Talk:SH Lecture - Lymphatic Structure and Organs: Difference between revisions
(→2011) |
|||
| Line 80: | Line 80: | ||
PMID 21555569 | PMID 21555569 | ||
http://www.pnas.org/content/108/21/8755.long | |||
==2012 Lecture Introduction== | ==2012 Lecture Introduction== | ||
Revision as of 07:37, 26 February 2013
2013
Mouse Model of Lymph Node Metastasis via Afferent Lymphatic Vessels for Development of Imaging Modalities
PLoS One. 2013;8(2):e55797. doi: 10.1371/journal.pone.0055797. Epub 2013 Feb 6.
Li L, Mori S, Sakamoto M, Takahashi S, Kodama T. Source Department of Biomedical Engineering, Graduate School of Biomedical Engineering, Tohoku University, Sendai, Japan ; Department of Diagnostic Radiology, Graduate School of Medicine, Tohoku University, Sendai, Japan. Abstract Animal studies of lymph node metastasis are constrained by limitations in the techniques available for noninvasive monitoring of the progression of lymph node metastasis, as well as difficulties in the establishment of appropriate animal models. To overcome these challenges, this study has developed a mouse model of inter-lymph-node metastasis via afferent lymphatic vessels for use in the development of imaging modalities. We used 14- to 18-week-old MRL/MpJ-/lpr/lpr (MRL/lpr) mice exhibiting remarkable systemic lymphadenopathy, with proper axillary lymph nodes (proper-ALNs) and subiliac lymph nodes (SiLNs) that are 6 to 12 mm in diameter (similar in size to human lymph nodes). When KM-Luc/GFP malignant fibrous histiocytoma-like cells stably expressing the firefly luciferase gene were injected into the SiLN, metastasis could be detected in the proper-ALN within 3 to 9 days, using in vivo bioluminescence imaging. The metastasis route was found to be via the efferent lymphatic vessels of the SiLN, and metastasis incidence depended on the number of cells injected, the injection duration and the SiLN volume. Three-dimensional contrast-enhanced high-frequency ultrasound imaging showed that the blood vessel volume and density in the metastasized proper-ALN significantly increased at 14 days after tumor cell inoculation into the SiLN. The present metastasis model, with lymph nodes similar in size to those of humans, has potential use in the development of ultrasound imaging with high-precision and high-sensitivity as well as other imaging modalities for the detection of blood vessels in lymph nodes during the progression of metastasis.
PMID 23405215
http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0055797
2012
A decade of imaging cellular motility and interaction dynamics in the immune system
Science. 2012 Jun 29;336(6089):1676-81. doi: 10.1126/science.1221063.
Germain RN, Robey EA, Cahalan MD. Source Laboratory of Systems Biology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892, USA. rgermain@nih.gov
Abstract
To mount an immune response, lymphocytes must recirculate between the blood and lymph nodes, recognize antigens upon contact with specialized presenting cells, proliferate to expand a small number of clonally relevant lymphocytes, differentiate to antibody-producing plasma cells or effector T cells, exit from lymph nodes, migrate to tissues, and engage in host-protective activities. All of these processes involve motility and cellular interactions--events that were hidden from view until recently. Introduced to immunology by three papers in this journal in 2002, in vivo live-cell imaging studies are revealing the behavior of cells mediating adaptive and innate immunity in diverse tissue environments, providing quantitative measurement of cellular motility, interactions, and response dynamics. Here, we review themes emerging from such studies and speculate on the future of immunoimaging.
PMID 22745423
Developmental heterogeneity in DNA packaging patterns influences T-cell activation and transmigration
PLoS One. 2012;7(9):e43718. doi: 10.1371/journal.pone.0043718. Epub 2012 Sep 5.
Gupta S, Marcel N, Talwar S, Garg M, R I, Perumalsamy LR, Sarin A, Shivashankar GV. Source National Centre for Biological Sciences, Tata Institute for Fundamental Research, Bangalore, Karnataka, India.
Abstract
Cellular differentiation programs are accompanied by large-scale changes in nuclear organization and gene expression. In this context, accompanying transitions in chromatin assembly that facilitates changes in gene expression and cell behavior in a developmental system are poorly understood. Here, we address this gap and map structural changes in chromatin organization during murine T-cell development, to describe an unusual heterogeneity in chromatin organization and associated functional correlates in T-cell lineage. Confocal imaging of DNA assembly in cells isolated from bone marrow, thymus and spleen reveal the emergence of heterogeneous patterns in DNA organization in mature T-cells following their exit from the thymus. The central DNA pattern dominated in immature precursor cells in the thymus whereas both central and peripheral DNA patterns were observed in naïve and memory cells in circulation. Naïve T-cells with central DNA patterns exhibited higher mechanical pliability in response to compressive loads in vitro and transmigration assays in vivo, and demonstrated accelerated expression of activation-induced marker CD69. T-cell activation was characterized by marked redistribution of DNA assembly to a central DNA pattern and increased nuclear size. Notably, heterogeneity in DNA patterns recovered in cells induced into quiescence in culture, suggesting an internal regulatory mechanism for chromatin reorganization. Taken together, our results uncover an important component of plasticity in nuclear organization, reflected in chromatin assembly, during T-cell development, differentiation and transmigration.
PMID 22957031
Figure 5. Schematic depicting the various stages in T-cell development and the observed differences in the nuclear organization.
2011
Ex vivo imaging of T cells in murine lymph node slices with widefield and confocal microscopes
J Vis Exp. 2011 Jul 15;(53):e3054. doi: 10.3791/3054.
Salmon H, Rivas-Caicedo A, Asperti-Boursin F, Lebugle C, Bourdoncle P, Donnadieu E. Source Institut Cochin, Université Paris Descartes, CNRS (UMR 8104). Abstract
Naïve T cells continuously traffic to secondary lymphoid organs, including peripheral lymph nodes, to detect rare expressed antigens. The migration of T cells into lymph nodes is a complex process which involves both cellular and chemical factors including chemokines. Recently, the use of two-photon microscopy has permitted to track T cells in intact lymph nodes and to derive some quantitative information on their behavior and their interactions with other cells. While there are obvious advantages to an in vivo system, this approach requires a complex and expensive instrumentation and provides limited access to the tissue. To analyze the behavior of T cells within murine lymph nodes, we have developed a slice assay, originally set up by neurobiologists and transposed recently to murine thymus. In this technique, fluorescently labeled T cells are plated on top of an acutely prepared lymph node slice. In this video-article, the localization and migration of T cells into the tissue are analyzed in real-time with a widefield and a confocal microscope. The technique which complements in vivo two-photon microscopy offers an effective approach to image T cells in their natural environment and to elucidate mechanisms underlying T cell migration.
PMID 21775968
B cells within germinal centers migrate preferentially from dark to light zone
Proc Natl Acad Sci U S A. 2011 May 24;108(21):8755-60. doi: 10.1073/pnas.1101554108. Epub 2011 May 9.
Beltman JB, Allen CD, Cyster JG, de Boer RJ. Source Theoretical Biology, Utrecht University, Padualaan 8, 3584 CH Utrecht, The Netherlands. J.B.Beltman@uu.nl
Abstract
One of the main questions in the field of imaging immune cell migration in living tissues is whether cells fulfill their functionality via random or nonrandom migration processes. For some applications, this issue has remained controversial even after publication of various imaging studies. A prime example is B-cell migration in germinal centers (GCs) where somatic hypermutation and clonal selection of B cells are thought to occur within morphologically distinct regions termed dark zone (DZ) and light zone (LZ). Here, we reanalyze a previously published dataset on GC B-cell migration, applying a sensitive analysis technique to detect directed migration and using a procedure to correct for a number of artifacts that frequently occur in time-lapse imaging experiments. Although B cells roughly perform a persistent random walk, we present evidence that they have a small preference (of on average about 0.2-0.3 μm min(-1)) to migrate from DZ to LZ, which is consistent with classical views of the GC reaction. This preference is most pronounced among a large subset of almost half of the B-cell population migrating along relatively straight tracks. Using a computational model to generate long-lasting B-cell tracks based on the experimental motility data (including the small directional preference), we predict a time course to travel from DZ to LZ of a few hours. This is consistent with experimental observations, and we show that at the observed cellular motility such a time course cannot be explained without the small preferential migration from DZ to LZ.
PMID 21555569
http://www.pnas.org/content/108/21/8755.long
2012 Lecture Introduction
While the structure of the lymphatic system (lympha = clear water) is well described, there is much to still learn about the complex development and function of this "system".
- Immune - “monitor” of body surfaces, internal fluids
- Extracellular fluid - returns interstitial fluid to circulation
- Gastrointestinal tract - carries fat and fat-soluble vitamins
Aim
This lecture will provide an overview of the histology of key lymphoid organs, including the lymph nodes, spleen and thymus, as well as extranodal lymphoid tissues including mucosal associated lymphoid tissues (MALT)
Key Concepts
- Lymphatic System
- Organs - Thymus, Spleen
- Lymph Nodes and Nodules
- Bone Marrow
- Extranodal Lymphoid Tissues
- Mucosal Associated Lymphoid Tissues (MALT)
Textbook References
- SH Laboratory Support
- Janeway’s Immunobiology NCBI Bookshelf | Publisher page
- Histology and Cell Biology - A.L. Kiersenbaum (2001) Chapter 6: Blood, Chapter 10: Immune-Lymphatic Cover
- Previous Lectures: 2010 | 2008
Two Cellular Systems
- Lymphoid System - three major types of lymphocytes (T, B, and NK), tissues, organs and vessels
- Mononuclear Phagocytic System (MPS, also called Lymphoreticular System or Reticuloendothelial System, RES) - circulating monocytes of peripheral blood and non-circulating (fixed) tissue macrophages found throughout the body
MBoC Figure 24-3 Human lymphoid organs
Lymphatic System
- Connective Tissue Embryonic origin- Mesoderm
- Consists of Cells, tissues and organs
Immune System Note: Immunity is covered in detail elsewhere in the course, current lecture is about Lymphoid Organ structure/location
- Tissues and Organs
- Thymus, spleen, lymph nodes, lymphatic nodules, diffuse lymphatic tissues, bone marrow
- Organs consist also of structural cells and extracellular matrix
- Lymphatic vessels connect system parts
- Cells are Lymphocytes
- B Lymphocytes and T Lymphocytes
- White blood cells, leukocytes
These are blood cells
Blood Cells
Lymphocyte Electron Micrographs
Central/Peripheral Lymphoid Organs
Central lymphoid organs
- Lymphocytes develop from precursor cells (see blood marrow image)
Peripheral lymphoid organs
- Lymphocytes respond to antigen
- lymph nodes or spleen
Mononuclear Phagocytic System
(Mononuclear Phagocytic System MPS, also called Lymphoreticular System or Reticuloendothelial System, RES)
Mononuclear Phagocytes 2 types:
- Circulating monocytes of peripheral blood (monocytes entering the connective tissue differentiate into macrophages)
- Non-circulating (fixed) tissue macrophages (MΦ) found throughout the body (Liver (Kuffler cells), spleen and other tissues)
Lymph
- Fluid portion of lymphatic circulation
- blood plasma will leave blood vessels into surrounding tissues
- adds to normal tissue interstitial fluid
- surplus of liquid needs to be returned to circulation
- Lymph vessels provide unidirectional flow of this liquid
Lymph Vessels
Three types based on size and morphology
- Lymph capillaries begin as blind-ending tubes in connective tissue, larger than blood capillaries, very irregularly shaped
- Lymph collecting vessels larger and form valves, morphology similar to lymph capillaries
- Lymph ducts 1 or 2 layers of smooth muscle cells in wall
(Remember anatomy acronym - NAVL = Nerve, Artery, Vein and Lymph)
Lymphocyte Circulation
- The circulation through a lymph node is shown.
- Microbial antigens are carried into the lymph node by dendritic cells, which enter via afferent lymphatic vessels draining an infected tissue.
- T and B cells, by contrast, enter the lymph node via an artery and migrate out of the bloodstream through postcapillary venules.
- Unless they encounter their antigen, the T and B cells leave the lymph node via efferent lymphatic vessels, which eventually join the thoracic duct.
- The thoracic duct empties into a large vein carrying blood to the heart.
- A typical circulation cycle takes about 12–24 hours.
Links: MBoC Chapter 24 - The Adaptive Immune System | MBoC Figure 24-14. The path followed by lymphocytes as they continuously circulate between the lymph and blood | Immunobiology
Diffuse Lymphatic Tissue
Alimentary canal, respiratory passage, urogenital tract
- Not enclosed by a connective tissue capsule
- Located in subepithelial tissue - Lamina propria
Lymphocytes
- travel to nodes and back again
- proliferation and differentiation
Effector cells
- B Cell secreting antibody = Plasma Cell
- T Cell = Memory Cell, Cytotoxic T cells, T helper cell
- Diffuse lymphatic tissue + nodules
- Reactive - enlarge when activated (by antigen)
MALT, BALT and GALT
Internal epithelia Associated Lymphoid Tissue - naming based upon the anatomical locations
|
Immune Responses
Adaptive immunity has 2 main classes
- Antibody-mediated - B Lymphocyte
- Cell-mediated - T Lymphocyte
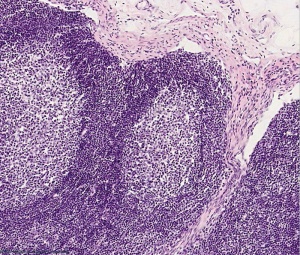
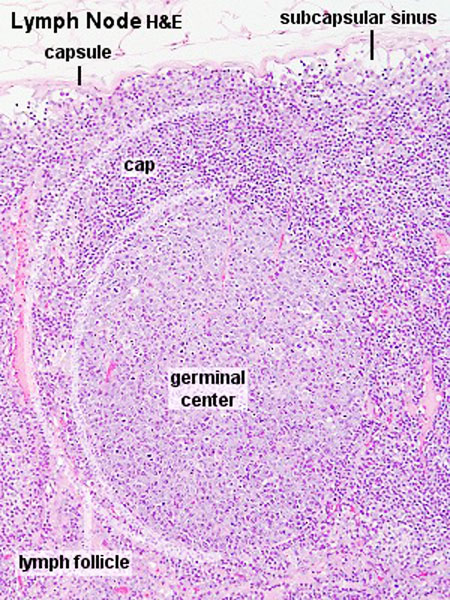
Lymph Nodules
- Organized concentrations of lymphocytes
- No capsule, covered by epithelia
- Nodules are also the unit structure seen in a node
- Oval concentrations in meshwork of reticular cells
Gastrointestinal Tract
- Oropharynx - Tonsils
- Distal small intestine (ilieum) - Peyer’s Patches
- Appendix, cecum
Nodule States
- Primary Nodule - Mainly small lymphocytes
- Secondary Nodule
- Central pale region (germinal centre) - Effector cells and macrophages
- Dark outer ring (small lymphocytes)
Links: Immnuobiology - Figure 1.10. Organization of typical gut-associated lymphoid tissue
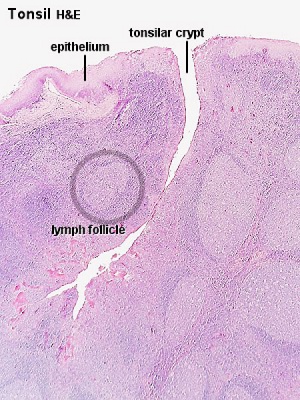
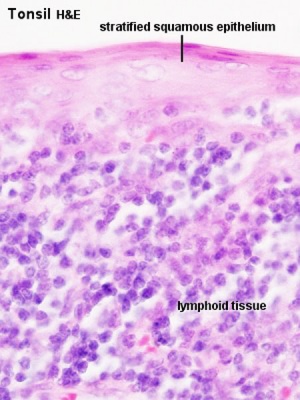
Tonsils
Anatomical location - Palatine (tonsils), Lingual and Pharyngeal ( adenoids )
Palatine Tonsils
- the "tonsils", lateral wall of oropharynx
- covered by stratified squamous epithelium
- numerous crypts (10-20) infolds of surface epithelium
- Afferent lymph vessels absent
- Efferent lymph vessels are present
Lingual Tonsils
- lamina propria root of tongue
- covered by stratified squamous epithelium
- salivary glands and skeletal muscle are directly adjacent
Pharyngeal Tonsils
- adenoids or nasopharyngeal tonsils, upper posterior part of throat
- covered by a pseudostratified ciliated epithelium with goblet cells
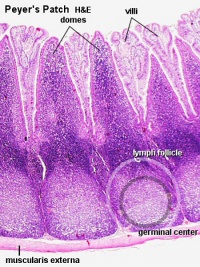
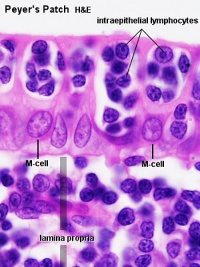
Peyer's Patch
Peyer's Patch, Ileum
microfold cells or M-cells
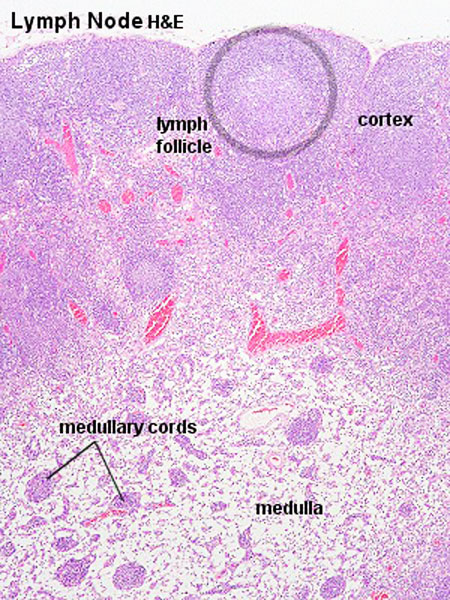
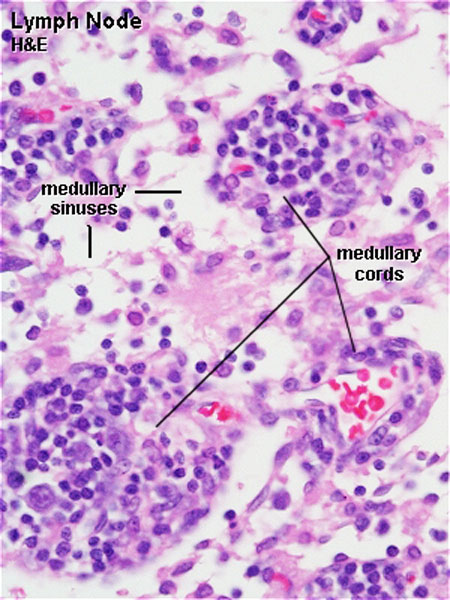
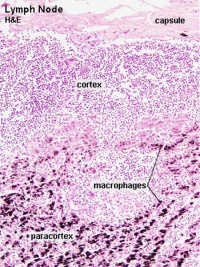
Lymph Nodes
MBoC Figure 24-16. A simplified drawing of a human lymph node
* Encapsulated organ (1 mm - 2 cm)
|
|
Lymph Node Structure
Connective Tissue
- Capsule - dense connective tissue (irregular CT, some adipocytes))
- Trabeculae - dense connective tissue
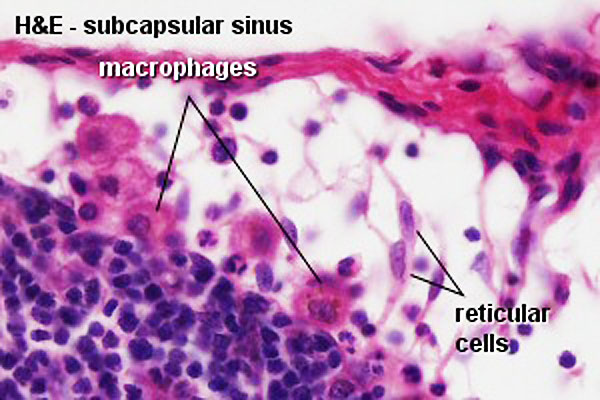
- Reticular Tissue - Reticular cells and fibers, supporting meshwork (collagen type III)
- Reticular cell produces reticular fibers (collagen type III) and surrounds the fibers with its cytoplasm
- reticular fibbers can also be produced by fibroblasts
Lymph
- enters the node through afferent vessels
- filters through the sinuses
- leaves through efferent vessels
Subcapsular sinus = marginal sinus
Continuation of trabecular sinus
Lymphocyte (T and B) Traffic
- Enter from high endothelial venules (HEVs also called post-capillary venules)
- Spend 8 to 24 h in the lymph node interstitium.
- Enter a network of medullary sinuses.
- Drain from sinuses into efferent lymphatic vessels.
Links: Immunobiology - Figure 1.8. Organization of a lymph node
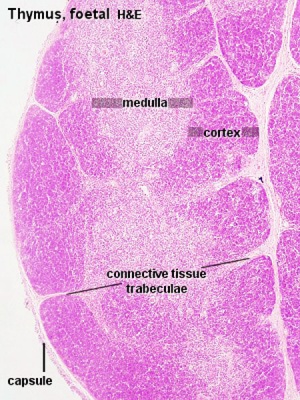
Thymus
| MBoC Figure 24-6. The development and activation of T and B cells
Figure 24-7. Electron micrographs of nonactivated and activated lymphocytes |
Development Changes
Changes with age Overall Size
- birth 10-15 g
- puberty 30-40 g
- after puberty - involution
- Replaced by adipose tissue
- middle-aged 10 g
Thymus Anatomy
- Superior mediastinum, anterior to heart
- Bilobed lymphoepithelial organ
- Contains reticular cells but no fibers
- Stem lymphocytes
- proliferate and differentiate
- forms long-lived T- lymphocytes
Thymus Cells
- Reticular cells
- Abundant, eosinophilic, large, ovoid and light nucleus 1-2 nucleoli
- sheathe cortical capillaries
- form an epitheloid layer
- maintain microenvironment for development of T-lymphocytes in cortex (thymic epitheliocytes)
- Macrophages
- cortex and medulla
- difficult to distinguish from reticular cells in H&E
- Lymphocytes
- cortex and medulla - more numerous (denser) in cortex
- majority of them developing T-lymphocytes (= thymic lymphocytes or thymocytes)
Fetal/Young Thymus
Thymic corpuscle
Hassall’s corpuscle - Mass of concentric epithelioreticular cells
Adult Thymus
- Cortical lymphoid tissue is replaced by adipose tissue
- Increase in size of thymic corpuscles
- Thymus Histology: Fetal Thymus overview | Fetal Thymus Medulla | Fetal Thymus Cortex | Adult Thymus | unlabeled fetal overview | unlabeled fetal medulla |unlabeled fetal thymic corpuscle |unlabeled fetal cortex | unlabeled adult overview | Category:Thymus | Immune System Development
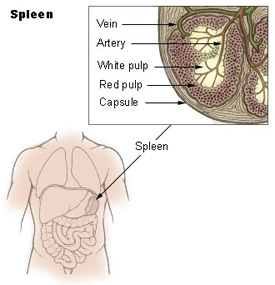
Spleen
Functions
1. Immune
- filters blood in much the way that the lymph nodes filter lymph.
- Lymphocytes in the spleen react to pathogens in the blood and attempt to destroy them.
- Macrophages then engulf the resulting debris, the damaged cells, and the other large particles.
2. Red Blood Cell Removal
- The spleen (and liver) removes old and damaged erythrocytes from the circulating blood.
- Like other lymphatic tissue, it produces lymphocytes, especially in response to invading pathogens.
3. Blood Reservoir
- The sinuses in the spleen also act as a reservoir for blood.
- In emergencies, such as hemorrhage, smooth muscle in the vessel walls and in the capsule of the spleen contracts.
- This squeezes the blood out of the spleen into the general circulation.
Structure
- Capsule, trabeculae (dense connective tissue)
- Splenic pulp White pulp, red pulp - based on appearance and cell content
White Pulp
- lymphocytes surround central arteries
- as periarterial lymphoid sheath (PALS)
Red Pulp
- Red blood cells
- Splenic sinuses
- Splenic cords
Reticular Fibers
| Spleen Development: SH Lecture Spleen | SH Adult Histology | Overview Red and White Pulp | Overview Red and White Pulp | Cords and Sinuses | Reticular Fibre overview | Reticular Fibre detail | unlabeled red and white pulp | unlabeled red pulp and macrophages | unlabeled white pulp germinal centre | unlabeled reticular fibre | unlabeled white pulp reticular | unlabeled red pulp reticular | Structure cartoon | Cartoon and stain | Category:Spleen | Histology Stains | Immune System Development |
Links: Immunobiology - Figure 1.9. Organization of the lymphoid tissues of the spleen
B Cell Development
- Bone marrow
- blood
- Lymph node, nodule
- Lymphatic vessel
- Bone marrow
Germinal Centres
- Bone Marrow
- Medullary cords contain plasma cells
Plasma cells
- secrete antibody directly into blood for distribution to all body
- in local extrafollicular sites are short lived 2–4 days
- longer-lived plasma cells in bone marrow 3 weeks to 3 months+
Additional Information
The following is not part of the lecture and is for reference purposes only.
Immunobiology Textbook
Immunobiology 5th edition The Immune System in Health and Disease Charles A Janeway, Jr, Paul Travers, Mark Walport, and Mark J Shlomchik.
Part I. An Introduction to Immunobiology and Innate Immunity
- Chapter 1. Basic Concepts in Immunology
- The components of the immune system
- Figure 1.3 All the cellular elements of blood, including the lymphocytes of the adaptive immune system, arise from hematopoietic stem cells in the bone marrow
- Figure 1.4 Myeloid cells in innate and adaptive immunity
- Figure 1.5 Lymphocytes are mostly small and inactive cells
- Figure 1.6 Natural killer (NK) cells
- Figure 1.7 The distribution of lymphoid tissues in the body
- Figure 1.8 Organization of a lymph node
- Figure 1.9 Organization of the lymphoid tissues of the spleen
- Figure 1.10 Organization of typical gut-associated lymphoid tissue
- Figure 1.11 Circulating lymphocytes encounter antigen in peripheral lymphoid organs
- Summary to Chapter 1
- The components of the immune system
Part III. The Development of Mature Lymphocyte Receptor Repertoires
- Chapter 7. The Development and Survival of Lymphocytes
- Generation of lymphocytes in bone marrow and thymus
- Figure 7.3 The early stages of B-cell development are dependent on bone marrow stromal cells
- Figure 7.5 The development of a B-lineage cell proceeds through several stages marked by the rearrangement and expression of the immunoglobulin genes
- Figure 7.7 The cellular organization of the human thymus
- Figure 7.13Thymocytes at different developmental stages are found in distinct parts of the thymus
- Survival and maturation of lymphocytes in peripheral lymphoid tissues
- Summary to Chapter 7
- Generation of lymphocytes in bone marrow and thymus
- Associated Practical support page - SH Practical - Lymphatic Structure and Organs
Blood Cell Numbers
The adult ranges of cells / 1 litre (l), total blood volume is about 4.7 to 5 litres. Blood Development | Blood Histology
Red Blood Cells
- Male: 4.32 - 5.66 x 1012/l
- Female: 3.88 - 4.99 x 1012/l
Leukocytes (white blood cells)
- Male: 3.7 - 9.5 x 109/l
- Female: 3.9 - 11.1 x 109/l
Granulocytes
- 1.8 - 8.9 x 109/l
- Neutrophils: 1.5 - 7.4 x 109/l
- Eosinophils: 0.02 - 0.67 x 109/l
- Basophils: 0 - 0.13 x 109/l
Non-Granulocytes
- Monocytes 0.21 - 0.92 x 109/l
Lymphocytes
- 1.1 - 3.5 x 109/l
- B-cells: 0.06 - 0.66 x 109/l
- T-cells: 0.77 - 2.68 x 109/l
- CD4+: 0.53 - 1.76 x 109/l
- CD8+: 0.30 - 1.03 x 109/l
- NK cells: 0.20 - 0.40 x 109/l
Platelets
- 140 - 440 x 109/l
- not a cell, a cell fragment.
Anatomy of the Human Body Gray (1918 Historic anatomy is good, there are there are some functional inaccuracies).
Additional Images
Reciprocal interactions of the intestinal microbiota and immune system http://www.nature.com/nature/journal/v489/n7415/full/nature11551.html
Todays Lecture in the Online Textbooks
--Mark Hill 10:32, 24 February 2012 (EST) The following link to text and figures that relate to lymphatic structure and organs. Remember the Lecture is about structure/function and not a description of immunity (that will be covered elsewhere in the course).
Immunobiology
Immunobiology 5th edition The Immune System in Health and Disease Charles A Janeway, Jr, Paul Travers, Mark Walport, and Mark J Shlomchik.
Part I. An Introduction to Immunobiology and Innate Immunity
- Chapter 1. Basic Concepts in Immunology
- The components of the immune system
- Figure 1.3 All the cellular elements of blood, including the lymphocytes of the adaptive immune system, arise from hematopoietic stem cells in the bone marrow
- Figure 1.4 Myeloid cells in innate and adaptive immunity
- Figure 1.5 Lymphocytes are mostly small and inactive cells
- Figure 1.6 Natural killer (NK) cells
- Figure 1.7 The distribution of lymphoid tissues in the body
- Figure 1.8 Organization of a lymph node
- Figure 1.9 Organization of the lymphoid tissues of the spleen
- Figure 1.10 Organization of typical gut-associated lymphoid tissue
- Figure 1.11 Circulating lymphocytes encounter antigen in peripheral lymphoid organs
- Summary to Chapter 1
- The components of the immune system
Part III. The Development of Mature Lymphocyte Receptor Repertoires
- Chapter 7. The Development and Survival of Lymphocytes
- Generation of lymphocytes in bone marrow and thymus
- Figure 7.3 The early stages of B-cell development are dependent on bone marrow stromal cells
- Figure 7.5 The development of a B-lineage cell proceeds through several stages marked by the rearrangement and expression of the immunoglobulin genes
- Figure 7.7 The cellular organization of the human thymus
- Figure 7.13Thymocytes at different developmental stages are found in distinct parts of the thymus
- Survival and maturation of lymphocytes in peripheral lymphoid tissues
- Summary to Chapter 7
- Generation of lymphocytes in bone marrow and thymus
Molecular Biology of the Cell
- Lymphocytes and the Cellular Basis of Adaptive Immunity
- The Adaptive Immune System
- Innate Immunity
- MBoC Figure 24-7. Electron micrographs of nonactivated and activated lymphocytes
Medical Microbiology
Pubmed Bookshelf
Immunobiology
5th edition The Immune System in Health and Disease Charles A Janeway, Jr, Paul Travers, Mark Walport, and Mark J Shlomchik.
- Preface to the Fifth Edition
- Acknowledgments
- Icons Used Throughout the Book
Part I. An Introduction to Immunobiology and Innate Immunity
- Chapter 1. Basic Concepts in Immunology
- The components of the immune system
- Figure 1.3 All the cellular elements of blood, including the lymphocytes of the adaptive immune system, arise from hematopoietic stem cells in the bone marrow
- Figure 1.4 Myeloid cells in innate and adaptive immunity
- Figure 1.5 Lymphocytes are mostly small and inactive cells
- Figure 1.6 Natural killer (NK) cells
- Figure 1.7 The distribution of lymphoid tissues in the body
- Figure 1.8 Organization of a lymph node
- Figure 1.9 Organization of the lymphoid tissues of the spleen
- Figure 1.10 Organization of typical gut-associated lymphoid tissue
- Figure 1.11 Circulating lymphocytes encounter antigen in peripheral lymphoid organs
- Principles of innate and adaptive immunity
- The recognition and effector mechanisms of adaptive immunity
- Summary to Chapter 1
- General references
- The components of the immune system
- Chapter 2. Innate Immunity
- The front line of host defense
- The complement system and innate immunity
- Receptors of the innate immune system
- Induced innate responses to infection
- Summary to Chapter 2
- General references
- Section references
Part II. The Recognition of Antigen
- Chapter 3. Antigen Recognition by B-cell and T-cell Receptors
- The structure of a typical antibody molecule
- The interaction of the antibody molecule with specific antigen
- Antigen recognition by T cells
- Summary to Chapter 3
- General references
- Section references
- Chapter 4. The Generation of Lymphocyte Antigen Receptors
- The generation of diversity in immunoglobulins
- T-cell receptor gene rearrangement
- Structural variation in immunoglobulin constant regions
- Summary to Chapter 4
- General references
- Section references
- Chapter 5. Antigen Presentation to T Lymphocytes
- The generation of T-cell receptor ligands
- The major histocompatibility complex and its functions
- Summary to Chapter 5
- General references
- Section references
Part III. The Development of Mature Lymphocyte Receptor Repertoires
- Chapter 6. Signaling Through Immune System Receptors
- General principles of transmembrane signaling
- Antigen receptor structure and signaling pathways
- Other signaling pathways that contribute to lymphocyte behavior
- Summary to Chapter 6
- General references
- Section references
- Chapter 7. The Development and Survival of Lymphocytes
- Generation of lymphocytes in bone marrow and thymus
- Figure 7.3 The early stages of B-cell development are dependent on bone marrow stromal cells
- Figure 7.5 The development of a B-lineage cell proceeds through several stages marked by the rearrangement and expression of the immunoglobulin genes
- Figure 7.7 The cellular organization of the human thymus
- Figure 7.13Thymocytes at different developmental stages are found in distinct parts of the thymus
- The rearrangement of antigen-receptor gene segments controls lymphocyte development
- Interaction with self antigens selects some lymphocytes for survival but eliminates others
- Survival and maturation of lymphocytes in peripheral lymphoid tissues
- Summary to Chapter 7
- General references
- Section references
- Generation of lymphocytes in bone marrow and thymus
Part IV. The Adaptive Immune Response
- Chapter 8. T Cell-Mediated Immunity
- The production of armed effector T cells
- General properties of armed effector T cells
- T cell-mediated cytotoxicity
- Macrophage activation by armed CD4 TH1 cells
- Summary to Chapter 8
- General references
- Section references
- Chapter 9. The Humoral Immune Response
- B-cell activation by armed helper T cells
- The distribution and functions of immunoglobulin isotypes
- The destruction of antibody-coated pathogens via Fc receptors
- Summary to Chapter 9
- General references
- Section references
- Chapter 10. Adaptive Immunity to Infection
- Infectious agents and how they cause disease
- The course of the adaptive response to infection
- The mucosal immune system
- Immunological memory
- Summary to Chapter 10
- General references
- Section references
Part V. The Immune System in Health and Disease
- Chapter 11. Failures of Host Defense Mechanisms
- Pathogens have evolved various means of evading or subverting normal host defenses
- Inherited immunodeficiency diseases
- Acquired immune deficiency syndrome
- Summary to Chapter 11
- General references
- Section references
- Chapter 12. Allergy and Hypersensitivity
- The production of IgE
- Effector mechanisms in allergic reactions
- Hypersensitivity diseases
- Summary to Chapter 12
- General references
- Section references
- Chapter 13. Autoimmunity and Transplantation
- Autoimmune responses are directed against self antigens
- Responses to alloantigens and transplant rejection
- Self-tolerance and its loss
- Summary to Chapter 13
- General references
- Section references
- Chapter 14. Manipulation of the Immune Response
- Extrinsic regulation of unwanted immune responses
- Using the immune response to attack tumors
- Manipulating the immune response to fight infection
- Summary to Chapter 14
- General references
- Section references
- Chapter 15. Afterword
- Evolution of the innate immune system
- Evolution of the adaptive immune response
- The importance of immunological memory in fixing adaptive immunity in the genome
- Future directions of research in immunobiology
- Summary of the Afterword
Appendices
- Appendix I. Immunologists' Toolbox
- Appendix II. CD Antigens
- Appendix III. Cytokines and Their Receptors
- Appendix IV. Chemokines and Their Receptors
- Appendix V. Immunological Constants
- Biographies
- Glossary
Molecular Biology of the Cell
Medical Microbiology
Search PubMed Databases immune system
2011
Start Time/End Time: 10am to 11am Monday 28 February 2011 Clancy Auditorium eMed Link to Learning Activity - Lymphatic organs histology
- UNSW Virtual Slide set http://vslides.unsw.edu.au/VirtualSlideV2.nsf/id/FA8942
- Blue Histology - Lymphoid Tissues I
- Blue Histology - Lymphoid Tissues II
Bookshelf links
old - http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A4419&rendertype=figure&id=A4423 MBoC Figure 24-3 Human lymphoid organs
new - http://www.ncbi.nlm.nih.gov/books/NBK26921/figure/A4423 MBoC Figure 24-3 Human lymphoid organs
Recent Research
Defining the quantitative limits of intravital two-photon lymphocyte tracking
Proc Natl Acad Sci U S A. 2011 Jul 26;108(30):12401-6. Epub 2011 Jul 6.
Textor J, Peixoto A, Henrickson SE, Sinn M, von Andrian UH, Westermann J. Source Institute for Theoretical Computer Science, University of Lübeck, 23562 Lübeck, Germany. textor@tcs.uni-luebeck.de
Abstract
Two-photon microscopy has substantially advanced our understanding of cellular dynamics in the immune system. Cell migration can now be imaged in real time in the living animal. Strikingly, the migration of naive lymphocytes in secondary lymphoid tissue appears predominantly random. It is unclear, however, whether directed migration may escape detection in this random background. Using a combination of mathematical modeling and experimental data, we investigate the extent to which modern two-photon imaging can rule out biologically relevant directed migration. For naive T cells migrating in uninfected lymph nodes (LNs) at average 3D speeds of around 18 μm/min, we rule out uniform directed migration of more than 1.7 μm/min at the 95% confidence level, confirming that T cell migration is indeed mostly random on a timescale of minutes. To investigate whether this finding still holds for longer timescales, we use a 3D simulation of the naive T cell LN transit. A pure random walk predicts a transit time of around 16 h, which is in good agreement with experimental results. A directional bias of only 0.5 μm/min-less than 3% of the cell speed-would already accelerate the transit twofold. These results jointly strengthen the random walk analogy for naive T cell migration in LNs, but they also emphasize that very small deviations from random migration can still be important. Our methods are applicable to cells of any type and can be used to reanalyze existing datasets.
PMID 21734152
http://www.pnas.org/content/108/30/12401.full
Stromal cell contributions to the homeostasis and functionality of the immune system
Nat Rev Immunol. 2009 Sep;9(9):618-29. Epub 2009 Jul 31.
Mueller SN, Germain RN. Source Department of Microbiology and Immunology, The University of Melbourne, Parkville, 3010 Victoria, Australia. smue@unimelb.edu.au Abstract A defining characteristic of the immune system is the constant movement of many of its constituent cells through the secondary lymphoid tissues, mainly the spleen and lymph nodes, where crucial interactions that underlie homeostatic regulation, peripheral tolerance and the effective development of adaptive immune responses take place. What has only recently been recognized is the role that non-haematopoietic stromal elements have in many aspects of immune cell migration, activation and survival. In this Review, we summarize our current understanding of lymphoid compartment stromal cells, examine their possible heterogeneity, discuss how these cells contribute to immune homeostasis and the efficient initiation of adaptive immune responses, and highlight how targeting of these elements by some pathogens can influence the host immune response.
PMID 19644499
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2785037
http://www.nature.com/nri/journal/v9/n9/abs/nri2588.html
http://www.microbiol.unimelb.edu.au/research/immunology/s_mueller.html
Lymphatic vessels: structure and function
Isr Med Assoc J. 2011 Dec;13(12):762-8.
Rovenská E, Rovenský J. Source National institute of Rheumatic Diseases, Piestany, Slovak Republik. rovensky.jozef@nurch.sk l ymphatic vessels are part of the lymphatic system. The ves- sels evolved phylogenetically only after it became necessary for multicellular organisms to remove fluids and proteins from tissue and return them to the bloodstream. In humans, the lymphatic system begins to develop between the sixth and seventh week of embryonic development, at a time when the cardiovascular system is already functioning.
PMID 22332449
http://www.ima.org.il/imaj/dynamic/web/ArtFromPubmed.asp?year=2011&month=12&page=762