Paper - Phases of maturation, fertilization and early development in man: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| (22 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
| [[File:Mark_Hill.jpg|90px|left]] This 1943 historic paper by Hamilton describes the earliest stages of development following fertilisation. | | [[File:Mark_Hill.jpg|90px|left]] This 1943 historic paper by Hamilton describes the earliest stages of development following fertilisation. | ||
* Embryo 1 - 17th day of the menstrual cycle, ovulation probably occurred less than 12 hours before. | |||
:'''Modern Notes:''' | * Embryo 2 - 16th day of the cycle; coitus had taken place 2 and 4 days previously. | ||
<br><br> | |||
:'''Modern Notes:''' {{fertilization}} | |||
<br> | |||
{{Fertilization Links}} | |||
<br> | <br> | ||
{{Week 1 Links}} | {{Week 1 Links}} | ||
| Line 42: | Line 46: | ||
==Introduction== | ==Introduction== | ||
Aaccount is given by Allen and others’ of attempts that have been made in the past to secure human tubal ova. They recovered 5 ova by reverse irrigation _of the uterine tubes from the uterus. An unfertilized ovum was recovered by Lewis“ by flushing the uterine tube which had been previously removed with a myomatous uterus; two other ova were recovered by Pincus and Saunders.“ Rock and Hertig‘ report the recovery of an unfertilized ovum, but do not give any details concerning it. | |||
attempts that have been made in the past | |||
to secure human tubal ova. They recovered | |||
5 ova by reverse irrigation _of the uterine | |||
tubes from the uterus. An unfertilized | |||
ovum was recovered by Lewis“ by flushing | |||
the uterine tube which had been previously | |||
removed with a myomatous uterus; two | |||
other ova were recovered by Pincus and | |||
Saunders.“ Rock and Hertig‘ report the | |||
recovery of an unfertilized ovum, but do | |||
not give any details concerning it | |||
Until the publication by Dible and West,‘ and that by Hertig and Rock,“ and more recently the account of young embryos given by Rock and Hertig,‘ the youngest human embryo was the “ Miller” described by Miller? and Streeter“ ; unfortunately only 5 sections were preserved. | |||
of the | |||
The | The present communication gives a description of an unfertilized ovum showing the second maturation spindle, an unsegmented ovum at an early stage of fertilization, and a young chorionic vesicle partially implanted in the endometrium. | ||
==Description== | |||
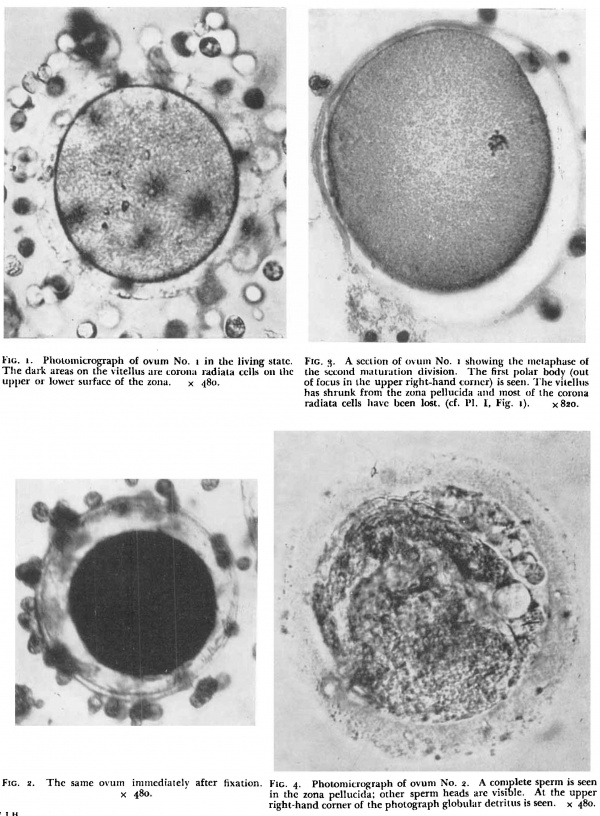
The first specimen was obtained from a woman who was sterilized on account of severe mitral stenosis. The ovum was recovered by flushing the uterine tube after its removal on the 17th day of the menstrual cycle; ovulation probably occurred less than 12 hours before. The cycle previous to that from which the ovum was recovered was of 30 days duration. At the operation the right ovary showed a recently ruptured follicle with a "small amount of blood still escaping. When examined in the fresh state the ovum was surrounded by several layers of corona radiata cells which were attached to a homogenous zona pellucida. (Plate I, fig. I.) The vitellus was yellowish in colour, uniformly finely granular and completely filled the zonal cavity. It did not show the three types of yolk bodies described by Lewis,” but resembled more closely the second ovum described by Pincus and Saunders.“ | |||
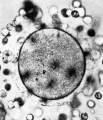
After fixation the ovum was found on histological section to be at the stage of the second maturation spindle. (Plate I, fig. 3.) The polar body showed scattered chromosomes in its cytoplasm. Dixon” concluded from an examination of an oocyte in the ovary that the second maturation spindle was completed before the oocyte left the ovary but the appearances found in this specimen and in the descriptions given by Allen and others‘ for two of their shed ova and by Pincus and Saunders“ do not support Dixon’s opinion. Whether the second maturation division is completed in the absence of fertilization cannot be stated at present. | |||
radiata cells | The second specimen was obtained from a woman who was sterilized as her last 2 pregnancies terminated as miscarriages on account of severe toxaemia. Her menstrual cycles previously were of the 28 day type. The ovum was recovered by flushing the tube removed on the 16th day of the cycle; coitus had taken place 2 and 4 days previously. At the operation the ovary showed a recently ruptured follicle with some blood still escaping. When examined in the fresh state the ovum was found to be free of corona radiata cells. The zona pellucida appeared as a homogenous membrane in which many completed sperms were found. (Plate I, fig. 4.) The vitellus did not completely fill the zonal cavity; at one side there were a number of granules, probably remains of the polar bodies. The vitellus showed a clearer zone in the centre and its general appearance was that of early degeneration. | ||
The third specimen (the “ Barnes ” embryo), an early chorionic vesicle partially implanted in the endometrium, was found after hysterectomy on the 25th day of the menstrual cycle. The patient had an ovarian cyst and was suffering from excessive menstrual loss. Coitus had taken place on the Ioth and Izth days previous to the date of operation. | |||
At the operation a considerable amount of free fluid was found in the peritoneal cavity. The left ovary contained a cyst about 4 inches in diameter and also a corpus luteum. The uterus was slightly enlarged and soft. As the nature of the ovarian cyst was in doubt a total hysterectomy, bilateral salpingectomy, and left oophorectomy was performed. The appendix was also removed. | |||
with | The uterus was incised along the attachment of the broad ligament on each side and was then opened, separating the anterior and posterior walls from each other. The endometrium in the cervix and in the lower part of the body was hypertrophic and polypoidal. The endometrium lining the remainder of the uterine cavity appeared to be normal; it showed, in approximately the middle of the posterior wall, a small elevation which was just visible to the naked eye. On examination with a low power binocular microscope the elevation appeared as a slightly raised translucent area which was clearly demarcated from the surrounding endometrium. The elevation was tentatively diagnosed as a young implanting blastocyst or a retention cyst of a uterine gland. After fixation with Bouin-Allen the area became opaque but was still raised above the level of the surrounding endometrium. (Plate II, fig. 5.) A block of the uterine wall containing the swelling was removed and after dehydration, clearing and double embedding was cut at 714. Microscopical examination of the sections showed an early implanted blastocyst. fig. 7.) The chorionic vesicle is not completely implanted in the endometrium as part of the elevation that projects into the uterine lumen is not yet covered by the uterine epithelium. This relatively superficial position of the chorionic vesicle in the present specimen resembles closely that of “ovum ’ ’ No. {{CE7700}} of Hertig and Rock.“ The trophoblastic shell varies in thickness; it is thickest over the deep embryonic region of the vesicle and .thinnest in the abembryonic region where the uterine epithelium is absent. The trophoblast is differentiated into a cytotrophoblast and a syncytiotrophoblast. The calculated dimensions of the vesicle are approximately 0.931 x 0.770 x 0.737 mm. In some areas the syncytiotrophoblast has. destroyed the wall of the blood vessels, allowing the blood to escape to a slight extent into the intercommunicating lacunae of the syncytiotrophoblast. The general appearance resembles very closely ‘those described and illustrated by Hertig and Rock.“ The peripheral edge of the syncytiotrophoblast, however, seems to be more sharply demarcated from the endometrium than in their specimens Nos. {{CE7699}} and {{CE7700}} and resembles more closely the appearance in their specimen No. {{CE7950}}, fig. 4 (I942). The cytotrophoblast is composed of cuboidal cells which in some situations have proliferated to form irregular masses which project into the syncytio-trophoblast ; these projections are the forerunners of the primary villi. | ||
[[File:Hamilton1943 plate01.jpg|600px]] | |||
'''PLATE i.''' | |||
'''Fig. 1.''' Photomicrograph of ovum No. 1 in the living state. | |||
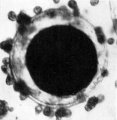
The | '''Fig. 2.''' The same ovum immediately after fixation. | ||
'''Fig. 3.''' A section of ovum No. 1 showing the metaphase of The dark areas on the vitellus are corona radiata cells on the the second maturation division. The first polar body (out upper or lower surface of the zona. x .180. of focus in the upper right-hand corner) is seen. The vitellns has shrunk from the zona pellucida and most of the corona radiata cells have been lost. (cf. P]. I, fig. I). x820. | |||
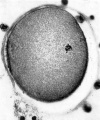
'''Fig. 4.''' Photomicrograph of ovum No. 2. A complete sperm is seen x 480. in the zona pellucida: other sperm heads are visiblle. At the upper right-hand corner of the photograph globular detritus is seen. x 480. w..H. | |||
<gallery> | |||
File:Hamilton1943 fig01.jpg|Fig 1 | |||
File:Hamilton1943 fig02.jpg|Fig 2 | |||
File:Hamilton1943 fig03.jpg|Fig 3 | |||
File:Hamilton1943 fig04.jpg|Fig 4 | |||
</gallery> | |||
[[File:Hamilton1943 plate02.jpg|600px]] | |||
'''PLATE II.''' | |||
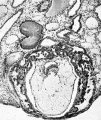
' | '''Fig. 5.''' A surface view of the endometrium of specimen No. 3. The smooth elevation produced by the implanting embryo is seen. The endometritnn shows fissures :md crct'iccs which for the most part are associated with the mouths of the uterine glands. x 15. | ||
the | |||
'''Fig. 6.''' A high power view of the embryonic disc showing the columnar nature of the embryonic ectoderm and the arrange ment of the endoderm. with Heuser’s membrane. The cells of the latter are continuous x 400. | |||
disc of | |||
'''Fig. 7.''' A general low power View of the implanting embryo and surrounding endometrium. The latter is oedematous and in it there are many dilated bloodvessels. The extent of the sync_vtiotrophoblast and cytotrophohlast in different parts of the vesicle is shown. The extra embryonic rnesoderm has been artificially separated from the cytolrophoblast except at the embryonic pole. The embryonic rudiment has difl'erentiated into a columnar ectodermal layer, which at the edge of the embryonic disc is continuous with the embryonic eetoderm of the amnion. and a mass of endodermal cells which with Heuser's membrane enclose a cavity, the primitive yolk sac. x no. | |||
<gallery> | |||
File:Hamilton1943 fig05.jpg|Fig 5 | |||
File:Hamilton1943 fig06.jpg|Fig 6 | |||
File:Hamilton1943 fig07.jpg|Fig 7 | |||
</gallery> | |||
The endometrial tissue shows marked oedema, but there is no evidence of a decidual reaction. The glands of the endometrium are dilated and tortuous. Their epithelium seems to be resistant, at this stage, to the action of the syncytiotrophoblast and the glands appear to be pressed aside by the expanding vesicle (cf. Streeter, 1926). | |||
The embryonic disc is convex towards the ‘amniotic cavity and is composed of tall columnar epithelial cells which are continuous at the periphery of the disc with the amniotic ectoderm. (Plate II, fig. 6.) This for the most part consists of a layer of flattened cells but in one situation these cells are cuboidal. The endoderm is composed of from one to several layers of large cuboidal cells with vacuoles in their cytoplasm. In the younger embryo No. {{CE7699}} described by Hertig and Rock the endodermal cells are heaped up to- form a mass as many as three layers thick but in their other specimens the endoderm forms a single layer. An endodermal yolk sac has not yet been formed but a cavity lined with endodermal cells and flattened mesodermal cells (Heuser’s, or exocoelomic, membrane) encloses a cavity (fig. 7) which has been designated by Hertig and Rock“ in their specimens as the exocoelomic space. In this cavity there is a precipitated coagulum. The outer aspect of the cells of Heuser’s membrane is continuous with a mesenchymal reticulum. which fills the trophoblastic vesicle except where it has become artificially separated from the cytotrophoblast. | |||
==Probable Age of the Specimen== | |||
As already stated coitus occurred 12 and 10 days before the operation. The last menstrual period began on the 25th May and, assuming that the present cycle would have been of 28 days duration, the next period was due on the 22nd June. Coitus occurred on the 6th and 8th June, i.e., I6 and 14 days before the expected next period. The operation was performed on the 18th June. If ovulation occurs (see later) 14.5 (i.e. 13 to 15) days before the next menstrual period then ovulation occurred on the 7th to 9th June. A comparison of the present specimen with those of Hertig and Rock shows that No. {{CE8004}} which is estimated to be 9.5; days old, is much less developed. The embryonic disc of No. {{CE7699}}, estimated age II days, is less developed but the syncytiotrophoblast has invaded the endometrium more than has that in the present specimen. It seems probable that ovulation occurred at the earliest on the 7th — 8th June, and that the fruitful coitus was that of the 6th June. The age of the embryo is thus estimated to be from 10 to 11 days, mean age Io% days. | |||
==Time of Ovulation== | |||
On the time of ovulation in women there is now an extensive literature which has been reviewed by a number of investigators (Knaus,‘° Dickinson," Latz and Reiner,” and others). The evidence which has been accumulated during the past few years from a number of different methods of investigation seems to show that ovulation is more closely related to the succeeding menstrual period than to the beginning of the preceding menstruation. Ogino” Pryde,” Smith,” and Latz and Reiner” base this assumption on their investigations on the so-called “ safe period.” Schroeder ”’ and Shaw” deduce the time of ovulation from the examination of the endometrium and related corpora lutea, and conclude that it occurs at about the middle of the cycle. Rock and Hertig,‘ by comparing the ages of young embryos with the associated endometrial histology, conclude that ovulation occurs 13 to 15 days before the next menstruation. | |||
It is thus presumed that ovulation occurs about 14.5 days before the beginning of the next menstrual period. The day, however, cannot always be accurately predicted since there is some variation in the length of the menstrual cycle in individual women. Gunn and others” state that no cases were found which did not vary at least 2.75 days between the shortest and longest interval, and Haman” states that there was no instance of absolute regularity in his series. Further, Rock” has suggested that about 3 per cent of the women in his series of 88 cases do not ovulate regularly 13 to 15 days before the next menstrual period and, therefore, do not fit into the general pattern. | |||
The recovery of ova at a definite period of the menstrual cycle gives the most reliable information on the time of ovulation. Specimen No. I of the present series which was presumed to be recently shed since it was surrounded by many corona radiata cells was recovered 14 days before the presumed beginning of the next menstrual cycle. Table I gives the estimated age of all ova described up to the present and the approximate day of their recovery in the cycle. There is some evidence from these few cases to show that the time of ovulation is more closely related to the beginning of the next menstruation than of the preceding one. | |||
==Table 1== | |||
{{Hamilton1943 table1}} | |||
==References== | |||
<references/> | |||
I. Allen, E., J. P. Pratt, Q. U. Newell, and L. J. Bland. Contrib. Embryol., Carnegie Inst, Wash., 1930, xxii, 45. | |||
2. Lewis, W. H. johns Hopkins Hosp. Bull” 1931, xlviii, 368. | |||
3. Pincus, G., and B. Saunders. 1937, xlix, I63. | |||
3 | |||
4 | 4. {{Ref-RockHertig1942}} | ||
5. {{Ref-Dible1941}} | |||
6. {{Ref-RockHertig1941}} | |||
7. Miller, J. W. Berliner klin Wochenschr., 1913, xix I. | |||
8. {{Ref-Streeter1926}} | |||
9. {{Ref-Dixon1927}} | |||
I0. Knaus, H. “Periodic Fertility and Sterility in Woman.” Vienna. | |||
11. Dickinson, R. L. Joun. Contraception, 1938, iii, 219. | |||
12. Latz, L. J., and E. Reiner. Amer. ]ourn. Obstet. and Gynecol., 1942, xliii, 74. | |||
13. Ogino, K. "onception period in Women” (trans. Y. Mujagawa). Harrisburg Medical Arts Co. | |||
14. Pryde, J. Brit. Med. ]oum., 1941, i, 12. | |||
15. Smith, G. P. Brit. Med. ]oum., 1942, ii, 38. | |||
16. Schroeder, R. Arch. f. Gym, 1914, ci, | |||
. | |||
I7. Shaw, W. journ Physiol., 1925, lx, I93. | |||
I8. Gunn, D. L., P. M. Jenkin, and A. L. Gunn. journ. Obstet. and Gynaecol. Brit. Emp., 1937, xliv, 839. | |||
19. Haman, J. O. Amer. joum. Obstet. and Gynecol., 1942, xliii, 870. | |||
20. {{Ref-Rock1940}} | |||
{{Footer}} | |||
Latest revision as of 13:15, 5 May 2018
| Embryology - 24 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Hamilton WJ. Barnes J. and Dodds GH. Phases of maturation, fertilization and early development in man. (1943) J. Obstet. Gynaecol, Brit. Emp., 50: 241-245.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Phases of Maturation, Fertilization and Early Development in Man
By
W. J. HAMILTON, M.D.,
D.Sc., F.R.S. (Edin.),
Anatomy Department, St. Bartholomew’s Hospital Medzcal College, London;
Josephine Barnes, M.A., D.M., F.R.C.S., M.R.C.P., M.R.C.O.G.,
and
Gladys H. Donns, M.D., F.R.C.S. (Eng. and Edin.), F.R.C.O.G., Obstetric Unit, University College Hospital, London.
Introduction
Aaccount is given by Allen and others’ of attempts that have been made in the past to secure human tubal ova. They recovered 5 ova by reverse irrigation _of the uterine tubes from the uterus. An unfertilized ovum was recovered by Lewis“ by flushing the uterine tube which had been previously removed with a myomatous uterus; two other ova were recovered by Pincus and Saunders.“ Rock and Hertig‘ report the recovery of an unfertilized ovum, but do not give any details concerning it.
Until the publication by Dible and West,‘ and that by Hertig and Rock,“ and more recently the account of young embryos given by Rock and Hertig,‘ the youngest human embryo was the “ Miller” described by Miller? and Streeter“ ; unfortunately only 5 sections were preserved.
The present communication gives a description of an unfertilized ovum showing the second maturation spindle, an unsegmented ovum at an early stage of fertilization, and a young chorionic vesicle partially implanted in the endometrium.
Description
The first specimen was obtained from a woman who was sterilized on account of severe mitral stenosis. The ovum was recovered by flushing the uterine tube after its removal on the 17th day of the menstrual cycle; ovulation probably occurred less than 12 hours before. The cycle previous to that from which the ovum was recovered was of 30 days duration. At the operation the right ovary showed a recently ruptured follicle with a "small amount of blood still escaping. When examined in the fresh state the ovum was surrounded by several layers of corona radiata cells which were attached to a homogenous zona pellucida. (Plate I, fig. I.) The vitellus was yellowish in colour, uniformly finely granular and completely filled the zonal cavity. It did not show the three types of yolk bodies described by Lewis,” but resembled more closely the second ovum described by Pincus and Saunders.“
After fixation the ovum was found on histological section to be at the stage of the second maturation spindle. (Plate I, fig. 3.) The polar body showed scattered chromosomes in its cytoplasm. Dixon” concluded from an examination of an oocyte in the ovary that the second maturation spindle was completed before the oocyte left the ovary but the appearances found in this specimen and in the descriptions given by Allen and others‘ for two of their shed ova and by Pincus and Saunders“ do not support Dixon’s opinion. Whether the second maturation division is completed in the absence of fertilization cannot be stated at present.
The second specimen was obtained from a woman who was sterilized as her last 2 pregnancies terminated as miscarriages on account of severe toxaemia. Her menstrual cycles previously were of the 28 day type. The ovum was recovered by flushing the tube removed on the 16th day of the cycle; coitus had taken place 2 and 4 days previously. At the operation the ovary showed a recently ruptured follicle with some blood still escaping. When examined in the fresh state the ovum was found to be free of corona radiata cells. The zona pellucida appeared as a homogenous membrane in which many completed sperms were found. (Plate I, fig. 4.) The vitellus did not completely fill the zonal cavity; at one side there were a number of granules, probably remains of the polar bodies. The vitellus showed a clearer zone in the centre and its general appearance was that of early degeneration.
The third specimen (the “ Barnes ” embryo), an early chorionic vesicle partially implanted in the endometrium, was found after hysterectomy on the 25th day of the menstrual cycle. The patient had an ovarian cyst and was suffering from excessive menstrual loss. Coitus had taken place on the Ioth and Izth days previous to the date of operation.
At the operation a considerable amount of free fluid was found in the peritoneal cavity. The left ovary contained a cyst about 4 inches in diameter and also a corpus luteum. The uterus was slightly enlarged and soft. As the nature of the ovarian cyst was in doubt a total hysterectomy, bilateral salpingectomy, and left oophorectomy was performed. The appendix was also removed.
The uterus was incised along the attachment of the broad ligament on each side and was then opened, separating the anterior and posterior walls from each other. The endometrium in the cervix and in the lower part of the body was hypertrophic and polypoidal. The endometrium lining the remainder of the uterine cavity appeared to be normal; it showed, in approximately the middle of the posterior wall, a small elevation which was just visible to the naked eye. On examination with a low power binocular microscope the elevation appeared as a slightly raised translucent area which was clearly demarcated from the surrounding endometrium. The elevation was tentatively diagnosed as a young implanting blastocyst or a retention cyst of a uterine gland. After fixation with Bouin-Allen the area became opaque but was still raised above the level of the surrounding endometrium. (Plate II, fig. 5.) A block of the uterine wall containing the swelling was removed and after dehydration, clearing and double embedding was cut at 714. Microscopical examination of the sections showed an early implanted blastocyst. fig. 7.) The chorionic vesicle is not completely implanted in the endometrium as part of the elevation that projects into the uterine lumen is not yet covered by the uterine epithelium. This relatively superficial position of the chorionic vesicle in the present specimen resembles closely that of “ovum ’ ’ No. 7700 of Hertig and Rock.“ The trophoblastic shell varies in thickness; it is thickest over the deep embryonic region of the vesicle and .thinnest in the abembryonic region where the uterine epithelium is absent. The trophoblast is differentiated into a cytotrophoblast and a syncytiotrophoblast. The calculated dimensions of the vesicle are approximately 0.931 x 0.770 x 0.737 mm. In some areas the syncytiotrophoblast has. destroyed the wall of the blood vessels, allowing the blood to escape to a slight extent into the intercommunicating lacunae of the syncytiotrophoblast. The general appearance resembles very closely ‘those described and illustrated by Hertig and Rock.“ The peripheral edge of the syncytiotrophoblast, however, seems to be more sharply demarcated from the endometrium than in their specimens Nos. 7699 and 7700 and resembles more closely the appearance in their specimen No. 7950, fig. 4 (I942). The cytotrophoblast is composed of cuboidal cells which in some situations have proliferated to form irregular masses which project into the syncytio-trophoblast ; these projections are the forerunners of the primary villi.
PLATE i.
Fig. 1. Photomicrograph of ovum No. 1 in the living state.
Fig. 2. The same ovum immediately after fixation.
Fig. 3. A section of ovum No. 1 showing the metaphase of The dark areas on the vitellus are corona radiata cells on the the second maturation division. The first polar body (out upper or lower surface of the zona. x .180. of focus in the upper right-hand corner) is seen. The vitellns has shrunk from the zona pellucida and most of the corona radiata cells have been lost. (cf. P]. I, fig. I). x820.
Fig. 4. Photomicrograph of ovum No. 2. A complete sperm is seen x 480. in the zona pellucida: other sperm heads are visiblle. At the upper right-hand corner of the photograph globular detritus is seen. x 480. w..H.
PLATE II.
Fig. 5. A surface view of the endometrium of specimen No. 3. The smooth elevation produced by the implanting embryo is seen. The endometritnn shows fissures :md crct'iccs which for the most part are associated with the mouths of the uterine glands. x 15.
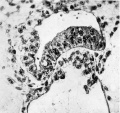
Fig. 6. A high power view of the embryonic disc showing the columnar nature of the embryonic ectoderm and the arrange ment of the endoderm. with Heuser’s membrane. The cells of the latter are continuous x 400.
Fig. 7. A general low power View of the implanting embryo and surrounding endometrium. The latter is oedematous and in it there are many dilated bloodvessels. The extent of the sync_vtiotrophoblast and cytotrophohlast in different parts of the vesicle is shown. The extra embryonic rnesoderm has been artificially separated from the cytolrophoblast except at the embryonic pole. The embryonic rudiment has difl'erentiated into a columnar ectodermal layer, which at the edge of the embryonic disc is continuous with the embryonic eetoderm of the amnion. and a mass of endodermal cells which with Heuser's membrane enclose a cavity, the primitive yolk sac. x no.
The endometrial tissue shows marked oedema, but there is no evidence of a decidual reaction. The glands of the endometrium are dilated and tortuous. Their epithelium seems to be resistant, at this stage, to the action of the syncytiotrophoblast and the glands appear to be pressed aside by the expanding vesicle (cf. Streeter, 1926).
The embryonic disc is convex towards the ‘amniotic cavity and is composed of tall columnar epithelial cells which are continuous at the periphery of the disc with the amniotic ectoderm. (Plate II, fig. 6.) This for the most part consists of a layer of flattened cells but in one situation these cells are cuboidal. The endoderm is composed of from one to several layers of large cuboidal cells with vacuoles in their cytoplasm. In the younger embryo No. 7699 described by Hertig and Rock the endodermal cells are heaped up to- form a mass as many as three layers thick but in their other specimens the endoderm forms a single layer. An endodermal yolk sac has not yet been formed but a cavity lined with endodermal cells and flattened mesodermal cells (Heuser’s, or exocoelomic, membrane) encloses a cavity (fig. 7) which has been designated by Hertig and Rock“ in their specimens as the exocoelomic space. In this cavity there is a precipitated coagulum. The outer aspect of the cells of Heuser’s membrane is continuous with a mesenchymal reticulum. which fills the trophoblastic vesicle except where it has become artificially separated from the cytotrophoblast.
Probable Age of the Specimen
As already stated coitus occurred 12 and 10 days before the operation. The last menstrual period began on the 25th May and, assuming that the present cycle would have been of 28 days duration, the next period was due on the 22nd June. Coitus occurred on the 6th and 8th June, i.e., I6 and 14 days before the expected next period. The operation was performed on the 18th June. If ovulation occurs (see later) 14.5 (i.e. 13 to 15) days before the next menstrual period then ovulation occurred on the 7th to 9th June. A comparison of the present specimen with those of Hertig and Rock shows that No. 8004 which is estimated to be 9.5; days old, is much less developed. The embryonic disc of No. 7699, estimated age II days, is less developed but the syncytiotrophoblast has invaded the endometrium more than has that in the present specimen. It seems probable that ovulation occurred at the earliest on the 7th — 8th June, and that the fruitful coitus was that of the 6th June. The age of the embryo is thus estimated to be from 10 to 11 days, mean age Io% days.
Time of Ovulation
On the time of ovulation in women there is now an extensive literature which has been reviewed by a number of investigators (Knaus,‘° Dickinson," Latz and Reiner,” and others). The evidence which has been accumulated during the past few years from a number of different methods of investigation seems to show that ovulation is more closely related to the succeeding menstrual period than to the beginning of the preceding menstruation. Ogino” Pryde,” Smith,” and Latz and Reiner” base this assumption on their investigations on the so-called “ safe period.” Schroeder ”’ and Shaw” deduce the time of ovulation from the examination of the endometrium and related corpora lutea, and conclude that it occurs at about the middle of the cycle. Rock and Hertig,‘ by comparing the ages of young embryos with the associated endometrial histology, conclude that ovulation occurs 13 to 15 days before the next menstruation.
It is thus presumed that ovulation occurs about 14.5 days before the beginning of the next menstrual period. The day, however, cannot always be accurately predicted since there is some variation in the length of the menstrual cycle in individual women. Gunn and others” state that no cases were found which did not vary at least 2.75 days between the shortest and longest interval, and Haman” states that there was no instance of absolute regularity in his series. Further, Rock” has suggested that about 3 per cent of the women in his series of 88 cases do not ovulate regularly 13 to 15 days before the next menstrual period and, therefore, do not fit into the general pattern.
The recovery of ova at a definite period of the menstrual cycle gives the most reliable information on the time of ovulation. Specimen No. I of the present series which was presumed to be recently shed since it was surrounded by many corona radiata cells was recovered 14 days before the presumed beginning of the next menstrual cycle. Table I gives the estimated age of all ova described up to the present and the approximate day of their recovery in the cycle. There is some evidence from these few cases to show that the time of ovulation is more closely related to the beginning of the next menstruation than of the preceding one.
Table 1
| Table 1 | ||||||
|---|---|---|---|---|---|---|
| Ovum | Corona Cells | Polar bodies | Day of cycle ovum recovered | Length of | Presumed number of days between ovulation and onset of next cycle | Remarks |
| Allen and others | ||||||
| 1 | Nil | 2 (10μ) | 15th | Not stated | — | Ovum lost |
| 2 | Present | 1 | 15th | Not stated | — | Twins—one from each ovary |
| 3 | Partly dispersed | 1 | 15th | Not stated | — | each ovary |
| 4 | Partly dispersed | 1 | 16th | Not stated | — | |
| 5 | 3 layers | — | 14th | Not stated | — | |
| Lewis | Nil | - | 22nd | Formerly 28 days, last 35 | 14-15 | Ovum possibly 1 day old |
| Pincus and Saunders | ||||||
| 1 | Nil | - | 19th | 28 days | 12 | Ovum possibly 2 days old |
| 2 | Nil | 1 | 20th | 26-30 days | 9-13 | Ovum possibly 2 days old |
| Present paper | ||||||
| 1 | Present | 1 | 17th | Formerly 28, last 30 | 14 | Not more than 12 hours old |
| 2 | Nil | ?Polar debris | 16th | 28 days | 14-15 | Ovum lost; possibly 1-2 days old |
References
I. Allen, E., J. P. Pratt, Q. U. Newell, and L. J. Bland. Contrib. Embryol., Carnegie Inst, Wash., 1930, xxii, 45.
2. Lewis, W. H. johns Hopkins Hosp. Bull” 1931, xlviii, 368.
3. Pincus, G., and B. Saunders. 1937, xlix, I63.
4. Rock J. and Hertig AT. Some aspects of early human development. (1942) Amer. f. Obstet. Gynecol, 44: 973-983.
5. Dible JH. and West CM. A human ovum at the previllous stage. (1941) J Anat. 75(3): 269–281. PMID 17104860
6. Rock J. and Hertig AT. Two human ova of the previous stage, having an ovulation age of about eleven and twelve days respectively. (1941) Contrib. Embryol., Carnegie Inst. Wash. Publ.525, 29: 127-156.
7. Miller, J. W. Berliner klin Wochenschr., 1913, xix I.
8. Streeter GL. The "Miller" ovum—the youngest normal human embryo thus far known. (1926) Carnegie Instn. Wash. Publ 363, Contrib. Embryol., 18: 31-48.
9. Dixon AF. Human oocyte showing first polar body and metaphase stage in formation of second polar body. (1927) Irish Jour. Med. Sci., 53: 149-151.
I0. Knaus, H. “Periodic Fertility and Sterility in Woman.” Vienna.
11. Dickinson, R. L. Joun. Contraception, 1938, iii, 219.
12. Latz, L. J., and E. Reiner. Amer. ]ourn. Obstet. and Gynecol., 1942, xliii, 74.
13. Ogino, K. "onception period in Women” (trans. Y. Mujagawa). Harrisburg Medical Arts Co.
14. Pryde, J. Brit. Med. ]oum., 1941, i, 12.
15. Smith, G. P. Brit. Med. ]oum., 1942, ii, 38.
16. Schroeder, R. Arch. f. Gym, 1914, ci,
I7. Shaw, W. journ Physiol., 1925, lx, I93.
I8. Gunn, D. L., P. M. Jenkin, and A. L. Gunn. journ. Obstet. and Gynaecol. Brit. Emp., 1937, xliv, 839.
19. Haman, J. O. Amer. joum. Obstet. and Gynecol., 1942, xliii, 870.
20. Rock J. TBA. (1940) New England J. Med. 223: 1020.
Cite this page: Hill, M.A. (2024, April 24) Embryology Paper - Phases of maturation, fertilization and early development in man. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Phases_of_maturation,_fertilization_and_early_development_in_man
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G