RG Test01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 112: | Line 112: | ||
|} | |} | ||
===Glands=== | |||
{| style="width:75%" border="1" align="center" style="text-align:left" | |||
|- | |||
! Gland Type !! Description !! Image | |||
|- | |||
| '''Sebaceous Glands''' || Sebaceous glands develop from the epithelial wall of the hair follicle. They secrete the '''vernix caseosa''' | |||
Vernix caseosa is a material secreted by sebaceous glands in the foetus in the last trimester of development (Week 21)<ref name="PMID15830002"><pubmed>15830002</pubmed></ref> and is characterised by it’s cheese-like appearance around the neonate at birth. The functions of vernix caseosa include: | |||
*thermal regulation <ref name="PMID15830002"><pubmed>15830002</pubmed></ref> | |||
*barrier to water loss (to keep fetal skin hydrated)<ref name="PMID21504444"><pubmed>21504444</pubmed></ref><ref name="PMID15830002"><pubmed>15830002</pubmed></ref> | |||
*prevents the epidermis from water contact while epidermal cornification and formation of the stratum corneum occurs<ref name="PMID21504444"><pubmed>21504444</pubmed></ref> | |||
*antioxidant<ref name="PMID19881987"><pubmed>19881987</pubmed></ref> | |||
*anti-infective<ref name="PMID19881987"><pubmed>19881987</pubmed></ref> | |||
*moisturises the skin<ref name="PMID19881987"><pubmed>19881987</pubmed></ref> | |||
*assists in wound-healing<ref name="PMID19881987"><pubmed>19881987</pubmed></ref> | |||
|| [[Image:Newborn - vernix caseosa.jpg|frame|center|middle|250x187px|Vernix caseosa on a neonate.<ref>Image source: JazlynRoseVernixByPhilKonstantin.jpg http://en.wikipedia.org/wiki/File:JazlynRoseVernixByPhilKonstantin.jpg</ref>]] | |||
|- | |||
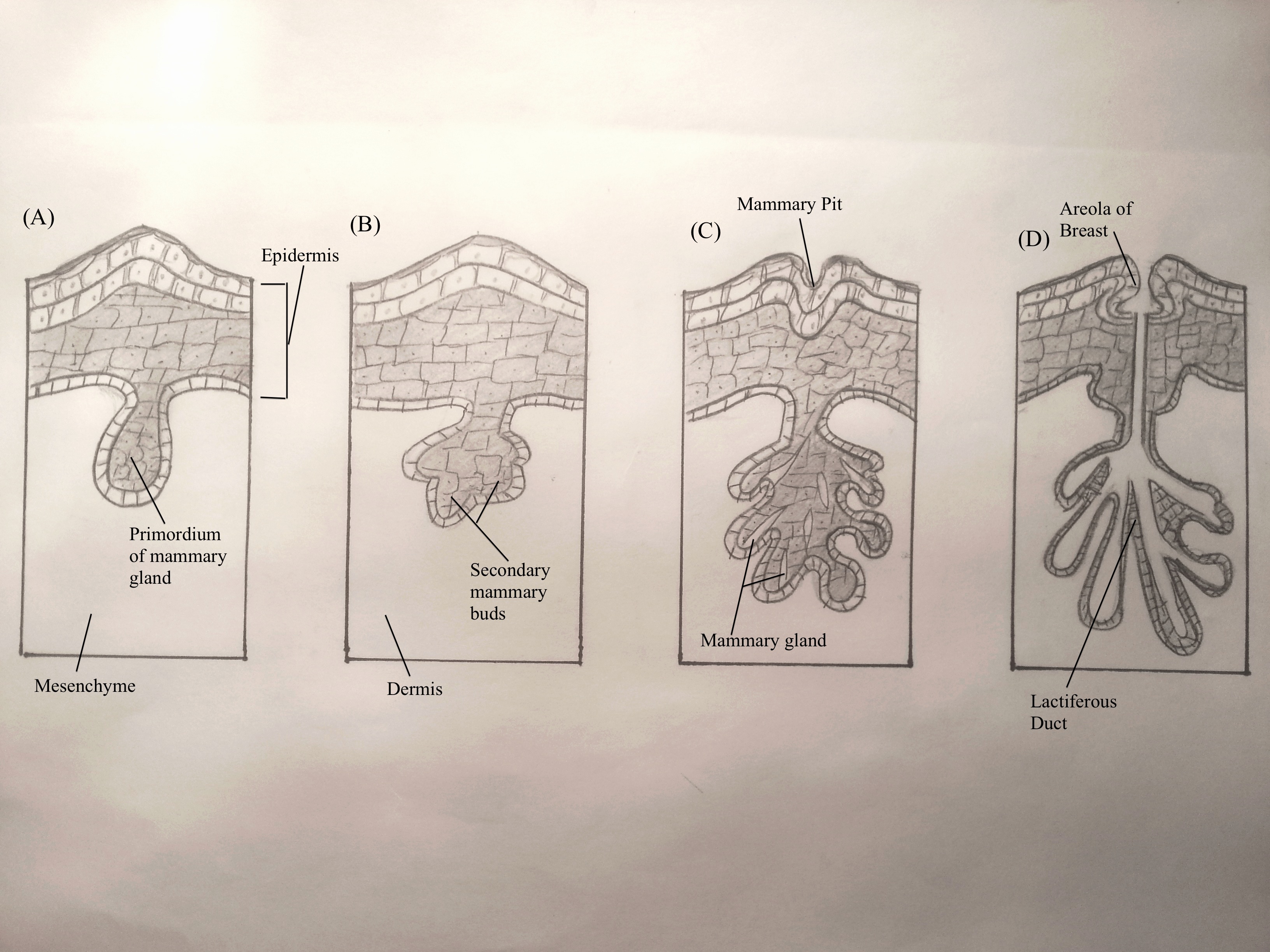
| '''Mammary Glands''' || Mammary glands develop from the mammary ridge- a downgrowth of the epidermis (ectoderm) into the underlying dermis (mesoderm). This occurs at about week 6 of development. Mammary glands first develop into primary mammary buds<ref name="Moore & Persaud"/>, which successively grow in length and complexity. Prior to puberty, the mammary glands are anatomically indistinguishable.<ref name= PMID23720046><pubmed>23720046</pubmed></ref> || [[Image:Hand-drawn_mammary_gland_during_fetal_development.jpg|frame|center|middle|250x187px|Mammary gland development during the fetal stage]] | |||
|- | |||
| '''Sweat Glands''' || | |||
There are two major kinds of sweat glands present in humans, both of which develop from downgrowths of the epidermis into the underlying dermis. Sweat glands have been histologically identified in studies from week 21 of development. They begin as cellular buds<ref name="Moore & Persaud"/>, which proliferate as solid, cylindrical down growths into mesenchyme. Central cells degenerate to form a lumen, while the terminal region coils to eventually form the body of the gland. As fetal development continues, peripheral cells eventually differentiate into secretory and myoepithelial cells<ref name="Moore & Persaud"/>. | |||
Eccrine Sweat Glands | |||
* Located in the skin with distributions throughout most of the body<ref name="Moore & Persaud"/>. | |||
* Function in thermoregulation and excretion of excess electrolytes and water <ref name="Bolognia">Bolognia, J.L., Jorizzo, J.L. & Schaffer J.V. (2012). Dermatology (3rd ed.). Elsevier Limited.</ref>. | |||
* Present at birth with function initiating shortly after birth<ref name="Moore & Persaud"/>. | |||
Apocrine Sweat Glands | |||
*Located in the skin of the axilla, pubic and perianal areas and nipple areolae<ref name="Moore & Persaud"/>. | |||
* May function in a form of olfactory communication <ref name="Bolognia"/>. | |||
* Present at birth with function originating at puberty<ref name="Moore & Persaud"/>. | |||
|| [[Image:Hand-drawn_sweat_gland_development.jpg|frame|center|middle|250x187px|Sweat gland development during the fetal stage]] | |||
|} | |||
Revision as of 14:30, 27 October 2014
| 2014 Student Projects | ||||
|---|---|---|---|---|
| 2014 Student Projects: Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | ||||
| The Group assessment for 2014 will be an online project on Fetal Development of a specific System.
This page is an undergraduate science embryology student and may contain inaccuracies in either description or acknowledgements. | ||||
Integumentary
Introduction
This page concerns the development of the integumentary system in the fetal stage of development, particularly its organs i.e. the skin, glands, hair, teeth, and nails. It explores the mechanism of development as well as the timeline of development. This page also outlines some recent findings on the development of the integumentary system, as well as historic findings. Finally, this page also explores some of the congenital abnormalities of the integumentary system, its mechanism or pathogenesis, clinical manifestations, and how they are treated or managed.
| Objectives |
|---|
|
Development Overview
Skin
The skin consists of 2 layers: the outer layer (epidermis) and a deeper connective tissue layer (dermis)[1].
- The epidermis is derived from the ectoderm. Initially it exists as only a single layer of ectodermal cells at 7-8 days of gestation[2]. However, by about 13-14 weeks after gestation, a 3- layered structure of fetal epidermis exists[3]- consisting of the stratum basale, 1 or 2 intermediate layers and the periderm[4]. The peridermal cells eventually become desquamated and form part of the vernix cervix.
- The somatic mesoderm is the embryonic origin of the dermis. The mesoderm of the dermatones of the body, also contribute to the development of the dermis. Specifically though, in the head and neck region of the body, the dermis is derived from neural crest cells[7].
- The dermis is initially composed of just mesenchymal cells- loosely aggregated mesodermal cells. These mesenchymal cells later develop into fibroblasts- which function to secrete collagen and lay-down elastic fibers into the extracellular matrix[7].
3 other specialised cells of the epidermis also exists[7] - these include melanoblasts, Langherhan cells and Merkel cells.
- Melanoblasts- are derived from neural crest cells that have migrated into the stratum basale. Mid-pregnancy, melanosomes are observed, differentiating the melanoblasts into melanocytes[7].
- Langheran cells- are derived from bone marrow (originally form mesoderm) and migrate into the epidermis[7]. They have the function of antigen presentation.
- Merkel cells- still have an uncertain origin. They have a function related to mechanoreception.
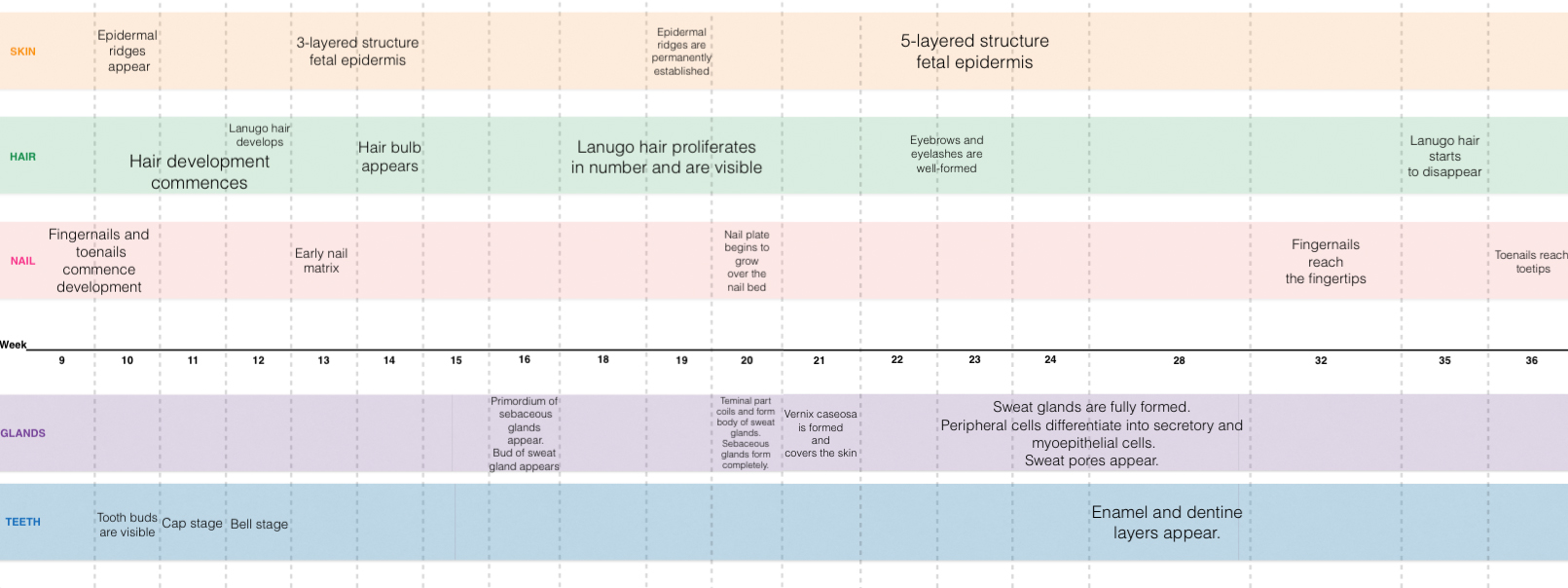
| Week | Description | Phase Diagram |
|---|---|---|
| Weeks 6-8 | In an electron micrograph study of the epidermis[3], the periderm and and basal layer of the developing skin was observed. The basal cell keratins K5 and K14 were also observed from 8 weeks onwards[3] | 
|
| Weeks 7-9 | In an electron micrograph study of the epidermis at weeks 7-9 of development, the stratified three-layer structure of the epidermis was observed[3]; with the stratum intermedium forming between the basal and periderm layers . Kertain filaments, such as K8 and K19 have been encircled- they feature during fetal skin development but are absent in the adult epidermis. | 
|
| Weeks 14-16 | By week 14, the basal layer, the intermediate layer/s and the periderm 3-layered structure can be observed in the fetus[3]. By week 14, K17 can also be found in the basal and intermediate layers of the epidermis (In adult skin, K17 is not observed) [2]. Developing blood vessels were observed at the end of week 16[2]. | 
|
| Weeks 20-22 | By week 20, hair follicles can be already be seen in the epidermis. The total number of intermediate layers has also increased[2]. In an electron micrograph study at week 22 of development[3], kertanised epidermis was analysed. It was observed that glycogen was abundantly present throughout all epidermal layers. (The included arrows, highlight the keratin filament bundles, which are now organised and peripherally placed.) | 
|
| Adult | In adult skin- a greater diversity of cells can be seen as more cells differentiate. Basal, spinous, granular and cornified cells are all example of such[4][6]. The fetal extra-cellular matrix also differs from that of the adult- mainly in terms of the collagen type[8]. and amount of glycosaminoglycans present[9]. | 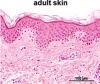
|
Hair
Hair originates from the ectoderm. At about 12 weeks, it is believed that specific signals from the dermis are released- signaling for the induction hair follicle formation [10][11]
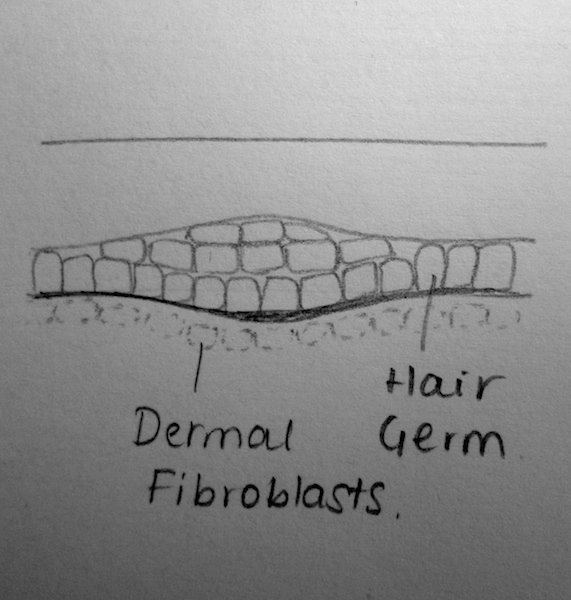
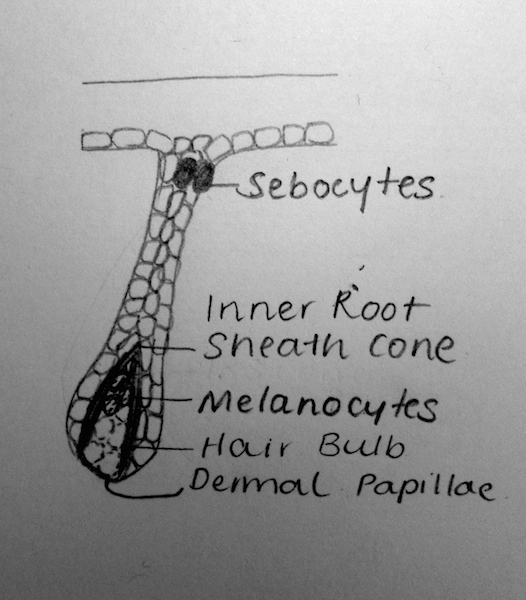
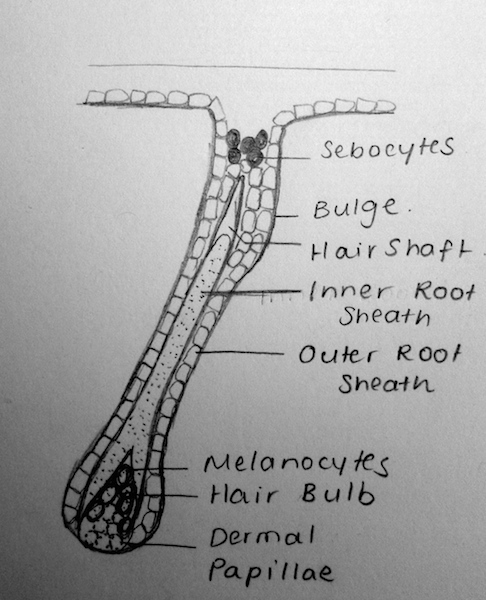
| Stage | Weeks | Description |
|---|---|---|
| (a) Undifferentiated Epithelium | Weeks 8-12 | Through reciprocal interactions and ‘first dermal signaling’, cells from the stratum basale grow into the underlying dermis. The signaling pathway, however, has not been fully identified[5] |
| (b) Placode | Weeks 12-14 | The ‘first dermal signals’ influence epithelial cells to develop a placode- a thickening of the columnar cells. It is theorised that varying intrinsic dermal signals lead to the expression of various placodes and consequently, the differences in the expression of hair thickness/size throughout the body[12]. The specific combination of promoter and repressor activators for hair development, is also theorised to characterise for the regional differences in eventual hair expression[13] |
| (c) Germ | Weeks 13-16 | WNT Signalling is believed to have a role in the induction of the dermal condesate[5]. Platelet-derived growth factor-A molecules from the placode, also contributes to the induction of the dermal condensate. The development of the dermal condensate helps further induce the downward growth of the placode.
Through secreted proteins such as Sonic Hedgehog, the placode continues to proliferate and enclose the dermal condensate. This eventually forms a deep, club-shaped hair bud, with an invaginated dermal papillae [5]. These dermal papillae are rapidly infiltrated by blood vessels and nerve endings. |
| (d) Peg | Weeks 19-21 | Sonic Hedgehog and the induction of a ‘secondary dermal signal’ (characterisation unknown) leads to a significant down-growth and proliferation of the follilular epithelium[14].
In this stage, it is also believed that the polarity of the hair follicle (the angle at which hair-follicles grow in relation to skin) and the architecture of the hair follicle itself (straight hair, wavy hair, etc) is regulated in part by Sonic Hedgehog and TGF-a signaling respectively[5]. |
| (e) Bulbous Peg | Weeks 23-28 | This stage is characterised by the appearance of the hair follicle bulb. Further and significant differentiation of the inner root sheeth and the hair shaft also characterises this stage[5].
The epithelial cells within the hair bulb, begin to differentiate into the germinal matrix – which grow, proliferate and keratinise to form the hair shaft and internal root sheet[5]. Other epithelial cells outside of the hair bud, form the external hair sheeth. Mesodermal cells of the dermis that surround the invaginating hair follicle form the dermal root sheeth and the arrecrtor pili muscles for hairs. Proteins such as Notch1 are believed to help regulate the phenotype of keratinocytes as they differentiate[15]. |
Lanugo Hair[1]
- Appear at the end of week 12
- Abundant from weeks 17-20
- Shed 4 weeks before birth [16]
- Lanugo Hairs are the first fetal hairs. They are characterised by their soft, fine and unpigmented nature.
- Lanugo Hairs have a role in keeping the vernix caseosa intact to the fetus[1]
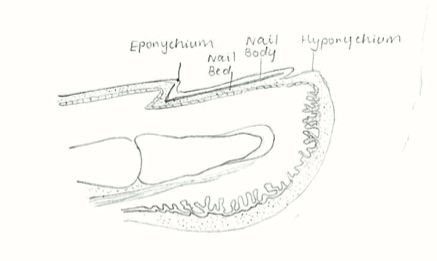
Nail
Together, fingernails and toenails are modifications of the epidermis which are derived from the same embryonic origin, the ectoderm. [17]
| Week | Event |
|---|---|
| Week 9 | The primitive finger nail beings to from. Preceding the morphological development, molecular signalling molecules being patterning the ectodermal layer. Signal molecules such as Bone Morphogentic Proteins (BMPs) allow communication between tissue layers and are involved in the initiating development of the nail. [18] |
| Week 10 | The primary nail field is establish, marked by a localised thickening of the epithelium. This primary nail fields initial from on the ventral surface of the digits and are repositioned to the dorsal side during development. [1] The LIM-homeodomain protein (Lmx1b) is a signalling molecule involved in this process, it's localised expression allows the dorsal-vetral limb axis to be established. A mutation in the gene coding for Lmx1b correlates with abnormal development of the nail and other bony structures. [19] |
| Week 11 | On the lateral edges of the primary nail field, ectodermal cells proliferate to from the shallow lateral nail folds. Similarly proliferation of the cells on the proximal end of the nail field gives rise to the deeper proximal nail fold. The nail field now appears as a distinct region on the digits. [17]. In addition, the distal ridges of nail bed keratinise. |
| Week 13 | Seen in cross-section, the early nail matrix begins to from, this marked region with in the proximal nail fold which undergoes localised cornification. The nail plate grows from the nail matrix as kertaised cells are flattened and compacted into dense nail tissue. |
| Week 14 | The primitive toe nails being to from. This event usually occurs 4 weeks after development of the finger nails. The differential timing of these events is established by signalling molecules that establish the rostro-cauda sequence of development in the embryo and fetus. |
| Week 20 | Nail plate begins to grow over the nail bed from the proximal nail matrix towards the distal direction. |
| Week 24 | Free nail plate is visible to the naked eye. Initially the developing nail is covered by a thin layer of epidermis known as the eponychium (corneal layer of epidermis). At this stage in fetal development the eponychium declines, the cuticle remains over the proximal nail plate. Below the free end of the nail, epidermal cells aggregate to form the mass known as the hyponychium[1]. |
| Week 32-36 | The finger nails and toe nails respectively reach the tips of the digits and the toes. |
Glands
| Gland Type | Description | Image |
|---|---|---|
| Sebaceous Glands | Sebaceous glands develop from the epithelial wall of the hair follicle. They secrete the vernix caseosa
Vernix caseosa is a material secreted by sebaceous glands in the foetus in the last trimester of development (Week 21)[20] and is characterised by it’s cheese-like appearance around the neonate at birth. The functions of vernix caseosa include: |
 Vernix caseosa on a neonate.[23] |
| Mammary Glands | Mammary glands develop from the mammary ridge- a downgrowth of the epidermis (ectoderm) into the underlying dermis (mesoderm). This occurs at about week 6 of development. Mammary glands first develop into primary mammary buds[1], which successively grow in length and complexity. Prior to puberty, the mammary glands are anatomically indistinguishable.[24] | |
| Sweat Glands |
There are two major kinds of sweat glands present in humans, both of which develop from downgrowths of the epidermis into the underlying dermis. Sweat glands have been histologically identified in studies from week 21 of development. They begin as cellular buds[1], which proliferate as solid, cylindrical down growths into mesenchyme. Central cells degenerate to form a lumen, while the terminal region coils to eventually form the body of the gland. As fetal development continues, peripheral cells eventually differentiate into secretory and myoepithelial cells[1]. Eccrine Sweat Glands
Apocrine Sweat Glands |
-snip-
Hypohidrotic Ectodermal Dysplasia

Hypohidrotic ectodermal dysplasia (HED) is the most of all ectodermal dysplasias, caused by an abnormality in the development of ectodermal tissues, which inlude skin, hair, teeth, sweat glands, and nails.[27][28] Patients with ectodermal dysplasia often have sparse hair and oligodontia, which is a condition where teeth are missing and are poorly developed.[27][28] Sweating is a very important function in the body in terms of thermoregulation. HED is mainly characterised by hypohidrosis due to the lack of sweat glands in the skin, which could lead to hyperpyrexia and sometimes death. In neonates, the mortality rate of HED reaches up to 30%, with the first year of life having the highest risk. [27] HED is caused by a genetic abnormality of the ectodysplasin A gene (EDA) and passed on by X-linked inheritance. The mutations of this gene results in the poor sweating ability or none at all in a person. The effects of this abnormality is usually more severe in males than in females. [29][28]
There is currently no pharmacological therapies for HED but there are methods applied to prevent the disease from aggravating. Neonates with HED are placed in incubators and monitored to prevent them from overheating. Management of this disease gets easier as the patient ages. Adults with HED can control their thermoregulation by staying in cool environments or drinking cold drinks to lower the body temperature. Currently, there are studies that aim to find a cure for this abnormality, e.g. gene replacement therapy in animal models.[28]
| More Abnormalities |
|---|
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Moore, K.L., Persaud, T.V.N. & Torchia, M.G. (2011). The developing human: clinically oriented embryology (9th ed.). Philadelphia: Saunders.
- ↑ 2.0 2.1 2.2 2.3 <pubmed>19701759</pubmed>
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 <pubmed>2413039</pubmed>
- ↑ 4.0 4.1 4.2 <pubmed>168272</pubmed>
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 <pubmed>11841536</pubmed>
- ↑ 6.0 6.1 <pubmed>17039717</pubmed>
- ↑ 7.0 7.1 7.2 7.3 7.4 Dudek, R.W. (2011). BRS Embryology (5th ed.). Lippincott Williams & Wilkins
- ↑ <pubmed>8292556</pubmed>
- ↑ <pubmed>2027330</pubmed>
- ↑ <pubmed>1566372</pubmed>
- ↑ <pubmed>20590427</pubmed>
- ↑ <pubmed>10529418</pubmed>
- ↑ <pubmed>10431226</pubmed>
- ↑ <pubmed>9768360</pubmed>
- ↑ <pubmed>10804183</pubmed>
- ↑ Cite this page: Hill, M.A. (2014) Embryology 2009 Lecture 18. Retrieved October 23, 2014, from https://php.med.unsw.edu.au/embryology/index.php?title=2009_Lecture_18
- ↑ 17.0 17.1 Pansky, B. (1982). Review of Medical Embryology. Embryome Sciences, Inc 1301 Harbor Bay Parkway, Alameda, CA, 94502
- ↑ <pubmed>21387539</pubmed>
- ↑ <pubmed>9590288</pubmed>
- ↑ 20.0 20.1 20.2 <pubmed>15830002</pubmed>
- ↑ 21.0 21.1 <pubmed>21504444</pubmed>
- ↑ 22.0 22.1 22.2 22.3 <pubmed>19881987</pubmed>
- ↑ Image source: JazlynRoseVernixByPhilKonstantin.jpg http://en.wikipedia.org/wiki/File:JazlynRoseVernixByPhilKonstantin.jpg
- ↑ <pubmed>23720046</pubmed>
- ↑ 25.0 25.1 Bolognia, J.L., Jorizzo, J.L. & Schaffer J.V. (2012). Dermatology (3rd ed.). Elsevier Limited.
- ↑ <pubmed>21165248 </pubmed>
- ↑ 27.0 27.1 27.2 <pubmed>20682465</pubmed>
- ↑ 28.0 28.1 28.2 28.3 <pubmed>24678015</pubmed>
- ↑ <pubmed>21357618</pubmed>