2012 Group Project 5
Abnormal Vision
Introduction
This page will be discussing Abnormal development of vision. The purpose of this page is to give a brief overview of the development of the eye, association between genes and abnormalities that can occur under environmental influence. By understanding the factors that contribute to abnormal vision development, treatments or cures maybe developed in the future and these conditions can be better managed.
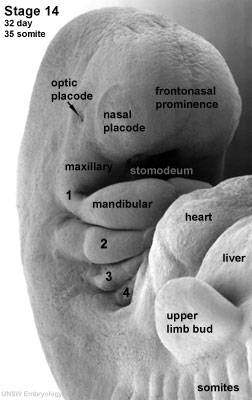
The development of the eye is very sensitive and requires accurate, co-ordinated associations of many different factors, both genetic and environmental. The development of the eye occurs with the optic placode at a Carnegie stage 12 embryo (week 4). During this time any malfunction of development or disturbance of developmental factors such as gene mutations will create an abnormality of the eye.
Abnormalities in vision can be acquired by environmental means, like that of Fetal Alcohol Syndrome or by Genetics such as Leber Congenital Amaurosis and others discussed on this page. People with congenital vision abnormalities experience a lot of inconvenience and current technology generally cannot replace the abnormal eye for example in the case of Microphthalmia (development of abnormally small eye) and Anophthalmia (the absence of eyeball in the orbit). Normally, treatment or management of these conditions focus heavily on improving the appearance of the patients rather than improving their vision.
Normal Eye Development
In order to fully comprehend abnormal development, an understanding of the normal early development of the eye is important. It begins with the optic primordium and sulcus developing in the neural folds at Carnegie stage 10 or around 22 days. Stage 11, or 24 days, sees the rostral neuropore closing and the optic vesicle forming from the optic sulcus. Only at stage 12 will you see the beginning formation of the optic placode as the optic vesicle sits near the surface ectoderm.[1][2][3]At stage 13, 28 days, a thickened surface ectoderm layer has developed on top of the optic vesicle, we know this to be the lens disc. The retinal disc (soon to be optic cup) has appeared on the wall of the optic vesicle.[2] As development continues through to Carnegie stage 14, the retinal disc is invaginated to form then optic cup and the lens pit forms. This lens pit closes in stage 15 and the optic cup and lens vesicle seem to bulge and press against the surface as the primary vitreous body begins to form.[2] [1][3] This contour of the optic cup progresses until it is quite noticeable in stage 16, small grooves can also be seen above and below the eye. The retina differentiates at around stage 17 and the primary lens fibers obliterate the cavity of the lens vesicle filling the space at stage 18. The pupillary membrane and the layers of the cornea develop from stages 19-21. At stage 20 the retinal nerve fiber layer appears and grow towards the brain.[2] As the grooves above and below the eye develop and deepen in stage 17-19, eyelid folds develop, which soon turn into actual eyelids at around stage 19 and continue to develop further and grow slowly at stage 22. The eyelids close completely at stage 23.[1]
For more information on normal development please click here
Abnormal Development
Abnormalities can occur in different parts of the eye. In the section below, we have focused on abnormalities that developed in the lens, cornea and retina and have included genes that are associated with the abnormalities. Also, a general overview on the role of each genes and what is their impact on the structure of the eye when mutated is given.
Abnormal Lens Development
Overview of Normal Lens Development
As stated above, the lens originates from the ectoderm on top of the optic vesicle, it also requires a large portion of the head ectoderm surrounding this area before interacting with the optic vesicle. The vesicle is more involved with the correct positioning and lens formation at this stage.[4] [5]As the optic vesicle grows rapidly, the lens placode moves almost on top of the vesicle just close enough for a small gap to show. From this a network of fibrin adheres one surface to the other and the lens placode thickens and both sufaces start to invaginate becoming the lens pit and optic vesicle. The lens pit then detatches after deepening further and becomes the lens vesicle. From here the cells of the lens differentiate into primary fibres in the posterior half of the vesicle and epithelium in the anterior half. Rapid growth occurs with cell division occurring mostly in the epithelium region called the germinative zone with the daughter cells moving to the transitional zone where they mature and differentiate into fibre cells which continues throughout life. [4]
- Pax-6 Genes
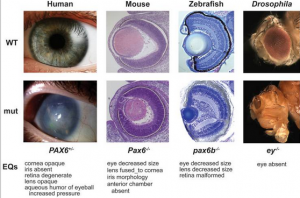
Most of the development of the lens occurs as a result of the Pax-6 gene. [5][4] It is expressed in early developing optic vesicle and lens placode which is during the 4th and 5th week of human eye development. [6]It is responsible for the embryonic and postnatal development of the lens epithelium, and in particular, the ectoderm in embryonic development. And a variety of abnormalities can occur as a result of a mutation from this gene, Peters' anomaly (Corneal Opacity) and aniridia (lack of Iris) [7] are just some, as well as an abnormal cortical plate formations.[5] Within a few days of development, it is easy to distinguish an abnormality with the forebrain and the shape of the optic vesicle, as development continues there is an absence of a thickened ectodermal surface, lens placode, lens pit and the optic vesicle becomes distorted. As the ectodermal surface did not thicken, there is then no developing lens thus, Pax-6 gene is absolutely essential in the development of the lens. [5]
- FOX genes
Another gene that may have an important role in the development of the lens is FOXe3 (Forkhead box protein E3) and FOXe4 (Forkhead box protein E4). They are expressed in the lens and they aid in the formation of the lens. Mutation of FOXe3 gene will cause anterior segment dysgenesis and result in cataracts formation as part of the dysgenesis.[8][9]FOXe3 can be detected in the first embryonic days 8.5, in two distinctive area of the cephallic neural folds. Both FOXe3 and FOXe4 are not as important as the Pax-6 gene, but it plays a very essential role in the control of proliferation and differentiation of the anterior len epithelium and it is evident that, the loss of these two genes will lead to anterior segment dysgenesis. [9]
- Crystalline genes
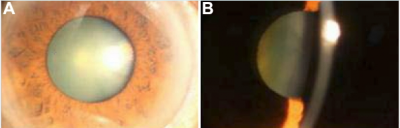
Images of congenital hereditary cataracts from mutations of crystallin genes
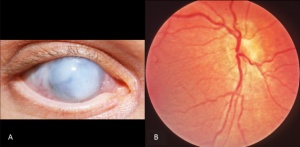
Cataracts of the eye can be defined as any opacity of the crystalline lens in which congenital cataract is especially important because it has the potential for inducing abnormal visual development and resulting in permanent blindness. Inherited cataracts contribute the most to congenital cataracts especially in developed countries.[8][10] Crystalline genes in the human body codes for major structural proteins in the lens, they are considered important due to their high level of expression in the lens and their functions in maintaining lens transparency. There is a strong relationship between their mutation and the development of congenital cataracts.[10] One example of crystalline gene is βγ-crystallins. When this gene is mutated, major abnormalities in the protein structure can occur and result in the presence of an unstable protein that is able to precipitate from solution and protein denaturation and precipitation that will eventually lead to cataract formation.[8]Mutations in the αA-crystallin gene have also been implicated both in autosomal recessive and autosomal dominant cataract. One would expect that αB-crystallin would have a similar effect on cataracts formation but experiments suggested that cataracts formation is dominated by mutations in αA-crystallin gene with the abnormal protein having a toxic effect on lens cells. The mutant protein also inhibit the functioning of normal αA-crystallin protein.[8]
Abnormal Corneal Development
Overview of Normal Corneal Development
The corneal Development begins at around Carnegie Stage 15 with the surface ectoderm differentiating into the anterior epithelieum of the cornea, at this stage it has its own basement membrane. At stage 18 the posterior epithelium of the cornea begins to form and by stage 19, is easily recogniseable. [3] Stage 20 sees the developing cornea with an anterior epithelium, a postepithelial layer, and a posterior epithelium. The postepithelial layer will develop further to become the substantia propria of the cornea in stage 21 via cells invading the layer. This process is finished in stage 22 and at stage 2 the cornea consists of an anterior epithelium and a basement membrane, the substntia propria and the posterior epithelium. [3]
- SCL4A11 gene
Congenital Autosomal recessive corneal endothelial dystrophy type 2(CHED2) is associated with mutations in SLC4A11, Solute Carrier family 4 (sodium borate cotransporter) member 11). This gene is located on chromosome 20p13-12. Mutation of this gene cause disorder of the cornea that is characterised by diffuse bilateral corneal clouding and they are often edematous and have a ground glass appearance that is evident at birth or in the neotal period and can cause the impairment of vision and require corneal transplantation.[11][12][13]
SLC4A11 is an electrogenic Na/borate cotransporter and it can stimulate cell growth and proliferation by increasing intracellular borate and activating the MAPK pathway.[11]The ion transporter SLC4A11 promote sodium-dependent transport of borate as well as flux of sodium and hydroxyl ions. It has been shown that SLC4A11 is expressed in the endothelial cells of the cornea, and mutation of the gene will cause increased sodium concentrations in the stroma, thus resulting in morphological changes of the cornea. The mutated gene also result in the death of endothelial cells and loss of barrier function, eventually secondary corneal edema. [14].Some other studies have shown that borate can lead to the phosphorylation of both MAP-kinase and extracellular signalling kinases, and these are part of the mitogen activated protein kinase cascade (MAPK). MAPKs are important in the regulation of cell cycle and growth, a less regulated MAPK pathway can lead to major characteristics that are presented in CHED2 such as the morphology of the eye. [14]
Abnormal Retinal Development
Overview of Normal Retinal Development
The retina is of neuro-ectodermal origin[15] and is derived from the optic cup (stage 17). The optic cup is divided into the neural retina and the retinal pigmental epithelium. The neural retina is light sensitive, the region from which the photoreceptors and cell bodies of the neurons are located. As the retina is the part of the eye from which the brain receives the most visual information there are many abnormalities which can occur here during development. In various research avenues for Leber Congenital Amaurosis (to be discussed later) the following genes have been found to be involved in retinal development, they are as follows : RPE65 for retinoid metabolism, GUCY2D phototransduction, CRX photoreceptor outer segment development, RPGRIP1 disk morphogenesis, CRB1 zonula adherens formation and AIPL1 cell-cycle progression.[15]
- CRX gene
An example of one of these gene expressions is CRX. CRX is expressed abundantly in photoreceptor cells and is an important regulator of various photoreceptor specific genes and key enzymes for melanin synthesis. [16] While it has been determined that CRX is important in terminal differentiation of the photoreceptors, using a mouse model, in which the otx2 was conditionally knocked out there was a complete loss of retinal photoreceptors and thus it has been found that otx2 is essential for CRX transcription as it is an upstream regulator of CRX expression[16]. Otx2 expression covers most of the fore and midbrain neuro-epithelium and subsequently the retinal region[16]
- RPE65
RPE65 is an enzyme located in the retinal pigment epithelium (RPE)which is a catalyst during a crutial part of the visual cycle. It permits photoreceptors pigments to absorb photons which maintain sight[17].
- Retinal Pigment Epithelium and Albinism
Some research suggests that Retinal Pigment Epithelium (RPE) has a regulatory effect upon neural retina development[18]. A melanin related agent, has been suggested to be important in retinal development and maintenance during maturity as in albinism there is a reduction in melanin and retinal abnormalities are present. [18]The Tyrosinase gene controls melanin production and acts a catalyst in DOPA production from tyrosine and is a regulator of cell cycles.[18] Although there is evidence for a relationship between DOPA and retinal mitosis the mechanisms responsible are not so easily identifiable.[18] When melanin is absent a variety of retinal disorders such as abnormal connection between the eye and brain, undeveloped central retina and rod defects can occur, this reduction and/or absence of melanin is commonly caused by Albinism which also has reduced cell density is also abnormally low with ganglion cells of the retina decreased by 25%.[18] Other eye defects caused by Albinism are absent Fovea, undeveloped Macula region and abnormal chiasmatic projections to name a few[18]
Ocular Disorders
Major ocular disorders can be split into two separate sections based on the way in which they originated.
- Genetic disorders are cause when there is a mutation to genes which allow for ocular development.
- Environmental disorders are caused when external factors disrupt the mechanisms or genes involved in ocular development
Genetic Ocular Disorders
Leber Congenital Amaurosis
Leber Congenital Amaurosis (LCA) is an inherited retinal degenerative disorder that causes blindness or loss of sight at birth.[19] LCA has an Autosomal recessive pattern of inheritance where there is a 25% chance for a child to contract LCA.[19]
LCA was first recognized by Dr Theodor Leber in 1869. He published the paper Ueber Retinitis pigmentosa und angeborene Amaurosa (About Retinitis pigmentosa and congenital Amaurosa) in Archiv fur Ophthalmologie (now known as Graefes Archive of clinical and experimental ophthalmology).[20] In his research Leber describes a set of clinical manifestations in children he studied at the Ilvesheim School for the blind in Germany with Retinitis Pigmentosia which are used still today as a diagnostic technique for LCA [15]. In his paper Leber classified this disease as being a part of the Retinitis Pigmentosia group of optical disorder, placing great emphasis upon the high incidence of hereditary factors[15]. As stated in the section on Abnormal Retinal Development out of the eighteen genes [21] found that contribute to LCA, six of them have been linked to specific sections to retinal development[15]. As a result making a differential diagnosis on congential blindness can be difficult as LCA can overlap with many other disorders such as Bardet-Biedl syndrome and Senior-Loken syndrome for example[21]. An important diagnostic technique was recognised by Franeschetti and Dieterle of non-detectable or severely reduced Electroretinogram (ERG) measured in early progression of LCA[15]. The measurement of ERG has become a prerequisite diagnostic protocol for LCA[15].
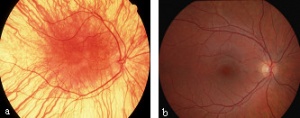
CEP290 gene mutation is the most common cause of LCA and accounts for 6-22% of all cases[21]. This mutation is a slow progressive form of LCA and has a relatively normal optic disc with sclera ring. Abnormalities associated with CEP290 mutation include salt and pepper aspect of fundus with Macular degeneration causes typical Retinitis Pigmentosa appearance in second decade and Mild lobar retinal pigment epithelium atrophy[21]. Clinical signs associated with CEP290 include sluggish pupillary reflexes and juvenile cataracts[21].
Clinical Manifestation
As stated above Leber was the first to identify the clinical appreance of LCA which are still used today. While sufferers of LCA can be blind from birth or suffer sever visual loss during infancy, other manifestations found in sufferers of LCA can include all or some of the following[15]:
Research Timeline of LCA
From 1869-2009, the major contributers to LCA research and consequently to retinal regeneration therapy research: "Courtesy: National Eye Institute, National Institutes of Health (NEI/NIH)."
- 1869- Dr. Theodor Leber (1840-1917), German ophthalmologist, first describes what is now known as Leber congenital amaurosis, an inherited retinal disease that causes severe visual impairment early in childhood [22]
- 1965- Human adeno-associated virus (AAV) was discovered. This discovery is important as it has become an important research avenue for retinal regeneration therapies[22]
- 1984- Drs. Nicolas Muzyczka and Paul Hermonat publish an article on adeno-associated virus (AAV) expresses that it can be used to introduce foreign DNA into human and murine tissue culture cells.[22]
- 1990- Dr. T. Michael Redmond of the NEI's Laboratory of Retinal Cell and Molecular Biology, Section on Gene Regulation began work on RPE-specific monoclonal antibody.[22]
- 1993- Dr. T. Michael Redmond of the NEI's Laboratory, cloned RPE65, a protein necessary for processing vitamin A in the visual cycle.[22]
- 1997- RPE65 gene mutations identified as the cause of congenital blindness in some children with Leber congenital amaurosis.[22]
- 1998- Dr. T. Michael Redmond's team in the NEI's Laboratory use the knockout mouse model to establish RPE65’s role in vitamin A metabolism. RPE65 gene mutation was discovered as causing congenital blindness in Briard dogs.[22]
- 2001- Gene transfer RPE65 therapy used to restore vision in a Briard Dog by researchers at the University of Pennsylvania and University of Florida supported by the NEI.[22]
- 2005- Restored vision in Briard dog persists longer than four years following RPE65 gene transfer [22]
- 2007- NEI-supported RPE65 human clinical trial began. This study was designed to assess the safety of using a modified adeno-associated viral vector (rAAV2-hRPE65) to deliver the normal RPE65 gene to the retina.[22]
- 2009- One-year results from the NEI-supported RPE65 human clinical trial published in Human Gene Therapy and the New England Journal of Medicine. All three patients remained healthy and maintained previous visual gains. One patient also noticed a visual improvement that helped her perform daily tasks. Results from early LCA clinical studies represent one of the first steps toward the use of gene transfer therapy for an inherited form of blindness.[22]
Treatment
To date there is no effective treatment for LCA [23]
New Research Development for LCA
A current field of research for LCA and in turn retinal restoration is that of using an altered version of the adeno-associated virus. the following papers go into depth, the research taking place to restore retinal function.
Gene therapy restores vision in a canine model of childhood blindness.
This proof of concept study being the first to show gene therapy can restore vision[24]. In 2001 study on dogs with early and a similar impairment to that of LCA in children[24]. The virus was injected intra ocular, into 3 dogs with LCA and the results were assessed by ERG[24]. As a result of this experiment retinal function improved in eyes compared with the same eyes before treatment[24].
Treatment of Leber Congenital Amaurosis Due to RPE65 Mutations by Ocular Subretinal Injection of Adeno-Associated Virus Gene Vector: Short-Term Results of a Phase I Trial
This Paper had three adult patients with LCA from RPE65 mutation. The recombinant adeno-associated virus serotype 2 (rAVV2) vector to carry the human RPE65 has restored vision in this model.[25]. The eye with the worst function was used in this study with the other left for comparison. During this procedure a core and peripheral vitrectomy was performed.[25] All patients in this study noticed light sensitivity in the eye but to differing degrees. This paper calls for a greater understanding of positive and negative effects of subretinal injection. It also states that the dismal results are due to the advance stage of the disease and suggests that these trials should be conducted on children.[25]
Effect of gene therapy on visual function in Leber's congenital amaurosis
This article was a study on clinical administration of gene therapy on patients with early onset severe retinal dystrophy aged between 17-23 years, undertaken in 2008.[23] The procedure in this paper was subretinal injection of AAV RPE65 vector and the results showed an improvement in visual function. This study reported that AAV produced no adverse effects on patients. It also stated that while the results were not perfect it is suggested that these procedures would benefit children with LCA than adults[23].
Gene Therapy Rescues Cone Structure and Function in the 3-Month-Old rd12 Mouse: A Model for Midcourse RPE65 Leber Congenital Amaurosis
This article was investigating whether the remaining cones in late LCA can be rescued using AAV. In the test subject (mouse with natural LCA) there was early cone degeneration by RPE65 mutation with some peripheral M cones remaining.[26] The outcome of this trial strengthened the results of previous experiments that AAV gene therapy can help to restore S and M cone function and morphology. It also explained that if treatment is delayed AAV can also help to restore M cone function in late LCA patients.[26]
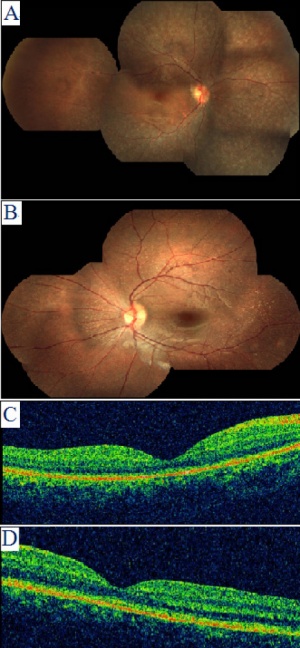
Anophthalmia and Microphthalmia
The condition of anophthalmia refers to complete absence of the globe in the presence of ocular adnexa (eyelids, conjunctiva, and lacrimal apparatus). Microphthalmia is defined as a globe with a total axial length (TAL) that is at least two standard deviations below the mean for age.[27][28][29]Anophthalmia and Micropthalmia, these two ocular manifestations have a combined incidence of approximately 2/10000 births and can be developed as unilateral or bilateral. [28] The development of both Anaophthalmia and Microphthalmia can be isolated, associated with other abnormalities or can be part of a well defined syndrome.The aetiology of these two conditions can be very complex with genetic, environmental causes both identified.[27][28][29]Some risks factors for these conditions are idenitifed such as maternal age over 40, multiple births, infants of low birth weight and low gestational age.[30][31][32]There are many causes of these two conditions including chromosomal, part of a syndrome, single gene disorder and environmental. Only single gene disorder as an aetiology of these two conditions will be discussed in detail, where the accumulative mutations of several genes eventually result in the development of anophthalmia and microphthalmia.
Clinical Manifestation
Anophthalmia is a condition that result in the absence of ocular tissue in the orbit. Simple microphthalmia is condition where patient present with structurally normal, small eyes. In both conditions, there is increased incidence of uveal effusions and choroidal detachments, and this is thought to be due to the increased thickness of the sclera and changes in blood flow that results from anophthalmia and microphthalmia. Complex micropthlamia occurs when microphthalmia is complicated with other ocular disorders. Both Anterior and posterior segment can be affected by complex microphthalmia [29]
Genetic cause (Single gene disorders)
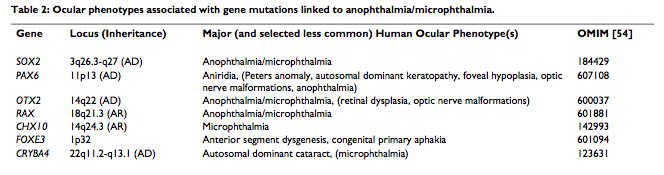
There are various causes to the development of Anophthalmia and microphthalmia, the roles of several genes involved in the ocular development have been identified. Also, some well-defined syndrome have been proved to be related with these two conditions, such as matthew-wood syndrome and Fraser syndrom. Genes that are responsible for these conditions include, PAX6, RAX, CHX10, SOX2, OXT2 and FOXE3. These are the main contributor to the development of anophthalmia and microphthalmia. [28][29]
Summary of genes associated with anophthalmia and microphthalmia
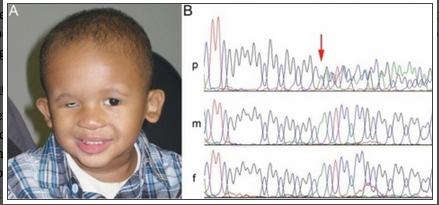
SOX-2
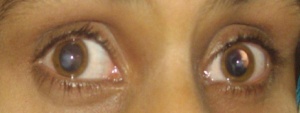
A patient with microphthalmia with deletion of SOX2 gene
The image above shows a patient with microphthalmia and from his gene sequence, it was discovered that the SOX2 gene has been deleted and it is indicated by the red arrow on the image. His parents gene sequence is shown underneath his and shows the presence of the normal SOX2 gene. [33]The SOX-2 gene has been identified as the major causative gene of the two conditions, it is located on the location 3q26.3-q27. It is an important transcription factor responsible for the maintaining self-renewal, or pluripotency, of undifferentiated embryonic stem cells.[29]Although, SOX2 gene mutation is quite rare with an estimated frequency of 1 in 250,000 births, they are still considered the most common genetic cause of anophthalmia and microphthalmia to date. [34]Mutations in the SOX-2 gene is often associated with ocular malformations, they are variable in types but mostly bilateral and severe, it can also lead to mild dysmorphic facial features, genital abnormalities in males, learning disabilities, and motor delay as part of anaophthalmia syndrome. Some addtional features of SOX2 mutation are cataracts, coloboma, optic nerve hypoplasia. [35]This gene is highly expressed during lens induction and play an important role in the growth and maintenance of the developing lens. [36]SOX2 expression in humans are also seen in area of neural retina, optic stalk and lens, further suggesting its importance to the development of the eye.[34]
SOX2 gene also co-operates with other genes to ensure normal lens development such as the PAX 6 gene and they mutually induce each other.[34]It has been demonstrated in chicks, the co-regulation of these two gene drive lens induction in chicks, suggesting that lens induction failure in humans can be due to these genes. The lens induction is also mediated by the interaction of the two genes through their action on the γ-crystallin gene. The three genes SOX2, PAX6 and FOXE3 are all highly express in the retinal area of the developing eye, their accumulated mutations may cause failure of retinal differentiation and related to the development of anophthalmia and microphthalmia.[29]
PAX6
The PAX6 gene mentioned before in the abnormal lens development section has already been stated of its importance in lens development, located on chromosome 11p13.The mutation of this gene cause alterantion to the developing lens and the pit of the optic vesicle. Through the use of differentiation marker, this gene has also been proved to be expressed by corneal epithelial cells and can regulate their differentiation.[34][37]As mentioned in the section before, PAX6 co-regulates with SOX-2 for lens induction and the induction is also mediated through their interaction on the γ-crystallin gene.[29] Overall, the PAX6 gene is a major gene involved in the normal development of the eye, any mutation of this gene will cause abnormalities to the eye one way or the other.
OTX2
OTX2 is a homeobox-containing trancription factor gene that plays a key role in the development of head structures in vertebrates. The protein products of OTX2 and SOX2 have been shown to co-regulate the expression of the RAX gene which is also essential in eye development. Mutations in OTX2 is associated with unilateral or bilateral anophthalmia and microphthamia, along with other abnormalities such as pituitary defects and significant developmental delays. [28]
RAX
The establishment and proliferation of retinal progenitor cells are mainly regulated by the RAX gene. [29] The transcription of this gene starts in the anterior neural plate and then simultaneously in the eye field and the ventral forebrain. [35] Mutation of this gene will bring failure of lens induction in eye development, along with other genes such as PAX6, CHX10, OTX2.[29] [28]
CHX10
The role of this gene is the proliferation of neuroretinal progenitor cells and the mutation of this gene will lead to microphthalmia, cataract and iris coloboma. [28]
FOXE3
THe FOXE3 gene is responsible for lens development in vertebrates and a mutation of this gene will lead to lens agenesis, it is a transcription factor that is critical for the regulation of lens fibre differentiation, also in promoting the growth and survival of the lens epithelium cells. [38] [29] [28]In humans, mutations of this gene is associated with variable phenotypes such as anterior segment abnomalities, cataracts and microphthalmia. [39]
Treatment
Conservative
In unilateral cases of both anophthalmia and microphthalmia, the good eye must be protected with any visual deficit managed well. [29]
Surgical
Congenital anophthalmia and microphthalmia result in small volume orbit when compared to age-related controls and this will potentially lead to the appearance of hemifacial asymmetry. And the removal of the globe will produce a reduction in orbital volume. Mild or moderate micropthalmia is generally managed with insertion of a conformer which is a prosthetic eye , not painted, increasing in size periodically to ensure normal orbit growth. There are many others implants of the orbits that are inflatable or expandable, often increase in size periodically to ensure the normal development of the orbital bone such as endo-orbital volume replacement, volume replacement and static orbital implants. [29]
Ideal treatment for anophthalmic patients would be simultaneous expansion of the eyelids, socket and orbital bones, and this type of treatment should begin right after birth. Socket expansion with self-inflating expanders is also a useful technique, along with conformers.[40]
Prognosis
The therapy tries to maximise vision for patients with micropthalmia, and rather to enhance the appearance rather than improving the sight.[29]
Environmental Ocular Disorders
Chorioretinal Scars Caused by Congenital Varicella Syndrome
Chorioretinal Scars are scars within the eye that have penetrated the choroid and the retina giving the back of the eye a white, black or pinky appearance rather than that of a healthy eye on examination. This scar or scars damage significant parts of the retina and can cause a cloudiness or even a substantial lack of vision. They are usually described in three sites, Juxapapillary, Peripheral or Macular with macular being the most common. After possible finding of these scars at birth, it is extremely common for the scars to worsen and enlarge with age.
Chorioretinal scars can be caused by a number of diseases during development within the uterus, most commonly seen after contracting the congenital varicella syndrome, rubella. syphilis, and toxoplasmosis. In rarer cases it can be seen after contraction of the herpes simplex. The Congenital Varicella Syndrome or Fetal Varicella Syndrome is the common chicken pox virus which is transferred from the mother to the fetus after she contracts the syndrome. [41] The risk of this transference is around 1.3%[42] if the mother has it during the 1st and half way through the 2nd trimester. in 1996 , seven in every 10000 pregnancies were affected by the Varicella Syndrome. [43] The reason for such a low number is because most women have usually already had the virus as a child, so their chances of getting it again are greatly reduced if not totally reduced. Fetal death caused by congenital varicella syndrome is very low at 0.8%. [43] Premature delivery is increased by 12.4% also. [43]
However, pregnancies that are affected by Congenital Varicella Syndrome are usually left with some morphological anomalies such as growth retardation(39%), gastro-intestinal lesions(23%), problems with skeletal development (68%), ocular abnomalities (68%) and many more. [43] For those born with ocular problems, the most obvious abnormality is the presence of chorioretinal scarring, but there are also many other abnormalities that are sometimes overlooked. Including atrophy of the optic discs, congenital cataracts, and Horner's Syndrome. [41]
Fetal alcohol syndrome
Fetal alcohol syndrome (FAS) is caused by maternal alcohol consumption chronically during pregnancy, eye abnormalities have been shown to occur in over 90% of children that were exposed to FAS prenatally.[44] FAS has been estimated with a frequency of 0.97 cases in 1000 births[45] The aetiology of FAS can be both genetic and nutritional, a list of genes have been identified as potential targets for the teratogenic effects of alcohol on humans. Some animals studies have suggested nutritional issues are important risk factors that may contribute to FAS. It is evident that plasma zinc and copper concentration are lower in pregnant women that have high alcohol intake, compared to women who only drink lightly or not at all. Zinc can influence on the risk of FAS in humans. [46]
Prenatal exposure to alcohol can cause profound effects and impacts on fetal development. These include alterations to somatic growth and specific minor malformation of facial structures.The most significant and important effect that fetal alcohol syndrome have on fetal development is on brain which may leads to substantial problems with neurobehavioral development. And defect in brain development an cause decreased IQ, hyperactivity, behavioural and adaptive difficulties. Also, there can be deficits in motor function, attention, verbal language and visuo-spatial skills.[46]
FAS can also cause alterations to facial structure such as coloboma, hypoplasia of the optic nerve resulting in an abnormal small size of the nerve, increasing abnormal shaping of retinal vessels, short palpebral fissure length, anterior segment abnormalities and microphthalmia. It is also an environmental aetiology for the condition, microphthalmia.[44][47] Alcohol may produce its effect by interacting with cell membranes and receptors of cells and by changing the morphology and function of the proteins that regulate signal transduction, gene expression, cell differentiation and proliferation, impacting on the development of the prenatal eye. Demonstrated in some animal studies, malformations result from FAS are caused by the effects of ethanol on embryos prior to or during gastrulation and neurulation during embryonic development, inducing the development of small neural plates, abnormal migration of mesodermal cells and malformations of the cranial- facial area. [48]Another study on animal suggested, ethanol can alter the normal patterns of recruitment and loss of neural progenitor (Stem cells) which is especially important in neurogenesis. A strong correlation has been shown between ethanol dose being exposed to neuroblasts at their highest activity level and the damage to neuroretinogenesis and optic nerve development. [45]
Glossary
- Adeno-Assocaited virus (AAV) - A small virus which affects humans and primates. It causes mild immune response and is an important part of gene therapy research.
- Adnexa - Accessory anatomical parts
- Albinism - Congenital disorder characterized by an absence of pigmentation in skin, eye and hair. This is due to a non expression of Melanin
- Anterior segment dysgenesis - Failure of the normal development of the tissues in the anterior compartment of the eye including the cornea, iris, ciliary body, sclera, conjunctiva and lens.
- Autosomal recessive inheritance - gene inheritance by which the gene is carried on one of the 22 non sex determining chromosomes and both parents must be carriers of the gene for a child to inherit. There is a 1 in 4 chance of inheritance and a 2 in 4 chance of becoming a unaffected carrier
- Bardet-Biedl Syndrome - ciliopathic genetic disorder which main clinical feature id rod-cone dystrophy onset during childhood.
- Choroidal detachment - A separation of the choroid from the sclera
- Choroid fissure - Middle, vascular coat of the eye which resides between the sclera and the retina.
- Coloboma - Failure of closure of choroid fissure that should be closed during the 7th week of development
- Electroretinography (ERG) - technique by which the electrical response of the retina is measured.
- Hemifacial asymmetry - also known as hemifacial microsomia, there are marked three dimensional asymmetry of various facial structure: the mandible, the ear, the maxilla, the zygoma and the orbit.
- Retinitis Pigmentosa (RP) - an inherited degenerative eye disorder which causes impairment and blindness and is characterised by many symptoms including Night Blindness.
- Senior-Løken Syndrome - Rare Congenital recessive inherited eye disorder. Discovered in 1961 and is a progressive disorder
- Teratrogenic - Substances or agents that can interfere with normal embryonic development
- Uveal effusion - An abnormal accumulation of serous fluid in the outer layer of the choroid and ciliary body
References
- ↑ 1.0 1.1 1.2 <pubmed>PMC1233106</pubmed>
- ↑ 2.0 2.1 2.2 2.3 <pubmed>1100417</pubmed>
- ↑ 3.0 3.1 3.2 3.3 <pubmed>6650859</pubmed>
- ↑ 4.0 4.1 4.2 <pubmed>10627820</pubmed>
- ↑ 5.0 5.1 5.2 5.3 <pubmed>7789273</pubmed>
- ↑ Mihelec, M, St Heaps, L, Flaherty, M Chromosomal rearrangements and novel genes in disorders of eye development, cataract and glaucoma.Twin Research and Human Genetics 2008 vol:11 pp.412-21 [1]
- ↑ Glaser, T Et al Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene Nature Genetics 1992 vol:2:3 pp.232 [2]
- ↑ 8.0 8.1 8.2 8.3 <pubmed>18035564</pubmed>
- ↑ 9.0 9.1 <pubmed>15855758</pubmed>
- ↑ 10.0 10.1 <pubmed>17460281 </pubmed>
- ↑ 11.0 11.1 <pubmed>16825429 </pubmed>
- ↑ <pubmed>18474783</pubmed>
- ↑ <pubmed>21855542</pubmed>
- ↑ 14.0 14.1 <pubmed>16767101 </pubmed>
- ↑ 15.00 15.01 15.02 15.03 15.04 15.05 15.06 15.07 15.08 15.09 15.10 <pubmed>15231395</pubmed>
- ↑ 16.0 16.1 16.2 <pubmed>14625556</pubmed>
- ↑ <pubmed>PMC2940541</pubmed>
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 <pubmed>9775209</pubmed>
- ↑ 19.0 19.1 Evans, J. (2012). Leber Congenital Amaurosis. Retrieved 2012, from Foundation Fighting Blindness[3]
- ↑ Leber, T. (1869). Ueber Retinitis pigmentosa und angeborene Amaurosa. Archiv fur Ophthalmologie , 1-25.[4]
- ↑ 21.0 21.1 21.2 21.3 21.4 <pubmed>PMC3283211</pubmed>
- ↑ 22.00 22.01 22.02 22.03 22.04 22.05 22.06 22.07 22.08 22.09 22.10 http://www.nei.nih.gov/lca/timeline.asp
- ↑ 23.0 23.1 23.2 <pubmed>18441371</pubmed>
- ↑ 24.0 24.1 24.2 24.3 <pubmed>11326284</pubmed>
- ↑ 25.0 25.1 25.2 <pubmed>PMC2940541</pubmed>
- ↑ 26.0 26.1 <pubmed>PMC3053305</pubmed>
- ↑ 27.0 27.1 Bardakjian T, Weiss A, Schneider AS. Anophthalmia / Microphthalmia Overview. 2004 Jan 29 [Updated 2007 Feb 15]. In: Pagon RA, Bird TD, Dolan CR, et al., editors. GeneReviews™ [Internet]. Seattle (WA): University of Washington, Seattle; 1993 http://www.ncbi.nlm.nih.gov/books/NBK1378/
- ↑ 28.0 28.1 28.2 28.3 28.4 28.5 28.6 28.7 <pubmed>21825993</pubmed> Cite error: Invalid
<ref>tag; name 'PMID21825993' defined multiple times with different content - ↑ 29.00 29.01 29.02 29.03 29.04 29.05 29.06 29.07 29.08 29.09 29.10 29.11 29.12 <pubmed>18039390</pubmed>
- ↑ <pubmed>16007635</pubmed>
- ↑ <pubmed>8921488</pubmed>
- ↑ <pubmed>16498668</pubmed>
- ↑ <pubmed>20454695</pubmed>
- ↑ 34.0 34.1 34.2 34.3 <pubmed>22005280</pubmed>
- ↑ 35.0 35.1 <pubmed>19921648</pubmed>
- ↑ <pubmed>15812812</pubmed>
- ↑ <pubmed>19347868</pubmed>
- ↑ <pubmed>20216939</pubmed>
- ↑ <pubmed>20140963 </pubmed>
- ↑ <pubmed>21730840</pubmed>
- ↑ 41.0 41.1 <pubmed>2910286</pubmed>
- ↑ Boussault, P et al Chronic Varicella-Zoster Skin Infection Complicating the Congenital Varicella Syndrome Pediatric Dermatology 2007 vol:24:4 pp. 429 [5]
- ↑ 43.0 43.1 43.2 43.3 <pubmed>8735731</pubmed>
- ↑ 44.0 44.1 <pubmed>19907681</pubmed>
- ↑ 45.0 45.1 <pubmed>11825849</pubmed>
- ↑ 46.0 46.1 <pubmed>21425437</pubmed>
- ↑ <pubmed>18571671</pubmed>
- ↑ <pubmed>8857698</pubmed>
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Albanism and Hypopigmentation Organisation
Genetic Alliance - advocacy, education & empowerment
--Mark Hill 12:22, 15 August 2012 (EST) Please leave the content listed below the line at the bottom of your project page.
2012 Projects: Vision | Somatosensory | Taste | Olfaction | Abnormal Vision | Hearing