Paper - On the implantation of the human ovum and the early development of the trophoblast (1925)
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Teacher JH. On the implantation of the human ovum and the early development of the trophoblast. (1925) J Obst. Gynaecol. 31(2); 166-217.
| Online Editor | ||
|---|---|---|
| Note this paper was published in 1925 and our understanding of early development has improved since this historic human study. This embryo (Teacher-Bryce Ovum No. 1) was later classified as Carnegie stage 6
See also the earlier paper by the same author Bryce TH. and Teacher JH. Contributions To The Study Of The Early Development And Imbedding Of The Human Ovum 1. An Early Ovum Imbedded In The Decidua. (1908) James Maclehose and Sons. Glasgow.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
On the Implantation of the Human Ovum and the Early Development of the Trophoblast
By John H. Teacher, M.A., M.D. (Glas.),
St. Mungo (Notman) Professor of Pathology in the University of Glasgow, and Pathologist to Glasgow Royal Infirmary.
Introduction
The process by which the human ovum becomes enclosed within the decidua is still uncertain. William Hunter recognized that the membrane to which he gave the name decidua was the endometrium modified to serve the needs of pregnancy. How the decidua reflexa came to envelope the ovum he confessed he did not know, and he left on record no theory. This defect in his last work was supplied by printing as his the views of his brother. These were founded on John Hunter’s theory of the nature of inflammation and healing of wounds, and, while in gross they were erroneous, they have their place in the present day conception of the nature of the implantation of the ovum which he compared to the encapsulation of a foreign body in the tissues.
The idea that the mammalian ovum attains its access to the stores of nutriment borne in the maternal circulation by destruction of maternal tissue, which is followed by reaction on the part of the maternal organism, is now based upon many studies of human and comparative embryology. In 1899 the monograph of Hubert Peters gathered all the available data together.[1]
The famous Peters’ ovum, although only about two millimetres in diameter, was already embedded in a little swelling of the decidua. There was a gap in the roof of the decidual cavity about eight-tenths of a millimetre in diameter, which was closed by a mushroom-like mass of fibrin and blood-clot involving some of the outer cells of the ovum.
This suggested a process of implantation like that of the ovum of the hedgehog, as described by Huhrecht in 1889. The works of Hensen and V. Spee on the embedding of the ovum of the guinea-pig showed a different mode of implantation. But these two modes are in essential correspondence, inasmuch as there is destruction of the maternal tissue by the ovum itself, and the definitive connexion is effected by means of a great proliferation of the outer layer of the ovum (called trophoblast by Hubrecht, ectoplacenta by Duval). This unites with the reacting maternal tissues and opens the maternal vessels in such a way that a circulation of maternal blood becomes established in it, and later the development of a foetal circulation completes the placenta.
The theory of Peters that the human ovum implants itself in the decidua and that the gap over it is closed by a coagulum (Gewebspilz of Peters) still prevails, although it has been criticized. Several apparently normal ova, nearly as young, showed no “closing coagulum” (Bonnet’s name for the Gewebspilz), but what they did show could well be derived from it. In 1908 the writer and Professor T. H. Bryce in collaboration described the ovum T.B.I, younger than that of Peters, in which instead of the wide gap there was only a little circular aperture about one—eighth of a millimetre in diameter. The lips of the aperture were thick and composed of living decidua like the r-est of the roof of the cavity. The aperture was occupied by a little mass of what was regarded as fibrin and leucocytes. We held that this ovum revealed the real aperture of entrance an-d that the wide gap and closing coagulum of the Peters’ ovum were secondary and due to the destructive action of the trophoblast on the roof of the cavity. Between 1908 and 1922 much new material had become available, a number of valuable ova had been very fully worked up and the problems had been discussed by many writers. We, therefore, felt that the time was ripe for a fresh review of the “ Imbedding and early development of the human ovum." Then, in ]une 1923, Professor Teacher discovered a new ovum a little larger than that of Peters and excellently preserved. In the present memoir the writer takes as his province the implantation of the ovum and the development of the trophoblast, and in a contemporary memoir Professor Bryce ‘deals with the embryo proper and the mesoderm. There are now in existence at least six ova younger than that of Peters, viz., “ Miller,” “ Kleinhans,” “Teacher-Bryce,” Von Mollendorff’s “Sch.,” “Leopold (19o6)” and “Linzenmeier.” The first two of these are certainly younger than “ Teacher-Bryce.” It cannot be denied that any or all of these ova may be pathological, but all of them are valuable. Used as a series of specimens the facts which they reveal, in spite of some apparent contradictions, go far towards the construction of a normal line of development in the period which they cover. On resuming critical study of the Teacher-Bryce ovum, the authors were delighted to find that they had in no way exaggerated the beauty and clearness of the specimen. Considering “its adventures,” as Johnstone tactfully puts it, the preservation is wonderful. Compared with other young ova it is good, and in the light of our fuller knowledge to-day, the writer and Professor Bryce are more confident than before that it is normal. It is still a unique specimen, although “Sch.” of Von Mollendorff is very like it in some respects. The central subject, therefore, of the present communication is the original Teacher—Bryce ovum (“ T.B.1.”).
The new ovum, which we have called Teacher-Bryce No. 2 (T.B.2.) shows more clearly than any previously described specimen adhesion of the blastocyst to the roof of the cavity of the decidua at the aperture of entrance and the filling of the aperture by an outgrowth of primitive ectoderm. A similar structure was described by Herzog (1909) and by Linzenmeier (1914) without recognition of its special significance, but in 1916 Schlagenhaufer and Verocay identified it as a mechanism by which the last entering parts of the ovum itself close the aperture by which it entered the decidua. Before the writer was aware of the work of these authors he had already arrived at the same conclusion. He believes that their interpretation is absolutely sound, and congratulates them on having solved an important problem of the relations between the ovum and the mother. For this apparatus, which they called the Verschluss (“ lock,” “ seal" or “plug”), I suggest the name “ Operculum Deciduee.”
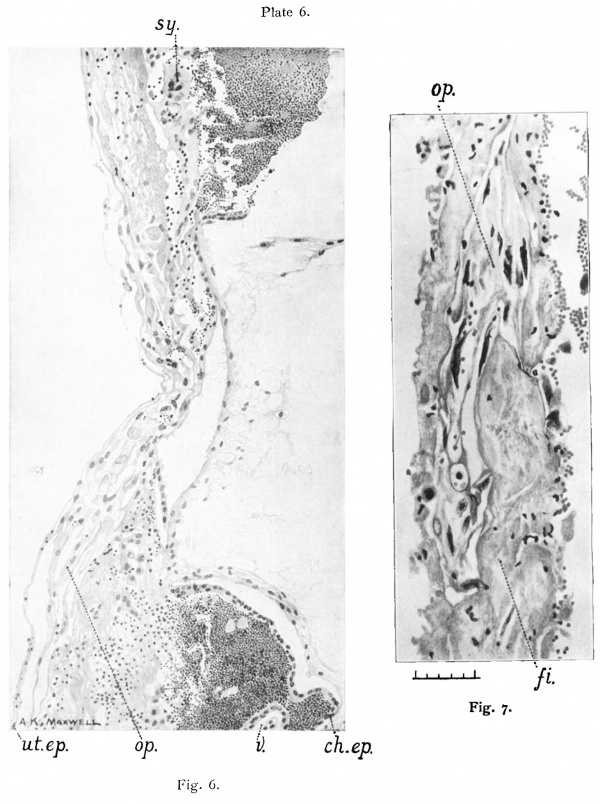
Attention may be drawn here to the illustrations from the pencil of Mr. A. K. Maxwell. These are the most faithful interpretations of the microscopic pictures and represent the finer histological details more accurately than any photograph which I have been able to produce.
Part I. The Closure of the Decidua Capsularis (Reflexa)
Description of the Teacher-Bryce Ovum No. 2.
It has seemed to the writer best to approach the subject by a description of the new ovum as it belongs to a group in which there is a considerable number of well described specimens, and regarding the main features of which there is now comparatively little difference of opinion.
The new ovum, although considerably larger than that of Peters, presents a very close resemblance to it in many particulars. The villi are longer and the intervillous space is, therefore, more developed. The characters of the trophoblast are practically identical in the two specimens. The most striking points of difference are the extent to which traces of a necrotic or at least degenerate layer of the decidua are recognizable in places almost all round the new ovum and the presence of the structure at the aperture of entrance referred to above.
The closing cap (“ Gewebspilz ” or “ Schluss-coagulurn ”) of the Peters ovum is absent, but there is one little pear-shaped clot on the surface of the decidua which is very suggestive as to the origin of that structure. It lies close to the aperture of entrance overlapping the operculum, measures 0.36 mm. in length, and seems to have arisen as the result of haemorrhage from. the implantation cavity through the thin degenerate roof. (Compare fig. 2, Pl. 2 and Text fig. 1 on p. 174.)
History of Ovum T.B. 2. It was found at autopsy at Glasgow Royal Infirmary on the 12th June, 1923.
History. P.M. No. 10902, June 12th, 1923, M. S., 36 years of age, was taken ill with severe pains iii the joints on the evening of Thursday, 7th June, 1923. In the evening of the following day she was admitted to the Royal Infirmary as a case of acute articular rheumatism and was at once put on sodium salicylate and sodium bicarbonate. The joint condition subsided rapidly and on the third day she complained of slight buzzing in the ears. The salicylate treatment was, therefore, discontinued. On the evening of Sunday the 10th she became very ill with air hunger, restlessness and tendency to delirium. This became very acute and violent and death took place on Monday, June 11th. There was a very bad history of alcoholism.
Data as to coitus and menstruation could not be obtained, but from the statement by the husband that he had no idea his wife was pregnant it seems reasonable to infer that there had been no missed period.
The post-mortem examination was made about 19 hours after death. The body was that of a big, stout woman. There were a few small petechial haemorrhages into the skin. The mammce were large but not notably pigmented. The joints were not swollen. There was practically no sign of post-mortem change. The organs generally were those of a healthy subject but showed considerable cloudy swelling and fatty degeneration and many small haemorrhages. The liver was of distinctly alcoholic type. The uterus was slightly enlarged, and on examining the appendages a large corpus luteum was found in the left ovary.[2]
The uterus was then removed with great care and opened up the middle of the anterior aspect. On cutting through the muscle it was recognized that there was a thick congested decidua. This was opened carefully and on drawing the two portions of the anterior wall apart, characteristic decidua was seen covering the posterior wall. Near the fundus and to the left of the middle line there was a small thickening of the decidua, the right margin of which overhung a deep furrow. In this thickening a clear spot was recognized indicating the presence of a very small ovum. There was a sort of halo of radially arranged blood vessels around the clear spot. The appearances suggested an ovum about 4 mm. in diameter—~a good guess. The uterus and appendages were immediately placed in Kaiserling’s formalin and salts solution.
Head. There was marked congestion of the membranes and substance of the brain and some chronic thickening of the pia arachnoid. Portions of liver and kidney were preserved in order to obtain some estimate of the degree of post-inortem change which was present.
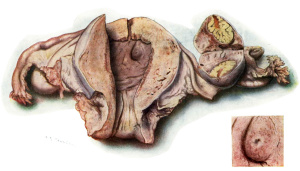
After 30 hours in Kaiserling the uterus was well washed in water and transferred to alcohol. Next day the colour l1ad come up very well, and Mr. A. K. Maxwell made the picture of the whole uterus and appendages, Plate 1, fig. I, after which the portion of diecidua containing the ovum was cut out by Dr. Teacher. An oblong about I cm. in length was taken and the knife was passed under the ovum so as to take a very thin layer of uterine muscle along with the decidua. The block of tissue included the whole of the swelling and the furrow. Plate 2, fig. 2, was painted by Mr. Maxwell at a magnification of almost exactly 16 when the specimen was in absolute alcohol. During the -clearing in xylol, the red marks on the surface of the lobule appeared distinctly as blood vessels arranged around the lighter area which corresponds to the situation of the blastocyst. Text fig. 1 shows this in a camera lucida drawing originally at X40. The paraffin block was cut by Dr. Teacher and the sections mounted in a practically perfect series by Mr. N. H. W. McLaren of the Anatomy Department. The cutting was done with an old-fashioned Cambridge Rocking Microtome, and the thickness of the section is 6.25 microns, that is 160 sections to the millimetre. Most of the sections were stained with haemalurn and eosin. Iron haematoxylin, Carmalum and Gram’s stain, Pappenheim, van Gieson and Leishman’s stain were also tried.
Description of the Ovum
On examining the decidua with a hand lens a slight depression was recognized which was taken to be the point of entrance. At the side of this depression furthest from the furrow there was a tiny blood—clot, referred to above, which became an important landmark.
During the cutting of the sections an air-bell was found in the ovum. From various considerations it was made out that this must have got in after the fixation. It has caused tearing of part of the blastocyst wall from the blood—clot in the intervillous space beside the operculum, but it does not interfere with the interpretation of the specimen (see Pl. 3). The series starts at the furrow and traverses the block in what was the sagittal plane of the uterus. The point of entrance was found to lie nearer the furrow and lower margin of the lobule than the centre of the ovum. This eccentric position is quite usual. The ovum was measured in Slide No. 36, which was approximately the equator. Over the ends of the villi its diameter is about 3.75 mm., and including the trophoblast about 4.5 mm. The blastocyst itself measures 2.6 mm. in the sagittal plane by 2.25 mm. in depth and 2.8 mm. in the third diameter calculating from the number of the sections in which the blastocyst appeared. The ovum is, therefore, slightly larger than that of Jung and Von Spee’s 1905 ovum, but slightly smaller than that of Strahl-Beneke.
The blastocyst is roughly globular in shape, but shows slight irregularities. It has a large cavity, at the borders of which there is a thin layer of mesoderm, except in the neighbourhood of the embryonic rudiment where there is a considerable thickening. The cavity has contain-ed a richly albuminous fluid, the more solid parts of which had settled towards the deep si-de corresponding to the dorsal decubitus of the body. Coagulation apparently took place after settling had occurred, and as a result of these two processes the rudiment was torn at the junction of the vesicles. Nevertheless, a very successful reconstruction was made by Bryce and McLaren. The embryo resembles closely that of Strahl and Beneke, but is distinctly younger. It is situated towards one side of the blastocyst and runs aslant along the wall of that cavity next the decidua basalis and is attached at the distant. ends of amnio-embryonic sac and yolk-sac.
The blastocyst is covered with short villi some of which show the commencement of branching. The intervillous space is full of blood which is well preserved. The cytotrophoblast extends between the tips of the villi forming a thick and fairly complete shell practically all round the ovum. There has been considerable congestion which has caused a certain amount of breaking up of the trophoblast and infiltration of blood between the cells. In places, however, notably in the neighbourhood of the point of entrance, the trophoblast shell is practically undamaged. This breaking up by the blood brings into prominence the polyhedral shape of the cells of the cytotrophoblast. At many points the trophoblast and the decidua come into intimate union, so that it is impossible to distinguish exactly where the one ends and the other begins, but over large areas of the periphery the two structures are delimited from one another by a zone of degeneration or actual necrosis of the maternal tissue. It may be said, therefore, that t_here is a transition zone (Umlagerungszone), as in Peters’ ovum, but likewise there is in places a necroticlayer asin T.B.I. fig. 3, 4 and 14. The principal feature of the decidua is the great development of the glands. The decidua cells are distinctly less advanced than around T.B.I.
At many points blood vessels open into the implantation cavity. As a general rule the agent which opened them appears to be a mass or masses of the primitive syncytium (plasmoditrophoblast). The blood vessels are of capillary size and run round the periphery of the ovum. As a consequence they usually appear as slit-lilzie cavities lined by trophoblast on the ovum side and by, endothelium on the decidual side, and they open obliquely into the cavity. Vessels of larger size have usually undergone thrombosis of their contents, but this condition is not peculiar to the neighbourhood of the ovum. It is seen throughout the decidua. figs. 3, 12, 13 and 15.
The trophoblast differs strikingly in its character on the wall of the blastocyst and stems of the villi and at the ends of the latter. In the former situation it consists of the usual two layers. The inner (the cytotrophoblast representing the future layer of Langhans or “cell layer”) Consists of protoplasm with very little indication of differentiation into individual cells. The nuclei are placed at very regular intervals and are of globular form. The second layer (plasmoditrophoblast or syncytium),definitely syncytial in character, forms a thin border in some places so delicate as to resemble endothelium. Nuclei in this syncytial layer are mostly of elongated form and lie parallel to the surface. They are of much larger size than those of the endothelium of maternal vessels and confusion between the «two structures is practically impossible. There is no sharp demarcation of the two layers from one another. The appearance in this respect differs very strikingly from what is seen in the fully developed chorionic epithelium of even very slightly later stages. figs. 6, I3 and 14.
At the ends of the villi the cytotrophoblast spreads out into masses of polyhedral cells which are considerably larger than those of the inner layer on the stem of the villus and are well defined from one another. In a villus cut lengthwise the syncytium (plasrnoditrophoblast) ends a little beyond the tip of the villus leaving the cytotrophoblast uncovered, and the latter fuses with that of neighbouring villi forming a complex structure tunnelled here and there by passages for the maternal blood. The walls of the passages thus may consist not of syncytium but of slightly flattened cytotrophoblast cells. This direct apposition between the cytotrophoblast and the maternal blood was observed by Peters, and can be quite clearly recognized in some of his figures.
The appearances generally may be interpreted in the sense that at this stage of development the two layers have become well differentiated although not sharply demarcated. We are inclined to believe that they both increase by independent proliferation. At the same time further formation of syncytium from the inner layer cannot be excluded.[3] It is clear from T.B.I that they both arise from the ectoderm, the undifferentiated cytotrophoblast of that stage being the mother-layer of both.
Karyokinetic figures were recognized in the cytotrophoblast but were not numerous. On the whole, despite the damage by congestion, the trophoblast and neighbouring maternal tissues are excellently preserved, and the whole object is exceedingly beautiful.
In all respects the villi and trophoblast present a close resemblance to those of the very well preserved ovum of Jung.
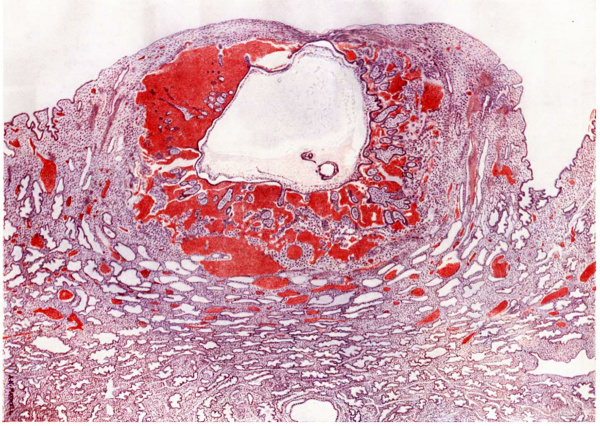
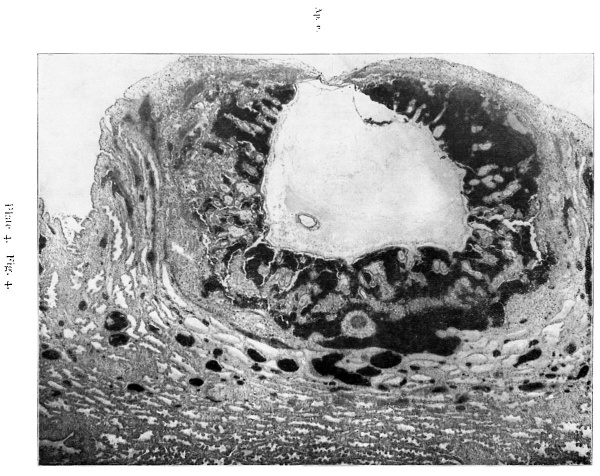
The ovum, compared with other specimens of a similar stage of development can be described as deeply implanted; that is to say, that the lobule in which it is embedded did not project much above the level of the surrounding decidua, and the lateral walls of the cavity are composed of thick, well-preserved maternal tissue. The implantation cavity occupies the compact layer and pushes down into the spongy layer. The decidua, as usual, is thinner on the top than at the sides. A considerable oentral, rather irregular, area of the roof of the implantation cavity is necrotic, while a wider area round that presents signs of degeneration.
The thin roof corresponds to the flat-looking surface shown in fig. 2. The extent of the avascular degenerate decidua is fairly accurately shown by text fig 1. Some capillaries extend further towards the centre than the blood-vessels which are represented, but only for a very short distance. In section 28.2.2 (corresponding to the cross marking the centre of the aperture) the avascular area is about 2.5 mm. and the dead area about 1.5 mm. in diameter.
The sides of the lobule are covered with uterine epithelium which becomes more and more flattened towards the top. It has disappeared from much of the degenerated area, but even where the epithelium itself has disappeared there is a continuous lamella consisting of the basement membrane, and on this there are traces of epithelium often associated with leucocytes and thin fibrinous exudate. As the point of entrance is approached this lamella becomes separated from the decidua by the outlying processes of the operculum. figs. 5, 6, and I0. Practically throughout the area of the roof, except jus_t under the aperture of entrance, the implantation cavity is lined by a necrotic layer similar to that bounding the implantation cavity of T.B.I. It is composed partly of dead decidua and partly of fibrin. Immediately internal to this in places there are mononuclear cells with deeply-staining protoplasm and slightly shrivelled appearance, which are decidua cells that have been loosened from the necrotic zone by a process. of lysis or digestion of the dead tissue. Leucocytes are present in large numbers.
In places, notably near the point of entrance, there are masses of rather lumpy fibrin with entangled leucocytes. This does not give the characteristic staining reaction of fibrin, but its nature is quite clear, transition stages from it to characteristic fibrin network being readily recognizable. Masses of the primitive syncytium are seen here and there, sometimes apparently eroding the necrotic zone. In other parts the cytotrophoblast is seen in intimate union with the necrotic layer. The general picture, apart from the peculiarity of the point of entrance, is very like what is seen (unfortunately under a very low magnification) in the beautiful photograph by Schmorl of the whole section of the Strahl-Beneke ovum.
The aperture of entrance and the Operculum Deciduce
The accompanying line figure gives the key to the description. The small, heavily outlined area is the blood-clot which is seen in fig. 2. The heart—shaped area, which it overlaps, was seen as a white figure during the clearing of the specimen, and microscopic investigation proved that it is the apex of the cone of blastocyst. which is adherent to the decidua. The cross in this area represents, as nearly as could be -diet-ermined, the centre of the operculum in section 28.2.2.
For measurement this section, which is the subject of figs. 4 and 6, was chosen. It is difficult to recognize the limits of the aperture as the margins of it are thin and degenerate, and confusion arises between fibrin. and the remains of decidua. In this there is marked contrast with the conditions in the T.B.1, in which the lips of the aperture of entrance are composed of thick, living decidua right up to the operculum nearly all round. The dimensions of the aperture of entrance, therefore, are really determined by the measurements of the narrowest part of the operculum, viz. near its base on the mesoderm. fig. 6.
As nearly as can be determined, the real orifice in T.B.2 is roughly circular in outline, and measures about 0.3 (three-tenths) of a millimetre in diameter contrasting with that of T.B.1, which is 0.125 (one-eighth) of a millimetre in diameter. Superficially it widens out crater-wise, corresponding to the spreading of the outer parts of the operculum.
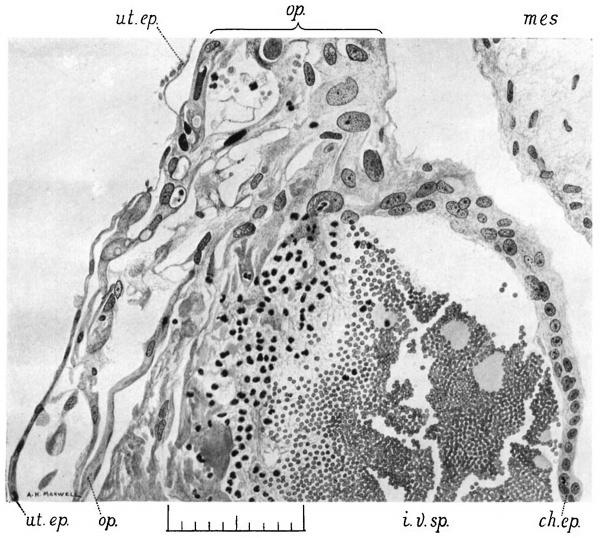
The operculum is clearly an outgrowth of the ectoderm. The wall of the blastocyst is adherent to the roof of the cavity over a roughly circular area from five- to six-tenths of a millimetre in diameter, at the margins of which it falls steeply into the implantation cavity, so that the attachment forms the flat top of a blunt conical projection of the blastocyst.
In places the ectoderm on the sloping sides of the cone has undergone slight proliferation. The characteristic chorionic epithelium becomes thicker and the nuclei become irregular in size and oval in shape, figs. 5 and 6. At other places there is no such overgrowth. At the edges of the cone and in some places within them, the ectoderm has disappeared or become degenerate, fig. 6, and there is union between the mesodermic wall of the blastocyst and the dead material forming the roof of the implantation cavity. The appearance suggests that degeneration overtook the two tissues together, so that they became fused with one another. At other points, however, the ectoderm is living and well preserved, and can be followed from the side of the cone round the edge and along the flat top into the structure in the centre. The continuity of the trophoblast, that is to say, of the ectoderm of the blastocyst wall with the operculum is, therefore, quite clear.
The operculum is composed of cells of irregular shape and size with rather ill—defined boundaries. Their character is best seen in a section passing somewhat to one side of the centre. figure 5 shows one half of the operculum in_ section 26.1.5.
The mesoderm has retracted from the ectoderm. The cells of the operculum, which were set upon it, are large and fleshy and contain large oval nuclei. Both cells: and nuclei vary much in size. This layer presents a close resemblance to the primitive cytotrophoblast of T.B. T. The cells also resemble the elongated cells of the ectoderm of the hedgehog ovum in Hubrecht’s fig. 39, which is reproduced on Plate 14.
The outer parts of the operculum consist of long sprawling cells more like the syncytium in character. They contain large elongated nuclei and there are also a few vacuoles. The outermost strands are somewhat degenerate and there are lumps of coagulated material in the vacuoles. The large size, both of the cells and of the nuclei, is realized when they are compared with the uterine epithelium.
The uterine epithelium or its basement membrane can be traced from the sides of the lobule of decidua right to the centre of the operculum. ' It. is seen in figs. 5, 6, 8 and 10 as a thin, well defined lamella separated by a narrow space from the «ectodermic cells of the operculum. In places it is difficult to distinguish it, and in the centre it appears as irregular lamellae, the nature of which can only be inferred from their likeness to the basement membrane in the better preserved region. In the figures the nature of the lamella is clearly recognizable where it consists of flattened cells with oval nuclei which are exactly like the flattened cells of the epithelium on the sides of the lobule, and just where it joins the decidua three quite definite low cubical epithelial cells are seen. fig. 10. Under these, but separated from them by a spindle-shaped decidua oell, is seen the extreme edge of the operculum in the form of a long thin cell with a long, deeply stained nucleus of large size.
From the relations between the epithelial lamella and the ectoderm, it is clear that the latter has spread out not over the surface of the decidua but into the decidua below the epithelium. This is an important observation as it provides the foundation for the theory that the last entering cells of the ovum unite with the uterine epithelium and close the breach in it in the same manner as occurs in the implantation of the ovum of the guinea—pig. See part III, section 2.
The adhesion of the blastocyst to the decidua capsularis looks quite strong at some points of its circumference, but in some sections it has the appearance of becoming loosened either by mechanical separation with formation of fibrin in the gap or by the agency of masses of syncytium growing in between it and the decidua. fig. 9, Plate 7.
It is impossible to say whether the apex would eventually have become free or not’. It does not appear to me that adhesion is necessarily pathological. In most young ova which have been described there is no such adhesion, and it seems probable that freedom of the blastocyst from the decidua capsularis is the more common result of development. It is the type which is seen in T.B. I. and Johnstone’s ovum.
The Operculum in T.B.1
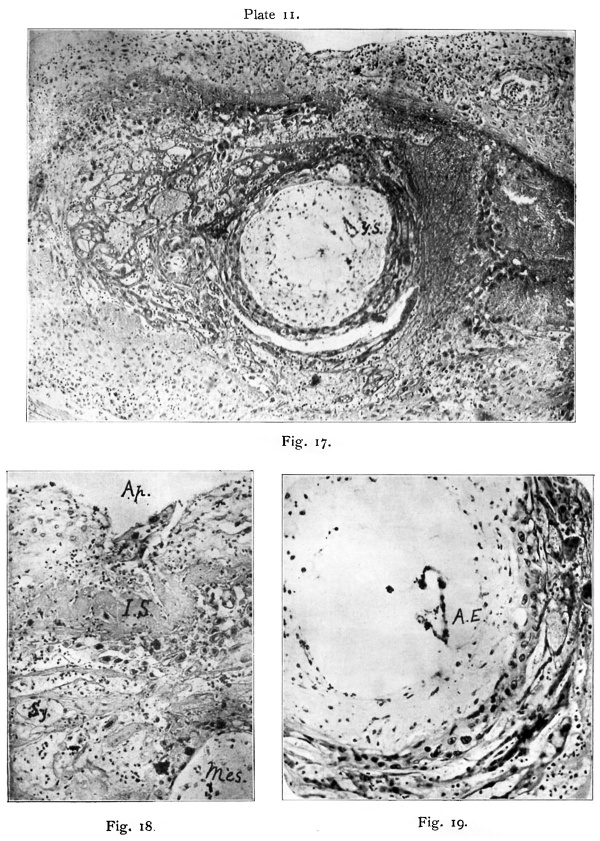
At various times in studying the original Teacher-Bryce ovum we were struck by the resemblance of part of the mass in the aperture of entrance to the vacuolated syncytium of the ovum. This, in fact, had been noted when the ovum was originally described, but Bryce and I were unable to come to a decision as to its nature or significance and eventually left it unremarked. Recent «examination, however, enables me to say with certainty that it is a mass of primitive ectoderm, and that it represents the structure in T.B. 2 which has just been described. The blastocyst of T.B. 1, as can be seen in fig. 3 of the 1908 memoir, is separated from the aperture of entrance by a thick mass of dead material, fibrinous in nature, which extends in a peculiar blunt point down into the implantation cavity. fig. 16, Plate 10. It is dense and lumpy like slowly formed thrombus, and this suggests that it was formed from slowly circulating blood doubtless when the blastocyst was being separated from the operculum. A similar plate of fibrin is found in the corresponding position in Johnstone’s ovum, and it is probably a structure which regularly forms to strengthen the decidua capsularis at and about the aperture of entrance. We will refer to it as the “internal shield ” of fibrin. The character of the tissue occupying the aperture of entrance is seen in figs. 16 and 18. It is an irregular mass of protoplasm enclosing several nuclei. There are also some vacuoles. The loose cells clinging to its outer end are rather suggestive of the remains of the uterine epithelium. The mass of protoplasrn on careful examination with high powers was found to be similar to that tissue of the operculum in T.B.2, and no doubt is felt as to its nature. ‘
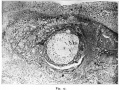
After a prolonged Search I found the corresponding point of the blastocyst at the place where I should have looked for it at first, viz., opposite the pointed end of the endodermic vesicle, as shown in fig. 17, Plate 11, and text fig. 4. It has retracted to some little distance from the aperture of entrance in an oblique direction. The photograph fig. 17 shows a little depression in the necrotic zone which is suggestive of an inner, crater-like mouth of the aperture of entrance, but is in reality an area of solution of the internal shield by syncytium. The supposed point of attachment on the wall of the blastocyst is marked by a slight protrusion of the ectoderm, but there is no thickening. An appearance suggestive of thickening is present, but is due to the fact that the area is situated near the apex of a bulging of the blastocyst wall and has been cut somewhat obliquely. In the wax model this bulging is seen to be a fairly narrow ridge, and is so represented in the transverse section in text fig. 4, pl. 205. Th-e only clear difference between this point and the rest of the blastocyst wall is the manner in which the syncytium leaves bare a minute area of the cytotrophoblast. Although there is so little characteristic histologically there can be little doubt, from the relations of this area, that it. represents the point at which the blastocyst was attached in the aperture of entrance.
On examining all the sections in which the operculum occurred it was found to be of quite considerable dimensions. It extends through I2 sections of 7 ,u thickness, and, therefore, measure o.o84 mm. in diameter. It has a flat base of roughly semi-circular shape resting on the internal shield of fibrin, Possibly it represents only about half of the operculum, the rest having been detached and its place taken by the fibrinous exudate which occupies the rest of the aperture. fig‘. 16 on P1. 10.
2 mm of Schlagenhaufer and Verocay
To finish the account of the operculum the ovum described by Schlagenhaufer and Verocay must be given first place. It is slightly larger than that of Peters, the blastocyst measuring 2 mm. by 1.6 mm. by 1 mm. The embryo is very similar, and the authors group their ovum with that of Peters, whereby only those of Miller and Linzenmeier are reckoned as younger, while the Teacher—Bryce ovum is cast out of the list of normal ova as “ vollig pathologisch.” Schlagenhaufier never did deal in half measures.
The villi are very short, and they terminate in long-drawn—out processes of trophoblast which is largely syncytial, and stretches out through a relatively large implantation cavity which is distended with blood. The ovum was obtained at the post—mortem examination of a married woman, 35 years of age, who committed suicide by swallowing lysol. Death occurred three hours later, and the examination was made 14 hours after death. The mucous membrane presented the usual thick, furrowed appearance. From the posterior wall projected a small prominent lobe about 5 mm. in diameter and of dark red colour. fixation in 10 per cent. formalin.
The illustrations show a fine specimen, but it is quite clear that there has been enormous congestion which seems to have distended the intervillous spaces and stretched the trophoblast to an extreme degree instead of breaking it up, as occurred in T.B.2 in which the congestion was much less. One would doubt whether sucli long drawn out processes represent the normal picture of the trophoblast at any stage.
The authors comment that there is one feature of the ovum which stamps it as a remarkable specimen. In sections which occur towards the centre of the ovum, the blastocyst, which had been of elliptical outline, assumes the form of an amphora. This change of shape is produced through adhesion of the mesodermal and ectodermal wall to an area at the outer pole of the implantation cavity. “On both sides this——-to continue comparison with the amphora—ampho1'a-neck is bounded by the pre-villous blood spaces. One sees quite clearly that the tissue filling the narrow area has arisen from the ectoderm. The series shows that the ectoderm, which heretofore had consisted of a single layer of cells, passes over into this plug of peculiar epithelial cells which have a strange nail-like shape rising vertically [from the blastocyst wall] and without definite cell boundaries. 'I‘he nuclei are mostly large and clear, the protoplasm homogeneous. A few Ieucocytes are scattered about. This remarkable structure is shut off from the cavity of the uterus by a clot of fibrin, leucocytes and red blood corpuscles. It is further remarkable that in this area, which is found in 41 sections, and measures 0.328 mm. broad, 0.36 mm. long, and is about 0.2 mm. thick, there is no uterine epithelium. Also, the mesoderm takes no part in the formation of this plug of tissue.” Elsewhere the “ ectoblast,” which apparently is here used with the meaning “ cytotrophoblast,” consists of a one, two or many celled layer surrounding the whole blastocyst. The discussion of this remarkable structure is worth quoting in full. “ Before all must be considered the little area which stamps our ovurn as remarkable. There is no doubt that we have here before us the portal of entrance of the ovum into the uterine tissue, and that here is clearly revealed to us how the aperture due to the invasion by the ovum is closed. We see clearly that the ovum itself and in special its ectoderm attends to this closure by the formation of a plug of epithelial cells. As the stopper of a bottle, as the keystone of a vault, so this outgrowth of the ectoderm closes the entrance to the implantation cavity with its precious contents, and only like the sealing wax over the cork there follows a formation of fibrin and blood clot on the surface as a sort of caulking.” In a footnote the authors mention how the accidental crushing of a section by the lens showed the strength of the “ Verschluss.” The whole slice of ovum went to pieces and left the operculurn and the process of the blastocyst together like the neck of a broken flask. The firmness of the adhesion is also shown in TB. 2 by the way it resisted detachment by the air bell.
The authors ask why other investigators have not seen this kind of closure of the decidua; and reply that it is partly because their ova were too old, and partly because, once the ovum has sunk below the surface and become completely Covered by decidua the seal or “ Verschluss ” is no longer necessary and disappears or becomes so much altered as to be no longer recognizable. They point out that most probably Herzog had seen the “ Verschluss,” but they have evidently found some difficulty in understanding Herzog who did not recognize the structure which is so clearly shown in his illustrations as the seal of the point of entrance. Herzog, on the contrary, believed that the ovum entered by quite a different channel, vi7.., a long tunnel which started outside the boundaries of the implantation cavity and passed along under the surface of the decidua and almost parallel to it until it reached the place where the ovum was going to settle, They also refer to the occurrence of very similar structures in the ova of Jung and Linzenmeier, and in an ovum belonging to Professor Peters which has not been published in detail. In conclusion they say, “ we might, therefore, believe that the discovery which we have set forth is not a peculiarity of our own but that this Schutz-deckel [safety cover], which the ectoderm itself prepares, is probably of normal occurrence in the development of the ovum and persists perhaps only until the ovum is entirely surrounded by blood lacunae.” This discovery is brushed aside by von Mollendorff as “ etwas hypothetisch,” but in our opinion, it is of the highest importance and is the solution of the problem of the closure of the decidua reflexa (capsularis). It will be referred to again under Implantation,
The Ovum of Lmzenmeier
The ovum of Linzenmeier is at a stage between T.B.1 and Peters but nearer to the latter. It is remarkable for the very small size of the blastocyst and the structure at the aperture of entrance. The measurements of the blastocyst, taken from the junctions of mesoderm and trophoblast, are practically the same as those of T.B.I, viz., 0.75 mm.xo.6I5 mm. ><o.525 mm. The external measurements are about 1.05 mm.>< 09 mm. It was found in site in the uterus which had been excised on account of persistent metrorrhagia in a multipara. There are no accurate data as to possible age. It was fixed immediately in IO per cent. formalin solution. The ovum was situated on the posterior wall somewhat to the left of the middle line. The endometrium was thick and showed the usual furrows. In section, there was no definite division into compact and spongy layers and there were no proper decidua cells, but the decidual reaction was distinct around some of the blood vessels. (Edema of the superficial layers was the chief feature. The glands, however, were greatly hypertrophied and were in full secretory activity. The author points out particularly that the secretion was not mucous but albuminous and apparently contained glycogen, there being much red staining with Best’s glycogen stain.
The ovum was embedded in the usual somewhat prominent lobule. The epithelium is flattened over the surface of the capsularis, but is absent only from an area 0.525 mm. in diameter in which alts the “ closing coagulum-.” This is shaped like a biconvex lens 0.225 mm. in thickness and “its edges are fitted into the opening like a lens in its mounting.” The outer convexity projects above the level of the mucous membrane. Its inner surface is continuous with the trophoblast. It consists of fibrin and blood clot. “From the edges spindle-shaped and stellate cells have grown in—the beginning of organization—and the base is in part permeated by trophoblast cells.” The wall of the blastocyst rises to it in a broad, flat conical projection very similar to that in the T.B.2. Linzenmeier apparently regards the closure as essentially due to the coagulum, and the relation of that structure to the wall of the blastocyst is an interesting peculiarity which he does not attempt to interpret.
For purposes of Part II the following is noted here.
The mesoderm projects in a few short villi which are covered on the sides by the usual two—layered epithelium. “ From the tips of the villi project broad columns of cells which spread out laterally so that they frequently unite with neighbouring columns." The outer surface of the trophoblast shell, which is thus formed, is in contact with maternal tissue. The spaces between it and the villi and surface of the blastocyst contain well-preserved blood and constitute the beginnings of the intervillous space which communicates with maternal capillaries through gaps in the trophoblast shell.
7 mm of Johnstone
All of the ova above described as exhibiting an operculum, except the original Teacher-Bryce specimen, show it with a broad adhesion of the blastocyst to the decidua capsularis. I, therefore, approached Dr. Johnstone, whose uterine ovum has the apex of the blastocyst free from the decidua. Dr. Johnstone most kindly placed his specimens at my disposal. The little ovum is poorly preserved from having been obtained too long post—mortem, and the decidua capsularis and the blastocyst itself had both been ruptured by pressure during removal of the uterus. Nevertheless, it is a valuable ovum and thoroughly reliable conclusions can be drawn from it upon many points in spite of these defects.
The ovum is about the same size as T.B.2. It is embedded in a small prominent lobule of decidua which is deeply congested. The decidua capsularis is thin and degenerate. The oval blastocyst, which measures 2.72 x 1.66 x .84 mm., is ruptured towards the base of the lobule. This involves the immediate neighbourhood of the embryonic rudiments, and has probably caused the separation of the two sacs, which was one of the difficulties Johnstone found in interpreting the embryonic structures. The blastocyst is oval in shape, covered all over by villi of considerable length with occasional branching, and has a very thick trophoblast shell like that of the Strahl-Beneke ovum. Post-mortem changes had produced detachment of the villi from the decidua through the trophoblast, but the usual features, such as infiltration of the decidua and connexion with maternal vessels are readily made out.
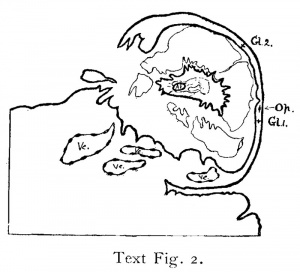
The embryonic rudiment consists of two vesicles, both of which are damaged. It was not felt possible to distinguish which was amnio-embryonic and which yolk-sac. One of the suggested explanations was that they represented twins. On examination of the vesicles with high power, the writer found that one of them, the second in Johnstone’s description, was provided with a well defined basement membrane (membrana prima of Hensen), the cells inside which could be recognized as columnar in shape. In T.B.2 and Strahl-Beneke the amnio—embryonic vesicle likewise is provided with a basement membrane, while the yolk-sac is not. It is in my opinion, therefore, clear that Johnstone’s No. 2 vesicle is amnio-embryonic. The vesicles are attached to the wall of the blastocyst by their opposite ends (see diagram) in the position which Bryce shows to be normal. On the strength of the relationship observed between the end of the yolk-sac and the operculum in both T.B.I and T.B.2, search for the operculum was directed to the area of the capsularis opposite the end of the yolk-sac with immediately successful result. A mass of long, flattened cells with large deeply—staining nuclei, degenerate but characteristic, was found. This is embedded in the thin degenerate capsularis, and is mostly cover-ed externally by a layer of degenerate material enclosing a few leucocytes. The internal appearance is, however, more significant, the remains of the operculum being shut off from the implantation cavity by a thin layer of lumpy material which quite clearly corresponds to the shield of fibrin which is such a characteristic object underneath the aperture of entrance of T.B.1-. The structure extends through 33 sections (I7.2.5 to 20.1.1), and, therefore measures in this direction 0.33 of a millimetre. In section 18.2.5 it measures 0.4 mm. In spite of its degenerate character there can be no reasonable doubt as to the correctness of the identification of it as the operculum. It is easily distinguished under high powers from invading processes of syncytium. The latter never extend through more than three or four or at most six sections and are better preserved. Its position relative to the blastocyst and embryonic vesicles is shown in text fig. 2, which is traced from a photograph of section 17.2.5. It lies very diagonally across the capsularis as if it had be-en drawn out unevenly and much flattened. Without reference to the opercular structures in T.B.2 its nature could not possibly have been made out.
The importance of this specimen lies in the fact that the apex of the blastocyst is free from the decidua capsularis, and, to judge by the degeneration of the operculum, it has clearly been separated from it for some time. Probably the detachment occurred at much the same stage as in the case of the T.B.1. It was doubtless a larger operculum. No trace of the point of attachment could be made out on the blastocyst,
Thus in three specimens of which a complete series of sections has been available three stages of the operculum have been found. Confirmation is thereby provided for the theory of Schlagenhaufer and Verocay that the “ Verschluss ” or operculum is an invariable constituent of the human ovum, and that it is a temporary structure which commences to disappear at a very early period. Most probably what is seen in T.B.1 and Johnstone’s ovum represents the normal behaviour of the blastocyst, viz., that it is early separated from the decidua capsularis. Under the remains of the operculum a pad or shield of fibrin is formed which seals the inner mouth of the aperture of entrance securely. This, like the cellular operculum itself, becomes flattened out and disappears in the degenerating capsularis, and also no mark is left on the blastocyst. The usual bald area at the apex might be due to the separation in such cases as the T.B.2 or Schlagenhaufer and Verocay, but it seems more probable that it is due to atrophy of the villi, which, where they are in contact with the degenerating centre of the decidua capsularis soon cease to grow.
The adhesion of the blastocyst to the decidua capsularis is not necessarily an abnormality. It is rather to be regarded as one of those variations which are so commonly observed in the early ovum.
Ovum “ Sch.” of Mollendorff
Description of the operculum leads up to consideration of the ovum “ Sch.” of von Mollendorff, which is in some respects the most interesting of all the new specimens. The account of the ovum is most painstakingly and thoughtfully worked out and the full details which are given form most valuable data for comparison. There are also discussions of other human ova and of the comparative anatomy of embedding and development.
The ovum “ Sch.” is described as the youngest known abortive ovum. It was found in a decidual cast of the uterus.
History. The patient was a healthy I-para. On the 5th of January, 1921, between 5 and 6 p.m., abortion occurred after about half an hour of pains in the abdomen. The whole specimen was placed in formalin solution 24 hours later and subsequently fixed again in sublimate, and the result is quite good.
Menstruation was regular and of four weeks’ type. Last period began Dec. 2, 1920; next was due 011 Dec. 30, 1920, and was missed; abortion occurred on Jan. 5, 1921.
The history of coitus given by the husband was Dec. 6, 1920, that is 30 days before abortion; Dec. 23, 1920, that is 13-14 days before abortion; Jan. 2-3, 1921, that is 3-4 days before abortion.
Following the reckoning of Bryce and Teacher (1908), V011 Mollendorff deducts 24 hours for the period up to fertilization and 24 hours for the abortion, and gives the-age of the ovum as 11-12 days, that is two days younger than TB. 1. Fertilization occurred 20-21 days after the start of the last period, and for the date of implantation he accepts the opinion of Graf von Spec, 1915, that a free stage of 10 days’ duration is most probable. The history, therefore, closely resembles that of T.B. 1.
The ovum was found in a little oval area slightly projecting from the decidua which was marked by shallow furrows. It is enclosed in decidua which shows practically identical characters with that of TB. 1, the decidua cells being young but characteristic and more advanced close to the ovum. Little more than the compact layer had been shed and the condition of the glands is also like that in T.B. 1. The ovum lies in a rounded cavity in the compact decidua which is bounded by a narrow necrotic zone. The deciclua capsularis is relatively thick and well preserved and there is an aperture of entrance very similar to but rather broader and shallower than that of T,B. 1. At the deep end of this crater-like depression a cellular mass forming the pole of the ovum is attached to the decidua by a mass of fibrin in which it is embedded as in a collar.
The author regards the cellular mass as the embryonic rudiment. From it the blastocyst projects as an elongated body of thick club-shape obliquely into the implantation cavity which is filled with blood and trophoblast. Maternal vessels open into it at various points. The trophoblast is composed largely of well defined cells having the character of cytotrophoblast. There is a considerable amount of syncytium which is principally at the periphery.
The dimensions of the cavity in the decidua are 1.44 mm, by 1,00 mm. compared with 1.9 mm. by .9 mm. by 1.1 mm. in T.B.1. The long narrow‘ blastocyst measures 0.64 mm. in length by 0.32 mm. in breadth, and 0.14 mm. in the third dimension estimated from the sections. The blastocyst in 'l‘.B.1 measures 0.77 mm. in the longest diameter which could be found, but this is greatly affected by an irregular projection at one end of the blastocyst, and probably the shorter measure given, viz., 0.63 mm. would more fairly represent its real diametric measurement.
In its dimensions, therefore, the ovum “Sch.” is considerably smaller than T.B.r, and Von Mollendorff assumes that it is considerably younger. He also regards it as younger than “ Miller ” and “ Kleinhans.”
The most striking resemblances between T.B.1 and “Sch.” are (I) the presence of a complete necrotic zone which forms the lining of the implantation cavity; (2) the large size of that cavity compared with the blastocyst; the presence of an irregular trophoblast straggling through that cavity from the blastocyst wall to the decidua; and (4) the absence of invasion of the decidua by the foetal elements. The points of contrast are (1) the shape of the blastocyst; (2) the absence from its interior of a structure comparable to the embryonic rudiment; (3) the structure of the mesoderm; and (4) the differ-ent cellular character of the trophoblast. The long club-shaped blastocyst has some resemblance to the long thin blastocyst of the guinea-pig about the 9th or 10th day. It is completely filled with mesoderm, which presents a denser and more fibrous Structure than the mesoderm of the T.B.i Von Mollendorff interprets this as possibly the result of pressure of the surrounding tissues upon the invading ovum. The shape of the blastocyst. however, would have been altered into conformity with the globular shape of the later stages as the pressure was released by development of the implantation cavity. He, therefore, regards this as the younger stage of the mesoderm, and the evolution _to the later stage would have been accompanied by a loosening of the structure and an increase of fluid in its meshes. The cells of the mesoderm are of two types—(I) embryonic connective-tissue cells, and (2) round cells having peculiar foam-like structure which suggests that they had contained fat.
Von Mollendorff refers to the amnio-embryonic vesicle and yolk-sac of .T.B.I as “ Zell-strange,” from which the embryonic structures of later stages could not be derived, and specifies that there are no such “ Zell-strange ” in the mesoderm of his ovum. Von Mollendorff, like some other critics of the Teacher-Bryce ovum who have not seen the specimen, has obviously quite failed to appreciate the striking and definite character of the structures thus referred to.
The body which Von Mollendorff regards as the embryonic rudiment (Embryonalknoten) is _situated in the deep part of the aperture of entrance embedded in a collar-like mass of somewhat dense lump-y fibrin. It is composed of relatively large cells of varying size, and showing some difference in staining reaction and nuclear form. It is situated on the extreme outer pole of the blastocyst, and is continuous at its margins with the trophoblast covering the rest of the blastocyst. It is roughly of hemispherical shape and very well defined. The cells Composing it are somewhat degenerate, and Von Mollendorff regards it as perhaps a somewhat degenerate remains of the embryonic rudiment. To the writer it is clearly the remains of the operculurn, and the Collar of fibrin around it is the “ internal shield ” of fibrin.
The trophoblast of the “Sch.” ovum consists principally of well-defined cells somewhat irregular in size and shape, but generally polyhedral. These form thick columns. stretching out from the wall of the blastocyst through the implantation cavity. In places they are mingled with masses of syncytium (plasmoditrophoblast), and at the outer margin of the ovum there is more abundant syncytium both in the form of solid masses and spun- out into vacuolated networks like those of T.B.I. Some of the syncytium shows the striated border as it is seen at later‘ stages of development.
As to the cytotrophoblast, fig. 15 shows a mass of cells not very different from those forming the trophoblast shell of T.B.2 or Peters. The photograph fig. 8 shows the cytotrophoblast projecting from the blastocyst in thick columns with syncytium on either side of them. There are even suggestions of mesodermic villi. Von Mollendorff claims, and in this he is followed by Grosser, that this is a first generation of cytotrophoblast which changes into plasmoditrophoblast producing the condition seen in T.B.I. To me this is a reversal of the order of things. The cytotrophoblast in ovum “ Sch.” is a more differentiated tissue than that of T.B.1. The picture, which is produced, is strongly suggestive of the result which would be attained by the formation of cellular villi from the primitive cytotrophoblast of T.B.1 according to Bryce’s theory of 1908, as will be discussed below.
The writer is forced to the conclusion that Von Mollendorff has erred in taking for the embryonic rudiment a structure having a totally different function and situated at a totally different part of the blastocyst from that which the embryo would naturally occupy. In our opinion, the ovum is rightly described as an abortive ovum, the embryo being entirely absent, doubtless having failed to be formed.
The history presents some difficulty if one would regard “ Sch.” as older than T.B.I, but it is probable that all the stages from Miller and Kleinhans to T.B.1 and “ Sch.” are gone through very rapidly, perhaps at most within 24. hours, and that great variations occur in the rate of development. The exact age of the specimens, therefore, does not matter so much as their relative ages, and these should be judged not by considerations of size hr history only, but as Von Mollendorff indicates, by the state of development of the component tissues. In conclusion, the structure of the trophoblast and mesoderm strongly suggests to us that the Von Mollendorff ovum is slightly the older, and is, in fact, one of the much desired stages between T.B.1 and Peters. As that it is an ovum of great value, and probably reveals a normal stage in the development of the cytotrophoblast following the actively destructive plasmoditrophoblastic stage of T.B.1.
Part II. The Trophoblast
The Teacher-Bryce Ovum N0. 1 (T.B.I).
Prior to the discovery of von Mollendorff’s “ Sch.” the original Teacher-Bryce ovum was the only one of the stages earlier than Peters, of which there was a complete series excepting Leopold’s (1906), in which there is no embryo.
The history of the ovum has been often quoted. It was found in a decidual cast aborted 10% days after the missed period. Fertilization appears clearly to have resulted from a single coitus 16% days before its expulsion, and the abortion seems to have been precipitated by coitus about 1% days before expulsion. Bleeding, like the commencement of the period, was noticed about seven hours after this coitus. About 14 months later a healthy boy was born to the parents. The ovum was fixed in alcohol. It had lain for about 22 hours in a mixture of blood-clots and urine. This has produced consi-derable yet surprisingly little maceration of the decidua. It is a very striking fact that the ovum itself and the decidua Close to it are much better preserved than the rest, and present most lively pictures of cellular activity.
The principal features were summarized in 1908 as follows :—
- The blastocyst is completely enclosed in decidua except at one point, where there is a small gap closed by a mass of fibrin and leucocytes. The wide gap closed by the mushroom-like mass of fibrin and blood-clot seen in Peters’ ovum is entirely absent.
- The ovum lies, bathed in blood, in a relatively large implantation chamber, with the walls of which it is not united. There is no interlocking or mixing of maternal and foetal tissues. The innermost layer of the decidua lining the cavity is in a state of advanced coagulation necrosis, and this, together‘ with a certain amount of fibrinous deposit, forms a layer of dead material which is practically complete except at one or two points where blood vessels have been opened up, and at one end where a haemorrhage has broken into the implantation chamber.
- The wall of the blastocyst consists of an inner lamella (cytotrophoblast or cell layer) composed of cells rather ill-defined from one another, and continuous externally with an extremely irregular formation which has definitely plasmodial characters (plasmodi-trophoblast). This forms a straggling reticulum, the meshes of which are filled with maternal blood (primitive blood lacunae). There is no protrusion of the cytotrophoblast into the plasmodi—trophoblast strands.
- The cavity of the blastocyst is filled by a delicate tissue having the characters of mesenchyme. There is no cleavage of this early mesoblast into a parietal and a visceral layer; it does not form a distinct lamella round the wall of the cavity; and there are no protrusions from it representing future mesoblastic villi.
- The embryonic rudiment is represented by two eccentrically placed vesicles slung in the mesenchyme by fine protoplasmic threads. They are quite separated from the wall of the blastocyst by mesenchyme, and the cells formingthe two sacs have definite and different characters, but inter Se show no differentiation. The cells of the larger (amnio—embryonic) vesicle are cubical; those of the smaller (endodermic) vesicle are flattened.
To this description, which in the main is correct, a few emendations and many additions must now be made. The aperture of entrance has already been described. As regards the implantation cavity, there is perhaps rather more union of the ovum with its walls than we believed. In places, e.g., at the lower end of fig. 3 of the original memoir, the syncytium of the ovum is seen in continuity with the necrotic border of the maternal tissues. In view of the firmness of the somewhat similar adhesion between the operculum and the maternal tissue, it seems possible that this is a fairly strong adhesion between the ovum and the wall of the cavity —an anchoring strand. In other parts, however, similar masses of syncytium appear to have separated from the necrotic layer when vacuolation took place, and it is probably a temporary adhesion.
There is no inter-locking or mixing of living maternal and foetal tissues as in the border zone of later stages. The only penetration of the maternal tissue by the foetal is by the processes of syncytium seeking blood-vessels, as will be described in detail below.
We have rather exaggerated the amount of the spun-out vacuolated syncytium or plasmoditrophoblast to the neglect of the solid masses. The latter predominate at the upper end of the implantation cavity, the former at the lower end, probably indicating that the vacuolation was taking place at different times in different parts of the specimen. The vacuoles are seen in all stages in the syncytium. They appear first-as little rounded bodies lightly stained blue, and not unlike nuclei but easily distinguished from them. These become cavities containing granular debris, and, lastly, cavities containing blood. -Our interpretation of this as the evolution of the secretion within the syncytium has been generally accepted. These cavities, however, have nothing to do with the intervillous space, an opinion which various commentators have erroneously attributed to the writers.
The striated border could not be made out on the syncytium. It is certainly absent from the masses which are invading the decidua in search of vessels;—these have clean-cut margins.
Much of the highly vacuolated and spun—out syncytium shows by its excessive affinity for eosin and the poor staining properties of the nuclei that degenerative changes have occurred.
The formation of the inter-villous space. The origin of the inter—villous space is bound up with the development from the stage of the Teacher-Bryce ovum to that of Peters’ ovum.
In 1908 Bryce examined carefully all the sections for the beginning of villi, and all that he found was a few little bud-like processes of the cellular layer of the trophoblast, but the important fact which he observed was that these were not growing out into the existing syncytial processes, but rather between them. His conclusion was that the great syncytial (plasmoditrophoblastic) formation had nothing to do with the villi and intervillous.space of the later stages, but was in all probability a temporary formation which disappeared when its function of excavating the implantation cavity had been performed. Masses of vacuolated syncytium are seen in the Peters’ ovum and others of similar age, but they are small relatively to the amount of the cytotrophoblast. They are situated-mostly at the periphery of that structure, and signs of degeneration have been observed in them, just as in T.B.I. The purpose of the excavation of the large space filled with maternal blood is presumably to provide room for the next development, viz., the formation of a thick layer of trophoblast principally cytotrophoblastic in type.
This takes place by proliferation and differentiation occurring practically all over the blastocyst. Proliferation would occur especially at the points where the mesodermic villi were going to form, while-on the areas of the blastocyst between these differentiation would predominate. In the latter areas the very primitive and irregular cytotrophoblast of the Teacher-Bryce stage would differentiate in the direction of Langhans’ layer with a thin Covering of syncytium, and this two-layered epithelium would extend along the sides of the developing mesodermic villi. The primitive cellular villi would develop as masses of cells having the general cellular characteristics of the primitive cytotrophoblast, although tending to become more regular. This would explain the character of -the extensive trophoblast shell which consists of polyhedral cells presenting greater resemblance to the cytotrophoblast of the Teacher-Bryce ovum than to the actual Langhans’ layer of the later stages. The primitive cellular (cytotrophoblastic) villi then would push the primitive syncytium before them and eventually burst through it, and, growing out laterally, would coalesce with one another. By this lateral growth a shell of cellular trophoblast is formed in which many openings are left corresponding to the orifices of the maternal blood-vessels. At first considerable spaces are left outside it to which the name “ pre-villous spaces ” used by Schlagenhaufer and Verocay would be not inappropriate. As union and the formation of the border zone (“ Umlagerungszone ” of Peters) took place these spaces would be reduced to narrow cavities lined on the outside by maternal endothelium and on the inside by the trophoblast. In the later stages, when the trophoblast becomes reduced to the masses at the tips of the anchoring villi, the distinction between the pre-villous and intervillous spaces would disappear.
The plasmoditrophoblast or syncytium probably differentiates all over the surface of the blastocyst from the outer parts of the primitive cytotrophoblast and comes to form the characteristic thin syncytial sheath of the villi and the internal floor of the intervillous space. This would be the definitive or permanent[4] syncytium in contra-distinction to the primitive or destructive syncytium,[5] which gradually disappears.
The Peters’ ovum shows the stage when the intervillous spaces are very small. Further development follows automatically as the villi grow. The account here given of the formation of the intervillous space agrees in principle with those given by Frassi, Jung, Linzenmeier and johnstone, except in so far as they were not under the necessity of explaining the first advance from the stage of T.B.1.
On the question of a circulation around the young ovum. There can be no doubt that a gentle but efficient circulation flows through the intervillous space, but what occurs in the implantation cavity or pre-villous space? An attempt was made to answer this question by further study of T.B.1.
In the original memoir it is mentioned that blood-vessels actually opened into the implantation cavity only at a few points. On re-examination, this was found to be correct, but there are also many points at which blood-vessels enter into relations with the cavity but open connexion between their lumen and that of the cavity cannot be made out. At such points the wall of the implantation cavity, including both the necrotic layer and the living decidua immediately outside, presents the appearance of having been opened up and dissolved, and in the gaps thus created a mass or masses of syncytium are seen which appear to be burrowing towards, and in some cases actually attacking, the vessels (figs. 20 and 21. Compare photos 22 and 23). The picture varies very greatly, but these two figures show two clear types or stages of the process. In one the endothelial tube is seen to be separated from the other decidual tissue so that it lies free among the débris as if dissected out by a process of histolysis. The lumen of the endothelial tube is occupied by a thrombus. In the other figure the endothelial tube is seen with a mass of syncytium pressing into it, but not yet breaking through, and the blood in the lumen is fluid, but at the end of the figure the endothelium has disappeared, and, as can be confirmed from other sections, there is a gap in the wall of the capillary which is filled by thrombus.
Between the two masses of syncytium in this figure a streak-like arrangement of the blood is seen. This appearance is seen in the neighbourhood of a number of invaded vessels. fig. 25. There seems to have been a flow of blood from the vessel into the implantation cavity pushing the syncytium aside. To a pathologist these are clearly hemmorrhages taking place into that cavity. Except in a very few instances thrombosis has followed at the gap in the vessel wall, but it has not occurred within the implantation cavity.
The absence of an open persist-ent communication between the vessels and the implantation cavity is difficult to understand, and the writer suggests that it indicates that the opening of the blood vessels is very like a morbid process at this stage, and that the natural reaction against invasion by an alien body takes place, viz., thrombosis. The result, on this theory, is a blood chamber filled by hemmorrhages rather than a circulation.
Yet this is not the full explanation, for there is one large and distinct open mouth of a blood—vessel. This is the vessel whose communication with the implantation cavity is shown as fig. 5 on Plate 5 in the original memoir, and as fig. 24, Plate 13, in the present one. The picture shows the vessel mouth towards one side. Actually in the middle it is considerably wider and forms a relatively large gap in the necrotic layer. It is shown in correct proportion in text figs. 4and 5, p. 205. Following this orifice through the series, it is found that it is the opening between the implantation cavity and the large venous sinus which is such a characteristic structure underlying most young ova. A mass of syncytium, surrounded by uncoagulated blood, hangs down into the mouth of the sinus. The endothelium at one side of the mouth is well preserved, but has disappeared from the other side where the syncytium lies in apposition to the necrotic tissue. There is no suggestion at this point of a flow of blood, into the implantation cavity.
If‘ my interpretation is right, this vessel represents the main drain of the implantation cavity into the maternal veins. The blood was flowing into the implantation cavity in jets from many little vessels situated at various points on the roof and sides of the cavity, and was flowing out by the large sinus at the base. As these vessels are all very small it is impossible to be sure from their microscopic characters whether they are arterial or venous capillaries, but the direction of the blood flow from them is clear. The heemorrhage referred to in paragraph 2 quoted on page 187 as having broken into one end of the implantation cavity is really the inflow from several vessels in that neighbourhood. figs. 17 and 25.
The venous sinus is filled up to quite near the implantation cavity with red thrombus, and strands of it extend into the implantation cavity. The explanation of this is that the sinus has burst a little distance below the margin of the implantation cavity, producing a relatively extensive haemorrhage into the decidua below the upper half of the implantation cavity, text fig. 5. There is no sharp break in the vessel wall, but rather an opening up of a considerable area of it. Doubtless the sinus had been greatly distended by congestion. l\Iost probably thrombosis followed immediately upon this rupture and effusion into the tissues, and it finished the circulation such as it was. The haemorrhages which have occurred into the surrounding decidua seem to indicate that there had been a rising congestion of the uterus of considerable intensity. Text fig. 5 (p. 205) and fig. :7.’
It seems to the writer most probable that the ovum was in a very early stage of its connexion with the maternal circulation. Only two or three vessels, or perhaps even only one, were actually .in free open communication with the implantation cavity, that one being, however, the main outgoing channel to the underlying venous sinus. Then the congestion due to the untimely coitus led to heemorrhage from, and thrombosis in, the great sinus, and abortion commenced.
This is, of course, a speculation, but it is in line with the phenomena of abortion due to trauma at later stages of development. If it be correct, it would mean that the circulation stopped suddenly, and all activity came to a standstill in the ovum-. This would. explain the stirprisingly good preservation of the ovum and the signs of lively activity in it such as the karyoltinetic figures and the phenomena of invasion of blood—vessels by the syncytium which have remained fixed for us to see as they had been just prior to its death. Likewise. it suggests the probability that the ovum is normal.
On comparing the relations of the trophoblast to blood-vessels and glands in T.B.1 and T.B.2 very striking contrasts emerge. In T.B.1 the invasion is by primitive syncytium which dissolves an area of the necrotic zone and opens up and destroys the vessel and the living tissue around it. On the other hand the gland No. 1 in text fig. 3, p. 204 is allowed to stand out unbroken into the cavity. Invasion of gland is not essential at this stage. Invasion of vessels is purposive, the opening of blood-vessels and bleeding from them into the implantation cavity being essential to the survival of the ovum.
In later stages glands are destroyed, but this is also incidental.
In T.B.2 the relations of the trophoblast to the vessel are very different. In most instances the vessel is opened by syncytium, but in some cases the cytotrophoblast seems to be the active agent. In either case there is no appearance of solution of tissue round the endothelial tube as in T.B.I, but instead there is a growing of the trophoblast along and around the endothelial tube and a quiet replacement of the endothelium by the foetal tissue, figs. 12 and 13. The result of this is not hzemorrhage but the establishment of new channels for circulation between the intervillous space and the maternal blood-vessels as has often been described before.
It is of interest to note that the invasion of vessels by chorionepithelioma resembles the later type, but in the tumour both layers of the chorionic epithelium attack the maternal tissues and their growth is highly irregular. In my opinion, the tumour activity is something new and different from that of the normal ovum in spite of the resemblances between them.
Ova younger than T.B.1.
The ovum described by John Willoughby Miller in 1913 is clearly considerably younger than T.B.1. It was discovered in curetted material at the Heidelberg Clinic for Diseases of Women. Only five sections -exist, three of which traverse approximately the largest diameter of the ovum, and in them the embryonic rudiment is recognizable. The history does not give accurate data as to the age of the specimen. The whole ovum measures 83 mm. in diameter and the cavity of the blastocyst .44 mm. As the embryo is usually situated towards one side of the blastocyst it seems probable that the section is not equatorial, and that the greatest dimensions of the ovum would have been slightly larger.
The cavity of the blastocyst is filled with a mass of delicate finely granular material lightly stained with eosin which has retracted considerably from the ectoderm. Especially in the neighbourhood of the rudiment this is pervaded with scattered cells of the type of “young fibroblasts still relatively poor in protoplasm.” Some of them show very "beautiful and distinct karyokinetic figures. The mass of mesoderm is clothed round the edges with a thin layer of similar cells. There is no trace of mesodermal villi. About the middle of the mesoderm, but somewhat eccentrically placed, lies the embryonic rudiment which is described by Miller as the rudiment of amnion and yolk-sac together, but clearly it is only the former, and it is probably cut tangentially. It is a little round mass of cells with the commencement of a cavity. (See Bryce’s memoir.)
The ectoderm, according to the author, “already shows differentiation into cytotrophoblast (Langhans’ layer) and plasmoditrophoblast (syncytium). There exists, however, throughout no fundamental diflerence in the nuclear forms or in the coloration of nucleus and protoplasm so that one receives the distinct impression of a continuous transition between the two cell types, as expressly stated also by Peters, Leopold and Jung, for their cases. The regular arrangement of Langhans’ cells as a basal layer of the trophoblast is not everywhere distinct, and the differentiation of the single cell individuals from one another is in places quite indistinct. Mitoses are fairly frequent in the cytotrophoblast.’ ’
“The syncytium which shows the well-known lacunae, although not a honeycomb-like arrangement, occupies not nearly so much space as in the next older ovum of Bryce and Teacher, and the large blood spaces are not present in greater number than one dozen per section.”
In several places the maternal capillaries are seen to have been opened and blood and leucocytes are present in the spaces of the plasmoditrophoblast.
The ovum is closely surrounded by the maternal tissues which in a very limited area show commencing transformation of the stroma cells into decidua cells. The necrotic zone, such as is seen around. T.B.1 and von Mollendorff’s “ Sch.,” is completely absent. Rather the syncytium “ everywhere and throughout touches tissue with well-preserved nuclei.” The limits of the ovum in relation to the maternal tissue in part follow wavy outlines in which there are large lacunae at the periphery of the syncytium. In parts that structure seems to run radially towards the mucous membrane. Along the border line polymorpho-nuclear leucocytes are present in small numbers.
“The cell complexes of the two organisms can, however, be easily distinguished from one another. There can be no question of a zone in which there is mutual penetration of foetal and maternal elements (Umlagerungszone of Peters).”
“The small mass of blood in the immediate neighbourhood of the ovum deserves mention.”
Destruction of the uterine glands’ by the trophoblast is not seen in the sections, although at points the two kinds of epithelium touch one another. There is little change in the shape of the glands. The mucous membrane further removed from the ovum is oedematous and presents the picture of the pre—menstrual or early pregnancy reaction,
Miller’s paper has only one illustration, which is rather poorly reproduced. It represents one of the principal sections of the ovum, and the original picture must be an excellent drawing. As reproduced the magnification is rather low, and one cannot help feeling that there must be a great deal of detail which it does not show. To Professor Bryce and the writer the resemblances to our young ovum are more striking than the differences. The cellular characters of the trophoblast and the mesoderm are in general very similar in the two specimens. The striking differences are (1) the much less Vacuolated condition of the plasmoditrophoblast in Miller’s ovum; (2) the absence of the large blood-filled implantation cavity, and (3) the living condition of the maternal tissues around the ovum in contrast to the necrotic condition in the later stage. The significance of these differences will be dealt with later. In the meantime it is sufficient to say that the Miller ovum is a completely consistent earlier stage of the T.B.1.
Aft-er describing the large blood spaces in the syncytium Miller continues as follows :—“I have the impression that they do not owe their formation to a vacuolization of cell heaps primarily solid through a proteolytic ferment, also not to a kind of selfdigestion, but that the syncytium, after erosion of the dilated capillaries with displacement and destruction of the endothelium, takes over the lining of the blood spaces.” With this interpretation we agree generally. The spaces in the outer layer of Miller’s ovum are not those of the Vacuolated network of T.B.1 but the larger spaces between the solid masses of syncytium. As already explained, there can be no doubt that the spun—out sponge work of syncytium does originate by the formation of vacuoles presumably secretory in character in the solid masses of protoplasm. As the next phase develops and the secretion is shed, maternal blood flows into the vacuoles producing the well-known picture. This, in my opinion, is the natural evolution of the more solid syncytium of the stage of the Miller ovum. Vacuolation is seen in the syncytium of the Kleinhans ovum, which is probably intermediate between Miller and T.B.1.
The Ovum of Klreinhans
It was shown by its discoverer in 1911, but only described fully by Grosser in 1.922, an-d presents a very close resemblance to that of Miller. It was found in the uterus of a young woman who had committed suicide by drowning. The presence of an ovum was not suspected, but a single section of it was found in one of the two large celloidin sections of half the uterus which had been prepared. The section is almost whole, and shows a beautiful picture of a very superficial embedding between the mouths of two glands. The preservation is exceedingly good, but unfortunately the mesoderm is absent from the section. Grosser regards this as due to shrinkage during the celloidin embedding. The external dimensions of the ovum are 0.8 mm. by 0.65 mm., and the cavity of the blastocyst measures .035 by .015. Grosser regards the section as being probably near the meridian but obliquely cut. Comparing it with the T.B.1, we are inclined to regard it as more probably a considerable distance towards one side. A plane passing a little above the line between glands I and 2 in text fig. 3 p. 204 might represent it. The wall of the blastocyst presents a close resemblance to that of the T.B.1, and the cavity in the decidua is occupied by a large amount of straggling syncytium, among the masses of which there are cavities full of blood and leucocytes. The syncytium is more vacuolated than that of the Miller ovum, and probably represents a stage between it and T.B.1. There is no necrotic zone, but there are early degenerative changes in some of the maternal cells close to the syncytium. The pole of the ovum is almost bare, probably as the result of post—mortem changes or through accident of the processes of preparation. Grosser regards this specimen as re-habilitating the Teacher—Bryce ovum, which many investigators have “ regarded as wholly pathological and struck out from the list of normal objects.” I agree with Grosser’s estimate of the value of Kleinhans’s ovum and its relation to the Miller and Teacher—Bryce specimens.
Summary
The relations between the three may be summarized as follows :—
The Miller ovum represents the completion of a period of growth of the trophoblast during which its two layers become clearly differentiated. The relations between it and the maternal tissues are those of contact with little provocation to reaction. In Kleinhans twe have the secretory activity of the syncytium commencing, and therewith irritation of the maternal tissue becomes more pronounced and emigration of leucocytes more abundant, and degenerative changes commence.
T.B.I and von Mollendorff represent the results of the full activity of the primitive syncytium, most of which becomes vacuolated and spent. On the maternal side the necrotic zone attains its full development and blood-vessels are violently invaded and hamorrhages occur into the implantation cavity.
The establishment of an open connexion with the great venous sinus below the ovum marks the commencement of a true circulation in that cavity. It is probably effected during the quiet phase represented by Miller's ovum.
Part III. The Implantation Of The Human Ovum
Everyone who has studied the comparative anatomy and physiology of reproduction must feel that in attempting to apply data thus obtained to the human ovum great caution must be exercised. For in every order of animals, and even in different members of the same order, the Connexion between the ovum and the mother is achieved in very diverse ways. Naturally one would turn to the primates for suitable comparisons, but so far as we are aware, rather less is known of the early stages in the apes and monkeys than of those in man, and the lemurs and the primate tarsius spectrum do not present the close analogy that might have been expected. Among the rodents, conditions are so different in the rabbit as to make comparison useless, and after consideration of the literature we have decided again that the best analogies are obtained from the guinea-pig and hedgehog. For the guineapig we rely on the classical descriptions of Von Spee, a new abstract of which has been made emphasizing certain points which the present study has shown to be important. There has also been available for study and comparison with this a series of preparations made by Mr. N. H. W. McLaren, an account of which will appear elsewhere.
Von Spee's account of the ovum of the guinea-pig just before fixation to the uterus is so concise that it is better to quote it :—
"‘ From examination of the fresh intact ovum of the guineapig we some years ago determined that on the sixth day after fert'ilization it consists of a little oval blastocyst about one-tenth of a millimetre in its longest diameter lying free in the cavity of the uterus and still enclosed in the zona pellucida. The cellular wall consists in the equatorial region of a single layer of very flat cells. At both poles of the ovum it appears thicker. At one pole (at which the embryonic mass and the rudiment of the placenta will be formed) at a certain stage the cellular wall of the blastocyst is many cells thicker and forms a. little mass projecting into the Cavity of the blastocyst (Hensen's Keimhugel). I call this pole henceforth the placental _pole of the ovum. At the other pole the wall consists_ of a single layer of cells which, in contrast to the very flat cells of the equatorial region are cubical (Spee’s Gegenpol).
This pole I call in the first place the implantation pole. This, after implantation, is the free end of the ovum into which the embryonic mass later passes and in which the embryo is eventually formed. The cubical cells of the ‘ implantationspol ’ drive processes of their bodies through the zona pellucida, and thus, before the latter has been separated from the ovum, they enter into direct relations with the lining epithelium of the uterus and initiate the first fixation leading up to the implantation of the ovum in the uterine wall.”
The rest of von Spee’s observations are made on fixed material cut into serial sections.
The ovum is usually embedded near the anti-mesometral end of the slit-like cavity of the uterus. Embedding begins about six days and eight to ten hours after fertilization, and the ovum lies quite within the maternal tissues in two or three hours thereafter. The fate of the zona is not clear, but it is not carried into the implantation cavity. The cells of the ovum are so much larger than the maternal cells that there is never in the early stages any difficulty in telling the one from the other.
In the first known stage of embedding the cells of the “ implantation spol ” destroy as by a “sort of digestion” a few of the surface epithelial cells and substitute themselves for them. The ovum passes in and the underlying connective-tissue cells are next attacked and some of them are dissolved. There is a little cavity in the ovum at this stage on the attacking side of the embryonic mass, so that the thin line of outer layer cells seems like a mobile line of attack resting on fluid. Soon after the ovum has passed completely through the epithelium the embryonic mass increases and the ovum becomes more solid.
The last entering cells of the placental pole, as they pass through, become firmly united with the epithelial cells around the margin of the aperture of entry. That this union is a strong one is shown by the fact that through all the subsequent stage of destruction of maternal tissue the adhesion holds fast. A little later they grow along the deep aspect of the epithelium. in a form very suggestive of the human operculum.
Degenerative changes quickly appear in the maternal mesodermic cells which become swollen and tightly pressed against one another throughout a wide area (Implantation spot) round the ovum so as to look somewhat like epithelial cells. It" may also be remarked that they are like the young decidua cells close round the Teacher-Bryce ovum (T.B.I).
This swelling of the cells is the first change which is observed in the endometrium, which up to the attack by the ovum had remained as in the unfertilized uterus. Embedding takes place on a perfectly healthy area free from congestion, oedema and furrows. Karyokinetic figures are remarkable by their absence near the ovum, and von Spee rightly regards this as proving that the epithelium is quite passive and that the changes in the mesodermic cells are degenerative. In the stage of figs. 11 and 13, which are reproduced on Plate 14, the degeneration has progressed rapidly. Cytolysis has produced a cavity filled with a “thin emulsion,” and the damaged cells in part have broken down into a deeply staining homogeneous mass, enclosing some vacuoles, which he calls “symplasma.” Outside the region of degeneration reactive changes are just beginning. There are at this stage no migration of leucocytes (although he notes that there is one in fig. 11) and no haemorrhage. The blood-vessels indeed show a much higher resistance than the cells, which he attributes to the influence of the circulating blood in them hindering the cytolysis.
In the next stage widespread destruction of the maternal tissue occurs and a great irregular cavity is formed in which the long straggling blastocyst develops. The epithelium is stripped from its base, but it and its adhesion to the ovum hold fast and keep this cavity distinct from the uterine cavity. Already also reaction of the inflammatory type has begun outside the zone of destruction and degeneration and in from 10 to 16 hours from the beginning of implantation a “sort of granulation tissue” has formed which
“hinders or limits” the destructive activities of the ovum.
As to the force by which the ovum embeds, to Von Spee it is clear that it is nothing but the living force of the ovum itself, which, “like an alien parasite,” attacks and burrows into the maternal tissue. For a comparison which is both apt and suggestive one may take the penetration of the human skin or mucous membrane by the blunt-ended cercaria of Bilharzia.
The implantation cavity in the guinea-pig is never filled with maternal blood and the nutrition of the ovum is of the embryotrophic type up to a stage of development far more advanced than is the case with the human ovum considering the difference in the length of the period of gestation. The placenta of the guinea-pig also where alone a maternal and foetal circulation come together, is formed only where the ovum has remained attached, viz., in the neighbourhood of the point of entrance. The form of the blastocyst is one which could hardly come into human development.
The ovum of the guinea-pig, therefore, diverges early and radically from what seems probable for the human ovum. While agreeing, therefore, with the accepted view that the penetration of the human ovum into the maternal tissue is probably similar to that of the guinea-pig ovum, the writer believes that, for the later phases of implantation and for attachment, there is closer analogy with the hedgehog.[6]
The ovum of the hedgehog, after developing into a small blastocyst, comes to rest in a deep groove formed by decidual swellings from either side of the uterus. At this stage it measures about one—tenth of a millimetre in diameter, and it grows slightly and develops considerably before it adheres to the endometrium. Definite adhesion and the penetration of the trophoblast by blood are seen when it is about 0.2 mm. in diameter, fig. 30. Whether the decidual formation begins prior to the ovum reaching its site of implantation or not Hubrecht was unable to -determine, but certainly the development of the decidual swelling occurs before there are any signs of the attack by the ovum upon the uterine epithelium. This is an important difference from what happens in the case of the guinea-pig, and is most probably what occurs in the human species.
The embryo destroys the uterine epithelium in its immediate neighbourhood and the underlying tissues to an extent sufficient to open the maternal vessels. Resink finds no evidence that the ovum “ eats its way into” the maternal tissues. At the stage at which this erosion takes place the extra-embryonic ectoderm has developed all over the ovum into the thick layer of cells for the description of which Hubrecht invented the term “ trophoblast ” or “ trophic epiblast ” and the embryonic knob and the beginningsof endoderrn are distinct, figs. 30 and 32. Resink describes this as the growth stage or pre-placental stage of the trophoblast. He prefers to use Duval’s term “ ectoplacenta,” and speaks of it as “ primitive ectoplacenta.” The maternal blood flows from the opened vessels into spaces in the primitive ectoplacenta but more into a space between it and the decidua. Probably it does not circulate in the spaces of the primitive ectoplacenta but returns from the larger space directly into the maternal vessels. There is, therefore, some circulation, but it is not comparable with the later true placental (“euplacental”) circulation. It is rather like the condition in T.B.1.
The groove in the neighbourhood of the ovum becomes occupied by a plug of clotted blood which Resink regards as having flowed not directly from the maternal vessels but from spaces in the ectoplacenta, a considerable area of which projects at this stage beyond the margin of the destroyed epithelium into the uterine cavity. figs. 31 and 32. The ovum at this stage, therefore, is of the incompletely embedded type and the mode of embedding recalls the old circumvallation theory of the formation of the decidua capsularis. The closure is achieved when the blood clots between the lips of the decidual swellings and adheres to them in consequence of superficial necrosis. The growing together of the lips of the decidua, which Hubrecht originally described, apparently does not take place, but there is a firm union of the degenerate decidual tissue and blood clot as if they had melted into one another. There is considerable variation in the size of the space between the lips of decidua and of the plug of clot. There is also considerable variation in the size of the ova in the same uterus.
After this a secondary development of trophoblast occurs and mesoderm and blood vessels are brought to it first by the yolk sac. the great development of which contrasts with its small size in the human ovum, and then by the allantois. The definitive placenta is formed from the allantois. Most of the primitive ectodermic -formation, which includes the original trophospongia of Hubrecht and the large multinucleate phagocytic cells (deciduofracts) which occupied the space between the trophoblast and the decidual membrane, become degenerate and are pushed by the growing ovum against the wall of the uterus and to a great extent disappear.
In the later account of the embedding and placentation of the hedgehog a very important point is the recognition of two generations of ectoplacenta (trophoblast), the first of which is temporary and concerned with nutrition of the embryotrophic type. The second is that in which the maternal blood really circulates and in which the type of nutrition becomes heemotrophic through the proximity of the maternal‘ blood to the allantoic vessels. A fundamental difference from the embedding in the rodents is that at all stages the decidua of the hedgehog forms a close fitting envelope around the growing ovum. Other fundamental features are that (1) the embryonic ectoderm projects as a knob into the cavity of the blastocyst and never spreads out on its surface; and (2) the definitive (allantoic) placenta forms at the same pol-e of the ovum as the embryo. The embryonic rudiment in the. hedgehog goes with the implantation pole of the embedding ovum not with the outer pole as in the guinea—pig. That it probably behaves in the human ovum as in the hedgehog is shown in Bryce’s memoir.
Implantation of the Human Ovum
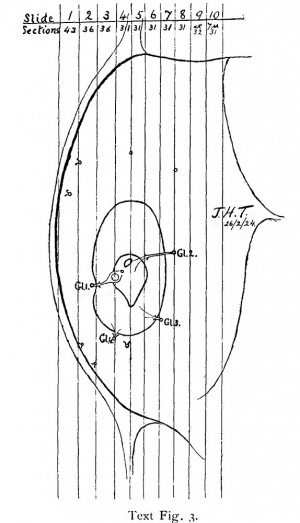

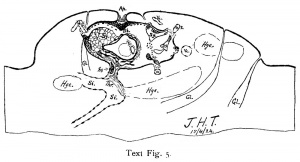
Before attempting to describe this, certain peculiarities of human anatomy must be noted. The human unfertilized ovum is relatively large compared with those of the animals we have chosen for comparison. The newly embedded ovum of the guinea-pig, considered " as a sphere, is" less than one-tenth of a millimetre in diameter. The ovum of the hedgehog is about the same size as that of the guinea-pig, but is considerably larger by the time of embedding, as already mentioned. Da Costa’s fig. 13, reproduced as fig. 29, shows its structure and state of development and the commencing destruction of the maternal epithelium by the implantation pole of the ovum.
The ripe human ovarian ovum measures on the average .25 of a millimetre according to Waldeyer, and other authors have given the limits of size as from .22 to .32 of a millimetre. Doubtless it embeds itself as a blastocyst, and most probably it is still about that size. The discovery of the operculum and its relations to the embryo warrant us in asserting that it will have two poles——an entering or implantation pole and an adhering or closing pole.
The Fallopian tubes of the human subject are enormously longer than those of the guinea-pig or hedgehog, and, assuming that fertilization occurs in the outer part of them, it seems probable that the period occupied by the ovum in passing through the tube to the uterus would be longer than in the small animals. Newer investigation, however, according to George W. Corner, shows that the length of the Fallopian tube does not matter as in most animals the progression through it occupies three to four days.
Grosser, in Keibel and Mall’s Embryology, suggests that implantation of the human ovum takes place about nine or ten days after fertilization instead of seven or eight as formerly suggested by Von Spee, who also now regards the tenth day as the likeliest date of implantation. If these views are correct, the greater part of the free life of the human ovum is spent, not in the tube, but in the uterus, the structure of which seems to be very suitable for its reception and nourishment. As in origin so also in cellular character the glands of the body of the human uterus differ from those of the cervix. The latter are of the mucous type, while the- glands of the body resemble serous gland.
The cavity of the body of the uterus is very large, and is lined by a relatively thick mucous membrane which undergoes periodic and- considerable thickening in the pre-menstrual stage of the menstrual cycle. On the one hand, the stroma cells show considerable development in the direction of becoming decidua cells, and-, on the other, there is great glandular hyperplasia.
In relation to early pregnancy, the state of development of decidua cells is found to differ greatly; for example, in T.B.I and T.B.2. But in all cases in which the young ovum has been found in the uterus, whether extirpated during life or removed at autopsy, the leading feature has been the great thickness of the endometrium, and- on microscopic examination the enormous hyperplasia of the uterine glands. This feature of the uterus would appear very significant if we are to admit the possibility that the ovum lies free in it for four or five days. The proliferation of the glands is doubtless connected with the provision of abundant pabulum for the ovum (uterine milk; embryotrophe of German authors). The secretion of the glands of the body of the uterus is albuminous, and, as already noted, glycogen is said by Linzenmeier to be present in it.[7] It seems, therefore, probable that the essential feature of the decidua in the strict sense of William Hunter, viz., the membrane which develops to serve the need-s of pregnancy, is, in the first place, the proliferation of the glandular elements for purposes of nutrition of the free ovum, the real decidua cells not being required until a later stage. Whether the swollen endometrial cells of the “menstrual decidua ” may in some cases undergo some retrogression on the occurrence of pregnancy, as suggested by von Mollendorff, seems to me doubtful. If so, they soon advance again.
It seems to me possible that the greater development of them in the cases of his ovum “Sch.” and T.B.1, which were fertilized towards the end of the menstrual cycle, as compared with older ova fertilized early in the cycle, is due to that difference, and can be explained on the supposition that in them the already well-developed cells of the pre-menstrual phase carried on without pause into young decidua cells.
Much has been written about the causes which bring about the implantation of the ovum and the character of the site at which it becomes implanted. The opinion of von Spee has been quoted. The determining causes of implantation doubtless are the attainment by the ovum of that stage of development in which the cells of the implantation pole become capable of penetrating into the maternal tissues and the necessity for a different mode of nutrition. Normally things would be so arranged that this would occur when the ovum was in the cavity of the uterus, and in the usual situation, viz., the upper part of that cavity.. If this stage is reached before the attainment of the normal situation an extra-uterine embedding would result, and, on the other hand, if the ovum had travelled too fast and too far, implantation in the lower segment of the uterus and placenta praevia might result. This is well brought out by Johnstone, and illustrated by the circumstances of his second specimen which is embedded in the infundibulum of the Fallopian tube. “ In the ovary removed along with the pregnant tube there is no recent corpus luteum. On the ovary of the other side there was observed at the time of operation what we took without hesitation to be a recent corpus luteum. The ovum must, therefore, have travelled from the one side of the uterus to the other. Further, assuming that it was fertilized on the surface of the ovary from which it arose and that the journey to the mouth of the opposite tube occupied» several days, it is possible by the time it arrived at this point it was ready to implant itself.” This is speculative but it seems. to us an extremely probable theory.
As regards embedding within the uterus, controversy principally concerns the question whether or not there is any physical character of the mucous membrane which determines the site of implantation; for example, whether the ovum becomes implanted in the mouth of a gland or whether it comes to rest in a furrow of the decidua. As Gross-er has pointed out, the human ovum is far too large to enter the mouth of a gland which measures from 20 to 30 microns in diameter as against the 250 microns of the ovum even before fertilization. In the case of the ovum of Kleinhans the mucous membrane was smooth and free from furrows. Hitschmann and L-indenthal also have shown that the menstrual swelling does not produce furrows in the endometrium like those which are seen in the decidua. The evidence, especially when extrauterine implantation is included, goes far to prove that there is no need for any special furrow, depression or lobule.
The ovum of Kleinhans, as shown in fig. 5 of Grosser’s memoir, is set squarely between the mouths of two glands, having been implanted in the surface between them. To investigate the relation of the ovum to the glands an attempt was made to reconstruct the whole lobule of decidua in which the original Teacher-Bryce ovum was implanted. This was found to be impracticable with reference to such small structures as glands and blood-vessels, but plans of the lobule (reproduced below as text figs. 3 and 4) were prepared by plotting off the position of various structures on lines representing planes traversing the lobules through certain points. The spaces between the lines represent the thickness of all the sections on each microscopic slide of the series. This means roughly the combined thickness of 30 to 40 sections, as is shown in the figures. The text figs. 3 and 4 show in horizontal plan and transverse section the results which were arrived at by plotting out the outline of the lobule, the outline of the implantation cavity and the outline of the blastocyst approximately in equatorial section, and as if they were on one plane and placing the other structures in relation to them as nearly as could be determined by direct measurement or by counting the number of sections. The plans are, therefore, rough graphic reconstructions and comparison with wax plate reconstructions certified that the relations were sufficiently accurate for the purpose in view. The figures are, therefore, really graphic reconstructions. The third plane is represented by the photomicrograph, fig. 17 and text fig. 5, which represents the various structures on one plane in the same manner as in text figs. 3 and 4.
In the transverse section of the lobule, text fig. 4., the aperture of entrance is seen to be close to the mouth of a gland, otherwise the picture resembles that of the ovum of Kleinhans.
Text fig. 3 shows the aperture of entrance of T.B.1. to be situated near the apex of a triangle the angles of which are the gland mouths Nos. 1, 2 and 3. In the case of glands I and 2, the mouth on the side next the aperture of entrance is drawn out into a narrow fissure, the extent of which is indicated- on the plan by the heavy lines terminating in a splay mouth. The lighter lines indicate that there was a tiny groove traceable from gland I to the aperture of entrance and beyond that almost without a break to the stretched out mouth of gland 2. Only in the very centre was this groove completely flattened out. My interpretation of the relation of the ovum to the two glands is as follows :—
Gland No. I stands out slightly into the implantation Cavity (as was mentioned in the original memoir) having presented distinctly more resistance to the destructive activities of the trophoblast than the other tissues of the endometrium. (Actually it is not the gland but the layers of decidual cells ensheathing the gland which have presented the resistance.) The ovum, possibly taking advantage of the tiny groove which extends from the mouth of gland- I to the mouth of gland 2, entered close to gland No, I. Probably it burrowed vertically through the epithelium and superficial layers of decidua and then turned into the oblique position relatively to the aperture of entrancewhich is characteristic of the two Teacher-Bryce specimens and “ Sch.” of von Mollendorff, see Plate XV. Whether this is a characteristic obliquity dependent on the ovum or whether it is accidental and dependent upon the resistance of the gland causing the ovum to develop into the more easily penetrated endometrium towards the more distant glands, it is not possible to say.
Indications of similar relations to glands were found in T.B.2
and ]ohnstone’s ovum, but so many glands have become involved
in the extending capsularis and so much destruction of them
has occurred that a clear picture could not be obtained. See text
figs. I and 2. The relations of the ovum and the glands in this
phase of development require further study.
Summary
Implantation of the human ovum and development of the trophoblast. Plate XV, figs. 33 to 37. From analogy with comparative embryology it may be concluded that the human ovum is fiertilized in the outer part of the Fallopian tube and reaches the uterine cavity in three or four days. There it lies, probably in little bays (not preformed). in the surfaces of the soft endometrium, nourished by the secretion of the uterine glands. fig. 33.
About the tenth day after fertilization it attains a state of development when it must either enter the maternal tissu-es or perish. At this
stage it is a small blastocyst showing a definitely bi-polar structure.
fig. 34. It then behaves like the ovum of the guinea-pig and
attacks the endometrium and burrows into it by means of cytolytic
activity of the extra-embryonic ectoderm at the implantation pole.
The ectod-ermic cells of the opposit-e pole, which are last to enter,
adhere to the uterine epithelium and close the breach. Later they
grow out beneath the epithelium, uniting with the maternal tissues,
and forming a closing apparatus which shuts off the implantation
cavity from the cavity of the uterus. fig. 35. Destruction of
maternal tissue is probably limited in amount as in the hedgehog,
and a close-fitting decidua capsularis is developed, as in Miller’s
ovum :—-—fig. 36. A period of growth follows in which the relations to the maternal organism continue to resemble those seen in the
hedgehog, and a primitive or first generation of trophoblast is
developed in which the two types of cell structure appear. Probably
some reaction occurs limiting and controlling the growth of the
ovum and nourishment provided for by the opening of vessels
and flowing of blood into the plasmoditrophoblast. At this stage
doubtless the connexion with the great venous sinus is effected,
which survives through the succeeding phase of destructive activity.
Figures not unlike the ovum of Miller occur among the early
stages of the hedgehog, but nothing like T.B.1 :~fig. 37. The
behaviour of the embryo proper cannot be considered here, but it
too seems already to have taken a strong line of its own. fig.35+—37.
T.B.I shows the destructive phase in full activity. The primitive plasmoditrophoblast or syncytium has excavated a relatively large implantation cavity with necrotic walls. The vacuolated syncytium, which is already commencing to degenerate, is the agent by which this was effected. The smaller masses of syncytium are now seeking for and opening the maternal blood-vessels, and the cavity becomes filled with blood, which, although extravasated, does not coagulate but drains into the large venous sinus. This marks the beginning of a circulation of blood round the ovum.
Probably towards the end of this period the blastocyst becomes
detached from the operculum, and at the point of rupture thrombosis occurs in the implantation cavity and the “ internal shield ” of
fibrin is formed to strengthen the capsularis at the aperture of
entrance. The operculum would then degenerate and soon cease
to be recognizable.
The operculum must be regarded as a special apparatus. it is not a part of the trophoblast, although it originates from the same tissue, as it does not exercise the functions which characterizes the rest of the extra-embryonic ectoderm. Neither is it a “ Traeger ” in the sense in which that term is used in rodent embryology as it takes no part in the formation of the placenta.
The next stage is characterized by the g'1'owth of the second-ary or permanent trophoblast which destroys the primitive syncytium and replaces the primitive cytotrophoblast. Destruction gives place to a more orderly process, the principal features of which are attachment of the ovum to the decidua and the quiet opening of blood-vessels and the formation of the intervillous space through which a circulation becomes established. In T.B.2 the rudiments of the chorionic vessels appear. Their development will be dealt with elsewhere by Dr. Donald McIntyre. As to the embryo, it may be repeated that its position in the blastocyst, as seen in the Teacher-Bryce specimens, is characteristic and of fundamental significance. It follows, therefore, that if other ova are searched, keeping in mind the relations of the yolk-sac to the operculum, that structure should be found at any rate in specimens about the stage of Peters.
A number of other problems, such as the reckoning of the age
of ova, and the relations of fertilization and implantation to the
menstrual cycle, and the development of the decidua and the
influence of the corpus luteum and ductless glands still await full
investigation.
Conclusions
(1) The human ovum burrows into thependornetrium like a vigorous parasite destroying maternal tissue and provoking inflammatory and reparative reaction.
(2) The closure of the decidua capsularis (reflexa) is effected by an apparatus developed from the last entering cells of the ectoderm of the ovum. This becomes united with the uterine epithelium and later with the other tissue of the lips of the aperture of entrance. For it the term “ operculum deciduae ” is suggested.
(3) The recognition of this apparatus has led to th-e discovery that in the human ovum there is a polar structure similar to that seen in the guinea—pig and hedgehog. The ovum is therefore described as having an entering or implantation and an adhering or closing pole. This polarity determines the position of the embryonic rudiment in the blastocyst and the situation of the placenta. The operculum differs from the Traeger of the rodent in taking no part in the formation of the placenta.
(4) The operculum usually becomes detached from the blastocyst and degenerates when its functions of closure of the aperture of entrance and temporary fixation of the ovum have been fulfilled. When the separation occurs, an “internal shield” of fibrin is formed by thrombosis in the implantation cavity and closes the aperture of entrance from within. In some cases the operculum remains attached to the blastocyst and an external “closing coagulum ” (Gewebspilz) may form over it.
(5) The conception (1908) that there are two generations of trophoblast (a primitive or implantation trophoblast and a secondary or attaching or placental trophoblast) is found to be justified.
(6) Much of the syncytial part of the primitive trophoblast disappears when its functions of forming the implantation cavity and openingthe maternal vessels have been discharged.
(7.) The rest of it and the primitive cytotrophoblast develop into the placental trophoblast, and, in the first place, are concerned with the attachment of the ovum and the formation of the int-ervillous space and proper channels between it and the maternal circulation, eventually becoming the chorionic epithelium.
(8) The original Teacher—Bryce Ovum (T.B.I) is found to demonstrate the r-elations of the maternal blood to the ovum prior to the formation of the int-ervillous space. The blood enters the implantation cavity as haemorrhages from minute vessels of the endometrium and leaves it by the large venous sinus which is so conspicuous an object underneath most young human ova. This sinus must be regarded as a normal feature of the surroundings of the young human ovum. Abortion in the case of T.B.1 appears to have been due to rupture of the sinus followed by thrombosis which arrested the circulation through the implantation cavity.
(9) In these early arrangements the human ovum exhibits fundamental differences from the ovum of any other species, the early development of which is known.
On account of the rarity and beauty of the obj-ects dealt with
in this communication it seemed fitting that the illustrations should
be abundant and of fine quality. I have much pleasure in
expressing my indebtedness to the Carnegie Trust of the Universities of Scotland for a grant in aid of research which has made it
possible to carry this out on a lavish scale.
Footnotes
- ↑ The position at 1903 was summarized fully by the writer in his work on Chorionepithelioma. The fullest account in English of the literature and position up to 1910 is that of Keibel and Grosser in Keibel and Mall’s Embryology, and Johnstone’s memoir of 1914 and Strachan’s recent review in this Journal are well worthy of study.
- ↑ The ovaries of all women of child-bearing age ought to be examined for the presence of corpora lutea before the body of the uterus is touched. The uterus should not be grasped but should be steadied by the broad ligament during removal.
- ↑ In this connexion the beautiful illustrations of Delporte should be studied.
- ↑ “ Zotten Syncytium ” of Grosser.
- ↑ “ Implantations-syncytiurn ” of Grosser.
- ↑ It is necessary to take the classic work of Hubrecht along with the account given by Resink, who re-did the work under the supervision and with the co-operation of Hubrecht, as there are many changes of interpretation. Unfortunately, Resink’s publication is rather inaccessible. It is advisable also to read it along with the original monograph as there are numerous references to the old illustrations. For the embryo later papers such as those of da Costa and Baurneister must be consulted.
- ↑ Glycogen is present in the uterine secretion of monkeys. - G. W. Corner.
Literature Cited in the Text
Baumeister. Zeitschr.f . Wzss. Zoologie, 1913, 150, I.
Beneke, see Strahl.
Bilharzia, Manson—Bahr and N. Hamilton Fairley. Parasitology, Vol. 12, No. 1, p. 33, and personal communication from Prof. R. T. Leiper, F.R.S.
Bryce TH. and Teacher JH. Contributions To The Study Of The Early Development And Imbedding Of The Human Ovum 1. An Early Ovum Imbedded In The Decidua. (1908) James Maclehose and Sons. Glasgow.
Corner, George W. “ Oestrus, ovulation and menstruation.” Physiological Reviews, 1923, iii, 457.
da Costa. “ Libro en honor de Santiago Ramon y Cajal.” Madrid, 1922, 11, 215.
Cova, Ercole. Arch. f. (§ym'z'kol., 1907, lxxxiii, 83,.
Delporte. “Etude de la Nidation de 1’oef hurnain et de la Physiologie du trotphoblaste.” Brussells, 1912. '
Frassi. Archiv. f. Mik. Anat., 1907 and 1908, 70 and 71.
Grosser, Otto. “Lehrbuch der Vergleichende Anatomie und Entwickelungsgeschichte der Eihaute und der Placenta.” Vienna, 1909.
—. Chapter VII in Keibel and Mall’s “ Human Embryology,” vol. i, on “ The development of the egg membranes and the placenta; menstruation,” 91——179.
—. Trophoblastschale bei jungen inenschlichen Eiern. Ei “ Kleinhans ’’ und Ei “Sch. 3.” Zcitschr. f. Amt. 14 Entwickelungsgeschichte 66, 1922, 179.
Hitschmann and Lindenthal. On Menstruation. Monatsschr. f. Geburtsh. u. Gym'z‘kol., 1908, 27, 1.
Herzog MA. A contribution to our knowledge of the earliest known stages of placentation and embryonic development in man. (1909) Amer. J Anat., 9(3): 361-400.
Hubrecht. “ Placentation of Erinaceus Europaeus.” Quart. foum. Micros. Sci, 1889, XXX, 283.
Johnstone, R. W. fawn. of Obstet. and Gymecol. of the Brit. Emp., 1914, Xxx, 231.
Jung. “ Beitrage zur fruhesten Ei-einbettung beim menschlichen Weibe.” Berlin: S. Karger, 1908.
Keibel F. and Mall FP. Manual of Human Embryology I. (1910) J. B. Lippincott Company, Philadelphia. Chap. II. 2 Fertilization; Chap, III. 1 Segmentation; Chap. IV. : Young human ova and embryos up to the formation of the first primitive segment.
Linzenmeier. Archiv. f. Gym'1'k0l., 1914, cii, 1.
Miller, John W. Berl. Klin. Wochenschr, 1913, 1, 866.
v. Mollendorfi, W. Zeitschr. J‘. Anat, u. Entwrickelungsgeschichte, 1921, lxii, 352. A
Peters, H. “ Einbettung des Menschlichen Eies.” Vienna : Deutiche. 1899.
Resink. Tijdschr. d. Ned Dierk. Vereen (a) D1. 7, Afl. 3 en 4, 1902.
Schlagenhaufer u. Verocay. Archiv. f. Gync'ik0l., 1916, cv, 151.
V. Spee. “ Implantation des Meerschweinchen Eies. Zeitschr. f. Morphvol u. A1/Lthropol, III, 1901, 130.
—. Verhandl. Deutschien Ges. f. Gym'ik0l.., Leipsic, 1906, 421.
~. Article in Doederl-ein’s “ Handbuch der Geburtsh,” 1915, Wiesbaden.
Stolper. Monatsschr. f. Gcbmtsh. u. Gym'ik0l., 1906, xxiv.
Strahl u. Beneke. “ Ein jungen Menschlichen Embryo.” Wiesbaden, 1910. Bergmann.
Teacher, see Bryce.
Teacher. “ Chorionepithelioma.” ]0m'/n. Obstet. and Gjmmcol. of Brit Emp., 1903, iv, 1.
Verocay, sue Schlagenhaufer.
Description of Plates
- Figure Links: text-fig. 1 | text-fig. 2 | text-fig. 3 | text-fig. 4 | text-fig. 5 | plate 1 | plate 2 | plate 3 | plate 4 | plate 11 | fig. 17 | fig. 18 | fig. 19 | Teacher 1925 | Carnegie stage 5 | Carnegie stage 6
Plate 1 Teacher-Bryce Ovurn No. 2
Fig. 1. Teacher-Bryce Ovurn No. 2
The uterus laid open in front showing the lobule in which the ovum was imbedded situated in the left upper quarter of the posterior wall, The left ovary contains a large corpus luteum. Inset is the lobule enlarged to about four diameters. Drawn by A. K. Maxwell.
Plate 2, fig. 2. Teacher-Bryce Ovum No. 2.
The lobule enlarged to about X15. This should be examined along with text figure No. 1 on page 174.
The text figure shows the principal structures in outline as they appeared during clearing in Xylol. The landmark is the oval blood clot (marked “ clot ”). The cross beside it indicates the centre of the aperture of entrance and operculum. The area enclosing the cross represents the extent of the adherent cone of blastocyst. The operculum is considerably more extensive. The oval bodies below it are the ends of chorionic villi and the dark bodies Show the masses of uncleared blood in the superficial vessels. The clear area of the figure represents fairly accurately the avascular decidua capsularis. G1. 1 and G1. 2 indicate the nearest glands. Both figures drawn by A. K. Maxwell.
Plate 3, fig. 3. Teacher-Bryce Ovum No. 2
General View of the whole ovum in Section 26.1.1. In the cavity of the blastocyst are seen the ainnio-embryonic rudiment and yolk—sac. Ap.e. Aperture of entrance occupied by the operculum cut considerably to one side of the centre. The section for purpose of the drawing was projected without an eye-piece. Consequently the drawing is reversed from the usual microscopic picture which is shown in the photomicrograph fig. 4.
X about 21. Drawn by A. K. Maxwell.
Plate 4, fig. 4. Teacher-Bryce Ovum No. 2
X about 25.
Photomicrograph of Section 28.2.2 for comparison with the drawing. This section passes through the centre of the operculum. Only the yolksac appears in it.
Note the tearing of the blastocyst wall from the blood of the intervillous space close to the operculum, the coaguluin and its situation at the dependent aspect of the blastocyst (dorsal decubitus of body) and the large venous sinus below the ovum. Photograph by J. H. T.
Plate 5, fig. 5. Teacher-Bryce Ovum No. 2
One half of the operculuin (Op.) in Section N0. 25.2.1. X 350. The mesoderm (mes.) is retracted from the base of the operculurn. Ch. ep. The chorionic epithelium which becomes more primitive in character with nuclei of irregular shape and size as the operculum is approached.
The cells and nuclei of the operculum are large and vary in size and shape. Those further from the blastocyst wall Show signs of degeneration. There is some Vacuolation of their protoplasin and a few leucocytes are seen among them. The thin membrane with small elongated nuclei on the surface of the operculurn is the remains of the uterine epithelium—ut.ep. Note the diflerent size of the cells of the two structures. Between the operculum and the chorionic epithelium covering the blastocyst there are remains of decidua, thrombus-like material with many leucocytes and the blood in the intervillous space—i.v.sp. The pale granular masses in the blood occur frequently throughout the intervillous space. I am not prepared to make a definite statement as to their nature. Compare fig. 7 and Photos figs. 8 to 10.
Drawn by A. K. Maxwell. Scale : hundredths of millimetre.
Plate 6
fig. 6. Teacher-Bryce Ovum No. 2
The operculum in Section 28.2.2. x about 120. It shows the broad, flat and the tearing of the specimen by the air bubble. Ch.ep. Chorionic epithelium.
Sy. Portion of syncytiuni which is also seen in fig. 9, apparently separating the blastocyst from the operculum. V. Villus.
Ut. ep. Remains of uterine epithelium passing in over the surface of the operculum. Compare figs. 8 to 10, photographs. Drawn by A. K.Maxwell.
Fig. 7. Dr. Johnstone’s Ovum No. 1. (Uterine)
The decidua capsularis showing the remains of the operculum (op.) in section 18.2.5. X 320. The blood corpuscles indicate the inner aspect of the clecidua. The operculum is seen as a well defined structure with dark shrivelled nuclei occupying a space in the remains oi‘the decidua. The solid looking mass (fi.) on the inner aspect is part of the internal shield of fibrin. Compare text fig. 2, p. I82, and fig. 11, photograph. Drawn by A. K. Maxwell. Scale: hundredths of mm.
Plate 7 Teacher-Bryce Ovum No. 2
Fig. 8. The operculum and cone of blastocyst in Section 28.2.2. X about 80. For comparison with fig. 6. Sy. Primitive syncytiutn. Mes. Mesoderm of blastocyst. Op. Centreof the operculum. Photograph by J. H. T.
Fig. 9. Teacher-Bryce Ovum No. 2. One half of the operculum with adjacent structures to show the longpointed end of a mass of primitive syncytium (Sy.) stretching in between the operculum (op.) and the wall of the blastocyst. X I 56. Photograph by J. H. T.
Fig. I0. Teacher-Bryce Ovum No. 2.
The lower edge of the operculum in Section 28.2.2. X about 360. Op. The end of the long sprawling cells imbedded in the decidua. D. A decidua cell lying between it and U.E. remains of the uterine epithelium.
Note the thin coagulum overlying the three well preserved epithelial cells. Photograph by J. H. T.
fig. 11. Dr. Johnstone’s Ovum No. 1 (uterine).
Decidua capsularis and margin of the trophoblast showing the remains of the operculum (op.) in Section 18.1.5 x about 150. Photograph by J.H.T.
Plate 8 Teacher-Bryce Ovum No. 2
Fig. 12. Teacher-Bryce Ovum No. 2.
A blood vessel of the decidua capsularis opening into the intervillous space. Section 47.1.5 X 270. The vessel, although it appears to enter vertically, really lies obliquely to the implantation cavity, the section being almost tangential. The cytotrophoblast is seen in contact with the maternal blood. About the centre of the picture two endothelial cells are seen separatedfrorn the decidua by cytotrophoblast which has grown along under it and replaced it.
Compare the necrotic layer of the deciclua with the pictures of T.B. 1. Compare fig. 15, photograph. Drawn by A. K. Maxwell Scale : hundredths of mm.
fig. 13. Teacher-Bryce Ovum No. 2.
The mouth of a blood-vessel comrnunicating with the intervillous space. Section 36.2.2. X about 120. Scale : hunclreclths of mm.
On both" sides of the mouth the syncytium is seen in continuity with the endothelium which is practically perfect. In some sections there is a small area of degenerate tissue between the lower mass of syncytium and the endotheliurm. Over all it is nevertheless clear that the syncytium does not originate from the maternal endothelinm. Drawn by A. K. Maxwell.
Plate 9 Teacher-Bryce Ovum No. 2
Fig. I4. Teacher-Bryce Ovum No. 2.
The deciduacapsularis, trophoblast and villi in Section 2I.2.5. X 156.
The figure shows the border between the fully living and the degenerating decidua and the necrotic inner zone of the decidua like in T.B. 1. The ends of two villi are seen. The cytotrophoblast nearer to them is edged with syncytium. Further out it may lie naked to the maternal blood. Masses" of primitive syncytium are seen and above them is a blood Vessel (Ve.) which has almost been opened by syncytium as in T.B. I. The serial sections show that actually it has not beenopenecl. The area is shut off from the implantation cavity by thrombosis. Photograph by J, H. T,
fig. 15. Teacher-Bryce Ovum No. 2.
Decidua capsularis at one side of the ovum showing the characters of the decidua and the blood vessel (Ve.) shown in fig. 12, Plate 8. Section 47.1.5. X 156. Photograph by J. H. T.
Plate 10, fig. 16. Teacher-Bryce Ovum No. 1.
Section 3.5.3. X 3,40. The aperture of entrance filled by the remains of the operculum (op.) and some thrombus—like material. Beneath this is seen the internal shield of fibrin (i.s.) with a conical point stretching down almost to the blastocyst. The wall of the blastocyst shows the primitive syncytium (plasmoditrophoblast) sy. and the cytotrophoblast (cy.tr.). Along the junction between the two there are signs of degeneration and some dead material. On the right of the conical point of the internal shield of fibrin a mass of syncytium (Sy.) which is attacking and dissolving the fibrin. Compare figs. 20 and 21, Plate 12. Drawn by A. K. Maxwell. Scale: hundredths of mm.
Plate 11
Fig. 17. Teacher—Bryce Ovum No. I.
The whole ovum in Section 4,2,4. X 40. General view showing the decidua capsularis with necrotic zone next the blood in the implantation cavity and the various tissues of the ovum. Compare text fig. 5.
Y.S. The yolk-sac is seen towards one side of the blastocyst imbedded in the mesoderm. Right above it is th-e bulge of the blastocytt wall which is supposed to have been the site of the operculum.
On the left the implantation cavity is occupied by a mass of spun-out spongy syncytium. On the right the spent remains of this are seen flattened against the blastocyst and further out is a line of liberated decidua cells which have been driven towards the middle of the cavity by an inrush of blood. Compare fig. 24, Plate 13. Photograph by I. H. T., 1908.
Fig. 18. The aperture of entrance (Ap.) occupied by remains of the operculum. Section 4.5.4a. Gram-Weigert’s fibrin stain. X 156.
I.S. Internal shield of fibrin with a layer of liberated decidua cells below it. Mes. Mesoderm. Sy. Mass of vacuolated syncytium. Photograph by J. H. T.
Fig. 19. Portion of the blastocyst in Section 4.4.6. X 156 to show.
A.E. The aninio-embryonic sac which is torn. In the photograph masses of deposit from the haemalum complicate the picture. Also shows the mesoderrn, cytotrophoblast and the syncytium (plasmoditrophoblast). Photograph by J. H. T.
Plate 12
Fig. 20. Teacher-Bryce Ovum No. 1.
Part of the decidua capsularis showing a stage in the opening of maternal blood vessels by the ovum. Section 3.3.2. >< zro. On the left there is living decidna with blood vessels. In the centre the endothelial tube of a small blood vessel is seen dissected out from the surrounding decidua, some of the cells of which lie more or less free in the cavity around it. Below there is a mass of young syncytium adhering to the necrotic layer of decidua. To the right and higher up is seen the spent syncytium with its vacuoles full of blood. Between this and the decidna the solution of the necrotic layer and liberation of the decidua cells into the implantation cavity can be seen. Drawn by A. K. Maxwell.
fig. 21. Opening of maternal blood vessel. Section 2.5.2. X about 290. In the centre is seen a small blood vessel running round the margin of the iiriplaiitatioii cavity. There is a gap in the necrotic layer of the decidua Implantation of the Human Ovum 215
which is filled with blood and two masses of syncytium, one of which is seen pressing into the endothelial wall of a capillary. To the right of it the capillary appears to have been opened and the aperture and the lumen of the capillary are occupied by a small thrombus. The blood in the gap differs in appearance from that in the implantation cavity. Compare photographic figs. 23 and 25. Drawn by A. K. Maxwell.
Plate 13. Teacher-Bryce Ovum No. 1.
fig. 22. Decidua and implantation cavity at lower end in Section 3.3.2. >< about 125, for comparison with fig. 20.
In the centre is seen the endothelial tube of a small blood vessel filled with thrombus. The decidua and necrotic zone around it appear to have been dissolved. The character of the (lecidua well shown. Compare fig. 14 of T.B. 2, Plate 9. Photograph by J. H. T.
fig. 23. End of implantation cavity in Section 2.5.2. X 125, for comparison with fig. 21 and to show three openings of maternal blood vessels. The necrotic layeizappears to have been dissolved at each of these.
The dark strands in the blood are mostly remains of the vacuolated syncytium. Photograph by J. H. T. Compare text fig. 5 on p.
fig. 24. The mouth of the venous sinus with part of the blastocyst. Section 5.4.3. X 125. From a section near that figured in Plate V, fig. 5, of 1908 Memoir. Mes. Mesoblast. Tr. The cytotrophoblastic wall of the blastocyst. Sy. Mass of primitive syncytium hanging down into the sinus. Thr. The thrombus in the sinus. Compare the text fig, 5, p. 205, for the position of the rupture of the sinus and the haemorrhage. Photograph by J. H. T.
fig. 25. Margin of implantation cavity in Section 4.4.5. X 125. To show a wide gap in the necrotic layer and haemorrhage into the cavity on either side of a large mass of primitive syncytium. The released
decidua cells are driven towards the blastocyst by the inrushing blood. Photograph by J. H. T.
Plate I4, fig. 26.
Ovum of guinea-pig at an early stage of implantation. It has destroyed an area of the uterine epithelium and is commencing the attack upon the underlying tissue. The embryonic mass is attached to the pole of the ovum which is the last to enter. v. Spee, fig. 7, Pl. 1, reduced to about X 400.
fig. 27. Ovum of guinea-pig implanted in the endometrium and surrounded by a considerable area of degenerate maternal tissue and a cavity (H) which was filled with “ a very thin emulsion ” (V. Spee, p. 161, line 31). Ue. The uterine epithelium is seen in contact with (but they were sharply defined from) the last entering cells of the ovum. P. “Placentarpol ” and p. l. “ Implantationspol.”
The site of imbeclrling at one side of the slit-like cavity of the uterus is more usual than that shown in the next figure. V. Spee, fig. II, Plate VIII.
fig. 28. Ovum of guinea-pig implanted and attached to the uterine epithelium by the “ Placentarpol.” Around it there is a relatively large cavity (H) lined by necrotic tissue outside which the cells of the endometriunl are swollen and closely packed against one another. Note the 216 Journal of Obstetrics and Gynaecology
separation of the uterine epithelium from the other maternal tissues and its adhesion to the ovum.
As. Cells in early stage of degeneration. C. Capillary. Y. The embryonic mass of cells. P. l. The “ Implantationspol.” U. Cavity of uterus. v. Spee, fig. 13, Plate X.
fig. 29. The ovum of the hedgehog at the commencement of implantation. The cells of the implantation pole, with which goes the embryonic mass, are destroying the uterine epithelium.
b.e. bouton embryonaire (embryonic cctodcrm). en. endoderm. ep.uterine epithelium. tr. trophoblast. v. blood vessels of decidua. da Costa, text fig. 13.
fig. 30. The ovum of the hedgehog (Erinaceus europczus) at an early stage of implantation. It consists of a thick outer shell of elongated darkly stained cells (the trophoblast) with a knob projecting inwards (the embryonic ectoderm) and a thin-walled lining to the cavity (the endoderm “ hy.”) The outer pole lies bare to the clot (coa) which fills the space between the lips of the decidual swellings. Hubrecht, fig. 39, much reduced.
fig. 3,1. The ovum of the hedgehog implanted in a groove between great decidual swellings, the lips of which are glued together by a mass of blood clot. Resink, fig. 1.
fig. 32. The‘ ovum of the hedgehog showing the bare outer pole, its relation to the uterine epithelium, the trophoblast and the uterine Vessels communicating with spaces in it. Resink, fig. 3.
Plate 15. Diagrams illustrating the Implantation of the Human Ovum. All the figures are magnified by about 70. The embryonic rudiment is proportionately too large.
Ap. e. Aperture of entrance. G G . Uterine glands corresponding to G1. I and G1. 2 in text fig. 3. Is. Internal shield of fibrin. Op. Operculum. Si. Mouth of venous sinus below ovum. Ue. Uterine epithelium shown by black line. Ve. Small blood vessels at periphery of implantation cavity.
fig. 33. The human ovum free in the uterus. Its shape is oval and the longest diameter is taken as o.3o of a millimetre.
fig. 34. The human ovum passing through the epithelium into the endometrium. The embryonic mass goes with the implantation pole. Note the larger size of the cells at the opposite pole.
fig. 35. The human embryo possibly about 24 hours after implantation which is taken as commencing on the tenth day after fertilization. There has been considerable growth and the endoderm and mesoderm have appeared. The large cells at the closing pole have multiplied and spread underneath the epithelium forming the operculum to the centre of which the epithelium it attached. Expansion is occurring in the direction of G. 2. and the embryo is assuming the characteristic oblique position.
fig. 36. The human ovum in the stage of Miller or Kleinhans, possibly
about 48 hours after implantation. The operculum has grown considerably.
It is still attached to the blastocyst. The differentiation of the trophoblast
into the two layers has taken place and the syncytium forms an irregular sponge work. Small maternal blood vessels have beeen opened and blood
has flowed into the spaces of the trophoblast. The orifice of the great sinus
underlying the ovum has been established. The decidna fits closely round
the ovum and there is no necrotic zone.
fig. 37. The human ovum in the stage of T.B.I., possibly 72 hours after implantation and towards the end of the destructive phase. On the left of the implantation cavity the Vacuolated syncytiurn which excavated the cavity is seen. On the right the condition is more that of masses of syncytium which are opening the blood vessels. The necrotic zone is fully developed. The large sinus has remained unaltered. The operculum has become detached and the internal shield of fibrin has formed to close the gap in the decidua which might have arisen.
Text Figures
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Reference
Teacher JH. On the implantation of the human ovum and the early development of the trophoblast. (1925) J Obst. Gynaecol. 31(2); 166-217.
Cite this page: Hill, M.A. (2024, April 19) Embryology Paper - On the implantation of the human ovum and the early development of the trophoblast (1925). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_On_the_implantation_of_the_human_ovum_and_the_early_development_of_the_trophoblast_(1925)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098GCarnegie Stage 5