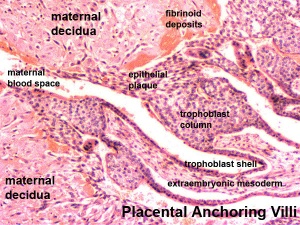
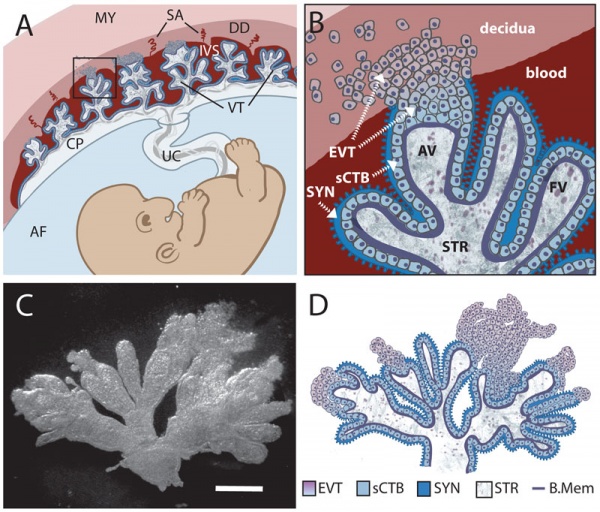
Trophoblast - Protein Expression
| Embryology - 23 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
This page relates to proteins expressed by trophoblast cells during syncitial development and implantation. This page is more detailed than a general description of trophoblast cells, see Trophoblast for a general introduction to this cell type. Many studies use animal models, term placenta or some new in vitro cell models.
Proteins expressed relate to differentiation, cell fusion, immune function, invasion processes.
- Links: HLA-G | Cytokeratin 8 | HSP27 | Reference Searches | Trophoblast | Implantation
Protein RNA and DNA Preparation
- Trizol
- http://www.sigmaaldrich.com/technical-documents/protocols/biology/tri-reagent.html tri-reagent]
Trophinin
Trophinin, tastin, and bystin identified as potentially involved in human embryo implantation.
- Trophinin-Associated Protein; Troap (tastin)
OVO-like 1
OVO-like 1 regulates progenitor cell fate in human trophoblast development[1]
- Cytotrophoblast cells either propagate or undergo a differentiation program fusing into an overlying syncytiotrophoblast.
- Syncytiotrophoblast is the primary barrier regulating the exchange of nutrients and gases between maternal and fetal blood and is the principal site for synthesizing hormones vital for human pregnancy.
- Transcription factor OVO-like 1 (OVOL1), a homolog of Drosophila ovo, regulates the transition from progenitor to differentiated trophoblast cells.
- OVOL1 is expressed in human placenta and is induced following stimulation of trophoblast differentiation.
- Disruption of OVOL1 abrogated cytotrophoblast fusion.
- OVOL1 was required to suppress genes that maintain cytotrophoblast cells in a progenitor state (MYC, ID1, TP63, and ASCL2) and bound specifically to regions upstream of each of these genes.
- Antibody: Rabbit Polyclonal anti-Human ABIN203039 | LSBiol
HLA-G
An acronym for histocompatibility antigen, class I, G (also called: Human Leukocyte Antigen G, (HLA-6.0; HLA60, T-CELL A LOCUS, TCA) and is expressed on placental cytotrophoblast cells and other adult tissues. This distinct tissue distribution differs from the other HLA antigens (HLA-A, HLA-B, HLA-C) leading to the description as a non-classical class I antigen. May have a role in protecting the fetus from the maternal immune response.
Human gene is located at 6p22.1 and there exist several protein isoforms from alternative splicing of messenger RNAs, membrane-bound isoforms (HLA-G 1-4) and soluble soluble (HLA-G 5-6)[2]. The molecule is a heterodimer consisting of both a heavy chain and a light chain (beta-2 microglobulin). The membrane-bound isoform heavy chain is anchored in the membrane and increased expression of the soluble form is related to higher implantation rates. Changes in HLA-G expression have been associated with increased miscarriage rates.[3] Killer cell immunoglobulin-like receptor (KIR) 2DL4 (KIR2DL4) has been shown to act as a receptor for the soluble HLA-G, leading to a stimulation of resting natural killer (NK) cells.[4]
- Links: HLA-G | OMIM142871 | OMIM 142871 | UniProt P17693 | Search PubMed
OMIM
- OMIM 142871 - HLAG HLA-6.0; HLA60, T-CELL A LOCUS; TCA
- Human leukocyte antigen (HLA)-G
- The HLA-G gene is monomorphic and the only MHC antigen expressed on cytotrophoblast cells of placenta.
- Extravillous trophoblasts from normal human placenta and the BeWo adherent human choriocarcinoma cell line express an unusual form of class I HLA molecule.
- nonclassic HLA-G class I molecules in inhibiting natural killer cell function.
- modulate the immunologic relationship between mother and fetus.
- alternatively spliced HLA-G isoforms in first trimester trophoblast cell populations. Several alternatively spliced HLA-G mRNA isoforms, including a 14-bp polymorphism in the 3-prime UTR end (exon 8) of the HLA-G gene, are expressed at a significantly lower level than the corresponding HLA-G mRNA isoforms with the 14-bp sequence deleted. Hviid et al. (2003) suggested that this finding may have functional implications in connection with reports of aberrant HLA-G expression and reproductive success.
- alternative splicing that generates seven HLA-G proteins.
UniProtein
- UniProt P17693 338 AA
- Involved in the presentation of foreign antigens to the immune system.
- Plays a role in maternal tolerance of the fetus by mediating protection from the deleterious effects of natural killer cells, cytotoxic T-lymphocytes, macrophages and mononuclear cells.
Immunohistochemical study of immunological markers: HLAG, CD16, CD25, CD56 and CD68 in placenta tissues in recurrent pregnancy loss
Histol Histopathol. 2014 Feb 21. [Epub ahead of print]
Papamitsou T1, Toskas A2, Papadopoulou K2, Sioga A2, Lakis S2, Chatzistamatiou M2, Economou Z3, Adriopoulou L2. Author information
Abstract
Introduction: Recurrent pregnancy loss (RPL) of unknown etiology is correlated with immunological alterations during pregnancy. Normally, changes in leukocyte subpopulations and HLA expression take place in pregnant uterus in order to tolerate the semi-allogenic embryo. Objective: Our research tries to enlighten the immunological changes that take place in the uterus of women with recurrent abortions of unknown etiology during first trimester of pregnancy. Materials and methods: The miscarriage group was obtained from 25 women who miscarried between the ages of 35 to 42 years and controls consisted of 25 healthy women between the ages of 27 to 39 years, who had electively terminated their pregnancies during the first trimester of pregnancy. The abortion was processed and specimens taken were studied using immunohistochemical methods. Specimens were taken from decidua basalis and decidua parietalis. Monoclonal antibodies were used against HLAG (Human Leukocyte Antigen G), CD68( Cluster of Differentiation 68), CD56, CD16 and CD25. The results were statistically analysed with Mann-Whitney test. Results: HLA-G expression in decidua basalis from miscarriage group was found to be decreased. CD25+ cell expression was found to be invariable in deciduas from both groups. CD16+ cell and CD68 + cell expression was found to be increased in deciduas from the miscarriage group. CD56+ cell expression was found to be increased in decidua parietalis from miscarriage group. Conclusion : Several differences in the immunological profile of deciduas from RPL group were observed. Changes in feto-protective HLA-G expression and a possible implication of macrophages and NK cells were found.
PMID 24557735
HLA-G molecule
Curr Pharm Des. 2009;15(28):3318-24. Kamishikiryo J1, Maenaka K. Author information
Abstract
Human leukocyte antigen-G (HLA-G) is a non-classical HLA class I molecule, which was first discovered in 1987 by Geraghty and colleagues. While classical HLA class I molecules are expressed on all nucleated cells, the expression of the HLA-G molecule is highly tissue-restricted, such as to placental trophoblast cells. HLA-G binds inhibitory receptors such as leukocyte immunoglobulin-like receptors B1 (LILRB1/ILT2/CD85j) and LILRB2 (ILT4/CD85d), which are widely expressed on immune cells, to suppress a broad range of immune responses. Thus, the expression of HLA-G in placenta protects the fetus from the maternal immune system. On the other hand, emerging studies have shown the relevance of the HLA-G molecule in pathologic conditions, such as transplantation rejection, autoimmunity, and cancer. HLA-G has other unique characteristics, in contrast with classical HLA molecules, including the existence of various forms of HLA-G: several splice variants, subunit-deficient conformations, homodimers, and their combinations have been found. In this review, we highlight the molecular basis for the tolerogenic ability of the HLA-G molecule, especially by LILR recognition of various forms of HLA-G. We also discuss the potential clinical applications of HLA-G molecules.
PMID 19860681
A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment
Proc Natl Acad Sci U S A. 1987 Dec;84(24):9145-9.
Geraghty DE1, Koller BH, Orr HT. Author information
Abstract
We have cloned genomic DNA encoding a non-HLA-A, -B, -C class I gene located within a HindIII-generated restriction fragment of 6.0 kilobase pairs. This gene, designated HLA-6.0, is as homologous to HLA-A and HLA-B as they are to each other. The HLA class I protein encoded by HLA-6.0 is similar in organization to the HLA-A-, -B-, and -C-encoded proteins except that an in-frame termination codon prevents translation of a majority of the cytoplasmic region of the HLA-6.0 polypeptide. Moreover, the promoter region of HLA-6.0 resembles the promoter region of a Qa region gene. These structural features of HLA-6.0 suggest that this non-HLA-A, -B, -C gene is a structural homolog of a murine Qa region class I gene.
PMID 3480534
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC299709
http://www.pnas.org/content/84/24/9145.long
HLAG Elisa Kits
Antibody Resource - HLAG Elisa Kits
Biovendor
- RD194070100R sHLA-G ELISA is a sandwich enzyme immunoassay for the quantitative measurement of soluble forms of human leukocyte antigen-G (sHLA-G).
- The total assay time is about 20 hours
- The kit measures shedded HLA-G1 and HLA-G5 in plasma-EDTA, amniotic fluid, or cell culture supernatant Calibrator is human native protein
- Assay format is 96 wells
- Components of the kit are provided ready to use, concentrated or lyophilised
- RD194070100R 96 wells (1 kit) 795 €
EXBIO Antibodies
- RD194070100R 874 USD
AMS Bio
- Human Leukocyte antigen G ELISA (HLA-G)
- AMS.E01H1255 Human USD 930
- All Species Kits
United States Biological
- Catalog H6098-71
- sHLA-G ELISA is a double monoclonal sandwich enzyme immunoassay for the quantitative measurement of soluble forms of Human Leukocyte Antigen-G (sHLA-G) in amniotic fluid, cell culture supernatant and plasma or serum.
Cytokeratin 8
- Links: OMIM148060
Trophoblast cell
- Type II keratin 8 (K8) and type I keratin 18 (K18) first intermediate filament proteins
- mouse - K8 provides trophoblast giant cells resistance to a maternal challenge (trophoblast barrier function failure leads to embryo death)
- intermediate filament protein, intracellular localisation, TROMA-1 antibody
- KRT8, 485 AA
- Endo A is the mouse equivalent. Endo B, which is the equivalent of human keratin 18
- Cytokeratin (feline)[5]
Melanocortin-1-Recepto
- Melanocortin-1-Receptor (MC1R) expressed on trophoblast cells.[6]
Laser-assisted blastocyst dissection and subsequent cultivation of embryonic stem cells in a serum/cell free culture system: applications and preliminary results in a murine model
J Transl Med. 2006 May 8;4:20.
Tanaka N, Takeuchi T, Neri QV, Sills ES, Palermo GD. Source Center for Reproductive Medicine and Infertility, Weill Medical College of Cornell University, New York, NY 10021, USA. not2003@med.cornell.edu Abstract BACKGROUND: To evaluate embryonic stem cell (ESC) harvesting methods with an emphasis on derivation of ESC lines without feeder cells or sera. Using a murine model, laser-assisted blastocyst dissection was performed and compared to conventional immunosurgery to assess a novel laser application for inner cell mass (ICM) isolation. METHODS: Intact blastocysts or isolated ICMs generated in a standard mouse strain were plated in medium with or without serum to compare ESC harvesting efficiency. ESC derivation was also undertaken in a feeder cell-free culture system. RESULTS: Although ICM growth and dissociation was comparable irrespective of the media components, an enhanced ESC harvest was observed in our serum-free medium (p < 0.01). ESC harvest rate was not affected by ICM isolation technique but was attenuated in the feeder cell-free group. CONCLUSION: Achieving successful techniques for human ESC research is fundamentally dependent on preliminary work using experimental animals. In this study, all experimentally developed ESC lines manifested similar features to ESCs obtained from intact blastocysts in standard culture. Cell/sera free murine ESC harvest and propagation are feasible procedures for an embryology laboratory and await refinements for translation to human medical research.
PMID 16681851
Reactivity of two monoclonal antibodies (Troma 1 and CAM 5.2) on human tissue sections: analysis of their usefulness as a histological trophoblast marker in normal pregnancy and trophoblastic disease
Int J Gynecol Pathol. 1986;5(4):345-56.
Sasagawa M, Watanabe S, Ohmomo Y, Honma S, Kanazawa K, Takeuchi S.
Abstract
In normal and molar pregnancy, a morphological discrimination between nonvillous trophoblasts which lie scattered in the placental bed and surrounding maternal cells is considered to be difficult. We examined the reactivity of two monoclonal antibodies (Troma 1 and CAM 5.2) against cytokeratin by an immunoperoxidase technique and analyzed their usefulness as a histological trophoblast marker. Materials were taken from 42 uteri with normal pregnancy, 7 uteri with hydatidiform mole, 2 uteri with gestational choriocarcinoma, 1 fallopian tube with nongestational choriocarcinoma, 5 delivered term placentae of normal pregnancy, and 5 nongestational uteri. The reactivities of Troma 1 on frozen sections and those of CAM 5.2 on paraffin sections were identical. They reacted with surface epithelium and gland epithelium in the nongestational uterine corpus. In the implantation site of normal and molar pregnancy, they reacted with villous and nonvillous trophoblasts as well as endometrial gland epithelium. In gestational and nongestational choriocarcinoma, they reacted with carcinoma cells specifically. Since the histological detection of gland epithelium may not be difficult, it was concluded that the two antibodies were very beneficial as a histological marker for trophoblasts in normal pregnancy and trophoblastic disease.
PMID 2433238
Keratin 8 protection of placental barrier function
J Cell Biol. 2003 May 26;161(4):749-56.
Jaquemar D1, Kupriyanov S, Wankell M, Avis J, Benirschke K, Baribault H, Oshima RG. Author information
Abstract
The intermediate filament protein keratin 8 (K8) is critical for the development of most mouse embryos beyond midgestation. We find that 68% of K8-/- embryos, in a sensitive genetic background, are rescued from placental bleeding and subsequent death by cellular complementation with wild-type tetraploid extraembryonic cells. This indicates that the primary defect responsible for K8-/- lethality is trophoblast giant cell layer failure. Furthermore, the genetic absence of maternal but not paternal TNF doubles the number of viable K8-/- embryos. Finally, we show that K8-/- concepti are more sensitive to a TNF-dependent epithelial apoptosis induced by the administration of concanavalin A (ConA) to pregnant mothers. The ConA-induced failure of the trophoblast giant cell barrier results in hematoma formation between the trophoblast giant cell layer and the embryonic yolk sac in a phenocopy of dying K8-deficient concepti in a sensitive genetic background. We conclude the lethality of K8-/- embryos is due to a TNF-sensitive failure of trophoblast giant cell barrier function. The keratin-dependent protection of trophoblast giant cells from a maternal TNF-dependent apoptotic challenge may be a key function of simple epithelial keratins. PMID 12771125
TROMA-I Antibody
http://dshb.biology.uiowa.edu/cytokeratin-Endo-A
http://dshb.biology.uiowa.edu/Price-list
Antigen: cytokeratin 8, Endo-A
Contributor: Brulet, P. / Kemler, R.
Cells Available: Yes
Host Species: rat
Isotype: IgG2a, kappa light chain
Antigen Species: mouse
Species Tested: mouse, human
Immunoblotting: Yes
References: Proc. Nat'l. Acad. Sci. 77, 4113-4117.; J. Embryol. Exp. Morph. 64, 45-60.; Exp. Cell Res. 154(1), 315-319.; J. Cell Sci. 109, 2789-2800.; J. Comp. Path. 119, 177-181.; Dev. Biol. 253, 258-263.; Int. J. Cancer 113, 525-532.; Cell 138, 592-603.
HSP27
The effect of heat shock protein 27 on extravillous trophoblast differentiation and on eukaryotic translation initiation factor 4E expression
Mol Hum Reprod. 2014 Feb 24. [Epub ahead of print]
Sadeh-Mestechkin D1, Epstein Shochet G, Pomeranz M, Fishman A, Drucker L, Biron-Shental T, Lishner M, Tartakover Matalon S. Author information
Abstract
Heat shock protein (HSP27) is expressed in human placentae. Previously, we showed that HSP27 is expressed in the villous cell column of first trimester placental explants and in extravillous trophoblast (EVT) cells. EVT differentiation is accompanied by increased motility, matrix metalloproteinase (MMP) activity, decreased proliferation and expression of specific markers such as HLAG and CD9. HSP27 regulates cell apoptosis, migration, protein stability and the availability of eukaryotic translation initiation factors, such as eukaryotic translation initiation factor 4E (eIF4E). eIF4E supports trophoblast cell proliferation and survival. We wanted to explore the effect of HSP27 silencing on trophoblast cell phenotype, EVT markers and eIF4E expression and regulators [4E-binding protein (4E-BP1) and MAP kinase-interacting kinase (MNK1)]. This study evaluated the effect of HSP27 siRNA on placental explant and HTR-8/SVneo migration, MMP activity/mRNA, cell death, cell cycle, HLAG/CD9 levels, and eIF4E and its regulators' total and phosphorylated levels. Furthermore, we evaluated HSP27 levels in placentae exposed to ribavirin, which triggers EVT differentiation. We found that HSP27 silencing increased cell death in HTR-8/SVneo and placental explants. Furthermore, it reduced HTR-8/SVneo migration and EVT outgrowth from the explants (P < 0.05), MMP2 activity and expression of EVT markers HLAG and CD9 (in placental explants and HTR-8/SVneo, respectively, P < 0.05). Induction of EVT differentiation by ribavirin elevated HSP27 levels. Finally, HSP27 silencing in both HTR-8/SVneo and placental explants reduced eIF4E levels (33 and 28%, respectively, P < 0.05) and the levels of its regulators 4E-BP1 and MNK1 (37 and 32%, respectively, done on HTR-8/SVneo only), but not their phosphorylated forms. Altogether, our results suggest that HSP27 contributes to EVT cell differentiation. KEYWORDS: HSP27, differentiation, eIF4E, placenta
PMID 24431103
HSP27 ELISA Kits
Alternative protein names: Heat shock 17 kDa protein / HSP 17 / HSP27 / HSPB3 / HSPL27 / Protein 3 / Q12988 / Q9QZ57 / Q9QZ58
- Heat shock protein beta-1 ELISA Kits 23 ELISA Kits
- Heat shock protein beta-3 ELISA Kits 7 ELISA Kits from 2 suppliers.
Ambio
- AMS.E09H0289 - Monkey
Boster Immunoleader
StressMarq
Sino Biological
- minimum detectable dose of Human HSP27 was determined to be approximately 9.4 pg/ml.
- SEK10351 5 Plates ($350)
- double antibody kit need to coat your own plates.
Abnova
- KA1065 USD $ 598
Geminin
The dual roles of geminin during trophoblast proliferation and differentiation
Dev Biol. 2014 Mar 1;387(1):49-63. doi: 10.1016/j.ydbio.2013.12.034. Epub 2014 Jan 9.
de Renty C1, Kaneko KJ1, Depamphilis ML2. Author information
Abstract
Geminin is a protein involved in both DNA replication and cell fate acquisition. Although it is essential for mammalian preimplantation development, its role remains unclear. In one study, ablation of the geminin gene (Gmnn) in mouse preimplantation embryos resulted in apoptosis, suggesting that geminin prevents DNA re-replication, whereas in another study it resulted in differentiation of blastomeres into trophoblast giant cells (TGCs), suggesting that geminin regulates trophoblast specification and differentiation. Other studies concluded that trophoblast differentiation into TGCs is regulated by fibroblast growth factor-4 (FGF4), and that geminin is required to maintain endocycles. Here we show that ablation of Gmnn in trophoblast stem cells (TSCs) proliferating in the presence of FGF4 closely mimics the events triggered by FGF4 deprivation: arrest of cell proliferation, formation of giant cells, excessive DNA replication in the absence of DNA damage and apoptosis, and changes in gene expression that include loss of Chk1 with up-regulation of p57 and p21. Moreover, FGF4 deprivation of TSCs reduces geminin to a basal level that is required for maintaining endocycles in TGCs. Thus, geminin acts both like a component of the FGF4 signal transduction pathway that governs trophoblast proliferation and differentiation, and geminin is required to maintain endocycles. Published by Elsevier Inc. KEYWORDS: Cdkn1a/p21/Cip1, Cdkn1c/p57/Kip2, Chk1, Differentiation, Endocycles, Endoreplication, Geminin, Trophectoderm, Trophoblast giant cells, Trophoblast stem cells
PMID 24412371
ADAM12
A disintegrin and metalloproteinase 12 (ADAM12) localizes to invasive trophoblast, promotes cell invasion and directs column outgrowth in early placental development
Mol Hum Reprod. 2014 Mar;20(3):235-49. doi: 10.1093/molehr/gat084. Epub 2013 Nov 15. Aghababaei M1, Perdu S, Irvine K, Beristain AG. Author information
Abstract
During pregnancy, stromal- and vascular-remodeling trophoblasts serve critical roles in directing placental development acquiring pro-invasive characteristics. The A Disintegrin and Metalloproteinase (ADAM) family of multifunctional proteins direct cellular processes across multiple organ systems via their intrinsic catalytic, cell adhesive and intracellular signaling properties. ADAM12, existing as two distinct splice variants (ADAM12L and ADAM12S), is highly expressed in the human placenta and promotes cell migration and invasion in several tumor cell lines; however, its role in trophoblast biology is unknown. In this study, ADAM12 was localized to anchoring trophoblast columns in first trimester placentas and to highly invasive extracellular matrix-degrading trophoblasts in placental villous explants. The importance of ADAM12 in directing trophoblast invasion was tested using loss-of and gain-of-function strategies, where siRNA-directed knockdown of ADAM12 inhibited trophoblast cell invasion while over-expression promoted migration and invasion in two trophoblastic cell models. In placental villous explant cultures, siRNA-directed loss of ADAM12 significantly dampened trophoblast column outgrowth. Additionally, we provide functional evidence for the ADAM12S variant in promoting trophoblast invasion and column outgrowth through a mechanism requiring its catalytic activity. This is the first study to assign a function for ADAM12 in trophoblast biology, where ADAM12 may play a central role regulating the behavior of invasive trophoblast subsets in early pregnancy. This study also underlines the importance of ADAM12L and ADAM12S in directing cell motility in normal developmental processes outside of cancer, specifically highlighting a potentially important function of ADAM12S in directing early placental development. KEYWORDS: A Disintegrin and Metalloproteinase 12, cell invasion, placenta, protease, trophoblast
PMID 24243624
Mucin Role
|
|
| Model with Mucin | Model without Mucin |
ATP-Binding Cassette, Subfamily A, Member 1
ABCA1 functions as a cholesterol efflux pump in the cellular lipid removal pathway.
<pubmed>29066252</pubmed>
To study the potential impact of ABCA1 on the function of the placenta.
Trophoblasts and macrophages were isolated from the placenta with enzymatic digestion; Immunofluorescence assay was used to detect the location of ABCA1 in cells; RT-PCR and Western-blot were used to detect the expression of ABCA1; The cholesterol efflux assays of primary trophoblasts was detected by Amplex Red cholesterol assay kit (Invitrogen);Inflammatory factor secretion from primary macrophages was detected by Elisa.
ABCA1 was mainly located on trophoblast membranes. Decreased ABCA1 expression in trophoblasts reduced the cholesterol efflux of trophoblasts (P<0.01). while increased ABCA1 expression in trophoblasts reduced the cholesterol efflux of trophoblasts (P<0.05). ABCA1 was uniformly expressed on the cell membrane, cytoplasm, and nucleus of macrophages. Decreased ABCA1 expression in macrophages, increased inflammatory factors but reduced IL-10 (P<0.01). While increased ABCA1 expression in macrophages, reduced inflammatory factors but increased IL-10 (P<0.01).
SIGNIFICANCE: ABCA1 may be a potential target for the prevention of gestation diseases.
Ectopic
- Endocannabinoids G protein-coupled cannabinoid receptor CB1 (ectopic pregnancy show down-regulation) PMID 19093002
Reference Searches
Note - This sub-heading shows an automated computer PubMed search using the listed sub-heading term. References appear in this list based upon the date of the actual page viewing. Therefore the list of references do not reflect any editorial selection of material based on content or relevance. In comparison, references listed on the content page and discussion page (under the publication year sub-headings) do include editorial selection based upon relevance and availability. (More? Pubmed Most Recent)
Trophoblast
<pubmed limit=5>Trophoblast</pubmed>
Syncitiotrophoblast
<pubmed limit=5>Syncitiotrophoblast</pubmed>
Cytotrophoblast
<pubmed limit=5>Cytotrophoblast</pubmed>
HLAG
<pubmed limit=5>HLAG</pubmed>
Secreted protein of Ly-6 domain 1 (SOLD1)
http://www.rbej.com/content/12/1/55/abstract
- regulation of the trophoblast invasiveness.
- secretory-type member of the Ly-6 superfamily
- expressed in both fetal and maternal tissues throughout gestation.
- SOLD1 mRNA is expressed in the endometrium and in trophoblast mononucleate and binucleate cells
Related References
2014
Expression and potential roles of HLA-G in human spermatogenesis and early embryonic development
PLoS One. 2014 Mar 25;9(3):e92889. doi: 10.1371/journal.pone.0092889. eCollection 2014.
Yao GD1, Shu YM2, Shi SL1, Peng ZF1, Song WY1, Jin HX1, Sun YP1.
Abstract
As one of the non-classical major histocompatibility complex(MHC)-1 antigens, Human Leukocyte Antigen G (HLA-G), has been suggested as a prognostic marker to identify the embryo developmental potential. In the present study, we investigated the potential roles of HLA-G in human spermatogenesis and early embryonic development. Quantitative real-time PCR analysis revealed that HLA-G's expression was increased with increased Johnsen score in testicular tissues. There was no significant difference in HLA-G mRNA expression between testicular tissues with Johnsen score of 8-9 and normal sperm from ejaculated semen. HLA-G mRNA expression was detected in human zygotes, embryos and blastocysts but not in unfertilized oocytes. In testicular tissues where sperm was obtained by testicular sperm extraction (Johnsen score was 8 to 9), there were no correlations between HLA-G mRNA expression and fertilization, cleavage and high-quality embryo rates. At 48-72 h post-fertilization, HLA-G expression was higher in fast growing embryos. HLA-G specific siRNA injection into zygotes not only slowed down embryonic cleavage rate at 48 h post-fertilization, but also down-regulated the expression of embryo metabolism related gene (SLC2A1) and cell cycle-regulated gene (CCND2). Taken together, our findings suggested that HLA-G plays significant roles in human spermatogenesis and early embryonic development.
- RNA Extraction - Frozen testicular tissues were diced and fully grinded in liquid nitrogen, and then mixed with lysis buffer of PureLink RNA Mini Kit (Life Technologies, Inc., Gaithersburg, MD). RNA was extracted using the PureLink RNA Mini Kit.
- PCR primers described for HLA-G;
PMID 24667226
http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0092889
Immunohistochemical study of immunological markers: HLAG, CD16, CD25, CD56 and CD68 in placenta tissues in recurrent pregnancy loss
Histol Histopathol. 2014 Feb 21. [Epub ahead of print]
Papamitsou T1, Toskas A2, Papadopoulou K2, Sioga A2, Lakis S2, Chatzistamatiou M2, Economou Z3, Adriopoulou L2. Author information
Abstract
Introduction: Recurrent pregnancy loss (RPL) of unknown etiology is correlated with immunological alterations during pregnancy. Normally, changes in leukocyte subpopulations and HLA expression take place in pregnant uterus in order to tolerate the semi-allogenic embryo. Objective: Our research tries to enlighten the immunological changes that take place in the uterus of women with recurrent abortions of unknown etiology during first trimester of pregnancy. Materials and methods: The miscarriage group was obtained from 25 women who miscarried between the ages of 35 to 42 years and controls consisted of 25 healthy women between the ages of 27 to 39 years, who had electively terminated their pregnancies during the first trimester of pregnancy. The abortion was processed and specimens taken were studied using immunohistochemical methods. Specimens were taken from decidua basalis and decidua parietalis. Monoclonal antibodies were used against HLAG (Human Leukocyte Antigen G), CD68( Cluster of Differentiation 68), CD56, CD16 and CD25. The results were statistically analysed with Mann-Whitney test. Results: HLA-G expression in decidua basalis from miscarriage group was found to be decreased. CD25+ cell expression was found to be invariable in deciduas from both groups. CD16+ cell and CD68 + cell expression was found to be increased in deciduas from the miscarriage group. CD56+ cell expression was found to be increased in decidua parietalis from miscarriage group. Conclusion : Several differences in the immunological profile of deciduas from RPL group were observed. Changes in feto-protective HLA-G expression and a possible implication of macrophages and NK cells were found.
PMID 24557735
Intermediate conductance Ca2+-activated k+ channels modulate human placental trophoblast syncytialization
PLoS One. 2014 Mar 3;9(3):e90961. doi: 10.1371/journal.pone.0090961. eCollection 2014.
Díaz P, Wood AM, Sibley CP, Greenwood SL. Author information
Abstract
Regulation of human placental syncytiotrophoblast renewal by cytotrophoblast migration, aggregation/fusion and differentiation is essential for successful pregnancy. In several tissues, these events are regulated by intermediate conductance Ca2+-activated K+ channels (IKCa), in part through their ability to regulate cell volume. We used cytotrophoblasts in primary culture to test the hypotheses that IKCa participate in the formation of multinucleated syncytiotrophoblast and in syncytiotrophoblast volume homeostasis. Cytotrophoblasts were isolated from normal term placentas and cultured for 66 h. This preparation recreates syncytiotrophoblast formation in vivo, as mononucleate cells (15 h) fuse into multinucleate syncytia (66 h) concomitant with elevated secretion of human chorionic gonadotropin (hCG). Cells were treated with the IKCa inhibitor TRAM-34 (10 µM) or activator DCEBIO (100 µM). Culture medium was collected to measure hCG secretion and cells fixed for immunofluorescence with anti-IKCa and anti-desmoplakin antibodies to assess IKCa expression and multinucleation respectively. K+ channel activity was assessed by measuring 86Rb efflux at 66 h. IKCa immunostaining was evident in nucleus, cytoplasm and surface of mono- and multinucleate cells. DCEBIO increased 86Rb efflux 8.3-fold above control and this was inhibited by TRAM-34 (85%; p<0.0001). Cytotrophoblast multinucleation increased 12-fold (p<0.05) and hCG secretion 20-fold (p<0.05), between 15 and 66 h. Compared to controls, DCEBIO reduced multinucleation by 42% (p<0.05) and hCG secretion by 80% (p<0.05). TRAM-34 alone did not affect cytotrophoblast multinucleation or hCG secretion. Hyposmotic solution increased 86Rb efflux 3.8-fold (p<0.0001). This effect was dependent on extracellular Ca2+, inhibited by TRAM-34 and 100 nM charybdotoxin (85% (p<0.0001) and 43% respectively) but unaffected by 100 nM apamin. In conclusion, IKCa are expressed in cytotrophoblasts and their activation inhibits the formation of multinucleated cells in vitro. IKCa are stimulated by syncytiotrophoblast swelling implicating a role in syncytiotrophoblast volume homeostasis. Inappropriate activation of IKCa in pathophysiological conditions could compromise syncytiotrophoblast turnover and volume homeostasis in pregnancy disease.
PMID 24595308
IgG expression in trophoblasts derived from placenta and gestational trophoblastic disease and its role in regulating invasion
Immunol Res. 2014 Jan 28. [Epub ahead of print]
Yang M, Ha C, Liu D, Xu Y, Ma Y, Liu Y, Nian Y. Author information
Abstract Immunoglobulin G (IgG) is an important humoral immune factor, which plays a role in innate immunity of the fetus. IgG immunoreactivity was often seen in trophoblasts of placenta. Traditionally, IgG in trophoblasts was believed to be transported from the maternal blood through neonatal Fc receptor (FcRn). Here, we explored the phenomenon of IgG expression and its role in regulating invasion in trophoblasts derived from normal placenta and gestational trophoblastic disease (GTD). IgG expression was detected with an emphasis on mRNA transcripts by using reverse transcription-polymerase chain reaction and hybridization in situ, besides evaluated at the protein level with immunohistochemistry and immunofluorescence. The migration and attachment of normal trophoblast cell line (TEV-1) and choriocarcinoma cell line (JAR) were inhibited with down-regulation of IgG expression. Methotrexate promoted the differentiation of JAR cell line; however, it had little effect on the differentiation of TEV-1 cell line. IgG expression, migration, and attachment of JAR and TEV-1 cell lines were decreased in the presence of methotrexate. Furthermore, statistical analysis showed that the differences in migration and attachment were significant (P < 0.05) for JAR cell line, while no significant difference was found for TEV-1 cell line. Collectively, these results confirmed that with the progression from normal placenta to GTD, the expression of IgG was increased in trophoblasts, which might actively promote the migration and attachment of trophoblasts as an important regulating factor.
PMID 24469916
2013
The proprotein convertase furin is required for trophoblast syncytialization
Cell Death Dis. 2013 Apr 18;4:e593. doi: 10.1038/cddis.2013.106.
Zhou Z, Zhang Q, Lu X, Wang R, Wang H, Wang YL, Zhu C, Lin HY, Wang H. Source 1] State Key Laboratory of Reproductive Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China [2] Graduate School of the Chinese Academy of Sciences, Beijing 100039, China. Abstract The multinucleated syncytial trophoblast, which forms the outermost layer of the placenta and serves multiple functions, is differentiated from and maintained by cytotrophoblast cell fusion. Deficiencies in syncytial trophoblast differentiation or maintenance likely contribute to intrauterine growth restriction and pre-eclampsia, two common gestational diseases. The cellular and molecular mechanisms governing trophoblast syncytialization are poorly understood. We report here that the proprotein convertase furin is highly expressed in syncytial trophoblast in the first trimester human placentas, and expression of furin in the syncytiotrophoblast is significantly lower in the placentas from pre-eclamptic patients as compared with their gestational age-matched control placentas. Using multiple experimental models including induced fusion of choriocarcinoma BeWo cells and spontaneous fusion of primary cultured cytotrophoblast cells or placental explants, we demonstrate that cytotrophoblast cell fusion and syncytialization are accompanied by furin expression. Furin-specific siRNAs or inhibitors inhibit cell fusion in BeWo cells, as well as trophoblast syncytialization in human placental explants. Furthermore, type 1 IGF receptor (IGF1R) is indicated in this study as a substrate of furin, and processing of IGF1R by furin is an essential mechanism for syncytialization. Finally, using lentivirus-mediated RNAi targeting to mouse trophectoderm, we demonstrate that furin function is required for the development of syncytiotrophoblast structure in the labyrinth layer, as well as for normal embryonic development.
PMID 23598405
Reduced syncytin-1 expression levels in placental syndromes correlates with epigenetic hypermethylation of the ERVW-1 promoter region
PLoS One. 2013;8(2):e56145. doi: 10.1371/journal.pone.0056145. Epub 2013 Feb 14.
Ruebner M, Strissel PL, Ekici AB, Stiegler E, Dammer U, Goecke TW, Faschingbauer F, Fahlbusch FB, Beckmann MW, Strick R. Source University-Clinic Erlangen, Department of Gynaecology and Obstetrics, Laboratory for Molecular Medicine, Erlangen, Germany. Matthias.Ruebner@uk-erlangen.de
Abstract
Terminal differentiation of villous cytotrophoblasts (CT) ends in formation of the multinucleated syncytiotrophoblast representing the fetal-maternal interface. Aberrations during this cell-fusion process are associated with Intrauterine Growth Restriction (IUGR), Preeclampsia (PE) and High Elevated Liver and Low Platelets (HELLP) Syndrome. Syncytin-1, the envelope gene of the human Endogenous Retrovirus ERVW-1, is one of the most important genes involved in cell-fusion and showed decreased gene expression during these pathological pregnancies. The aim of this study was to determine the methylation pattern of the entire promoter of ERVW-1 and to correlate these findings with the expression profile of Syncytin-1 in the placental syndromes. 14 isolated villous cytotrophoblasts from control (n = 3), IUGR (n = 3), PE (n = 3), PE/IUGR (n = 3) and HELLP/IUGR (n = 2) placentae were used to determine the mean methylation level (ML) for the ERVW-1 promoter region. ML rose significantly from 29% in control CTs to 49% in IUGR, 53% in PE, 47% in PE/IUGR and 64% in HELLP/IUGR indicating an epigenetic down-regulation of Syncytin-1 by promoter hypermethylation. DNA demethylation of the trophoblast like cell lines BeWo, JEG-3 and JAR with 5-AZA-2'desoxycytidine (AZA) showed an increased Syncytin-1 expression and fusion ability in all cell lines. Promoter activity of the 5'LTR could be inhibited by hypermethylation 42-fold using a luciferase based reporter-gene assay. Finally overexpression of the methyltransferases DNMT3a and LSH could be responsible for a decreased Syncytin-1 expression by promoter hypermethylation of ERVW-1. Our study linked decreased Syncytin-1 expression to an epigenetic hypermethylation of the entire promoter of ERVW-1. Based on our findings we are predicting a broad aberrant epigenetic DNA-methylation pattern in pathological placentae affecting placentogenesis, but also the development of the fetus and the mother during pregnancy.
PMID 23457515
2012
Epigenetic features of the mouse trophoblast
Reprod Biomed Online. 2012 Jul;25(1):21-30. doi: 10.1016/j.rbmo.2012.01.012. Epub 2012 Jan 25.
Rugg-Gunn PJ. Source Epigenetics Programme, The Babraham Institute, Babraham Research Campus, Cambridge, UK. peter.rugg-gunn@babraham.ac.uk Abstract Trophoblast cells are required for the growth and survival of the fetus during pregnancy, and failure to maintain appropriate trophoblast regulation is associated with placental insufficiencies and intrauterine growth restriction. Development of the trophoblast lineage is mediated by interactions between genetic and epigenetic factors. This review will focus on new insights that have been gained from analysis of mouse models into the epigenetic mechanisms that are required for the early establishment of the trophoblast lineage and for the development of specialized cell types of the fetal placenta. In particular, the importance of DNA methylation, 5-hydroxymethylcytosine and histone modifications in orchestrating trophoblast gene expression and functional outcome will be discussed. These insights are beginning to be extended towards human studies and initial results suggest that the causes and consequences of a variety of placental pathologies are related to epigenetic processes. Furthermore, the epigenetic landscape that regulates trophoblast cells seems to be particularly vulnerable to perturbation during development. This has major implications for diet and other environmental factors during pregnancy. Copyright © 2012 Reproductive Healthcare Ltd. Published by Elsevier Ltd. All rights reserved.
PMID 22578826
Inhibition of histone deacetylase activity in human endometrial stromal cells promotes extracellular matrix remodelling and limits embryo invasion
PLoS One. 2012;7(1):e30508. Epub 2012 Jan 26.
Estella C, Herrer I, Atkinson SP, Quiñonero A, Martínez S, Pellicer A, Simón C. Source Fundación Instituto Valenciano de Infertilidad, Valencia University, and Instituto Universitario IVI/INCLIVA, Valencia, Spain.
Abstract
Invasion of the trophoblast into the maternal decidua is regulated by both the trophoectoderm and the endometrial stroma, and entails the action of tissue remodeling enzymes. Trophoblast invasion requires the action of metalloproteinases (MMPs) to degrade extracellular matrix (ECM) proteins and in turn, decidual cells express tissue inhibitors of MMPs (TIMPs). The balance between these promoting and restraining factors is a key event for the successful outcome of pregnancy. Gene expression is post-transcriptionally regulated by histone deacetylases (HDACs) that unpacks condensed chromatin activating gene expression. In this study we analyze the effect of histone acetylation on the expression of tissue remodeling enzymes and activity of human endometrial stromal cells (hESCs) related to trophoblast invasion control. Treatment of hESCs with the HDAC inhibitor trichostatin A (TSA) increased the expression of TIMP-1 and TIMP-3 while decreased MMP-2, MMP-9 and uPA and have an inhibitory effect on trophoblast invasion. Moreover, histone acetylation is detected at the promoters of TIMP-1 and TIMP-3 genes in TSA-treated. In addition, in an in vitro decidualized hESCs model, the increase of TIMP-1 and TIMP-3 expression is associated with histone acetylation at the promoters of these genes. Our results demonstrate that histone acetylation disrupt the balance of ECM modulators provoking a restrain of trophoblast invasion. These findings are important as an epigenetic mechanism that can be used to control trophoblast invasion.
PMID 22291969 [PubMed - indexed for MEDLINE] PMCID: PMC3266920
Early Expression of Pregnancy-Specific Glycoprotein 22 (PSG22) by Trophoblast Cells Modulates Angiogenesis in Mice
Biol Reprod. 2012 Mar 14. [Epub ahead of print]
Blois S, Tirado-Gonzalez I, Wu J, Barrientos G, Johnson B, Warren J, Freitag N, Klapp B, Irmak S, Ergün S, Dveksler G.
Abstract
Mouse and human pregnancy-specific glycoproteins (PSG) are known to exert immunomodulatory functions during pregnancy by inducing maternal leukocytes to secrete anti-inflammatory cytokines that promote a tolerogenic decidual microenvironment. Many such anti-inflammatory mediators also function as pro-angiogenic factors, which, along with the reported association of -murine PSG - with the uterine vasculature, suggest that PSG may contribute to the vascular adaptations necessary for successful implantation and placental development. We observed that PSG22 is strongly expressed around the embryonic crypt on gestation day (Gd) 5.5, indicating that trophoblast giant cells are the main source of PSG22 during the early stages of pregnancy. PSG22 treatment up-regulated the secretion of transforming growth factor beta 1 (TGFB1) and vascular endothelial growth factor A (VEGFA) in murine macrophages, uterine dendritic cells and natural killer cells. A possible role of PSGs in uteroplacental angiogenesis is further supported by the finding that incubation of endothelial cells with PSG22 resulted in the formation of tubes in the presence and absence of VEGFA. We determined that PSG22, like human PSG1 and murine PSG17 and 23, binds to the heparan sulfate chains in syndecans. Therefore, our findings indicate that despite the independent evolution and expansion of human and rodent PSG, members in both families have conserved functions, which include their ability to induce anti-inflammatory cytokines and pro-angiogenic factors as well as to induce the formation of capillary structures by endothelial cells. In summary, our results indicate that PSG22, the most abundant PSG expressed during mouse early pregnancy, is likely a major contributor to the establishment of a successful pregnancy.
PMID 22423048
2010
Trisomy 21- affected placentas highlight prerequisite factors for human trophoblast fusion and differentiation
Int J Dev Biol. 2010;54(2-3):475-82.
Malassiné A, Frendo JL, Evain-Brion D. INSERM, U767, Paris, France.
Abstract Trophoblastic cell fusion is one essential step of the human trophoblast differentiation pathway and is a multifactorial and dynamic process finely regulated and still poorly known. Disturbances of syncytiotrophoblast formation are observed in numerous pathological clinical conditions such as preeclampsia, intrauterine growth retardation and trisomy 21. In this review, we summarize current knowledge of the different membrane proteins directly involved in trophoblastic cell fusion, which we identified by using the physiological model of primary culture of villous trophoblastic cells. Connexin 43 and gap junctional intercellular communication point to the role of molecular exchanges through connexin channels preceding membrane fusion. Zona occludens-1, which can interact with connexin 43, is also directly involved in trophoblast fusion. The recently identified fusogenic membrane retroviral envelop glycoproteins syncytin 1 (encoded by the HERV-W gene) and syncytin 2 (encoded by the FRD gene) and their receptors are major factors involved in human placental development . We describe the increasing number of factors promoting or inhibiting trophoblast fusion and differentiation and emphasize the role of human chorionic gonadotropin (hCG) and its receptor. Indeed, in trisomy 21 the dynamic process leading to membrane fusion is impaired due to an abnormal hCG signaling. This abnormal trophoblast fusion and differentiation in trisomy 21-affected placenta is reversible in vitro. Trisomy 21 trophoblastic cell culture may therefore be useful to identify the possible large number of prerequisite factors involved in trophoblast fusion, the limiting step of trophoblast differentiation.
PMID 19876835
Development and function of trophoblast giant cells in the rodent placenta
Int J Dev Biol. 2010;54(2-3):341-54.
Hu D, Cross JC.
Department of Comparative Biology and Experimental Medicine, Faculty of Veterinary Medicine and Graduate Program in Biochemistry and Molecular Biology, University of Calgary, Calgary, Alberta, Canada. Abstract Trophoblast giant cells (TGCs) are the first cell type to terminally differentiate during embryogenesis and are of vital importance for implantation and modulation of post-implantation placentation. TGCs are mononuclear and polyploid but are heterogenous and dynamic. At least four different subtypes of TGCs are present within the mature placenta that have distinct cell lineage origins. The development of TGCs is complex and requires transition from the mitotic to the endoreduplication cell cycle and is regulated by a wide variety of factors. During early gestation, TGCs mediate blastocyst attachment and invasion into the uterine epithelium, regulate uterus decidualization, and anatomosis with maternal blood spaces to form the transient yolk sac placenta. During later gestation, TGCs secrete a wide array of hormones and paracrine factors, including steroid hormones and Prolactin-related cytokines, to target the maternal physiological systems for proper maternal adaptations to pregnancy and the fetal-maternal interface to ensure vasculature remodeling. The large number of mouse mutants with defects in TGC development and function are giving us significant new insights into the biology of these fascinating cells.
PMID 19876834
http://www.ijdb.ehu.es/web/paper.php?doi=10.1387/ijdb.082768dh
Trophoblast phagocytic program: roles in different placental systems
Int J Dev Biol. Vol. 54 Nos. 2/3 (2010) Placenta
http://www.ijdb.ehu.es/web/contents.php?vol=54&issue=2-3
Int J Dev Biol. 2010;54(2-3):495-505.
Bevilacqua E, Hoshida MS, Amarante-Paffaro A, Albieri-Borges A, Zago Gomes S.
University of São Paulo, SP, Brazil. bevilacq@usp.br Abstract Although not belonging to the class of professional phagocytes, in many species trophoblast cells exhibit intense phagocytic activity. The complete range of physiological functions of trophoblast phagocytosis has not yet been fully characterized. Close association between the trophoblast and nutrition was determined many years ago. Hubrecht (1889) when proposing for the first time the name trophoblast to the external layer of the blastocyst, directly established the nutritive significance of this embryonic layer. Indeed, histotrophic phagocytosis, i.e. the internalization of maternal cells and secreted materials, is considered an important function of the trophoblast before the completion of the placenta. Recently, however, unexpected characteristics of the trophoblast have significantly enhanced our understanding of this process. Roles in acquisition of space for embryo development, in tissue remodeling during implantation and placentation and in defense mechanisms are highlighting how this cellular activity may be relevant for the maternal-fetal relationship beyond its nutritional function.
PMID 19757392
2009
Function of caspase-14 in trophoblast differentiation
Reprod Biol Endocrinol. 2009 Sep 14;7:98.
White LJ, Declercq W, Arfuso F, Charles AK, Dharmarajan AM.
School of Anatomy and Human Biology, Faculty of Life and Physical Sciences, The University of Western Australia, Perth, Western Australia, Australia. yornup@gmail.com Abstract BACKGROUND: Within the human placenta, the cytotrophoblast consists of a proliferative pool of progenitor cells which differentiate to replenish the overlying continuous, multi-nucleated syncytiotrophoblast, which forms the barrier between the maternal and fetal tissues. Disruption to trophoblast differentiation and function may result in impaired fetal development and preeclampsia. Caspase-14 expression is limited to barrier forming tissues. It promotes keratinocyte differentiation by cleaving profilaggrin to stabilise keratin intermediate filaments, and indirectly providing hydration and UV protection. However its role in the trophoblast remains unexplored.
METHODS: Using RNA Interference the reaction of control and differentiating trophoblastic BeWo cells to suppressed caspase-14 was examined for genes pertaining to hormonal, cell cycle and cytoskeletal pathways.
RESULTS: Transcription of hCG, KLF4 and cytokeratin-18 were increased following caspase-14 suppression suggesting a role for caspase-14 in inhibiting their pathways. Furthermore, hCG, KLF4 and cytokeratin-18 protein levels were disrupted.
CONCLUSION: Since expression of these molecules is normally increased with trophoblast differentiation, our results imply that caspase-14 inhibits trophoblast differentiation. This is the first functional study of this unusual member of the caspase family in the trophoblast, where it has a different function than in the epidermis. This knowledge of the molecular underpinnings of trophoblast differentiation may instruct future therapies of trophoblast disease.
PMID 19747408
Intrauterine fate of invasive trophoblast cells
Placenta. 2009 May;30(5):457-63. Epub 2009 Apr 2.
Rosario GX, Ain R, Konno T, Soares MJ.
Institute of Maternal-Fetal Biology, Division of Cancer and Developmental Biology, Department of Pathology and Laboratory of Medicine, University of Kansas Medical Center, Kansas City, KS 66160, USA. Abstract Invasion of trophoblast cells into the uterine spiral arteries and the uterine wall is characteristic of hemochorial placentation. In the rat, trophoblast cells penetrate through the uterine decidua and well into the metrial gland. In this report, we examined the fate of these invasive trophoblast cells following parturition. Invasive trophoblast endocrine cells were retained in the postpartum mesometrial uterus in the rat. The demise of invasive trophoblast cells was followed by the appearance of differentiated smooth muscle cells surrounding blood vessels previously lined by invasive trophoblast cells and an infiltration of macrophages. Regulation of intrauterine trophoblast cell fate was investigated following premature removal of the fetus or removal of the fetus and chorioallantoic placenta. The presence of the fetus affected the distribution of invasive trophoblast cells within the uterus but did not negatively impact their survival. Premature removal of all chorioallantoic placentas and associated fetuses from a uterus resulted in extensive removal of intrauterine trophoblast cells. In summary, the postpartum demise of intrauterine invasive trophoblast cells is a dynamic developmental event regulated in part by the removal of trophic signals emanating from the chorioallantoic placenta.
PMID 19344949
Trophoblast infection with Chlamydia pneumoniae and adverse pregnancy outcomes associated with placental dysfunction
Am J Obstet Gynecol. 2009 May;200(5):526.e1-7.
Gomez LM, Parry S.
Maternal and Child Research Program, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of Pennsylvania School of Medicine, Philadelphia, PA, USA. Abstract OBJECTIVE: We sought to determine whether Chlamydia pneumoniae impairs invasive trophoblast function and is associated with preeclampsia.
STUDY DESIGN: We conducted cell viability and invasion assays using primary extravillous trophoblast cells isolated from first-trimester placentas. We performed a case-control study to identify C pneumoniae in trophoblast cells dissected by laser capture microscopy from placentas in women with severe preeclampsia and control subjects who delivered at term.
RESULTS: Trophoblast cell viability and invasion through extracellular matrices were decreased after infection with C pneumoniae (both P < .05). C pneumoniae DNA was detected in trophoblast cells in 15/48 cases but only 3/30 controls (odds ratio, 4.1; P = .02). Positive and negative controls yielded expected results.
CONCLUSION: C pneumoniae infection can reduce trophoblast invasion into the uterine wall and is associated with preeclampsia. Further investigation of the mechanisms by which C pneumoniae induces trophoblast dysfunction, and the identification of therapies to prevent adverse outcomes attributed to trophoblast dysfunction, are warranted.
PMID 19375572
Chlamydophila pneumoniae is a species of Chlamydophila bacteria that infects humans and is a major cause of pneumonia.
While dysferlin and myoferlin are coexpressed in the human placenta, only dysferlin expression is responsive to trophoblast fusion in model systems
Biol Reprod. 2009 Jul;81(1):33-9. Epub 2009 Feb 18. Robinson JM, Ackerman WE 4th, Behrendt NJ, Vandre DD.
Departments of Physiology and Cell Biology and Obstetrics and Gynecology, The Ohio State University, Columbus, Ohio 43210, USA. robinson.21@osu.edu Abstract The syncytiotrophoblast is a specialized epithelium derived from mononuclear cytotrophoblasts that fuse to form this extensive syncytium. Dysferlin is expressed primarily in the apical plasma membrane of the syncytiotrophoblast in the human placenta. Here, we document the presence of another member of the ferlin family, myoferlin, in the placenta and show that it too is expressed primarily in the syncytiotrophoblast. Additionally, we examined the trophoblastic cell lines BeWo, JAR, and JEG-3 for the expression of dysferlin and myoferlin and determined the extent to which their expression was modulated by cell-cell fusion. In trophoblastic cells, there was a positive correlation between cell fusion and increased dysferlin expression but not myoferlin expression. Regarding expression, these trophoblastic cell lines recapitulate the distribution of dysferlin in mononuclear cytotrophoblasts and the syncytiotrophoblast in vivo.
PMID 19228595
2008
HOXA13 Is essential for placental vascular patterning and labyrinth endothelial specification
PLoS Genet. 2008 May 16;4(5):e1000073.
Shaut CA, Keene DR, Sorensen LK, Li DY, Stadler HS. Department of Molecular and Medical Genetics, Oregon Health & Science University, Portland, Oregon, United States of America.
Abstract
In eutherian mammals, embryonic growth and survival is dependent on the formation of the placenta, an organ that facilitates the efficient exchange of oxygen, nutrients, and metabolic waste between the maternal and fetal blood supplies. Key to the placenta's function is the formation of its vascular labyrinth, a series of finely branched vessels whose molecular ontogeny remains largely undefined. In this report, we demonstrate that HOXA13 plays an essential role in labyrinth vessel formation. In the absence of HOXA13 function, placental endothelial cell morphology is altered, causing a loss in vessel wall integrity, edema of the embryonic blood vessels, and mid-gestational lethality. Microarray analysis of wild-type and mutant placentas revealed significant changes in endothelial gene expression profiles. Notably, pro-vascular genes, including Tie2 and Foxf1, exhibited reduced expression in the mutant endothelia, which also exhibited elevated expression of genes normally expressed in lymphatic or sinusoidal endothelia. ChIP analysis of HOXA13-DNA complexes in the placenta confirmed that HOXA13 binds the Tie2 and Foxf1 promoters in vivo. In vitro, HOXA13 binds sequences present in the Tie2 and Foxf1 promoters with high affinity (K(d) = 27-42 nM) and HOXA13 can use these bound promoter regions to direct gene expression. Taken together, these findings demonstrate that HOXA13 directly regulates Tie2 and Foxf1 in the placental labyrinth endothelia, providing a functional explanation for the mid-gestational lethality exhibited by Hoxa13 mutant embryos as well as a novel transcriptional program necessary for the specification of the labyrinth vascular endothelia.
PMID 18483557
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 23) Embryology Trophoblast - Protein Expression. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Trophoblast_-_Protein_Expression
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G