Paper - The Organization and Cell-Lineage of the Ascidian Egg 2
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Conklin EG. The Organization and Cell-Lineage of the Ascidian Egg (1905) J. Acad., Nat. Sci. Phila. 13, 1.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Conklin 1905 TOC: I. The Ovarian Egg | II. Maturation and Fertilization | III. Orientation of Egg and Embryo | IV. Cell-Lineage | V. Later Development | VI. Comparisons with A.mphioxus and Amphibia | VII. The Organization of the Egg | Summary | Literature Cited | Explanation of Figures
II. Maturation And Fertilization
These processes are so intimately associated in the ascidian egg that it is difficult and perhaps inadvisable to treat them entirely separately. As in so many other eggs the entrance of the spermatozoon furnishes some stimulus to the egg which leads to the completion of the maturation divisions. Without this stimulus the egg may remain in the stage of the metaphase of the first polar spindle for hours or even days.
A. Maturation
1. Disappearance of Nuclear Membrane
The first steps in the formation of the polar spindle take .place before the entrance of the spermatozoon. Almost as soon as the egg is laid, and sometimes even before this, the wall of the germinal vesicle dissolves and the clear protoplasm contained within the germinal vesicle moves up to the animal pole of the egg where it may spread out into a cap or peripheral layer (Ciona), or may form merely a somewhat flattened disk (Cynthia). As soon as the nuclear membrane has dissolved the chromosomes, nucleolus and a granular mass from which the spindle fibres are formed gather together into the center of this area of nuclear protoplasm (figs. 62, 63, 77, 78) ; since the chromosomes lay at the periphery of the germinal vesicle before its membrane dissolved, this involves a considerable movement on the part of these various constituents. No distinct linin network is visible throughout the germinal vesicle, either before or after its membrane dissolves, and the drawing together of these scattered elements into a central mass must be due to something other than the contraction of the threads of such a network.
The chromosomes, when drawn together into a central mass, are connected by a faintly staining, finely granular substance, which is much denser than the surrounding nuclear protoplasm. In the further development of the polar spindle this mass gives rise to the spindle fibres, and from this fact, as well as from its staining reactions, it may be identified with linin (figs. 62, 77).
The question as to the cause of the dissolution of the nuclear membrane is an
interesting one. In a recent work, R. Hertwig (1904) suggests that it is due to
the fact that the cytoplasm attacks the nucleus after the cell has ceased to grow.
From such observations as I have made 1 should be inclined to think that the
cause was a quite different one, viz., the continued growth of the nucleus at a
iin ire rapid rate than the cytoplasm. ' In most if not all cases the nuclear membrane dissolves only alter the nucleus has exceeded in volume a certain ratio to
the cell bodv. In the ascidian egg the germinal vesicle does not begin to dissolve
as soon as the egg ceases to grow ; on the other hand, therv is a considerable period
after the maximum size has been reached before the nuclear membrane disappears;
during this period the germinal vesicle continues to enlarge, the test cells are
extruded, the secretion which gives rise to the chorion is poured out, the entire
egg shrinks in volume, and finally the nuclear membrane grows very thin and disappears. This process is in no wise complicated by the presence of a centrosome,
since, according to my observations, no centrosomes are present at any stage of the
maturation divisions.
2. Chromosomes
Even before the wall of the germinal vesicle dissolves the chromosomes may be distinguished as small deeply staining bodies, some of which at least are Vor Y-shaped (fig. 70). They are small and numerous, and I have not been able to count them with any assurance of accuracy. After they have been drawn together into the center of the nuclear area, as described above, they become a little larger and are plainly V-shaped (tigs. 62, 63, 77). When the spindle fibres appear they are at first widely scattered on or among these fibres (figs. 63, 79, SO), and only in the metaphase do they become arranged in an equatorial plate (fig. 66). In the splitting of the chromosomes the daughter halves first separate at the apex of the V and remain longest connected together by the two limbs ; this double V, with the apices pointing to the two poles of the spindle, is then stretched out until the two limbs of each V come to lie near together, thus forming a double Y, each with a long stem pointing to opposite poles; even the little space between the limbs of the Y may disappear, thus forming cross-shaped chromosomes (fig. 66). After the daughter chromosomes have separated they are plainly V-shaped (figs. 67, 68) ; and this shape may also be seen in the first polar body and in the second polar spindle (figs. 68, 69). In the second polar spindle each limb of the V is separated from the other, thus giving rise to rod-shaped chromosomes, which are found in all the stages of the anaphase and in the second polar body (figs. 70-73). Owing to the small size of the chromosomes it has not been possible to determine with certainty which of these maturation divisions is reducing and which equational. If the two limbs of the V's in the first maturation represent two individual chromosomes united at one end, then the first maturation division is equational and the second reducing, for these limbs of the V's are not separated until the second maturation ; if, on the other hand, the cleft in the original V's represents the splitting of two original chromosomes placed side by side (a thing which seems likely, since such parallel chromosomes without any cleft arc found in early stages ((-/. figs. iti. 62), then the first maturation division would separate whole chromosomes and hence be reducing, while the second would separate half chromosomes and therefore be equational. Only a careful investigation of the manner of origin of these \ -shaped chromosome- would finally solve this problem, and this material is unfavorable for such work.
3. Nucleolus
The nucleolus of the germinal vesicle is large and is frequently vacuolated; it usually lies eccentrically in the germinal vesicle, though its position hears no constant relation to the polarity of the egg. As is usually the ease, it begins to dissolve at the same time that the nuclear membrane does, and it disappears, with great rapidity, so that no trace of it is left by the time that the first maturation figure has reached the metaphase. In this respect it differs materially from the nucleolus of many other eggs, where its solution is so slow that it may not disappear until late in the first maturation division. In this case the solution of the nucleolus is hastened by its breaking up into many small fragments (figs. 62, 63, nl.).
4. Spindle Formation
My observations agree entirely with those of Boveri ( lS'.ldi. .1 ulin dS'JMi. Hill (1895), Castle (1896) and Crampton 1 in showing that there is no trace of a centrosome at either pole of either of the maturation spindles at any time in their historv. These results are directly opposed to those of Golski (1809). who found minute centrosomes at the poles of the maturation spindles of Ciona intestinalis. Not only are no centrosomes visible in my preparations at the poles of the spindle, but all evidences of astral radiations are also absent. Under these circumstances the formation of the spindle is of unusual interest. The spindle fibres first appear as lines of granules, which radiate in all directions from the finely granular mass of linin substance which unites the chromosomes in the middle of the nuclear area (fig. 62). These lines of granules are quickly transformed into fibres which run through the linin substance ; these fibres are never parallel at their first appearance and frequently radiate in all directions, though they sometimes run in the same general course (figs. 63, 64). As this mitotic figure with the surroundingnuclear plasm is moved nearer and nearer to the surface of the egg the fibres come to be more nearly parallel, becoming paratangential with the egg surface (fig. 65). In this rearrangement of the fibres they are at first farthesl apart at the ends, so that the spindle has an hour-glass shape (fig. 65). Then certain of these fibres unite at their ends into several groups or bundles, but the fibres which belong to one group at one pole may be associated with different fibres at the opposite pole (fig. CO). There is thus formed a kind of multipolar spindle, closely resembling the mitotic figure present in many plants [cf. Osterhout, 1897: Mottier, 1897:
- 1 I am indebted to Dr. Crampton for the privilege of seeing photographs of the beautiful plates of his completed but yet unpublished work on the maturation and fertilization of Molgula.
Nemec, 1899). Finally, in the metaphase all the spindle fibres are drawn together
at the poles; but even in this stage, though the spindle fibres lie close together,
they do not unite into a central body, and there are no astral rays (fig. 66). In
the anaphase a few rays may be seen running from the margin of the chromatic
plate toward the equator of the cell and lying on the periphery of the spindle (figs.
67, 81, 82). These are the only fibres which are not continuous from pole to pole,
and are therefore the only ones which bear even a remote resemblance to astral rays ;
that they are not such, however, is shown by the fact that they radiate from no
center but lie only around the periphery of the spindle. In this respect they more
closely resemble peripheral spindle fibres than astral rays. Still, if one considers
that one of the characteristics of peripheral spindle fibres is that they are attached
to chromosomes, it will be seen that these fibres do not belong in that category. Of
course, since centrosomes are not present, there can be no central spindle. We have
in this case, therefore, a mitotic figure in which are neither central spindle, peripheral spindle nor astral rays in the strict significance of those terms. The spindle
which is present arises wholly from nuclear linin, and consists almost exclusively of
fibres which are continuous from pole to pole.
The small size of the maturation spindles of the ascidian egg is notable as contrasted with the great size of the germinal vesicle. Among many annelids and mollusks the first maturation spindle is at least as long as the diameter of the germinal
vesicle, whereas among the ascidians it is scarcely more than one-quarter as long.
However, in those animals in which the spindle is very long in the prophase or
metaphase it undergoes a great shortening in the anaphase, e. g., in Crepidula it
is not more than half as long in the anaphase as in the metaphase (Conklin, 1902).
This is probably true of all cases in which the maturation spindle is a large one ;
for, since division of the cell body regularly takes place through the equator of the
spindle, the spindle must be relatively short at the time of the division of the cell
body, or the polar body will be relatively large. In all those cases in which the
first polar spindle is a long one, centrosomes are present near the periphery of the
germinal vesicle before its membrane disappears and the loose linin network of the
nucleus is transformed into the spindle fibres, thus forming a large, loosely constructed spindle. Later, by contraction of these fibres, the spindle shrinks in all
dimensions. In the ascidians, on the other hand, no centrosomes are present and
the shrinkage of the linin takes place before the spindle is formed, so that from the
first it occupies but a small part of the volume of the germinal vesicle, and is no
larger at the beginning of mitosis than at its close.
The second maturation spindle arises in part at least from the remains of the
first, and is about half as large. Here also there is no trace of centrosomes or astral
radiations at any stage. The spindle is barrel-shaped, and a few peripheral fibres
are found around it in the anaphase (figs. 69-72) ; in all respects it closely resembles the first maturation spindle.
Such a case of mitosis as this, in which we have the formation of a spindle, the
separation of chromosomes and the division of the cell body entirely without the
presence of centrosomes, offers a valuable opportunity for the study of the mechanics of indirect cell division. Inasmuch as sonic of the cleavages also throw lighl on
this problem, its further consideration will be postponed to the section which deals
with the first clca\ age.
5. Movements of Spindle and of Nuclear Plasm - formation of Polar Bodies
In Cynthia the tirst maturation spindle and the surrounding nuclear plasm remain indefinitely in the condition shown in figures 77 and 78 unless the egg be fertilized. In Ctona the stage at which the maturation processes come to rest is a little more advanced than in Cynthia, as is shown in figure 17li: the peripheral layer of protoplasm is here collected over the lower hemisphere of the egg, and the nuclear plasm which has escaped from the germinal vesicle forms a layer over the entire upper hemisphere. Unfertilized eggs may remain in this condition for at least three or four hours and still he capable of fertilization and normal development ; Imt if they remain unfertilized for ten or twelve hours the nuclear plasm spreads through the substance of the yolk in irregular masses {cf. text lig. I), and the eggs thereafter are not capable of normal fertilization. The maturation and further development of the egg are finally and forever halted in this early stage unless the eo-g be fertilized. As soon as a spermatozoon enters the egg active movements of the protoplasm begin and a localization of different ooplasmic materials occurs, which will be described later; at the same time the fust maturation spindle moves to the animal pole and is turned from a paratangential to a nearly radial position. The daughter chromosomes then separate and the first polar body is extruded (figs. 66-68 and 79-82).
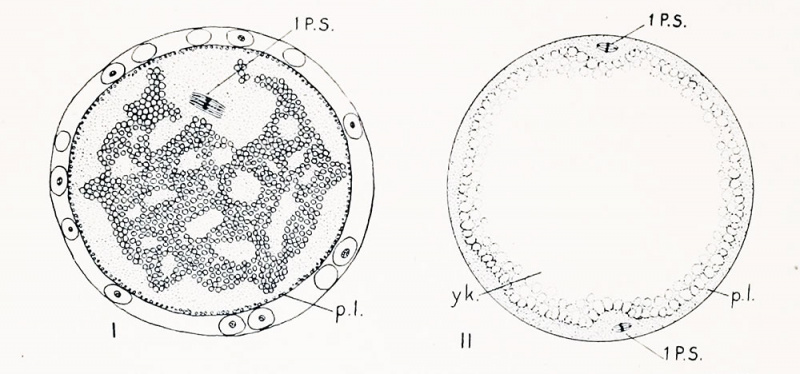
Fig. I. Section of an egg of Cynthia partita which had lain twelve hours without being fertilized. The first polar spindle (1 p. -. lies in tin- position in which it was first formed ; the peripheral layer of yellow protoplasm ip. l.i remains uniformly distributed over the surface, hut the clear protoplasm has spread throughout the yolk and broken it up into irregular masses (compare with figs. 77 and 78 showing unfertilized est:s in normal condition).
Fig. II. Stained preparation of an entire egg of Cynthia partita, showing small spindles at opposite poles (1. p. s.i. which are possibly two first maturation spindles, though more probably one of these is a precociously developed sperm spindle.
The second maturation spindle is smaller than the first, as Castle has shown. and. like the first, is paratangential in position in early stages and only later becomes radial. The second polar bod) is extruded close to or immediately under the first (figs. 71-73). The two polar bodies are of approximately the same size, and neither ever divides. They are at first composed of clear protoplasm in which the chromosomes are free; later the chromatin is dissolved and diffused throughout the cell body, so that they stain deeply and uniformly. They may at all times be distinguished from the test cells by this staining reaction as well as by their being closely attached to or imbedded in the egg. In Ciona they may further be distinguished from the test cells by the fact that they are larger than the latter. In many eggs of Cynthia and in almost all of Ciona the polar bodies remain attached to or imbedded in the egg at the point of their formation, and they thus constitute a most important landmark.
B. Fertilization
As has been said, the first maturation spindle remains in the metaphase until the egg is fertilized. The egg remains capable of fertilization for three or four hours at least after the first formation of this spindle. As Castle (1896) has shown, self-fertilization rarely if ever occurs in Ciona, though artificial cross-fertilization is most easily accomplished. In Cynthia, on the other hand, artificial cross-fertilization is successful in only a small proportion of the eggs.
I have so far been unable to find any artificial means which will cause the unfertilized eggs to develop beyond the metaphase of the first maturation division. Violent shaking, various degrees of concentration or dilution of sea water, solutions of sodium or magnesium chloride of varying strengths have all been without effect in this regard. My experience in this matter is similar to that of Lyon (1903). who reports that he was unable to cause parthenogenetic development among ascidians at Naples by any artificial means.
1. Entrance of Spermatozoon
Of the multitudes of spermatozoa which may be seen burrowing between the follicle cells outside of the chorion after spermatozoa have been mixed with the ova, only a few ever pass through that membrane. I have never seen a spermatozoon in process of passing through the chorion and do not know how it is accomplished. It is possible that there are one or more micropyles at the lower pole, though I have never seen them. In whatever manner the spermatozoa pass the chorion it is done very quickly and several frequently enter the perivitelline space; dispermy or polyspermy, however, is very unusual. A spermatozoon enters the egg in from two to five minutes after the spermatozoa are mixed with the ova, and the presence of supernumerary spermatozoa in the perivitelline space is shown by the fact that some of the test cells are occasionally fertilized (figs. SO, 85, sn).
The spermatozoon always enters the egg near the vegetal pole. I have not found it possible to determine in living eggs whether the point of entrance lies exactly at the vegetal pole or a little to one side of this. In stained preparations of entire eggs, as well as in sections, the entering spermatozoon is usually seen to lie eccentrically with reference to the vegetal pole (figs. 79, 173). In other cases, however, it lies almost exactly at that pole; in sections this appearance might be due to obliquity of the plane of section to the egg axis, but in preparations of entire eggs it can he seen that the spermatozoon does sometimes enter almost exactly at the vegetal pole. It is unquestionably true that the point of entrance is usually eccentric as Castle affirms, hut the degree of eccentricity just as certainly varies in different cases. It might he supposed that this eccentricity always lay in a single dehnite meridian, were it not for the fact that in cases of dispermy and polyspermy the various points of entrance lie in different meridians {cf. figs. 12, 94). I conclude therefore that the spermatozoon may enter at any point on the vegetal hemisphere within about 30 of the pole.
The fact that the spermatozoon always enters near the vegetal pole must be due to some structural peculiarity ; the peripheral layer of protoplasm is a little thicker at this pole than elsewhere at the time that the sperm enters, and this might be held to be the cause of the sperm's entering at this pole, were it not for the fact that the sperm enters at the vegetal pole in many other eggs, e.g. those of annelids and mollusks, in which there is no periperal layer. It is probable that this very general phenomenon is dependent upon some fundamental property, such as the polarity of the egg or the direction of movement of the egg substance.
2. Movements of Ooplasm
With the entrance of the sperm the most astonishing series of changes takes place in the egg. These changes are most striking in the living eggs of Cynthia, where, owing to the yellow color of the peripheral protoplasm, the movements of the egg substance can be directly observed ; but they may also be seen in the living eggs of Ciona, and a detailed study of these changes may be made on fixed and stained preparations. Almost immediately after the entrance of the spermatozoon the peripheral layer of protoplasm, which is nearly uniformly thick, and the great area of nuclear plasm, in which the first maturation spindle lies (figs. 77, 78), How around to the lower pole of the egg, leaving the first maturation spindle surrounded by only a small amount of protoplasm. Thus within some ten minutes after the entrance of the sperm the protoplasmic pole of the egg is transformed into the yolk pole and vice versa. Castle does not figure nor describe this flowing of the protoplasm from the animal to the vegetal pole, and it is probably owing to the fact that he had not observed the early stages in which this occurs that he describes the polar bodies as being formed at the yolk pole of the egg and the spermatozoon as entering at the protoplasmic pole. Although he says that the presence of a spermatozoon cannot be detected in the egg from which his figure 1 is drawn, I should suppose from the fact that the first polar body is being extruded that the sperm must already have entered {cf. my fig. 173).
a. Localization of Yellow Protoplasm
In Cynthia this downflow of protoplasm takes place so rapidly that it can be seen in the living egg and with such force that the test cells, which lie between the surface of the egg and the chorion, are sometimes carried down with the streaming protoplasm to the lower pole of the egg, where they are crowded together and heaped up in the perivitelline space (fig. 3, et seq.). While this flowing is most active, streamers of yellow surface protoplasm may be seen radiating toward the upper pole. The yellow protoplasm thus carried to the lower pole collects into a deep orange-yellow spot which surrounds the sperm nucleus (figs. 4-6); it frequently forms a prominence at the lower pole which recalls the polar lobe of the eggs of annelids and mollusks. The clear nuclear protoplasm also flows to the lower pole, where it lies beneath the yellow disk or spot and is visible around its periphery (figs. 4-6). The yellow protoplasm then gradually spreads again until it covers most of the lower hemisphere (figs. 6-10). Then the sperm nucleus moves to one side of this yellow cap, and a large part of the yellow protoplasm is drawn over with it until it forms a yellow band or crescent, in the middle of which the sperm nucleus lies. This crescent lies just below the equator of the egg and its middle point marks the posterior pole of the future embyro. while its two horns reach forward about half-way around the egg to the middle of the right and left sides.
b. Localisation of Clear Protoplasm and Yolk
At the same time that the yellow protoplasm is being formed into a crescent and moved up toward the equator on the posterior side of the egg, the clear protoplasm which surrounds the sperm nucleus and aster is also drawn entirely away from the lower pole to the posterior side of the egg and thence up to the equator (figs. 82-92). Up to this time the sperm nucleus and the clear and yellow protoplasm have remained near to the egg surface ; finally, after the meeting of the germ nuclei near the posterior pole of the egg, these nuclei and the clear protoplasm surrounding them move inward to the center of the egg, while the yellow protoplasm is largely left at the surface.
When the clear and yellow protoplasm are withdrawn from the upper pole the gray yolk is there exposed (figs. 4, 5, 11). After the protoplasm moves up to the posterior pole the yolk is exposed over the entire egg, except for the area of the yellow crescent and a narrow line of clear protoplasm, which comes to the surface just above the crescent (figs. 13-18).
In sections, small spherules which probably represent the yellow granules of the peripheral layer of protoplasm, may be seen heaped up around the entering sperm (fig. 74), this aggregation corresponding to the yellow spot of the living egg (fig. 6). This massing of the yellow spherules is most marked, while the sperm head lies in the peripheral layer ; when it passes through this layer into the deeper layer of clear protoplasm the yellow spherules again spread out into a flattened disk, as shown in figures 75 and 80, which correspond to figures 7 and 8 of the livingegg (Plate I). Later, when the sperm nucleus moves to the posterior pole and the yellow protoplasm is drawn over to that side to form the crescent, sections show that this crescent does not lie entirely on the surface, but that it extends for some distance inward toward the sperm nucleus (figs. 87, 90, 92).
In Ciona the same type of protoplasmic movement occurs as in t ynthia, 1 >ut
with certain minor differences. The peripheral layer is here decidedly thicker at
the lower pole than elsewhere, even before the fertilization of the egg; the nuclear
plasm or clear protoplasm is also at this stage distributed as a layer over the entire
upper hemisphere of the egg (fig. 172). After the entrance of the spermatozoon
the protoplasm of both these layers collects at the lower pole. The nuclear plasm
and peripheral protoplasm cannot easily be distinguished in living eggs of Ciona, but
in fixed and stained material the latter stains more deeply than the former (figs.
172. 173). A crescent of peripheral protoplasm is formed here in the same way as
in Cynthia (figs. 175, 170). and it occupies the same relative position (figs. 179
183). Though Castle did not observe the peripheral layer of protoplasm and its
movement to the lower hemisphere it is evident that he recognized at least a part
of the crescent. His figures 17 and 45-47 show the middle portion of the crescent in the 2-8 cell stages, and he describes this as an area of finely granular protoplasm, which is clear in the living egg, and out of which the small posterior
mesenchyme cells are formed. According to my observations these cells arise from
a small part only of the middle portion of this crescent, while the greater part of
the crescent gives rise to the muscle and mesenchyme cells of the tadpole. From
his figures, as well as his descriptions, it is evident that he recognized only a small
portion of the crescent, viz.. this median area of "clear protoplasm."
In many eggs of Ciona, if not in all, clear protoplasm, which is composed of large alveoles, surrounds the entering spermatozoon (fig. 173). Later, when the sperm nucleus moves to the posterior pole, this clear area moves with it. and in sections in the median plane (figs. 175, 170) forms a clear triangular area in the middle of the deeply staining crescent. There is here shown a marked differentiation of the substance of the crescent which continues to lie recognizable throughout most of the cleavage. I have not observed this clear median portion of the crescent in the 4-cell stage, but in the S-cell stage and thereafter it is plainly visible as a deeply staining cap of protoplasm on each side of the mid-line. It corresponds in the main to the 'clear protoplasm" described by Castle, which, as he discovered, marks the position of the sperm nucleus at the posterior pole and which ultimately gives rise to the '-small posterior mesenchyme" cells (B 7 ") at the posterior pole of the gastrula. This same clear protoplasm is present in the middle of the crescent in the Cynthia egg, although it is here obscured by the surrounding yellow pigment; in the unsegmented e^/ it forms a layer of transparent protoplasm over the surface of the crescent, and in the cleavage stages of prepared eggs it is visible as two deeply staining caps of protoplasm similar to those in the egg of Ciona ; it ultimately gives rise to the small posterior mesenchyme cells which are formed from the middle of the crescent and which are composed of clear protoplasm in which there is no yellow pigment (fig. 48. inch.). The substance of the crescent is therefore plainly differentiated from the first into these two substances, clear and yellow protoplasm, which remain distinct throughout the entire development.
3. Development of Sperm Nucleus and Aster
Immediately after it has entered the egg the sperm head is rod-shaped and is frequently coiled or twisted on itself (figs. 74, 79, 173). It decreases in length and increases in width very quickly, and soon appears pear-shaped, the pointed end being directed toward the sperm aster. At first densely staining throughout, it stains less and less* densely as it swells in volume, until finally the chromatic and achromatic constituents are easily distinguishable (figs. 80-87). During this process there are no evidences of chromosomal vesicles, the nucleus constituting a single vesicle. In some cases there is a faint line between the head of the sperm and the egg membrane which represents the middle piece and perhaps a portion of the tail (figs. 74. 79). Very soon after the spermatozoon has entered the egg a small aster, with central clear area and minute rays, appears in the position of the middle piece, between the sperm head and egg membrane (figs. 80, 173). The sperm aster then grows with great rapidity, the rays extend throughout the greater part of the clear protoplasm and even into the yolk and a minute body, the centrosome, becomes visible at the centre of the rays, while the whole aster stains moi'e deeply that the surrounding protoplasm (figs. 81-87).
4. Path of the Spermatozoon within the Egg
The spermatozoon usually enters the egg in a radial direction and keeps right on through the protoplasm at the lower pole until it reaches the deeper lying yolk (figs. 74, 75, 80). This may be known as the penetration path (Roux). The sperm nucleus and aster then rotate so that the aster is directed forward in all further movements, as is true in so many other cases (figs. 80-83). The path described after the rotation is the copulation path (Roux), and it always forms more or less of an angle with the penetration path. While the penetration path may apparently lie in any portion of the lower hemisphere within about 30 of the pole, the copulation path seems to be definitely determined by the structure of the egg. The sperm nucleus and aster move in this path from the neighborhood of the lower pole up to the equator of the egg on the posterior side, all the time keeping near to the surface of the egg (figs. 81-87). But this path is not always the shortest path to the equator; sometimes it is the longest, as in figures 81 and 85, in which the sperm having entered to the left of the lower pole moves across to the right side in the figure and then up to the equator. The point near the equator to which the sperm nucleus moves invariably marks the posterior pole of the egg and of the future embryo, and the copulation path by which the sperm nucleus reaches this posterior pole must lie along the posterior side of the egg ; but since the point of entrance of the sperm and the penetration path may lie near to or far from the posterior side, it is evident that they can have nothing to do in determining the position of the posterior pole. And since the copulation path is not always the shortest path to the equator, but may sometimes be the longest, it seems probable that the direction of the copulation path is not the cause but the result of the antero-posterior differentiation of the egg. A further consideration of this subject will be found in the general part of this paper.
5. The Egg Nucleus and its Movements
After the formation of the second polar bodv the chromosomes left in the egg form chromosomal vesicles which then unite to form the egg nucleus (fig. 73). The latter then moves away from the animal pole into the yolk, apparently in the direction of the axis of the second polar spindle (figs. 86, 87); it soon turns, however, and moves toward the sperm nucleus and aster at the posterior pole. A't first a few remnants of spindle fibres connect the egg nucleus with the animal pole (fig. 87), but these are soon lost and thereafter this nucleus, without any surrounding area of protoplasm or astral rays, is almost lost to view in the dense mass of yolk (fig. 89). Finally the egg nucleus emerges from this yolk into the clear protoplasm surrounding the sperm nucleus, and the two nuclei meet at the equator of the egg about half way between the posterior pole and the center. The relative positions of the two germ nuclei when they first meet is invariably the same ; the egg nucleus always lying on the central (anterior) and animal pole (ventral) side of the sperm nucleus (figs. 89-93).
6. Sperm Amphiaster and first Cleavage Spindle
About the time that the sperm nucleus has moved to the edge of the yellow cap (fig. 8) and some time before the union of the two germ nuclei, the sperm centrosome divides as shown in figures 88, 89. 1 have not observed all the details of this division, but it is evident that the centrosome here gives rise to a centrosomal spindle or netrum (Boveri L901 ). at the poles of which the daughter centrosomes lie. After the centrosome has divided, the sphere also divides (fig. 88), and a well-marked central spindle is left connecting the two daughter centrosomes (fig. 89). When these daughter centrosomes have moved to the poles of the sperm nucleus the central spindle is curved around that nucleus, and finally its fibres become indistinct (fig. 90) and then disappear altogether (fig. 91 ). The sperm aster, at the time of its division, invariably lies on the central side of the sperm nucleus, and the axis of the amphiaster thus formed is at right angles to the copulation path and to the plane of the first cleavage (figs. 88-91). Up to the time when the two germ nuclei meet, the sperm centrosomes lie at the poles of the sperm nucleus (fig. 91), whereas no trace of centrosomes are ever found in connection with the egg nucleus, and after the latter has moved away from the- animal pole, on its path to the sperm nucleus, no trace of radiations, spindle fibres or even of surrounding cytoplasm can he found near the egg nucleus. The cleavage centrosomes may be traced without a break back to the sperm amphiaster, to the sperm aster and finally to the middle piece of the spermatozoon. There could not possibly be a clearer ease of the origin of the cleavage centrosomes from the middle piece of the spermatozoon than is presented by the ascidian egg. This conclusion agrees with the work of all who have ever studied the fertilization in these eggs (Boveri 1890, Julin 1893, Hill 1895, Castle L896, Golski 1899, Crampton). In Ciona, Castle observed an archoplasmic mass in connection with each of the germ nuclei, though that found near the sperm nucleus was larger and more energetic than the one near the egg nucleus; the latter afterward completely degenerates, according to Castle, and takes no part whatever in the formation of the lirst cleavage spindle. I have been unable to find tins archoplasmic mass in connection with the ega; nucleus unless the remnants of the second polar spindle (fig. 87) maybe interpreted as such.
Any one who has studied the method of origin of the cleavage centrosomes in the eggs of ascidians and of mollusks cannot fail to be impressed with the profound differences between the two. In the one we have no centrosome or aster in connection with the egg nucleus at any stage, while the sperm centrosome and aster are visible at all stages alter the entrance of the spermatozoon, and give rise directly to the cleavage centrosomes; in the other, according to my observations, a centrosome and aster are found in connection with each of the germ nuclei, and coincidently with the union of these nuclei the asters or spheres also unite, while out of this fused sphere material a single centrosome arises in connection with each genu nucleus. It is recommended to those who maintain that in these details of fertilization all animals must conform to a single type that they study the fertilization of a gasteropod as compared with that of an ascidian.
7. Dispermy
Although it is a relatively rare thing for more than one spermatozoon to enter an egg. still ova are occasionally found into which two spermatozoa have- penetrated. The entrance of more than two spermatozoa, if it occurs at all. must be a very rare phenomenon. In stained preparations and in serial sections I have never seen an undoubted case of it; unsegmented eggs are sometimes found in which there are a number of nuclei, but in all cases it is possible that these may have arisen from the division of two sperm nuclei. In living eggs I have sometimes observed several yellow spots on the lower hemisphere. Such an egg is shown in figure 12; there are here four yellow spots, each about equidistant from the vegetal pole, and presumably there is a nucleus in each of these, though nuclei were actually observed in only two of them. It is possible that these may have arisen by division from two original nuclei, and that this is therefore a case of dispermy and not of polyspermy. It is an interesting fact that dispermic eggs never divide, though the nuclei may do so repeatedly, and of course they never develop normally.
Dispermic eggs have been repeatedly observed both in living and in fixed
material, in entire preparations and in serial sections. Such eggs afford a valuable
means of testing the question as to whether the point of entrance of the sperm is
predetermined, and more important still, as to whether the posterior pole of the
egg and the plane of the first cleavage is pre-existent in the egg or is established
by the entering spermatozoon. So far as I have observed, (lie two sperm nuclei
always enter the egg near the vegetal pole, and at lirst they lie in a common protoplasmic field. As they move toward the equator, however, they frequently separate, and when they have reached the equator and have each given rise to a spindle they are often found on opposite sides of the egg with the surrounding protoplasmic fields quite separate. The two spindles are usually parallel to each other
and are always entirely independent, the pules never being united into a triaster
or tetraster.
Sections of two dispermic eggs are shown in figures 94 and 95; in the former
the sperm nuclei, which have not yel reached the equator of the egg, occupy symmetrical positions on each side of the mid-line, and the protoplasmic field in which the}
lie is located on that side of the egg which corresponds to the posterior pole of normal eggs. The yellow protoplasm here forms a continuous crescent, and save for the
fact that the sperm nuclei do not lie at the middle of this crescent and that a small
tongue of yolk partly separates the two sperm asters, the egg is not unlike a normal
one. In figure 95 a later stage of a dispermic egg is shown, in which the sperm nuclei
have reached the equator and have moved in from the surface toward the center of
the egg, while one of these nuclei has united with the single egg nucleus. There
is here also a symmetrical arrangement of the sperm nuclei and of the (dear and yellow protoplasm on each side of the mid-line. The protoplasmic areas are here further separated than in the preceding figure, though they still lie nearer one pole of the
egg than the other. In this case also there can be little doubt that the more richly
protoplasmic pole corresponds to the posterior pole of normal eggs while the yolkladen pole corresponds to the anterior one.
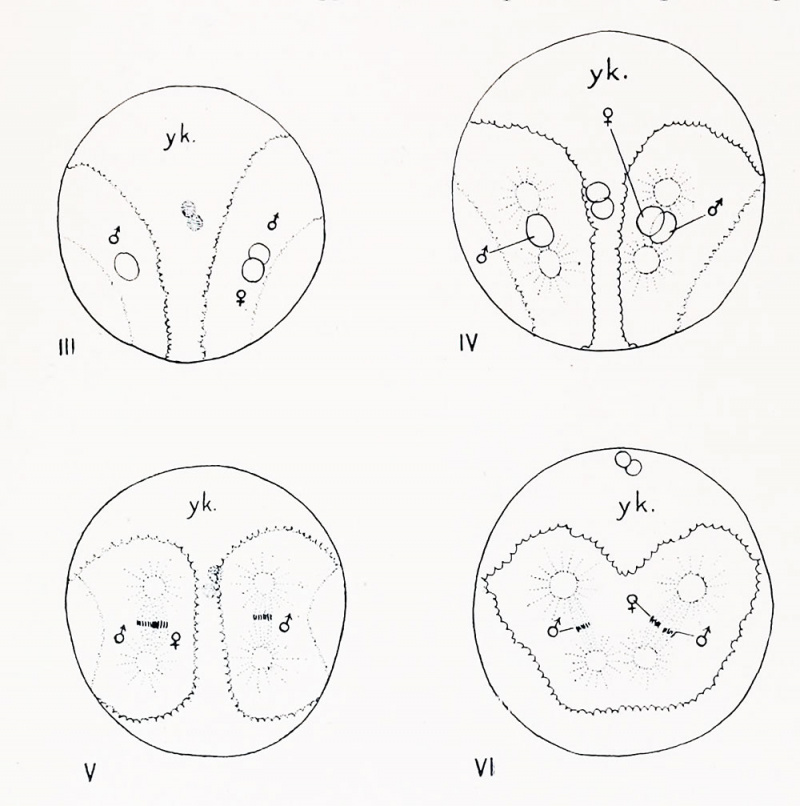
Figs. III-VI. Dispermic eggs of ('. partita; drawn from stained preparations of entire eggs. Figs. III. and V are viewed from the vegetal pole, the polar bodies being seen through the egg. Fig. IV is viewed from the animal pole and Fig. VI from the posterior pole. The boundary between the protoplasm and yolk is indicated by a crenated line; when seen through the egg this boundary is represented by a line of stipples.
Other dispermic eggs arc shown in text figures III-VI, and here also one hemisphere of the egg contains more protoplasm than the other, and may probably be identified with the posterior pole. In still other eggs, especially those in which the cleavage spindles are fully formed, the spindle and protoplasmic fields may lie on opposite sides of the egg [cf. text fig. V). In these cases neither pole can be certainly identified as anterior or posterior. In normal eggs the cleavage spindle always stands at right angles to the chief axis of the egg and to the plane of the first cleavage; in dispermic eggs the spindles are frequently not at right angles to the egg axis and if. as 1 believe, the plane between the two protoplasmic areas represents the normally median plane, they are more frequently parallel with this plane than perpendicular to it.
The phenomena of dispermy demonstrate that the point of entrance of the spermatozoon is not predetermined but that spermatozoa may enter at different points on the vegetal hemisphere; they also render probable the view that the plane of bilateral symmetry is not first established by the accidental path of the spermatozoon within the egg, but that this plane is structurally present before fertilization. This problem will be more fully discussed in the general part of this paper (Chap. VII).
Conklin 1905 TOC: I. The Ovarian Egg | II. Maturation and Fertilization | III. Orientation of Egg and Embryo | IV. Cell-Lineage | V. Later Development | VI. Comparisons with A.mphioxus and Amphibia | VII. The Organization of the Egg | Summary | Literature Cited | Explanation of Figures
Conklin EG. The Organization and Cell-Lineage of the Ascidian Egg (1905) J. Acad., Nat. Sci. Phila. 13, 1.
Cite this page: Hill, M.A. (2024, April 19) Embryology Paper - The Organization and Cell-Lineage of the Ascidian Egg 2. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_Organization_and_Cell-Lineage_of_the_Ascidian_Egg_2
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G