Paper - Human Embryo Horizons 19-23
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Streeter GL. Developmental Horizons In Human Embryos Description Or Age Groups XIX, XX, XXI, XXII, And XXIII, Being The Fifth Issue Of A Survey Of The Carnegie Collection. (1957) Carnegie Instn. Wash. Publ. 611, Contrib. Embryol., 36: 167-196.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Developmental Horizons In Human Embryos Description Or Age Groups XIX, XX, XXI, XXII, And XXIII
Being The Fifth Issue of a Survey of the Carnegie Collection
Late Research Associate of The Carnegie Institution Of Washington, John Hopkins University
Contributions To Embryology, No. 230
Prepared For Publication By Chester H. Heuser and George W. Corner

|

|
| Chester H. Heuser | George W. Corner |
Department of Embryology, Carnegie Institution Of Washington, Baltimore.
Prefatory Remarks
With the present installment of “Developmental horizons in human embryos," the undertaking passes into new hands. George L. Streeter, who began it in 1940 when he retired from the directorship of the Department of Embryology, died in July 1948 after a brief and unexpected illness. On his desk he left the illustrations which he had prepared for stages xix to xxiii and a few pages of notes for the text that was to accompany them. The article, had he finished it, would have completed the second half of his great project, which he had chosen to deal with first. Preferring to leave the earlier stages for attention later, when (as he hoped) more material might be available, he began with stage xi (13 to 20 paired somites) and planned to carry on his descriptions to the end of what he considered the embryonic period as distinguished from the fetal stage. In the first of his articles[1](1942, Contrib. to Embryol., vol. 30, pp. 211-245) he stated that he planned to establish twenty-five stages or “horizons,” the last of which would end at the time when the eyelids come together. Later he decided that stage xxiii could be considered to mark the ending of the embryonic period, stating in his fourth article[2] (1949, Contrib. to Embryol., vol. 33, p. 162) that the end of stage xxiii is clearly definable by the onset of marrow formation in the humerus: “If the onset can be recognized in a given specimen, that specimen is straightway classed as a fetus, and this places it beyond the bounds of our embryonic horizons."
After the beginning of the fetal stage, as thus demarcated, detailed descriptions of external form and structural development are no longer necessary to provide an adequate index of relative development, for the weekly increment of weight and length is then suliicicntly informative for the purpose. In volumes 30, 31, and 32 of the Contributions to Embryology[1][3][4] Dr. Streeter carried his descriptions on through stage xviii (14 to 16 mm. greatest length, age 37 2 I days). In volume 33 he permitted himself to digress from the stage-by—stage sequence in order to contribute an account of certain features of the development of bone and cartilage which are relevant to his concepts of the orderly progression of embryonic growth and organization. The work he left unfinished would have resumed the sequence and would have terminated at stage xxiii. No one else, of course, could hope to do this, from his scanty notes, exactly as Dr. Streeter would have done it, but it seems wise to fill the gap by publishing the illustrations and to attempt, at least, a summary statement of what Dr. Streeter might have written. The result, it is hoped, will serve the needs of embryologists who have found the earlier sections useful in the dating and comparison of human embryos.
That part of the program which Dr. Streeter had left untouched, i.e. horizons i to x, will be undertaken by Chester H. Heuser and will be published in subsequent volumes of the Contributions to Embryology.
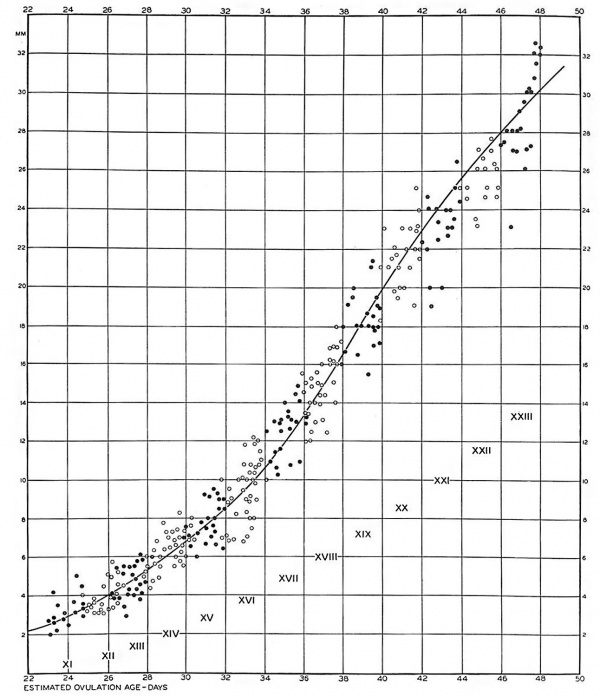
The accompanying growth curve (fig. 1) shows the number of specimens comprised in our material of the last five embryonic horizons, spaced according to their state of development. This growth curve is extended backward into the curve of horizons xi to xviii which was published in a previous issue.[4]In the earlier horizons the ovulation age could be accurately estimated by matching specimens with rhesus monkey embryos of known ovulation age. We have now, however, reached a developmental level at which the growth curves of monkey and man begin to draw apart and are no longer directly comparable. We do not again reach a satisfactory basis for estimating age until we come to the period covered by the Carnegie curves of fetal growth.[5] The last five horizons are adrift in this interval, but if the two-day intervals for the developmental horizons are tentatively continued along the base line, and if the same scale is used for the dimensions of the embryo, a plot of the specimens of horizons xix to xxiii produces the growth curve shown herewith. It proves to be satisfactory, blending at one end with the curve of the earlier horizons, and at the other end leading directly into the previously mentioned curves of Streeter, which continue through the remainder of prenatal life. The latter curves start at 8 weeks menstrual age. At this time the growth curve shows a mean sitting height of 23 mm.; by 9 weeks this has become 31 mm. Translating these menstrual ages into ovulation ages by subtracting 14 days, it is found by extrapolation that at 42 days ovulation age we are to expect an embryo of 23 mm, and at 49 days one of 31 mm, with due allowance for individual variation. This agrees closely with the curve of growth shown in figure 1, a fact which speaks favorably for the grouping into two—day developmental periods which was adopted by Dr. Streeter, as a feasible procedure in this kind of embryological analysis.
Fig. 1. Graphic plot of specimens in the Carnegie Embryological Collection which have been surveyed and assigned to horizons xi to xxiii. Embryos belonging to odd-numbered horizons are represented by black dots, and those of even-numbered horizons by white circles.
Point Scores of Development
The problem of placing embryos at their proper level of development, relative to other specimens, becomes more complicated as the number and complexity of the component organs increase. For setting up arbitrary horizons it proves that general impressions, which had sufficed with simpler specimens, are no longer adequate. As the embryo advances into the more specialized levels, the cataloguer is faced with increasing need of detailed histogenetic data. Only by ascertaining the developmental status of the various organs of an embryo can he be reasonably sure that it is older or younger than another specimen with similar characteristics. This situation was met by Dr. Streeter, as he undertook the study of horizons xix to xxiii, by a method which he devised of rating the development of selected organs on a system of point scores. For this purpose he chose key organs which during the period studied are undergoing marked transformations, readily recognizable under the microscope. For example, the number of half turns of the cochlear duct, with the transitional stages between them, affords seven recognizable points of advancement during the live horizons xix to xxiii. The least advanced cochlea was therefore credited with one developmental point and the most advanced with seven points. Other structures suitable for numerical evaluation in this way include the cornea, optic nerve, hypophysis, vomeronasal organ, submandibular gland, kidney, and skeleton. For each of these, Streeter arbitrarily selected morphological characters which could be recorded by camera lucida sketches from serial sections and which registered the progressive development of the organ. Other characters could probably have been equally well chosen, the only essential being that they could be uniformly applied from embryo to embryo. In the five horizons with which we are now concerned, all the embryos were so examined. The same key organs were recorded, each organ having its own scale, and in this manner the total development point score per embryo was established. The numbers of scoring points are given by horizons in the following table:
Number of Developmental Points for Embryo
| Horizon | Youngest number of points | Oldest number of points |
|---|---|---|
| XIX | 10 | 16.5 |
| XX | 19 | 29.5 |
| XXI | 30 | 39 |
| XXII | 40.5 | 46 |
| XXIII | 48 | 60.5 |
From this it can be seen that the total number of developmental points registered by its key organs could be utilized to determine not only the horizon to which the embryo belongs, but also its relative position within that horizon.
The tables accompanying this article, listing all the embryos used in compiling horizons xix to xxiii, were left in practically complete form by Dr. Streeter, and they include the total point score of each embryo. The following schedules give for each organ a list of the advancing characteristics and the numerical values assigned to them. The horizon number for each age group is placed at the approximate level of the average score of the embryos of that group.
Schedules of Scoring Points
Cornea
Horizon
1. A thin layer of loose mesoderm, the primitive corneal body, crosses the mid-line of the eye. XIX
2. Corneal body is being differentiated from the remnant of crossing mesoderm. XX
3. Corneal body is a compact mesodermal sheet, two to five cells thick. XXI
4. Cortical body is a thick mass delatninating into looser and more compact layers. Mesothelial layer (Descemetis) is forming.
5-6. A well defined cuboidal posterior surface layer (Descemet’s endothelium) is present. Corneal body sharply layered, with large nuclei. XXII
6-7. Corneal body thick, fibrous, with thinner, elongated nuclei; body 3 or more times as thick as epithelium. XXIII
8. Corneal body formed of thin, elongated (endothelioid) cells.
Optic Nerve
- Optic nerve small, slender. XIX
- Lumen practically whole length of stalk. Few or no fibers.
- Ependymal arrangement partially retained along stalk. XX
- Remnant of ependyma along whole length of stalk. Hyaloid groove at bulbar end. A few fibers arriving at brain.
- Remnant of ependyma present. XXI
- Sheath layer beginning to form. XXII
- Vascular canal present.
- Early nerve sheath. XXIII
- Reticular spongioblastic framework, striate arrangement of nuclei, bundles of fibers. Definite nerve sheath.
Cochlea
- L-shaped cochlear duct (condition in embryos belonging to horizon XVIII).
- Tip of cochlea turns up; short tip. XIX
- Long tip; transitional stage. XX
- Return down curve (tip turns down). XXI
- Transitional stage. XXII
- Tip turns up second time. XXIII
- Tip turns down second time (second return down curve).
Hypophysis
- Thick stalk with remnant of lumen (Rathke’s pouch); angiogenesis beginning. XIX
- Capillaries appearing in mesodcrm at rostral surface of anterior lobe.
- Long, slender stalk. XX
- Thread-like stalk; beginning absorption. XXI
- Remnant of incomplete stalk at either end. XXII
- Practically no trace of stalk remains. Oriented epithelial follicles. Abundant angioblasts and capillaries in vascular component of anterior lobe. XXIII
Vomeronaml Organ
- Essentially a groove or pit. XIX
- Opening at oral end broad. Caudal end a shallow, blind sac. XX
- Oral opening reduced in size. Short, narrow neck; caudal end expanded. XXI
- Intermediate stage. XXII
- Narrow canal in long, tapering duct. XXIII
- Retrogression; shrinking sac.
Submandibular Gland
1-2. Short, clublike duct entering mesenchymal primordium of gland. XIX
3. Longer, knobby duct well in gland. XX
4. Duct beginning to form knob-like branches.
5. Simple, stubby primary branching of duct. XXI
6. Secondary branching of duct. Practically solid duct; suggestion of lumen in distal part. XXII
7. Definite lumen in oral part of duct.
8. Long duct, much branched. Lumen deep in gland. XXIII
9. Lumina in many terminal branches of ducts.
10. Beginning orientation of epithelial tree. Angiogenesis beginning around epithelium. Mesoblast begins to form layer around gland.
Kidney
- Lack of orientation in metanephrogenic tissue. XIX
- Beginning formation of renal vesicles.
- S-shaped lumen in renal vesicles. XX
- Spoon-shaped capsules (Bowman’s).
- No large glomeruli. XXI
- Few large glomeruli. XXII
- Short secretory tubules. Numerous large glomeruli. XXIII
- Long secretory tubules. High epithelium in some tubules.
- Increased number and convolutions of tubules.
Cartilage and Bone
- Differentiation of cartilage cells has progressed to phase 3. XIX
- Clearing center in cartilage of bones. XX
- Fibrous zone not very distinct from osteoblast zone.
- Transitional stage. XXI
- Beginning osteoblast formation.
- Early shaft shell (osseous band). Borders of shell not sharp. XXII
- Shaft shell forming constriction cuff. XXIII
- All five phases of cartilage cells. Elongated shaft shell.
Organogenesis of Particular Organs in Horizons XIX to XXIII
In the earlier issues of this study the successive periods of development were reviewed separately, horizon by horizon. That could be done easily enough in the simpler stages, but as the organism becomes more highly specialized it acquires more structural parts. To meet this advance, Dr. Streeter had planned to simplify the description of horizons xix to xxiii by giving connected accounts of the development of some of the key organs through the five horizons. Such an account of one system, the skeleton, has already been published as the fourth issue[6] of the present series. For the remaining organs and systems, Dr. Streeter had prepared all the figures and plates needed for the present article, and his papers contained a portfolio of brief notes. With this material in hand, the following text has been prepared. It cannot, as has already been explained, represent the full discussion that Streeter intended to present. All that can be done is to assist the reader in using the illustrations and tables, by pointing out, as well as can be done from the notes and from memory of Dr. Streeter’s informal discussions in the laboratory, the details which he had chosen to use in mapping the progress of development during the last ten days of the embryonic period as he defined it.
Eye
Over and above its progressive increase in size, the eye of the human embryo undergoes several distinct structural claborations in the course of the closing ten days of the embryonic period (horizons xix to
(insert page 173)
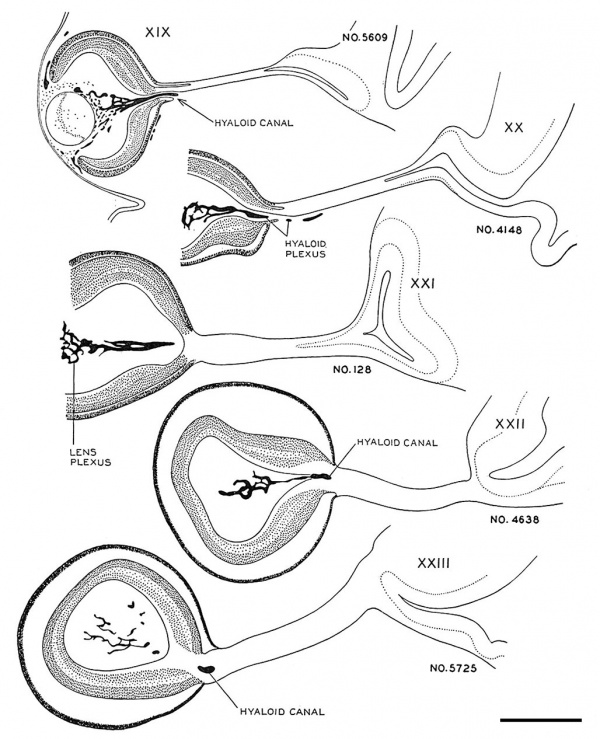
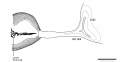
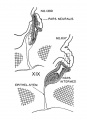
Fig. 4. Drawings of sections through the eye and optic nerve in age groups xix to xxiii. Several sections in each embryo were combined to show the form and dimensions of the optic nerve. x50.
- Eye and Optic Nerve (week 7-8)
of the stalk. They are arriving at the brain in the next age group. If the growth in length of nerve fibers seems remarkable, it should be remembered that the actual distance between eye and brain at this time is not great, only I mm. or less in a 41-day embryo. During the early steps in the transformation of the optic stalk into a nerve tract there is no definite condensation or alignment of the cells in the bordering mesenchyme. In age group xxii a sheath layer begins to form which becomes a distinct structure in horizon xxiii, at least in the older members of the group.
Fig. 5. Drawings of sections showing the form of the optic nerve and the progressive spread of nerve fibers from retina to brain. Few fibers have reached the optic nerve in embryos of horizon xix. Some fibers have grown through the whole tract and are arriving at the brain in embryos of group xx. In the older age groups many of the primitive cells of the optic tract are seen in longitudinal rows between bundles of nerve fibers. x100.
Cornea and Sclera
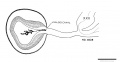
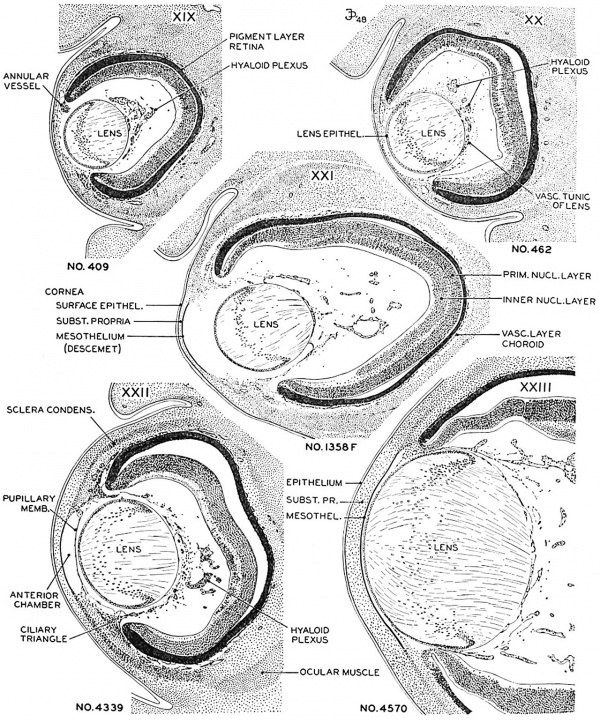
In embryos of horizon xviii mesoderm has already begun to invade the interval between the epithelium of the lens and the surface ectoderm. This is the first step in the formation of the cornea. A thin layer of mesoderm, one to two cells in thickness, crosses the mid-point of the future corneal region (pl. 1) in embryos of group xix. The layer becomes slightly thicker toward the end of xix or the beginning of xx, and the cells on the internal surface begin to arrange themselves in the form of a mesothelium. The mesothelium is a definite layer in the next group (xxi), as indicated in figure 6, and clelimits the cornea from the emerging anterior chamber of the eye. The mesoderm (substantia propria) is a stratum 6 or more cells thick with conspicuous nuclei in xxii. It more than doubles in thickness and the nuclei become elongated during the next two days.
Peripherally, the substantia propria of the cornea is continuous with a condensed band of mesenchyme which gradually spreads backward from the corneal margin to enclose the eyecup. This primitive scleral condensation is present in embryos belonging to all the last five horizons.
Fig. 6. Drawings of mid-section of the eyeball in embryos of horizons xix to xxiii. The sections were selected to show the organization and relations of parts of the eye in the various age groups. The lens remains nearly spherical throughout this period and occupies a relatively large part of the eye. In front of the lens the pupillary membrane develops and the anterior chamber emerges. The hyaloid plexus partly fills the primitive vitreous humor of the eye and forms a network on the posterior surface of the lens. X60.
- Human Embryo Lens (week 7-8)
Reference[7]
Cochlea
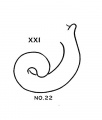
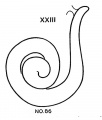
Progressive changes in the transformation of the otocyst——establishment of the semicircular canals especially——providerl criteria for classifying embryos of the two preceding age groups (horizons xvii and xviii). The emphasis now is shifted to the cochlear duct, which is the most rapidly changing part of the internal ear. The cochlear duct, showing the peculiarity of spiral growth, practically completes its characteristic form during the period covered by age groups xix to xxiii. Referring to figure 7, it is seen that embryos belonging to group xix have duets with short, upturned tips. In two days the cochlear duct grows upward and horizontally and may be almost ready to point downward for the first time. Embryos with this configuration of the cochlea belong to horizon xx. After further elongation, the duct points definitely downward as shown in figure 7 for group xxi. The tip of the duct does not begin to turn up for the second time until two days later. During this period it passes through a transitional phase in which it grows horizontally and then begins to point upward for the second time (horizon xxii). finally, when the tip of the cochlea is found to turn downward for the second time the terminal horizon is reached, and the duct is coiled nearly to its final extent of two and one-half turns.
- Cochlea (week 8)
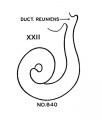
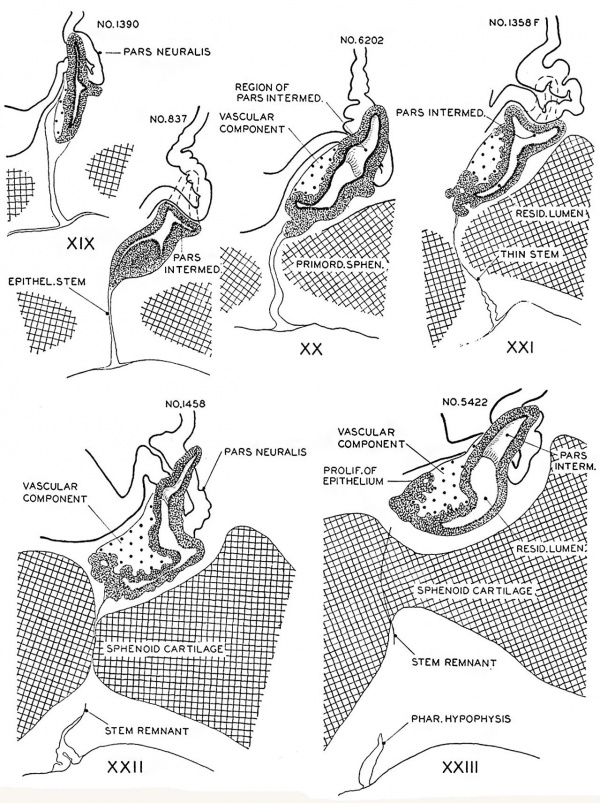
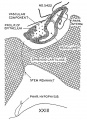
Hypophysis
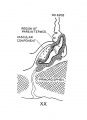
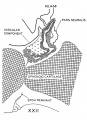
In the preceding age group (xviii) the hypophysis revealed an elongated and slightly folded neural lobe and an expanded anterior lobe with its stalk connected to the epithelium of the oral roof. The hypophysis makes considerable progress in its development during the ten—day period covered by age groups xix to xxiii. The changes in the pars neuralis are largely restricted to some increase in size and a deepening of the characteristic folding of the walls. Growth and differentiation of the anterior lobe, however, are more conspicuous. The epithelial stem gradually regresses until it is absorbed for the most part, leaving strandlike remnants in embryos of the two oldest horizons. A marked proliferation of the epithelium occurs, especially in the ventral part of the anterior lobe, and trabeculae invade the mesodermal component of the gland.
The pars intermedia is becoming established in group xix and can be readily identified in all older groups. The wall of this part of the gland remains thin, in contrast with the thick, ruffled wall of the body of the anterior lobe. The pars intermedia abuts against the neural lobe and partly covers it. The free surface of the pars intermedia, as shown in figure 3. is exposed to the residual lumen of Rathke’s pouch. Close to the attachment of the stalk to the gland on either side, there are thin epithelial shelves on the brim of the hypophyseal cup which later form small lateral lobes. These lobes, as shown by Atwell,[8] will fuse across the mid—line and develop into the pars tuberalis. The rudiment of this part of the gland is indicated by the processes attached to the stem in figure 9 (xix E). The pars tuberalis has not yet begun to grow forward in the terminal horizon (xxiii). The vascular component of the anterior lobe is largely confined to its ventral part and does not to any extent become associated with the pars intermedia. Angioblastic strands are already present in the mesoderm rostral to the anterior lobe in embryos belonging to group xix. but capillaries have not yet appeared. The gradual increase in size of the vascular component is shown graphically in figure 8. Histological details are well portrayed in the photographs in plate 2.
Fig. 8. Drawings of sagittal sections of the hypophysis in embryos of horizons xix to xxiii. Two or more sections were combined in preparing the drawings. Several sections were utilized in embryos no. 6202, xx, no. 1458, xxii, and no. 342, xxiii, to represent the lateral processes of the pars intermedia, which grow dorsnlward around the sides of the neural lobe.
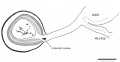
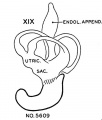
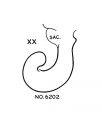
Vomeronasal Organ
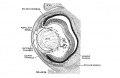
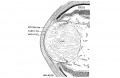
The vomeronasal organs are much less developed in man than in the lower vertebrates, but the changes in form which they show during the ten (lays covered by horizons xix to xxiii aid in classifying‘ the embryos. The thicltenings in the nasal epithelium which gives rise to the vomeronasal organs are seen as shallow grooves or pits in embryos of group xix. The deepening of these grooves results in the [ormation of long, tapering ducts which expand caudally into blind tubular sacs. The sections illustrated in figure I() show characteristics of the organ in the various horizons.
Submandibular Gland
A rudiment of each submandibular duct in the form of an epithelial thickening in the groove between the tongue and lower jaw is already present in embryos belonging to horizon xviii. Areas or fields of condensed mesoderm (fig. II and pl. 3) appear beneath the ducts in embryos of group six and fix the positions of the two glands. During the following ten days the ducts and fields grow apace, the latter being gradually invaded by the branching but still solid epithelial component of the glands. Lumina in the terminal subdivision of the duct system as well as early angioblastic tissue appear during the last one or two days of this period.
XX XXI No. 6202 No. 460 No. 7392
pelvis with attached calyces subdivided into primitive collecting tubules, advanced at least as far as the third order of branching, is seen in human embryos 35 to 37 days of age (horizon xvii or xviii). In embryos of horizon xix we find that the rudiments of the metanephric tubules are just appearing. Steps in the origin and diflerentiation of these tubules are illustrated with drawings and photographs in figures 12 and 13, and plate 4. In horizon xix the metanephro
Fig. 9. Semi-diagrammatic drawings of transverse sections of the hypophysis. One embryo was chosen for each horizon, and drawings were made of the hypophysis at five more or less evenly spaced levels. The changing pattern of the various parts of the gland can be followed stage by stage, by scanning the horizontal rows A to X20.
Kidney
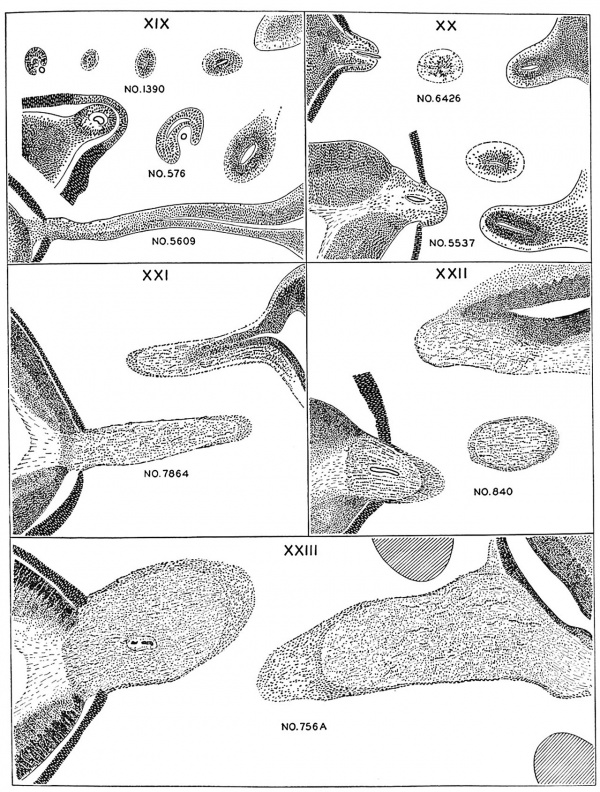
The kidney has passed through its early phases of development before embryos reach the stages now being considered. This is especially true as regards the duct system of the organ. The primary renal genic tissue surrounding the ampullar ends of collecting tubules in many cases remains undifferentiated. In others a group of cells lying in the angle between the stem of a collecting tubule and its enlarged end segregates from the metanephrogenic mantle. These nodules are the rudiments of future metanephric secretory tubules. Small cavities have appeared in some of these primordia, transforming them into renal vesicles. The first few generations of uriniferous tubules are provisional and vestigial, as shown by Kampmeierg" many of the primary vesicles never join the collecting ducts. During the next two days group xx) vesicles of the second order unite with collecting ducts, elongate, and assume the 5—shaped form characteristic of young secretory tubules. The greater part of the lower limit of the 5 curve is destined to become transformed into the capsule of a renal corpuscle. The foundation plan of the whole uriniferous tubule is thus already established, and by later differential growth its various parts gradually evolve from the segments of the primitive S—shaped structure. New generations of collecting and secreting tubules continue to develop, so that units of the fourth and fifth orders are found in specimens belonging to horizon xxiii.
50. F. Karnpmeier, The metanephros or so-called permanent kidney in part provisional and vestigial, Anat. Rec. .35. PP115-120, 1926.
Plates
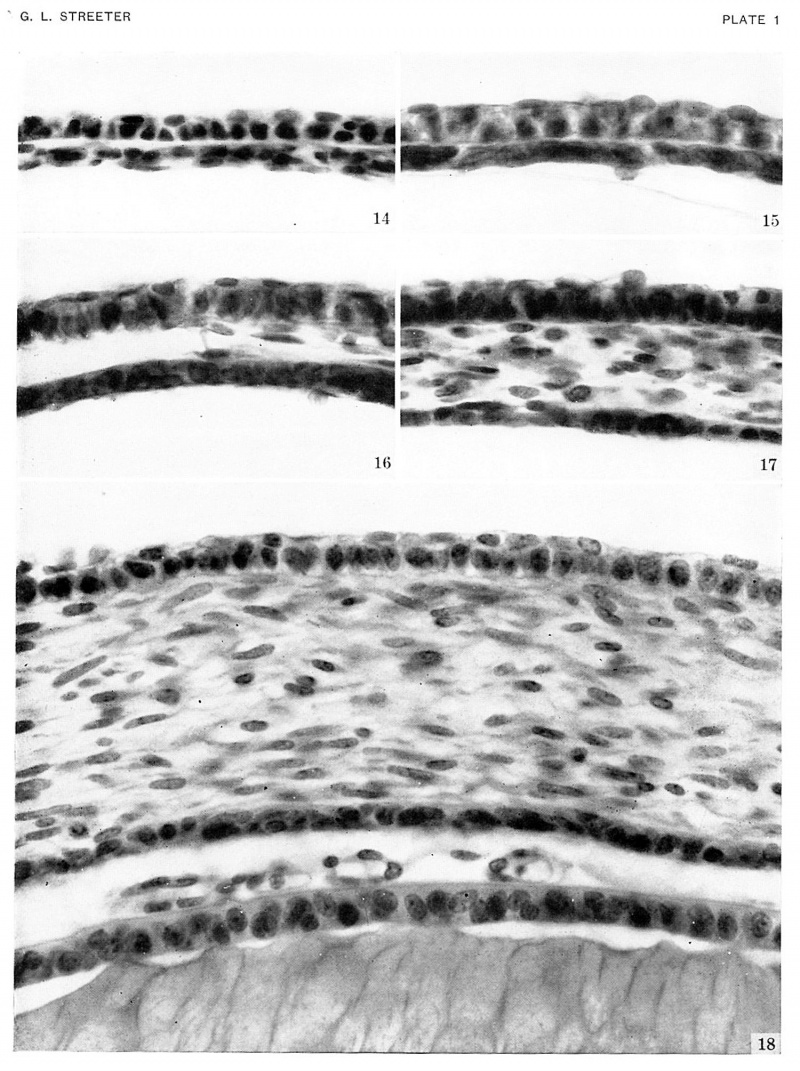
Plate 1. Photographs of the cornea in embryos stage 19-23
Photographs of the cornea in embryos belonging to horizons xix to xxiii. A segment of the cornea near the mid-line is shown in an embryo of each horizon. The mesoclermal component is a thin layer one or two cells thick in the youngest stage. The stroma gmclually increases in thickness, and the cells at the inner border form a mesothelium. In the oldest specimen (Hg. 13), :1 part of the lens included. The pupillary membrane is seen in the space between the mesothelium (Descement's) and the epithelium of the lens.
Fig. 14. No. 5609, xix, I5-I-I x 800.
Fig. 15. No. 431, xx, 37-2-2, x800.
Fig. 16. No. 1358F, xxi, 7-2-1, x800.
Fig. 17. No. 3681, xxii, 32-2-4, x800.
Fig. 18. No. 4570, xxiii, 70-3-4, x800.
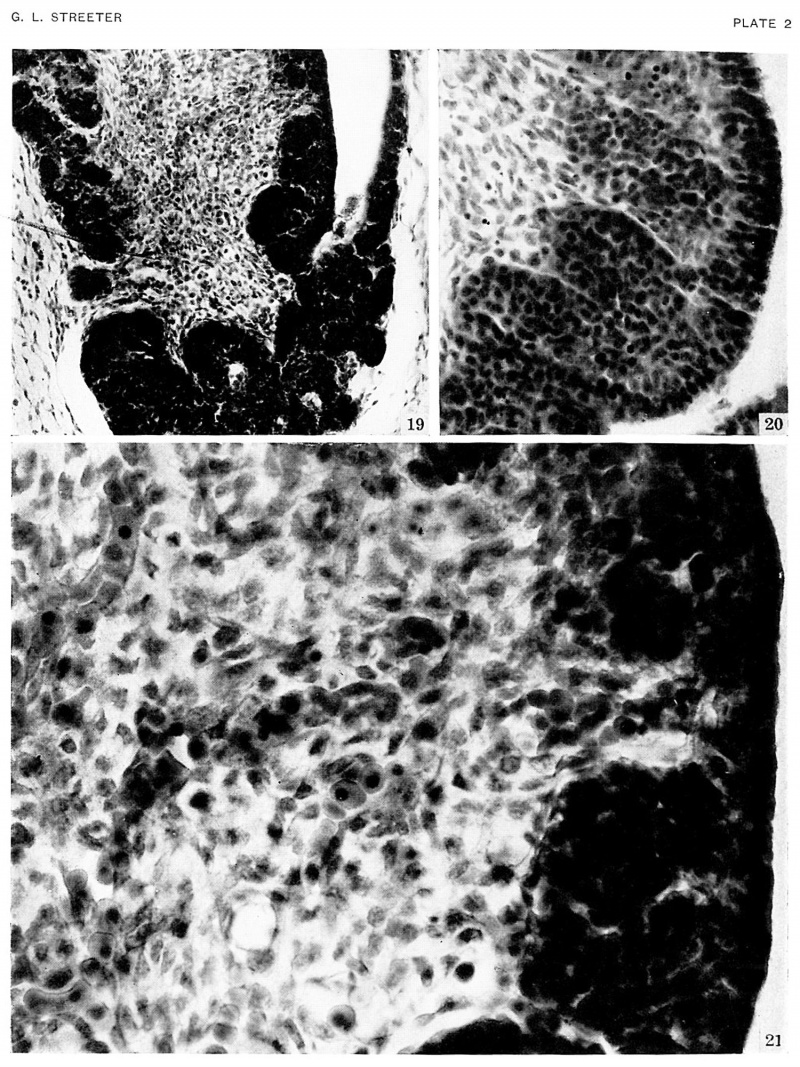
Plate 2. Photographs showing the histology of the hypophysis
In embryos belonging to horizon xxiii.
The epithelium of the anterior lobe has become partly subdivided into lobule which project into the mesodermal component of the gland. The abundant vascular elements are well shown in figure 21.
Fig. 19. No. 4289. 39-3-3. x200
Fig. 20. No. 4570. 62-1-3 x400
Fig. 21. No. 4289. 39-3-3 x800
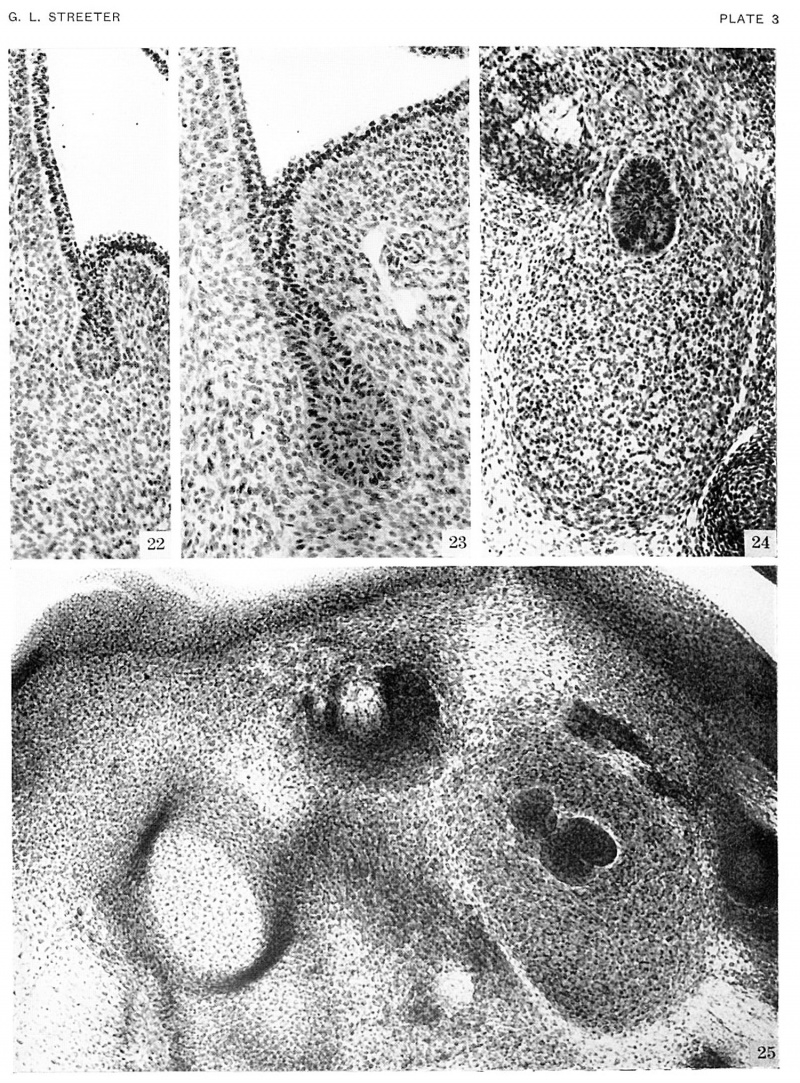
Plate 3. Photographs of the Submandibular gland in embryos of horizons xvii, xix, and xx
Rudiments of the ducts are present in the youngest age group. Figure 24 reveals a condensed field of mesoderm beneath the epithelial bud. The latter invades the mesodermal bed and by repeated branchings forms the epithelial component of the gland.
Fig. 22. No. 6524, xviii, 23-3-2 x200
Fig. 23. No. 7707, xviii, 21-1-3, x200
Fig. 24. No. 6824, xix, 12-1-4 x200
Fig. 25. No. 1134B, xx, 6-2-2 x150
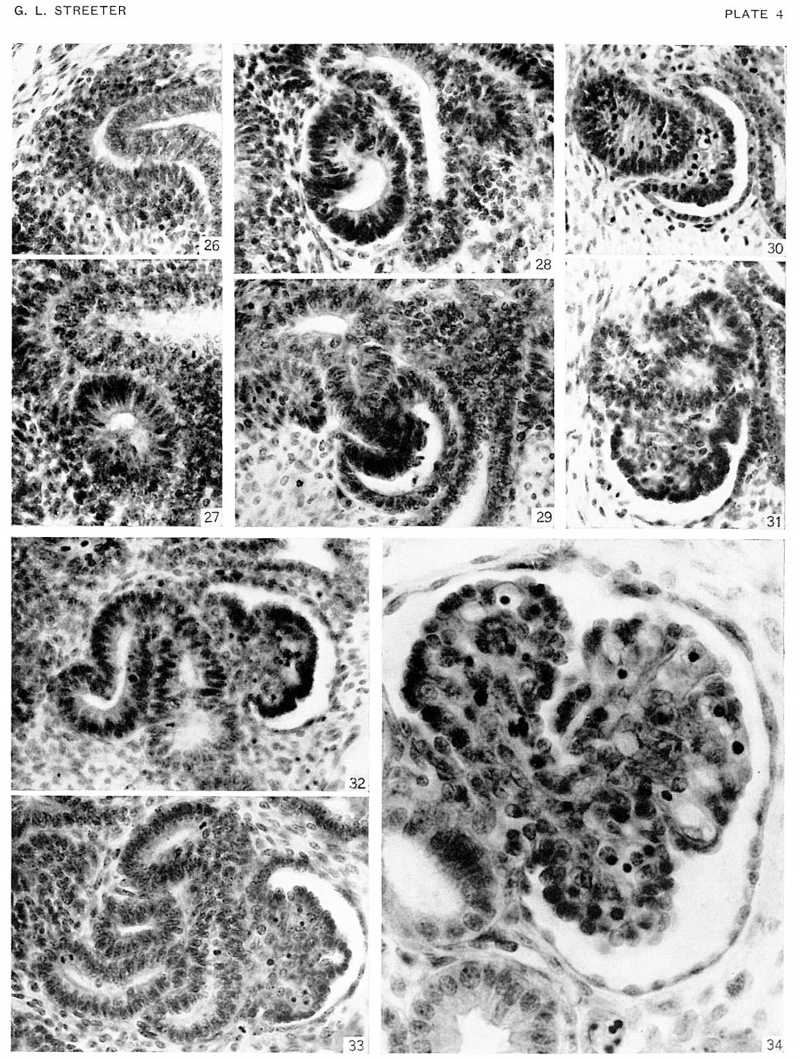
Plate 4. Photographs of sections illustrating uriniferous tubules in the embryos of horizons xix to xxiii
A renal vesicle will soon form in that part of the metanephric tissue (fig. 26) which lies beneath the ampullae end of a collecting tubule. Successive steps in the development of the tubules are shown in specimens of the several age groups.
Fig. 26. No. 576
Fig. 27. No. 8092
Fig. 28. No. 8157
Fig. 29. No. 5537
Fig. 30. No. 6531
Fig. 31.No. 7392
Fig. 32. No. 4638
Fig. 33. No. 8394
Fig. 34. No. 4570
Plate 1. Photographs of three embryos belonging to horizon xix
Comparing specimens of earlier age groups with those now being considered, it is seen that the trunk region is beginning to straighten out and the cervical angle is less acute. The coalescence of parts in the branchial region has altered the appearance of the external ear, and the hillocks are less conspicuous. The axes of the arms are almost at right angles to the dorsal line of the body of the embryo. x4.
Figs. 35-37 No. 8092.
Figs. 38-40 No. 6824.
Figs. 41-43 No. 4501.
Plate 1. Photographs of three embryos belonging to horizon xx
The upper extremities of embryos of this age group show a slight bend at the elbow, and the hands are curving inward over the heart. region. A characteristic feature of embryos of this and the succeeding horizon is the presence of a superficial vascular plexus in the head. The fringe-like border of the plexus is usually more sharply outlined than the photographs of this plate indicate (see text fig. 44). x4.
Figs. 45-47. No. 7274
Figs. 48-50. No. 7906
Figs. 51-53. No. 8157
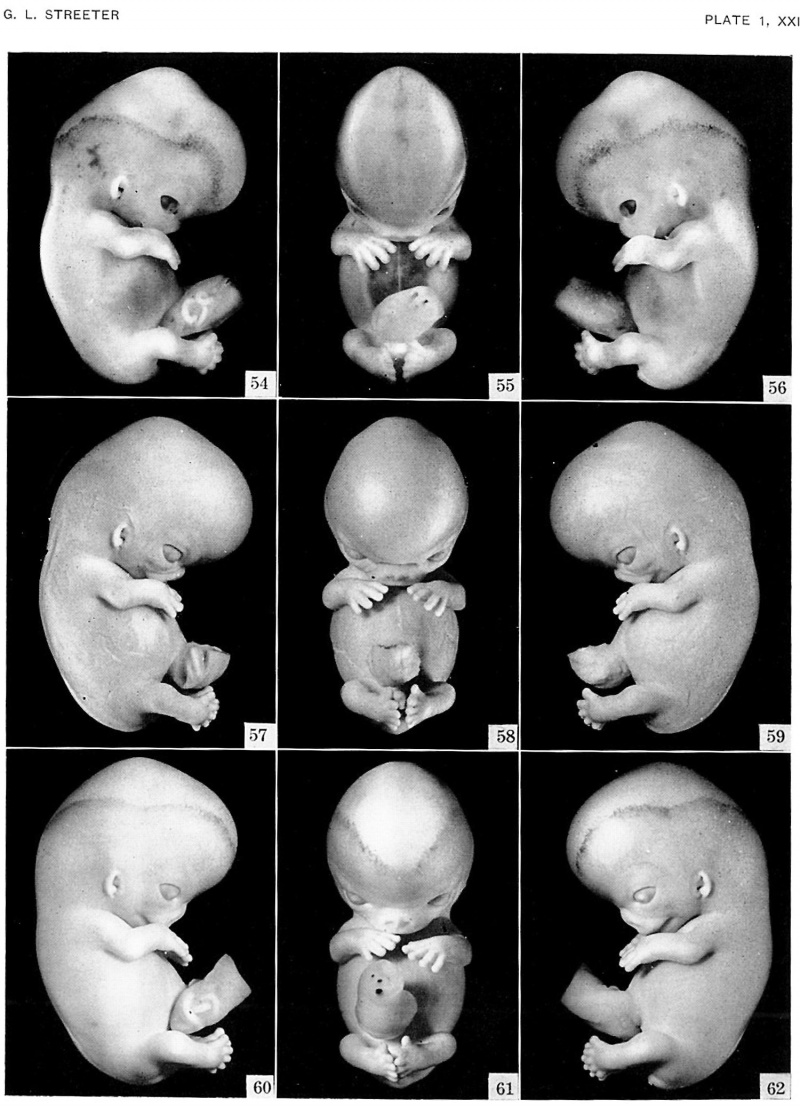
Plate 1. Photographs of three embryos belonging to horizon xxi
The superficial head plexus is plainly visible in embryos of this age group, and has spread to a level more than half the distance between the eye-ear plane and the vertex of the head. The fingers are longer and show an early stage in the development of touch pads. The hands are flexed at the wrists and are approaching each other over the heart region. The lower extremeties curve towards the median plane, and toes of the two feet make contact in some specimens. x3.
Figs. 54-56. No. 4090.
Figs. 57-59. No. 8553.
Figs. 60-62. No. 7392.
Plate 1. Photographs of three embryos belonging to horizon xxii
Evidence of growth of the eyelids is apparent in all the embryos of horizons xix to xxiii. The eyes are more than half covered by the lids in specimens of age group xxii. The extremities have increased in length, and the digits touch or overlap. x3.
Figs. 63-65 No. 6701.
Figs. 66-68 No. 6832.
Figs. 69-71 No. 8394.
Plate 1. Photographs of two embryos belonging to horizon xxiii
The superficial vascular plexus has spread nearly to the vertex of the head. A considerable growth in the length of the extremities has occurred in two days. The forearm is sometimes raised to a level above that of the shoulder, and the hands extend far out in front of the embryo. x2.5.
Figs. 72-75. No. 7425.
Figs. 76-79. No. 4570.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
References
- ↑ 1.0 1.1 Streeter GL. Developmental horizons in human embryos. Description of age group XI, 13 to 20 somites, and age group XII, 21 to 29 somites. (1942) Contrib. Embryol., Carnegie Inst. Wash. Publ. 541, 30: 211-245.
- ↑ Streeter GL. Developmental horizons in human embryos (fourth issue). A review of the histogenesis of cartilage and bone. (1949) Carnegie Instn. Wash. Publ. 583, Contrib. Embryol., 33: 149-169. PMID: 18144445
- ↑ Streeter GL. Developmental horizons in human embryos. Description of age group XIII, embryos about 4 or 5 millimeters long, and age group XIV, period of indentation of the lens vesicle. (1945) Carnegie Instn. Wash. Publ. 557, Contrib. Embryol., Carnegie Inst. Wash., 31: 27-63.
- ↑ 4.0 4.1 Streeter GL. Developmental horizons in human embryos. Description of age groups XV, XVI, XVII, and XVIII, being the third issue of a survey of the Carnegie collection. (1948) Contrib. Embryol., Carnegie Inst. Wash. 575, 32: 133-203.
- ↑ Streeter GL. Weight, sitting height, head size, foot length, and menstrual age of the human embryo. (1921) Carnegie Instn. Wash. Publ., 55 Contrib. Embryol., Publ. 274, 11: 143-170.
- ↑ Streeter GL. Developmental horizons in human embryos (fourth issue). A review of the histogenesis of cartilage and bone. (1949) Carnegie Instn. Wash. Publ. 583, Contrib. Embryol., 33: 149-169. PMID: 18144445
- ↑ Streeter GL. Developmental Horizons In Human Embryos Description Or Age Groups XIX, XX, XXI, XXII, And XXIII, Being The Fifth Issue Of A Survey Of The Carnegie Collection. (1957) Carnegie Instn. Wash. Publ. 611, Contrib. Embryol., 36: 167-196.
- ↑ Atwell WJ. The development of the hypophysis cerebri in man, with special reference to the pars tuberalis. (1926) Amer. J Anat. 37: 139-193.
Cite this page: Hill, M.A. (2024, April 19) Embryology Paper - Human Embryo Horizons 19-23. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Human_Embryo_Horizons_19-23
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G]]]
- Carnegie Embryo
- Carnegie Stage 19
- Carnegie Stage 20
- Carnegie Stage 21
- Carnegie Stage 22
- Carnegie Stage 23
- Carnegie Embryo 1134B
- Carnegie Embryo 1358F
- Carnegie Embryo 3681
- Carnegie Embryo 4090
- Carnegie Embryo 4289
- Carnegie Embryo 431
- Carnegie Embryo 4339
- Carnegie Embryo 4501
- Carnegie Embryo 4570
- Carnegie Embryo 460
- Carnegie Embryo 4638
- Carnegie Embryo 5537
- Carnegie Embryo 5609
- Carnegie Embryo 576
- Carnegie Embryo 6202
- Carnegie Embryo 6524
- Carnegie Embryo 6531
- Carnegie Embryo 6701
- Carnegie Embryo 6824
- Carnegie Embryo 6832
- Carnegie Embryo 7274
- Carnegie Embryo 7392
- Carnegie Embryo 7425
- Carnegie Embryo 7707
- Carnegie Embryo 7906
- Carnegie Embryo 8092
- Carnegie Embryo 8157
- Carnegie Embryo 8394
- Carnegie Embryo 8553
- Historic Embryology
- 1940's
- Draft
- George Streeter
- George Corner
- Carnegie Collection