Paper - The history of the prochordal plate in the rabbit: Difference between revisions
mNo edit summary |
m (→References) |
||
| (19 intermediate revisions by the same user not shown) | |||
| Line 4: | Line 4: | ||
! Online Editor | ! Online Editor | ||
|- | |- | ||
| [[File:Mark_Hill.jpg|90px|left]] This historic 1931 paper describes very early rabbit development during | | [[File:Mark_Hill.jpg|90px|left]] [[File:James Peter Hill.jpg|100px|right|alt=James Peter Hill|link=Embryology History - James Hill|James Peter Hill]] | ||
This historic 1931 paper describes very early rabbit development during the early period of gastrulation and somitiogenesis. | |||
Note that Aasar used rabbit histological material from the [[Hill Collection]]. | |||
<br><br> | <br><br> | ||
'''Modern Notes:'''{{rabbit}} | '''Modern Notes:''' {{rabbit}} | ||
<br><br> | <br><br> | ||
{{Rabbit_Links}} | {{Rabbit_Links}} | ||
| Line 17: | Line 22: | ||
Honorary Demonstrator, Department of Anatomy and Embryology, University College, London | Honorary Demonstrator, Department of Anatomy and Embryology, University College, London | ||
1931 | |||
* Thesis approved for the degree of Master of Science in the University of London. | |||
==Introductory== | ==Introductory== | ||
This work has been carried out under the supervision of [[Embryology History - James Hill|Prof. J. P. Hill]] to whom my sincere thanks are due, not only for his great help and criticism but also for allowing me access to the valuable series of {{rabbit}} embryos in his collection. | |||
In all, ninety embryos, most of which are in a very good state of preservation, | In all, ninety embryos, most of which are in a very good state of preservation, have been examined. The age, taken as the time lapsing between insemination and the removal of the embryo, ranges from eight to eleven days, but where possible the somites have been counted, and their number has been taken as indicating the degree of development. This method, however, does not seem to me to be the most exact. In fact the best criterion is a general survey of the degree of development of the structures present. The majority of the embryos were cut transversely, but a number were cut longitudinally. These have been of special value, and have enabled me to confirm the reconstructions made of transversely cut embryos. | ||
have been examined. The age, taken as the time lapsing between insemination | |||
and the removal of the embryo, ranges from eight to eleven days, but where | |||
possible the somites have been counted, and their number has been taken as | |||
indicating the degree of development. This method, however, does not seem | |||
to me to be the most exact. In fact the best criterion is a general survey of | |||
the degree of development of the structures present. The majority of the embryos were cut transversely, but a number were cut longitudinally. These have | |||
been of special value, and have enabled me to confirm the reconstructions | |||
made of transversely cut embryos. | |||
==Descriptive== | |||
I propose to describe shortly the stages illustrating the history of the | I propose to describe shortly the stages illustrating the history of the prochordal plate. The description will be made clearer by reference to the accompanying series of photomicrographs and reconstructions. | ||
prochordal plate. The description will be made clearer by reference to the | |||
accompanying series of photomicrographs and reconstructions. | |||
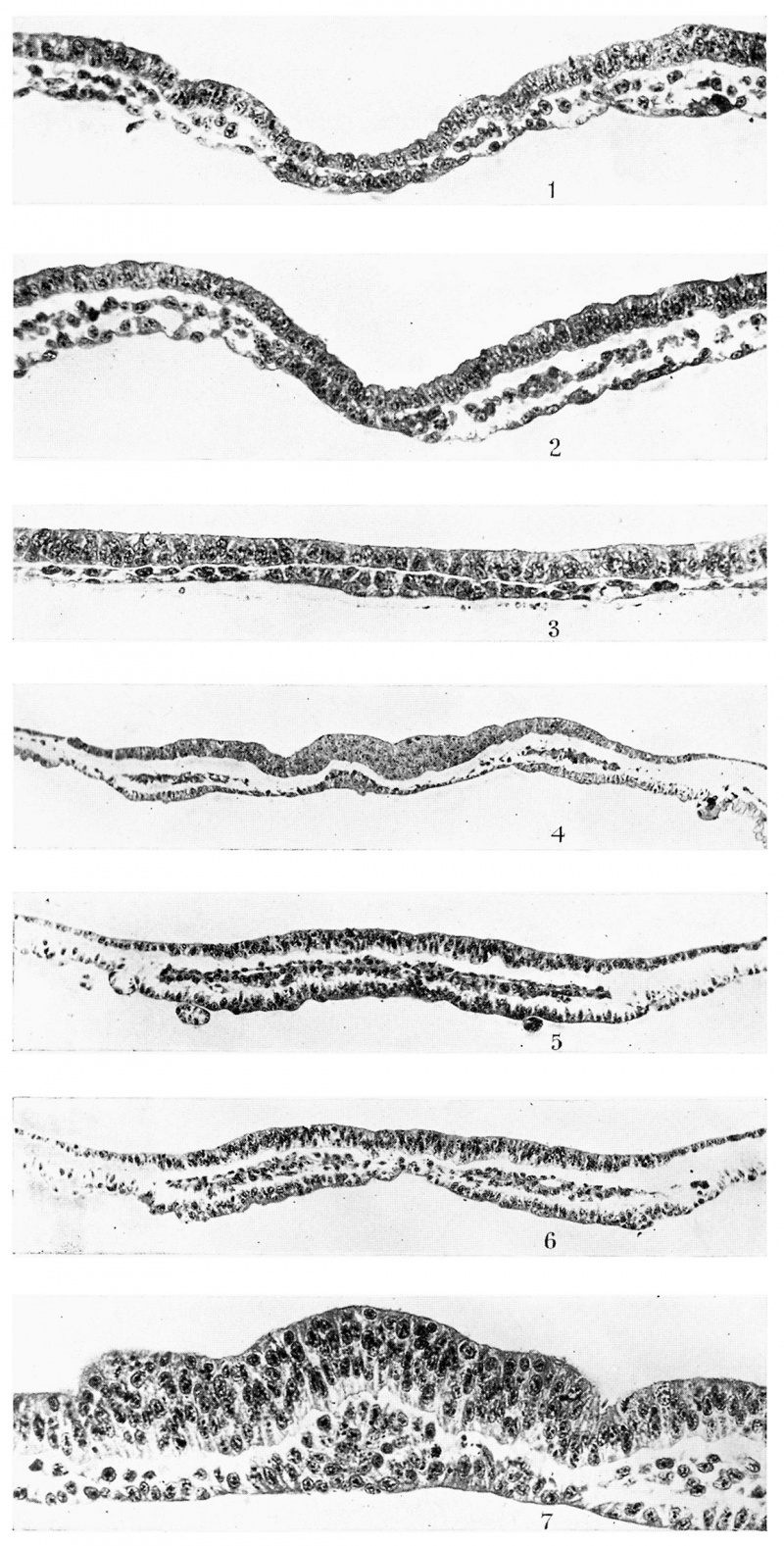
The earliest embryos examined are those of the head-process stage. In the two embryos representing this stage, the primitive knot, the primitive streak and the mesodermal sheet are already established. The prochordal plate presents the appearance shown in Plate I, fig. 1. It first makes its appearance 0-4 mm. behind the anterior border of the embryonal area and appears as a localised thickening of the endoderm. It does not seem to ‘contribute to the mesoderm which reaches up to its lateral edges. The cells composing it are robust and compactly arranged, so that the plate is easily distinguishable from the endoderm on either side. Here and there a few flattened cells are seen on its under-surface. | |||
to the mesoderm is concerned. This is the head-process region and must not be | If this figure is compared with Plate I, fig. 2, which represents the 48th section behind fig. 1, the difference stands out clearly in so far as the relation to the mesoderm is concerned. This is the head-process region and must not be confused with the prochordal plate. The presence of a mitotic figure on the right side and the apparent continuity with the mesoderm on the left seem to me to be very good evidence of the contribution of the head-process to the mesoderm. The appearance of this figure is also important, because later I shall produce figures, very similar to this, from an area between the definite prochordal plate and the definite chorda plate, which is marked (I .Z.) in the figures. | ||
confused with the prochordal plate. The presence of a mitotic figure on the | |||
right side and the apparent continuity with the mesoderm on the left seem to | |||
me to be very good evidence of the contribution of the head-process to the | |||
mesoderm. The appearance of this figure is also important, because later I | |||
shall produce figures, very similar to this, from an area between the definite | |||
prochordal plate and the definite chorda plate, which is marked (I .Z.) in the | |||
I should mention that in one of the embryos of the head-process stage the mesoderm is continuous across the middle line in front of the prochordal plate, while in the other, a rather poor specimen, it is not yet continuous, and we see the ectoderm overlying a thick patch of endoderm which extends across the whole breadth of the embryonal area in front of the prochordal plate. | |||
prochordal plate | |||
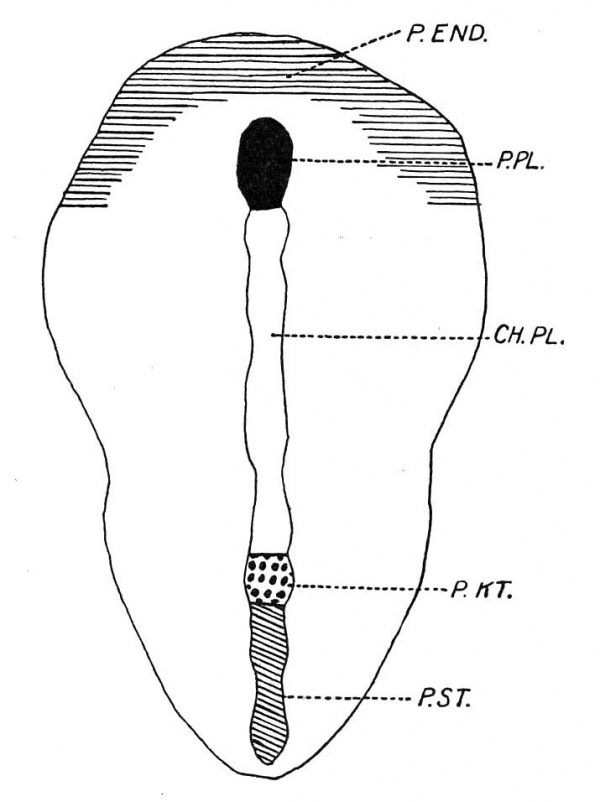
This patch (P.EN D.) is however better marked in the next stage, which is shown in text-fig. 1. This is a. dorsal view of an embryo with possibly one pair of somites and in which the axial structures are depicted. The crescentic shaded area in the figure (P.END.) represents a patch of thickened endoderm, the transverse part of which is co-extensive with the area between the prochordal plate (P.PL.) and the anterior border of the embryonal shield. It is prolonged backwards as two horns situated peripherally. The posterior limits are not easy to determine, but they extend some distance backwards. In this connection it is essential to state that Hubrecht has described in Screw vulgaris the presence of an annular zone of thickened endoderm which is in continuity with the lateral margins of the prochordal plate and which he states proliferates to form vascular mesoderm. Assheton describes a similar area, which is, however, deficient posteriorly and which he calls the “pericardial thickening,” in the rabbit. | |||
an embryo with possibly one pair of somites | |||
in | |||
[[File:Aasar1931 text-fig01.jpg|600px]] | |||
'''Text-fig. 1.''' A reconstruction dorsal view of embryo R352, 8 days 1 hour with possibly 1 somite, X 25. | |||
' | |||
The horseshoe-shaped zone of thickened endoderm (P.EN D.) here described, or rather its median part, lies cranially to the prochordal plate (P.PL.) as textfig. 2 clearly shows. This figure also shows the presence of mesoderm (P.M.) between the ectoderm and the median part of the horseshoe-shaped endodermal thickening (P.END.), but whether the thickened endoderm contributes to this mesoderm or whether the latter is solely derived from the primitive streak mesoderm, the horns of which grow forward and meet, cannot be decided in the stages examined. The further history of the antero-median part of the horseshoe-shaped endodermal thickening can be easily followed by the study of the reconstructed median longitudinal sections which are given here. It is important to note that with the forward growth of the brain accompanied by the development of the head-fold bay, the subsequent brain flexure and the backward growth of the pericephalic coelom, this antero-median area of thickened endoderm is seen to contribute to the floor of the fore-gut and to furnish the greater part of the endodermal element of the oral plate. Plate I, fig. 3, from an embryo with possibly one pair of somites, shows the prochordalplate to be in much the same condition as that shown in Plate I, fig. 1. It is also still the same, though shortened, in embryos possessing two somites and shown in textfigs. 2A. and 213. It is clear, however, that the mass of mesoderm (P.M.) is becoming thicker. I think it may rightly be called the “ pre-axial mesoderm,” since it is cranial to the prochordal plate (P.PL.), which is the most cranial axial structure. | |||
Text-fig. 2. A reconstructed median longitudinal section of anterior end of same embryo as text-fig. 1. x 100. | |||
Text- | Text-fig. 2A. A reconstructed median longitudinal section of anterior end of R 339, 8 days, 2 somites. x 100. | ||
R 339, 8 days, 2 somites. x 100. | |||
Text-fig. 2B. A reconstructed median longitudinal section of anterior end of R 338, 8 days, 2 somites. x 100. | |||
So far, the prochordal plate has been a one-layered structure, but in the stage possessing three pairs of somites it is several cells thick, cells having been proliferated on its upper side as is seen in Plate I, fig. 4«. This figure also shows the two lateral horns of the thickened horseshoe-shaped endoderm situated towards the periphery of the embryonal area. They are made up of large cylindrical endodermal cells and are connected with the central prochordal plate by thin flattened endoderm. The mesoderm overlying the thickened endodermal horns is more compact than elsewhere, and in this as well as in the next embryos this compact mesoderm is found to follow the course of the horseshoe-shaped endodermal thickening. It is in this mesoderm that the pericardio-peritoneal coelom makes its appearance first in the region of the lateral heart tubes in embryos with two pairs of somites. It extends forward and by the 5-somite stage it has become horseshoe-shaped with its transverse median part cranial to the prochordal plate. Van Beneden states that it is horseshoeshaped from the beginning. It should be pointed out, however, that the earliest cavity to appear is the extra-embryonal coelom. It develops very early immediately behind the posterior end of the embryonal shield. The pericardioperitoneal coelom is bilateral at first, and by the extension forward of its lateral halves and their union in front, the pericephalic coelom is established. | |||
The horseshoe—shape of the thickened endodermal zone, the overlying compact mesoderm and the independence of boththese structures from the prochordal plate are clearly shown in this embryo, but are illustrated from the next older stage—the 4-somite stage. I would like, however, to add that the horns of the horseshoe-shaped endodermal thickening become insignificant just before the region of the first pair of somites is reached. | |||
the | |||
Text-fig. 3. Superimposed axes of twelve embryos with the anterior end as the fixed point. From above down they are: R 336, 8 days, no somites. R 353, 8 days 1 hour, no somites. R 352, 8 days 1 hour, ? 1 somite. R 349, 8 days 3 hours, 2 somites. R 338, 8 days, 2 somites. R 339, 8 days, 2 somites. R 314, 8 days 172 hours, 4 somites. R 357, 8 days 1 hour, 4-5 somites. R 343, 8 days 4 hours, 5 somites. R 318 A, 8 days 17% hours, 6 somites. R 316, 8 days 17% hours, 6 somites. R 346, 8 days 3 hours, 7 somites. x 25. | |||
1 | |||
Before discussing the 4-somite stage I wish to refer briefly to text-figs. 3 and 4. These represent the structures in the median plane in each of the embryonal shields, which have been reconstructed in the same way as text-fig. 1. They are placed as shown in the figures with the anterior end as the fixed point in textfig. 3 and the middle of the primitive knot as the fixed point in text-fig. 4. They range from the presomitic stage to the 7-somite stage. In all the prochordal plate is flat and the fore-gut has not yet closed. | |||
Text-fig. 3 shows at a glance the remarkable fact that the prochordal plate (P.PL.) shortens with advance of age, while the chorda plate (CH .PL.) increases considerably in length. The significance of this will be discussed later, but it must be noted that this decrease in length of the prochordal plate is accompanied by an increase in thickness. In fact the first traces of this thickening are seen in Plate I, fig. 4. Any attempt, therefore, to explain the genuine shortening of the prochordal plate must not ignore the marked thickening it undergoes. | |||
The second point to attract attention in text-figs. 3 and 4 is the apparent forward shifting of the prochordal plate (P.PL.) towards the anterior end of the embryonal area. This may be due to the unequal rate of growth of the parts concerned. Whether or not the shortening of the prochordal plate means contribution to the chorda plate is a question to be discussed. | |||
. | |||
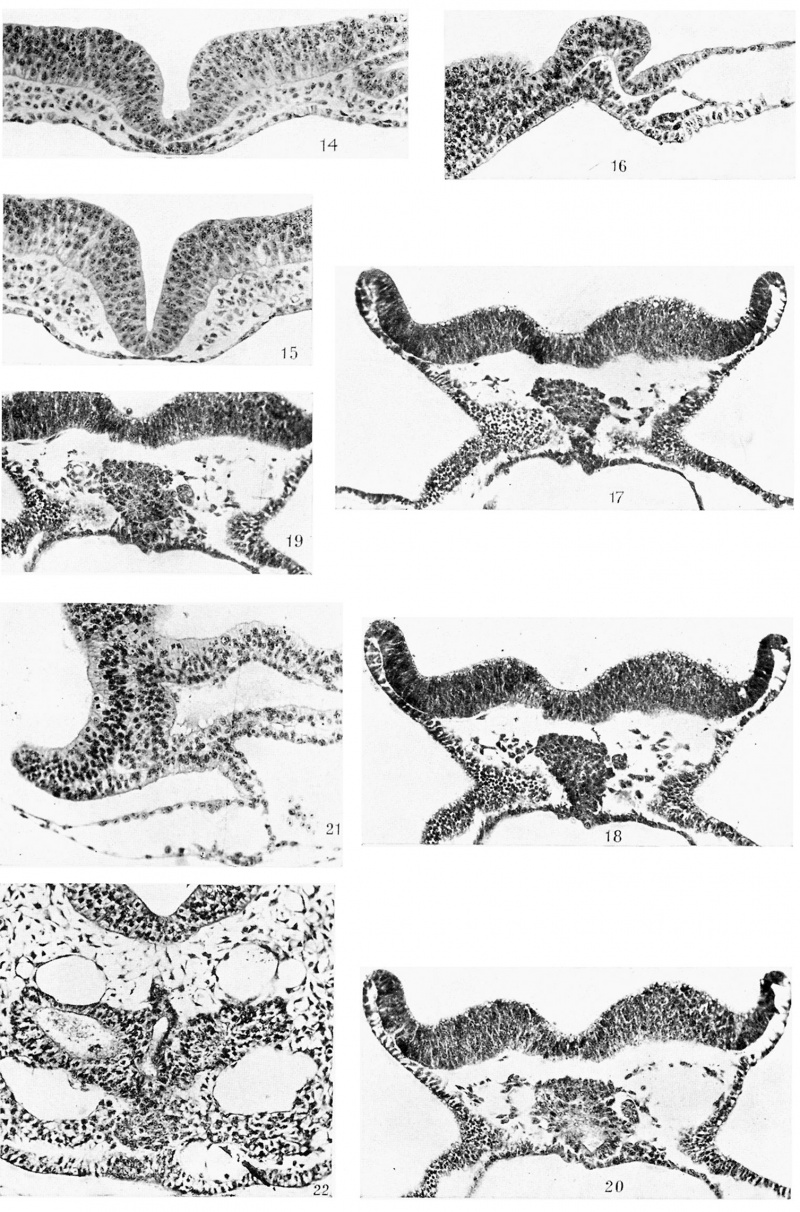
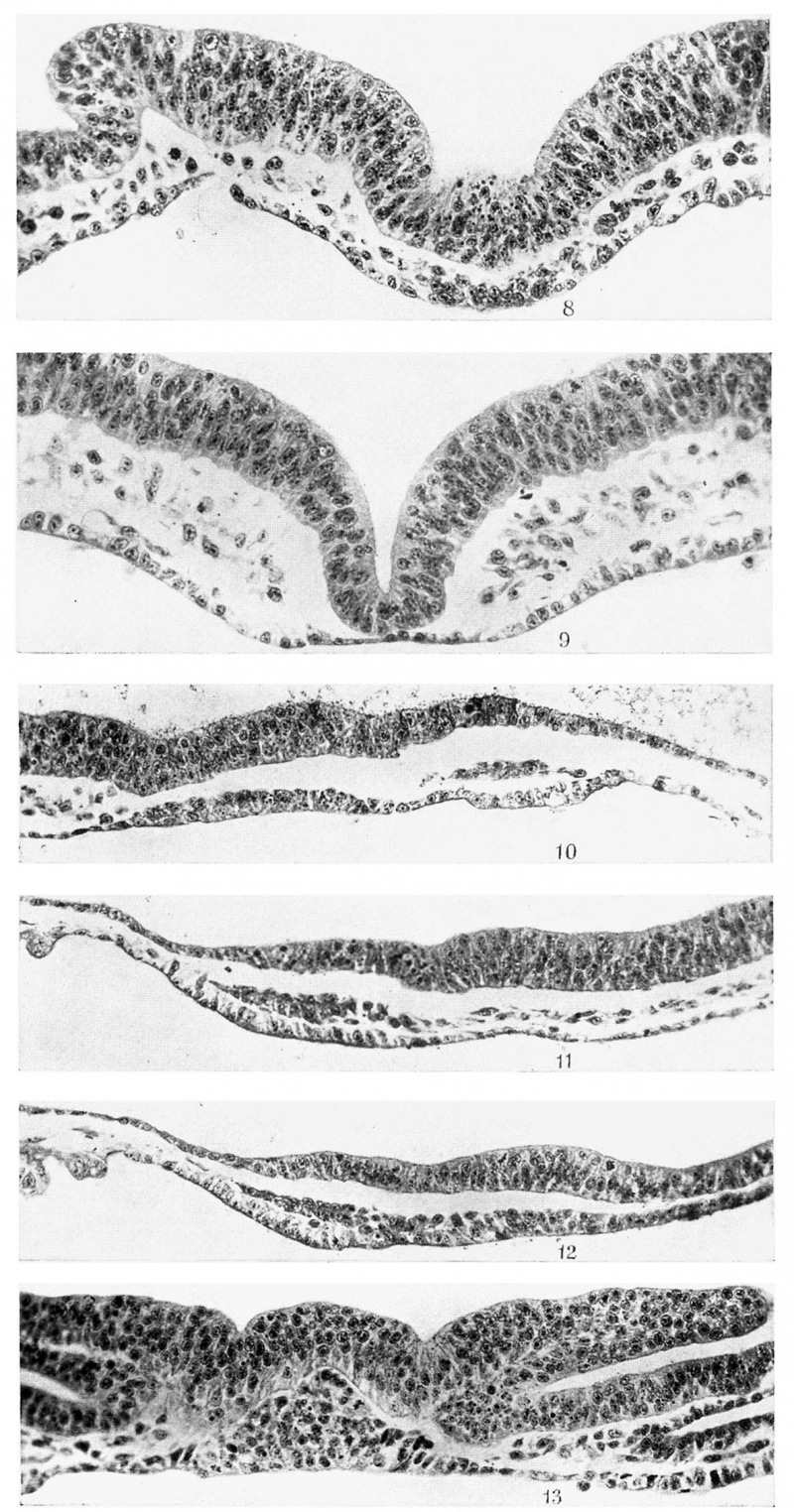
The anterior end of the prochordal plate is practically always well defined from the antero-median part of the horseshoe-shaped thickened endoderm and the overlying compact mesoderm, but its junction with the chorda plate is not so readily determinable. In text-figs. 3 and 4, in a number of embryos, I have indicated a short axial segment intervening between the prochordal plate and the definite chorda plate (I.Z.). Its structure and relation to the mesoderm are shown in Plate II, fig. 8, and Plate III, fig. 14:. Its cells are robust and closely packed, and at the sides are continuous with the mesodermal cells. The segment is usually one cell thick and contrasts markedly with the prochordal plate and the chorda plate. Inappearance it very much resembles Plate I, fig. 2, which passes through the head-process. I consider this segment, labelled I .Z., as the last remains of the undifferentiated head‘-process, in fact its anterior end. If this view is correct, then the prochordal plate is not of head-process origin. This segment is shown in five embryos out of twelve, but its frequency is greater than that because in some cases, after having come to the conclusion that it is part of the head-process, I have included it with the chorda plate. It disappears later in older embryos, having become converted into chorda plate which becomes properly intercalated in the gut wall. | |||
Text-fig. 4. Same as text-fig. 3, but middle of primitive knot is taken as the fixed point. x 25. | |||
The shortening of the prochordal plate is relatively insignificant in com - parison with the marked lengthening of the chorda plate. Even supposing the prochordal plate is diminished in length by reason of its transformation into chorda plate, a supposition which I believe is not true, the part added would be so small as to be insignificant. | |||
Text- | Text-fig. 4. shows that the primitive knot (P.K T.) is more or less constant in extent, but the primitive streak (P.S'T.) shortens as age advances. | ||
It is perhaps worthy of mention here that the cloacal membrane (CL.M appears very late at the 6-somite stage immediately behind the primitive streak and not actually in its substance. The area behind the primitive streak in which it appears gradually increases in extent as age advances. The cloacal membrane (CL.M.) is shown in text-fig. 4 in the two embryos with 6 somites, but it is not yet distinguishable in the 7-somite. embryo. | |||
The two small circles situated just in front of the primitive knot in two of the embryos depicted in text-figs. 3 and 4 represent the ventral opening of the chorda canal, which is poorly developed in rabbit embryos. | |||
in | |||
of the | |||
Now we can come back to the 4—somite stage. Amedian longitudinal section of its anterior end is shown in text-fig. 5 and a dorsal View in text-fig. 6. In the latter the outlines of the horseshoe-shaped mesoderm, in the anterior part of which the pericephalic coelom (P.COE.) is not yet fully developed, are shown. The horns of this mesoderm, if followed back, will be found continuous with the primitive streak mesoderm (P .S T .]|l.). The pericardio-peritoneal coelom (PP. C.) is well developed in the region of the lateral heart tubes (E .H T .), but anteriorly, especially in the pre-axial mesoderm, it is in the form of isolated clefts. The position of the la.teral heart tubes (E .H T .) leaves no doubt of their independence from the prochordal plate (P.PL.) which latter is also independent from the pre-axialmesoderm (P.ZVI.), as text-figs. 5 and 6 and Plate I, figs. 6 and 7, clearly show. When we examine text-figs. 2, 2A, 2 B and 5 we find that the mass of preaxial mesoderm (P.lM.) is gradually increasing, and that in the last figure the pericardio-peritoneal coelom has appeared in the middle line, although its lateral extension is better appreciated by examining Text-fig. 6. | |||
Text-fig. 5. A reconstructed median longitudinal section of R 314, 8 days 17;} hours, 4 somites. x 100. | |||
P. | The prochordal plate (P.PL.) has increased considerably in thickness and now appears in transverse section as a more or less triangular plate with cells piled on top of each other and Well packed. The mesoderm on either side stops short of its lateral sides, and its cells contain chromatophilic granules in their cytoplasm. Its blunt apex produces an elevation in the middle of the neural plate, and curiously enough this elevation is replaced by a groove as soon as the caudal end of the prochordal plate is reached and we pass into that area between it and the definite chorda plate, regarded as the anterior end of the undifferentiated head-process (I .Z.). This area is well developed in this embryo, as can be gathered from text-figs. 5 and 6 and also from Plate II, fig. 8, which also shows the last trace of the elevation caused by the prochordal plate. It also shows very clearly the continuity of the mesoderm with the remains of the head-process. Behind this area is the typical c chorda plate. It is intercalated in the endoderm and does not show any continuity with the mesoderm on either side of it. To appreciate these points, Plate I, fig. 7, and Plate II, figs. 8 and 9, should be examined. The first figure is a typical section of the prochordal plate, the second passes through the remains of the head-process and the third through a typical chorda plate region. | ||
In front of the prochordal plate the relations of the parts are well shown in Plate I, figs. 5 and 6. In the former we see three layers spreading across the whole width of the embryonal area in that region. The endoderm is thick but is really made up of a layer one cell thick. The cells are large and cylindrical. This is the antero-median part of the horseshoe-shaped endodermal thickening. In Plate I, fig. 6, only O-048 mm. behind fig. 5, the endoderm is beginning to resolve itself into the two horns connected here by a thinner patch of endoderm. The overlying mesoderm resolved into two horns, as the two figures under consideration clearly show. In the mesoderm referred to the pericephalic coelom has appeared, but is not well developed. It has, where present, a thin dorsal and a thicker ventral wall. | |||
Text-fig. 6. A reconstructed dorsal view behaves like the endoderm in so far as it is of same embryo as 1-,ext_fig_ 5, X 25, | |||
- | |||
A glance at the five figures——Plate I, figs. 5, 6, 7, and Plate II, figs. 8, 9will convince one of the independence, already referred to, of the endoderm of the horseshoe-shaped thickening and the pre-axial mesoderm from the prochordal plate. This statement is further confirmed by Plate II, fig. 10, of a 5-somite embryo. It is as median a longitudinal section as possible and clearly shows the thickened endoderm at the anterior end of the embryonal area overlain by the compact pre-axial mesoderm, in which no definite pericephalic coelom has appeared. It is connected to the prochordal plate posteriorly by a thinner stretch of endoderm. The prochordal plate is only two cells thick and is not as well developed as in the 4-somite stage. This is, however, an individual variation. | |||
in | |||
The relations of the prochordal plate to the pre-axial mesoderm, the underlying thickened endoderm and the lateral heart tubes should be borne in mind. There is an interval between the anterior end of the prochordal plate and the posterior border of the pre-axial mesoderm. This is seen in text-figs. 2, 2 A, 2 B and 5, and is confirmed by Plate I, fig. 6. Few cells occupy this interval and there is no indication that the prochordal plate contributes to the pre-axial mesoderm. It has, however, proliferated dorsally and has also shortened, a fact which may be explained by the contraction. of the plate rather than by its transformation into chorda plate. Again, there is no evidence that the prochordal plate is contributing to the mesoderm on either side of it. | |||
The | The endothelium of the lateral heart tubes may be derived either from the horns of the horseshoe-shaped endodermal thickening, or from the overlying compact mesoderm. The prochordal plate does not give any contribution to either the endothelium of the lateral heart tubes or the wall of the pleuropericardial coelom as was suggested by Hubrecht. | ||
the | |||
Embryos possessing five pairs of somites show little or no advance in the condition of the prochordal plate. Reference has already been made to Plate II, fig. 10. The pre-axial mesoderm is here clearly distinct both from the anteromedian part of the horseshoe-shaped endodermal thickening and the prochordal plate. The prochordal plate here is two cells thick but is less massive than that of the 3-somite stage described above, though it is decidedly more advanced than that of the 2-somite stage. | |||
of | |||
is | |||
Plate II, fig. 11, is a longitudinal section, about 0-128 mm. from the median plane of another 5-somite embryo. It shows very well the compact mass of pre-axial mesoderm tending to be arranged in two layers, a thinner dorsal and a thicker ventral with a potential cavity between them. The contrast between this mesoderm and the more loose mesenchyme with which it is continuous posteriorly is very marked. The thickened endoderm, at the anterior end of the embryonal area and underlying the pre-axial mesoderm, is also very distinct. The prochordal plate (Plate II, fig. 12) is 2-3 cells thick, whereas this anteromedian part of the horseshoe-shaped endodermal thickening is practically one cell thick, and while the latter is separated from the ectoderm by a compact darkly staining mass of pre-axial mesoderm, the former is not separated by any mass from the overlying ectoderm. There is in this specimen, as Plate II, fig. 12, shows, an apparent continuity between the anterior end of the prochordal plate and the pre-axial mesoderm, but as I have pointed out above this is not the usual arrangement, which is the more or less complete separation of the pre-axial mesoderm from the prochordal plate by an interval containing very few loose cells. I consider the condition seen here as simply an exaggeration of the usual arrangement. It must be remembered that the pre-axial mesoderm was already present in this region in the earliest embryos examined when the prochordal plate was one cell thick. | |||
Text-fig. | The next stage of a 5-6-somite embryo exhibits much the same condition as the preceding stages. Text-fig. '7 represents a reconstructed median longitudinal section of the anterior part of the embryo. It shows a well-developed prochordal plate (P.PL.) connected to the chorda plate (CH .PL.) by a segment, regarded as the remains of the undifferentiated head-process (I .Z.), but anteriorly it is separate from the pre-axial mesoderm (P.M.) in which the pericephalic coelom is not yet continuous across the middle line. The ectoderm is decidedly thicker and is elevated over the prochordal plate area in the middle line. The latter shows an isolated mass of cells in its caudal half of unknown significance. | ||
Beneath the anterior end of the prochordal plate there are three cells which are considered as degenerating cells of prochordal plate origin. Few mitotic figures are present in it, and again there is no evidence that it contributes to the mesoderm on either side of it. | |||
It is a noteworthy fact that, whilst the prochordal plate undergoes definite changes, particularly in thickness, the antero-median part of the horseshoe-shaped endodermal thickening (P.END.) does not show any change in form, thickness or extent. | |||
Text-fig. 8. Anterior part of a reconstructed median longitudinal section of R 346, 8 days 3 hours, 7 somites. x 100. ' | |||
Now we pass on to a slightly older stage which is represented by text-fig. 8, the anterior end of a reconstructed median longitudinal section of a 6-7-somite embryo. The anterior part of the prochordal plate is more massive than its posterior part. Its caudal extremity corresponds with the point where the elevation in the ectoderm, referred to above, gives place to a groove. The head-fold bay (HF.B.) is beginning to appear just cranial to this elevation in the middle of the neural plate over the extent of the prochordal plate. This latter shows chromatophilic granules, but there is no proliferation of mesoderm from its lateral margins. Mitotic figures are not specially abundant, and in this embryo some degenerated cells are scattered here and there beneath the main mass of the plate. There is a short segment, the remains of the head-process, which extends through 2-3 sections and which, in the figure, has been included with the chorda plate (CH .PL.). The distinctive features of the prochordal plate, apart from its thickness and position, are the presence of the chromatophilic granules and the compact arrangement of its cells. | |||
head- | |||
The | |||
Text-fig. 9 is a reconstruction of the dorsal view of a 6-somite embryo, and text-fig. 10 is a median longitudinal reconstruction of the anterior part of the same embryo. The pericephalic coelom (P.COE.) is well developed and the preaxial mesoderm (P.M.) in which it is developed is in close contact with the endoderm just in front of the anterior end of the prochordal plate (P.PL.). The latter is very well developed and it is now many cells in thickness. It is triangular in both the coronal and Sagittal planes, as is clearly seen in text-fig. 10 and Plate II, fig. 13. The elevation presumably produced by this plate in the middle of the neural plate is also well shown in both figures. The plate shows the characteristic chromatophilic granules (Plate II, fig. 13) and does not contribute to the mesoderm on either side. On the right of this figure one can see a thin endoderm layer connecting the prochordal plate with the right horn of the horseshoe-shaped endodermal thickening underlying the pericardioperitoneal coelom. The latter has a thin ‘ roof and a thick floor between which CL-"T . and the endoderm is a thin strand Of Text-fig. 9. Reconstructed dorsal view of angioblastic cells. The endothelial heart R 313A» 3 days 17% h011I‘S» 5 80miteStubes (E.HT.) are well developed, as " 25' can be seen in text-fig. 9. The head-fold bay (HF.B.) is well established, as seen in text-fig. 10. | |||
,9. | |||
Plate III, fig. 14, passes through the cranial segment of the head-process and shows very well its relations to the mesenchyme, especially on the right side. In this figure the dorsal furrow has replaced the median elevation which was seen in the region of the prochordal plate in Plate II, fig. 13. Plate II, fig. 13, and Plate III, figs. 14 and 15, show the prochordal plate, the remains of the cranial segment of the head-process and the chorda plate respectively, and should be compared with Plate I, fig. 7, and Plate II, figs. 8 and 9. | |||
In the dorsal view—text-fig. 9-—the outlines of the pericardio-peritoneal coelom (PP.C.) and its pericephalic part (P.COE.) are well shown. It communicates in the region of the first somite with the extra-embryonal coelom, and immediately behind the last somite its inner limit (M .B.) lies Very near the margin of the embryonal area. | |||
It may be noted that the cloacal membrane (CL.M.) makes its first appearance in this stage immediately behind the posterior end of the primitive streak and not in its substance as some observers claim. | |||
and | |||
Some progress has manifested itself in the next stage, although it possesses the same number of somites as the preceding. Text-fig. 11 shows a well developed head-fold bay (HF.B.) and an apparent caudal displacement of the pre-axial mesoderm (P.M.), its contained pericephalic coelom (P.COE.) and the underlying thickened endoderm of the horseshoe-shaped area (P.END.). The prochordal plate (P.PL.) is well marked and has a triangular shape in the sagittal plane. It occupies the angle between the neural plate and the upper layer of the head-fold bay and forms -the roof of the incipient fore-gut bay. This stage is importantvbecause it shows us the beginning of the establishment of the anterior position of the prochordal‘ plate, which is better seen in textfig. 12. | |||
Text-fig. 10. Anterior part of a reconstructed median longitudinal section of R 318 A. x 100. | |||
of | |||
Text-fig. ll. Anterior part of a reconstructed median longitudinal section of R 316, 8 days 17% hours, 6 somites. x 100. | |||
In preceding stages the pre-axial mesoderm was situated in practically the same plane as the prochordal plate, but with the establishment of the headfold bay and the forward growth of the neural plate, the pre-axial mesoderm and the underlying endoderm of the horseshoe-shaped area now occupy a more ventral plane than the prochordal plate, although they are still anterior to it. | |||
The tip of the angle between the neural plate and -the upper layer of the head-fold bay lies opposite the posterior third of the pre-axial mesoderm, whereas in the previous embryo it lies just behind its posterior end. These alterations in the relations of the prochordal plate are the outcome of the commencing formation of the fore-gut, and that, as K. M. Parker has pointed out in her paper on the early development of the heart in marsupials, would appear to be induced by the forward growth of the brain plate, the backward extension of the pre-axial mesoderm and its contained coelom, and the gradual incorporation into the latter of the bilateral pericardial cavities. | |||
Plate III, fig. 16, showing a median longitudinal section of a 7 -somite embryo, affords confirmation of the general correctness of the last four text-figures, which are reconstructions made from transverse series. It much resembles text-fig. 11, the projection formed by the main mass of the prochordal plate being a striking feature. The posterior limit of the prochordal plate is not so easy to make out here as in the transverse sections, in which the characteristic appearance of the cranial segment of the head-process serves as a good landmark. Its anterior end passes into the antero-median part of the horseshoeshaped endodermal thickening, overlying which is the pre-axial mesoderm with its pericephalic coelom. I have mentioned above, however, that there is a thinner patch of endoderm between the prochordal plate and the anteromedian thickened endoderm referred to. This is the usual arrangement. The elevation of the anterior part of the brain plate as well as the head-fold bay are well marked. | |||
Between the floor of the pericephalic coelom and the underlying thickened endoderm there are found two angioblastic cells, the origin of which is not easy to decide, but they appear to be mesodermal. There is no evidence of proliferation on part of the endoderm. | |||
The changes that have been described above have made further progress in the next stage possessing 8-9 pairs of somites. A reconstructed median longitudinal section is shown in text-fig. 12. Here the head-fold bay (H F.B.) is more marked than before, the brain plate (BR.PL.) has grown forward considerably and the pericephalic coelom (P .COE. ) now lies further back, its anterior or cranial wall lying a little caudal to the free tip of the prochordal plate (P.PL.) which still occupies the angle between the brain plate (BR.PL.) and the upper layer of the head-fold bay. The fore-gut (FG.) is established, its length being 0-112 mm. Examination of text-figs. 8, 10, 11 and 12 will convince one that its floor is formed by the antero-median part of the horseshoe-shaped endodermal thickening. The prochordal plate (P.PL.), however, forms the anterior wall of the foregut and a very small part of the adjoining roof. Perhaps this will be better understood if we were to unfold the bends caused by the head-fold bay and the closed gut. If we do that we should obtain a figure very much like text-fig. 10. In this way we can realise how the endoderm of the thickened zone, originally anteriorly situated, has come to form the floor of the fore-gut, with the result that the prochordal plate comes to be the most anterior or cranial endodermal structure. As the reconstruction shows there are no foldings or evaginations in the fore-gut wall where it is formed by the prochordal plate. | |||
the chorda plate (CH .PL.). The | Text-fig. 12 shows the relations of the massive prochordal plate. The plate lies mainly in the angle between the brain plate and the upper layer of the head-fold bay- Its tip is free and it gradually increases in depth as it is traced caudally. It forms the anterior wall of the fore-gut and the adjoining part of the roof, but does not enter into the formation of the floor of the fore-gut. Caudally it passes into the chorda plate (CH .PL.) without the intervention of a head-process segment. There is a distinct space between the dorsal surface of the prochordal plate and the neural plate (BR.PL.). The latter does not exhibit any elevation in the middle line such as was observed in earlier stages. The prochordal plate is solid and massive, measuring about 0-112 mm. in length, 0-088 mm. in greatest depth and O-088 mm. in greatest width. Its cells are compactly arranged (Plate III, figs. 18 and 19) and show a tendency, especially on the left side, to proliferate mesoderm. In Plate III, fig. 19, there is an apparent vesicular structure to the left of the prochordal plate and in apparent v | ||
Text-fig. 12. Median reconstruction of R. 324, 8 days 17:} hours, 8-9 somites. x 100. | |||
A further change has manifested itself in the next stage. Theprimary brain flexure is now quite prominent, as can be seen in text-fig. 13 and Plate III, fig. 21. The neural tube is closed from just behind the cephalic flexure to the region of the first somite. Text-fig. 13 is from a median longitudinal section of a 9-10-somite embryo. It shows how the flexure in the brain has influenced the form of the prochordal plate (P.PL.). It lies in close contact with the brain wall and with the upper layer of the head-fold bay. It is, therefore, still situated in the angle between the anterior part of the wall of the brain and the upper layer of the head-fold bay, as in text-fig. 12, in which the flexure was much less obvious. The prochordal plate forms the anterior Wall of the fore-gut (FG.), but only a very small part of the dorsal wall. Just at the angle where the very short dorsal moiety of the plate passes into the extreme anterior part, there is a slight evagination which I believe to be the homologue of Seessel’s pouch. Its Walls are accordingly formed by the prochordal plate. Besides this, there are no other foldings or evaginations in the region of the plate. Streeter observed several foldings in the pig and homologised the folded prochordal plate with the “ Gaumentasche” of Selenka. | |||
The oral plate (0.PL.) is now established, its ectodermal and endodermal elements being in contact but individually distinguishable. It lies immediately caudal to the prochordal plate and just cranial to the pericardial cavity. Its ectodermal element is formed by the caudal'part of the upper layer of the headfold bay. The endodermal element of the oral plate is directly continuous with that of the prochordal plate. The posterior two-thirds of the oral plate endoderm are to my mind derived from the antero-median part of the horseshoeshaped endodermal thickening. The cranial third, however, may receive contributions from the prochordal plate. These statements can be verified by examining the figures. ' | |||
Text-fig. 13. Median reconstruction of R 321, 8 days 172 hours, 9-10 somites. x 100. | |||
Text-fig. 13 shows that the ventral part of the prochordal plate (P.PL.) is more massive than its dorsal part. There is as yet no‘ trace of the buccal hypophysis, and the pericardial cavity has been pushed further back and is quite huge. The chorda plate ( CH .PL.) is still intercalated in the roof of the gut, and in many places is in very close contact with the floor of the neural tube. | |||
fold bay | The reconstruction shown in text-fig. 13 should be compared with Plate III, fig. 21, which is a photomicrograph of the anterior end of a median longitudinal section of an embryo possessing 9 somites. It is practically identical with text-fig. 13. Note the Very close contact between the brain wall and the thick prochordal plate. The latter is also in contact with the upper layer of the head-fold bay and forms the anterior wall of the fore-gut. It also forms the dorsal angle of the fore-gut where there is an evagination Seessel’s pouch—the wall‘ of which is of prochordal plate derivation. The ventral angle of the fore-gut shows the continuity of the prochordal plate with the thick endoderm of the oral plate. The latter is pale compared with the darker staining prochordal plate in which are chromatophilic granules, absent in the oral plate endoderm. Immediately behind Seessel’s pouch the prochordal plate runs into the chorda plate which is one cell thick and in contact with the floor of the neural tube. Caudal to the oral plate and the fundus of the headfold bay the pericardial cavity is seen with thick roof and thin floor. | ||
The | |||
Text-fig. 14. Median reconstruction of R 320, 8 days 17 ;% hours, 10-11 somites. x 100. | |||
Practically the same relations are shown in the next older stage which possesses 10-11 pairs of somites. Text-fig. 14 resembles text-fig. 13 in the salient points. The prochordal plate lies immediately behind the flexed brain wall and forms the anterior wall of the fore-gut which is considerably thicker than the other walls. It extends slightly laterally and shows chromatophilic granules in the cytoplasm of its cells. Its intimate relation with the flexed Wall of the fore-brain should be borne in mind, since in the next stage to be described this comes to an end and a space filled with mesenchyme is present between the two. The oral plate is not well seen, probably owing to the sectional plane. | |||
the next stage | |||
and the | |||
wall | |||
The notochord is not yet separated, at least in the area shown here. The foregut is extensive and the roof of the pericardial cavity (R.PC.C.) is now very thick, whereas the floor is very thin (F.PC.C.). | |||
The anterior end of the chorda plate ( CH .PL.) passes into the prochordal plate (P.PL.), but it is practically impossible to locate the limits of the latter accurately because there is always some difficulty about one or two sections at the ends of the plate where it passes into the oral plate endoderm and the chorda plate respectively. | |||
We may pass on to a stage with 14-15 somites, since there are no noteworthy changes in the interval. Important advances have now been made as text-fig. 15 shows. The chorda ( CH.) has separated from the endoderm but is still in close contact with both brain wall (BR.W.) and gut roof (EN D.), except at its most anterior end which terminates in the prochordal plate just cranial to the meeting of the roof of the gut and its anterior wall (W.PG.). I should state that it has started to separate in the 13-somite stage. | |||
The prochordal plate shows partial delamination into its two important derivatives which are the anterior wall of the gut (W.PG'.') and the prochordal mesoderm (P.PL.M.). These are still in close contact as the reconstruction shows, but can be identified as separate structures, ‘except perhaps dorsally, where the chorda passes into the prochordal plate. The ventral portion of the prochordal plate mesoderm appears as a thickened mass which is practically separate from the wall of the gut as Plate III, fig. 22, shows. It is situated between the oral plate (0.PL.) caudally and the brain wall cranially, and cranio-ventral to it lies the primordium of the buccal hypophysis (B.HP.). As Plate III, fig. 22, shows, it differs in appearance from the mesenchyme on either side of it, the cells stain more deeply, are more closely packed and frequently contain chromatophilic granules. This mass appears to be the homologue of that part of the prochordal mesoderm from which in other forms the premandibular somites arise. It may be referred to as the premandibular somitic mass or, shortly, as the premandibular primordium. Its position in front of the oralplate, here well developed, as well as its relation to the hypophysis, which will be better seen later, entitles it to be so homologised. It is more massive than the thinner strand of prochordal mesoderm which connects it with the anterior end of the chorda ( CH .). There are no cavities either in the mass itself or in its lateral extensions when these are present, and in putting forward this View of its homology, ‘I lay stress on its position, origin and, relations. Its fate will be described later. | |||
the | |||
in the | |||
Text-fig. 15. Median reconstruction of R 179, 8 days 19 hours, 14-15 somites. x 100. | |||
text-fig. 15’— | |||
The appearance of a space occupied by mesen chyme between the prochordal plate and the brain wall is another striking feature of this stage. In the pre_ceding stage the prochordal plate and the brain wall were in close contact. It may be that the appearance of this space is due to the fact that the brain is growing more rapidly than the adjoining structures, particularly the prochordal plate. The origin of the mesenchyme which fills the space is of interest. It is either proliferated from the prochordal plate or is formed by the medial growth of the laterally situated mesenchyme. The latter is the most probable origin, but it is probable that the prochordal plate contributes‘ to it in part. | |||
is more | |||
is | |||
The oral plate (0.PL.) is well established and its two layers are still quite distinct. The primordium of the buccalhypophysis (B.HP.) is just indicated cranial to the oral plate and ventral to the mass of presumed premandibular mesoderm. The anterior part of the chorda (CH .) is somewhat thickened, but there are no lateral proliferations in connection with it. | |||
the oral plate | |||
Text-fig. 16. Median reconstruction of R 253, 9 days 10 hours, 21 somites. x 100. | |||
It is accordingly in the stages between 14 and 16 somites that the changes above described manifest themselves. They are uniformly exhibited in the ten embryos of this stage which I have examined. | |||
Further progress in the same direction has taken place in the next stage, represented by an embryo aged 9 days 10 hours and possessing 21 pairs of somites. If, however, text-fig. 16, representing this stage, is compared with the preceding text-figure, it will be noticed that nothing of great importance has happened in the interval. | |||
The buccal hypophysis (B.HP.) is well established, is in close contact with the brain Wall, and is widely open. The oral plate (0.PL.) is Very thin but is still intact. The chorda, separate as before, ends anteriorly in the strand of prochordal mesoderm (P .PL.M .) which is practically completely separate from the anterior wall of the fore-gut (W.PG.), in fact a narrow space exists between them. The process which began in the 14-16-somite stage is here more or less complete and has resulted in the transformation of the prochordal plate into a strand of prochordal mesoderm (P.PL.M.) and the anterior wall of the foregut (W.PG.). The mesenchyme-filled space in front of the prochordal mesoderm is considerably increased in extent as compared with text-fig. 15. | |||
still in | |||
The prochordal | The structure and relations of the prochordal mesoderm are shown in Plate IV, figs. 23 and 24. In the former we see a median cellular strand situated between the brain walls. The Very short and thin upper part of this is formed by the chorda, whilst the remainder, much thicker and more or less irregular, is formed , by prochordal mesoderm in which the chorda ends. It contains granules which are, however, not well shown in this section. If we compare this figure with Plate III, fig. 18, we at once see the difference in size of the prochordal plate. This, together with the irregular appearance of the prochordal mesoderm, suggests that it contributes to the mesenchyme. Indeed, in the later stages we shall find that the whole prochordal mesoderm is converted into mesenchyme. The solid compact mass which lies to the right of the prochordal mesoderm is the cut wall of the fore-gut and has nothing to do with the prochordal plate. | ||
mesoderm | |||
prochordal | |||
Plate IV, fig. 24, is 0-112 mm. behind Plate IV, fig. 23. The separated chorda is clearly seen between the roof of the gut and the floor of the brain, whilst between the floor of the gut and the posterior wall of the buccal hypophysis is a horizontally disposed, flattened, plate-like strand of cells, readily distinguish able by its darkly staining character. If this strand is examined it will be seen. | |||
and | |||
the | |||
of | |||
, | that it really consists of a small median mass and two lateral extensions, slightly asymmetrical, the left one being the larger of the two and possessing a small lumen round which the cells are radially arranged. When the median part is followed in the series it is found to become continuous with the prochordal strand of mesoderm seen in Plate IV, fig. 23—see the reconstruction, text-fig. 16. This median mass is accordingly to be regarded as none other than the premandibular somitic mass seen in the preceding stage, whilst its lateral extensions, if that interpretation is correct, are to be regarded as rudimentary premandibular somites. " | ||
The | The net result, therefore, is the transformation of the prochordal plate into prochordal mesoderm and the anterior wall of the pre-oral part of the fore-gut. Dorsally the chorda ends in the slender median prochordal mesoderm, which is expanded ventrally to form the premandibular mass from which rudimentary premandibular somites may arise. This process takes place by delamination as can easily be made out by examining text-figs. 14, 15 and 16. | ||
plate and the | |||
The oral plate (0.PL.) is | The delamination is almost complete in the next stage, shown in text-fig. 17, which is a reconstruction made from the longitudinal sections, while Plate IV, fig. 25, shows a nearly median longitudinal section of the same embryo aged 9 days 16 hours and possessing 23-24 pairs of somites. The oral plate (0.PL.) is deficient in places, and it is now difficult to identify ectoderm and endoderm as separate layers. The prochordal mesoderm (P.PL.M.) is clearly seen in front of the anterior wall of the fore-gut (W.PG.) which is made up of columnar epithelium and is therefore thicker than the dorsal wall made of cubical epithelium (EN D.). Chromatophilic granules are seen in the cytoplasm of the cells of the prochordal mesoderm, particularly in its Ventral part, which extends out laterally on either side of the median plane, as can be ascertained by examining the sections. The dorsal part of the prochordal mesoderm, however, appears in 2-3 sections only and in it the notochord (CH ends cranially to the wall of the gut. The chorda ( CH .), quite separate from the brain wall and the roof of the fore-gut, shows a free anterior tip (T .CH .) which is better marked in later stages. There are no chromatophilic granules in the chorda at all. | ||
mesoderm. The | |||
It is not out of place here to state that the intercalated chorda plate separates from the gut wall either by evagination of the plate and the approximation of the edges of the endoderm, or simply by the approximation of these edges beneath the chorda plate. These two different methods may take place in one and the same embryo. | |||
Text-fig. 17. Composite outline drawing of anterior part of R 248, ‘ 9 days 16 hours, 23-24 somites. x 100. | |||
anterior | |||
N o cavities occur in the prochordal mesoderm, nor is there any definite proliferation from the slender strand which connects the swollen Ventral part with the chorda. The whole prochordal mesoderm (P.PL.M.) corresponds to what Oppel calls “Praechordalplatte,” and its swollen Ventral part has been here called the premandibular primordium. The space which lies cranially to the prochordal mesoderm and which is filled with loose mesenchyme is greater than before. | |||
The | |||
I referred above to the fact that the anterior end of the chorda extends forward as a free tip beyond the connection with the prochordal mesoderm (P.PL.M.). This is better seen in text-fig. 18, which is a composite outline drawing of the anterior end of an embryo aged 9 days 10 hours and possessing 24 somites. The median sections are shown in Plate IV, figs. 26 and 27. | |||
IV, figs. | |||
The prochordal mesoderm (P.PL.M occupies its usual position but is more irregular and much looser than in the preceding stage (compare Plate IV, figs. 25, 26 and 27). It is connected by a bridge to the anterior wall of the pre-oral part of the fore-gut (W.PG.), but apart from that it is quite free from both the gut wall (W.PG.) and the buccal hypophysis (B.HP.). Here again its ventral part extends laterally on either side of the median plane, whereas the strand connecting it with the chorda is quite thin, extending through only 2-3 sections in the median plane itself. Chromatophilic granules are present in the prochordal mesoderm (P.PL.M.) as well as in the anterior wall of the fore—gut (W.PG.), but only two mitotic figures were observed in the whole extent of the mass. If we examine text-fig. 18 and Plate IV, figs. 26 and 27, we see at once that the freely projecting cranial tip ( T .CH .) of the chorda is now more marked. It is in active growth as shown by the presence in it of mitotic figures. This is evidence of the independence of the chorda from the prochordal mesoderm. The latter reaches down to the apex of the angle between the anterior wall of the fore-gut (W.PG.) and the posterior wall of the buccal hypophysis (B.HP.). | |||
are, | |||
Plate | |||
This | |||
The | |||
the | |||
Text-fig. 18. Same as text-fig. 17 from R 255, 9 days '10 hours, 24 somites. x 100. | |||
In text-fig. 18 a projection of the anterior wall of the fore-gut ( W.PG.) is seen to bedirected towards the posterior wall of the buccal hypophysis (B.HP.) from which a similar projection arises but does not appear in the figure since the projections are separated by prochordal mesoderm. More laterally they meet but do not fuse together. Certain authors, Kupffer, Atwell, Miller and Parker, state that the endoderm of the fore-gut contributes to the hypophyseal primordium, but, beyond the relationship just described, I have seen no evidence of any contribution from the anterior wall of the fore-gut (W.PG.) to the hypophysis in the rabbit. ' | |||
the | |||
The next stage, an embryo possessing 27 pairs of somites and aged 10 days 18 hours, is represented by text-fig. 19, which is a composite outline drawing. The brain has increased greatly in size, so that the bay resulting from the cranial flexure is much enlarged and the oral plate has entirely disappeared. The cranial extremity of the chorda now presents a bifid appearance, but only the dorsal limb (T.CH.) belongs to the chorda and represents its freely projecting tip. The ventral limb is really formed by a pointed mass of prochordal mesoderm (P.PL.M.), all that is now left of it. The remainder of this structure, including its swollen ventral part, has been practically completely converted into mesenchyme. All the evidence is against the ventral part of the apparent bifid chorda being regarded as a chordal extension. Griinwald, Tourneux, and Keibel gave figures of apparent bifid chorda, but the explanation given above seems to me the only one possible. | |||
and the | |||
In text-fig. 19 it will be seen that the tip of the prochordal mesoderm remnant (P.PL.M.) nearly comes in contact with a projection from the posterior wall of the buccal hypophysis. There is an attempt at a third projection from the anterior wall of the pre-oral gut (W.PG.) which is here very well developed. It is still thicker than the rest of the fore—gut wall (END.). | |||
than | |||
Text-fig. 19. Same as text-fig. 17 from R 281, 10 days 18 hours, 27 somites. x 100. | |||
The next stage is shown in text-fig. 20 and is thatof an embryo possessing 27-28 somites and aged 9 days 16 hours. The prochordal mesoderm (P.PL.M.) is a thin strand of cells containing chromatophilic granules and extending between the chorda dorsally and the posterior wall of the buccal hypophysis ventrally. It is irregular in its outline and I believe it is contributing to the mesenchyme. Its ventral part is definitely mesenchymatous on one side of the median plane, while on the other it consists of more closely packed cells containing granules. We have here the last remnant of the premandibular mass. Note the free tip of the chorda (T.CH.) which shows mitotic figures. | |||
The | The oral plate has practically completely disappeared, leaving only a remnant (0.PL.) at the angle where the endoderm of the pre-oral part of the fore-gut passes into the ectoderm. The History of the Prochordal Plate tn the Rabbit 35 | ||
The | |||
Text-fig. 21 does not show much advance over the previous stage. It is a composite outline drawing of an embryo possessing 28-29 somites and aged 10 days 1 hour. Again the free tip of the chorda (T.CH.) shows mitotic figures indicative of growth at this point. The strand of prochordal mesoderm is not compact, is irregular in outline and seems to be changing into mesenchyme. Between its main mass (P.PL.M.) and the anterior wall of the pre-oral part of the fore-gut (W.PG.) there is a detached mass of prochordal mesoderm. It contains chromatophilic granules and much resembles the same mass in Plate IV, fig. 26, but its ventral part is not swollen; the whole mass extends through 2-3 sections. | |||
Text-fig. 20. Anterior part of a reconstructed median longitudinal section of R 246, 9 days 16 hours, 27-28 somites. x 100. | |||
Text-fig. 21. Composite outline drawing of anterior part of R 277, 10 days 1 hour, 28—29 somites. x 100. A | |||
So far the chorda has not come in direct relation with the hypophysis. They are linked together by the prochordal mesoderm (P.PL.M.). | |||
What is practically the last stage in the history of the prochordal mesoderm is shown in text-fig. 22 and Plate IV, figs. 28 and 29. The embryo from which these are taken possesses 30 pairs of somites and is 1021- days old. Text-fig. 22, which is a composite outline drawing, shows that the oral plate has completely disappeared and that the mesenchyme-filled space cranial to the pre-oral part of the fore-gut has increased in extent. Immediately in front of the gut wall (W.PG.) we come across the remnant of the prochordal mesoderm (P.PL.M.) in the form of a thin strand stretching from the anterior end of the chorda (which is devoid of a projecting free tip), down to the angle (0.PL.X.) bounded by the posterior wall of the buccal hypophysis and the anterior wall of the foregut (W.PG.). It extends through only two sections as a thin compact strand of cells. Its ventral part, which was homologised with the premandibular primordium, has been largely converted into mesenchyme. Close on either side of the middle line in the region of the Ventral swollen mass of earlier stages one encounters a blood Vessel surrounded by mesenchyme. | |||
pre-oral part of the fore-gut (W.PG.) | |||
mesoderm. | |||
Text-fig. 22. R 232, 10% days, 30 somites. x 100. | |||
The tip of the chorda lies cranially to the angle between the anterior ( W.PG.) and the dorsal (EN D.) walls of the fore-gut. No mitotic figures are Visible in it. | |||
Text-fig. 23 is a median reconstruction made from the longitudinal sections of the anterior end of an embryo of 11 days and possessing 37 somites. The chorda has now grown forward to a much greater extent than in any of the The H istory of the Prochordal Plate in the Rabbit 37 | |||
of | |||
of the | |||
A preceding embryos, as is indicated by the position of the remnant of the pro chordal mesoderm (P.PL.M.) which is in continuity with its ventral side some distance behind its tip (T.CH.). The prochordal mesoderm takes the form of a small more or less irregular strand composed of loosely arranged cells which extends through two sections, one of which is shown in Plate IV, fig. 30. | |||
Here again a projection of the posterior wall of the buccal hypophysis (B .H P .) is seen directed towards the prochordal mesoderm remnant (P.PL.M.). It is very similar to that shown in text-fig. 19. In fact the two stages closely resemble each other save for the greater forward extension of the chorda in text-fig. 23. | |||
of the | |||
The buccal hypophysis is still widely open (B.HP.), and the oral plate has completely disappeared. The anterior wall (W.PG.) of the pre-oral part of the fore-gut is still Very thick. | |||
Text-fig. 23. R 210, 11 days, 37 somites. x 100. | Text-fig. 23. R 210, 11 days, 37 somites. x 100. | ||
No definite trace of prochordal mesoderm was observed in any of the older | No definite trace of prochordal mesoderm was observed in any of the older embryos examined. Text-fig. 24 is a reconstructed median longitudinal section of an embryo decidedly older than the last one. No trace of the prochordal mesoderm was seen, and the chorda terminates in a recurved tip. Attention may be called to the curious flexures of the chorda seen in text-fig. 24 as well as in text-fig. 23. The latter is an actual tracing made from the median longitudinal section and the section on either side of it, so that in this ‘embryo at all events the notochordal kinks are actually present as such. The hypophysis (B.H P.) is well developed but still open. The anterior wall of the pre-oral part of the fore-gut (W.PG.) is not thicker than the roof (END.). | ||
embryos examined. Text-fig. 24 is a reconstructed median longitudinal section | |||
of an embryo decidedly older than the last one. No trace of the prochordal | |||
mesoderm was seen, and the chorda terminates in a recurved tip. Attention | |||
may be called to the curious flexures of the chorda seen in text-fig. 24 as | |||
well as in text-fig. 23. The latter is an actual tracing made from the median | |||
longitudinal section and the section on either side of it, so that in this ‘embryo | |||
at all events the notochordal kinks are actually present as such. The hypophysis (B.H P.) is well developed but still open. The anterior wall of the pre-oral | |||
part of the fore-gut (W.PG.) is not thicker than the roof (END.). | |||
After the above observations were completed, I examined a large number of | After the above observations were completed, I examined a large number of embryos aged between 9 and 10 days. They support the conclusions set forth above. The last embryo examined showed a very well-developed Seessel’s pouch, as seen in text-fig. 25. Here the chorda (CH .) ends as usual at this stage in the prochordal mesoderm (P.PL.M.). The very well-developed Seessel’s pouch marked S.P. arises from the dorsal angle of the fore-gut, and its distal end’ contains a closed cavity. It is asymmetrical and lies to the right of the middle line. It is clearly a derivative of the prochordal plate, since the wall of the fore-gut from which it arises (the dorsal angle) is itself derived from that plate. This is the only case of its kind among the very numerous embryos I have examined. | ||
embryos aged between 9 and 10 days. They support the conclusions set forth | |||
above. The last embryo examined showed a very well-developed Seessel’s | |||
pouch, as seen in text-fig. 25. Here the chorda (CH .) ends as usual at this stage in | |||
the prochordal mesoderm (P.PL.M.). The very well-developed Seessel’s pouch | |||
Text-fig. 24. Part of reconstructed median longitudinal /section of R 326, 11 days § hour. x 100. | |||
11 days § hour. x 100. | |||
Text- | Text-fig. 25. Reconstructed median longitudinal section of anterior end of an embryo, 10 days, 26 somites. x 100. | ||
Concerning the later history of the cranial end of the chorda, I may state that I have examined some forty embryos in order to try and determine the The H istory of the Prochorclal Plate in the Rabbit 39 | |||
in the | |||
the posterior wall of the buccal hypophysis. | relation of the tip of the chorda to the buccal hypophysis. The embryos range between 7 and 17-5 mm. in length, and in the later ones the chondrocranium is laid down. In thirty-nine embryos the tip of the chorda, although situated in the vicinity of the posterior wall of the buccal hypophysis, does not come in contact witl1 it. In fact there is nearly always a capillary in contact with that wall. In the fortieth and last examined embryo (Lepus, 10 mm., A.), however, the tip of the chorda (actually its investing sheath) lay in close contact with | ||
the posterior wall of the buccal hypophysis. Reference may be made to the paper of Huber(11) in which this relationship is discussed. | |||
==Summary== | |||
The preceding observations may be summarised as follows: | The preceding observations may be summarised as follows: | ||
The prochordal plate is not the most anterior part of the embryonal endoderm, since in front of it the latter is thickened over a crescentic area (P.END.), | The prochordal plate is not the most anterior part of the embryonal endoderm, since in front of it the latter is thickened over a crescentic area (P.END.), the horns of which are continued backwards as lateral thickenings. In the thickened mesoderm overlying this area the pleuro-pericardial coelom is eventually developed. Its median anterior part contributes to the oral plate endoderm. | ||
the horns of which are continued backwards as lateral thickenings. In the | |||
thickened mesoderm overlying this area the pleuro-pericardial coelom is eventually developed. Its median anterior part contributes to the oral plate | |||
endoderm. | |||
The prochordal plate (P.PL.)is at first a single-celled axial plate of thickened endoderm, and in the early stages there is no evidence of dorsal or lateral proliferation of cells from it. The plate is in continuity behind with the cranial segment of the l1ead—process (I .Z.) which is not yet differentiated into typical chorda plate. As development progresses the prochordal plate shortens, and at the 3-somite stage it thickens dorsally and takes the form of a compact cellular mass several cells in thickness. It is distinctly separate from the limbs of the horseshoe—shaped endodermal thickening. | |||
With the flexure of the brain and the formation of the fore—gut, the prochordal plate comes to form the cranial wall of the latter and probably also a very small part of the adjoining ventral wall. | |||
the prochordal | At the 13-somite stage the chorda, up till now intercalated in the gut roof and in continuity at its anterior end with the prochordal plate, separates from the gut roof. A little later, in embryos of 14-15 somites, the prochordal mesoderm delaminates from the anterior wall of the gut, but its dorsal end remains in continuity with the chorda. The ventral part of the prochordal mesoderm is swollen cranial to the oral plate and has been homologised with the premandibular primordium or somitic mass, but no cavities were observed, except in one case, on one side only. | ||
The | The chorda grows forward beyond its junction with the prochordal mesoderm as a free process in which mitotic figures are not infrequent. This relation explains the apparent bifid character of the anterior end of the chorda. | ||
prochordal | |||
The prochordal plate derivatives comprise the anterior wall of the fore—gut, the prochordal mesoderm including the homologue of the premandibular somitic mass and probably a small part of the oral plate endoderm. Ultimately the prochordal mesoderm is converted into mesenchyme. There is no evidence that the prochordal plate participates in the formation of the chorda. | |||
of | |||
==Discussion== | |||
The axial plate of endodermal cells, the history of which has been outlined here, has been designated differently by different observers. The name “prochordal plate ” suggested by Van Oordt (18) seems to be the most suitable. It has been approved by Hill and Tribe (9), who have shown that this thickened patch of endoderm proliferates mesenchyme in the dog long before the primitive streak is laid down. This observation is of great value, since it shows that the prochordal plate cannot be of head-process origin. They also identified the prochordal plate in the early blastocysts of the cat. Van Beneden, in his posthumous paper of 1912(5), also recognised the existence in the rabbit of a thickened patch of endoderm which was, however, one cell thick. He states that it indicates the bilateral symmetry of the embryo before the primitive streak makes its appearance. Whether this area is the prochordal plate or simply corresponds with the antero-median part of the horseshoe—shaped thickening, described here, it is not easy to decide, since he did not refer to it in his later stages. The same may be said of the thickened area described by Huber (10), who, however, denies the existence of the prochordal plate in the guinea-pig. Rabl (21) figured a similar area behind which, in older stages possessing a head-process, he figured a definite prochordal plate proliferating mesoderm from its dorsal surface, though he did not recognise it. | |||
shown | |||
primitive streak | |||
it | |||
the prochordal plate | |||
Hubrecht(12), however, was the first to distinguish a thickened patch of endoderm at the anterior end of the embryonal area in Screw which he called the “ protochordal plate ” on the supposition that it contributes to the anterior end of the chorda. He recognised also another mesoderm-producing endodermal zone—his annular zone—the antero-median part of which constitutes the protochordal plate. Whether or not the horseshoe—shaped zone of thickened endoderm, described here in the rabbit, corresponds with the anterior part of Hubrecht’s annular zone must remain an open question. This zone does not appear to give origin to mesoderm in the rabbit, and moreover the prochordal plate lies caudal to its antero-median part and in practically all the embryos is delimited from it. The general arrangement in the rabbit seems to resemble very much that in Manis described by Van Oordt(18), and his fig. 21 agrees with my text-fig. 1 . According to Van Oordt’s description and figures, however, his annular zone proliferates mesoderm like that of Hubrecht and eventually forms a complete ring by the junction of its lateral halves behind the primitive streak. The zone in question in the rabbit overlies the thickened band of mesoderm in which the pericephalic coelom arises, and a glance at text-figs. 1, 6 and 9 will convince one that the endothelial wall of the lateral heart tubes, which are the first constituents of the heart to be formed, cannot possibly be derived from the prochordal plate as suggested by Hubrecht(12,13). He also derived the pharyngeal membrane and the wall of the fore-gut from the protochordal plate. It is, however, only the anterior wall of the fore-gut which is of prochordal origin, as the observations of K. M. Parker (19) and my own have shown. | |||
mesoderm, | |||
in the rabbit the | |||
fore-gut. | |||
the | Assheton (4) described in the rabbit an endodermal zone corresponding with the anterior part of Van Oordt’s zone. He calls it the “ pericardial thickening,” but he does not describe any prochordal plate. | ||
Bonnet(6) described a similar endodermal thickening which he called “Erganzungsplatte des Urdarmstranges ”——the completion plate of the headprocess. There is no justification for the name. He did not describe it in his youngest stages of the dog, though it appears quite early as Hill and Tribe have shown, but gave a detailed interesting account of it in later stages in which the primitive streak an(l the head-process were already established. He pointed out that it actively proliferates mesoderm and supports Hubrecht’s view that it furnishes the anterior end of the chorda, but his argument is not convincing. The balance of opinion is against this view, and although I have shown that the prochordal plate shortens yet there is no evidence to show that that shortening is due to its conversion into head-process or chorda plate, rather is it to be regarded as the result of the considerable increase in thickness which it at the same time undergoes. Moreover the chorda grows forward in later stages beyond its point of contact with the prochordal mesoderm. As we are not examining the same embryo at different stages of its development but different embryos at different ages, it is quite possible that the apparent shortening may be due in part to individual variations in the degree of development of the plate. | |||
the | |||
Fig. 60 of Bonnet resembles my text-fig. 11. In his fig. 66, the chorda terminates in a mass of mesoderm proliferated from the anterior wall of the fore-gut. In principle, therefore, it resembles my text-fig. 15. Fig. 67 agrees with my Plate IV, fig. 23, which, however, exhibits no lateral extensions and a slender prochordal mesoderm. | |||
K. M. Parker(19) has dealt in some detail with the prochordal plate in her paper on the hypophysis of the marsupials. There can be no doubt that her prechordal plate (Oppel(17)) is the equivalent of the prochordal mesoderm described here. Fig. 1 in her paper suggests that the prochordal plate gives rise to the anterior wall of the fore-gut as well as a part of the adjoining dorsal wall. She describes the termination of the chorda in the prechordal plate of mesoderm, but this plate lies more or less dorsal to the fore-gut, and in the stage figured blends by its apex with the anterior wall of the latter, whereas in the rabbit the prochordal mesoderm lies cranial to the anterior wall of the fore-gut. This difference, however, is purely topographical and is probably due to the more rapid brain development in the rabbit. | |||
In the marsupials Miss Parker was able to trace the origin of the premandibular somites from the prechordal plate, whereas in the rabbit they do not apparently occur as definite structures, and all there is to represent them is the ventral thickened part of the prochordal mesoderm——the premandibular somitic mass. Apart from these differences in detail our observations are in agreement. | |||
Dorel1o(7), in reptiles, described a mass of endoderm in which the chorda terminates and which encloses the pre-oral gut. It gives rise to hollow budsthe head cavities. von Kupffer (15) found in Bdellostoma and Petromyzon that the chorda ends in a thickened endoderm in the wall of the pre-oral endoderm pocket. | |||
Streeter (23) showed in the pig that the mesoderm grows forward in the form of two horns from the primitive streak region. They meet anteriorly, leaving a “ bald ” area in which the head-process makes its appearance before there is any sign of the prochordal plate. Soon the cells of the latter become piled on each other and show foldings which produce clefts that open into the fore-gut. He homologises this folded plate with the “Gaumentasche” described by Selenka in a much older stage of Didelphys. No such foldings are seen in the rabbit, and K. M. Parker((19), p. 230) has suggested that the “ Gaumentasche ” “is nothing more than a prechordal plate in continuity with a well-developed Seessel’s pocket.” | |||
and | |||
Huber, Rabl, Kolliker and Keibel deny that the endoderm makes any contribution to the mesoderm. The thickened patch of endoderm recognised by Huber (10) in the guinea-pig is considered by that author as the primordium of the oral plate endoderm, in this respect agreeing with Keibel and Carius. | |||
with | |||
I | I have shown that the anterior end of the chorda does not come into direct contact with the buccal hypophysis, and that it is only the strand of prochordal mesoderm which, descending from its point of continuity with the chorda, terminates in the vicinity of the hypophyseal primordium. This is in agreement with Adelmann’s statements (1,2) for the chick. He appears to think that in mammals the chorda very early acquires contact with the buccal hypophysis, but considers that the relation is a secondary one. He agrees with K. M. Parker that the premandibular somites are derived from the prechordal plate. His figs. 33-36(2) closely agree with my text-figs. 15-23, but he does not describe any forward growth of the chorda beyond its contact with the prechordal plate. He points out that the brain grows more rapidly than the fore-gut and prechordal plate, thus leading to the formation of a mesenchyme-filled space in front of the prechordal plate and between it and the brain wall. On the whole my observations on the rabbit are in agreement ‘with his based on the chick (2). | ||
The chorda, in the stages examined, does not show any connection with the anterior wall of the fore-gut after its separation. In fact it grows forward beyond its union with the prochordal mesoderm. Griinwald and Staderini, however, have stated that the chorda comes into contact with Seessel’s pocket. However that may be, the primary relation in the rabbit is that described above, namely, the termination of the chorda in the prochordal mesoderm. | |||
Up to the stages examined there are no ventral extensions of the chorda connecting it with the dorsal wall of the fore-gut. Huber (11) described such extensions in the human embryo and Griinwald in the sheep. These connections must clearly be secondary. | |||
The apparent bifid anterior end of the chorda has already been explained. This condition has been figured by Gri'1nWald(8), Keibel (14), Huber (11) and others, but in view of my observations, the contention that the chorda contributes to the hypophysis (Reichert, His and Dursy) must be regarded as very doubtful. In this connection, Kolliker described in an 11 day rabbit a strand of cells passing from the hypophysis to the chorda, and thought that it represented a contribution by the ectoderm to the chorda. It is clear that this strand. is nothing but the remains of the prochordal mesoderm, as seen in Plate IV, figs. 28 and 29. | |||
Saint-Remy (22) concludes that in all amniotes the anterior end of the chorda is bent down so that it can maintain its insertion into the epithelium of the buccal hypophysis. He states, moreover, that the descending limb is converted into connective tissue. In view of Adelmann’s observations (1,2) on the chick and my own on the rabbit this statement must be regarded as incorrect so far as the insertion of the chorda is concerned. The descending limb referred to is not formed by chorda but by a prochordal mesodermal strand which is later transformed into mesenchyme, whilst the chorda can grow forward beyond it. | |||
Keibel’s figs. 38, 39, 40 a, 40 b (14)for the rabbit resemble my text-fig. 15, and his fig. 41 b seems to show an apparent bifid anterior end of the chorda, but the lower limb is very short and ends in the wall of the fore-gut in the region of Seessel’s pocket. Paulisch’s figs. 1, 2, 3 (20) of 9-11 days rabbits are very much like those of Keibel. ' | |||
Atwell (3) figured a longitudinal section through the head of a 6 mm. rabbit embryo in which he showed an epithelial bud from the anterior wall of the foregut which more or less fuses with the tip of the chorda. He thinks it is identical with Selenka’s “Gaumentasche” and Saint-Remy’s descending branch of the chorda. It, no doubt, represents part of the prochordal mesoderm. | |||
Some observers—von Kupffer, Saint-Remy and Miller (16)—hold the View that the anterior wall of the fore-gut contributes to the buccal hypophysis, but beyond the projections mentioned in the descriptive part of this paper I have no evidence that the anterior wall of the fore-gut contributes to the hypophysis in the rabbit. | |||
I hope later to be able to examine rabbit embryos of 6 and 7 days and so complete this study of the history of the prochordal plate. | |||
I wish to express my thanks to Mr A. K. Maxwell for touching up and lettering the text-figures and to Mr F. Pittock for the photomicrographs. | |||
==References== | |||
H | (1) ADELMANN, H. B. (1922). “The significance of the prechordal plate, an interpretative study.” Amer. J. Anat. vol. XXXI. | ||
(2) —— (1926). “The development of the premandibular head cavities and the relations of the anterior end of the notochord in the chick and robin.” J. Morph. and Phys. vol. XLII, No. 2. | |||
(3) ATWELL, W. J. (1916). “Relation of the chorda dorsalis to endodermal component of hypophysis.” Anat. Rec. vol. X. | |||
(4) ASSHETON, R. (1894-95). “The primitive streak of the rabbit, the causes which may determine its shape and the part of the embryo formed by its activity.” Quart. J. M tic. Sci. vol. XXXVII. | |||
( | (5) VAN BENEDEN, E. (1912). “Recherches sur l’embryologie des Mammiféres.” Arch. dc Biol. t. XXVII. | ||
( | (6) BONNET, R. (1901). “Beitrage zur Embryologie des Hundes. Erste Fortsetzung.” Anat. Hefte, 51 Heft, Bd. XVI. | ||
(7) DORELLO, P. (1900). “Studi embryologici sui Rettili.” Ricerche fatte nel Lab. di Anat. Norm. della R. Universita di Roma ed in altri Lab. biol., vol. VII, fasc. 3e 4, 1900. Quoted by Bonnet (6). | |||
(7) DORELLO, P. (1900). “Studi embryologici sui Rettili.” Ricerche fatte nel Lab. di Anat. | |||
Norm. della R. Universita di Roma ed in altri Lab. biol., vol. VII, fasc. 3e 4, 1900. | |||
Quoted by Bonnet (6). | |||
(8) GRI'iNWALD, L. (1910). “Eine Cyste der Chordascheide.” Anat. Anz. vol. XXXVII. | |||
(9) {{Ref-Hill1924}} | |||
(10) HUBER, G. C. (1917). “On the anlage and morphogenesis of the chorda dorsalis in the Mammalia, particularly the guinea-pig.” Anat. Rec. vol. XIV. | |||
(11) —- (1912). “On the relation of the chorda dorsalis to the anlage of the pharyngeal bursa or median pharyngeal recess.” Anat. Rec. vol. VI. | |||
(12) HUBRECHT, A. W. (1890). “Studies in mammalian embryology. II. The development of the germinal layers in Sorex vulgaris.” Quart. J. Mic. Sci. vol. XXXI. | |||
(13) —— (1909). “Early ontogenetic phenomena in mammals and their bearing on our interpretation of the phylogeny of the vertebrates.” Quart. J. 111 ic. Sci. vol. LIII. | |||
(14) KEIBEL, F. (1889). “Zur Entwicklungsgeschichte der Chorda bei Saugern (Meerschweinchen und Kaninchen).” Arch. Anat. u. Physiol. Anat. Abt. | |||
(15) VON KUPFFER (1894). “Die Deutung des Hirnanhanges.” Sitzber. der Gesellschaft f. Morph. u. Physiol. in Munchen. Quoted by Bonnet (6). | |||
(16) MILLER, M. M. (1916). “The hypophysis of the pig.” Anat. Rec. vol. X, No. 3. Journal of Anatomy, Vol. LX VI, PC!/7'5 1 | |||
( 17) OPPEL, A. (1890). “Uber Vorderkopfsomiten und die Kopfhohle von Anguis Fragilis.” Arch. f. M ikr. Anat. Bd. XXXVI. Quoted from Hill and Tribe (9) and K. M. Parker (19). | |||
(18) VAN OORDT, G. J. (1921). “Early developmental stages of Manis Javanica Desm.” Verhand. Kori. Akad. v. Wetensch. Amsterdam, Dl. XXI. | |||
(19) PARKER, K. M. (1917). “The development of the hypophysis cerebri, pre-oral gut and related structures in the marsupials.” J. Anat. vol. LI. | |||
( | (20) PAULISCH, O. (1887). “Das vordere Ende der Chorda Dorsalis und der Franksche Nasenkamm.” Arch. f. Anat. u. Physiol. Anat. Abt. | ||
Arch. f | |||
( | (21) RABL, C. (1915). “Edouard van Benden und der gegenwiirtige Stand der wichtigesten von ihm behandelten Probleme.” Arch. f. Milcr. Anat. Bd. LXXXVIII. | ||
( | (22) SAINT-REMY, G. (1895-96). “Recherches sur l’extrémité antérieure de la Chorda Dorsal chez les Amniotes.” Arch. de Biol. t. XIV. | ||
( | (23) {{Ref-Streeter1927}} | ||
==Explanation of Plates== | |||
The figures on Plates I and II have suffered a reduction of 1/3, those on Plates III and IV a reduction of 1/2. | |||
===Abbreviations=== | |||
{{Aasar1931 Abbreviations table}} | |||
===Plate I=== | |||
[[File:Aasar1931 plate1.jpg|800px]] | |||
Fig. 1. T.S. (9.1.2.) of R 335, 8 days, primitive streak and head-process stage. x 330. | |||
Fig. 2. T.S., 48th section behind fig. 1—same embryo as fig. 1. x 330. | Fig. 2. T.S., 48th section behind fig. 1—same embryo as fig. 1. x 330. | ||
| Line 1,368: | Line 477: | ||
Fig. 7. T.S., 41st section of same. x 330. | Fig. 7. T.S., 41st section of same. x 330. | ||
===Plate II=== | |||
[[File:Aasar1931 plate1.jpg|800px]] | |||
Fig. 8. T.S., 56th section of same as 5. x 330. | Fig. 8. T.S., 56th section of same as 5. x 330. | ||
| Line 1,374: | Line 485: | ||
Fig. 9. T.S., 95th section of same as 5. x 330. | Fig. 9. T.S., 95th section of same as 5. x 330. | ||
Fig. 10. Median longitudinal section (5.3.6.) of R 347, 8 days 3 hours, 5 somites. x 250. | Fig. 10. Median longitudinal section (5.3.6.) of R 347, 8 days 3 hours, 5 somites. x 250. | ||
Fig. 11. Longitudinal section (2.2.3.) of R 348, 8 days 3 hours, 5 somites. x 250. | Fig. 11. Longitudinal section (2.2.3.) of R 348, 8 days 3 hours, 5 somites. x 250. | ||
Fig. 12. Median longitudinal section, 16th section after fig. 11, same embryo. x 250. | Fig. 12. Median longitudinal section, 16th section after fig. 11, same embryo. x 250. | ||
Fig. 13. T.S., 35th section of R 318A, 8 days 17% hours, 6 somites. x 250. | Fig. 13. T.S., 35th section of R 318A, 8 days 17% hours, 6 somites. x 250. | ||
===Plate III=== | |||
[[File:Aasar1931 plate3.jpg|800px]] | |||
Fig. 14. 50th section of same as 13. x 250. | Fig. 14. 50th section of same as 13. x 250. | ||
| Line 1,396: | Line 510: | ||
Fig. 20. Next section behind 19. x 250. | Fig. 20. Next section behind 19. x 250. | ||
Fig. 21. Anterior part of a median longitudinal section (3.3.4.) of R 306, 8 days 16 hours, 9 somites. | Fig. 21. Anterior part of a median longitudinal section (3.3.4.) of R 306, 8 days 16 hours, 9 somites. x 250. | ||
Fig. 22. T.S. (18.5.1.) of R 179, 8 days 19 hours, 14-15 somites. x 250. Shows premandibular somitic mass. | |||
Fig. 22. T.S. (18.5.1.) of R 179, 8 days 19 hours, 14-15 somites. x 250. Shows premandibular | |||
===Plate IV=== | |||
[[File:Aasar1931 plate2.jpg|800px]] | |||
Fig. 23. T.S. (8.3.2.) of R 253, 9 days 10 hours, 21 somites. x 250. | Fig. 23. T.S. (8.3.2.) of R 253, 9 days 10 hours, 21 somites. x 250. | ||
| Line 1,410: | Line 523: | ||
Fig. 25. Median longitudinal section (6.3.2.) of R 248, 9 days 16 hours, 23-24 somites. x 180. | Fig. 25. Median longitudinal section (6.3.2.) of R 248, 9 days 16 hours, 23-24 somites. x 180. | ||
Fig. 26. Median longitudinal section (8.2.2.) of anterior part of R 255, 9 days 10 hours, 24 somites. | Fig. 26. Median longitudinal section (8.2.2.) of anterior part of R 255, 9 days 10 hours, 24 somites. x 180. | ||
x 180. | |||
Fig. 27. Next section (9.2.2.) of same. x 180. | Fig. 27. Next section (9.2.2.) of same. x 180. | ||
| Line 1,417: | Line 529: | ||
Fig. 28. Median longitudinal section (8.5.2.) of R 282, 10% days, 30 somites. x 180. | Fig. 28. Median longitudinal section (8.5.2.) of R 282, 10% days, 30 somites. x 180. | ||
Fig. 29. Section immediately after 28 (9.5.2.). x 180. - | Fig. 29. Section immediately after 28 (9.5.2.). x 180. | ||
Fig. 30. Median longitudinal section (anterior part) (6.2.3.) of R 210, 11 days, 6-5 mm., 37 somites. x 135. | |||
The magnifications of text-figures 17, 18, 19, 21, 22, 23 and 25 are correct as printed. | |||
{{Footer}} | {{Footer}} | ||
[[Category: | [[Category:Rabbit]][[Category:Historic Embryology]][[Category:1930's]] | ||
[[Category:Hill Collection]] | |||
Latest revision as of 09:06, 29 July 2019
| Embryology - 16 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Aasar YH. The history of the prochordal plate in the rabbit. (1931) J. Anat., 66(1):14-i3. PubMed 17104355
| Online Editor |
|---|
|
This historic 1931 paper describes very early rabbit development during the early period of gastrulation and somitiogenesis.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The History of the Prochordal Plate in the Rabbit
By Y. H. Aasar, M.Sc.
Honorary Demonstrator, Department of Anatomy and Embryology, University College, London
1931
- Thesis approved for the degree of Master of Science in the University of London.
Introductory
This work has been carried out under the supervision of Prof. J. P. Hill to whom my sincere thanks are due, not only for his great help and criticism but also for allowing me access to the valuable series of rabbit embryos in his collection.
In all, ninety embryos, most of which are in a very good state of preservation, have been examined. The age, taken as the time lapsing between insemination and the removal of the embryo, ranges from eight to eleven days, but where possible the somites have been counted, and their number has been taken as indicating the degree of development. This method, however, does not seem to me to be the most exact. In fact the best criterion is a general survey of the degree of development of the structures present. The majority of the embryos were cut transversely, but a number were cut longitudinally. These have been of special value, and have enabled me to confirm the reconstructions made of transversely cut embryos.
Descriptive
I propose to describe shortly the stages illustrating the history of the prochordal plate. The description will be made clearer by reference to the accompanying series of photomicrographs and reconstructions.
The earliest embryos examined are those of the head-process stage. In the two embryos representing this stage, the primitive knot, the primitive streak and the mesodermal sheet are already established. The prochordal plate presents the appearance shown in Plate I, fig. 1. It first makes its appearance 0-4 mm. behind the anterior border of the embryonal area and appears as a localised thickening of the endoderm. It does not seem to ‘contribute to the mesoderm which reaches up to its lateral edges. The cells composing it are robust and compactly arranged, so that the plate is easily distinguishable from the endoderm on either side. Here and there a few flattened cells are seen on its under-surface.
If this figure is compared with Plate I, fig. 2, which represents the 48th section behind fig. 1, the difference stands out clearly in so far as the relation to the mesoderm is concerned. This is the head-process region and must not be confused with the prochordal plate. The presence of a mitotic figure on the right side and the apparent continuity with the mesoderm on the left seem to me to be very good evidence of the contribution of the head-process to the mesoderm. The appearance of this figure is also important, because later I shall produce figures, very similar to this, from an area between the definite prochordal plate and the definite chorda plate, which is marked (I .Z.) in the figures.
I should mention that in one of the embryos of the head-process stage the mesoderm is continuous across the middle line in front of the prochordal plate, while in the other, a rather poor specimen, it is not yet continuous, and we see the ectoderm overlying a thick patch of endoderm which extends across the whole breadth of the embryonal area in front of the prochordal plate.
This patch (P.EN D.) is however better marked in the next stage, which is shown in text-fig. 1. This is a. dorsal view of an embryo with possibly one pair of somites and in which the axial structures are depicted. The crescentic shaded area in the figure (P.END.) represents a patch of thickened endoderm, the transverse part of which is co-extensive with the area between the prochordal plate (P.PL.) and the anterior border of the embryonal shield. It is prolonged backwards as two horns situated peripherally. The posterior limits are not easy to determine, but they extend some distance backwards. In this connection it is essential to state that Hubrecht has described in Screw vulgaris the presence of an annular zone of thickened endoderm which is in continuity with the lateral margins of the prochordal plate and which he states proliferates to form vascular mesoderm. Assheton describes a similar area, which is, however, deficient posteriorly and which he calls the “pericardial thickening,” in the rabbit.
Text-fig. 1. A reconstruction dorsal view of embryo R352, 8 days 1 hour with possibly 1 somite, X 25.
The horseshoe-shaped zone of thickened endoderm (P.EN D.) here described, or rather its median part, lies cranially to the prochordal plate (P.PL.) as textfig. 2 clearly shows. This figure also shows the presence of mesoderm (P.M.) between the ectoderm and the median part of the horseshoe-shaped endodermal thickening (P.END.), but whether the thickened endoderm contributes to this mesoderm or whether the latter is solely derived from the primitive streak mesoderm, the horns of which grow forward and meet, cannot be decided in the stages examined. The further history of the antero-median part of the horseshoe-shaped endodermal thickening can be easily followed by the study of the reconstructed median longitudinal sections which are given here. It is important to note that with the forward growth of the brain accompanied by the development of the head-fold bay, the subsequent brain flexure and the backward growth of the pericephalic coelom, this antero-median area of thickened endoderm is seen to contribute to the floor of the fore-gut and to furnish the greater part of the endodermal element of the oral plate. Plate I, fig. 3, from an embryo with possibly one pair of somites, shows the prochordalplate to be in much the same condition as that shown in Plate I, fig. 1. It is also still the same, though shortened, in embryos possessing two somites and shown in textfigs. 2A. and 213. It is clear, however, that the mass of mesoderm (P.M.) is becoming thicker. I think it may rightly be called the “ pre-axial mesoderm,” since it is cranial to the prochordal plate (P.PL.), which is the most cranial axial structure.
Text-fig. 2. A reconstructed median longitudinal section of anterior end of same embryo as text-fig. 1. x 100.
Text-fig. 2A. A reconstructed median longitudinal section of anterior end of R 339, 8 days, 2 somites. x 100.
Text-fig. 2B. A reconstructed median longitudinal section of anterior end of R 338, 8 days, 2 somites. x 100.
So far, the prochordal plate has been a one-layered structure, but in the stage possessing three pairs of somites it is several cells thick, cells having been proliferated on its upper side as is seen in Plate I, fig. 4«. This figure also shows the two lateral horns of the thickened horseshoe-shaped endoderm situated towards the periphery of the embryonal area. They are made up of large cylindrical endodermal cells and are connected with the central prochordal plate by thin flattened endoderm. The mesoderm overlying the thickened endodermal horns is more compact than elsewhere, and in this as well as in the next embryos this compact mesoderm is found to follow the course of the horseshoe-shaped endodermal thickening. It is in this mesoderm that the pericardio-peritoneal coelom makes its appearance first in the region of the lateral heart tubes in embryos with two pairs of somites. It extends forward and by the 5-somite stage it has become horseshoe-shaped with its transverse median part cranial to the prochordal plate. Van Beneden states that it is horseshoeshaped from the beginning. It should be pointed out, however, that the earliest cavity to appear is the extra-embryonal coelom. It develops very early immediately behind the posterior end of the embryonal shield. The pericardioperitoneal coelom is bilateral at first, and by the extension forward of its lateral halves and their union in front, the pericephalic coelom is established.
The horseshoe—shape of the thickened endodermal zone, the overlying compact mesoderm and the independence of boththese structures from the prochordal plate are clearly shown in this embryo, but are illustrated from the next older stage—the 4-somite stage. I would like, however, to add that the horns of the horseshoe-shaped endodermal thickening become insignificant just before the region of the first pair of somites is reached.
Text-fig. 3. Superimposed axes of twelve embryos with the anterior end as the fixed point. From above down they are: R 336, 8 days, no somites. R 353, 8 days 1 hour, no somites. R 352, 8 days 1 hour, ? 1 somite. R 349, 8 days 3 hours, 2 somites. R 338, 8 days, 2 somites. R 339, 8 days, 2 somites. R 314, 8 days 172 hours, 4 somites. R 357, 8 days 1 hour, 4-5 somites. R 343, 8 days 4 hours, 5 somites. R 318 A, 8 days 17% hours, 6 somites. R 316, 8 days 17% hours, 6 somites. R 346, 8 days 3 hours, 7 somites. x 25.
Before discussing the 4-somite stage I wish to refer briefly to text-figs. 3 and 4. These represent the structures in the median plane in each of the embryonal shields, which have been reconstructed in the same way as text-fig. 1. They are placed as shown in the figures with the anterior end as the fixed point in textfig. 3 and the middle of the primitive knot as the fixed point in text-fig. 4. They range from the presomitic stage to the 7-somite stage. In all the prochordal plate is flat and the fore-gut has not yet closed.
Text-fig. 3 shows at a glance the remarkable fact that the prochordal plate (P.PL.) shortens with advance of age, while the chorda plate (CH .PL.) increases considerably in length. The significance of this will be discussed later, but it must be noted that this decrease in length of the prochordal plate is accompanied by an increase in thickness. In fact the first traces of this thickening are seen in Plate I, fig. 4. Any attempt, therefore, to explain the genuine shortening of the prochordal plate must not ignore the marked thickening it undergoes.
The second point to attract attention in text-figs. 3 and 4 is the apparent forward shifting of the prochordal plate (P.PL.) towards the anterior end of the embryonal area. This may be due to the unequal rate of growth of the parts concerned. Whether or not the shortening of the prochordal plate means contribution to the chorda plate is a question to be discussed.
The anterior end of the prochordal plate is practically always well defined from the antero-median part of the horseshoe-shaped thickened endoderm and the overlying compact mesoderm, but its junction with the chorda plate is not so readily determinable. In text-figs. 3 and 4, in a number of embryos, I have indicated a short axial segment intervening between the prochordal plate and the definite chorda plate (I.Z.). Its structure and relation to the mesoderm are shown in Plate II, fig. 8, and Plate III, fig. 14:. Its cells are robust and closely packed, and at the sides are continuous with the mesodermal cells. The segment is usually one cell thick and contrasts markedly with the prochordal plate and the chorda plate. Inappearance it very much resembles Plate I, fig. 2, which passes through the head-process. I consider this segment, labelled I .Z., as the last remains of the undifferentiated head‘-process, in fact its anterior end. If this view is correct, then the prochordal plate is not of head-process origin. This segment is shown in five embryos out of twelve, but its frequency is greater than that because in some cases, after having come to the conclusion that it is part of the head-process, I have included it with the chorda plate. It disappears later in older embryos, having become converted into chorda plate which becomes properly intercalated in the gut wall.
Text-fig. 4. Same as text-fig. 3, but middle of primitive knot is taken as the fixed point. x 25.
The shortening of the prochordal plate is relatively insignificant in com - parison with the marked lengthening of the chorda plate. Even supposing the prochordal plate is diminished in length by reason of its transformation into chorda plate, a supposition which I believe is not true, the part added would be so small as to be insignificant.
Text-fig. 4. shows that the primitive knot (P.K T.) is more or less constant in extent, but the primitive streak (P.S'T.) shortens as age advances.
It is perhaps worthy of mention here that the cloacal membrane (CL.M appears very late at the 6-somite stage immediately behind the primitive streak and not actually in its substance. The area behind the primitive streak in which it appears gradually increases in extent as age advances. The cloacal membrane (CL.M.) is shown in text-fig. 4 in the two embryos with 6 somites, but it is not yet distinguishable in the 7-somite. embryo.
The two small circles situated just in front of the primitive knot in two of the embryos depicted in text-figs. 3 and 4 represent the ventral opening of the chorda canal, which is poorly developed in rabbit embryos.
Now we can come back to the 4—somite stage. Amedian longitudinal section of its anterior end is shown in text-fig. 5 and a dorsal View in text-fig. 6. In the latter the outlines of the horseshoe-shaped mesoderm, in the anterior part of which the pericephalic coelom (P.COE.) is not yet fully developed, are shown. The horns of this mesoderm, if followed back, will be found continuous with the primitive streak mesoderm (P .S T .]|l.). The pericardio-peritoneal coelom (PP. C.) is well developed in the region of the lateral heart tubes (E .H T .), but anteriorly, especially in the pre-axial mesoderm, it is in the form of isolated clefts. The position of the la.teral heart tubes (E .H T .) leaves no doubt of their independence from the prochordal plate (P.PL.) which latter is also independent from the pre-axialmesoderm (P.ZVI.), as text-figs. 5 and 6 and Plate I, figs. 6 and 7, clearly show. When we examine text-figs. 2, 2A, 2 B and 5 we find that the mass of preaxial mesoderm (P.lM.) is gradually increasing, and that in the last figure the pericardio-peritoneal coelom has appeared in the middle line, although its lateral extension is better appreciated by examining Text-fig. 6.
Text-fig. 5. A reconstructed median longitudinal section of R 314, 8 days 17;} hours, 4 somites. x 100.
The prochordal plate (P.PL.) has increased considerably in thickness and now appears in transverse section as a more or less triangular plate with cells piled on top of each other and Well packed. The mesoderm on either side stops short of its lateral sides, and its cells contain chromatophilic granules in their cytoplasm. Its blunt apex produces an elevation in the middle of the neural plate, and curiously enough this elevation is replaced by a groove as soon as the caudal end of the prochordal plate is reached and we pass into that area between it and the definite chorda plate, regarded as the anterior end of the undifferentiated head-process (I .Z.). This area is well developed in this embryo, as can be gathered from text-figs. 5 and 6 and also from Plate II, fig. 8, which also shows the last trace of the elevation caused by the prochordal plate. It also shows very clearly the continuity of the mesoderm with the remains of the head-process. Behind this area is the typical c chorda plate. It is intercalated in the endoderm and does not show any continuity with the mesoderm on either side of it. To appreciate these points, Plate I, fig. 7, and Plate II, figs. 8 and 9, should be examined. The first figure is a typical section of the prochordal plate, the second passes through the remains of the head-process and the third through a typical chorda plate region.
In front of the prochordal plate the relations of the parts are well shown in Plate I, figs. 5 and 6. In the former we see three layers spreading across the whole width of the embryonal area in that region. The endoderm is thick but is really made up of a layer one cell thick. The cells are large and cylindrical. This is the antero-median part of the horseshoe-shaped endodermal thickening. In Plate I, fig. 6, only O-048 mm. behind fig. 5, the endoderm is beginning to resolve itself into the two horns connected here by a thinner patch of endoderm. The overlying mesoderm resolved into two horns, as the two figures under consideration clearly show. In the mesoderm referred to the pericephalic coelom has appeared, but is not well developed. It has, where present, a thin dorsal and a thicker ventral wall.
Text-fig. 6. A reconstructed dorsal view behaves like the endoderm in so far as it is of same embryo as 1-,ext_fig_ 5, X 25,
A glance at the five figures——Plate I, figs. 5, 6, 7, and Plate II, figs. 8, 9will convince one of the independence, already referred to, of the endoderm of the horseshoe-shaped thickening and the pre-axial mesoderm from the prochordal plate. This statement is further confirmed by Plate II, fig. 10, of a 5-somite embryo. It is as median a longitudinal section as possible and clearly shows the thickened endoderm at the anterior end of the embryonal area overlain by the compact pre-axial mesoderm, in which no definite pericephalic coelom has appeared. It is connected to the prochordal plate posteriorly by a thinner stretch of endoderm. The prochordal plate is only two cells thick and is not as well developed as in the 4-somite stage. This is, however, an individual variation.
The relations of the prochordal plate to the pre-axial mesoderm, the underlying thickened endoderm and the lateral heart tubes should be borne in mind. There is an interval between the anterior end of the prochordal plate and the posterior border of the pre-axial mesoderm. This is seen in text-figs. 2, 2 A, 2 B and 5, and is confirmed by Plate I, fig. 6. Few cells occupy this interval and there is no indication that the prochordal plate contributes to the pre-axial mesoderm. It has, however, proliferated dorsally and has also shortened, a fact which may be explained by the contraction. of the plate rather than by its transformation into chorda plate. Again, there is no evidence that the prochordal plate is contributing to the mesoderm on either side of it.
The endothelium of the lateral heart tubes may be derived either from the horns of the horseshoe-shaped endodermal thickening, or from the overlying compact mesoderm. The prochordal plate does not give any contribution to either the endothelium of the lateral heart tubes or the wall of the pleuropericardial coelom as was suggested by Hubrecht.
Embryos possessing five pairs of somites show little or no advance in the condition of the prochordal plate. Reference has already been made to Plate II, fig. 10. The pre-axial mesoderm is here clearly distinct both from the anteromedian part of the horseshoe-shaped endodermal thickening and the prochordal plate. The prochordal plate here is two cells thick but is less massive than that of the 3-somite stage described above, though it is decidedly more advanced than that of the 2-somite stage.
Plate II, fig. 11, is a longitudinal section, about 0-128 mm. from the median plane of another 5-somite embryo. It shows very well the compact mass of pre-axial mesoderm tending to be arranged in two layers, a thinner dorsal and a thicker ventral with a potential cavity between them. The contrast between this mesoderm and the more loose mesenchyme with which it is continuous posteriorly is very marked. The thickened endoderm, at the anterior end of the embryonal area and underlying the pre-axial mesoderm, is also very distinct. The prochordal plate (Plate II, fig. 12) is 2-3 cells thick, whereas this anteromedian part of the horseshoe-shaped endodermal thickening is practically one cell thick, and while the latter is separated from the ectoderm by a compact darkly staining mass of pre-axial mesoderm, the former is not separated by any mass from the overlying ectoderm. There is in this specimen, as Plate II, fig. 12, shows, an apparent continuity between the anterior end of the prochordal plate and the pre-axial mesoderm, but as I have pointed out above this is not the usual arrangement, which is the more or less complete separation of the pre-axial mesoderm from the prochordal plate by an interval containing very few loose cells. I consider the condition seen here as simply an exaggeration of the usual arrangement. It must be remembered that the pre-axial mesoderm was already present in this region in the earliest embryos examined when the prochordal plate was one cell thick.
The next stage of a 5-6-somite embryo exhibits much the same condition as the preceding stages. Text-fig. '7 represents a reconstructed median longitudinal section of the anterior part of the embryo. It shows a well-developed prochordal plate (P.PL.) connected to the chorda plate (CH .PL.) by a segment, regarded as the remains of the undifferentiated head-process (I .Z.), but anteriorly it is separate from the pre-axial mesoderm (P.M.) in which the pericephalic coelom is not yet continuous across the middle line. The ectoderm is decidedly thicker and is elevated over the prochordal plate area in the middle line. The latter shows an isolated mass of cells in its caudal half of unknown significance.
Beneath the anterior end of the prochordal plate there are three cells which are considered as degenerating cells of prochordal plate origin. Few mitotic figures are present in it, and again there is no evidence that it contributes to the mesoderm on either side of it.
It is a noteworthy fact that, whilst the prochordal plate undergoes definite changes, particularly in thickness, the antero-median part of the horseshoe-shaped endodermal thickening (P.END.) does not show any change in form, thickness or extent.
Text-fig. 8. Anterior part of a reconstructed median longitudinal section of R 346, 8 days 3 hours, 7 somites. x 100. '
Now we pass on to a slightly older stage which is represented by text-fig. 8, the anterior end of a reconstructed median longitudinal section of a 6-7-somite embryo. The anterior part of the prochordal plate is more massive than its posterior part. Its caudal extremity corresponds with the point where the elevation in the ectoderm, referred to above, gives place to a groove. The head-fold bay (HF.B.) is beginning to appear just cranial to this elevation in the middle of the neural plate over the extent of the prochordal plate. This latter shows chromatophilic granules, but there is no proliferation of mesoderm from its lateral margins. Mitotic figures are not specially abundant, and in this embryo some degenerated cells are scattered here and there beneath the main mass of the plate. There is a short segment, the remains of the head-process, which extends through 2-3 sections and which, in the figure, has been included with the chorda plate (CH .PL.). The distinctive features of the prochordal plate, apart from its thickness and position, are the presence of the chromatophilic granules and the compact arrangement of its cells.
Text-fig. 9 is a reconstruction of the dorsal view of a 6-somite embryo, and text-fig. 10 is a median longitudinal reconstruction of the anterior part of the same embryo. The pericephalic coelom (P.COE.) is well developed and the preaxial mesoderm (P.M.) in which it is developed is in close contact with the endoderm just in front of the anterior end of the prochordal plate (P.PL.). The latter is very well developed and it is now many cells in thickness. It is triangular in both the coronal and Sagittal planes, as is clearly seen in text-fig. 10 and Plate II, fig. 13. The elevation presumably produced by this plate in the middle of the neural plate is also well shown in both figures. The plate shows the characteristic chromatophilic granules (Plate II, fig. 13) and does not contribute to the mesoderm on either side. On the right of this figure one can see a thin endoderm layer connecting the prochordal plate with the right horn of the horseshoe-shaped endodermal thickening underlying the pericardioperitoneal coelom. The latter has a thin ‘ roof and a thick floor between which CL-"T . and the endoderm is a thin strand Of Text-fig. 9. Reconstructed dorsal view of angioblastic cells. The endothelial heart R 313A» 3 days 17% h011I‘S» 5 80miteStubes (E.HT.) are well developed, as " 25' can be seen in text-fig. 9. The head-fold bay (HF.B.) is well established, as seen in text-fig. 10.
Plate III, fig. 14, passes through the cranial segment of the head-process and shows very well its relations to the mesenchyme, especially on the right side. In this figure the dorsal furrow has replaced the median elevation which was seen in the region of the prochordal plate in Plate II, fig. 13. Plate II, fig. 13, and Plate III, figs. 14 and 15, show the prochordal plate, the remains of the cranial segment of the head-process and the chorda plate respectively, and should be compared with Plate I, fig. 7, and Plate II, figs. 8 and 9.
In the dorsal view—text-fig. 9-—the outlines of the pericardio-peritoneal coelom (PP.C.) and its pericephalic part (P.COE.) are well shown. It communicates in the region of the first somite with the extra-embryonal coelom, and immediately behind the last somite its inner limit (M .B.) lies Very near the margin of the embryonal area.
It may be noted that the cloacal membrane (CL.M.) makes its first appearance in this stage immediately behind the posterior end of the primitive streak and not in its substance as some observers claim.
Some progress has manifested itself in the next stage, although it possesses the same number of somites as the preceding. Text-fig. 11 shows a well developed head-fold bay (HF.B.) and an apparent caudal displacement of the pre-axial mesoderm (P.M.), its contained pericephalic coelom (P.COE.) and the underlying thickened endoderm of the horseshoe-shaped area (P.END.). The prochordal plate (P.PL.) is well marked and has a triangular shape in the sagittal plane. It occupies the angle between the neural plate and the upper layer of the head-fold bay and forms -the roof of the incipient fore-gut bay. This stage is importantvbecause it shows us the beginning of the establishment of the anterior position of the prochordal‘ plate, which is better seen in textfig. 12.
Text-fig. 10. Anterior part of a reconstructed median longitudinal section of R 318 A. x 100.
Text-fig. ll. Anterior part of a reconstructed median longitudinal section of R 316, 8 days 17% hours, 6 somites. x 100.
In preceding stages the pre-axial mesoderm was situated in practically the same plane as the prochordal plate, but with the establishment of the headfold bay and the forward growth of the neural plate, the pre-axial mesoderm and the underlying endoderm of the horseshoe-shaped area now occupy a more ventral plane than the prochordal plate, although they are still anterior to it.
The tip of the angle between the neural plate and -the upper layer of the head-fold bay lies opposite the posterior third of the pre-axial mesoderm, whereas in the previous embryo it lies just behind its posterior end. These alterations in the relations of the prochordal plate are the outcome of the commencing formation of the fore-gut, and that, as K. M. Parker has pointed out in her paper on the early development of the heart in marsupials, would appear to be induced by the forward growth of the brain plate, the backward extension of the pre-axial mesoderm and its contained coelom, and the gradual incorporation into the latter of the bilateral pericardial cavities.
Plate III, fig. 16, showing a median longitudinal section of a 7 -somite embryo, affords confirmation of the general correctness of the last four text-figures, which are reconstructions made from transverse series. It much resembles text-fig. 11, the projection formed by the main mass of the prochordal plate being a striking feature. The posterior limit of the prochordal plate is not so easy to make out here as in the transverse sections, in which the characteristic appearance of the cranial segment of the head-process serves as a good landmark. Its anterior end passes into the antero-median part of the horseshoeshaped endodermal thickening, overlying which is the pre-axial mesoderm with its pericephalic coelom. I have mentioned above, however, that there is a thinner patch of endoderm between the prochordal plate and the anteromedian thickened endoderm referred to. This is the usual arrangement. The elevation of the anterior part of the brain plate as well as the head-fold bay are well marked.
Between the floor of the pericephalic coelom and the underlying thickened endoderm there are found two angioblastic cells, the origin of which is not easy to decide, but they appear to be mesodermal. There is no evidence of proliferation on part of the endoderm.
The changes that have been described above have made further progress in the next stage possessing 8-9 pairs of somites. A reconstructed median longitudinal section is shown in text-fig. 12. Here the head-fold bay (H F.B.) is more marked than before, the brain plate (BR.PL.) has grown forward considerably and the pericephalic coelom (P .COE. ) now lies further back, its anterior or cranial wall lying a little caudal to the free tip of the prochordal plate (P.PL.) which still occupies the angle between the brain plate (BR.PL.) and the upper layer of the head-fold bay. The fore-gut (FG.) is established, its length being 0-112 mm. Examination of text-figs. 8, 10, 11 and 12 will convince one that its floor is formed by the antero-median part of the horseshoe-shaped endodermal thickening. The prochordal plate (P.PL.), however, forms the anterior wall of the foregut and a very small part of the adjoining roof. Perhaps this will be better understood if we were to unfold the bends caused by the head-fold bay and the closed gut. If we do that we should obtain a figure very much like text-fig. 10. In this way we can realise how the endoderm of the thickened zone, originally anteriorly situated, has come to form the floor of the fore-gut, with the result that the prochordal plate comes to be the most anterior or cranial endodermal structure. As the reconstruction shows there are no foldings or evaginations in the fore-gut wall where it is formed by the prochordal plate.
Text-fig. 12 shows the relations of the massive prochordal plate. The plate lies mainly in the angle between the brain plate and the upper layer of the head-fold bay- Its tip is free and it gradually increases in depth as it is traced caudally. It forms the anterior wall of the fore-gut and the adjoining part of the roof, but does not enter into the formation of the floor of the fore-gut. Caudally it passes into the chorda plate (CH .PL.) without the intervention of a head-process segment. There is a distinct space between the dorsal surface of the prochordal plate and the neural plate (BR.PL.). The latter does not exhibit any elevation in the middle line such as was observed in earlier stages. The prochordal plate is solid and massive, measuring about 0-112 mm. in length, 0-088 mm. in greatest depth and O-088 mm. in greatest width. Its cells are compactly arranged (Plate III, figs. 18 and 19) and show a tendency, especially on the left side, to proliferate mesoderm. In Plate III, fig. 19, there is an apparent vesicular structure to the left of the prochordal plate and in apparent v
Text-fig. 12. Median reconstruction of R. 324, 8 days 17:} hours, 8-9 somites. x 100.
A further change has manifested itself in the next stage. Theprimary brain flexure is now quite prominent, as can be seen in text-fig. 13 and Plate III, fig. 21. The neural tube is closed from just behind the cephalic flexure to the region of the first somite. Text-fig. 13 is from a median longitudinal section of a 9-10-somite embryo. It shows how the flexure in the brain has influenced the form of the prochordal plate (P.PL.). It lies in close contact with the brain wall and with the upper layer of the head-fold bay. It is, therefore, still situated in the angle between the anterior part of the wall of the brain and the upper layer of the head-fold bay, as in text-fig. 12, in which the flexure was much less obvious. The prochordal plate forms the anterior Wall of the fore-gut (FG.), but only a very small part of the dorsal wall. Just at the angle where the very short dorsal moiety of the plate passes into the extreme anterior part, there is a slight evagination which I believe to be the homologue of Seessel’s pouch. Its Walls are accordingly formed by the prochordal plate. Besides this, there are no other foldings or evaginations in the region of the plate. Streeter observed several foldings in the pig and homologised the folded prochordal plate with the “ Gaumentasche” of Selenka.
The oral plate (0.PL.) is now established, its ectodermal and endodermal elements being in contact but individually distinguishable. It lies immediately caudal to the prochordal plate and just cranial to the pericardial cavity. Its ectodermal element is formed by the caudal'part of the upper layer of the headfold bay. The endodermal element of the oral plate is directly continuous with that of the prochordal plate. The posterior two-thirds of the oral plate endoderm are to my mind derived from the antero-median part of the horseshoeshaped endodermal thickening. The cranial third, however, may receive contributions from the prochordal plate. These statements can be verified by examining the figures. '
Text-fig. 13. Median reconstruction of R 321, 8 days 172 hours, 9-10 somites. x 100.
Text-fig. 13 shows that the ventral part of the prochordal plate (P.PL.) is more massive than its dorsal part. There is as yet no‘ trace of the buccal hypophysis, and the pericardial cavity has been pushed further back and is quite huge. The chorda plate ( CH .PL.) is still intercalated in the roof of the gut, and in many places is in very close contact with the floor of the neural tube.
The reconstruction shown in text-fig. 13 should be compared with Plate III, fig. 21, which is a photomicrograph of the anterior end of a median longitudinal section of an embryo possessing 9 somites. It is practically identical with text-fig. 13. Note the Very close contact between the brain wall and the thick prochordal plate. The latter is also in contact with the upper layer of the head-fold bay and forms the anterior wall of the fore-gut. It also forms the dorsal angle of the fore-gut where there is an evagination Seessel’s pouch—the wall‘ of which is of prochordal plate derivation. The ventral angle of the fore-gut shows the continuity of the prochordal plate with the thick endoderm of the oral plate. The latter is pale compared with the darker staining prochordal plate in which are chromatophilic granules, absent in the oral plate endoderm. Immediately behind Seessel’s pouch the prochordal plate runs into the chorda plate which is one cell thick and in contact with the floor of the neural tube. Caudal to the oral plate and the fundus of the headfold bay the pericardial cavity is seen with thick roof and thin floor.
Text-fig. 14. Median reconstruction of R 320, 8 days 17 ;% hours, 10-11 somites. x 100.
Practically the same relations are shown in the next older stage which possesses 10-11 pairs of somites. Text-fig. 14 resembles text-fig. 13 in the salient points. The prochordal plate lies immediately behind the flexed brain wall and forms the anterior wall of the fore-gut which is considerably thicker than the other walls. It extends slightly laterally and shows chromatophilic granules in the cytoplasm of its cells. Its intimate relation with the flexed Wall of the fore-brain should be borne in mind, since in the next stage to be described this comes to an end and a space filled with mesenchyme is present between the two. The oral plate is not well seen, probably owing to the sectional plane.
The notochord is not yet separated, at least in the area shown here. The foregut is extensive and the roof of the pericardial cavity (R.PC.C.) is now very thick, whereas the floor is very thin (F.PC.C.).
The anterior end of the chorda plate ( CH .PL.) passes into the prochordal plate (P.PL.), but it is practically impossible to locate the limits of the latter accurately because there is always some difficulty about one or two sections at the ends of the plate where it passes into the oral plate endoderm and the chorda plate respectively.
We may pass on to a stage with 14-15 somites, since there are no noteworthy changes in the interval. Important advances have now been made as text-fig. 15 shows. The chorda ( CH.) has separated from the endoderm but is still in close contact with both brain wall (BR.W.) and gut roof (EN D.), except at its most anterior end which terminates in the prochordal plate just cranial to the meeting of the roof of the gut and its anterior wall (W.PG.). I should state that it has started to separate in the 13-somite stage.
The prochordal plate shows partial delamination into its two important derivatives which are the anterior wall of the gut (W.PG'.') and the prochordal mesoderm (P.PL.M.). These are still in close contact as the reconstruction shows, but can be identified as separate structures, ‘except perhaps dorsally, where the chorda passes into the prochordal plate. The ventral portion of the prochordal plate mesoderm appears as a thickened mass which is practically separate from the wall of the gut as Plate III, fig. 22, shows. It is situated between the oral plate (0.PL.) caudally and the brain wall cranially, and cranio-ventral to it lies the primordium of the buccal hypophysis (B.HP.). As Plate III, fig. 22, shows, it differs in appearance from the mesenchyme on either side of it, the cells stain more deeply, are more closely packed and frequently contain chromatophilic granules. This mass appears to be the homologue of that part of the prochordal mesoderm from which in other forms the premandibular somites arise. It may be referred to as the premandibular somitic mass or, shortly, as the premandibular primordium. Its position in front of the oralplate, here well developed, as well as its relation to the hypophysis, which will be better seen later, entitles it to be so homologised. It is more massive than the thinner strand of prochordal mesoderm which connects it with the anterior end of the chorda ( CH .). There are no cavities either in the mass itself or in its lateral extensions when these are present, and in putting forward this View of its homology, ‘I lay stress on its position, origin and, relations. Its fate will be described later.
Text-fig. 15. Median reconstruction of R 179, 8 days 19 hours, 14-15 somites. x 100.
text-fig. 15’—
The appearance of a space occupied by mesen chyme between the prochordal plate and the brain wall is another striking feature of this stage. In the pre_ceding stage the prochordal plate and the brain wall were in close contact. It may be that the appearance of this space is due to the fact that the brain is growing more rapidly than the adjoining structures, particularly the prochordal plate. The origin of the mesenchyme which fills the space is of interest. It is either proliferated from the prochordal plate or is formed by the medial growth of the laterally situated mesenchyme. The latter is the most probable origin, but it is probable that the prochordal plate contributes‘ to it in part.
The oral plate (0.PL.) is well established and its two layers are still quite distinct. The primordium of the buccalhypophysis (B.HP.) is just indicated cranial to the oral plate and ventral to the mass of presumed premandibular mesoderm. The anterior part of the chorda (CH .) is somewhat thickened, but there are no lateral proliferations in connection with it.
Text-fig. 16. Median reconstruction of R 253, 9 days 10 hours, 21 somites. x 100.
It is accordingly in the stages between 14 and 16 somites that the changes above described manifest themselves. They are uniformly exhibited in the ten embryos of this stage which I have examined.
Further progress in the same direction has taken place in the next stage, represented by an embryo aged 9 days 10 hours and possessing 21 pairs of somites. If, however, text-fig. 16, representing this stage, is compared with the preceding text-figure, it will be noticed that nothing of great importance has happened in the interval.
The buccal hypophysis (B.HP.) is well established, is in close contact with the brain Wall, and is widely open. The oral plate (0.PL.) is Very thin but is still intact. The chorda, separate as before, ends anteriorly in the strand of prochordal mesoderm (P .PL.M .) which is practically completely separate from the anterior wall of the fore-gut (W.PG.), in fact a narrow space exists between them. The process which began in the 14-16-somite stage is here more or less complete and has resulted in the transformation of the prochordal plate into a strand of prochordal mesoderm (P.PL.M.) and the anterior wall of the foregut (W.PG.). The mesenchyme-filled space in front of the prochordal mesoderm is considerably increased in extent as compared with text-fig. 15.
The structure and relations of the prochordal mesoderm are shown in Plate IV, figs. 23 and 24. In the former we see a median cellular strand situated between the brain walls. The Very short and thin upper part of this is formed by the chorda, whilst the remainder, much thicker and more or less irregular, is formed , by prochordal mesoderm in which the chorda ends. It contains granules which are, however, not well shown in this section. If we compare this figure with Plate III, fig. 18, we at once see the difference in size of the prochordal plate. This, together with the irregular appearance of the prochordal mesoderm, suggests that it contributes to the mesenchyme. Indeed, in the later stages we shall find that the whole prochordal mesoderm is converted into mesenchyme. The solid compact mass which lies to the right of the prochordal mesoderm is the cut wall of the fore-gut and has nothing to do with the prochordal plate.
Plate IV, fig. 24, is 0-112 mm. behind Plate IV, fig. 23. The separated chorda is clearly seen between the roof of the gut and the floor of the brain, whilst between the floor of the gut and the posterior wall of the buccal hypophysis is a horizontally disposed, flattened, plate-like strand of cells, readily distinguish able by its darkly staining character. If this strand is examined it will be seen.
that it really consists of a small median mass and two lateral extensions, slightly asymmetrical, the left one being the larger of the two and possessing a small lumen round which the cells are radially arranged. When the median part is followed in the series it is found to become continuous with the prochordal strand of mesoderm seen in Plate IV, fig. 23—see the reconstruction, text-fig. 16. This median mass is accordingly to be regarded as none other than the premandibular somitic mass seen in the preceding stage, whilst its lateral extensions, if that interpretation is correct, are to be regarded as rudimentary premandibular somites. "
The net result, therefore, is the transformation of the prochordal plate into prochordal mesoderm and the anterior wall of the pre-oral part of the fore-gut. Dorsally the chorda ends in the slender median prochordal mesoderm, which is expanded ventrally to form the premandibular mass from which rudimentary premandibular somites may arise. This process takes place by delamination as can easily be made out by examining text-figs. 14, 15 and 16.
The delamination is almost complete in the next stage, shown in text-fig. 17, which is a reconstruction made from the longitudinal sections, while Plate IV, fig. 25, shows a nearly median longitudinal section of the same embryo aged 9 days 16 hours and possessing 23-24 pairs of somites. The oral plate (0.PL.) is deficient in places, and it is now difficult to identify ectoderm and endoderm as separate layers. The prochordal mesoderm (P.PL.M.) is clearly seen in front of the anterior wall of the fore-gut (W.PG.) which is made up of columnar epithelium and is therefore thicker than the dorsal wall made of cubical epithelium (EN D.). Chromatophilic granules are seen in the cytoplasm of the cells of the prochordal mesoderm, particularly in its Ventral part, which extends out laterally on either side of the median plane, as can be ascertained by examining the sections. The dorsal part of the prochordal mesoderm, however, appears in 2-3 sections only and in it the notochord (CH ends cranially to the wall of the gut. The chorda ( CH .), quite separate from the brain wall and the roof of the fore-gut, shows a free anterior tip (T .CH .) which is better marked in later stages. There are no chromatophilic granules in the chorda at all.
It is not out of place here to state that the intercalated chorda plate separates from the gut wall either by evagination of the plate and the approximation of the edges of the endoderm, or simply by the approximation of these edges beneath the chorda plate. These two different methods may take place in one and the same embryo.
Text-fig. 17. Composite outline drawing of anterior part of R 248, ‘ 9 days 16 hours, 23-24 somites. x 100.
N o cavities occur in the prochordal mesoderm, nor is there any definite proliferation from the slender strand which connects the swollen Ventral part with the chorda. The whole prochordal mesoderm (P.PL.M.) corresponds to what Oppel calls “Praechordalplatte,” and its swollen Ventral part has been here called the premandibular primordium. The space which lies cranially to the prochordal mesoderm and which is filled with loose mesenchyme is greater than before.
I referred above to the fact that the anterior end of the chorda extends forward as a free tip beyond the connection with the prochordal mesoderm (P.PL.M.). This is better seen in text-fig. 18, which is a composite outline drawing of the anterior end of an embryo aged 9 days 10 hours and possessing 24 somites. The median sections are shown in Plate IV, figs. 26 and 27.
The prochordal mesoderm (P.PL.M occupies its usual position but is more irregular and much looser than in the preceding stage (compare Plate IV, figs. 25, 26 and 27). It is connected by a bridge to the anterior wall of the pre-oral part of the fore-gut (W.PG.), but apart from that it is quite free from both the gut wall (W.PG.) and the buccal hypophysis (B.HP.). Here again its ventral part extends laterally on either side of the median plane, whereas the strand connecting it with the chorda is quite thin, extending through only 2-3 sections in the median plane itself. Chromatophilic granules are present in the prochordal mesoderm (P.PL.M.) as well as in the anterior wall of the fore—gut (W.PG.), but only two mitotic figures were observed in the whole extent of the mass. If we examine text-fig. 18 and Plate IV, figs. 26 and 27, we see at once that the freely projecting cranial tip ( T .CH .) of the chorda is now more marked. It is in active growth as shown by the presence in it of mitotic figures. This is evidence of the independence of the chorda from the prochordal mesoderm. The latter reaches down to the apex of the angle between the anterior wall of the fore-gut (W.PG.) and the posterior wall of the buccal hypophysis (B.HP.).
Text-fig. 18. Same as text-fig. 17 from R 255, 9 days '10 hours, 24 somites. x 100.
In text-fig. 18 a projection of the anterior wall of the fore-gut ( W.PG.) is seen to bedirected towards the posterior wall of the buccal hypophysis (B.HP.) from which a similar projection arises but does not appear in the figure since the projections are separated by prochordal mesoderm. More laterally they meet but do not fuse together. Certain authors, Kupffer, Atwell, Miller and Parker, state that the endoderm of the fore-gut contributes to the hypophyseal primordium, but, beyond the relationship just described, I have seen no evidence of any contribution from the anterior wall of the fore-gut (W.PG.) to the hypophysis in the rabbit. '
The next stage, an embryo possessing 27 pairs of somites and aged 10 days 18 hours, is represented by text-fig. 19, which is a composite outline drawing. The brain has increased greatly in size, so that the bay resulting from the cranial flexure is much enlarged and the oral plate has entirely disappeared. The cranial extremity of the chorda now presents a bifid appearance, but only the dorsal limb (T.CH.) belongs to the chorda and represents its freely projecting tip. The ventral limb is really formed by a pointed mass of prochordal mesoderm (P.PL.M.), all that is now left of it. The remainder of this structure, including its swollen ventral part, has been practically completely converted into mesenchyme. All the evidence is against the ventral part of the apparent bifid chorda being regarded as a chordal extension. Griinwald, Tourneux, and Keibel gave figures of apparent bifid chorda, but the explanation given above seems to me the only one possible.
In text-fig. 19 it will be seen that the tip of the prochordal mesoderm remnant (P.PL.M.) nearly comes in contact with a projection from the posterior wall of the buccal hypophysis. There is an attempt at a third projection from the anterior wall of the pre-oral gut (W.PG.) which is here very well developed. It is still thicker than the rest of the fore—gut wall (END.).
Text-fig. 19. Same as text-fig. 17 from R 281, 10 days 18 hours, 27 somites. x 100.
The next stage is shown in text-fig. 20 and is thatof an embryo possessing 27-28 somites and aged 9 days 16 hours. The prochordal mesoderm (P.PL.M.) is a thin strand of cells containing chromatophilic granules and extending between the chorda dorsally and the posterior wall of the buccal hypophysis ventrally. It is irregular in its outline and I believe it is contributing to the mesenchyme. Its ventral part is definitely mesenchymatous on one side of the median plane, while on the other it consists of more closely packed cells containing granules. We have here the last remnant of the premandibular mass. Note the free tip of the chorda (T.CH.) which shows mitotic figures.
The oral plate has practically completely disappeared, leaving only a remnant (0.PL.) at the angle where the endoderm of the pre-oral part of the fore-gut passes into the ectoderm. The History of the Prochordal Plate tn the Rabbit 35
Text-fig. 21 does not show much advance over the previous stage. It is a composite outline drawing of an embryo possessing 28-29 somites and aged 10 days 1 hour. Again the free tip of the chorda (T.CH.) shows mitotic figures indicative of growth at this point. The strand of prochordal mesoderm is not compact, is irregular in outline and seems to be changing into mesenchyme. Between its main mass (P.PL.M.) and the anterior wall of the pre-oral part of the fore-gut (W.PG.) there is a detached mass of prochordal mesoderm. It contains chromatophilic granules and much resembles the same mass in Plate IV, fig. 26, but its ventral part is not swollen; the whole mass extends through 2-3 sections.
Text-fig. 20. Anterior part of a reconstructed median longitudinal section of R 246, 9 days 16 hours, 27-28 somites. x 100.
Text-fig. 21. Composite outline drawing of anterior part of R 277, 10 days 1 hour, 28—29 somites. x 100. A
So far the chorda has not come in direct relation with the hypophysis. They are linked together by the prochordal mesoderm (P.PL.M.).
What is practically the last stage in the history of the prochordal mesoderm is shown in text-fig. 22 and Plate IV, figs. 28 and 29. The embryo from which these are taken possesses 30 pairs of somites and is 1021- days old. Text-fig. 22, which is a composite outline drawing, shows that the oral plate has completely disappeared and that the mesenchyme-filled space cranial to the pre-oral part of the fore-gut has increased in extent. Immediately in front of the gut wall (W.PG.) we come across the remnant of the prochordal mesoderm (P.PL.M.) in the form of a thin strand stretching from the anterior end of the chorda (which is devoid of a projecting free tip), down to the angle (0.PL.X.) bounded by the posterior wall of the buccal hypophysis and the anterior wall of the foregut (W.PG.). It extends through only two sections as a thin compact strand of cells. Its ventral part, which was homologised with the premandibular primordium, has been largely converted into mesenchyme. Close on either side of the middle line in the region of the Ventral swollen mass of earlier stages one encounters a blood Vessel surrounded by mesenchyme.
Text-fig. 22. R 232, 10% days, 30 somites. x 100.
The tip of the chorda lies cranially to the angle between the anterior ( W.PG.) and the dorsal (EN D.) walls of the fore-gut. No mitotic figures are Visible in it.
Text-fig. 23 is a median reconstruction made from the longitudinal sections of the anterior end of an embryo of 11 days and possessing 37 somites. The chorda has now grown forward to a much greater extent than in any of the The H istory of the Prochordal Plate in the Rabbit 37
A preceding embryos, as is indicated by the position of the remnant of the pro chordal mesoderm (P.PL.M.) which is in continuity with its ventral side some distance behind its tip (T.CH.). The prochordal mesoderm takes the form of a small more or less irregular strand composed of loosely arranged cells which extends through two sections, one of which is shown in Plate IV, fig. 30.
Here again a projection of the posterior wall of the buccal hypophysis (B .H P .) is seen directed towards the prochordal mesoderm remnant (P.PL.M.). It is very similar to that shown in text-fig. 19. In fact the two stages closely resemble each other save for the greater forward extension of the chorda in text-fig. 23.
The buccal hypophysis is still widely open (B.HP.), and the oral plate has completely disappeared. The anterior wall (W.PG.) of the pre-oral part of the fore-gut is still Very thick.
Text-fig. 23. R 210, 11 days, 37 somites. x 100.
No definite trace of prochordal mesoderm was observed in any of the older embryos examined. Text-fig. 24 is a reconstructed median longitudinal section of an embryo decidedly older than the last one. No trace of the prochordal mesoderm was seen, and the chorda terminates in a recurved tip. Attention may be called to the curious flexures of the chorda seen in text-fig. 24 as well as in text-fig. 23. The latter is an actual tracing made from the median longitudinal section and the section on either side of it, so that in this ‘embryo at all events the notochordal kinks are actually present as such. The hypophysis (B.H P.) is well developed but still open. The anterior wall of the pre-oral part of the fore-gut (W.PG.) is not thicker than the roof (END.).
After the above observations were completed, I examined a large number of embryos aged between 9 and 10 days. They support the conclusions set forth above. The last embryo examined showed a very well-developed Seessel’s pouch, as seen in text-fig. 25. Here the chorda (CH .) ends as usual at this stage in the prochordal mesoderm (P.PL.M.). The very well-developed Seessel’s pouch marked S.P. arises from the dorsal angle of the fore-gut, and its distal end’ contains a closed cavity. It is asymmetrical and lies to the right of the middle line. It is clearly a derivative of the prochordal plate, since the wall of the fore-gut from which it arises (the dorsal angle) is itself derived from that plate. This is the only case of its kind among the very numerous embryos I have examined.
Text-fig. 24. Part of reconstructed median longitudinal /section of R 326, 11 days § hour. x 100.
Text-fig. 25. Reconstructed median longitudinal section of anterior end of an embryo, 10 days, 26 somites. x 100.
Concerning the later history of the cranial end of the chorda, I may state that I have examined some forty embryos in order to try and determine the The H istory of the Prochorclal Plate in the Rabbit 39
relation of the tip of the chorda to the buccal hypophysis. The embryos range between 7 and 17-5 mm. in length, and in the later ones the chondrocranium is laid down. In thirty-nine embryos the tip of the chorda, although situated in the vicinity of the posterior wall of the buccal hypophysis, does not come in contact witl1 it. In fact there is nearly always a capillary in contact with that wall. In the fortieth and last examined embryo (Lepus, 10 mm., A.), however, the tip of the chorda (actually its investing sheath) lay in close contact with
the posterior wall of the buccal hypophysis. Reference may be made to the paper of Huber(11) in which this relationship is discussed.
Summary
The preceding observations may be summarised as follows:
The prochordal plate is not the most anterior part of the embryonal endoderm, since in front of it the latter is thickened over a crescentic area (P.END.), the horns of which are continued backwards as lateral thickenings. In the thickened mesoderm overlying this area the pleuro-pericardial coelom is eventually developed. Its median anterior part contributes to the oral plate endoderm.
The prochordal plate (P.PL.)is at first a single-celled axial plate of thickened endoderm, and in the early stages there is no evidence of dorsal or lateral proliferation of cells from it. The plate is in continuity behind with the cranial segment of the l1ead—process (I .Z.) which is not yet differentiated into typical chorda plate. As development progresses the prochordal plate shortens, and at the 3-somite stage it thickens dorsally and takes the form of a compact cellular mass several cells in thickness. It is distinctly separate from the limbs of the horseshoe—shaped endodermal thickening.
With the flexure of the brain and the formation of the fore—gut, the prochordal plate comes to form the cranial wall of the latter and probably also a very small part of the adjoining ventral wall.
At the 13-somite stage the chorda, up till now intercalated in the gut roof and in continuity at its anterior end with the prochordal plate, separates from the gut roof. A little later, in embryos of 14-15 somites, the prochordal mesoderm delaminates from the anterior wall of the gut, but its dorsal end remains in continuity with the chorda. The ventral part of the prochordal mesoderm is swollen cranial to the oral plate and has been homologised with the premandibular primordium or somitic mass, but no cavities were observed, except in one case, on one side only.
The chorda grows forward beyond its junction with the prochordal mesoderm as a free process in which mitotic figures are not infrequent. This relation explains the apparent bifid character of the anterior end of the chorda.
The prochordal plate derivatives comprise the anterior wall of the fore—gut, the prochordal mesoderm including the homologue of the premandibular somitic mass and probably a small part of the oral plate endoderm. Ultimately the prochordal mesoderm is converted into mesenchyme. There is no evidence that the prochordal plate participates in the formation of the chorda.
Discussion
The axial plate of endodermal cells, the history of which has been outlined here, has been designated differently by different observers. The name “prochordal plate ” suggested by Van Oordt (18) seems to be the most suitable. It has been approved by Hill and Tribe (9), who have shown that this thickened patch of endoderm proliferates mesenchyme in the dog long before the primitive streak is laid down. This observation is of great value, since it shows that the prochordal plate cannot be of head-process origin. They also identified the prochordal plate in the early blastocysts of the cat. Van Beneden, in his posthumous paper of 1912(5), also recognised the existence in the rabbit of a thickened patch of endoderm which was, however, one cell thick. He states that it indicates the bilateral symmetry of the embryo before the primitive streak makes its appearance. Whether this area is the prochordal plate or simply corresponds with the antero-median part of the horseshoe—shaped thickening, described here, it is not easy to decide, since he did not refer to it in his later stages. The same may be said of the thickened area described by Huber (10), who, however, denies the existence of the prochordal plate in the guinea-pig. Rabl (21) figured a similar area behind which, in older stages possessing a head-process, he figured a definite prochordal plate proliferating mesoderm from its dorsal surface, though he did not recognise it.
Hubrecht(12), however, was the first to distinguish a thickened patch of endoderm at the anterior end of the embryonal area in Screw which he called the “ protochordal plate ” on the supposition that it contributes to the anterior end of the chorda. He recognised also another mesoderm-producing endodermal zone—his annular zone—the antero-median part of which constitutes the protochordal plate. Whether or not the horseshoe—shaped zone of thickened endoderm, described here in the rabbit, corresponds with the anterior part of Hubrecht’s annular zone must remain an open question. This zone does not appear to give origin to mesoderm in the rabbit, and moreover the prochordal plate lies caudal to its antero-median part and in practically all the embryos is delimited from it. The general arrangement in the rabbit seems to resemble very much that in Manis described by Van Oordt(18), and his fig. 21 agrees with my text-fig. 1 . According to Van Oordt’s description and figures, however, his annular zone proliferates mesoderm like that of Hubrecht and eventually forms a complete ring by the junction of its lateral halves behind the primitive streak. The zone in question in the rabbit overlies the thickened band of mesoderm in which the pericephalic coelom arises, and a glance at text-figs. 1, 6 and 9 will convince one that the endothelial wall of the lateral heart tubes, which are the first constituents of the heart to be formed, cannot possibly be derived from the prochordal plate as suggested by Hubrecht(12,13). He also derived the pharyngeal membrane and the wall of the fore-gut from the protochordal plate. It is, however, only the anterior wall of the fore-gut which is of prochordal origin, as the observations of K. M. Parker (19) and my own have shown.
Assheton (4) described in the rabbit an endodermal zone corresponding with the anterior part of Van Oordt’s zone. He calls it the “ pericardial thickening,” but he does not describe any prochordal plate.
Bonnet(6) described a similar endodermal thickening which he called “Erganzungsplatte des Urdarmstranges ”——the completion plate of the headprocess. There is no justification for the name. He did not describe it in his youngest stages of the dog, though it appears quite early as Hill and Tribe have shown, but gave a detailed interesting account of it in later stages in which the primitive streak an(l the head-process were already established. He pointed out that it actively proliferates mesoderm and supports Hubrecht’s view that it furnishes the anterior end of the chorda, but his argument is not convincing. The balance of opinion is against this view, and although I have shown that the prochordal plate shortens yet there is no evidence to show that that shortening is due to its conversion into head-process or chorda plate, rather is it to be regarded as the result of the considerable increase in thickness which it at the same time undergoes. Moreover the chorda grows forward in later stages beyond its point of contact with the prochordal mesoderm. As we are not examining the same embryo at different stages of its development but different embryos at different ages, it is quite possible that the apparent shortening may be due in part to individual variations in the degree of development of the plate.
Fig. 60 of Bonnet resembles my text-fig. 11. In his fig. 66, the chorda terminates in a mass of mesoderm proliferated from the anterior wall of the fore-gut. In principle, therefore, it resembles my text-fig. 15. Fig. 67 agrees with my Plate IV, fig. 23, which, however, exhibits no lateral extensions and a slender prochordal mesoderm.
K. M. Parker(19) has dealt in some detail with the prochordal plate in her paper on the hypophysis of the marsupials. There can be no doubt that her prechordal plate (Oppel(17)) is the equivalent of the prochordal mesoderm described here. Fig. 1 in her paper suggests that the prochordal plate gives rise to the anterior wall of the fore-gut as well as a part of the adjoining dorsal wall. She describes the termination of the chorda in the prechordal plate of mesoderm, but this plate lies more or less dorsal to the fore-gut, and in the stage figured blends by its apex with the anterior wall of the latter, whereas in the rabbit the prochordal mesoderm lies cranial to the anterior wall of the fore-gut. This difference, however, is purely topographical and is probably due to the more rapid brain development in the rabbit.
In the marsupials Miss Parker was able to trace the origin of the premandibular somites from the prechordal plate, whereas in the rabbit they do not apparently occur as definite structures, and all there is to represent them is the ventral thickened part of the prochordal mesoderm——the premandibular somitic mass. Apart from these differences in detail our observations are in agreement.
Dorel1o(7), in reptiles, described a mass of endoderm in which the chorda terminates and which encloses the pre-oral gut. It gives rise to hollow budsthe head cavities. von Kupffer (15) found in Bdellostoma and Petromyzon that the chorda ends in a thickened endoderm in the wall of the pre-oral endoderm pocket.
Streeter (23) showed in the pig that the mesoderm grows forward in the form of two horns from the primitive streak region. They meet anteriorly, leaving a “ bald ” area in which the head-process makes its appearance before there is any sign of the prochordal plate. Soon the cells of the latter become piled on each other and show foldings which produce clefts that open into the fore-gut. He homologises this folded plate with the “Gaumentasche” described by Selenka in a much older stage of Didelphys. No such foldings are seen in the rabbit, and K. M. Parker((19), p. 230) has suggested that the “ Gaumentasche ” “is nothing more than a prechordal plate in continuity with a well-developed Seessel’s pocket.”
Huber, Rabl, Kolliker and Keibel deny that the endoderm makes any contribution to the mesoderm. The thickened patch of endoderm recognised by Huber (10) in the guinea-pig is considered by that author as the primordium of the oral plate endoderm, in this respect agreeing with Keibel and Carius.
I have shown that the anterior end of the chorda does not come into direct contact with the buccal hypophysis, and that it is only the strand of prochordal mesoderm which, descending from its point of continuity with the chorda, terminates in the vicinity of the hypophyseal primordium. This is in agreement with Adelmann’s statements (1,2) for the chick. He appears to think that in mammals the chorda very early acquires contact with the buccal hypophysis, but considers that the relation is a secondary one. He agrees with K. M. Parker that the premandibular somites are derived from the prechordal plate. His figs. 33-36(2) closely agree with my text-figs. 15-23, but he does not describe any forward growth of the chorda beyond its contact with the prechordal plate. He points out that the brain grows more rapidly than the fore-gut and prechordal plate, thus leading to the formation of a mesenchyme-filled space in front of the prechordal plate and between it and the brain wall. On the whole my observations on the rabbit are in agreement ‘with his based on the chick (2).
The chorda, in the stages examined, does not show any connection with the anterior wall of the fore-gut after its separation. In fact it grows forward beyond its union with the prochordal mesoderm. Griinwald and Staderini, however, have stated that the chorda comes into contact with Seessel’s pocket. However that may be, the primary relation in the rabbit is that described above, namely, the termination of the chorda in the prochordal mesoderm.
Up to the stages examined there are no ventral extensions of the chorda connecting it with the dorsal wall of the fore-gut. Huber (11) described such extensions in the human embryo and Griinwald in the sheep. These connections must clearly be secondary.
The apparent bifid anterior end of the chorda has already been explained. This condition has been figured by Gri'1nWald(8), Keibel (14), Huber (11) and others, but in view of my observations, the contention that the chorda contributes to the hypophysis (Reichert, His and Dursy) must be regarded as very doubtful. In this connection, Kolliker described in an 11 day rabbit a strand of cells passing from the hypophysis to the chorda, and thought that it represented a contribution by the ectoderm to the chorda. It is clear that this strand. is nothing but the remains of the prochordal mesoderm, as seen in Plate IV, figs. 28 and 29.
Saint-Remy (22) concludes that in all amniotes the anterior end of the chorda is bent down so that it can maintain its insertion into the epithelium of the buccal hypophysis. He states, moreover, that the descending limb is converted into connective tissue. In view of Adelmann’s observations (1,2) on the chick and my own on the rabbit this statement must be regarded as incorrect so far as the insertion of the chorda is concerned. The descending limb referred to is not formed by chorda but by a prochordal mesodermal strand which is later transformed into mesenchyme, whilst the chorda can grow forward beyond it.
Keibel’s figs. 38, 39, 40 a, 40 b (14)for the rabbit resemble my text-fig. 15, and his fig. 41 b seems to show an apparent bifid anterior end of the chorda, but the lower limb is very short and ends in the wall of the fore-gut in the region of Seessel’s pocket. Paulisch’s figs. 1, 2, 3 (20) of 9-11 days rabbits are very much like those of Keibel. '
Atwell (3) figured a longitudinal section through the head of a 6 mm. rabbit embryo in which he showed an epithelial bud from the anterior wall of the foregut which more or less fuses with the tip of the chorda. He thinks it is identical with Selenka’s “Gaumentasche” and Saint-Remy’s descending branch of the chorda. It, no doubt, represents part of the prochordal mesoderm.
Some observers—von Kupffer, Saint-Remy and Miller (16)—hold the View that the anterior wall of the fore-gut contributes to the buccal hypophysis, but beyond the projections mentioned in the descriptive part of this paper I have no evidence that the anterior wall of the fore-gut contributes to the hypophysis in the rabbit.
I hope later to be able to examine rabbit embryos of 6 and 7 days and so complete this study of the history of the prochordal plate.
I wish to express my thanks to Mr A. K. Maxwell for touching up and lettering the text-figures and to Mr F. Pittock for the photomicrographs.
References
(1) ADELMANN, H. B. (1922). “The significance of the prechordal plate, an interpretative study.” Amer. J. Anat. vol. XXXI.
(2) —— (1926). “The development of the premandibular head cavities and the relations of the anterior end of the notochord in the chick and robin.” J. Morph. and Phys. vol. XLII, No. 2.
(3) ATWELL, W. J. (1916). “Relation of the chorda dorsalis to endodermal component of hypophysis.” Anat. Rec. vol. X.
(4) ASSHETON, R. (1894-95). “The primitive streak of the rabbit, the causes which may determine its shape and the part of the embryo formed by its activity.” Quart. J. M tic. Sci. vol. XXXVII.
(5) VAN BENEDEN, E. (1912). “Recherches sur l’embryologie des Mammiféres.” Arch. dc Biol. t. XXVII.
(6) BONNET, R. (1901). “Beitrage zur Embryologie des Hundes. Erste Fortsetzung.” Anat. Hefte, 51 Heft, Bd. XVI.
(7) DORELLO, P. (1900). “Studi embryologici sui Rettili.” Ricerche fatte nel Lab. di Anat. Norm. della R. Universita di Roma ed in altri Lab. biol., vol. VII, fasc. 3e 4, 1900. Quoted by Bonnet (6).
(8) GRI'iNWALD, L. (1910). “Eine Cyste der Chordascheide.” Anat. Anz. vol. XXXVII.
(9) Hill JP. and Tribe M. The early development of the cat (Felis domestica). (1924) Quart. J. Microsc. Sci., 68: 513-602.
(10) HUBER, G. C. (1917). “On the anlage and morphogenesis of the chorda dorsalis in the Mammalia, particularly the guinea-pig.” Anat. Rec. vol. XIV.
(11) —- (1912). “On the relation of the chorda dorsalis to the anlage of the pharyngeal bursa or median pharyngeal recess.” Anat. Rec. vol. VI.
(12) HUBRECHT, A. W. (1890). “Studies in mammalian embryology. II. The development of the germinal layers in Sorex vulgaris.” Quart. J. Mic. Sci. vol. XXXI.
(13) —— (1909). “Early ontogenetic phenomena in mammals and their bearing on our interpretation of the phylogeny of the vertebrates.” Quart. J. 111 ic. Sci. vol. LIII.
(14) KEIBEL, F. (1889). “Zur Entwicklungsgeschichte der Chorda bei Saugern (Meerschweinchen und Kaninchen).” Arch. Anat. u. Physiol. Anat. Abt.
(15) VON KUPFFER (1894). “Die Deutung des Hirnanhanges.” Sitzber. der Gesellschaft f. Morph. u. Physiol. in Munchen. Quoted by Bonnet (6).
(16) MILLER, M. M. (1916). “The hypophysis of the pig.” Anat. Rec. vol. X, No. 3. Journal of Anatomy, Vol. LX VI, PC!/7'5 1
( 17) OPPEL, A. (1890). “Uber Vorderkopfsomiten und die Kopfhohle von Anguis Fragilis.” Arch. f. M ikr. Anat. Bd. XXXVI. Quoted from Hill and Tribe (9) and K. M. Parker (19).
(18) VAN OORDT, G. J. (1921). “Early developmental stages of Manis Javanica Desm.” Verhand. Kori. Akad. v. Wetensch. Amsterdam, Dl. XXI.
(19) PARKER, K. M. (1917). “The development of the hypophysis cerebri, pre-oral gut and related structures in the marsupials.” J. Anat. vol. LI.
(20) PAULISCH, O. (1887). “Das vordere Ende der Chorda Dorsalis und der Franksche Nasenkamm.” Arch. f. Anat. u. Physiol. Anat. Abt.
(21) RABL, C. (1915). “Edouard van Benden und der gegenwiirtige Stand der wichtigesten von ihm behandelten Probleme.” Arch. f. Milcr. Anat. Bd. LXXXVIII.
(22) SAINT-REMY, G. (1895-96). “Recherches sur l’extrémité antérieure de la Chorda Dorsal chez les Amniotes.” Arch. de Biol. t. XIV.
(23) Streeter GL. Development of the mesoblast and notochord in pig embryos. (1927) Contrib. Embryol., Carnegie Inst. Wash. Pub. no. 380. 19: 73-92.
Explanation of Plates
The figures on Plates I and II have suffered a reduction of 1/3, those on Plates III and IV a reduction of 1/2.
Abbreviations
| Abbreviations |
|---|
|
B.H P. Buccal hypophysis primordium. BR.F. Brain flexure. BR.PL. Brain plate. BR. W. Brain wall. C.FL. Cephalic flexure. CH. Notochord. CH.PL. Chorda plate. CL.M. Cloacal membrane. E.H T. Endothelial heart tube. END. Endoderm. FG. Fore-gut. F.FG. Floor of fore-gut. F.PC.C. Floor of pericardial coelom. H F.B. Head-fold bay. P.PL.M. Prochordal mesoderm. I.Z. Cranial remains of head process. M.B. Medial boundary of pericardio-peritoneal coelom. M.S. Mesodermal somite. O.PL.X. Angle where ectoderm runs into endoderm. O.PL. Oral plate. PAR. M. Paraxial mesoderm. P.C0E. Pericephalic coelom. P.EN D. Horseshoe-shaped or pericephalic zone of thickened endoderm. P.PL. Prochordal plate. P.K T. Primitive knot. P.ST. Primitive streak. P.ST. M. Primitive streak mesoderm. PP.C’. Pericardio-peritoneal coelom. P. M. Pre-axial mesoderm. PC.C. Pericardialcoelom. PR. G. Pre-oral part of fore-gut. R.PC'.C'. Roof of pericardial coelom. S. P. Seessel’s pouch. T.OH. Free tip of notochord. W .PG. Anterior wall of pre-oral part of foregut. |
Plate I
Fig. 1. T.S. (9.1.2.) of R 335, 8 days, primitive streak and head-process stage. x 330.
Fig. 2. T.S., 48th section behind fig. 1—same embryo as fig. 1. x 330.
Fig. 3. T.S. (4.2.2.) of R 352, 8 days 1 hour, ?I somite. x 330.
Fig. 4. T.S. (25.l.2.) of rabbit C’, 8 days, 3 somites. x 150.
Fig. 5. T.S., 22nd section of R 314, 8 days 17% hours, 4 somites. x 150.
Fig. 6. T.S., 28th section of same. x 150.
Fig. 7. T.S., 41st section of same. x 330.
Plate II
Fig. 8. T.S., 56th section of same as 5. x 330.
Fig. 9. T.S., 95th section of same as 5. x 330.
Fig. 10. Median longitudinal section (5.3.6.) of R 347, 8 days 3 hours, 5 somites. x 250.
Fig. 11. Longitudinal section (2.2.3.) of R 348, 8 days 3 hours, 5 somites. x 250.
Fig. 12. Median longitudinal section, 16th section after fig. 11, same embryo. x 250.
Fig. 13. T.S., 35th section of R 318A, 8 days 17% hours, 6 somites. x 250.
Plate III
Fig. 14. 50th section of same as 13. x 250.
Fig. 15. 67th section of same as 13. x 250.
Fig. 16. Median longitudinal section (6.4.5.) of R 359, 8 days 3 hours, 7 somites. x 250.
Fig. 17. T.S. (20.4.l.) of R 324, 8 days 17% hours, 8-9 somites. x 250.
Fig. 18. Next section of same. x 250.
Fig. 19. Next section behind 18 of same. x 250.
Fig. 20. Next section behind 19. x 250.
Fig. 21. Anterior part of a median longitudinal section (3.3.4.) of R 306, 8 days 16 hours, 9 somites. x 250.
Fig. 22. T.S. (18.5.1.) of R 179, 8 days 19 hours, 14-15 somites. x 250. Shows premandibular somitic mass.
Plate IV
Fig. 23. T.S. (8.3.2.) of R 253, 9 days 10 hours, 21 somites. x 250.
Fig. 24. 14th section behind 23. x 180.
Fig. 25. Median longitudinal section (6.3.2.) of R 248, 9 days 16 hours, 23-24 somites. x 180.
Fig. 26. Median longitudinal section (8.2.2.) of anterior part of R 255, 9 days 10 hours, 24 somites. x 180.
Fig. 27. Next section (9.2.2.) of same. x 180.
Fig. 28. Median longitudinal section (8.5.2.) of R 282, 10% days, 30 somites. x 180.
Fig. 29. Section immediately after 28 (9.5.2.). x 180.
Fig. 30. Median longitudinal section (anterior part) (6.2.3.) of R 210, 11 days, 6-5 mm., 37 somites. x 135.
The magnifications of text-figures 17, 18, 19, 21, 22, 23 and 25 are correct as printed.
Cite this page: Hill, M.A. (2024, April 16) Embryology Paper - The history of the prochordal plate in the rabbit. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_history_of_the_prochordal_plate_in_the_rabbit
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G