2009 Group Project 2
FLY
Drosophila Melanogaster
Drosophila melanogaster, the common fruit fly, plays a major role in embryological and genetic research. There are a number of factors which make drosophila a very useful tool for genetic research. These factors include their size, short lifespan, their short generation time, and high reproduction rate (females can lay upwards of 100 eggs a day). They are cost effective for schools and universities, allowing easy replication of studies for on going years, and large populations mean statistical analysis can be reliable, and easy to obtain. The complete genome was sequenced and published in 2000 by Adams et al.
The object of this page is to examine the embryological development of Drosophila. However, the embryological development of Drosophila is just the beginning, as this development occurs within the female parent fly before birthing. Post-natal development has two separate periods in which the embryo develops to become a fully grown adult fly. These periods are the larval stage, and the pupal stage. In these stages the embryo goes through a metamorphosis changing its external appearance, and growing appendages such as wings, legs, and antennae, becoming a fully functional adult fly.
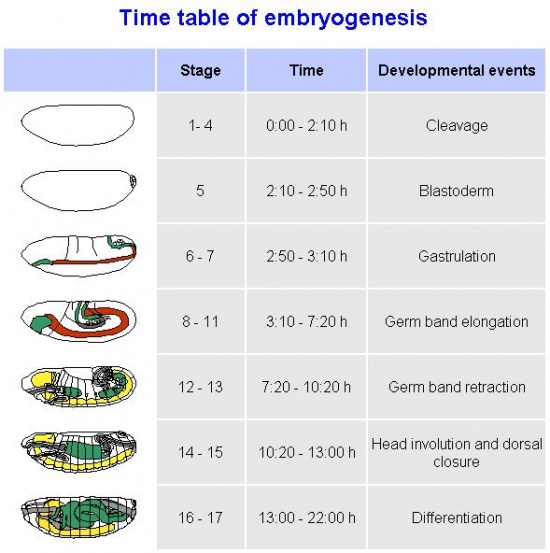
The time line of Drosophila development will be discussed, as will the stages of development, as adapted from the Bowne's stages by Campos-Ortega and Hartenstein . The genetics of the fly will be examined, as will the history of the use of Drosophila as an embryological model. The future direction of the use of Drosophila as an embryological model will also be looked at.
Timeline of Drosophila Development
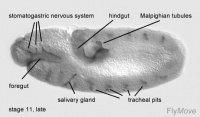
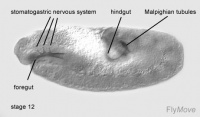
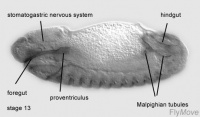
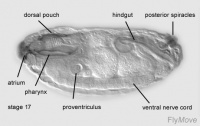
Development of the foregut
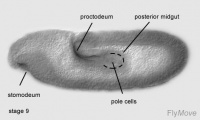
Stage 9 – a layer of basophilic cells cylindrical in shape forms from the primordium of the stomodeum.
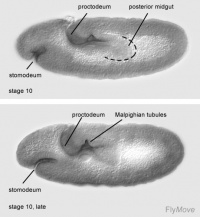
Stage 10- cells invaginate. Cells undergo mitotic division. The oesophagus and distal pharynx is derived from these cells.
Stage 13- stomodeum continues to develop caudally
Stage 13- 15 – promixal pharynx floor and pharyngeal arch becomes incorporated into the foregut.
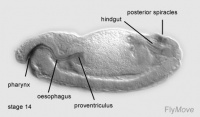
Stage 14-17- median tooth, mouth hooks and maxillary cirri develops
Development of midgut
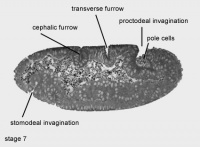
Stage 7- endodermal part of anterior midgut primordium invaginates ventrally.
Stage 9- second mitotic division occurs for all cells in the midgut primordium.
Stage 10- cells of anterior midgut attaches to stomodeum.
Stage 15- 16- midgut constrictions appear.
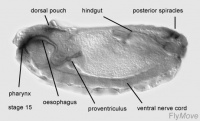
Stage 16-17- proventriculus arises from stomodeal cells.
Stage 17- the gastric valve is formed.
Development of the hindgut
Stage 8- first mitotic division occurs in the hindgut primordium.
Stage 10- the first phase of germ band extension is completed. Distinctive regions of the hindgut can be seen. A transversal depression forms the Malpighian tubules.
Stage 12- the hindgut changes its shape due to germ band shortening. The hindgut is brought to the caudal end and is quickly bent.
Development of somatic and visceral musculature
Stage 8- germ band extension commences. Cells inside the mesodermal tube undergoes first postblastodermal mitosis.
Stage 9- mesodermal cells becomes cuboidal in shape and forms a monostratified epithelium. These cells lies along the germ band in the ectoderm. The germ band at this stage consists of consistent bulges.
Stage 10- cells in the mesoderm undergoes third postblastodermal mitosis. Affects all the cells within the germ layer.
Stage 12- germ band shortening happens. Cells in the splanchnopleura and cells from the somatopleura separates from each other. Cells in splanchopleura and somtopleura adjusts themselves into an epithelium of tightly packed slim cells. These cells then comes into contact with the anterior and posterior midgut. Visceral musculature is developed from splanchopleura cells.
Stage 13- 15 – single somatic mesodermal cells fuses to form syncytial cells. Formation of muscle occurs due to this process.
Stage 16- final pattern of somatic musculature is developed.
Stages of Drosophila Embryological Development
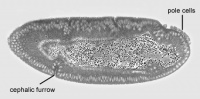
The stages of Drosophila embryological development are listed below. The larval and pupal stages in post embryologic development are separate to these, and are mentioned in the timeline of development. The stages listed below are adapted from Bownes stages (1975) by Ortega and Hartenstein (1985), as there were some dissimilarities and inconsistencies observed in Bownes stages. The following times given for the stages are an average recorded room temperature (25°C).
History of Drosophila Embryological Model Use
- In 1900, Ectomologist Charles W. Woodworth was the first to breed the Drosophila at Harvard university and suggested to W.E. Castle they could be used in studies of genetics.
- In 1908 T.H Morgan, an American geneticist and embryologist, was looking for an inexpensive that could be breed quickly and in limited space and Castle suggested the drosophila. Through Morgan’s studies of heredity he discovered the white-eyed mutation in the drosophila.
- By 1910, at Columbia University T.H Morgan and his students work on the top floor of the Schermerhorn Hall and t became known as the fly room. Students of the Fly Room were A.H. Sturtevant, C.B Bridges and H.J. Muller.
- In 1913 Sturtevant published a paper with the first genetic map and clearly laid out the logic for genetic mapping.
- In 1927 Muller ionized radiation and found it caused genetic damage.
- 1930’s feasability of generating deficiences and duplications were exploited in 1970 to generate duplicates and initiating whole-genome scanning.
- In 1933 Morgan won the Nobel Prize for his discovery that genes are carried on chromosomes and are the mechanical basis of hereditary.
- Also in 1933 Heitz and Bauer fly Bibio hortulanus studies discovered salivary gland chromosomes.
- 1934 saw the 1st published drawings of Drosophila melanogaster polytene chromosomes by T.S painter.
- In 1935 and 1938 Bridges publish polytene maps that are still used today.
- In 1946 Muller received the Nobel prize for his work, showing mutation induced by x-rays.
- In, 1971, ‘Clock mutants of Drosophila Melanogaster’ was published by Ron Konopka and Seymour Benzer. This paper described the first mutations that affected an animal’s behaviour.
- In 1972 D.S. Hogness of Stanford Universtiy put in a grant application for modern genome reseach and in 1974 he made the 1st random clone of any organism.
- In 1975, clone libraries representing the entire genome had been generated and screened for clones carrying specific sequences.
- In 1980 C. Nusseiln-Volhard and E. Wieschaus attempt to identify all genes involved fundamental processes leading to the discovery of major signalling pathways. In 1995 they shared a Nobel Prize for their work.
- In 1981 a breakthrough of first rescue of a mutant phenotype in an animal by gene transfer. This led to the use of enhancer traps to screen for genes based on pattern of expression.
- In 1987, enhancer traps were developed, making it possible to screen for genes based on their pattern of expression
- In 1990, Milicki, J et al introduced a mouse gene into a Drosophila embryo, establishing that, in animals that have been evolving independently for hundreds of millions of years, genes will generate products that function interchangeably.
- In 1992, was the first year the Drosophila embryos could be frozen for future use.
- In 2000 the Drosophila melanogaster genome was published by Adams MD, et al.
Genetics of Drosophila
The Drosophila melanogaster genome was fully sequenced in the year 2000. It genomic size is relatively small as it is a simple organism with few complex genes needed to be expressed. The simplicity of its genome allows researchers to pinpoint exact gene locations and discrepancies with relative ease. This ability gives it an advantage in not only reproducing quickly and making fewer mistakes, but also allowing us to trial new gene expressions in its genome.
The size of the Drosophila genome is about 165 million pairs and estimated to contain about 14000 genes. In comparison, humans have 3.4 billion base pairs with about 22500 gene sequences and yeast has about 5800 genes in 13.5 million base pairs.
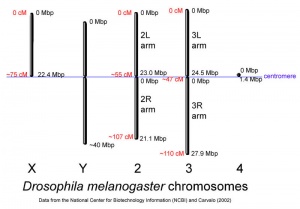
The Drosophila melanogaster fly has four pairs of chromosomes: the X/Y sex cells and the autosomes 2, 3 and 4. The fourth chromosome is so small that it is usually overlooked. The comparison of the insignificant 4th chromosome to the other three pairs are shown in the image to the right. Also, according to research done by T.H.Morgan et al. in his book The Genetics of Drosophila the Y sex chromosome has no factors that affect the influence of recessive factors carried by the X chromosomes. In other words, the Y chromosomes has no effect on the sex of the Drosophila fly, and it is unaccounted for in gene expression.
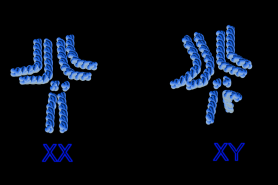
Drosophila, like all other insects, is a gynandromorph. This means that some of their anatomy is male and some female. This results from an X chromosome being lost from one embryonic nucleus. Insects do not possess sex hormones to regulate these actions and so each cell "decides" to be male or female. Thus, the sex chromosomes become XO (male) not XY (male) or XX (female) as seen in most other organisms. XX cells display female characteristics while the XO cells display male characteristics.
As you can see in the blue image to the right, depicting the female and male chromosome maps, the Y sex chromosome is bent almost in half. This bending restricts the effect the chromosome has on gene expression and thus the Y chromosome is usually ignored.
Although there are many varieties of the Drosophila fly, only the melanogaster has been shown here.
Current Embryology Research on Drosophila
In the past Drosophila had many different avenues for which it was studied, however the majority of studies focused mainly on genetics. After the genome was sequenced in 2000, studies moved towards different areas and the majority of studies are now in developmental biology and embryology development.
Genetics of Postnatal Cardiac Function.
Wolfe et al. (2008), Drosophila melanogaster as a model system for genetics of postnatal cardiac function. PMID 19802348
Most of the genetic pathways studied in the drosophila melanogaster for heart conditions are in development of the heart. Recent research by MJ Wolf and HA Rockman focus on structure and function of the adult heart to hopefully try and understand human heart failure more. In the Wolf experiment they looked at the adult drosophila melanogaster for genes and pathways which cause dilated cardiomyopathy.
Drosophila and Parkinson's disease
Cox RT, Spradling AC. (2009) Clueless, a conserved Drosophila gene required for mitochondrial subcellular localization, interacts genetically with parkin Disease Models and Mechanisms, 2(9-10); 490-499 PMID 19638420
Experiments conducted by Cox et. al. have located a gene in Drosophila, called the clueless gene (clu), which encodes a protein that is related to proteins in humans. It has been found that clu mutations affects the clustering of mitochondria within the cell. These mutations resemble the mutations scene in human cells of sufferers with Parkinson's disease. This discovery provides a basis for the further analysis of similar genes in humans.
The evolution of visual systems
Erclik T, Hartenstein V, McInnes RR, Lipshitz HD (2009) Eye evolution at high resolution: the neuron as a unit of homology. Developmental Biology, 332(1); 70-79 PMID 19467226
Erclik et. al. have identified similarities in the neuronal cell types of the visual systems of vertebrates and Drosophila. This identification provides a basis for the study of the evolution of complex visual systems, as the origin of such a system is more easily traced back down the evolutionary tree. This could potentially provide a basis for the future treatments of genetic damage or physical traumas to the eye, as retinas could potentially be grown from derivatives of Drosophila visual neuronal cells.
Alzheimer's model of disease from Drosophila
Crowther DC, Page R, Chandraratna D, Lomas DA (2006) A Drosophila model of Alzheimer's disease. Methods in Enzymology, 412; 243-255 PMID 17046662
One of the underlying factors that induce the onset of Alzheimer's in humans is the excess production of proaggregatory peptides. A secretion signal peptide has been identified by Crowther et. al. in Drosophila that controls the synthesis of this peptide. The identification of such a gene means that researchers can manipulate different pathological pathways to observe the phenotypes produced. This means that the progression of the disease in Drosophila can help us to understand the progression of the disease in human patients suffering from Alzheimer's disease.
The use of Drosophila as a model for the development of human traits
Mackay TFC, Annholt RRH (2006) Of Flies and Man: Drosophila as a Model for Human Complex Traits Annual Review of Genomics and Human Genetics, 7;339-367 PMID 1675648
There are a number of genes that are shared between Drosophila and humans. The phenotypes of variations in a variety of genes of Drosophila are easily observed, and as a result of the closeness of some genes, it is relatively easy to observe the effect of a variety of factors on the expression of some genes. Mackay et. al. are examining how genetic mechanisms affect the expression of some genes in humans producing defined phenotypic traits.
Using the Drosophila genome and colon cancer
Firestein R, Hahn WC (2009) "Revving the throttle on an oncogene: CDK8 takes the driver seat." Cancer research, Oct 6. PMID 19808961
This study establishes that the WNT/beta catenin as a key initiator in colon cancers. The researchers in this study have used Drosophila models of WNT/beta catenin activation of transcription in the nucleus to establish that CDK8 is a key oncogene, regulating WNT/beta catenin transcription in colon cancers. In this study, the similarity of the drosophila and human methods of transcription activation have been used to the researchers advantage, resulting in this critical finding. This discovery of the role of CDK8 in the regulation of transcription means that new therapeutic approaches to the treatment of colon cancers can be developed.
Drosophila and neurodegenerative diseases
http://www.sciencedaily.com/releases/2009/06/090622171501.htm#
Fruit flies have been used to study neurodegenerative diseases in humans. A genetically modified fruit fly was created by the scientists from The Scripps Research Insitute, Katholeike University Leuven, and the University of Antwerp, Belgium. This genetically modified fly has features found in a common neurodegenerative disease in humans called the Charcot- Marie- Tooth disease. This inherited neurodegenerative disease occurs in 1 out of every 2500 people in the United States.
Studies have shown that suffers of the Charcot-Marie-Tooth disease all have mutations in the genes that constructs an important protein called tyrosyl-tRNA synthetase. Causing inefficiency in the functioning of this protein. Ribosomes uses tRNA to bring corresponding amino acids to make proteins. tRNA synthetase are basically messengers that bring tRNA in contact to amino acids as it attaches tRNA to the amino acids.
The question that scientists were debating in regards to the disease is whether symptoms are present because the mutation in the gene prevents the normal functioning of tRNA synthetase, inhibiting the formation of certain proteins from genes or whether the symptoms caused were due to another function of the protein.
Scientists then discovered that the symptoms of this disease is not caused by the obstruction of synthetase’s role in protein synthesis from genes. They used the fruit fly and mutated the equivalent protein to the protein in humans. The fruit fly presented symptoms similar to the symptoms that occurred in humans that had the disease. This shows that the disease is not due to tyrosyl-tRNA synthease’ inability to make proteins. The disease is caused by another function of the protein which affects the proper development of the nervous system.
This other function is not known yet but further research is being done to find out. Further research on these genetically modified fruit flies enables scientists to hopefully discover how the neurodegenerative disease develops and possibly the treatment for it.
Helpful links
http://flybase.bio.indiana.edu/ This site is a database for anything Drosophila related. It contains an exceptional image and movie catalogue which can be very helpful.
http://www.sdbonline.org/fly/aimain/1aahome.htm The Interactive Fly has a lot of information of the embryogenesis, pupal development and larval devlopment of Drosophila
http://flymove.uni-muenster.de/Homepage.html Flymove is a very easy to use database about all things drosophila. The main focus is on the stages, with some very explanatory movies.
http://en.wikipedia.org/wiki/Drosophila_melanogaster This wiki page provides a very easy to read and understand, albeit basic explanation of Drosophila.
http://www.ncbi.nlm.nih.gov/sites/gquery?itool=toolbar&cmd=search&term=DROSOPHILA+EMBRYOLOGY This is a database search of PUBMED providing useful links to drosophila embryology related papers.
http://www.google.com.au/search?q=Timeline+of+Drosophila+Development&hl=en&sa=G&tbs=tl:1&tbo=u&ei=S9e6SrPrK8SAkQWdgr2cDQ&oi=timeline_result&ct=title&resnum=11 This is a helpful link in giving an in depth timeline of the drosophila model, its history of use and research papers throughout the last century.
References
1. Ashburner M, Bergman CM (2005) Drosophila melanogaster: A case study of a model genomic sequence and its consequences. Genome Research, 15(12); 1661-1667 PMID 16339363
2. Adams MD, et al. (2000). The genome sequence of Drosophila melanogaste. Science, 287(5461): 2185–95. PMID 10731132
3. Campos-Ortega, Hartenstein (1985) The Embryonic Development of Drosophila melanogaster. Springer-Verlag, Berlin Heidelberg. pp9-84
4. Crowther DC, Page R, Chandraratna D, Lomas DA (2006) A Drosophila model of Alzheimer's disease. Methods in Enzymology, 412; 243-255 PMID 17046662
5. Cox RT, Spradling AC. (2009) Clueless, a conserved Drosophila gene required for mitochondrial subcellular localization, interacts genetically with parkin Disease Models and Mechanisms, 2(9-10); 490-499 PMID 19638420
6. Erclik T, Hartenstein V, McInnes RR, Lipshitz HD (2009) Eye evolution at high resolution: the neuron as a unit of homology. Developmental Biology, 332(1); 70-79 PMID 19467226
7. Firestein R, Hahn WC (2009) "Revving the throttle on an oncogene: CDK8 takes the driver seat." Cancer research, Oct 6. PMID 19808961
8. Konopka RJ, Benzer S. (1971) Clock mutants of Drosophila melanogaster.PMID 5002428
9. Mackay TFC, Annholt RRH (2006) Of Flies and Man: Drosophila as a Model for Human Complex Traits Annual Review of Genomics and Human Genetics, 7;339-367 PMID 16756480
10. Morgan TH, Bridges CB, Sturtevant AH (1919), The Genetics of Drosophila Gaarland Publications, New York.
11. Rubin GM, Lewis EB (2000) A brief history of Drosophila's contributions to genome research. Science, 287 (5461); 2216-2218 PMID 10731135
12. Sturtevant AH (1913), The linear arrangement of six sex-linked factors in Drosophila, as shown by their mode of association. Journal of Experimental Zoology, 14: 43-59.
13. Weigmann K, Klapper R, Strasser T, Rickert C, Technau G, Jäckle H, Janning W, and Klämbt C: FlyMove – a new way to look at development of Drosophila.Trends Genet. In press. http://flymove.uni-muenster.de
14. Wolfe et al. (2008), Drosophila melanogaster as a model system for genetics of postnatal cardiac function. PMID 19802348
15. http://www.sciencedaily.com/releases/2009/06/090622171501.htm#
Glossary
Amnioproctodeal Invagination - The posterior ectodermal invagination, giving rise to portion of the digestive system
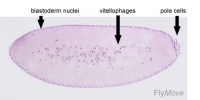
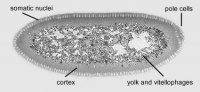
Blastoderm - The layer of cells inside of the blastula which forms the embryo.
Gynandromorphs - Animals in which certain regions of the body are male and other regions are female.
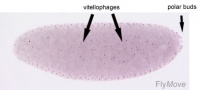
Polar bud - Cells of the posterior pole giving rise to germ cells
ANAT2341 group projects
Project 1 - Rabbit | Project 2 - Fly | Project 3 - Zebrafish | Group Project 4 - Mouse | Project 5 - Frog | Students Page | Animal Development