User:Z3333038
Individual Assessments
Lab 1 Assessment
1. Identify the origin of In Vitro Fertilization and the 2010 nobel prize winner associated with this technique and add a correctly formatted link to the Nobel page.
The History of In Vitro Fertilisation
In vitro fertilisation (IVF) refers to the process of artificial fertilisation conducted ex vivo. The IVF technique was first described for non-human use. The earliest known research conducted was by Walter Heape from Cambridge University in the 1890s who reported the first known case of embryo transplantation in rabbits. In 1959, Dr. Min Chueh Chang published his work in Nature describing the first successful mammalian live birth (rabbits) after IVF therapy.
Eventually, the use of IVF for humans became a possibility and then a reality: in 1978, the first successful birth from IVF occurred in England. The success of this IVF birth is credited to Patrick Steptoe and Robert Edwards. In 2010, Edwards was awarded the Nobel Prize in Medicine for the development of human IVF therapy. Because of IVF, many couples have been given a chance to conceive. However, the history of IVF is still in the making with constant improvements in the technology being developed and applied.
2. Identify and add a PubMed reference link to a recent paper on fertilisation and describe its key findings (1-2 paragraphs).
Research in Fertilisation
In order for fusion between mammalian gametes to occur, a spermatozoon must first pass through the external layers surrounding the oocyte: the cumulus oophorus and the zona pellucida (ZP). It is believe that the acromosome reaction (AR) of the spermatozoa starts upon contact with the zona pellucida. Consequently, the cumulus cell layer is typically removed in studies of mouse sperm-oocyte interactions in order to facilitate fertilisation. The recent experiments of Jin et al. [1] sought to answer the question: "Where does the fertilising mouse spermatozoon begin the AR - in the cumulus [of the oocyte] or the zona pellucida?" Jin et al. [1] utilised fluorescence microscopy and transgenic mouse spermatozoa to conduct their investigation. Additionally, Jin et al. [1] used cumulus-free oocytes and cumulus-enclosed oocytes to study the role of the cumulus cells in fertilisation.
From the experiment, Jin et al. [1] found that most fertilising spermatozoa begin the AR before their first contact with the ZP. The significance of this finding was that the spermatozoa with intact acromosomes at the ZP seldom had the ability to penetrate through [1]. In contrast, spermatozoa which had already began the AR could easily penetrate the ZP. In regards to the role of the cumulus cells, it was found that cumulus-enclosed oocytes had a higher incidence of fertilisation compared to cumulus-free oocytes [1]. Moreover, cumulus-free oocytes had an increased incidence of in vitro fertilisation when incubated with other cumulus-enclosed cells; this finding suggests that cumulus cells harbour an important role in fertilisation [1]. However, it is notable that when cumulus-free oocytes were incubated in a cumulus-conditioned medium, no increase in fertilisation rate was noted[1]. Overall, two conclusions were made: firstly, that the AR is required by the spermatozoa prior to meeting the ZP for effective fertilisation[1]. Secondly, the cumulus oophorus confers benefit in increasing the chance of fertilisation[1].
References
Lab 2 Assessment
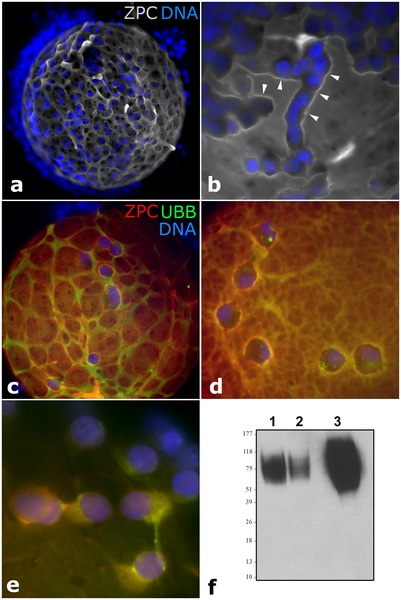
1. Upload an image from a journal source relating to fertilization or the first 2 weeks of development as demonstrated in the practical class. Including in the image “Summary” window: An image name as a section heading, Any further description of what the image shows, A subsection labeled “Reference” and under this the original image source, appropriate reference and all copyright information and finally a template indicating that this is a student image.
Patterns of ZPC Deposition in Porcine Oocyte-Cumulus Complexes
Immunofluorescence Detection for ZPC and Ubiquitin in a Porcine Oocyte [1]
References
- ↑ <pubmed>21383844</pubmed>
2. Identify a protein associated with the implantation process, including a brief description of the protein's role (1-2 paragraphs).
Trophinin and Implantation
Trophinin is a membrane protein expressed in chorionic villi trophoblasts and in the maternal endometrium. In the early stages of pregnancy, trophinin is strongly expressed along with tastin and bystin, which form a complex; this complex mediates apical cell adhesion between the trophoblasts and the endometrial epithelial cells[1]. The time frame in which trophinin is expressed on the apical aspect of the endometrial cells coincides with the "implantation window"[1]; the period in which successful implantation is possible. Trophonin-trophonin adhesion during implantation occurs via signal transduction with bystin and tastin[2]. As a consequence of trophinin-trophinin adhesion, trophectoderm cells become activated for implantation[2]. Moreover, there have been reports that endometrial epithelial cells undergo apoptosis upon blastocyst adhesion; human trophoectoderm cells express the Fas ligand which interacts with Fas expressed on the endometrium[2]. However, other studies have shown that trophinin-mediated cell adhesion can induce endometrial cell apoptosis through mechanisms other than the Fas/FasL cascade[2].
In regards to ectopic pregnancies located within the fallopian tube, research has shown that trophinin is strongly expressed by both the embryonic trophoblasts and maternal fallopian tube epithelium, induced by human chorionic gonadotrophin (hCG)[1]. These findings highlight the function of trophonin in facilitating implantation in conjunction with its role in the pathogenesis of ectopic pregnancies.
References
Lab 3 Assessment
1. Identify the difference between "gestational age" and "post-fertilisation age" and explain why clinically "gestational age" is used in describing human development.
Gestational Age versus Post-Fertilisation Age
Gestation is the period of time between conception and birth (Kaneshiro, 2011; Vishton, 2011). Gestational age is the developmental age of the conceptus based on the presumed first day of the last normal menstrual period to the current date, measured in weeks (Kaneshiro, 2011; Vishton, 2011). In contrast, post-fertilisation age refers to the age of the conceptus expressed in elapsed time since fertilisation (Vishton, 2011). Gestational age is approximately two weeks greater than post-fertilization age (Kaneshiro, 2011; Vishton, 2011). Gestational age is used in human development because its start date can be determined by asking the mother when was the presumed first day of the last normal menstrual period (Kaneshiro, 2011; Vishton, 2011). In contrast, the moment of fertilization must be inferred (Vishton, 2011).
References
Kaneshiro, N. K. (2011). Gestational age. Retrieved from http://www.umm.edu/ency/article/002367.htm Vishton, P. M. (2011). Embryo Foetus Development Stages. Retrieved from http://www.livestrong.com/article/92683-embryo-fetus-development-stages/
2.Identify using histological descriptions at least 3 different types of tissues formed from somites.
Tissues Derived From Somites
References </references>
Lab Attendance
Lab 1 --Z3333038 11:49, 25 July 2012 (EST)
Lab 2 --Z3333038 10:05, 1 August 2012 (EST)
Lab 3 --Z3333038 10:01, 8 August 2012 (EST)