Paper - Normal haemopoiesis in intra-uterine and neonatal life: Difference between revisions
mNo edit summary |
m (→Material) |
||
| (28 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Header}} | {{Header}} | ||
{{Ref-Gilmour1941}} | {{Ref-Gilmour1941}} | ||
{| class="wikitable mw-collapsible mw-collapsed" | |||
! Online Editor | |||
|- | |||
| [[File:John Ernest Frazer.jpg|thumb|150px|alt=File:John Ernest Frazer.jpg|J. Ernest Frazer (1870-1946)]][[file:Mark_Hill.jpg|90px|left]] This historic 1941 paper by Gilmour describes early human embryo blood formation. | |||
<br> | |||
Some of the embryos in this study were from [[Embryology History - Ernest Frazer|Ernest Frazer]]. | |||
<br><br><br><br> | |||
<br> | |||
'''Modern Notes:''' {{blood}} | |||
<br> | |||
{{Heart Links}} | |||
<br> | |||
|} | |||
{{Historic Disclaimer}} | {{Historic Disclaimer}} | ||
=Normal Haemopoiesis in Intra-Uterine and Neonatal Life= | |||
J. R. Gilmour | |||
From the Bernhard Baron Institute of the London Hospital | |||
Plates VII—X (1941) | |||
( | ==Introduction== | ||
During a study of the morbid histology of erythroblastosis foetalis I realised that the literature upon foetal haemopoiesis in man was insufficient for a reliable control. The classical Works of Van der Stricht (1891 and 1892), Saxer (1895-96) and Maximow (1909) on haemopoiesis in small mammals were valuable, but the latter differs from that in man in certain respects. The present work was therefore undertaken to establish a control but was extended to cover early embryonic life and the first weeks of extra-uterine life. | |||
==Material== | |||
Embryos and foetuses | Embryos and foetuses | ||
(1) Presomite embryo (A), (Frazer). Ovum 2 | (1) Presomite embryo (A), (Frazer). Ovum 2.1 x 1.95 x 1 mm (measured from the chorionic epithelium excluding the rudimentary villi). Amniotic vesicle 0.17 x 0.143 x 0.1 mm. Entodermic vesicle 0-127 x 0-11 mm. Embryonic plate 0.065 x 0.045 mm. Age about 16 days, probably slightly younger than Peters’ embryo (1899). | ||
from the chorionic epithelium excluding the rudimentary villi). Amniotic | |||
vesicle 0 | |||
Embryonic plate 0 | |||
younger than Peters’ embryo (1899). | |||
(2) Presomite embryo (B), (Frazer). Ovum 2 | (2) Presomite embryo (B), (Frazer). Ovum 2.2 x 2.1 X 1.7 mm. Yolk sac 0.225 x 0.190 mm. Embryonic plate 0-323 mm. Age about 19 days, probably between the Jung (1908) and Meyer (1924) embryos in development. | ||
0 | |||
probably between the Jung (1908) and Meyer (1924) embryos in development. | |||
(3) Presomite embryo (C), (Frazer). Ovum 4 | (3) Presomite embryo (C), (Frazer). Ovum 4.66 x 4.5 x 3 mm. Amnion 0.55 x 0.5 mm. Yolk sac 1.2 x 0.75 mm. Embryonic plate 0-55 X0-36 mm. Age about 19 days, probably between the Von Spee (1896) and Jones-Brewer (1935) embryos in development. | ||
0 | |||
Age about 19 days, probably between the | |||
(1935) embryos in development. | |||
(4) Embryo of about 20 pairs of somites, (Frazer). Yolk sac only. Age | (4) Embryo of about 20 pairs of somites, (Frazer). Yolk sac only. Age 3 to 4 weeks. (5-43) Embryos and foetuses 3-200 mm. crown-rump, 4-5 weeks to 23 weeks. Nine from [[Embryology History - Ernest Frazer|Professor Frazer’s collection]]. Yolk sac only in the 12.5, 15.5 and 26.9 mm specimens. | ||
(44-57) Foetuses 320-546 mm. crown-heel, 24-25 weeks to full term. One post-mature. | |||
( | |||
(58-68) New-born infants, 2-21 days old, 406-533 mm crown-heel. Five premature. | |||
The ages of the presomite embryos are derived from Grosser (1924), those of the older specimens from the table of Mall (1910) according to the crown-rump measurement in embryos and foetuses up to 200 mm, and the crown-heel measurement in longer foetuses. The specimens under 9 mm long were measured by serial section when embedded, the others when fresh or fixed. The specimens are referred to according to their length, whether crown-rrump (C-R) or crown-heel (C-H). | |||
==Methods of preparation== | |||
My specimens were fixed in 4 per cent. saline-formaldehyde, dehydrated in alcohol, cleared in chloroform and embedded in paraffin. | |||
The number of tissues examined and the stains applied varied with each specimen. Much reliance had to be placed upon Ehrlich’s heematoxylin and eosin, but most specimens were stained also by Jenner’s method according to Turnbull (1931). The prussian-blue reaction was done on sections from most specimens. The tissues examined are mentioned in the description of haemopoiesis. | |||
The | |||
The 13 Frazer embryos had been treated with clove oil between the alcohol and chloroform and in some instances a little celloidin had been added to the clove oil. Most of the sections had been stained by Heidenhairfs. iron haematoxylin. | |||
==General Description of Haemopoiesis, with Nomenclature== | |||
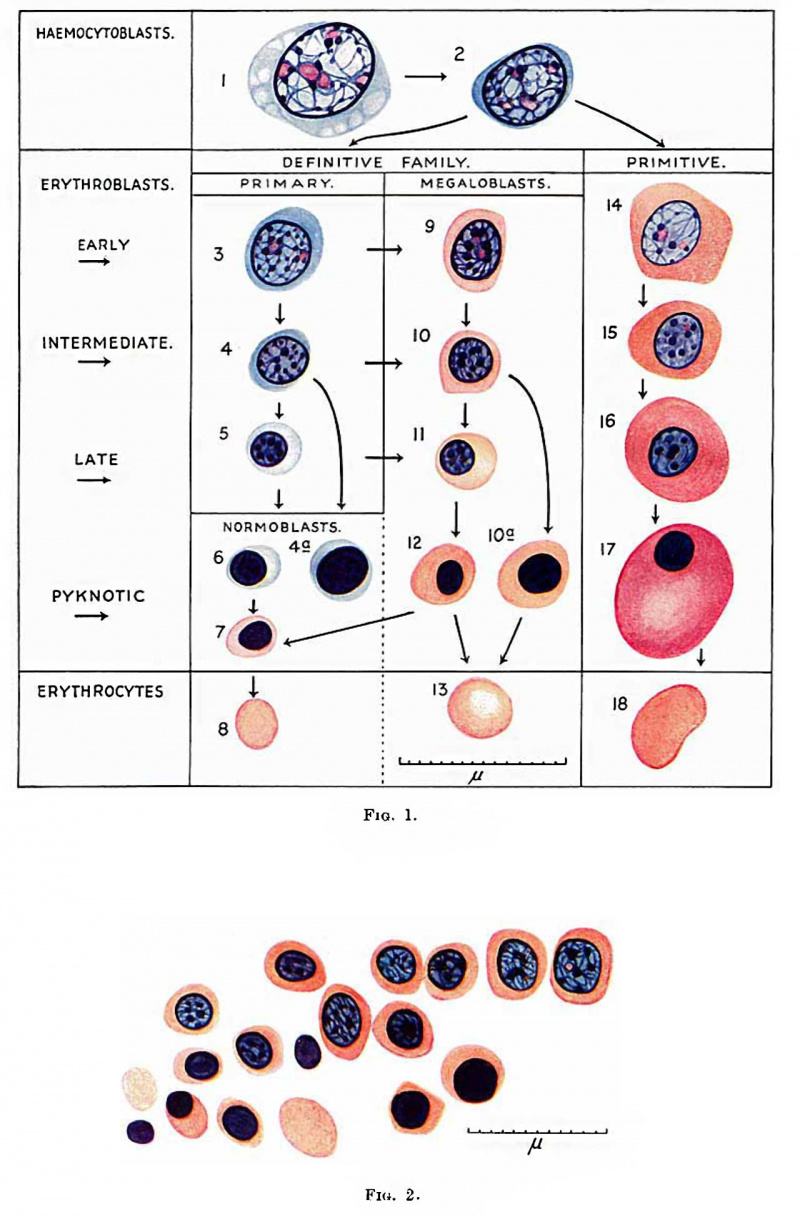
[[File:Gilmour1941 fig01.jpg|thumb|Fig. 1. Normal Haemopoiesis]] | |||
In embryos and foetuses there is a system of mesodermal cells of many potentialitie corresponding to the reticulo—endothelia1 system of post-foetal life. Within this system in embryos must be included the vascular endothelium and, to a lesser extent, the interstitial mesoderm of the yolk sac. From the fixed cells of this system free cells arise in vessels or solid tissues. | |||
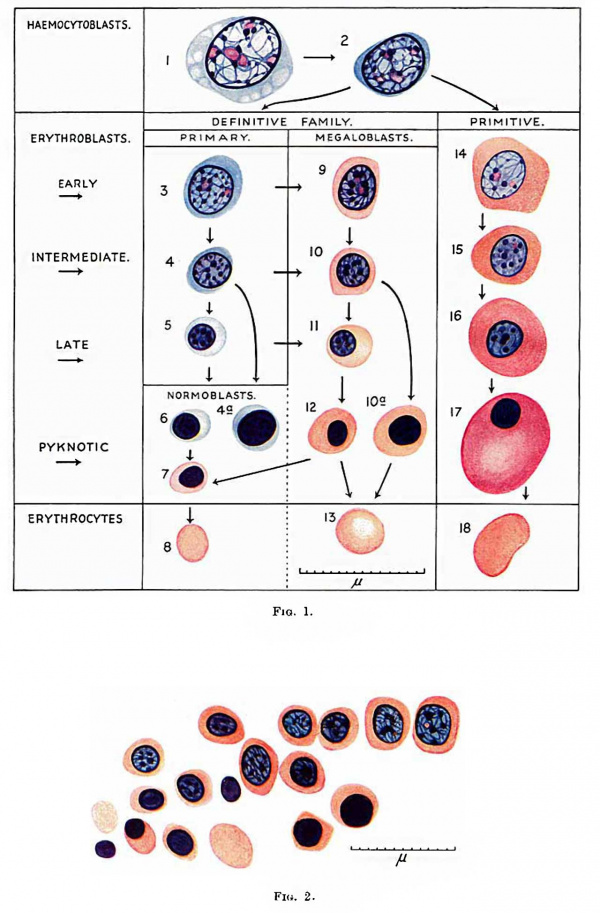
One type is the haemocytoblast ([[:File:Gilmour1941 fig01.jpg|Fig. 1]]). It is the most primitive cell, whose potentiality of differentiation is directed to haemopoiesis only. It forms red blood corpuscles, granular leucocytes, lymphocytes and megakaryocytes. Its appearance has been described by Turnbull (1934). In foetal and postfcetal life the heemocytoblast does not show evidence of migration but in early embryos it is frequently amoeboid. Occasionally in blood Vessels and very often in the connective tissues of early embryos it shows pseudopodia and in the process of migration in the tissues may appear distorted. Some of these early haemocytoblasts are slightly smaller and more lymphocytoid in appearance than those in fig. 1, but still larger than the large lymphocyte. The early haemocytoblast appears also to be able to form histiocytes. These slight differences in the early heemocytoblasts do not warrant giving them a special name. They correspond to the primary wandering cell of Saxer (1895-96), the lymphocytoid wandering cell of Maximow (1909, 1927) and the mesamoeboid cell of Minot (1912). Maximow (1927) regarded his lymphocytoid wandering cell as identical with the haemocytoblast and sometimes called it such. | |||
Turnbull ( | |||
The other type of cell that may arise directly from the reticulo-endothelial cell is the free kistéocyte. As already mentioned, it appears to arise also from early heemocytoblasts in embryonic life. It has a round, oval or kidneyshaped nucleus and a moderate or abundant amount of pale-staining cytoplasm which may be vacuolated. Its chief potentiality is to take up Various substances or cells into its cytoplasm and become a phagocyte. In the cedematous rarefied mesoderm of the chorion and in places in the embryo are to be found cells not unlike histiocytes. They differ in having a more abundant, more Vacuolated pale-staining cytoplasm. These are the same as the Hofbauer cells of the placenta (Hofbauer, 1905). The potentiality of this cell is not obvious. It is probably connected in some way with the tissue fluids, as they are numerous in oedernatous tissues. | |||
In embryos and | In young embryos the megalca/ryocytes differ from those in older specimens and I call these early megakaryocytes. They are smaller and have one, two or three separate nuclei or one bi- or trilobed nucleus. They do not possess pseudopodia, whereas in later embryonic and middle foetal life those in the tissues are often surrounded by numerous minute pseudopodia. | ||
The | The nomenclature in connection with the development of the red blood eorpuscles is, with minor differences, that used by Tumbull (1934). | ||
The | The term erythroblast ([[:File:Gilmour1941 fig01.jpg|Fig. 1]]) is used for any nucleated cell whose potentiality of differentiation is directed only towards the formation of erythrocytes. It is not used in the sense of Lowit (1886), who introduced the term for haemoglobin-free precursors of nucleated red blood cells. The term erythrocyte is used for a nucleus-free cell containing haemoglobin stainable by eosin, not, as is sometimes used, for an eosinophilic red blood corpuscle Whether nucleated or not. | ||
During the differentiation of the erythroblast the nuclei show all gradations of diminution in size, condensation of chromatin structure and diminution in size and number of nucleoli from that of the haemocytoblast, till eventually the nucleus becomes shrunken and pyknotic. The pyknotic nucleus is then extruded and the cell becomes an erythrocyte. The erythroblasts may be subdivided into early, intermediate and late, those with pyknotic "nuclei having a similar size of cell and nucleus to those in the intermediate or late stage. Nuclei do not as a rule become pyknotic until they have become very small and reached the late stage. Sometimes larger nuclei in the intermediate stage of development become pyknotic (fig. 1, cells 4a and 10a). During their development, erythroblasts multiply by mitotic division. In a late stage of development they probably do not multiply and those with pyknotic nuclei certainly do not. Mitotic division is usually accompanied by variable shrinkage in size of the cell. The nucleus of the erythroblast is usually round, but late and pyknotic forms are frequently deforIned-Tu1'nbull’s crenated, moniliform or rosette-like distortion, probably from lowering of the surface tension between nucleus and cell body, as suggested by Albrecht (Weidenreich, 1904). | |||
is | |||
During the | During the development of the erythroblasts, haemoglobin stainable by eosin appears in the cytoplasm. The nucleus is not lost until haemoglobin is thus made visible. The stage of development of the erythroblast at which visible haemoglobin appears varies, and accordingly there are two types of erythrocyte formation. In one type haemoglobination is late and begins in small erythroblasts with pyknotic or almost pyknotic nuclei and the resulting erythrocyte is within the normal size for adult blood. This is nownoblaetelc erythropoiesis and a normocyte is formed (fig. 1, cells 3-8). In the other type heemoglobination occurs in larger erythroblasts with larger nuclei having still a Well preserved nuclear structure. This is megaloblastic erythropoicsis (fig. 1, cells 9-13 or 14-18). The resulting erythrocyte is usually a megalocyte, larger than the normocyte found in normal adult blood, but during the evolution of a megaloblast so great a reduction in size may occur that a normocyte is formed. An erythroblast with basophil cytoplasm and its nuclear structure still preserved can develop along either the normoblastic or the megaloblastic series. Such a cell has been called by Turnbull a primary erythroblast. The term had been applied previously by Schridde (1907) to the erythroblasts in embryos of 1-9 mm., later called by Maximow “ primitive ”. Since MaximoW’s terminology in this respect has been generally adopted Schridde’s priority to the use of the term primary erythroblast has been waived. The remaining erythroblasts develop from the primary and may be called secondary erythroblasts. Schridde used the term “ secondary erythroblasts ” for those first appearing in the liver in a 12-5 mm. embryo, corresponding to Maximow’s definitive erythroblasts, and Maximow used the term as an alternative for his definitive erythroblasts. ' The term is not now used for these cells and is available for erythroblasts which are not primary. It is useful to call secondary erythroblasts nomwblasts when they are scarcely larger than a norrnocyte and develop only into normocytes, and megaloblasts when haemoglobin is visible in larger cells which will form as a rule megalocytes but sometimes norinoeytes. The nucleus of the normoblast is always small and pyknotic or almost pyknotic, while that of the megaloblast has variable degrees of preservation of nuclear structure (Ehrlich and Lazarus, 1909) or is pylmotic. | ||
having a | |||
The normoblasts are small cells with pyknotic or almost pyknotic nuclei and are subdivided into basophil if there is no visible heemoglobination of the cytoplasm, and polychromatic or orthochromatic if there is incomplete or complete heemoglobination as revealed by eosin staining. The normoblast in foetal tissues which have been dehydrated in alcohol, cleared in chloroform and embedded in paraffin is not more than 5 [L and the normocyte not more than 4-5 p, in diameter. They are slightly smaller than the corresponding cells in adults. | |||
small | |||
or | |||
the | |||
Megaloblasts are either polychromatic or orthochromatiti/»——there are no basophil megaloblasts. The megaloblasts may be divided into early, intermediate and late according to the degree of preservation of nuclear structure, corresponding to similar stages of the primary erythroblast. The final stage is one with a pyknotic nucleus and may be called in short a pyknotic megaloblast (Turnbull, 1934). The megaloblasts are larger than the normoblasts. A late megaloblast can either develop from an intermediate megaloblast or a late primary erythroblast. An intermediate megaloblast can either develop from an early megaloblast or from an intermediate primary orythroblast. As has been mentioned, a megaloblast during maturation of its nucleus may show considerable reduction of its cytoplasm, so that when the nucleus is pyknotic the cell is small and identical with a normoblast. This may be referred to as megalo-normoblastic erythropoiesis (fig. 1—change from cell 12 to cell 7——and fig. 2). | |||
After the first two or three weeks of post-foetal life normal erythropoiesis is entirely normoblastic. In embryoni.c life "it is entirely megaloblastic ; in early foetal life it is megaloblastic and megalo-normoblastic, while in later foetal life it is partly megaloblastic and partly normoblastic. | |||
is | |||
The terms primitive and definitive erythroblaets are used in the sense of Maximow to describe the erythroblasts of two distinct erythropoietic families in the embryo. The term primitive erythroblast was first used by Bryce (1906) for the first erythroblasts in Lepidosiren paradoxa but these differ from those in man. As has been mentioned, Schridde used the terms primary and secondary erythroblasts for the cells Maximow later called primitive and definitive erythroblasts. The primitive erythroblasts (fig. 1, cells 14-17) are first formed in the yolk sac and later are present and multiply in the blood of early embryos but soon disappear. The definitive erytlmoblasts are formed in the yolk sac and embryo later than the primitive. There is no essential difference between the two types of erythroblasts. The primitive erythroblast and the megalocyte it forms are, however, as a rule considerably larger than the definitive forms, though small varieties of about the same size as the latter occur. Late and pyknotic late primitive erythroblasts often swell up to a large size, apparently by imbibition of fluid, and usually have an elliptical shape. The nuclei of the primitive erythroblasts usually differ slightly from those of the definitive. They are paler and the cl'J.romatin threads more delicate in the primitive early and intermediate erythroblasts than in the definitive, and nucleoli may be seen in the primitive intermediate erythroblasts but never in the definitive. The primitive erythroblasts are all heemoglobinated and megaloblasts. The cytoplasm is usually more deeply stained by eosin than in definitive secondary erythroblasts. Primitive erythroblasts arise from hsemocytoblasts, probably directly. I have not been able to recognise primitive primary erythroblasts. | |||
in early | |||
===Plate VII=== | |||
[[File:Gilmour1941 plate07.jpg|800px]] | |||
'''Fig. 1.''' Red blood cells and their precursors. 1 and 2, haemocytoblasts ; 3, 4 and 5, early, intermediate and late primary erythroblasts; 6 and 7, basophil and erthochromatic normoblasts; 8, normocyte; 9, 10, l1 and 12, early, intermediate, late and pyknotic megaloblasts; 13, megalocyte; 14, 15, 16 and 17 early, intermediate, late and pyknotic primitive megaloblasts; 18, primitive erythrocyte; 4a, basophil normoblast with prematurely pyknotic nucleus; 10a, megaloblast with prematurely pyknotic nucleus. Jenner. | |||
5, early, intermediate and late primary | |||
early, intermediate, late and pyknotic primitive | |||
erythrocyte; 4a, basophil | |||
10a, | |||
'''Fig. 2.''' 125 mm foetus. Focus of megalo-normoblaetic erythropoiesis in neck. One early and several intermediate megaloblasts forming pyknotic megaloblasts and - with loss of nucleus - a Megalocyte, and — from shrinkage in size — orthochromatic normoblasts and — by loss of nucleus - a normocyte. Jenner. | |||
One early and several intermediate | |||
The primitive erythroblasts were first seen by Erb (1865), who likened them to the blood cells of the frog because of their great size and elliptical shape. Howell (1891) also recognised them and called them ancestral oorpuscles because they resembled those of reptilia and amphibia. | |||
==Development of Blood Vessels and Cells Before a Complete Circulation is Established (Embryos up to 3 mm)== | |||
The origin and development of the first blood vessels is very obscure owing to the small number of well preserved and stained very young human embryos available for study by any one observer and the scant attention given to the vascular development in many of the original descriptions. The three presomite embryos of Frazer which I have examined are by themselves insufficient for me to draw conclusions ; the following account therefore takes into consideration the observations in the literature. | |||
The | The presomite embryos that will be mentioned are the Teacher-Bryce no. 1 (1908)*, Miller (1913, quoted by Streeter, 1920), Linzenmeier (1914)*, Frazer (A), Peters (1899), Mollendorff (l921)*, Fetzer (l9l0)*, Sch1agenhauferVerocay (1916), Tea-cher-Bryce no. 2 (1908)*, Jung (1908), Frazer (B), Meyer (1924), Strahl-Beneke (l9l0)*, Herzog (1909), von Spec (1896), Frazer (C), Jones-Brewer (1-935), Minot (1912), Streeter (1920), Grosser (1931), Heuser (1932), Grosser (1913), Ingalls (1918), McIntyre (1926-28), Eternod (1898-99), Boerner-Schwarzacher (1923), von Spec (1889) and Triepel (1917). They are placed in order of development according to Grosser (1924), if included in his list, and according to the individual author in specimens subsequently described. I have placed the Frazer embryos according to their probable development. The somite embryos are those of Ingalls (1920) 2-3 pairs of somites, Jagerroos (1934) about 6 pairs of somites, Dandy (1910) 7 pairs of somites, Low (1907-08) 13-14 pairs of somites, 2-6 mm., Thompson (1906-07) 23 pairs of somites, 2'5 mm. and Frazer, about 20 pairs of somites. Descriptions of other embryos were examined but found to be of no value. | ||
and | |||
of Frazer | |||
* Quoted by McIntyre (1926-98). | |||
Yolk | ===Yolk sac=== | ||
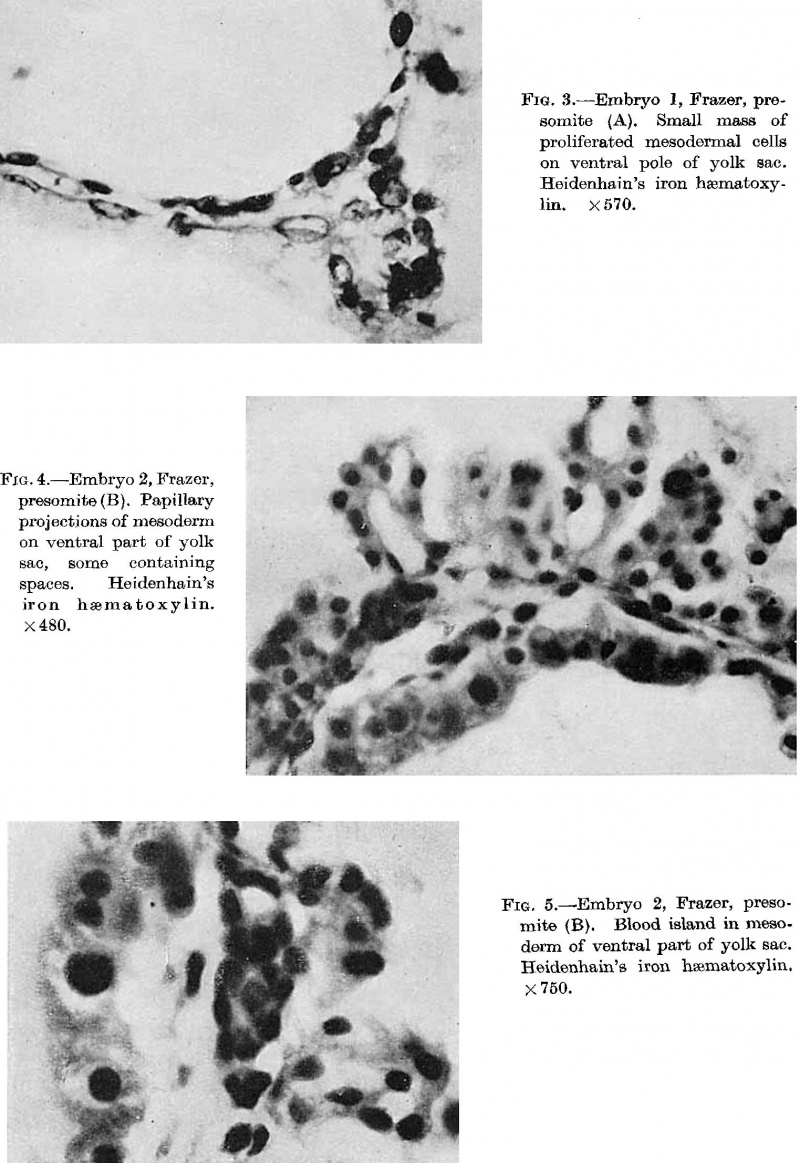
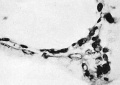
The first vessels arise from solid cellular masses which have | The first vessels arise from solid cellular masses which have proliferated from the mesothelium. The latter is the single layer of flattened mesodermal cells which first covers the yolk sac after the formation of the chorionic cavity. They had not appeared in the Teacher—Bryce (no. 1), Linzenmeier or Fetzer embryos according to McIntyre, the Miller embryo according to Streeter, Peters’s embryo according to Lewis (1912) or the Jung or Herzog embryos. A single mass of this kind is first seen in the Frazer presomite (16-day) embryo. In this the yolk sac consists of two layers of endothelial-like cells, the inner entodermal, the outer mesodermal. On the ventral aspect of the sac there is a solid rounded proliferation of mesodermal cells about 4 cells wide, projecting about 40 p. high into the chorionic cavity (fig. 3). This is probably angeioblastic. | ||
proliferated from the mesothelium. The latter is the single layer | |||
of flattened mesodermal cells which first covers the yolk sac after | |||
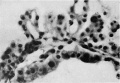
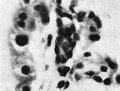
the formation of the chorionic cavity. They had not appeared in | Mesodermal cell masses were present in the Mollendorif, Teacher Bryce (no. 2) and Strahl-—Beneke embryos according to McIntyre and in the Schlagenhaufer—Verocay embryo. They are present in the Frazer presomite 19-day embryos. Here the masses project as papillae into the chorionic cavity. I believe vessels arise from the masses by loss of cells in the centre while the peripheral cells become endothelial. From the endothelial cells hmmoglobin-free basophil cells arise and become free in the lumen. These are haemocytoblasts. Isolated vessels containing free cells constitute the blood islands of Pander (1817), observed by him in chick embryos. One of the Frazer presomite 19-day embryos appears to be the youngest with blood islands. In it several proliferations of mesodermal cells on the ventral aspect of the yolk sac project as papillae from the outer surface. Some papillae contain empty spaces, like vessels, some of which are lined with flattened cells (fig. 4). The spaces appear to have arisen by vacuolation and loss of cells in the centre of the papillae. Deeper in the mesoderm beneath the papillae are a few well formed endothelial-lined vessels. Four of these contain groups of free cells in the lumen and constitute blood islands (fig. 5). The cells are basophil and most can be identified as haemocytoblasts. Others cannot be identified because of crowding. The cells probably arose from the vessel wall, since some plump endothelial cells are present as if in process of becoming free. It appears certain that blood vessel precedes blood cell formation. Connections between the different spaces and vessels could not be made out but in the absence of reconstructions they cannot be excluded.) In the Frazer presomite 19-day embryo (0) similar appearances are present except that the papilla and blood islands are more numerous and occupy about the ventral half of the yolk sac. Further, while some of the free cells are typical haemocytoblasts others are slightly smaller and more lymphocytoid. Blood islands are present in the Meyer (1924) and von Spee ( 1896) embryos and are probably constant in all older embryos. The haemocytoblasts differentiate into haemoglobinated primitive megaloblasts, but a few persist and a few apparently differentiate into histiocytes. The Jones-Brewer embryo is the youngest containing haemoglobinated cells; Bloom ( 1938) describes some of the free cells as haemoglobinated primitive erythroblasts. When haen1o— globinated cells become constantly present is diflicult to estimate. | ||
the Teacher—Bryce (no. 1), Linzenmeier or Fetzer embryos according | |||
to McIntyre, the Miller embryo according to Streeter, Peters’s | ===Plate VIII=== | ||
[[File:Gilmour1941 plate08.jpg|800px]] | |||
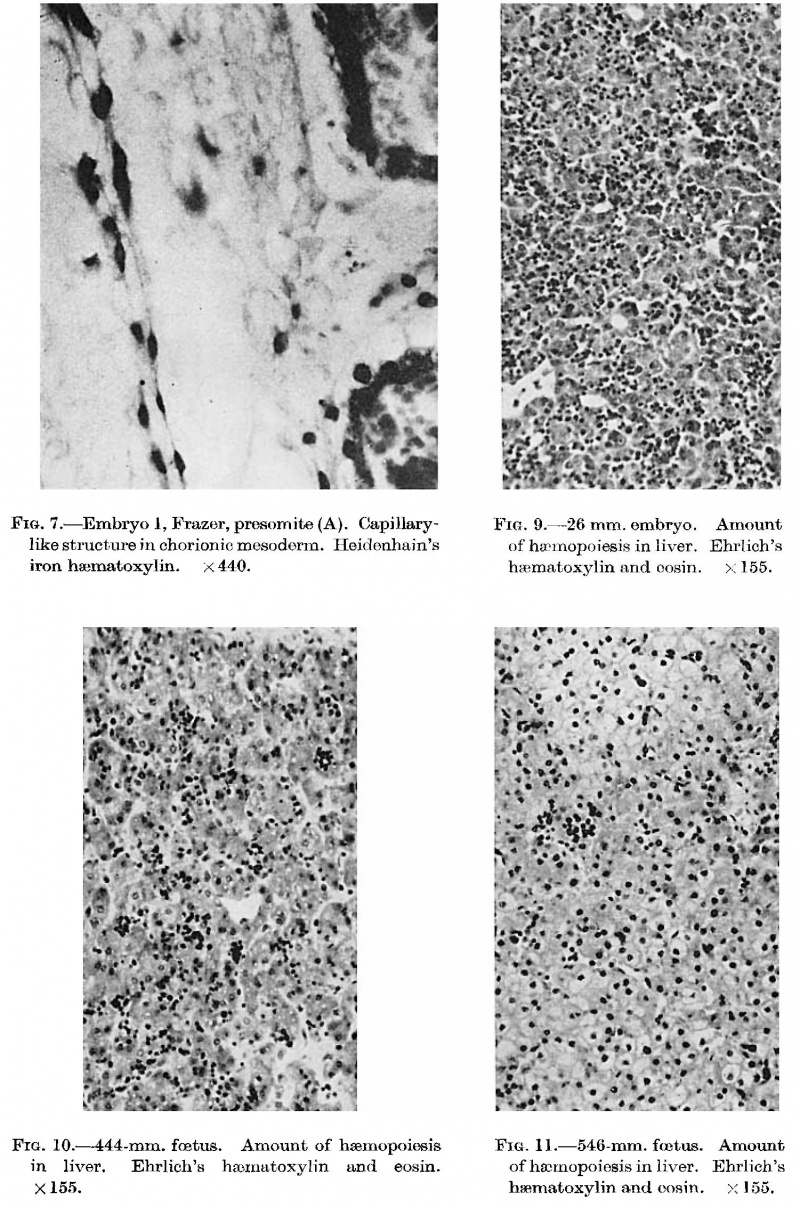
'''Fig. 3.''' Embryo 1, Frazer, presomite (A). Small mass of proliferated mesodermal cells on Ventral pole of yolk sac. Heidenhain's iron haematoxylin. x570. | |||
of mesodermal cells on | |||
'''Fig. 4.''' Embryo 2, Frazer, presomite (B). Papillary projections of mesoderm on ventral part of yolk sac, some containing spaces. Heidenhain's iron haematoxylin. x480. | |||
on | |||
'''Fig. 5.''' Embryo 2, Frazer, prosomite (B). Blood island in mesoderm of ventral part of yolk sac. Heidenhain's iron haematoxylin. x750. | |||
sac | |||
* {{Online Editor}} - Heidenhain's iron haematoxylin - an iron alum hematoxylin stain used for staining muscle striations and mitotic structures blue-black. Named after Rudolph Heidenhain (1834-1897) a German histologist and physiologist. (More? [[Histology stains]]) | |||
iron haematoxylin. | |||
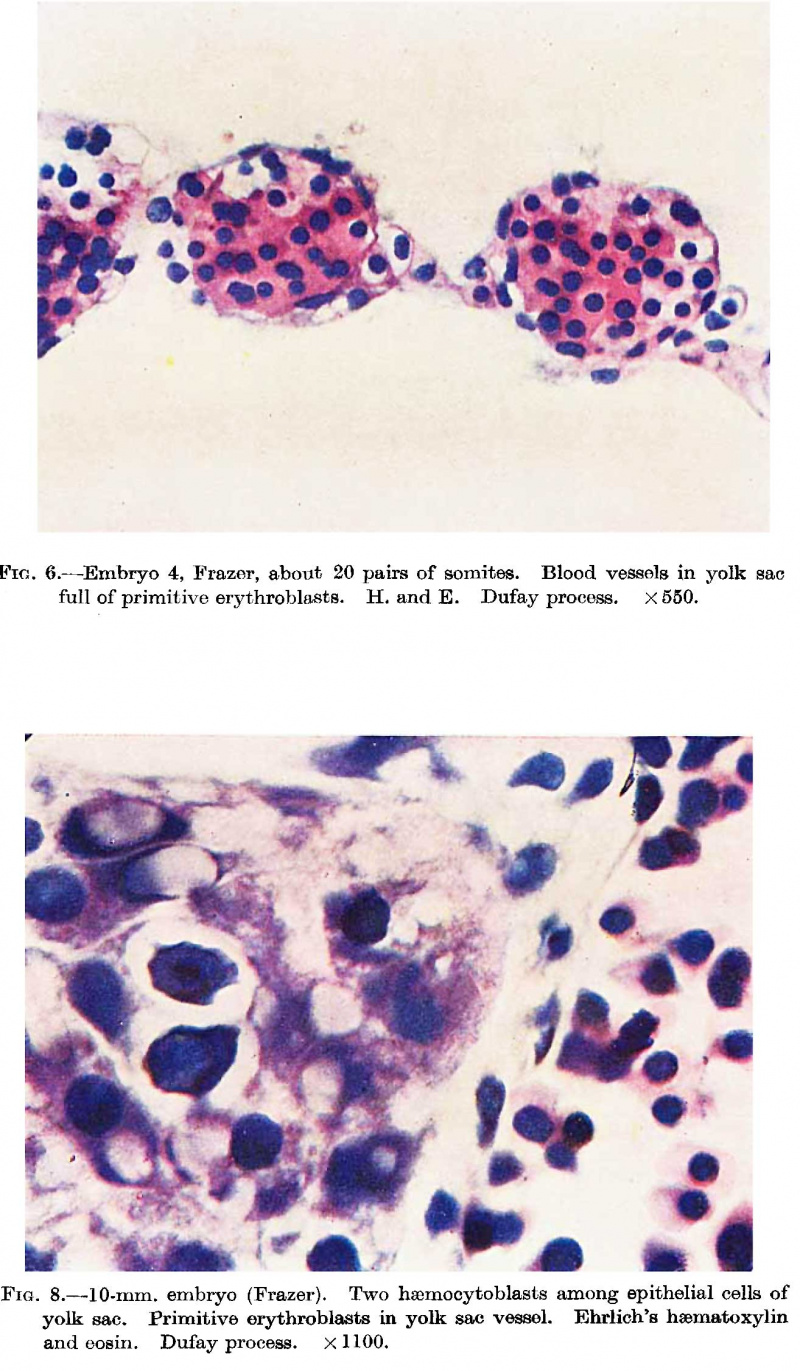
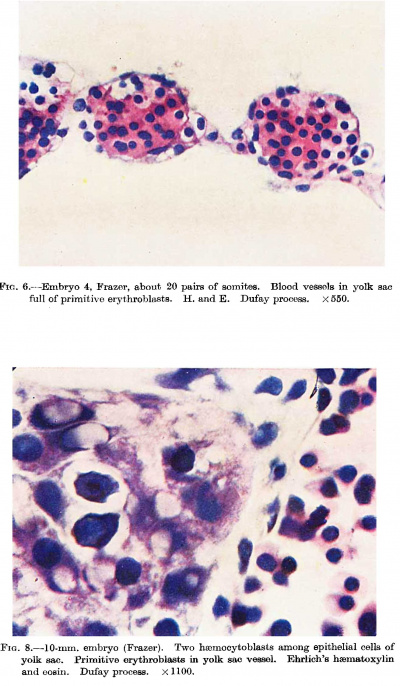
cells the centres of which become haemoglobinated and subsequently | They are present in the McIntyre and probably in all older embryos but are undoubtedly present in some of the embryos intermediate in development between the J ones-Brewer and McIntyre, such as the Minot, Grosser (1931) and Heuser embryos. The persistence of hsemocytoblasts after the formation of erythroblasts is mentioned only in the Jones—Brewer embryo by Bloom (1938), and in the Minot embryo, where they are called mesamoeboids. They are also present in the Frazer embryo of about 20 pairs of somites. Their presence, however, can be presumed in embryos of intermediate development. The first appearance of histiocytes is apparently in the Frazer embryo of about 20 pairs of somites, but no doubt they are present in younger somite and perhaps some presomite embryos. In this embryo the yolk sac in rather more than its ventral half shows numerous vessels distended with cells (fig. 6). The vessels project from both the outer and inner aspects of the sac, especially the former. The great majority of the cells are haemoglobinated early and intermediate megaloblasts, and less numerous late and pyknotic late megaloblasts, all of the primitive type. The cells stain deeply eosinophil with haematoxylin and eosin, but Jennerstained sections are not available to show the degree of chromatophilia. Many are in mitosis. Besides these there are a very few hsemocytoblasts and phagocytic histiocytes. The vessels are probably united to each other to a considerable degree. The blood vessels and islands first appear at the ventral pole of the yolk sac and later involve the ventral half of the sac. Fewer and smaller islands may be found at a later date in the other hemisphere of the sac. Little can be concluded about the union of the separate vessels to form a net. Probably union of blood islands occurs early but the net is not complete till late, possibly not till the yolk sac vessels have joined the embryonic. The above view of the histiogenesis of blood islands differs from that of Jones and Brewer, Streeter, and McIntyre. In their embryos they describe mesodermal cell masses in which central cells become in part free cells while the peripheral cells become endothelial. McIntyre describes syncytial masses of mesodermal cells the centres of which become haemoglobinated and subsequently break up into free cells. | ||
break up into free cells. | |||
===Chorion and body stalk=== | |||
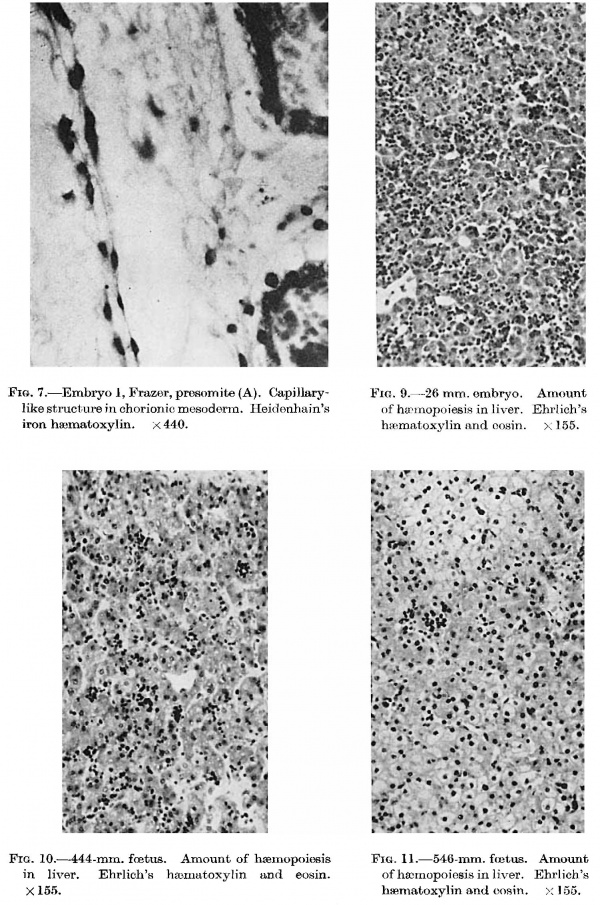
In the chorion, Bremer’s theory (1914) of the histiogenesis of vessels has not been disproved. | In the chorion, Bremer’s theory (1914) of the histiogenesis of vessels has not been disproved. | ||
He states that the Vessels are derived from the mesothelium of the body stalk. The mesothelium is at first limited to the yolk sac, as in the Frazer presomite (16-day) embryo, but later spreads as a continuous or interrupted | He states that the Vessels are derived from the mesothelium of the body stalk. The mesothelium is at first limited to the yolk sac, as in the Frazer presomite (16-day) embryo, but later spreads as a continuous or interrupted layer over the body stalk and finally lines the chorionic cavity completely. From the mesothelium funnel-shaped ingrowths pass into the body stalk mesoderm. By closure of the lumen. of a funnel at some point near its opening into the chorionic cavity, isolated spaces lined with mesothelium are formed inside the body stalk. The space may remain connected by solid cellular cords with the orifice of the funnel or with the surface mesothelium. From the inner ends of funnels or from the spaces formed from them, multiple nets of tubes pass out into the body stalk and extend into the remainder of the chorion. The tubes have no definite endothelial lining and he calls them unli_ned spaces. By a process which he calls delamination, solid cellular cords—-his angioblastic cords_arise from the walls of the unlined spaces and lie in the lumen. Angioblastic cords may also grow out from the inner ends of the mesothelial funnels and no_t be enclosed in unlined spaces. At an early stage spaces, called angiocysts, may form in places in the angioblastic cords and later a continuous lumen changes the cords into vessels. The separate nets unite to form a continuous vascular net in the body stalk and remainder of the chorion. His theory was formed from a study chiefly of the Grosser (1913), Minot, and Herzog embryos. McIntyre found similar structures in his own and the Teacher-Bryce (no. 2) embryo and Grosser in his 1931 embryo. | ||
layer over the body stalk and finally lines the chorionic cavity completely. | |||
From the mesothelium funnel-shaped ingrowths pass into the body stalk mesoderm. By closure of the lumen. of a funnel at some point near its opening | The scantiness of the material I have examined does not permit me to dispute Bremer’s theory but two observations appear to disagree with it. Firstly, angioblastic activity is first present in the Frazer presomite (16~day) embryo. The chorionic mescderm is in general poor in cells, stellate and spindle, but adjacent to the embryo, in the part that would become the body stalk, it is more cellular than elsewhere and contains a few small groups, either syncytial or bounding very small spaces. There are also a few strands of elongated cells, in single or double layer, which pass out into the neighbouring mesoderm. One of the strands (fig. 7), separated from the chorionic cavity by only a very few cells, has developed a lumen and resembles a capillary. As the mesothelium is limited to the yolk sac these angioblastic structures probably arose from undifferentiated mesodermal cells. Secondly, in the Frazer presomite ( 19-day) embryos, lined capillary-like empty tubes are present in the body stalk and chorion, very few in the first and many in the second embryo. Some strands two cells wide without lumina are also present but no solid cellular cords enclosed in unlined spaces. In the body stalk of these embryos a few larger end0thelial—lined spaces are also present. On the other hand in the Frazer embryo of about 20 pairs of somites, in addition to lined capil1ary—like empty tubes, there are several unlined spaces containing short cellular cords either solid or with minute lumina. | ||
into the chorionic cavity, isolated spaces lined with mesothelium are formed | |||
inside the body stalk. The space may remain connected by solid cellular | |||
cords with the orifice of the funnel or with the surface mesothelium. From | |||
the inner ends of funnels or from the spaces formed from them, multiple | |||
nets of tubes pass out into the body stalk and extend into the remainder of | |||
the chorion. The tubes have no definite endothelial lining and he calls them | |||
unli_ned spaces. By a process which he calls delamination, solid cellular | |||
cords—-his angioblastic cords_arise from the walls of the unlined spaces and | |||
lie in the lumen. Angioblastic cords may also grow out from the inner ends | |||
of the mesothelial funnels and no_t be enclosed in unlined spaces. At an early | |||
stage spaces, called angiocysts, may form in places in the angioblastic cords | |||
and later a continuous lumen changes the cords into vessels. The separate | |||
nets unite to form a continuous vascular net in the body stalk and remainder | |||
of the chorion. His theory was formed from a study chiefly of the Grosser | |||
(1913), Minot, and Herzog embryos. McIntyre found similar structures in | |||
his own and the Teacher-Bryce (no. 2) embryo and Grosser in his 1931 | |||
embryo. | |||
Angioblastic activity in the body stalk and chorion is absent from the Teacher-Bryce (no. 1), Linzenmeier and Fetzer embryos according to McIntyre, from the Miller embryo according to Streeter and from the Peters and Schlagenhaufer-Verocay embryos. In the Mollendorff embryo according to McIntyre there are channels lined with flattened cells in the chorionic mesoderm which do not join up to form a continuous system. Angioblastic activity is present in the Teacher-Bryce (no. 2) embryo according to McIntyre and is probably constant in older embryos. | |||
Heemopoiesis occurs in the body stalk independently of that in the yolk sac. In the present state of knowledge this cannot be said to be constant. In the Minot, Grosser (1931), Ingalls (1918), 1VIcIntyre and Triepel presomite embryos and Ingalls’s (1920) embryo of 2-3 pairs of somites and in Dandy’s embryo of 7 pairs of somites free cells are present in some body stalk vessels which have no connection with yolk sac vessels. The cells are probably for the most part haemoglobinated primitive erythroblasts but the presence of haemoglobin is 11ot stated in all instances. It is probable that the cells arose from heemocytoblasts derived from the endothelium but their histiogenesis is not described. Dandy and McIntyre describe blood cell formation in chorionic vessels apparently apart from the body stalk. J agerroos in his embryo of about 6 pairs of somites describes blood cells in isolated chorionic vessels, the cells having been formed extravascularly and subsequently included in vessels. These are however only isolated observations of haemo 'poiesis in the chorion apart from the body stalk. Besides the intravascular formation of blood cells in the body stalk the Minot, Heuser, Grosser (1913), McIntyre and Dandy embryos are said to show blood islands consisting of groups of cells apparently free in the body stalk mesoderm. McIntyre describes the cells as haemoglobinated and perhaps haemoglobin was present in the cells of some of the others. The nature of these so-called blood islands is very doubtful as they are very poorly described. I doubt whether they represent an extravascular formation of blood cells derived from the mesodermal cells. I think also that the term blood island should be reserved for blood cell formations inside isolated vessels. If blood cells were present inside body stalk vessels then perhaps some of the extravascular groups of cells could be explained by continued multiplication of cells which had escaped from the vessels. This undoubtedly occurs in older embryos as I will describe. In the J ones—Brevver and Boerner—SchWarzacker embryos blood islands are described in the body stalk but their relation to vessels is not mentioned. | |||
McIntyre | |||
mesoderm | |||
===The embryo=== | |||
The number of observations on the histiogenesis of vessels Within the embryo itself is few. The appearances in von Spee’s (1889, 1896) and Triepel’s presomite embryos and Ingalls’s (1920) embryo of 2-3 pairs of somites suggest that cardiac and vascular endothelium arises in situ from mesodermal cells. Ingalls believed that the vessels arise in multiple sites. In the present state of knowledge it must be presumed that blood islands consisting of blood cell formation in isolated vessels do not occur. The blood island Ingalls describes in the posterior end of the right aorta of his embryo of 2-3 pairs of somites is an isolated observation and the nature of the cells and their mode of origin are not described. | |||
vessels. | |||
The | ===The establishment of the circulation=== | ||
It is certain that an umbilical circulation between the body stalk and the embryo is established before a vitelline between the yolk sac and the embryo. This is seen in Eternod’s presomite embryo, somewhat precociously, and in Dandy’s. The blood in the circulation I was probably derived from blood islands in the body stalk. In Ingal.ls’s embryo of 2-3 pairs of somites a similar condition is developing, the yolk sac vessels being still unconnected with the embryo while the right umbilical artery communicates with the right aorta. Whether or not this is the rule cannot be stated but there are no observations of the establishment of the vitelline before the umbilical circulation. Both umbilical and vitelline circulations were established in Low’s embryo of 13-14 pairs of somites (2-6 mm.) and Thompson’s embryo of 23 pairs of somites (2-5 mm.). At this time it may be presumed that the cells of the blood are for the most part derived from the yolk sac but they will have joined cells from the body stalk in embryos with blood islands in this site. In a few embryos some may have come from chorionic vessels apart from the body stalk and if Ingalls’s observation is correct some may have been derived from. the embryo itself. It is not possible to state the time when the chorionic vessels fill with blood but it is probably about the time of the formation of the complete circulation. | |||
embryo of 2-3 pairs of somites | |||
blood | |||
===Summary=== | |||
It is | It may be concluded that vessels arise from mesodermal cells independently in three areas, the yolk sac, the chorion——-perhaps at first limited to the body stalk—and the embryo. The vessels in each of these areas unite to form nets or systems. The three systems later unite with each other and the complete circulation is established. In some if not all embryos the circulation between body stalk and embryo begins before that between yolk sac and embryo. Blood islands form constantly in the yolk sac and consist of separate vascular units containing blood cells. The cells are first hsemocytoblasts which arise from the vessel wall. Later the cells are almost entirely primitive erythroblasts but a few haemocytoblasts persist and a few differentiate into histiocytes. Intravascular blood formation occurs frequently, perhaps constantly in the body stalk, and when the umbilical but not the vitelline circulation has been established it will supply the embryonic vessels with blood. Doubtful blood formation has been recorded in the chorionic and embryonic vessels and in the mesoderm of the body stalk. The cytology and histiogenesis of blood formation outside the yolk sac at this period has unfortunately been neglected or very inadequately described. | ||
stalk and | |||
the | |||
body stalk | |||
vitelline | |||
with blood | |||
===Plate IX=== | |||
[[File:Gilmour1941 plate09.jpg|800px]] | |||
'''Fig. 6.''' Embryo 4, Frazer, about 20 pairs of somites. Blood vessels in yolk sac full of primitive erythroblasts. {{HE}} Dufay process. x550. | |||
'''Fig. 8.''' 10 mm embryo (Frazer). Two haemocytoblasts among epithelial cells of yolk sac. Primitive erythroblasts in yolk sac vessel. Ehrlich’s haematoxylin and eosin. Dufay process. x1100. | |||
This theory of the histiogenesis of blood cells and vessels is in opposition | This theory of the histiogenesis of blood cells and vessels is in opposition to that of His who in 1900, according to Bloom, deri.ved the blood cells and vessels from a special angioblastic tissue which arose from the yolk sac ontoderm. Minot (1912) accepts this theory and states that the angioblast maintains its independence throughout life. According to this theory the embryonic and chorionic vessels are extensions from those of the yolk sac. Minot derived the cells of the reticulo-endothelial system from the angioblast but the work of Maximow has shown that these cells are mesodermal and their potentialities can be assumed by undifferentiated mesodermal cells. | ||
to that of His who in 1900, according to Bloom, deri.ved the blood cells and | |||
vessels from a special angioblastic tissue which arose from the yolk sac | |||
ontoderm. Minot (1912) accepts this theory and states that the angioblast | |||
maintains its independence throughout life. According to this theory the | |||
embryonic and chorionic vessels are extensions from those of the yolk sac. | |||
Minot derived the cells of the reticulo-endothelial system from the angioblast but the work of Maximow has shown that these cells are mesodermal | |||
and their potentialities can be assumed by undifferentiated mesodermal cells. | |||
==Development of the Blood After Establishment of the Complete Circulation (Embryos from 3 to 12 mm)== | |||
In embryos of 3-9 mm., after the circulation is established, | In embryos of 3-9 mm., after the circulation is established, new blood cells are formed everywhere in the circulation equally by mitotic division of blood cells previously present. Perhaps to a very slight extent erythroblasts are formed by differentiation of haemocytoblasts in the circulation but the great majority are derived from preformed erythroblasts. At this stage almost all the blood cells are primitive erythroblasts in early, intermediate, late or pyknotic late stages: most are intermediate. With haematoxylin and eosin they appear fully haemoglobinated but Jenner sections are not available to show the degree of chromatophilia. A very few erythroblasts are binucleate. Very rarely one may be seen with vacuolated cytoplasm. Mitoses are numerous. In a 3—mm. embryo the erythroblasts average about 7-5 pt in diameter. The other embryos of this group (3-9 mm.) belong to Professor Frazer’s collection and in them the erythroblasts average about 10-5 p, in diameter. A few are small-—about 6 p.—or very large——about 16 u. The difference in average diameters is probably due to difl°erent methods of embedding. Some of the small erythroblasts might form erythrocytes within the normal variation of diameter for adult blood, 23.6. normocytes. This would constitute megalo—normoblastic erythropoiesis. Knoll (1929) describes a small proportion of definitive erythroblasts in the blood at this stage, calling them second generation cells, but these are undoubtedly only mall primitive erythroblasts. Besides the erythroblasts there are a few megalocytes, histiocytes—~—some phagocytic-———and haemoeytoblasts, most of which are slightly smaller and more lymphocytoid than the typical hsemocytoblast- The histiocytes contain nuclear fragments or brown granular pigment. In the aorta, groups of histiocytes may be present. The cells forming a cluster in the aorta of a 9-4.—mm. embryo of Minot (his figure 368) I would call histiocytes but he calls them mesamoeboids, which I believe correspond to heemocytoblasts. In the Frazer 4-5~mm. embryo (fig. 11) there are many more heemocytoblasts and histiocytes in the yolk sac vessels than in the embryonic vessels but in the other embryos at this stage this diiference is very slight. | ||
new blood cells are formed everywhere in the circulation equally | |||
by mitotic division of blood cells previously present. Perhaps to | |||
a very slight extent erythroblasts are formed by differentiation | |||
of haemocytoblasts in the circulation but the great majority are | |||
derived from preformed erythroblasts. At this stage almost all | |||
the blood cells are primitive erythroblasts in early, intermediate, | |||
late or pyknotic late stages: most are intermediate. With | |||
haematoxylin and eosin they appear fully haemoglobinated but | |||
Jenner sections are not available to show the degree of chromatophilia. A very few erythroblasts are binucleate. Very rarely one | |||
may be seen with vacuolated cytoplasm. Mitoses are numerous. | |||
In a 3—mm. embryo the erythroblasts average about 7-5 pt in | |||
diameter. The other embryos of this group (3-9 mm.) belong to | |||
Professor Frazer’s collection and in them the erythroblasts average | |||
about 10-5 p, in diameter. A few are small-—about 6 p.—or very | |||
large——about 16 u. The difference in average diameters is probably | |||
due to difl°erent methods of embedding. Some of the small | |||
erythroblasts might form erythrocytes within the normal variation | |||
of diameter for adult blood, 23.6. normocytes. This would constitute | |||
megalo—normoblastic erythropoiesis. Knoll (1929) describes a small | |||
proportion of definitive erythroblasts in the blood at this stage, | |||
calling them second generation cells, but these are undoubtedly | |||
only mall primitive erythroblasts. Besides the erythroblasts there | |||
are a few megalocytes, histiocytes—~—some phagocytic-———and haemoeytoblasts, most of which are slightly smaller and more lymphocytoid | |||
than the typical hsemocytoblast- The histiocytes contain nuclear | |||
fragments or brown granular pigment. In the aorta, groups of | |||
histiocytes may be present. The cells forming a cluster in the | |||
aorta of a 9-4.—mm. embryo of Minot (his figure 368) I would call | |||
histiocytes but he calls them mesamoeboids, which I believe correspond to heemocytoblasts. In the Frazer 4-5~mm. embryo | |||
(fig. 11) there are many more heemocytoblasts and histiocytes in | |||
the yolk sac vessels than in the embryonic vessels but in the other | |||
embryos at this stage this diiference is very slight. | |||
In all the embryos of this stage (3-9 mm.) the chorionic vessels | In all the embryos of this stage (3-9 mm.) the chorionic vessels are filled with blood. In all, primitive erythroblasts are scattered singly or in groups in the mesoderm of the embryo, chorion and umbilical cord. These undoubtedly result from hsemorrhage, but continued multiplication occurs, as is shown by the presence of mitotic figures. Haemorrhage is present in the yolk sac cavity in two and chorionic cavity in one. | ||
are filled with blood. In all, primitive erythroblasts are scattered | |||
singly or in groups in the mesoderm of the embryo, chorion and | |||
umbilical cord. These undoubtedly result from hsemorrhage, but | |||
continued multiplication occurs, as is shown by the presence of | |||
mitotic figures. Haemorrhage is present in the yolk sac cavity in | |||
two and chorionic cavity in one. | |||
The Frazer | The Frazer 10 mm. embryo is similar as regards the circulating blood and the presence of haemorrhages in the mesoderm, but there is, in addition, activity of the reticulo—endothelial system, chiefly directed to the formation of a new haemopoietic family, the definitive. Hsemocytoblasts, isolated or in groups, appear in the liver among the liver cells outside the sinusoids. Some lie in deep bays (lacunae of Neumann, 1874) within liver cells. In the sinusoids besides primitive erythroblasts there are a few haemocytoblasts and one early trilobed megakaryocyte is seen, while scattered or in groups are numerous histiocytes, many containing brown granular pigment or less commonly nuclear fragments or degenerated primitive erythroblasts. In the yolk sac are a few haemocytoblasts, singly or in groups, among the large entodermal epithelial cells (fig. 8). Some appear to lie in lacunae, as in the liver. Two early megakaryocytes are also present among the epithelial cells. In the yolk sac vessels, besides primitive erythroblasts and a few haemocytoblasts and histiocytes, there are a very few early megakaryocytes, groups of early and intermediate primary erythroblasts and intermediate megaloblasts, all of definitive type. | ||
blood and the presence of haemorrhages in the mesoderm, but there | |||
is, in addition, activity of the reticulo—endothelial system, chiefly | |||
directed to the formation of a new haemopoietic family, the definitive. | |||
Hsemocytoblasts, isolated or in groups, appear in the liver among | |||
the liver cells outside the sinusoids. Some lie in deep bays (lacunae | |||
of Neumann, 1874) within liver cells. In the sinusoids besides | |||
primitive erythroblasts there are a few haemocytoblasts and one | |||
early trilobed megakaryocyte is seen, while scattered or in groups | |||
are numerous histiocytes, many containing brown granular pigment | |||
or less commonly nuclear fragments or degenerated primitive | |||
erythroblasts. In the yolk sac are a few haemocytoblasts, singly | |||
or in groups, among the large entodermal epithelial cells (fig. 8). | |||
Some appear to lie in lacunae, as in the liver. Two early megakaryocytes are also present among the epithelial cells. In the yolk | |||
sac vessels, besides primitive erythroblasts and a few haemocytoblasts | |||
and histiocytes, there are a very few early megakaryocytes, groups | |||
of early and intermediate primary erythroblasts and intermediate | |||
megaloblasts, all of definitive type. | |||
The brain and spinal cord are surrounded by a zone of very | The brain and spinal cord are surrounded by a zone of very delicate connective tissue rich in young capillaries. These contain histiocytes and heemocytoblasts of the early, smaller and more lymphocytoid type in greater number than the general circulation. A few of the heemocytoblasts have small pseudopodia. Some of the histiocytes contain brown granular pigment, one a pyknotic primitive megaloblast. Some early forms of megakaryocytes are also present. Some of these are no larger than a hsemocytoblast, others are 2-3 times as large. They have one or two nuclei which are round, oval or bilobed. The cytoplasm is eosinophil and as a rule abundant. Outside the capillaries there are many amoeboid haemocytoblasts——some with pseudopodia, many histiocytes———some pigmented, and a few early megakaryocytes. This activity is especially marked at the base of the brain. | ||
delicate connective tissue rich in young capillaries. These contain | |||
histiocytes and heemocytoblasts of the early, smaller and more | |||
lymphocytoid type in greater number than the general circulation. | |||
A few of the heemocytoblasts have small pseudopodia. Some of | |||
the histiocytes contain brown granular pigment, one a pyknotic | |||
primitive megaloblast. Some early forms of megakaryocytes are | |||
also present. Some of these are no larger than a hsemocytoblast, | |||
others are 2-3 times as large. They have one or two nuclei which | |||
are round, oval or bilobed. The cytoplasm is eosinophil and as a | |||
rule abundant. Outside the capillaries there are many amoeboid | |||
haemocytoblasts——some with pseudopodia, many histiocytes———some | |||
pigmented, and a few early megakaryocytes. This activity is | |||
especially marked at the base of the brain. | |||
In the Frazer 12-mm. embryo the distribution is similar except | In the Frazer 12-mm. embryo the distribution is similar except that extravascular heemopoiesis is more extensive in the liver. | ||
that extravascular heemopoiesis is more extensive in the liver. | |||
Besides many hsemocytoblasts there are numerous cells of the | Besides many hsemocytoblasts there are numerous cells of the definitive series, namely early and intermediate primary erythroblasts, some intermediate and fewer early and late megaloblasts and a few early megakaryocytes. The latter lie outside and inside the sinusoids. | ||
definitive series, namely early and intermediate primary erythroblasts, some intermediate and fewer early and late megaloblasts | |||
===Summary=== | |||
In embryos of 3-9 mm. the blood cells in the vessels are of the primitive series ; almost all are formed by mitosis of erythroblasts previously formed ; some of the erythroblasts are of the diameter of normoblasts. A few erythroblasts, have reached the tissues by haemorrhage and multiply there by mitosis. In embryos of 10-12 mm. the blood vessels still contain cells of the primitive series but in the liver and yolk sac there is now active proliferation of haemocytoblasts, early megakaryocytes and erythroblasts of the definitive series. | |||
==Further Development of the Blood (Specimens over 12 mm)== | |||
mm | |||
===1. General circulation, excluding capillaries=== | |||
In the 18- and 19-5-mm. embryos the blood cells are almost entirely erythroblasts and erythrocytes in about equal numbers. Of the former, all or almost all are primitive, all are megaloblasts and the majority orthoohromatic ; a very few are early, many are intermediate but the majority late or pyknotic. Mitotic figures are numerous. A few heemocytoblasts, histiocytes and phagocytic histiocytes are also present. The first are usually slightly smaller than typical hsemocytoblasts and are lymphocytoid in appearance but larger than large lymphocytes. A few show small pseudopodia. | |||
In the | In the 26- and 28—mm. embryos about a quarter of the red blood corpuscles are nucleated. They are megaloblasts and the majority are primitive. A very few are early, a few intermediate, the majority late or pyknotic. Mitotic figures are very sparse. Haemocytoblasts are absent but there are a few lymphocytes which were probably formed directly from haemocytoblasts. Histiocytes are absent. In the 35-min. embryo the blood is similar except that there are no early and only a few intermediate megaloblasts and only one mitotic figure was seen. | ||
and the majority | |||
intermediate | |||
are | |||
In the | In one of the 48—mm. embryos about 10 per cent. only of the red blood corpuscles are nucleated. Most are late and pyknotic primitive megaloblasts. The great majority are fully haemoglobinated; a few of the definitive are polychromatic. Mitotic figures are absent. Besides these cells there are a very few lymphocytes and neutrophil myelocytes and leucocytes. | ||
corpuscles are nucleated. | |||
In | In the 65-mm. and all subsequent embryos the blood cells appear to be definitive. Not more than 5 per cent. of the red cells are nucleated. Most are late and pyknotic megaloblasts, a few are intermediate megaloblasts and normoblasts (4-5 p. in diameter). A few are polychromatic. Mitotic figures are absent. There are a Very few lymphocytes and neutrophfl myeloeytes and leucocytes. In the 7 6—mm. embryo the blood is similar except that a Very few intermediate primary erythroblasts are present. A very few of these are in mitosis. One eosinophil leucocyte was seen. | ||
red | |||
figures are absent. | |||
lymphocytes and | |||
In the | In the lO4.—mm. embryo the blood is similar except that now there are Very few early primary erythroblasts : a mitotic figure in a megaloblast was seen. In the 120- and 125-mm. embryos the blood is the same except that no early primary erythroblasts are present. | ||
that | |||
In the | In the 146—mm. embryo a film of fresh blood from the umbilical cord shows about 0-6 per cent. of the red cells to be nucleated. In 200 nucleated cells 5 per cent. are intermediate, 18 per cent. late and 34-5 per cent. pyknotic megaloblasts, 10 per cent. are normoblasts (not larger than 8 )u. in diameter), 2 per cent. inter~ mediate primary erythroblasts, 30 per cent. lymphocytes, 1 per cent. eosinophil and 0-5 per cent. neutrophil myelocytes. Almost all the erythrocytes are megalocytes, a few are normocytes (6-5 to 7-5 p, in diameter). Some of the nucleated and non-nucleated red cells are polychromatic. Two megaloblasts showed mitotic figures. Some leucocytes are present in the blood in sections but not in the film. | ||
in | |||
blood | |||
In the | In the 170-mm. embryo the blood in sections is similar to that in the 120- and 125-mm. embryos, except that only a very few intermediate megaloblasts are present and primary erythroblasts and mitoses are absent. In longer foetuses the blood was not studied in detail. There appeared to be no marked alteration and probably there was a gradual change to the state at birth. | ||
not in the | |||
===2. Yolk sac=== | |||
In the Frazer 12.5 mm embryo the vessels contain a few early, some late and pylmotic and many intermediate megaloblasts and a very few megaloeytes, all of the primitive series. A very few and small vessels contain small groups of early and intermediate primary erythroblasts and intermediate megaloblasts of the definitive generation. A very few small haemocytoblasts are also present: one early bilobed megakaryocyte was seen. Only three haemocytoblasts were seen, lying outside vessels among the epithelial cells. | |||
The Frazer 15 | The Frazer 15.5 mm embryo is similar except that a few late megaloblasts are present in the few small groups of definitive erythroblasts. Six early megakaryocytes and one histiocyte containing a pyknotic megaloblast were also seen. Amongst the epithelial cells outside vessels are a few groups of about a dozen cells consisting of early and intermediate primary erythroblasts of the definitive generation and one or two heemocytoblasts. | ||
megaloblasts are present in the few small groups of definitive | |||
erythroblasts. Six early megakaryocytes and one histiocyte | |||
containing a pyknotic megaloblast were also seen. Amongst the | |||
epithelial cells outside vessels are a few groups of about a dozen cells consisting of early and intermediate primary erythroblasts | |||
of the definitive generation and one or two heemocytoblasts. | |||
The 26.9 mm embryo is similar except that there are now numerous megalocytes. There are several histiocytes and phagocytic histiocytes but no megakaryocytes. The histiocytes contain pyknotic nuclear fragments or nucleated red cells : one contained brown granular pigment. A few scattered groups of early and intermediate primary erythroblasts with a very few intermediate megaloblasts are present outside vessels among the epithelial cells. | |||
are | |||
In | In a 48 mm embryo both the mesoderm and entoderm of the yolk sac are very atrophic and the wall of the sac is usually very thin. The blood in the vessels consists of megalocytes with a few late and pyknotic megaloblasts and a very few lymphocytes. There are no histiocytes and no megakaryocytes. There are now no foci of heemopoiesis in the vessels or among the epithelial cells. | ||
of | |||
pyknotic megaloblasts | |||
among the | |||
===3. Liver=== | |||
[[File:Gilmour1941 fig09.jpg|thumb|200px|Fig. 9. 26 mm embryo haemopoiesis in liver]] | |||
In | In an 18 mm embryo there is a conspicuous increase in amount of haemopoiesis from that in the l2 mm Frazer embryo and the blood formation is definitive. The second 18 mm, 19.5 mm and 26 mm ([[:File:Gilmour1941 fig09.jpg|fig. 9]]) embryos show further increases and the maximum amount found is in the last. The liver now shows great diffuse infiltration with hsemopoietic cells, with numerous foci of increased density of infiltration. There are numerous haemocytoblasts, early and intermediate primary erythroblasts and intermediate megaloblasts, and a few late primary erythroblasts and early, late or pyknotic megaloblasts. The primary erythroblasts and haemocytoblasts outnumber the megaloblasts. The cells for the most part lie in the parenchyma outside the sinusoids and many, especially heemocytoblasts, lie in bays or lacunae in the liver cells. Similar cells are found in the sinusoids, scattered or in small groups among the Inegalocytes or megaloblasts of the primitive series. A few eosinophil and neutrophil myelocytes and leucooytes and megakaryocytes lie within thesinusoids or scattered outside in the parenchyma. The portal systems are not formed but some neutro~ phil and eosinophil myelocytes and leucocytes are present in the connective tissue of the hilum. | ||
primary erythroblasts | |||
late megaloblasts | |||
the | |||
The degree of haemopoiesis relative to the parenchyma in the 26~mm. embryo is maintained in the 20 longer specimens up to 190 mm. in which the liver was examined. The heemopoiesis shows slight changes with increasing age. The early megaloblasts soon disappear and towards the end of this group the late megaloblasts outnumber the intermediate. Primary erythroblasts and heen1ocytoblasts still outnumber the haemoglobinated cells. Orthochromatic normoblasts (about 4-5 pr. in diameter) appear in scanty numbers in the 48 mm and longer embryos. Basophil normoblasts do not appear until the 190—mm. embryos and are then very scanty. In embryos of 48 - 180 mm inclusive, therefore, the normoblasts are derived from megaloblasts by considerable shrinkage of cytoplasm during development, that is, by megalo —norm oblastic erythropoiesis. The amount of leucopoiesis in the parench yma shows little increase, but in the connective tissue of the hilum it is increased and it is abundant in the portal systems, which are flrst well developed in the 48—mm. embryos. In six specimens of 65 - 190 mm, focal erythropoiesis similar to that in the parenchyma is present in the portal systems. In six specimens of 76 - 190 mm, scattered tissue mast cells lie in the portal systems and in four (65 - 190 mm) foci of lymphocytes are also present there. In the 170 mm foetus a very few tissue mast cells lie free in the sinusoids. | |||
[[File:Gilmour1941 fig10.jpg|thumb|200px|Fig. 10. 444 mm foetus haemopoiesis in liver.]] | |||
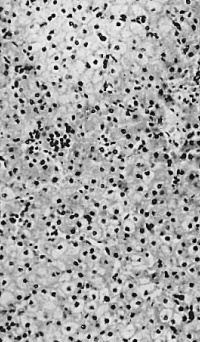
In five foetuses of 200-457 mm. ([[:File:Gilmour1941 fig10.jpg|fig. 10]]) a progressive decrease in the amount of heemopoiesis relative to parenchyma is apparent. It is still diffuse, with foci of increased density, but the density of the infiltration becomes progressively less. Intermediate and late primary erythroblasts increase relatively to the early forms and late megaloblasts outnumber intermediate forms. Basophil and orthochromatic normoblasts are scanty. The leucopoiesis remains the same in the portal systems and parenchyma. A few scattered tissue mast cells are present in the sinusoids of the 444 and 457 mm foetuses and in the portal systems of all. In the 200, 343 and 457 mm foetuses foci of erythropoiesis, and in the 330, 343 and 457 mm foetuses foci of lymphopoiesis, occupy portal systems. | |||
[[File:Gilmour1941 fig11.jpg|thumb|200px|Fig. 11. 546 mm foetus haemopoiesis in liver.]] | |||
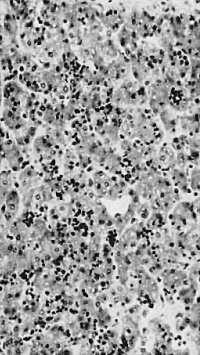
In nine older (470 - 546 mm) foetuses the haemopoiesis in the liver has decreased conspicuously ([[:File:Gilmour1941 fig11.jpg|fig. 11]]). It is new entirely focal. In the parenchyma outside the sinusoids are to be found widely separated small foci of intermediate and late primary erythroblasts, occasionally including some early forms or normoblasts and late and pyknotic megaloblasts. Some foci consist entirely of normoblasts and late and pyknotic megaloblasts. A very few heemocytoblasts are present outside the sinusoids. In the sinusoids are a few isolated or small groups of primary erythroblasts, normoblasts or late megaloblasts and a few isolated heemocytoblasts. A very few eosinophil myelocytes and leucocytes are present in the parenchyma or in the sinusoids. Megakaryocytes, constantly present in the younger foetuses, are now found in only 3 of the 9 older specimens and then in very scanty numbers. Haemopoiesis is considerably reduced in the portal systems. A few scattered or small groups of lymphocytes and eosinophil myelocytes and leucocytes and a few scattered tissue mast cells are present in most systems. In two specimens (508 and 533 mm) one or two tissue mast cells lie free in sinusoids. A 546 mm foetus is considerably post-mature but the amount of haemopoiesis is the same as in the others of the group. In all but two (495 and 540 mm), the liver cells are to a variable degree water-clear. This is undoubtedly due to glycogenic infiltration. | |||
===Plate X=== | |||
[[File:Gilmour1941 plate10.jpg|800px]] | |||
in | '''Fig. 7.''' Embryo I, Frazer, presomite (A). Capillary-like structure in chorionic mesoderm. Heidenhain’s iron haematoxylin. x440. | ||
'''Fig. 9.''' 26 mm embryo. Amount of haemopoiesis in liver. Ehrlich’s {{HE}}. x155. | |||
'''Fig. 10.''' 444 mm fetus. Amount of haemopoiesis in liver. Ehrlich’s {{HE}}. x155. | |||
'''Fig. 11.''' 546 mm fmtus. Amount of haemopoiesis in liver. Ehrlich’s {{HE}}. x156. | |||
mm. | |||
In the 11 newly born infants the amount of haemopoiesis in the liver shows a considerable diminution from that in late foetal life. A very few leucocytes and lymphocytes are present in some portal systems in most cases. In the 4-day infants and one of the 5-day infants some portal systems contain numerous myelocytes and leucocytes. Two or three small foci of late primary erythroblasts and normoblasts are present outside the sinusoids in the 2-day, one of the 3-day and the 4-day infants. In the remainder, including all infants over five days, erythropoiesis is absent. In the. 4- and 15-day infants a few usually degenerate -looking megakaryocytes are present. The water-clear appearance of the liver cells seen in the full-term foetuses is absent in the infants. A very few small bile thrombi are present in dilated intercellular canaliculi in one of the 5- and one of the 14-day infants, neither of which was jaundiced. Bile thrombi are not present in the liver of the 4-day infant, which was jaundiced. | |||
Free iron is present in the liver cells of all the embryos of 18 mm. and over except two. It is present either in the form of brown granular haemosiderin or as a diffuse prussian blue reaction. Both forms occur together except in the 70-mm. embryo, in which granules are absent. Only a minority of liver cells are affected. In very young embryos, before the formation of portal systems, the iron is localised as a rule to the cells around veins. In older subjects the usual site is in the cells bordering the portal systems and the cells of the bile ductules near their junction with the liver columns. The iron may be confined to these sites or may also occupy scattered cells anywhere in the lobule. In a few specimens in which intercellular bile canaliculi are sharply outlined, the iron favours that part of the liver cell which lies between the canalicular lumen and the nucleus. The total amount of intracellular iron is never great and does not show much variation in different subjects. However in nine, all 28-17 O-mm. specimens, the amount of iron is more abundant than in the remaining 30 livers examined. Towards the end of pregnancy (457 mm." onwards) iron is relatively scanty. In seven infants of 2-15 days the amount of iron in the liver cells is in keeping with that in late foetal life ; in the remainder, it is slightly more abundant. | |||
and | |||
The | |||
Several foetuses of 146 mm. or more show some intra- and extracellular haemosiderin granules in the portal systems. | |||
Iron pigment is present in a variable number of Kupffer cells in all but three (80-, 190- and 343-mm.) embryos and foetuses. It is usually in the form of granular hsemosiderin, but in some it shows as a diffuse prussian blue reaction in the cytoplasm. In all but two (2- and 4-day) infants the Kupffer cells contain more iron than in foetal life. The cells are often enlarged and sharply defined by a deep prussian blue reaction in the cytoplasm. Granules or globules of iron pigment are also present in some. In the other three infants the amount of iron in the Kupffer cells is about the same as in foetal life. This intense iron staining of Kupffer cells is probably related to the normal post-natal heemolysis. | |||
are also present in the | |||
the | |||
Phagocytic Kupffer cells containing erythrocytes, nucleated red cells or free nuclei are present in the sinusoids of all the livers of embryos, foetuses and infants. Sometimes only one, sometimes many such cells are seen. The number in infants does not differ from that in foetuses. | |||
of the | |||
===4. Connective tissue, including its capillaries=== | |||
In | In the two 18-mm. embryos and in the 19-5 mm. embryo, foci of a few intermediate and more late and pyknotic primitive megaloblasts and megalocytes are scattered in many connective tissues. These are undoubtedly similar to those seen in the earlier embryos and probably resulted from multiplication of primitive megaloblasts which had escaped from blood vessels. These foci are also present in the 26-, 28~ and 35-mm. embryos, but contain increasing numbers of erythrocytes, so that nearly all the cells in the last embryo are erythrocytes. | ||
In | In four embryos of 18-26 mm. many amoeboid haemocytoblasts, megakaryocytes and some histiocytes, most of them containing brown granular iron pigment and a few containing free nuclei or nucleated red cells, lie scattered in connective tissues. They are most numerous in delicate tissues such as the very vascular meninges, especially at the base of the brain. The megakaryocytes are usually early, with one, two or three nuclei or bi- or trilobed nuclei, and rarely have pseudopodia. Similar cells are present in greater number in the meningeal capillaries than in the general circulation. Several megakaryocytes are present in the capillaries of various tissues and organs throughout these embryos. The intravascular megakaryocytes do not have pseudopodia. There are also a few megakaryocytes, haemocytoblasts and histiocytes within the lumen of and in the tissue around small veins forming plexuses in the retroperitoneal tissue and neck in the two 18- and the 19-5—mm. but not in older embryos. In the 28- and 35—mm. embryos the capillaries and connective tissue of the meninges contain fewer of these cells, but iron-containing phagocytes are still found outside the capillaries. In the 48-mm. embryos these cells are absent from the meninges, while megakaryocytes are absent from the connective tissues and capillaries elsewhere and are confined to special sites—-—liver, marrow, spleen, lymph glands and stroma of lymph plexuses. In some connective tissues, especially the loose tissues of the neck, some amceboid haemocytoblasts and iron—containing histiocytes are present in decreasing numbers in foetuses up to 190 mm. Thereafter no special examination was made of the connective tissues in general. | ||
In one 18 - | In one of the 18-mm. embryos in the dense connective tissues of the head below the brain there are a few small scattered groups of heemocytoblasts and early primary erythroblasts of the definitive series. In the other 18-mm. embryo in the same site, especially about the eyes and cranial nerves, are more numerous and larger groups of either hsemocytoblasts and early primary crythroblasts alone or of intermediate and late megaloblasts alone or of mixtures of these cells. Many groups of megaloblasts with occasional intermediate primary erythroblasts are present in the meninges at the base of the brain. In the 19-5-mm. embryo there are similar groups in the meninges and a few elsewhere ; one was seen in the mesentery and two near the vertebral column. In the 26- and 28mm. embryos there are several scattered foci of primary erythroblasts of all stages, some having in addition intermediate and late megaloblasts, and more numerous foci of intermediate, late and pyknotic megaloblasts. Most lie in the tissues of the head and neck below the brain, fewer in the meninges at the base of the brain and in other tissues such as the choroid plexus, mesentery and limbs. In six 35-125—mm. specimens small foci of intermediate and late primary erythroblasts alone, or of intermediate, late and pyknotic megaloblasts alone or mixtures of these cells, are present in connective tissues in various places. In this group a very few orthochromatic cells of 4-5 p. diameter with pyknotic nuclei and thus identical with normoblasts are present in association with megaloblasts in embryos of 48 mm. or longer. As there are no basophil normoblasts, they undoubtedly arose from megaloblasts (megalo—normoblastic erythropoiesismfig. 2). In the 170- and one of the 190—mm. foetuses the number of erythropoietic foci in the connective tissues is considerably reduced and only a very few are present, consisting of late and pyknotic megaloblasts and orthochromatic normoblasts, the latter derived from the former. In the other 190-mm. and longer foetuses only special organs were examined, but probably erythropoiesis in connective tissues soon disappeared, as the foci in the 170- and first of the 190-mm. foetuses are very few and composed of erythrocytes and secondary erythroblasts only ; there are no groups of primary erythroblasts to suggest recent formation of foci. | ||
present in the | |||
in the | |||
Histiocytes and haemocytoblasts are not present in the capillaries | In five 18-28-mm. embryos a few eosinophil and neutrophil myelocytes and leucocytes lie scattered or in small groups in various connective tissues, especially the meninges, mesentery and retroperitoneal tissue. In the longer specimens up to 190 mm. it is rarer to find similar cells scattered in any connective tissue. | ||
in the lymph plexuses in the 48-mm. and longer embryos. In five | |||
28—125—mm. specimens a very few small foci of erythropoiesis | In five 26-48—mm. embryos a few small haemocytoblasts and eosinophil and neutrophil myelocytes and leucocytes lie in the adventitia of a few arteries in various parts of the body. In six 65-125-mm. specimens a few lymphocytes are present as well. Similar cells are also present in the carotid bodies of some of these foetuses. In the 170- and one of the 190-mm. foetuses these infiltrations are absent. | ||
occupy the connective tissue of cervical lymph plexuses. In the | |||
===5. Lymph plexuses=== | |||
In one 18 -«mm. embryo a few neutrophil and eosinophil myelocytes and leucocytes and amoeboid haemocytoblasts are present in the connective tissue of developing lymph plexuses in the neck, and a few haemocytoblasts and histiocytes lie in blood capillaries therein. In the 19 '5 — mm. embryo this infiltration is increased and the stroma of cervical lymph plexuses shows many eosinophil and neutrophil myelocytes and leucocytes, amoeboid haemocytoblasts, histiocytes—some containing brown granular iron pigmcnt—and a few megakaryocytes. A few hjstiocytes and haemocytoblasts are also present in the blood capillaries. In seven 26-75 - mm. embryos there are also a few lymphocytes. | |||
Histiocytes and haemocytoblasts are not present in the capillaries in the lymph plexuses in the 48-mm. and longer embryos. In five 28—125—mm. specimens a very few small foci of erythropoiesis occupy the connective tissue of cervical lymph plexuses. In the | |||
I 170- and one of the 190-mm. foetuses there is no special haemopoietic activity in the plexuses. | I 170- and one of the 190-mm. foetuses there is no special haemopoietic activity in the plexuses. | ||
===6. Lymph glands=== | |||
In one 48 mm. embryo definite lymph glands have formed and consist of local proliferations of stellate and spindle cells in the connective tissue stroma of lymph plexuses. The areas of pro1ifera~ tion are infiltrated with lymphocytes, amoeboid haemocytoblasts and a very few histiocytes and eosinophil and neutrophil myelocytes and leucocytes. A very few lymphocytes have entered the lymph vessels. In the 65- and 70-mm. embryos the glands are further developed and lymphocytes have increased greatly. They now contain numerous myelocytes and leucocytes and a few megakaryo— cytes. Some lymph vessels contain numerous lymphocytes. In the 75-, 76- and 120—mm. embryos several of the glands contain in addition small foci of late megaloblasts and normoblasts or of intermediate primary erythroblasts and late megaloblasts. In the 125 — mm. foetus leucopoiesis is considerably less, and in seven 17 0-546-mm. foetuses it is still less, amounting to a few eosinophil and fewer neutrophil myelocytes and leucocytes in the sinuses or gland tissue, especially in the peripheral parts. Megakaryocytes are absent in one 190 - mm. and longer foetuses examined. One small focus of megaloblasts is present in a lymph gland in the 125- and 170—mm. foetuses but all erythropoiesis is absent from glands in one of the 190-mm. and longer foetuses. A few tissue mast cells lie free in the sinuses or in the gland tissue in four 470-546—mm. foetuses. | |||
Erythrophages are rare in lymph glands in foetal life. Two were present in sinuses in one 546 mm. foetus. Some free sinus cells in full—term foetuses contain diffuse brown pigment which does not give the prussian blue reaction. | |||
===7. Marrow=== | |||
A clavicle was examined in 13 specimens (18-120 mm.), a femur in 26 (18-540 mm.), a humerus in 9 (18-75 mm.) and a rib in 8 (1041-5340 mm.). Heemopoiesis made its first appearance in the skeletal system in the clavicle in the 43—mm. embryo. It then appeared in the humerus in the 57-mm. embryo and in the femur in the 75-mm. embryo. Cytological studies were difficult because of the effect of decalcification upon staining. | |||
In the 43-min. embryo in one place between capillary sinuses in the loose connective tissue marrow of the clavicle there are a very few amoeboid heemocytoblasts and neutrophil myelocytes and leucocytes and in another place a few am oeboid haemocytoblasts alone. In the clavicular marrow of the 48-mm. embryo a few hsemocytoblasts, lymphocytes and neutrophil myelocytes and leucocytes and one or two histiocytes are present. In the clavicle of the 57 —mm. embryo a few eosinophil myelocytes and leucocytes and a small focus of definitive erythroblasts, apparently intermediate primary I erythroblasts and megaloblasts, are present in addition. In the humerus of this embryo there is a small group of amoeboid haemocytoblasts. In the clavicle in the 65—mm. embryo haemopoiesis has developed considerably, forming larger and denser areas between the sinuses, most dense around the arteries. There are many haemocytoblasts, numerous eosinophil and neutrophil myelocytes and leucooytes, a few early and intermediate primary erythroblasts and intermediate and late megaloblasts and a few lymphocytes. There are also a few scattered well developed megakaryocytes. In the "3'5—mm. foetus the marrow in the clavicle, humerus, femur, radius, ulna, tibia, fibula and pelvis presents a similar appearance. The haemopoietic tissue occupies only part of the marrow and in the long bones there is no haemopoiesis for quite a distance from the cartilaginous epiphyses. The areas of haemopoiesis, although often diffuse between the sinuses, tend to surround the developing arteries. In older foetuses the maximum density is reached in the 160~mm. foetus. The cytology of the marrow was not followed. It is probable, however, that erythropoiesis was of the same type as elsewhere, becoming in part normoblastic in middle foetal life and entirely normoblastic in post-foetal life. | |||
In the femur in six foetuses of 80—170 mm. no heemopoiesis is present for distances of 0-8 to 1-1 mm. from the zones of provisional calcification of cartilage. In the 320- and 343—mm. foetuses hwmopoiesis reaches to 0-3 mm. from the zone. In five foetuses of 444-540 mm. it reaches up to the zone but is less dense and in places absent in the metaphysis just below the zone. In the rib the haemopoietic marrow has not reached the zone in one 508and in the 540—mm. foetus. No haemopoiesis has occurred in any specimen in primary medullary spaces, periosteum or chondral canals. Towards the end of foetal life some adipose tissue cells appear in the marrow in the lower half of the femur in some specimens. | |||
cells in | |||
===8. Spleen=== | |||
The spleen was examined in 28 embryos and foetuses and in 7 infants. It is first present in the 28-mm. embryo. In the 28and 35-mm. embryos it consists of a mass of undifferentiated cells. In the 35—mm. embryo one or two early megakaryocytes are present in capillaries in this mass. In four specimens of 55-80 mm. the spleen consists of vessels surrounded by spindle cells, lying in a pulp of widely meshed delicate connective tissue with an occasional recognisable sinus. In the pulp is a lake of blood. A few early or well formed megakaryocytes are present, usually in sinuses. In the lake of blood are a very few haemocytoblasts, eosinophil myelocytes and leucocytes, a few orthochromatic normoblasts and scattered megaloblasts, usually late and pyknotic, but a few intermediate. The erythroblasts were probably not formed in s-itu from precursors but carried in from the blood stream and probably most are definitive. Five specimens of 70-162 mm. show in addition a few scattered foci of definitive erythropoiesis, some in sinuses, most in the pulp. They consist of early and intermediate primary erythroblasts with occasionally one or two haemocytoblasts, or of intermediate and late and sometimes one or two early megaloblasts, or of primary erythroblasts and megaloblasts mixed. A few normoblasts formed from megaloblasts are also present. In the 146-mm. foetus a very few heemocytoblasts are present in the cellular connectivet issue around the arteries. In the 162—mm. foetus heemocytoblasts are more numerous and many lymphocytes have been formed from them, constituting small Malpighian bodies. In the remaining foetuses there is very little variation. The total amount of haemopoiesis is always slight and near the end of pregnancy is very slight. Early megaloblasts are absent and intermediate forms less numerous. Megakaryocytes decrease but one or more are always‘ found. In the 470-mm. and longer foetuses a very few neutrophil myelocytes and leucocytes have appeared in the pulp. A few tissue mast cells lie in the trabeculae in the 444- and 533—mm. and in the pulp in the 457 - and 533-mm. foetuses. The spleen at full term shows a few scattered heemocytoblasts, intermediate and late megaloblasts, normoblasts, eosinophil (and fewer neutrophil) myelocytes and leucocytes, a few small groups of primary erythroblasts and one or two megakaryocytes, often appearing degenerated. These cells lie chiefly in the pulp but are also present in the sinuses. Quite frequently intermediate megaloblasts were absent from the liver at full term but were readily found in the spleen. | |||
in | |||
appeared in the | |||
in the | |||
In | In five infants of 3-5 days a few myelocytes, leucocytes, normoblasts and late megaloblasts are present scattered in the pulp. In one 14-day and in the 15-day infant a few leucocytes but no myelocytes, megaloblasts or normoblasts are present. One megakaryocyte was seen in a 3-day infant but none in the other four. | ||
Erythrophages are visible in the pulp in a third of the embryos and foetuses and in some of the infants. Never numerous, they are difficult to see because of the large number of red blood cells free in the pulp. | |||
In eleven 55-190-mm. specimens and in the 320- and 330-mm. foetuses, free iron pigment is present in the pulp in small amount, either free or in reticulum cells in the form of haemosiderin granules and spheres. In one 190- and in the 343-mm. foetus no iron pigment is present. In the 444-mm. and older foetuses iron is present in granules or globules or diffusely in the cytoplasm of reticulum cells and sometimes in sinus cells. It varies greatly in amount. In four cases (470-508 mm.) the amount is abundant, but in three others (495-546 mm.) it is very slight. This considerable variability in iron content in older foetuses probably. depends upon the degree of congestion of the pulp. In infants the amount of iron varies considerably, as in older foetuses, and probably for the same reason. In one of the 3-day infants and in the 15-days infant the amount is slight. In three infants of 3-14 days it is very abundant and in two of 4 and 5 days a moderate amount is present. | |||
===9. Kidneys=== | |||
In | The kidneys were examined in 26 embryos and foetuses and in 7 infants. In the 104-mm. foetus one small group, and in three foetuses of 146-180 mm. a few small groups of primary erythroblasts and megaloblasts or of megaloblasts and normoblasts are present about the tubules in the upper part of the medulla. In one 190 mm. foetus several very small foci of late megaloblasts and normoblasts and one or two myelocytes lie around tubules and vessels in the intermediate zone between cortex and medulla. In the 200—mm. fcetus two foci, and in the 320- and 495-mm. foetuses single small foci of erythropoiesis occupy the intermediate zone. In one 3-day infant there is one focus of normoblasts in the peripelvic tissue ; in the very premature 15-days infant there are three in the connective tissue of the intermediate zone. | ||
In one | |||
In one of the 180-mm. foetuses and in the 200-mm. foetus a few h2em0siderin—containing phagocytes are present in the connective tissue of the upper part of the medulla and intermediate zone. These are probably the result of slight extravasations of blood. No free iron is present in the epithelium of the kidney in any specimen. | |||
are | |||
free in the | |||
===10. Thymus=== | |||
This was examined in 22 embryos and foetuses. In the 28-mm. embryo a few amoeboid haemocytoblasts infiltrate the otherwise epithelial thymus. In the 35-mm. and longer specimens lymphopoiesis is established. Leucopoiesis begins in the 55-mm. embryo, and in this and in the four specimens of 75-120 mm. is shown by the presence of a Very few small groups of eosinophil promyelocytes and myelocytes in the interlobular septa. In the 70- and 140-mm. specimens, besides these cells there are a few eosinophil myelocytes in the medulla and a few tissue mast cells in the capsule and interlobular tissue. In the 125—mm. foetus a few eosinophil myelocytes and leucocytes are present in the capsule and interlobular tissue and in the cortex‘ ; two mast cells were present in the capsule. In the 162-mm. foetus leucopoiesis has considerably increased. There are numerous eosinophil and a few neutrophil myelocytes and leucocytes and a few promyelocytes in several interlobular septa, while many eosinophil myelocytes and leucocytes infiltrate the medulla adjacent to the septa and a few lie in the cortex. | |||
Tissue mast cells are also present in the interlobular septa and capsule. In eight foetuses of 17 0-540 mm. there is similar leucopoiesis. | |||
In eleven specimens of 28-170 mm., but not in longer, a little erythropoiesis is represented in the younger specimens by a few small groups of intermediate and late megaloblasts and in the older by late megaloblasts and normoblasts. The groups lie in | |||
the interlobular septa or in the perithymic tissue. Erythropoiesis is never present within the thymic tissue. | |||
==Summary of Different Types of Blood Cells from their Earliest Appearance== | |||
===Haemocytoblasts and histiocytes=== | |||
the | Heemocytoblasts first arose from the endothelium of the yolk sac Vessels and were first seen in the Frazer embryo (B). For a time they were the only free cells in the blood islands but soon most differentiated into erythroblasts—first in the Jones—Brewer presomite embryo, a few into histiocytes. The latter were first seen in the yolk sac vessels in the Frazer embryo of about 20 pairs of somites but were probably present in some younger embryos. After the establishment of the circulation, hwmocytoblasts and histiocytes, some of them phagocytic, persisted in the yolk sac vessels and were present in the general circulation up to the 196mm. embryo ; a few histiocytes however persisted in the yolk sac vessels in the 26-9—mm. embryo. In the 26—mm. and longer specimens the heemocytoblasts had become smaller and were identical with lymphocytes. | ||
Beginning in the 10-mm. embryo and persisting up to the 35-mm. embryo there was a concentration of haemocytoblasts and histiocytes in the tissues around the central nervous system, especially at the base of the brain. Similar cells were present in many connective tissues—especial.ly loose tissuesmin embryos of 18-190 mm. and in and about small veins forming plexuses in the neck and retroperitoneal tissue in the 18- and 19-5-mm. embryos, in the connective tissue in lymph plexuses in embryos of 18-75 mm. and in the capillaries of this connective tissue in embryos of 18-48 mm. Heemocytoblasts were also present in the adventitia of scattered small arteries in embryos of 26-125 mm. They were present in the developing lymph glands in embryos of 48 mm. or more and were associated with histiocytes. Some haemocytoblasts appeared in the connective tissue of the clavicular marrow in the 43—mm. embryo. Infiltration of the epithelial thymus by heemocytoblasts occurred in the 28-mm. embryo. The haem0cytoblasts in these sites differentiated into the various blood cells. In certain sites such as connective tissue, especially that around the central nervous system and in lymph plexuses, the haemocytoblasts appeared also to form histiocytes. In young embryos hsemocytoblasts migrating in connective tissues or the thymus were often distorted from their usual more or less rounded shape and often showed pseudopodia. Small pseudopodia were also occasionally seen on the rounded forms in the blood. | |||
===Erythropoiesis=== | |||
In early embryos the erythroblasts belonged to the primitive family. They appeared first in the J ones-Brewer presomite embryo in the blood islands of the yolk sac. They were megaloblasts and were derived directly from haemocytoblasts without passing through a stage comparable with the primary erythroblasts in the definitive family. According to the literature erythroblasts are also formed in many older presomite and younger somite embryos in isolated vessels in the body stalk and possibly in a few embryos in the chorion. Blood islands are also described in the mesoderm of the body stalk in a few embryos and in one embryo one island was found in the embryo itself. Since the cellular content and histiogenesis of these formations were not described they must be regarded as very doubtful. With the establishment of the circulation the primitive erythroblasts entered the embryonic vessels and continued to multiply there. With increasing age the number of early and intermediate primitive erythroblasts decreased and the late and pyknotic late forms and megalocytes increased, accompanied by diminished multiplication. Mitotic division of primitive erythroblasts had ceased in the 35-mm. embryo, and in the 48-mm. embryo these cells were all in a late or pyknotic late stage. In older embryos no primitive erythroblasts were recognisable in the blood, but primitive megalocytes undoubtedly persisted longer. In embryos of 3-35 mm. multiplication and development of primitive erythroblasts continued in places in many connective tissues, the original cells having reached the tissues by haemorrhage. In the 35mm. embryo most of these cells had developed into megalocytes and the erythroblasts were all late. Apart from this observation and the doubtful description in the literature of blood islands in the mesoderm of the body stalk, primitive erythropoiesis was essentially intravascular. Primitive erythropoiesis was also essentially megaloblastic but in embryos of 3 mm. or more a very few small erythroblasts of the size of normoblasts had been formed from megaloblasts (megalo-normoblastic erythropoiesis). | |||
Definitive erythropoiesis appeared first in the 10-mm. embryo in the yolk sac vessels as groups of primary erythroblasts and megaloblasts. These groups persisted but had disappeared in the 48—mm. embryo. Heemocytoblasts had appeared among the hepatic and yolk sac epithelial cells in the 10-mm. embryo but extravascular definitive erythropoiesis did not begin in the liver until the 12-mm. embryo and in the yolk sac till the 15-5-mm. embryo. In the yolk sac it was still present in the 26-9- but had disappeared in the 48—mm. embryo. In the liver it increased in amount and reached a maximum relative to the parenchyma in the 26-mm. embryo. This maximum persisted until in foetuses of 200-457 mm. there was a progressive decrease to a slight amount which remained constant in foetuses of 470 mm. and more. In infants 5 or more days old such erythropoiesis was absent. Definitive erythropoiesis was also present in embryos and foetuses of 18 mm. or more in the sinusoids of the liver, in amounts that were relatively slight but in proportion to the amounts outside the sinusoids. In some foetuses of 65-457 mm. focal definitive erythropoiesis was also present in the connective tissue of the portal systems. In many connective tissues it was present focally in specimens of 18-190 mm. and probably did not persist in longer foetuses. The sites of election, especially in the younger embryos, were the dense tissue at the base of the brain and in the upper part of the neck, the delicate tissue surrounding the central nervous system, especially at the base of the brain, and the mesentery, but any connective tissue in the body or limbs sometimes showed it. In the spleen focal definitive erythropoiesis in the pulp and to a less degree in the sinuses began in the 70-mm. embryo and first disappeared in infants over 5 days old. It was always slight and decreased in late foetal life. In the connective tissue of the marrow definitive erythropoiesis began in the clavicle in the 57—mm. embryo and subsequently increased considerably in amount. In the connective tissue in lymph plexuses it formed a few foci in some specimens of 28-125 mm. It was also present in slight amount in a few lymph glands of a few foetuses of 75-170 mm. In the kidney a few foci were found in some foetuses and infants of 104 mm. or more and were last observed in the 15-days-old infant. In the thymus there were a few foci in the connective tissue of the capsule and interlobular septa in some specimens of 28-170 mm. In the blood definitive megaloblasts were present in small numbers in the 28and 35-mm. embryos and were almost as numerous as the primitive in the 48—mm. embryo. All erythroblasts in the blood were definitive in the 65~mm. and older specimens. In foetuses of 76-146 mm. there appeared to be exceptional erythropoietic activity in the blood, as some intermediate and in one a few early primary erythroblasts appeared and mitotic figures reappeared. In the circulation primary erythroblasts were not seen previously nor subsequently. | |||
===Table - Stages of erythropoiesis=== | |||
{{Gilmour1941 table1}} | |||
It is safe to state that after the 15th day of life erythropoiesis is confined to the marrow. Definitive erythropoiesis was essentially extravascular apart from that in the sinusoids of the liver and sinuses of the spleen and that in the circulation in foetuses of 76-146 mm. It was entirely megaloblastic in specimens up to 48 mm. and partly megaloblastic subsequently up to the infant of 5 days old. In specimens of 48-180 mm. orthochromatic but no basophil normoblasts were present in some sites and had been formed from megaloblasts (megalo-normoblastic erythropoiesis). Such formation of normoblasts occurred also in older foetuses to a less degree. In the 190-mm. and older specimens basophil normoblasts were present so that the erythropoiesis became in part truly normoblastic. With increasing age the number of earlier forms of megaloblasts decreased. Early megaloblasts were very rarely seen after 48 and never after 125 mm. and towards the end of foetal life the intermediate megaloblasts usually disappeared. The last secondary erythroblasts seen in the tissues examined microscopically were in the kidneys of the 15-day infant. They’ were normoblasts and it is probable that erythropoiesis in the marrow is, as found by Turnbull (1934), entirely normoblastic in older infants and children as in adults. | |||
days old | |||
erythropoiesis | |||
were | |||
in the | |||
as | |||
Erythropoiesis can be divided into five stages in embryos, foetuses and post-foetal life according to its site and character. The stages are summarised in the table (p. 51). | |||
===Leucopoiesis=== | |||
Leucopoiesis with the formation of eosinophil and neutrophil myelocytes and leucocytes was first seen in one of the 18 mm. embryos in the parenchyma of the liver and in various connective tissues such as the meninges, mesentery and stroma of lymph plexuses. It persisted up to birth, never in great amount, in the hepatic parenchyma and in various connective tissues up to 190 mm., and in the lymph plexuses up to 125 mm. It was fairly abundant in the hilum of the liver in early embryos such as the 18 mm. and in the portal systems in 48--495—mm. foetuses, after which it became reduced. It was present in some portal systems in infants up to 5 days old, and a very few leucocytes but no myelocytes persisted in the older infants. A little was seen in the adventitia of a few scattered arteries in 26-125-mm. specimens. It was present in lymph glands from 48mm. embryos up to fullterm foetuses, fairly abundantly in the 65- and 70-mm. embryos, but at other times in scanty amount. It began in the interlobular septa of the thymus in the 55-mm. foetus and in the glandular tissue in the 7 0-mm. foetus ; it was scanty but increased considerably in amount in the 162-mm. foetus and subsequently remained about the same until birth. In the spleen slight leucopoiesis was present in infants over 5 days old. Leucopoiesis began in the clavicular marrow in the 43-mm. embryo and subsequently increased con siderably. Leucopoiesis in the yolk sac, as observed by Maximow (1927) and figured by Bloom, was not seen. Myelocytes and leucocytes first appeared in the blood stream in scanty numbers in the 48-mm. embryo. | |||
===Lymphopoiesis=== | |||
Lymphopoiesis began in the connective tissue of lymph plexuses in very scanty amount in the 26—mm. embryo and in the adventitia of a few arteries in the 65-mm. embryo and was continued in these sites until the 125-mm. embryo. It began in lymph glands in the 48-mm. embryo, at which time lymphatic vessels contained some lymphocytes. It began in the thymus of the 35-mm. embryo and in the Malpighian bodies of the spleen in the 162-mm. foetus. It was present in some portal systems in the liver in several foetuses of 65-457 mm., and some portal systems contained a few lymphocytes in older foetuses and infants. Lymphocytes were present in the marrow in embryos of 48-65 mm. and probably persisted but could not be recognised because of the effect of decalcification on staining and because of the abundance of erythropoiesis and leucopoiesis. No lymphopoiesis occurred in the yolk sac. In the blood, lymphocytes were first seen in the 26-mm. embryo and they probably arose directly from the haemocytoblasts in the circulation. | |||
===Formation of megakaryocytes=== | |||
Intravasoular and a little extravascular formation of megakaryocytes was present in the yolk sac in the 10-mm. embryo. Intravascular but not extravascular formation continued in the 12-5- and 15-5—mm. embryos but not in older. Intravascular or extravascular formation of megakaryocytes occurred in the liver of all specimens from 10 mm. up to 457 mm. but in only 3 of 9 older foetuses. Megakaryocytes, usually degenerated, were present in the liver of 3 of the 12 infants, the oldest being 15 days. In the spleen they were constant in specimens from 35 mm. to birth; at full time they were often degenerated ; only one was seen after birth, in an infant of 3 days. They were constantly present in the marrow of foetuses of 65 mm. or more. In the capillaries and connective tissues they were especially numerous at the base of the brain, where they were first seen in the 10-mm. embryo ; in the rest of the body and limbs they appeared in the 18-mm. embryos; in both sites they persisted to the 35—mm. embryo. Some were present in small veins forming plexuses in the neck and retroperitoneal tissue in the 18- and 19-5—mm. embryos, in the connective tissues in lymph plexuses in specimens of 195-125 mm. and in lymph glands in foetuses of 65-170 mm. No megakaryocytes were seen in the general circulation in any specimen. In the early embryos (10-35 mm.) the megakaryocytes usually differed from the typical form of adults in being smaller and having one, two or three separate nuclei. These early forms did not have the pseudopodia which were often abundant in the well developed forms of early and middle foetal life. | |||
===Summary=== | |||
The development of the blood vessels and cells until a complete circulation has been established is described from 3 presomite and 1 somite embryo kindly lent by Professor J. E. S. Frazer, supplemented by 30 presomite and somite embryos selected from the literature. The subsequent development of the blood cells in the Vessels and tissues is described from the examination of 53 embryos and foetuses ranging from between 4 and 5 weeks to full term, and of 11 new-born infants 221 days old. The development of histiocytes and phagocytes and the distribution of iron are included. | |||
I wish to thank Professor H. M. Turnbull for help in the preparation of this paper. I am deeply indebted to Professor J. E. S. Frazer for permission to examine some of his young embryos. | |||
embryos | |||
==References== | |||
BLOOM, ‘W. . . . . . . In Downey’s Handbook of hematology, London, 1938, vol. ii, p. 865. | |||
BOERNER-PATZELT, D., AND Z. gee. Anat., I Abt., 1923, lxviii, 204. SOHWARZACHER, W. | |||
BREMER, J. L. . . . . . Amer. J. Anat., 1914, xvi, 447. | |||
BRYCE, T. H. . . . . . Trans. Roy. Soc. Edinb., 1906, Xli, 435. | |||
DANDY, VV. E. . . . . . Amer. J. Anat., 1910, x, 85. | DANDY, VV. E. . . . . . Amer. J. Anat., 1910, x, 85. | ||
E1-IRLIOI-I, P., AND LAZARUS, A.‘ Die Anaemie, 2nd ed., by A. Lazarus and | E1-IRLIOI-I, P., AND LAZARUS, A.‘ Die Anaemie, 2nd ed., by A. Lazarus and O. Naegele, Vienna and Leipzig, 1909, Abt. I, Teil I, S. 52. | ||
O. Naegele, Vienna and Leipzig, 1909, | |||
Abt. I, Teil I, S. 52. | |||
EBB, W. . . . . . . . Arch. path. Anaz‘.., 1865, xxxiv, 138. | EBB, W. . . . . . . . Arch. path. Anaz‘.., 1865, xxxiv, 138. | ||
| Line 1,509: | Line 385: | ||
GROSSER, O. . . . . . . Anal. Hefle, 1913, xlvii, 649. | GROSSER, O. . . . . . . Anal. Hefle, 1913, xlvii, 649. | ||
,, . . . . . . Ergebn. Anat. E'm$wichel., 1924, xxv, 391. | ,, . . . . . . Ergebn. Anat. E'm$wichel., 1924, xxv, 391. | ||
. . . . . . Z. ges. A7iat., Abt. I, 1931, xciv, 275. | |||
HERZOG, M. . . . . . . Amer. J. Anat., 1909, ix, 361. | HERZOG, M. . . . . . . Amer. J. Anat., 1909, ix, 361. | ||
HEUSER, C. H. . . . . . Contributions to embryology, Carnegie | HEUSER, C. H. . . . . . Contributions to embryology, Carnegie Institution of Washington, 1932, Xxiii, 251. | ||
Institution of Washington, 1932, Xxiii, | |||
251. | |||
HOFBAUER, J. . . . . . Grrundziige einer Biologie den menschlichen | HOFBAUER, J. . . . . . Grrundziige einer Biologie den menschlichen Plazenta, Vienna, 1905. | ||
Plazenta, Vienna, 1905. | |||
HOWELL, W. H. . . . . J. Morph., 1891, iv, 57. | HOWELL, W. H. . . . . J. Morph., 1891, iv, 57. | ||
INGALLS, N. W. . . . . . Contributions to embryology, Carnegie | INGALLS, N. W. . . . . . Contributions to embryology, Carnegie Institution of Washington, 1918, vii, 111. | ||
,, . . . . . Ibid.,_1920, xi, 61. | ,, . . . . . Ibid.,_1920, xi, 61. | ||
JAGERROOS, B. H. . . . . Acta 800. med. fennicae “ Duodecim,” sect. | JAGERROOS, B. H. . . . . Acta 800. med. fennicae “ Duodecim,” sect. B, fasc. 2 and 4, 1934, xix, 1. | ||
B, fasc. 2 and 4, 1934, xix, 1. | |||
JONES, H. 0., AND BREWER, S/wrg., Gym. and Obstet, 1935, 1):, 657. | JONES, H. 0., AND BREWER, S/wrg., Gym. and Obstet, 1935, 1):, 657. | ||
| Line 1,535: | Line 407: | ||
J. I. | J. I. | ||
J UNG, P. J. . . . . . . Beitrage zur friihesten Ei-Einbettung beim | J UNG, P. J. . . . . . . Beitrage zur friihesten Ei-Einbettung beim menschlichen Weibe, Berlin, 1908. | ||
menschlichen Weibe, Berlin, 1908. | |||
KNOLL, VV. . . . . . . Morph. Jah/rb., Abt. II, 1929, xviii, 199. | KNOLL, VV. . . . . . . Morph. Jah/rb., Abt. II, 1929, xviii, 199. | ||
| Line 1,542: | Line 413: | ||
LEWIS, F. T. . . . . . I n Keibel and Mall’s Manual of human | LEWIS, F. T. . . . . . I n Keibel and Mall’s Manual of human | ||
embryology, Philadelphia and London, | embryology, Philadelphia and London, 1912, vol. ii, p. 295. | ||
1912, vol. ii, p. 295. | |||
Low, A. . . . . . . . J. Anat., 1907-08, X111, 237. | Low, A. . . . . . . . J. Anat., 1907-08, X111, 237. | ||
LCWIT, M. . . . . . . Sitzungsber. d. Kais. Akad. d. Wissen. z. | LCWIT, M. . . . . . . Sitzungsber. d. Kais. Akad. d. Wissen. z. Wien, Abt. III, 1886, Xcii, 22. | ||
Wien, Abt. III, 1886, Xcii, 22. | |||
MALL, F. P. . . . . . . In Keibel and Ma11’s Manual of human embryology, Philadelphia and London, 1910, vol. i, p. 199. | MALL, F. P. . . . . . . In Keibel and Ma11’s Manual of human embryology, Philadelphia and London, 1910, vol. i, p. 199. | ||
| Line 1,556: | Line 425: | ||
3! | 3! | ||
MCINTYRE, D. | MCINTYRE, D. MEYER, P. . Mnsroaz, C‘. S. . | ||
MEYER, P. . | |||
Mnsroaz, C‘. S. . | |||
NEUMANN, E . | NEUMANN, E . PANDEB, C. | ||
PANDEB, C. | |||
PETERS, H. | PETERS, H. | ||
SAXER, F. . . . . . | SAXER, F. . . . . . SOHLAGENHAUFER AND VEROGAY SCHBIDDE, H. . VON SPEE, F. GRAF . | ||
SOHLAGENHAUFER AND VEROGAY | |||
SCHBIDDE, H. . | |||
VON SPEE, F. GRAF . | |||
33 3.’! | 33 3.’! | ||
| Line 1,578: | Line 441: | ||
35 93 93 | 35 93 93 | ||
THOMPSON, P. | THOMPSON, P. TBIEPEL, H. . . TURNBULL, H. M. | ||
TBIEPEL, H. . . | |||
TURNBULL, H. M. | |||
)9 | )9 | ||
| Line 1,588: | Line 449: | ||
Arch. mikros. Arlal., 1909, lxxiii, 444. | Arch. mikros. Arlal., 1909, lxxiii, 444. | ||
I rl V011 M<'5llendorff’s Handbuch der mikr0skopischen Anatomic der Menschen, Berlin, | I rl V011 M<'5llendorff’s Handbuch der mikr0skopischen Anatomic der Menschen, Berlin, 1927, V01. ii, part I, p. 232. | ||
1927, V01. ii, part I, p. 232. | |||
Trans. Roy. Soc. Ealinb., 1926-28, lv, 77. | Trans. Roy. Soc. Ealinb., 1926-28, lv, 77. | ||
| Line 1,595: | Line 455: | ||
Arch. Gymilc., 1924, cxxii, 38. | Arch. Gymilc., 1924, cxxii, 38. | ||
I re. Keibel and Ma.11’s Manual of human | I re. Keibel and Ma.11’s Manual of human embryology, Philadelphia and London, 1912, vol. ii, p. 498. | ||
embryology, Philadelphia and London, | |||
1912, vol. ii, p. 498. | |||
Arch. Hcilla, 1874, xv, 441. | Arch. Hcilla, 1874, xv, 441. | ||
Beitriige zur Entwickelungsgeschichte des | Beitriige zur Entwickelungsgeschichte des Hiihnchens irn Eye, Wilrzburg, 1817. | ||
Hiihnchens irn Eye, Wilrzburg, 1817. | |||
fiber die Einbettung des menschlichen | fiber die Einbettung des menschlichen Eies, Leipzig and Vienna, 1899-. | ||
Eies, Leipzig and Vienna, 1899-. | |||
Anal. Hefle, 1895-96, Vi, 347. | Anal. Hefle, 1895-96, Vi, 347. | ||
| Line 1,613: | Line 469: | ||
Verh. cllsch. path. G'es., 1907 (1908), xi, 360. | Verh. cllsch. path. G'es., 1907 (1908), xi, 360. | ||
Arch. Anal. Phys-iol., Anat. Abt., 1889, | Arch. Anal. Phys-iol., Anat. Abt., 1889, p. 159. | ||
p. 159. | |||
Ibid., 1896, p. 1. | Ibid., 1896, p. 1. | ||
| Line 1,630: | Line 485: | ||
This Journal, 1931, xxxiv, 277. | This Journal, 1931, xxxiv, 277. | ||
I rl The anaemias, by J. M. Vamtghan, | I rl The anaemias, by J. M. Vamtghan, London, 1934, p. 8, (2nd ed., 1936, p. 11). | ||
London, 1934, p. 8, (2nd ed., 1936, p. 11). | |||
Ergcbn. Anal. E’nlwiclccl., 1904, xiv, 345. | Ergcbn. Anal. E’nlwiclccl., 1904, xiv, 345. | ||
==Plates== | |||
===Plate XII=== | |||
[[File:Gilmour1941 plate07.jpg|600px]] | |||
{| width=610px| | |||
! Plate 7 Figures | |||
|- | |||
| [[File:Gilmour1941 fig01.jpg|300px|Fig. 1]] | |||
| [[File:Gilmour1941 fig02.jpg|300px|Fig. 2]] | |||
|- | |||
| '''Fig. 1.''' Red blood cells and their precursors. 1 and 2, haemocytoblasts ; 3, 4 and 5, early, intermediate and late primary erythroblasts; 6 and 7, basophil and erthochromatic normoblasts; 8, normocyte; 9, 10, l1 and 12, early, intermediate, late and pyknotic megaloblasts; 13, megalocyte; 14, 15, 16 and 17 early, intermediate, late and pyknotic primitive megaloblasts; 18, primitive erythrocyte; 4a, basophil normoblast with prematurely pyknotic nucleus; 10a, megaloblast with prematurely pyknotic nucleus. Jenner. | |||
| '''Fig. 2.''' 125 mm foetus. Focus of megalo-normoblaetic erythropoiesis in neck. One early and several intermediate megaloblasts forming pyknotic megaloblasts and - with loss of nucleus - a Megalocyte, and — from shrinkage in size — orthochromatic normoblasts and — by loss of nucleus - a normocyte. Jenner. | |||
|} | |||
===Plate XIII=== | |||
[[:File:Gilmour1941 fig03.jpg|'''Fig. 3.''']] Embryo 1, Frazer, presomite (A). Small mass of proliferated mesodermal cells on Ventral pole of yolk sac. Heidenhain's iron haematoxylin. x570. | |||
[[:File:Gilmour1941 fig04.jpg|'''Fig. 4.''']] Embryo 2, Frazer, presomite (B). Papillary projections of mesoderm on ventral part of yolk sac, some containing spaces. Heidenhain's iron haematoxylin. x480. | |||
[[:File:Gilmour1941 fig05.jpg|'''Fig. 5.''']] Embryo 2, Frazer, prosomite (B). Blood island in mesoderm of ventral part of yolk sac. Heidenhain's iron haematoxylin. x750. | |||
{{Online Editor}} - Heidenhain's iron haematoxylin is an iron alum hematoxylin stain used for staining muscle striations and mitotic structures blue-black. Named after Rudolph Heidenhain (1834-1897) a German histologist and physiologist. (More? [[Histology Stains]]) | |||
<gallery caption="Plate 8"> | |||
File:Gilmour1941 fig03.jpg|Fig. 3 | |||
File:Gilmour1941 fig04.jpg|Fig. 4 | |||
File:Gilmour1941 fig05.jpg|Fig. 5 | |||
</gallery> | |||
===Plate IX=== | |||
[[File:Gilmour1941 plate09.jpg|400px]] | |||
{| width=610px| | |||
! Plate 7 Figures | |||
|- | |||
| [[File:Gilmour1941 fig06.jpg|300px|Fig. 6]] | |||
| [[File:Gilmour1941 fig08.jpg|300px|Fig. 8]] | |||
|- | |||
| '''Fig. 6.''' Embryo 4, Frazer, about 20 pairs of somites. Blood vessels in yolk sac full of primitive erythroblasts. {{HE}} Dufay process. x550. | |||
| '''Fig. 8.''' 10 mm embryo (Frazer). Two haemocytoblasts among epithelial cells of yolk sac. Primitive erythroblasts in yolk sac vessel. Ehrlich’s haematoxylin and eosin. Dufay process. x1100. | |||
|} | |||
===Plate X=== | |||
[[File:Gilmour1941 plate10.jpg|600px]] | |||
<gallery caption="Plate 10"> | |||
File:Gilmour1941 fig07.jpg|Fig. 7 | |||
File:Gilmour1941 fig09.jpg|Fig. 9 | |||
File:Gilmour1941 fig10.jpg|Fig. 10 | |||
File:Gilmour1941 fig10.jpg|Fig. 11 | |||
</gallery> | |||
[[Category:Draft]] | {{Footer}} | ||
[[Category:Blood]][[Category:Draft]] | |||
Revision as of 12:29, 17 May 2018
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Gilmour JR. Normal haemopoiesis in intra-uterine and neonatal life. (1941) J. Pathol. Bacteriol. 52: 25-55.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Normal Haemopoiesis in Intra-Uterine and Neonatal Life
J. R. Gilmour
From the Bernhard Baron Institute of the London Hospital
Plates VII—X (1941)
Introduction
During a study of the morbid histology of erythroblastosis foetalis I realised that the literature upon foetal haemopoiesis in man was insufficient for a reliable control. The classical Works of Van der Stricht (1891 and 1892), Saxer (1895-96) and Maximow (1909) on haemopoiesis in small mammals were valuable, but the latter differs from that in man in certain respects. The present work was therefore undertaken to establish a control but was extended to cover early embryonic life and the first weeks of extra-uterine life.
Material
Embryos and foetuses
(1) Presomite embryo (A), (Frazer). Ovum 2.1 x 1.95 x 1 mm (measured from the chorionic epithelium excluding the rudimentary villi). Amniotic vesicle 0.17 x 0.143 x 0.1 mm. Entodermic vesicle 0-127 x 0-11 mm. Embryonic plate 0.065 x 0.045 mm. Age about 16 days, probably slightly younger than Peters’ embryo (1899).
(2) Presomite embryo (B), (Frazer). Ovum 2.2 x 2.1 X 1.7 mm. Yolk sac 0.225 x 0.190 mm. Embryonic plate 0-323 mm. Age about 19 days, probably between the Jung (1908) and Meyer (1924) embryos in development.
(3) Presomite embryo (C), (Frazer). Ovum 4.66 x 4.5 x 3 mm. Amnion 0.55 x 0.5 mm. Yolk sac 1.2 x 0.75 mm. Embryonic plate 0-55 X0-36 mm. Age about 19 days, probably between the Von Spee (1896) and Jones-Brewer (1935) embryos in development.
(4) Embryo of about 20 pairs of somites, (Frazer). Yolk sac only. Age 3 to 4 weeks. (5-43) Embryos and foetuses 3-200 mm. crown-rump, 4-5 weeks to 23 weeks. Nine from Professor Frazer’s collection. Yolk sac only in the 12.5, 15.5 and 26.9 mm specimens.
(44-57) Foetuses 320-546 mm. crown-heel, 24-25 weeks to full term. One post-mature.
(58-68) New-born infants, 2-21 days old, 406-533 mm crown-heel. Five premature.
The ages of the presomite embryos are derived from Grosser (1924), those of the older specimens from the table of Mall (1910) according to the crown-rump measurement in embryos and foetuses up to 200 mm, and the crown-heel measurement in longer foetuses. The specimens under 9 mm long were measured by serial section when embedded, the others when fresh or fixed. The specimens are referred to according to their length, whether crown-rrump (C-R) or crown-heel (C-H).
Methods of preparation
My specimens were fixed in 4 per cent. saline-formaldehyde, dehydrated in alcohol, cleared in chloroform and embedded in paraffin.
The number of tissues examined and the stains applied varied with each specimen. Much reliance had to be placed upon Ehrlich’s heematoxylin and eosin, but most specimens were stained also by Jenner’s method according to Turnbull (1931). The prussian-blue reaction was done on sections from most specimens. The tissues examined are mentioned in the description of haemopoiesis.
The 13 Frazer embryos had been treated with clove oil between the alcohol and chloroform and in some instances a little celloidin had been added to the clove oil. Most of the sections had been stained by Heidenhairfs. iron haematoxylin.
General Description of Haemopoiesis, with Nomenclature
In embryos and foetuses there is a system of mesodermal cells of many potentialitie corresponding to the reticulo—endothelia1 system of post-foetal life. Within this system in embryos must be included the vascular endothelium and, to a lesser extent, the interstitial mesoderm of the yolk sac. From the fixed cells of this system free cells arise in vessels or solid tissues.
One type is the haemocytoblast (Fig. 1). It is the most primitive cell, whose potentiality of differentiation is directed to haemopoiesis only. It forms red blood corpuscles, granular leucocytes, lymphocytes and megakaryocytes. Its appearance has been described by Turnbull (1934). In foetal and postfcetal life the heemocytoblast does not show evidence of migration but in early embryos it is frequently amoeboid. Occasionally in blood Vessels and very often in the connective tissues of early embryos it shows pseudopodia and in the process of migration in the tissues may appear distorted. Some of these early haemocytoblasts are slightly smaller and more lymphocytoid in appearance than those in fig. 1, but still larger than the large lymphocyte. The early haemocytoblast appears also to be able to form histiocytes. These slight differences in the early heemocytoblasts do not warrant giving them a special name. They correspond to the primary wandering cell of Saxer (1895-96), the lymphocytoid wandering cell of Maximow (1909, 1927) and the mesamoeboid cell of Minot (1912). Maximow (1927) regarded his lymphocytoid wandering cell as identical with the haemocytoblast and sometimes called it such.
The other type of cell that may arise directly from the reticulo-endothelial cell is the free kistéocyte. As already mentioned, it appears to arise also from early heemocytoblasts in embryonic life. It has a round, oval or kidneyshaped nucleus and a moderate or abundant amount of pale-staining cytoplasm which may be vacuolated. Its chief potentiality is to take up Various substances or cells into its cytoplasm and become a phagocyte. In the cedematous rarefied mesoderm of the chorion and in places in the embryo are to be found cells not unlike histiocytes. They differ in having a more abundant, more Vacuolated pale-staining cytoplasm. These are the same as the Hofbauer cells of the placenta (Hofbauer, 1905). The potentiality of this cell is not obvious. It is probably connected in some way with the tissue fluids, as they are numerous in oedernatous tissues.
In young embryos the megalca/ryocytes differ from those in older specimens and I call these early megakaryocytes. They are smaller and have one, two or three separate nuclei or one bi- or trilobed nucleus. They do not possess pseudopodia, whereas in later embryonic and middle foetal life those in the tissues are often surrounded by numerous minute pseudopodia.
The nomenclature in connection with the development of the red blood eorpuscles is, with minor differences, that used by Tumbull (1934).
The term erythroblast (Fig. 1) is used for any nucleated cell whose potentiality of differentiation is directed only towards the formation of erythrocytes. It is not used in the sense of Lowit (1886), who introduced the term for haemoglobin-free precursors of nucleated red blood cells. The term erythrocyte is used for a nucleus-free cell containing haemoglobin stainable by eosin, not, as is sometimes used, for an eosinophilic red blood corpuscle Whether nucleated or not.
During the differentiation of the erythroblast the nuclei show all gradations of diminution in size, condensation of chromatin structure and diminution in size and number of nucleoli from that of the haemocytoblast, till eventually the nucleus becomes shrunken and pyknotic. The pyknotic nucleus is then extruded and the cell becomes an erythrocyte. The erythroblasts may be subdivided into early, intermediate and late, those with pyknotic "nuclei having a similar size of cell and nucleus to those in the intermediate or late stage. Nuclei do not as a rule become pyknotic until they have become very small and reached the late stage. Sometimes larger nuclei in the intermediate stage of development become pyknotic (fig. 1, cells 4a and 10a). During their development, erythroblasts multiply by mitotic division. In a late stage of development they probably do not multiply and those with pyknotic nuclei certainly do not. Mitotic division is usually accompanied by variable shrinkage in size of the cell. The nucleus of the erythroblast is usually round, but late and pyknotic forms are frequently deforIned-Tu1'nbull’s crenated, moniliform or rosette-like distortion, probably from lowering of the surface tension between nucleus and cell body, as suggested by Albrecht (Weidenreich, 1904).
During the development of the erythroblasts, haemoglobin stainable by eosin appears in the cytoplasm. The nucleus is not lost until haemoglobin is thus made visible. The stage of development of the erythroblast at which visible haemoglobin appears varies, and accordingly there are two types of erythrocyte formation. In one type haemoglobination is late and begins in small erythroblasts with pyknotic or almost pyknotic nuclei and the resulting erythrocyte is within the normal size for adult blood. This is nownoblaetelc erythropoiesis and a normocyte is formed (fig. 1, cells 3-8). In the other type heemoglobination occurs in larger erythroblasts with larger nuclei having still a Well preserved nuclear structure. This is megaloblastic erythropoicsis (fig. 1, cells 9-13 or 14-18). The resulting erythrocyte is usually a megalocyte, larger than the normocyte found in normal adult blood, but during the evolution of a megaloblast so great a reduction in size may occur that a normocyte is formed. An erythroblast with basophil cytoplasm and its nuclear structure still preserved can develop along either the normoblastic or the megaloblastic series. Such a cell has been called by Turnbull a primary erythroblast. The term had been applied previously by Schridde (1907) to the erythroblasts in embryos of 1-9 mm., later called by Maximow “ primitive ”. Since MaximoW’s terminology in this respect has been generally adopted Schridde’s priority to the use of the term primary erythroblast has been waived. The remaining erythroblasts develop from the primary and may be called secondary erythroblasts. Schridde used the term “ secondary erythroblasts ” for those first appearing in the liver in a 12-5 mm. embryo, corresponding to Maximow’s definitive erythroblasts, and Maximow used the term as an alternative for his definitive erythroblasts. ' The term is not now used for these cells and is available for erythroblasts which are not primary. It is useful to call secondary erythroblasts nomwblasts when they are scarcely larger than a norrnocyte and develop only into normocytes, and megaloblasts when haemoglobin is visible in larger cells which will form as a rule megalocytes but sometimes norinoeytes. The nucleus of the normoblast is always small and pyknotic or almost pyknotic, while that of the megaloblast has variable degrees of preservation of nuclear structure (Ehrlich and Lazarus, 1909) or is pylmotic.
The normoblasts are small cells with pyknotic or almost pyknotic nuclei and are subdivided into basophil if there is no visible heemoglobination of the cytoplasm, and polychromatic or orthochromatic if there is incomplete or complete heemoglobination as revealed by eosin staining. The normoblast in foetal tissues which have been dehydrated in alcohol, cleared in chloroform and embedded in paraffin is not more than 5 [L and the normocyte not more than 4-5 p, in diameter. They are slightly smaller than the corresponding cells in adults.
Megaloblasts are either polychromatic or orthochromatiti/»——there are no basophil megaloblasts. The megaloblasts may be divided into early, intermediate and late according to the degree of preservation of nuclear structure, corresponding to similar stages of the primary erythroblast. The final stage is one with a pyknotic nucleus and may be called in short a pyknotic megaloblast (Turnbull, 1934). The megaloblasts are larger than the normoblasts. A late megaloblast can either develop from an intermediate megaloblast or a late primary erythroblast. An intermediate megaloblast can either develop from an early megaloblast or from an intermediate primary orythroblast. As has been mentioned, a megaloblast during maturation of its nucleus may show considerable reduction of its cytoplasm, so that when the nucleus is pyknotic the cell is small and identical with a normoblast. This may be referred to as megalo-normoblastic erythropoiesis (fig. 1—change from cell 12 to cell 7——and fig. 2).
After the first two or three weeks of post-foetal life normal erythropoiesis is entirely normoblastic. In embryoni.c life "it is entirely megaloblastic ; in early foetal life it is megaloblastic and megalo-normoblastic, while in later foetal life it is partly megaloblastic and partly normoblastic.
The terms primitive and definitive erythroblaets are used in the sense of Maximow to describe the erythroblasts of two distinct erythropoietic families in the embryo. The term primitive erythroblast was first used by Bryce (1906) for the first erythroblasts in Lepidosiren paradoxa but these differ from those in man. As has been mentioned, Schridde used the terms primary and secondary erythroblasts for the cells Maximow later called primitive and definitive erythroblasts. The primitive erythroblasts (fig. 1, cells 14-17) are first formed in the yolk sac and later are present and multiply in the blood of early embryos but soon disappear. The definitive erytlmoblasts are formed in the yolk sac and embryo later than the primitive. There is no essential difference between the two types of erythroblasts. The primitive erythroblast and the megalocyte it forms are, however, as a rule considerably larger than the definitive forms, though small varieties of about the same size as the latter occur. Late and pyknotic late primitive erythroblasts often swell up to a large size, apparently by imbibition of fluid, and usually have an elliptical shape. The nuclei of the primitive erythroblasts usually differ slightly from those of the definitive. They are paler and the cl'J.romatin threads more delicate in the primitive early and intermediate erythroblasts than in the definitive, and nucleoli may be seen in the primitive intermediate erythroblasts but never in the definitive. The primitive erythroblasts are all heemoglobinated and megaloblasts. The cytoplasm is usually more deeply stained by eosin than in definitive secondary erythroblasts. Primitive erythroblasts arise from hsemocytoblasts, probably directly. I have not been able to recognise primitive primary erythroblasts.
Plate VII
Fig. 1. Red blood cells and their precursors. 1 and 2, haemocytoblasts ; 3, 4 and 5, early, intermediate and late primary erythroblasts; 6 and 7, basophil and erthochromatic normoblasts; 8, normocyte; 9, 10, l1 and 12, early, intermediate, late and pyknotic megaloblasts; 13, megalocyte; 14, 15, 16 and 17 early, intermediate, late and pyknotic primitive megaloblasts; 18, primitive erythrocyte; 4a, basophil normoblast with prematurely pyknotic nucleus; 10a, megaloblast with prematurely pyknotic nucleus. Jenner.
Fig. 2. 125 mm foetus. Focus of megalo-normoblaetic erythropoiesis in neck. One early and several intermediate megaloblasts forming pyknotic megaloblasts and - with loss of nucleus - a Megalocyte, and — from shrinkage in size — orthochromatic normoblasts and — by loss of nucleus - a normocyte. Jenner.
The primitive erythroblasts were first seen by Erb (1865), who likened them to the blood cells of the frog because of their great size and elliptical shape. Howell (1891) also recognised them and called them ancestral oorpuscles because they resembled those of reptilia and amphibia.
Development of Blood Vessels and Cells Before a Complete Circulation is Established (Embryos up to 3 mm)
The origin and development of the first blood vessels is very obscure owing to the small number of well preserved and stained very young human embryos available for study by any one observer and the scant attention given to the vascular development in many of the original descriptions. The three presomite embryos of Frazer which I have examined are by themselves insufficient for me to draw conclusions ; the following account therefore takes into consideration the observations in the literature.
The presomite embryos that will be mentioned are the Teacher-Bryce no. 1 (1908)*, Miller (1913, quoted by Streeter, 1920), Linzenmeier (1914)*, Frazer (A), Peters (1899), Mollendorff (l921)*, Fetzer (l9l0)*, Sch1agenhauferVerocay (1916), Tea-cher-Bryce no. 2 (1908)*, Jung (1908), Frazer (B), Meyer (1924), Strahl-Beneke (l9l0)*, Herzog (1909), von Spec (1896), Frazer (C), Jones-Brewer (1-935), Minot (1912), Streeter (1920), Grosser (1931), Heuser (1932), Grosser (1913), Ingalls (1918), McIntyre (1926-28), Eternod (1898-99), Boerner-Schwarzacher (1923), von Spec (1889) and Triepel (1917). They are placed in order of development according to Grosser (1924), if included in his list, and according to the individual author in specimens subsequently described. I have placed the Frazer embryos according to their probable development. The somite embryos are those of Ingalls (1920) 2-3 pairs of somites, Jagerroos (1934) about 6 pairs of somites, Dandy (1910) 7 pairs of somites, Low (1907-08) 13-14 pairs of somites, 2-6 mm., Thompson (1906-07) 23 pairs of somites, 2'5 mm. and Frazer, about 20 pairs of somites. Descriptions of other embryos were examined but found to be of no value.
- Quoted by McIntyre (1926-98).
Yolk sac
The first vessels arise from solid cellular masses which have proliferated from the mesothelium. The latter is the single layer of flattened mesodermal cells which first covers the yolk sac after the formation of the chorionic cavity. They had not appeared in the Teacher—Bryce (no. 1), Linzenmeier or Fetzer embryos according to McIntyre, the Miller embryo according to Streeter, Peters’s embryo according to Lewis (1912) or the Jung or Herzog embryos. A single mass of this kind is first seen in the Frazer presomite (16-day) embryo. In this the yolk sac consists of two layers of endothelial-like cells, the inner entodermal, the outer mesodermal. On the ventral aspect of the sac there is a solid rounded proliferation of mesodermal cells about 4 cells wide, projecting about 40 p. high into the chorionic cavity (fig. 3). This is probably angeioblastic.
Mesodermal cell masses were present in the Mollendorif, Teacher Bryce (no. 2) and Strahl-—Beneke embryos according to McIntyre and in the Schlagenhaufer—Verocay embryo. They are present in the Frazer presomite 19-day embryos. Here the masses project as papillae into the chorionic cavity. I believe vessels arise from the masses by loss of cells in the centre while the peripheral cells become endothelial. From the endothelial cells hmmoglobin-free basophil cells arise and become free in the lumen. These are haemocytoblasts. Isolated vessels containing free cells constitute the blood islands of Pander (1817), observed by him in chick embryos. One of the Frazer presomite 19-day embryos appears to be the youngest with blood islands. In it several proliferations of mesodermal cells on the ventral aspect of the yolk sac project as papillae from the outer surface. Some papillae contain empty spaces, like vessels, some of which are lined with flattened cells (fig. 4). The spaces appear to have arisen by vacuolation and loss of cells in the centre of the papillae. Deeper in the mesoderm beneath the papillae are a few well formed endothelial-lined vessels. Four of these contain groups of free cells in the lumen and constitute blood islands (fig. 5). The cells are basophil and most can be identified as haemocytoblasts. Others cannot be identified because of crowding. The cells probably arose from the vessel wall, since some plump endothelial cells are present as if in process of becoming free. It appears certain that blood vessel precedes blood cell formation. Connections between the different spaces and vessels could not be made out but in the absence of reconstructions they cannot be excluded.) In the Frazer presomite 19-day embryo (0) similar appearances are present except that the papilla and blood islands are more numerous and occupy about the ventral half of the yolk sac. Further, while some of the free cells are typical haemocytoblasts others are slightly smaller and more lymphocytoid. Blood islands are present in the Meyer (1924) and von Spee ( 1896) embryos and are probably constant in all older embryos. The haemocytoblasts differentiate into haemoglobinated primitive megaloblasts, but a few persist and a few apparently differentiate into histiocytes. The Jones-Brewer embryo is the youngest containing haemoglobinated cells; Bloom ( 1938) describes some of the free cells as haemoglobinated primitive erythroblasts. When haen1o— globinated cells become constantly present is diflicult to estimate.
Plate VIII
Fig. 3. Embryo 1, Frazer, presomite (A). Small mass of proliferated mesodermal cells on Ventral pole of yolk sac. Heidenhain's iron haematoxylin. x570.
Fig. 4. Embryo 2, Frazer, presomite (B). Papillary projections of mesoderm on ventral part of yolk sac, some containing spaces. Heidenhain's iron haematoxylin. x480.
Fig. 5. Embryo 2, Frazer, prosomite (B). Blood island in mesoderm of ventral part of yolk sac. Heidenhain's iron haematoxylin. x750.
- Online Editor - Heidenhain's iron haematoxylin - an iron alum hematoxylin stain used for staining muscle striations and mitotic structures blue-black. Named after Rudolph Heidenhain (1834-1897) a German histologist and physiologist. (More? Histology stains)
They are present in the McIntyre and probably in all older embryos but are undoubtedly present in some of the embryos intermediate in development between the J ones-Brewer and McIntyre, such as the Minot, Grosser (1931) and Heuser embryos. The persistence of hsemocytoblasts after the formation of erythroblasts is mentioned only in the Jones—Brewer embryo by Bloom (1938), and in the Minot embryo, where they are called mesamoeboids. They are also present in the Frazer embryo of about 20 pairs of somites. Their presence, however, can be presumed in embryos of intermediate development. The first appearance of histiocytes is apparently in the Frazer embryo of about 20 pairs of somites, but no doubt they are present in younger somite and perhaps some presomite embryos. In this embryo the yolk sac in rather more than its ventral half shows numerous vessels distended with cells (fig. 6). The vessels project from both the outer and inner aspects of the sac, especially the former. The great majority of the cells are haemoglobinated early and intermediate megaloblasts, and less numerous late and pyknotic late megaloblasts, all of the primitive type. The cells stain deeply eosinophil with haematoxylin and eosin, but Jennerstained sections are not available to show the degree of chromatophilia. Many are in mitosis. Besides these there are a very few hsemocytoblasts and phagocytic histiocytes. The vessels are probably united to each other to a considerable degree. The blood vessels and islands first appear at the ventral pole of the yolk sac and later involve the ventral half of the sac. Fewer and smaller islands may be found at a later date in the other hemisphere of the sac. Little can be concluded about the union of the separate vessels to form a net. Probably union of blood islands occurs early but the net is not complete till late, possibly not till the yolk sac vessels have joined the embryonic. The above view of the histiogenesis of blood islands differs from that of Jones and Brewer, Streeter, and McIntyre. In their embryos they describe mesodermal cell masses in which central cells become in part free cells while the peripheral cells become endothelial. McIntyre describes syncytial masses of mesodermal cells the centres of which become haemoglobinated and subsequently break up into free cells.
Chorion and body stalk
In the chorion, Bremer’s theory (1914) of the histiogenesis of vessels has not been disproved.
He states that the Vessels are derived from the mesothelium of the body stalk. The mesothelium is at first limited to the yolk sac, as in the Frazer presomite (16-day) embryo, but later spreads as a continuous or interrupted layer over the body stalk and finally lines the chorionic cavity completely. From the mesothelium funnel-shaped ingrowths pass into the body stalk mesoderm. By closure of the lumen. of a funnel at some point near its opening into the chorionic cavity, isolated spaces lined with mesothelium are formed inside the body stalk. The space may remain connected by solid cellular cords with the orifice of the funnel or with the surface mesothelium. From the inner ends of funnels or from the spaces formed from them, multiple nets of tubes pass out into the body stalk and extend into the remainder of the chorion. The tubes have no definite endothelial lining and he calls them unli_ned spaces. By a process which he calls delamination, solid cellular cords—-his angioblastic cords_arise from the walls of the unlined spaces and lie in the lumen. Angioblastic cords may also grow out from the inner ends of the mesothelial funnels and no_t be enclosed in unlined spaces. At an early stage spaces, called angiocysts, may form in places in the angioblastic cords and later a continuous lumen changes the cords into vessels. The separate nets unite to form a continuous vascular net in the body stalk and remainder of the chorion. His theory was formed from a study chiefly of the Grosser (1913), Minot, and Herzog embryos. McIntyre found similar structures in his own and the Teacher-Bryce (no. 2) embryo and Grosser in his 1931 embryo.
The scantiness of the material I have examined does not permit me to dispute Bremer’s theory but two observations appear to disagree with it. Firstly, angioblastic activity is first present in the Frazer presomite (16~day) embryo. The chorionic mescderm is in general poor in cells, stellate and spindle, but adjacent to the embryo, in the part that would become the body stalk, it is more cellular than elsewhere and contains a few small groups, either syncytial or bounding very small spaces. There are also a few strands of elongated cells, in single or double layer, which pass out into the neighbouring mesoderm. One of the strands (fig. 7), separated from the chorionic cavity by only a very few cells, has developed a lumen and resembles a capillary. As the mesothelium is limited to the yolk sac these angioblastic structures probably arose from undifferentiated mesodermal cells. Secondly, in the Frazer presomite ( 19-day) embryos, lined capillary-like empty tubes are present in the body stalk and chorion, very few in the first and many in the second embryo. Some strands two cells wide without lumina are also present but no solid cellular cords enclosed in unlined spaces. In the body stalk of these embryos a few larger end0thelial—lined spaces are also present. On the other hand in the Frazer embryo of about 20 pairs of somites, in addition to lined capil1ary—like empty tubes, there are several unlined spaces containing short cellular cords either solid or with minute lumina.
Angioblastic activity in the body stalk and chorion is absent from the Teacher-Bryce (no. 1), Linzenmeier and Fetzer embryos according to McIntyre, from the Miller embryo according to Streeter and from the Peters and Schlagenhaufer-Verocay embryos. In the Mollendorff embryo according to McIntyre there are channels lined with flattened cells in the chorionic mesoderm which do not join up to form a continuous system. Angioblastic activity is present in the Teacher-Bryce (no. 2) embryo according to McIntyre and is probably constant in older embryos.
Heemopoiesis occurs in the body stalk independently of that in the yolk sac. In the present state of knowledge this cannot be said to be constant. In the Minot, Grosser (1931), Ingalls (1918), 1VIcIntyre and Triepel presomite embryos and Ingalls’s (1920) embryo of 2-3 pairs of somites and in Dandy’s embryo of 7 pairs of somites free cells are present in some body stalk vessels which have no connection with yolk sac vessels. The cells are probably for the most part haemoglobinated primitive erythroblasts but the presence of haemoglobin is 11ot stated in all instances. It is probable that the cells arose from heemocytoblasts derived from the endothelium but their histiogenesis is not described. Dandy and McIntyre describe blood cell formation in chorionic vessels apparently apart from the body stalk. J agerroos in his embryo of about 6 pairs of somites describes blood cells in isolated chorionic vessels, the cells having been formed extravascularly and subsequently included in vessels. These are however only isolated observations of haemo 'poiesis in the chorion apart from the body stalk. Besides the intravascular formation of blood cells in the body stalk the Minot, Heuser, Grosser (1913), McIntyre and Dandy embryos are said to show blood islands consisting of groups of cells apparently free in the body stalk mesoderm. McIntyre describes the cells as haemoglobinated and perhaps haemoglobin was present in the cells of some of the others. The nature of these so-called blood islands is very doubtful as they are very poorly described. I doubt whether they represent an extravascular formation of blood cells derived from the mesodermal cells. I think also that the term blood island should be reserved for blood cell formations inside isolated vessels. If blood cells were present inside body stalk vessels then perhaps some of the extravascular groups of cells could be explained by continued multiplication of cells which had escaped from the vessels. This undoubtedly occurs in older embryos as I will describe. In the J ones—Brevver and Boerner—SchWarzacker embryos blood islands are described in the body stalk but their relation to vessels is not mentioned.
The embryo
The number of observations on the histiogenesis of vessels Within the embryo itself is few. The appearances in von Spee’s (1889, 1896) and Triepel’s presomite embryos and Ingalls’s (1920) embryo of 2-3 pairs of somites suggest that cardiac and vascular endothelium arises in situ from mesodermal cells. Ingalls believed that the vessels arise in multiple sites. In the present state of knowledge it must be presumed that blood islands consisting of blood cell formation in isolated vessels do not occur. The blood island Ingalls describes in the posterior end of the right aorta of his embryo of 2-3 pairs of somites is an isolated observation and the nature of the cells and their mode of origin are not described.
The establishment of the circulation
It is certain that an umbilical circulation between the body stalk and the embryo is established before a vitelline between the yolk sac and the embryo. This is seen in Eternod’s presomite embryo, somewhat precociously, and in Dandy’s. The blood in the circulation I was probably derived from blood islands in the body stalk. In Ingal.ls’s embryo of 2-3 pairs of somites a similar condition is developing, the yolk sac vessels being still unconnected with the embryo while the right umbilical artery communicates with the right aorta. Whether or not this is the rule cannot be stated but there are no observations of the establishment of the vitelline before the umbilical circulation. Both umbilical and vitelline circulations were established in Low’s embryo of 13-14 pairs of somites (2-6 mm.) and Thompson’s embryo of 23 pairs of somites (2-5 mm.). At this time it may be presumed that the cells of the blood are for the most part derived from the yolk sac but they will have joined cells from the body stalk in embryos with blood islands in this site. In a few embryos some may have come from chorionic vessels apart from the body stalk and if Ingalls’s observation is correct some may have been derived from. the embryo itself. It is not possible to state the time when the chorionic vessels fill with blood but it is probably about the time of the formation of the complete circulation.
Summary
It may be concluded that vessels arise from mesodermal cells independently in three areas, the yolk sac, the chorion——-perhaps at first limited to the body stalk—and the embryo. The vessels in each of these areas unite to form nets or systems. The three systems later unite with each other and the complete circulation is established. In some if not all embryos the circulation between body stalk and embryo begins before that between yolk sac and embryo. Blood islands form constantly in the yolk sac and consist of separate vascular units containing blood cells. The cells are first hsemocytoblasts which arise from the vessel wall. Later the cells are almost entirely primitive erythroblasts but a few haemocytoblasts persist and a few differentiate into histiocytes. Intravascular blood formation occurs frequently, perhaps constantly in the body stalk, and when the umbilical but not the vitelline circulation has been established it will supply the embryonic vessels with blood. Doubtful blood formation has been recorded in the chorionic and embryonic vessels and in the mesoderm of the body stalk. The cytology and histiogenesis of blood formation outside the yolk sac at this period has unfortunately been neglected or very inadequately described.
Plate IX
Fig. 6. Embryo 4, Frazer, about 20 pairs of somites. Blood vessels in yolk sac full of primitive erythroblasts. (Stain - Haematoxylin Eosin) Dufay process. x550.
Fig. 8. 10 mm embryo (Frazer). Two haemocytoblasts among epithelial cells of yolk sac. Primitive erythroblasts in yolk sac vessel. Ehrlich’s haematoxylin and eosin. Dufay process. x1100.
This theory of the histiogenesis of blood cells and vessels is in opposition to that of His who in 1900, according to Bloom, deri.ved the blood cells and vessels from a special angioblastic tissue which arose from the yolk sac ontoderm. Minot (1912) accepts this theory and states that the angioblast maintains its independence throughout life. According to this theory the embryonic and chorionic vessels are extensions from those of the yolk sac. Minot derived the cells of the reticulo-endothelial system from the angioblast but the work of Maximow has shown that these cells are mesodermal and their potentialities can be assumed by undifferentiated mesodermal cells.
Development of the Blood After Establishment of the Complete Circulation (Embryos from 3 to 12 mm)
In embryos of 3-9 mm., after the circulation is established, new blood cells are formed everywhere in the circulation equally by mitotic division of blood cells previously present. Perhaps to a very slight extent erythroblasts are formed by differentiation of haemocytoblasts in the circulation but the great majority are derived from preformed erythroblasts. At this stage almost all the blood cells are primitive erythroblasts in early, intermediate, late or pyknotic late stages: most are intermediate. With haematoxylin and eosin they appear fully haemoglobinated but Jenner sections are not available to show the degree of chromatophilia. A very few erythroblasts are binucleate. Very rarely one may be seen with vacuolated cytoplasm. Mitoses are numerous. In a 3—mm. embryo the erythroblasts average about 7-5 pt in diameter. The other embryos of this group (3-9 mm.) belong to Professor Frazer’s collection and in them the erythroblasts average about 10-5 p, in diameter. A few are small-—about 6 p.—or very large——about 16 u. The difference in average diameters is probably due to difl°erent methods of embedding. Some of the small erythroblasts might form erythrocytes within the normal variation of diameter for adult blood, 23.6. normocytes. This would constitute megalo—normoblastic erythropoiesis. Knoll (1929) describes a small proportion of definitive erythroblasts in the blood at this stage, calling them second generation cells, but these are undoubtedly only mall primitive erythroblasts. Besides the erythroblasts there are a few megalocytes, histiocytes—~—some phagocytic-———and haemoeytoblasts, most of which are slightly smaller and more lymphocytoid than the typical hsemocytoblast- The histiocytes contain nuclear fragments or brown granular pigment. In the aorta, groups of histiocytes may be present. The cells forming a cluster in the aorta of a 9-4.—mm. embryo of Minot (his figure 368) I would call histiocytes but he calls them mesamoeboids, which I believe correspond to heemocytoblasts. In the Frazer 4-5~mm. embryo (fig. 11) there are many more heemocytoblasts and histiocytes in the yolk sac vessels than in the embryonic vessels but in the other embryos at this stage this diiference is very slight.
In all the embryos of this stage (3-9 mm.) the chorionic vessels are filled with blood. In all, primitive erythroblasts are scattered singly or in groups in the mesoderm of the embryo, chorion and umbilical cord. These undoubtedly result from hsemorrhage, but continued multiplication occurs, as is shown by the presence of mitotic figures. Haemorrhage is present in the yolk sac cavity in two and chorionic cavity in one.
The Frazer 10 mm. embryo is similar as regards the circulating blood and the presence of haemorrhages in the mesoderm, but there is, in addition, activity of the reticulo—endothelial system, chiefly directed to the formation of a new haemopoietic family, the definitive. Hsemocytoblasts, isolated or in groups, appear in the liver among the liver cells outside the sinusoids. Some lie in deep bays (lacunae of Neumann, 1874) within liver cells. In the sinusoids besides primitive erythroblasts there are a few haemocytoblasts and one early trilobed megakaryocyte is seen, while scattered or in groups are numerous histiocytes, many containing brown granular pigment or less commonly nuclear fragments or degenerated primitive erythroblasts. In the yolk sac are a few haemocytoblasts, singly or in groups, among the large entodermal epithelial cells (fig. 8). Some appear to lie in lacunae, as in the liver. Two early megakaryocytes are also present among the epithelial cells. In the yolk sac vessels, besides primitive erythroblasts and a few haemocytoblasts and histiocytes, there are a very few early megakaryocytes, groups of early and intermediate primary erythroblasts and intermediate megaloblasts, all of definitive type.
The brain and spinal cord are surrounded by a zone of very delicate connective tissue rich in young capillaries. These contain histiocytes and heemocytoblasts of the early, smaller and more lymphocytoid type in greater number than the general circulation. A few of the heemocytoblasts have small pseudopodia. Some of the histiocytes contain brown granular pigment, one a pyknotic primitive megaloblast. Some early forms of megakaryocytes are also present. Some of these are no larger than a hsemocytoblast, others are 2-3 times as large. They have one or two nuclei which are round, oval or bilobed. The cytoplasm is eosinophil and as a rule abundant. Outside the capillaries there are many amoeboid haemocytoblasts——some with pseudopodia, many histiocytes———some pigmented, and a few early megakaryocytes. This activity is especially marked at the base of the brain.
In the Frazer 12-mm. embryo the distribution is similar except that extravascular heemopoiesis is more extensive in the liver.
Besides many hsemocytoblasts there are numerous cells of the definitive series, namely early and intermediate primary erythroblasts, some intermediate and fewer early and late megaloblasts and a few early megakaryocytes. The latter lie outside and inside the sinusoids.
Summary
In embryos of 3-9 mm. the blood cells in the vessels are of the primitive series ; almost all are formed by mitosis of erythroblasts previously formed ; some of the erythroblasts are of the diameter of normoblasts. A few erythroblasts, have reached the tissues by haemorrhage and multiply there by mitosis. In embryos of 10-12 mm. the blood vessels still contain cells of the primitive series but in the liver and yolk sac there is now active proliferation of haemocytoblasts, early megakaryocytes and erythroblasts of the definitive series.
Further Development of the Blood (Specimens over 12 mm)
1. General circulation, excluding capillaries
In the 18- and 19-5-mm. embryos the blood cells are almost entirely erythroblasts and erythrocytes in about equal numbers. Of the former, all or almost all are primitive, all are megaloblasts and the majority orthoohromatic ; a very few are early, many are intermediate but the majority late or pyknotic. Mitotic figures are numerous. A few heemocytoblasts, histiocytes and phagocytic histiocytes are also present. The first are usually slightly smaller than typical hsemocytoblasts and are lymphocytoid in appearance but larger than large lymphocytes. A few show small pseudopodia.
In the 26- and 28—mm. embryos about a quarter of the red blood corpuscles are nucleated. They are megaloblasts and the majority are primitive. A very few are early, a few intermediate, the majority late or pyknotic. Mitotic figures are very sparse. Haemocytoblasts are absent but there are a few lymphocytes which were probably formed directly from haemocytoblasts. Histiocytes are absent. In the 35-min. embryo the blood is similar except that there are no early and only a few intermediate megaloblasts and only one mitotic figure was seen.
In one of the 48—mm. embryos about 10 per cent. only of the red blood corpuscles are nucleated. Most are late and pyknotic primitive megaloblasts. The great majority are fully haemoglobinated; a few of the definitive are polychromatic. Mitotic figures are absent. Besides these cells there are a very few lymphocytes and neutrophil myelocytes and leucocytes.
In the 65-mm. and all subsequent embryos the blood cells appear to be definitive. Not more than 5 per cent. of the red cells are nucleated. Most are late and pyknotic megaloblasts, a few are intermediate megaloblasts and normoblasts (4-5 p. in diameter). A few are polychromatic. Mitotic figures are absent. There are a Very few lymphocytes and neutrophfl myeloeytes and leucocytes. In the 7 6—mm. embryo the blood is similar except that a Very few intermediate primary erythroblasts are present. A very few of these are in mitosis. One eosinophil leucocyte was seen.
In the lO4.—mm. embryo the blood is similar except that now there are Very few early primary erythroblasts : a mitotic figure in a megaloblast was seen. In the 120- and 125-mm. embryos the blood is the same except that no early primary erythroblasts are present.
In the 146—mm. embryo a film of fresh blood from the umbilical cord shows about 0-6 per cent. of the red cells to be nucleated. In 200 nucleated cells 5 per cent. are intermediate, 18 per cent. late and 34-5 per cent. pyknotic megaloblasts, 10 per cent. are normoblasts (not larger than 8 )u. in diameter), 2 per cent. inter~ mediate primary erythroblasts, 30 per cent. lymphocytes, 1 per cent. eosinophil and 0-5 per cent. neutrophil myelocytes. Almost all the erythrocytes are megalocytes, a few are normocytes (6-5 to 7-5 p, in diameter). Some of the nucleated and non-nucleated red cells are polychromatic. Two megaloblasts showed mitotic figures. Some leucocytes are present in the blood in sections but not in the film.
In the 170-mm. embryo the blood in sections is similar to that in the 120- and 125-mm. embryos, except that only a very few intermediate megaloblasts are present and primary erythroblasts and mitoses are absent. In longer foetuses the blood was not studied in detail. There appeared to be no marked alteration and probably there was a gradual change to the state at birth.
2. Yolk sac
In the Frazer 12.5 mm embryo the vessels contain a few early, some late and pylmotic and many intermediate megaloblasts and a very few megaloeytes, all of the primitive series. A very few and small vessels contain small groups of early and intermediate primary erythroblasts and intermediate megaloblasts of the definitive generation. A very few small haemocytoblasts are also present: one early bilobed megakaryocyte was seen. Only three haemocytoblasts were seen, lying outside vessels among the epithelial cells.
The Frazer 15.5 mm embryo is similar except that a few late megaloblasts are present in the few small groups of definitive erythroblasts. Six early megakaryocytes and one histiocyte containing a pyknotic megaloblast were also seen. Amongst the epithelial cells outside vessels are a few groups of about a dozen cells consisting of early and intermediate primary erythroblasts of the definitive generation and one or two heemocytoblasts.
The 26.9 mm embryo is similar except that there are now numerous megalocytes. There are several histiocytes and phagocytic histiocytes but no megakaryocytes. The histiocytes contain pyknotic nuclear fragments or nucleated red cells : one contained brown granular pigment. A few scattered groups of early and intermediate primary erythroblasts with a very few intermediate megaloblasts are present outside vessels among the epithelial cells.
In a 48 mm embryo both the mesoderm and entoderm of the yolk sac are very atrophic and the wall of the sac is usually very thin. The blood in the vessels consists of megalocytes with a few late and pyknotic megaloblasts and a very few lymphocytes. There are no histiocytes and no megakaryocytes. There are now no foci of heemopoiesis in the vessels or among the epithelial cells.
3. Liver
In an 18 mm embryo there is a conspicuous increase in amount of haemopoiesis from that in the l2 mm Frazer embryo and the blood formation is definitive. The second 18 mm, 19.5 mm and 26 mm (fig. 9) embryos show further increases and the maximum amount found is in the last. The liver now shows great diffuse infiltration with hsemopoietic cells, with numerous foci of increased density of infiltration. There are numerous haemocytoblasts, early and intermediate primary erythroblasts and intermediate megaloblasts, and a few late primary erythroblasts and early, late or pyknotic megaloblasts. The primary erythroblasts and haemocytoblasts outnumber the megaloblasts. The cells for the most part lie in the parenchyma outside the sinusoids and many, especially heemocytoblasts, lie in bays or lacunae in the liver cells. Similar cells are found in the sinusoids, scattered or in small groups among the Inegalocytes or megaloblasts of the primitive series. A few eosinophil and neutrophil myelocytes and leucooytes and megakaryocytes lie within thesinusoids or scattered outside in the parenchyma. The portal systems are not formed but some neutro~ phil and eosinophil myelocytes and leucocytes are present in the connective tissue of the hilum.
The degree of haemopoiesis relative to the parenchyma in the 26~mm. embryo is maintained in the 20 longer specimens up to 190 mm. in which the liver was examined. The heemopoiesis shows slight changes with increasing age. The early megaloblasts soon disappear and towards the end of this group the late megaloblasts outnumber the intermediate. Primary erythroblasts and heen1ocytoblasts still outnumber the haemoglobinated cells. Orthochromatic normoblasts (about 4-5 pr. in diameter) appear in scanty numbers in the 48 mm and longer embryos. Basophil normoblasts do not appear until the 190—mm. embryos and are then very scanty. In embryos of 48 - 180 mm inclusive, therefore, the normoblasts are derived from megaloblasts by considerable shrinkage of cytoplasm during development, that is, by megalo —norm oblastic erythropoiesis. The amount of leucopoiesis in the parench yma shows little increase, but in the connective tissue of the hilum it is increased and it is abundant in the portal systems, which are flrst well developed in the 48—mm. embryos. In six specimens of 65 - 190 mm, focal erythropoiesis similar to that in the parenchyma is present in the portal systems. In six specimens of 76 - 190 mm, scattered tissue mast cells lie in the portal systems and in four (65 - 190 mm) foci of lymphocytes are also present there. In the 170 mm foetus a very few tissue mast cells lie free in the sinusoids.
In five foetuses of 200-457 mm. (fig. 10) a progressive decrease in the amount of heemopoiesis relative to parenchyma is apparent. It is still diffuse, with foci of increased density, but the density of the infiltration becomes progressively less. Intermediate and late primary erythroblasts increase relatively to the early forms and late megaloblasts outnumber intermediate forms. Basophil and orthochromatic normoblasts are scanty. The leucopoiesis remains the same in the portal systems and parenchyma. A few scattered tissue mast cells are present in the sinusoids of the 444 and 457 mm foetuses and in the portal systems of all. In the 200, 343 and 457 mm foetuses foci of erythropoiesis, and in the 330, 343 and 457 mm foetuses foci of lymphopoiesis, occupy portal systems.
In nine older (470 - 546 mm) foetuses the haemopoiesis in the liver has decreased conspicuously (fig. 11). It is new entirely focal. In the parenchyma outside the sinusoids are to be found widely separated small foci of intermediate and late primary erythroblasts, occasionally including some early forms or normoblasts and late and pyknotic megaloblasts. Some foci consist entirely of normoblasts and late and pyknotic megaloblasts. A very few heemocytoblasts are present outside the sinusoids. In the sinusoids are a few isolated or small groups of primary erythroblasts, normoblasts or late megaloblasts and a few isolated heemocytoblasts. A very few eosinophil myelocytes and leucocytes are present in the parenchyma or in the sinusoids. Megakaryocytes, constantly present in the younger foetuses, are now found in only 3 of the 9 older specimens and then in very scanty numbers. Haemopoiesis is considerably reduced in the portal systems. A few scattered or small groups of lymphocytes and eosinophil myelocytes and leucocytes and a few scattered tissue mast cells are present in most systems. In two specimens (508 and 533 mm) one or two tissue mast cells lie free in sinusoids. A 546 mm foetus is considerably post-mature but the amount of haemopoiesis is the same as in the others of the group. In all but two (495 and 540 mm), the liver cells are to a variable degree water-clear. This is undoubtedly due to glycogenic infiltration.
Plate X
Fig. 7. Embryo I, Frazer, presomite (A). Capillary-like structure in chorionic mesoderm. Heidenhain’s iron haematoxylin. x440.
Fig. 9. 26 mm embryo. Amount of haemopoiesis in liver. Ehrlich’s (Stain - Haematoxylin Eosin). x155.
Fig. 10. 444 mm fetus. Amount of haemopoiesis in liver. Ehrlich’s (Stain - Haematoxylin Eosin). x155.
Fig. 11. 546 mm fmtus. Amount of haemopoiesis in liver. Ehrlich’s (Stain - Haematoxylin Eosin). x156.
In the 11 newly born infants the amount of haemopoiesis in the liver shows a considerable diminution from that in late foetal life. A very few leucocytes and lymphocytes are present in some portal systems in most cases. In the 4-day infants and one of the 5-day infants some portal systems contain numerous myelocytes and leucocytes. Two or three small foci of late primary erythroblasts and normoblasts are present outside the sinusoids in the 2-day, one of the 3-day and the 4-day infants. In the remainder, including all infants over five days, erythropoiesis is absent. In the. 4- and 15-day infants a few usually degenerate -looking megakaryocytes are present. The water-clear appearance of the liver cells seen in the full-term foetuses is absent in the infants. A very few small bile thrombi are present in dilated intercellular canaliculi in one of the 5- and one of the 14-day infants, neither of which was jaundiced. Bile thrombi are not present in the liver of the 4-day infant, which was jaundiced.
Free iron is present in the liver cells of all the embryos of 18 mm. and over except two. It is present either in the form of brown granular haemosiderin or as a diffuse prussian blue reaction. Both forms occur together except in the 70-mm. embryo, in which granules are absent. Only a minority of liver cells are affected. In very young embryos, before the formation of portal systems, the iron is localised as a rule to the cells around veins. In older subjects the usual site is in the cells bordering the portal systems and the cells of the bile ductules near their junction with the liver columns. The iron may be confined to these sites or may also occupy scattered cells anywhere in the lobule. In a few specimens in which intercellular bile canaliculi are sharply outlined, the iron favours that part of the liver cell which lies between the canalicular lumen and the nucleus. The total amount of intracellular iron is never great and does not show much variation in different subjects. However in nine, all 28-17 O-mm. specimens, the amount of iron is more abundant than in the remaining 30 livers examined. Towards the end of pregnancy (457 mm." onwards) iron is relatively scanty. In seven infants of 2-15 days the amount of iron in the liver cells is in keeping with that in late foetal life ; in the remainder, it is slightly more abundant.
Several foetuses of 146 mm. or more show some intra- and extracellular haemosiderin granules in the portal systems.
Iron pigment is present in a variable number of Kupffer cells in all but three (80-, 190- and 343-mm.) embryos and foetuses. It is usually in the form of granular hsemosiderin, but in some it shows as a diffuse prussian blue reaction in the cytoplasm. In all but two (2- and 4-day) infants the Kupffer cells contain more iron than in foetal life. The cells are often enlarged and sharply defined by a deep prussian blue reaction in the cytoplasm. Granules or globules of iron pigment are also present in some. In the other three infants the amount of iron in the Kupffer cells is about the same as in foetal life. This intense iron staining of Kupffer cells is probably related to the normal post-natal heemolysis.
Phagocytic Kupffer cells containing erythrocytes, nucleated red cells or free nuclei are present in the sinusoids of all the livers of embryos, foetuses and infants. Sometimes only one, sometimes many such cells are seen. The number in infants does not differ from that in foetuses.
4. Connective tissue, including its capillaries
In the two 18-mm. embryos and in the 19-5 mm. embryo, foci of a few intermediate and more late and pyknotic primitive megaloblasts and megalocytes are scattered in many connective tissues. These are undoubtedly similar to those seen in the earlier embryos and probably resulted from multiplication of primitive megaloblasts which had escaped from blood vessels. These foci are also present in the 26-, 28~ and 35-mm. embryos, but contain increasing numbers of erythrocytes, so that nearly all the cells in the last embryo are erythrocytes.
In four embryos of 18-26 mm. many amoeboid haemocytoblasts, megakaryocytes and some histiocytes, most of them containing brown granular iron pigment and a few containing free nuclei or nucleated red cells, lie scattered in connective tissues. They are most numerous in delicate tissues such as the very vascular meninges, especially at the base of the brain. The megakaryocytes are usually early, with one, two or three nuclei or bi- or trilobed nuclei, and rarely have pseudopodia. Similar cells are present in greater number in the meningeal capillaries than in the general circulation. Several megakaryocytes are present in the capillaries of various tissues and organs throughout these embryos. The intravascular megakaryocytes do not have pseudopodia. There are also a few megakaryocytes, haemocytoblasts and histiocytes within the lumen of and in the tissue around small veins forming plexuses in the retroperitoneal tissue and neck in the two 18- and the 19-5—mm. but not in older embryos. In the 28- and 35—mm. embryos the capillaries and connective tissue of the meninges contain fewer of these cells, but iron-containing phagocytes are still found outside the capillaries. In the 48-mm. embryos these cells are absent from the meninges, while megakaryocytes are absent from the connective tissues and capillaries elsewhere and are confined to special sites—-—liver, marrow, spleen, lymph glands and stroma of lymph plexuses. In some connective tissues, especially the loose tissues of the neck, some amceboid haemocytoblasts and iron—containing histiocytes are present in decreasing numbers in foetuses up to 190 mm. Thereafter no special examination was made of the connective tissues in general.
In one of the 18-mm. embryos in the dense connective tissues of the head below the brain there are a few small scattered groups of heemocytoblasts and early primary erythroblasts of the definitive series. In the other 18-mm. embryo in the same site, especially about the eyes and cranial nerves, are more numerous and larger groups of either hsemocytoblasts and early primary crythroblasts alone or of intermediate and late megaloblasts alone or of mixtures of these cells. Many groups of megaloblasts with occasional intermediate primary erythroblasts are present in the meninges at the base of the brain. In the 19-5-mm. embryo there are similar groups in the meninges and a few elsewhere ; one was seen in the mesentery and two near the vertebral column. In the 26- and 28mm. embryos there are several scattered foci of primary erythroblasts of all stages, some having in addition intermediate and late megaloblasts, and more numerous foci of intermediate, late and pyknotic megaloblasts. Most lie in the tissues of the head and neck below the brain, fewer in the meninges at the base of the brain and in other tissues such as the choroid plexus, mesentery and limbs. In six 35-125—mm. specimens small foci of intermediate and late primary erythroblasts alone, or of intermediate, late and pyknotic megaloblasts alone or mixtures of these cells, are present in connective tissues in various places. In this group a very few orthochromatic cells of 4-5 p. diameter with pyknotic nuclei and thus identical with normoblasts are present in association with megaloblasts in embryos of 48 mm. or longer. As there are no basophil normoblasts, they undoubtedly arose from megaloblasts (megalo—normoblastic erythropoiesismfig. 2). In the 170- and one of the 190—mm. foetuses the number of erythropoietic foci in the connective tissues is considerably reduced and only a very few are present, consisting of late and pyknotic megaloblasts and orthochromatic normoblasts, the latter derived from the former. In the other 190-mm. and longer foetuses only special organs were examined, but probably erythropoiesis in connective tissues soon disappeared, as the foci in the 170- and first of the 190-mm. foetuses are very few and composed of erythrocytes and secondary erythroblasts only ; there are no groups of primary erythroblasts to suggest recent formation of foci.
In five 18-28-mm. embryos a few eosinophil and neutrophil myelocytes and leucocytes lie scattered or in small groups in various connective tissues, especially the meninges, mesentery and retroperitoneal tissue. In the longer specimens up to 190 mm. it is rarer to find similar cells scattered in any connective tissue.
In five 26-48—mm. embryos a few small haemocytoblasts and eosinophil and neutrophil myelocytes and leucocytes lie in the adventitia of a few arteries in various parts of the body. In six 65-125-mm. specimens a few lymphocytes are present as well. Similar cells are also present in the carotid bodies of some of these foetuses. In the 170- and one of the 190-mm. foetuses these infiltrations are absent.
5. Lymph plexuses
In one 18 -«mm. embryo a few neutrophil and eosinophil myelocytes and leucocytes and amoeboid haemocytoblasts are present in the connective tissue of developing lymph plexuses in the neck, and a few haemocytoblasts and histiocytes lie in blood capillaries therein. In the 19 '5 — mm. embryo this infiltration is increased and the stroma of cervical lymph plexuses shows many eosinophil and neutrophil myelocytes and leucocytes, amoeboid haemocytoblasts, histiocytes—some containing brown granular iron pigmcnt—and a few megakaryocytes. A few hjstiocytes and haemocytoblasts are also present in the blood capillaries. In seven 26-75 - mm. embryos there are also a few lymphocytes.
Histiocytes and haemocytoblasts are not present in the capillaries in the lymph plexuses in the 48-mm. and longer embryos. In five 28—125—mm. specimens a very few small foci of erythropoiesis occupy the connective tissue of cervical lymph plexuses. In the
I 170- and one of the 190-mm. foetuses there is no special haemopoietic activity in the plexuses.
6. Lymph glands
In one 48 mm. embryo definite lymph glands have formed and consist of local proliferations of stellate and spindle cells in the connective tissue stroma of lymph plexuses. The areas of pro1ifera~ tion are infiltrated with lymphocytes, amoeboid haemocytoblasts and a very few histiocytes and eosinophil and neutrophil myelocytes and leucocytes. A very few lymphocytes have entered the lymph vessels. In the 65- and 70-mm. embryos the glands are further developed and lymphocytes have increased greatly. They now contain numerous myelocytes and leucocytes and a few megakaryo— cytes. Some lymph vessels contain numerous lymphocytes. In the 75-, 76- and 120—mm. embryos several of the glands contain in addition small foci of late megaloblasts and normoblasts or of intermediate primary erythroblasts and late megaloblasts. In the 125 — mm. foetus leucopoiesis is considerably less, and in seven 17 0-546-mm. foetuses it is still less, amounting to a few eosinophil and fewer neutrophil myelocytes and leucocytes in the sinuses or gland tissue, especially in the peripheral parts. Megakaryocytes are absent in one 190 - mm. and longer foetuses examined. One small focus of megaloblasts is present in a lymph gland in the 125- and 170—mm. foetuses but all erythropoiesis is absent from glands in one of the 190-mm. and longer foetuses. A few tissue mast cells lie free in the sinuses or in the gland tissue in four 470-546—mm. foetuses.
Erythrophages are rare in lymph glands in foetal life. Two were present in sinuses in one 546 mm. foetus. Some free sinus cells in full—term foetuses contain diffuse brown pigment which does not give the prussian blue reaction.
7. Marrow
A clavicle was examined in 13 specimens (18-120 mm.), a femur in 26 (18-540 mm.), a humerus in 9 (18-75 mm.) and a rib in 8 (1041-5340 mm.). Heemopoiesis made its first appearance in the skeletal system in the clavicle in the 43—mm. embryo. It then appeared in the humerus in the 57-mm. embryo and in the femur in the 75-mm. embryo. Cytological studies were difficult because of the effect of decalcification upon staining.
In the 43-min. embryo in one place between capillary sinuses in the loose connective tissue marrow of the clavicle there are a very few amoeboid heemocytoblasts and neutrophil myelocytes and leucocytes and in another place a few am oeboid haemocytoblasts alone. In the clavicular marrow of the 48-mm. embryo a few hsemocytoblasts, lymphocytes and neutrophil myelocytes and leucocytes and one or two histiocytes are present. In the clavicle of the 57 —mm. embryo a few eosinophil myelocytes and leucocytes and a small focus of definitive erythroblasts, apparently intermediate primary I erythroblasts and megaloblasts, are present in addition. In the humerus of this embryo there is a small group of amoeboid haemocytoblasts. In the clavicle in the 65—mm. embryo haemopoiesis has developed considerably, forming larger and denser areas between the sinuses, most dense around the arteries. There are many haemocytoblasts, numerous eosinophil and neutrophil myelocytes and leucooytes, a few early and intermediate primary erythroblasts and intermediate and late megaloblasts and a few lymphocytes. There are also a few scattered well developed megakaryocytes. In the "3'5—mm. foetus the marrow in the clavicle, humerus, femur, radius, ulna, tibia, fibula and pelvis presents a similar appearance. The haemopoietic tissue occupies only part of the marrow and in the long bones there is no haemopoiesis for quite a distance from the cartilaginous epiphyses. The areas of haemopoiesis, although often diffuse between the sinuses, tend to surround the developing arteries. In older foetuses the maximum density is reached in the 160~mm. foetus. The cytology of the marrow was not followed. It is probable, however, that erythropoiesis was of the same type as elsewhere, becoming in part normoblastic in middle foetal life and entirely normoblastic in post-foetal life.
In the femur in six foetuses of 80—170 mm. no heemopoiesis is present for distances of 0-8 to 1-1 mm. from the zones of provisional calcification of cartilage. In the 320- and 343—mm. foetuses hwmopoiesis reaches to 0-3 mm. from the zone. In five foetuses of 444-540 mm. it reaches up to the zone but is less dense and in places absent in the metaphysis just below the zone. In the rib the haemopoietic marrow has not reached the zone in one 508and in the 540—mm. foetus. No haemopoiesis has occurred in any specimen in primary medullary spaces, periosteum or chondral canals. Towards the end of foetal life some adipose tissue cells appear in the marrow in the lower half of the femur in some specimens.
8. Spleen
The spleen was examined in 28 embryos and foetuses and in 7 infants. It is first present in the 28-mm. embryo. In the 28and 35-mm. embryos it consists of a mass of undifferentiated cells. In the 35—mm. embryo one or two early megakaryocytes are present in capillaries in this mass. In four specimens of 55-80 mm. the spleen consists of vessels surrounded by spindle cells, lying in a pulp of widely meshed delicate connective tissue with an occasional recognisable sinus. In the pulp is a lake of blood. A few early or well formed megakaryocytes are present, usually in sinuses. In the lake of blood are a very few haemocytoblasts, eosinophil myelocytes and leucocytes, a few orthochromatic normoblasts and scattered megaloblasts, usually late and pyknotic, but a few intermediate. The erythroblasts were probably not formed in s-itu from precursors but carried in from the blood stream and probably most are definitive. Five specimens of 70-162 mm. show in addition a few scattered foci of definitive erythropoiesis, some in sinuses, most in the pulp. They consist of early and intermediate primary erythroblasts with occasionally one or two haemocytoblasts, or of intermediate and late and sometimes one or two early megaloblasts, or of primary erythroblasts and megaloblasts mixed. A few normoblasts formed from megaloblasts are also present. In the 146-mm. foetus a very few heemocytoblasts are present in the cellular connectivet issue around the arteries. In the 162—mm. foetus heemocytoblasts are more numerous and many lymphocytes have been formed from them, constituting small Malpighian bodies. In the remaining foetuses there is very little variation. The total amount of haemopoiesis is always slight and near the end of pregnancy is very slight. Early megaloblasts are absent and intermediate forms less numerous. Megakaryocytes decrease but one or more are always‘ found. In the 470-mm. and longer foetuses a very few neutrophil myelocytes and leucocytes have appeared in the pulp. A few tissue mast cells lie in the trabeculae in the 444- and 533—mm. and in the pulp in the 457 - and 533-mm. foetuses. The spleen at full term shows a few scattered heemocytoblasts, intermediate and late megaloblasts, normoblasts, eosinophil (and fewer neutrophil) myelocytes and leucocytes, a few small groups of primary erythroblasts and one or two megakaryocytes, often appearing degenerated. These cells lie chiefly in the pulp but are also present in the sinuses. Quite frequently intermediate megaloblasts were absent from the liver at full term but were readily found in the spleen.
In five infants of 3-5 days a few myelocytes, leucocytes, normoblasts and late megaloblasts are present scattered in the pulp. In one 14-day and in the 15-day infant a few leucocytes but no myelocytes, megaloblasts or normoblasts are present. One megakaryocyte was seen in a 3-day infant but none in the other four.
Erythrophages are visible in the pulp in a third of the embryos and foetuses and in some of the infants. Never numerous, they are difficult to see because of the large number of red blood cells free in the pulp.
In eleven 55-190-mm. specimens and in the 320- and 330-mm. foetuses, free iron pigment is present in the pulp in small amount, either free or in reticulum cells in the form of haemosiderin granules and spheres. In one 190- and in the 343-mm. foetus no iron pigment is present. In the 444-mm. and older foetuses iron is present in granules or globules or diffusely in the cytoplasm of reticulum cells and sometimes in sinus cells. It varies greatly in amount. In four cases (470-508 mm.) the amount is abundant, but in three others (495-546 mm.) it is very slight. This considerable variability in iron content in older foetuses probably. depends upon the degree of congestion of the pulp. In infants the amount of iron varies considerably, as in older foetuses, and probably for the same reason. In one of the 3-day infants and in the 15-days infant the amount is slight. In three infants of 3-14 days it is very abundant and in two of 4 and 5 days a moderate amount is present.
9. Kidneys
The kidneys were examined in 26 embryos and foetuses and in 7 infants. In the 104-mm. foetus one small group, and in three foetuses of 146-180 mm. a few small groups of primary erythroblasts and megaloblasts or of megaloblasts and normoblasts are present about the tubules in the upper part of the medulla. In one 190 mm. foetus several very small foci of late megaloblasts and normoblasts and one or two myelocytes lie around tubules and vessels in the intermediate zone between cortex and medulla. In the 200—mm. fcetus two foci, and in the 320- and 495-mm. foetuses single small foci of erythropoiesis occupy the intermediate zone. In one 3-day infant there is one focus of normoblasts in the peripelvic tissue ; in the very premature 15-days infant there are three in the connective tissue of the intermediate zone.
In one of the 180-mm. foetuses and in the 200-mm. foetus a few h2em0siderin—containing phagocytes are present in the connective tissue of the upper part of the medulla and intermediate zone. These are probably the result of slight extravasations of blood. No free iron is present in the epithelium of the kidney in any specimen.
10. Thymus
This was examined in 22 embryos and foetuses. In the 28-mm. embryo a few amoeboid haemocytoblasts infiltrate the otherwise epithelial thymus. In the 35-mm. and longer specimens lymphopoiesis is established. Leucopoiesis begins in the 55-mm. embryo, and in this and in the four specimens of 75-120 mm. is shown by the presence of a Very few small groups of eosinophil promyelocytes and myelocytes in the interlobular septa. In the 70- and 140-mm. specimens, besides these cells there are a few eosinophil myelocytes in the medulla and a few tissue mast cells in the capsule and interlobular tissue. In the 125—mm. foetus a few eosinophil myelocytes and leucocytes are present in the capsule and interlobular tissue and in the cortex‘ ; two mast cells were present in the capsule. In the 162-mm. foetus leucopoiesis has considerably increased. There are numerous eosinophil and a few neutrophil myelocytes and leucocytes and a few promyelocytes in several interlobular septa, while many eosinophil myelocytes and leucocytes infiltrate the medulla adjacent to the septa and a few lie in the cortex.
Tissue mast cells are also present in the interlobular septa and capsule. In eight foetuses of 17 0-540 mm. there is similar leucopoiesis.
In eleven specimens of 28-170 mm., but not in longer, a little erythropoiesis is represented in the younger specimens by a few small groups of intermediate and late megaloblasts and in the older by late megaloblasts and normoblasts. The groups lie in
the interlobular septa or in the perithymic tissue. Erythropoiesis is never present within the thymic tissue.
Summary of Different Types of Blood Cells from their Earliest Appearance
Haemocytoblasts and histiocytes
Heemocytoblasts first arose from the endothelium of the yolk sac Vessels and were first seen in the Frazer embryo (B). For a time they were the only free cells in the blood islands but soon most differentiated into erythroblasts—first in the Jones—Brewer presomite embryo, a few into histiocytes. The latter were first seen in the yolk sac vessels in the Frazer embryo of about 20 pairs of somites but were probably present in some younger embryos. After the establishment of the circulation, hwmocytoblasts and histiocytes, some of them phagocytic, persisted in the yolk sac vessels and were present in the general circulation up to the 196mm. embryo ; a few histiocytes however persisted in the yolk sac vessels in the 26-9—mm. embryo. In the 26—mm. and longer specimens the heemocytoblasts had become smaller and were identical with lymphocytes.
Beginning in the 10-mm. embryo and persisting up to the 35-mm. embryo there was a concentration of haemocytoblasts and histiocytes in the tissues around the central nervous system, especially at the base of the brain. Similar cells were present in many connective tissues—especial.ly loose tissuesmin embryos of 18-190 mm. and in and about small veins forming plexuses in the neck and retroperitoneal tissue in the 18- and 19-5-mm. embryos, in the connective tissue in lymph plexuses in embryos of 18-75 mm. and in the capillaries of this connective tissue in embryos of 18-48 mm. Heemocytoblasts were also present in the adventitia of scattered small arteries in embryos of 26-125 mm. They were present in the developing lymph glands in embryos of 48 mm. or more and were associated with histiocytes. Some haemocytoblasts appeared in the connective tissue of the clavicular marrow in the 43—mm. embryo. Infiltration of the epithelial thymus by heemocytoblasts occurred in the 28-mm. embryo. The haem0cytoblasts in these sites differentiated into the various blood cells. In certain sites such as connective tissue, especially that around the central nervous system and in lymph plexuses, the haemocytoblasts appeared also to form histiocytes. In young embryos hsemocytoblasts migrating in connective tissues or the thymus were often distorted from their usual more or less rounded shape and often showed pseudopodia. Small pseudopodia were also occasionally seen on the rounded forms in the blood.
Erythropoiesis
In early embryos the erythroblasts belonged to the primitive family. They appeared first in the J ones-Brewer presomite embryo in the blood islands of the yolk sac. They were megaloblasts and were derived directly from haemocytoblasts without passing through a stage comparable with the primary erythroblasts in the definitive family. According to the literature erythroblasts are also formed in many older presomite and younger somite embryos in isolated vessels in the body stalk and possibly in a few embryos in the chorion. Blood islands are also described in the mesoderm of the body stalk in a few embryos and in one embryo one island was found in the embryo itself. Since the cellular content and histiogenesis of these formations were not described they must be regarded as very doubtful. With the establishment of the circulation the primitive erythroblasts entered the embryonic vessels and continued to multiply there. With increasing age the number of early and intermediate primitive erythroblasts decreased and the late and pyknotic late forms and megalocytes increased, accompanied by diminished multiplication. Mitotic division of primitive erythroblasts had ceased in the 35-mm. embryo, and in the 48-mm. embryo these cells were all in a late or pyknotic late stage. In older embryos no primitive erythroblasts were recognisable in the blood, but primitive megalocytes undoubtedly persisted longer. In embryos of 3-35 mm. multiplication and development of primitive erythroblasts continued in places in many connective tissues, the original cells having reached the tissues by haemorrhage. In the 35mm. embryo most of these cells had developed into megalocytes and the erythroblasts were all late. Apart from this observation and the doubtful description in the literature of blood islands in the mesoderm of the body stalk, primitive erythropoiesis was essentially intravascular. Primitive erythropoiesis was also essentially megaloblastic but in embryos of 3 mm. or more a very few small erythroblasts of the size of normoblasts had been formed from megaloblasts (megalo-normoblastic erythropoiesis).
Definitive erythropoiesis appeared first in the 10-mm. embryo in the yolk sac vessels as groups of primary erythroblasts and megaloblasts. These groups persisted but had disappeared in the 48—mm. embryo. Heemocytoblasts had appeared among the hepatic and yolk sac epithelial cells in the 10-mm. embryo but extravascular definitive erythropoiesis did not begin in the liver until the 12-mm. embryo and in the yolk sac till the 15-5-mm. embryo. In the yolk sac it was still present in the 26-9- but had disappeared in the 48—mm. embryo. In the liver it increased in amount and reached a maximum relative to the parenchyma in the 26-mm. embryo. This maximum persisted until in foetuses of 200-457 mm. there was a progressive decrease to a slight amount which remained constant in foetuses of 470 mm. and more. In infants 5 or more days old such erythropoiesis was absent. Definitive erythropoiesis was also present in embryos and foetuses of 18 mm. or more in the sinusoids of the liver, in amounts that were relatively slight but in proportion to the amounts outside the sinusoids. In some foetuses of 65-457 mm. focal definitive erythropoiesis was also present in the connective tissue of the portal systems. In many connective tissues it was present focally in specimens of 18-190 mm. and probably did not persist in longer foetuses. The sites of election, especially in the younger embryos, were the dense tissue at the base of the brain and in the upper part of the neck, the delicate tissue surrounding the central nervous system, especially at the base of the brain, and the mesentery, but any connective tissue in the body or limbs sometimes showed it. In the spleen focal definitive erythropoiesis in the pulp and to a less degree in the sinuses began in the 70-mm. embryo and first disappeared in infants over 5 days old. It was always slight and decreased in late foetal life. In the connective tissue of the marrow definitive erythropoiesis began in the clavicle in the 57—mm. embryo and subsequently increased considerably in amount. In the connective tissue in lymph plexuses it formed a few foci in some specimens of 28-125 mm. It was also present in slight amount in a few lymph glands of a few foetuses of 75-170 mm. In the kidney a few foci were found in some foetuses and infants of 104 mm. or more and were last observed in the 15-days-old infant. In the thymus there were a few foci in the connective tissue of the capsule and interlobular septa in some specimens of 28-170 mm. In the blood definitive megaloblasts were present in small numbers in the 28and 35-mm. embryos and were almost as numerous as the primitive in the 48—mm. embryo. All erythroblasts in the blood were definitive in the 65~mm. and older specimens. In foetuses of 76-146 mm. there appeared to be exceptional erythropoietic activity in the blood, as some intermediate and in one a few early primary erythroblasts appeared and mitotic figures reappeared. In the circulation primary erythroblasts were not seen previously nor subsequently.
Table - Stages of erythropoiesis
| Stage of development | Within embryo or extra-embryonic tissues | Within or outside vessels | Haemopoietic family | Type of erythropoiesls |
|---|---|---|---|---|
| Jones-Brewer presomite embryo till establishment of circulation (about 2.5 mm) | In blood islands in yolk sac, in some of cases in body stalk in the chorion or ' the embryo | Intravascular: possibly extravascular in body stalk in a few cases | Primitive | Megaloblastic |
| From establishment of circulation (about 2-5 mm) to 9 mm. | In general circulation (of embryo, yolk sac and chorion) | Intravascular except for continued multiplication of cells in hemorrhages | Primitive | Megaloblastic |
| 10 mm up to less than 48 mm. | In embryo, yolk sac and general circulation | Intra- and extra~vascular (marrow first in 57 mm embryo) | Partly primitive, partly definitive in general circulation; definitive elsewhere | Megaloblastic |
| 48 mm to between 15 and 21 days after birth | Intracorporeal in many organs and tissues | Extravascular, except sinuses of liver spleen and multiplication of cells in circulation (70 - 160 mm.) | Definitive | Partly megaloblastic, partly megalo-normoblastic (48-190 mm.); partly megaloblastic, partly normoblastic (190 mm. onwards) |
| 21 days or more after birth | Intracorporeal in marrow only | Extravascular | Definitive | Normoblastic |
It is safe to state that after the 15th day of life erythropoiesis is confined to the marrow. Definitive erythropoiesis was essentially extravascular apart from that in the sinusoids of the liver and sinuses of the spleen and that in the circulation in foetuses of 76-146 mm. It was entirely megaloblastic in specimens up to 48 mm. and partly megaloblastic subsequently up to the infant of 5 days old. In specimens of 48-180 mm. orthochromatic but no basophil normoblasts were present in some sites and had been formed from megaloblasts (megalo-normoblastic erythropoiesis). Such formation of normoblasts occurred also in older foetuses to a less degree. In the 190-mm. and older specimens basophil normoblasts were present so that the erythropoiesis became in part truly normoblastic. With increasing age the number of earlier forms of megaloblasts decreased. Early megaloblasts were very rarely seen after 48 and never after 125 mm. and towards the end of foetal life the intermediate megaloblasts usually disappeared. The last secondary erythroblasts seen in the tissues examined microscopically were in the kidneys of the 15-day infant. They’ were normoblasts and it is probable that erythropoiesis in the marrow is, as found by Turnbull (1934), entirely normoblastic in older infants and children as in adults.
Erythropoiesis can be divided into five stages in embryos, foetuses and post-foetal life according to its site and character. The stages are summarised in the table (p. 51).
Leucopoiesis
Leucopoiesis with the formation of eosinophil and neutrophil myelocytes and leucocytes was first seen in one of the 18 mm. embryos in the parenchyma of the liver and in various connective tissues such as the meninges, mesentery and stroma of lymph plexuses. It persisted up to birth, never in great amount, in the hepatic parenchyma and in various connective tissues up to 190 mm., and in the lymph plexuses up to 125 mm. It was fairly abundant in the hilum of the liver in early embryos such as the 18 mm. and in the portal systems in 48--495—mm. foetuses, after which it became reduced. It was present in some portal systems in infants up to 5 days old, and a very few leucocytes but no myelocytes persisted in the older infants. A little was seen in the adventitia of a few scattered arteries in 26-125-mm. specimens. It was present in lymph glands from 48mm. embryos up to fullterm foetuses, fairly abundantly in the 65- and 70-mm. embryos, but at other times in scanty amount. It began in the interlobular septa of the thymus in the 55-mm. foetus and in the glandular tissue in the 7 0-mm. foetus ; it was scanty but increased considerably in amount in the 162-mm. foetus and subsequently remained about the same until birth. In the spleen slight leucopoiesis was present in infants over 5 days old. Leucopoiesis began in the clavicular marrow in the 43-mm. embryo and subsequently increased con siderably. Leucopoiesis in the yolk sac, as observed by Maximow (1927) and figured by Bloom, was not seen. Myelocytes and leucocytes first appeared in the blood stream in scanty numbers in the 48-mm. embryo.
Lymphopoiesis
Lymphopoiesis began in the connective tissue of lymph plexuses in very scanty amount in the 26—mm. embryo and in the adventitia of a few arteries in the 65-mm. embryo and was continued in these sites until the 125-mm. embryo. It began in lymph glands in the 48-mm. embryo, at which time lymphatic vessels contained some lymphocytes. It began in the thymus of the 35-mm. embryo and in the Malpighian bodies of the spleen in the 162-mm. foetus. It was present in some portal systems in the liver in several foetuses of 65-457 mm., and some portal systems contained a few lymphocytes in older foetuses and infants. Lymphocytes were present in the marrow in embryos of 48-65 mm. and probably persisted but could not be recognised because of the effect of decalcification on staining and because of the abundance of erythropoiesis and leucopoiesis. No lymphopoiesis occurred in the yolk sac. In the blood, lymphocytes were first seen in the 26-mm. embryo and they probably arose directly from the haemocytoblasts in the circulation.
Formation of megakaryocytes
Intravasoular and a little extravascular formation of megakaryocytes was present in the yolk sac in the 10-mm. embryo. Intravascular but not extravascular formation continued in the 12-5- and 15-5—mm. embryos but not in older. Intravascular or extravascular formation of megakaryocytes occurred in the liver of all specimens from 10 mm. up to 457 mm. but in only 3 of 9 older foetuses. Megakaryocytes, usually degenerated, were present in the liver of 3 of the 12 infants, the oldest being 15 days. In the spleen they were constant in specimens from 35 mm. to birth; at full time they were often degenerated ; only one was seen after birth, in an infant of 3 days. They were constantly present in the marrow of foetuses of 65 mm. or more. In the capillaries and connective tissues they were especially numerous at the base of the brain, where they were first seen in the 10-mm. embryo ; in the rest of the body and limbs they appeared in the 18-mm. embryos; in both sites they persisted to the 35—mm. embryo. Some were present in small veins forming plexuses in the neck and retroperitoneal tissue in the 18- and 19-5—mm. embryos, in the connective tissues in lymph plexuses in specimens of 195-125 mm. and in lymph glands in foetuses of 65-170 mm. No megakaryocytes were seen in the general circulation in any specimen. In the early embryos (10-35 mm.) the megakaryocytes usually differed from the typical form of adults in being smaller and having one, two or three separate nuclei. These early forms did not have the pseudopodia which were often abundant in the well developed forms of early and middle foetal life.
Summary
The development of the blood vessels and cells until a complete circulation has been established is described from 3 presomite and 1 somite embryo kindly lent by Professor J. E. S. Frazer, supplemented by 30 presomite and somite embryos selected from the literature. The subsequent development of the blood cells in the Vessels and tissues is described from the examination of 53 embryos and foetuses ranging from between 4 and 5 weeks to full term, and of 11 new-born infants 221 days old. The development of histiocytes and phagocytes and the distribution of iron are included.
I wish to thank Professor H. M. Turnbull for help in the preparation of this paper. I am deeply indebted to Professor J. E. S. Frazer for permission to examine some of his young embryos.
References
BLOOM, ‘W. . . . . . . In Downey’s Handbook of hematology, London, 1938, vol. ii, p. 865.
BOERNER-PATZELT, D., AND Z. gee. Anat., I Abt., 1923, lxviii, 204. SOHWARZACHER, W.
BREMER, J. L. . . . . . Amer. J. Anat., 1914, xvi, 447.
BRYCE, T. H. . . . . . Trans. Roy. Soc. Edinb., 1906, Xli, 435.
DANDY, VV. E. . . . . . Amer. J. Anat., 1910, x, 85.
E1-IRLIOI-I, P., AND LAZARUS, A.‘ Die Anaemie, 2nd ed., by A. Lazarus and O. Naegele, Vienna and Leipzig, 1909, Abt. I, Teil I, S. 52.
EBB, W. . . . . . . . Arch. path. Anaz‘.., 1865, xxxiv, 138.
ETERNOD, A. C. F. . . . . Anat. Anz., 1898-99, xv, 181.
GROSSER, O. . . . . . . Anal. Hefle, 1913, xlvii, 649.
,, . . . . . . Ergebn. Anat. E'm$wichel., 1924, xxv, 391.
. . . . . . Z. ges. A7iat., Abt. I, 1931, xciv, 275.
HERZOG, M. . . . . . . Amer. J. Anat., 1909, ix, 361.
HEUSER, C. H. . . . . . Contributions to embryology, Carnegie Institution of Washington, 1932, Xxiii, 251.
HOFBAUER, J. . . . . . Grrundziige einer Biologie den menschlichen Plazenta, Vienna, 1905.
HOWELL, W. H. . . . . J. Morph., 1891, iv, 57.
INGALLS, N. W. . . . . . Contributions to embryology, Carnegie Institution of Washington, 1918, vii, 111.
,, . . . . . Ibid.,_1920, xi, 61.
JAGERROOS, B. H. . . . . Acta 800. med. fennicae “ Duodecim,” sect. B, fasc. 2 and 4, 1934, xix, 1.
JONES, H. 0., AND BREWER, S/wrg., Gym. and Obstet, 1935, 1):, 657.
J. I.
J UNG, P. J. . . . . . . Beitrage zur friihesten Ei-Einbettung beim menschlichen Weibe, Berlin, 1908.
KNOLL, VV. . . . . . . Morph. Jah/rb., Abt. II, 1929, xviii, 199.
LEWIS, F. T. . . . . . I n Keibel and Mall’s Manual of human
embryology, Philadelphia and London, 1912, vol. ii, p. 295.
Low, A. . . . . . . . J. Anat., 1907-08, X111, 237.
LCWIT, M. . . . . . . Sitzungsber. d. Kais. Akad. d. Wissen. z. Wien, Abt. III, 1886, Xcii, 22.
MALL, F. P. . . . . . . In Keibel and Ma11’s Manual of human embryology, Philadelphia and London, 1910, vol. i, p. 199.
MAXIMOW, A.
3!
MCINTYRE, D. MEYER, P. . Mnsroaz, C‘. S. .
NEUMANN, E . PANDEB, C.
PETERS, H.
SAXER, F. . . . . . SOHLAGENHAUFER AND VEROGAY SCHBIDDE, H. . VON SPEE, F. GRAF .
33 3.’!
STEEETEE, G. L.
VAN DER STRICHT, O.
35 93 93
THOMPSON, P. TBIEPEL, H. . . TURNBULL, H. M.
)9
WEIDENEEICH, F.
Arch. mikros. Arlal., 1909, lxxiii, 444.
I rl V011 M<'5llendorff’s Handbuch der mikr0skopischen Anatomic der Menschen, Berlin, 1927, V01. ii, part I, p. 232.
Trans. Roy. Soc. Ealinb., 1926-28, lv, 77.
Arch. Gymilc., 1924, cxxii, 38.
I re. Keibel and Ma.11’s Manual of human embryology, Philadelphia and London, 1912, vol. ii, p. 498.
Arch. Hcilla, 1874, xv, 441.
Beitriige zur Entwickelungsgeschichte des Hiihnchens irn Eye, Wilrzburg, 1817.
fiber die Einbettung des menschlichen Eies, Leipzig and Vienna, 1899-.
Anal. Hefle, 1895-96, Vi, 347.
Arch. Gyra.alc., 1916, cv, 151.
Verh. cllsch. path. G'es., 1907 (1908), xi, 360.
Arch. Anal. Phys-iol., Anat. Abt., 1889, p. 159.
Ibid., 1896, p. 1.
Contributions to embryology, Carnegie Institution of Washington, 1920, ix, 389.
Arch. Biol., 1891, xi, 19.
Ibid., 1892, xii, 199.
J. Anal, 1906-07, xli, 159.
Anal. Hcftc, 1917, liv, 149.
This Journal, 1931, xxxiv, 277.
I rl The anaemias, by J. M. Vamtghan, London, 1934, p. 8, (2nd ed., 1936, p. 11).
Ergcbn. Anal. E’nlwiclccl., 1904, xiv, 345.
Plates
Plate XII
| Plate 7 Figures | |
|---|---|
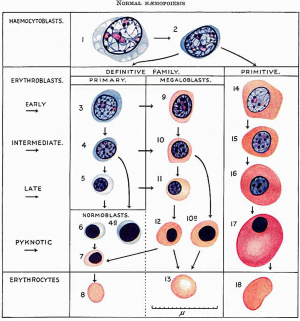
|
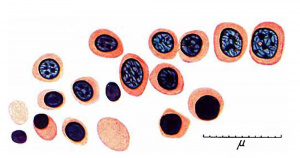
|
| Fig. 1. Red blood cells and their precursors. 1 and 2, haemocytoblasts ; 3, 4 and 5, early, intermediate and late primary erythroblasts; 6 and 7, basophil and erthochromatic normoblasts; 8, normocyte; 9, 10, l1 and 12, early, intermediate, late and pyknotic megaloblasts; 13, megalocyte; 14, 15, 16 and 17 early, intermediate, late and pyknotic primitive megaloblasts; 18, primitive erythrocyte; 4a, basophil normoblast with prematurely pyknotic nucleus; 10a, megaloblast with prematurely pyknotic nucleus. Jenner. | Fig. 2. 125 mm foetus. Focus of megalo-normoblaetic erythropoiesis in neck. One early and several intermediate megaloblasts forming pyknotic megaloblasts and - with loss of nucleus - a Megalocyte, and — from shrinkage in size — orthochromatic normoblasts and — by loss of nucleus - a normocyte. Jenner. |
Plate XIII
Fig. 3. Embryo 1, Frazer, presomite (A). Small mass of proliferated mesodermal cells on Ventral pole of yolk sac. Heidenhain's iron haematoxylin. x570.
Fig. 4. Embryo 2, Frazer, presomite (B). Papillary projections of mesoderm on ventral part of yolk sac, some containing spaces. Heidenhain's iron haematoxylin. x480.
Fig. 5. Embryo 2, Frazer, prosomite (B). Blood island in mesoderm of ventral part of yolk sac. Heidenhain's iron haematoxylin. x750.
Online Editor - Heidenhain's iron haematoxylin is an iron alum hematoxylin stain used for staining muscle striations and mitotic structures blue-black. Named after Rudolph Heidenhain (1834-1897) a German histologist and physiologist. (More? Histology Stains)
- Plate 8
Plate IX
| Plate 7 Figures | |
|---|---|
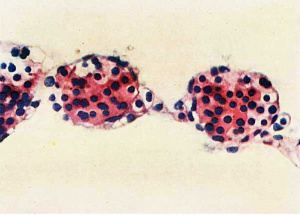
|
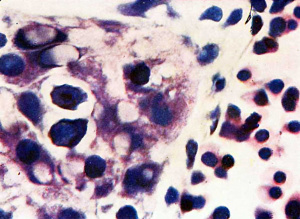
|
| Fig. 6. Embryo 4, Frazer, about 20 pairs of somites. Blood vessels in yolk sac full of primitive erythroblasts. (Stain - Haematoxylin Eosin) Dufay process. x550. | Fig. 8. 10 mm embryo (Frazer). Two haemocytoblasts among epithelial cells of yolk sac. Primitive erythroblasts in yolk sac vessel. Ehrlich’s haematoxylin and eosin. Dufay process. x1100. |
Plate X
- Plate 10
Cite this page: Hill, M.A. (2024, April 19) Embryology Paper - Normal haemopoiesis in intra-uterine and neonatal life. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Normal_haemopoiesis_in_intra-uterine_and_neonatal_life
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G