Paper - Notes on the development of the prepuce (1935)
| Embryology - 25 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Hunter RH. Notes on the development of the prepuce. (1935) J Anat. 70: 68-75. PMID 17104576
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Notes on the Development of the Prepuce
By Richard H. Hunter
Department of Anatomy, Queen’s University, Belfast.
Introduction
The differentiation of the prepuce was described by Retterer and Neuville[1] to be brought about by a “ glandulo-preputial epithelial invagination of cells ” which, towards the end of intra-uterine life, breaks down to form a slit-like space between the preputial skin and the glans penis. The breaking down of the epithelial cells, according to them, is due to the sudden enlargement of the glans at this time, the degenerating cells first forming isolated intra-epithelial lacunae which then run together and become the glandulo-prcputial space.
Berry Hart[2] in an earlier paper had described the process differently. The prepuee, according to him, grows forward to cover a previously naked glans penis. He states that, in his opinion, the prcpuce does not grow forward as a free unattached sheath, but that the fold rolls forward to become inverted “so that what was superficial is now deep, facing the glans surface”. He then states that the glans and the fold become blended and continuous as a tissue, the future preputial space being filled with epidermal cells, the central of which correspond to the superficial epidermal layers. These central cells, Berry Hart states, “desquamate”, and by this desquamation produce the prcputial space. Berry Hart studied only two stages of development (2.; months foetus and at birth), but he believes, he says, that the View which he advanced “may be inferred with some safety from the earlier state”.
This account of the development of the prepuce appears to have been generally accepted, and since Berry Hart’s time has been reproduced in most text-books of embryology.
Frazer[3] in his Manual of Embryology follows it. He writes: “About the end of the second month a low ridge appears round the coronary suleus marking off the glans. This ridge is the beginning of an upgrowth of the superfidial tissues proximal to the suleus, which continuing its growth leads to the formation of the prepuce. As it grows the epithelial cells covered by it form a solid layer deep to it: they break down subsequently, leaving the structure applied to the underlying glans without any adhesion between their surface other than remnants of the epithelial attachments.” Neither Berry Hart nor Frazer offers any explanation of the mode of formation of the frenulum.
A different account of the development of the prepucc was given in 1921 by Spaulding.[4] He (lescribes the prepuce to be formed from a pair of swellings at the sides of the urethral opening, which fusing over the dorsurn of the shaft of the penis, form a flattened ridge of skin whose distal margin envelops the proximal margins (corona glandis) of the penis. The subsequent growth of this ridge of skin, Spaulding states, results in the progressive inclusion of the glans by the distally migrating fold of skin until in foetuses of 100 mm. C.R. lengths the originally naked glans is entirely covered. Spaulding in his paper then admits that his observations “are based only upon the external examination of the genitalia of older embryos, and have not been confirmed by histological study.” “For these reasons”, he continues, “they must be considered merely as suggestions of what apparently takes place, and may later be corroborated or contradicted.”
The purpose of the present paper is to supply the histological studies omitted by Spaulding, and to discuss how far they support or contradict his work, and to compare his conclusions, which broadly agree with those of Berry Hart, with those of Retterer and Neuville.
Material
The material used in these studies was entirely human. Serial sections 10 micron in thickness were cut in the sagittal plane of the external genitalia of male foetuses of 40 mm, 70 mm, 100 mm and 170 mm C.R. length, and a second series of sections from specimens of the same size were cut at right-angles to the long axis of the penis. These sections were stained with Erlich’s haematoxylin and cosin, and wax-plate reconstructions were made from them.
Results
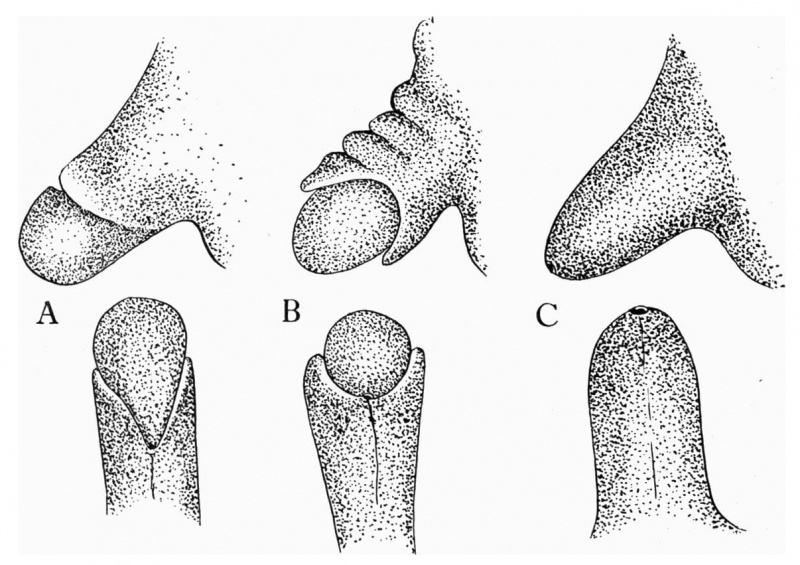
The penis at the 1.0 mm. stage presents a naked rounded extremity on the surface of which is a thin layer of dcsquamating epithelial cells. The terminal portion of the penis is demarcated from the shaft by a shallow groove, and a low ridge of epithelial tissue is present just behind this groove (text-fig. 1). The cavernous tissue of the. penis is represented by a clearly marked condensation of mesodermal tissue which is stained more deeply than the surrounding tissue.
A reconstruction of the penis at this stage shows that the ridge of epithelial tissue behind the groove which demarcatcs the glans from the body of the penis does not uniformly surround it in the form of a circle (text-fig. 5 A), but is rather disposed in an oblique direction, radiating out on either side of the penis from the point of the urethral opening placed well back on the ventral surface of the organ. It appears as a kind of “hood” on the dorsum, gradually becoming less well marked as its ventrally continued folds approach the urethral opening.
The penis of the 70 mm stage (text-fig. 2) shows a more definite condensation of the mesodermal tissues and a beginning of differentiation into cavernous tissue. A thick layer of desquamating cells is spread over the surface of the glans, and is in continuity with tl1e epitrichiall layer of the general skin. The skin on the dorsal aspect of the penis is thrown into folds, as if it were developing at a much more rapid rate of growth than the differentiating mesoderm into cavernous tissue. At the same time it appears as if flowing over the dorsum of the glans which at this stage is clearly separated from the shaft of the penis by a well-marked sulcus. On the ventral aspect of the organ the urethral opening is placed farther towards the extremity of the penis than in the 40 mm. stage (text-fig. 5 B), and the folds of ectodermal tissue passing downwards and forwards, from the dorsal prepuee, gradually become less well marked as they approach the urethral opening.

|
|
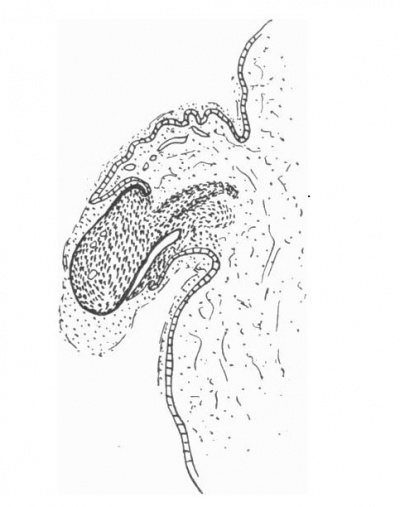
| Text-Fig. 1. L.S. Penis of human foetus 40 mm. C.R. length. Note the ridge of ectoderm behind the corona and the glans, and the thin layer of desquamating cells on the surface. | Text-Fig. 2. L.S. Penis of human foetus 70 mm. C.R. length. Note the dorsal ectodermal tissues thrown into loose folds, and the distribution of the desquamating cells. |

|

|
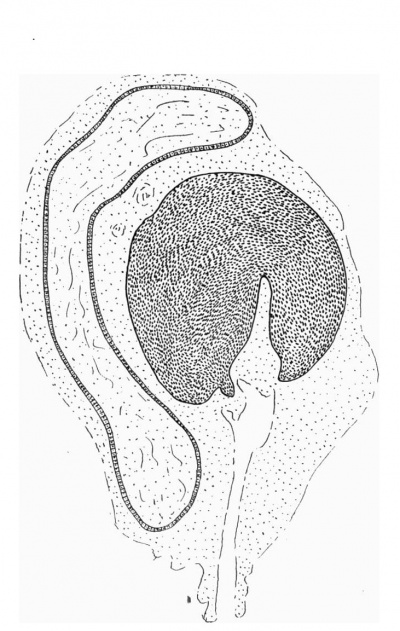
| Text-Fig. 3. L.S. Penis of human foetus 100 mm C.R. length. The prepuce is here seen “overflowing” the glans and enclosing a layer of desquamating cells between it and the glans. | Text-Fig. 4. L.S. Penis of human foetus 170 mm C.R. length. This section shows the prepuce covering the whole of the glans, with the layer of desquamating cells connecting the two. Note the “plug” of desquamating cells closing the opening of the urethra. |
Text-fig. 5. Shows the lateral and ventral aspects of wax-plate reconstructions of three stages in the development of the prepuce. A, 40 mm C.R. length; B, 70 mm. C.R. length; C, 170 mm C.R. length.
The genital area of the 100 mm. stage (text-fig. 3) shows a still further differentiation in form and structure. The ectodermal tissue of the body of the penis has the appearance of flowing over the clearly differentiated glans. The layer of dcsquamating cells on the surface of the glans is irregularly thickened, the ectodermal tissue of the developing prepuee encloses a layer of the desquamating cells, and the prepuee more nearly encircles the glans. The wax-plate reconstruction shows the urethral opening in a position still more advanced towards the tip of the glans than in any of the earlier stages.
- 1 The term “epitrichial” is an unfortunate one, as it implies the presence of hair, and this layer is found in the human embryo at a stage long before there is any trace of the epithelial cords from which the hair is developed. In addition, it is developed to an even greater extent in those areas of the skin, as for example the prepuee, in which there is a complete absence of hair follicles than in the hair bearing areas. This growth of the epitrichial layer in such areas suggests that there may be some connection between it and the absence of hair development; that is in those regions where the epithelial hair shafts do not develop, the ectodermal growth expresses itself in a generalised proliferation as against the specialised growth of the anlagen of the hair.
At the 170 mm. stage (text-fig. 4) the prepuce completely encloses the whole surface of the well-defined glans and a well-marked layer of desquamating cells connects the two parts, prepuce and glans. Sagittal sections of the penis at this stage appear as if an invaginating layer of cells had insinuatcd itself between the glans an(l the prepuce, as suggested by Retterer and Neuville.[1]
|

|
| Text-Fig. 6. C.S. Glans region of penis from human foetus 100 mm. C.R. length. Note the two sub-glans folds closing off a portion of the desquamating epithelium within the developing glans portion of the urethra. | Text-Fig. 7. C.S. Glans region of penis from human foetus 170 mm. C.R. length. The glans urethra is here seen closed off enclosing within it a. plug of desquamating epithelium. Note the continuity of the glans tissue with the mesodermal tissue of the prepuce at the frenulum, and the V-shaped line of fusion between the two sides of the prepuee epithelium on its ventral aspect. |
These cells in addition form a kind of plug closing off the fossa navicularis and the external opening of the urethra. The cavernous tissue at this stage is well differentiated. The wax-plate reconstruction shows the external opening of the urethra to have advanced to its adult terminal position (text-fig. 5 C). Coronal sections of the glans region of the penis show in the 100 mm. stage, that the portion of the urethra which is developed by the closing of the genital folds on the under surface of the penis ends just behind the glans region. Serial sections of this region near the tip of the penis show that the under surface of the glans presents a well-marked groove. This groove is formed by the growing downwards of two narrow but distinct folds of cavernous tissue (text-fig. 6). As the sections were traced in a backward direction these folds were seen to become longer and thicker and presently to fold towards each other in the middle line, meet, and fuse to form that part of the urethra which lies within the glans. In forming this part of the urethral canal the two folds enclose a small mass of the desquamating epithelial cells which forms the thick layer on the whole of the outer surface of the glans. When the folds completely join and the glans portion of the urethra is formed, the desquamating cells thus caught within the canal appeared as a plug of cells as described in the sagittal sections within the fossa navicularis of the 170 mm. C.R. stage (text-fig. 4). The sections at this stage also show the line of fusion of the ventral margins of the overflowing prepuce and a continuity between these skin folds and the sub-glans groove; this continuity forms the frenulum.
The further development of this region is seen in the 170 mm. stage (textfig. 7) cut in coronal section. Here the urethral canal is found completely formed within the cavernous tissue of the glans. The glans is connected on its ventral side by a fold of tissue to the prepuce. This is the frenulum. The cavernous tissue, as in the earlier stages, appears to be continuous with the connective tissue elements of the prepuce, and the two are not separated from one another by any epithelial or other ectodermal tissue.
Discussion
A study of the sections described shows a series of clearly marked changes in the development of the glans penis and prepuce from a naked terminal glans without a urethral canal to one completely surrounded by a prepuce with an apical urethral opening. At first ectodermal_and mesodermal portions of the penis appear to increase in size at approximately the same rate, but from the 40 mm. stage onward, when a slowing in the rate of growth occurs in the condensation of the mesoderm which precedes the differentiation into cavernous tissue, the ectodermal tissues increase greatly in size; and by this inequality of growth they flow over the dorsum of the glans, the lateral margins of the folds becoming less evident as they converge on the ventral surface of the penis to the point of the urethral opening.
The part of the urethral tube which lies within the glans portion of the penis, and which has its long axis disposed in a vertical direction as opposed to the cavernous portion which has its long axis in a transverse plane, and known as the fossa navicularis, is developed as a separate entity from that portion of the urethra which lies within the shaft of the organ. It begins as two folds on the under aspect of the glans, and these meet in the middle line, then by their fusion they close off a canal, the fossa navicularis. The fusion of these two folds, which are continuous behind with the apical portion of the skin folds of the prepuce, causes the skin folds of this region to be drawn together in the middle line. They meet and fuse there with the result that the loose dorsal folds of prepuce are drawn over the sides down to the under surface of the glans in line with the fusion of the sub-glans folds. The fusion of these two sets of folds gives rise to the frenulum. But if these two folds fail to meet and fuse, the cleft prepuce found in cases of hypospadius of the glans results.
As the closure of the sub-glans folds occurs, they enc.lose what appears to be a plug of the desquamating epithelial cells. The presence of such a plug of cells at this stage of development has long been recognised, but its origin has been explained in quite a different way. Wood-Jones[5] describes it as a “ cell invasion, which burrows into the glans itself, and forms the urethra (at first as a solid plug) within the glans. The urethral plug is broken down by the seventh month of foetal life and the fossa navicularis and lacuna magna are f0I‘lIlC( .”
The sections studied in the present series of observations at no stage showed such a “cell invasion” and from them there appears to be no doubt but that the solid plug is simply a mass of desquamating epithelial cells on the surface of the differentiating glans which have been cut off, trapped as it were, between the margins of the sub-glans folds.
At first the terminal part of the differentiating glans has disposed on its surface a thin layer of desquamating ectodermal cells, which are continuous with those of the so-called epitriehial layer of the general skin. This layer of desquamating cells becomes thicker, and when the skin overflows the glans, it encloses a layer of these cells, giving sections of this part the appearance of an “invaginating layer”, as described by Retterer and Neuville.[1]
The breaking down of the cells of this layer was studied by Edington[6], who drew attention to their changing form in the later stages of foetal life. The cells of the glans are columnar in shape with deeply stained nuclei, rapidly merging into the cubical-shaped cells of the epithelium of the prepuce at the frenulum. The layer of cells between the glans and prepuce are large and irregularly polygonal in outline, with faintly stained nuclei, while in places they become flattened, and form “nests” of cells arranged in “whorls”. The central cells of these nests degenerate and break down, leaving clefts and cavities, and by the extension of these clefts and their ultimate fusion the preputial space is formed.
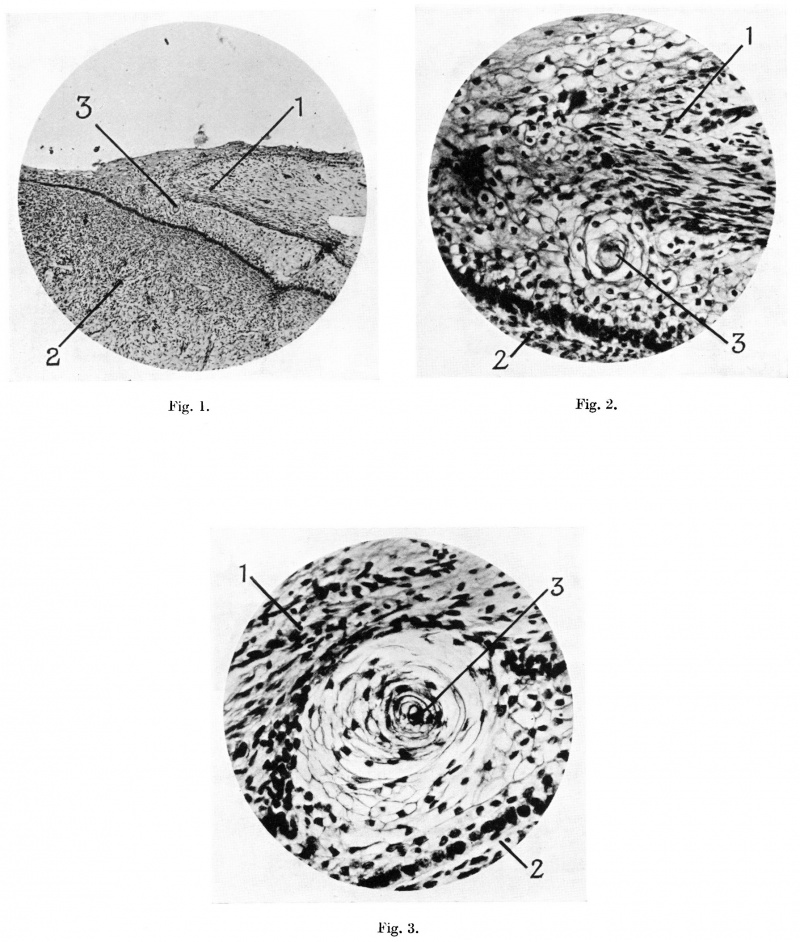
A study of the microphotograph shown in Plate I, fig. 1, of a section of the terminal portion of the penis from a foetus of 100 mm. C.R. length shows the continuity of the epitriehial layer of cells on the external surface of the prepuce with the invaginating—like layer of cells between the prepuce and the glans. A “eell—nest” seen in this photograph is shown in higher magnification in Plate I, fig. 2. This shows the “whorled” arrangement of the cells which form the nest, and the degenerative changes in the cell which lies at its central portion. The epitriehial and invaginating-like layer of cell are seen to be composed of the same irregularly flattened polyhedral type. The mierophotograph of one of these eell-nests in the invaginating-like layer between the prepuee and glans from a foetus of 170 mm. C.R. length (Plate I, fig. 3) shows a further stage in the degenerative process of its cells, a process which results in the formation of the elefts within this cell layer, which in turn run together to form the definite preputial space.
I should like to acknowledge my indebtedness to Prof. Thomas Walmsley for his kindly criticism and advice in the preparation of this paper.
Summary
- The glans penis is at first naked and uncovered by a prepuee. It is demarcated from the shaft of the organ by a shallow groove.
- During differentiation of the cavernous tissue of the penis, growth of this tissue does not occur at so rapid a rate as that of the ectodermal tissues, and the latter becomes thrown into folds on the dorsum of the penis.
- The folds of ectodermal tissue then appear to flow over the dorsum of the glans as the beginning of the prepuee. The margins of the prepuee are obliquely disposed along the sides of the glans towards the position of the urethral opening, which at this time is placed well back on the ventral surface of the penis.
- By the further growth of the prepuee its margins flow over the sides of the glans. and are drawn together on its ventral surface by two sub-glans folds, so that the glans is completely surrounded by the prepuee. The line of fusion between the sub-glans folds and the margins of the prepuee form the frenulum.
- During these changes (lesquamation of the general ectodermal tissue occurs, and the glans becomes covered by a layer of desquamating cells which is continuous with the epitriehial layer of eetoderm. The prepuee as it flows over the glans enfolds this layer of desquamating cells, and these at a later date degenerate, leaving elefts which in turn run together and form the definitive preputial space.
References
- ↑ 1.0 1.1 1.2 Retterer Ed. And Neuville H. (1915). C.R. Soc. Biol., Paris, t. XXVIII, p. 388.
- ↑ Hart DB. On the role of the developing epidermis in forming sheaths and lumina to organs, illustrated specially in the development of the prepuce and urethra. (1907) J Anat Physiol. 42(1): 50–56.
- ↑ Frazer JE. A Manual of Embryology. (1940) Bailliere, Tindall and Cox, London. 1st ed. p. 44.
- ↑ Spaulding MH. The development of the external genitalia in the human embryo. (1921) Contrib. Embryol., Carnegie Inst. Wash. Publ. 81, 13: 69 – 88. p. 85.
- ↑ Wood-Jones, F. (1910). Brit. med. J. vol. I, p. 138.
- ↑ Edington GH. (1910). Glasgow med. J. (Reprint)
Explanation of Plate I
Fig. 1. Microphotograph of terminal part of penis from human foetus of 100 mm. C.R. length, to show the continuity of the cells of the epitriehial layer of the prepuee with those of the layer between the prepuee and glans. Pointers: 1, prepuce; 2, glans penis; 3, cell nest.
Fig. 2. Microphotograph of a “cell-nest” in the layer of cells between prepuce and glans, from a human foetus of 100 mm. C.R. length. Pointers as fig. 1.
Fig. 3. Microphotograph of a “cell-nest” in the layer of cell between prepuce and glans, from a human foetus of 170 mm. C.R.. length. Pointers as fig. 1.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 25) Embryology Paper - Notes on the development of the prepuce (1935). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Notes_on_the_development_of_the_prepuce_(1935)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G