Paper - The development of synovial joints: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 8: | Line 8: | ||
<br> | <br> | ||
'''Modern Notes:''' [[Musculoskeletal System - Joint Development|Joint Development]] | '''Modern Notes:''' [[Musculoskeletal System - Joint Development|Joint Development]] | ||
<br> | |||
{{Joint Links}} | |||
<br> | |||
{{Musculoskeletal Links}} | |||
|} | |} | ||
{{Historic Disclaimer}} | {{Historic Disclaimer}} | ||
| Line 19: | Line 21: | ||
==Introduction== | ==Introduction== | ||
Most of the descriptions of the development of synovial joints give the impression that the synovial cavity is formed by a process of absorption. It is the purpose of this paper to review the factors concerned in the formation and extension of the synovial cavity as seen in the development of the human embryo | Most of the descriptions of the development of synovial joints give the impression that the synovial cavity is formed by a process of absorption. It is the purpose of this paper to review the factors concerned in the formation and extension of the synovial cavity as seen in the development of the human embryo and the white rat. | ||
and the | |||
Observations have been made on the interphalangeal joints of the foot in a series of human embryos varying in length from 30 to 125 mm. Much material of this kind available had to be rejected because of shrinkage due to inadequate fixation and this led to a supplementary study of rat embryos, where the age could be determined with accuracy and fixation adequately controlled. | |||
For the rat embryos used in the investigation I am indebted to Mr T. McKeown of Guy’s Hospital. | |||
The human material was fixed in formalin and the rat limbs were fixed in Bouin’s solution. After fixation and clearing, the tissues were first infiltrated with wax of 45° melting point at a temperature of 50° C. and then transferred to 60° wax at 62° C. for a further period of 1 hour. | |||
The appearances seen in the human specimens will be described first and their significance discussed later. | |||
===30 mm Stage=== | |||
(fig.1) | |||
A section of one of the interphalangeal joints at this stage shows the two chondrifying elements separated by relatively undifferentiated mesenchymal cells constituting the “joint disk”. The cells of the disk are arranged in arcs parallel with the surfaces of the cartilage elements. In that part of the disk in the region of the future articular surfaces the cells are flattened, very closely packed and disposed with their long axes in the joint line. At the extreme periphery of the disk the mesenchymal cells are disposed longitudinally and here the mesenchyme is continuous with the condensation forming the perichondrium. In the peripheral part of the disk, where there is a change from the arc to the longitudinal arrangement of the cells, the mesenchyme is much looser than in the central part of the disk where the cells are very closely packed. | |||
where the | |||
===45 mm Stage=== | |||
(fig. 2) | |||
The | The cartilage elements are developing their shape, a convex surface proxi mally, and a concave surface distally. The joint disk of mesenchyme is reduced in thickness and the loose arrangement of the cells at the periphery is still more evident, and cavities are appearing among them. The central part of the disk is formed of flattened closely packed cells with their long axes parallel with the future joint surfaces. | ||
===63 mm Stage=== | |||
(fig. 3) | |||
Further reduction of the thickness of the joint disk has occurred and it is now represented by a layer two cells in thickness between the two cartilage elements. The synovial cavity is present in the circumferential part and, in serial sections, can be traced all round the joint. The mesenchymal cells nearby are becoming arranged parallel with the boundaries of the synovial cavity. | |||
===72 mm Stage=== | |||
(fig.4) | |||
The joint disk is represented by a single layer of flattened cells. The circumferentially placed synovial cavity is well defined and lined with flattened cells. On the left of the section, the mesenchyme is forming a rudimentary meniscus in continuity laterally with the lining of the synovial cavity and medially with the joint disk. The shape of the future articular surfaces is well defined. | |||
===125 mm Stage=== | |||
(fig. 5) | |||
The joint disk between the cartilages has entirely disappeared and the two elements are united across the joint line by primitive cartilage, or “precartilage ”. The matrix of this primitive cartilage has a staining reaction rather like that of collagen with haematoxylin and eosin, but with thionin, although not so dark as the more mature matrix, it gives the typical reaction of cartilage. Near the margin of the area of union the matrix shows little aflinity for stains and is apparently undergoing liquefaction. There is, at this stage, no extension of the circumferential synovial cavity between the two cartilage elements. On the right of the section the meniscus formed by the remains of the original mesenchymal disk is free of the cartilage elements; on the left, the meniscus is attached to the surface of the proximal cartilage and contains some young chondroblasts. Menisci of this type are found at this stage in nearly all the joints examined. | |||
==Discussion== | |||
It is convenient first to review the development of joints up to the point where the elements are united across the joint line by primitive cartilage and the joint cavity is limited to the circumferential region, and to consider later the solution of this bond and the extension of the cavity between the two cartilages. | |||
cavity. | |||
The | The work of Fell (1925) has shown that at an early stage the mesenchymal core in the limb bud is determined in respect of the pattern of future chondrification in the process of laying down of the skeletal elements. Experiments by Fell & Canti (1934) show, moreover, that the early stages of joint formation described above take place in almost identical fashion in the avian knee joint cultivated in vitro. There is progressive diminution in the thickness of the mesenchymal disk between the developing cartilages, loosening of the mesenchyme in the circumferential part of the disk, and finally union of the two skeletal elements by primitive cartilage. These experiments show conclusively that the mesenchyme of the joint disk does chondrify, and dispose of the theory that the joint is formed because of the interposition of non-chondrifying tissue between the two bones. Carey (1922) described the tissue uniting the cartilages as “mucoid”, but Fell’s experiments and observations on normal embryonic joints have demonstrated that it is very young cartilage. | ||
in | |||
the joint disk. | |||
Recent experiments by Gliicksmann (1938) have shown that undifferentiated mesenchyme subjected to pressure between two developing cartilages itself undergoes chondrification. The progressive chondrification of the joint disk occurring in normal development is, therefore, consistent with the disk mesenchyme being subjected to pressure. The fact that there are many mitotic figures in the cells of the cartilage in the immediate vicinity of the disk during this period of development probably indicates that the progressive diminution in the thickness of the disk is due partly to the conversion of mesenchymal cells into chondroblasts and partly to the invasion of the disk by actively multiplying chondroblasts from the ends of the cartilages. | |||
of the | |||
mesenchymal | |||
chondroblasts | |||
To postulate pressure on the mesenchymal disk it must be assumed that the cartilage elements are growing very much faster than the sleeve of tissues surrounding them in the limb bud. The assumption is reasonable because the cartilage is growing not only by multiplication of its cells but also by the addition of a relatively large amount of intercellular substance. | |||
the | |||
the | |||
That the progressive chondrification of the joint disk is due in part to the effect of pressure is, however, supported by evidence available when the disk mesenchyme is protected from such pressure. The region of the wrist affords the first example of this. | |||
A coronal section of the wrist region of a human embryo of 30 mm. is shown in fig. 6. The early stages of joint formation are evident between all the cartilage elements. The cartilage of the ulnar styloid is long and curved, and is separated from the triquetral cartilage by a well-marked joint disk which resembles in every way those seen where synovial joints will later be formed. The presence of the early stages of joint formation between the ulnar styloid and the triquetral in the human embryo is of special interest because in the macaque monkey a large synovial joint is present in this region (Wood Jones, 1920). It will be obvious from reference to fig. 6 that the mesenchyme between the head of the ulna and the triquetral will be protected from counterpressure exerted by the growing cartilages because of the apposition of the ulnar styloid to the triquetral. fig. 7 shows the appearance at the 125 mm. stage. The styloid process of the ulna is much smaller relative to the size of the head than it is at the 30 mm. stage. It is separated from the triquetral by a considerable interval and is not so curved as in the earlier stage of its formation. A comparison between figs. 6 and 7, moreover, shows that the growth of the radius has apparently pushed the carpal elements distally away from the ulna which seems to be growing rather more slowly than the radius. Between the individual carpal elements, and between the lower end of the radius and the carpus, the joint disks have chondrified and the elements are united across the joint lines by primitive cartilage. Between the head of the ulna and the triquetral, however, where the previous apposition of the ulnar styloid to the carpus has protected the mesenchyme from pressure, it has not chondrified but has become modified to form the articular disk of the inferior radio-ulnar joint. Where the withdrawal of the ulnar styloid from the triquetral has resulted in relief of pressure on the joint disk originally present between the two elements, the disk mesenchyme has not chondrified but has become stretched and incorporated in the medial ligament of the wrist joint. | |||
The features seen at the two ends of the developing clavicle afford similar evidence. The appearance, very early in development, of the centres of ossification in the clavicle leads to a slowing of the growth of the precartilage of which it is formed at a period when the mesenchyme at the two ends is relatively undifferentiated. The relief of pressure on this mesenchyme removes the stimulus for its further chondrification and it becomes modified to form the articular disks of the sterno-clavicular and acromio-clavicular joints. | |||
the | |||
The | |||
If there be pressure on the joint disk in the earlier stages of development, such pressure would assist in the formation of the circumferential synovial cavity. fig. 8 shows a disk formed of laminae of sheet rubber subjected to pressure by a clamp. Compression of the central part leads to a reduction in thickness, whilst in the part not subjected to pressure the sheets tend to separate. The orientation of the mesenchymal cells in the joint disk in fig. 3 bears a striking resemblance to the arrangement of the rubber sheets in the model. | |||
The way in which the solution of the temporary continuity between the | The way in which the solution of the temporary continuity between the bone elements is brought about must now be considered. | ||
bone elements is brought about must now be considered. | |||
The fact that the union of the two skeletal elements by primitive cartilage persists in the human embryo up to the fourth or fifth month, and is not observed after that period, makes it tempting to assume that foetal movements are the determining factor in the completion of the process of joint formation. The evidence that this is so is, however, scanty and unconvincing. In the first place, the facts might just as well be explained by assuming that movement does not occur because joint formation is not complete as by assuming that it is movement that completes the formation of the joint. Moreover, there is very definite evidence that movement cannot be the sole factor in the extension of the joint cavity between the bone elements. Hamburger (1928) has demonstrated the formation of normal joints in the hindlimbs of frogs in which the muscles had been paralysed by denervation. This observation in itself does not, however, exclude movement completely as a factor in joint formation. first, Hamburger describes, in two cases, complete cartilaginous union between the femur and the hip bone and similar fusion in the tarsal joints in a few cases. | |||
the | |||
the | |||
Secondly, the possibility of passive movements cannot be excluded in these experiments. A description by Nicholson (1937) of the formation of perfect synovial joints in a hand-like appendage occurring in a teratoma does, however, show quite definitely that normal joints can develop in the complete absence of movement. In the appendage, very carefully examined and described by him, no trace of muscle could be found. Experiments by Straus (1938) on the occurrence of movements in rat embryos on stimulation over the region of the brachial plexus showed that movement could be elicited in the proximal joints of the upper limb at the 14 mm. stage but not in the region of the carpus or digits. This conforms with the fact that the differentiation of the joints proceeds proximo-distally but, as the movements occurred under abnormal stimulation, it cannot be taken as evidence that, in normal conditions, even the proximal joints were really capable of movement. Indeed, the section of the elbow joint shown in fig. 9 demonstrates union of the humerus and the ulna by primitive cartilage after birth, and no movement of the elbow region was detected in the rat from which the section was taken. Straus’s experiments do show that in conditions of abnormal stimulation the muscles, which are capable of contracting, may break the bond of union so that movements can take place at a time when they do not normally occur. This therefore suggests that movement, although not the sole factor, may be one of the factors in normal joint formation. In the tissue culture experiments conducted by Fell and Canti passive movements of the two cartilage elements failed in every case to prevent their subsequent cartilaginous fusion. This confirms the evidence that some additional factor is essential in the formation of the joint. | |||
It will be noted that the only factor which is constantly associated with | In the search for other factors which may be concerned it is interesting to note that it is only in tissue explants cultivated in vitro that the cartilaginous union between the two bone elements persists in all cases. In the other examples which have been considered, viz., normal development, denervated limbs, and the teratoma, there is solution of this continuity and the formation of the completed synovial joint. A tabulation of the conditions present in the cases under review is given below: | ||
persistence of union between the two elements is absence of calcification in the | <br> | ||
cartilage. On this evidence it would seem that the completion of joint formation occurs at a time when the cartilages have reached a certain stage of development, and that a further accompaniment of this stage is the occurrence | {| | ||
of calcification. The failure of both calcification and joint formation in the | | width=150px| | ||
tissue explants occurs because they are not, at this stage, in a normal environment. | ! Nerves | ||
! Vessels | |||
! Muscles | |||
! Calcification | |||
! Persistent union | |||
|- | |||
| Embryo | |||
| Yes | |||
| Yes | |||
| Yes | |||
| Yes | |||
| No | |||
|- | |||
| Teratoma | |||
| Yes | |||
| Yes | |||
| No | |||
| Yes | |||
| No | |||
|- | |||
| Denervated limb | |||
| No | |||
| Yes | |||
| Paralysed | |||
| Yes | |||
| Unusual | |||
|- | |||
| Explant | |||
| No | |||
| No | |||
| No | |||
| No | |||
| Always | |||
|} | |||
<br> | |||
It will be noted that the only factor which is constantly associated with persistence of union between the two elements is absence of calcification in the cartilage. On this evidence it would seem that the completion of joint formation occurs at a time when the cartilages have reached a certain stage of development, and that a further accompaniment of this stage is the occurrence of calcification. The failure of both calcification and joint formation in the tissue explants occurs because they are not, at this stage, in a normal environment. | |||
Carey, in 1922, stated that the joint was completed by liquefaction of the tissue (termed by him “mucoid”) uniting the two elements. This observation has been confirmed in the present investigation by the examination of the joints of the upper limb, particularly the elbow, in rats during the few days following birth. At this period, when the joint surfaces are separating, the matrix of the primitive cartilage uniting the two bone elements loses its staining reaction. The process is most marked near the edges where the matrix is exposed to the synovial fluid present in the circumferential cavity. Separation occurs by progressive liquefaction of the degenerate matrix from~the margins inwards. fig. 9 represents the appearances seen in the central part of the bond of union between the ulna and the humerus in a newly born rat. It can be seen that the young cartilage is here normal and gives the typical staining reactions. fig. 10 represents a section through the marginal part of the same joint nearer the synovial cavity. Here the matrix is degenerated and stains with great difficulty. fig. 11 shows a section through the wrist joint where the liquefaction of the matrix and ingress of synovial fluid have occurred almost to the centre of the joint. These processes take place in the white rat during the few days immediately after birth, and in the human embryo about the fifth month. The cartilage cells exposed in the process of liquefaction become flattened where a considerable part of their surface is in relation to the synovial fluid, but where they are still protected by matrix over the greater part of their surface they remain spherical for some time. The reason for the liquefaction of the matrix along the joint line at this period is unknown. It occurs on the attainment of a certain stage of development of the cartilage which is, in some situations, associated with the appearance of calcification. It is possible that attempts at movement in the joint at this critical period may be of assistance in the separation of the two bones. | |||
This investigation has directed attention to further problems on which | This investigation has directed attention to further problems on which work is now being carried out. The first is the explanation of the fact that the costal elements of the vertebrae in the thoracic region form joints with the other elements, whereas in the other regions they become incorporated in the vertebrae. Secondly it seems likely that the behaviour of undifferentiated mesenchyme under various stresses and strains in controlled experimental conditions such as tissue culture offers a method for elucidating further the factors which determine the appearance of the cavity in synovial joints. | ||
work is now being carried out. The first is the explanation of the fact that the | |||
costal elements of the vertebrae in the thoracic region form joints with the | |||
other elements, whereas in the other regions they become incorporated in the | |||
vertebrae. Secondly it seems likely that the behaviour of undifferentiated | |||
mesenchyme under various stresses and strains in controlled experimental | |||
conditions such as tissue culture offers a method for elucidating further the | |||
factors which determine the appearance of the cavity in synovial joints. | |||
In conclusion I must record my thanks to my technical assistant Mr G. A. Walker who has been responsible for the preparation of the serial sections which have been necessary in this investigation and who has prepared for me the photographic illustrations. | |||
==Summary== | |||
# The initial stages in the formation of synovial joints show that counterpressure of the two cartilage elements on the intervening mesenchymal joint disk may be responsible for the progressive chondrification of the disk and for the appearance of the circumferentially placed synovial cavity. Where pressure on the disk is removed it does not chondrify and this accounts for the presence of intra-articular disks at the wrist and at the two ends of the clavicle. | |||
for a time by primitive cartilage. | # After chondrification of the disk, the two elements in the joint are united for a time by primitive cartilage. | ||
# The solution of continuity in the later stages of joint formation is accomplished by liquefaction of the matrix of the primitive cartilage uniting the two bones. The cause of the liquefaction is not explained. | |||
Movement alone does not cause breakdown of the bond of union between | Movement alone does not cause breakdown of the bond of union between the two cartilage elements, but it may play an ancillary part in the process. | ||
the two cartilage elements, but it may play an ancillary part in the process. | |||
==References== | |||
CAREY, E. J. (1922). J. Morph. 37, l. | CAREY, E. J. (1922). J. Morph. 37, l. | ||
| Line 306: | Line 155: | ||
FELL, H. B. (1925). J. Morph. 40, 417. | FELL, H. B. (1925). J. Morph. 40, 417. | ||
FELL, H. B. & CANTI, R. G. (1934). Proc. ray. Soc. B, 118, 316. | FELL, H. B. & CANTI, R. G. (1934). Proc. ray. Soc. B, 118, 316. GLiicKsMANN, A. (1938). Anat. Rec. 73, 39. | ||
GLiicKsMANN, A. (1938). Anat. Rec. 73, 39. | |||
HAMBURGER, V. (1928). Ram: Arch. Entw. Mach. Organ. 114-, 272. | HAMBURGER, V. (1928). Ram: Arch. Entw. Mach. Organ. 114-, 272. HARRIS, H. A. (1933). Bone Growth in Health and Disease. | ||
HARRIS, H. A. (1933). Bone Growth in Health and Disease. | |||
NICHOLSON, G. W. (1937). Guy’: Hosp. Rep. 87, 46. | NICHOLSON, G. W. (1937). Guy’: Hosp. Rep. 87, 46. | ||
| Line 318: | Line 165: | ||
WOOD JONES, F. (1920). The Principles of Anatomy as seen in the Hand. | WOOD JONES, F. (1920). The Principles of Anatomy as seen in the Hand. | ||
==Explanation of Plates I—III== | |||
===Plate I=== | |||
[[File:Whillis1940 plate01.jpg|600px]] | |||
fig. l. Coronal section of an interphalangeal joint at the 30 mm. stage. x200. a, joint disk; | fig. l. Coronal section of an interphalangeal joint at the 30 mm. stage. x200. a, joint disk; b, looser mesenchyme in circumferential part. | ||
b, looser mesenchyme in circumferential part. | |||
fig. 2. Interphalangeal joint at 45 mm. stage. x 180. a, joint disk; | fig. 2. Interphalangeal joint at 45 mm. stage. x 180. a, joint disk; b, synovial cavity forming in circumferential part of disk. | ||
in circumferential part of disk. | |||
fig. 3. Interphalangeal joint at 63 mm. stage. x 150. a, joint disk reduced to two cells in thickness; b, synovial cavity. | fig. 3. Interphalangeal joint at 63 mm. stage. x 150. a, joint disk reduced to two cells in thickness; b, synovial cavity. | ||
fig. 4. Interphalangeal joint at 72 mm. stage. x185. a, disk reduced to single layer of cells; | fig. 4. Interphalangeal joint at 72 mm. stage. x185. a, disk reduced to single layer of cells; b, synovial cavity. | ||
b, | |||
===Plate II=== | |||
[[File:Whillis1940 plate02.jpg|600px]] | |||
Fig. 6. Interphalangeal joint at 125 mm. stage. x60. 11., Liquefaction of matrix in marginal part of bond of union. | |||
Fig. 6. Wrist region, 30 mm. stage. x 55. a, joint disk between ulnar styloid and triquetral. | |||
Fig. 7. Wrist region, 125 mm. stage. x 20. | |||
Fig. 8. Model illustrating elfect of pressure on a laminated disk formed of sheets of rubber. | |||
===Plate III=== | |||
[[File:Whillis1940 plate03.jpg|600px]] | |||
Fig. 9. Section of elbow of newly born rat. (Thionin.) X340. a, continuity of matrix across joint line; b, liquefaction of matrix at edge near synovial cavity. | |||
Fig. 10. Section of elbow of newly born rat, nearer the edge of the bond of union than fig. 9. x 340. a, degenerating matrix. | |||
Fig. 11. The process of separation almost complewd. x 340. a, liquefaction of the matrix. | |||
{{Footer}} | {{Footer}} | ||
[[Category:Human]][[Category:Joint]][[Category:Draft]][[Category:1940's]] | [[Category:Human]][[Category:Joint]][[Category:Draft]][[Category:1940's]] | ||
Revision as of 18:47, 16 August 2017
| Embryology - 16 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Whillis J. The development of synovial joints. (1940) J Anat. 74(Pt 2): 277-283. PMID: 17104813
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of Synovial Joints
By James Whillis
Department of Anatomy, Guy’s Hospital Medical School
Introduction
Most of the descriptions of the development of synovial joints give the impression that the synovial cavity is formed by a process of absorption. It is the purpose of this paper to review the factors concerned in the formation and extension of the synovial cavity as seen in the development of the human embryo and the white rat.
Observations have been made on the interphalangeal joints of the foot in a series of human embryos varying in length from 30 to 125 mm. Much material of this kind available had to be rejected because of shrinkage due to inadequate fixation and this led to a supplementary study of rat embryos, where the age could be determined with accuracy and fixation adequately controlled.
For the rat embryos used in the investigation I am indebted to Mr T. McKeown of Guy’s Hospital.
The human material was fixed in formalin and the rat limbs were fixed in Bouin’s solution. After fixation and clearing, the tissues were first infiltrated with wax of 45° melting point at a temperature of 50° C. and then transferred to 60° wax at 62° C. for a further period of 1 hour.
The appearances seen in the human specimens will be described first and their significance discussed later.
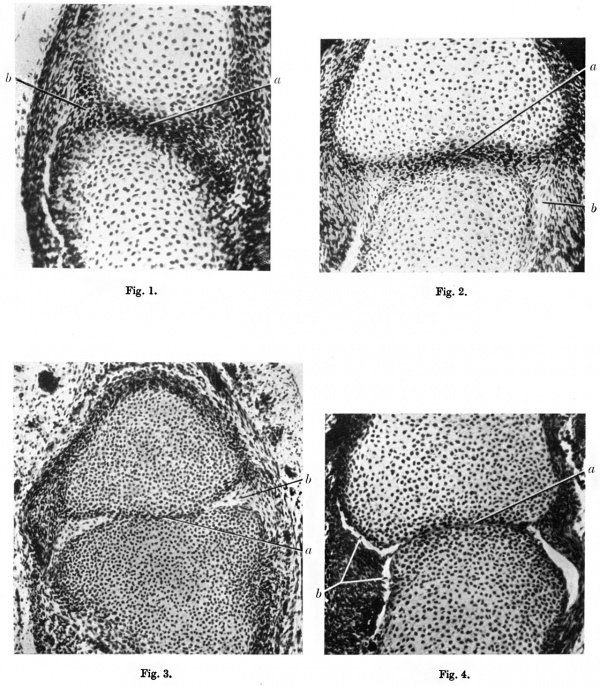
30 mm Stage
(fig.1)
A section of one of the interphalangeal joints at this stage shows the two chondrifying elements separated by relatively undifferentiated mesenchymal cells constituting the “joint disk”. The cells of the disk are arranged in arcs parallel with the surfaces of the cartilage elements. In that part of the disk in the region of the future articular surfaces the cells are flattened, very closely packed and disposed with their long axes in the joint line. At the extreme periphery of the disk the mesenchymal cells are disposed longitudinally and here the mesenchyme is continuous with the condensation forming the perichondrium. In the peripheral part of the disk, where there is a change from the arc to the longitudinal arrangement of the cells, the mesenchyme is much looser than in the central part of the disk where the cells are very closely packed.
45 mm Stage
(fig. 2)
The cartilage elements are developing their shape, a convex surface proxi mally, and a concave surface distally. The joint disk of mesenchyme is reduced in thickness and the loose arrangement of the cells at the periphery is still more evident, and cavities are appearing among them. The central part of the disk is formed of flattened closely packed cells with their long axes parallel with the future joint surfaces.
63 mm Stage
(fig. 3)
Further reduction of the thickness of the joint disk has occurred and it is now represented by a layer two cells in thickness between the two cartilage elements. The synovial cavity is present in the circumferential part and, in serial sections, can be traced all round the joint. The mesenchymal cells nearby are becoming arranged parallel with the boundaries of the synovial cavity.
72 mm Stage
(fig.4)
The joint disk is represented by a single layer of flattened cells. The circumferentially placed synovial cavity is well defined and lined with flattened cells. On the left of the section, the mesenchyme is forming a rudimentary meniscus in continuity laterally with the lining of the synovial cavity and medially with the joint disk. The shape of the future articular surfaces is well defined.
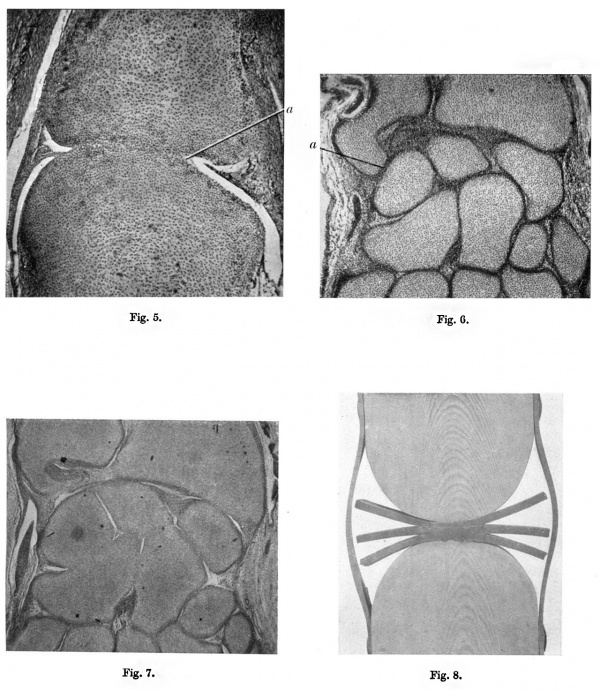
125 mm Stage
(fig. 5)
The joint disk between the cartilages has entirely disappeared and the two elements are united across the joint line by primitive cartilage, or “precartilage ”. The matrix of this primitive cartilage has a staining reaction rather like that of collagen with haematoxylin and eosin, but with thionin, although not so dark as the more mature matrix, it gives the typical reaction of cartilage. Near the margin of the area of union the matrix shows little aflinity for stains and is apparently undergoing liquefaction. There is, at this stage, no extension of the circumferential synovial cavity between the two cartilage elements. On the right of the section the meniscus formed by the remains of the original mesenchymal disk is free of the cartilage elements; on the left, the meniscus is attached to the surface of the proximal cartilage and contains some young chondroblasts. Menisci of this type are found at this stage in nearly all the joints examined.
Discussion
It is convenient first to review the development of joints up to the point where the elements are united across the joint line by primitive cartilage and the joint cavity is limited to the circumferential region, and to consider later the solution of this bond and the extension of the cavity between the two cartilages.
The work of Fell (1925) has shown that at an early stage the mesenchymal core in the limb bud is determined in respect of the pattern of future chondrification in the process of laying down of the skeletal elements. Experiments by Fell & Canti (1934) show, moreover, that the early stages of joint formation described above take place in almost identical fashion in the avian knee joint cultivated in vitro. There is progressive diminution in the thickness of the mesenchymal disk between the developing cartilages, loosening of the mesenchyme in the circumferential part of the disk, and finally union of the two skeletal elements by primitive cartilage. These experiments show conclusively that the mesenchyme of the joint disk does chondrify, and dispose of the theory that the joint is formed because of the interposition of non-chondrifying tissue between the two bones. Carey (1922) described the tissue uniting the cartilages as “mucoid”, but Fell’s experiments and observations on normal embryonic joints have demonstrated that it is very young cartilage.
Recent experiments by Gliicksmann (1938) have shown that undifferentiated mesenchyme subjected to pressure between two developing cartilages itself undergoes chondrification. The progressive chondrification of the joint disk occurring in normal development is, therefore, consistent with the disk mesenchyme being subjected to pressure. The fact that there are many mitotic figures in the cells of the cartilage in the immediate vicinity of the disk during this period of development probably indicates that the progressive diminution in the thickness of the disk is due partly to the conversion of mesenchymal cells into chondroblasts and partly to the invasion of the disk by actively multiplying chondroblasts from the ends of the cartilages.
To postulate pressure on the mesenchymal disk it must be assumed that the cartilage elements are growing very much faster than the sleeve of tissues surrounding them in the limb bud. The assumption is reasonable because the cartilage is growing not only by multiplication of its cells but also by the addition of a relatively large amount of intercellular substance.
That the progressive chondrification of the joint disk is due in part to the effect of pressure is, however, supported by evidence available when the disk mesenchyme is protected from such pressure. The region of the wrist affords the first example of this.
A coronal section of the wrist region of a human embryo of 30 mm. is shown in fig. 6. The early stages of joint formation are evident between all the cartilage elements. The cartilage of the ulnar styloid is long and curved, and is separated from the triquetral cartilage by a well-marked joint disk which resembles in every way those seen where synovial joints will later be formed. The presence of the early stages of joint formation between the ulnar styloid and the triquetral in the human embryo is of special interest because in the macaque monkey a large synovial joint is present in this region (Wood Jones, 1920). It will be obvious from reference to fig. 6 that the mesenchyme between the head of the ulna and the triquetral will be protected from counterpressure exerted by the growing cartilages because of the apposition of the ulnar styloid to the triquetral. fig. 7 shows the appearance at the 125 mm. stage. The styloid process of the ulna is much smaller relative to the size of the head than it is at the 30 mm. stage. It is separated from the triquetral by a considerable interval and is not so curved as in the earlier stage of its formation. A comparison between figs. 6 and 7, moreover, shows that the growth of the radius has apparently pushed the carpal elements distally away from the ulna which seems to be growing rather more slowly than the radius. Between the individual carpal elements, and between the lower end of the radius and the carpus, the joint disks have chondrified and the elements are united across the joint lines by primitive cartilage. Between the head of the ulna and the triquetral, however, where the previous apposition of the ulnar styloid to the carpus has protected the mesenchyme from pressure, it has not chondrified but has become modified to form the articular disk of the inferior radio-ulnar joint. Where the withdrawal of the ulnar styloid from the triquetral has resulted in relief of pressure on the joint disk originally present between the two elements, the disk mesenchyme has not chondrified but has become stretched and incorporated in the medial ligament of the wrist joint.
The features seen at the two ends of the developing clavicle afford similar evidence. The appearance, very early in development, of the centres of ossification in the clavicle leads to a slowing of the growth of the precartilage of which it is formed at a period when the mesenchyme at the two ends is relatively undifferentiated. The relief of pressure on this mesenchyme removes the stimulus for its further chondrification and it becomes modified to form the articular disks of the sterno-clavicular and acromio-clavicular joints.
If there be pressure on the joint disk in the earlier stages of development, such pressure would assist in the formation of the circumferential synovial cavity. fig. 8 shows a disk formed of laminae of sheet rubber subjected to pressure by a clamp. Compression of the central part leads to a reduction in thickness, whilst in the part not subjected to pressure the sheets tend to separate. The orientation of the mesenchymal cells in the joint disk in fig. 3 bears a striking resemblance to the arrangement of the rubber sheets in the model.
The way in which the solution of the temporary continuity between the bone elements is brought about must now be considered.
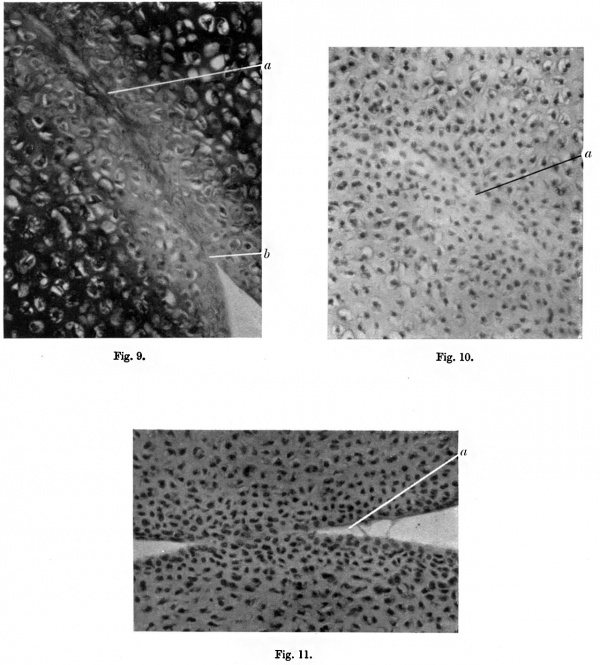
The fact that the union of the two skeletal elements by primitive cartilage persists in the human embryo up to the fourth or fifth month, and is not observed after that period, makes it tempting to assume that foetal movements are the determining factor in the completion of the process of joint formation. The evidence that this is so is, however, scanty and unconvincing. In the first place, the facts might just as well be explained by assuming that movement does not occur because joint formation is not complete as by assuming that it is movement that completes the formation of the joint. Moreover, there is very definite evidence that movement cannot be the sole factor in the extension of the joint cavity between the bone elements. Hamburger (1928) has demonstrated the formation of normal joints in the hindlimbs of frogs in which the muscles had been paralysed by denervation. This observation in itself does not, however, exclude movement completely as a factor in joint formation. first, Hamburger describes, in two cases, complete cartilaginous union between the femur and the hip bone and similar fusion in the tarsal joints in a few cases.
Secondly, the possibility of passive movements cannot be excluded in these experiments. A description by Nicholson (1937) of the formation of perfect synovial joints in a hand-like appendage occurring in a teratoma does, however, show quite definitely that normal joints can develop in the complete absence of movement. In the appendage, very carefully examined and described by him, no trace of muscle could be found. Experiments by Straus (1938) on the occurrence of movements in rat embryos on stimulation over the region of the brachial plexus showed that movement could be elicited in the proximal joints of the upper limb at the 14 mm. stage but not in the region of the carpus or digits. This conforms with the fact that the differentiation of the joints proceeds proximo-distally but, as the movements occurred under abnormal stimulation, it cannot be taken as evidence that, in normal conditions, even the proximal joints were really capable of movement. Indeed, the section of the elbow joint shown in fig. 9 demonstrates union of the humerus and the ulna by primitive cartilage after birth, and no movement of the elbow region was detected in the rat from which the section was taken. Straus’s experiments do show that in conditions of abnormal stimulation the muscles, which are capable of contracting, may break the bond of union so that movements can take place at a time when they do not normally occur. This therefore suggests that movement, although not the sole factor, may be one of the factors in normal joint formation. In the tissue culture experiments conducted by Fell and Canti passive movements of the two cartilage elements failed in every case to prevent their subsequent cartilaginous fusion. This confirms the evidence that some additional factor is essential in the formation of the joint.
In the search for other factors which may be concerned it is interesting to note that it is only in tissue explants cultivated in vitro that the cartilaginous union between the two bone elements persists in all cases. In the other examples which have been considered, viz., normal development, denervated limbs, and the teratoma, there is solution of this continuity and the formation of the completed synovial joint. A tabulation of the conditions present in the cases under review is given below:
| Nerves | Vessels | Muscles | Calcification | Persistent union | |
|---|---|---|---|---|---|
| Embryo | Yes | Yes | Yes | Yes | No |
| Teratoma | Yes | Yes | No | Yes | No |
| Denervated limb | No | Yes | Paralysed | Yes | Unusual |
| Explant | No | No | No | No | Always |
It will be noted that the only factor which is constantly associated with persistence of union between the two elements is absence of calcification in the cartilage. On this evidence it would seem that the completion of joint formation occurs at a time when the cartilages have reached a certain stage of development, and that a further accompaniment of this stage is the occurrence of calcification. The failure of both calcification and joint formation in the tissue explants occurs because they are not, at this stage, in a normal environment.
Carey, in 1922, stated that the joint was completed by liquefaction of the tissue (termed by him “mucoid”) uniting the two elements. This observation has been confirmed in the present investigation by the examination of the joints of the upper limb, particularly the elbow, in rats during the few days following birth. At this period, when the joint surfaces are separating, the matrix of the primitive cartilage uniting the two bone elements loses its staining reaction. The process is most marked near the edges where the matrix is exposed to the synovial fluid present in the circumferential cavity. Separation occurs by progressive liquefaction of the degenerate matrix from~the margins inwards. fig. 9 represents the appearances seen in the central part of the bond of union between the ulna and the humerus in a newly born rat. It can be seen that the young cartilage is here normal and gives the typical staining reactions. fig. 10 represents a section through the marginal part of the same joint nearer the synovial cavity. Here the matrix is degenerated and stains with great difficulty. fig. 11 shows a section through the wrist joint where the liquefaction of the matrix and ingress of synovial fluid have occurred almost to the centre of the joint. These processes take place in the white rat during the few days immediately after birth, and in the human embryo about the fifth month. The cartilage cells exposed in the process of liquefaction become flattened where a considerable part of their surface is in relation to the synovial fluid, but where they are still protected by matrix over the greater part of their surface they remain spherical for some time. The reason for the liquefaction of the matrix along the joint line at this period is unknown. It occurs on the attainment of a certain stage of development of the cartilage which is, in some situations, associated with the appearance of calcification. It is possible that attempts at movement in the joint at this critical period may be of assistance in the separation of the two bones.
This investigation has directed attention to further problems on which work is now being carried out. The first is the explanation of the fact that the costal elements of the vertebrae in the thoracic region form joints with the other elements, whereas in the other regions they become incorporated in the vertebrae. Secondly it seems likely that the behaviour of undifferentiated mesenchyme under various stresses and strains in controlled experimental conditions such as tissue culture offers a method for elucidating further the factors which determine the appearance of the cavity in synovial joints.
In conclusion I must record my thanks to my technical assistant Mr G. A. Walker who has been responsible for the preparation of the serial sections which have been necessary in this investigation and who has prepared for me the photographic illustrations.
Summary
- The initial stages in the formation of synovial joints show that counterpressure of the two cartilage elements on the intervening mesenchymal joint disk may be responsible for the progressive chondrification of the disk and for the appearance of the circumferentially placed synovial cavity. Where pressure on the disk is removed it does not chondrify and this accounts for the presence of intra-articular disks at the wrist and at the two ends of the clavicle.
- After chondrification of the disk, the two elements in the joint are united for a time by primitive cartilage.
- The solution of continuity in the later stages of joint formation is accomplished by liquefaction of the matrix of the primitive cartilage uniting the two bones. The cause of the liquefaction is not explained.
Movement alone does not cause breakdown of the bond of union between the two cartilage elements, but it may play an ancillary part in the process.
References
CAREY, E. J. (1922). J. Morph. 37, l.
FELL, H. B. (1925). J. Morph. 40, 417.
FELL, H. B. & CANTI, R. G. (1934). Proc. ray. Soc. B, 118, 316. GLiicKsMANN, A. (1938). Anat. Rec. 73, 39.
HAMBURGER, V. (1928). Ram: Arch. Entw. Mach. Organ. 114-, 272. HARRIS, H. A. (1933). Bone Growth in Health and Disease.
NICHOLSON, G. W. (1937). Guy’: Hosp. Rep. 87, 46.
STRAUS, W. J. (1938). J. Anat., L¢md., '12, 470.
WOOD JONES, F. (1920). The Principles of Anatomy as seen in the Hand.
Explanation of Plates I—III
Plate I
fig. l. Coronal section of an interphalangeal joint at the 30 mm. stage. x200. a, joint disk; b, looser mesenchyme in circumferential part.
fig. 2. Interphalangeal joint at 45 mm. stage. x 180. a, joint disk; b, synovial cavity forming in circumferential part of disk.
fig. 3. Interphalangeal joint at 63 mm. stage. x 150. a, joint disk reduced to two cells in thickness; b, synovial cavity.
fig. 4. Interphalangeal joint at 72 mm. stage. x185. a, disk reduced to single layer of cells; b, synovial cavity.
Plate II
Fig. 6. Interphalangeal joint at 125 mm. stage. x60. 11., Liquefaction of matrix in marginal part of bond of union.
Fig. 6. Wrist region, 30 mm. stage. x 55. a, joint disk between ulnar styloid and triquetral.
Fig. 7. Wrist region, 125 mm. stage. x 20.
Fig. 8. Model illustrating elfect of pressure on a laminated disk formed of sheets of rubber.
Plate III
Fig. 9. Section of elbow of newly born rat. (Thionin.) X340. a, continuity of matrix across joint line; b, liquefaction of matrix at edge near synovial cavity.
Fig. 10. Section of elbow of newly born rat, nearer the edge of the bond of union than fig. 9. x 340. a, degenerating matrix.
Fig. 11. The process of separation almost complewd. x 340. a, liquefaction of the matrix.
Cite this page: Hill, M.A. (2024, April 16) Embryology Paper - The development of synovial joints. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_development_of_synovial_joints
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G