Paper - The structure of the blastoderm, and the continuity of the cell-elements during the early stages of development (1916)
| Embryology - 16 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Cameron J. and Gladstone RJ. The structure of the blastoderm, and the continuity of the cell-elements during the early stages of development. (1916) J Anat. Physiol. 50(3): 207-27. PubMed 17233062
| Online Editor | |
|---|---|
| This 1916 paper by Cameron and Gladstone describes early human embryology during week 1 of development.
Modern Notes:
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Structure of the Blastoderm, and the Continuity of the Cell-Elements During the Early Stages of Development
By
J. Cameron, M.D., D.Sc., Professor of Anatomy, Dalhousie University, Halifax, Nova Scotia;
and
R. J. Gladstone, M.D., F.R.C.S., Senior Demonstrator and Lecturer on Anatomy, King’s College, University of London. (1916)
1. Introductory
The conception of a general continuity of the protoplasmic elements which form the tissues of all living organisms is by no means new. It preceded the “cell theory,” and although “ cells” have for many years been regarded as “independent units, each having its separate life—history,” there has always existed a considerable number of observers who, while not questioning the theory of separate cells, have described organic continuity of the cell-elements in the particular organ or tissue which they have specially studied. Thus Sedgwick (28) in Peripatus and in chick embryos, Grah m Kerr (17) in tracing the continuity of developing nerves with muse in Lepidosiren, Bernard in his researches on the structure of the retina, Studincka (30) and F. E. Schultze (26) in studying the structure of epithelial cells, have all been led to the same conclusion, namely, that there is a general continuity of the cell-elements in these tissues. Some of these writers, notably Sedgwick and Bernard, have extended the conception of continuity of the cell-elements in the tissues which they specially examined to continuity of the cell-elements in general.
Wilson (33) has pointed out and emphasised the very important fact that in Amphioxus and Echinoderms “the results of experiments on the early stages of cleavage are diflicult to explain save under the assumption that there must be a structural continuity from cell to cell.” This conclusion is supported by the work of Hammar (10) on the ova of Echinus.
More recently, Hardesty (11) in the nervous system, Godlewski (9) and M‘Gill (20) in muscle tissue, His (14) in epithelia, and Spalteholz (29) in the connective tissues, have demonstrated the continuity of the cellelements in these different forms of tissues. Notwithstanding the evidence brought forward by these and by many other observers, the theory that all living tissues are built up of independent units called “cells” is still almost universally held, and that the “cell” is in Briicke’s words “an elementary organism” (3).
In tracing the history of the cell, - theory which was promulgated by Schleiden (25) and Schwann (27) in 1838 and 1839, we find that Heitzmann (13), so long ago as 1873, contended that cell-division is incomplete in nearly all forms of tissue, and that even when cell walls are formed, they are traversed by strands of protoplasm, by means of which the cell bodies remain in organic continuity. The Whole body was thus conceived by him as a syncytium, the cells being no more than nodal points in a general reticulum, the tissues thus forming a continuous protoplasmic mass.
It has been conclusively demonstrated that in nearly all plant tissues the cell walls are traversed by delicate intercellular protoplasmic bridges, whilst in the case of animal tissues many observers have described the existence of similar bridges, one of the most familiar examples being the so-called “prickle cells” of the stratum Malpighi of the skin. These will be described later in detail when we come to deal with the structural features of epithelial tissues. A more important example is furnished by the protoplasmic connexions of the cell-elements of the corona radiata, not only with each other but actually with the ovum itself. This structural continuity, which has been already demonstrated by Flemming (8), Heape (12), and Retzius (23), is illustrated in fig. 1, which shows two stages in the evolution of the ovarian ovum of the cat. In the early stages (A) it will be noticed that the ooplasm is directly continuous with the cytoplasm of the surrounding cell-elements. This appearance is made more manifest by the fact that the ovum possesses no zona pellucida at this stage, though the continuity is still distinct even in the later stages (B). This observation is of the utmost importance in relation to the question of the inheritance of acquired characters, for it is not in agreement with the theory of the immutability of the germ plasm, postulated by Weismann (32), and upon which his theory with regard to the non—transmissibility of acquired characters has been largely founded. It seems to us that the organic continuity we have described between the germ plasm and the cytoplasm of the “somatic cells” must surely mean that the former is liable to be influenced by the latter, apart from the nutritive function, which is generally admitted. We hope to prove in a subsequent paper that the “germ cells” have a similar relation to the surrounding “somatic cells” in the male also.
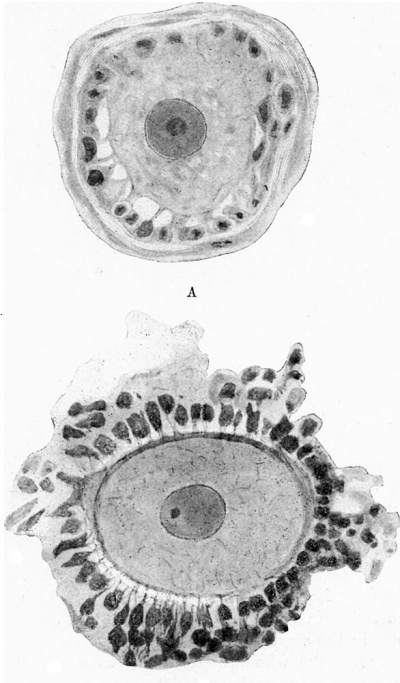
|
Fig. 1. Two stages illustrating the development of the ovarian ovum and Graafian follicle in the cat.
A - shows an early stage, before the complete development of the zona pellucida. It will be observed that the cytoplasm of the follicle cells is continuous, not only with that of the ovum, but a so with that of the adjacent cells of the same layer, and of the ovarian stroma.
B - later stage, in which it will be noted that the zona pellucida now forms a distinct boundary zone, but does not completely separate the ovum from the cells of the corona radiata. Processes from those cells pass through the zona pellucida, and are continuous with the cyto-reticulum of the ooplasm. |
It will be our aim in this memoir (to which the present paper is merely an introduction) to demonstrate that—
- It is the nuclei with their contained chromatic material which should be regarded as units, rather than the cell—elements.
- There is an organic continuity between the cell-elements of the connective tissues, the cell-elements of the central and peripheral nervous systems, and in certain epithelial layers.
- This continuity is in most cases primary, and not secondary.
- The so-called intercellular substance‘ forms an essential part of this continuous living tissue, which with its contained nuclei forms a plasmodium.
The connexion of cell-elements together by means of delicate filaments continuous with the cyto-reticulum and nuclear network of the individual cells was demonstrated in the retina by the late Mr H. M. Bernard (2). This author afterwards extended his observations to other tissues, and described a continuous network of linin threads, uniting and passing through the cell—elements. This he termed a protomitomic system. Further, he considered nuclei to be aggregations of chromatin at the intersections of the linin network.
Our views in the main accord with those of Bernard. Our observations, however, indicate that the mode of continuity between neighbouring cellelements is not necessarily through the linin network alone, but also by a direct continuity of the cytoplasm, which has existed from the very first.
2. The Early Ovum
It is well recognised that in the early stages of segmentation of amphibian and avian ova the division is incomplete. For example, the divisional planes in the segmenting cicatricula of the bird’s ovum do not involve anything like the whole thickness of the cicatricula; and what is more significant still, it may be noted that these planes do not extend peripherally into the periblast, as is well shown in the excellent microphotographs by Miss Blount in Lillie’s book on the development of the chick (18). fig. 2 is a drawing of a small portion of the ectoderm near the yolk wall, from a transverse section through a chick embryo, incubated eighteen hours. The section passes through the primitive plate, a. short distance behind the primitive groove, and shows, above, the ectoderm.
1 We do not regard as living material, calcareous matter deposited in the matrix of bone, or other inert substances eposited in the ectoplasm.
This consists of fairly well defined cell—elements, which are mapped off by thin partitions of darkly stained protoplasm, directly continuous with the cyto—reticulum of the adjoining cell-elements, and also with the protoplasm filling in the angular spaces between these. There are no clefts in these partitions separating adjacent “cells,” nor can the partitions be regarded as cement substance, for, as is clearly shown in the large underlying entodermal ce1l—elements, they are continuous with and have the same structure as the strands of the cyto-reticuluin or spongioplasm.
Fig. 2. is a drawing of a small portion of the ectoderm and entoderm, near the yolk wall, from a transverse section through an eighteenth-hour chick embryo. The section passes through the primitive plate, a short distance behind the primitive groove. The ectoderm is shown above. This consists of well-defined cell-elements separated by thin partitions of darkly stained protoplasm, which is directly continuous with the cyto-reticulum of adjoining “cells,” and also with the protoplasm filling in the angular spaces between the cell-elements. There are no clefts in these partitions, nor can the partitions be regarded as cement substance; for, as is clearly shown in the large underlying entodermal cell-elements, they are continuous with and have the same structure as the strands of the cyto-reticulum or spongioplasm. The _faintly stained entodermal cell-elements have a remarkable appearance in contrast with the more deeply stained ectodermal layer. They appear to be distended with a clear achromatic cytoplasm, almost identical in its physical characters with the nucleoplasm. After a careful study of this material we are convinced that 1l2.1S a protoplasm extruded from the nuclei in a nascent condition, in virtue of which it is achromatic in‘ its reaction to staining agents. Drawn under an apochromatic objective with compensating ocular x 7.
The faintly stained entodermal cell—elements in fig. 2 have a remarkable appearance, which contrasts with the more deeply stained ectodermal layer. They appear to be distended with a clear achromatic cytoplasm, almost identical in appearance with the nucleoplasm. After a careful study of this material, we are convinced that it is a protoplasm extruded from the nuclei in a nascent condition, in virtue of which it is achromatic in its reaction to staining reagents; This nascent non—staining cytoplasm can be demonstrated also in the entypy stage of the mouse embryo (fig. 10), where it exhibits itself in the form of clear achromatic zones immediately surrounding the nuclei or as clear refractile globules in the general protoplasm. This appearance is likewise characteristically shown in the deeper layers of the trophoblast of the chorionic villi (fig. 14). One of us has previously demonstrated the extrusion of this nascent achromatic cytoplasm from the nuclei of the developing retina (4 and 5) and brain (6).
Fig. 3. Segmenting ovum of Echinus esculemtus, sixteen-cell stage, showing blastomeres surrounded by a. continuous ‘ ‘ ectoplasmic layer,” which forms a connecting medium between the cells, and, in conjunction with these, constitutes a “ complete organism, " or “individual,” as distinguished from a “cell-colony,” united by an extraneous “cement substance.”
The continuity of the cell-elements of the morula mass may also be observed in invertebrate ova. For example, fig. 3 is a drawing which one of us executed whilst studying the fertilisation and segmentation of the ova of Echinus esculentus at the Millport Marine Biological Laboratory in 1903. In the morula stage of development of this species it can be clearly recognised that the blastomeres are held together by a thin layer of homogeneous protoplasm which exhibits itself as a very delicate achromatic zone surrounding the periphery of the morula mass. Hammar (10) has, moreover, shown that in another species of Echinus (E. mili/arts) the segmenting ova possess what he terms an ectoplasmic layer which not only surrounds the blastomeres, but can also betraced deeply between these elements. He was able to demonstrate this “ectoplasm” in ova from the two—cell stage up to that of the blastula, and he regards it as establishing a structural continuity between the-cell-elements, and forming an essential part of the organism. The achromatic perimorular zone shown in fig. 3 thus clearly corresponds to the ectoplasm described by Hammar in Echinus miliamls, and we consider it to be a vital constituent of the organism, and by no means‘ the inert substance it is generally considered to be. It probably forms the medium through which one blastomere influences another during development.
Fig. 4. Frog’s ovum, showing an early stage in segmentation. The segmentation is incomplete, and the cell-elements at the “animal pole” are seen to be continuous with the deutoplasm of the “ vegetative pole,” in which the lines of cleavage are only just visible.
It is a well-known fact that in centrolecithal ova, segmentation is incomplete, and it has been shown by Morin (22) that the daughter nuclei which at first lie in the middle of the ovum, subsequently migrate freely towards the surface along the radiating strands of the protoplasmic reticulum.
This direct structural continuity of the cell-elements of the morula. mass appears to have been recognised by Wilson (33), who in his standard book on The Cell makes the following significant statement: “The unity of the embryo is not caused by a mere juxtaposition of the cells . . . this unity is physiological, and the facts point towards the conclusion that there must be a structural continuity from cell to cell, which is the medium of coordination.” It follows that if this structural continuity be broken or interrupted by mechanical displacement of the blastomeres, the unity of the organism is likewise broken up.
One of the most convincing proofs of the direct structural continuity of the blastomeres is shown by the following experiments. Roux (24), in an extensive series of articles covering the period between 1883 and 1903, elaborated a theory of development known as the germinal localisation theory of self-differentiation. According to this, certain portions of the ovum are early set apart for the formation of a definite organ or tissue of the embryo. The result of this idea is that by the time the morula stage is reached certain groups of cells are set aside for this purpose. It is evident, then, that according to this theory each of the two primary blastomeres ought to produce a hemi—embryo, and Roux at first considered that he had succeeded in producing from the blastomeres of frogs ova such a hemi—embryo, passing through the stages of hemi—morula, hemiblastula, and hemi-gastrula.
Subsequent experiments by Wilson (33) and Morgan (21) on Amphioxus, and J enkinson (16) on the frog, gave results diametrically opposed to those of Roux. They found that if the two primary blastomeres were completely separated from one another, each produced a complete embryo, though only half the size of the normal. Even a quarter blastomere was found to give rise to a normal blastula, gastrula, and sometimes even to the stage of an embryo, but only one-quarter the normal size. An eighth blastomere produced a morula, but this did not gastrulate. finally, a one-sixteenth blastomere produced merely an irregular clump of cells. Further experiments by Driesch (7), Jenkinson (16), and others on the ova of Echinus gave almost identical results.
The conclusion one arrives at as a result of these foregoing experiments is, that if the two primary blastomeres did normally separate from one another completely, each ‘ought always to give rise to a complete embryo in the natural process of development, but of course this is not so. The only inference, then, is that the continuity of the protoplasm of the two primary blastomeres is not completely severed, and this permits some mutual influence or interaction between the two, the result being a single complete embryo.
On studying later stages in the segmentation of the amphibian ovum, we discovered that the cyto-reticulmn of the blastomeres could be traced through the cleavage planes as a direct connexion between the cellelements. This appearance is rendered more striking by the fact that these strands cross the above-mentioned planes at right angles (fig. 5). We thus found a direct continuity in the morula stage which presumably must have resulted from a ol2'm'sio/n of nuclei in a mass of protoplasm the whole constituting a plaswwdium. The latter term is the only one which appears to us to meet the case, and we will therefore continue to employ it throughout this paper. It is certainly a more accurate title than syncytium, a term which suggests that the cell-elements were at one time separate and had become fused together secondarily. We hold, that protoplasmic continuity must have existed from the very first.
Fig. 5. Later stage in the segmentation of a frog’s ovum. The divisional planes between the cell-elements are now distinctly visible, and in the section a pear as a protoplasmic network surrounding the cells, and characterised by a rich eposit of pigment granules. The cyto-reticulum of the enclosed cells is also pigmented, and its filaments will be seen to be continuous, not only with the pigmented planes of intercellular protoplasm, but also with the cyto-reticulum of neighbouring cell-elements.
The protoplasm of the blastodermic plasmodium can be conveniently divided into two kinds. That immediately surrounding the nuclei constitutes the endoplasm, and is of course readily recognisable. It is not so easy, however, to recognise the second kind, which we have decided (by contrast) to term the ectoplasm. The latter Will be found not only to intervene between the cell-elements,-but also to form a continuous zone surrounding the whole blastula mass. If this ectoplasmic laiyer increase in amount, the cell-elements become more or less completely separated; but even then it still forms,a connecting medium. At this point We wish to emphasise the importance of recognising this differentiation of the proto.-. plasm of the blastula mass into a clear homogeneous ectoplasm and a perinuclear endoplasm; for we hope to be able to demonstrate subsequently a similar process of differentiation during the stages of development of practically all the tissues of the body, which will be found to constitute an important phase in their life-history. Thus, if one studies tissue-ontogeny from this new standpoint, one will be provided with remarkable evidences of the persistence of this primitive plasmodial condition even in the adult, the result of which, We venture to hope, will be a much more simplified conception of the histological structure of the body, than that provided by the cell theory, as usually understood. In the blastula stage of mammals the separation of the cell-elements is also incomplete, and divisional lines are often absent altogether.
Fig. 6. is a microphotograph of a section through the free blastuln. of a mouse, which is at one part of its circumference becoming attached by fibrinous material to the uterine mucosa. The cell-elements are incompletely separated from one another. Some of the nuclei are undergoing karyokinesis and appear to be imbedded in a continuous protoplasmic matrix. The wall of the blastula must therefore be regarded as a. “ plasmodium,” since the dividing nuclei are contained in a continuous matrix, and are not situated in separate “ cells.” From a specimen kindly lent by Professor Dendy. The section was stained with “ picro-indigo-carmine.”
fig. 6, which represents a. critical stage in the development of "the blastoderm of the mouse, simply shows a. nucleated layer of protoplasm enclosing the cavity of the blastocyst, and constituting its wall. It will be observed that the outlines of the individual cell-elements, if visible at all, are only very imperfectly shown, so that there is an obvious continuity of the protoplasm. The chromatin of some of the nuclei is in a‘ disturbed condition (fig. 6), indicating that they are in one of the stages of karyokinesis. After studying this microphotograph one fails to recognise any resemblance to the conventional diagrams of the blastodermic vesicle which have been handed on from text—book to text-book. To represent cells sharply marked off from one another by strongly marked boundary lines, is to give to students of embryology an inaccurate impression of the structure of the blastoderm, and it is to be hoped that this conventionality will soon be finally disposed of. This continuity is well maintained in the entypy stage of the mouse embryo, as shown infigs. 8,’ 9, and 10. We have represented in fig. 10 a very highly magnifiedyiew of a group of the cell-elements shown in fig. 9. At first sight these appear to be mapped off from each other by divisional planes in the most definite manner. A more intimate examination clearly shows, however,.that this appearance is due to the differentiation of the protoplasm of the plasmodium into ectoplasm and nascent endoplasm. The latter forms the very definite zone immediately surrounding the nuclei, whilst the ectoplasm which at first sight appears to constitute the “partitions” between the individual cell-elements forms one continuous bond of union for the whole mass.
Fig. 7. is a higher-power view of the blestuls shown in the previous figure in order to demonstrate its structure more clearly.. Drawn under an spochromstic objective with compensating ocular x 7.
Uterine wall.
Fig. 8. is a low-power view of the entypy stage of a mouse embryo, which will be recognised in the centre of the microphotograph.
Take another example from a higher type of mammal. The plasmodial structure of the blastoderm has been very clearly represented by Hubrecht (15) in his well-known figure of the blastocyst of Tarsius spectrum
Fig. 9. is a high-power view of the entypy stage shown in fig. 8.
(fig. 11). In this both the “formative cell mass” and the trophoblast appear to be a plasmodium. The fluid contained within. the blastocyst seems to be a secretion from its plasmodial wall. The wall itself is represented as being mainly composed of a mass of protoplasm containing nuclei one of which is shown in the act of dividing. Here and there in the figure are cell—elements which have become mapped off in a greater or lesser degree from the general plasmodium.
In the blastocyst of the bat, as figured by van Beneden (1), the contained fluid appears first in the form of small vacuoles Within the plasmodial mass. As these increase in size they coalesce.to form the fluid filling the central cavity.
Fig. 10. Portion of the endoderm and underlying ectoderm from the preceding specimen. The nuclei, some of which are undergoing mitosis, are seen to contain a clear nucleo-plasm. In the surrounding protoplasm is a similar clear material which appears to have been extruded in dro lets from the nuclei, and sometimes forms a clear zone surrounding the nue ear membrane. This clear rotoplasm we speak of as “ endoplasm,” and we believe it to be recently formed, 13.9. in a nascent condition. The surrounding granular protoplasm is stained, and forms a continuous matrix. This granular protoplasm corresponds to the ‘ ‘ ectoplasm,” and it will be noted that where it forms a partition between adjacent nuclei, there is no indication of this partition consisting of two cell membranes lying in contact with one another, nor is there any indication of a space separating adjacent cell—elements. Drawn under an § apochromatic objective with compensating ocular x 7.
The relation of the two central cavities to the mesenchyme invthe Teacher-Bryce (31) ovum also suggests that in the human subject the cavities of the blastocyst are formed within a plasmodium much in the same way as in lower mammals.
3. Continuity of the Ectoderm, Mesoderm, and Endoderm
A study of the three-layered condition of the embryo shows that there still exists at this stage a direct continuity of the cell-elements. Those of the mesoderm usually exhibit a multipolar character, and it has been taught that this is due to an outward growth of processes from originally rounded cells which unite with those of neighbouring cells to form a network, or, in other words, a secondary continuity (fig. 12). A close examination of this stage of embryonic development shows, however, that these processes have been in direct continuity with one another from the very outset, the connecting strands of protoplasm being drawn out as a result of the separation of the nuclei. Moreover, the processes of the mesodermic cell-elements lying next the ectoderm and endoderm are frequently seen to be in direct continuity with the protoplasm of these, and all three layers are distinctly continuous with one another atthe primitive streak. Here also the cell-elements are imperfectly separated from one another, so that the actively growing tissue in this region must therefore be regarded as a plasmodium.
Fig. 11. is a drawing of I-Iubrecht’s well-known figure of the blastecyst of Tarsius spectrum. In this, both the formative cellmass and the trophoblast appear to form a plasmodium.
On tracing the ectoderm outward from the primitive streak the cellelements certainly do appear to be marked off from one another by definite divisional planes. Upon closer examination, however, they are seen to be connected and held together by a thin ectoplasmic layer on the surface. This is most readily visible in the angles between the cell-elements, as in the entypy stage of the mouse embryo. It is probable that these elements are also connected with one another laterally, for they hold closely together, and are not separated by the action of the reagents employed for fixing the tissue, as they most certainly would be if the cells were merely in a condition of contiguity instead of organic continuity. One can thus trace this ectoplasmic bond of union from the early developmental stages. Moreover, the intercellular material spoken of by histologists as inert “cement,” and described by them as a secretion of the cells, we interpret as ectoplasm, and thus as an integral part of the living tissue.
Fig. 12. Transverse section through the primitive goove, and three primary layers of a chick embryo, incubated eighteen hours. The illustration shows one lip of the groove on the left-hand side; the ectoderm (ect.) is above, the endoderm (end.) below; between these two layers is the mesoderm, which consists of irregular strands of protoplasm connected by bridges of varying thickness, and containing nuclei, some of which exhibit karyokinetic figures. The cell-elements of the mesoderm are not only continuous with one another, but are continuous also with the ectoderm and endoderm. The continuity of the cell-elements of the mesoderm appears to be primary, for it will be noted that those nuclei which show mitotic figures (see nucleus to left in illustration) are situated in thickenings of the general reticulum, and are usually connected by protoplasmic bridges with several of the neighbouring nuclei. They do not appear to us to conform to the usual description, namely, that isolated cells of a rounded form wander out between the ectoderm and endoderm, and afterwards send out rotoplasmic processes which join to form a reticulum. We seldom find isolat cells undergoing karyokinesis, either at the growing edge of the mesoderm or in any part of its extent between this and the primitive streak. Where such isolated cells are present, we believe that the protoplasmic bridges which have connected them with the neighbouring cell-elements from which they have originated have been broken in the “preparation of the specimen, and the “cell” thus liberated has assumed a round form.
Blood vessel.
Fig. 13. Section through a villus from the chorionic vesicle of a 9-mm. human embryo. The mesenchymatous core consists of a scanty meshwork of lightly stained protoplasm with nuclei at the nodes, and continuous externally with the granular protoplasm of the surface layers. The mesenchyme of the villus is also in direct continuity with the walls of the developing blood-vessels, one of which is shown cut transversely, the other longitudinally. Drawn with a 5 objective.
The behaviour of the plasmodium during the three-layered stage ex emplifies an interesting tendency it is constantly displaying, namely, the surface cell-elements always arrange themselves in more or less definite layers, thus giving rise to the so-called ectoderm and entoderm. Another point which has impressed us strongly during these observations is, that the mesoderm is not produced wholly by a proliferation of the cell-elements in the region of the primitive streak, but also by.a multiplication of the nuclei throughout the general plasmodial mass; that is to say, a generalised instead of an entirely localised proliferation. fig. 12 exhibits this generalised multiplication of the nuclei during the three-layered stage.
Fig. 14. A portion of the surface layer of the villus shown in fig. 13 drawn under an § apochromatic objective with compensating ocular x 7. The drawing shows an outer layer of oval, darkly stained nuclei, imbedded in a granular >rotoplasm. Next to this nuterplasmodial stratum is “ Langhans’ layer,” the nuclei of which are approximately spherical and less deeply stained. They are surrounded by a clear zone of unstained protoplasm similar in appearance to the clear nucleoplasm. This perinuclear protoplasm we speak of as nascent cndoplasm, neighbouring zones of which are separated from one another by septa of granular stained proto lasm continuous externally with that of the outer layer, and internally wit the mesenchymatous core. These septa at first give one the im ression of separate cell-elements, but a closer examination shows that there is direct structural continuity throughout the specimen.
4. Plasmodial Structure of a Placental Villus
The central core of the villus (fig. 13) corresponds to the mesenchyme. It consists of a delicate protoplasmic network with nuclei at the nodes, and thus exhibits the typical plasmodial structure. The surface of the villus (shown highly magnified in fig. 14) exhibits the characteristic tendency referred to in the previous section towards a layering of the cell-elements; Thus it shows an inner single stratum—the “absorptive layer of Langhans ”—whilst the outermost coating permanently maintains its plasmodial character, constituting the “ syncytium” of the chorionic villus. The plasmodial structure of this tissue has never been questioned, and has, in fact, been recognised for years. Still later, Langhans’ layer merges with the syncytial coating of the villus, which then becomes spread out to form a single stratum of nucleated cell-elements connected at certain points with the protoplasm of the decidua basalis, and forming with it a continuum. The core of the villus consists of the typical mesenchymatous network. In the trabeculae of the meshwork are spaces Which, as we hope to describe later, develop into the blood-vessels of the villus. fig. 13 shows two of these primitive blood-vessels, one cut transversely, the other longitudinally. An examination of figs. 13 and 14 will show that the cellelements covering the surface of the villus are in direct continuity with the mesenchymatous tissue of the core. The chorionic villus in all its features thus affords an instructive study of the structural continuity of developing tissue.
Fig. 16. Section through the placenta of a 7-mm. guinea-pig embryo. The'illustration shows part of a trabecula between two of the cotyledons. It consists of a plasmodisl network, in the substance of which large oval nuclei are imbedded. Round each nucleus is a zone of lightly stained protoplasm, similar to the endoplasm surrounding the nuclei of Langhans’ layer in the chorionic villi. Drawn under an § apochromatic objective with compensating ocular x 7.
The maternal portion of the placenta likewise exhibits very strikingly its plasmodial character. The tissue of the decidua basalis becomes greatly hypertrophied, and indeed reverts to its primitive plasmodial condition. Fig. 15 shows this characteristic tissue in process of being transformed into maternal blood sinuses.
5. Summary and Conclusions
- In the developing blastoderm the nuclei with their contained chromatic material ought to be regarded as the structural units rather than the cell-elements as a Whole.
- There is an organic continuity between the cell-elements of the developing blastoderm both in vertebrates and invertebrates. In the present paper We have traced this continuity up to the three—layered stage in mammals and in the chick embryo, in the chorionic villi and in the placenta.
- This continuity is in most cases primary, and not secondary (plasmodial rather than syncytial).
- Protoplasm may be differentiated into endoplasm and ectoplasm. The nascent endoplasm forms a clear, highly refractile zone immediately surrounding the nucleus. This merges into a more mature endoplasm, which in its turn undergoes transition into a granular ectoplasm.
- The nascent endoplasm, the more mature endoplasm, and the ectoplasm, represent three stages in the genesis of protoplasm. It is a well-recognised fact that the protoplasm of every living tissue has a limited period of activity during which its vitality is constantly being revived and rejuvenated by regulated supplies of nascent material. The latter is apparently a derivative of the nucleus, and is discharged from this in the form of nascent endoplasm. It would appear, therefore, that nutritive material ingested by the cytoplasm receives its final elaboration in the nucleus. From this standpoint we would argue further that the nascent endoplasm possesses the greatest activity, whilst the functions of the ectoplasm are more of a passive nature and are mainly in the direction of maintaining structural continuity between neighbouring cell-elements.
- The ectoplasm corresponds to the so-called intercellular substance, and forms an essential part of the continuous living tissue which with its contained nuclei constitutes a plasmodiam.
References to Literature
(1) VAN BENEDEN, E., “Recherches sur les premiers stades du développement du Murin,” Anat. Anz., xvi., 1899.
(2) BERNARD, HENRY M., Some Neglected Factors in Evolution, G. Putnam and Sons, 1911. Also in Quart. Journ. Micros. Science, vols. xliii. to xlvn. 61
(3)BRUCKE, C., “ Die Elementarorganismen,” Wiener Silzben, x1iV., 18 .
(4) CAMERON, J., Journ. of Anal, 1905.
(5) CAMERON, J., Journ. o_f'Anat., 1912.
(6) CAMERON, J., Brain, 1906.
(7) DRIESCH, H., Arch. Enl. 11[ech., x., 1900, and xvi., 1903.
(8) FLEMMING, W., Zellsubslanz, Kern, u. Zelltheilung, Leipzig, 1882.
(9) GODLEWSKI, Archivf. Mihro-Anat., Bd. 121., 1902.
(10) HAMMAR, J. A., “ Ueber eine allgemein vorkommende priméire Protoplasma verbindnng zwischen den Blastomeren,” Arch. f. Micro-Anat., Upsala, xlvii. p. 14, and xlix.
(11) HARDESTY, J., Amer. Journ. of Anal, vol. iii., 1904.
(12) HEAPE, W., Quart. Journ. Jlllcros. Sci, vol. xxiii., 1883.
(13) HEITZMANN, J., .Sz'tz. d. /r. Ahaol. Wiss. Wien, xlvii.
(14) HIS, WILHELM, “Ueber Syncytien, Epithelien, u. Endothelien,” Verh. Ges. d Nalfi Leipzig/, L.\'.\’1I., ii. 2, 1901 (273-276).
(15) HUBRECHT, A. A. W., Verhand. d. Kane”/Lklz'jhe A/cad. van Wetterschappen te Amsterdam, viii., 1902.
(16) JENKINSON, J. \V., E';cpe1‘i7ne7ltal Embryology, 1909.
(17) KERR, GRAHAM, Trans. Roy. Soc. Il'din., vol. x1i., Part I.
(18) LILLIE, F. 11., Development of the Chick, 1908, pp. 38-52.
(19) McBRIDE, E. W., Te.ct—l;oo/c of Embrg/olog_'/, V01. i., 1914.
(20) McGILL, CAROLINE,, Internal. Zllonatsschrlft. Anal. u. Phg/siol., Bd. xxiv., 1907.
(21) MORGAN, T. 11., Anal. Anz., x., 1895, and Arch. Enl. M€C]L., xix., 1905.
(22) MORIN, Development of Astacus, Korschelt & Heider.
(23) RETZIUS, G., “Die Intercellularbriicken des Eierstockeies, und der F0l1ike17.e11en,” Verh. Anal. (}es., 1889.
(24) Roux, W., Vlrchow’s Archiv, 1889.
(25) SCHLEIDEN, M. J., “ Beitmge zur 1’hytogenesis,” ]l[dller’s Archiv, 1838.
(26) SCHULTZE, F. E., Sz'tznngsI;erz'chte der Kiln. Pr. Alcad. der Wissenschaflen zn Berlin, 1896.
(27) SCHWANN, TH., All/rros. Untersnch. fiber (lie Uebereinstimmung in der Strnlclur nnd dem Wachslxzmv (ler Tie/‘e und Pfianzen, Berlin, 1839.
28) SEDGWICK, ADAM, “On the Inadequacy of the Cellular Theory of Developments,” ( uart. Jonrn. 11/1,'c7'os. Sci, xxxvii.
(29) SPALTEHOLZ. W., Ver/mzmll. Anal. (lesellsch, Rostock, 1906.
(30) STUDINCKA, F. K., Site. (ler /r. hlihm. (Ies. dew 1Viss. in Pmg, 1897.
“Ueber Stachzellcn und sternfiirniige Zellen in Epithelien,” S1512. der /c. D()7L7ll. Ges. rlcr 1Vi.s-5. in I’7‘a5/, 1902.
(31) TEACHER and BRYCE, The Early Development and Imbedding of the Human Ovmn, Glasgow, 1908.
(32) WEISMANN, A., The G1/I‘lIL Plasm, transl. by W. N. Parker, 1893.
(33) WILsoN, E. B , The Cell in Development and Inheritance, 1906, pp. 58-61.
Reference
Cameron J. and Gladstone RJ. The structure of the blastoderm, and the continuity of the cell-elements during the early stages of development. (1916) J Anat. Physiol. 50(3): 207-27. PubMed 17233062
Cite this page: Hill, M.A. (2024, April 16) Embryology Paper - The structure of the blastoderm, and the continuity of the cell-elements during the early stages of development (1916). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_structure_of_the_blastoderm,_and_the_continuity_of_the_cell-elements_during_the_early_stages_of_development_(1916)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G

