Paper - Development of the otic capsule 2
| Embryology - 18 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Bast TH. Development of the otic capsule II. The origin, development and significance of the fissula ante fenestram and its relation to otosclerotic foci. (1933) Arch. Otolaryng. 18(1):
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Development of the Otic Capsule II. The Origin, Development and Significance of the Fissula Ante Fenestram and its Relation to Otosclerotic Foci
T. H. Bast, Ph.D. Madison, Wis.
These studies were made for the Research Council of the American Otological Society.
Introduction
The fissula ante fenestram is an irregular projection of the periotic connective tissue extending from the angle of junction of the vestibule and the scala vestibuli, through the otic bony capsule to the periosteum of the middle ear just below the pulley of the tensor tympani muscle. Little significance has been attached to this structure. The common view held by former authors is that it is a synchondrosis or possibly a place for expansion of the otic capsule. However, further studies have shown that this structure is constant in human otic capsules even into old age; that the capsule in this region is the usual locus or otosclerotic foci; that the tissue of the fissula resembles and is continuous with the loose periotic tissue of the vestibule at its junction with the scala vestibuli, and that the fissula becomes apparent and its character definite in the same manner and at the same time that the periotic tissue differentiates from the precartilage. These facts lead one to- question whether the fissula ante fenestram can be dismissed by simply considering it a synchondrosis. The purpose of this paper is to present developmental and anatomic data regarding this structure which indi cate that it has a significance other than that which was formerly attributed to it.
Review of the Literature
In my earlier account of the “Ossification of the Otic Capsule in Human Fetuses,”[1] an extended historical account of the fissula ante fenestram and the fissula post fenestram was given. Only enough of the literature will be reviewed here to summarize the views held regarding it.
The earliest reference to the fissula to which I have had access is that of Huschke[2] published in 1884. He merely referred to it and stated that it extends from the vestibule toward the groove of the tensor tympani. In 1845 Hyrtl[3] published a picture of corrosion of the ear of a new-born infant which showed a bulblike outpunching from the vestibule in front of the oval window, but he did not say anything about it. In 1873 Hyrtl, in describing the ear of Hyaena spelaea, wrote: “Before the oval window one finds in the outer wall of the vestibulum a small evagination which in the corrosion appears as a low stumplike process.” He said that it is absent in most animals, but he found it in a young antelope where a diverticulum from the sacculus projected into it. In 1890 Siebenmann,‘ after describing the inconstant fissula po-st fenestrarn, wrote: “Another opening, which is more slitlike, occurs immediately in front of the oval window, the fissula ante fenestram, where the vestibule unites with the upper wall of the scala vestibuli.” He compared this structure with the evagination containing the diverticulum of the sacculus which Hyrtl described, and considered it in man as a rudiment of the evagination found in the hyena and antelope. Siebenmann was the first to call this structure the fissula ante fenestram.[4] Of course he, as also did the earlier writers, described the structure as it appeared in corrosions and did not describe its histologic nature. However, Siebenmann located it accurately as being a slitlike outpunching from the periotic labyrinth at the point where the vestibule unites with the upper wall of the scala vestibuli.
In 1916 Perozzi,[5] after working in Siebenmann’s laboratory, gave a histologic account of the otic capsule and in particular of the evagination of the vestibule in front of the oval window and named it, in honor of his uncle and teacher, Cozzolino’s zone. The following year 0. Mayer[6] referred to it as Cozzolino’s zone, and described it as a synchondrosis. In 1920 and 1921 Perozzi[7] wrote a series of five articles in which he described in detail the fissula (Cozzolino’s zone) as shown by sections from a comprehensive series of fetal, postnatal and adult ears. He described it as a fissure-like band of connective tissue which extends from the point where the vestibule joins with the upper wall of the scala vestibuli through the otic capsule to the middle car just in front of the oval window. He noted the structure first in fetuses at the time the capsule became cartilaginous». Other workers state that the cochlear and vestibular capsules chondrify separately, and that the union of these two cartilaginous anlagen is not complete, thus resulting in a synchondrosis in the capsule anterior to the stapes. Perozzi, however, considered this zone as a place whichpermitted growth or expansion of the capsule. Thus he wrote: “I believe that through this layer of perichondrium, placed in the thickness of the capsule, the cavity of the cochlea can more greatly augment and permit the cochlear canal to describe its spirals. In fact, at the moment the cochlear canal augments the most, this zone is larger and the internal layer of cartilaginous young cells is thicker; this would prove a greater activity.” Regarding the histologic structure o-f the zone, he stated that at first it consists of a peculiar loose connective tissue continuous with the loose tissue which fills the periotic space in young fetuses. This tissue extends from the vestibule to the middle ear. In older fetuses the tissue becomes denser and resembles perichondrium. The tissue is surrounded by the cartilage of the capsule. Later when the capsule ossifies, the part surrounding this tissue remains cartilaginous even in adult ears.
In 1922, Kosokabe[8] also a student of Siebenmann, used the term Cozzolino’s zone. In describing the zone in a 1.5 to 2 month fetus, he said that there is a raphe-like line between the cartilaginous medial part of the cochlear capsule and the capsule of the canal which is no-t cartilaginous. He said: “In it one can see closely packed embryonic tissue with irregularly placed cells, which have the appearance of perichondrial cells. One sees in it no blood vessels. This zone, already present in the precartilaginous state, appears from a develo-pmental standpoint as the incomplete slitlike union of the vestibule and the cochlea. Later the area becomes smaller; the form and structure remain the same until the period of ossification.” Claoué[9] pictured the fissula ante fenestram as seen in section. He regarded it, as did Mayer and Perozzi, as a place where two processes of chondrification meet but are separated by means of a perichondrium. They call this a synchondrosis.
In a recent article Guggenheim[10] advanced a phylogenetic interpretation of the fissula. He regarded it as a rudiment of the ductus fenestra ovalis of the frog. He wrote: “The fissula ante fenestram, in which otosclerosis often b-egins, is a vestigial structure which may show cartilage throughout life and is therefore predisposed to mesenchymal invasion. The primary involvement being of a vestigial structure suggests its regressive character. The homologue of the fissure is the canalis fenestrae ovalis of the frog.”
Histologic Findings
This study was made on serial sections and reconstructions of the internal ears of fifty-five human fetuses ranging from 8 weeks to full term. In this series the youngest fetus to show a fissula was a human fetus of 43 mm. crown-rump length. In the next younger fetus which I studied, a human fetus of 30 mm. crown-rump length, the fissula could not be seen. The fissula therefore must become differentiated some time between the 30 mm. and the 43 mm. stage. In the latter stage it was not fully formed, but could be clearly seen only at its vestibular end. In the two youngest fetuses which I studied, one of 25 mm. and one of 30 mm., the otic capsule was for the most part precartilage, including the area which later becomes the periotic labyrinth, scala vestibuli, scala tympani and fissula ante fenestram. In the posterior part of the capsule in the canal region and around the internal auditory meatus, some of the precartilage had changed into young cartilage (figs. 1 and 2). This metamorphosis of precartilage to true cartilage occurs between the 25 and 50 mm. stage, or between the ages of 8 and 10 weeks (figs. 3 and 4).
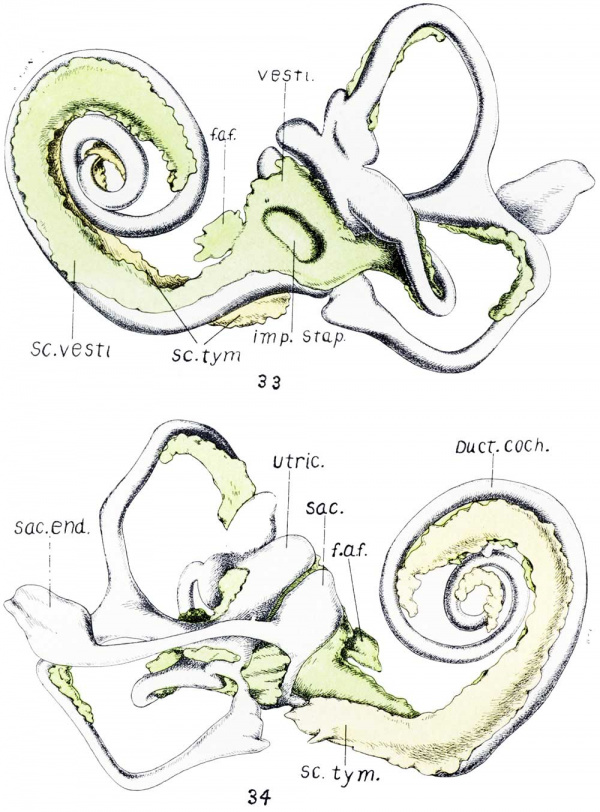
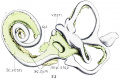
During the same period that metamorphosis takes place, the precartilage immediately surrounding the otic cavity begins a retrogressive change which eventually results in the periotic spaces such as the vestibule, scala tympani and scala vestibuli. This process has been described by Streeter[11]and Foley[12]. My observations agree with theirs as to the formation of the periotic spaces. figures 33 and 34 are drawings of a reconstruction of a 50 mm. human fetal inner ear, showing the extent of the formation of the periotic tissue.[13]
The earliest site of the formation of periotic tissue is between the saccule and the stapes foot-plate. From this place it spreads to form the vestibule and the scala vestibuli. The scala tympani originates in the region of the round window about the same time.
figures 1 to 27 are photomicrographs of sections through otic capsules in the region of the fissula a.nte fenestram. fig. _l.—Embryo 87; size, 25 mm. crown-rump length; age, about 8 weeks. The capsule is mostly precartilage, and the fissula
13 not yet Seen‘ fior. 2.—Embryo 92; size, 30 mm. crown—rump length; age, about 8% weeks.
figs. 3 and 4.—Embryo 86; size, 50 mm. crown—rurnp length; age, about 10 weeks. figure 3 shows the fissula ante fenestram (F.A.F.) as it communicates with the vestibule (V). figure 4 shows the fissula a little higher up where the fissula commnicates with the stapes joint. The area outlined in figure 3 is magnified in figure 29.
In figures 1 to 4, M indicates middle ear; S, stapes; V, vestibule; S, saccule ; U, utriculus; P.C., precartilage; CA, cartilage; C, cochlea; f.F.A.F., junction of fissula ante fenestram and the vestibule; F.A.F., fissula ante fenestram; P.T., periotic tissue.
Origin of the Fissula Ante Fenestram
At the angle of junction of the vestibule and the scala vestibuli there occurs an evagination of the periotic tissue into the capsule.
It is directed upward and toward the middle ear and is known as the fissula ante fenestram (f.a-.f. in figs. 33 and 34). In the 25 and 30 mm. fetuses this area is occupied by precartilage (figs. 1 and 2). Between the 30 and 50 mm. stage the precartilaginous capsule changes to cartilage except around the otic labyrinths, where the periotic tissue spaces form, and where the fissula ante fenestram occurs (figs. 3 and 4). In these places the precartilage undergoes a regressive dedifferentiation. The rounded precartilage cells become more stellate, and small spaces form between the cells; these spaces become larger and larger, coalesce and thus form the periotic spaces. In the fissula ante fenestram the early space formation is marked at its vestibular end, but in the main part of the fissula the stellate cells become more spindle—shaped to form a cellular embryonic connective tissue (figs. 3 and 29). As in the younger fetuses the site of the fissula is occupied by precartilage (figs. 1 and 2), the structure cannot be considered a synchondrosis. But as a regressive change takes place in the precartilage similar to that which occurs in the formation of the periotic spaces and as the fissula is continuous with the periotic tissue of the vestibule, one must conclude that the fissula ante fenestram has its origin in relation and in manner similar to the periotic labyrinth. The relation and origin suggest that the function of the fissula may be related to the function of the periotic labyrinth.
Development of the Fissula and Surrounding Capsule
The story of the development of the fissula and the surrounding capsule is pictured in figures 3 to 28. These seven stages are representative of the fifty-five fetal ears studied. figures 3 and 4 are photomicro-graphs of two horizontally cut sections at different levels through the otic capsule of a 50 mm. or 10 week old human fetus. They show the early stage in the development of the fissula ante fenestram. In figure 3, a section near the lower part of the fissure, the latter is seen as a narrow cellular tissue budding out from the vestibule and extending into the newly formed cartilaginous otic capsule. The tissue of the fissula is a somewhat altered precartilage. Many of the cell nuclei are necrotic, and tissue spaces are forming between the cells. This formation of tissue spaces. is well marked in the region of the junction of the fissula with the vestibule. The main part of the fissula is still quite cellular. The histologic detail is better shown in figure 29, which is an enlargement of the area outlined in figure 3. In figure 4, which is a section about 150 microns above the one shown in figure 3, the fissula is directed toward and almost meets the stapes joint. It is not so well difierentiated as in lower sections. In sections above this level it gradually fades out, and in this fetus no communication is made with the middle ear. The region where the communication with the middle ear later occurs consists of very young cartilage just beyond the precartilage stage. These facts again show that the fissula is not a synchondrosis, but that it develops as an evagination of the periotic tissue. It is well formed at its junction with the vestibule, but has not yet pushed through the capsule to its communication with the middle ear. Again the site of its junction with the middle ear at this stage is cartilage, which, according to the theory of synchondrosis, should neverforrn.
Figs. 5, 6 and 7. Embryo 22; size, 100 mm.; age, about 14 weeks. Sections at three. levels through the region of the fissula ante fenestram. figure 5 shows the communications of the fissuia with the vestibule, Around the fissula the cartilage is very poorly stained. The area outlined is enlarged in figure 30, figure 6 shows the fissula in the region of the stapes joint. figure 7 shows the opening of the fissula into the middle car. A blood vessel is entering the fissula from the middle ear,
fig. 8. An enlargement of the area outlined in figure 6.
In figures 5 to 8, M indicates middle ear; V, vestibule; S, saccule.; F.A.F., fissula ante fenestram; C.D., cochlear duct; S'.J., stapes joint.
figs. 9, 10 and 11.—Fetus 13; size, 161 mm. crown-rump length; age, about 19% weeks. Sections at three levels through the region of the fissula ante fenestram. At this stage the fissula reaches its greatest dimensions. Ossification of the capsule progresses to-ward the fissula. The area outlined in figure 9 is enlarged in figure 31. Small blood vessels are seen in the fissula in figures 9 and 11' fig. 12.—An enlarged view of the area outlined in figure 10.
In figures 9 to 12, M indicates middle ear; V, vestibule; C, cochlea; I7.A.F., fissula ante fenestram; 5.1., stapes joint.
During the next few weeks there occurs a. gradual enlargement of the fissula. This is shown in figures 5 to 8, which are sections from a 100 mm. or 14 week old human fetus. The loose periotic tissue from the vestibule dips deeper into it, and the cartilage around it is poorly stained and appears necrotic. The tissue in the fissula shows many necrotic nuclei and a marked formation of tissue spaces. This is shown in figure 30, which is an enlargement of the area outlined in figure 5. The tissue cells in the fissula a little farther removed from the vestibule show a tendency toward a return to the early precartilage cell type, but the cells are far apart with tissue spaces between them, and the whole tissue is poorly stained (fig. 6 and fig. 8, which is an enlargement of area outlined in fig. 6). In figures 6 and 8 it will also be noted that the tissue of the fissula as it approaches the stapes joint retains the more cellular appearance similar to the tissue of the fissula in the earlier stage shown in figures 3, 4 and 29. It is a late part of the fissula to develop and in most cases retains a cellular nature much like the tissue of the stapes joint. As will be noted from figures 4, 8, 12, 16, 20, 24 and 28, the fissula in most cases extends to the stapes joint. The long axes of the connective tissue cells of the fissula are continuous with and lie in the same plane as those of the stapes joint.
figure 7 is a photomicrograph of a section through the upper part of the fissula (F./l.F.), at the point where it communicates with the middle ear. In the 50 mm. fetus this communication was not yet formed. In the 100 mm. fetus (fig. 7) a blood vessel will be seen entering the fissula from the side of the middle ear. The tissue of the fissula around this entering vessel is poorly stained necrotic young cartilage similar to that around the fissula in figure 6. This stage shows: (1) that the fissula is enlarging, (2) that the fissula is developed furthest at its vestibular end, where the formation of tissue spaces of the vestibule extends a considerable way into it, (3) that the cartilage around the early fissula is poorly stained and (4) that the fissula comrnunicates with the middle ear from whence it receives a blood vessel. It appears, further, that two processes are at Work in the formation of the fissula:
figs. 13, 14 and 15. Fetus 51; size, 210 mm. crown-rump length; age, about 24 weeks. Sections at three levels through the region of the fissula ante fenestram. The capsule at this stage in the region of the fissula is intracartilaginous bone and an internal and external rim of perichondral bone. An irregular rim of cartilage, which remains throughout life, separates the fis sula from the capsule of the bone. Small blood vessels are seen in the fissula. The area outlined in figure 13 is enlarged in figure 32.
fig. 16. An enlargement of the area outlined in figure 14.
In figures 13 to 16, M indicates middle ear; V, vestibule; F./1.17., fissula ante fenestram; 0.F.A.F., opening of the fissula ante fenestram into the middle ear; C/1., cartilage; B, intrachondral bone; 5.1., stapes joint; Y.B.M., young bone marrow.
Figs. 17, 18 and 19.~Fetus 66; size, 290 mm. crown-rump length; age, about 31% weeks. This fetus, judging from the degree of bone formation, is very likely a good deal older than 311; weeks, which is the age determined from the size. The dark areas in the Capsule are intrachondral bone (cartilage islands of other authors); the lighter areas of bone around the dark areas are endochondral bone. fig. 2(}.—An enlargement of the area outlined in figure 18.
In figures 17 to 20, M indicates middle ear; V, vestibule; S, sacculc; C, cochlea; SJ, stapes joint; ]7.A.F., fissula ante
(1) the process of the formation of tissue spaces, Continuous with and like that which forms the periotic labyrinth, and (2) the formation of a vascular bud at the end of the fissula where the middle ear is situated, which results in a destruction of cartilage similar to that found in the cartilage of the canal region described in a recent paper.”
It is difficult to determine whether the fissula enlarges at the expense of the surrounding cartilage or whether the enlargement is entirely due to the spreading of the cells within the fissula owing to the formation of tissue spaces. However, the facts that there are necrotic nuclei in the fissula, indicating nongrowth, and that the cartilage around the fissula is light-staining and necrotic indicate that the fissula enlarges at least in part at the expense of the surrounding cartilage. This enlargement of the fissula has been correlated with the rapid expansion of the entire internal ear and its capsule at this fetal period. Perrozi thought that the enlarging fissula permitted the enlargement of the cochlea and its capsule. I incline toward the view that the fissula enlarges at this period of growth not merely to permit the cochlea and capsule to enlarge, but for the same reason that the rest of the internal ear enlarges. In other words, the fissula is a structure of other importance than a mere means for permitting augmentation of the capsule and cochlea.
During the next few weeks of fetal life, the fissula continues its growth, to reach its maximum size and definite delamination from the surrounding cartilage in fetuses of from about 4 to 4% months and of from 126 to 140 mm. crown-rump length. This is also the period when the cochlea and vestibule attain full size and when the early ossification of the capsule begins.‘ The definite delamination of the fissula is accomplished at the vestibular end by the endochondrium of the vestibule, which dips into- the fissula and separates it from the surrounding cartilage. Near the middle ear the vascular bud which eroded the cartilage brings with it connective cells which line up along the eroded cartilage to form a sort of endochondrium continuous with the endochondrium of the middle ear, which definitely outlines the fissula. The clear delamination and maximum size of the fissula are attained at about the same time that the cochlea reaches its maximum size. If the fissula serves as a center of growth for the expanding cochlear capsule, one would expect that it would be almost used up and small at the time the maximum growth of the cochlea and the capsule was attained. This, however, is not the case. figures 9, 10, 11, 12 and 31 show various views and magnifications of the fissula of a 161 mm. or 19.5 week old
12. Bast, T. H.: Development of the Otic Capsule: I. Resorption of the Cartilage in the Canal Portion of the Otic Capsule in Human Fetuses and Its Relation to the Growth of the Semicircular Canals, Arch. Otolaryng. 16:19 (July) 1932.
figs. 21, 22 and 23. Fetus 60; size, 305 mm. crown-rump length; age, about 34V weeks. In this fetus the middle ear open ing of the fissula a.nte fenestram is small and very near the stapes joint. The opening into the vestibule is prominent. The dark areas of bone are intrachondral bone and are surrounded by endochondral bone.
fig. 24. An enlargement of the area outlined in figure 22. It shows the rim of cartilage separating the fissula from the surrounding bone.
In figures 21 to 24, M indicates middle ear; S.J., stapes joint; V, vestibule; S, saccule; F./1.17., fissula ante fenestrarn; C, cochlea; O.F.A.F., opening of the fissula in the middle ear.
usual. The opening into the middle ear is large, but the vestibular end of the fissula is small.
fig. 28. An eniargement of the area outlined in figure 26. In figures 25 to 28, M indicates middle ear; V, vestibule; S, saccule; C, cochlea; F.A.F., fissula ante fenestram; 5.1., stapes
joint.
figs. 25, 26 and 27. Fetus 67' size, 370 mm. crown-rump length; age, full term. In this fetus the fissula is smaller than
human fetus at the time the cochlea and fissula reach their maximum size. From these pictures it will be noted that the character of the tissue in the fissula is quite different from that shown in figures 5, 6, 7, 8 and 30. In place of the necrotic tissue there is an actively growing tissue (fig. 31). This change from degenerating to growing tissue within the fissula is brought about by the advent of the entrance of blood vessels into the fissula from the middle ear. In the 100 mm. fetus (fig. 7) a blood vessel just entered the fissula from the middle ear, but in the rest of the fissula no vessels are seen. In the 161 mm. fetus (figs. 9 to 12 and 31), numerous vessels are seen throughout it, and the connective tissue cells show marked activity.
Ossification of the capsule is progressing rapidly at this stage, as seen in figure 9. During the subsequent three or four weeks the cartilaginous capsule surrounding the fissula becomes completely ossified, as shown in figures 13 to 15, which are from a 210 mm. or 24 week old human fetus. The fissula thus is completely surrounded by bone except where it communicates with the vestibule and where it butts against the annular ligament of the stapes.
The tissue of the fissula has also changed its character from that seen in the 161 mm. stage. At that stage the connective tissue of the fissula showed actively growing fibroblasts, but in the 210 mm. fetus the tissue is again quiescent, the cells are small with round nuclei, some finely fibrillar intercellular substance is present, and around a number of the cells vacuolation is marked (fig. 32). Blood vessels are numerous, and in figures 14 and 16 one is seen entering the annular ligament of the stapes from the fissula.
In the subsequent development of the fissula, represented by figures 17 to 28, two changes occur: 1. The connective tissue of the fissula bordering the fissula next to the surrounding bony capsule changes to cartilage; this cartilage normally remains as cartilage throughout life. 2. The connective tissue of the central part of the fissula becomes somewhat looser than that shown in figure 32 and remains as a fine reticular connective tissue containing small blood vessels. By means of these two processes the fissula becomes narrower because the peripheral part of the fissula changes to cartilage. The thus somewhat narrowed fissula remains throughout life. The shape and size of the fissula, however, vary in different persons. This is already seen in fetuses. Thus in the 305 mm. fetus (figs. 21 to 24), the fissula is large, but its communication with the middle ear is small, whereas in the fu1l—term fetus (figs. 25 to 28) the fissula is very narrow, almost obliterated in spots, but the communication with the middle ear is large. Again in some fetuses the opening to the middle ear is very near the stapes joint; in others, it is quite distant from the oval window.
fig. 29. Enlarged View of area outlined in figure 3. It shows the vestibular end of the fissula. The loose vacuolated tissue at PT is periotic tissue. This loose vacuolated tissue extends into the fissula for a little way. The rest of the tissue of the fissula ante fenestrani (F.A.F.) is cellular, but many of the nuclei are necrotic. CA indicates cartilage.
fig. 30. Enlarged View of the area outlined in figure 5. The fissula is much larger in this fetus than in the one shown in figure 29. The tissue is very necrotic and vacuolated. The fissula enlarges by destruction of the surrounding cartilage shown at lower left corner of the figure.
fig. 31. Enlarged View of the area outlined in figure 9. Active fibroblasts are seen invading the fissula. A blood vessel (B.V.) is seen.
fig. 32. Enlarged Vi€W of the area outlined in figure 13. The tissue of the fissula is denser, with fine intercellular fibers between the fibrohlasts. Vacuolated areas around some of the cells are noted. A large blood vessel (B.V.) is seen near the upper part of the figure and several smaller vessels at the bottom.
Fig. 33. Fetus 86; size, 50 mm. crown-rump length; age, about 10 weeks. Anterior view of a model of the otic labyrinth showing the development of the periotic tissue and spaces and the fissula ante fenestram.
Fig. 34. Posterior View of the same model shown in figure 33.
In figures 33 and 34, vesti. indicates vestibule; f.a.f., fissula ante fenestram; S c. 'vesti., scala vestibuli; Sc. tym., scala tympani; imp. Sta.p., impression of stapes; Sac. end., endolyrnphatic duct; Sm:., sacculus; Ut1'z'c., utriculus; Duct. coch-., cochlear duct.
Summary
The tissue of the fissula arises, as does the periotic tissue, from precartilage. 1. The precartilage undergoes a regressive change to form a vacuolated reticular tissue. 2. With the entrance of blood vessels from the middle ear, the tissue undergoes new growth, filling the fissula with fibroblasts which form a fine fibrillar connective tissue. 3. At the periphery of the fissula this connective tissue changes to cartilage, whereas in the center of the fissula a loose vacuolated vascular connective tissue is formed.
Relation of the Fissula Ante Fenestram to the Annular Ligament of the Stapes
From the accompanying figures it will be noted that one end of the middle part of the fissula comes to lie in close relation to the annular ligament of the stapes foot~plate. In most cases the tissue of the stapes ligament is continuous with the tissue of the fissula. The connective tissue cells of the annular ligament are arranged in a radial direction, or the long axes of the fibroblasts lie at right angles to and stretch across the stapes joint. These cells retain this same direction as they continue into the fissula, which communicates at a right angle with the stapes joint. This is well shown in figures 8 and 28. In a small percentage of cases the communication with the stapes joint does not occur. This is shown in figure 12. In figure 20 the communication is poorly formed.
No attempt is made in this paper to explain this communication of the fissula with the stapes joint; it is intended only to present this relationship as an anatomic fact. However, it may be well to bear this relationship in mind in studying otosclerosis, since it usually occurs in this region and often causes ankylosis of the stapes.
Development of the Capsule in the Region of the Fissula
It has already been stated that the otic capsule changes from precartilage to cartilage about from the eighth to tenth week of intra~ uterine life. Ossification starts at certain designated areas at about 16 weeks. By 24 weeks the capsule is well ossified, except certain areas in the region of the canals. (For the complete history of ossification see my previous articles”)
The portion of the capsule surrounding the fissula ante fenestram ossifies at about from 22 to 23 weeks. It is one of the last areas to ossify. The only areas which ossify later are in the canal region and a ring around the oval window. The structure of the newly ossified otic capsule around the fissula is shown in the 210 mm. fetus (figs. 13 to 16). The only bone formed at this time is intrachondral bone 1 and some perichondral bone (fig. 16). It is at this time that the peripheral portion of the tissue of the fissula changes to cartilage. Intrachondral bone (so-called cartilage islands) grows no more after once being formed, but endochondral bone forms around it in subsequent development. figures 21 to 24, taken from a 305 mm. fetus, show the further endochondral ossification around the fissula. The dark-staining intrachondral bone is surrounded by the light areas of endochondral bone. It is this endochondral bone and its subsequent growth that complete the ossification. In a full—term fetus, as shown in figures 25 to 28, the bone marrow spaces are greatly reduced owing to the growth of the endochondral bone around the intrachondral bone. The formation of periChondral bone also goes on and accounts for the thickening of the wall of the capsule.
Fig. 35. Model of part of the internal ear showing an otosclerotic area (08). The area lies anterior to the stapes (ST) and caps over the vestibular end of the fissula ante fenestram (1’.z'1.F.).
Fig. 36. Scction through the oto-sclerotic area shown i11 figure 35. The dark bone (O.B.) is otosclerotic bone. It surrounds the fissula ante fenestram (F./1.F.). The intrachondral hone (I.B.) is more resistant to the otosclerotic invasion than the endochondral bone (E.B.).
The serial sections from which the model was made (figure 35) and the photograph was taken (fig. 36) belong to Dr. J. Gordon \=’Vils0n, who loaned them to me for study.
The completion of the ossification varies considerably in different fetuses, but the 210, 305 and 370 mm. fetuses show representative stages in the process. In the 290 mm. fetus shown in figures 17 to 20, the ossification is much further advanced and is physiologically more mature than that in the 305 mm. fetus. Either the 290 mm. fetus was a much older fetus than its size indicated, or else the ossification progressed much more rapidly than in other fetuses, for structurally the bone looks like that in a full-term fetus.
The otic capsule around the fissula, then, is not different from the otic capsule around the cochlea or vestibule, except that the cartilage lining the bone around the fissula does not ossify but remains throughout life.
Comment
A review of the literature reveals three interpretations regarding the fissula ante fenestram: 1. Mayer, Kosokabe and others regarded the fissula as a synchondrosis or a place where the two processes of chondrification of the vestibular and cochlear capsule meet but are separated by perichondrium. 2. Perozzi regarded the perichondrium as a place for growth, which thus permits the cochlea to describe its spiral. 3. Guggenheim regarded the fissula as a vestige of the canalis fenestrae ovalis of the frog and therefore as “predisposed to mesenchymal invasion.”
The embryologic findings outlined in this paper do not suggest the first and second views. Certainly the tissue of the fissula is not of a simple perichondral type. VVhile there is condensation of the tissue of the fissula where it borders the cartilage, the central part varies from a loose vascular reticular type to a loose periotic type.
The third View is arrived at, so far as can be ascertained, from Guggenheim’s papers, through philosophical deduction. This interpretation may be correct. However, I have never found a fissula either in the embryo or in the adult of any of the laboratory animals, from rats to monkeys, or in pigs and cows. Hyrtl claimed to have found something 1ike.the fissula in an antelope and a hyena. If the fissula .is a rudimentary structure, it should be present at least in some of the intermediate animals between the frog and man.
The second point made by Guggenheim, that the fissula is a rudimentary structure and is predisposed to mesenchymal invasion, is based on the fact that otosclerosis usually attacks the otic capsule in the region of the fissula. This concept is open to question. While it is a fact that otosclerosis is very apt to start in the region of the fissula, it does not as a rule attackthe tissue of the fissula or the supposedly more primitive intrachondral bone (so-called cartilage islands) first, but rather the bone surrounding these. I have had occasion to examine a number of Dr. J. G. Wilson’s preparations of otic capsules with pathologic changes in the bone; the patholo-gic process had eroded enlarged areas of endochondral bone, and the so—called cartilage islands were slowly effected and were protruding into the excavated area. This is in part shown in figure 36, in which a process of the intrachondral bone (I.B.) is seen projecting into the otosclerotic area. In one of Dr. Wilson’s specimens of otosclerosis in which the otosclerotic patch involved most of the capsule in the region of the fissula, the fissula near the middle ear was destroyed, but the vestibular end of the fissula was unchanged and extended a considerable way into the oto-sclerotic area. This is pictured in figures 35 and 36. It appears that the perichondral bone and the endochondral bone are most predisposed to pathologic change, whereas the fissula and the intrachondral bone are more resistant. This observed pathologic sequence is contrary to- what one would deduce from philosophic reasoning and shows that abstract reasoning will not necessarily reveal scientific fact.
Conclusion
While I do not intend entirely to discredit the former views regarding the fissula, I wish merely to present that my observations on fifty-five human fetuses show that the vestibular end of the fissula ante fenestram is an outpouching from and develops in a manner similar to the periotic tissue. As this developing fissula pushes through the capsule toward the middle ear its development is somewhat altered and is speeded up by the entrance of blood vessels irom the side of the capsule near the middle ear. Whether the, blood vessels within the fissula of the fetus throw any light on the function of the fissula cannot be asserted until it has been established whether these vesselsremain throughout adult life.
References
- ↑ Bast TH. Ossification of the otic capsule in human fetuses. (1930) Contrib. Embryol., Carnegie Inst. Wash. 121, Publ. 407, 53-82.
- ↑ Huschke, E., in Sommerringz Lehre von den Eingeweiden und Sinnesorganen, Leipzig, Leopold Voss, 1844, vol. 5, p. 828.
- ↑ Hyrtl, Joseph: Verglichend-anatomische Untersuchungen iiber das innere Gehiirorgan des Menschen und der Saugethiere, Prag, F. Ehrlich, 1845.
- ↑ Siebenmann, Friedrich: Die Korrosions~Anatomie des menschlichen Ohres, Munich, I. F. Bergmann, 1890.
- ↑ Perozzi, Luigi: Contributo all’etiologia dell’otospongiosi, Arch. ital. di otol. 27:85, 1916.
- ↑ Mayer, Otto: Untersuchungen iiber die Otosklerose, Vienna, Alfred Holder, 1917.
- ↑ Perozzi, Luigi: Ricerche anatomica intorno alla capsula del labirinto, Arch. ital. di otol. 31:214, 321 and 353, 1920; 32:46 and 65, 1921.
- ↑ Kosokabe, H.: Ueber die Knorpelflugen in der Labyrinths Kapsel, Stuttgart, Alfred Kernen, 1922.
- ↑ Claoué, R.: Oreille interne: Etude anatomopathologique, clinique, microscopique et expérimentale, Paris, Norbert Malo-ine, 1927.
- ↑ Guggenheim, L. K.: The Cause of Otosclerosis, Ann. Otol., Rhin. & Laryng. 4l:1l49 (Dec.) 1932.
- ↑ Streeter, George L.: The Histogenesis and Growth of the Otic Capsule and Its Contained Periotic Tissue—Spaces in the Human Embryo, Contrib. Emb-ryol. (no. 20) 7:5, 1918.
- ↑ Foley, I. 0.: The Cytological Process Involved in the Formation of the Scalae of the Internal Ear, Anat. Rec. 49:1, 1931.
- ↑ A word of explanation is necessary. Although the extent of the vestibule, scala tympani and scala vestibuli in my model is similar to- that pictured by Streeter, the ages are different. Streeter’s specimen was from a 100 mm. fetal ear and mine, from a 50 mm. fetal inner ear. Streeter modeled the periotic spaces when quite fully formed, whereas I modeled all the periotic tissues which showed only a small degree of space formation. Had Streeter modeled the young periotic tissue in his 100 mm. human fetal ear, his model would have shown much more of the periotic labyrinth. Or had I modeled only the fully excavated areas as Streeter did, my model would show only a small space between the stapes and the saccule.
Cite this page: Hill, M.A. (2024, April 18) Embryology Paper - Development of the otic capsule 2. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Development_of_the_otic_capsule_2
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G