Paper - The Formation of the Connecting Stalk and the Extension of the Amniotic Cavity towards the Tissue of the Connecting Stalk in Young Human Embryos
| Embryology - 23 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Florian J. The formation of the connecting stalk and the extension of the amniotic cavity towards the tissue of the connecting stalk in young human embryos. (1930) J. Anat., 64: 454-476.
| Online Editor | ||
|---|---|---|
| This historic 1930 paper by Florian describes several early human embryos.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Formation of the Connecting Stalk and the Extension of the Amniotic Cavity towards the Tissue of the Connecting Stalk in Young Human Embryos
By J. Florian, M.D. Masaryk University, Brno
(1930)
From the Department of Anatomy and Embryology, University College, London
V. Mollendorff first drew attention to the relation of the ectoderm to the mesoderm of the stalk in very young human embryos; in the description of the embryo OP (1921) he describes a "Zerfallende Epithelwucherung des Amnions" (1921, pp. 416, 417; fig. 9, p. 415) behind the caudal end of the embryonic plate, in which the epithelium degenerates and so the amniotic cavity is enlarged at its cost.
In the course of the study of a number of young human embryos I have had the opportunity of convincing myself of the correctness of the above mentioned discovery, and shall now try to give a description of my own observations.
In the Fetzer embryo, the detailed description of which I shall shortly publish in collaboration with Dr Fetzer, as well as in the embryo Bi I (Florian, 1927) and T.F. (Florian, 1927, 1928 b) I was able to observe, in the neighbourhood of the tip of the amnion, granules in the mesoderm which can only be interpreted as the result of the dissolution of tissue proving the correctness of v. Mollendorff's observation. In the embryo BiI, I shall describe the region in question in detail; in the present paper I shall occupy myself only with the description of the relations between the stalk ectoderm (I use this name in contradistinction to the amniotic ectoderm of which it is the direct continuation) and the stalk mesoderm in the embryo Bi II (with four paired somites) and BiXI (with ten paired somites).The stalk ectoderm is separated from the ectoderm of the embryonal disc by the cloacal membrane; it wil, therefore, also be necessary to take into consideration the surroundings of the cloacal membrane in our description.
In order that the reader may be able to follow my observations more easily, I shall begin with a short description of the early development of the human embryo and its connecting stalk.
|
|
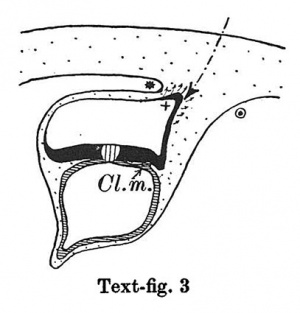
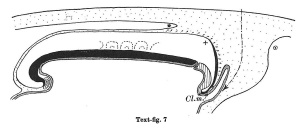
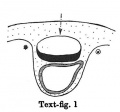
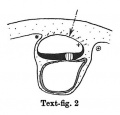
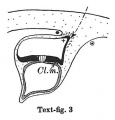
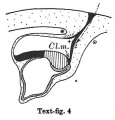
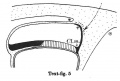
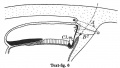
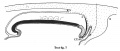
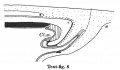
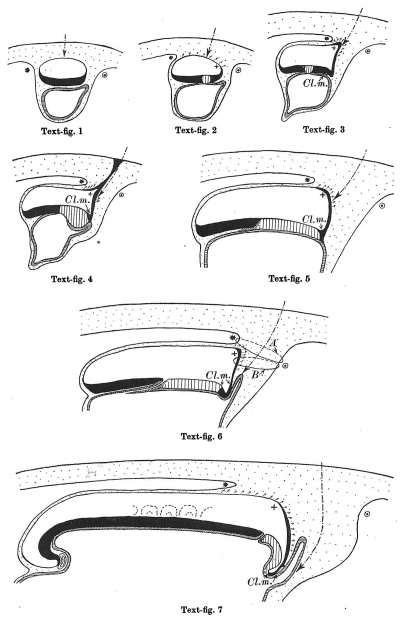
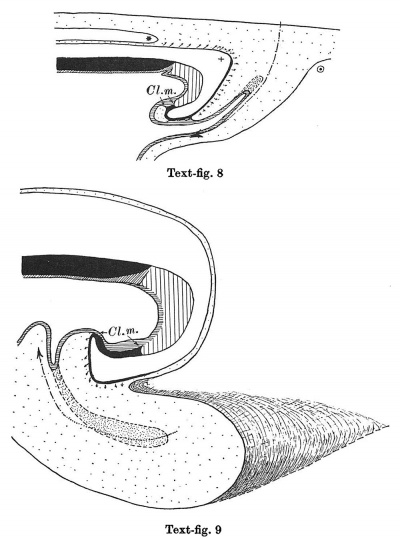
Text-figs.1-9. Schemes of the chief stages in the development of the human embryo. Ectoderm black; entoderm lined horizontally, primitive streak vertically, head process and chorda plate obliquely. Mesoderm dotted. The axis of the connecting stalk is marked by an arrow. The limits of the connecting stalk are marked by 1: and Q, the limits of the umbilical stalk by + and G). The direction of the extension of the amniotic cavity towards the connecting stalk is marked by arrows. The parts of the allantois and of the axis of the connecting stalk situated out of the median plane are finely dotted. A. Connecting stalk; B. umbilical stalk; Cl.m. cloacal membrane.
Stage 1
(text-fig. 1) corresponds with the Peters embryo (1899; Grosser, 1913): the primitive streak primordium and the stalk are not yet present; the embryo is situated in a thickening of the chorionic mesoderm. The future limits of the stalk are designated by a ® and a *. The axis of that part of the chorionic mesoderm, which will be transformed into the stalk in the course of further development, is marked by an arrow: it is perpendicular to the middle of the embryonal plate.
During the further development, the amniotic cavity and the yolk-sac enlarge so rapidly that the mesoderm covering them is not able to keep pace. Whilst in stage 1 (text-fig. 1) the mesoderm of the amnion and that covering the dorsal marginal part of the yolk-sac are similar in structure to the chorionic mesoderm,
Stage 2
In stage 2 (text-fig. 2) the mesoderm covering the whole yolk-sac and the cranial end of the amniotic cavity has taken on a different structure, and is becoming transformed into a "mesothelium." The cells of the mesoderm in this region are connected with the cells of the amniotic ectoderm and with those of the yolk-sac entoderm by means of cytodesmata, and some of the cells of the yolk-sac entoderm appear to have entered the mesoderm. The transition of the mesodermal layer which covers the cranial end of the amnion into the chorionic mesoderm (text-fig. 2*) represents the cranial limit of the connecting stalk, which first begins to be formed in this stage. The first traces of the stalk appear, therefore, in the stage when the primitive streak primordium is represented by a localised fusion of the embryonal ectoderm with the entoderm of the yolk-sac (embryo OP v.Mollendorff).The yolk-sac (in this stage) runs out towards the connecting stalk in the form of a slight diverticulum. Already in this stage v. Mollendorff described (1921,1925) an active extension of the amniotic cavity towards the stalk tissue (in a dorsal and caudal direction from the embryonal plate). If we regard the caudal limit of the connecting stalk (in text-figs. 1-8) as a fixed point, we may say that the embryonal plate grows in the cranial direction, while the amniotic cavity expands caudally and dorsally. The yolk-sac correspondingly grows forwards below the embryonic plate almost up to the cranial end of the latter, and also grows in the caudal direction. Its growth in this direction, however, encounters the resistance of the connecting stalk tissue and so, not being able to extend freely, it grows towards the connecting stalk in the form of a diverticulum.
Already in this stage we can distinguish two parts in the connecting stalk which I propose to term the amnio-embryonal stalk and the umbilical stalk respectively. The amnio-embryonal stalk (between + and * in text-figs. 1-8) attaches the embryonal formation (amnio-embryonal and yolk-sac vesicles) as a whole to the chorionic mesoderm and continues on the one hand into the amniotic mesoderm, and on the other into the umbilical stalk.
The umbilical stalk (between + and - in text-figs. 2-8) represents a partial continuation of the amnio-embryonal stalk; on its cranial surface it is covered by the stalk ectoderm which is continuous with the ectoderm of the embryonic plate and with that of the amnion, and its whole surface can only be seen if the amnion is opened up. The most important difference between the amnio-embryonal stalk and the umbilical stalk lies in the fact that the latter is in all stages covered on its cranial surface by ectoderm and that it is transformed into the umbilical cord during later stages of development. The amnio-embryonal stalk on the other hand persists for a time but finally disappears when the amniotic mesoderm fuses with that of the chorion. The axis of the connecting stalk (marked by an arrow) begins already in stage 2 (text-fig.2)to form an acute angle (openc audally) with the embryonic plate and has moved slightly towards the caudal end of the embryo.
Stage 3
In stage 3 (text-fig.3, corresponding approximately with the Fetzer embryo (Fetzer-Florian, 1929)) both the amnio-embryonal and the umbilical stalks are quite distinct. The axis of the connecting stalk has now come to liebehind the caudal end of the embryonic plate and it is beginning to form a curve with the convexity directed caudally. The amniotic cavity runs out into a distinct amniotic tip caudally and dorsally, a feature which is to be seen in all following stages. The extension of the amniotic cavity towards the tissue of the connecting stalk takes place in an opposite direction to that of the embryonic plate, the result being, that the distance between the amniotic tip on the one handandthe chorionic ectoderm and the caudal limit of the amnio-embryonal stalk on the other increases at a much slower rate than the distance between the cranial end of the embryonic plate and the above-mentioned structures. In this stage, approximately in the middle of the embryonic plate, there is present a distinct primordium of the primitive streak and, close to the caudal end of the plate,the cloacal membrane is already developed. Itis noteworthy that these two structures are in no way connected in their origin in the human embryo. The primitive streak primordium seems to correspond in its position with Hensen's knot. in this stage,theyolk-sacdoesnotsend any distinct diverticulum towards the stalk.
Stage 4
In stage 4, represented by the embryo Bi I, of which a schema is shown in text-fig. 4, the bending of the axis of the stalk has become more distinct, and its tip forms a sharper angle with the embryonic plate than in the preceding stage. In text-fig.4 I have shown an amniotic duct connecting the amniotic ectoderm with the chorionic ectoderm, because two human embryos (BiI, Florian, 1927 and Strahl-Beneke, 1910) belonging to this stage possess it. It is not certain whether it is only a chance that the amniotic duct is not present in the embryos represented by text-figs. 1-3. The knot which represented the primitive streak primordium in the preceding two stages has now become transformed into a true "streak," at the end of which there is a distinct "end-knot of the primitive streak." The thickened end-knot produces a ventral down-bulging of the underlying wall of the yolk-sac and so a diverticulum is produced, at the caudal end of which the cloacal membrane is situated. This diverticulum resembles an allantoic outgrowth-with which, however, it has nothing to do, because it disappears during the later development by opening out into the yolk-sac cavity (Florian-Volker, 1929).
Stage 5
In stage 5 (text-fig.5, corresponding approximately with the Stieve embryo "Hugo," 1926) the tip of the bent axis of the stalk has passed still further caudally. The primitive streak occupies the whole caudal half of the embryonic plate and the primordium of the head process has appeared. The end-knot of the primitive streak has disappeared, and the above-mentioned caudal diverticulum has become merged in the yolk-sac cavity.
Stage 6
In stage 6 (text-fig. 6, corresponding approximately with the embryo Peh. 1-Hochstetter, Rossenbeck, 1923) a new diverticulum is seen extending almost vertically upwards from the caudo-dorsal extremity of the yolk-sac in to the stalk mesoderm. I propose to speak of this as the allanto-enteric diverticulum since its proximal part represents the later hind-gut, its distal portion the entodermal allantoic canal. The proximal part coincides in position with the cloacal membrane ,furnishing its entodermal layer,whilst the allantoic canal is situated caudally to that membrane. The allanto-enteric diverticulum in this stage appears at first sight to be very similar to the diverticulum described in stage 4, which has been regarded by many authors, including myself, as the allantoic primordium, but there is a very important difference between the two, apart from the fact that the one is transitory, the other permanent. The cloacal membrane is situated in stage 4 at the end of the diverticulum there present, but in stage 6 it lies at the opening of the diverticulum into the yolk-sac cavity. The part of the diverticulum caudal to the cloacal membrane (the above-mentioned "allantoic" canal) is, therefore, in stage 4 not yet present. The axis of the stalk is now situated caudally to the amniotic cavity and its tip is directed to the upper or caudal end of the allantoic canal.
Stage 7
In stage 7 (text-fig. 7, corresponding approximately with the embryo Bi II) the axis of the stalk lies still more caudally, being now situated behind the allanto-enteric diverticulum. The cloacal membrane is divided into two parts: one of them lies at the orifice of the diverticulum, the other is situated more caudally.
Stage 8
In stage 8 (text-fig. 8, corresponding approximately with the Sternberg embryo (1927a), with four paired somites) the cranial part of the cloacal membrane is situated in the now very distinct primordium of the hind-gut. The other part lies close behind the orifice of the allanto-enteric diverticulum into the yolk-sac; it is now in process of degeneration and has become separated into three pieces. Whereas in stage 7, the allanto-enteric diverticulum lies wholly in the median plane of the embryo, in this stage the lengthening of the stalk has permitted a slight rotation of the embryo around its long axis, with the result that the end of the allantoic canal is no longer in the median plane. The mesoderm between the allantoic canal and the stalk ectoderm is now much thicker than in the preceding stage. The axis of the stalk now makes such a sharp curve that its tip runs nearly parallel to the long axis of the embryo.
Stage 9
In stage 9 (text-fig. 9, embryo Bi XI with ten paired somites) certain important advances have been made in the region under discussion, (a) the caudal end of the embryo now projects freely backwards as the result of the developmental processes already initiated in stage 7, (b) the connecting stalk has increased greatly in length and, as the result of the growth of the tail-end of the embryo and the development of the hind-gut,has been carried ventrally so that it now arises from the ventral side of the embryo, in front of the freely projecting caudal end of the latter, (c) as the result of the lengthening of the stalk,the embryo has turned its right side towards the chorion,(d)the opening of the allantoic canal is now situated on the floor of the hind-gut. The tip of the axis of the connecting stalk is once more perpendicular to the axis of the embryonic body, but it has made a rotation of 1800 in comparison with the stage1. We can see,therefore,that the migration of the insertion of the umbilical stalk from the dorsal-caudal to the ventral aspect of the body of the embryo is the result partly of the active growth of the stalk and partly of the growth of the tail-end of the embryo in the caudal direction and the correlated extension of the amniotic cavity in the same direction. We see further that the embryo need not change its position during this process. The definitive cloacal membrane is now established in the floor of the hind-gut. It is formed by the cranial part of the cloacal membrane of stages 7 and 8. The whole allantoic diverticulum of this stage corresponds to that part of the allanto-enteric diverticulum in stage 7 which is situated behind the definitive cloacal membrane . Only the part of the allantoic diverticulum which is situated close to its opening into the hind-gut is situated in the median plane as the result of the rotation of the embryonic body round its long axis.
If we compare the cloacal membrane in text-figs. 7 and 8, we notice that the definitive part of the cloacal membrane is situated in stage7 (text-fig.7) and the same is true for stage 6 (text-fig. 6)-partly in the allanto-enteric diverticulum, but in stage 8 (text-fig. 8) the definitive cloacal membrane is now situated in the hind-gut. This fact justifies the conclusion that the part of the allanto-enteric diverticulum close to its opening in to the yolk-sac really belongs not to the allantois,but to the hind-gut. In this connection I call attention to the characters of the entoderm in this region of the embryonic body (p. 467), which support my interpretation. If that interpretation is correct, it is clear that we cannot determine with certainty the cranial limit of the allantoic canal in stages prior to stage 8. This is one of the most remarkable differences between the development of the Man (and no doubt that of all the higher Primates) and the development of lower Mammals, and it is conditioned by the presence of the connecting stalk and the very early differentiation of the primordia of the cloacal membrane and the allantois. Only when the insertion of the umbilical stalk to the ventral part of the embryonic body (stage 9, text-fig. 9) is attained and the hind-gut is formed can the human embryo,with regard to the cloacal membrane and the allantois, be compared with that of a lower Mammal.
Now I shall try to support my views by an account of my own observations.
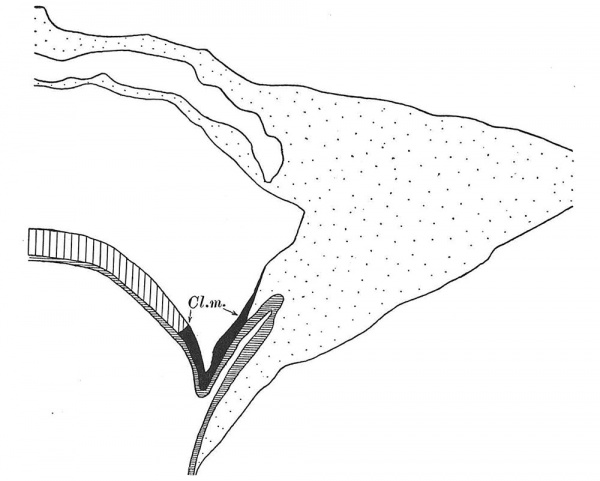
I shall begin my remarks with the description of the cloacal membrane in the Embryo Bi II, in which I have previously described the chorda canal (1928 a). The embryo was cut cranio-caudally. The graphic reconstruction of the caudal end of this embryo (text-fig. 11) shows that the hind-gut is already forming,but is still very shallow.The cloacal membrane is not found in this hind-gut primordium, but in that region where the yolk-sac passes into the allanto-enteric diverticulum, the greater part of which corresponds with the allantois of later stages. Caudally to the true cloacal membrane I found a connection between the amniotic ectoderm and the entoderm of the allanto- enteric diverticulum (between sections 315 and 314), the significance of which I shall discuss later. Here I wish only to call attention to the region between the cloacal membrane and this connection. In this region, the embryo Bi II shows nearly the same relations as the Sternberg embryo (1927 a), with which it agrees in the number of somites.
Text-fig. 10. Embryo Peh.1-Hochstetter (Rossenbeck, 1923) according to Sternberg (1927b). x113. Ectoderm black; entoderm lined horizontally, primitive streak vertically. Mesoderm dotted.
Text-fig. 11. Embryo Bi(ttmann) II with four paired somites. x 113. Ectoderm black; entoderm lined horizontally, primitive streak vertically, head-process and chorda-plate obliquely. Mesoderm dotted. The outline of that part of the allanto-enteric diverticulum which is not in the median plane is broken. Sectional planes indicated above the figure.
Sternberg has described in his specimen (text-fig. 12) behind the cloacal membrane (which is found in the hind-gut)three separate connections between the amniotic ectoderm and the entoderm of the allantois, and has regarded these as the remains of the original cloacal membrane . I suggested in1929 (Florian and V8lker) the possibility that these connections are the result of secondary fusion of the amniotic ectoderm and the entoderm of the allantois, for the reason that the distance of the points of fusion from the actual cloacal membrane in the Sternberg embryo seemed to be much too great but, more recently, I believe that I have found a distinct transitional stage in the graphic reconstruction of the embryo Bi II (text-fig. 11) between the embryo Peh.1-Hochstetter (text-fig. 10) and the Sternberg embryo (text-fig. 12) and accordingly accept the view of Sternberg that they are actually remnants of the original much more extensive cloacal membrane.
In the region between the actual cloacal membrane and the connection of the amniotic ectoderm and the entoderm of the allantois, the relations between the embryonal layers are very difficult to study, although the tissue is well preserved and the embryonal layers can be easily distinguished elsewhere. I, therefore, think it is necessary to describe this region somewhat more in detail.
Text-fig.12. Embryo Sternberg (1927a,b). x113. Ectoderm black,entoderm lined horizontally, primitive streak,vertically, head-process and chorda-plate,obliquely. Mesoderm dotted.
In section 330 (Plate I, fig. 1) the cloacal membrane has its typical form; it is sharply separated from the mesoderm by a fairly broadspace. The various sized granules stained with haematoxylin, which are to be observed in great number in section 229 and which are possibly to be interpreted as a sign of cell destruction,are here present only in minimal quantities. We can accordingly describe this section as the most caudal in which the cloacal membrane shows no noticeable sign of degeneration. in the next( in the caudal direction) section(No.229)the tissue composing the cloacal membrane seems to be much less compact, especially close to the ectoderm. (It seems that we can see here the limit between the ectodermal and entodermal part of the cloacal membrane, which was not possible in section 330.) in the entodermal part of a cloacal membrane there is a cavity filled with variously sized granules; the granules contain enclosures stained with haematoxylin. They are possibly products of cell dissolution. On the right the cloacal membrane is separated from the mesoderm by a wide space;on the left,on the contrary,the mesoderm approaches much nearer to the cloacal membrane and is connected to it by fine but quite distinct cytodesmata. in the next section (328) the ectoderm and the entoderm are connected by a single cell in the region of the cloacal membrane. Otherwise the ectoderm is separated from the entodermbya distinct space, which is bridged over by fine cytodesmata. The granules referred to as products of cell dissolution are only visible in the entoderm. On the left, the mesoderm is quite near to the cloacal membrane, but one can easily distinguish it from the cloacal membrane . in the next section (327, Plate I,fig. 2) the ectoderm shows a fairly wide connection with the entoderm- we have,therefore,before us a distinct cloacal membrane but if we compare this section with the section 330, we can note certain differences. The dorsal surface of the cloacal membrane is not flat as in section 330, but shows a distinct pit, whilst its left border is much less distinct than the right,because the mesoderm approaches very close to the cloacal membrane . Products of cell dissolution are not to be observed in the cloacal membrane , but they seem to be present in the mesoderm at a certain distance from it. In section 326 (PlateI,fig.3)the relations of the embryonal layers are very indistinct. We can observe a cell cord which seems to connect the entoderm and the ectoderm, but the limit between this and the mesoderm is so in distinct that it can scarcely be compared with the,well-developed cloacal membrane seen in section 330 (Plate I, fig. 1). Degeneration products are not visible in the cloacal membrane, but they are quite distinct in the mesoderm (on the left). In section 325 (Plate I, fig. 4) the limits between the cloacal membrane and the mesoderm are not much more distinct than in section 326, whilst degeneration products are very clearly visible in the ectoderni near to the amniotic cavity. In section 324 the limits of the cloacal membrane are not more distinct, and there are present in it very many degeneration granules. In section 323 the ectoderm sends out towards the entoderm a narrow cell cord, between the end of which and the entoderm a degenerated cell is present. In section 322 this cell cord nearly reaches the entoderm; a very narrow space, possibly an artefact, lying between the two. On the left of this cell cord,the ectoderm is thickened and the limit towards the mesoderm is very indistinct. In section 321(PlateI, fig. 5) there are only very indistinct traces of the above-mentioned cell cord. On the left the ectoderm is again thickened as in sections 324-2, and contains numerous granules. The limit between the ectoderm and the mesoderm in this section is non-existent. in the following sections ( in the caudal direction) there is no cell cord visible. The above-mentioned thickening of the ectodermi comes to occupy its position and assumes a triangular form; it contains many granules and its limit towards the mesoderm is indistinct. In section 316 the tip of this triangular thickening passes into a very distinct cell cord containing granules. These latter are especially distinct in section 315 (Plate I, fig.6) in a narrow cord whichcanbetraced from the ectoderm towards the entoderm. Already in this section, but still more distinctly in section314 (PlateI,fig.7), the entoderm sends out a pointed process towards the ectoderm, which isvery likely connected with the ectodermic cell cord seen in section 315 and which, as indicated above, shows distinct signs of degeneration. (Similar signs of degeneration are also to be seen in degenerating amniotic ducts.)
In order to understand the development of the hind-gut,itisofimportance to know the conditions at the cranial end of the cloacal membrane. The cloacal membrane shows no signs of degeneration in the cranial direction up to section332. In section 333 a cloacal membrane is still present, but in its centre there is an oval cavity containing numerous granules, staining similarly to those in the caudal region of the membrane. In section 334 the latter is much narrower than in the preceding section. The hinder end of the amniotic cavity extends as a narrow horse-shoe shaped diverticulum ventral to the caudal end of the embryo, immediately in front of the cloacal membrane. The ectoderm in this section is distinct and is separated from the mesoderm, except over a very narrow region in the middle line, where these two layers are fused; we have to do here with the caudal end of the primitive streak, which streak is still to be seen also in section 335.The amniotic cavity is still present in this section, but appears as two separated clefts, representing the continuation of the limbs of the horse-shoe shaped diverticulum in the preceding section. The ectoderm and entoderm are connected in the middle line by a cell mass, in which are present numerous granules, so that we have here the cranial extremity of the cloacal membrane, but the ectoderm is very indistinct, because it is fused dorsally with the mesoderm of the primitive streak and ventrally with the entodermn in the region of the cloacal membrane. In section 336 (Plate I, fig. 11) we find the same relations, but there are no granules in the cloacal membrane . In section 337 the amniotic cavity is still present as a single small, but sharply limited cleft. The ectoderm is quite indistinct. In section 338 (PlateI,fig.12) neither an amniotic cavity nor the ectodermn can be distinguished. The entoderm is here fused with what is certainly the mesoderm of the primitive streak. in the mesoderm,close to the entoderm, there are present granules of variable size. In section 339 the entodermn of the hind-gut is also fused with the primitive streak mesoderm.
The observations recorded above justify us in drawing the following conclusions: the cloacal niembrane shows very distinct signs of degeneration over a considerable extent of its caudal region. Productsofceldissolutionare also present in the cranial region of the cloacal membrane, but they are here much less abundant than in its caudal end. At its cranial end the cloacal membrane passes over without any limit in to the primitive streak. Cranial to the cloacal membrane the mesoderm is in continuity with both the ectoderm and the entoderm and a migration of cells from the entoderm into the mesoderm seems to be very probable in several places.
In this connection we would call attention to the form of the entoderm cells in the neighbourhood of the cloacal membrane . The study of the entoderm in this region is not quite easy, because the entoderm is cut in most places obliquely; nevertheless we can conclude that the entoderm, cranial to the posterior intestinal portal ( in the mid-gut as well as ilthe yolk-sac), coIsists usuallyofcubicalcells,though in places the cells are flattened. in the posterior intestinal portal and in the hind-gut the entoderm cells assume a higher, even cylindrical form, and their cytoplasm stains deeply. The entoderm of the yolk-sac, elsewhere low, is higher in the region of the cloacal membrane and wheretheyolk-sac passes over in to the allanto-enteric diverticulum. in the most caudal part of the hind-gut the entoderm is lower again, but it cannot be studied easily because of the obliquity of the sectional plane.
The entoderm is especially high in the caudal part of the cloacal membrane, where there are signs of the degeneration of the cloacal membrane . in the ventral part of the orifice of the allanto-enteric diverticulum the entoderm contains numerous granules.
The above-mentioned allanto-enteric diverticulum of the yolk-sac has in transverse section a circular outline near its opening into the yolk-sac. Caudally to section 322 it is oval with its long axis runmiing dorso-ventrally. Starting from this section. the entodermal cells in the ventral wall of the diverticulum contain small basally situated vacuoles; the nuclei lie towards the free surfaces of the cells where the cytoplasm stains deeply like that of thecells in the dorsalwall of the diverticulum. Caudallythecavity of the allanto-enteric diverticulum assumes an elongated oval form (Plate I, fig. 7); the basal vacuoles appear also in the cells of the dorsalwall. in this caudal region of the allanto-enteric diverticulum, the cells become reduced in height. The form of the entoderin cells in the caudal region of the allanto-enteric diverticulum of embryo Bi II corresponds with that of the cells over the whole extent of the allantois in embryo BiXI (Plate I, figs. 9,10).
The significance of these observations I have discussed above (p. 460); now we may pass to the description of the cloacal membrane in embryo Bi XI.
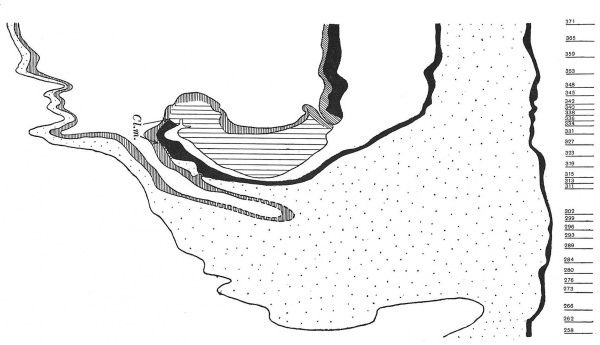
The embryo Bi(ttmann) XI, of whose caudal end a graphic reconstruction is shown in text-fig. 13, was obtained by an operation by Dr 0. Bittmann in Brno and was fixed intact in the chorionic vesicle in Zenker's solution immediately after the operation. About four hours later the chorionic vesicle was carefully opened. The specimen was imbedded in paraffin and cut nearly transversely in the cranio-caudal direction (the sections are 7,u thick). The entire embryo is well preserved and without any deformation; it possesses ten paired somites. I intend to describe it in detail later, and now I shall direct attention only to the cloacal membrane and the relations between the ectoderm and the mesoderm of the stalk.
The cranial end of the cloacal membrane is in embryo Bi XI indicated already in section 272, where there is no mesoderm between the ectoderm and the entoderm in the median plane and the ectoderm nearly reaches the entoderm. In sections 273 and 274 a triangular process of the ectoderm meets the entoderm and fusion of the two layers has probably been effected. Butthe cloacal membrane is quite definitely formed in section 276. There are no granules regarded as products of cell dissolution to be observed in sections 276 to 279, and no other signs of degeneration of the cloacal membrane are present. Here and there in the cranial end of the cloacal membrane the ectoderm is loosened from the entoderm, but such places are always very small, and they are also to be seen in mid-region of the membrane and accordingly are not to be regarded as indications of degeneration of the membrane. In section 280 cell granules first appear, but they are always very few in number and much smaller than those in embryo Bi IL.
The cloacal membrane can be followed up to section 294 without any difficulty (in section 293 there is a mitosis in one of its cells). In section 295 the cloacal membrane is much more indistinct than in the preceding section, because the mesoderm on both sides comes close up to it and the area of fusion of the ectoderm and entodermis greatly reduced. in the following section there is a cellmass which connects the ectoderm and entoderm, but its cells seem to belong to the mesoderm; we have, therefore, reached the transition region where the cloacal membrane passes into the primitive streak. From the cloacal membrane up to the head-process the three embryonal layers are in continuity with each other in the median plane.
It is not necessary here to enter into detailed comparison of the condition of the cloacal membrane in my specimens with that described in other human embryos. Ineedonlyreferthereader to the paperbyFlorian-Vdlker,1929, in which the cloacal membrane in other very young human embryos is described, and the papers of Sternberg (1927 a, 1927 b). It would be superfluous to repeat the details here.
Before I proceed to discuss the significance of my observations, I shall describe the conditions behind the cloacal membrane and, more particularly, the relations between the ectoderm and mesoderm of the connecting stalk in both my specimens, beginning with the embryo Bi II. In this embryo the interpretation of the sections is made difficult by the obliquity of the sectional plane. The result of this is that the limit between the ectoderm and mesoderm is in some places obliterated. But it seems very probable that the indistinct limit of the mentioned layers is not simply the result of the mentioned obliquity, but that there is actually no distinct limit between them. The ectoderm of the stalk consists of a layer of flattened or low cubical cells, which exhibits only rarely a distinct limit towards the mesoderm and, even in these places, the ectodermal cells are connected with the mesodermal by means of numerous fine cytodesmata. We may divide the stalk into two regions: a ventral one extending to the level of the caudal end of the allantois, and a dorsal one found dorsally to the latter.
I shall describe the ventral region of the stalk first. In many places the stalk epithelium consists here of three or more cell layers, of which the cells of the basal layer are connected with the underlying mesodermal cells by means of processes. It is impossible to prove to which germ layer the single cells of this epithelium belong. One possibility is that we have here to do with anectodermconsistingofmany celllayers,and that the cells of the basallayer are connected with the mesodermal cells; in several places the axes of these basal cells are so directed as to suggest the passage of ectodermic cells in to the mesoderm. But there is also another possibility: the cells of the basallayer may belong to the mesoderm, although they are so closely related to the ectoderm. It is especially difficult to find the limit between the ectoderm and mesoderm close to the median plane in the region between the ectoderm and the allanto-enteric divertieulum, as was mentioned in the description of the cloacal membrane . We can see this region (close to the median plane) on the right of fig.8 (Plate I). On the left of the same figure there is a cell to be seen, which seems to belong to the stalk ectoderm and which is connected by means of a very long and distinct process with the mesodermal cells (Plate I, fig. 8 +).
As we have mentioned above, the indistinct limit between the ectoderm and the mesoderm in this region is not only a result of the oblique plane of sections; it is very possible that some of the ectodermal cells pass into the mesoderm.
Also in the dorsal part of the connecting stalk (dorsal to the level of the caudal end of the allanto-enteric divertieulum) the limit between the ectoderm and the mesoderm is very indistinct. The amniotic ectoderm close to the insertion of the amnion into the connecting stalk is distinguishable by its characters; whilst the amniotic ectoderm far from that insertion consists of a single layer of very flattened cells, the amniotic ectoderm close to its transition in to the stalk ectoderm is formed by several layers. Its basal layer represents the direct continuation of the amniotic ectoderm of that part of the amnion
remote from the insertion on the connecting stalk, and consists of a single, continuous layer of cells which has stained very deeply with cosin. The cells are cubical and their limits are very distinct. On this basal layer are situated polygonal or round cells whose cytoplasm has stained only faintly with haematoxylin. In their cytoplasm there are distinct vacuoles especially close to the cel surface next the amniotic cavity. These cells are rather large and contain a large, round nucleus, which is larger and rounder than the nuclei of the basal layer. These cells are vcry often separated from each other by distinct spaces, and accordingly they do not form a continuous layer. The connection of the cells of this layer with the cells of the basal layer is in some places only very loose, and there is no doubt that they can be shed into the amniotic cavity where they undergo dissolution. The products of this cell dissolution can very easily be found in the amniotic cavity.
The two above-mentioned cel layers of the amniotic ectoderm can be followed into the stalk ectoderm. The upper layer of the stalk ectoderm shows no particular differences as compared with the amnion in its neighbourhood, but the cells of the basal layer (deeply stained with cosin) enter into very complicated relations with the surrounding mesoderm. The form of cells of the basal layer is very irregular: some of them are cubical like the cells in the basal layer of the amnion, and there are many transitions between them and the upper layer of the stalk ectoderm; it seems to be very likely that the se cells will be shed into the amniotic cavity during the further development, and there dissolved.
The other cells in the basallayerreta in their eosinophil character. Close to the insertion of the amnion into the connecting stalk, these cells send out long and broad processes-into the mesoderm and, as the result, they present peculiar forms and their nuclei become oval (Plate II, fig.14). in the tissue close to the ectoderm there are in some places very distinct granules(PlateII, fig. 15). These seem to be a certain proof of the extension of the amniotic cavity at the expense of the stalk mesoderm; the ectoderm grows here deeply in to the mesoderm and compresses it or brings about its dissolution. in this way the amniotic cavity is enlarged towards the tissue of the connecting stalk, this enlargement being specially marked and proceeding most rapidly close to the insertion of the amnion into the connecting stalk.,The convex outline of the surface of the connecting stalk directed towards the amniotic cavity (in the sections) is the result of this process (Plate I,figs.13 and 16).
Even in the region remote from the insertion of the amnion into the connecting stalk there are present, underneath the stalk ectoderm, products of cell dissolution, so that even here the mesoderm undergoes an involution. The form of the ectoderm also differs very much in this same region from that close to the insertion (Plate III, fig. 17). The ectodermal cells are in places very flattened (like an endothelium) and vaulted towards the amniotic cavity so that the axis of the celforms here a curve convex towards the amniotic cavity. Even in this region there is evidence that some of the ectodermalcells are eliminated into the amniotic cavity and dissolved. Such cells contain, even before they are eliminated, large vacuoles and their cytoplasm stains only with haematoxylin. It is of some interest that also the mesodermal cells close underneath the ectoderm contain vacuoles.
In this same region there are other places where it is impossible to prove the existence of an ectodermal layer, the mesoderm alone appearing to bound the amniotic cavity. Suchplacesare in the embryo BiII never very large. In other places the ectoderm is very distinct; its cells are very deeply stained with eosin in contrast to the mesodermal cells, but the limit between the ectoderm and the mesoderm isnot distinct,sincethecells of the formerare connected with those of the latter by means of cytodesmata.
(In section 332 I happened to find a cell in the mesoderm which was much larger than the surrounding mesodermal cells, and deeply stained with eosin. It seems very likely that we have here to do with an ectodermal cell which has emigrated in to the mesoderm.)
We may now pass to the description of the relations between the ectoderm and the mesoderm of the connecting stalk in embryo BiXI (text-figs.9,13). The flattened amniotic ectoderm in embryo BiXI passes in to the ectoderm of the connecting stalk, which consists of much higher cells; these cells cannot be shortly designated as cubical or cylindrical, because their form is too irregular and we must, therefore, describe them more in detail.
As in the embryo Bi II, we can, even in the embryo Bi XI, find in the stalk ectoderm cells which are eliminated into the amniotic cavity and dissolved. They appear to lie on a single layer of flattened cells (Plate III, figs. 22 and 23). These flattened cells in some places become higher and assume an irregular form. The line formed by the basal surface of these cells is not rectilinear as in a cubical or cylindrical epithelium, but quite uneven. The reason for this is to be found in the migration, in many places, of some of the ectodermal cells into the mesoderm (through the basal line of the ectoderm). They are to be found in varying stages of passage into the mesoderm (Plate III, fig. 22). We can very often observe cells with half of their bodies below the basal line of the ectoderm and connected with the mesodermal cells by means of wide processes. In one place (Plate III, fig. 21), where the ectoderm forms a comparatively regular layer and where the basal line isrectilinear, we find a cell,
with one half of its body situated above the basal line, the other half below it, the two halves being connected by a very narrow bridge. Even the nucleus is divided into two halves connected by a very narrow, but quite distinct, bridge. Juston the right of this cell there is another cell which ends a broad process into the mesoderm, the sharpened end of its nucleus lying below the basal line.
In other places the ectodermal cells form litle groups, penetrating into the mesoderm. We can see in Plate III,fig.18 such a group in the formof a triangle, the base of which lies at the upper surface of the ectoderm, whilst the tip is directed towards the mesoderm. In such a group the cells undergo a dissolution in such a way that finally only those cells remain which cover the mesoderm; the inner cells disappear and in the place there arises a small cavity connected with the anmniotic cavity (Plate III, fig. 19). This isone way in which the amniotic cavity is enlarged towards the connecting stalk.
In order to elucidate further the mode of extension of the amniotic cavity towards the stalk mesoderm, I must describe a very interesting observation in embryo Bi XI. As is to be seen in Plate III, figs. 20 and 24, the right side of the embryo (in the younger embryos it is the dorsal side) is turned towards the chorionic membrane and the insertion of the amnion into the connecting stalk is ventral to, and on the right (in the figure) of, the caudal end of the embryo. The amniotic vesicle runs out towards the mesoderm of the connecting stalk in theform ofa wide bay, inwhich a freecellmass issituated. This cell mass, according to its structure, belongs without any doubt to the mesoderm, but it is completely separated from the stalkmesoderm andliesfree in the amniotic fluid. As is to be seen especially in Plate III, fig. 24, the mesodermal cells
are comparatively well preserved in the middle of the mass, in contrast to its surface, where no cells arc visible. We have here, without any doubt, to do with a mass of mesoderm in course of dissolution, from the surface inwards.
That this is so is indicated by the presence of numerous granules on the whole surface of the mass, and especially in that part of the surface which is turned towardsthestalkectoderm;here butonlyhere very slight traces of the ectoderm are present on the surface of the mass which, in some places, touches the stalk cetoderm.
In my opinion we can explain the presence of such a quite separated mesodermal mass in the amniotic cavity and its dissolution only in the following way. The stalk ectoderm penetrates into the mesoderm as above described and brings about its dissolution, where it is in direct contact with it, but it also can grow round a comparatively large mass of mesoderm and separate it completely from its surroundings. Such a sequestered mass remains for a time in the amniotic fluid, until it is completely dissolved. This is another and very intensive way, in which the amniotic cavity increases in extent at the expense of the connecting stalk.
The observations recorded above justify the opinion that the extension of the amniotic cavity first mentioned by v.M6llendorff in the veryyoung embryos OP and XVO takes place even in the embryo Bi XI with ten paired somites and that itiseven in this much more developed specimenin active progress. A knowledge of the above-mentioned processes is very important for the understanding of the development of the connecting stalk in Mlan, as I have attempted to describe by means of the schematic median sections (text-figs. 1-9).
In this connection I must make some mention of the literature bearing on this question. I have already had the opportunity (Florian, 1928 b, pp. 537-8) of describing my observations on the stalk ectoderm of some young human embryos. I was then inclined to accept the opinion of Fahrenholz (1927) that the amniotic cavity is not enlarged towards the tissue of the connecting stalk, but that, on the contrary, it grows out of the connecting stalk. But the sequestration of a part of the stalk mesoderm, described in embryo Bi XI, cannot be explained by Fahrenholz's view and, therefore, I am obliged to mention the Fahrenholz's observations more in detail.
Here I cite the most important part of Fahrenholz's account (1927): "Auch bei dem Ei Ho sind die Degenerationserscheinungen im Amnionzipfel nachweisbar....Es listsichjedochindiesemFallzeigen,dassdieseVorgange nicht zu einer Erweiterung, sondern umgekehrt zu einer Verkleinerung der Amnionhdhle ffihren. Das Amnionepithel whchst nicht in den IHaftstiel hinein, sondern es zieht sich daraus zurick" (pp. 301-2). "Wir haben hier also einen Ilohlraum im Bindegewebe vor uns, der in der Nachbarschaft des Amnionepithels noch gut erhalten, an seinem distalen Ende aber bereits im Schwinden begriffen ist und noch Reste des Amnionepithels enthilt, das ihn friiher auskleidete. Der Spalt ist fiber neun Schnitte von 7 5,u Dicke zu verfolgen und reicht stellenweise bis an die Chorionmembran heran.....Ich muss der Meinung von Grosser (1924) und Bryce (1924 )beipflichten, dass Amniongang usw. Reste eines primaren Zusammenhanges zwischen Amnion-und Chorionepithel sind. Diese Annahme allein bringt die Befunde unter einen Hut und gibt eine Erkldrung nicht nur fuirden Amnionzipfel, den Amniongang und die Zelldegenerationen, sondern auch fur den Amnionnabel und die Choriongange des Ilaftstieles, denen gegenuiber die v. Mollendorffs Theorieversagt. Eineigentlicher Amniion gangfehltbeimEiHo. Die Zellen des oben (S. 268) erwhihnten Epithelstranges im IHaftstiel (Fig. 30 und 31) unterscheiden sich durch ihren hellen, scharf umgrenzten Plasmaleib deutlich vondendunkelgefdirbtenZellendesAmnionepithels. Siegleichendagegen vollkommen den Elementen der Langhansschen Zcllschicht. Ich halte den Strang daher fireinen Chorionstrang" (p. 303). The difference between v.MCllendorff's observations on embryos OP (1921) and WO (1925) on the one hand, and Fahrenholz's observations on the other, could possibly be put in this way, that v. AMullendorff studied the stalk ectoderm in that region, where an extension of the amniotic cavity towards the connecting stalk takes place, whilst Fahrcnholz devoted particular attention to the place of the insertion of the amniotic duct into the stalk ectoderm. The fact that the epithelial cord, described in embryo Ho by Fahrenholz, consists of Langhan's cells, is not in contradiction with the opinion that we haveheretodowith theremainsofanamnioticduct. Ihadtheopportunity to convince myself in embryo Bi I, where there is a continuous connection between the ectoderm of the chorion and that of the amnion, that the caudal part of the amniotic duct consists of Langhan's cells. Stump (1929) made the same observation in an embryo, in which there is the same continuous connection between the ectoderm of the chorion and the amnion, but which is much older than the embryo Bi I; it is the oldest embryo in which such a continuous connection has been described. I believe, therefore, that the "chorionic duct" described by Fahrenholz in embryo I-lo represents remains of an amniotic duct, and that Fahrenholz was dealing with the junction of the amniotic duct with the stalk ectoderm.
v. Mollendorff's opinion possibly does not explain the amniotic duct so well as Fahrenholz's view, but there are, I believe, other and more important difficulties raised by the view of Fahrenholz: it is not able, for example, to explain our observations on the stalk ectoderm of the embryo Bi ll and Bi XI. XVe ought to count also on the possibility of a combination of an involution of the amniotic cavity in the neighbourhood of the insertion of the amniotic duct and of an extension of the same in other parts of the stalk ectoderm.
I may cite here another important observation made by Rossenbeck in the embryo Peh.1-Hochsteter (1923, p. 353): "Auffallend ist, dass funf Schnitte vor dem caudalen Ende der Amnionhohle ihre dorsale Wand wie zersprengterscheint, sodassman den Eindruckhat, als wiirdesichier direkt von dem Mesoblastgewebe des Bauchstieles begrenzt (vgl.Taf. XLI, Abb. 46)." I have already described places (p. 460) in the embryo Bi I, where there is also no ectoderm visible, but this region in the embryo Peh.1-IHochstetter seems to be much larger. In this connection I call attention to the outline of the connecting stalk towards the amniotic cavity in the sections of embryo Peh.1-I1-ochsteter; it is convex towards the amniotic cavity as it is also in the embryo Bi II. XVe could easily imagine that the stalk ectoderm could grow round and isolate a part of the stalk mesoderm with resulting sequestration of the latter during the further development.
If we compare the early development of the Man with the development of the other Mammals on the basis of the observations and interpretations set forth in the preceding pages, we reach the following conclusions:
The most remarkable difference-the reason of al later-mentioned differences-between the development of Man and that of other Mammals, is to be seen in the very early development in the former of the mesoderm of the chorion and the very early differentiation of the connecting stalk and the structures related to it, viz. the primitive streak primordium, the cloacal membrane and the allanto-enteric diverticulum. Further,thepresence of the ectoderm on the cranial surface of the umbilical stalk must be regarded as a preparation for the development of the umbilical cord.
The existence of the stalk offers a certain resistance in the development of the caudal parts of the embryonic body and complicates that development in the following respects: (1) The extension of the amniotic cavity towards the tissue of the stalk (in the caudal and dorsal direction) can be effected only by means of an activity of the stalk ectoderm by which the mesoderm of the stalk is partially destroyed. (2) The yolk-sac penetrates in to the connecting stalk in the form of a narrow diverticulum which enlarges and eventually opens out again into the cavity of the yolk-sac. This process may probably be repeated several times. (3) The very early primordium of the allantois does not arise directly from the hind-gut (as in the lower Mammals) but from an allanto-enteric diverticulum. The orifice of this diverticulutm later comes to formapart of the hind-gut. Itisonly in the stage when the insertion of the umbilical stalk has reached the ventral wall of the embryonic body that the allantois can be said to arise directly from the hind-gut, and so in this way the conditions characteristic of the lower Mammals are realised.
I desire to express my grateful thanks to Prof. J. P. Hill for his advice and criticism and for reading the manuscript. The photo-micrographs have been prepared in the Department of Anatomy and Embryology and I desire to thank Mr F. Pittock for his great help.
Description of Plates I-III
Figs. 1-24. The figures are orientated so that the dorsal side is uppermost, unless otherwise stated. Al. allanto-enteric diverticulum; Amn. amniotic cavity; Amn'. diverticulum of the amniotic cavity below caudal end of embryo in the median plane; Cl. cloacal membrane; Bet. ectoderm; Emb. caudal end of the embryo; Ent. entoderm; Ent'. entoderm of the hind-gut; Ent". entoderm close cranial to the orifice of the allanto-enteric diverticulum; Ch. chorion; Ch.c. chorionic cavity (extra-embryonal coelom); Mes. mesodermal mass lying free in the amniotic cavity.
Plate I
Fig.1.Embryo BiII, section 330. x300. cloacal membrane initsbestdevelopedpart.Dorsal sideontheright,ventralsideontheleft.
Fig.2.Embryo BiII, section 327. x400.Thelimits of the cloacal membrane towardsthe mesoderm are becoming indistinct. The limits of the ectoderm towards the mesoderm are indistinct laterally to the cloacal membrane. Text on p. 465.
Fig.3.Embryo BiII,section 326. x400.Theprocessgivenoffbytheectodermtowardsthe entoderm of the allanto-enteric diverticulum is very indistinctly marked off from the meso- derm. Text on p. 465.
Fig.4.Embryo BiI, section 325. x400.Thelimits of the remnant of the cloacal membrane are very indistinct. Underneath + are distinct granules (sign of cell-dissolution). Text on p.465.
Fig.5.Embryo BiII, section 321. x400.Theectodermisconnectedwith theentodermbya singlespindle-shapedcel. in the placemarkedbyanarrowtheectodermisthickenedin a knot. Text on p. 465.
Fig.6.Embryo BiII, section 315. x400.The ectodermgivesoffacell-cordwhichnearlyreaches theentoderm. Betweenitsendandtheentodermtherearenumerousgranules(possibly products of cell-dissolution); such are also visible in the ectoderm and entoderm. The dorsal Bide of this section on the right, the ventral on the left. Text on pp. 465, 466.
Fig.7.Embryo BiII, section 314. x400.The allanto-enteric diverticulum has a form of a flattened canal. in the dorsal direction( to the right) its ends a short process towards the ectoderm, in which granules are to be seen. in the ventralwall of the allantois(ontheleft) theentodermalcellscontainvacuoles in the basal part; the nucleiaresituatedtowardsthe freesurface of the cells.Textonpp.466,467.
Fig.8. Embryo BiI, section 320. x520. The limit betweentheectodermandthemesodermis very indistinct, especially in the thickened knot (*) close to the median plane. Below + there isanectodermalcelconnectedwith themesodermbyadistinct,longprocess.Textonp.469.
Plate II
Fig.9. Embryo BiXI, section 261. x400.Theallantoisclosebeneathitsorificein to the hind-gut. Entoderm of the allantoisconsistsofvacuolatedcells. Dorsalsideontheright,ventralon the left. Text on p. 467.
Fig.10. Embryo BiXI, section 276. x400.Allantoisfarcaudaltoitsorificein to the hind-gut. Otherwiseasinfig.9.
Fig.11. Embryo BiII, section 336. x161.Textonp.466.
Fig.12.Embryo BiII, section 338. x361.Textonp.466.
Fig.13. Embryo BiII, section 326. x1661.The penetration of the ectoderm in to the mesoderm (especially distinct at +). Text on p. 470.
Fig.14. Embryo BiII, section 326. x520.Thedetail of the place marked by + in fig.13. Text on p. 470.
Fig.15. Embryo BiIT, section 322. x520. Numerous granules in the mesoderm underneath the ectoderm (close to the insertion of the amnion to the connecting stalk).
Fig.16.EmbryoBiII,section335. x161.Text on p.470.
Plate III
Fig.17. Embryo BiII, section 335. x480. Detail from fig.16 (the place between the two arrows in fig. 16). The ectoderm is apparently absent in some places.
Fig.18. Embryo BiXI, section 301. x6131.The penetration of the stalk ectoderm in to the mesoderm in the form of a thickened knot. Text on p. 471.
Fig.19. Embryo BiXI, section 295. x613k. A place similar to that in fig.18.Textonp.471.
Fig.20. Embryo BiXI, section 304. x52.View of the amniotic cavity (Amn.) with the caudal end of the embryo, the chorionic membrane (Ch.) and the connecting stalk (on the right). In an outpocketing of the amniotic cavity there is a sequestered mesodermic mass (Mes.).
Fig.21.Embryo BiXI, section 284. x613k. Ectodermal cells penetrating in to the mesoderm of the connecting stalk (+,*). Textonp.471.
Fig.22. Embryo BiXI, section 300. x6131.Flattene dectodermal cells penetrating in to the mesoderm of the connecting stalk (for example at +). Some of them have been shed into the amniotic cavity (marked by an arrow).
Fig.23. Embryo BiXI, section 298. x613k. The flattened cells of the stalk-ectoderm are connected with the mesodermal cells by means of cytodesmata (+, *). Text on p. 471.
Fig.24. Embryo BiXI, section 315. x93k.The sequestered mesodermal mass (Mes.) in an outpocketing of the amniotic cavity. This figure is turned through 900 in comparison with fig.20.
Text-Figures
Ectoderm black; entoderm lined horizontally, primitive streak vertically, head process and chorda plate obliquely. Mesoderm dotted. The axis of the connecting stalk is marked by an arrow. The limits of the connecting stalk are marked by * and ʘ, the limits of the umbilical stalk by + and ʘ). The direction of the extension of the amniotic cavity towards the connecting stalk is marked by arrows. The parts of the allantois and of the axis of the connecting stalk situated out of the median plane are finely dotted. A. Connecting stalk; B. umbilical stalk; Cl.m. cloacal membrane.
References
FAHRENHOLZ, C.(1927). "EinjungesmenschlichesAbortiv-Ei." Zeitschr. f. mikr.-anat. Forschung, vol.vy.
FETZER (1910). "Tber ein durch Operation gewonnenes menschliches Ei." Anat. Anz. vol. xxxvii, Erganz.-Heft. (Verh.d.anat.Ges.,Bruissel.)
FETZER, M. and FLORIAN, J. (1929). "Der jiingste menschliche Embryo (Embryo Fetzer) mit bereitsentwickelter Kloakenmembran." Anat. Anz. vol. LxVII.
FLORIAN, J. (1927). "Uber zwei junge menschliche Embryonen." Anat. Anz. vol. LXIII, Ergainz.- Heft. (Verh. d. anat. Ges., Kiel.)
- (1928 a). "La gouttiere primitive, le canal de Lieberkiihn et la plaque chordale chez deux Embryons humains (BiIL, BiIII) avec quatre somites." Compt.rendus del' Assoc.des Anat. xxiumc R6union, Prague.
- (1928b). "Ein junges menschliches Ei in situ (Embryo T.F. mit Primitivstreifen ohne Kopffortsatz)." Zeitschr. f. mikr. -anat. Forsch. vol. xiii.
FLORIAN, J. and VOLKER, 0. (1929) . "tberdie Entwicklungdes Primitivstreifens,der Kloaken- membran und der Allantoisbeim Menschen." Zeitschr. f. mikr. -anat. Forsch. vol. xvi.
GROSSER, 0. (1913). "Ein menschlicher Embryo mit Chordakanal." Anat. Hefte, vol. xLVII. v. MOLLENDORFF, W. (1921). "Tber einen jungen operativ gewonnenen menschlichen Keim (EiOP.)." Zeitschr. f .Anat. u. Entwickl. vol. Lxii.
- (1925)."DasmenschlicheEiWO(lfring).Implantation,Verschluss der Implantations offnung und Keimesent wicklung beim Menschen vor Bildung des Primitivstreifens." Zeitschr. f. Anat. u. Entwickl.vol.LxxVI.
PETERS, H. (1899). uber die Ein bettungdes menschlichen Eies und da8 frihestefisherbekannte menschliche Plazentations stadium. Leipzig and Wien.
ROSSENBECK, H. (1923). "Ein junges menschliches Ei. Ovum humanum Peh.,-Hochsteter." Zeitschr .f. Anat. u. Entwickl. vol. LXVm.
STERNBERG, H. (1927a). "Beschreibung eines menschlichen Embryos mit vier Ursegment paaren, etc." Zeitschr. f. Anat. u. Entwickl. vol. Lxxxia.
- (1927b)."Zurformalen Geneseder Bauchblasenspalte (Exstrophiavesicae)." Virchows
Archiv,vol.ccLxmi.
STIEVE, H. (1926). "Ein 13k Tage altes, in der GeebArmutter erhaltenes und durch Eingriff gewonnenes menschliches Ei." Zeitschr. f. mikr.-anat. Forsch. vol. Vi.
STRAHL, H. and BENEKE, R. (1910). Ein junger menschlicher Embryo. Wiesbaden.
STUMP, C. WITHERINGTON (1929). "A human Blastocyst insitu."Transactions of the Royal Society of Edinburgh, vol. LVI, ptI (No.10).
Reference
J Florian The Formation of the Connecting Stalk and the Extension of the Amniotic Cavity towards the Tissue of the Connecting Stalk in Young Human Embryos. J. Anat.: 1930, 64(Pt 4);454-476.5 PMID 17104291 | PMC1250149
Cite this page: Hill, M.A. (2024, April 23) Embryology Paper - The Formation of the Connecting Stalk and the Extension of the Amniotic Cavity towards the Tissue of the Connecting Stalk in Young Human Embryos. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_Formation_of_the_Connecting_Stalk_and_the_Extension_of_the_Amniotic_Cavity_towards_the_Tissue_of_the_Connecting_Stalk_in_Young_Human_Embryos
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G