Sertoli cell: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 13: | Line 13: | ||
{| | {| | ||
| [[File:Enrico Sertoli.jpg| | | [[File:Enrico Sertoli.jpg|100px|alt=Enrico Sertoli (1842 - 1910)]] | ||
Enrico Sertoli (1842 - 1910) | Enrico Sertoli (1842 - 1910) | ||
| Sertoli cell are named after Enrico Sertoli (1842 - 1910), an Italian physiologist and histologist. | | Sertoli cell are named after Enrico Sertoli (1842 - 1910), an Italian physiologist and histologist. | ||
| Line 48: | Line 48: | ||
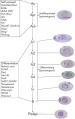
==Development Overview== | ==Development Overview== | ||
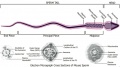
[[File:Mouse- gonadal supporting cell development.jpg|thumb|alt=Gonadal supporting cell development|Gonadal supporting cell development]] | [[File:Mouse- gonadal supporting cell development.jpg|thumb|alt=Gonadal supporting cell development|Gonadal supporting cell development]] | ||
Ultrastructural description of human Sertoli cells{{#pmid:11732573|PMID11732573}} | |||
* '''7 weeks''' - first morphologically recognised in testicular cords, organised as primordial germ cells surrounded by pre-Sertoli cells. | |||
* '''7 to 8 weeks''' - basal lamina of the cords becomes distinguishable, pre-Sertoli cells the rough endoplasmic reticulum develops. | |||
* '''14 to 20 weeks''' - pre-Sertoli cells maintain their general morphology whereas the most significant change is the maximum development of Leydig cells. | |||
{{Seminiferous Tubule puberty table}} | {{Seminiferous Tubule puberty table}} | ||
| Line 67: | Line 72: | ||
(see also review{{#pmid:7711190|PMID7711190}}) | (see also review{{#pmid:7711190|PMID7711190}}) | ||
Molecular factors: | Molecular factors: | ||
| Line 79: | Line 80: | ||
:'''Links:'''[[Sertoli cell]] | :'''Links:'''[[Sertoli cell]] | ||
==Blood-Testis Barrier == | ==Blood-Testis Barrier == | ||
Within the testis seminiferous tubules the Sertoli cells located near the basement membrane act as an initial cellular barrier with many functions, but often described as forming a "blood-testis barrier". (see | Within the testis seminiferous tubules the Sertoli cells located near the basement membrane act as an initial cellular barrier with many functions, but often described as forming a "blood-testis barrier". (see reviews{{#pmid:22039149|PMID22039149}}{{#pmid:21134990|PMID21134990}} The BTB physically divides the seminiferous epithelium into two compartments: the basal and apical (adluminal). | ||
Functions: | Functions: | ||
| Line 90: | Line 89: | ||
* establish a basal and adluminal (apical) compartment (specialized microenvironment) | * establish a basal and adluminal (apical) compartment (specialized microenvironment) | ||
* provide an immunological privilege status of the testis (anti-sperm antibodies are not developed) | * provide an immunological privilege status of the testis (anti-sperm antibodies are not developed) | ||
{| | |||
|+ '''Rat Blood–Testis Barrier''' | |||
|- | |||
| colspan=2|A model depicting the migration of developing preleptotene spermatocytes across the blood–testis barrier (BTB}.{{#pmid:17698604|PMID17698604}} | |||
|- | |||
| [[File:Rat blood–testis barrier 02.jpg|400px]] | |||
| [[File:Rat blood–testis barrier 03.jpg|400px]] | |||
|- | |||
| Movement of developing germ cells in the seminiferous epithelium of the adult testis. | |||
Germ cells first have to break through the tight junctions at the BTB with minimal disruptions. After the opening of the BTB, the progression of germ cells relies solely on series of transient adherens junctions at the Sertoli–germ cell interface. Assembly and disassembly of junctional complexes at the basal and apical ectoplasmic specialization occurs continuously. | |||
| When germ cells move along Sertoli cells, the original JAM/CAR/nectin trans-homodimers between Sertoli cells are replaced by the Sertoli–germ cell junctions composed of CAR–CAR/CAR–JAM-C/nectin-2–nectin-3/necl-5(PVR)–necl-2/JAM-B–JAM-C complexes plausibly through competitive binding. It is noted that CAR–CAR adhesion has not been directly demonstrated for Sertoli cell–germ cell interaction, but remains an attractive possibility. | |||
|- | |||
| colspan=2|Text from original figure legend. | |||
|} | |||
==Hormones== | |||
These cells secrete several hormones, anti-Müllerian hormone ({{AMH}}), inhibin and activin. | |||
Inhibin and activin provide positive and negative feedback respectively on follicle-stimulating hormone ({{FSH}}) secretion from the {{pituitary}}. | |||
* activins - dimers of beta-A and/or beta-B subunits encoded by the genes INHBA and INHBB | |||
* Follicle-stimulating hormone (FSH)-releasing protein (FRP) subunit is identical in structure to the beta-A subunit of inhibin. | |||
===Anti-Mullerian Hormone=== | |||
Anti-Mullerian Hormone (Anti-Müllerian Hormone, {{AMH}}) is a glycoprotein that is produced by immature Sertoli cells. | |||
Anti-Mullerian hormone (AMH) is a glycoprotein belonging to the transforming growth factor-beta (TGF-beta) family. During development, immature Sertoli cells produce AMH{{#pmid:1292986|PMID1292986}} to aid regression of Müllerian (paramesonephric) ducts in male sex developement.{{#pmid:28851672|PMID28851672}} | |||
Follicle-stimulating hormone (FSH) promotes AMH transcription in the absence of androgen signaling, while {{Testosterone}} has been shown to inhibit the transcriptional activation of AMH. | |||
:'''Links:''' {{Anti-Mullerian Hormone}} | |||
==Abnormalities== | ==Abnormalities== | ||
| Line 185: | Line 220: | ||
{{#pmid:19339813}} | {{#pmid:19339813}} | ||
{{#pmid:22039149}} | |||
===Articles=== | ===Articles=== | ||
{{#pmid:21147849}} | {{#pmid:21147849}} | ||
| Line 197: | Line 234: | ||
===Books=== | ===Books=== | ||
{{#pmid:25905331}} | {{#pmid:25905331}} | ||
Skinner M. and Griswold M. [https://www.elsevier.com/books/sertoli-cell-biology/skinner/978-0-12-647751-1 '''Sertoli Cell Biology'''] (2004) Academic Press | |||
===Search PubMed=== | ===Search PubMed=== | ||
Latest revision as of 22:53, 2 May 2019
| Embryology - 16 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
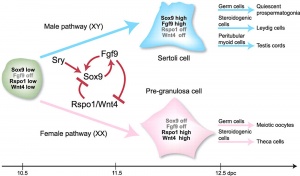
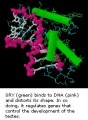
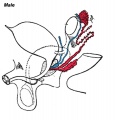
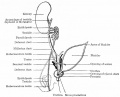
The sertoli cells are the first cells to be differentiated in development by SRY expression. Post-puberty these are the "support" cells for spermatozoa development and transport from the periphery to lumen of the seminiferous tubule. Sertoli cells form a barrier with cell junctions at the Sertoli cell-cell and Sertoli-germ cell interface.
The initial difference in male and female gonad development are dependent on testis-determining factor (TDF) the protein product of the Y chromosome SRY gene expression in the developing Sertoli cell. Recent studies have indicated that additional factors may also be required for full sex differentiation. The seminiferous tubules are considered the parenchyma of the testis. Within the developing testis the three main differentiating cell types are: gamete forming cells (spermatogonia), support cells (Sertoli cell) and hormone secreting cells (Leydig cell or interstitial cell).
Sertoli cell postnatal proliferation may be regulated by thyroid status. An animal model study of postnatal transient hypothyroidism has demonstrated Sertoli cell proliferation (6 to 8 fold increase) 2 days after the diet switch and remained elevated the next days.[1]

Enrico Sertoli (1842 - 1910) |
Sertoli cell are named after Enrico Sertoli (1842 - 1910), an Italian physiologist and histologist.
His historic paper "The structure of seminiferous tubules and the development of (spermatids) in rats" have recently been translated from the original Italian into English.[2] See also a biography written in 2002.[3] |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Sertoli cell development | Sertoli cell barrier |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Development Overview
Ultrastructural description of human Sertoli cells[8]
- 7 weeks - first morphologically recognised in testicular cords, organised as primordial germ cells surrounded by pre-Sertoli cells.
- 7 to 8 weeks - basal lamina of the cords becomes distinguishable, pre-Sertoli cells the rough endoplasmic reticulum develops.
- 14 to 20 weeks - pre-Sertoli cells maintain their general morphology whereas the most significant change is the maximum development of Leydig cells.
| Testis - Seminiferous Tubule | |
|---|---|
| Pre-puberty | Post-puberty |
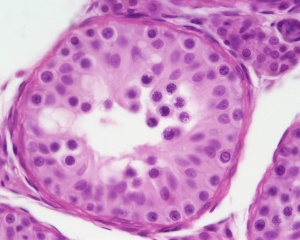
|
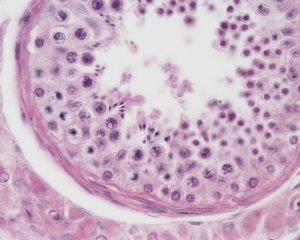
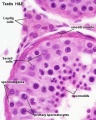
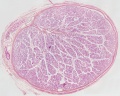
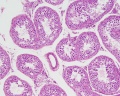
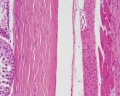
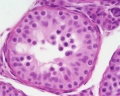
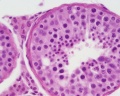
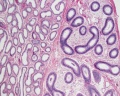
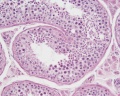
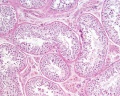
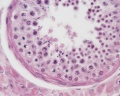
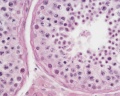
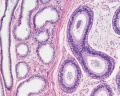
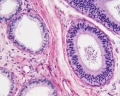
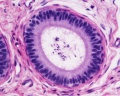
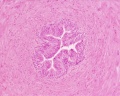
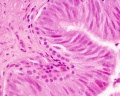
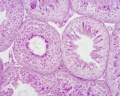
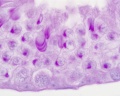
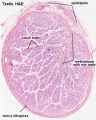
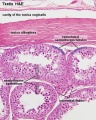
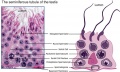
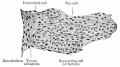
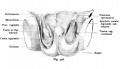
|
| Cross-sectional view of the seminiferous tubule histology before and after puberty. | |
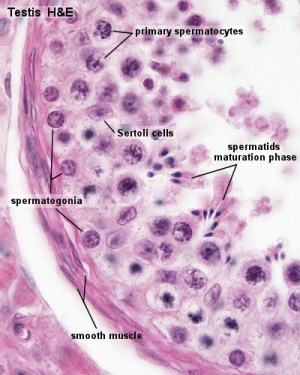
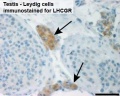
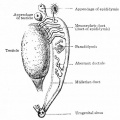
The sertoli cell cells provide support for spermatozoa development within the seminiferous tubule. In the mouse, these cells are derived from the coelomic epithelium along with other testis somatic cells.[9] Their differentiation is regulated by the presence of a Y chromosome and in turn regulates Leydig cell differentiation. Sertoli cells direct testis morphogenesis, organizing testis cord formation, establishing testis vasculature and inducing differentiation of peritubular myoid cells and fetal Leydig cells. At puberty the immature Sertoli cells cease to proliferate and differentiate. Activin A acts upon Sertoli cells to promote their embryonic proliferation[10]
Sertoli cells express the androgen receptor and receptors for follicle stimulating hormone (FSH).
Sertoli cell functions include:
- regulation of spermatogenesis through endocrine FSH and testosterone
- regulation of the intratubular and intercellular environment adluminal to the tight junctional complexes
- meiotic and post-meiotic germ cells are sequestered by Sertoli-Sertoli junctional complexes
- generate adluminal compartment isolated from both serum and lymph
- attachment of germ cells through unique intermediate filament (desmosome-like junctions) and microfilament (actin-ectoplasmic specializations, ESs) junctions[11]
- to prevent premature sloughing of immature germ cells from the seminiferous epithelium
- desmosome-like junctions are initially present (up to step 8 spermatids)
- ectoplasmic specializations then replace this junction (in step 8 spermatids)
(see also review[12])
Molecular factors:
- Follicle Stimulating Hormone (FSH) -> Krüppel-like factor 4 (KLF4)
- Krüppel-like factor 4 (KLF4) - zinc finger transcription factor, terminal differentiation of epithelial cells.
- Epidermal Growth Factor (EGF)
- Transforming Growth Factor-beta (TGFbeta)
- Links:Sertoli cell
Blood-Testis Barrier
Within the testis seminiferous tubules the Sertoli cells located near the basement membrane act as an initial cellular barrier with many functions, but often described as forming a "blood-testis barrier". (see reviews[13][14] The BTB physically divides the seminiferous epithelium into two compartments: the basal and apical (adluminal).
Functions:
- prevent substances reaching the developing spermatozoa (through drug transporters)
- establish a basal and adluminal (apical) compartment (specialized microenvironment)
- provide an immunological privilege status of the testis (anti-sperm antibodies are not developed)
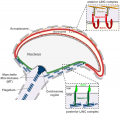
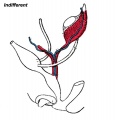
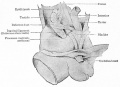
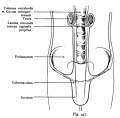
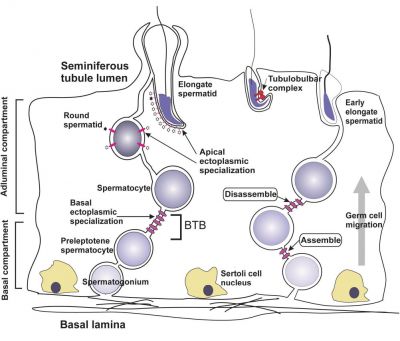
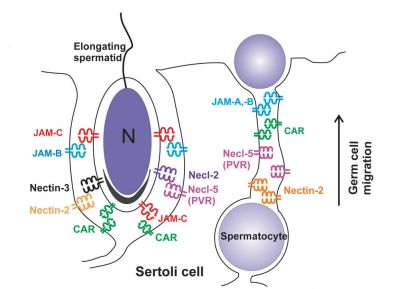
| A model depicting the migration of developing preleptotene spermatocytes across the blood–testis barrier (BTB}.[15] | |
|
|
| Movement of developing germ cells in the seminiferous epithelium of the adult testis.
Germ cells first have to break through the tight junctions at the BTB with minimal disruptions. After the opening of the BTB, the progression of germ cells relies solely on series of transient adherens junctions at the Sertoli–germ cell interface. Assembly and disassembly of junctional complexes at the basal and apical ectoplasmic specialization occurs continuously. |
When germ cells move along Sertoli cells, the original JAM/CAR/nectin trans-homodimers between Sertoli cells are replaced by the Sertoli–germ cell junctions composed of CAR–CAR/CAR–JAM-C/nectin-2–nectin-3/necl-5(PVR)–necl-2/JAM-B–JAM-C complexes plausibly through competitive binding. It is noted that CAR–CAR adhesion has not been directly demonstrated for Sertoli cell–germ cell interaction, but remains an attractive possibility. |
| Text from original figure legend. | |
Hormones
These cells secrete several hormones, anti-Müllerian hormone (AMH), inhibin and activin.
Inhibin and activin provide positive and negative feedback respectively on follicle-stimulating hormone (FSH) secretion from the pituitary.
- activins - dimers of beta-A and/or beta-B subunits encoded by the genes INHBA and INHBB
- Follicle-stimulating hormone (FSH)-releasing protein (FRP) subunit is identical in structure to the beta-A subunit of inhibin.
Anti-Mullerian Hormone
Anti-Mullerian Hormone (Anti-Müllerian Hormone, AMH) is a glycoprotein that is produced by immature Sertoli cells.
Anti-Mullerian hormone (AMH) is a glycoprotein belonging to the transforming growth factor-beta (TGF-beta) family. During development, immature Sertoli cells produce AMH[16] to aid regression of Müllerian (paramesonephric) ducts in male sex developement.[17]
Follicle-stimulating hormone (FSH) promotes AMH transcription in the absence of androgen signaling, while testosterone has been shown to inhibit the transcriptional activation of AMH.
- Links: Anti-Mullerian Hormone
Abnormalities
Histology
Testis Histology Links: Testis Development | Spermatozoa Development | Histology
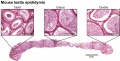
- Human (young): overview labeled | overview unlabeled | convoluted seminiferous tubules x10 | x40 | x40 | tunica albuginea x20
- Human (adult): overview x2 | convoluted seminiferous tubules labeled | x10 | x20 | x40 | x40 | epididymis ductulus efferens | ductus epididymidis | epithelium | overview x4 | x10 | x20 | x40 | ductus deferens labeled overview | epithelium | overview x2 | x10 | x40
- Human Stage 22: Testis - labeled overview | Testis - unlabeled overview | Testis - unlabeled detail | Testis - labeled detail | testis | Carnegie stage 22 | Movie - Urogenital stage 22
- Mouse: postnatal epididymis | 14 days postnatal | 33 days postnatal | 45 days postnatal | 2 months postnatal
| Spermatozoa Development (expand to see terms) | ||
|---|---|---|
|
Note there are additional glossaries associated with genital, spermatozoa, oocyte and renal.
See also: Spermatozoa Terms collapse table
|
Genital Links: genital | Lecture - Medicine | Lecture - Science | Lecture Movie | Medicine - Practical | primordial germ cell | meiosis | endocrine gonad | Genital Movies | genital abnormalities | Assisted Reproductive Technology | puberty | Category:Genital
| ||||
|
Molecular
Sry
- Y chromosome gene for a transcription factor
- member of the high mobility group (HMG)-box family of DNA binding proteins
- human - 204 amino acid protein[18]
- Links: OMIM - Sry
Sox9
- autosomal transcription factor
- Development of XY females - presence of only a single functional copy of the transcription factor encoding genes SOX9, SF1, or WT1 (Note- not all XY humans are sex-reversed if only a single copy of a normal SF1 or WT1 allele is present)
- A nuclear export signal within the high mobility group domain regulates the nucleocytoplasmic translocation of SOX9 during sexual determination[19]
Other roles
- Cartilage - essential for chondrocyte differentiation
- Hearing - otic placode formation, maintenance of progenitors in the otic epithelium
- Links: Sox9 | Cartilage Development | Inner Ear Development
Fog2
- transcription factor, named Friend of Gata2
- human - (8q23) 1,151 amino acid nuclear protein that contains 8 zinc finger motifs[20]
- dosage critical for fetal testis development in mice[21]
- Links: OMIM - Fog2
Gadd45g
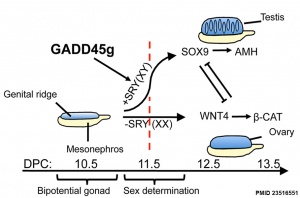
Growth Arrest- And Dna Damage-Inducible Gene (GADD45, GAMMA; GADD45G)
A Recent mouse study[6] has shown that Gadd45g protein has a role in primary sex differentiation. Knockout mice (Gadd45g(-/-) XY gonads) resulted in a a sex reversal.
- Links: OMIM - Gadd45g
Gata4
- transcription factor
- dosage critical for fetal testis development in mice[21]
Eif2s3y
References
- ↑ Rijntjes E, Gomes MLM, Zupanič N, Swarts HJM, Keijer J & Teerds KJ. (2017). Transient Hypothyroidism: Dual Effect on Adult-Type Leydig Cell and Sertoli Cell Development. Front Physiol , 8, 323. PMID: 28588502 DOI.
- ↑ Sertoli E. (2018). The structure of seminiferous tubules and the development of [spermatids] in rats. Biol. Reprod. , 99, 482-503. PMID: 29961830 DOI.
- ↑ Baratelli GM, Lanzani A & Sacco RN. (2002). Biography of Enrico Sertoli. Urology , 60, 196-8. PMID: 12100962
- ↑ Wen Q, Wang Y, Tang J, Cheng CY & Liu YX. (2016). Sertoli Cell Wt1 Regulates Peritubular Myoid Cell and Fetal Leydig Cell Differentiation during Fetal Testis Development. PLoS ONE , 11, e0167920. PMID: 28036337 DOI.
- ↑ Rebourcet D, O'Shaughnessy PJ, Pitetti JL, Monteiro A, O'Hara L, Milne L, Tsai YT, Cruickshanks L, Riethmacher D, Guillou F, Mitchell RT, van't Hof R, Freeman TC, Nef S & Smith LB. (2014). Sertoli cells control peritubular myoid cell fate and support adult Leydig cell development in the prepubertal testis. Development , 141, 2139-49. PMID: 24803659 DOI.
- ↑ 6.0 6.1 6.2 Johnen H, González-Silva L, Carramolino L, Flores JM, Torres M & Salvador JM. (2013). Gadd45g is essential for primary sex determination, male fertility and testis development. PLoS ONE , 8, e58751. PMID: 23516551 DOI.
- ↑ Hiramatsu R, Harikae K, Tsunekawa N, Kurohmaru M, Matsuo I & Kanai Y. (2010). FGF signaling directs a center-to-pole expansion of tubulogenesis in mouse testis differentiation. Development , 137, 303-12. PMID: 20040496 DOI.
- ↑ Heyn R, Makabe S & Motta PM. (2001). Ultrastructural morphodynamics of human Sertoli cells during testicular differentiation. Ital J Anat Embryol , 106, 163-71. PMID: 11732573
- ↑ Karl J & Capel B. (1998). Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev. Biol. , 203, 323-33. PMID: 9808783 DOI.
- ↑ Archambeault DR & Yao HH. (2010). Activin A, a product of fetal Leydig cells, is a unique paracrine regulator of Sertoli cell proliferation and fetal testis cord expansion. Proc. Natl. Acad. Sci. U.S.A. , 107, 10526-31. PMID: 20498064 DOI.
- ↑ Kopera IA, Bilinska B, Cheng CY & Mruk DD. (2010). Sertoli-germ cell junctions in the testis: a review of recent data. Philos. Trans. R. Soc. Lond., B, Biol. Sci. , 365, 1593-605. PMID: 20403872 DOI.
- ↑ Griswold MD. (1995). Interactions between germ cells and Sertoli cells in the testis. Biol. Reprod. , 52, 211-6. PMID: 7711190
- ↑ Cheng CY & Mruk DD. (2012). The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. , 64, 16-64. PMID: 22039149 DOI.
- ↑ Su L, Mruk DD & Cheng CY. (2011). Drug transporters, the blood-testis barrier, and spermatogenesis. J. Endocrinol. , 208, 207-23. PMID: 21134990 DOI.
- ↑ Wang CQ & Cheng CY. (2007). A seamless trespass: germ cell migration across the seminiferous epithelium during spermatogenesis. J. Cell Biol. , 178, 549-56. PMID: 17698604 DOI.
- ↑ Josso N. (1992). Anti-müllerian hormone and Sertoli cell function. Horm. Res. , 38 Suppl 2, 72-6. PMID: 1292986 DOI.
- ↑ Rehman ZU, Worku T, Davis JS, Talpur HS, Bhattarai D, Kadariya I, Hua G, Cao J, Dad R, Farmanullah T, Hussain L & Yang. (2017). Role and mechanism of AMH in the regulation of Sertoli cells in mice. J. Steroid Biochem. Mol. Biol. , 174, 133-140. PMID: 28851672 DOI.
- ↑ Su H & Lau YF. (1993). Identification of the transcriptional unit, structural organization, and promoter sequence of the human sex-determining region Y (SRY) gene, using a reverse genetic approach. Am. J. Hum. Genet. , 52, 24-38. PMID: 8434602
- ↑ Gasca S, Canizares J, De Santa Barbara P, Mejean C, Poulat F, Berta P & Boizet-Bonhoure B. (2002). A nuclear export signal within the high mobility group domain regulates the nucleocytoplasmic translocation of SOX9 during sexual determination. Proc. Natl. Acad. Sci. U.S.A. , 99, 11199-204. PMID: 12169669 DOI.
- ↑ Holmes M, Turner J, Fox A, Chisholm O, Crossley M & Chong B. (1999). hFOG-2, a novel zinc finger protein, binds the co-repressor mCtBP2 and modulates GATA-mediated activation. J. Biol. Chem. , 274, 23491-8. PMID: 10438528
- ↑ 21.0 21.1 Bouma GJ, Washburn LL, Albrecht KH & Eicher EM. (2007). Correct dosage of Fog2 and Gata4 transcription factors is critical for fetal testis development in mice. Proc. Natl. Acad. Sci. U.S.A. , 104, 14994-9. PMID: 17848526 DOI.
Reviews
Rotgers E, Jørgensen A & Yao HH. (2018). At the crossroads of fate - somatic cell lineage specification in the fetal gonad. Endocr. Rev. , , . PMID: 29771299 DOI.
Barton LJ, LeBlanc MG & Lehmann R. (2016). Finding their way: themes in germ cell migration. Curr. Opin. Cell Biol. , 42, 128-137. PMID: 27484857 DOI.
De Felici M. (2016). The Formation and Migration of Primordial Germ Cells in Mouse and Man. Results Probl Cell Differ , 58, 23-46. PMID: 27300174 DOI.
Virtanen HE & Toppari J. (2014). Embryology and physiology of testicular development and descent. Pediatr Endocrinol Rev , 11 Suppl 2, 206-13. PMID: 24683945
Svingen T & Koopman P. (2013). Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes Dev. , 27, 2409-26. PMID: 24240231 DOI.
Hinton BT, Galdamez MM, Sutherland A, Bomgardner D, Xu B, Abdel-Fattah R & Yang L. (2011). How do you get six meters of epididymis inside a human scrotum?. J. Androl. , 32, 558-64. PMID: 21441421 DOI.
Griswold SL & Behringer RR. (2009). Fetal Leydig cell origin and development. Sex Dev , 3, 1-15. PMID: 19339813 DOI.
Cheng CY & Mruk DD. (2012). The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. , 64, 16-64. PMID: 22039149 DOI.
Articles
Kaftanovskaya EM, Feng S, Huang Z, Tan Y, Barbara AM, Kaur S, Truong A, Gorlov IP & Agoulnik AI. (2011). Suppression of insulin-like3 receptor reveals the role of β-catenin and Notch signaling in gubernaculum development. Mol. Endocrinol. , 25, 170-83. PMID: 21147849 DOI.
Stukenborg JB, Colón E & Söder O. (2010). Ontogenesis of testis development and function in humans. Sex Dev , 4, 199-212. PMID: 20664245 DOI.
Hutson JM, Balic A, Nation T & Southwell B. (2010). Cryptorchidism. Semin. Pediatr. Surg. , 19, 215-24. PMID: 20610195 DOI.
Adham IM & Agoulnik AI. (2004). Insulin-like 3 signalling in testicular descent. Int. J. Androl. , 27, 257-65. PMID: 15379965 DOI.
Heyns CF. (1987). The gubernaculum during testicular descent in the human fetus. J. Anat. , 153, 93-112. PMID: 2892824
Books
De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A & Hutson JM. (2000). Cryptorchidism and Hypospadias. , , . PMID: 25905331
Skinner M. and Griswold M. Sertoli Cell Biology (2004) Academic Press
Search PubMed
Search Pubmed: Testis Development | Epididymis Development | Sry | Sox9 |
Additional Images
Historic Images
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 16) Embryology Sertoli cell. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Sertoli_cell
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G