Paper - Developmental defects at the foramen ovale (1938): Difference between revisions
m (→Bibliography) |
m (→Bibliography) |
||
| Line 324: | Line 324: | ||
Rostan, A. Contn'bution -£1 1’étude de l’embolie croiseé consécutive 5. la persistance du trou de Botal. These Genéve, Rivera & Dubois, 1884. (Cited by Herxheimer, p. 378.) | Rostan, A. Contn'bution -£1 1’étude de l’embolie croiseé consécutive 5. la persistance du trou de Botal. These Genéve, Rivera & Dubois, 1884. (Cited by Herxheimer, p. 378.) | ||
{{Ref- | {{Ref-ScammonNorris1918}} | ||
Seib, George A. Incidence of the patent foramen ovale cordis in adult American whites and American negroes. Am. J. Anat., 1934, 55, 511-525. | Seib, George A. Incidence of the patent foramen ovale cordis in adult American whites and American negroes. Am. J. Anat., 1934, 55, 511-525. | ||
Latest revision as of 18:41, 3 March 2020
| Embryology - 25 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Patten BM. Developmental defects at the foramen ovale. (1938) Am J Pathol. 14(2):135-162. PMID 19970381
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Developmental Defects at the Foramen Ovale
Bradley M. Patten, PHD.
(From the Department of Anatomy, University of Michigan Medical School, Ann Arbor, Mich.)
Received for publication November 3, 1937.
Introduction
Many individual instances of patent foramen ovale are already on record in the literature. These cases, however, do not seem to have been studied as a group, either with a view to differentiating the types of malformation encountered in this location, or ascertaining for the different kinds of defects the possible range of their variation in extent. A long-standing interest in the normal and defective development of the heart has, through the generous cooperation of colleagues, brought to me for study more material of this type than one person would ordinarily encounter. Recently a leave of absence afforded the further opportunity of studying the specimens accumulated in a group of pathological institutes with records covering a total of over 500,000 autopsies. Naturally not all the congenitally defective hearts from these autopsies had been preserved, but the extensiveness and variety of the material available was exceptional. Using drawings made directly from my own or museum specimens as a basis, and supplementing this material from a study of the literature, I have attempted to assemble a brief, but freely illustrated, survey of the defects that may be encountered at the foramen ovale. Being not a clinician but an embryologist, I have approached the subject from a morphological standpoint. It is hoped, however, that the material may prove a useful foundation for those interested in attacking the clinical problems associated with such defects.
Literature
Publications dealing with failure of the foramen ovale to close have been appearing for more than three centuries. Many of the papers are so old that their viewpoint has become almost unintelligible to us of today. Botalli in I 56 5, for example, seized on cases exhibiting an open foramen ovale as offering an improvement on Galen’s idea that the blood entered the left side of the heart from the right by way of spaces between the trabeculae of the interventricular septum (Dalton, 1884, p. I 37). The weight of Botalli’s name behind this erroneous conception delayed for many years the acceptance of Servetus’ contention that the passage of blood from right to left “does not take place through the median wall of the heart as commonly believed; but, by a grand defice, the refined blood is driven from the right ventricle of the heart, in a long course through the lungs.” The language in which Servetus elaborated his ideas well indicates the curious mixture of keen observation and dogma that pervaded the work of this period. “By the lungs it (the blood) is prepared, assuming a bright color. It is mingled with the inspired air and purged of its fulginous matter by expiration and so at length the left ventricle of the heart attracts by its diastole the whole mixture, a suitable . . . material that . . . may become vital spirit.” (Translation from Dalton, I884, p. ns.)
Unfortunately the old papers are by no means the only ones in the literature that throw little light on the subject. Many comparatively recent articles are but superficial descriptions of isolated cases. An idea of the frequency with which papers based on r or 2 cases appear in the literature may be gathered from the fact that in 205 references cited by Poynter (r9r9) only 225 cases are involved. Many of these were merely clinical diagnoses of “open foramen ovale” with no confirmation by autopsy. Among the enormous number of papers on the subject disappointingly few contain both a good clinical history of the case and an adequate record of the autopsy findings.
Viewing the literature as a whole there seem to have been three factors primarily responsible for the often contradictory and unsatisfactory information it contains. First is the deep rooted tradition that the foramen ovale closes immediately following birth. Thus, in the absence of other findings accounting for death, an open foramen ovale in a young infant is frequently unjustly accused. This has led to much misapprehension 3 to both the frequency of occurrence, and the functional significance, of an unclosed foramen ovale during the neonatal period. There has long been ample evidence that the foramen ovale is not closed immediately after birth, but that its closure is a gradual process spreading over most of the first year (Aleksieyefi, Igor; Alvarenga, I869; Elsasser, I852; Hinze, I893; Patten, I930, I931; Scammon and Norris, I918). Familiarity with this fact would have eliminated from the literature many papers describing as instances of “abnormal patency of the foramen ovale” conditions perfectly normal for the age at which they were observed. For example, a paper published comparatively recently in a well known medical journal is based on the heart of an infant that lived but 6 hours after birth. Death was attributed to an open foramen ovale and an unclosed ductus arteriosus!
A second cause of confusion commonly encountered in the literature is the failure to between conditions in which the foramen ovale is adequately covered by a valve which is not completely adherent to the septum, and conditions in which a structural defect of the valve or the septum makes it impossible for the foramen to be functionally closed. Incomplete adhesion of the valvula to the septum, with a resulting “probe-patency,” is so common that it must be regarded as a variant of the normal rather than as an abnormality. The combined figures of ten different observers compiled from over .4000 autopsies in which this condition was an object of special attention show that probe-patency exists in one out of every four or five adult hearts (see Table I). As long 3 the valvula foraminis ovalis adequately overlaps the limbus fossae ovalis, probe-patency appears to be no functional handicap to an otherwise normal individual. The inclusion in the literature of a large number of cases where the “defect at the foramen ovale” was mere probe-patency has led to the impression that functionally significant defects in this region are much more common than is actually the case.
Still a third underlying difficulty in arriving at any clear interpretation of the significance of defects at the foramen ovale is one that seems inherent in the entire subject of congenital defects of the heart. There appears to have been a sort of co1lector’s instinct obsessing contributors to this field. The more bizarre and complicated the case, the more interest it appears to arouse. From either the practical or the scientific standpoint this is unfortunate. The clinical picture especially is most confusing when several defects co-exist in the same heart. The only hope of arriving at any sound interpretation of such cases lies in better understanding of the developmental conditions responsible for, and the clinical manifestations of, uncomplicated cases of specific defects in which the major characteristics of the condition stand out unequivocally.
To attempt to give a systematic survey of all the articles in a field where such a large proportion of the material is either antiquated or uncritical would not be profitable. In the course of preparing this paper about 3ooo references on congenital defects of the heart were culled. Some 300 of these purported to deal with an open foramen ovale. Even this burdensome list undoubtedly fails to constitute a complete bibliography, for the literature is scattered among journals dealing with clinical medicine, pathology, physiology, anatomy, embryology, and even general biology. It has, therefore, seemed wiser to dismiss the literature as a whole with the foregoing general comments and deal only with a rela tively few selected references in connection with matters on which they were found helpful.
The Development of the Interatrial Septal System
The growth processes leading toward the establishment of conditions am they appear in the heart of a newborn infant and the changes in the heart following birth are fairly well covered in the embryological literature (Born, 1889; Keibel and Mall, 1910; Mall, 1912; Odgers, I935; Patten, Sommerfield and Paff, I929; Tandler, 1912 and 1913; Waterston, 1918). Much of this information, however, is so widely scattered and so uncorrelated that it is not readily utilizable by those working in other fields. For this reason, and also for the sake of emphasizing certain points especially pertinent to an understanding of the defective conditions under discussion, the following brief summary of the normal prenatal and postnatal development of the interatrial septa is given.
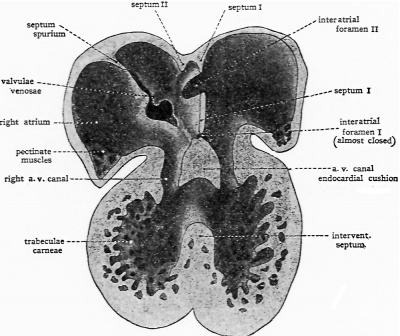
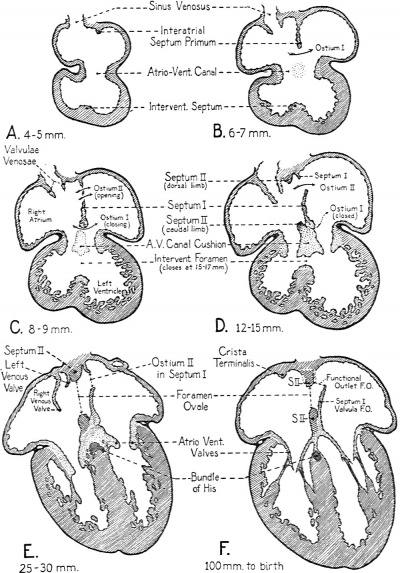
In the separation of the primitive common atrium into right and left chambers two septa are directly involved. These, on the basis of their sequential appearance, are commonly called septum primum and septum secundum. The partitioning process starts in very young embryos, indications of the formation of septum primum being recognizable as early as the 5th week * of development. Starting as a crescentic ridge on the dorsocephalic part of the atrial well, septum primum grows toward the atrioventricular canal (Text-Figs. I, A and 4, A).
- Ages as here given are approximate fertilization ages, for “menstrual age” add 14 days.
At about the same time that septum primum is making its appearance, the first indications of the impending division of the original common atrioventricular canal into a right and a left channel become evident. Two local thickenings, one dorsally, the other ventrally located, appear in the walls of the canal. These thickenings are the so-called endocardial cushions of the atrioventricular canal. Each cushion consists of a plastic mass of embryonal connective tissue, of the type characteristically appearing in the developing heart at points where septa will fuse, or where elaborate connective tissue structures such as the cardiac valves are destined to be moulded. During the 6th week of development the dorsal and ventral cushions are brought into contact with each other by their own growth and fuse to form a common mass dividing the atrioventricular canal (cf. Text-Figs. 1 and 2, and Text-Fig. 4, C and D).
Text-Fig.1. Semischematic drawings of the interior of the heart to show the steps in its partitioning. (From Embryology, Patten, B. M., courtesy P. Blakiston’s Son and Co.)
- A. The cardiac septa are represented at the stage reached in human embryos early in the 5th week of development. Note especially the primary relations of interatrial septum primum. Based on original reconstructions of the heart of a 3.7 mm. pig embryo, and on Tandler’s reconstructions of corresponding stages of the human heart.
- B. The cardiac septa as they appear in human embryos of the 6th week. Note the restriction of interatrial foramen primum by the growth of interatrial septum primum. Based on original reconstructions of the heart of a 6 mm. pig embryo, on Born’s reconstructions of the rabbit heart, and Tand1er’s reconstructions of corresponding stages of the human heart.
Between the concave margin of septum primum and the growing atrioventricular canal cushions is a progressively diminishing opening known as the interatrial foramen primum, or ostium primum (Text-Fig. I, B and 4, B). About when it seems as if the closure of the ostium primum would shut ofi the left atrium from the right (Text-Fig. 4, C) a secondary opening develops in septum primum near its origin from the dorsocephalic atrial wall. This new aperture first appears as multiple small perforations which soon coalesce to form a single opening known as the interatrial foramen secundum, or more briefly, as ostium secundum (Text-Fig. 2, and TextFig.4, C and D).
Text-Fig. 2. Semi-schematic drawing of the interior of the heart to show the start of interatrial septum sectmdum and the appearance of interatrial foramen secundum in septum primum. Based on original reconstructions of the heart of a 9.4 mm. pig embryo and on Tandler’s reconstructions of the heart of human embryos of the 7th week. (From Embryology, Patten, B. M., courtesy P. Blakiston’s Son & Co.)
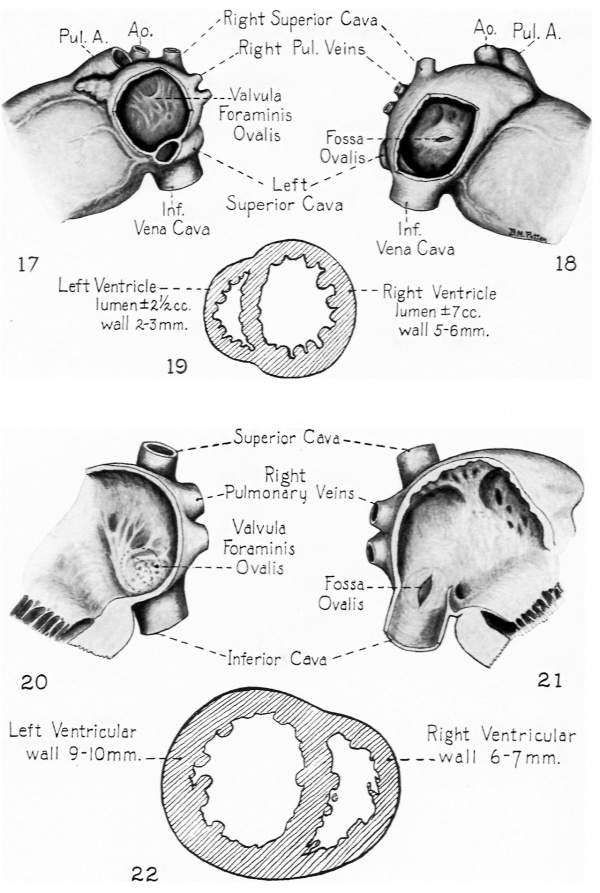
The appearance of a second interatrial communication just as the initial one is closing is of fundamental functional significance. In early embryonic life, when the lungs are as yet undeveloped, the left atrium lacks any considerable direct intake of its own. The constant presence of an interatrial communication makes it possible for the left atrium to receive without interruption a contribution from the blood entering the right atrium. More than the atrial part of the heart is involved in this matter of balanced atrial intakes for, 5 we have seen, the atrioventricular canal is divided by the 10 mm. stage, and at about the 15-17 mm. stage the interventricular septum separates the right and left ventricles from each other. After these partitions are completed, if the atrial intakes were unbalanced the ventricular intakes would inevitably be correspondingly disturbed. That this is a matter of more than theoretical importance is clearly shown by the conspicuously defective development of the left side of the heart which is encountered when, as occasionally happens, abnormal development prematurely closes or markedly narrows the interatrial communication of the fetal heart. (A case of this type is presented later in this paper, see Figs. 17, I8 and 19.)
About the time the secondary interatrial opening is formed in septum primum, another septum begins to develop. The second septum is usually first readily recognized in embryos of about 12 mm. (end of 6th week), although occasionally its beginnings may be made out somewhat earlier. Like septum primum, septum secundum is crescentic in shape, but the open part of the crescent is directed more dorsally—toward the sinus inlet rather than toward the atrioventricular canal as was the case with septum primum. In reconstructions of the developing heart septum secundum can be seen lying just to the right of septum primum (TextFig. 2). Its cephalodorsal limb extends along the dorsal wall of the atrium with its tip lying in close association with the left valve of the sinus venosus. The ventrocaudal limb of septum II extends along the ventral walls of the atrium, sweeps caudally and merges with the atrioventricular canal cushion just to the right of the place where septum primum fuses with the canal cushion to obliterate the primary interatrial foramen (ostium I) (Text-Fig. 2 and Text-Fig. 4, D). The extreme tip of the ventrocaudal limb of the septum extends to meet the tip of the cephalodorsal limb at the base of the left valve of the sinus venosus (Text-Fig. 2).
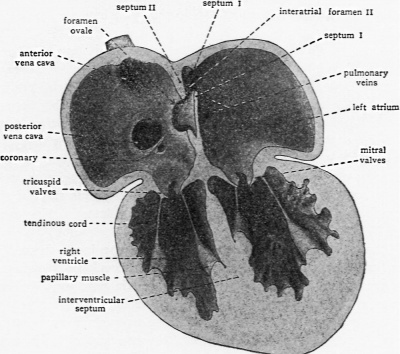
As septum secundum grows, its concave margin for a time cuts progressively farther into the atrial lumen; but septum II is not destined to become a complete partition. Its extension gradually ceases, leaving a characteristic oval aperture which is the foramen ovale (Text-Fig. 3 and Text-Fig. 4, E, F). The margin of septum secundum thus constitutes what in adult anatomy is called the limbus or annulus fossae ovalis.
Text-Fig. 3. Schematic drawing to show the interrelations of septum primum and septum secundum during the latter part of fetal life. Note especially the way in which the lower part of septmn primum is situated so it acts as a one-way valve at the oval foramen in septmn sectmdum. (From Embryology, Patten, B. M., courtesy P. Blal:iston’s Son & Co.)
The relations of septum primum to the oval foramen persisting in septum secundum are of vital importance. The secondary opening in septum primum is formed so near the cephalic wall of the atrium that the unresorbed lower part of septum primum lies as a loose flap covering, on its left atrial side, the oval opening in septum secundum (Text-Fig. 3 and Text-Fig. 4, F). In this position it acts as one-way valve, permitting the filling of the left atrium from the right but effectively shutting off return flow. In the fully formed fetal heart this flap is commonly known as the valvula foraminis ovalis rather than by its embryological name, septum primum.
Text-Fig. 4. Sectional plans of the embryonic heart in the frontal plane, showing extent of growth of the various cardiac septa at several stages of development. These diagrams give specifically for the human embryo a more precise picture of the rate of progress of partitioning than do the schematic drawings of Text-Figs. 1-3.
Stippled areas in the diagrams indicate the distribution of endocardial connective tissue, muscle is shown in diagonal hatching, and the epicardium in solid black. The lightly stippled areas in the atrioventricular canal in B and C indicate the lomtion of the dorsal and ventral endocardial cushions of the atrioventricular canal before they have grown suficiently to fuse with each other in the plane of the diagram.
Thus during intrauterine life we find a succession of three morphologically distinct interatrial communications, the first below septum primum, the second in septum primum, and the final one in septum secundum. This permits the left atrium, throughout fetal life, to receive a contribution of blood from the right atrium by a transseptal flow which compensates for the relatively small amount of blood entering the left atrium by way of the pulmonary circuit, and maintains an approximate balance of intake into the right and left sides of the heart. The amount of this compensatory interatrial flow changes in relative volume at different ages. Early in development, before the lungs have been formed, the flow from the right atrium through the interatrial ostium primum constitutes the entire intake of the left atrium. After ostium primum is closed and while the lungs are but little developed, flow through the interatrial ostium secundum must still be the major part of the blood entering the left atrium. During the latter part of fetal life the foramen ovale in septum secundum becomes the transseptal route. As the pulmonary circulation increases in volume, a progressively smaller proportion of the left atrial intake appears to come by way of the foramen ovale and a progressively larger amount from the vessels of the growing lungs. At the time of birth, on the basis of orifice measurements, somewhat more than half the blood entering the left side of the heart appears to come from the lungs and less than half from the right atrium by way of the foramen ovale (Patten, 1930).
The progressively diminishing transatrial flow and the progressive increase in the volume of the pulmonary circulation during the latter part of fetal life seem to have been largely overlooked. Unfortunately, no one has as yet solved the difficult problem of obtaining from living embryos pressure and volume determinations such as would permit a quantitatively accurate evaluation of the situation. But, if we may judge anything from the size of the vessels concerned, the volume of the pulmonary circulation of a term fetus is far from the negligible quantity commonly assumed. On the contrary, it is probably already sufiicient to take care of gaseous interchange as soon as the lungs are ventilated, for the pulmonary arteries of a term fetus are of approximately the sa.me size as its umbilical arteries, and the total cross sectional area of the pulmonary veins is approximately equivalent to the cross section of the umbilical vein (Patten and Toulmin, 1930). If we recognize the fact that pulmonary vessels as large as the umbilical vessels can carry a volume of blood sufficient for gaseous interchange we are at once relieved of the necessity of postulating the traditional revolutionary changes in circulation at the moment of birth. Postnatal circulatory changes can then be interpreted on the basis of gradual readjustments which are more in harmony with what we know of other processes of change in living organisms. Following birth, the lumen of the ductus arteriosus is gradually occluded by an overgrowth of its intimal tissue. The histological picture presented is somewhat suggestive of the changes seen in endarteritis obliterans. This process in the wall of the ductus is as characteristic and regular a feature of the development of the circulatory system as the formation of the cardiac septa. Its earliest phases begin to be recognizable in the fetus as the time of birth approaches, and after birth continue at an accelerated rate to terminate in complete occlusion of the lumen of the ductus about 6 to 8 weeks after birth. This progressive closure of the ductus arteriosus reduces the shunt from the pulmonary circuit to the aorta and, acting together with the newly assumed respiratory activity of the lungs themselves, gradually raises the pulmonary circulation to full functional level. Barcroft (personal communication) is inclined to believe on the basis of recent experiments that there is also contraction of the smooth muscle in the wall of the ductus following birth. If this proves to be the case it would mean that the increase in pulmonary circulation is accelerated by a physiological mechanism which begins to act more promptly than the mechanism of morphological closure. It is diflicult to conceive of such muscular action closing the ductus immediately and completely and being efiectively maintained during the 6 to 8 weeks occupied by morphological closure. Nevertheless such a vasoconstriction might well reduce flow through the ductus sufliciently to accelerate the increase in blood flow to the lungs and to facilitate the ultimate closure of the ductus arteriosus by the growth of its own intimal tissue.
The results of increased pulmonary circulation with the concomitant increase in the direct intake of the left atrium are manifested secondarily at the foramen ovale. Even before birth —in the latter part of fetal life as the lungs attained considerable development—we noted that a reduction in transseptal flow was beginning to be evidenced. Following birth, as the pulmonary return increases still more, compensatory blood flow from the right atrium to the left decreases correspondingly. This is indicated anatomically by a progressive reduction in the looseness of the valvula foraminis ovalis and the consequent diminution of the interatrial communication to a prognesively narrower slit between the valvula and the septum. This first phase in the closure of the foramen ovale occupies approximately the 1st postnatal month, during which time the pulmonary return is mounting toward equivalence with the right atrial intake. Wh this equalization has occurred, the compensating one-way valve at the foramen ovale falls into disuse. Although a probe can still be passed freely behind the valvula, the foramen ovale may be regarded as functionally closed when this new intracardiac balance has been attained.
Then follows a period of 6 to 8 months in which the connective tissue of the valvula increases from 600 to 7oo per cent (Patten, 1931). Probe-patency still persists but the size of the slit through which a probe may be passed progressively diminishes and the resistance to its pasage increases with the increase in the thickness of the valvula. This second phme in the closure of the foramen ovale with its characteristic histological alteration is essentially the conversion of an originally movable, flap-like valve into a fixed septal structure.
Finally, coming leisurely in the wake of functional abandonment and as a culmination of the period of connective tissue proliferation, is the adhesion of the valvula to become an integral part of the interatrial septum. There is great individual variability in the age at which this final step in the closure of the foramen ovale occurs. A usual range, rather than a specific time of final anatomical closure, is all that can be specified. Substantiated cases of the fibrous adhesion of the valvula to the septum becoming complete under 3 months are exceedingly rare. The usual time of complete anatomical closure appears to be not earlier than the last 3rd of the 1st year after birth, and is frequently much later (Patten, 1931).
In 20 to 25 per cent of adult individuals the fibrous adhesion is never entirely completed (Table I). Provided the valvula amply overlaps the foramen ovale such failures of complete adhesion appear to be no functional handicap to an otherwise normal individual. Because of this fact and the frequency with which they occur, these cases may well be regarded as variations of the normal rather than as abnormalities. Such an attitude, however, must be tempered by the realization that in the event of disturbances in the pulmonary circuit sufficiently severe to unbalance intra-atrial pressures, an area of incomplete adhesion may again become a path for transseptal flow. The interesting experimental work of Gross (19 34), in which he observed the behavior of interatrial septa obtained at autopsy and clamped between artificial atria in which the pressures could be varied at will, clearly demonstrates that this is more than a mere theoretical possibility.
Table 1
| Records as to Completeness of Closure of Foramen Ovale, from a Large Series of Individuals Beyond Childhood | ||
|---|---|---|
| Observer | No. of cases examined | Not completely closed |
| Adami-Abbott, 1915 | 1374 (adults) | 199 |
| Bizot, 1837 | 155 (mostly adults) | 44 |
| Brit. Anal. Soc., 1897 (Parsons and Keith) | 316 (all above 10 yrs.) | 76 |
| Fawcett and Blachford, 1901 | 306 (all over 6 yrs.) | 96 |
| Hinze, 1893 | 359 (all over 20 yrs.) | 82 |
| Ogle, 1857 | 62 (adults) | 13 |
| Rostan, 1884, and Zahn, 1889 | 711 (661 over 20 years) | 139 |
| Seib, 1934 | 500 (all over 20 yrs.) | 85 |
| Wallmann, 1859 | 300 (291 over 20 yrs.) | 130 |
| Totals | 4083 | 864 |
Foramen ovale not completely closed in 21.2 per cent of cases
The exact percentage incidence of undosed foramen ovale obtained by compiling such data naturally varies with the length of the series of cases and the criteria used in selecting acceptable data. In a previously compiled table for about 4000 cases, “mostly adult” but not rigidly selected for age, the per cent obtained was 24.6 (Patten, 1931). In a compilation of 2648 cases in which all cases under 20 years were excluded, Seib (1934) arrived at a figure of 23.1 per cent. The present table showing 21.2 per cent difiers from my own previous one in the substitution of Seib’s new series of 500 cases in which the ages were all known to be above 20 years, for the 500 cases of Klob in which no account was taken of ages, and which one suspects from the 45 per cent of non-closures must have included many very young individuals. The present table differs from Seib’s in containing a considerably greater number of cases because of less rigid age selection. The point to be emphasized is the essential consistency of these three tabulations, rather than their minor variations. For all practical purposes we may say that in individuals beyond childhood we may expect 1 case out of every 4 or 5 to show an incompletely closed (i.e. “probe-patent”) foramen ovale.
With this brief sketch of prenatal and neonatal conditions as a background we may turn to a consideration of the various types of developmental defects which may manifest themselves at the foramen ovale.
Congenital Defects at the Foramien Ovale
Congenital defects of the heart are commonly attributed to one of two alleged causes: to “developmental arrests” which are said to leave some essential cardiac structure in an “underdevelo ” condition characteristic of a phase of its formation during embryonic life; or to the damaging effects on local growth of some inflammatory process that becomes established during fetal life. Neither of these factors adequately accounts for the wide variety of congenital defects encountered either at the foramen ovale or other locations in the heart.
As far as the evidence from any material that I have seen is concerned, pathological lesions rarely, if ever, appear to play a part in the primary causation of a developmental defect at the site of the lesion. Inflammatory reactions resembling those caused in the adult by endocarditis undoubtedly do occur occasionally at the site of congenital defects. There is, however, no reason to believe that such a process is the cause of the congenital defect. On the contrary, the lack of any semblance of constancy in the association of such lesions with developmental defects in general points very strongly to the conclusion that the association, when it does occur, is fortuitous. Possibly a defect of such a nature that it constitutes a point of local stress, as for example pulmonary stenosis, may furnish a site of predilection for an inflammatory process, once the causative agent ha become established in the fetal blood stream. That a localized inflammatory process causes a developmental defect at the site of the lesion appears to be unsupported by any valid evidence.
While local pathological lesions may be discounted, or even dismissed altogether, as direct causative agents, congenital defects which might be interpreted as developmental arrests unquestionably occur. If, for example, septum secundum does not grow to the usual extent, the orifice to be occluded by the valvula foraminis ovalis remains abnormally large and, therefore, may be inadequately guarded by a valvula which is itself perfectly normal (Fig. 5). In such a case we might properly employ the expression “developmental arrest,” for growth progressing along its normal course has fallen short of completion.
There are, however, defects at the foramen ovale that are in no sense the result of the cessation of a growth process short of its usual culmination. If, for example, the normal process of resorption which is concerned in the establishment of the secondary opening in septum primum (Text-Fig. 4, C) does not cease at the proper point, septum primum may be so extensively destroyed that it fails to occlude efiectively a foramen ovale of normal size (Figs. 3 and 4). This is a radically different process from a developmental arrest. Instead of dealing with a growth process which ha not gone to completion, we are dealing with a process of resorption that has gone too far.
Another condition which is a variant of the process just considered occurs not infrequently. The resorption of septum primum may take place in abnormal areas as well as to an abnormal degree. Instead of being limited to the quadrant in which the secondary opening in septum primum is ordinarily established, the resorption may occur in several places and progress to such an extent that the remains of septum primum can not possibly act as an efficient valve at the foramen ovale (Fig. 6). This abnormal resorption may start from the margins, 5 in the heart shown in Figure 6, or it may appear also in the form of multiple small openings reminiscent of the manner in which ostium II is first formed in septum I (TextFig. 4, C). The openings may be formed near the normal site of ostium II or at various other parts of the valvula as shown in Figures 7, 8, IO, 12. In such cases we are dealing with the distortion of a resorptive process instead of with the “arrest” of a growth process. When it progresses and terminates normally, this process of resorption plays just as important a part in moulding an efficient valve as do the growth processes with which it is correlated.
In rare instances hearts are encountered that show no trace of a valve covering the foramen ovale (Fig. 16). While it is impossible to be certain that this represents a defect due to secondary resorption of a once present septum primum, circumstantial evidence points to that conclusion. When septum primum is primarily defective there remains a very characteristically shaped opening just above the atrioventricular valves. Usually the valves themselves are notched at the point where septum primum would have fused with the atrioventricular canal cushions. The absence of such a condition, in this heart, and the existence in other hearts of a whole series of conditions grading toward complete destruction of the free part of the valve (Figs. 1-14) point strongly toward secondary absorption of a once present septum primum as the correct interpretation.
The most common type of defect at the foramen ovale appears to be that in which there has been just a little too much resorption of septum primum at the normal site of ostium II (Fig. 3). This condition is surprisingly frequent in newborn infants. In a series of mo consecutive cases studied with special reference to this condition its incidence was above 20 per cent. Apparently such slight failures of the valvula to overlap the foramen are rapidly compensated for in some manner because, except in newborn infants, they are not strikingly common. It may be that septum secundum grows somewhat after birth thereby reducing the extent of the foramen ovale and eliminating the small unguarded area. It is possible, also, that the marked fibrous development characteristic of the valvula from the 2nd to the 9th month after birth may account for the elimination of this defect in some cases. In I unusual case, secondary growth of the tissue around the limbus fossae ovalis was very marked and there had been, also, as far as one could judge by looking at the completed process, some secondary filling in of small multiple defects in the valvula (Figs. 20, 21, 22). How common such repair may be it is impossible to guess. The case mentioned is the only one of the kind I have seen but it seemed unmistakable in its significance.
Probably the rarest of anomalies occurring at the foramen ovale is congenital atresia (Corvisart, 1818; Smith, 1846; Osler, 1880; and Lehman, 1927). Through the courtesy of Dr. Howard T. Karsner I had the opportunity of seeing the additional case illustrated in Figures I7, 18 and I9. Such cases throw an interesting side light on the functional significance of the foramen ovale during fetal life, for in every instance the left ventricle was developed to only about half its normal size. The muscular development of the ventricles being largely influenced by the volume of blood that they handle during their period of growth, one must infer that the half-norrnal development the left ventricle acquires in cases where the foramen ovale is prematurely closed depends on the blood returning to the left heart through the fetal lungs. The condition seen in these cases seems to corroborate the interpretation given above on the basis of orifice memurements, that approximately half the blood entering the left side of the heart in a term fetus comes by way of the lungs and half by way of the foramen ovale.
From the morphogenetic standpoint, congenital stenosis or atresia of the foramen ovale presents yet another different type of departure from the normal. It is not the result of inhibited growth, nor yet of exaggerated or distorted resorption. On the contrary, it is the continuation of a normal constructive process “beyond the point specified in the plans.” Septum secundum fails to cease growing when its margins have reached the usual boundaries of the foramen ovale. Its growth continues abnormally until it has closed an opening without which the left side of the heart develops so defectively that it cannot long maintain the load imposed on it after birth.
That congenital defects at a given location may arise in such fundamentally different ways would seem to have significant implications. Cases have been here presented which show abnormal interatrial openings appearing as the result of: (I) underdevelopment of septum secundum; (2) resorption of septum primum starting in the normal location but going too far; (3) resorption of septum primum taking place in abnormal locations; and (4) overgrowth of septum secundum. Such radical differences in the immediate mechanisms concerned should give us pause in considering any “blanket explanation” of congenital defects. Certainly the ultimate solution of the intricate problem of their causation will not be advanced by overemphasizing the developmental arrest concept when congenital defects may equally possibly be the result of a resorptive process which has gone astray, or a growth process which has failed to stop soon enough. Pending the acquisition of more satisfactory knowledge as to etiology, we would be on sounder ground if we were more restrained in our use of “developmental arrest” with its often false implications as to causation, and employed some such non-committal expression as developmental distortion or developmental defect.
Clinical Significance of Defects at the Foramen Ovale
It would carry me out of my province to undertake any extensive discussion of the clinical problems presented by individuals with congenital defects at the foramen ovale. There are, nevertheless, certain things that stand out from a study of the records of a large number of cases that it might not be out of place to mention.
The still rather widespread practice of attributing otherwise unaccounted for deaths of young infants to “an open foramen ovale” is utterly unsound. In the first place, anatomical closure of the foramen ovale does not ordinarily take place until toward the close of the 1st postnatal year. Secondly, from 20 to 25 per cent of all adults show incomplete fusion of the valvula to the septum without the slightest evidence that this condition is any functional handicap, provided the valvula adequately covers the foramen and there are no other concomitant circulatory disturbances. Finally, the way individuals with extensively unguarded interatrial openings frequently live into maturity and even old age clearly shows the absurdity of regarding a mere unclosed foramen ovale as the immediate cause of a fatal circulatory disturbance.
While an unclosed but competent valvula foraminis ovalis cannot be regarded as a causative factor in circulatory disturbances, it frequently is the result of disturbances elsewhere. If, during the period in which the valvula would normally fuse to the septum, there is any condition operative that reduces the left atrial intake from the lungs, transseptal blood flow from right to left will continue to take place postnatally as it did prenatally and the valvula will thereby be prevented from fusing to the septum. The most striking cause of such a situation is congenital pulmonary stenosis. Almost without exception when there is a pulmonary stenosis of embryological origin, an unfused and slack valvula persists at the foramen ovale. This is the logical sequel of the failure to accomplish the balancing of direct atrial intakes which normally comes with the attainment of full functional activity by the pulmonary circuit.
While it may not invariably be the case, there is usually a recognizable morphological difference between the condition exhibited by a valvula which ha been kept open by circulatory unbalance and one which, although subjected to no such disturbance, has failed to fuse with the septum. In the fortuitous failure of complete adhesion which is encountered in 20 to 25 per cent of all adults the valvula tends to lie tight against the septum and the areas of incomplete fusion may be entirely overlooked unless one meticulously explores all parts of the valve margins with a fine flexible probe. When transseptal blood flow has persisted, the valvula tends to retain a certain fullness like that characteristically present in the fetus. It is likely for the same reasons to lie less closely against the septum, thus readily revealing the presence of an interatrial communication. These differences appear most clearly when a fresh heart is examined under water, with the aid of a current from a syringe.
There is a rather neglected corollary to the proposition that a foramen ovale with a freely opening valve should be regarded as part of the picture one would expect to find with congenital pulmonary stenosis. Cases of pulmonary stenosis not infrequently come to autopsy in which conditions at the pulmonary outlet alone do not give satisfactory evidence as to whether the stenosis was congenital or acquired. In such cases conditions at the foramen ovale may furnish valuable collateral evidence. In the absence of some other circulatory by-pass of similar functional significance, a completely closed foramen ovale in a case of pulmonary stenosis is strong evidence that the stenosis developed after the fetalneonatal period.
Thus it should be emphasized that, when the foramen ovale possesses a competent valve, the question of whether or not this potential by-pass is closed should be considered in the light of the factors involved in establishing and maintaining a balanced atrial intake. Phrased in another way, an open foramen ovale of this type is not to be thought of as a cause of circulatory disturbances but as a result of them. Equally obviously, the question of the condition of the pulmonary circuit should be the first consideration from the standpoint of diagnosis. The fundamental importance of maintaining a. right to left transatrial blood flow during fetal life is correlated with the low volume of the pulmonary circuit while the lungs are not functioning in respiration. The cessation of transatrial blood flow and the balancing of direct atrial intakes, which occurs following birth, is dependent on the attainment of full functional level in the pulmonary circuit. And finally there is no single factor a likely as some disturbance of the pulmonary circulation to upset this balance during postnatal life and thus reopen a foramen ovale which has happened to remain unsealed.
A heart with a foramen ovale which is inadequately guarded by a malformed valvula (Figs. 14 and I 5), or one which is completely unguarded (Fig. 16) presents quite different possibilities from cases of the type just discussed. In hearts with a competent but unfused valvula, transseptal flow takes place readily from right to left but is inhibited from left to right. When there is an unguarded foramen ovale, transseptal flow can take place just as readily from left to right as from right to left. Of course if there is an associated pulmonary stenosis causing a compensatory flow from right to left, the mechanism of the circulation will be quite similar to that just discussed for a heart with a competent but unfused valvula. If, on the other hand, the pulmonary circuit is normal a radically different picture is presented. The clinical manifestations of "such cases have recently been presented very ably and in considerable detail by Roesler (1934). When, as Roesler has done, all cases of interatrial defect in which there is an associated pulmonary stenosis are ruled out, a very characteristic picture remains. Its outstanding features, summarized from Roesler’s findings, are essentially as follows: The heart tends to be enlarged, often becoming of enormous size and causing a marked precordial bulge. The enlargement involves primarily the right side of the heart and especially the right ventricle which is usually both extensively dilated and hypertrophied. The left ventricle remains strikingly uninvolved by the enlargement (Abbott’s Case 1, 1915, with hypertrophy involving the left 3 well as the right ventricle is exceptional). The pulmonary artery is consistently larger than the aorta, the average ratio being 3:2. The aorta tends to be below normal size and thin walled. In upwards of threefourths of the long-standing cases valvular lesions were found which affected predominantly the mitral orifice (see also McGinn and White, 1933). In contrast with certain other types of congenital defects, subacute bacterial endocarditis is strikingly absent, and chronic pericardial disease, crossed embolism, and pulmonary tuberculosis are noticeably rare concomitants.
The critical factor in this picture again appears to involve relative atrial intakes and pressures. In normal adults the pressure in the left atrium is believed to be slightly greater than that in the right. Under such conditions an unguarded interatrial opening would permit the backing of blood from the left atrium into the right, thus causing the right side of the heart to handle an increased amount of blood. This situation acting over a long period of time would account for the dilatation and hypertrophy of the right ventricle and the large pulmonary arteries which are so characteristic in these hearts. The same transatrial flow which overloads the right heart, by reducing the blood entering the left ventricle, would account for the fact that it is consistently uninvolved by dilatation or hypertrophy, and also for the fact that the aorta tends to be relatively small.
Detailed physiological evidence for this interpretation is admittedly scanty but the circumstantial evidence is convincingly consistent. For a fuller discussion from a clinical standpoint reference should be made to Roesler’s excellent paper. There are, however, two points of special interest which it might be pertinent to mention here. One is the striking absence of cros@ emboli in cases of frankly unguarded interatrial openings where the flow is presumably taking place from left to right, in contrast with the comparative frequency of crossed emboli in association with pulmonary stenosis where the interatrial communication may be much smaller but where the leakage is from right to left. The other point concerns cyanosis. Characteristically, u long 3 the individual is subject to no complicating factors, cyanosis will be absent as one would expect on the basis of the above interpretation of a left to right shunt. A sudden terminal cyanosis (“cyanose tardive,” Bard and Curtillet, 1889) is, however, very likely to occur. What apparently happens in such cases is a reversal of the direction of the shunt due either to intercurrent pulmonary dilficulty, or to breakdown of the long overloaded right ventricle.
Many individuals, even with large interatrial defects, live to advanced years with surprisingly little handicap. Perhaps the most interesting are cases in which individuals have performed hard work for many years or otherwise lived an active life. Caton (1878) wrote of a man, powerfully built, who had been a seaman for 20 years and finally died of an acute respiratory infection at the age of 40. His interatrial defect was 3 inches in diameter. The heart here illustrated in Figure 14 was from a charwoman who lived to the age of 52, and that illustrated in Figure 16 was from a day laborer who lived to the age of 44. Gibier (1880) records the case of a man who showed no cardiac symptoms during life, withstood anesthesia for a cancer operation and lived to the age of 70. Firket (1880) reported on a woman who had II children, and lived to an age of 74 years. Tamower and Woodrufi (1936) have published the clinical findings and detailed autopsy report on a woman who lived to be 77 years of age although she had a patent foramen ovale measuring 4 cm. in diameter. Why this tolerance of the defect is so striking in some cases and why other individuals with similar defects are incapacitated and go on to a relatively early death from cardiac failure is diflicult to explain. One significant fact brought out by Roesler, is that in three-fourths of the 62 cases of Imguarded interatrial defects which he reviewed chronic valvular lesions of some degree were fotmd. This is considerably above the average incidence of such lesions in all types of congenital defects, which is placed by Abbott (1932) at 17.6 per cent. The mitral orifice seems to be the one most frequently afiected in individuals with interatrial defects. While there is no clue as to why this is the case the aggravating results of its occurrence are self-evident. Either mitral insufliciency or stenosis will tend to exaggerate the flow from left atrium to right which is the critical factor in producing the characteristic cardiac changes seen with an interatrial defect, whether it be at an unguarded foramen ovale or at the site of interatrial foramen primum. Apparently when this back flow becomes extreme there is a breakdown of the compensation previously maintained and the individual begins to show marked cardiac symptoms which are likely to increase rather rapidly in severity.
As far as it is possible to generalize, the situation would appear to be that even a large interatrial defect is by itself not necessarily incompatible with a long and active life. It does, however, produce striking and characteristic changes in the proportions of the heart. Moreover, such hearts appear to be more than normally vulnerable. Individuals who have carried the defect for years without particular handicap may suddenly show signs of cardiac failure. What part is played in such cases by the frequently concomitant valvular disease, why valvular diseae has such a high incidence in these hearts, and why it shows a predilection for the mitral orifice are all matters that need further study.
Summary
This paper aims to present a survey of the various types of developmental defects encountered at the foramen ovale, and to make a re-evaluation of their significance. As a foundation for this study the formation of the interatrial septal complex in the embryonic heart is reviewed. In this review special attention is given to the functional significance of the three different interatrial communications which appear during intrauterine life.
The mechanism of the closure of the foramen ovale following birth, and the time at which its closure may be expected to occur, are presented in the light of recent work which points to the conclusion that the postnatal changes in circulation are far less abrupt and immediate than traditionally postulated.
The various types of developmental defects which may occur at the foramen ovale are illustrated and the embryological processes which have been distorted in the production of each type of defect are discussed. It is pointed out that the “developmental arrest” concept is inadequate as an interpretation, in view of the several fundamentally different ways in which the developmental defects may arise.
Finally, brief comment is made on certain things of clinical interest that emerge from the review of a large number of cases. Reasons are given for regarding as unsound the still widespread practice of attributing otherwise unaccounted for deaths of neonatal infants to “an open foramen ovale.”
Emphasis is placed on the necessity of discriminating more critically between an open foramen ovale with a competent but unfused valve, and a frankly unguarded foramen ovale with an incompetent valve. With an unfused but competent valve transseptal leakage, if it occurs at all, is limited to one directionright to left. The common cause of such leakage is some disturbance of the pulmonary circuit which results in relative lowering of left atrial intake and pressure. What occurs at the foramen ovale in such cases should be regarded 3 a result of disturbances elsewhere and not as a cause.
In sharp contrast with such cues are those in which the foramen ovale is inadequately guarded by an incompetent valve. In these cases, provided the pulmonary circuit is normal, transseptal flow appears to take place quite consistently from left to right. This overloads the right heart at the expense of the left and causes characteristic changes from the normal cardiac proportions. There tends to be a marked dilatation and hypertrophy resulting in a great increase in heart weight. The right side of the heart, especially the right ventricle, is most conspicuously involved, whereas the left ventricle remains strikingly uninvolved. Consonant with the relative ventricular development, the pulmonary artery is markedly larger than the aorta, which tends to be below normal size and thin walled. In these cases with an unguarded foramen ovale the characteristic and clinically recognizable changes in cardiac structure are clearly the result of the defect. Even in these cases the prognosis should not be unduly pessimistic as many individuals support such defects surprisingly well and live to an advanced age.
Bibliography
Abbott, Maude E. Two cases of widely patent foramen ovale. Internat. A. M. Museums Bull. No. V, 1915, 129-134.
Abbott ME. Congenital Cardiac Disease (1915) Osler & Mccrae's Modern Medicine 6, 2nd Edition. Ed. 2, 4, 355.
Abbott, Maude E. Congenital heart disease. Nelson’s Looseleaf Medicine. Thos. Nelson & Sons, New York, 1932, 4, 207-321.
Aleksieyeff, Aleksiei Ivanovich. O foramen ovale cordis u dietei [. . . in children]. Diss. St. Petersburg, P. P. Soikin, S.-Peterburg, 1901. (Cited by Scammon and Norris.)
Alvarenga, Pedro Francisco Da Costa. Considerations et observations sur l’époque de l’occlusion du trou ovale et du canal artériel. Imprimerie nationale, Lisbonne, 1869.
Bard, L., and Curtillet, J. Contribution it l’étude de la physiologic pathologique de la maladie bleue; forme tardive de cette afiection. Rev. de méd., 1889, 9, 993-1017 Bizot, J. Recherches sur le coeur et le systéme artériel chez l’homme. Mém. Soc. méd. d’ob.rervation, 1837, 1, 262-411.
Born, G. Beitrige zur Entwicklungsgeschichte des Siugethierherzens. Arch. f. mikr. Anat., 1889, 33, 284-378.
Caton, Richard. Case of absence of the inter-auricular septmn, without cyanosis in a man aged forty. Lancet, 1878, 2, 252.
Corvisart, J. N. Essai sur les maladies et les lésions organiques du coeur. Méquignon-Marvis, Paris, 1818.
Dalton, J. C. Doctrines of the Circulation. Henry C. Lea’s Son & Co., Philadelphia, 1884.
Elsisser. Uber den Zustand der Fiituskreislaufwege bei neugeborenen Kindern. Ztschr. f. Staatsarzneih, 1852, 32, 247-261.
Fawcett, E., and Blachford, J. V. The frequency of an opening between the right. and left auricles at the seat of the foetal foramen ovale. J. Anat., 1901, 35, 67-70 Firket, Ch. Examen anatomique dem cas de persistance du trou ovale de
Botal, avec lésions valvulaires considerable: du coeur gauche, chez tme femme de 74 ans. Ann. de Sac. méd.-chit, Liege, 1880, 19, 188-197.
Gibier. Note sur un cas de persistance du trou de Botal chez un homme de 70 ans, ne s’étant revélée par aucun symptome pendant la vie. Union méd., 1880, 30, 349
Gross, Paul. The patency of the so-called “anatomically open but functionally closed” foramen ovale. Am. Heart 1., 1934, 10, 101-109.
Herxheimer, G. Missbildimgen des Herzens und der grossen Gefisse. Morphologie der Missbildungen des Menschen und der Tiere, Schwalbe, E. G. Fischer, Jena, 1910, 3, Pt. 2, Chapt. 4, 339-504.
Hinze, Friedrich. Ueber den Verschluss des Foramen ovale des Herzens. Inaug. Diss., G. Schade, Berlin, 1893.
Keibel, Franz, and Mall, Franklin P. Manual of Hinnan Embryology. J. B. Lippincott Company, Philadelphia, 1910-12.
Lehman, Edward. Congenital atresia of the foramen ovale; report of a case, classification and comment on ftmction. Am. J. Dis. Child., 1927, 33, 585539 Mall, Franklin P. On the development of the human heart. Am. J. Anat., 1912, 13, 249-298.
McGinn, Sylvester, and White, Paul D. Interauricular septal defect associated with mitral stenosis. Am. Heart 1., 1933, 9, 1-13.
Odgers PNB. The formation of the venous valves, the foramen secundum and the septum secundum in the human heart. (1935) J. Anat., 69: 412-422. PMID 17104548
Ogle, John W. On certain cases in which the foramen ovale was still patent in the adult. Brit. M. 1., 1857, 1, 5oo—5o1.
Osler, William. Cases of cardiac abnormalities. Montreal Gen. Hosp. Rep., 1880, 1, 177-192.
Parsons, F. G., and Keith, A. Seventh report of the committee of collective investigation of the Anatomical Society of Great Britain and Ireland, for the year 1896-97. J. Anat., 1897, 32, 164-136.
Patten BM. The changes in circulation following birth. Am. Heart 1., 1930, 6, 192-205.
Patten BM. The closure of the foramen ovale. Am. J. Anat., 1931, 48. 19-44
Patten, Bradley M. Changes in the fetal circulation following birth. Obstetrics and Gynecology, Curtis, Arthur Hale. W. B. Satmders Company, Philadelphia. 1933, 1. Chapt 27. 906-929
Patten, Bradley M., Sommerfield, William A., and Pafi, George H. Functional limitations of the foramen ovale in the human foetal heart. Anat. Rec., 1929, 44, 165-178.
Patten, Bradley M., and Toulmin, Kathryn. Certain measurements of the foetal heart and their significance. Abstr. Anat. Rec., 1930, 45, 235. (Paper in preparation.)
Poynter, C. W. M. Congenital anomalies of the heart. Univer. Nebraska Studies, 1919, 19, 1-102.
Roesler, H. Interatrial septal defect. Arch. Int. Med., 1934, 54, 339-380.
Rokitansky, Carl. Die Defecte der Scheidewhde des Herzens. W. Braumfiller, Wien, 1875.
Rostan, A. Contn'bution -£1 1’étude de l’embolie croiseé consécutive 5. la persistance du trou de Botal. These Genéve, Rivera & Dubois, 1884. (Cited by Herxheimer, p. 378.)
Scammon RE. and Norris EH. On the time of the post-natal obliteration of the fetal blood-passages (foramen ovale, ductus arteriosus, ductus venosus). (1918) Anat. Rec. 15(4): 166-180.
Seib, George A. Incidence of the patent foramen ovale cordis in adult American whites and American negroes. Am. J. Anat., 1934, 55, 511-525.
Smith, Ebenezer. Premature occlusion of the foramen ovale; large pulmonary artery and duct, and contracted left heart. Tr. Path. Soc. London, x846—48, 1, 25-28.
Tandler J. The Development of the Heart. (1912) Sect. II, chapt. 18, vol. 2, in Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia., pp. 534-570.
Tandler, Julius. Anatomie des Herzens. Handbuch der Anatomie des Menschen, von Bardeleben Karl. Gustav Fischer, Jena, 1913, 3, Pt. 1.
Tarnower, Herman, and Woodrufi, I. Ogden. Widely patent foramen ovale: case report with discussion of diagnosis. Am. Heart J., 1936, 12, 358-364.
Wallmann, Heinrich. Ueber das Ofienbleiben des Forarnen ovale cordis bei Erwachsenen. Viertljahrsschr. f. d. pralzt. Hei1k., I859, 62, 20-35.
Waterston, D. The development of the heart in man. Tr. Roy. Soc. Edinburgh, 1918—r9, 52, 257-302.
Zahn, F. Wilh. Ueber paradoxe Embolie und ihre Bedeutung fiir die Geschwulstmetastase. Virchows Arch. f. Path. Anat., 1889, 115, 71-80. (Zahn reported, with some difierences in figures, the same series of autopsies as Rostan, 1884.)
Description Of Plates
Plate 30
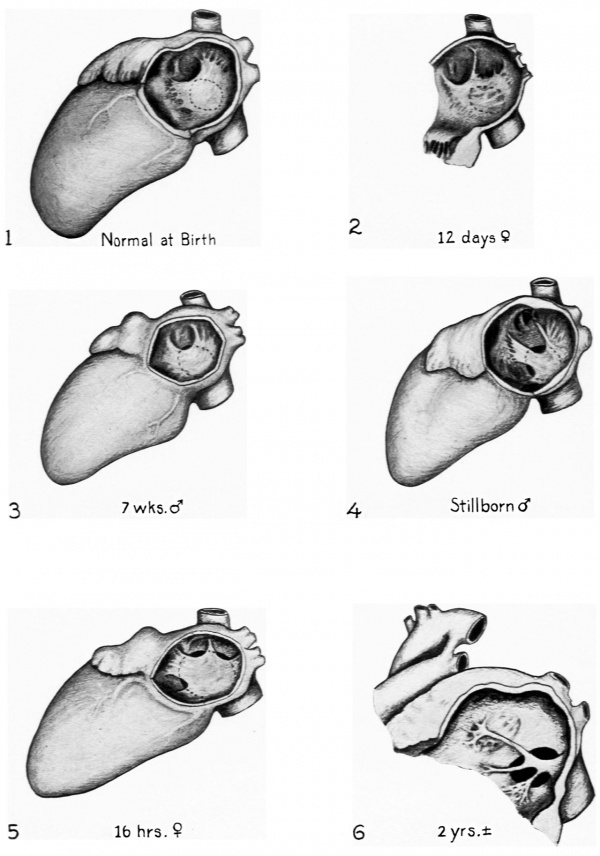
Fig. 1. Usual appearance of the valvula foraminis ovalis as seen from the left in the hart of a newborn infant. Note the fullness of the valvula which is represented in the bulged out position it assmnes when subjected to excess fluid pressure from the right atrium through the foramen ovale. The size and position of the foramen ovale are indicated by the broken line.
Fig. 2. Resorption of septum primum in an abnormal area dorsal to the usual site of ostium secundum. In this instance the abnormality is of no functional significance since it does not unguard the foramen ovale.
Fig. 3. Slight incompetence of valvula foraminis ovalis due to excess resorption of septum primtnn at the normal site of ostium secundum. This is a. common condition, occurring in some 20 per cent of newborn infants. It is apparently “corrected” in most cases by postnatal changes either in septum primmn or septum secundum and probably has no functional significance.
Fig. 4. Incompetence of valvula due to excess resorption at the normal site of ostium secundum combined with resorption at an abnormal site. This defect is definitely more extensive than the apparently correctable type shown in Fig. 3, and xmdoubtedly would persist throughout life.
Fig. 5. The valvula is abnormally resorbed in two small areas but these defects are so slight that they would be of no significance were they not combined with an abnormally large foramen ovale. The large foramen ovale due to defective development of septum secundum is the condition of primary importance in this case.
Fig. 6. Extensive defects of the valvula due to over-resorption in the normal and in several abnormal locations.
Plate 31
Fig. 7. Multiple small perforations of valvula. The formation of ostium secundum normally starts with the appearance of small openings which later coalesce. Here such openings have appeared in a definitely abnormal location.
Fig. 8. Case similar to that shovm in Fig. 7. except that the openings are larger and more vddely distributed.
Figs. 9. 10 and 11. Various combinations of marginal over resorption of the types shown in Figs. 1-6 with small multiple perforations similar to those shown in Figs. 7 and 8.
Fig. 12. Extreme resorption of valvula combined with abnormally large foramen ovale due to defective development of septum secundum. There is also in this heart unbalanced development of the ventricles. the left ventricle being very small, correlated probably with a defective puhnonary circuit as indicated by the marked stenosis of the pulmonary veins.
Plate 32
Fig. 13. Valvula foraminis ovalis markedly sacculated toward left atriinn and showing multiple perforations of considerable size. No clinical history. Dissecting room specimen “from an old man” sent in by Dr. John Donaldson. University of Pittsburgh.
Fig. 14. Drawn from specimen No. 3027, Pathologisch-Anatomisches Institut. Vienna. The heart was from a charwoman who died suddenly of pulmonary thrombosis at the age of 52 years. Rokitansky (I 75, p. 52) gives a brief unillustrated record of the case. The heart was “very large, 90 mm. long and 115 mm. broad” with rormded apex. The similarity of the morphological picture presented by this adult heart and the infant heart shown in Fig. 11 is interesting.
Plate 33
Fig. 15. Drawn from specimen No. 2410. Pathologischa-Anatomisches Institut. Vienna. Case briefly described by Rokitansky (1875. p. 47). Day laborer 21 years old. admitted to the hospital with “the itch” (Kriitze). Died following an unexpected attack of dyspnoea. Heart very large. “100 mm. lang und ebenso breit"; right ventricle and conus "erweitert.” Heart weight not recorded.
Fig. 16. Drawn from specimen No. 2225. Pathologisch-Anatornisches Institut. Vienna. Case mentioned briefly by Rokitansky (I87 5. p. 45). Male. day laborer. 44 years old. Had purulent bronchitis and gangrene of oral mucous membrane. No cyanosis noted. Immediate cause of death appeared to be primarily pulmonary. although the clinical information given is too meager to be certain. The heart was greatly enlarged and showed a fibrinous pericarditis. No trace of a valvula foraminis ovalis could be seen and the unguarded foramen ovale was of enormous size measuring 40 by 47 mm. in the fixed specimen.
Plate 34
Figs. 17. 18 and 19. Heart of a 1 month old infant showing the conditions encountered in premature closure of the foramen ovale. (Babies and Childrens Hospital. Autopsy No. A—374. made available through the courtesy of Dr. Howard T. Karsner. Western Reserve University Medical School.) Although there is no way of being certain whether the closure as seen at autopsy had been fully established in utero. there seems no doubt that a marked ante natal stenosis. if not an atresia. must have existed. This is indicated: (1) by the slit-like fossa ovalis which is but a small fraction of the oval opening left when septum secundum normally ceases further growth: ( 2) by the complete adhesion of the valvula which does not ordinarily occur until several months after the cessation of transseptal flow: and (3) by the deficiently developed left ventricle which seems clearly attributable to lessened left atrial intake due to a foramen ovale closed. or greatly narrowed. during the growth of the fetal heart.
Figs. 20. 21 and 22. Possible case of postnatal repair of congenital defect at the foramen ovale. The appearance of the narrowed fossa ovalis is superficially somewhat similar to the case of premature closure illustrated above. but there are two associated conditions which indicate that in this case the narrowing occurred postnatally. First is the normal development of the ventricles. If the fossa ovalis had been of its present abnormally small size during intrauterine life the left ventricle would have been undersized. Second is the faint depression which sketches the contours of a fossa ovalis of the normal size. This seems to suggest that the fossa was. at the time of birth. of the size outlined by this depression, and that the difierently disposed tissue now narrowing it was formed later. There is yet another interesting condition pointing in the same direction. In the valnila foraminis ovalis one sees an arrangement of robust strands which suggest that it might once have appeared not unlike the defective valve in Fig. 9. Between these heavy strands there is a more delicate tissue which might conceivably have been secondarily formed. Of course this entire interpretation must be regarded as tentative. but postnatal repair of a congenital defect. if it does occur. is of so much interest that it seemed worth while presenting this unique case in the hope of stimulating further observations bearing on such a possibility.
Cite this page: Hill, M.A. (2024, April 25) Embryology Paper - Developmental defects at the foramen ovale (1938). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Developmental_defects_at_the_foramen_ovale_(1938)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G