2012 Group Project 1
Vision Development
Introduction
Eyes are an important sensory organ shared across many different species and allow organisms to gather useful visual information from their environment. The visual system uses light from the environment and processes this information in the brain for visual perception. The visual system is complex, and is made up of various structures that work together to form vision. Each of the structures in the eye have specific tasks which contribute to the visual system. Knowledge of how the eye develops extends as far back as Aristotle more than 2000 years ago, and current knowledge shows that most of the crucial events of eye development occur in the embryological stage. The eye is an interesting model for studying the development of tissues in organisms, as it consists of cells from several parts of the embryo including the head ectoderm, neural ectoderm and mesoderm. From its many origins the cells come together and differentiate to produce the complex organ that is the eye. During this period there are many examples of inductive signaling, as the tissues coordinate their development throughout this elegant process.
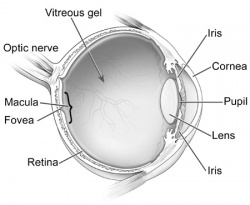
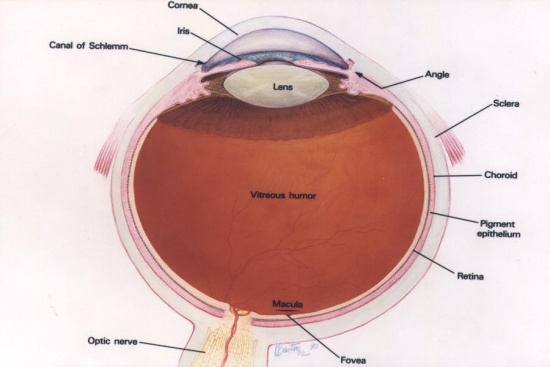
Basic Anatomy of the eye
The main anatomical structures of the eye are as follows:
|
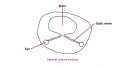
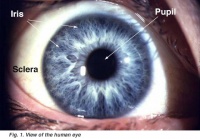 Illustration of the front of the eye, showing the sclera, iris and pupil. Credits: Webvision [1] |
The cornea is a transparent section in the anterior of the eye which acts as a window over the pupils, and is involved with refracting light as it enters the eye. It consists of 5 layers: anterior epithelium, bowman's layer, stroma, descemet's layer, and endothelium. [2]
The pupil is an opening in the anterior part of the eye, which controls how much light enters the eye. [2]
The iris is A circular shaped muscle which controls the opening and contraction of the pupil. [2]
The sclera is the white external anterior surface of the eye, which envelopes the eyeball to give it support and protection of its internal contents. [2]
The lens is a structure inside the eye which refracts light as it enters the eye for clear vision. [2]
Optic Nerve is the nerve which carries visual information from the retina to the brain for processing. [2]
The choroid is the middle coat of the eye, located between the sclera and retina, which contains blood vessels that nourish the structures in the eye. [2]
The ciliary body is a structure located behind the iris which secretes aqueous humour. It contains ciliary muscle, which is involved with changing the shape of the lens for accommodation. [2]
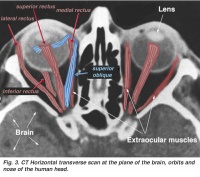
Extraocular muscles are the six muscles that control the movement of the eyeball. They are lateral rectus, medial rectus, superior rectus, inferior rectus, superior oblique, inferior oblique. [2]
Anterior chamber is the fluid-filled area located between the iris and cornea. [2]
Posterior chamber is the fluid-filled area located between the iris and lens. [2]
Vitreous Chamber is the area located between the lens and retina, which contains vitreous (a gel like substance) whose function is to maintain the shape of the eye. [2]
The retina is a light-sensitive layer located towards the back of the internal surface of the eye, which contains photoreceptors (rods and cones) which detects visual information and transmits it to the brain through the optic nerve. The retina is made up of approximately 10 layers as follows: retinal pigment epithelium, photoreceptor cell layer, external limiting membrane, outer nuclear layer, outer plexiform layer, inner nuclear layer, inner plexiform layer, ganglion cell layer, nerve fiber layer, and internal limiting membrane. [2]
Macula is a pigmented oval region in the central area of the retina, important for maintaining visual acuity. Fovea is the central point in the macula, which is concentrated with cones for sharp colour vision. [2]
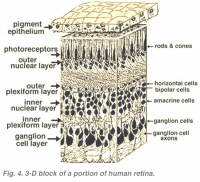 A diagram of the layers of the retina. Credits: Webvision [1] |
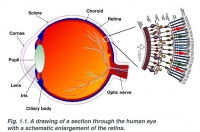 The layers of the retina magnified, showing the direction of the layers of the retina in the back of the eye. Credits: Webvision [1] |
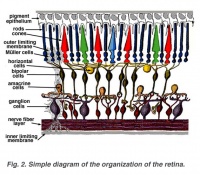 A diagram of the components of the retina. Credits: Webvision [1] |
Research History
Brief Timeline of Historical Developments on the Eye and its Embryology
| Time | Discovery |
| Ancient Egyptians | First to document cataracts. It is described as being 'the white disease of the eye' or 'darkening of the pupil.' [3] The Egyptians had some knowledge of the eye, however it is not known how much of the anatomy of the eye was known in their era. |
| 535 BC
|
Ancient Greek philosopher Alcmaeon conducted dissection of humans for the first time in recorded history. This included dissection of the eye. However, not much is known about which anatomical features he discovered. [4]
|
| 384- 322 BC
|
Aristotle performed dissections of animal embryos.[4] When Aristotle described the embryo of a ten day old chicken, he wrote "The eyes about this time, if taken out, are larger than beans and black; if their skin is removed the fluid inside is white and cold, shining brightly in the light, but nothing solid." [6] Aristotle believed that the eyes started forming during early embryogenesis, however, he also believed that the eyes are the last organs to form completely, and he incorrectly thought that the eyes shrink in later embryonic development. [7] .[4] |
| 340 BC
|
Lens is thought to have been discovered by Hippocrates, due to his descriptions of the contents of the internal eye There has been studies in chick development later on by followers of Hippocrates. They claimed that eyes were visible in early embryogenesis. .[4] |
| 25 BC - 50 AD
|
Aulus Cornelius Celsus wrote a Roman medical text called 'De Medicina' in which he wrote that the lens was the part of the eye from which vision originated. [8] Celsus also incorrectly drew the lens in the center of the globe in his diagram of the eye. [8] |
| 23-79 AD |
Pliny the Elder said that the eye is the last of the organs to develop in the womb [6] |
| 98-117 AD
|
|
| 130-200 AD
|
 The eye according to Galen. [5]
Claudius Galen practised medicine in Rome. He wrote: "1. Within the eye the principal orgran of sensation is the crystalline lens. 2. The sensation potential comes from the brain and is conducted via the optic nerves. 3. All other parts of the eyeball are supporting structures." [9] Galen thought that the lens was produced from the vitreous. He also believed that the retina’s function was to give nourishment to the lens and vitreous, and to carry visual information to the brain from the lens. [8] |
| 1514-1564
|
Andreas Vesalius published his anatomy book "De Humani Corporis Fabrica in 1543. He had the misconception that the lens was located in the centre of the eyeball. .[4] He also wrote that the lens functioned "like a convex lens made of glass" [10] pp. 48 |
| 1535-1606
|
Georg Bartisch correctly drew a diagram of the lens placed behind the iris in his book 'Ophthalmodouleia: das ist Augendienst'. [10] |
| 1537-1619
|
Fallopio Hieronymus Fabricius ab Aquapendente studied anatomy and embryology. He studied chicken embryos, and thought that chalazae (which comes from egg white) gives rise to the eyes. He also drew the lens directly behind the iris in a diagram in is book 'Tractatus de Oculo Visuque Organo. [10] |
| 1583
|
Felix Platter published his book 'De corporis Humani Structura et Usu, after he performed dissections of human bodies. He believed that the retina is the primary visual organ in the eye. .[4] |
| 1619 | Scheiner is given credit to be the first person to correctly draw the diagram of the anatomy of the eye. [4] |
| 1672 | Marcello Malpighi described the embryonic development of the chicken. He drew many detailed diagrams of the chick eye. [7] |
| 1665 | Nicolaus Steno identified the choroid fissure in his study of a developing embryo of a chicken. [7] |
| 1754
|
Albrecht von Haller studied the embryology of the eye. With help from his student Johann Gottfried Zinn, he contributed to the understanding of the development of the ciliary body, ciliary zonule, and their relationship with the lens and vitreous. [7] |
| 1817
|
Christian Pander discovered the three embryonic germ layers, which he wrote about in his book. [7] Pander was the first to think of 'the optic vesicles as lateral evaginations' of the 'prosencephalon'; however, he was incorrect about the details regarding how 'the eye develops from these evaginations'. [7] |
| 1828-1837
|
Karl Ernst von Baer studied embryology. He discovered that the optic vesicles were 'outgrowths of the embryonic forebrain' [4] which he believed was caused by pressure from fluids in the central nervous system. Von Baer also believed that the optic vesicle opens to form the pupil, and that fluid in the optic vesicle coagulates to form the vitreous body and lens. [7] |
| 1830
|
Emil Huschke discovered that the lens forms from the invagination of the surface ectoderm. He concluded that the lens hence does not form ‘from the fluid of the optic vesicle’ [4] as previously thought. |
| 1832
|
Emil Huschke wrote in his manuscript ‘Ueber die erste Entwinkenlung des Auges und die damit zusammenhängende Cyklopie’ that the lens capsule forms from the outer surface ectoderm, which detaches and moves back inward, which is later enclosed again by several membranes, such as by the cornea. [7]
Huschke also described how the optic cup and choroid fissure forms. He discovered that the optic vesicles are produced from the two-layered optic cup. However, he incorrectly described the destiny of the ‘individual optic cup layers’. [4] [7] |
| 1838 | Matthias Jakob Schleiden and Theodor Schwann formulated the ‘cell theory’: “All living things are formed from cells, the cell is the smallest unit of life, and cells arise from pre-existing cells.” [4] |
| 1839
|
Theodor Schwann contributed a better understanding of the development of the lens through studying the foetus of a pig, which he wrote about in his book ‘Mikroskopische Untersuchungen Über Die Uebereinstimmung in Der Struktur Und Dem Wachsthum Der Thiere Und Pflanzen’. He wrote that the lens is made of ‘concentric layers’ of fibres which proceeds from an anterior to posterior direction. [7] |
| 1842 | Robert Remak gave the current names to the three embryonic germ layers: ectoderm, mesoderm and endoderm. [4] |
| 1843 | Wilhelm Werneck published his book ‘Beiträge zur Gewebelehre des Kristallkörpers’. He wrote that the contents inside of the lens is not made of fluids, as was previously believed. [7] Werneck also discovered that the fibers of the lens continues to grow from the outside to the centre during embryogenesis. [7] |
| 1855
|
Robert Remak wrote his book ‘Untersuchungen über die Entwickelung der Wirbelthiere’. He wrote about what he discovered in his studies of the development of the eye in the embryos of chickens, frogs, and rabbits. He wrote very descriptively about the embryology of lens formation, amongst other topics. He discovered that the ectoderm gives rise to the lens placode. [7] |
| 1858 | Henry Gray published his book 'Anatomy, Descriptive and Surgical'. He had also previously studied the embryonic development of the optic nerve and retina of chickens. |
| 1877 | Paul Leonhard Kessler wrote about the embryonic development of the lens in mice in his book ‘Zur Entwickelung des Auges der Wirbelthiere. [4] |
| 1891 | Vincenzo Colucci studied newts and discovered their ability to regenerate the lens.[11] |
| 1892 | Dr. Oscar Hertwig published his book ‘Text-Book of the Embryology of Man and Mammals. [12] It contains a very detailed description of the development of the eye, according to the findings at that time. [1] |
| 1895 | Gustav Wolff also independently studied newts and discovered their ability to regenerate the lens. .[13] |
| 1900 | Carl Rabl published his book ‘Uber den Bau und die Entwicklung der Linse’. He wrote about the development of the lens in mammals, fish, birds, reptiles, and amphibians. [4] |
| 1901 | Hans Spemann published his findings from his experimental studies about the formation of the lens in the frog. He found that the optic cup needed to be in contact with the ectoderm for normal development of the eye. [14] [15] |
| 1906 | Brown ‘s book “The Embryology Anatomy and Histology of the Eye” was published. It contained detailed descriptions of the embryonic development of the eye according to the knowledge current at that time, mainly based on observations from embryos of rabbits and chickens. [16] |
| 1907
|
John Clement Heisler published his book ‘A Text-book of embryology’. It contains a chapter detailing the embryonic development of the eye, according to the knowledge current at that time. The book’s copyright has expired, so it can be viewed free online: [2]
Julius Kollmann also published his book 'Atlas of the Development of Man'. It contained very detailed description and illustrations showing the embryonic development of the human according to the knowledge current at that time. His illustrations were reused by many others after his time and built upon for further refined understanding of the embryology of the human. Here are examples of Julius Kollman's excellent illustrations showing eye development in various stages: Formation of Primary Optic Vesicle: Development of Lens:
|
| 1921 | Bailey and Miller published their textbook “Text-Book of Embryology “. [17] It contains detailed description of the development of the embryonic eye according to the knowledge current at that time. [3] |
| 1925
|
Mann published his research article, in which he gives a detailed account of the development of the human iris. He divided the development of the iris into four stages: weeks 4-7 (before the ectodermal iris forms or before the anterior chamber forms); weeks 7-11 (anterior chamber appears, and mesodermal iris forms); weeks 11-12 (ectodermal iris forms); 3-8 months (muscles of the pupil forms from ectodermal iris, and the central portion of the mesodermal iris atrophies to make the pupil clear). [18]
O Leser also published an article detailing the development of extraocular muscles in mammals he studied. [19] |
| 1939 | Holtfreter [20] studied amphibians and observed that that the development of the eye stops at the ‘optic vesicle stage’ if there is no contact ‘with the epidermis and neural crest driven mesenchyme’. [21] |
| 1955 | Barber published his book ‘Embryology of the human eye’. [22] In contains detailed descriptions of the embryological development of the human eye according to the knowledge current at that time. It contains many photographs of the eye at different stages of development. |
| 1957 | Coulombre studied a chicken embryo to find the role of intraocular pressure in the development of the chick’s eye, especially in regards to its control of the size of the eye structures. [23] |
| 1958 | Coulombre studied the development of the cornea and how it develops its transparency. [24] He also studied the development of corneal curvature. [25] |
| 1962 | Coulombre studied the development of the conjunctival papillae and scleral ossicles. [26] |
| 1963 | Coulombre studied the development of lens fibers and their orientation. [27] He also studied the development of pigmented epithelium. [28] |
| 1964
|
Coulombre further studied the development of the lens to determine the role of the lens in eye growth. [29] He also studied the role of thyroid in the development of the cornea and the development of corneal transparency. [30] Mann also published his work called ‘The development of the human eye’, which contains detailed description of the embryonic development of the eye according to current knowledge at that time. [31] |
| 1965 | Coulombre published his findings regarding the regeneration of the neural retina from pigmented epithelium in the embryo of chickens. [32] Smelser also published his findings on the embryological development and morphology of the lens. [33] |
| 1966 | Formation of the face and orbit occurs from the differentiation of neural crest cells. [34] O’Rahilly also published findings of the development of the eye in the early stages of human embryos. [35] |
| 1968 | Findings of the postnatal development of the retina of rats was published. [36] |
| 1969
|
Mann again published his work called ‘The development of the human eye’. He stated that that the lens in humans forms completely from the ectoderm. Cite error: Invalid <ref> tag; invalid names, e.g. too many Coulombre also studied the development of the lens, and took note of its size, shape and orientation throughout its developmental stages. [37]
|
| 1970 | Coulombre again further studied the regeneration of the neural retina from pigmented epithelium of embryos of chickens. [38] |
| 1971
|
Coulombre further studied the development of the lens. This time he focused on analysing the histological mechanisms in the reconstitution of the lens from implanted lens epithelium. [39] |
| 1973 | A research article was published, detailing the embryonic development of the retina of humans. [40] |
| 1976
|
Geeraets published his observations of the closure of the embryonic optic fissure in golden hamsters, using the electron microscope. [41] Kornneef also published an article based on his studies of the development of connective tissue in the human orbit. [42] |
| 1981 | A research article was published detailing how myelin forms in the optic nerve of humans. [43] |
| 1983 | O’Rahilly’s further research developments was published, reporting the timing and sequence of events in the development of the embryonic human eye. [40] |
| 1990
|
Van Driell et al. [44] studied the manner in which amacrine, bipolar, retinal ganglion cells, and Muller cells differentiate in the developing fovea of the retina of a 15-week old human foetus. [45] Tripathy also published an article providing evidence that the lacrimal glands in humans originates from the neuroectoderm. [46]
|
Development, Structure and Function of Ocular Components
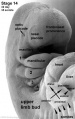
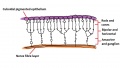
The eye itself is formed from several components; notably the optic placode of the head ectoderm, the optic vesicle from the neural tube, and mesenchyme from the mesoderm and neural crest cells. The optic placode contributes the lens to the eye, the optic vesicle gives rise to layers of the retina, while the mesenchyme will produce the ciliary body, iris, choroid and sclera.[47] Cells from the neural tube will also produce the optic nerve, which receives nerve impulses from the retina of the eye. Eyes initially form as laterally paired structures and migrate medially in the human embryo. In other animals such as birds and lizards, the eyes do not migrate and develop laterally on the head. The optic placodes become prominent on the surface of the embryo at approximately Stage 14 of development.
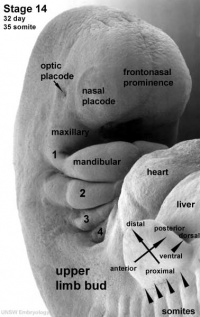
Optic Nerve
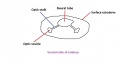
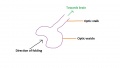
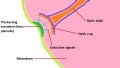
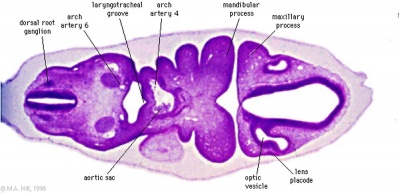
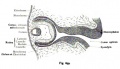
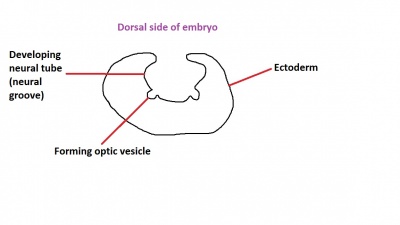
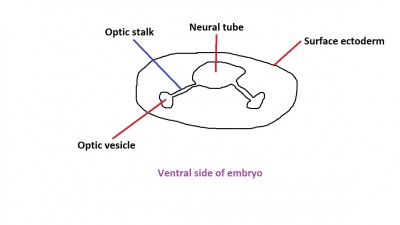
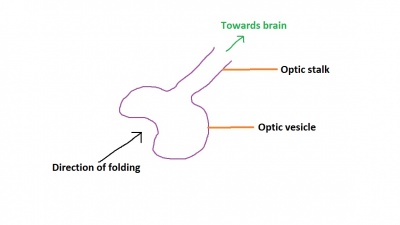
The optic nerve consists of nerve fibres that transmit information from the retinal photoreceptor cells to the brain. The optic nerve is formed from the optic stalk, which develops as the optic vesicle migrates from its origin in the neural tube to its destination - the surface ectoderm - where it will fuse with the optic placode (also known as the lens placode, which will contribute the lens to the eye).[49]
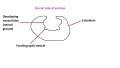
As can be seen in Figure 1 above, the optic vesicle forms from the neural tube. However, note that the neural tube has not yet closed, and is still the neural groove at this point. Figure 2 then shows the optic vesicle at slightly later stage in the same simplified cross-section of the embryo, as it migrates from the neural tube to the surface ectoderm. Note the presence of the optic stalk which links the optic vesicle to the neural tube. Later in development, this primitive structure will become the optic nerve, which will link the eye to the brain.
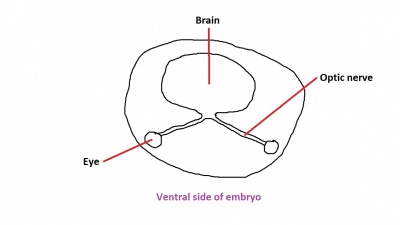
The nerve fibres themselves will initially originate from the retinal ganglion cells in the eye during week 6.[50] After two weeks, these fibers will have grown along the inner wall of the optic stalk and have reached the brain. They grow both in length and width, with the nerve fibres filling the hollow optic stalk to form the solid optic nerve. More than one million nerve fibers will eventually make up the optic nerve, along with glial cells which arise from the inner wall of the optic stalk itself.[51] Myelinisation of the optic nerve begins much later in development at around 7 months, beginning at the optic chiasm and moving towards the eye.[52] The optic chiasm forms just before the nerves reach the brain, and is where half the nerve fibres from each eye will cross over to the opposite side of the brain. This is demonstrated in Figure 3. Note the crossing over of the optic nerves just before they enter the brain, at the optic chiasm. This organisation is now much more familiar, with the eyes near the ectoderm and the optic nerve leading through the mesoderm to the brain buried deep in the embryo.
Retina
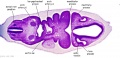
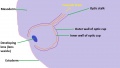
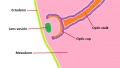
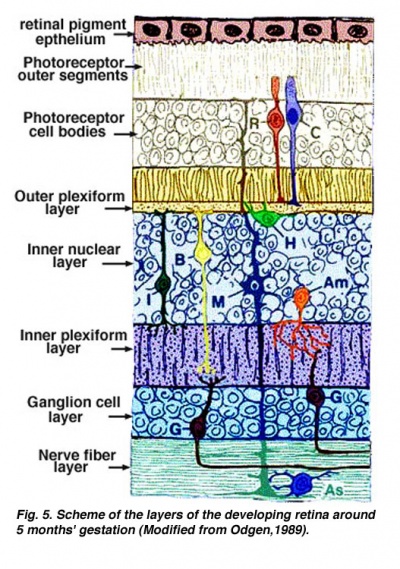
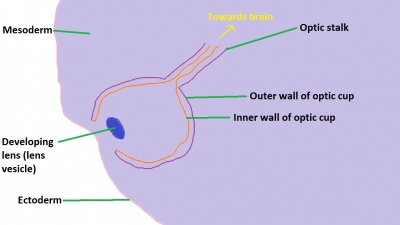
The retinal component of the eye is formed when the optic vesicle folds in upon itself, forming the optic cup (see Figure 4). In doing so it creates two layers - an inner wall and an outer wall of the optic cup (Figure 5). These two layers of the optic cup will give rise to the two layers of the retina - the inner neural retina, and the outer pigmented epithelium.[49] Note the existence of the space between the two layers of the retina. This is known as the intraretinal space and disappears by the 7th week of development, however the two layers never completely fuse and can become separated as a result of physical trauma to the head - leading to a detached retina and loss of vision.[50]
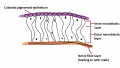
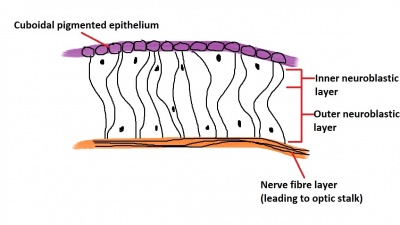
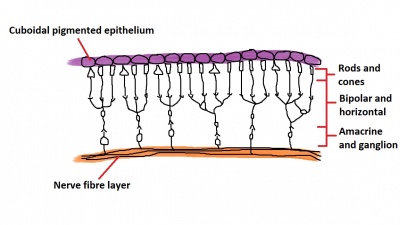
The inner wall of the optic cup, which will give rise to the neural retina, consists of a layer of pseudostratified cells (see Figure 6) that later differentiate into rod, cone, bipolar, ganglion, horizontal, amacrine and glial cells of the retina (Figure 7).[53] The outer wall of the optic cup consists of a layer of cuboidal cells that contain melanin - the light absorbing pigment. The function of this layer is to absorb light and prevent internal reflection of light within the eye, which would impair our ability to form distinct images. Interestingly, in some animals such as cats, this layer actually reflects light intentionally to increase the amount of light available to the eye in low-light conditions. This is why cats seem to have eyes that glow in the dark.[54]
The inner wall itself is divided into two components - the inner neuroblastic layer and the outer neuroblastic layer (see Figure 6). The outer neuroblastic layer forms the rod and cone cells while the inner neuroblastic layer forms the remaining cell types found in the retina - the bipolar, ganglion, horizontal, amacrine and glial cells (Figure 7).[50] The organisation of the retina is interesting in that incoming light passes through several layers of these neural retina cells before it is detected by rod and cone cells at the back of the retina, and then nerve signals are passed back through the layers of neural retina cells that the light just passed through moments before - a seemingly strange design that the eye does not share with man-made light-capturing devices such as a camera (imagine putting the wires in front of the image sensor!).
Differentiation of the neuroblastic layers into neural retina cells occurs in a pattern both within the layers and across the retina. Cells differentiate from the inner neuroblastic layer to the outer neuroblastic layer, and differentiate from the central retina to the peripheral retina.[53] The macula is first identifiable in week 22 when ganglion cells start to form multiple rows, and the primitive fovea begins to form at approximately the same time as a depression in the macula.[55] It is not until 15-45 months after birth that this area becomes exclusively populated by cone cells and becomes the fovea centralis - the area of the retina with the highest visual acuity.
Ciliary Body
The ciliary body consists of ciliary processes and three portions of fibres that constitute the ciliary muscles. It functions to maintain normal eye physiology as well as playing a direct role in accommodation.
During development, the ciliary processes form slightly posterior to the iris, developing from part of the anterior rim of the optic cup. It is thought that the folded structure of the ciliary processes is brought about by intraocular pressure and specific signalling pathways.[56] While the ciliary muscles and the endothelial cells of the ciliary blood vessels are chiefly formed by mesenchymal cells[57], the neural crest and neuroectoderm also contribute to their development.[58] The normal development of the ciliary body is dependent on the correct expression of bone morphogenetic protein (BMP)-4, which is a member of the transforming growth factor-β superfamily.[59] Napier and Kidson (2007) summarised numerous genes that have been associated with ciliary body development, however their direct roles have not been well documented.[56]
Iris
The iris is a thin layer that develops at the end of the third month of development and is derived from the anterior rim of the optic cup. The stroma of the iris develops from cells of neural crest cell origin.[50] The muscles that are responsible for the dilation and constriction of the pupil (dilator pupillae and sphincter pupillae muscles) form from the neuroectoderm of the optic cup. These cells are initially epithelial cells that then transform into smooth muscle cells. [60]. The invagination of the optic vesicle which creates the optic cup, also causes the formation of the optic cup lip. This is the region of the where the epithelium doubles back, separating the outer pigmented layer and the inner nonpigmented layer. This is the edge of the iris that borders on the pupil[61].
The final colour of the iris is not evident until the postnatal period. It is determined by a number of genes including IRF4, SLC24A4 and MATP[62]. Other features such as crypt frequency, furrow contractions, presence of peripupillary pigmented ring, and number of nevi also become evident during development[63]. Mutations in Pax6 have been shown to cause partial or complete loss of the iris [64].
Cornea
The cornea is the transparent, avascular, most anterior portion of the eye. It is responsible for conducting light into the eye and focusing it on to the retina, as well as maintaining the rigidity of the eyeball. It consists of 5 layers- the epithelium, Bowman’s layer, stroma, Descemet’s membrane and the endothelium.
The epithelium and endothelium of the cornea first appear during the 5th week of gestation. The epithelium of the external surface of the cornea is derived from surface ectoderm, while the mesenchyme is derived from the mesoderm[60]. The endothelium is a two-cell cuboidal layer which is made up of differentiated neural crest cells that were initially from the optic cup. By week 8 the endothelial cells begin to secrete a basement membrance which later forms Descemet’s membrane[65]. At approximately 16 weeks gestation the Bowman’s membrane begins to form from the thickening of the stroma that is located under the corneal epithelium[66]. During the third month glycosaminoglycans secreted by fibroblasts form the ground substance of the cornea, with collagen fibrils and keratan sulphate also appearing around this time. Shortly after this tight junctions form between the endothelial cells[67]. Fibroblast growth factor causes the epithelial cells to proliferate[68].
Towards the end of the gestational period the cornea becomes larger due to the production of aqueous humor[69]. The final transparent structure develops because hyaluronidase removes hyaluronic acid, thyroxine causes dehydration of the stroma, and the entire structure becomes avascular[50]. Numerous genes have been implicated in the development of the cornea, these include, but are not limited to, PAX6, PITX2, FOXC1, MAF, TMEM114, SOX2, OTX2 and BMP4[70]. Pax6 and Pax6(5a) isoforms are essential for the normal development of the eye. Over or under expression can both lead to major structural abnormalities[71].
Lens
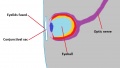
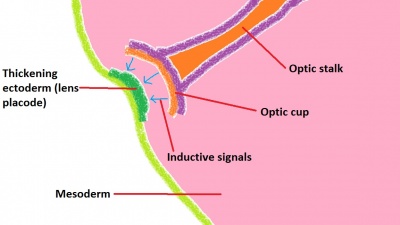
The lens has its origin from the optic placode, which develops on the ectodermic surface of the embryo and migrates both medially and inwards into the embryo. The lens allows accommodation of the eye, and adjusts its thickness in order to focus on near or far objects. The study of lens development was one of the first to highlight the importance of inductive signaling in development, with Spemann's pioneering work at the start of the 20th century, finding that the absence of retinal development resulted in the absence of lens formation.[49] Indeed, it has been consistently shown that the interaction of the migrating optic vesicle with the surface ectoderm of the head is vital in producing differentiation of the lens.[72] The mechanism of interaction is complex but basically involves upstream genes switching on downstream genes, with the genes eventually producing specialised proteins which constitute the lens. The whole process starts with the signaling molecules from the optic cup initiating a thickening of the surface ectoderm of the head (Figure 8). It is thought that this region of specific ectoderm is responsive to the signaling molecules, as lens formation is incomplete or absent when ectoderm from the lateral portion of the embryo (i.e. non-head ectoderm) is exposed to the same inductive signaling processes.[73] Pax6 has been shown to be one of the major genes required for differentiation of the lens, which in turn switches on transcriptional genes such as Sox 1, 2 and 3 among others - producing water-soluble proteins called crystallins - responsible for giving the lens its transparency and refractive properties.[74]
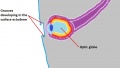
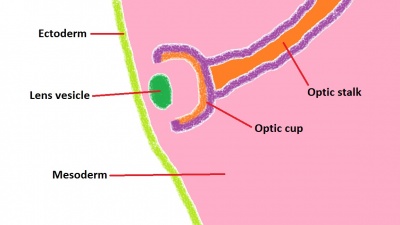
The lens placode invaginates from the head ectoderm and migrates into the mesoderm (Figure 9). Once this structure (now known as the lens vesicle) is in place opposite the optic cup, the combined structure is referred to as the optic globe and resembles a recognisable eye structure. The lens continues to differentiate further, as mentioned above, through the formation of crystallin proteins, which give the lens its unique properties and allows for the fine control over the degree of refraction that takes place.
Aqueous Chambers
There are both anterior and posterior aqueous chambers of the eye which contain aqueous humour. A space develops in the mesenchyme situated between the lens and cornea to form the anterior aqueous chamber. The mesenchyme located superficially to this chamber forms the mesothelium as well as the transparent portion of the cornea.
The posterior chamber develops from a similar space in the mesenchyme, however it is located between the iris and the lens. The anterior and posterior chambers are able to communicate with one another once the papillary membrane vanishes and the pupil is formed. This channel is known as the scleral venous sinus.[60]
Contained within the aqueous chambers is aqueous humor. The production of aqueous humor is dependant on the development of the ciliary body. It is produced in the ciliary processes and it’s production is a metabolic process driven by the delivery of oxygen and the removal of wastes via the ciliary circulation[75].
Vitreous
The primary vitreous originates from the ectoderm and mesenchyme. [2] Vitreous starts to build up within the primary vitreous space during the time the lens develops. [76] The developing lens produces ‘fibrils’ which contribute to the components of the primary vitreous. [77] Hyalocytes from the primary vitreous produces the secondary vitreous. [45] [2] The neural retina also produces the secondary vitreous. [45] [2] The secondary vitreous thickens at three months. [45]
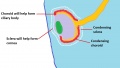
Choroid and Sclera
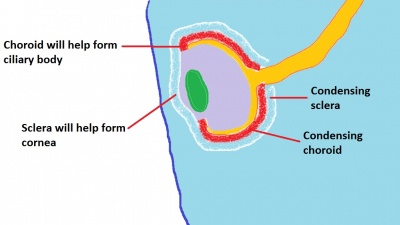
The choroid and sclera are adjacent layers that surround the eye and act to vascularise and protect the eye respectively. They are formed from neural crest and mesoderm-derived mesenchyme which condenses around the optic cup and lens vesicle between weeks 5 and 7 of development to form a primitive eyeball structure known as the optic globe.[50] Blood vessels first start to appear in the choroid layer at approximately week 15, and arteries and veins can be distinguished by week 23.[78] Inductive processes are thought to play a vital role during formation of the choroid and sclera; with the retinal pigmented epithelium inducing differentiation of the surrounding mesenchyme while at the same time the neural crest-derived mesenchyme contributing components to the retinal pigmented epithelium such as melanocytes.[45] In addition to having functional roles themselves, the primitive choroid and sclera also contribute components to the developing ciliary body and cornea (Figure 10). In the adult eye, the choroid is continuous with the ciliary body and the sclera with the cornea.
Eyelids
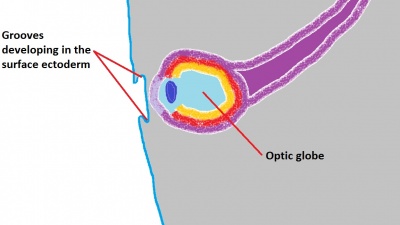
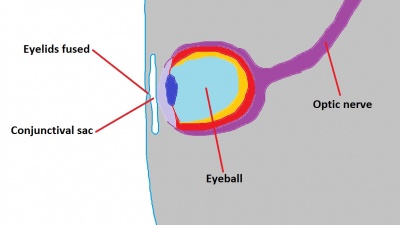
The eyelids are ectodermal and mesodermal in origin and are an extension of the skin which covers and protects the eye. The surface ectoderm gives rise to the conjunctiva, skin epithelium, hair follicles, cilia, Zeis glands, glands of Moll, and meibomian glands. [79] The mesenchyme gives rise to the tarsal plates, levator muscles, orbicularis muscles, and tarsal muscle of Muller. [79] Eyelid formation can be first noted during week 5 when small grooves develop in the surface ectoderm (Figure 11).[80] These small grooves deepen and extend into the mesoderm and the primitive eyelid structures grow towards one another, eventually fusing together during week 8.[60] It is not until week 26-28 that the eyelids will separate again. The anterior surface of the eyelid becomes covered by two layers of epithelium; this forms the epidermis of the eyelids. [81] Tarsal plates then begin to develop, which eventually leads to the formation of meibomian glands. [45] The ectoderm reflects over the developing cornea to form the conjunctival sac, a space that is filled by secretions from the lacrimal gland in order to allow smooth motions of the eyelid over the eye and also to clean the cornea and prevent accumulation of particles on the eye that may disrupt vision. By the time the eyelids separate, the eye has all its major components present (Figure 12), and further development consists mainly of growth and vascularisation.
Lacrimal Glands
There are three stages of lacrimal gland development. The first is the presumptive glandular stage in which the superior conjunctival fornix epithelium thickens and the surrounding mesenchymal cells condense. These mesenchymal cells are of neural crest origin[82]. The second stage sees the development of nodular formations around the superior conjunctival fornix and the formation of lumina within the epithelial buds, this stage is therefore known as the bud stage. Innervation and vascularisation also occur during this stage. The final morphological changes occur during the glandular maturity stage which occurs in weeks 9-16 when the lacrimal glands begin to resemble the mature glands. During the 13th week the lacrimal and zygomatic nerves anastomose[83].
These glands are responsible for the production of tears however they do not start to function until 1-3 months after birth. The mature lacrimal gland is made up of two lobes- the palpebral and orbital lobes.
Extraocular Muscles
The extraocular muscles originates from the mesenchyme. [2] The neural crest gives rise to the connective tissue of the extraocular muscles, while the mesoderm gives rise to the muscle cells. [45] [84] The first pair of somites gives rise to the medial rectus, superior rectus, inferior rectus, and inferior oblique muscles at day 26. [2] At day 27, the mesenchyme gives rise to the lateral rectus muscle. [2] On day 29, the second pair of somites gives rise to the superior oblique muscle. [45] It takes 18 months for the tendinous sheath which attaches the extraocular muscles to the sclera to completely take formation. [45]
Current Research
Not only are there still many important processes and components of eye development that we would like to understand, this knowledge also contributes to the development of treatments for eye disorders and technologies such as the bionic eye.
Below are summaries of some current research articles.
The impact of visible light on the immature retina
Article Source: <pubmed>22405869</pubmed>
The authors mentioned in this article [85] that they were interested in investigating the effect of light on postnatal eye development in mice, because mice are born with fused eyelids, which separate 12 days after birth. Before the eyelids separate, the retina develops in mice with very little radiation from light. It is believed that the darkness plays a role in the development of the retina in mice, which is why their eyelids are fused for 12 days after birth. Therefore the authors were interested to see what effect light would have on postnatal retinal development of mice, with special interest in retinal ganglion cells (RGC). In their experiment, they surgically opened the eyelids on the right eyes of some of the mice to expose them to visible light 12 hours per day, while they left some other mice in the dark after surgical separation of their eyelids. They also kept the left eyes of the mice naturally fused as controls in the experiment. Their results showed that early light exposure in mice causes a decrease in retinal ganglion cells because it affects cellular apoptosis in the retina. The authors also observed that early exposure to light in mice causes lumican mRna transcription to resume and to quickly increase. (Lumican normally stays silent in retina after birth). [85]
GABA Maintains the Proliferation of Progenitors and Non-Pigmented Ciliary Epithelium
Article Source: <pubmed>22590629</pubmed>
| GABA is an ‘inhibitory neurotransmitter’ in the central nervous system of adults. [86] It is responsible for controlling proliferation of stem cells and progenitor cells. The authors of this article [86] was interested to find the effects of GABA on proliferation of progenitor cells and non-pigmented ciliary epithelial cells (NPE) in the retina. Their study focused on progenitor cells and non-pigmented epithelium of the ciliary body in chickens. Non-pigmented epithelial cells in chickens arise from the neuroepithelium of the optic cup. They share similar functions as progenitors of the early retina, such as expression of Chx10 and Pax6 genes. It is not agreed upon whether epithelial cells of the ciliary body have stem cell properties. However, it has been found that these cells can be cultured and transplanted into retinas that are injured, in order to replace neurons that were previously lost. [86] However, there is not much known about what factors regulate the proliferation of stem cells. Hence the authors were interested in finding the effects of GABA on proliferation of retinal cells. Their results showed that non-pigmented epithelial cells in chickens ‘express extrasynaptic-like GABAA receptors’ that have the ability to regulate cell proliferation. It has been found that inhibiting these ‘GABAA receptors’ also causes a decrease in proliferation of retinal progenitor cells and non-pigmented epithelial cells in 'the intact E8 retina’. [86]
|
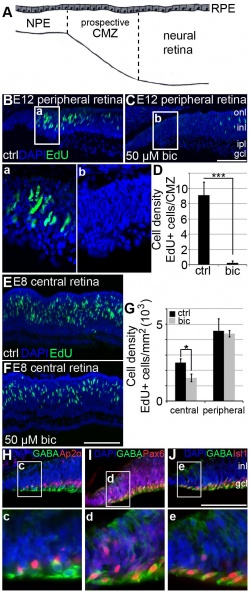 "GABAA receptor mediated effects on retinal progenitor cell proliferation" [86] |
Stem Cells
Advanced Cell Technology is a biotechnology company which is currently running two clinical trials that utilise human embryonic stem cell derived retinal pigmented epithelial cells. These trials are examining the possibility of using these cells to treat stargardt's macular dystrophy and dry age-related macular degeneration.
Despite the discovery of human embryonic stem cells (hESCs) 13 years ago, these trials are the first to describe the subretinal transplantation of hESCs into humans. The participants in these trials were sufferers of Stargardt's macular dystrophy or dry age-related macular degeneration, which is the chief cause of blindness in the developed world.
The trials were relatively successful in the sense that the hESC-derived retinal pigment epithelium cells that were implanted integrated well into the existing tissue, and there were no signs of hyperproliferation, abnormal growth, or rejection. The authors hope that in future this technique will be applied to patients in the earlier stages of disease, preventing disease progression[87].
Bionic Eye
Bionic Vision Australia are the first organisation to implant a bionic eye. In 2012 a prototype made up of a retinal implant with 24 electrodes was implanted into 3 different patients with retinitis pigmentosa.
A camera is used to capture images which are transferred to an external data processing unit. From here the data is processed and transmitted via a wire to the implanted receiver, which in turn sends the signal to the retinal implant. The retinal implant is then able to stimulate the visual pathways in the brain.
Bionic Vision Australia hopes that in 2013, trials for a wide-view device that consists of 98 electrodes will be in progress. This prototype will be inserted into the suprachoroidal space in order to prevent mechanical damage to the retina. Trials for a more advanced high-acuity device with 1024 electrodes are planned for 2014. The electrode array contained in this device will be made of diamond to prevent irritation of surrounding tissues. These devices are expected to be suitable for patients with retinitis pigmentosa and age-related macular degeneration. The eventual goal will be to provide a completely wireless device which gives the patient high visual acuity.
"MIP/Aquaporin 0 Represents a Direct Transcriptional Target of PITX3 in the Developing Lens"
Article Source: <pubmed>21698120</pubmed>
| The authors in this article [88] mentioned that PITX3 plays a siginificant role in the development of lens in vertebrates. If there is a deficiency is PITX3, it causes a range of problems in humans such as microphthalmia, Peter’s anomaly, or isolated cataracts. Mutation of PITX3 also causes degeneration of the lens in zebrafish and mice. It is therefore important to understand what factors may affect the decrease in PITX3, as a normal level of PITX3 is needed to maintain normal eye development. The authors wanted to investigate specific genes which are affected by PITX3. Previous research has shown that MIP and Aquaporin causes defects in the lens in both mice and humans. MIP and Aquaporin are targeted by PITX3, so their imbalance is interrelated in the cause of defects in the lens. Therefore it has been previously proven that PITX3 is needed for normal development of the lens. However, there has not been much information previously known regarding the exact effect that PITX3 has, or the specific genes it targets. Since MIP and Aquaporin is common genes found in humans, mice and zebrafish, the authors chose to study these genes to understand the pathway that PITX3 takes and its exact involvement in the development of the lens. Their results proved that deficiency in MIP and Aquaporin indeed affects normal development of the lens, and it is indeed related to deficiency in PITX3. However, there is still more research needed to understand PITX3 and the genes it interacts with, and their effect in ocular development.
|
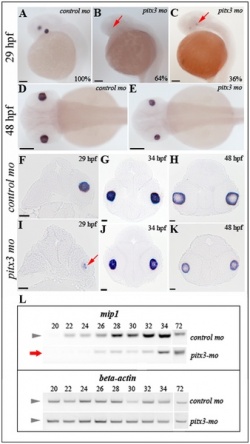 "Analysis of mip1 expression in pitx3-mo and control embryos via in situ hybridization and RT-PCR" [88] |
"Activation of c-Jun N-terminal kinase (JNK) during mitosis in retinal progenitor cells."
Article Source: <pubmed>22496813</pubmed>
| In the past, most studies about c-Jun N-terminal kinase (JNK) in the retina have been in relation to neurodegeneration; therefore the authors in this article were interested in investigating the function of c-Jun N-terminal kinase in the retinal progenitor cells in neonatal rats. [89] In the experiment, they took retinal tissue from newborn rats and fixed them, and subsequently examined them using confocal microscopy and fluorescence to discover c-Jun N-terminal kinase ‘phosphorylation by immunohistochemistry’. [89] Mitotic cells in the retina were identified during the experiment. The results of their experiment revealed that c-Jun N-terminal kinase is phosphorylated in the developing retina of neonatal rats during the mitosis of progenitor cells. This shows that c-Jun N-terminal kinase can control the proliferation of progenitor cells in the developing retina. Their experiment also revealed that inhibiting c-Jun N-terminal kinase causes disruptions to the mitotic cell cycle by reducing the cell numbers in anaphase. However, inhibiting c-Jun N-terminal kinase did not change the cell numbers in metaphase or prophase. [89]
|
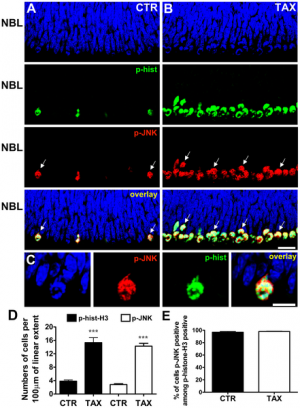 "JNK is phosphorylated during mitosis of retinal progenitor cells." [89] |
"LRP5 is required for vascular development in deeper layers of the retina"
Article Source: <pubmed>20652025</pubmed>
|
The authors in this article [90] mentioned that lipoprotein receptor-related protein 5 (LRP5) has a significant function in the development of retinal vasculature. Research has shown that mutations of the LRP5 causes loss of function, due to incomplete development of retinal vessel network, in both humans and mice. The authors investigated how mutations occur in the LRP5, which leads to abnormal development of the retinal vasculature. They have studied retinal endothelial cells in mutant mice in their study. Their results showed that in retina with mutated LRP5, endothelial cells in the retinal vasculature primarily produced cell clusters in the inner-plexiform layer instead of migrating into deeper layers of the retina to form normal retinal vasculature. The authors also discovered that there was a decrease in Slc38a5, which is “a Müller cell-specific glutamine transporter”, in mice with mutated LRP5. [90] Their results lead the authors to conclude that normal LRP5 is very important in the development of normal retinal vasculature due to their role in causing migration of retinal endothelial cells in the deeper layers of the retina. LRP5 is also important for retinal interneurons and Müller cells to function correctly.
|
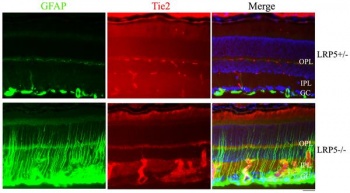 "Endothelial cells form thick clusters in the LRP5 mutant retina" [90] |
Astrocyte-Derived Vascular Endothelial Growth Factor
Article Source: <pubmed>20686684</pubmed>
|
The authors in this article mentioned that "vascular endothelial growth factor" (VEGF) has an important role in normal development of retinal vasculature. [91] The authors explained that in the process of vascularisation of the retina, the retinal astrocytes (both vascularised and not yet vascularised) expresses the vascular endothelial growth factor. This fact indicates that vascular endothelial growth factor that are derived from astrocytes of the retina plays an important role in vessel maturation and angiogenesis. Therefore the authors wanted to test the role of vascular endothelial growth factor that are derived from astrocytes to find further confirmation. ‘Cre-lox technology’ was used in the experiment to remove the vascular endothelial growth factor from mice retinal astrocytes in the developmental period. [91] The results showed that removing vascular endothelial growth factor that are derived from astrocytes caused ‘the regression of smooth muscle cell-coated radial arteries and veins’ from the effects of hyperoxia. [91] Hence, this result indicates that vascular endothelial growth factor plays an important role in stabilising blood vessels during the development of the retinal vasculature. It has been suggested that this finding may be of relevance to retinopathy in premature neonatal humans. [91] 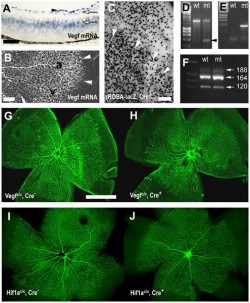 "Astrocyte specific deletion of VEGF." [91] |
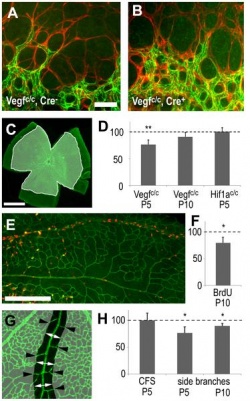 "Effects of astrocyte-derived VEGF on retinal vascular development." [91] 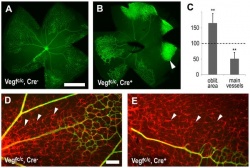 "Astrocyte-derived VEGF protects vessels from hyperoxia." [91] |
Useful Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Visualisation of eye development in the embryo
Brief Video on Embryonic development of the eyes
Embryonic Development of the eye
Webvision free online textbook
Free basic online book about the eyes
Simple eye embryology explanation
Progress in retinal and eye research journal
Animation showing the visual pathway
Video describing the layers of the retina
Video on visual processing in the retina
Development of the vertebrate eye
Easy-to-understand descriptions of the development of vision after birth
John Clement Heisler's historic textbook on Embryology (1907)
Glossary
Accommodation - changing the focal length of the lens in order to focus on an object.
Amacrine cells - interneurons located in the retina
Anterior chamber - Fluid-filled area located between the iris and cornea.
Choroid - The middle coat of the eye, located between the sclera and retina, which contains blood vessels that nourish the structures in the eye.
Ciliary body - Structure located behind the iris which secretes aqueous humour. It contains ciliary muscle, which is involved with changing the shape of the lens for accommodation.
Cornea- a transparent section in the anterior of the eye which acts as a window over the pupils, and is involved with refracting light as it enters the eye.
Downstream genes - genes that are activated by other "upstream genes".
Ectoderm - outermost layer of germ cells in an early embryo.
Endoderm - innermost layer of germ cells in an early embryo.
Extraocular muscles - Muscles that control the movement of the eyeball.
Glial cells - non-neuronal cells that provide structure and protection to neurons as well as producing myelin.
Inductive signaling - a process whereby the secretion of factors from one cell or tissue triggers a response in another.
Iris- A circular shaped muscle which controls the opening and contraction of the pupil.
Lens- A structure inside the eye which refracts light as it enters the eye for clear vision.
Lens vesicle - the cavity of invaginated ectoderm from the optic placode that will form the lens.
Macula - a highly pigmented, oval-shaped area located near the centre of the retina. Important for visual acuity.
Mesenchyme - undifferentiated, loose connective tissue.
Mesoderm - middle layer of germ cells in an early embryo.
Mesothelium - the epithelial layer of the mesoderm.
Myelinisation - development of a myelin sheath around a nerve fibre.
Neural crest - a portion of the ectoderm situated next to the neural tube.
Neural groove - a large invagination on the dorsal surface of the embryo which will close off and form the neural tube.
Neural tube - hollow structure that results from the folding of the neural plate and eventually forms the central nervous system.
Neuroblastic layer - a layer of immature cells that differentiate to form either glial cells or neurons. The retina has two of these (an inner and outer).
Neuroectoderm - portion of the ectoderm that develops to form the central and peripheral nervous systems.
Optic chiasm - the point at which the optic nerves meet and cross over.
Optic cup - the structure that is formed after the optic vesicle folds in upon itself. This will form the retina.
Optic globe - a term that refers to the optic cup, lens vesicle and surrounding mesenchyme collectively.
Optic Nerve - The nerve which carries visual information from the retina to the brain for processing.
Optic placode - area of thickened ectoderm that gives rise to the lens of the eye.
Optic stalk - a long, narrow cavity that will produce the optic nerve.
Optic vesicle - a cavity that buds off from the neural tube and gives rise to the optic cup.
Posterior chamber- Fluid-filled area located between the iris and lens.
Pupil- opening in the anterior part of the eye, which controls how much light enters the eye.
Retina - Light-Sensitive portion located towards the back of the internal surface of the eye, which contains photoreceptors (rods and cones) which detects visual information and transmits it to the brain through the optic nerve.
Retinal bipolar cells - specialised neurons that transmit signals between the photoreceptors and ganglion cells in the retina
Retinal ganglion cells - transmit visual information from the retina to the brain
Sclera- white part of the external anterior surface of the eye, which envelopes the eyeball to give it support and protection of its internal contents.
Upstream genes - genes that activate one or more other "downstream genes".
Vascularise - to invade with blood vessels.
Vitreous Chamber- Area located between the lens and retina, which contains vitreous (a jelly like substance) whose function is to maintain the shape of the eye.
Image Gallery
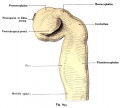
Illustration of the front of the eye, showing the iris, sclera and pupil. Credits: Webvision PMID:21413389 [PubMed]
A CAT scan with illustrations to show the extraocular muscles from the back view of the eye. Credits: Webvision PMID:21413389 [PubMed]
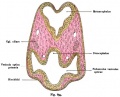
A diagram of the layers of the retina. Credits: Webvision PMID:21413389 [PubMed]
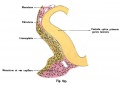
The layers of the retina magnified, showing the direction of the layers of the retina in the back of the eye. Credits: Webvision PMID:21413389 [PubMed]
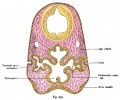
A diagram of the components of the retina. Credits: Webvision PMID:21413389 [PubMed]
The bulging lateral wall of the primary optic vesicle is covered by a fairly well demarcated lens plate, a direct continuation of the ectoderm. Between the optic vesicle and the lens pit are some flattened spindle-shaped cells. In the adjoining mesoderm are cross-sections of capillaries. Credits: Kollmann, J. (1907)
4th Week of development. The internal organisation shows the secondary optic vesicle. A: The rear wall of lens is noticeable and is enveloped by mesoderm. B: The edges of the lens pit is already grown and the lens vesicles are formed, which is still related to the remaining ectoderm. Credits: Kollmann, J. (1907)
The lens has now cut off from the ectoderm, but is still very superficial. Between it and the lateral lamina of the optic cup, there is a considerable space. The eye stalk has become longer and is enclosed together with the optic cup and lens of the mesoderm. The cornea, sclera and choroid make gradual development. Credits: Kollmann, J. (1907)
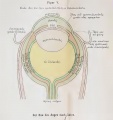
The layers of the retina in the fifth month of development. Credits: Webvision PMID:21413389 [PubMed]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Kolb H, Fernandez E, Nelson R. The Organization of the Retina and Visual System (Online Book). PMID:21413389 [PubMed]
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 Remington L.A. (2012) Clinical Anatomy of the Visual System. 3rd Ed. Elsevier 2012.
- ↑ Edwards, D.D. (1996). Ophthalmology before Hippocrates. In the History of Ophthalmology, ed. D.M. Albert and D.D. Edwards. Cambridge, Mass.: Blackwell Science.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 Lovicu, F.J., & Robinson, M.L. (2004), Chapter 1: The Lens: Historical and Comparative Perspectives, Development of the Ocular Lens, Cambridge University Press, pp. 3-17.
- ↑ 5.0 5.1 5.2 5.3 Magnus,H., (1901). Die Augenheilkunde der Alten, Breslau
- ↑ 6.0 6.1 Magnus, H. (1998). Ophthalmology of the ancients. In J. Hirschberg (Ed.), The History of Ophthalmology: The monographs, Vol. 4, Part 1 (F.C. Blodi, Trans.) Bonn: Wayenborgh.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 Adelmann, H.B. (1966). Marcello Malpighi and the Evolution of Embryology. Ithaca: Cornell University Press.
- ↑ 8.0 8.1 8.2 8.3 Albert, D.M. (1996). Greek, Roman and Arabian Ophthalmology. In D.M. Albert and D.D. Edwards (Eds.), The History of Ophthalmology. Cambridge, MA: Blackwell Science.
- ↑ Hirschberge, J. (1982). Antiquity, Vol. X in the History of Ophthalmology (F.C. Blodi, Trans.) Bonn: Wayenborgh. pp. 280
- ↑ 10.0 10.1 10.2 Albert, D.M. (1996). Discovering the anatomy of the eye. In D.M. Albert and D.D. Edwards (Eds.), The History of Ophthalmology. Cambridge, MA: Blackwell Science.
- ↑ Tsonis, P. A. (2001). Regeneration of the Vertebrate Lens and Other Eye Structures. eLS. (Online Publication). DOI: 10.1038/npg.els.0001102
- ↑ Hertwig, O. Text-book of the embryology of man and mammals. S. Sonnenschein 1901. (Translated from the 3d German ed. by Edward L. Mark.)
- ↑ Tsonis, P. A. (2001). Regeneration of the Vertebrate Lens and Other Eye Structures. eLS. (Online Publication). DOI: 10.1038/npg.els.0001102
- ↑ Spemann, H. (1901). Über Correlationen in der Entwicklung des Auges. Verhand. Anat. Ges. 15: 61-79.
- ↑ Saha, M. (1991). Spemann seen through a lens. In Gilbert, S. F. (ed.). A Conceptual History of Modern Embryology. Plenum Press, NY. pp. 91-108.
- ↑ Brown, E.J. (1906). The Embryology Anatomy and Histology of the Eye. Chicago: Hazlitt & Walker. 1906
- ↑ Bailey, F.R. and Miller, A.M. (1921). Text-Book of Embryology. New York: William Wood and Co. (Note- This book is only at an early edited stage)
- ↑ <pubmed>18168466</pubmed>
- ↑ <pubmed>18168498</pubmed>
- ↑ Holtfreter, J. (1939). Gewebeaffinitat, ein Mittel der embryonalen Formbildung. Arch. Exp. Zellforsch. 23, 169-209.
- ↑ <pubmed>11023863</pubmed>
- ↑ Barber AN: Embryology of the human eye. St. Louis. CV Mosby 1955
- ↑ <pubmed>13469954</pubmed>
- ↑ <pubmed>13563560</pubmed>
- ↑ <pubmed> 13519969</pubmed>
- ↑ <pubmed> 14023393</pubmed>
- ↑ <pubmed>14077035</pubmed>
- ↑ <pubmed>14023394</pubmed>
- ↑ <pubmed>14189921</pubmed>
- ↑ <pubmed>14211912</pubmed>
- ↑ Mann I. The development of the human eye. New York: Grune and Stratton 1964
- ↑ <pubmed>5833111</pubmed>
- ↑ <pubmed>14340157</pubmed>
- ↑ <pubmed>5969670</pubmed>
- ↑ O'Rahilly, R. 1966 The early development of the eye in staged human embryos. Contr. Embry. Carnegie Inst., Wash., 38: 1–42
- ↑ <pubmed>5640327</pubmed>
- ↑ <pubmed> 5772716</pubmed>
- ↑ <pubmed> 5472476</pubmed>
- ↑ <pubmed> 4925671</pubmed>
- ↑ 40.0 40.1 <pubmed> 6650859</pubmed>
- ↑ <pubmed> 1266776</pubmed>
- ↑ <pubmed> 1020699</pubmed>
- ↑ <pubmed> 7224936</pubmed>
- ↑ Driell, D. Van; Provis, J.M.; Billson, F.A.: Early differentiation of ganglion, amacrine, bipolar and Muller cells in the developing fovea of the human retina. J. Comp. Neurol. 291: 203-219.
- ↑ 45.0 45.1 45.2 45.3 45.4 45.5 45.6 45.7 45.8 <pubmed>1628748</pubmed>
- ↑ <pubmed>2406219</pubmed>
- ↑ http://www.vetmed.vt.edu/education/curriculum/vm8054/eye/EMBYEYE.HTM
- ↑ 48.0 48.1 http://embryology.med.unsw.edu.au/embryology/index.php?title=ANAT2341_Lab_6_-_Early_Embryo
- ↑ 49.0 49.1 49.2 <pubmed>11687490</pubmed>
- ↑ 50.0 50.1 50.2 50.3 50.4 50.5 Schoenwolf: Larsen’s Human embryology, 4th ed. Churchill Livingstone, An Imprint of Elsevier. 2008
- ↑ <pubmed>1451666</pubmed>
- ↑ <pubmed>7224936</pubmed>
- ↑ 53.0 53.1 <pubmed>18168748</pubmed>
- ↑ http://dialspace.dial.pipex.com/agarman/bco/fact4.htm
- ↑ <pubmed>6462623</pubmed>
- ↑ 56.0 56.1 <pubmed>16959249</pubmed>
- ↑ <pubmed>16249499</pubmed>
- ↑ <pubmed>12127103</pubmed>
- ↑ <pubmed>1222340</pubmed>
- ↑ 60.0 60.1 60.2 60.3 Moore: The Developing Human, 9th ed. Saunders, An Imprint of Elsevier. 2011
- ↑ <pubmed> Retinal and anterior eye compartments derive from a common progenitor pool in the avian optic cup</pubmed>
- ↑ <pubmed>19710684</pubmed>
- ↑ <pubmed>21835309</pubmed>
- ↑ <pubmed>12386935</pubmed>
- ↑ <pubmed>6511224</pubmed>
- ↑ Riordan-Eva P, Whitcher JP. Vaughn and Asbury's General Ophthalmology, Lange Medical Books/McGraw Hill. 2004:25–27
- ↑ <pubmed>19481138</pubmed>
- ↑ <pubmed>20105280</pubmed>
- ↑ Yanoff M, Duker JS. Ophthalmology. Mosby; St. Louis, MO: 2004
- ↑ <pubmed>18637741</pubmed>
- ↑ <pubmed>18386822</pubmed>
- ↑ <pubmed>15558475</pubmed>
- ↑ <pubmed>9216064</pubmed>
- ↑ <pubmed>9609835</pubmed>
- ↑ <pubmed>20801226</pubmed>
- ↑ <pubmed>805092</pubmed>
- ↑ <pubmed>5542135</pubmed>
- ↑ Development of the Choroid and Related Structures, K. Sellheyer, Eye (1990) 4, 255-261
- ↑ 79.0 79.1 Cook CS, Ozanics V, Jakobiec FA. (1994) Prenatal development of the eye and its adnexa. In Tasman W, Jaeger EA, editors: Duane’s foundations of clinical ophthalmology, vol 1, Philadelphia, 1994, Lippincott.
- ↑ <pubmed>7364662</pubmed>
- ↑ Kikkawa DO, Lucarelli MJ, Shovlin JP, et al: Ophthalmic facial anatomy and physiology. In Kaufman PL, Alm A, editors: Adler’s physiology of the eye, St Louis, 2003, Mosby, pp 16.
- ↑ <pubmed>9882499</pubmed>
- ↑ <pubmed>14635806</pubmed>
- ↑ <pubmed>16249499</pubmed>
- ↑ 85.0 85.1 <pubmed>22405869</pubmed>
- ↑ 86.0 86.1 86.2 86.3 86.4 <pubmed>22590629</pubmed>
- ↑ <pubmed>22281388</pubmed>
- ↑ 88.0 88.1 <pubmed>21698120</pubmed>
- ↑ 89.0 89.1 89.2 89.3 <pubmed>22496813</pubmed>
- ↑ 90.0 90.1 90.2 <pubmed>20652025</pubmed>
- ↑ 91.0 91.1 91.2 91.3 91.4 91.5 91.6 <pubmed>20686684</pubmed>
--Mark Hill 12:22, 15 August 2012 (EST) Please leave the content listed below the line at the bottom of your project page.
2012 Projects: Vision | Somatosensory | Taste | Olfaction | Abnormal Vision | Hearing




![Illustration of the front of the eye, showing the iris, sclera and pupil. Credits: Webvision PMID:21413389 [PubMed]](/embryology/images/thumb/c/c4/Eye-pupil-sclera-iris.jpg/120px-Eye-pupil-sclera-iris.jpg)
![A CAT scan with illustrations to show the extraocular muscles from the back view of the eye. Credits: Webvision PMID:21413389 [PubMed]](/embryology/images/thumb/9/93/Extraocular-muscles-scan.jpg/120px-Extraocular-muscles-scan.jpg)
![A diagram of the layers of the retina. Credits: Webvision PMID:21413389 [PubMed]](/embryology/images/thumb/d/d6/Retina-layers-diagram2.jpg/120px-Retina-layers-diagram2.jpg)
![The layers of the retina magnified, showing the direction of the layers of the retina in the back of the eye. Credits: Webvision PMID:21413389 [PubMed]](/embryology/images/thumb/8/8f/Eye-retina-layers.jpg/120px-Eye-retina-layers.jpg)
![A diagram of the components of the retina. Credits: Webvision PMID:21413389 [PubMed]](/embryology/images/thumb/5/57/Retina-layers-diagram.jpg/120px-Retina-layers-diagram.jpg)


![The layers of the retina in the fifth month of development. Credits: Webvision PMID:21413389 [PubMed]](/embryology/images/thumb/c/cd/5months-gestation-retina.jpg/84px-5months-gestation-retina.jpg)