REI - Reproductive Medicine Seminar 2018
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
This is the partial online draft version of my presentation, as there was no internet I switched to powerpoint fversion for the final presentation. I will though update this online version as it will include full access to molecular data and all animations presented in my talk. Please feel free to contact me at any time with your embryology questions.
Seminar Topics
- Preimplantation
- Implantation
- Urogenital
- Endocrine
Powerpoint Slides: 1 slide/page PDF |
| 3.1 Fetal Medicine Content |
|---|
|
General Aim Candidates should understand and describe normal and abnormal human development and the principles of implantation, developmental embryology and early pregnancy maintenance. Specific Objectives 3.1.1 Implantation Understand and describe;
3.1.2 Developmental Embryology Understand and describe;
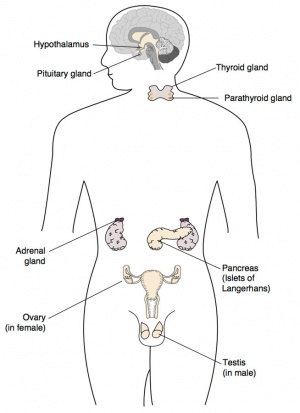
� Embryology of the hypothalamic/pituitary, adrenal and thyroid endocrine systems
|
Background
- Uterine Tube Biobank (Prof Ledger)
- Molecular aspects of ectopic implantation
- Trophoblast differentiation
- Control of implantation and early placentation
- Digital Embryology Consortium
- The objective of this international partnership is to digitise, preserve, and make available for researchers the major embryology histological collections.
- Kyoto Collection(eBook)
- with Shiota, Yamada and Ho.
- Human SEM (eBook)
- in preparation with Sulik, Vekemans and Attié-Bitach.
- UNSW Embryology
Reproductive
Timeline
| Gestational Age GA Week |
Event | Fertilization Age Week |
|---|---|---|
| 5-6 | primordial germ cells migrate during gastrulation | 3-4 |
| 6 | intermediate mesoderm, pronephros primordium | 4 |
| 7 | mesonephros and mesonephric duct (Wolffian duct) | 5 |
| 8 | ureteric bud, metanephros, genital ridge | 6 (35 days) |
| 9 | cloacal divison, gonadal primordium - indifferent to first appearance of testis cords | 7 (42 days) |
| 10 | paramesonephric duct (Mullerian duct), clear gonadal differentiation | 8 (49 days) |
| 11 | paramesonephric duct fusion (female) | 9 (56 days) |
| 17 | primary follicles (ovary) | 15 (100 days) |
Primordial Germ Cells
Primoridal Germ Cell Migration
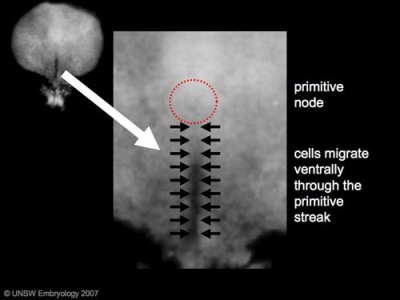
Primordial Germ Cells (PGCs) are thought to be the first population of cells to migrate through the primitive streak in early gastrulation.
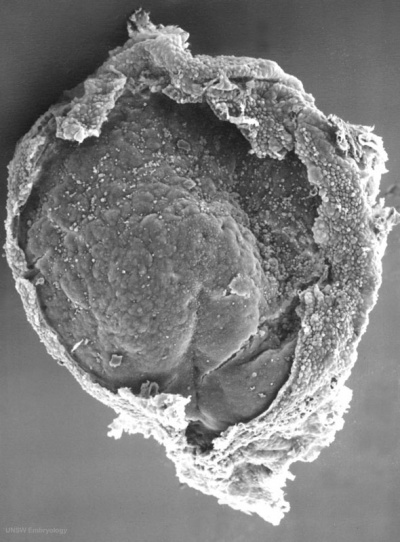
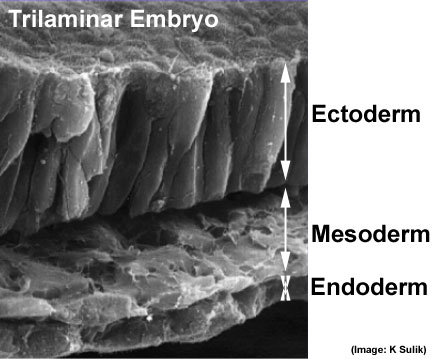
Human Embryonic Disc (Stage 7 GA week 5)
GA Week 5
- Human embryonic disc showing the primitive streak region where gastrulation occurs, generation the trilaminar embryo.
- Arrows indicate direction of cell migration through the streak.
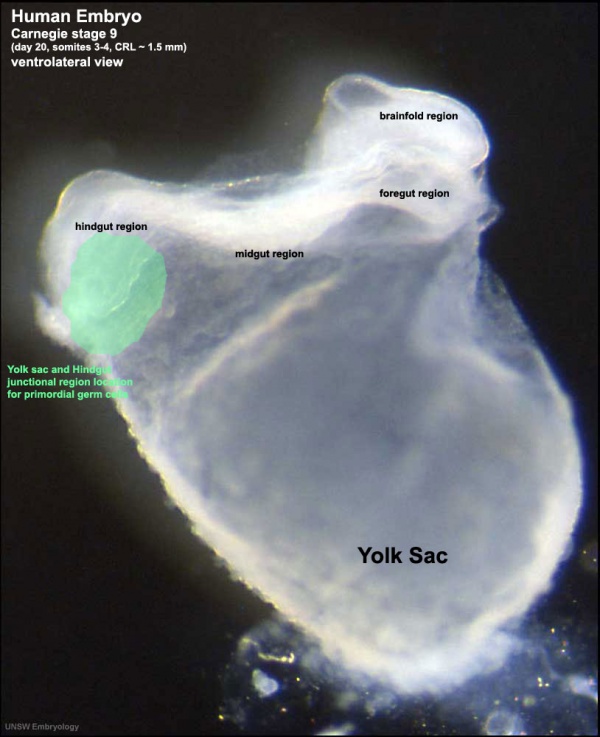
This population of cells then lie at the hindgut and yolk sac junctional region and later migrate into the germinal ridge in early embryonic development.
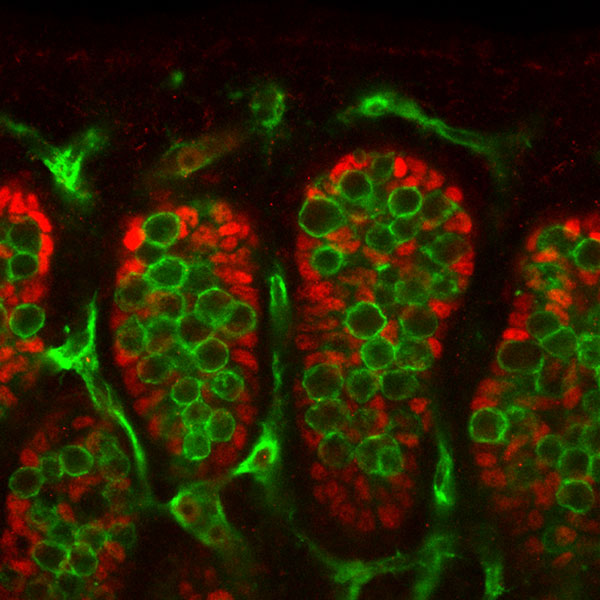
Human Embryo (Stage 9 GA week 5) primordial germ cell region (green)
- Sacrococcygeal teratomas - Remnant primitive streak cells (most common solid tumor in newborn infants)
- Germline teratoma - (Germinoma) abnormally differentiated/located PGCs fail to die.
Mouse PGC Migration
| E9.5 | E10.5 |
|---|---|
|
<html5media height="400" width="400">File:Primordial_germ_cell_002.mp4</html5media> |
<html5media height="400" width="400">File:Primordial_germ_cell_003.mp4</html5media> |
PGC motility 3 phases - initiation, migration and stopping
- based upon normal actin cell motility.
- no sex-specific differences
PGC Chemoattraction
Chemoattraction
|
Stromal Derived Factor 1 (SDF-1, CXCL12) chemotaxis
<html5media width="400" height="300">https://www.youtube.com/embed/tqlc_YAtWRE</html5media> |
Intermediate Mesoderm
| Mesoderm Generation (chicken)[3]
<html5media height="700" width="300">File:Chicken presomitic mesoderm 03.mp4</html5media> |
Human trilaminar embryo {"Germ layers")
Intermediate mesoderm derives genital and renal systems. |
|
<html5media height="520" width="360">File:Urogenital_sinus_001.mp4</html5media> GA week 6-7 Urogenital Sinus Movie |
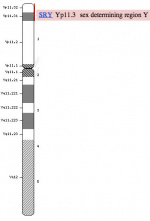
It is not the primordial germ cells which respond to SRY presence or absence, but the supporting cells within the developing gonad.
Urogenital Septum
| <html5media height="440" width="420">File:Urogenital_septum_001.mp4</html5media> |
GA Week 8
|
Mouse Gonad
Sertoli and Germ Cells - showing PGCs are not responsive to SRY signaling.
Sry Signaling[4]
- red - Sertoli cells, showing Fgf9 expression (following Sry expression FGF9 is a downstream signaling molecule).
- green - Germ cells and endothelial cells, showing PECAM expression.
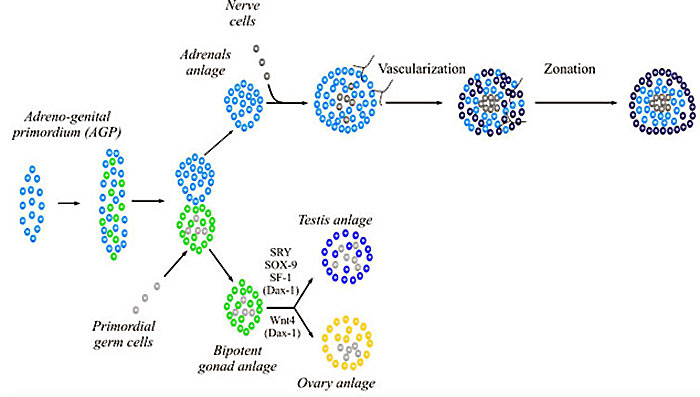
Genital Ridge (early embryo)
- green - gonadal supporting cells
- blue - Sertoli cells (male)
- pink - pre-granulosa cells (female)
RSPO1 R-Spondin Family, Member 1
Sex Development Genes
| Gene (OMIM) | Protein Function | Gonad Phenotype of Null Mice | Human Syndrome | |
| Bipotential gonad | ||||
| Wt1 | Transcription factor | Blockage in genital ridge development | Denys-Drash, WAGR, Frasier syndrome | |
| Sf1 | Nuclear receptor | Blockage in genital ridge development | Embryonic testicular regression syndrome | |
| Lhx9 | Transcription factor | Blockage in genital ridge development | a | |
| Emx2 | Transcription factor | Blockage in genital ridge development | a | |
| M33 | Transcription factor | Gonadal dysgenesis | a | |
| Testis-determining pathway | ||||
| Gata4/Fog2 | Transcription/cofactor | Reduced Sry levels, XY sex reversal | a | |
| Sry | Transcription factor | XY sex reversal | XY sex reversal (LOF); XX sex reversal (GOF) | |
| Sox9 | Transcription factor | XY sex reversal | Campomelic dysplasia, XX sex reversal (GOF) | |
| Sox8 | Transcription factor | XY sex reversal in combination with partial loss of Sox9 function | a | |
| Fgf9 | Signaling molecule | XY sex reversal | a | |
| Dax1 | Nuclear receptor | Impaired testis cord formation and spermatogenesis | Hypogonadism | |
| Pod1 | Transcription factor | XY sex reversal | a | |
| Dhh | Signaling molecule | Impaired differentiation of Leydig and PM cells | XY gonadal dysgenesis | |
| Pgdra | Receptor | Reduction in mesonephric cell migration | a | |
| Pgds | Enzyme | No phenotype | a | |
| Arx | Transcription factor | Abnormal testicular differentiation | X-linked lissencephaly with abnormal genitalia | |
| Atrx | Helicase | ND | ATRX syndrome | |
| Insl3 | Signaling factor | Blockage of testicular descent | Cryptorchidism | |
| Lgr8 | Receptor | Blockage of testicular descent | Cryptorchidism | |
| Hoxa10 | Transcription factor | Blockage of testicular descent | Cryptorchidism | |
| Hoxa11 | Transcription factor | Blockage of testicular descent | Cryptorchidism | |
| Amh | Hormone | No Müllerian duct degeneration | Persistent Müllerian duct syndrome | |
| Misrl1 | Receptor | No Müllerian duct degeneration | Persistent Müllerian duct syndrome | |
| Pax2 | Transcription factor | Dysgenesis of mesonephric tubules | a | |
| Lim1 | Transcription factor | Agenesis of Wolffian and Müllerian ducts | a | |
| Dmrt1 | Transcription factor | Loss of Sertoli and germ cells | XY femaleb | |
| Ovary-determining pathway | ||||
| Wnt4 | Signaling molecule | Müllerian duct agenesis, testosterone synthesis, and coelomic vessel formation | XY female (GOF) | |
| FoxL2 | Transcription factor | Premature ovarian failure | BPES | |
| Dax1 | Nuclear receptor | XY sex reversal (GOF) | XY sex reversal (GOF) | |
| RSPO1 | Signaling molecule | XX sex reversal (LOF) | XX sex reversal (LOF) | |
| Table Legend | ||||
|
a No mutations in human sexual disorders identified to date.
b Candidate gene for 9p deletion, XY sex reversal. | |||
| Table data modified[5] | ||||
Note new HUGO Gene Nomenclature Committee (HGNC)
- Male sex - SF-1 (NR5A1) Nuclear Receptor Subfamily 5, Group A, Member 1
- Female sex - DAX-1 (NR0B1) Nuclear Receptor Subfamily 0, Group B, Member 1
Stages
- Development of the indifferent gonad - (genital ridge) early embryo
- Differentiation of gonad - (testis or ovary) late embryo, defining event in sexual differentiation
- Differentiation of internal genital organs and ducts - late embryo to fetal
- Differentiation of external genitalia - fetal
- Development of secondary sexual characteristics - puberty
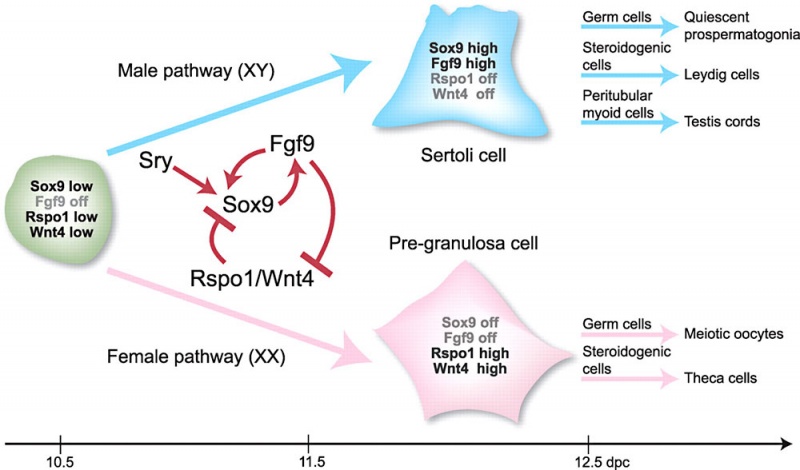
Male – Sertoli Cells
- Human SRY activation not known
- Mouse SRY activation - Wilms tumor 1 (Wt1), GATA binding protein 4 (Gata4), zinc fnger protein, fog family member 2 (Zfpm2), chromobox homolog 2 (Cbx2), mitogen-activated protein kinase 4 (Map3k4), insulin receptors.
- SRY activates key transcription factor SOX9 in Sertoli cells
- SOX9 requires positive regulatory loop
- Sox9 and FGF9 feed-forward loop upregulates Fgf9 expression
- repression of a WNT4
- Retinoic acid (RA) - required later for Sertoli to germ cells essential for adult mammalian spermatogenesis.
| Human SOX Family | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Female - Granulosa Cells
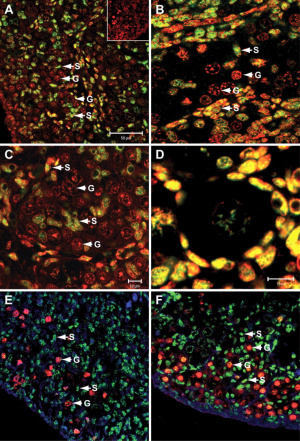
- Wnt4 (1p36.12) secreted protein, inhibit testis-specific processes
- mesonephros migration of endothelial cells
- repress steroidogenesis by Sf1 or blocking recruitment of steroidogenic cell precursors
- RSPO1 (1p34.3) secreted protein
- agonist WNT4 signalling
- FOXL2 (3q22.3) transcription factor
- repress Sox9 by synergistic interaction with estrogen receptors α and -β (ER-α-β)
- postnatal follicle development and female fertility maintenance
- FOXL2 gene are associated with syndromic and non-syndromic ovarian failure.
- Mutations in FOXL2 result in blepharophimosis/ptosis/epicanthus inversus syndrome (BPES).
| Human WNT Family | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Gonad Differentiation
Animations summarising the early morphological development of the male and female gonad.
| Male | Female |
|---|---|
| <html5media height="600" width="300">File:Testis 001.mp4</html5media> | <html5media height="600" width="300">File:Ovary_001.mp4</html5media> |
|
|
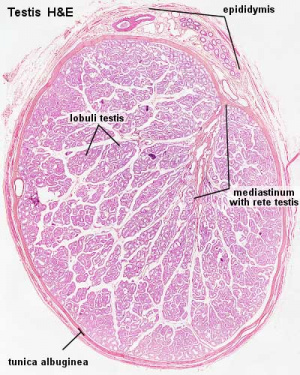
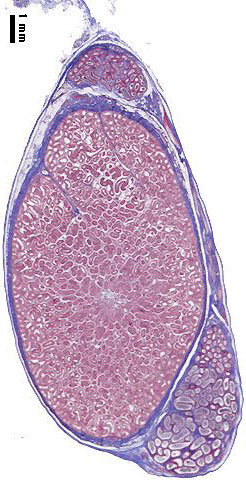
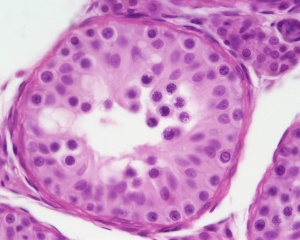
Testis
| Testis Pre-Puberty | Testis Post-Puberty |
|---|---|
|
|
|
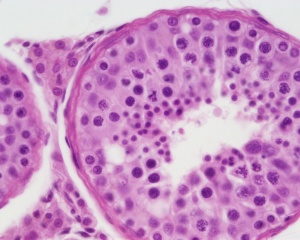
|
Ovary
Ovary Pre-Puberty
Internal Genital
Male
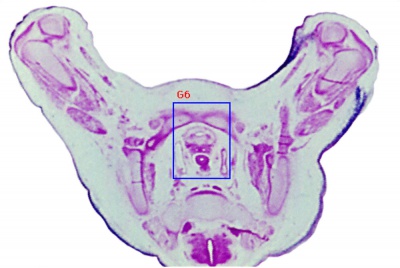
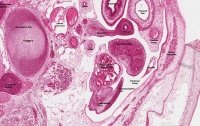
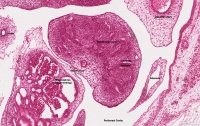
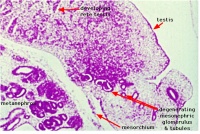
| Human Embryo (GA week 10, stage 22) pelvic level cross-section. | |
|---|---|
|
|
| G5 urogenital | urogenital |
|
|
|
| peritoneal cavity | rete tesits | G3 testis |
- week 8 - males Sertoli cells secrete anti müllerian hormone (AMH), which causes regression of the paramesonephric ducts between the 8th and 10th weeks.
- week 9 to 10 - gonadal cells begin to produce testosterone, which maintains the mesonephric ducts.
- mesonephric ducts go on to form the internal genital tract:
- rete testis
- ductuli efferentes
- vas deferens
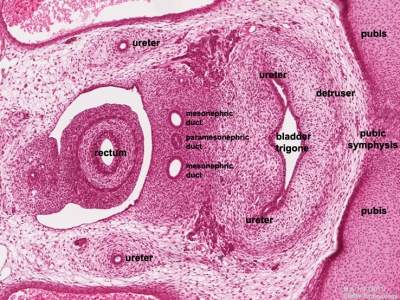
Trigone - note trigone does not include mesonephric contribution, though the ducts do shift location to open into teh male urethra.
<html5media height="380" width="450">File:Trigone_001.mp4</html5media>
Female
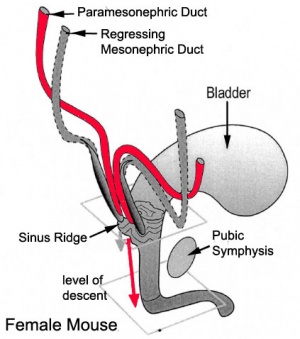
Mouse paramesonephric duct (Müllerian duct)[7] |
This mouse image shows the relationship between the mesonephric and paramesonephric ducts opening into the urogenital sinus.
|
| Female Uterus and Vagina (between GA week 11 and 22) | |
|---|---|
| <html5media height="500" width="490">File:Uterus_002.mp4</html5media> | Note - the entire vagina is formed from the paramesonephric (Müllerian) duct and does not have a contribution from the urogenital endoderm.
The uterus and broad ligament will eventulaly divide the pelvic cavity into two separate pouches.
|
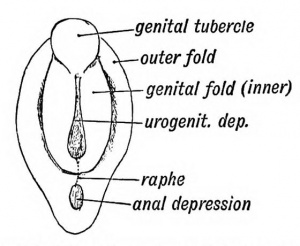
External Genital
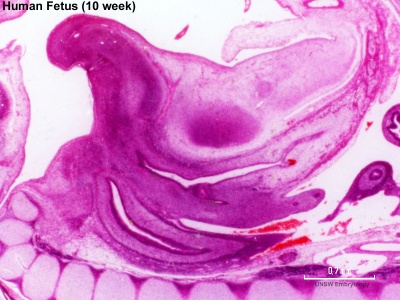
The unisex genital tubercle (GA week 12) is a major contributor to both sexes external genitalia.
| Male | Female |
|---|---|
| <html5media height="380" width="270">File:Male_external_001.mp4</html5media> | <html5media height="380" width="270">File:Female_external_001.mp4</html5media> |
| Animation showing the development of external male genitalia from the indifferent external structure ((GA week 11 to 14 approximately).
The reduction of Testosterone to active metabolite, 5α-dihydrotestosterone (DHT) is carried out by the enzyme 5α-reductase expressed in the region or male external genitaila and prostate gland. Note that there are several 5α-reductase isoforms that differ in both tissue distribution and kinetics.
|
Animation showing the development of external female genitalia from the indifferent external structure (GA week 11 to 14 approximately).
|
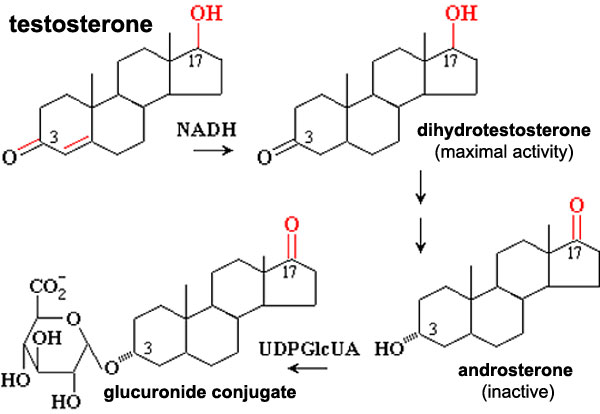
Testosterone metabolism
Biochemical pathway showing testosterone metabolism, to a more active form, then inactivation and conjugation for elimination.
5-alpha reductase - enzyme that convert testosterone into the more potent dihydrotestosterone (DHT).
Testes Descent
| <html5media height="400" width="600">File:Testis_Descent_001.mp4</html5media> | The animation shows the descent of the testes (between GA week 9 to 38, birth).
Descent of the testes into the scrotal sac begins generally during week 26 and may take several days.
Incomplete or failed descent can occur unilaterally or bilaterally, is more common in premature births, and can be completed postnatally. (see also cryptorchidism). |
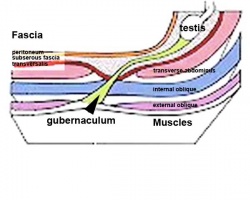 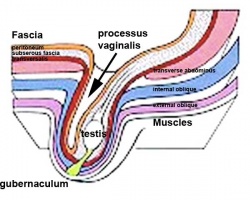
|
Data from a study of male human fetal (between 10 and 35 weeks) gonad position[8]
|
Human Embryo Development
These sagittal MRI scans of GA week 8-10 show overall embryo development during this period.
| Week 8 | Week 10 |
|---|---|
| <html5media height="500" width="400">File:Stage16 MRI S01.mp4</html5media> | <html5media height="600" width="500">File:Stage23 MRI S02.mp4</html5media> |
Kidneys
<html5media height="400" width="500">File:Nephron_development.mp4</html5media>| This animation shows the process of early renal (kidney) development.
Legend
| |
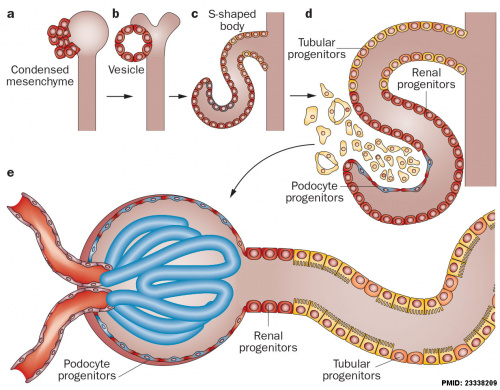
|
The morphological events and localization of renal progenitors occurring during nephron development in the adult human kidney.[9]
Mesenchymal cells near the tips of the branching ureteric bud undergo epithelial transition and differentiate through a series of forms made up of renal progenitors (red)
|
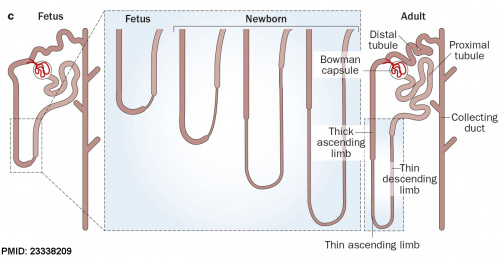
|
After nephron development has completed and concomitant with the development of the renal papilla in the newborn, the thin ascending limb of Henle’s loops is generated as an outgrowth from the S3 segment of the proximal tubule and from the distal tubule anlage of the nephron. (birds and mammals) |
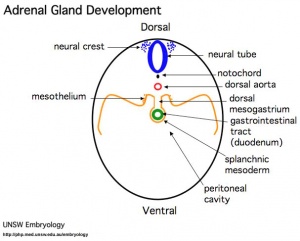
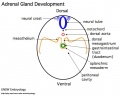
Adrenals
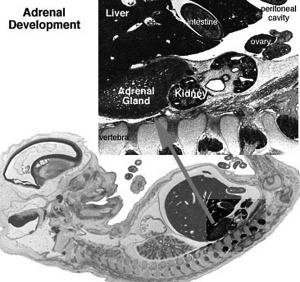
|
- Week 6 - fetal cortex, from mesothelium adjacent to dorsal mesentery; Medulla, neural crest cells from adjacent sympathetic ganglia
- Fetal Adrenals - fetal cortex later replaced by adult cortex
- Adult cortex - mesothelium mesenchyme encloses fetal cortex
Adrenal Cortex
- Late Fetal Period - differentiates to form cortical zones
- Birth - zona glomerulosa, zona fasiculata present
- Year 3 - zona reticularis present
|
Adrenal Medulla
|
|
| Adrenal gland development and steroid hormone synthesis | |
|---|---|
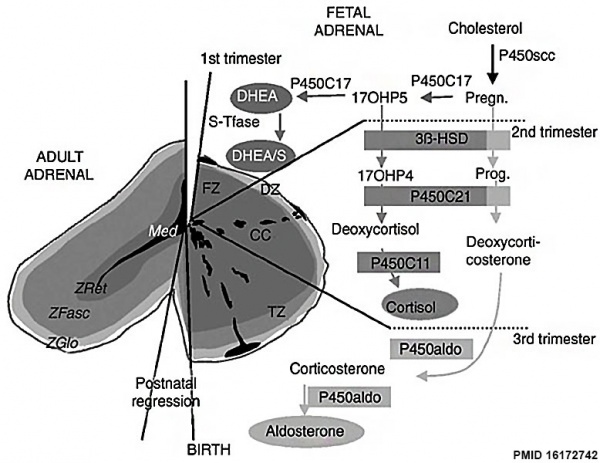
Overview cartoon of changes in adrenal gland structure and steroid hormone syntesis[10] |
Ontogenesis of steroidogenic enzymes in the human fetal adrenal gland. This schematic representation is divided into portions showing the fetal adrenal gland (right) at the first, second and third trimesters of pregnancy, and the adult adrenal gland (left). During the first trimester, the fetal gland is composed of a definitive zone (DZ, light grey) and a fetal zone (FZ, dark grey).
Fetal zone (FZ) - expressing the P450C17 cytochrome, is responsible for massive secretion of DHEA and DHEA/S, used by the placenta as estrogen precursors. Second trimester - chromaffin cells (CC, black) originating from the neural crests migrate through the fetal cortex to progressively colonize the center of the gland to form the future medulla (Med). Third trimester - the newly constituted transitional zone (TZ, medium grey) acquires the enzyme 3ß-HSD while the expression of P450C17 remains, thus allowing the production of fetal cortisol. Near birth, cells of the definitive zone which express only 3ß-HSD, acquire the P450aldo and begin to secrete mineralocorticoids such as aldosterone. Neonatal - the fetal adrenal regresses strongly (mainly due to the regression of the fetal zone) and recovers progressively during the first years of extra-uterine life. Adult - adult adrenal gland is composed of the zona glomerulosa (ZGlo, light grey), zona fasciculata (ZFasc , medium grey) and zona reticularis (ZRet, dark grey) responsible for the production of mineralocorticoids (aldosterone), glucocorticoids (cortisol) and androgens (DHEA-DHEA/S), respectively. |
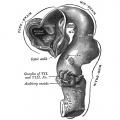
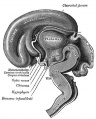
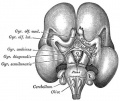
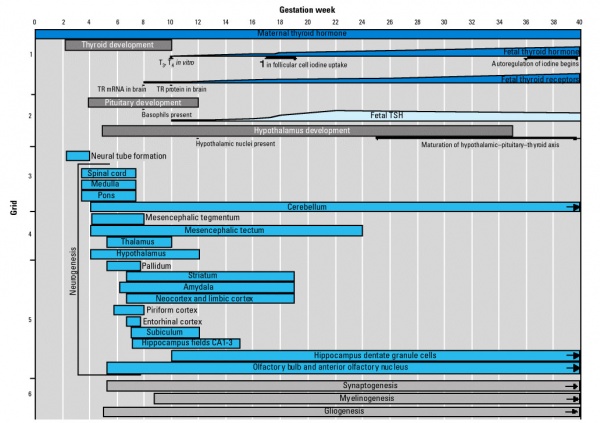
Hypothalamus
Hypothalamus is a neuroendocrine organ linking the brain to the endocrine system. There are also hypothalamic cells that hormone-responsive during development and in the adult. Recently there has been shift in describing neurological development from the traditional morphological model of “primary and secondary vesicles” with developmental origin and gene expression model “prosomeric development”
- Neuroectoderm - prosenecephalon then diencephalon after induction by the underlying prechordal plate.
- Prosomeric model - hypothalamus and telencephalon are part of the secondary prosencephalon
- ventro-lateral wall intermediate zone proliferation
- Mamillary bodies - form pea-sized swellings ventral wall of hypothalamus
• Sonic hedgehog (Shh) - initially expressed in prechordal plate, is essential for inductive process.
|
|
|
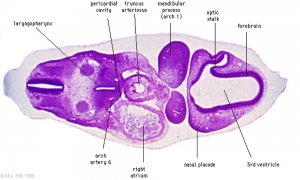
| Diencephalon region, shown by optic stalk (Stage 13) |
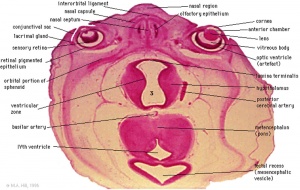
Late embryonic hypothalamus (Stage 22) |
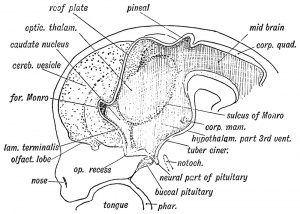
Hypothalamus embryonic position |
Hormones - Corticotrophin releasing hormone (CRH), Thyrotrophin releasing hormone (TRH), Arginine vasopressin (AVP), Gonadotrophin releasing hormone (GnRH), Growth hormone releasing hormone (GHRH), Somatostatin, Prolactin relasing factor (PRF), Dopamine
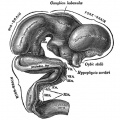
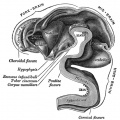
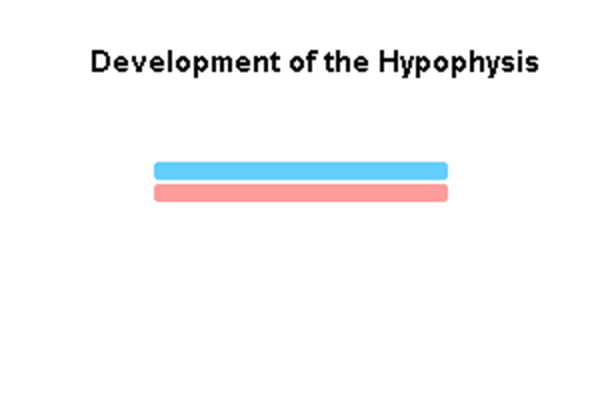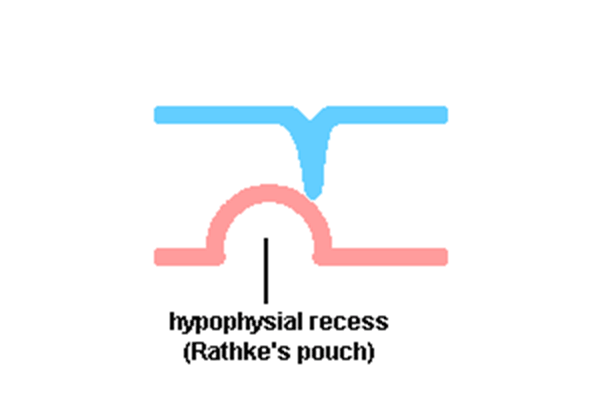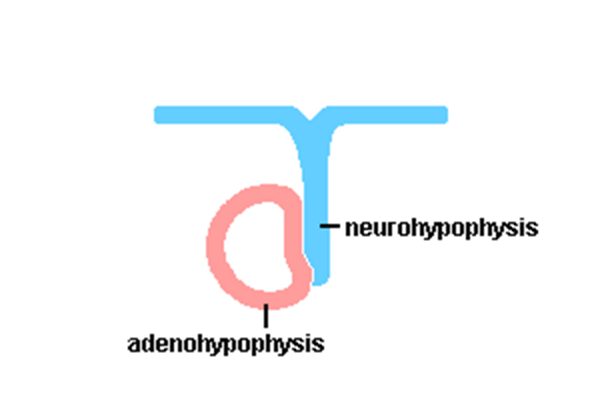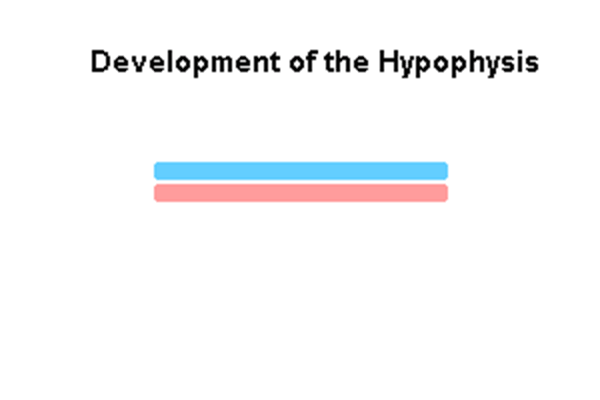
Pituitary
| Blue - neural tube ectoderm | Dual ectoderm origins
|
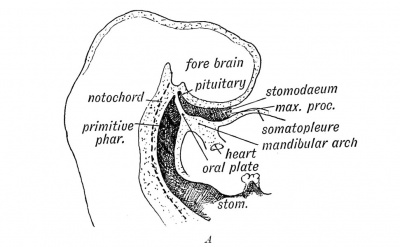
|
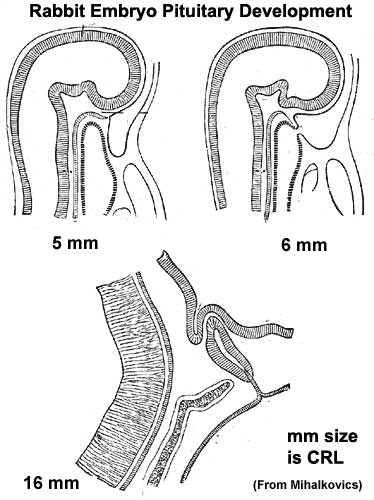
|
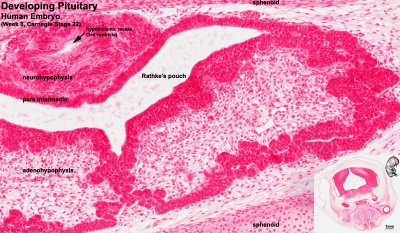
Adenohypophysis
- Anterior wall proliferates - pars distalis
- Posterior wall little growth – pars intermedia
- Rostral growth around infundibular stem – pars tuberalis
Neurohypophysis
- Infundibulum – median eminence, infundibulum, pars nervosa
Pituitary Timeline
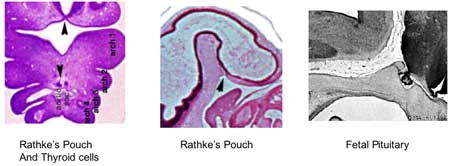
- Week 4 - hypophysial pouch, Rathke’s pouch, diverticulum from roof
- Week 5 - elongation, contacts infundibulum, diverticulum of diencephalon
- Week 6 - connecting stalk between pouch and oral cavity degenerates
- Week 8 - basophilic staining cells appear
- Week 9 - acidophilic staining cells appear
- Week 10 - growth hormone and ACTH detectable
- Week 16 - adenohypophysis fully differentiated and TSH increases to peak at 22 weeks
- Week 20 to 24 - growth hormone levels peak, then decline
- Birth - second TSH surge and decreases postnatally
Thyroid
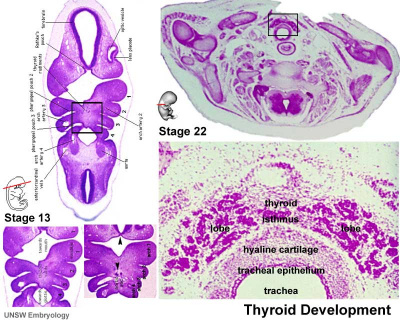
|
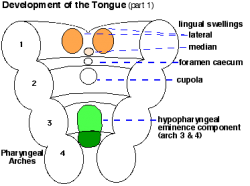
|
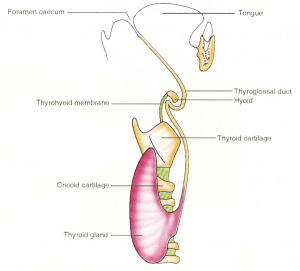
|
| Embryo Stage 13 and 22 thyroid (GA week 6 and 10) | Tongue foramen caecum | Thyroglossal duct |
- thyroid median endodermal thickening in the floor of pharynx, outpouch – thyroid diverticulum.
- tongue grows, cells descend in neck.
- thyroglossal duct - proximal end at the foramen caecum of tongue.
- thyroid diverticulum - hollow then solid, right and left lobes, central isthmus.
Thyroid Timeline
- GA week 6 - thyroid median endodermal thickening in the floor of pharynx, outpouch – thyroid diverticulum (FA 24 days)
- GA week 13 - colloid appearance in thyroid follicles, iodine and thyroid hormone (TH) synthesis. Growth factors (insulin-like, epidermal) stimulates follicular growth.
- Fetal TH - initial secreted biologically inactivated by modification
- GA 18-20 weeks - fully functional
- late fetal secretion develops brown fat
- Birth - TSH levels increase, thyroxine (T3) and T4 levels increase to 24 h, then 5-7 days postnatal decline to normal levels.
Maternal TH - iodine/thyroid status can affect development.
- studies show that both high and low maternal thyroid hormone can impact on neural development [11])
- Iodine deficiency - during prenatal period, leads to low fetal TH and neurological defects (cretinism).
Human thyroid system and neural development
Parathyroid - different embryonic origin
- Endoderm - third and fourth pharyngeal pouches, could also have ectoderm and neural crest
- 3rd Pharyngeal Pouch - inferior parathyroid, initially descends with thymus
- 4th Pharyngeal Pouch - superior parathyroid
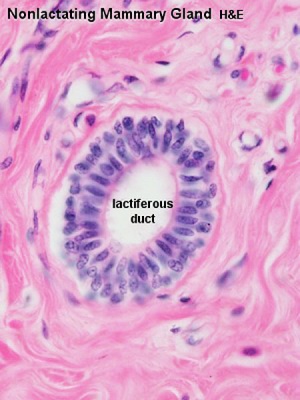
Breast
- Week 8 - epidermis placode down-growth into the dermis, modified sweat glands
- Key Molecular Factors - WNT10B, GLI3, [Hh
- epithelia/mesenchyme inductive interaction, mesenchyme forms connective tissue and fat
- mammary ridges - mammary bud formation, pair of ventral regions axilla to inguinal
- buds branch to form lactiferous ducts, only main duct formed at birth
- prior to puberty male and female glands the same
Lactiferous duct
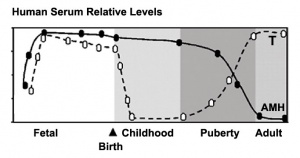
Puberty Changes
|
The Tanner scale is an anatomical staging system for measuring male/female sexual development at puberty. Stages were based upon genital and secondary sex characteristic development and named after James M. Tanner who, along with W.A. Marshall, published the stages for both girls (1969 [13]) and boys (1970 [14]). It is not a system for determining age. (More? Puberty Development | Tanner stages) |
References
- ↑ Molyneaux K & Wylie C. (2004). Primordial germ cell migration. Int. J. Dev. Biol. , 48, 537-44. PMID: 15349828 DOI.
- ↑ Barton LJ, LeBlanc MG & Lehmann R. (2016). Finding their way: themes in germ cell migration. Curr. Opin. Cell Biol. , 42, 128-137. PMID: 27484857 DOI.
- ↑ Denans N, Iimura T & Pourquié O. (2015). Hox genes control vertebrate body elongation by collinear Wnt repression. Elife , 4, . PMID: 25719209 DOI.
- ↑ Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R & Capel B. (2006). Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. , 4, e187. PMID: 16700629 DOI.
- ↑ Wilhelm D, Palmer S & Koopman P. (2007). Sex determination and gonadal development in mammals. Physiol. Rev. , 87, 1-28. PMID: 17237341 DOI.
- ↑ Duffin K, Bayne RA, Childs AJ, Collins C & Anderson RA. (2009). The forkhead transcription factor FOXL2 is expressed in somatic cells of the human ovary prior to follicle formation. Mol. Hum. Reprod. , 15, 771-7. PMID: 19706741 DOI.
- ↑ Drews U, Sulak O & Schenck PA. (2002). Androgens and the development of the vagina. Biol. Reprod. , 67, 1353-9. PMID: 12297555
- ↑ Sampaio FJ & Favorito LA. (1998). Analysis of testicular migration during the fetal period in humans. J. Urol. , 159, 540-2. PMID: 9649288
- ↑ Romagnani P, Lasagni L & Remuzzi G. (2013). Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol , 9, 137-46. PMID: 23338209 DOI.
- ↑ Chamoux E, Otis M & Gallo-Payet N. (2005). A connection between extracellular matrix and hormonal signals during the development of the human fetal adrenal gland. Braz. J. Med. Biol. Res. , 38, 1495-503. PMID: 16172742 DOI.
- ↑ Korevaar TI, Muetzel R, Medici M, Chaker L, Jaddoe VW, de Rijke YB, Steegers EA, Visser TJ, White T, Tiemeier H & Peeters RP. (2016). Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol , 4, 35-43. PMID: 26497402 DOI.
- ↑ Howard B & Ashworth A. (2006). Signalling pathways implicated in early mammary gland morphogenesis and breast cancer. PLoS Genet. , 2, e112. PMID: 16933995 DOI.
- ↑ Marshall WA & Tanner JM. (1969). Variations in pattern of pubertal changes in girls. Arch. Dis. Child. , 44, 291-303. PMID: 5785179
- ↑ Marshall WA & Tanner JM. (1970). Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. , 45, 13-23. PMID: 5440182
Cite this page: Hill, M.A. (2024, April 19) Embryology REI - Reproductive Medicine Seminar 2018. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/REI_-_Reproductive_Medicine_Seminar_2018
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G