Somatic Cell Nuclear Transfer: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| Line 14: | Line 14: | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| | | | ||
* '''Review - Artificial cloning of domestic animals'''<ref name=PMID26195770><pubmed>26195770</pubmed></ref> "Domestic animals can be cloned using techniques such as embryo splitting and nuclear transfer to produce genetically identical individuals. Although embryo splitting is limited to the production of only a few identical individuals, nuclear transfer of donor nuclei into recipient oocytes, whose own nuclear DNA has been removed, can result in large numbers of identical individuals. Moreover, clones can be produced using donor cells from sterile animals, such as steers and geldings, and, unlike their genetic source, these clones are fertile. In reality, due to low efficiencies and the high costs of cloning domestic species, only a limited number of identical individuals are generally produced, and these clones are primarily used as breed stock. In addition to providing a means of rescuing and propagating valuable genetics, somatic cell nuclear transfer (SCNT) research has contributed knowledge that has led to the direct reprogramming of cells (e.g., to induce pluripotent stem cells) and a better understanding of epigenetic regulation during embryonic development. In this review, I provide a broad overview of the historical development of cloning in domestic animals, of its application to the propagation of livestock and transgenic animal production, and of its scientific promise for advancing basic research." | |||
* '''Inheritance of mitochondrial DNA in serially recloned pigs by somatic cell nuclear transfer (SCNT)'''<ref name=PMID22809505><pubmed>22809505</pubmed></ref> "Somatic cell nuclear transfer (SCNT) has been established for the transmission of specific nuclear DNA. However, the fate of donor mitochondrial DNA (mtDNA) remains unclear. Here, we examined the fate of donor mtDNA in recloned pigs through third generations. ... These results indicate that heteroplasmy that originate from donor and recipient mtDNA is maintained in recloned pigs, resulting from SCNT, unlike natural reproduction." | * '''Inheritance of mitochondrial DNA in serially recloned pigs by somatic cell nuclear transfer (SCNT)'''<ref name=PMID22809505><pubmed>22809505</pubmed></ref> "Somatic cell nuclear transfer (SCNT) has been established for the transmission of specific nuclear DNA. However, the fate of donor mitochondrial DNA (mtDNA) remains unclear. Here, we examined the fate of donor mtDNA in recloned pigs through third generations. ... These results indicate that heteroplasmy that originate from donor and recipient mtDNA is maintained in recloned pigs, resulting from SCNT, unlike natural reproduction." | ||
* '''Number of blastomeres and distribution of microvilli in cloned mouse embryos during compaction'''<ref><pubmed>20735894</pubmed></ref> "We concluded that: (i) the cleavage of blastomeres in cloned embryos was slow at least before compaction; (ii) the distribution of microvilli in cloned, normal, parthenogenetic, and tetraploid embryos was coherent before and after compaction; and (iii) the initiation of compaction in somatic cell nuclear transfer (SCNT) embryos was delayed compared with that of intracytoplasmic sperm injection (ICSI) embryos." | * '''Number of blastomeres and distribution of microvilli in cloned mouse embryos during compaction'''<ref><pubmed>20735894</pubmed></ref> "We concluded that: (i) the cleavage of blastomeres in cloned embryos was slow at least before compaction; (ii) the distribution of microvilli in cloned, normal, parthenogenetic, and tetraploid embryos was coherent before and after compaction; and (iii) the initiation of compaction in somatic cell nuclear transfer (SCNT) embryos was delayed compared with that of intracytoplasmic sperm injection (ICSI) embryos." | ||
Revision as of 09:16, 28 July 2016
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
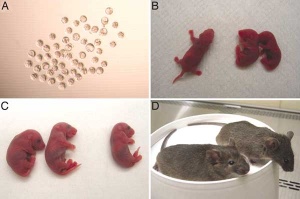
In 1996 Dolly the sheep was the first animal to be produced by somatic cell nuclear transfer (SCNT) using an adult-derived somatic cell as nuclear donor. A somatic cell refers to the fact that a cell that is not a germ cell (spermatozoa, oocyte) is used to generate a zygote from which the embryo develops. This topic is closely related to Stem Cells.
A range of different cell types has now been successfully applied to a range of species (cattle, mice, goats, pigs, cats, rabbits, horses, rats, dogs and ferrets. (see review[2])
- SCNT Links: Introduction | Stem Cells - Induced | Stem Cells - SCNT | Epigenetics | Stem Cells | ART | Fertilization | Week 1 | Category:Zygote
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Somatic Cell Nuclear Transfer <pubmed limit=5>Somatic Cell Nuclear Transfer</pubmed> |
SCNT Animal Timeline
Process
- Somatic nucleus cell source - culture of somatic cells from nucleus donor.
- Oocyte - nucleus and the polar body are removed from oocyte by aspiration giving an enucleated oocyte.
- Injection - of a somatic cell between the zona pellucida and the membrane of the enucleated oocyte.
- Electrofusion - Introduction of the somatic cell nucleus (and cytoplasm) into the oocyte cytoplasm.
- Embryo clone - formed by an oocyte cytoplasm and a somatic cell nucleus containing two copies of chromosomes.
- Embryo transfer - into a surrogate dam generating clone (F0) with coat colour similar to that of the nucleus source.
- Clone offspring - (F1) generated by the sexual reproduction of the clone (F0) with a normal partner.
Oocyte Enucleation
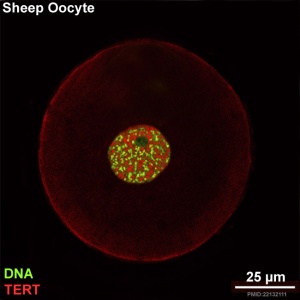
Before a somatic cell nuclei can be introduced into an oocyte, the oocyte's own nucleus needs to be removed. This process of oocyte nuclear removal is described as "oocyte enucleation". A recent study in cattle found oocyte imaging had a higher efficiency. [12]
The oocyte nucleus can be identified by:
- Hoechst staining and UV irradiation.
- Oocyte imaging.
Somatic Cell Source
A number of different tissues have been used as the somatic cell nucleus source including:
- ovarian cumulus cells
- fibroblasts
- mammary epithelium
- lymphocytes
- neural stem cells
- olfactory
- myoblasts
Epigenetics
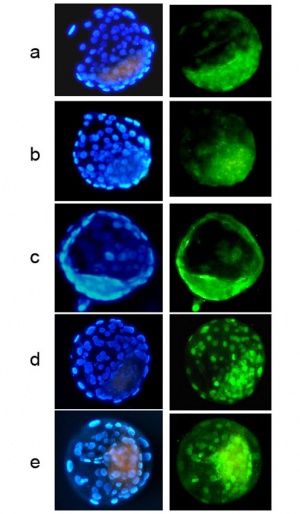
Several studies have reported that introduction of the somatic nuclei leads to deleterious epigenetic changes including DNA methylation and histone acetylation.[14][15][16]
Mitochondria
Typically mitochondria are maternally inherited and any paternal (spermatozoa) mitochondria either do not enter the oocyte or are destroyed. A recent SCNT study in pig[4] has demonstrated a mixed inheritance pattern.
Inheritance of mitochondrial DNA in serially recloned pigs by somatic cell nuclear transfer (SCNT)[4] "Somatic cell nuclear transfer (SCNT) has been established for the transmission of specific nuclear DNA. However, the fate of donor mitochondrial DNA (mtDNA) remains unclear. Here, we examined the fate of donor mtDNA in recloned pigs through third generations. ... These results indicate that heteroplasmy that originate from donor and recipient mtDNA is maintained in recloned pigs, resulting from SCNT, unlike natural reproduction."
Legislation
This technique essentially results in a clone of the original animal and therefore has been regulated by different countries in many different ways.
Australia
2010 - Cloning Legislative Review Committee Established
An independent committee has been established by the Federal Government to review cloning legislation in Australia (22 December 2010).
The independent Legislation Review Committee for the review of the Prohibition of Human Cloning for Reproduction Act 2002 and the Research Involving Human Embryos Act 2002 was announced today by the Federal Minister for Mental Health and Ageing, Mark Butler.
2006 - Prohibition of Human Cloning for Reproduction and the Regulation of Human Embryo Research Amendment Act 2006 (formerly known as the Patterson Bill) came into effect in June 2007.
2002 - Research Involving Human Embryos Act 2002 and the Prohibition of Human Cloning Act 2002 were passed by Parliament in December 2002.
USA
2012 - Human somatic cell nuclear transfer and cloning by The Ethics Committee of the American Society for Reproductive Medicine.[17]
- "This document presents arguments that conclude that it is unethical to use somatic cell nuclear transfer (SCNT) for infertility treatment due to concerns about safety; the unknown impact of SCNT on children, families, and society; and the availability of other ethically acceptable means of assisted reproduction. This document replaces the ASRM Ethics Committee report titled, "Human somatic cell nuclear transfer (cloning)," last published in Fertil Steril 2000;74:873-6."
Europe
2008 - Food Safety, Animal Health and Welfare and Environmental Impact of Animals derived from Cloning by Somatic Cell Nucleus Transfer (SCNT) and their Offspring and Products Obtained from those Animals [18] The committee included a number of recommendations in the paper.
- "At present there is no indication that clones or their progeny would pose any new or additional environmental risks compared with conventionally bred animals."
References
- ↑ <pubmed>17299040</pubmed>| PMC1815251
- ↑ <pubmed>22681293</pubmed>
- ↑ <pubmed>26195770</pubmed>
- ↑ 4.0 4.1 4.2 <pubmed>22809505</pubmed>
- ↑ <pubmed>20735894</pubmed>
- ↑ <pubmed>8598906</pubmed>
- ↑ <pubmed>9596577</pubmed>
- ↑ <pubmed>9690471</pubmed>
- ↑ <pubmed>10331804</pubmed>
- ↑ <pubmed>10993078</pubmed>
- ↑ <pubmed>22132111</pubmed>| PLoS One.
- ↑ <pubmed>22816525</pubmed>
- ↑ <pubmed>18154666</pubmed>| BMC Dev Biol.
- ↑ <pubmed>22039959</pubmed>
- ↑ <pubmed>22393450</pubmed>
- ↑ <pubmed>20677935</pubmed>
- ↑ <pubmed>22795681</pubmed>
- ↑ The European Food Safety Authority Journal (2008) 767, 1-49 PDF
Reviews
<pubmed>26195770</pubmed> <pubmed>22536140</pubmed> <pubmed>22000472</pubmed> <pubmed>21982229</pubmed> <pubmed>21555407</pubmed>
<pubmed></pubmed> <pubmed></pubmed> <pubmed></pubmed>
Articles
Search Pubmed
August 2012 "somatic cell nuclear transfer" All (1883) Review (360) Free Full Text (585) Published in 2012 (125)
Search Pubmed: somatic cell nuclear transfer
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 19) Embryology Somatic Cell Nuclear Transfer. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Somatic_Cell_Nuclear_Transfer
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G

