Talk:Mouse Timeline Detailed
<pubmed>13207763</pubmed>
<pubmed>22899949</pubmed>| PMC3415261 | Clin Dev Immunol.</ref>
2017
A staging system for correct phenotype interpretation of mouse embryos harvested on embryonic day 14 (E14.5)
J Anat. 2017 Feb 9. doi: 10.1111/joa.12590. [Epub ahead of print]
Geyer SH1, Reissig L1, Rose J1, Wilson R2, Prin F2, Szumska D3, Ramirez-Solis R4, Tudor C4, White J4, Mohun TJ2, Weninger WJ1.
Abstract
We present a simple and quick system for accurately scoring the developmental progress of mouse embryos harvested on embryonic day 14 (E14.5). Based solely on the external appearance of the maturing forelimb, we provide a convenient way to distinguish six developmental sub-stages. Using a variety of objective morphometric data obtained from the commonly used C57BL/6N mouse strain, we show that these stages correlate precisely with the growth of the entire embryo and its organs. Applying the new staging system to phenotype analyses of E14.5 embryos of 58 embryonic lethal null mutant lines from the DMDD research programme (https://dmdd.org.uk) and its pilot, we show that homozygous mutant embryos are frequently delayed in development. To demonstrate the importance of our staging system for correct phenotype interpretation, we describe stage-specific changes of the palate, heart and gut, and provide examples in which correct diagnosis of malformations relies on correct staging.
© 2017 Anatomical Society.
KEYWORDS: Deciphering the Mechanisms of Developmental Disorders; embryo; high-resolution episcopic microscopy; knock out; morphology; mutant
PMID 28185240 DOI: 10.1111/joa.12590
2015
4D atlas of the mouse embryo for precise morphological staging
Development. 2015 Oct 15;142(20):3583-91. doi: 10.1242/dev.125872.
Wong MD1, van Eede MC2, Spring S2, Jevtic S2, Boughner JC3, Lerch JP4, Henkelman RM4.
Abstract
After more than a century of research, the mouse remains the gold-standard model system, for it recapitulates human development and disease and is quickly and highly tractable to genetic manipulations. Fundamental to the power and success of using a mouse model is the ability to stage embryonic mouse development accurately. Past staging systems were limited by the technologies of the day, such that only surface features, visible with a light microscope, could be recognized and used to define stages. With the advent of high-throughput 3D imaging tools that capture embryo morphology in microscopic detail, we now present the first 4D atlas staging system for mouse embryonic development using optical projection tomography and image registration methods. By tracking 3D trajectories of every anatomical point in the mouse embryo from E11.5 to E14.0, we established the first 4D atlas compiled from ex vivo 3D mouse embryo reference images. The resulting 4D atlas comprises 51 interpolated 3D images in this gestational range, resulting in a temporal resolution of 72 min. From this 4D atlas, any mouse embryo image can be subsequently compared and staged at the global, voxel and/or structural level. Assigning an embryonic stage to each point in anatomy allows for unprecedented quantitative analysis of developmental asynchrony among different anatomical structures in the same mouse embryo. This comprehensive developmental data set offers developmental biologists a new, powerful staging system that can identify and compare differences in developmental timing in wild-type embryos and shows promise for localizing deviations in mutant development.
© 2015. Published by The Company of Biologists Ltd.
KEYWORDS: 3D; 4D; Development; Embryo; Imaging; OPT; Staging PMID 26487781 DOI: 10.1242/dev.125872
2009
Histology atlas of the developing mouse heart with emphasis on E11.5 to E18.5
Toxicol Pathol. 2009 Jun;37(4):395-414. Epub 2009 Apr 9.
Savolainen SM, Foley JF, Elmore SA. Source NIEHS, Cellular and Molecular Pathology Branch, Research Triangle Park, North Carolina 27709, USA.
Abstract
In humans, congenital heart diseases are common. Since the rapid progression of transgenic technologies, the mouse has become the major animal model of defective cardiovascular development. Moreover, genetically modified mice frequently die in utero, commonly due to abnormal cardiovascular development. A variety of publications address specific developmental stages or structures of the mouse heart, but a single reference reviewing and describing the anatomy and histology of cardiac developmental events, stage by stage, has not been available. The aim of this color atlas, which demonstrates embryonic/fetal heart development, is to provide a tool for pathologists and biomedical scientists to use for detailed histological evaluation of hematoxylin and eosin (H&E)-stained sections of the developing mouse heart with emphasis on embryonic days (E) 11.5-18.5. The selected images illustrate the main structures and developmental events at each stage and serve as reference material for the confirmation of the chronological age of the embryo/early fetus and assist in the identification of any abnormalities. An extensive review of the literature covering cardiac development pre-E11.5 is summarized in the introduction. Although the focus of this atlas is on the descriptive anatomic and histological development of the normal mouse heart from E11.5 to E18.5, potential embryonic cardiac lesions are discussed with a list of the most common transgenic pre- and perinatal heart defects. Representative images of hearts at E11.5-15.5 and E18.5 are provided in Figures 2-4, 6, 8, and 9. A complete set of labeled images (Figures E11.5-18.5) is available on the CD enclosed in this issue of Toxicologic Pathology. All digital images can be viewed online at https://niehsimages.epl-inc.com with the username "ToxPath" and the password "embryohearts."
PMID 19359541 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2773446 http://tpx.sagepub.com/content/37/4/395.long
<pubmed>19359541</pubmed>| PMC2773446 | Toxicol Pathol.
E11.5 (TS19)
- Progressive septation of the outflow tract
- Progressive septation of the atria and ventricles
E12.5 (TS21)
- Progressive septation of the outflow tract
- Initiation of atrioventricular canal septation
E13.5 (TS22)
- Bilaterally asymmetrical aortic arch system
- Completely septated outflow tract and ventricles
- Remodeling of the atrioventricular cushions
E14.5 (TS23)
- Atrial septation complete
E15.5 (TS24) - E18.5 (TS27)
- Definitive external prenatal configuration achieved
- Atrioventricular valve leaflets are being modified
- Coronary arteries are being modified
Urogenital Development
A high-resolution description of the developing murine genitourinary tract from Theiler stage (TS) 17 (E10.5) through to TS27 (E19.5) and then to postnatal day 3 was published in 2007.[1]
The GenitoUrinary Development Molecular Anatomy Project (GUDMAP) http://www.gudmap.org/About/Tutorial/DevMUS.html
Renal
TS12 (about 8 dpc): The first appearance of the intermediate plate mesoderm is seen, and this approximately corresponds with the first appearance of the somites.
TS13-15 (about 8.5-9.5 dpc): First evidence of nephrogenic cord formation and the differentiation of its most rostral part to form the pronephros. Later, the nephrogenic cord differentiates into the urogenital ridge. Mesonephric tubules are first recognised (at about TS15), and these drain into the mesonephric duct that is clearly seen to be patent along its length. The duct extends caudally towards the urogenital sinus.
TS16-17 (about 10-10.5 dpc): Mesonephric tubules are now clearly seen, as are mesonephric vesicles. The first evidence of the urorectal septum is seen, and will soon separate the hindgut from the urogenital sinus, and divide the cloacal septum into hindgut and urogenital components.
TS18-19 (about 11-11.5 dpc): The first evidence of differentiation of the metanephric region (caudal component) of the nephrogenic cord is first seen, although much of the mesonephros with its mesonephric ducts and vesicles are still present. The ureteric buds (sometimes termed the metanephric ducts) are also first seen at this stage. By TS19, the first evidence of branching of the ureteric bud derivatives within the nephrogenic interstitium (peripheral blastema) is also noted.
TS20-21 (about 12-13 dpc): The future renal cortex of the metanephros (now clearly the definitive kidney) is recognizable, and shows evidence of peripheral blastema and early nephrons. It should be noted that the primitive S-shaped bodies are more readily seen at TS21 of development than at TS20. The medullary component of the future kidney is now also delineating. As far as the “drainage” component of the future kidney is concerned, branching of the ureteric bud tissue is now clearly seen. This gives rise to the renal pelvis associated with the presence of a number of primitive renal collecting ducts within the hilar region of the kidney. As the general location of the kidneys rise rostrally within the peritoneal (or abdominal) cavity, the ureters correspondingly increase in length, and are clearly seen to be patent from an early stage of their differentiation. The mesonephros at this stage has now almost completely regressed.
TS22-23 (about 14-15 dpc): Early nephrons are now seen in the cortical region of the kidney as are numbers of maturing glomerular tufts, while medullary interstitium (stromal cells) are dispersed throughout much of the renal medulla. By TS23, the outermost region of the kidney now shows evidence of differentiation into a well-defined renal capsule, while the rest of the kidney is clearly subdivided into an outer renal cortex, subjacent to which is a zone that represents the region occupied by the cap mesenchyme tissue. There is also an inner medullary region. With regard to the ureteric bud-derived tissue, the renal pelvis is now clearly seen. The primitive collecting ducts and the ureter are also showing increased evidence of differentiation.
TS24-25 (about 16-17 dpc): In the cortical region of the kidney, proximal and distal tubules are first recognised, as well as glomerular tufts and Bowman’s capsules. In relation to the drainage system of the kidney, collecting ducts are also now clearly recognised.
TS26 (about 18 dpc): Within the renal cortex, the region subjacent to the capsule contains all components of the excretory system, including large numbers of glomerular tufts and the associated proximal and distal tubules (although these may occasionally be seen at earlier stages of development). The associated ascending and descending components of the loops of Henle, also termed the immature loops of Henle at this stage, may also be seen within the renal medulla. This region is now seen to contain considerably less undifferentiated mesenchyme tissue than formerly appeared to be the case. All of the components of the drainage system are now present and readily recognised, including the renal pelvis and the collecting tubules. They also now appear to possess a distinct endothelial lining. The ureter is also clearly seen, although readily recognised from about TS21. At all stages, the ureters are surrounded by a substantial amount of mesenchyme tissue.
Genital
http://www.gudmap.org/About/Tutorial/DevMRS.html
Observations on reproductive development obtained from the analysis of staged histologically sectioned mouse embryos. These have principally been obtained from the detailed analysis of serially sectioned mouse embryos isolated at sequential stages of development.
TS10 (about 7 days post coitum (dpc)): The first evidence of the primordial germ cells are seen histologically in the mesodermal component of the wall of the secondary yolk sac towards the end of this stage.
TS11 (about 7.5 dpc): Towards the end of this stage the number of primordial germ cells present in the wall of the secondary yolk sac is in the region of 150. They are by now mostly located close to the allantois, although some are located at the caudal end of the primitive streak. The first evidence of a gonadal primordium is seen at about this stage. They have yet to be invaded by the primordial germ cells..
TS12 (about 8 dpc): The hindgut diverticulum is first recognised at this stage.
TS13 (about 8.5 dpc):[for reference only] The blind-ending primitive foregut makes contact with the surface indentation termed the oral pit (or stomatodaeum) at about this stage, or during the early part of the following stage. A bilayered membrane, termed the buccopharyngeal membrane initially separates the two, and this usually breaks down during TS14.
TS14 (about 9 dpc): The first evidence of differentiation of the pronephros from the intermediate plate mesoderm is seen at this stage, and slightly later the pronephric duct develops. Shortly afterwards, the pronephros regresses, and is replaced during the next stage by the mesonephros. The pronephric duct is then taken over by the mesonephros, and is then termed the nephric duct (or Wolffian duct). The buccopharyngeal membrane breaks down.
TS15 (about 9.5 dpc): Some primordial germ cells have reached the future gonadal region of the urogenital ridge by this stage. However, most of the primordial germ cells are observed at some stage along their migratory pathway. These cells are either in the endoderm of the wall of the hindgut, or in the dorsal mesentery of the hindgut. They may also be seen passing across the region of the medial coelomic bay. Those in the latter location would shortly enter the future gonadal region.
TS16 (about 10 dpc): The genital (or gonadal) ridge is first clearly evident as a longitudinally running ridge or elevation that extends along the majority of the medial aspect of the urogenital ridge at this stage. Vesicles are first recognised in the mesonephros as well as mesonephric tubules, and the former are connected via the tubules to the mesonephric portion of the nephric ducts.
TS17 (about 10.5 dpc): With regard to the development of the future bladder, the first evidence of the urorectal septum is noted, and by about TS19/20 this will separate the cloaca into a dorsal (hindgut) and a ventral (urogenital sinus) component. An endodermal invagination comparable to the oral pit (see TS13) is observed in the region of the cloaca at this stage. The bilayered membrane that separates it from the endodermally-lined cloaca is then termed the cloacal membrane.
TS18 (about 11 dpc): The earliest evidence of the metanephric mesenchyme is seen at this stage, within the most caudal part of the nephric component of the urogenital ridge, and is induced by the ureteric bud. The first evidence of the so-called gonadal primordium is also seen at this stage, and replaces the genital primordium (or gonadal ridge). Towards the end of this stage and during the first part of the following stage, the cloacal region will become divided into two parts by the down growth of the urorectal septum to form the hindgut dorsally, and the urogenital sinus ventrally. Similarly, the cloacal membrane will also become divided into two parts.
TS19 (about 11.5 dpc): The first wave of cortical activity in the surface region of the future testis occurs at this stage to form the cortical cords, and these will in due course subdivide the testes perpendicularly into discrete longitudinal units that are destined to form the testicular cords. Towards the end of this stage, and during the early part of the following stage, the urorectal septum will completely separate the cloaca into dorsal and ventral components. These represent the primitive hindgut, and urogenital sinus, respectively. The metanephric mesenchyme is first clearly evident at this stage, with early evidence of both excretory and drainage components (future collecting duct system), the latter differentiating from the rostral part of the ureteric bud. Towards the end of this stage, the first evidence of the differentiation of the ureteric bud to form the definitive ureter is seen. The first appearance of the genital tubercle is noted at this stage. This will in due course give rise to the penis in the male and the clitoris in the female.
TS20 (about 12 dpc): The majority of the primordial germ cells have reached the future gonadal region of the urogenital ridge. The first evidence of a Barr body may be seen in appropriately stained female germ cells. The increased down growth of the urorectal septum towards the end of this stage now completely separates the cloacal membrane into a dorsally located anal membrane, and a ventrally located urogenital membrane. These two membranes then become separated in the midline by a fibrous structure that is called the perineal body in humans. Much of the rostral part of the mesonephros shows evidence of regression, and regression of the entire mesonephros becomes increasingly apparent during the next few stages.
TS21 (about 13 dpc): The gonads are first histologically recognisable as being either testes or ovaries at this stage. In the testis (male embryos only), the cortical cords have now developed into the testicular cords, and have further differentiated into the seminiferous cords. The germ cells undergo active mitotic division, so that prespermatogonia and occasionally spermatogonia may be recognised. Some of the mesenchymal cells that are located within the seminiferous cords (seminiferous tubules) are now recognised as developing Sertoli cells. In the ovary (female embryos only), the first evidence of rete ovarii may be seen in the hilar region of the ovary at this stage, as well as a distinct paramesonephric (or Müllerian) duct in association with the most lateral part of the mesonephros. Once the gonads are recognised as either testes or ovaries, their gonadal mesenteries are thereafter termed either the mesorchium or mesovarium, respectively. The urogenital sinus (the future bladder) now possesses a distinct vesical part, with an urachus at its apex that is directed towards the umbilical region.
TS22 (about 14 dpc): During this stage, the first evidence of the definitive bladder is seen, (although the region of the trigone cannot yet be recognised). Early evidence of the pelvic and phallic parts of the urogenital sinus is also noted. The earliest evidence of the labial or scrotal folds is seen, and these will give rise to some of the components of the external genitalia in both sexes, although the presence of the genital tubercle has already been noted at TS19. In the male, the ventral part of the scrotal swellings fuse across the midline to form the scrotum, while in the female the equivalent labial swellings remain separate. The labial swellings will give rise to the labia. The caudal part of the mesonephros shows almost complete evidence of regression, and has largely been replaced by the metanephros.
TS23 (about 15 dpc): The first evidence of differentiation of the distal part of the genital tubercle is seen, with the differentiation of the glans penis. The preputial swelling is evident at this stage. It basically reaches to the distal region around E 15.5.The testis appears to be divided into a cortical region and a medullary region. It is within the latter that the primitive seminiferous cords (seminiferous tubules) continue to differentiate. A gubernaculum testis is also first recognised at this stage.
TS24 (about 16 dpc): The trigone region of the definitive bladder is first recognised at this stage. It is at the apex of the caudal part of this region where the mesodermally derived mesonephric ducts insert into the future prostatic region of the urethra, and at this location, they will give rise to the ejaculatory ducts. Both the appendix testis and appendix epididymis is first recognised during the latter part of this stage. The caudal parts of the ureteric buds (the future ureters) insert into the upper and outer part of the trigone region. Within the ovary, some of the germ cells have achieved the oogonial stage of their differentiation, while others may achieve the primary oocyte stage. The oviduct, uterine horn and upper part of the vagina display early evidence of differentiation at this stage.
TS25 (about 17 dpc): It has been calculated that there may be as many as 25,000 germ cells in the gonads at this stage. Unlike the buccopharangeal membrane that breaks down during TS14, the anal membrane usually breaks down during the latter part of this stage. The rostral and dorsal surfaces of the ovary begin to be embraced by the flattened tissue of paramesonephric duct origin. Early differentiation of the cervical region of the uterus is noted at this stage, as well as differentiation of the muscular layer of the uterine horns (the myometrium).
TS26 (about 18 dpc): Early differentiation of the prostate gland has been noted at this stage and, within the bulbar region of the urethra, the bulbo-urethral glands. Within the interstitium of the testis, the occasional fetal Leydig cell may be recognised, while Sertoli cells envelop the seminiferous cords (seminiferous tubules). Early evidence of differentiation may also be seen within the fibrous capsule of the testis, to form the definitive tunica albuginea. In relation to the external genitalia in the male, both penis differentiation and development of the scrotum is also apparent. By the end of TS26, and the period shortly before term, the flattened tissue of paramesonephric duct origin that began to embrace the ovary in TS26 completely surrounds it and forms the thin ovarian bursa that completely surrounds the ovary.
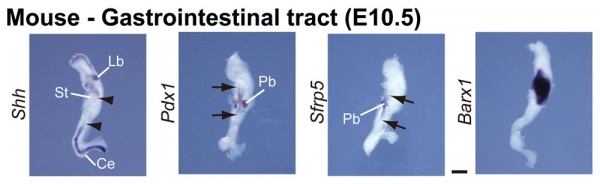
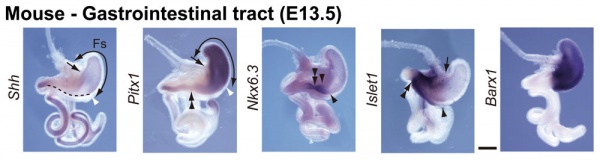
GIT Development
The images below show differential gene expression of some selected markers during development (E10.5 and E13.5) of the mouse gastrointestinal tract.[2]
- Links: Mouse Development | Full original figure | E10.5 | E13.5
Taste Development
Timeline of mouse morphological development in the taste system.
| Gustatory ganglion | Placode formation | Placode delamination migration | Initial axon outgrowth | Peak cell production | Axons reach tongue | Peak cell death | Target innervation |
|---|---|---|---|---|---|---|---|
| E8.5 | E9.5 | E9.5 | E10.5 | E12 | E14.5 | E14-15 | |
| Tongue and taste buds | Tongue | Fungiform papillae (placode) (SEM) | Full no. of fungiform papillae | Taste bud differentiation begins | Taste pores | Full no. of vallate taste buds | |
| E12 | E13-13.5 | E14.5 | E16.5 | postnatal | adult | ||
A general timetable of major morphological changes is provided for rats and mice. The first sperm/plug positive day is considered day 0.5. Mice typically develop two days earlier than rats. The bold time points have been determined experimentally, non-bold numbers are estimated values based on this two-day difference.
Table and Data from: Krimm RF. Factors that regulate embryonic gustatory development. BMC Neurosci. 2007 Sep 18;8 Suppl 3:S4. Review. PMID: 17903280 | BioMed Central Link
Week 1
| Theiler Stage | ||
|---|---|---|
| Theiler Stage 1
Theiler Stage 2 |
Fertilization
Dividing egg stage 2-4 cells. Zona pellucida present. First cleavage occurs at about 24 hours. Embryonic age = 1 dpc (range 1-2.5 dpc) | |
| Theiler Stage 3 | Morula (early to fully compacted) 4-16 cells. Zona pellucida present. Usually found in the oviduct towards the utero-tubal junction. Embryonic age = 2 dpc (range 1-3.5 dpc) | |
| Theiler Stage 4 | Blastocyst (ICM apparent) 16-40 compacted cells. Zona pellucida present. Embryo progresses from morula to the blastocyst. Early evidence of the blastocoelic cavity.
In the blastocyst stage (zona-intact) there is a distinct inner cell mass and an outer layer of trophectoderm cells. Usually located in the uterine lumen. Embryonic age = 3 dpc (range 2-4 dpc) | |
| Theiler Stage 5
Theiler Stage 6 |
Blastocyst (Zona pellucida absent) Zona free blastocyst. Invariably located within the uterine lumen. Embryonic age = 4 dpc (range 3-5.5 dpc) Theiler Stage 6 - Attachment of blastocyst. Blastocyst implants, first evidence of embryonic endoderm cells covering the blastocoelic surface of the inner cell mass. Embryonic age = 4.5 dpc (range 4-5.5 dpc) | |
| Theiler Stage 7 | Implantation and formation of egg cylinder. Ectoplacental cone appears. Rapid increase in the number of inner cell mass cells leading to the formation of the epiblast with subsequent growth to form the egg cylinder. The proximal or visceral cells (opposite side from the trophoblastic cap) are cuboidal in shape. Primary endoderm lines the mural trophectoderm. Embryonic age = 5 dpc (range 4.5-6 dpc) | |
| Theiler Stage 8
Theiler Stage 9 |
Differentiation of egg cylinder. Implantation site 2x3mm.The maternal tissue is invaded by trophoblast (primary) giant cells and the ectoplacental cone is invaded by maternal blood. Differentiation of the egg cylinder into embryonic and extra-embryonic regions and the formation of the pro-amniotic cavity. Reichert's membrane, which is non-cellular and secreted by the distal endoderm, first appears. Embryonic age = 6 dpc (range 5-6.5 dpc)
Stage 9a Advanced Endometrial Reaction. Advanced egg-cylinder stage with the first evidence of an embryonic axis. Clear morphological distinction between the embryonic and extra-embryonic ectoderm. The ectoplacental cone is further invaded by maternal blood and the original lumen of the uterine crypt has disappeared. Equivalent Downs and Davies Stage : PS (pre-streak) Stage 9b Advanced Endometrial Reaction. Late in this stage gastrulation begins, producing the first mesodermal cells. Equivalent Downs and Davies Stage : ES (early streak) | |
| Theiler Stage 10
Theiler Stage 11 |
Stage 10a Amnion. Tissue at the posterior end of the primitive streak bulges into the pro-amniotic cavity and forms the amniotic fold (Equivalent Downs and Davies stage: MS, mid-streak)
Stage 10b Amnion. In the mesoderm of the posterior amniotic fold small cavities coalesce to form a single cavity, the exocoelom Embryonic age = 7.0 dpc (range 6.5-7.5 dpc) Stage 10c . Amnion. The allantoic bud first appears, gastrulation continues and the node becomes visible. Embryonic age = 7.0 dpc (range 6.5-7.5 dpc) (Equivalent Downs and Davies stages: MS - LS, mid-streak to late streak) Stage 11a Neural Plate, Presomite stage The amniotic cavity is now sealed off into three distinct cavities - the amniotic cavity, the exocoelom and the ectoplacental cleft. The neural plate is defined anteriorly and the head process is developing. In the midline, subjacent to the neural groove, the notochodal plate is visible. Embryonic age = 7.5 dpc (range 7.25-8 dpc) Equivalent Downs and Davies stages: OB-EB (no allantoic bud to early allantoic bud); LB-EHF-LHF (late allantoic bud to early head fold to late head fold) Stage 11b Neural Plate, Presomite stage. The allantoic bud elongates. Embryonic age = 7.5 dpc (range 7.25-8 dpc) . Equivalent Downs & Davies stages: OB-EB (no allantoic bud to early allantoic bud); LB-EHF-LHF (late allantoic bud to early head fold to late head fold) Stage 11c Neural Plate, Presomite stage. The rostral part of the neural plate begins to enlarge to form the head folds. The neural groove is visible. Embryonic age = 7.5 dpc (range 7.25-8 dpc) Equivalent Downs and Davies stages: OB-EB (no allantoic bud to early allantoic bud); LB-EHF-LHF (late allantoic bud to early head fold to late head fold) Stage11d Neural Plate, Presomite stage. Head folds continue to enlarge and the foregut pocket begins to form. Embryonic age = 7.5 dpc (range 7.25-8 dpc) ; LB-EHF-LHF (late allantoic bud to early head fold to late head fold) . Equivalent Downs & Davies stages: OB-EB (no allantoic bud to early allantoic bud) |
Week 2
| Theiler Stage | ||
|---|---|---|
| Theiler Stage 12
Theiler Stage 13 |
Theiler Stage 12a First Somites Unturned embryo with first appearance of somite pairs 1-4 somites. The allantois extends further into the exocoelom and the maxillary components of the 1st branchial arch become prominent. The preotic sulcus is visible in the 2-3 somite embryo. The cardiogenic plate begins to form and the foregut pocket is clearly visible. Embryonic age = 8 dpc (range 7.5-8.75 dpc) 1-7 somite pairs
Theiler Stage 12b First Somites Unturned embryo with first appearance of somite pairs 5-7 somites. The headfolds are particularly prominent and neural closure occurs in the region of the 4th and 5th somites, extending in both directions from this site. The otic placode appears at 9 somite pairs stage (between E8.5 to 8.75). The optic placodes are first evident and become indented to form the optic pits. The heart rudiment develops rapidly. The allantois contacts the chorion at the end of this stage. Absent: The 2nd branchial arch and >7 somites. Embryonic age = 8 dpc (range 7.5-8.75 dpc) 1-7 somite pairs Theiler Stage 13 Turning of the embryo. This is a short period with turning initiated in embryos with 6-8 pairs of somites and usually completed in embryos with 14-16 pairs of somites. The first branchial arch has maxillary and mandibular components but the maxillary process is not visible until later (TS16). A second branchial arch is now evident. There is evidence of regionalisation of the heart and the neural tube is closed from a point opposite the outflow tract to the proximal part of the tail. Absent: 3rd branchial arch. Embryonic age = 8.5 dpc (range 8-9.25 dpc) 8-12 somite pairs | |
| Theiler Stage 14
Theiler Stage 15 |
Neural - Formation and closure of anterior neuropore. The rostral extremity of the neural tube closes in embryos with usually about 15-18 somite pairs and defines this stage. The otic pit becomes progressively more indented but not closed, the mandibular process of the 1st branchial arch is clearly visible. The 3rd branchial arch becomes visible late in the stage.
Limb - An increasingly prominent ridge on the lateral body wall, approximately at the level of the 8th-12th somite, indicates the site of the future forelimb bud. Absent: forelimb bud. Embryonic age = 9 dpc (range 8.5-9.75 dpc) 13-20 somite pairs Formation of posterior neuropore, forelimb bud. The posterior neuropore forms and the condensation of the forelimb bud becomes apparent near the 8th-12th somite pairs. A distinct condensation of the hind limb bud appears just at the end of the stage.The forebrain vesicle subdivides into telencephalic and diencephalic vesicles. Absent: hindlimb bud, Rathke's pouch. Embryonic age = 9.5 dpc (range 9-10.25 dpc) 21-29 somite pairs | |
| Theiler Stage 16
Theiler Stage 17 |
Closure of posterior neuropore. Hind limb bud and tail bud. The hind limb bud becomes visible at the level of the 23rd-28th somites. The tail bud appears as a short stump and the 3rd and 4th branchial arches are distinctly concave. Rathke's pouch and the nasal processes start to form. At the end of this stage the posterior neuropore begins to close. Absent: thin and long tail. Embryonic age = 10 dpc (range 9.5-10.75 dpc) 30-34 somite pairs
Stage 17 - Deep Lens Indentation The most obvious distinguishing features are the deepening of the lens pit, with a narrowing of its outer pore-like opening, and the first appearance of the physiological umbilical hernia. The 1st branchial arch is conspicuously divided into maxillary and mandibular components. There is advanced development of the brain tube and the tail elongates and thins. Absent: nasal pits. Embryonic age = 10.5 dpc (range 10-11.25 dpc) 35-39 somite pairs Hearing and Balance - Formation of vestibular (otic) ganglion cells (E10-12) Afferent processes of vestibular ganglion cells invade the macula utricle and saccule and cristae of the semicircular canals (10.5)[3] | |
| Theiler Stage 18
Theiler Stage 19 |
Closure of Lens Vesicle. The primary externally recognisable feature is the progressive closure of the lens vesicle. The somites in the cervical region are no longer visible and the rapid growth of the brain is striking. The nasal pits start to form. Absent: auditory hillocks, anterior footplate. Embryonic age = 11 dpc (range 10.5-11.25 dpc) 40-44 somite pairs
Stage 19 - Lens vesicle completely separated from surface. The lens vesicle becomes completely closed and detached from the ectoderm. The peripheral margins of the eye become well defined. The forelimbs are seen to be divided into two regions, the proximal part consisting of the future limb-girdle and 'arm' and the more peripheral part which forms a circular or paddle-shaped 'handplate' (anterior footplate). The medial and lateral margins of the otic pit are coming together reducing the entrance to a narrow slit and the auditory hillocks become visible. Absent: retinal pigmentation, signs of 'fingers'. Embryonic age = 11.5 dpc (range 11-12.25 dpc) 45-47 somite pairs Gonad - 11.5 - coelomic epithelium basement membrane is discontinuous, supporting cell migration. 11.2-11.4 - (somite 15-17 stages) coelomic epithelial cells of both sexes migrated into the gonad. In XY gonads, the migrating coelomic epithelial cells became Sertoli cells, as well as interstitial cells. This ability of the coelomic epithelium to give rise to Sertoli cells was developmentally regulated. 11.5-11.7 - (somite 18-20 stages) coelomic epithelial cells no longer became Sertoli cells. Instead, cells that migrated into the gonad stayed outside testis cords, in the interstitium. [4] Hearing and Balance - Efferent nerve endings first approach hair cells (E11-12)[3] | |
| Theiler Stage 20 | Earliest signs of fingers. The 'handplate' (anterior footplate) is no longer circular but develops angles which correspond to the future digits. The posterior footplate is also distinguishable from the lower part of the leg. It is possible to see the pigmentation of the pigmented layer of the retina through the transparent cornea. The tongue and brain vesicles are clearly visible. Absent:5 rows of whiskers, indented handplate. Embryonic age = 12 dpc (range 11.5-13 dpc) 48-51 somite pairs
Gonad - 12.5 - (somite 30 stage) cell migration finished. Coelomic epithelium basement membrane thickens to form the tunica albuginea.[4] Joints - 12.5 -13.5 interzone forms in cartilage of digits which is the precursor to synovial joint formation. | |
| Theiler Stage 21 | Anterior footplate indented, marked pinna. The distal borders of the anterior and posterior footplates are now indented and the digit widths and locations can be discerned. The 'elbow' and 'wrist' are now identifiable. The pinna rapidly develops and forms a crest at right angles to the head. Five rows of vibrissae are visible as well as a prominant hair follicle over the eye and another over the ear. The lens vesicle has lost its lumen. The physiological umbilical hernia is prominent. Absent: hair follicles, distally separate fingers. Embryonic age = 13 dpc (range 12.5-14) 52-55 somite pairs
Ovary - E13.5-16.5 ovarian cord structure formation required for oocyte development[5] Liver - E13.5-15.5 single layer of hepatoblasts forms close to the portal mesenchyme (ductal plate) and expresses bile duct-specific cytokeratins. By E16.5, the ductal plate partially becomes bi-layered and, around E17.5, enters a phase of profound remodeling during which time focal dilations appear between the two cell layers. Hearing and Balance - Peak hair cell mitosis in crista ampullaris, maculae of saccules, and utricles (E13-17)[3] | |
| Theiler Stage 22 | Fingers separate distally. Individual 'fingers' are visible in the anterior footplate and there are deep indentations between the 'toes' which are not yet separated.The long bones of the limbs are present and there are hair follicles in the pectoral, pelvic and trunk regions. The pinna is turned forwards and the umbilical hernia is conspicuous. Absent: hair follicles in the cephalic region. Embryonic age = 14 dpc (range 13.5-15 dpc) 56-60 somite pairs |
Week 3
| Theiler Stage | ||
|---|---|---|
| Theiler Stage 23 | Toes separate. The 'toes' separate and are clearly divergent, not becoming parallel until later. Hair follicles are present in the cephalic region but not at the periphery of the vibrissae. The pinna covers more than half of the external auditory meatus and the eyelids are still open. Absent: nail primordia, 'fingers' 2-5 parallel. Embryonic age = 15 dpc >60 somite pairs
Hearing and Balance - Afferent synaptogenesis with hair cells begins (E15)[3] | |
| Theiler Stage 24 | Reposition of umbilical hernia 'Fingers' 2-5 are nearly parallel. Nail primordia are visible on the 'toes'. The eyelids have fused in most cases by the end of the stage and the pinna almost completely covers the external auditory meatus. The umbilical hernia is disappearing and there is a corresponding increase in the size of the peritoneal sac. Absent: 'fingers' and 'toes' joined together. Embryonic age = 16 dpc > 60 Somite pairs
Liver - E16.5 ductal plate becomes partially bi-layered. | |
| Theiler Stage 25 | Skin wrinkled The skin has thickened and formed wrinkles and the subcutaneous veins are less visible. The 'fingers' and 'toes' have become parallel and the umbilical hernia has disappeared. The eyelids have fused. Whiskers are just visible. Absent: ear extending over auditory meatus, long whiskers. Embryonic age = 17 dpc
Liver - E17.5 bilayered ductal plate remodelled with focal dilations between the two cell layers.Prostate - E17.5 urogenital sinus epithelial cells grow into mesenchyme to form prostate buds. | |
| Theiler Stage 26 |
Long whiskers The whiskers that were present at stage 25 are definitely longer and the skin has thickened. The pinna is larger and such that virtually none of the lumen of the auditory meatus is visible. The eyes are barely visible through the closed eyelids. Embryonic age = 18 dpc Hearing and Balance - Morphological differentiation into Type I and Type II hair cells (E18-P10)[3] | |
| Theiler Stage 27
Theiler Stage 28 |
New born Mouse
Post-natal development | |
|
Hearing and Balance - Fully mature morphological and physiological innervation of vestibular system (P28)[3] |
Table Data
The main table data is modified from several different sources[6] [7] [8] and includes additional sources as listed within the table.
- ↑ <pubmed>17452023</pubmed>| PMC2117077
- ↑ <pubmed>19300477</pubmed>| PLoS Genet.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 <pubmed>18298265</pubmed>
- ↑ 4.0 4.1 <pubmed>9808783</pubmed>
- ↑ <pubmed>20064216</pubmed>
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedThe House Mouse - ↑ Edinburgh Mouse Atlas Appendix 4 Theiler Staging | Staging Criteria | Standard Anatomical Nomenclature
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedPMID8269852
1996
An Internet atlas of mouse development
Comput Med Imaging Graph. 1996 Nov-Dec;20(6):433-47.
Williams BS1, Doyle MD.
Abstract
A prototype two- and three-dimensional color atlas of mouse development is described. The prototype has been developed using two embryos, a 13.5 d normal mouse embryo and a PATCH mutant embryo of the same age. Serial sections of the embryos, with an external registration marker system, introduced into the paraffin embedding process, were prepared by standard histological methods. For the 2D atlas, color images were digitized from 100 consecutive sections of the normal embryo. For the 3D atlas, 300 gray scale images digitized from the mutant embryo were conformally warped and reconstructed into a 3D volume dataset. The external fiducial system facilitated the three-dimensional reconstruction by providing accurate registration of consecutive images and also allowed for precise spatial calibration and the correction for warping artifacts. The atlases, with their associated anatomical knowledge base, will be integrated into a multimedia on-line information resource via the Internet's World Wide Web (WWW) using an enhanced (patent pending, Eòlas Technologies) version of the Mosaic WWW browser program from the National Center for Supercomputer Applications. These programs will provide research biologists with a set of advanced tools to analyze normal and abnormal development.
PMID 9007211