2011 Group Project 5
| Note - This page is an undergraduate science embryology student group project 2011. |
Fragile X Syndrome
Introduction
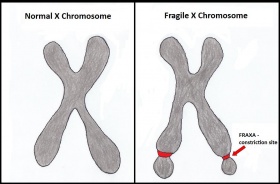
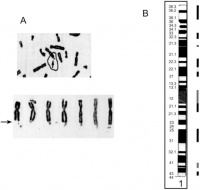
Fragile X syndrome (FXS) is a congenital disorder resulting from an abnormality on the X-chromosome. FXS is named for the fragile site on the X chromosome found in those affected with this syndrome. The fragile site is known as FRAXA (Fragile site on the X chromosome site A) which appears as a gap, constriction or break in metaphase chromosomes as shown in figure 1[1]. Specifically it is caused by methylation of the CGG triplet resulting in its amplification. The expansion of the nucleotide triplet (CGG) causes the extremity of the long arm of chromosome X to appear elongated,[2] creating a site of constriction which becomes fragile and is prone to breaking[3]. The amplification of this codon also results in the silencing of the FMR1 gene.
This genetic anomaly manifests itself as a number of phenotypic abnormalities including; physical appearance (long narrow face, prominent ears, a prominent jaw, and macroorchidism), social deficits, intellectual retardation (Low IQ, decreased working memory), speech deficits and often extreme, child-like emotions.
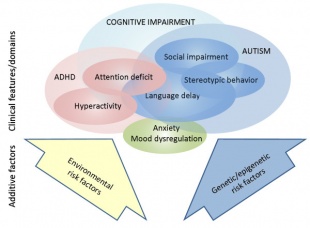
FXS has a strong correlation with Autism, with approximately 25-30% of FXS afflicted males meeting the diagnostic criteria for Autism.
As a genetic disorder, direct treatment of the cause is unavailable. As such, the majority of treatments target the individual symptoms associated with the disorder. These include drug treatments for Attention Deficit Hyperactivity Disorder (ADHD), aggression and seizures, as well as non-drug treatments such as counseling, environmental changes and behavioural interventions.
History of the disease
| Year | Discovery |
| 1910 | Thomas Hunt Morgan demonstrated that when a gene is located on the X chromosome of a fruit fly, the characteristic appears more frequently in males than in females[4]. |
| 1943 | Martin and Bell first reported that the excess number of retarded males in the population was due to a sex linked inheritance. The two also noted that there was a lack of unusual physical features related to the mental retardation, including the shape of the head and face. Therefore Martin and Bell were also the first to report sex linked mental retardation without microcephaly or microphthalmia[3]. For this reason, Fragile X syndrome is also referred to as Martin-Bell syndrome. |
| 1969 | Herbert Lubs first documented and reported the existence of the marker X chromosome.[3]. Lubs developed the chromosomal test for Fragile X, however it was not used extensively until the late 1970's[5]. |
| 1977 | Sutherland showed Herbert Lubs' findings to be accurate and reliable. This was done through cells cultured in a folate deficient medium[5].
Herbert and Lubs found that cytogenetically, fragile X syndrome is characterized by the presence of a folate-sensitive fragile site at Xq27.3 (FRAXA) in the lymphocytes of affected individuals[5]. |
| 1980 | Turner and colleagues recognized the combination of macroorchidism and mental retardation in males in conjunction with a fragile site on the X chromosome to be a separate clinical entity[5]. |
| 1991 | Verkerk discovered and described the FMR1 gene and encouraged the medical and psychopedagogic research of Fragile X Syndrome[2]. |
| 1992 | Sutherland and baker discovered two other rare folate-sensitive fragile sites distal to FRAXA at Xq28, these include; FRAXE and FRAXF. Both of these fragile sites are also caused by the expansion and subsequent methylation of the CGG nucleotide triplet, however, they have not been associated with any phenotype[5]. |
| 2001 | Hagerman and colleagues first recognized Fragile X-associated tremor/ ataxia syndrome (FXTAS).[6] FXTAS is a disorder that is under-recognized because the first published report was only in 2001 and the presentation of the disorder is variable and mainly consists of a combination of signs common in the elderly.[7] The core clinical symptoms of FXTAS are prominent tremors, ataxia and cognitive decline. Cognitive decline occurs in at least 50% of the patients with neurological symptoms usually at a later age (>70 years).[8] |
| 2010 | Paul and colleagues deduced that the elevated mRNA in FXS premutation carriers are vulnerable to neurotoxin, leading to early cell death and brain disease, consistent with FXTAS symptoms[5]. |
Epidemiology
Recent data suggests that Fragile X Syndrome (FXS) approximately affects 1 in 2,500 individuals of which there is approximately equal prevalence in males and females.[9]
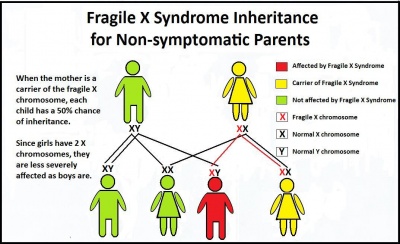
The incidence of the premutation carriers is significantly higher with approximations of up to 1 in 251 males and 1 in 100 females noted as carriers[9]. Since FXS is an X-linked neurodevelopmental disorder, females are less likely to show severe signs of physical, cognitive and behavioural phenotypes of Fragile X due to the presence of one normal allele [9].
There are an inadequate number of studies conducted on Fragile X Syndrome of different populations. However, those studies that have been done, suggests that there is a difference in prevalence across populations.[10] For example:
- Figures from Israel find the frequency of the premutation carrier state in females to be approximately 1 in 130 and the full mutation to be present in 1 in 2,500 females[9] .
- Data from Canada, suggest that the incidence of premutation carriers to be 1 in 800 males and 1 in 260 females[9].
- Data from Taiwan find the frequency of premutation male carriers to be much lower at approximately 1 in 1,670[9].
- One study proposed that the prevalence of FXS is higher amongst Tunisian Jews compared with Caucasians by as much as 10 times more[10].
- Another study established a lack of large CGG repeats in Native American populations to suggest a lower prevalence of the syndrome amongst this population[10].
- Similarly, the Spanish Basque population has reported a lower prevalence of males with the FXS of pure Basque origin in a mentally retarded population and a lower frequency of large CGG repeats[10].
- Studies carried out on Indian Fragile X patients have shown a frequency of 7% among the mentally retarded population.[11]
A study in the United States has found variability amongst the frequency of premutation carriers of different ethnicities within their population.[12]
- Caucasian Americans had a frequency of 1 in 169[12]
- Hispanics had a frequency of 1 in 287[12]
- African Americans had a frequency of 1 in 124[12]
- Ashkenazi Jewish had a frequency of 1 in 134[12].
Genetic Counseling
Genetic counseling is advised for individuals with FXS in their family history. The genetic counselor will identify individuals that are at risk for carrying FMR1 mutations by reviewing the individual’s inheritance pattern for FXS. The counselor will also assess the chances of having offspring affected by FXS and review reproductive options for future pregnancies. [13]
Etiology
Involving methylation-induced silencing of the FMR1 gene on the X chromosome, Fragile X Syndrome is an entirely genetic disease. The disease is not necessarily hereditary; given the location of the gene on a particularly fragile segment of Xq27.3, the disease commonly occurs in people without a family history. Nonetheless, the disease is X-linked and thus likely to be passed down between generations.
At times Fragile X Syndrome may occur due to partial or complete deletion of the FMR1 gene, however it most typically occurs due to amplification of a CGG triplet repeat on Xq27.3. This amplification can take 4 forms, each of whose increasing amplification correlates with varying degrees of the disease: common, intermediate, premutation and full mutation[10].
- Common amplification typically presents with anywhere between 6 and 40 repeats; 30 repeats is most common[14]. This form presents with no signs or symptoms of the disease.
- Intermediate amplification features between 41 and 60 repeats. Parents with intermediate amplification will similarly typically produce asymptomatic offspring. However, the risk of full mutation Fragile X Syndrome in their offspring is increased.
- Premutation amplification refers to repeats between 61-200. While children born with premutation amplification are largely asymptomatic, studies have shown that it predisposes to Parkinson's disease, intention tremor and brain atrophy, as well as ovarian failure in women.[15][6][16]
- Full mutation refers to any amplification of >200 repeats. Children born with full mutation Fragile X Syndrome present with the classical symptoms of the disease.
Elongation of the FMR1 gene beyond 200 repeats results in methylation of the CpG island that typically regulates its expression, the loss of which inhibits the binding of transcription factors, effectively silencing the gene and causing fragile X mental retardation protein 1 (FMRP) to be under-expressed. Transcription is similarly inhibited by methylation-induced chromatin condensation.[17] Repression of FMRP expression interferes with nervous system functioning[18]. Studies have also suggested FMRP playing a role in synapse formation and function[19][20], being significantly expressed in the brain and testes.[21] The prominence of FMR1 mRNA in the normal developing fetal central and peripheral nervous systems strongly suggests that its absence brings about the mental retardation typical of sufferers of the disease.[22] FMRP itself has been found to play a role in synaptic plasticity via its deactivation of gene expression by forming a complex with a RISC subunit containing Dicer enzyme.[23][24] Its role in RNA metabolism sees FMRP repressing translation of mRNA at synapses via interaction with proteins required for miRNA function.[25]
It is worth noting that a person with a full mutation gene may nevertheless be able to synthesise FMRP in some of their cells due to differing degrees of repeats between cells of the body, consequently presenting with milder symptoms than full mutation patients[26].
Development of the Disease
Developmentally, mental retardation is the most common evidence of Fragile X Syndrome, with patients typically evincing IQs of 20-70[27][28]. Nonetheless there are certain other phenotypes to be observed at various stages of development of a child with Fragile X Syndrome.
Fetal Development
During fetal development, rostral structures exhibit abnormal proportions. Facial structure reminiscent of macrocephaly is expressed: ears, the mandible and the forehead are prominent. Given the hypothesized role of FMRP in proper neuronal development[29] and the role of neural crest migration in fetal facial skeleton development[30], silencing of FMRP production may consequently result in imperfect neural crest migration and subsequent facial skeleton development.
Postnatally
From birth to puberty, other phenotypes of Fragile X Syndrome become evident. Impairments in working memory, selective attention, inhibition and spatial cognition begin to be exhibited as the child misses developmental milestones[31][32]. Hoeft et al. (2010) identified a period in early brain development wherein typical neurodevelopment was not seen, correlating with observations of the above-mentioned impairments[33]. Coincidentally, enlargement of the caudate nucleus and thalamus have been noted in such patients, a feature hypothesized to be related to inadequate repression of translation and protein expression within tissue of the central nervous system by FMRP[34].
The development of macrocephalic features continues post-natally; elongation of the face becomes more noticeable. Additionally, reports of mitral valve prolapse, reduced joint stability, flat feet, arched high palate and Postural hypotension have been noted[35][36]. Joint hyper flexibility, protruding ears and the macroorchidism evident after puberty are examples of deformities related to connective tissue dysplasia[36], which is emblematic of uncoordinated communication between cells within a tissue.
Postpubescent
Macroorchidism and mental retardation are the two main symptoms of Fragile X Syndrome evinced in postpubescent patients. From the age of 30 onwards, impairment of inhibition is significantly disparate in premutation and full mutation genotypes against a cohort of normal genotypes[37], likely as a result of previously impaired neuronal development and continuing inadequate synaptic messenger degradation. Similarly, the significant levels of expression of FMRP in normal development of the testes[38] during puberty mean that in post-pubescent male patients, macroorchidism is evident. Increased proliferation of Sertoli cells has been noted in these cases[39] and, although the exact molecular pathogenesis is as of yet unknown, FMRP’s failure in its role as a posttranscriptional regulator of mRNA export has been hypothesized to be significant either as a causative or correlative factor[40].
Signs and Symptoms
Fragile X syndrome is characterised by mental retardation, a variety of cognitive, physical and behavioural signs. Most males with the full FMR mutation exhibit the clinical features of fragile X displayed below. Furthermore, males affected tend not to reproduce, but this is possibly due to the severity of mental retardation.
Physical phenotype
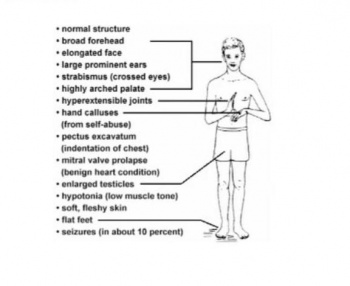
Before puberty, children with Fragile-X tend to have no discernible differences in physical appearance. They may have a broad forehead or a slightly larger size head. At puberty, these children begin to develop the physical signs recognized with Fragile-X, such as longer faces, larger jaws and ears[41]. Furthermore, they tend to have impaired growth, and will not achieve a height that one might expect (based on familial relations, or population averages). Males may also develop macroorchidism: enlargement of the testicles. Fragile-X patients may also have loose connective-tissues[42], allowing their joints to be more flexible than normal. This may cause complications arising from increased risk of hernia as well as problems associated with other connective tissues such as: heart-valve weaknesses resulting in murmur. Later in life, these men may develop a tremor and experience difficulty walking.
Physically, adult males often have a long narrow face, prominent ears, a prominent jaw, and macroorchidism. Other common physical features include a high arched palate, hyperextensible finger joints, double jointed thumbs, single palmar crease, hand calluses, velvet-like skin, flat feet[41][42]. Mitral valve prolapse has been shown to in late adolescence[43]. Males with the fragile X syndrome also tend to exhibit behavioral features such as hyperactivity, social anxiety, perseverative speech and language, tactile defensiveness, stereotypes (e.g., hand-flapping), and hand biting.
Social interaction
Children with Fragile-X tend to experience social anxiety, feeling awkward and uncomfortable in new environments and situations. Often, they may avoid social interactions, due to the anxiety, and tend not to seek contact with others[44]. Their anxiety often manifests itself as discontinuous speech and a lack of eye contact.
Studies into the social cognition network underlying face encoding, show profound causation relating to cortical activation [45] Individuals with FXS show decreased activation of prefrontal regions that are shown to affect social cognition. These areas include the medial and superior frontal cortex. The data suggests that social anxiety in Fragile-X patients may be related to the inability to successfully activate higher level social cognition regions during the early phases of memory formation. [45]
Intellectual development
Males with FXS tend to exhibit developmental delays in childhood. By age 3, these males will test in the mentally retarded range. As a generalisation, the majority of Fragile-X patients have an IQ defined as moderately retarded (IQ 40-54). However, the mental retardation ranges from profound (IQ<20) to mild. Females however, show lower impairment, with only one-third having IQs within the ‘mental retardation’ range.
In sibling studies, children with Fragile-X syndrome obtain lower percentage correct scores in all subtests of the WISC (Wechsler Intelligent Scale for Children) [46]. Over time, the gap between the FXS afflicted and the non-affected siblings grew dramatically. The unaffected children had a rate of intellectual development approximately 2.2x that of their FXS counterparts.
Specifically, a deficit in working memory has been attributed to the FXS mutation. When studied, they exhibit a weakness for tasks that reflect function of the central executive subcomponent of working memory[47]. Furthermore, the extent of the central executive deficit is correlated significantly with larger CGG section repeat. This correlation between repeat length and working memory (specifically in asymptomatic carrier males) suggests that carrier males have greater neuropathology with larger expansions in FMR1 mRNA transcripts[47].
Generally, Fragile-X patients have trouble with forming abstract ideas, planning and problem solving. Conversely, they tend to have a good memory for pictures and visual patterns, and may be better adept at following instructions if presented in picture format.
Autistic-like behavior is also described in these males, with as many as 25% of males with the fragile X syndrome meeting the diagnostic criteria for autism.
Behavioural characteristics
There are a number of of phenotypic behavioural characteristics associated with Fragile-X syndrome. It is important to note that a number of these are purely observational. Many do not have scientific studies associated with proof of the characteristic nor explanations of by which mechanism they occur. The information below is found at National Institute of Child Health & Human Development and The National Fragile X Foundation.
Males with the fragile X syndrome also tend to exhibit behavioral features such as hyperactivity, social anxiety, perseverative speech and language, tactile defensiveness, stereotypes (e.g., hand-flapping), and hand biting. ADHD and a short attention span are associated with Fragile-X patients. Girls are less likely to be diagnosed with hyperactivity, however, they do tend to exhibit an inattentiveness and a failure to focus on long, difficult tasks.
Emotional characteristics
Fragile-X children often are easily upset or overwhelmed. New situations can easily frighten them. Upon entering an unfamiliar situation, some tend to cry, whilst others may become tense. These may often lead to tantrums or repetitive tics. During puberty and teen years, hormone levels may exaggerate this, making the tantrums more violent and the patients largely more aggressive. Furthermore, the usual anxiety experienced with difficult tasks may take longer to abate, meaning the patient may take longer to calm-down.
Language and Speech
Often these children have problems with coherence, word pronunciation and correct grammar use. This impairs their ability to properly communicate meaning. More serious speech problems are associated with vocal processing, such as: moderating tone, pitch or loudness as well as coordinating the movements needed to vocalize sounds. Furthermore, they may have difficulties processing spoken information and, as shown above, will be better at following instructions if presented in picture format. These children may stutter, omit sounds out of their words, repeat themselves, or restart the same sentence many times. They may also speak fast and/or mumble.
It is important to note, that some of their disability to communicate can be attributed to the shyness and social anxiety, while specific deficits may be due to sensory overload, rather than specific neural problems with control of speech and language.
Screening/Population testing
The screening of FXS is aimed at newborns and women of reproductive age. Screening of newborns will help to recognize those affected by FXS to enable early treatment before the onset of symptoms whilst the screening of women of reproductive age is to identify those at risk of having a child born with FXS and to influence their life choices when starting a family[48].
Population screening for Fragile X Syndrome has been largely debated[48].
Arguments supporting the use of population testing include:[48]
- The severity of the condition
- High occurrence in the general population
- The burden the condition places on the families and society as well as the individual
Arguments against the use of population testing include:[48]
- FXS is very complex resulting in a variable degree of severity
- FXS has a complicated pattern of inheritance
- The health risks associated with being a premutation carrier;
- FXTAS: Fragile X-associated tremor/ ataxia syndrome is a late onset neurodegenerative disease. It is often encountered in older men who are carriers of the FMR1 premutation and is believed to be RNA-mediated and not due to the reduction or absence of FMRP unlike FXS[49]
- FXPOI: Fragile X-associated primary ovarian insufficiency. Approximately 25% of female premutation carriers have fragile X-associated primary ovarian insufficiency[49]
- Increased risk of mild learning or emotional disabilities
- These health risks will require counseling and education
- There is a concern for the availability of resources
Diagnosis
Modern-day approaches to diagnosing FXS involve the use of immunocytochemical and molecular techniques. [50] Such techniques include:
| Diagnostic Technique | Description | Related Videos |
| Southern Blot |
This is known to be the most popular procedure in most laboratories as it allows for the detection of mutations and determination of methylation status in the one test. [50] For instance, Southern Blot analysis of the fragile site on the 5.1 kb EcoRl fragment revealed a “complete correlation between total methylation at a BssHll site within the CpG island and absence of FMR-1 expression in Fragile-X patients.” [51] |
Southern Blot Steps [1] |
| Modified PCR techniques |
An example of such modified techniques is PCR that is methylation specific. This modified PCR-based method is used for the analysis of methylation of the FMR1 gene in Fragile X patients. Methylation-specific PCR is based on the bilsulfite modification of DNA where uracil is formed by the conversion of unmethylated cytosine residues, with methylated residues remaining unconverted. “The subsequent change in the sequence of the FMR1 promoter of fragile X affected and unaffected individuals after bisulfite treatment is monitored by PCR.” [52] These techniques are an option but they are not yet as popular as other techniques for diagnosing FXS as they possess difficulty amplifying CGG repeats, interpreting results in female patients and differentiating between mosaic patterns. [50] |
Methylation-Specific PCR [2]
|
| FMRP antibody test |
This test is suitable for the screening of large populations of mentally retarded people and neonates for patients with Fragile-X Syndrome who have no CGG expansion. An advantage of this test is that it is non-invasive and only requires 1 or 2 drops of blood. [53] This test is still, however, not widely used. [50] |
No video available for this technique |
Studies have shown that the most reliable method of diagnosing FXS is by using PCR as an initial pre-screening test followed by the Southern blot test. [50]
Treatment
Several medications have been proposed to treat the symptoms of FXS, some of which are more successful than others. However, in order to increase the efficacy of the treatments, further research and testing is imperative.
The following is a summary of the major treatment options related to specific symptoms and behavioural problems for individuals affected by FXS.
| Signs & Symptoms | Treatment Option and Description |
| Attention-Deficit/Hyperactivity Disorder (ADHD) |
The prevalence of ADHD symptoms in individuals with FXS is much higher than that of other individuals with either genetic conditions or non-specific intellectual disability. [54] [55] Stimulants have been shown to improve ADHD symptoms in FXS patients. These drugs are distributed in addition to individualized therapies and behavioural intervention. [56] [13] Some associated problems of using stimulants when treating symptoms of ADHD in younger children (such as 5 years of age and under) is that they may induce irritability and other behavioural problems. In this case, administration of non-stimulant medications may be more beneficial. Such alternative medications include adrenergic receptor agonists, such as clonidine and guanfacine. [13] Clonidine has shown to be helpful for children with ADHD who have sleep disturbances (a asymptom often present in FXS patients). [57] Guanfacine can also improve ADHD symptoms, such as “including hyperactivity and frustration intolerance, as well as hyperarousal.” [13] [58] |
| Mood Instability and Aggression |
Antipsychotic drugs, such as Risperidone and Aripiprazole, are proven to be helpful in treating mood instability, aggression, perseverative behaviours and irritability in patients with FXS. [13] Risperidone was the most popular and clinically effective antipsychotic drug in the past for treatment of aggression and mood instability in patients of FXS. [59] “The typical risperidone dose range for children with FXS is 1 to 2.5 mg/day.” [13] Aripiprazole was the second most popular atypical antipsychotic agent for targeting multiple behaviour difficulties in patients with FXS. “Typically, low doses of aripiprazole (2.5–5.0 mg for adolescents and even lower doses for younger children) work best for patients with FXS.” [13] |
| Seizures |
FXS patients are known to be at an increased risk for seizures, with rates of 5% for girls and 13% to 18% for boys. [60] Many types of seizures have been reported in individuals with FXS; the most common type being complex partial seizures. [61] A single anticonvulsant is usually the treatment of choice to control seizures in FXS. Following administration of anticonvulsant, general health and blood-specific monitoring is required. [13] |
| Excessive mGluR5 (metabotropic glutamate receptor 5 pathway)Signaling |
Enhancement of mGluR-mediated processes and excessive mGluR5 signaling that normally would be inhibited by FMRP has been shown to contribute to many of the phenotypic features of FXS, such as cognitive deficits, behavioural abnormalities, enhanced anxiety, seizures, coordination problems and more. [62] [63] [13] mGluR5 antagonists have been studied in animal models of FXS and have demonstrated benefits in decreasing problems associated with excessive mGluR5 signaling, such as reducing seizures, enhancing cognitive skills and improving behaviour. [64] mGluR5 antagonists trials are beginning with FXS individuals. [13] |
| Behavioural Deficits |
Studies have shown that environmental variations impact on behaviour. For instance, fewer autistic behaviours as well as enhanced IQ scores in children with FXS are associated with a higher-quality home environment. [65] [66] Educational services are also known to improve behaviour and decrease autistic symptoms in FXS patients. [67] Higher-functioning individuals with FXS may also benefit from counseling or psychotherapy. [13] |
Recent/ Future Research
Autism and Fragile X Syndrome (FXS)
Autism is a developmental disorder that usually occurs in childhood. It is part of a spectrum that is known as autism spectrum disorder (ASD).
Rapin & Tuchman, (2008),[68] have characterized autism into three key manifestations:
- Poor communication skills, impaired sociability and difficulty in developing relationships.
- Perseveration, rigidity and resistance to change.
- Impairment in language, difficulty in using abstract concepts and delayed symbolic play.
Thus it can be seen that many of the phenotypic characteristics of autism are synonymous to those of FXS, further emphasizing the interlinked nature of the two disorders.
Autism is a disorder whose etiology is not very well understood. Its diagnosis is mainly behavioral and the disorder does not present with any population-wide biomarkers. This is contrasted with FXS, a single-gene disorder whose etiology is well known and understood. Despite this difference, the two disorders share many characteristics and behavioral similarities which are suggestive of some of their commonly associated features. [69]
In their study on the link between FXS and autism, Hagerman et al., (2010), [70] state that FXS is the most common single gene cause of autism, accounting for 2% to 6% of all autism cases. Based on the criteria of the Autism Diagnostic Observation Scale (ADOS) and the Autism Diagnostic Interview (ADI-R), on average 25-30% of males with FXS have complete autism, furthermore another 30% of boys present with a pervasive developmental disorder and the remaining FXS patients have at least one autistic characteristic, such as “poor eye contact and tactile defensiveness.”
It is clinically advised that all individuals diagnosed with an autism spectrum disorder should carry out the FX DNA test (involving both the Southern blot and PCR) when the etiology of their autism is ambiguous. [70]
Fragile X Syndrome and the Amygdala
Current knowledge of the cellular and molecular mechanisms underlying the symptoms of Fragile-X Syndrome is mainly centered on the studies of the hippocampus and cortex. However, it is known that this syndrome is also associated with strong emotional symptoms. These emotional symptoms are likely to involve changes in the amygdala. Unfortunately, the synaptic basis of amygdala dysfunction in Fragile X Syndrome has yet to be explored. [71]
A study conducted by Suvrathan and Chattarji, (2011),[71] describes recent findings from mouse models of Fragile X Syndrome. These findings have identified synaptic defects in the basolateral amygdale that are in many ways different from those found earlier in the hippocampus. This studies’ main findings reveal that “long-term potentiation and surface expression of AMPA-receptors are impaired. Further, presynaptic defects are seen at both excitatory and inhibitory synapses. Remarkably, some of these synaptic defects in the amygdala are also amenable to pharmacological rescue.” [71] Thus, future research in this area is of great importance.
These results also highlight the importance of modifying the current hippocampus-centric framework in order to more accurately explain Fragile X Syndrome-related synaptic dysfunction in the amygdala.
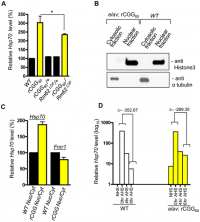
Nuclear Accumulation of Stress Response mRNAs Contributes to the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
Fragile X premutation carriers present with a neurodegenerative disorder known as Fragile X–associated tremor/ ataxia syndrome (FXTAS). Past studies identified that rCGG repeats in Fragile X are enough to cause neurodegeneration and that Pur α and hnRNP A2/B1 (rCGG repeat-binding proteins) can modulate rCGG–mediated neuronal toxicity. [72]
This study conducted by Qurashi et al., (2011),[72] further explores the role of Pur α in rCGG–mediated neurodegeneration by identifying over 100 proteins that interact with Pur α, focusing on Rm62 which could modulate neurodegeneration mediated by rCGG. This study shows that “rCGG repeats decreased the expression of Rm62 post-transcriptionally, leading to the nuclear accumulation of Hsp70 transcript, as well as additional mRNA's involved in stress and immune responses.” These findings are a significant part of Fragile X recent research as they suggest that the abnormal accumulation of these mRNA's in the nucleus (most likely due to impaired nuclear export) could be a contributing factor to the pathogenesis of FXTAS.
Related Links
- Abnormal Development - Fragile X - This page describes Fragile X Syndrome as an example of abnormal development. It includes an introduction, some recent findings, symptoms, the FMR protein responsible for Fragile X, diagnostic testing and www links.
- Abnormal Genetic Development - This page provides a good understanding of how genetic abnormal development occurs; especially in regards to it’s relationship with age, ethnicity, inheritance and prenatal testing.
- Abnormal Neural Development - This page is related to our group page. It describes examples of abnormalities associated with the nervous system which are congenital and environmentally derived. Two such examples which are of interest to us are Fragile X Syndrome and Autism.
Glossary
Allele: is a form of gene or genetic locus when in a group of genes. These alleles result in different observable traits such as eye colour.
Amygdala: almond-shaped mass of grey matter located in the anterior part of the temporal lobe of the cerebellum. It is part of the limbic system and plays an important role in emotional behavior and motivation.
Asymptomatic: if a patient is a carrier for a disease or infection but experiences no symptoms.
Ataxia: is a neurological sign and symptom that consists of a huge lack of coordination of muscle movements.
Atrophy: the partial or complete wasting away of a part of the body.
Caudate Nucleus: a nucleus in the basal ganglia which plays a role in learning and memory.
Chromatin: the combination of DNA and proteins that make up the contents of the nucleus of a cell.
Congenital disorder: a condition existing at birth or often before birth or one that develops during the first month of life.
Cortex: the outer layer of the cerebellum (i.e. cerebral cortex) while plays a key role in consciousness.
Cytogenetically: the branch of biology that deals with heredity and the cellular components, particularly chromosomes, associated with heredity.
Evincing: be evidence of; indicate.
Expression: the production of a polypeptide as a result of gene transcription and translation.
Hernia: the protrusion of an organ or the fascia of an organ through the wall of the cavity that normally contains it.
Hippocampus: component of the human brain located on the floor of each lateral ventricle. It is part of the limbic system and is thought to have a central role in emotion and formation of memories.
Immunocytochemical: common laboratory technique that uses antibodies that target specific peptides or protein antigens in the cell via specific epitopes.
Macrocephaly: a condition characterised by a head of abnormally large proportions, including those of the scalp, cranium and its contents.
Macroorchidism: abnormally large testes.
Metaphase: stage of mitosis in the eukaryotic cell cycle (where the chromosomes separate into two identical sets in two separate nuclei) in which condensed & highly coiled chromosomes, carrying genetic information, align in the middle of the cell before being separated into each of the two daughter cells.
Methylation: chemical science of the addition of a methyl group to a substrate or the substitution of an atom or group by a methyl group.
Microcephaly: neuro-developmental disorder in which the circumference of the head is more than two standard deviations smaller than average for the person's age and sex.
Microphthalmia: is a developmental disorder of the eye characterised by small eye/s.
mRNA: messenger RNA, the key transition in gene expression, translating the DNA's genetic code into the amino acids that make up proteins.
Neurodegeneration: the progressive loss of function/ structure or death of neurons within the nervous system.
Neurotoxin: a toxin or poisonous substance that acts specifically on nerve cells (neurons).
Nucleotide triplet: is a genetic code that is a set of rules by which information encoded in genetic material (DNA or mRNA sequences) is translated into proteins; in this case it is CGG (cytosine guanine guanine).
Perseveration: is the repetition of a particular movement, activity or response despite the cessation of a stimulus.
Phenotypic abnormality: a deviation from the normal or differing from the typical, in regards to the observable characteristics or traits of the organism.
Prolapse: literally means “to fall out” and is a condition where organs, such as the uterus, fall down or slip out of place.
Psychopedagogic: combination of two main branches of study; pedagogy and psychology. Pedagogy is the study of the process of teaching and psychology is the science of behaviour and mental processes.
Sign: objective evidence of the presence of a disease or disorder, as opposed to a symptom, which is subjective.
Silencing: gene silencing describes the "switching off" of a gene by a mechanism other than genetic modification. That is, a gene which would usually be expressed (turned on) under normal circumstances but is switched off by machinery in the cell.
Symptom: a departure from normal function or feeling which is noticed by a patient, indicating the presence of disease or abnormality.
Synaptic: relating to a synapse- a junction between two nerve cells. This junction permits a neuron to transmit a chemical/ electrical signal to another cell.
Synaptic plasticity: is the ability of the connection (synapse) between two neurons to change in strength in response to either use or disuse of transmission over synaptic pathways.
Thalamus: a centrally located structure in the brain whose function includes relaying sensation, spatial sense, and motor signals to the cerebral cortex, along with the regulation of consciousness, sleep, and alertness.
Transcription: the process by which a segment of genome is read by various proteins in order to produce an mRNA strand complementary to the genome, which is then translated by ribosomes.
Transcription Factors: proteins, molecules and/or ions that assist in facilitating transcription.
Translation: the process by which mRNA produced from transcription is converted by ribosomes into polypeptides.
References
- ↑ Kumari D, Somma V, Nakamura AJ, Bonner WM, D'Ambrosio E & Usdin K. (2009). The role of DNA damage response pathways in chromosome fragility in Fragile X syndrome. Nucleic Acids Res. , 37, 4385-92. PMID: 19465392 DOI.
- ↑ 2.0 2.1 Ridaura-Ruiz L, Quinteros-Borgarello M, Berini-Aytés L & Gay-Escoda C. (2009). Fragile X-syndrome: literature review and report of two cases. Med Oral Patol Oral Cir Bucal , 14, e434-9. PMID: 19718005
- ↑ 3.0 3.1 3.2 Hagerman RJ, McBogg P & Hagerman PJ. (1983). The fragile X syndrome: history, diagnosis, and treatment. J Dev Behav Pediatr , 4, 122-30. PMID: 6348096
- ↑ http://www.fragilex.org/html/history.htm
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 <pubmed>7541938</pubmed>
- ↑ 6.0 6.1 Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B & Hagerman PJ. (2001). Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology , 57, 127-30. PMID: 11445641
- ↑ Leehey MA. (2009). Fragile X-associated tremor/ataxia syndrome: clinical phenotype, diagnosis, and treatment. J. Investig. Med. , 57, 830-6. PMID: 19574929 DOI.
- ↑ Loesch DZ, Sherwell S, Kinsella G, Tassone F, Taylor A, Amor D, Sung S & Evans A. (2012). Fragile X-associated tremor/ataxia phenotype in a male carrier of unmethylated full mutation in the FMR1 gene. Clin. Genet. , 82, 88-92. PMID: 21476992 DOI.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Gallagher A & Hallahan B. (2012). Fragile X-associated disorders: a clinical overview. J. Neurol. , 259, 401-13. PMID: 21748281 DOI.
- ↑ 10.0 10.1 10.2 10.3 10.4 Crawford DC, Acuña JM & Sherman SL. (2001). FMR1 and the fragile X syndrome: human genome epidemiology review. Genet. Med. , 3, 359-71. PMID: 11545690 DOI.
- ↑ Dutta S, Das M, Bhowmik AD, Sinha S, Chattopadhyay A & Mukhopadhyay K. (2009). Screening of rural children in West Bengal for fragile-X syndrome. Indian J. Med. Res. , 130, 714-9. PMID: 20090132
- ↑ 12.0 12.1 12.2 12.3 12.4 Hantash FM, Goos DM, Crossley B, Anderson B, Zhang K, Sun W & Strom CM. (2011). FMR1 premutation carrier frequency in patients undergoing routine population-based carrier screening: insights into the prevalence of fragile X syndrome, fragile X-associated tremor/ataxia syndrome, and fragile X-associated primary ovarian insufficiency in the United States. Genet. Med. , 13, 39-45. PMID: 21116185 DOI.
- ↑ 13.00 13.01 13.02 13.03 13.04 13.05 13.06 13.07 13.08 13.09 13.10 <pubmed>19117905</pubmed>
- ↑ http://www.fragilex.org/html/premutation.htm
- ↑ Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, Hudson R, Gorwill H, Nolin SL, Glicksman A, Jenkins EC, Brown WT, Howard-Peebles PN, Becchi C, Cummings E, Fallon L, Seitz S, Black SH, Vianna-Morgante AM, Costa SS, Otto PA, Mingroni-Netto RC, Murray A, Webb J & Vieri F. (1999). Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study--preliminary data. Am. J. Med. Genet. , 83, 322-5. PMID: 10208170
- ↑ Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R, Berry-Kravis E, Brown WT, Schaeffer S, Kissel J, Tassone F & Hagerman PJ. (2003). Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am. J. Hum. Genet. , 72, 869-78. PMID: 12638084 DOI.
- ↑ Penagarikano O, Mulle JG & Warren ST. (2007). The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet , 8, 109-29. PMID: 17477822 DOI.
- ↑ http://ghr.nlm.nih.gov/condition/fragile-x-syndrome
- ↑ O'Donnell WT & Warren ST. (2002). A decade of molecular studies of fragile X syndrome. Annu. Rev. Neurosci. , 25, 315-38. PMID: 12052912 DOI.
- ↑ Gao FB. (2008). Posttranscriptional control of neuronal development by microRNA networks. Trends Neurosci. , 31, 20-6. PMID: 18054394 DOI.
- ↑ Devys D, Lutz Y, Rouyer N, Bellocq JP & Mandel JL. (1993). The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat. Genet. , 4, 335-40. PMID: 8401578 DOI.
- ↑ Abitbol M, Menini C, Delezoide AL, Rhyner T, Vekemans M & Mallet J. (1993). Nucleus basalis magnocellularis and hippocampus are the major sites of FMR-1 expression in the human fetal brain. Nat. Genet. , 4, 147-53. PMID: 8348153 DOI.
- ↑ Caudy AA, Myers M, Hannon GJ & Hammond SM. (2002). Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. , 16, 2491-6. PMID: 12368260 DOI.
- ↑ Ishizuka A, Siomi MC & Siomi H. (2002). A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev. , 16, 2497-508. PMID: 12368261 DOI.
- ↑ Barbee SA, Estes PS, Cziko AM, Hillebrand J, Luedeman RA, Coller JM, Johnson N, Howlett IC, Geng C, Ueda R, Brand AH, Newbury SF, Wilhelm JE, Levine RB, Nakamura A, Parker R & Ramaswami M. (2006). Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron , 52, 997-1009. PMID: 17178403 DOI.
- ↑ http://www.nichd.nih.gov/publications/pubs/fragileX/sub2.cfm
- ↑ <pubmed>12058838</pubmed>
- ↑ <pubmed>11459752</pubmed>
- ↑ <pubmed>15475576</pubmed>
- ↑ <pubmed>14523380</pubmed>
- ↑ <pubmed>18472033</pubmed>
- ↑ <pubmed> 16754531</pubmed>
- ↑ <pubmed> 20439717</pubmed>
- ↑ <pubmed> 21802660</pubmed>
- ↑ <pubmed>6348096</pubmed>
- ↑ 36.0 36.1 <pubmed>3953647</pubmed>
- ↑ <pubmed>18472033</pubmed>
- ↑ <pubmed>9259278</pubmed>
- ↑ <pubmed>9421410</pubmed>
- ↑ <pubmed>17548835</pubmed>
- ↑ 41.0 41.1 <pubmed>2018070</pubmed>
- ↑ 42.0 42.1 <pubmed>2195034</pubmed>
- ↑ <pubmed>8464655</pubmed>
- ↑ <pubmed>11773805</pubmed>
- ↑ 45.0 45.1 <pubmed>18778781</pubmed>
- ↑ <pubmed>18347972</pubmed>
- ↑ 47.0 47.1 <pubmed>19114290</pubmed>
- ↑ 48.0 48.1 48.2 48.3 <pubmed>20548240</pubmed>
- ↑ 49.0 49.1 <pubmed>19804849</pubmed>
- ↑ 50.0 50.1 50.2 50.3 50.4 <pubmed>19099346</pubmed>
- ↑ <pubmed> 1878973</pubmed>
- ↑ <pubmed> 10464640</pubmed>
- ↑ <pubmed> 7723547</pubmed>
- ↑ <pubmed> 15257661</pubmed>
- ↑ <pubmed> 10865102</pubmed>
- ↑ <pubmed> 3052064</pubmed>
- ↑ <pubmed>15756514</pubmed>
- ↑ <pubmed> 7860456</pubmed>
- ↑ <pubmed>14994287</pubmed>
- ↑ <pubmed>11181100</pubmed>
- ↑ <pubmed>10448821</pubmed>
- ↑ <pubmed>15219735</pubmed>
- ↑ <pubmed>16098137</pubmed>
- ↑ <pubmed>18093519</pubmed>
- ↑ <pubmed>12548736</pubmed>
- ↑ <pubmed>11886017</pubmed>
- ↑ <pubmed>11694672</pubmed>
- ↑ <pubmed>18929056</pubmed>
- ↑ <pubmed>17001341</pubmed>
- ↑ 70.0 70.1 <pubmed>20858229</pubmed>
- ↑ 71.0 71.1 71.2 <pubmed>21555214</pubmed>
- ↑ 72.0 72.1 <pubmed>21655086</pubmed>
2011 Projects: Turner Syndrome | DiGeorge Syndrome | Klinefelter's Syndrome | Huntington's Disease | Fragile X Syndrome | Tetralogy of Fallot | Angelman Syndrome | Friedreich's Ataxia | Williams-Beuren Syndrome | Duchenne Muscular Dystrolphy | Cleft Palate and Lip