Talk:Fetal ECHO Meeting 2012
| About Discussion Pages |
|---|
On this website the Discussion Tab or "talk pages" for a topic has been used for several purposes:
Glossary Links
Cite this page: Hill, M.A. (2024, April 25) Embryology Fetal ECHO Meeting 2012. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Talk:Fetal_ECHO_Meeting_2012 |
2012
Defining the spatial relationships between eight anatomic planes in the 11+6 to 13+6 weeks fetus: a pilot study
Prenat Diagn. 2012 Sep;32(9):875-82. doi: 10.1002/pd.3924. Epub 2012 Jun 19.
Abu-Rustum RS, Ziade MF, Abu-Rustum SE. Source Center For Advanced Fetal Care, Tripoli, Lebanon.
Abstract OBJECTIVE: Our study aims at investigating the spatial relationships between eight anatomic planes in the 11+6 to 13+6 weeks fetus. METHODS: This is a retrospective pilot study where three-dimensional and four-dimensional stored data sets were manipulated to retrieve eight anatomic planes starting from the midsagittal plane of the fetus. Standardization of volumes was performed at the level of the transverse abdominal circumference plane. Parallel shift was utilized and the spatial relationships between eight anatomic planes were established. The median and the range were calculated for each of the planes, and they were evaluated as a function of the fetal crown-rump length. P < 0.05 was considered statistically significant. RESULTS: A total of 63 volume data sets were analyzed. The eight anatomic planes were found to adhere to normal distribution curves, and most of the planes were in a definable relationship to each other with statistically significant correlations. CONCLUSION: To our knowledge, this is the first study to describe the possible spatial relationships between eight two-dimensional anatomic planes in the 11+6 to 13+6 weeks fetus, utilizing a standardized approach. Defining these spatial relationships may serve as the first step for the potential future development of automation software for fetal anatomic assessment at 11+6 to 13+6 weeks. © 2012 John Wiley & Sons, Ltd. © 2012 John Wiley & Sons, Ltd.
PMID 22711455
An image-based model of the whole human heart with detailed anatomical structure and fiber orientation
Comput Math Methods Med. 2012;2012:891070. Epub 2012 Aug 17.
Deng D, Jiao P, Ye X, Xia L. Source Key Lab of Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, Zhejiang University, Hangzhou 310027, China.
Abstract
Many heart anatomy models have been developed to study the electrophysiological properties of the human heart. However, none of them includes the geometry of the whole human heart. In this study, an anatomically detailed mathematical model of the human heart was firstly reconstructed from the computed tomography images. In the reconstructed model, the atria consisted of atrial muscles, sinoatrial node, crista terminalis, pectinate muscles, Bachmann's bundle, intercaval bundles, and limbus of the fossa ovalis. The atrioventricular junction included the atrioventricular node and atrioventricular ring, and the ventricles had ventricular muscles, His bundle, bundle branches, and Purkinje network. The epicardial and endocardial myofiber orientations of the ventricles and one layer of atrial myofiber orientation were then measured. They were calculated using linear interpolation technique and minimum distance algorithm, respectively. To the best of our knowledge, this is the first anatomically-detailed human heart model with corresponding experimentally measured fibers orientation. In addition, the whole heart excitation propagation was simulated using a monodomain model. The simulated normal activation sequence agreed well with the published experimental findings.
PMID 22952559
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3431151
http://www.hindawi.com/journals/cmmm/2012/891070
Copyright © 2012 Dongdong Deng et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
2011
Simple targeted arterial rendering (STAR) technique: a novel and simple method to visualize the fetal cardiac outflow tracts
Ultrasound Obstet Gynecol. 2011 May;37(5):549-56. doi: 10.1002/uog.8841. Epub 2011 Mar 2.
Yeo L, Romero R, Jodicke C, Kim SK, Gonzalez JM, Oggè G, Lee W, Kusanovic JP, Vaisbuch E, Hassan S. Source Perinatology Research Branch, NICHD/NIH/DHHS, Bethesda, MD and Detroit, MI 48201, USA. lyeo@med.wayne.edu
Abstract
OBJECTIVE: To describe a novel and simple technique—simple targeted arterial rendering (STAR)—to visualize the fetal cardiac outflow tracts from dataset volumes obtained with spatiotemporal image correlation (STIC) and applying a new display technology (OmniView). METHODS: We developed a technique to image the outflow tracts by drawing three dissecting lines through the four-chamber view of the heart contained in a STIC volume dataset. Each line generated the following plane: (a) Line 1: ventricular septum en face with both great vessels (pulmonary artery anterior to the aorta); (b) Line 2: pulmonary artery with continuation into the longitudinal view of the ductal arch; and (c) Line 3: long-axis view of the aorta arising from the left ventricle. The pattern formed by all three lines intersecting approximately through the crux of the heart resembles a star. The technique was then tested in 50 normal fetal hearts at 15.3–40.4 weeks' gestation. To determine whether the technique could identify planes that departed from the normal images, we tested the technique in four cases with proven congenital heart defects (ventricular septal defect (VSD), transposition of great vessels, tetralogy of Fallot and pulmonary atresia with intact ventricular septum). RESULTS: The STAR technique was able to generate the intended planes in all 50 normal cases. In the abnormal cases, the STAR technique allowed identification of the VSD, demonstrated great vessel anomalies and displayed views that deviated from what was expected from the examination of normal hearts. CONCLUSIONS: This novel and simple technique can be used to visualize the outflow tracts and ventricular septum en face in normal fetal hearts. Inability to obtain expected views or the appearance of abnormal views in the generated planes should raise the index of suspicion for congenital heart disease involving the great vessels and/or the ventricular septum. The STAR technique may simplify examination of the fetal heart and could reduce operator dependency.
PMID 20878672
Four-chamber view and 'swing technique' (FAST) echo: a novel and simple algorithm to visualize standard fetal echocardiographic planes
Ultrasound Obstet Gynecol. 2011 Apr;37(4):423-31. doi: 10.1002/uog.8840. Epub 2011 Mar 2.
Yeo L, Romero R, Jodicke C, Oggè G, Lee W, Kusanovic JP, Vaisbuch E, Hassan S.
Source
Perinatology Research Branch, NICHD/NIH/DHHS, Department of Obstetrics and Gynecology,Wayne State University/Hutzel Women's Hospital, Detroit, MI 48201, USA. lyeo@med.wayne.edu Abstract OBJECTIVE: To describe a novel and simple algorithm (four-chamber view and 'swing technique' (FAST) echo) for visualization of standard diagnostic planes of fetal echocardiography from dataset volumes obtained with spatiotemporal image correlation (STIC) and applying a new display technology (OmniView). METHODS: We developed an algorithm to image standard fetal echocardiographic planes by drawing four dissecting lines through the longitudinal view of the ductal arch contained in a STIC volume dataset. Three of the lines are locked to provide simultaneous visualization of targeted planes, and the fourth line (unlocked) 'swings' through the ductal arch image (swing technique), providing an infinite number of cardiac planes in sequence. Each line generates the following plane(s): (a) Line 1: three-vessels and trachea view; (b) Line 2: five-chamber view and long-axis view of the aorta (obtained by rotation of the five-chamber view on the y-axis); (c) Line 3: four-chamber view; and (d) 'swing line': three-vessels and trachea view, five-chamber view and/or long-axis view of the aorta, four-chamber view and stomach. The algorithm was then tested in 50 normal hearts in fetuses at 15.3-40 weeks' gestation and visualization rates for cardiac diagnostic planes were calculated. To determine whether the algorithm could identify planes that departed from the normal images, we tested the algorithm in five cases with proven congenital heart defects. RESULTS: In normal cases, the FAST echo algorithm (three locked lines and rotation of the five-chamber view on the y-axis) was able to generate the intended planes (longitudinal view of the ductal arch, pulmonary artery, three-vessels and trachea view, five-chamber view, long-axis view of the aorta, four-chamber view) individually in 100% of cases (except for the three-vessels and trachea view, which was seen in 98% (49/50)) and simultaneously in 98% (49/50). The swing technique was able to generate the three-vessels and trachea view, five-chamber view and/or long-axis view of the aorta, four-chamber view and stomach in 100% of normal cases. In the abnormal cases, the FAST echo algorithm demonstrated the cardiac defects and displayed views that deviated from what was expected from the examination of normal hearts. The swing technique was useful for demonstrating the specific diagnosis due to visualization of an infinite number of cardiac planes in sequence. CONCLUSIONS: This novel and simple algorithm can be used to visualize standard fetal echocardiographic planes in normal fetal hearts. The FAST echo algorithm may simplify examination of the fetal heart and could reduce operator dependency. Using this algorithm, inability to obtain expected views or the appearance of abnormal views in the generated planes should raise the index of suspicion for congenital heart disease. Copyright © 2011 ISUOG. Published by John Wiley & Sons, Ltd.
PMID 20878671
2010
Reference values for variables of fetal cardiocirculatory dynamics at 11-14 weeks of gestation
Ultrasound Obstet Gynecol. 2010 May;35(5):540-7.
Rozmus-Warcholinska W, Wloch A, Acharya G, Cnota W, Czuba B, Sodowski K, Skrzypulec V. Source Department of Obstetrics and Gynecology, Woman's Health Chair, Medical University of Silesia, Katowice, Poland.
Abstract
OBJECTIVE: Despite the increasing popularity of first-trimester fetal echocardiography, the evaluation of fetal heart function during this period remains challenging. The parameters of normal cardiac function at 11-14 weeks' gestation are not well defined and appropriate reference values have not yet been established. The purpose of this study was to evaluate the fetal cardiocirculatory dynamics during routine first-trimester screening and establish cross-sectional reference ranges for 11-14 weeks' gestation. METHODS: Fetal echocardiography was performed on 202 women with singleton pregnancies at 11 + 0 to 13 + 6 weeks' gestation. Global cardiac function was evaluated using the heart : chest area ratio and Tei index of the left (LV) and right (RV) ventricles. The proportion of isovolumic contraction (ICT%) and ejection (ET%) times of the cardiac cycle, and the outflow velocities described the systolic function. Diastolic function was evaluated by the proportion of relaxation (IRT%) and filling (FT%) times, the ratio of the blood velocity through the atrioventricular valves during early filling (E) and atrial contraction (A) phases of the cardiac cycle, and ductus venosus pulsatility index for veins (DV-PIV). All participants had additional fetal echocardiography in the second trimester and neonatal clinical examination after birth to confirm normality. RESULTS: The mean heart : chest area ratio (0.203 +/- 0.04) and the Tei indices of both ventricles did not vary significantly during weeks 11-14, but the mean Tei index of the LV (0.375 +/- 0.092) was significantly higher than that of the RV (0.332 +/- 0.079) (P = 0.001). The fetal heart rate (FHR) decreased with increasing crown-rump length (CRL) (P < 0.00001). The LV-ICT% did not vary significantly (P = 0.27), LV-IRT% (P = 0.03) and LV-ET% decreased (P = 0.01), whereas the LV-FT% increased (P = 0.02) with CRL. The RV-ET% (P = 0.84) and RV-FT% (P = 0.60) remained relatively stable. The LV-ET% was lower than the RV-ET% (P = 0.0001). The LV (P = 0.004) and RV (P < 0.00001) outflow velocities and E : A ratios of both ventricles (P < 0.0001) increased with advancing gestation. The E-velocity of the LV (P = 0.003) and RV (P = 0.002) increased significantly but the increase in A-velocity was not significant. The outflow velocity (P = 0.008) and E-velocity (P = 0.005) of the RV were higher than that of the LV but the A-velocities were similar (P = 0.066). The mean DV-PIV was 0.97 +/- 0.23 and did not change significantly (P = 0.95) during weeks 11-14. The FHR and DV-PIV did not correlate with the Tei index of either ventricle. CONCLUSION: We have established reference ranges for the noninvasive evaluation of fetal cardiocirculatory dynamics at 11-14 weeks' gestation. Copyright 2010 ISUOG. Published by John Wiley & Sons, Ltd.
PMID 20178107
http://onlinelibrary.wiley.com/doi/10.1002/uog.7595/full
2009
Fetal aortic arch measurements at 14 to 40 weeks' gestation derived by spatiotemporal image correlation volume data sets
J Ultrasound Med. 2009 Dec;28(12):1651-6.
Udomwan P, Luewan S, Tongsong T. Source Department of Obstetrics and Gynecology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand. Abstract OBJECTIVE: The purpose of this study was to establish reference ranges for the transverse aortic arch diameter (TAD) and distal aortic isthmus diameter (DAID) in normal singleton pregnancies (14-40 weeks) based on the 3-vessel/trachea (3VT) view of cardio-spatiotemporal image correlation (STIC) volume data sets. METHODS: A prospective descriptive study was conducted on uncomplicated singleton pregnancies with healthy fetuses and an accurate gestational age (GA). Cardio-STIC examinations were performed by experienced sonographers using a high-resolution ultrasound machine, and the volume data sets were manipulated to obtain the 3VT view and measured for the TAD and DAID. RESULTS: A total of 554 measurements were performed, ranging from 13 to 30 for each gestational week. The best regression models were as follows: TAD (in millimeters) = -1.01 + 1.69 (GA, in weeks) (r(2) = 0.93; P < .001), and DAID (in millimeters) = -0.85 + 1.54 (GA, in weeks) (r(2) = 0.92; P < .001). A table of nomograms for 5th, 50th, and 95th percentile ranges was constructed. CONCLUSIONS: Normative data for the TAD and DAID at each gestational week from 14 to 40 weeks were constructed by a new technique of measurement based on cardio-STIC. These reference ranges may be useful tools for assessment of fetal aortic arch abnormalities.
- "in utero detection rate of coarctation of the aorta (CoA) is very low, and it remains one of the most difficult structural cardiac abnormalities to prenatally diagnose although it accounts for 7% of all congenital heart defects."
PMID 19933478
Three and four dimensional ultrasound: a novel method for evaluating fetal cardiac anomalies
Prenat Diagn. 2009 Jul;29(7):645-53.
Gindes L, Hegesh J, Weisz B, Gilboa Y, Achiron R. Source Department of Obstetrics and Gynecology, The Chaim Sheba Medical Center, Tel Hashomer, Ramat Gan, Israel. gindesl@zahav.net.il
Abstract
OBJECTIVE: To evaluate the role of various new models of 3- and 4-dimensional (3D and 4D) ultrasound (US) applications in prenatal assessment of fetal cardiac anomalies. METHODS: Volume data sets of 81 fetuses with fetal cardiac anomalies, as previously diagnosed by 2D US, were acquired by 3D and cine 4D using spatiotemporal image correlation (STIC) software. Various additional rendering tools were applied. Color, power, high definition Doppler and B-flow were added to the volumes acquired. A retrospective offline analysis of the cardiac defects was performed. RESULTS: The mean gestational age at diagnosis was 24 weeks (range 13-38); 128 anomalies were detected and were classified into the following categories: I, Situs anomalies in 8 cases; II, abnormal four-chamber view in 63 cases; III, outflows tract anomalies in 27 cases; IV, arches anomalies in 21 cases; and V, veins anomalies in 9 cases. Rendering tools differed in each groups of anomalies. CONCLUSIONS: Fetal cardiac anomalies can be evaluated adequately by the information gained by 3D and 4D volumes obtained by STIC. Since no single module is sufficiently accurate for the diagnosis of all cardiac anomalies, each of the cardiac anomaly categories requires different and appropriate module of visualization.
PMID 19340842
2008
Persistent left superior vena cava: a case report and review of literature
Cardiovasc Ultrasound. 2008 Oct 10;6:50. Goyal SK, Punnam SR, Verma G, Ruberg FL. Source Department of Medicine, Section of Cardiology, Boston University School of Medicine, Boston, MA, USA. sandeep.goyal@bmc.org
Abstract
Persistent left superior vena cava is rare but important congenital vascular anomaly. It results when the left superior cardinal vein caudal to the innominate vein fails to regress. It is most commonly observed in isolation but can be associated with other cardiovascular abnormalities including atrial septal defect, bicuspid aortic valve, coarctation of aorta, coronary sinus ostial atresia, and cor triatriatum. The presence of PLSVC can render access to the right side of heart challenging via the left subclavian approach, which is a common site of access utilized when placing pacemakers and Swan-Ganz catheters. Incidental notation of a dilated coronary sinus on echocardiography should raise the suspicion of PLSVC. The diagnosis should be confirmed by saline contrast echocardiography.
PMID 18847480
2007
Changes in fetal cardiac geometry with gestation: implications for 3- and 4-dimensional fetal echocardiography
J Ultrasound Med. 2007 Apr;26(4):437-43; quiz 444.
Espinoza J, Gotsch F, Kusanovic JP, Gonçalves LF, Lee W, Hassan S, Mittal P, Schoen ML, Romero R. Source Perinatology Research Branch, National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland/Detroit, Michigan, USA.
Abstract
OBJECTIVE: Three- and 4-dimensional fetal echocardiography can be performed using novel algorithms. However, these algorithms assume that the spatial relationships among cardiac chambers and great vessels are constant throughout gestation. The objective of this study was to determine whether changes in fetal cardiac geometry occur during gestation. METHODS: A cross-sectional study was conducted by reviewing 3- and 4-dimensional volume data sets from healthy fetuses obtained between 12 and 41 weeks of gestation. Volume data sets were examined using commercially available software. Parameters measured included angles between: (1) the ductal arch and fetal thoracic aorta; (2) the ductal arch and aortic arch; and (3) the left outflow tract and main pulmonary artery, as seen in the short axis of the heart. The mean angle from the left outflow tract to the short axis was calculated. Nonparametric statistics were used for analysis. RESULTS: Eighty-five fetuses were included in the study. The angle between the ductal arch and the fetal thoracic aorta decreased with gestational age (Spearman rho coefficient: -0.39; P < .001). In contrast, the angle between the ductal arch and aortic arch, and the mean angle between the left outflow tract and the short axis of the heart increased with gestational age (Spearman rho coefficients: 0.45 and 0.40, respectively; P < .001). CONCLUSIONS: (1) Changes in fetal cardiac geometry were shown with advancing gestational age. (2) Proposed algorithms for the examination of the fetal heart with 3-dimensional ultrasonography may need to be adapted to optimize visualization of the standard planes before 26 weeks of gestation.
PMID 17384040
http://www.jultrasoundmed.org/content/26/4/437.full
2003
Spatio-temporal image correlation (STIC): new technology for evaluation of the fetal heart
Ultrasound Obstet Gynecol. 2003 Oct;22(4):380-7.
DeVore GR, Falkensammer P, Sklansky MS, Platt LD. Source Fetal Diagnostic Center of Pasadena, Pasadena, CA 91105, USA. fetalecho@fetalecho.com
Abstract
Spatio-temporal image correlation (STIC) is a new approach for clinical assessment of the fetal heart. It offers an easy to use technique to acquire data from the fetal heart and to aid in visualization with both two-dimensional and three-dimensional (3D) cine sequences. The acquisition is performed in two steps: first, images are acquired by a single, automatic volume sweep. Second, the system analyzes the image data according to their spatial and temporal domain and processes an online dynamic 3D image sequence that is displayed in a multiplanar reformatted cross-sectional display and/or a surface rendered display. The examiner can navigate within the heart, re-slice, and produce all of the standard image planes necessary for a comprehensive diagnosis. The advantages of STIC for use in evaluation of the fetal heart are as follows: the technique delivers a temporal resolution which corresponds to a B-mode frame rate of approximately 80 frames/s; it provides the examiner with an unlimited number of images for review; it allows for correlation between image planes that are perpendicular to the main image acquisition plane; it may shorten the evaluation time when complex heart defects are suspected; it enables the reconstruction of a 3D rendered image that contains depth and volume which may provide additional information that is not available from the thin multiplanar image slices (e.g. for pulmonary veins, septal thickness); it lends itself to storage and review of volume data by the examiner or by experts at a remote site; it provides the examiner with the ability to review all images in a looped cine sequence. Copyright 2003 ISUOG. Published by John Wiley & Sons, Ltd.
PMID 14528474
2002
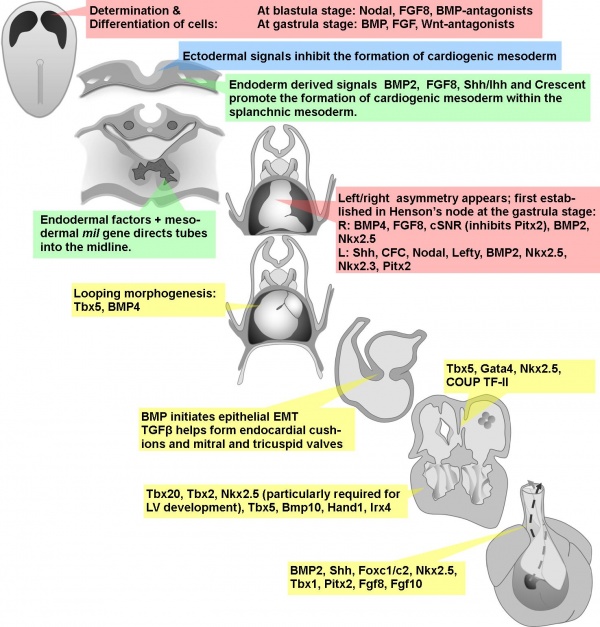
Early signals in cardiac development
Circ Res. 2002 Sep 20;91(6):457-69.
Zaffran S, Frasch M. Source Mount Sinai School of Medicine, Brookdale Department of Molecular, Cell and Developmental Biology, New York, NY 10029, USA.
Abstract
The heart is the first organ to form during embryogenesis and its circulatory function is critical from early on for the viability of the mammalian embryo. Developmental abnormalities of the heart have also been widely recognized as the underlying cause of many congenital heart malformations. Hence, the developmental mechanisms that orchestrate the formation and morphogenesis of this organ have received much attention among classical and molecular embryologists. Due to the evolutionary conservation of many of these processes, major insights have been gained from the studies of a number of vertebrate and invertebrate models, including mouse, chick, amphibians, zebrafish, and Drosophila. In all of these systems, the heart precursors are generated within bilateral fields in the lateral mesoderm and then converge toward the midline to form a beating linear heart tube. The specification of heart precursors is a result of multiple tissue and cell-cell interactions that involve temporally and spatially integrated programs of inductive signaling events. In the present review, we focus on the molecular and developmental functions of signaling processes during early cardiogenesis that have been defined in both vertebrate and invertebrate models. We discuss the current knowledge on the mechanisms through which signals induce the expression of cardiogenic transcription factors and the relationships between signaling pathways and transcriptional regulators that cooperate to control cardiac induction and the formation of a linear heart tube.
PMID 12242263
2000
Biometry of the fetal heart between 10 and 17 weeks of gestation
Fetal Diagn Ther. 2000 Jan-Feb;15(1):20-31.
Gembruch U, Shi C, Smrcek JM. Source Division of Prenatal Medicine, Department of Obstetrics and Gynecology, Medical University of Lübeck, Germany.
Abstract
OBJECTIVES: Assessment of the dimensions of the cardiac chambers and the great arteries in the human fetus may be helpful in the prenatal diagnosis of congenital heart disease. The purpose of this prospective cross-sectional study was to compile normative data in fetal cardiac measurements in early pregnancy. The structure of the fetal heart was examined in 136 normal singleton fetuses between 10 and 17 weeks of gestation. METHODS: The transversal heart diameter, both ventricular dimensions, interventricular septal thickness, heart area, heart circumference, thoracic diameter, thoracic circumference and thoracic area were measured in the four-chamber view during diastole. Diameters of the pulmonary trunk and ascending aorta were obtained in the short axis and long axis view during systole. Ultrasound examinations were performed with a 5.0-MHz transvaginal and/or transabdominal phased-array sector scanner. RESULTS: The four-chamber view and the cross-over of the pulmonary artery and the aorta were adequately visualized in 44% of the fetuses at 10 weeks of gestation, in 75% at 11 weeks of gestation, in 93% at 12 weeks of gestation and in 100% of the fetuses at 13-17 weeks of gestation. Before 14 weeks of gestation transvaginal sonography was superior to the transabdominal sonography in visualization of the fetal heart and great arteries. After 14 weeks of gestation transabdominal sonography accurately demonstrated the structure of the fetal heart. The ratio of right and left ventricle (RV/LV) and the ratio of the pulmonary trunk and aorta (PT/AO) were constant during this period of gestation (approximately 1.00 and 1. 10, respectively). The ratio of the cardiac and thoracic area showed only a slight increase with advancing gestational age, but with significant correlation. The fetal heart rate showed a slow decrease from 167 to 150 bpm in this period of gestation. The transversal heart diameter, both ventricular dimensions, interventricular septal thickness, heart area, cardiothoracic diameter ratio, aortic diameter and the pulmonary trunk diameter showed a highly significant linear correlation to the gestational age and the biparietal diameter. CONCLUSION: The advancing quality of ultrasound images allows fetal echocardiography in the first and early second trimester. Our normative data could be the basis of studying the development of cardiac structures in congenital heart disease and it might be helpful in the detection of some congenital heart defects in early pregnancy. Copyright 2000 S. Karger AG, Basel.
PMID 10705210
Reference ranges for two-dimensional echocardiographic examination of the fetal ductus arteriosus
Ultrasound Obstet Gynecol. 2000 Mar;15(3):219-25.
Mielke G, Benda N. Source Department of Obstetrics and Gynaecology, University of Tuebingen, Germany. grmielke@med.uni-tuebingen.de
Abstract
OBJECTIVES: To establish reference ranges for 2D-echocardiographic examination of the fetal ductus arteriosus and its relationship to the main pulmonary artery and the aorta. METHODS: A prospective cross-sectional echocardiographic study was performed in 222 normal fetuses from 13 to 41 weeks of gestation using high resolution/color Doppler ultrasound equipment. RESULTS: Gestational age-specific reference ranges are given for the diameter of the pulmonary valve anulus, diameter of the ductus arteriosus at its beginning, middle, and end, ductal length, ductal diameter-to-pulmonary valve anulus diameter ratio, and the spatial relationship of the ductus arteriosus to the main pulmonary artery and to the aorta. CONCLUSIONS: The presented data derived from a study group of 222 normal fetuses provide in-vivo insights into the morphology of the ductus arteriosus and its relationship to the adjacent vessels. The reference ranges may be helpful in prenatal diagnosis of cardiac malformations and abnormalities of the ductus arteriosus, such as obstruction or aneurysm from 13 to 41 weeks of gestation.
PMID 10846778
1990
The structural organization of the human myocardium: the interrelation between myocardial fibers and interstitial connective tissue
Cardiologia. 1991 Jul;36(7):541-8.
[Article in Italian] Serio G, Caruso G, Serio R, Pennella A, Masciandaro A, Favia A, Lozupone E. Source Cattedra di Patologia Cardiovascolare, Università degli Studi, Bari.
Abstract
The complex tridimensional structure of the human ventricular myocardium has rarely been studied in the past. In the normal heart, in the lapse of time of few weeks of the embryonic life, a radical transformation from a chaotic plexiform organization to a complex tridimensional structure occurs. From then on, the ulterior growth of the myocardial fibres will only be dimensional and quantitative, because the spatial geometry is to be considered definitive. The role of the interstitial connective tissue in following the development of the myocardium, possibly inducing or influencing it, is still unclear. We have performed an histologic study on serial sections of the ventricular mass of 7 human embryos and fetuses, from 5 to 20 weeks of gestational age. The sections have been evaluated for their morphologic characteristics as evidentiated by histochemical (PAS, trichrome, Gomori silver technique) and immunohistochemical (myosin, actin, desmin, myoglobin, vimentin, fibronectin, smooth muscle cell, endothelial factor VIII) stainings. The results show that myocardial growth is mantellar, proceeding from the epicardium toward the endocardium, with progressive structural organization in strata, variably related one to the other depending upon the considered site of the ventricular mass. The interstitium grows in parallel to the myocardial growth, beginning with a thin network surrounding each fibre that progressively in time is transformed in a complexly arranged and more densely packed structure. The collagen fibres appear initially at epicardial level, particularly around the coronary vessels.(ABSTRACT TRUNCATED AT 250 WORDS)
PMID 1790536
Timeline
Molecular
| Factors and Signalling |
|---|
Transcription Factors
Signalling Molecules
|