User talk:Z3289829
Reason for low contribution in Group 3 project: Klinefelter's Syndrome
The reason may be due to me uploading my work in chunks. Below is my contributions to two sections in the project;
History
Klinefelter syndrome (KS) was first described by Harry F. Klinefelter and his colleagues in 1942. Their observations of nine patients where characterised by a number of peculiar symptoms; gynecomastia, azoospermia, hyalinised and small testes, absent spermatogenesis, elevated levels of follicle-stimulating hormone (FSH) and hypogonadism. [1] [2] In 1956, an investigation was carried out with 7 patients with Klinefelter’s syndrome that had the buccal smears that demonstrated Barr bodies . However, the cause of the syndrome remained unknown until 1959, when Jacobs and Strong discovered that a patient with KS had 47 chromosomes, including an extra X chromosome in the karotype of the patient [1]. As recorded in their article 'A case of human intersexuality having a possible XXY sex-determining mechanism';
| “…There are strong grounds, both observational and genetic, for believing that human beings with chromatin-positive nuclei are genetic females having two X chromosomes. The fact that this patient is chromatin-positive and has an additional chromosome within the same size range as the X, as well as an apparently normal Y, makes it seem likely that he has the genetic constitution XXY”[3] |
This discovery confirmed that the Barr bodies seen in patients with KS corresponds to an extra X chromosome[3]. In 1966, Harry F. Klinefelter reported that the extra X chromosome results from either meiotic nondisjunction or anaphase lag. Anaphase lag describes a chromosome which is not incorporated into the new cell in the second stage of mitosis(anaphase) due to it ‘lagging’, resulting in gametes lacking a sex chromosome [4].
The ‘prototypic’ man with KS was initially described as tall, with narrow shoulders, broad hips, sparse body hair, gynecomastia, small testes, androgen deficiency and reduced intelligence[1]. However, a few years after the syndrome was described Heller and Nelson reported that the gynacomastia was not a necessary part of the syndrome, even though it occurred in about 75% of the patients which they observed. The hallmarks of the syndrome were then thought small testes, sterility and increased excretion of follicle stimulating hormone[4]. Extensive studies of these patients during their adolescence illustrated the various personality traits, which can be handled by proper counselling. Similar to today, Harry Klinefelter reported that most patients with this condition were not diagnosed until early adult life, when counselling may be less rewarding.
Below is a timeline highlighting all the major advancements and developments in Klinefelter’s Syndrome.
Timeline
| Year | Discovery |
| 1942 | Dr. Harry Klinefelter and his co-workers at Massachusetts General Hospital in Boston published a report on 9 men describing the signs and symptoms of Klinefelter’s Syndrome. The ‘prototypic’ man with Klinefelter’s syndrome was described as tall, with narrow shoulders, broad hips, sparse body hair, gynecomastia, small testes, androgen deficiency and reduced intelligence. [1] |
| 1949 | Barr and Bertram noticed positive chromatin material in KS patients.It was a dense chromatin mass which they later termed, Barr body. |
| 1956 | The discovery of Barr bodies as a result of the new development of Buccal smears |
| 1959 | Jacobs and Strong discovered that it was a chromosomal disorder, with the appearance of an extra X chromosome. |
| 1960s | Development of chromosome banding techniques. [5] |
| 1962 | Maclean and Mitchell’s studies on inmates in prisons and institutions for mental health revealed an increased risk of psychiatric disorders, mental retardations and criminal behaviour in patients with Klinefelter syndrome. [1] |
| 1966 | Dr. Harry Klinefelter reports that the extra X chromosome results from either meiotic non-disjunction or anaphase lag. [4] |
| 1970 | Rozen et al reported that approximately 1% of all individuals institutionalised with mental retardation have an XXY karotype. [6] |
| 1970s | A number of centres began screening newborns for sex chromatin abnormalities. |
| 1986 | Dr. Harry Klinefelter described that the hallmarks of the syndrome are now small testes, sterility and increased excretion of follicle stimulating hormone. [4]
He also reported that most patients with this condition were not diagnosed until early adult life, when counselling may be less rewarding. [4] Treated hypogonadism with injected testosterone, and thought this also aided personality abnormalities in adolescent patients. [4] |
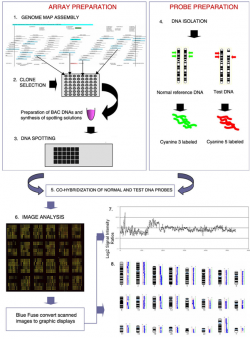
| 1992 | The Comparative Genomic Hybridization (CGH) analysis was developed as a genome wide screening strategy for detecting DNA copy number imbalances.[7] A classic array-CGH experiment is shown figure 2. |
| 1995 | Reiss et al., found that half all mental retardation in males originates from a defective gene on the X chromosome. Thus, the X chromosome comprises the genes involved in human cognition. [8] |
| 1998 | Smyth and Bremmer concluded that XXY karotypes occurs in 1 in 500 live male births and is the most common type of human chromosome anomaly. [9]
They also hypothesised that the characteristics features of Klinefelter’s syndrome originate from genes escape inactivation and are expressed in excess. |
| 2002 | Crow et al., suggested that at least one gene or genes in the X-Y homologous regions of the sex chromosomes which escape’s normal X- inactivation are crucial for language functioning.[10] |
| 2010 | Stefano Gambardella et al., designed a targeted array called GOLD (gain or loss detection) Chip which detects unexpected major chromosome imbalances. [11] |
Aetiology
Chromosome abnormalities have a high incidence in humans. The most common type is aneuploidy, which is the loss (monosomy) or gain (trisomy) of an entire chromosome [12]. Aneuploidy’s occur in approximately 5% of pregnancies which survive long enough to be seen and approximately 10-25% of all fertilised human oocytes are either monosomic or trisomic [12]. Trisomies are incapable of normal development, the consequences are less severe for the sex chromosomes than the autosomes, leading to enhanced sex chromosomes among live-borns in comparison with autosomal trisomies. Therefore, the 47,XXY condition, termed Klinefelter’s Syndrome (KS) is identified in almost 1 of every 1000 male births, causing it to be one of the most commonly identified chromosome abnormality among live-born individuals.
Non-Disjunction
Non-disjunction is the failure of chromosome pairs to separate during the first and second meiotic divisions. Maternal XXY can be caused by non-disjunction during the first and second meiotic divisions, however, XXY of paternal origin can only occur during the first meiotic division. In the great majority of trisomies the additional chromosome is of maternal origin and results from an error during the first meiotic division as seen in figure 1. However, the 47,XXY has been extensively studied and around one half of all cases are paternally derived. The nature of the errors by which maternal and paternal XXYs can arise are extremely diverse. The process of meiosis serves to generate haploid gametes through a specialised cell division process, consisting of two stages of cell division, MI and MII. During prophase of MI the pairs of homologous chromosomes synapse and undergo recombination, with chiasmata being formed at the sites of exchange. Their purpose is to link together the homologues and therefore play a crucial role in the proper disjunction of chromosomes in the first meiotic division[12].
Animation Meiotic Non-disjunction - Meiosis I
Animation Meiotic Non-disjunction - Meiosis II
Genetics
Although many other genes are important for proper sexual development of the male foetus, the SRY (sex-determining region of Y chromosome) gene located on the Y chromosome directs the development of the foetal gonads into testes. Typically, when two or more X chromosomes are present in a cell, as in healthy females or sex chromosome disorders like 47,XXY, only one is active. The additional X chromosome is mostly inactive and the X chromatin is perceived as a Barr body in the periphery of the cell nucleus[13]. In addition to specific regions, both sex chromosomes carry short regions of homology termed pseudoautosomal regions (PAR) [14] which remain active in men and woman [15]. They behave as an autosome and function to allow X and Y chromosomes to pair and properly segregate during meiosis in males. Some genes in the X chromosome which are not homologous to the Y chromosome can escape inactivation and are functionally duplicated in KS males[15].
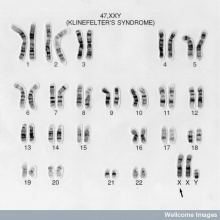
I also found this image for the introduction.
- ↑ 1.0 1.1 1.2 1.3 1.4 <pubmed>17415352</pubmed>
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedPMID21397196 - ↑ 3.0 3.1 <pubmed>13632697</pubmed>
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 <pubmed>3529433</pubmed>
- ↑ <pubmed>5640698</pubmed>
- ↑ <pubmed>4398603</pubmed>
- ↑ <pubmed> 1359641</pubmed>
- ↑ <pubmed>7585014</pubmed>
- ↑ <pubmed>9645824</pubmed>
- ↑ <pubmed> 15729733</pubmed>
- ↑ Stefano G., Erika C., Francesca M., Giusy S., Francesca G., Michela B., Anna M. N., Antonio N., Ercole B., Laura B. and Giuseppe N.(2010) Design, Construction and Validation of Targeted BAC Array-Based CGH Test for Detecting the Most Commons Chromosomal Abnormalities. ‘’Genomic Insights.’’ 3:9-21
- ↑ 12.0 12.1 12.2 <pubmed>12926525</pubmed>
- ↑ 21521364</pubmed>
- ↑ <pubmed> 20228051</pubmed>
- ↑ 15.0 15.1 <pubmed>21521364</pubmed>