the caudate nucleus stretching along the lateral ventricle from the foramen of Monro to the end of the descending horn which represents the posterior or caudal pole of the embryonic brain.
Fig. 106. The expansion of the right Cerebral Vesicle and the formation of the Corpus Striatum on its floor, during the 6th week of development.
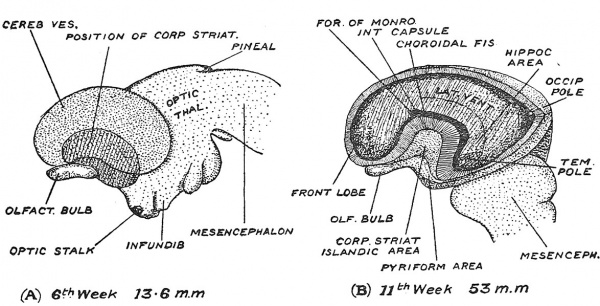
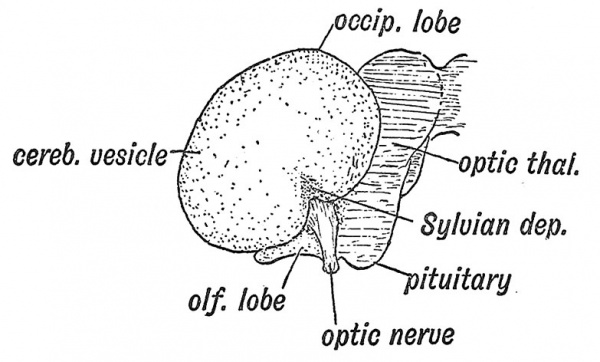
It is also instructive to note the expansion of the cerebral vesicle as seen on its lateral aspect (Fig. 107). At the 6th week the bean-shaped vesicle still leaves the greater part of the thalamencephalon exposed (Fig. 107) ; it then shows only a frontal and temporal pole, but by the end of the 3rd month, the expansion has reached the mesencephalon, and now there has appeared a third or occipital pole (Fig. 107, B) ; by the end of the 5th month the occipital region overlaps and covers the hind-brain. On embryological grounds alone one could infer that the dominance of the cerebrum is one of the more recent products of evolution. In the lateral aspect we again see how the corpus striatum forms the basis or fixed area from which the cerebral expansion is produced. The three primary constituents of the cerebral vesicle are indicated in Fig. 107, A, the small olfactory area, the large mantle formation and the position of the striate element at the junction of these two. The position of the corpus striatum determines the non-expansion of the overlying cortex — which later becomes differentiated to form the Island of Reil. The position and relationships of the islandic region towards the end of the 3rd month are shown in Fig. 107, B.
The Velum Interpositum
It is during the growth backwards of the cerebral hemispheres over the thalamencephalon that the basis of that complex structure — the velum interpositum — is formed. The basis of this structure is really that area of the pia mater — the mesodermic and vascular capsule of the brain— which is enclosed between the thalamencephalon and expanding cerebral vesicles (Fig. 108). The essential parts of the velum are its lateral edges, which project within the lateral ventricles and its lower surface lying over the third ventricle — parts which are covered by reflections of those areas of the neural tube which have been converted into a glandular or secretory epithelium. These parts form the choroidal villi — or plexuses — covered by the ependymal epithelium, which secrete the cerebro-spinal fluid.
Fig. 107. The Expansion of the left Cerebral Vesicle as seen on its lateral aspect. A, at the 6th week ; B, at the 11th week ; in J5, a window has been cut to expose the lateral ventricle, the corpus striatum and the choroidal gap. (After His.)
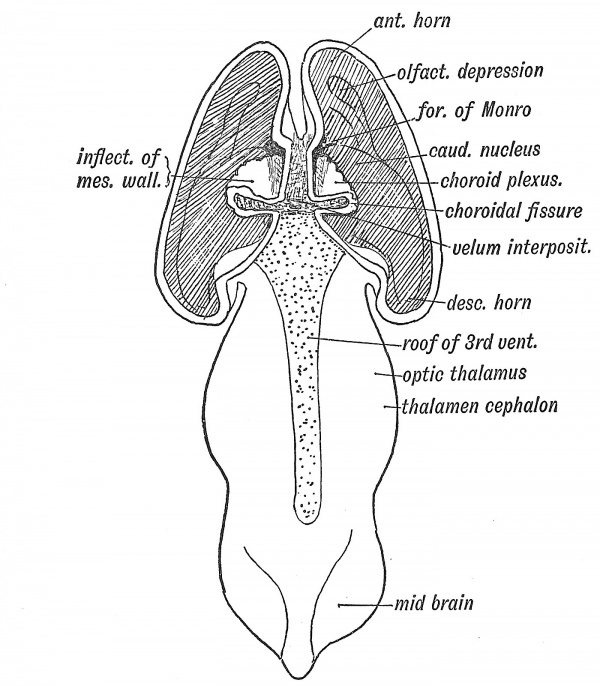
We have seen that in the anterior part of the roof plate of the -Ith ventricle the cerebellum is developed, while its posterior half becomes the inferior medullary velum — a secretory membrane (Fig. 86, p. 91). The roof plate of the third ventricle, from the foramina of Monro backwards to the stalk of the pineal body, becomes modified in a similar manner (Fig. 108). It merely forms the ependymal covering of the lower surface of the velum interpositum, thus clothing the choroid plexus on the roof of the 3rd ventricle (Fig. 109). The anterior part of the roof plate is produced into the cerebral vesicles at the foramina of Monro, and covers the apex of the velum interpositum (Fig. 108). The mesial wall of eacli cerebral vesicle from the foramen" of Monro back to the posterior extremity of the vesicle (Figs. 108, 109), which becomes the tip of the descending horn, is also inflected and forms a secretory ependyma, covering the velmn interpositum and choroid plexus within the lateral ventricles. Into this inflection — the choroidal fissure of the embryonic neural wall — spreads the mesoderm, carrying vessels with it. The velum interpositum is thus composed of a basis of mesoderm, while its intraventricular parts have an ependymal covering derived from the neural wall. When the velum interpositum is withdrawn from the foetal brain (Fig. 107, B) a linear opening is seen extending from the foramen of Monro to the temporal end of the cerebral vesicle.
Fig. 108. A dorsal view of the Fore- and Mid-Brain at the 6th week of development to show the formation of the Velum Interpositum. The Cerebral Vesicles are laid open and the inflection of the roof of the Fore-Brain shown on the ingrowing Velum. The Roof Plate of the 3rd Ventricle is also exposed. Modified from Wilhelm His (1831-1904)
The ependymal covering of the entire velum is derived from :
- The roof j)late of the 3rd ventricle (lower surface) ;
- The roof plate of the foramina of Monro ;
- An inflection of the mesial wall of the cerebral vesicle.
The choroid plexus, which merely fringes the velum in the adult, completely fills the cavities of the embryonic lateral ventricles. These for the first three months are relatively very large and their containing walls thin. The velum and choroid plexus must play an important part in the development of the cerebral vesicle in the early period of growth. The spread of the vesicles backwards and downwards over the optic thalami obscures the original simple relationship of the velum to the brain ; but, when withdrawn from the transverse fissure, the velum is seen to rest on the optic thalami and project within the ventricle from the foramen of Monro to the tip of the descending horn. This stretch marks the line at which the choroidal inflection took place (Fig. 109). The taenia semicircularis, in the groove between the optic thalamus and caudate nucleus (Fig. 100), marks the line at which the mesial wall of the cerebral vesicle was primarily attached.
The fibrous substance of the velum interpositum is continuous with the pial covering of the brain, and also with the edge of the tentorium cerebelli, for as the cerebral vesicles expand not only do they evaginate their proper mesodermal covering, the pia-arachnoid, but also the inner or dural stratum of the primitive cranial capsule. The corpus callosum and cerebral vesicles, as they develop, grow backwards and enclose, between the optic thalami below and the pillars of the fornix above, the fibrous basis of the velum interpositum (Fig. 109). The veins of Galen are developed in the velum and join the straight sinus in the tentorium.
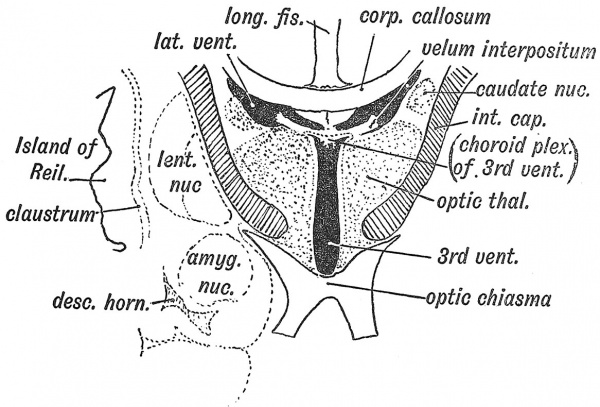
Fig. 109. Diagrammatic Section across the 3rd and lateral Ventricles of the Adult to show the Structures formed in their Walls.
Corpus Striatum
When a coronal section is made of the adult brain (Fig. 109) to exjDose the connections of the velum interpositum, it is clear that a mere overgrowth of the cerebral vesicles will not account for all the relationships shown. We shall see that the development of the commissures — particularly of the fornix and corpus callosum — introduces elements not seen in the simple brain of the embryo ; but, besides the commissural, another change has come about in the relationship of the corpus striatum to the optic thalamus. So intimate and extensive has the union between them become that the corpus striatum now forms a cap upon the lateral aspect of the optic thalamus (Fig. 109). Although developed in the wall of the cerebral vesicle the lenticular nucleus of the corpus striatum, and the islandic cortex are now constituents of the lateral wall of the 3rd ventricle — the cavity of the original thalamencephalon (Fig. 109). The intimate union of the corpus striatum with the optic thalamus we must regard as the result of two developmental processes : the formation of the nerve tracts of the internal capsule — which begin to appear in the 3rd month — and the neurobiotactic attraction which exists between the neuroblastic centres of the two great basal ganglia.
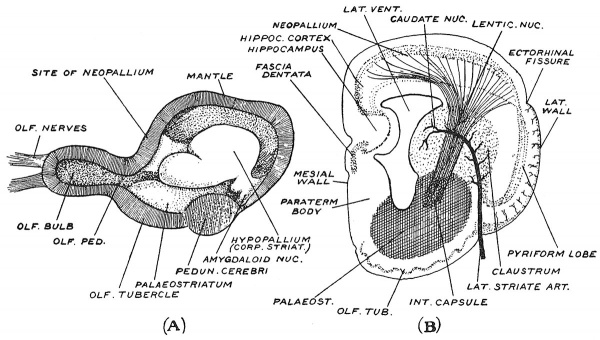
Two figures (Figs. 110, A, B) taken from a recent paper by Professor Elliot Smith[1] throw a new light on the nature and origin of that complex product of the cerebral vesicle— the corpus striatum. When the cerebral hemisphere of a turtle's brain is laid open, as in Fig. 110, A, we see the same three elementary parts as in the cerebral vesicle of the human embryo : (1) the hollow olfactory bulb, containing an extension of the ventricular cavity, (2) a mass occupying the floor and lateral wall representing the corpus striatum, (3) the vesicular wall, mantle or pallium. We see that the basal mass is made up of three projections, (1) the hypopallium representing for the greater part the caudate nucleus ; (2) at its hinder or temporal end the amygdaloid nucleus ; (3) the oldest part of all lying over the olfactory bulb (Fig. 110, A), and continuous with the hypothalamic region — the palaeostriate body. From Fig. 110, A, it is clear that the corpus striatum has a close connection with the olfactory region of the cerebral vesicle. The coronal section in Fig. 110, B shows the relationship of the corpus striatum to the remaining parts of the wall of the cerebral vesicle of a primitive mammal — the type of organ from which the human cerebrum has been evolved. An artery —the lateral striate[2] —one of the perforating branches of the anterior cerebral, is seen to enter the wall of the brain between the pyriform lobe above and the olfactory tubercle below and end in the corpus striatum. At the point where the artery enters, the cortex of the pyriform lobe is also inflected, although no fissure is present, and forms a stratum — the claustrum — on the outer aspect of the basal mass (Fig. 109). The cortical stratum, after forming the claustrum, bends inwards to become continuous with the nuclei of the corpus striatum. On the mesial wall of the vesicle (Fig. 110, B) the hippocampus arises in a somewhat similar way — by an inward growth of a cortical stratum— without the production of an open fissure. Elliot Smith, therefore, regards the corpus striatum as a cortical derivative ; growth has taken place towards the cavity of the ventricle tending to fill up the cavity with a cortical product. In the bird's brain the cortical growth is chiefly intraventricular.
Fig. 110, A, Sagittal Section of Turtle's Cerebral Vesicle made along the mesial plane so as to expose the cavity of the vesicle — the lateral ventricle. (Elliot Smith.)
Fig. 110, B. Coronal Section of a Primitive Mammalian Cerebrum, made across its anterior part, in front of the 3rd ventricle, to show the origin of the constituents of the Corpus Striatum. (Elliot Smith.)
The Mantle or Pallium
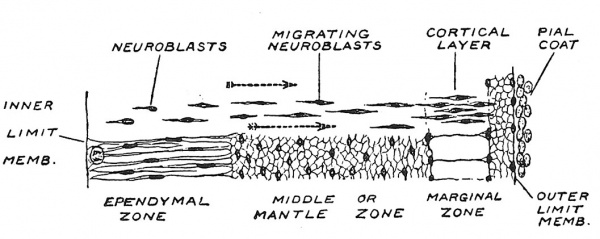
We have followed the fate of the striate area of the embryonic cerebral vesicle ; the differentiation of the olfactory area will be dealt with when we come to consider the nose and sense of smell ; there remains for consideration the third or paUial area of the cerebral vesicle. Even up to the end of the 3rd month the pallial wall of the vesicle remains thin ; it then measures only about one millimetre in thickness. Originally the pallial wall shows the same three strata or zones as were seen in other parts of the neural tube — namely an inner or ependymal zone, in which neuroblasts are produced ; a middle or mantle zone in which they are differentiated and an outer or marginal zone (Fig. 111). In the spinal cord the masses of neuroblasts were differentiated within the middle zone, where they remained, but in the cerebellum — and the same is true of the pallial wall — they invade the marginal zone. It is within the neuroglial scaffolding of the marginal zone that the grey cortical matter of the cerebral hemispheres is formed. In the 2nd month the migration of the neuroblasts to form a cortical layer has already commenced ; the process is particularly active in the 3rd and 4th months. Not only is there a migration from the ependymal to the marginal layer, but the production is particularly abundant where the mantle joins the corpus striatum. The middle zone, wMch contains grey matter in the spinal cord, is here the highway for the fibres developed from the cortical cells ; it forms the white medullary mass of the cerebral hemispheres.
Fig. 111. Diagram to show the differentiation of the Pallial Wall of the Cerebral Vesicle. After Wilhelm His (1831-1904)
Evolution of the Neopallium
Nothing could be more humble than the origin of man's master organ ; it was evolved in connection with the sense of smell.[3] The cerebral hemispheres, as we know them in the lowest vertebrates, are for the reception and interpretation of impulses from the olfactory end organs. Connections are established between the olfactory brain and the motor centres in the cord and in the hind- and mid-brain ; olfactory impressions can thus lead to action. Further, it became advantageous that there should be a nervous mechanism for the blending of impressions from the nose with impulses derived from sight, hearing and touch, and hence there arose connecting tracts by which stimuli streaming in from the various senses could be combined and their reactions coordinated with those streaming in from the nose. In the stem of vertebrates which became mammalian the supreme co-ordinating mechanism was evolved in that part of the neural system connected with smell — the telencephalon.
Fig. 112. Section across the Left Hemisphere of the Brain of a primitive vertebrate brain anterior to the Lamina Terminalis, to show the small extent of the Neopallium and the relatively great development of the Corpus Striatum and Bhinencephalon. (After Elliot Smith.)
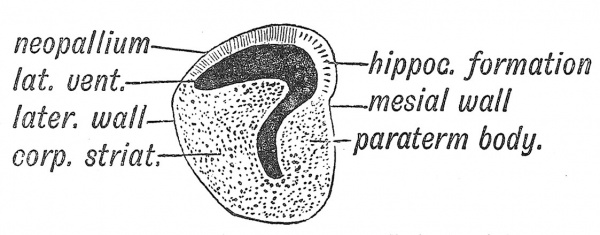
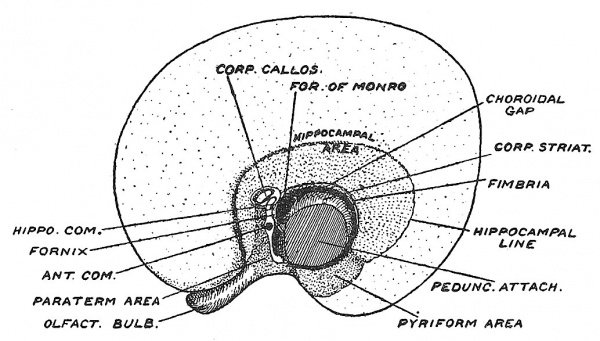
In Fig. 112 is represented a diagrammatic section across the anterior part of the cerebral vesicle of one of the lower vertebrate types — such a one as we may suppose preceded the modern mammalian form of cerebral hemisphere. There is a cavity within it — the lateral ventricle. The inner or mesial wall is formed of two parts : (1) the hippocampus or hippocampal formation — true cerebral cortex or mantle ; (2) below the hippocampus, the paraterminal body — a nuclear mass connected with the hippocampal formation by nerve tracts. The lateral or outer wall of the primitive hemisphere is made up of two parts — the corpus striatum — a nuclear mass partly covered by the cortex of the pyriform lobe (see Fig. 110, B). The pyriform lobe receives fibres from the outer root of the olfactory tract. These four parts — hippocampus, paraterminal body, pyriform lobe, corpus striatum — are connected with smell, and form the primitive mantle (archipallium) of the brain. In the roof of the ventricle an expansion of the mantle appears between the hippocampal formation on the inner side and the pyriform lobe on the outer side (Fig. 117) ; to this expansion Elliot Smith, whose account is followed here, gave the name of neopallium. It is this new mantle which becomes the basis for the higher combination of the sensory impressions coming in from all the organs of sense. It becomes the seat of consciousness and memory, and in man assumes enormous proportions ; hence the great and rapid expansion of the cerebral vesicles in the human foetus.
As may be seen from Fig. 117 the primitive mantle— all the cortical formation directly connected with the sense of smell — is arranged around the peduncular attachment, which may be described as the cerebral hilum. In Fig. 118 is shown how greatly the distribution of the primitive mantle is altered when the great commissures become developed.
Projection Fibres to the Neopallium
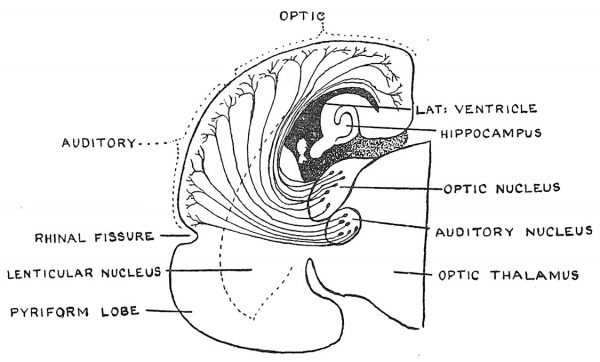
A transverse section of a mammalian brain of a primitive type— made further back and in a more advanced stage of development than that represented in Fig. 112 — is shown in Fig. 113. The section illustrates the manner in which projection fibres arise from two of the sensory nuclei in the optic thalamus— those connected with the nerves of sight and of hearing — and spread outwards into the neopallium — each set streaming into the area which lies nearest to it. In this way the mantle of the telencephalon becomes a higher sensorium for the reception, blending and storing of all sensory impressions. Other illustrations of the cortical afferent tracts are given in Figs. 98 and 110, B.
Fig. 113. Coronal section of the right half of the Cerebral Vesicle of a Primitive Type of Mammal, showing the termination of projection fibres arising in the optic thalamus, in the neopallium. (Elliot Smith.)
Localization of Function in the Neopallium
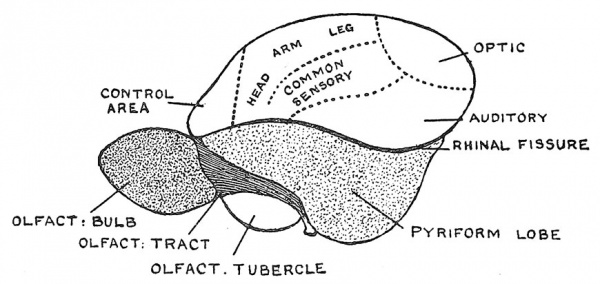
In Fig. 114 the brain of a primitive mammal is represented on its lateral aspect. The major part is seen to be made up of pyriform lobe, olfactory bulb and tubercle, all of them parts of the rhinencephalon. The rhinal fissure marks the junction of the neopallium with the older parts of the mantle on the outer or lateral aspect of the hemisphere. The areas adjacent to the various nuclei of the optic thalamus receive projection fibres from these nuclei. Thus it comes about that the lower and most posterior part of the neopallium, which forms the basis of the temporal lobe, receives fibres from the auditory centre ; in the upper posterior part the fibres from the optic nuclei end ; this area becomes the main part of the occipital lobe. Anterior to these two areas terminate the projection fibres connected with the sensory nuclei of the Vth nerve and with the nuclei of common sensation — receiving impulses from the leg, trunk, arm and head. Hence the surface areas of the body are represented in the neopallium. Naturally it is in connection with this area — the area of common sensation — that the cortical fibres which control the lower somatic motor nuclei arise. Anterior to the motor areas — occupying the region of the frontal pole — is an area connected with the control of the higher centres. These are the primary areas of the neopalhuni. In the course of evolution, secondary or associated zones have appeared round the primary areas, separating them widely and giving rise to the great mass of the human cerebrum.
Fig. 114. Lateral Aspect of the Cerebrum of a Primitive Mammal to show the Ehinal Fissure which separates the Neopallium above, from the older parts of the mantle below, represented by the Pyriform Lobe. The areas of the Neopallium in which the projection tracts from the optic thalami terminate are also shown. (Elliot Smith.)
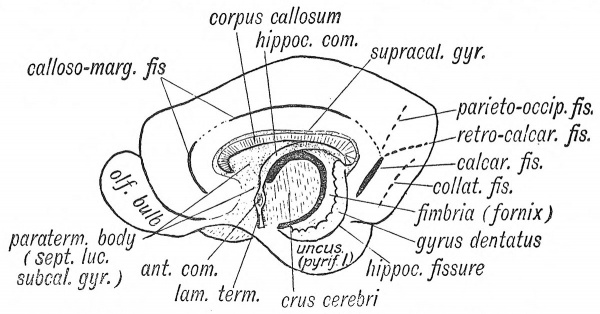
Fig. 115. The Anterior, Hippocampal and Callosal Commissures, with the primary fissures on the Mesial Aspect of a typical Mammalian Cerebrum. (Elliot Smith.)
Development of Cerebral Commissures
In order to secure a coordinated action of the whole brain, it is necessary not only that the cerebral centres of each hemisphere should be linked up by association p,nd projection fibres, but that the centres of one hemisphere should be united by transverse or commissural tracts with the corresponding centres of the other hemisphere. The lamina terminalis (see Figs. 97, 116, 117) affords a natural bridge for the formation and passage of the commissures. In the most primitive vertebrates, in all of which the cerebral hemispheres are chiefly olfactory in nature, the anterior commissure is already present. The next to appear is a dorsal or hippocampal commissure which unites the hippocampal areas on the mesial surfaces of the cerebrum (Figs. 115, 117). The last and greatest to be formed is the corpus callosum ; it appears in the true mammals— not in the monotr ernes and marsupials. Its development is commensurate with the size of the neopallium ; hence it is largest in man.
The cerebral hemispheres are thus connected by fibres which cross in the lamina terminalis, and form three commissures. (1) The anterior or ventral commissure, which connects the olfactory tracts, and afterwards parts of the temporal lobes ; (2) the dorsal or hippocampal commissure also formed in the lamina terminalis ; in man this commissure becomes the fornix ; (3) the corpus callosum, which unites the neopallium of one side with that of the other. It is formed in the lamina terminalis above the dorsal or hippocampal commissure (Figs. 114, 115, 116). The middle or grey commissure (Fig. 100) is merely an adhesion between the ependymal coverings of the optic thalami ; the optic chiasma (p. 207), the habenular or superior commissures (p. 110) need only be again mentioned. The posterior commissure is formed in the roof plate at the junction of the midand fore-brains (Figs. 94, 100).
Fig. 116. Mesial Aspect of the Brain of a Human Foetus in 4th month of development, showing the Lamina Terminalis and positions at which Commissures are formed. (After Goldstein.)
1 The Anterior Commissure
The Anterior Commissure (Figs. 116, 117) is developed in the lamina terminalis — the primitive anterior wall of the fore-brain. The commissure crosses in the lamina terminalis below and rather anterior to the foramen of Monro.
2 Hippocampal Commissure
Four parts are recognized in the fprnix of the human brain (Fig. 118) : (1) the body, adherent to the under surface of the corpus callosum ; (2) the posterior pillars, which are continuous with (3) the fimbriae and fibres of the alveus, covering the ventricular aspect of the hippocampus ; (4) the anterior pillars which end in the corpora mammilaria and optic thalami. The fornix contains two systems of fibres : (1) those which cross in the body and connect the hippocampal cortex of one side with that of the other, and form the true dorsal or hippocampal commissure, (2) fibres which connect together the various parts of the rhinencephalon of the same side, and with the corpora mammilaria and optic thalami.
Fig. 117. Mesial aspect of the Cerebral Vesicle of a Foetus about 3 months old, showing the commissures developing in the lamina terminalis and the distribution of the cortical areas which belong to the rhinencephalon. (After Streeter.)
To understand the development of this system it is necessary to obtain a clear conception of the relationships of the lamina terminalis to the various parts which have been distinguished in the rhinencephalon (Figs. 115, 117). On each side the lamina terminalis is continuous with the paraterminal body — that part of the rhinencephalon which lies immediately in front of the lamina terminalis. The paraterminal body becomes the subcallosal gyrus and septum lucidum in the mature brain (Fig. 118). The hippocampal formation, which includes the hippocampus and fascia dentata, bounds the choroidal fissure above (Figs. 117, 118). Fibres developed in the hippocampal formation cross to the opposite side in the lamina terminalis above the anterior or ventral commissure, thus forming the dorsal commissure (Fig. 116). It becomes included in the body of the fornix. The posterior pillar is developed in the hippocampal cortex, which forms the lip of the choroidal fissure. The anterior pillar lies in the paraterminal body and lamina terminalis.
Fig. 118. Diagram to show the structures formed in the Lamina Terminalis and Primitive Callosal Gyrus. (After Elliot Smith.)
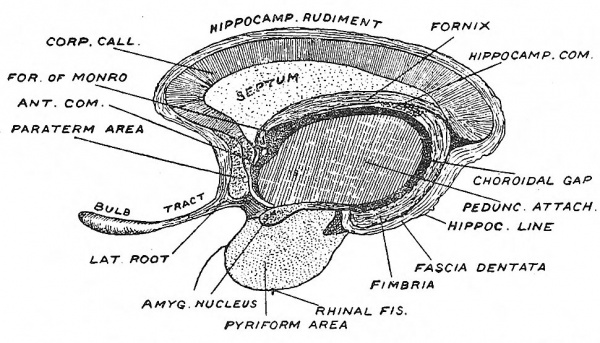
3 Corpus Callosum
The corpus callosum is the commissure of the neopallium, and hence in man, in whom the neopallium forms by far the greatest part of the cerebrum, this commissure attains an enormous development. The commissural fibres begin to form towards the end of the third month, crossing in the lamina terminalis with the fibres of the hippocampal commissure, but situated on their upper or dorsal aspect (Figs. 116, 117). As the corpus callosum rapidly increases within the lamina terminalis, it presses backwards on the hippocampal formation, and forwards on the paraterminal body. The hippocampal commissure is stretched, and forms the body and anterior pillars of the fornix. The hippocampal formation becomes (1) the supra-callosal gyrus, the hippocampus and fascia dentata (compare Figs. 117 and 118). The velum interpositum is overwhelmed and buried during the growth backwards of the corpus callosum and fornix. The paraterminal body is stretched to form the septum lucidum and subcallosal gyrus (Fig. 118). Thus by the development of the corpus callosum those two parts of the rhinencephalon — the paraterminal body and hippocampal formation — originally in close union, become widely separated. The supra-callosal and subcallosal gyri are vestiges of their former union. The corpus callosum may not be developed — a rare occurrence ; it is remarkable that this condition cannot be detected during the life of the individual.[4]
Formation of Fissures
Until the 5th month the surface of the cerebral vesicle is comparatively smooth. Up till then the three strata of the cerebral vesicle, the ependymal layer within, the cortical or nerve-cell layer on the surface and the medullary or nerve-fibre layer between, have increased at an equal rate. In the 6th and 7th months certain areas of the cortex increase rapidly, the increase affecting the superficial area to a very much greater extent than the deep, and affecting the cortex much more than the medulla, with the result that the surface of the cerebrum becomes raised into certain definite eminences or gyri, separated by depressions or fissures. The chief fissures are already well differentiated in the foetus of the 7th month ; during the last two months of intra-uterine development the secondary and tertiary sulci appear. The process of fissuration and convolution-formation are thus practically finished at birth. In the spinal cord the tracts of nerve fibres are formed outside the masses of grey matter ; in the cerebral vesicle the tracts are formed beneath the grey matter — between the grey matter and the ependyma (see p. 80). The neuroblasts in the cortex have reached nearly their full number by the 7th month ; after then it is their dendrites and collateral fibres that continue to develop (His).
Development of the Cortex
The mantle or wall of the cerebral vesicle of the brain becomes differentiated into a thin outer grey layer or cortex, containing the nerve cells, and an inner deep stratum — the medulla — of great thickness and made up of nerve fibres and tracts associated with the nerve cells of the cortex. The cortex is the substratum of consciousness, memory and mind. We naturally expect the great mental development which takes place in the earlier years of life to be accompanied by a corresponding change in the microscopic structure of the cortex. There is such a change, but it is difficult to measure for two reasons : (1) every area of cortex has its own peculiar structure and thickness ; Elliot Smith[5] has distinguished 28 areas in the cortex, each having its own peculiar structure ; (2) Dr. Joseph Bolton[6] observed that in some cases a newly born child may show a more mature development than a child of 3 months, there being as much variation in structure of cortex as in degree of ability. The latter observer noted that the cortex began to laminate or divide into three strata of nerve cells at the beginning of the 6th month, when the fissures and convolutions are in process of formation. He also made the important observation that the outer or pyramidal stratum was the latest in growth, and that the great development of this layer is the characteristic of the human cortex.
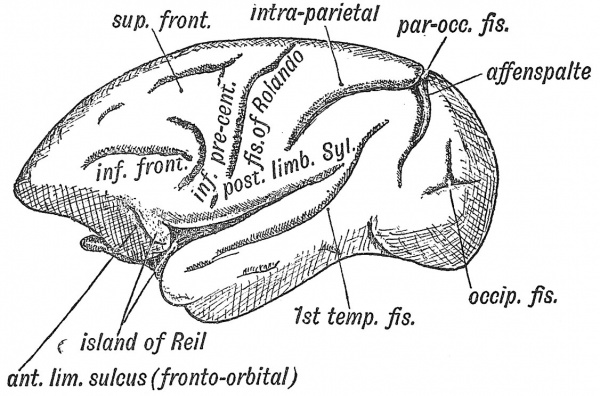
The Principal Fissures
The principal fissures of the brain are : (1) those connected with the rhinencephalon — the rhinal fissure (Figs. 114, 118) and the pseudo-hippocampal fissure — a mere linear depression (Elliot Smith) ; (2) those connected with the isolation of the Island of Reil — the fissure of Sylvius, the superior, inferior and anterior limiting fissures ; (3) those in the occipital cortex connected with the sense of sight — the calcarine, retro-calcarine, lunate sulcus (Affenspalte) parieto-occipital and collateral, (4) the callo so -marginal of uncertain import, (5) the fissure of Rolando, which is formed between motor and sensory areas of the cortex, (6) the orbital, (7) the sulcus rectus, (8) the intra-parietal, (9) the 1st temporal or parallel, which partially demarcates the auditory cortex. In the 7th month the fissures on the human brain have a remarkable correspondence to those on the cerebrum of an ape (Figs. 121, 123). We have already seen that the so-called choroidal fissure is formed by an inflection of the vesicular wall to form the choroidal villi of the lateral ventricles (Fig. 108).
Significance of Convolutions
There is some circumstance which limits the thickness of the cortex. If the cortical cells increase in number, accommodation is obtained, not by adding to the thickness of the cortex, but by enlarging the superficial area of the cerebrum. The cortex is correlated in its extent with the bulk of the body and with the area of the integumentary covering. Hence large animals such as whales and elephants have much convoluted brains. The rich convolutions of man's brain may be in some degree related to the nude and sensitive skin of his body (Elliot Smith). The most satisfactory explanation of the number and arrangement of the convolutions of the human brain is to be found in a study of the evolution of its various functional areas. The cortex was originally composed of primary sensory areas — connected with sight, touch, hearing, smell, etc. When secondary and higher zones were produced in connection with the primary areas, the surface of the brain was necessarily thrown into folds and fissures to provide the increase of surface required. Hence we find that the principal fissures are distinctly related to certain cortical areas. Elliot Smith distinguishes three kinds of fissures : (1) those like the calcarine and central fissures which separate one cortical area from another (being limiting fissures or sulci) ; (2) those like the lunate (Fig. 126), where the line of cortical demarcation lies, not at the bottom of the fissure, as in the last, but at the brink of the fissure. These are named operculate, because the convolution or operculum, which causes the fissure or depression, arises at the junction of two areas ; (3) a developing area may fold inwards, thus giving rise to a depression in the centre of an area, like the retro-calcarine in the midst of the visuo-sensory area (Fig. 127). The hippocampal linear depression and Sylvian fossa, as we have already seen (pp. 113, 117), are peculiar in their formation. Two fissures — the retro-calcarine and the collateral — actually cause an infolding of the whole thickness of the mantle, and give rise to two elevations in the posterior and descending horns of the lateral ventricle.
Fig. 119. Lateral Aspect of the Cerebral Hemisphere at the end of the 2nd month.
Formation of the Island of Reil and Fissure of Sylvius
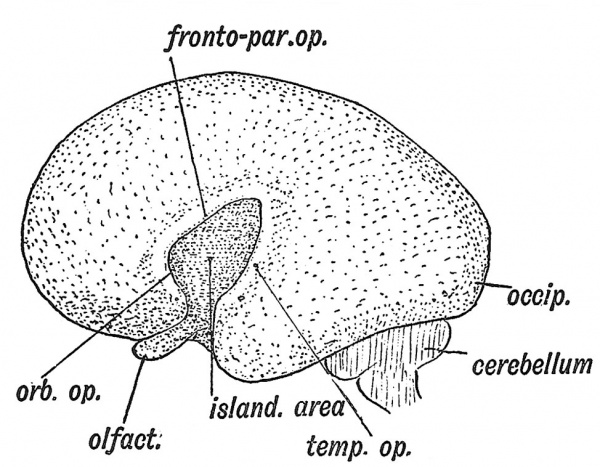
When the lateral wall of the cerebral vesicle is examined at the 5th month (Fig. 120) an area of cortex is seen to be rapidly becoming submerged by the overgrowth of the surrounding cortex. The submerged area is the Island of Reil ; it covers that part of the wall of the cerebral vesicle which is thickened by the corpus striatum (Figs. 107, B, 109). The submerged area becomes triangular in shape, the apex being directed backwards ; it is bounded by three limiting sulci — an anterior, superior and inferior. The rising lips of cortex, which bound the limiting sulci, form the temporal, fronto-parietal and orbital opercula, and ultimately meet over the submerged area (Fig. 122). The fissure of Sylvius separates the opercula. It will be readily grasped that the development of the corpus striatum prevents the expansion of the insular part of the vesicle, whereas the thinwalled mantle, out of which the other lobes of the brain are developed, expands readily and overwhelms the thickened area. The corpus striatum begins to form during the 2nd month, hence as early as that date the insular depression is visible on the lateral wall of the hemisphere (Fig. 119). This mechanical explanation of the origin of the fissure of Sylvius is probably only partially true ; the relatively great growth of the cortex which forms the opercula is due in the main to its great functional importance. By comparing Figs. 119 and 121 it will be realized that the formation of the Sylvian fossa is connected with the expansion and downward growth of the temporal lobe. The growth of the temporal lobe and the differentiation of the occipital pole (see Fig. 120) give the impression that there has been an actual rotation downwards of the cerebral vesicle on the Island of Reil. The lower end of the stem of the Sylvian fissure also indents the rhinencephalon, separating the uncinate gyrus from the anterior parts of the rhinencephalon (Figs. 118, 120).
Fig. 120. The same Aspect during the 5th month.
Fig. 121. The same Aspect during the 7th month.
The student is already familiar with the fact that the Island of Reil forms a cortical cap to the corpus striatum. The structures between the islandic cortex and the foramen of Monro rejDresent a section of the thickened wall of the cerebral vesicle (Fig. 109). Convolutions appear on it at the 7th month, when the rest of the cortex also becomes convoluted.
Further, the larger the area of cerebral cortex in any primate, the larger is the Island of Reil ; the more convoluted the cortex, the more convoluted the Island. Flechsig has shown that the cortex of the Island is joined to all the cortical areas of the mantle by bands of association fibres. Hence the Island must be regarded as playing a highly important part in coordinating the functions of the brain.
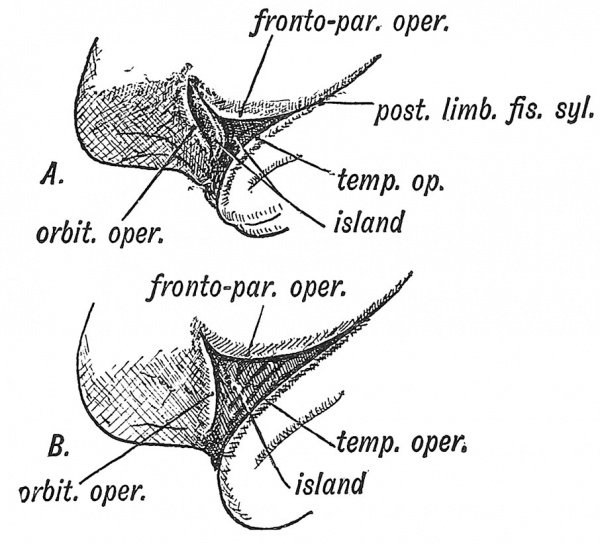
The Opercula
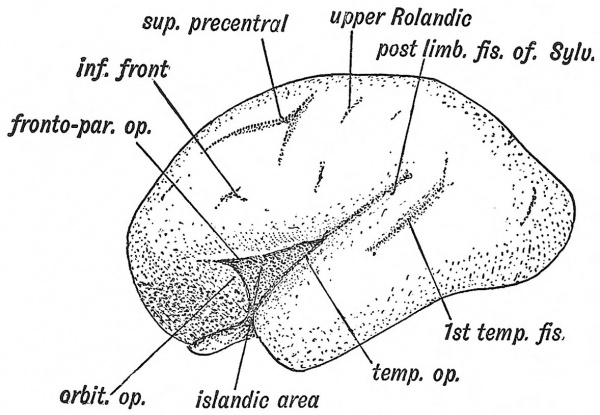
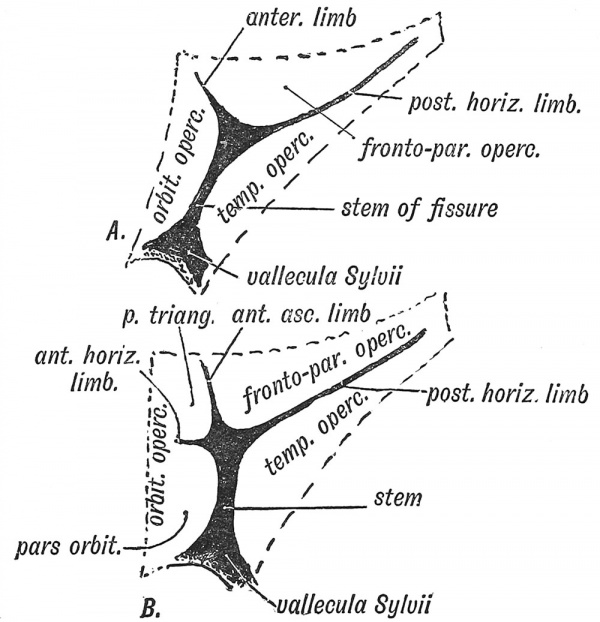
Three opercula grow up and cover the Island of Reil (see Figs. 121 and 122) : (1) the temporal, (2) the fronto-parietal, (3) the orbital. The late Professor D. J. Cunningham found that during the later months (7-9) of foetal life an opercular part, known as the pars triangularis (Fig. 122, B) appeared in quite 50 % of brains and was more frequently present on the left side than on the right, probably owing to the dominant centre for speech being situated on the left side. The jDars triangularis is the anterior part of the upper or dorsal operculum (labelled fronto-parietal in Figs. 120, 121), the horizontal limb of the fissure of Sylvius being the anterior continuation of the ujDper limiting sulcus of the Island of Reil (Elliot Smith). The pars triangularis is cut off from the dorsal operculum by the formation of the ascending limb of the fissure of Sylvius (Fig. 122, B). The temporal operculum rises first (5tli month), the others a month later. The opercula which bound the posterior horizontal limb of the fissure of Sylvius are the first to come in contact. By the end of the first year after birth all three opercula meet over the Island and completely Mde it. At birth there is still a part of the Island exposed behind the orbital operculum and in lower human races this is frequently the condition throughout life. The anterior opercula (pars triangularis and pars orbitalis) become part of the centre of speech and represent later additions to the human brain. If the pars triangularis be not separated from the dorsal operculum, which is commonly the condition on the right hemisphere, then the anterior limb of the fissure of Sylvius is not subdivided into anterior horizontal and ascending parts (Fig. 122, A and B). The posterior limb of the fissure of Sylvius is a limiting fissure ; it separates the audito-sensory area, situated in the first temporal gyrus — especially in the annectant convolutions of this gyrus buried in the posterior part of the Sylvian fissure, from the sensory-motor areas above the fissure.
Fig. 122. Diagram of the Opercula and Fissure of Sylvius. In A the orbital operculum is undivided ; in B it is subdivided. (After Cunningham.)
Comparative Anatomy of the Opercula and Island
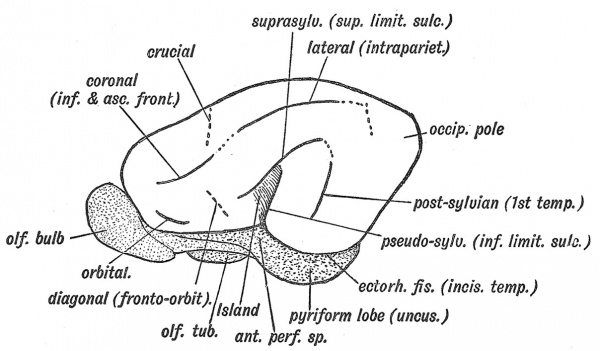
The Island of Reil and its opercula are only well developed in the higher primates. In the typical mammalian brain the upper limiting sulcus of the Island of Reil is represented by the supra-Sylvian fissure (Fig. 125), the inferior limiting sulcus by the pseudo-Sylvian fissure, the anterior limiting by the fronto-orbital fissure (Elliot Smith). There are no opercula — the cortex corres]3onding to the Island of Reil forms part of the surface of the brain. Figs. 123 and 124, A, B, represent stages in the evolution of the Island and opercula in the primates. In Fig. 123 the condition in dog-like apes is represented. Only the upper and lower limiting sulci of the Island are hid by opercula, the anterior limiting sulcus (fronto-orbital) being still freely exposed. The Island, which is small, is continuous anteriorly with the frontal lobe. In anthropoids (the gorilla, chimpanzee, orang and gibbon) the Island is larger ; the upper and lower limiting sulci are buried ; an imperfect anterior limiting sulcus (fronto-orbital fissure) partially separates the Island from the orbital surface of the frontal lobe. In man all three limiting sulci are covered by' opercula and completely isolate the Island, and occasionally this is the condition (Fig. 124, B) in the higher anthropoids, but it is in man only that the orbital operculum grows up and meets with the other opercula. This can be the more easily understood when it is remembered that the orbital part of the 3rd frontal convolution is connected with speech.
Fig. 123. The Island of Reil and Fissures on the Lateral Aspect of the Brain of a dog-like Ape.
Hippocampal and Ectorhinal Fissures
The hippocampal linear depression, which demarcates the hippocampal cortex (Figs. 117, 118) from the neopallial, we have already seen to be a mere superficial indication of a cortical ingrowth (p. 113). The incisura temporalis (Figs. 125 and 118), all that remains of the ectorhinal fissure of the typical mammalian brain, separates the uncus — part of the rhinencephalon — from the cortex fronto-par. open or neopallium of the temporal lobe. The ectorhinal, or rhinal fissure, as it is usually named, is thus a limiting fissure between olfactory and temporal cortex.
Fig. 124, A. The more common condition of the Island of Reil in Anthropoids. B. The complete isolation of the Island of E-eil, the condition seen constantly in the Human Brain and occasionally in the Anthropoid.
The Calloso-Marginal Fissure
This fissure on the mesial aspect of the brain arises from the fusion of the genual and intercalary fissures of the typical mammalian brain (Fig. 115). Its origin is probably the result of a pressure due to the growth of the cortex surrounding the corpus callosum, for if that structure be absent, the usual form of this fissure is completely altered. It separates one set of cortical areas from another (Elliot Smith).
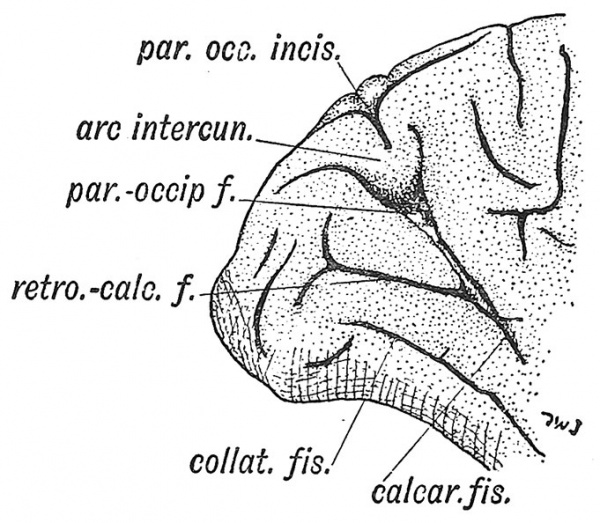
In the typical mammalian brain the calcarine fissure[7] forms part of the same arcuate system as the genual and intercalary (Fig. 115). The part of the cerebral wall in which it is formed projects within the posterior horn of the lateral ventricle (Fig. 226, B, p. 220). The cortex on the lower or posterior lip of the fissure shows the stria of Gennari which characterizes the cortex in which the optic radiations end. The calcarine fissure is thus a limiting fissure formed between striate and non-striate cortex. The retro-calcarine fissure or depression, which continues the calcarine sulcus backwards to the occipital pole, is formed by the growth and involution of the striate cortex (Fig. 127). In the ape's brain the striate cortex on the lateral aspect of the occipital pole increases rapidly and rises up as a lip or operculum over the cortex of the parietal lobe. The depression in front of the operculum is known as the simian fissure (Afienspalte) or sulcus lunatus (Fig. 123). In the human brain the great increase of the parietal cortex, a seat of association centres, has pushed the sulcus lunatus almost to the occipital pole (Fig. 126), or it may, especially in the more civilized races, be completely obliterated. Further the Y-shaped occipital sulcus (external calcarine) on the lateral aspect of the occipital pole (Figs. 123, 126) may join the retro-calcarine sulcus. More recently Elliot Smith has distinguished not only the striate area, in which the optic radiations end, but two surrounding areas, an outer zone — the peristriate, and an intermediate — between the outer zone and the striate area. Certain sulci have arisen in connection with the evolution of these two association or visuo-psychic areas. The collateral fissure below the calcarine (Fig. 127) probably results from a mechanical pressure exercised by the growth of the striate area. The parieto-occipital fossa or dejDression on the mesial aspect of the brain results from the inflection of an area of cortex between the calcarine areas of growth behind, and the area of association centres on the mesial aspect of the parietal lobe in front (Fig. 127). The production of the parieto-occipital fossa, with its complex of buried convolutions and sulci, is also related to the growth backwards of the corpus callosum. In human brains where this structure is absent the buried convolutions and sulci are superficial.
Fig. 125. The Fissures on the Lateral Aspect of a typical Mammalian Brain. (Elliot Smith.) The Fissures to which these correspond in the Human Brain are indicated within brackets. The parts of the Rhinencephalon are stippled ; the cortex corresponding to the Island of Hail, shaded.
Orbital Fissure
This fissure is present in most mammalian brains, but its significance is still doubtful.
Fissure of Rolando or Central Fissure, appears during the sixth month as an upper and lower linear depression, which join together in the course of development (Fig. 121). The fissure appears between the motor areas of cortex in front of it, and the sensory areas behind it, and is therefore a limiting fissure. The upper part does not quite correspond to the crucial sulcus of the brain of the cat and dog, for in them that sulcus forms the anterior limit of the motor areas (Elliot Smith) (Fig. 125) ; the lower part may represent part of the coronal fissure. The fissure of Rolando reaches its fullest development in man ; it is found only in the higher primates (monkeys and anthropoids).
Fig. 126. The Lateral Aspect of the Occipital Lobe of a Human Brain, showing the Sulcus Lunatus (Affenspalte). (Elliot Smith.)
Fig. 127. The Mesial Aspect of the Occipital Lobe of a Human Brain, showing the complex nature of the Parieto-Occipital Fissure. (Elliot Smith.)
Sulcus Rectus
The sulcus rectus, or straight fissure, appears before that of Rolando, and is found in primate brains in which the Rolandic fissure is absent (Figs. 123, 125). It forms in the adult brain (1) part of the inferior frontal fissure, (2) the lower part of the precental fissure (ascending frontal). It lies between two areas of frontal cortex which are of different structure, and corresponds to the coronal fissure of the cat's brain (Fig. 125).
Intra-parietal Fissure
The intra-parietal fissure appears between three areas of growth : (1) the cortex of the inferior parietal lobule below, chiefly consisting of association areas related to the visual and auditory and perhaps also to the areas of common sensation ; (2) the occipital cortex posteriorly ; (3) the cortex behind the upper end of the fissure of Rolando above and in front. It corresponds to the lateral fissure of the cat's brain (Fig. 125), while the whole of the intra-parietal fissure of the ape's brain (Fig. 123) may be regarded as equivalent to the ascending rami in the human brain (Jefferson). The ascending, horizontal and occipital limbs of this fissure arise independently in connection with separate areas. They may or may not become conjoined. All the parts of the fissure are limiting in nature.[8]
Parallel or First Temporal Fissure
The first temporal fissure separates the first temporal gyrus, in which the auditory centres are situated, from the neighbouring cortex (Figs. 123, 125). As the first temporal gyrus rises to form the inferior operculum of the island of Reil, part of it, in the form of a number of gyri which connect it with the island, are buried in the fissure of Sylvius. In these gyri Campbell has located the terminations of the auditory tracts, the superficial part of the first temporal convolution forming association areas for the auditory centre (Fig. 246). The first temporal fissure corresponds to the post-Sylvian fissure of the typical mammalian brain (Fig. 125).
Secondary Sulci and Gyri
During the eighth and ninth months the remaining sulci and convolutions of the brain are formed. For the greater part these are peculiar to the human brain.
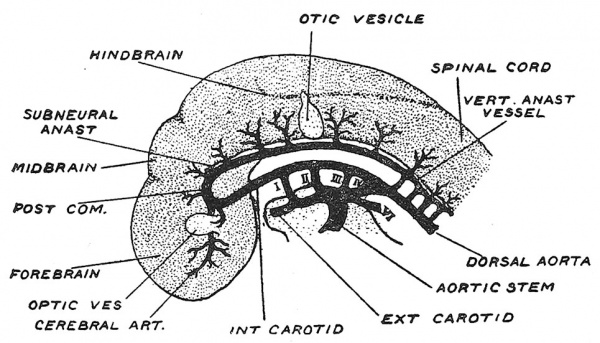
Fig. 128. Diagrammatic Representation of the Arteries of the Brain at the end of the first month of development. (After Evans.)
Vessels of the Brain
The embryonic arteries from which the cerebral and vertebral arteries become evolved, are shown in Fig. 128. The dorsal aorta, in which the aortic arches end, is continued forward to the fore-brain, where at the root of the optic vesicle and near the site of the future vallecula of Sylvius it divides into anterior and posterior branches ; the anterior branch will become the stem of the middle and anterior cerebral arteries as the cerebral vesicles begin to expand, while the posterior branch becomes continuous with the subneural anastomotic vessel, from which the posterior communicating and basilar arteries will become differentiated and from which the posterior cerebral will arise. The subneural anastomotic chain is fed by segmental vertebral branches of the dorsal aorta (Fig. 128). From this segmental network is formed the vertebral arteries. The right and left anastomotic vessels fuse under the hind-brain during the 6th week to form the. basilar artery.[9]
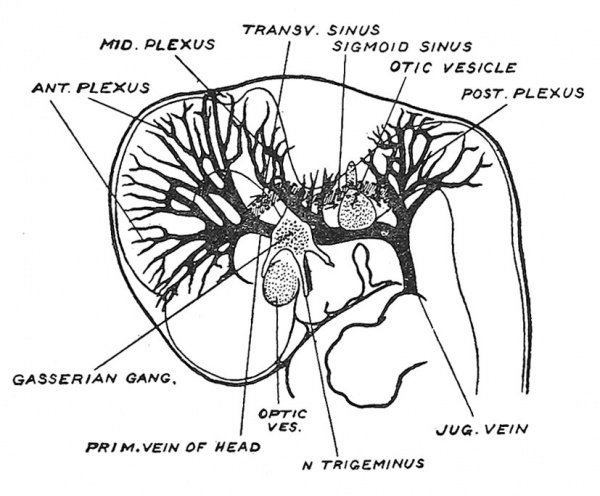
The embryological basis out of which the venous sinuses and cerebral veins are developed, is shown in Fig. 129. At the middle of the 2nd month the veins of the fore- and mid-brains unite behind the stalk of the optic vesicle to form the primitive vein of the head, which passing backwards internal to the Gasserian ganglion leaves the interior of the cranial cavity just in front of the internal ear, passes through in the region of the middle ear to become the jugular or anterior cardinal vein. Before leaving the interior of the skull it receives a cerebellar venous trunk (Fig. 129) and after its exit a medullary trunk — which escapes by the jugular foramen. With the expansion backwards of the cerebral vesicles during the 3rd, 4th and 5th months the system of the longitudinal and transverse sinus becomes evolved by the union of the venous network included in the longitudinal fissure between the cerebral vesicles and between the cerebral vesicles and cerebellum. The main changes are indicated in Fig. 129 ; only part of the primitive vein persists — the part lying internal to the Gasserian ganglion which becomes the cavernous sinus ; the extra-cranial part disappears towards the end of the 2nd month, but it occasionally persists as an emissary vein opening near the root of the zygomatic process. Two important anastomotic channels open up : (1) the precerebellar which drains the tributaries of the fore- and mid-brain into the primitive cerebellar trunk ; (2) the post-cerebellar which unites the cerebellar trunk with the veins of the hind-brain ; the hind-brain trunk escapes by the jugular foramen. Thus, as the cerebral vesicles grow back their veins are transferred first from the primitive vein to the cerebellar and then to the venous system of the hind-brain. From the anastomic channel thus opened up are fashioned the transverse and sigmoid sinuses[10] (Fig. 129).
Fig. 129. The Primitive Vein of the Head and its tributaries in the 6th week of development, with indications of the new anastomotic channels opened up during the 3rd and 4th months. (After Streeter.)
Membranes of the Brain
Even before the cephalic part of the neural tube is enclosed, mesodermal cells spread in between it and the surrounding ectoderm to form a primitive covering. Out of the covering become differentiated the capsule proper of the brain — the pia-arachnoid with its vessels, the membranous cranial capsule, from which are differentiated the dura mater, enclosing bones and pericranium, and the connective tissues of the scalp. The differentiation of the membranes of the brain and spinal cord is closely related to the establishment of a cerebrospinal fluid system. We have seen that the choroid plexuses of the ventricles of the brain become developed in the 7th week when the human embryo is about 15 mm. long. Dr. L. H. Weed found that at this stage of development in the pig, the cerebro-spinal fluid formed in the 4th ventricle began to escape through a localized area in the inferior medullary velum and to collect in the overlying mesodermal tissue.[11] At the site of escape an opening is formed in the medullary velum, the foramen of Magendie, arising in this way. The foramen formed in each lateral recess of the 4th ventricle are produced in a similar manner. The subarachnoid spaces thus commence over the 4th ventricle and round the medulla oblongata and from the region of the hind-brain the system extends proximally and distally until, by the middle of the 3rd month of development, the entire neural tube is enclosed by the arachnoid. The mesodermal condensation which bounds the subarachnoid system becomes the arachnoid ; the pia mater represents the subarachnoid tissue. At the same time as the subarachnoid spaces are being formed another plane of cleavage sets in external to the arachnoid, the arachnoid being thus separated from the dural layer of the cranial capsule and a potential space produced — the subdural. These spaces, particularly the subarachnoid, do not represent parts of the lymphatic system ; lymphatic vessels, we shall see, arise like blood vessels ; nor does the cerebro-spinal fluid represent a species of lymph.
- ↑ Journ. of Anat. 1919, vol. 53, p. 271.
- ↑ See paper by Col, J. L, Sbellshear, Journ. of Anat. 1921, vol. 55, p, 27,
- ↑ See Prof. Elliot Smith's "Arris and Gale Lectures," Lancet, 1910, Jan. 1st, 15th, 22nd.
- ↑ See cases described by Elliot Smith and by Cameron, J. Anat. and Physiol. 1907, vol. 41, pp. 234, 293.
- ↑ Prof. Elliot Smith, Journ. of Anat. and Physiol 1907, vol. 41, p. 237.
- ↑ Pr, Joseph S. Bolton, Brain, 1910, vol. 32, p. 26.
- ↑ See Prof. Elliot Smith, Journ. A)iat. and Physiol. 1907, vol. 41, p. 198.
- ↑ As to nature of the intra-parietal complex see Geoffrey Jefferson, Journ. Anat. 1913, vol. 47, p. 365.
- ↑ For further details see Bertha de Vriese, Archiv de Biol. 1904, vol. 21, p. 357 ; F. P. Mall, Amer. Journ. Anat. 1905, vol. 4, p. 1 ; H. M. Evans, Keibel and Mall's Manual of Human Embryology, 1912, vol. 2, p. 570 ; G. L. Streeter, Contributions to Embryology, 1918, vol. 8, p. 5.
- ↑ See G. L. Streeter, Amer. Journ. Anat. 1915, vol. 18, p. 145 ; 1916, vol. 19, p. 67.
- ↑ In connection with the development of the cerebro-spinal fluid system consult : Dr. L. H. Weed, Anat. Rec. 1916, vol. 10, p. 475 ; Contributions to Embryology, 1917, vol. 5, p. 3 ; 1920, vol. 9, p. 425 (production of Hydrocephalus) ; Percival Bailey, Journ. Comp. Neur. 1916, vol. 26, p. 79.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Human Embryology and Morphology: 1 Early Ovum and Embryo | 2 Connection between Foetus and Uterus | 3 Primitive Streak Notochord and Somites | 4 Age Changes | 5 Spinal Column and Back | 6 Body Segmentation | 7 Spinal Cord | 8 Mid- and Hind-Brains | 9 Fore-Brain | 10 Fore-Brain Cerebral Vesicles | 11 Cranium | 12 Face | 13 Teeth and Mastication | 14 Nasal and Olfactory | 15 Sense OF Sight | 16 Hearing | 17 Pharynx and Neck | 18 Tongue, Thyroid and Pharynx | 19 Organs of Digestion | 20 Circulatory System | 21 Circulatory System (continued) | 22 Respiratory System | 23 Urogenital System | 24 Urogenital System (Continued) | 25 Body Wall and Pelvic Floor | 26 Limb Buds | 27 Limbs | 28 Skin and Appendages | Figures