Vagina Development: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| Line 3: | Line 3: | ||
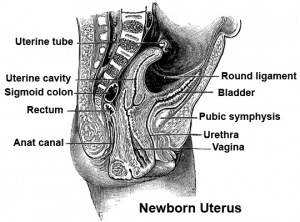
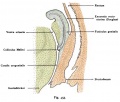
[[Image:newborn_uterus.jpg|thumb|300px|Section through newborn female]] | [[Image:newborn_uterus.jpg|thumb|300px|Section through newborn female]] | ||
The embryonic origin of the vagina has been a historically hotly debated issue with several different contributions and origins described. Current molecular studies show the whole vagina epithelium is derived from the paramesonephric (Müllerian) duct with bone morphogenic protein 4 (BMP4) reshaping the intermediate mesoderm-derived Müllerian duct into the vaginal primordium. | The embryonic origin of the vagina has been a historically hotly debated issue with several different contributions and origins described. Current molecular studies show the whole vagina epithelium is derived from the paramesonephric (Müllerian) duct with bone morphogenic protein 4 (BMP4) reshaping the intermediate mesoderm-derived Müllerian duct into the vaginal primordium.{{#pmid:19598112|PMID19598112}} Transgenic studies in mice also identified the developmental origin of vaginal epithelium derived solely from Müllerian duct epithelium.{{#pmid:20638775|PMID20638775}} Vaginal development is also under negative control of androgens. | ||
<center>''Paramesonephric duct = Müllerian duct and Mesonephric duct = Wolffian duct.''</center> | <center>''Paramesonephric duct = Müllerian duct and Mesonephric duct = Wolffian duct.''</center> | ||
| Line 10: | Line 10: | ||
See also for external genitalia [[Integumentary System Development]]. | See also for external genitalia [[Integumentary System Development]]. | ||
===History=== | ===History=== | ||
An earlier description gave the vagina arising by downward growth of the Wolffian and Mullerian ducts, with the sinovaginal bulbs located at the caudal ends of the Wolffian ducts. An earlier understanding was that the upper part of the vagina derived from Müllerian ducts and the lower part from the sinovaginal bulbs (formed by fusion form the vaginal plate) all derived from the urogenital sinus. The terms sinovaginal bulbs and vaginal plate were first coined by Koff in 1933. | An earlier description gave the vagina arising by downward growth of the Wolffian and Mullerian ducts, with the sinovaginal bulbs located at the caudal ends of the Wolffian ducts. An earlier understanding was that the upper part of the vagina derived from Müllerian ducts and the lower part from the sinovaginal bulbs (formed by fusion form the vaginal plate) all derived from the urogenital sinus. The terms sinovaginal bulbs and vaginal plate were first coined by Koff in 1933.{{#pmid:12332362|PMID12332362}} Acién's hypothesis, related to abnormalities and the embryology of the human vagina as deriving from the Wolffian ducts and the Müllerian tubercle. | ||
| Line 19: | Line 19: | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| | | | ||
* '''Normal and abnormal epithelial differentiation in the female reproductive tract''' | * '''Normal and abnormal epithelial differentiation in the female reproductive tract'''{{#pmid:21612855|PMID21612855}} "In mammals, the female reproductive tract (FRT) develops from a pair of paramesonephric or Müllerian ducts (MDs), which arise from coelomic epithelial cells of mesodermal origin. During development, the MDs undergo a dynamic morphogenetic transformation from simple tubes consisting of homogeneous epithelium and surrounding mesenchyme into several distinct organs namely the oviduct, uterus, cervix and vagina." | ||
* '''Vaginal microbiome of reproductive-age women''' | |||
* '''Vaginal microbiome of reproductive-age women'''{{#pmid:20534435|PMID20534435}} "The means by which vaginal microbiomes help prevent urogenital diseases in women and maintain health are poorly understood. To gain insight into this, the vaginal bacterial communities of 396 asymptomatic North American women who represented four ethnic groups (white, black, Hispanic, and Asian) were sampled and the species composition characterized by pyrosequencing of barcoded 16S rRNA genes. The communities clustered into five groups: four were dominated by ''Lactobacillus iners'', '''L. crispatus''', '''L. gasseri''', or '''L. jensenii''', whereas the fifth had lower proportions of lactic acid bacteria and higher proportions of strictly anaerobic organisms, indicating that a potential key ecological function, the production of lactic acid, seems to be conserved in all communities. The proportions of each community group varied among the four ethnic groups, and these differences were statistically significant [χ(2)(10) = 36.8, P < 0.0001]. Moreover, the vaginal pH of women in different ethnic groups also differed and was higher in Hispanic (pH 5.0 ± 0.59) and black (pH 4.7 ± 1.04) women as compared with Asian (pH 4.4 ± 0.59) and white (pH 4.2 ± 0.3) women." | |||
|} | |} | ||
{| class="wikitable mw-collapsible mw-collapsed" | {| class="wikitable mw-collapsible mw-collapsed" | ||
| Line 63: | Line 64: | ||
| [[File:Female reproductive tract Wnt4.jpg|400px]] | | [[File:Female reproductive tract Wnt4.jpg|400px]] | ||
Female reproductive tract Wnt4 | Female reproductive tract Wnt4{{#pmid:26721931|PMID26721931}} | ||
|} | |} | ||
:'''Links:''' [[Developmental Signals - Wnt]] | :'''Links:''' [[Developmental Signals - Wnt]] | ||
===Retinoic acid === | ===Retinoic acid === | ||
In mice, the epithelial fate of female reproductive organs is determined by factors secreted from the stroma. Retinoic acid-Retinoic acid Receptor signaling in the Müllerian duct determines the fate of stroma to form the future uterus and vagina. | In mice, the epithelial fate of female reproductive organs is determined by factors secreted from the stroma. Retinoic acid-Retinoic acid Receptor signaling in the Müllerian duct determines the fate of stroma to form the future uterus and vagina.{{#pmid:27911779|PMID27911779}} | ||
:'''Links:''' [[Developmental Signals - Retinoic acid]] | :'''Links:''' [[Developmental Signals - Retinoic acid]] | ||
| Line 96: | Line 97: | ||
==Adult Dimensions== | ==Adult Dimensions== | ||
A recent study using magnetic resonance imaging (MRI) has accurately measured the dimensions of the adult vagina. | A recent study using magnetic resonance imaging (MRI) has accurately measured the dimensions of the adult vagina.{{#pmid:16478763|PMID16478763}} | ||
:"Seventy-seven MRI scans were performed on 28 women before gel application to establish baseline vaginal measurements. Average dimensions were calculated for each woman and for the population. The influence of potential covariates (age, height, weight and parity) on these dimensions was assessed. ...Mean vaginal length from cervix to introitus was 62.7 mm. Vaginal width was largest in the proximal vagina (32.5 mm), decreased as it passed through the pelvic diaphragm (27.8 mm) and smallest at the introitus (26.2 mm)." | :"Seventy-seven MRI scans were performed on 28 women before gel application to establish baseline vaginal measurements. Average dimensions were calculated for each woman and for the population. The influence of potential covariates (age, height, weight and parity) on these dimensions was assessed. ...Mean vaginal length from cervix to introitus was 62.7 mm. Vaginal width was largest in the proximal vagina (32.5 mm), decreased as it passed through the pelvic diaphragm (27.8 mm) and smallest at the introitus (26.2 mm)." | ||
| Line 102: | Line 103: | ||
==Other Species== | ==Other Species== | ||
Female cetaceans (whales, dolphins, and porpoises) and hippopotamuses have unusual vaginal folds of tissue that are of unknown function(s). | Female cetaceans (whales, dolphins, and porpoises) and hippopotamuses have unusual vaginal folds of tissue that are of unknown function(s).{{#pmid:28362830|PMID28362830}} | ||
Female waterfowl have vaginal morphology involving complex convolutions and also a number of dead-end sacs. | Female waterfowl have vaginal morphology involving complex convolutions and also a number of dead-end sacs. | ||
| Line 110: | Line 112: | ||
===Mayer- Rokitansky-Kuster-Hauser syndrome=== | ===Mayer- Rokitansky-Kuster-Hauser syndrome=== | ||
(MRKH) Abnormality of development of the female genital tract: partial or complete absence (agenesis) of the [[U#uterus|uterus]]; absent or hypoplastic vagina; normal fallopian tubes, ovaries, normal external genitalia and normal female chromosome pattern (46, XX). Has an incidence of approximately 1 in 4500 newborn girls and has been associated with a microdeletion at 17q12. | (MRKH) Abnormality of development of the female genital tract: partial or complete absence (agenesis) of the [[U#uterus|uterus]]; absent or hypoplastic vagina; normal fallopian tubes, ovaries, normal external genitalia and normal female chromosome pattern (46, XX). Has an incidence of approximately 1 in 4500 newborn girls and has been associated with a microdeletion at 17q12.{{#pmid:1988902|PMID1988902}} | ||
===OHVIRA Syndrome=== | ===OHVIRA Syndrome=== | ||
| Line 124: | Line 126: | ||
==Endocrine Disruptors== | ==Endocrine Disruptors== | ||
Endocrine disruptors in female reproductive tract development and carcinogenesis. | Endocrine disruptors in female reproductive tract development and carcinogenesis.{{#pmid:19709900|PMID19709900}} | ||
| Line 142: | Line 144: | ||
===Reviews=== | ===Reviews=== | ||
{{#pmid:19598112}} | |||
{{#pmid:16208476}} | |||
{{#pmid:15467266}} | |||
===Articles=== | ===Articles=== | ||
{{#pmid:24172012}} | |||
{{#pmid:17532316}} | |||
{{#pmid:17070514}} | |||
{{#pmid:14695376}} | |||
{{#pmid:12740945}} | |||
{{#pmid:12449044}} | |||
{{#pmid:15086027}} | |||
{{#pmid:19598112}} | |||
{{#pmid:15821572}} | |||
{{#pmid:18391520}} | |||
===Search PubMed=== | ===Search PubMed=== | ||
Revision as of 17:00, 21 February 2018
| Embryology - 23 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The embryonic origin of the vagina has been a historically hotly debated issue with several different contributions and origins described. Current molecular studies show the whole vagina epithelium is derived from the paramesonephric (Müllerian) duct with bone morphogenic protein 4 (BMP4) reshaping the intermediate mesoderm-derived Müllerian duct into the vaginal primordium.[1] Transgenic studies in mice also identified the developmental origin of vaginal epithelium derived solely from Müllerian duct epithelium.[2] Vaginal development is also under negative control of androgens.
See also for external genitalia Integumentary System Development.
History
An earlier description gave the vagina arising by downward growth of the Wolffian and Mullerian ducts, with the sinovaginal bulbs located at the caudal ends of the Wolffian ducts. An earlier understanding was that the upper part of the vagina derived from Müllerian ducts and the lower part from the sinovaginal bulbs (formed by fusion form the vaginal plate) all derived from the urogenital sinus. The terms sinovaginal bulbs and vaginal plate were first coined by Koff in 1933.[3] Acién's hypothesis, related to abnormalities and the embryology of the human vagina as deriving from the Wolffian ducts and the Müllerian tubercle.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Vagina Embryology <pubmed limit=5>Vagina Embryology</pubmed> |
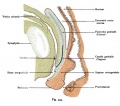
Paramesonephic Duct
The paired paramesonephic ducts (Müllerian ducts) go through a series of developmental changes recently identified as regulated by a number of molecular factors.
| Female Uterus and Vagina (between week 9 and 20) | ||||
|---|---|---|---|---|
|
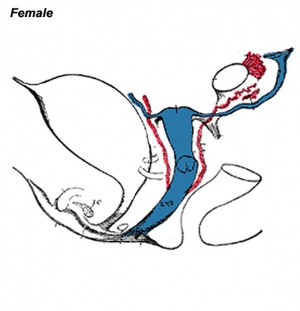
The entire vagina is formed from the paramesonephric (Müllerian) duct (red) and does not have a contribution from the urogenital endoderm (yellow). |
Molecular
BMP4
Wnt 4
| Processes regulated by Wnt4 during female reproductive tract development
Wnt4 is required during prenatal and postnatal development of female reproductive tract. The initial MD primordium occurs independently of Wnt4 function (E11.5, the MD primordium in red). After initiation of the process, differentiation of the MD tip cells, prenatal elongation of the MD and postnatal formation of the endometrial gland (eG) all depend on Wnt4 signalling. The daughter cells that are initially Wnt4+ contribute to MD and eG formation. (text from figure legend)
|
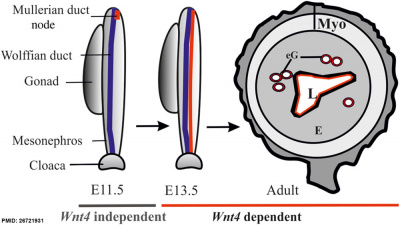
Female reproductive tract Wnt4[6] |
- Links: Developmental Signals - Wnt
Retinoic acid
In mice, the epithelial fate of female reproductive organs is determined by factors secreted from the stroma. Retinoic acid-Retinoic acid Receptor signaling in the Müllerian duct determines the fate of stroma to form the future uterus and vagina.[7]
Initiation
Coelomic epithelium Lim1 expressing cells are specified to a duct fate.
- Lim - proteins named for 'LIN11, ISL1, and MEC3,' are defined by the possession of a highly conserved double zinc finger motif called the LIM domain.
- LIM domain-binding factors - interact with the LIM domains of nuclear proteins are capable of binding to a variety of transcription factors.
Invagination
Duct invagination induced by Wnt4 to reach the mesonephric (Wolffian)
Elongation
Cells at the leading tip proliferate and form the duct elongating to reach the cloaca (urogenital sinus). Mesonephric secretes WNT9b to guide duct elongation. Pax2 also acts in elongation and duct maintenance.
- WNT9b - member of the WNT protein family that encode cysteine-rich secreted glycoproteins that act as extracellular signaling factors.
- Pax2 - member of the paired box protein family.
Postnatal Development
A study in mouse has identified Dicer, a riboendonuclease required for microRNA biosynthesis, to be required for postnatal growth if the female reproductive tract.[8]
Adult Dimensions
A recent study using magnetic resonance imaging (MRI) has accurately measured the dimensions of the adult vagina.[9]
- "Seventy-seven MRI scans were performed on 28 women before gel application to establish baseline vaginal measurements. Average dimensions were calculated for each woman and for the population. The influence of potential covariates (age, height, weight and parity) on these dimensions was assessed. ...Mean vaginal length from cervix to introitus was 62.7 mm. Vaginal width was largest in the proximal vagina (32.5 mm), decreased as it passed through the pelvic diaphragm (27.8 mm) and smallest at the introitus (26.2 mm)."
Other Species
Female cetaceans (whales, dolphins, and porpoises) and hippopotamuses have unusual vaginal folds of tissue that are of unknown function(s).[10]
Female waterfowl have vaginal morphology involving complex convolutions and also a number of dead-end sacs.
Abnormalities
Mayer- Rokitansky-Kuster-Hauser syndrome
(MRKH) Abnormality of development of the female genital tract: partial or complete absence (agenesis) of the uterus; absent or hypoplastic vagina; normal fallopian tubes, ovaries, normal external genitalia and normal female chromosome pattern (46, XX). Has an incidence of approximately 1 in 4500 newborn girls and has been associated with a microdeletion at 17q12.[11]
OHVIRA Syndrome
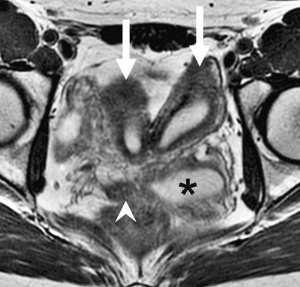
Obstructed HemiVagina and Ipsilateral Renal Anomaly with uterine didelphysis is a syndrome due to lateral non-fusion of the Mullerian ducts with asymmetric obstruction. The presence of vaginal septum also gives rise to other clinical conditions.
OHVIRA Syndrome Magnetic Resonance Images
Endocrine Disruptors
Endocrine disruptors in female reproductive tract development and carcinogenesis.[12]
Additional Images
Historic
References
- ↑ Cai Y. (2009). Revisiting old vaginal topics: conversion of the Müllerian vagina and origin of the "sinus" vagina. Int. J. Dev. Biol. , 53, 925-34. PMID: 19598112 DOI.
- ↑ Kurita T. (2010). Developmental origin of vaginal epithelium. Differentiation , 80, 99-105. PMID: 20638775 DOI.
- ↑ Koff AK. (1933). Development of the vagina in the human fetus. Contrib Embryol , 24, 59-91. PMID: 12332362
- ↑ Kurita T. (2011). Normal and abnormal epithelial differentiation in the female reproductive tract. Differentiation , 82, 117-26. PMID: 21612855 DOI.
- ↑ Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L & Forney LJ. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U.S.A. , 108 Suppl 1, 4680-7. PMID: 20534435 DOI.
- ↑ Prunskaite-Hyyryläinen R, Skovorodkin I, Xu Q, Miinalainen I, Shan J & Vainio SJ. (2016). Wnt4 coordinates directional cell migration and extension of the Müllerian duct essential for ontogenesis of the female reproductive tract. Hum. Mol. Genet. , 25, 1059-73. PMID: 26721931 DOI.
- ↑ Nakajima T, Iguchi T & Sato T. (2016). Retinoic acid signaling determines the fate of uterine stroma in the mouse Müllerian duct. Proc. Natl. Acad. Sci. U.S.A. , 113, 14354-14359. PMID: 27911779 DOI.
- ↑ <pubmed>19197916</pubmed>
- ↑ Barnhart KT, Izquierdo A, Pretorius ES, Shera DM, Shabbout M & Shaunik A. (2006). Baseline dimensions of the human vagina. Hum. Reprod. , 21, 1618-22. PMID: 16478763 DOI.
- ↑ Orbach DN, Marshall CD, Mesnick SL & Würsig B. (2017). Patterns of cetacean vaginal folds yield insights into functionality. PLoS ONE , 12, e0175037. PMID: 28362830 DOI.
- ↑ Brambati B, Tului L, Simoni G & Travi M. (1991). Genetic diagnosis before the eighth gestational week. Obstet Gynecol , 77, 318-21. PMID: 1988902
- ↑ Ma L. (2009). Endocrine disruptors in female reproductive tract development and carcinogenesis. Trends Endocrinol. Metab. , 20, 357-63. PMID: 19709900 DOI.
Reviews
Cai Y. (2009). Revisiting old vaginal topics: conversion of the Müllerian vagina and origin of the "sinus" vagina. Int. J. Dev. Biol. , 53, 925-34. PMID: 19598112 DOI.
Farage M & Maibach H. (2006). Lifetime changes in the vulva and vagina. Arch. Gynecol. Obstet. , 273, 195-202. PMID: 16208476 DOI.
Cummings AM & Kavlock RJ. (2004). Function of sexual glands and mechanism of sex differentiation. J Toxicol Sci , 29, 167-78. PMID: 15467266
Articles
Pechriggl EJ, Bitsche M, Blumer MJ, Zwierzina ME & Fritsch H. (2013). Novel immunohistochemical data indicate that the female foetal urethra is more than an epithelial tube. Ann. Anat. , 195, 586-95. PMID: 24172012 DOI.
Deutscher E & Hung-Chang Yao H. (2007). Essential roles of mesenchyme-derived beta-catenin in mouse Müllerian duct morphogenesis. Dev. Biol. , 307, 227-36. PMID: 17532316 DOI.
Guioli S, Sekido R & Lovell-Badge R. (2007). The origin of the Mullerian duct in chick and mouse. Dev. Biol. , 302, 389-98. PMID: 17070514 DOI.
Kobayashi A, Shawlot W, Kania A & Behringer RR. (2004). Requirement of Lim1 for female reproductive tract development. Development , 131, 539-49. PMID: 14695376 DOI.
Hashimoto R. (2003). Development of the human Müllerian duct in the sexually undifferentiated stage. Anat Rec A Discov Mol Cell Evol Biol , 272, 514-9. PMID: 12740945 DOI.
Mornex F. (2002). [Combined gemcitabine and radiotherapy]. Bull Cancer , 89 Spec No, S127-33. PMID: 12449044
Shapiro E, Huang H & Wu XR. (2004). New concepts on the development of the vagina. Adv. Exp. Med. Biol. , 545, 173-85. PMID: 15086027
Cai Y. (2009). Revisiting old vaginal topics: conversion of the Müllerian vagina and origin of the "sinus" vagina. Int. J. Dev. Biol. , 53, 925-34. PMID: 19598112 DOI.
Sebe P, Fritsch H, Oswald J, Schwentner C, Lunacek A, Bartsch G & Radmayr C. (2005). Fetal development of the female external urinary sphincter complex: an anatomical and histological study. J. Urol. , 173, 1738-42; discussion 1742. PMID: 15821572 DOI.
Drews U. (2007). Helper function of the Wolffian ducts and role of androgens in the development of the vagina. Sex Dev , 1, 100-10. PMID: 18391520 DOI.
Search PubMed
Search Pubmed: Vagina Embryology | Vagina Development | vaginal plate development | Mullerian duct
NCBI - Policies and Guidelines | PubMed | Help:Reference Tutorial
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- The Australian and New Zealand Vulvovaginal Society
- International Society for the Study of Vulvovaginal Disease
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 23) Embryology Vagina Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Vagina_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G