Talk:SH Lecture - Respiratory System Development: Difference between revisions
m (→2019) |
|||
| (47 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==2019== | |||
== | <html5media height="500" width="856">File:Rat respiratory 01.mp4</html5media> | ||
The flight starts by entering a transitional bronchiole. Domes of Club cell are visible on the surface of the bronchiole. Turning left an alveolar duct is entered. Various alveoli and the entrance of few alveolar ducts are visible. Shortly before the end of first alveolar duct, the fight turns left again and flies down another alveolar duct. After a short distance it ends in front of an alveolus which is subdivided by a low ridge representing a still forming new alveolar septum. Rat lung at postnatal day 36. Surface rendering of a sample scanned by SRXTM for Schittny et al. (Schittny et al. 2008) using the software Imaris 4.1 (Bitplane, Zürich, Switzerland). Because the magnification is changing during the flight a scale bar could not be easily shown. However, the entrance of the bronchiole has a diameter of ~100 µm | |||
(text from suppliers. information) | |||
{{#pmid:30390117}} | |||
[https://emed.med.unsw.edu.au/Map.nsf/wLAFByUniqueKey/LBMT-5SCBD7?OpenDocument&login eMed LAF] | |||
Paramothayan, S. (2018). Essential respiratory medicine. Retrieved from https://ebookcentral.proquest.com/lib/unsw/detail.action?docID=5571252 | |||
[https://ebookcentral.proquest.com/lib/unsw/reader.action?docID=5571252&ppg=21 CHAPTER 2 Embryology, anatomy, and physiology of the lung] | |||
Paramothayan, Shanthi. Essential Respiratory Medicine, John Wiley & Sons, Incorporated, 2018. ProQuest Ebook Central, http://ebookcentral.proquest.com/lib/unsw/detail.action?docID=5571252. | |||
Created from unsw on 2019-01-28 16:07:27. | |||
==Reviews== | |||
===Airway and blood vessel interaction during lung development=== | |||
J Anat. 2002 Oct;201(4):325-34. | |||
Hislop AA. | |||
Author information | |||
Abstract | |||
In the adult lung the pulmonary arteries run alongside the airways and the pulmonary veins show a similar branching pattern to the arteries, though separated from them. During early fetal development the airways act as a template for pulmonary blood vessel development in that the vessels form by vasculogenesis around the branching airways. In later lung development the capillary bed is essential for alveolar formation. This paper reviews evidence for the interaction of the airways and blood vessels in both normal and abnormal lung development. | |||
Histology images of lung stages | |||
PMID 12430957 | |||
===Development of the right outflow tract and pulmonary arterial supply=== | |||
Ann R Coll Surg Engl. 1975 Oct;57(4):186-97. | |||
Skidmore FD. | |||
Abstract | |||
The branchial arch vessels of the human embryo have been studied by histological and radiographic methods and the modelling that occurs during the period Day 25-Day 52 postfertilization is described. It has been shown that the myoendocardial reticulum is reamed out by blood flow and it is suggested that hydrodynamic force is the fundamental factor which determines chamber structure of the heart and flow pattern in the outflow tracts and great vessels. The sixth aortic arch vessels contribute tissue to the pulmonary trunk and proximal pulmonary arteries. The 'postbranchial pulmonary arteries' are morphologically distinct and form the pulmonary arteries at the lung hila. The primitive pulmonary plexus around the tips of the developing tracheobronchial primordia is formed from segmental vessels arising from the dorsal aorta. Bronchial arteries can be demonstrated only late in intrauterine life. The numerous bronchopulmonary precapillary anastomoses which are found in the fetus at this time have been demonstrated radiographically. | |||
PMID 1103698 | |||
==Deleted== | |||
:'''Links:''' [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2920089/figure/f2-ehp.0901856/ Principal stages of lung development in humans] | |||
File:Gitbpm.jpg|stage 11 foregut | File:Gitbpm.jpg|stage 11 foregut | ||
File:Gray0982a.jpg|week 4 early respiratory endodermal bud | File:Gray0982a.jpg|week 4 early respiratory endodermal bud | ||
File:Stage_22_image_167.jpg|Stage 22 trachea | |||
File:Head_arches_cartoon.jpg|Head arches cartoon | |||
week 4 - 5 embryonic | |||
week 5 - 17 pseudoglandular | |||
week 16 - 25 canalicular | |||
week 24 - 40 terminal sac | |||
late fetal - 8 years alveolar | |||
== | == Postnatal== | ||
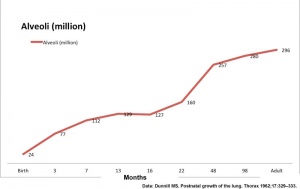
[[File: | [[File:Postnatal alveoli number.jpg|thumb|300px|Postnatal alveoli number]] | ||
{| | {| | ||
| | |-bgcolor="CEDFF2" | ||
| '''Age''' (months) | |||
| '''Alveoli''' (million) | |||
| '''Respiratory Airways''' (million) | |||
| '''Generations of Airways''' | |||
| Alveoli (million) | |||
| Respiratory Airways (million) | |||
| Generations of Airways | |||
|- | |- | ||
|Birth | |Birth | ||
| Line 254: | Line 75: | ||
| 1.5 | | 1.5 | ||
| | | | ||
|- bgcolor="F5FAFF" | |||
| 3 | | 3 | ||
| 86 | | 86 | ||
| Line 263: | Line 85: | ||
| 2.5 | | 2.5 | ||
| 21 | | 21 | ||
|- | |- bgcolor="F5FAFF" | ||
| 3 | | 3 | ||
| 73 | | 73 | ||
| Line 273: | Line 95: | ||
| 3.7 | | 3.7 | ||
| | | | ||
|- | |- bgcolor="F5FAFF" | ||
| 13 | | 13 | ||
| 129 | | 129 | ||
| Line 283: | Line 105: | ||
| 4.7 | | 4.7 | ||
| | | | ||
|- | |- bgcolor="F5FAFF" | ||
| 22 | | 22 | ||
| 160 | | 160 | ||
| Line 293: | Line 115: | ||
| 7.9 | | 7.9 | ||
| | | | ||
|- | |- bgcolor="F5FAFF" | ||
| 98 | | 98 | ||
| 280 | | 280 | ||
| Line 302: | Line 124: | ||
| 296 | | 296 | ||
| 14.0 | | 14.0 | ||
|23 | | 23 | ||
|} | |} | ||
Dunnill MS. '''Postnatal growth of the lung.''' Thorax 1962;17:329–333. [http://ajrccm.atsjournals.org/cgi/ijlink?linkType=PDF&journalCode=thoraxjnl&resid=17/4/329 PDF] | Dunnill MS. '''Postnatal growth of the lung.''' Thorax 1962;17:329–333. [http://ajrccm.atsjournals.org/cgi/ijlink?linkType=PDF&journalCode=thoraxjnl&resid=17/4/329 PDF] | ||
<pubmed>139844</pubmed>| [http://www.annualreviews.org/doi/pdf/10.1146/annurev.ph.39.030177.001345 PDF] | |||
<pubmed>1094872</pubmed> | |||
==Terms== | ==Terms== | ||
| Line 316: | Line 143: | ||
* '''Congenital Diaphragmatic Hernia''' (CDH) disorder with an incidence of 1 in 2500 live births. | * '''Congenital Diaphragmatic Hernia''' (CDH) disorder with an incidence of 1 in 2500 live births. | ||
* '''fetal breathing-like movements''' (FBMs) or Fetal respiratory movements are thought to be regular muscular contrations occurring in the third trimester, preparing the respiratory muscular system for neonatal function and to have a role in late lung development. | * '''fetal breathing-like movements''' (FBMs) or Fetal respiratory movements are thought to be regular muscular contrations occurring in the third trimester, preparing the respiratory muscular system for neonatal function and to have a role in late lung development. | ||
* '''FEV''' - Forced Expiratory Volume | |||
* '''forced expiratory volume''' - (FEV) Spirometry term for the fraction of the forced vital capacity that is exhaled in a specific number of seconds. Abbreviated FEV with a subscript indicating how many seconds the measurement lasted. | |||
* '''glucocorticoid treatment''' - antenatal therapy to promote the maturation of the human fetal lung. Given as a synthetic glucocorticoid between 24 and 32 weeks of pregnancy to promote lung maturation in fetuses at risk of preterm delivery. | * '''glucocorticoid treatment''' - antenatal therapy to promote the maturation of the human fetal lung. Given as a synthetic glucocorticoid between 24 and 32 weeks of pregnancy to promote lung maturation in fetuses at risk of preterm delivery. | ||
* '''lamellar bodies''' the storage form of surfactant in type II alveolar cells, seen as centrically layered "packages" of phospholipid. A count of lamellar bodies can be used as an assay for measuring fetal lung maturity. | * '''lamellar bodies''' the storage form of surfactant in type II alveolar cells, seen as centrically layered "packages" of phospholipid. A count of lamellar bodies can be used as an assay for measuring fetal lung maturity. | ||
| Line 326: | Line 155: | ||
* '''Respiratory distress syndrome''' (RDS) due to a surfactant deficiency at birth, particulary in preterm birth. | * '''Respiratory distress syndrome''' (RDS) due to a surfactant deficiency at birth, particulary in preterm birth. | ||
* '''secondary alveolar septa''' formed during the alveolar stage and are formed by projections of connective tissue and a double capillary loop. | * '''secondary alveolar septa''' formed during the alveolar stage and are formed by projections of connective tissue and a double capillary loop. | ||
* '''Spirometry''' - clinical measure of respiratory airflow. | |||
*''' surfactant''' produced by alveolar type II cells is a mixture of lipids and proteins that both maintains alveolar integrity and plays a role in the control of host defense and inflammation in the lung. | *''' surfactant''' produced by alveolar type II cells is a mixture of lipids and proteins that both maintains alveolar integrity and plays a role in the control of host defense and inflammation in the lung. | ||
* '''Surfactant therapy''' ([http://aappolicy.aappublications.org/cgi/content/full/pediatrics;121/2/419 American Academy of Pediatrics Policy] | [http://www.cps.ca/english/statements/fn/fn05-01.htm Canadian Paediatric Society Recommendations]) | * '''Surfactant therapy''' ([http://aappolicy.aappublications.org/cgi/content/full/pediatrics;121/2/419 American Academy of Pediatrics Policy] | [http://www.cps.ca/english/statements/fn/fn05-01.htm Canadian Paediatric Society Recommendations]) | ||
*''' thyroid hormone''' involved in the regulation of fetal lung development. | *''' thyroid hormone''' involved in the regulation of fetal lung development. | ||
* '''vascular endothelial growth factor''' (VEGF) a secreted growth factor acting through receptors on endothelial cells to regulate vasculogenesis through their development, growth and function. | * '''vascular endothelial growth factor''' (VEGF) a secreted growth factor acting through receptors on endothelial cells to regulate vasculogenesis through their development, growth and function. | ||
==Virtual Slides== | |||
===UNSW Virtual Slides=== | |||
http://vslides.unsw.edu.au/VirtualSlideV2.nsf/id/F51ED7 | |||
===Virtual Slidebox=== | |||
http://www.path.uiowa.edu/virtualslidebox/nlm_histology/content_index_db.html | |||
http://www.path.uiowa.edu/cgi-bin-pub/vs/fpx_browse.cgi?cat=o_lung&div=nlm | |||
===Blue Histology=== | |||
http://www.lab.anhb.uwa.edu.au/mb140/CorePages/Respiratory/respir.htm | |||
==2012== | |||
===Building and maintaining the epithelium of the lung=== | |||
J Clin Invest. 2012 Aug 1;122(8):2724-30. doi: 10.1172/JCI60519. Epub 2012 Aug 1. | |||
Rackley CR, Stripp BR. | |||
Source | |||
Pulmonary, Allergy and Critical Care, Department of Medicine, Duke University Medical Center, Durham, NC, USA. | |||
Abstract | |||
Airspaces of the lung are lined by an epithelium whose cellular composition changes along the proximal-to-distal axis to meet local functional needs for mucociliary clearance, hydration, host defense, and gas exchange. Advances in cell isolation, in vitro culture techniques, and genetic manipulation of animal models have increased our understanding of the development and maintenance of the pulmonary epithelium. This review discusses basic cellular mechanisms that regulate establishment of the conducting airway and gas exchange systems as well as the functional maintenance of the epithelium during postnatal life. | |||
PMID 22850882 | |||
===Fetal and infant origins of asthma=== | |||
Eur J Epidemiol. 2012 Jan;27(1):5-14. doi: 10.1007/s10654-012-9657-y. Epub 2012 Feb 18. | |||
Duijts L. | |||
Source | |||
Department of Epidemiology, Erasmus Medical Center, Rotterdam, The Netherlands. l.duijts@erasmusmc.nl | |||
Abstract | |||
Previous studies have suggested that asthma, like other common diseases, has at least part of its origin early in life. Low birth weight has been shown to be associated with increased risks of asthma, chronic obstructive airway disease, and impaired lung function in adults, and increased risks of respiratory symptoms in early childhood. The developmental plasticity hypothesis suggests that the associations between low birth weight and diseases in later life are explained by adaptation mechanisms in fetal life and infancy in response to various adverse exposures. Various pathways leading from adverse fetal and infant exposures to growth adaptations and respiratory health outcomes have been studied, including fetal and early infant growth patterns, maternal smoking and diet, children's diet, respiratory tract infections and acetaminophen use, and genetic susceptibility. Still, the specific adverse exposures in fetal and early postnatal life leading to respiratory disease in adult life are not yet fully understood. Current studies suggest that both environmental and genetic factors in various periods of life, and their epigenetic mechanisms may underlie the complex associations of low birth weight with respiratory disease in later life. New well-designed epidemiological studies are needed to identify the specific underlying mechanisms. This review is focused on specific adverse fetal and infant growth patterns and exposures, genetic susceptibility, possible respiratory adaptations and perspectives for new studies. | |||
PMID 22350146 | |||
==2011== | |||
===Planar polarity: A new player in both lung development and disease=== | |||
Organogenesis. 2011 Jul-Sep;7(3):209-16. doi: 10.4161/org.7.3.18462. Epub 2011 Jul 1 | |||
Yates LL, Dean CH. | |||
Source | |||
Peter MacCallum Cancer Institute, Melbourne, Australia. | |||
Abstract | |||
The clinical burden of both adult and neonatal lung disease worldwide is substantial; in the UK alone, respiratory disease kills one in four people. It is increasingly recognized that genes and pathways that regulate lung development, may be aberrantly activated in disease and/or reactivated as part of the lungs' intrinsic repair mechanisms. Investigating the genes and signaling pathways that regulate lung growth has led to significant insights into the pathogenesis of congenital and adult lung disease. Recently, the planar cell polarity (PCP) pathway has been shown to be required for normal lung development, and data suggests that this signaling pathway is also involved in the pathogenesis of some lung diseases. In this review, we summarize current evidence indicating that the PCP pathway is required for both lung development and disease. | |||
PMID 22030785 | |||
==Lung Development Stages== | |||
Text from: <pubmed>10852845</pubmed>| [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1637815 PMC1637815] | [http://ehpnetl.niehs.nih.gov/docs/2000/suppl-3/457462pinkerton/abstract.html Environ Health Perspect.] | |||
Embryogenesis | |||
The lungs in humans first appear at the end of the first month of gestation as an evagination of epithelium from the foregut. The bud rapidly divides as a series of branching tubes in a dichotomous pattern. These tubular branches invade and interdigitate with mesenchymal tissues. Branching morphogenesis during this period forms the most proximal portions of the future tracheobronchial tree. As these tissues grow, they push into the future pleuroperitoneal cavity of the embryo. During embryogenesis, transcription factors play an important role in gene expression and regulation. Transcription factors are essential in both the stimulation and inhibition of gene expression to regulate the proper temporal and spatial patterning of lung development. Hepatocyte nuclear factor- 3 (12) and the homeobox gene TTF-1 (13) are examples of transcription factors serving as important regulators of early differentiation of the pulmonary epithelium during this period. Lung development is also highly dependent on interactions between the epithelium and mesenchyme. This dual origin of lung tissues is critical in development. Removal of mesenchyme from the tip of a lung bud during early phases of development with transplantation to the side of a higher ordered segment abolishes further branching at the site of removal while stimulating growth of a | |||
Pseudoglandular stage. | |||
Tubular branching of the human lung airways continues from the fifth to the seventeenth week of gestation. As early as 2 months of gestational age, all segmental bronchi are present. During this period, the lungs take on the appearance of a glandlike structure. This stage is the most critical for the formation of all conducting airways. During this period, the airway tubular structures are lined with tall columnar epithelium, whereas the more distal structures are lined with cuboidal epithelium. A number of signals arising from epithelial mesenchymal interactions during this time continue to modulate cellular proliferation temporally as well as spatially (4). These regulatory signals lead to further branching morphogenesis by affecting the rate of cellular proliferation (15). The presence of extracellular matrix molecules, including collagen, fibronectin, laminin, glycosaminoglycans, and proteoglycans, as well as cell membrane-bound integrins, also plays an important role in directing lung development by influencing the rates of cellular proliferation and differentiation (3,16,1/). Mechanical distention exerted on the lung as well as on specific cell types can also significantly affect gene expression and, ultimately, lung growth and development (4). A variety of growth factors and growth factor receptors are also important in controlling cellular functions (3). Epidermal growth factor, transforming growth factor-a, and retinoic acid all act to affect branching morphogenesis and cellular differentiation (18,19). Epithelial differentiation of ciliated, goblet, and basal cells first appears in the most central airways during this stage of development. Cartilage and smooth muscle cells are also first noted in the trachea and extend more peripherally with progressive growth of the lungs. During this stage of | |||
Canalicular stage. | |||
This stage lasts from week 16 to week 24 in the human fetus. Lung morphology changes dramatically during this time because of differentiation of the pulmonary epithelium, resulting in the formation of the future air-blood tissue barrier. Surfactant synthesis and the canalization of the lung parenchyma by capillaries begin. During this stage, the future gas exchange regions can be easily distinguished from the future conducting airways of the lungs. | |||
Saccular stage. | |||
The saccular stage of lung development in humans lasts from week 24 to near term. The most peripheral airways form widened airspaces, termed saccules. These saccules widen and lengthen the airspace, in large measure by the addition of new generations. During this stage, the future gas exchange region expands significantly. Populations of fibroblastic cells also undergo differentiation during this stage. These fibroblast-like cells are responsible for the production of the extracellular matrix, collagen, and elastin. It is also presumed that they play an important role in epithelial differentiation and control of surfactant secretion in connection with the growth of the gas exchange region during this stage. The vascular tree also grows in length and diameter during this time. | |||
Columnar cells that are undifferentiated characterize the first epithelial cells lining fetal lung tubules. The first epithelial cells to differentiate in the trachea are neuroendocrine cells, followed closely by ciliated cells, and finally basal and secretory cells in rapid sequence. This process of differentiation covers a developmental period ranging from days to months. In rodents including the mouse, rat, and hamster, complete epithelial differentiation of the trachea occurs in as little as 2 days. In primate trachea, cellular differentiation takes up to 6 months to be complete. In most species, epithelial cell differentiation of the trachea usually is not complete until just before birth. For more peripheral airway generations, cellular differentiation is likely to continue into the early postnatal period. Fetal epithelial cells are typically filled with glycogen that is gradually replaced with a granular cytoplasm filled with numerous organelles during cellular differentiation. These glycogen-filled cells are found throughout the tracheobronchial tree as well as into the most peripheral saccules. Differentiation of the epithelium is highly site specific, giving rise to more than 10 different cell types. For example, within the saccules of the lungs, cells lining these surfaces differentiate to form both squamous type 1 cells as well as cuboidal type 2 cells. The presence of glycogen within these cells may persist into early postnatal life. | |||
Latest revision as of 15:41, 19 February 2019
2019
<html5media height="500" width="856">File:Rat respiratory 01.mp4</html5media>
The flight starts by entering a transitional bronchiole. Domes of Club cell are visible on the surface of the bronchiole. Turning left an alveolar duct is entered. Various alveoli and the entrance of few alveolar ducts are visible. Shortly before the end of first alveolar duct, the fight turns left again and flies down another alveolar duct. After a short distance it ends in front of an alveolus which is subdivided by a low ridge representing a still forming new alveolar septum. Rat lung at postnatal day 36. Surface rendering of a sample scanned by SRXTM for Schittny et al. (Schittny et al. 2008) using the software Imaris 4.1 (Bitplane, Zürich, Switzerland). Because the magnification is changing during the flight a scale bar could not be easily shown. However, the entrance of the bronchiole has a diameter of ~100 µm
(text from suppliers. information)
Schittny JC. (2018). How high resolution 3-dimensional imaging changes our understanding of postnatal lung development. Histochem. Cell Biol. , 150, 677-691. PMID: 30390117 DOI.
Paramothayan, S. (2018). Essential respiratory medicine. Retrieved from https://ebookcentral.proquest.com/lib/unsw/detail.action?docID=5571252
CHAPTER 2 Embryology, anatomy, and physiology of the lung
Paramothayan, Shanthi. Essential Respiratory Medicine, John Wiley & Sons, Incorporated, 2018. ProQuest Ebook Central, http://ebookcentral.proquest.com/lib/unsw/detail.action?docID=5571252. Created from unsw on 2019-01-28 16:07:27.
Reviews
Airway and blood vessel interaction during lung development
J Anat. 2002 Oct;201(4):325-34.
Hislop AA. Author information
Abstract In the adult lung the pulmonary arteries run alongside the airways and the pulmonary veins show a similar branching pattern to the arteries, though separated from them. During early fetal development the airways act as a template for pulmonary blood vessel development in that the vessels form by vasculogenesis around the branching airways. In later lung development the capillary bed is essential for alveolar formation. This paper reviews evidence for the interaction of the airways and blood vessels in both normal and abnormal lung development.
Histology images of lung stages
PMID 12430957
Development of the right outflow tract and pulmonary arterial supply
Ann R Coll Surg Engl. 1975 Oct;57(4):186-97.
Skidmore FD.
Abstract
The branchial arch vessels of the human embryo have been studied by histological and radiographic methods and the modelling that occurs during the period Day 25-Day 52 postfertilization is described. It has been shown that the myoendocardial reticulum is reamed out by blood flow and it is suggested that hydrodynamic force is the fundamental factor which determines chamber structure of the heart and flow pattern in the outflow tracts and great vessels. The sixth aortic arch vessels contribute tissue to the pulmonary trunk and proximal pulmonary arteries. The 'postbranchial pulmonary arteries' are morphologically distinct and form the pulmonary arteries at the lung hila. The primitive pulmonary plexus around the tips of the developing tracheobronchial primordia is formed from segmental vessels arising from the dorsal aorta. Bronchial arteries can be demonstrated only late in intrauterine life. The numerous bronchopulmonary precapillary anastomoses which are found in the fetus at this time have been demonstrated radiographically. PMID 1103698
Deleted
File:Gitbpm.jpg|stage 11 foregut
File:Gray0982a.jpg|week 4 early respiratory endodermal bud
File:Stage_22_image_167.jpg|Stage 22 trachea
File:Head_arches_cartoon.jpg|Head arches cartoon
week 4 - 5 embryonic
week 5 - 17 pseudoglandular
week 16 - 25 canalicular
week 24 - 40 terminal sac
late fetal - 8 years alveolar
Postnatal
| Age (months) | Alveoli (million) | Respiratory Airways (million) | Generations of Airways |
| Birth | 24 | 1.5 | |
| 3 | 86 | 1.8 | |
| 3 | 77 | 2.5 | 21 |
| 3 | 73 | 2.0 | |
| 7 | 112 | 3.7 | |
| 13 | 129 | 4.5 | 22 |
| 16 | 127 | 4.7 | |
| 22 | 160 | 7.1 | |
| 48 | 257 | 7.9 | |
| 98 | 280 | 14.0 | 23 |
| Adult | 296 | 14.0 | 23 |
Dunnill MS. Postnatal growth of the lung. Thorax 1962;17:329–333. PDF
<pubmed>139844</pubmed>| PDF
<pubmed>1094872</pubmed>
Terms
- antenatal before birth.
- alveoli number at birth - from 20 - 50 million and eventually in the adult 300 million.
- Bronchopulmonary dysplasia - (BPD) the most common serious sequela of premature birth.
- Bronchiolitis - is a viral infection of the lower respiratory tract and most common lower respiratory tract infection in infants. Respiratory syncytial virus (RSV) is responsible for 70 percent of all cases overall and Parainfluenza, adenovirus and influenza account for most of the remaining cases. (HSTAT Management of Bronchiolitis in Infants and Children)
- Chronic obstructive pulmonary disease (COPD) causes include smoking (85–90 percent of all cases), genetic factors (alpha-1 antitrypsin deficiency), passive smoking (children), occupational exposures, air pollution, and hyperresponsive airways. (HSTAT Management of Acute Exacerbations of Chronic Obstructive Pulmonary Disease)
- Clara cells non-ciliated cell found in the small airways (bronchioles) consisting of ciliated simple epithelium, these cells secrete glycosaminoglycans (Clara cell secretory protein, CCSP) to protect the bronchiole lining.
- Congenital Diaphragmatic Hernia (CDH) disorder with an incidence of 1 in 2500 live births.
- fetal breathing-like movements (FBMs) or Fetal respiratory movements are thought to be regular muscular contrations occurring in the third trimester, preparing the respiratory muscular system for neonatal function and to have a role in late lung development.
- FEV - Forced Expiratory Volume
- forced expiratory volume - (FEV) Spirometry term for the fraction of the forced vital capacity that is exhaled in a specific number of seconds. Abbreviated FEV with a subscript indicating how many seconds the measurement lasted.
- glucocorticoid treatment - antenatal therapy to promote the maturation of the human fetal lung. Given as a synthetic glucocorticoid between 24 and 32 weeks of pregnancy to promote lung maturation in fetuses at risk of preterm delivery.
- lamellar bodies the storage form of surfactant in type II alveolar cells, seen as centrically layered "packages" of phospholipid. A count of lamellar bodies can be used as an assay for measuring fetal lung maturity.
- maternal diabetes if not controlled in pregnancy may delay fetal pulmonary maturation.
- Persistent Pulmonary Hypertension of the Newborn (PPHN) serious newborn condition due to due to the failure of closure one of the prenatal circulatory shunts, the ductus arteriosus. Occurs in about 1-2 newborns per 1000 live births and results in hypoxemia. (More? Respiratory Development - Birth)
- Pharyngitis inflammation of the pharynx involving lymphoid tissues of the posterior pharynx and lateral pharyngeal bands.
- pneumocyte or alveolar type I and type II cells.
- pulmonary hypoplasia can be due to anencephaly, renal hypoplasia or abnormalities of the thoracic cage
- pulmonary neuroendocrine cells (PNEC) single or innervated clusters of cells (neuroepithelial bodies) that line the airway epithelium, thought to have a role in regulating fetal lung growth and differentiation. At birth may also act as airway oxygen sensors involved in newborn adaptation. These cells synthesis and release amine (serotonin, 5-HT) and a several neuropeptides (bombesin).
- Respiratory distress syndrome (RDS) due to a surfactant deficiency at birth, particulary in preterm birth.
- secondary alveolar septa formed during the alveolar stage and are formed by projections of connective tissue and a double capillary loop.
- Spirometry - clinical measure of respiratory airflow.
- surfactant produced by alveolar type II cells is a mixture of lipids and proteins that both maintains alveolar integrity and plays a role in the control of host defense and inflammation in the lung.
- Surfactant therapy (American Academy of Pediatrics Policy | Canadian Paediatric Society Recommendations)
- thyroid hormone involved in the regulation of fetal lung development.
- vascular endothelial growth factor (VEGF) a secreted growth factor acting through receptors on endothelial cells to regulate vasculogenesis through their development, growth and function.
Virtual Slides
UNSW Virtual Slides
http://vslides.unsw.edu.au/VirtualSlideV2.nsf/id/F51ED7
Virtual Slidebox
http://www.path.uiowa.edu/virtualslidebox/nlm_histology/content_index_db.html
http://www.path.uiowa.edu/cgi-bin-pub/vs/fpx_browse.cgi?cat=o_lung&div=nlm
Blue Histology
http://www.lab.anhb.uwa.edu.au/mb140/CorePages/Respiratory/respir.htm
2012
Building and maintaining the epithelium of the lung
J Clin Invest. 2012 Aug 1;122(8):2724-30. doi: 10.1172/JCI60519. Epub 2012 Aug 1.
Rackley CR, Stripp BR. Source Pulmonary, Allergy and Critical Care, Department of Medicine, Duke University Medical Center, Durham, NC, USA.
Abstract
Airspaces of the lung are lined by an epithelium whose cellular composition changes along the proximal-to-distal axis to meet local functional needs for mucociliary clearance, hydration, host defense, and gas exchange. Advances in cell isolation, in vitro culture techniques, and genetic manipulation of animal models have increased our understanding of the development and maintenance of the pulmonary epithelium. This review discusses basic cellular mechanisms that regulate establishment of the conducting airway and gas exchange systems as well as the functional maintenance of the epithelium during postnatal life.
PMID 22850882
Fetal and infant origins of asthma
Eur J Epidemiol. 2012 Jan;27(1):5-14. doi: 10.1007/s10654-012-9657-y. Epub 2012 Feb 18.
Duijts L. Source Department of Epidemiology, Erasmus Medical Center, Rotterdam, The Netherlands. l.duijts@erasmusmc.nl
Abstract
Previous studies have suggested that asthma, like other common diseases, has at least part of its origin early in life. Low birth weight has been shown to be associated with increased risks of asthma, chronic obstructive airway disease, and impaired lung function in adults, and increased risks of respiratory symptoms in early childhood. The developmental plasticity hypothesis suggests that the associations between low birth weight and diseases in later life are explained by adaptation mechanisms in fetal life and infancy in response to various adverse exposures. Various pathways leading from adverse fetal and infant exposures to growth adaptations and respiratory health outcomes have been studied, including fetal and early infant growth patterns, maternal smoking and diet, children's diet, respiratory tract infections and acetaminophen use, and genetic susceptibility. Still, the specific adverse exposures in fetal and early postnatal life leading to respiratory disease in adult life are not yet fully understood. Current studies suggest that both environmental and genetic factors in various periods of life, and their epigenetic mechanisms may underlie the complex associations of low birth weight with respiratory disease in later life. New well-designed epidemiological studies are needed to identify the specific underlying mechanisms. This review is focused on specific adverse fetal and infant growth patterns and exposures, genetic susceptibility, possible respiratory adaptations and perspectives for new studies.
PMID 22350146
2011
Planar polarity: A new player in both lung development and disease
Organogenesis. 2011 Jul-Sep;7(3):209-16. doi: 10.4161/org.7.3.18462. Epub 2011 Jul 1
Yates LL, Dean CH. Source Peter MacCallum Cancer Institute, Melbourne, Australia. Abstract The clinical burden of both adult and neonatal lung disease worldwide is substantial; in the UK alone, respiratory disease kills one in four people. It is increasingly recognized that genes and pathways that regulate lung development, may be aberrantly activated in disease and/or reactivated as part of the lungs' intrinsic repair mechanisms. Investigating the genes and signaling pathways that regulate lung growth has led to significant insights into the pathogenesis of congenital and adult lung disease. Recently, the planar cell polarity (PCP) pathway has been shown to be required for normal lung development, and data suggests that this signaling pathway is also involved in the pathogenesis of some lung diseases. In this review, we summarize current evidence indicating that the PCP pathway is required for both lung development and disease.
PMID 22030785
Lung Development Stages
Text from: <pubmed>10852845</pubmed>| PMC1637815 | Environ Health Perspect.
Embryogenesis
The lungs in humans first appear at the end of the first month of gestation as an evagination of epithelium from the foregut. The bud rapidly divides as a series of branching tubes in a dichotomous pattern. These tubular branches invade and interdigitate with mesenchymal tissues. Branching morphogenesis during this period forms the most proximal portions of the future tracheobronchial tree. As these tissues grow, they push into the future pleuroperitoneal cavity of the embryo. During embryogenesis, transcription factors play an important role in gene expression and regulation. Transcription factors are essential in both the stimulation and inhibition of gene expression to regulate the proper temporal and spatial patterning of lung development. Hepatocyte nuclear factor- 3 (12) and the homeobox gene TTF-1 (13) are examples of transcription factors serving as important regulators of early differentiation of the pulmonary epithelium during this period. Lung development is also highly dependent on interactions between the epithelium and mesenchyme. This dual origin of lung tissues is critical in development. Removal of mesenchyme from the tip of a lung bud during early phases of development with transplantation to the side of a higher ordered segment abolishes further branching at the site of removal while stimulating growth of a
Pseudoglandular stage.
Tubular branching of the human lung airways continues from the fifth to the seventeenth week of gestation. As early as 2 months of gestational age, all segmental bronchi are present. During this period, the lungs take on the appearance of a glandlike structure. This stage is the most critical for the formation of all conducting airways. During this period, the airway tubular structures are lined with tall columnar epithelium, whereas the more distal structures are lined with cuboidal epithelium. A number of signals arising from epithelial mesenchymal interactions during this time continue to modulate cellular proliferation temporally as well as spatially (4). These regulatory signals lead to further branching morphogenesis by affecting the rate of cellular proliferation (15). The presence of extracellular matrix molecules, including collagen, fibronectin, laminin, glycosaminoglycans, and proteoglycans, as well as cell membrane-bound integrins, also plays an important role in directing lung development by influencing the rates of cellular proliferation and differentiation (3,16,1/). Mechanical distention exerted on the lung as well as on specific cell types can also significantly affect gene expression and, ultimately, lung growth and development (4). A variety of growth factors and growth factor receptors are also important in controlling cellular functions (3). Epidermal growth factor, transforming growth factor-a, and retinoic acid all act to affect branching morphogenesis and cellular differentiation (18,19). Epithelial differentiation of ciliated, goblet, and basal cells first appears in the most central airways during this stage of development. Cartilage and smooth muscle cells are also first noted in the trachea and extend more peripherally with progressive growth of the lungs. During this stage of
Canalicular stage.
This stage lasts from week 16 to week 24 in the human fetus. Lung morphology changes dramatically during this time because of differentiation of the pulmonary epithelium, resulting in the formation of the future air-blood tissue barrier. Surfactant synthesis and the canalization of the lung parenchyma by capillaries begin. During this stage, the future gas exchange regions can be easily distinguished from the future conducting airways of the lungs.
Saccular stage.
The saccular stage of lung development in humans lasts from week 24 to near term. The most peripheral airways form widened airspaces, termed saccules. These saccules widen and lengthen the airspace, in large measure by the addition of new generations. During this stage, the future gas exchange region expands significantly. Populations of fibroblastic cells also undergo differentiation during this stage. These fibroblast-like cells are responsible for the production of the extracellular matrix, collagen, and elastin. It is also presumed that they play an important role in epithelial differentiation and control of surfactant secretion in connection with the growth of the gas exchange region during this stage. The vascular tree also grows in length and diameter during this time.
Columnar cells that are undifferentiated characterize the first epithelial cells lining fetal lung tubules. The first epithelial cells to differentiate in the trachea are neuroendocrine cells, followed closely by ciliated cells, and finally basal and secretory cells in rapid sequence. This process of differentiation covers a developmental period ranging from days to months. In rodents including the mouse, rat, and hamster, complete epithelial differentiation of the trachea occurs in as little as 2 days. In primate trachea, cellular differentiation takes up to 6 months to be complete. In most species, epithelial cell differentiation of the trachea usually is not complete until just before birth. For more peripheral airway generations, cellular differentiation is likely to continue into the early postnatal period. Fetal epithelial cells are typically filled with glycogen that is gradually replaced with a granular cytoplasm filled with numerous organelles during cellular differentiation. These glycogen-filled cells are found throughout the tracheobronchial tree as well as into the most peripheral saccules. Differentiation of the epithelium is highly site specific, giving rise to more than 10 different cell types. For example, within the saccules of the lungs, cells lining these surfaces differentiate to form both squamous type 1 cells as well as cuboidal type 2 cells. The presence of glycogen within these cells may persist into early postnatal life.